- 1Department of Pathology, Kamuzu University of Health Sciences, Blantyre, Malawi
- 2Molecular Core Laboratory, Blantyre Malaria Project, Blantyre, Malawi
- 3Department of Infectious Diseases, The Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia
- 4Department of Microbiology and Immunology, The Peter Doherty Institute for Infection and Immunity, Melbourne, VIC, Australia
Antibodies against Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) on infected erythrocytes (IEs) play a central role in naturally acquired protection against cerebral malaria (CM), yet the determinants of effective humoral immunity remain incompletely defined. We review evidence from seroepidemiological, functional, and mechanistic studies demonstrating that antibodies to endothelial protein C receptor (EPCR)‐binding cysteine-rich interdomain regions (CIDR)α1 and Duffy binding-like (DBL)β domains associated with dual EPCR and intercellular adhesion molecule 1 (ICAM1) binding correlate with reduced risk of CM, while responses to rosetting‐associated domains (DBLα, CIDRγ) and other domains are less well characterized. We synthesize findings on antibody kinetics—early, durable responses to Group A variants versus delayed, transient responses to Groups B and C—and on effector mechanisms including opsonic phagocytosis, complement activation, and Fc glycosylation. We highlight methodological challenges in measuring PfEMP1‐specific immunity, such as antigenic switching, differences between assays using single domains and native protein on IEs, and the need for physiologically relevant 3D vascular models. Finally, we identify key research priorities: mapping immunodominant epitopes across variant repertoires; longitudinal cohort studies to track antibody maturation and post‐translational modifications; and the development of broadly inhibitory monoclonal antibodies. Addressing these gaps will be critical for designing vaccines and therapeutics that harness protective antibody functions to prevent CM.
1 Introduction
Malaria remains a significant global health challenge, with over 200 million cases annually, disproportionately affecting resource-constrained regions, mostly in sub-Saharan Africa (1). Vulnerable populations, including children under 5 and pregnant women, bear the greatest burden, with severe consequences like high mortality rates, neurological complications, and adverse pregnancy outcomes. Malaria presents across a broad clinical spectrum, ranging from asymptomatic parasite carriage to uncomplicated malaria (UM), and progressing to severe, life-threatening disease. Severe malaria (SM) includes complications such as severe malarial anemia (SMA) and neurological syndromes like cerebral malaria (CM). CM, the most severe form of Plasmodium falciparum (P. falciparum) malaria, is clinically defined as unarousable coma not attributable to other causes in the presence of parasitemia (1).
P. falciparum, the most virulent species of malaria parasites infecting humans, is responsible for most of the severe disease and mortality (1, 2). A key contributor to the virulence of P. falciparum is the binding of infected erythrocytes (IEs) to the vascular endothelium, causing sequestration of the parasite in the microvasculature of various tissues. By preventing splenic clearance, sequestration aids parasite survival. Sequestration of IEs in the brain’s microvasculature is a defining feature of CM pathogenesis (3).
Sequestration is mediated by Plasmodium falciparum Erythrocyte Membrane Protein 1 (PfEMP1), a species-specific, highly polymorphic protein that is predominantly expressed on the surface of IEs during the blood stage of infection (4, 5). PfEMP1 proteins are encoded by approximately 60 var genes per genome that undergo frequent recombination to enhance their antigenic diversity and immune evasion capabilities (4, 6). Though diverse, the var genes that encode most PfEMP1s can be grouped based on upstream promoter sequences (UPS), chromosomal location, and transcription direction (Figure 1A) into 3 main groups: Group A, B, C, and 2 intermediary groups: Group B/A and UPS B/C.
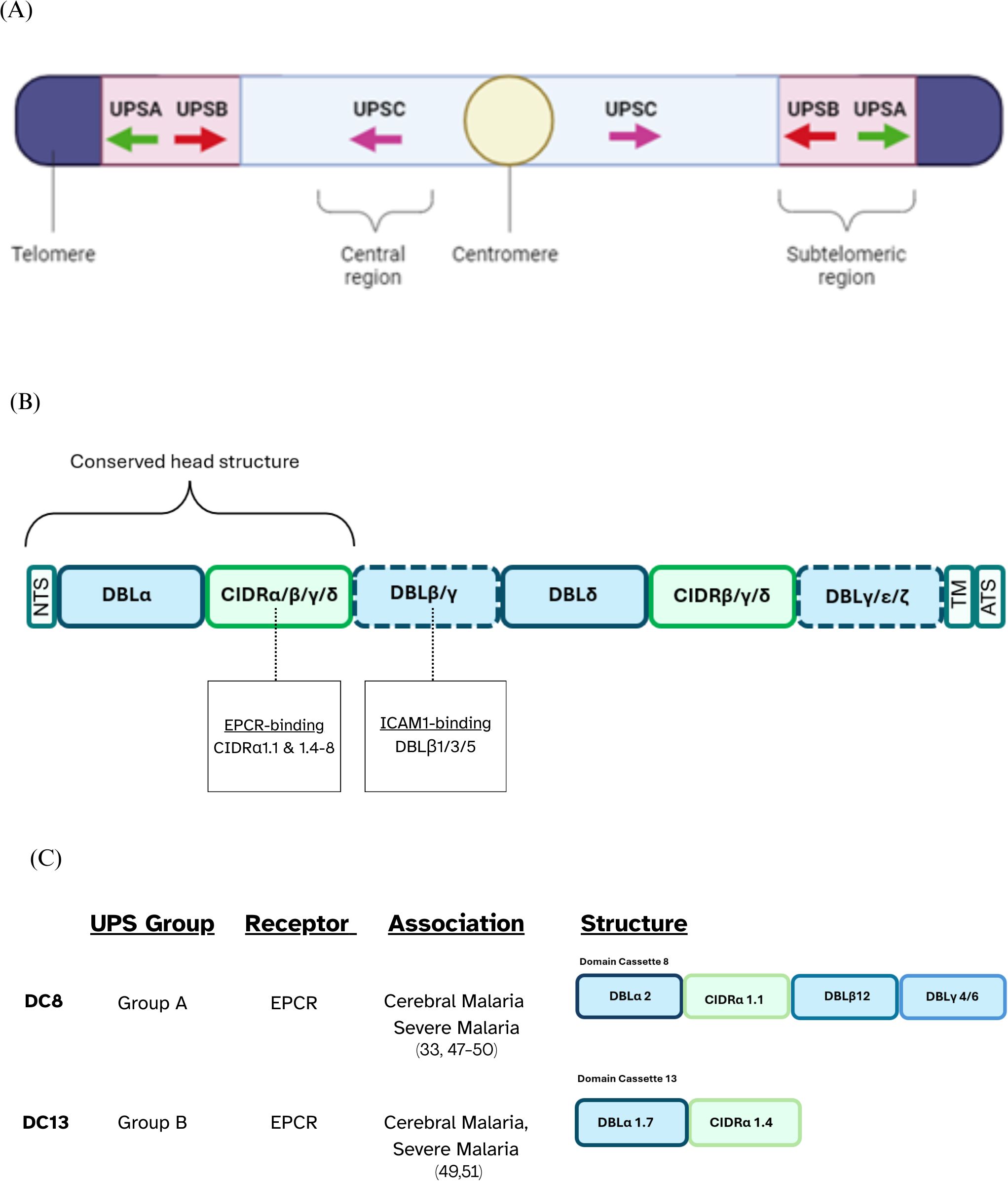
Figure 1. PfEMP1 classification overview. (A) UPS classification illustrating the chromosomal locations of var genes along with the direction of their transcription, depicted using arrows. (B) Schematic representation of the typical PfEMP1 structure, with dotted lines indicating domains that may or may not be present depending on the specific variant. (C) Schematic representation of domain cassettes 8 and 13 and their UPS group and binding phenotype.
The extracellular ectodomain of PfEMP1 has a modular structure, primarily composed of 2–10 tandemly arranged protein domains named the Duffy Binding-Like domains (DBL) and the Cysteine-Rich Interdomain Regions (CIDR). PfEMP1’s antigenic diversity is driven by the variation in the number, arrangement and sequences of the DBL and CIDR domains present in the ectodomain of different PfEMP1s (4, 7). Sequence similarities allow for the classification of DBL domains into distinct classes, including α, β, γ, δ, ϵ, and ζ, while CIDR domains are grouped into classes such as α, β, γ, δ, and pam. Each of these major classes is further subdivided into subclasses denoted by numbers, e.g., DBLα1 (5). Over 95% of PfEMP1s feature a head structure composed of tandem DBLα and CIDR domains adjacent to the N-terminal segment (NTS) (5). The central region of PfEMP1 often contains multiple, alternating DBL and CIDR domains, followed by a transmembrane region and an acidic-tail segment at the C-terminus (4, 5, 8) (Figure 1B).
There are specific combinations of domains which are seen in different PfEMP1s. Domain Cassettes (DCs) are defined as structural alignment of two or more adjacent DBL and CIDR domains within PfEMP1 proteins that frequently occur together in at least three P. falciparum genomes (5). Some DCs are known to bind to specific endothelial receptors, and it is possible that these conserved arrangements have evolved to facilitate a survival advantage for the parasite. For example, DC8 which consists of DBLα2 – CIDRα1.1 – DBLβ12 – DBLγ4/6 binds to Endothelial Protein C Receptor (EPCR) via a conserved CIDRα1.1 domain (Figure 1C).
The clinical manifestations of P. falciparum infection are influenced by the parasite’s ability to bind specific host receptors, which differ in abundance and distribution across tissues. While many of these receptors are found on the vascular endothelium, others involved in rosetting or placental malaria are located on uninfected erythrocytes and placental syncytiotrophoblasts, respectively. For instance, intercellular adhesion molecule 1 (ICAM1) is abundant in the brain, chondroitin sulfate A (CSA) is found on placental syncytiotrophoblasts, and Cluster of Differentiation 36 (CD36) is widely distributed throughout many tissues in the body (9–11). The ability of a particular PfEMP1 variant to bind to specific receptors determines where IEs sequester, driving organ-specific complications. This selective binding is critical to the manifestation of CM, in which PfEMP1 variants with high affinity for brain-expressed receptors promote sequestration in the microvasculature of the brain (4, 12, 13).
1.1 The pathogenesis of cerebral malaria
Despite standardized diagnostic criteria, distinguishing true CM from other causes of coma remains difficult in high-transmission settings, where incidental parasitemia is prevalent. The clinical definition of CM (Blantyre coma score ≤2, parasitemia, and exclusion of other causes (1)) misclassifies approximately a quarter of cases, which have alternative causes like meningitis, highlighting the prevalence of incidental parasitemia (3). Pathophysiologically, CM can be categorized into 3 subtypes: sequestration only, sequestration with microvascular pathology, and no sequestration—the latter likely representing non-malarial causes of comas (3, 14). Retinal findings, such as hemorrhages, vascular whitening, and other vascular changes, are strongly associated with sequestration in true CM, while the absence of these points to non-malarial causes of coma (3). These features make fundoscopic examination a practical, non-invasive tool to confirm CM (15, 16).
In CM, pathology is partly driven by blood-brain barrier breakdown, a process involving multiple interrelated mechanisms. Sequestration of IEs, inflammation from inflammatory cytokines, endothelial activation, and dysregulated coagulation leading to microvascular thrombosis can all contribute to tight junction disruption (Figure 2) [Reviewed in Jensen et al. (17)]. The expression of endothelial adhesion receptors is upregulated by two responses to pathogen associated molecular patterns (PAMPS) released during schizont rupture, namely the sensing of PAMPs by Toll-like receptors and TNF production by macrophages. Thus, the upregulation of endothelial adhesion receptors leads to further sequestration of IEs (18, 19). Sequestration activates endothelial cells, which in turn produce chemokines that recruit leukocytes. These leukocytes further amplify local inflammation by releasing additional chemokines (20). Cytotoxic T-cells are also recruited and can recognize endothelial-bound antigens via MHC-I sensing (21). They induce endothelial cell apoptosis through Granzyme B (22), compromising the integrity of the blood–brain barrier. During pathogenesis, a procoagulatory microenvironment develops due to two main factors: first, sequestration reduces the availability and activation of Protein C (23); second, activated endothelial cells release Von Willebrand factor, which activates platelets (24). These activated platelets then aggregate and bind to endothelial receptors or IEs (25). In vitro and ex vivo data suggests that IEs expressing ICAM1 and EPCR dual-binding PfEMP1 variants are internalized by brain endothelial cells via an ICAM1–dependent mechanism, leading to endothelial cell swelling and impaired BBB integrity (26). Additionally, focal hemorrhages further weaken the blood-brain barrier, causing plasma and protein leakage into brain tissue, which promotes cerebral swelling, a severe and potentially fatal complication of CM (27, 28). Sahu et al. (29) showed that increased parasite biomass (as indicated by higher PfHRP2 levels) and the elevated expression of EPCR-binding PfEMP1 variants are key determinants driving brain swelling in CM.
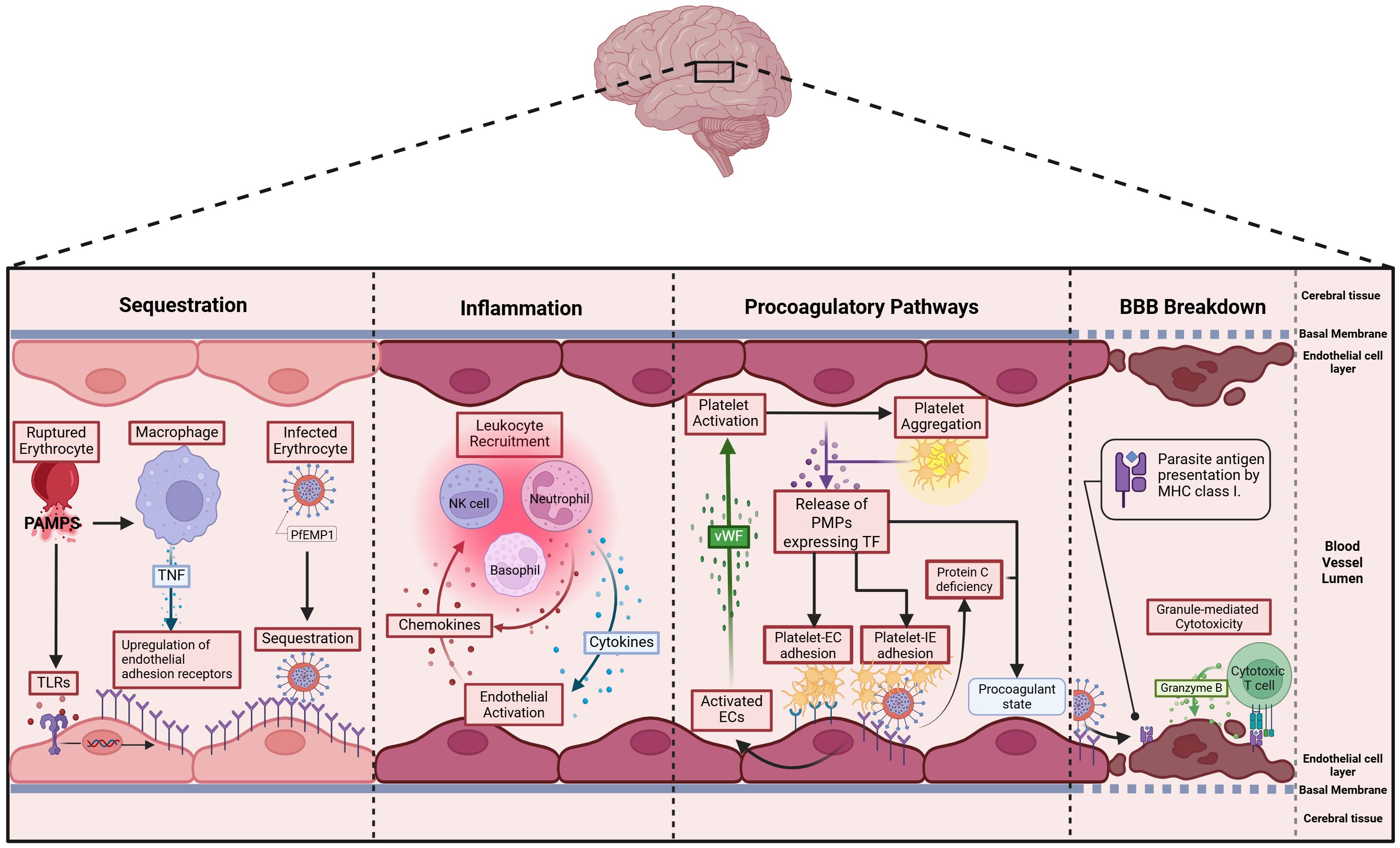
Figure 2. Postulated pathophysiology of cerebral malaria: 1. Sequestration – P. falciparum pathogen-associated molecular patterns (PAMPs) activate Toll-like receptors (TLRs) on endothelial cells and macrophages, upregulating endothelial adhesion molecules. TNF from macrophages amplifies this, promoting PfEMP1-mediated IE binding and further endothelial activation in the brain (17, 18). 2. Inflammation – Activated endothelial cells (ECs) secrete chemokines that recruit leukocytes, which in turn release inflammatory cytokines like TNF, further promoting sequestration. This cycle amplifies inflammation as recruited immune cells continue to produce cytokines and chemokines, sustaining the response (19). 3. Procoagulatory pathways – Activated ECs release von Willebrand Factor (vWF), which binds to glycoproteins on platelets, triggering their activation (20). Activated platelets aggregate and express various surface receptors—including tissue factor—that enhance adhesion to both ECs and IEs, forming multimeric complexes (21). Additionally, sequestration reduces the abundance and activation of protein C (22). 4. Blood-Brain Barrier (BBB) Breakdown - Cytotoxic T cells infiltrate the brain’s microvasculature, recognize antigens on endothelial cells via MHC-I (23), and release granzyme B to trigger apoptosis (24). This cytotoxic activity disrupts the blood-brain barrier, contributing to vasogenic oedema and neurological damage, and may result in brain swelling (25).
2 Structural diversity of PfEMP1s associated with cerebral malaria and their host receptor interactions
2.1 Sequestration
Sequestration in the cerebral microvasculature is a hallmark feature of CM. There isn’t a single PfEMP1 variant exclusively linked to the development of CM. Instead, multiple PfEMP1 variants are differentially transcribed in SM and CM (30–32). Given the high diversity of PfEMP1, research has focused on the binding phenotypes of different PfEMP1. Among these, EPCR and ICAM1-binding variants can bind brain endothelial cells in vitro and often associated with CM, making them the focus of the following discussion. However, they are not always expressed in CM and may not be the only PfEMP1 variants involved in its pathology.
Many studies have observed an association between infections caused by P. falciparum parasites that express var genes encoding PfEMP1 proteins from Group A and B/A and SM including CM (26, 30, 33–36). The receptor-binding phenotypes of some Group A and B/A PfEMP1 proteins can be directly linked to the pathogenesis of CM. Some PfEMP1 variants are capable of simultaneously binding to both EPCR and ICAM1 (37, 38) and CM is associated with P. falciparum expressing dual EPCR- and ICAM1-binding PfEMP1 variants, as shown by both upregulation of the corresponding var genes (39, 40) and IEs which bind microvascular endothelium (38, 41). Notably, these “dual-binding” proteins are primarily found in Group A and Group B/A (38).
EPCR is a receptor located on vascular endothelial cells, and PfEMP1 variants with CIDRα1 domains can bind EPCR, a feature associated with SM and CM (33, 40, 42). Phylogenetically, CIDRα1 sequences are grouped into subclasses (CIDRα1.1–1.8), of which CIDRα1.1 and 1.4–1.8 bind EPCR, while CIDRα1.2 and 1.3 do not and may occur only in pseudogenes.
DBLβ1/3/5 domains are known to bind ICAM1 (38, 43–45). In dual-binding PfEMP1 variants, ICAM1-binding DBLβ domains are downstream of EPCR-binding CIDRα1 domains (38). The “DBLβ motif”, a short amino acid sequence within DBLβ domains of specific PfEMP1 proteins, is associated with a dual binding PfEMP1 to EPCR and ICAM1. This motif is present in Group A and some Group B/A variants and is transcribed by isolates from children with CM (26, 46). Although transcript levels of var genes that encode both Group A and Group B PfEMP1 were elevated in CM cases (41), it remains unclear what fraction of these transcripts correspond to EPCR–ICAM1 dual binders versus ICAM1–only binders. In other words, it is not yet known whether ICAM1–binding PfEMP1 that lack EPCR binding are associated with disease. To address this, future studies should directly compare var gene transcription and surface expression of ICAM1–only versus dual-binding PfEMP1 in cerebral and non-cerebral cases, and evaluate these isolates' adhesive capacity under physiological flow conditions.
DC13 is an EPCR-binding domain cassette found in certain Group A PfEMP1 proteins, which is composed of DBLα1.7 and CIDRα1.4 (Figure 1C) (9, 43). High transcription of var genes encoding DC13 is observed in both African children and Indian adults with SM, including CM, suggesting that this association is not geographically restricted (47–50). Additionally, DC13-encoding var genes show higher transcription levels in CM compared to SMA, reinforcing their stronger link to CM (33, 48, 50–52). DC8 is an EPCR-binding PfEMP1 variant, primarily found in Group B PfEMP1 proteins, consisting of four domains: DBLα2, CIDRα1.1, DBLβ12, and DBLγ4/6 (4). Although DC8 transcription is elevated in CM it is also upregulated in individuals with broader SM manifestations (49, 51).
In CM, the transcription of DBLα from Group A var genes is upregulated compared to UM (30, 36). This includes DBLα1.1, which is typically adjacent to a CIDRα1.4/6/7 domain that facilitates binding to EPCR (4, 53) and DBLα1.6/8 domains that typically feature in rosetting types, which will be discussed in the following section (4, 53).
Platelet and endothelial cell adhesion molecule 1 (PECAM-1) binds PfEMP1 on infected erythrocytes. In vitro studies demonstrate adhesion to recombinant and transfected PECAM-1, and many field isolates, including those from children with severe malaria, show measurable but generally low binding (54). PECAM-1 is also expressed in the microvasculature of the human brain (55), providing a biologically plausible site for such interactions. Genetic association studies have further linked PECAM-1 polymorphisms with susceptibility to cerebral malaria, supporting its potential relevance to severe disease (56, 57). However, unlike EPCR, direct evidence from human autopsy material demonstrating colocalization of sequestered parasites with PECAM-1 in brain microvessels is lacking (23). This gap highlights PECAM-1 as a plausible but unconfirmed contributor to cerebral sequestration, and an important target for future exploration.
2.2 Rosetting
PfEMP1 not only mediates sequestration but can bind IEs to uninfected erythrocytes, forming clusters in a process known as rosetting. Isolates from individuals with CM have been shown to form rosettes in vitro (58–60). However, the contribution of rosetting to pathogenesis remains unclear due to conflicting findings, which may reflect geographical differences. In sub-Saharan African cohorts, significantly higher rosetting rates were observed in CM compared to UM (58, 61), and rosetting was elevated in SM compared to UM, regardless of syndrome subtype (59, 60). In contrast, a study from Papua New Guinea found similar rosetting rates in CM and UM (62), while a Thai study reported the highest rosetting frequencies in CM compared to both UM and non-cerebral SM (63).
The mechanisms by which rosetting might contribute to malaria pathogenesis remain unclear. One hypothesis is that rosettes impede phagocytosis of IEs. The larger size and complex structure of rosettes can physically hinder phagocytes from engulfing infected cells (64, 65). When rosetting is disrupted, parasites become more susceptible to phagocytosis, but intact rosettes require multiple phagocytes for clearance, potentially leading to phagocyte exhaustion in hyperparasitemic malaria patients (66).
Assessing the exact contribution of rosettes to disease severity is challenging, as they are difficult to observe in autopsy samples, making it hard to confirm their presence and impact in affected organs. Furthermore, rosetting involves multiple host factors, such as ABO blood group and complement components (67–69), which vary between individuals and populations. There may not be a universal mechanism linking rosetting to severe disease, as both host and parasite factors contribute to its heterogeneity. These limitations underscore the need for innovative approaches, such as 3D microvessels with precise control over vessel architecture and blood flow (70), to better replicate in vivo rosette dynamics.
Most rosetting variants are encoded by Group A var genes and typically feature domain combinations—such as DBLα1.5/6/8 paired with CIDRβ/γ/δ—that form the rosetting-associated head structure (4, 71–75). Given that transcription of these Group A var genes is upregulated in CM (26, 33, 35), this supports the idea that rosetting may contribute to pathogenesis. However, several knowledge gaps remain. First, not all var genes that give rise to the rosetting phenotype have been definitively identified, and it is unclear whether currently known associations capture the full diversity of rosetting PfEMP1 variants. Second, transcriptomic data provide only partial insight into protein expression; mRNA levels don’t always translate to surface-expressed PfEMP1, and the relationship between var gene transcription and PfEMP1 display remains incompletely understood. Third, parasites sampled from peripheral blood may not reflect the phenotype of sequestered IEs in critical organs, where rosetting is presumed to exert its pathological effects. The second and third challenges, although discussed in this section, represent broader obstacles to understanding all PfEMP1 and not only rosetting variants.
3 Antibodies to PfEMP1 in cerebral malaria
Antibodies targeting PfEMP1 are likely essential for malaria immunity as they could prevent the sequestration of IEs and facilitate IE clearance. PfEMP1 appears to be the immunodominant surface antigen on IEs, as suppression of PfEMP1 expression significantly reduced IgG binding by plasma from malaria-exposed individuals (76). Effective immunity may result from acquisition of antibodies to a broad range of PfEMP1 variants, or from the acquisition of strain-transcending antibodies against specific binding phenotypes (75, 77).
Individuals living in malaria-endemic regions develop naturally acquired immunity to P. falciparum through repeated infections, leading to the production of antibodies against key parasite antigens, including PfEMP1 (78, 79). This immunity is associated with reduced disease, with adults and older children developing a broader, more robust antibody response to PfEMP1 variants due to cumulative exposure (80, 81) The best evidence for the protective effect of PfEMP1 antibodies comes from placental malaria. Pregnant women develop antibody to the PfEMP1 VAR2CSA in a gravidity dependent manner, and development of these antibodies is associated with declining prevalence and density of placental malaria infection (Reviewed in Rogerson et al. (82)).
Studies indicate that PfEMP1-specific antibodies develop sequentially in response to different parasite variants. The earliest acquired antibodies target Group A PfEMP1 variants, which are associated with SM, followed by Group B and C variants, which are linked to UM (78, 79, 83). For example, Tessema et al. (84) demonstrated that young Papuan New Guinean children, age 1–3 years old, mainly develop antibodies to Group A PfEMP1, indicating early infections involve these variants. With age and repeated exposure, their immunity broadens to include Group B/C PfEMP1s, potentially reflecting progressive immunity to SM (84).
Sequential acquisition of antibodies toward Group A, B and C variants is true for CIDRα domains that bind to EPCR and CD36. In malaria-endemic regions, antibodies to CIDRα1 are present at birth, due to maternal antibody transfer, but decline by around six months of age (85). Children then begin to acquire IgG antibodies against EPCR-binding CIDRα1 variants, such as CIDRα1.7 and CIDRα1.8, earlier than IgG targeting CD36-binding variants (86, 87). Transcription of CIDRα1.7 was particularly associated with brain swelling in Malawian children (40). CIDRα1.7 elicited the highest IgG antibody levels among all the CIDR domain variants tested in young children and a larger proportion of children in the cohort had detectable IgG to CIDRα1.7 compared to other variants (86). This early antibody acquisition may reflect a parasite fitness advantage from expression of EPCR‐binding PfEMP1 variants, which mediate microvascular adhesion (33, 42, 88). PfEMP1 variants that are both common in circulating parasites and initially unopposed by antibodies are favored in early infection. As children develop specific IgG (such as against CIDRα1.7) these antibodies target the most widely circulating variants, gradually eroding their fitness advantage (89, 90).
Young children are highly vulnerable to CM, likely because of the limited antigenic breadth and functional capacity of their antibodies, predisposing them to high parasite burdens (90). Travassos et al. (91) highlight syndrome-specific differences: children with CM exhibited more frequent and wider gaps in seroreactivity than those with SMA and UM.
3.1 Antibody responses to EPCR-binding PfEMP1
Children recovering from SM —including CM and SMA—exhibit increased PfEMP1 antibody levels during convalescence, particularly targeting EPCR-binding CIDRα1 domains (91, 92). These findings suggest that episodes of severe disease may drive the acquisition of immunity to EPCR-binding PfEMP1 variants (91). Notably, Nunes-Silva et al. (93) reported that children with CM failed to boost antibody responses against parasites expressing the EPCR-binding PfEMP1 VAR19 or recombinant proteins containing VAR19’s EPCR binding CIDRα1.1 domain following infection. While this appears to contrast with previous findings of post-infection increases in antibody to EPCR-binding PfEMP1 variants, the difference may reflect the use of a single EPCR-binding PfEMP1 in the study (93). The apparent lack of boosted immunity to that variant may simply reflect antigenic differences between the PfEMP1s circulating in Benin and the variant tested. However, while convalescent children with CM develop a boost in IgG to EPCR-binding PfEMP1 domains (91, 92), they remain at increased risk of subsequent SM episodes (94). This may reflect immunological gaps, as Travassos et al. (91) demonstrated that children with CM lack IgG breadth to certain PfEMP1 subsets, and Rambhatla et al. (92) showed that convalescent boosts can be non-broadly reactive or transient. The persistence of these antibody “blind spots” may underlie why even boosted responses do not necessarily translate into durable protection from recurrent SM.
Across diverse populations, antibodies to the EPCR-binding CIDRα1 domains of PfEMP1 are significantly higher in UM than in SM, including CM, suggesting that these antibodies may play a protective role (76, 95, 96).
Antibody specificity plays a critical role in mediating protection against severe disease. In one study of IgG levels to 32 PfEMP1 domains—selected based on their differential transcription in SM compared to UM— individuals with UM had significantly higher IgG against 15 of 22 SM-associated PfEMP1 domains compared to those with SM (95). Of the domains eliciting significantly higher IgG responses, CIDRα1.6 was one of the three PfEMP1 domains that most effectively distinguished uncomplicated from severe cases (95). This suggests that IgG to CIDRα1.6 may contribute to protection from SM. Another study, which focused specifically on EPCR-binding DC13 (DBLα1.7-CIDRα1.4) found no significant differences in IgG1 nor IgG3 responses to DC13 between CM and UM (96). However, these IgG1 and IgG3 levels to DC13 did significantly increase in the CM cohort from admission to convalescence (96). Similarly, Kessler et al. (97) assessed IgG seroreactivity to 61 3D7-derived PfEMP1 domains using a proteome microarray in children with retinopathy-positive CM or UM and found no differences in antibody responses to EPCR-binding DC8 domains between the groups (97). This suggests that not all antibodies to EPCR−binding domains confer equal protection and echoes the “gaps in seroreactivity” described by Travassos et al. (91).
Retinopathy in CM provides a non-invasive window into brain pathology, reflecting both microvascular sequestration and hemorrhagic events occurring in the cerebral microvasculature (3, 16). Additionally, Joste et al. (98) reported that among children with CM, those with retinopathy had significantly lower IgG responses to the EPCR-binding CIDRα1.4 than children without retinopathy, even though the expression of var genes encoding CIRDα1.4 binding domains and in vitro cytoadherence levels of isolated IEs to EPCR were similar between the groups (98). Although antibodies to EPCR-binding CIDRα1.4 domains did not differ overall between all CM cases compared to UM (98), their lower levels in CM children with retinopathy compared to those without suggest a potential role in modulating severity, rather than conferring outright protection from CM.
The CIDRα1.1 domain of DC8 is known to bind to EPCR, however, other domains of DC8 may also contribute to vascular adhesion, via additional receptors. Recombinant DC8 exhibited stronger binding to EPCR and to an immortalized human microvascular endothelial cell line compared to the CIDRα1.1 domain of DC8 alone (93). Notably, antibodies targeting the multi-domain DC8 protein fully blocked binding to recombinant EPCR but only partially inhibited endothelial cell adhesion (93). This implies that domains of DC8 other than CIDRα1.1 could interact with unknown receptors, highlighting alternative adhesion pathways contributing to IE binding in CM (93). This notion of alternative adhesion pathways is further supported by findings from a separate study, which reported that children with UM had significantly higher IgG reactivity to DBLα2 and DBLγ6 domains (both of which are part of DC8) compared to those with CM (76). In contrast, IgG responses to CIDRα1 (the EPCR-binding domain) and DBLβ12 did not differ between the groups (76). These findings suggest that antibodies targeting only CIDRα1.1 may not block all adhesion mechanisms in vivo, and that antibodies against adjacent domains such as DBLα2 and DBLγ6 may contribute to protection against CM by interfering with alternative binding interactions beyond EPCR. However, further research is required to confirm these hypotheses.
3.2 Antibody responses to ICAM1-binding PfEMP1
Acquisition of antibodies to ICAM1–binding PfEMP1 domains differs between Group A and Groups B and C. Olsen et al. (79) report that in healthy children from Ghana, Group A DBLβ domains, which are more conserved and often associated with CM pathogenesis, tend to elicit early and sustained antibody responses, as seen in longitudinal cohorts (79, 99). These responses are characterized by prompt seroconversion and evidence of long-lived antibody memory, while responses to Groups B and C develop later and increase gradually with repeated exposure (79).
The role of DBLβ domains, particularly those encoding ICAM1-binding regions, as targets of the immune response in CM is complex and multifaceted.
In a study using an ICAM1-binding DBLβ3 domain from a Group A PfEMP1 variant with an EPCR-binding CIDRα1 domain, higher IgG levels against the DBLβ3 at enrolment were significantly associated with a reduced risk of high‐density clinical malaria (fever + ≥10,000 parasites/µL) and of progression to SM during follow-up (69 weeks) (84). While this longitudinal evidence suggests that antibodies to this DBLβ3 may protect against malaria, it remains unclear whether this protection extends to CM. Future studies should stratify SM cases by clinical phenotype to determine if these antibodies confer similar protection against CM, considering the potential for distinct pathogenic mechanisms between CM and other SM manifestations.
While children with CM and UM of similar age show no significant differences in DBLβ-specific IgG titers (98, 100, 101), they do differ in antibody function. Two separate studies investigated phagocytic activity against ICAM1-binding PfEMP1. In Benin, where the cohort included children with SM including CM, and in Malawi, which focused exclusively on CM, individuals with UM showed greater phagocytic activity against DBLβ-coated beads and ICAM1-binding IEs, respectively (101, 102). Additionally, among the four recombinant ICAM1-binding domains assessed, antibodies from children with UM showed significantly higher inhibition of ICAM1 binding to a Group A DBLβ domain, compared to antibodies from children with SM or CM (101). These findings suggest that antibody-mediated inhibition of ICAM1-binding and enhanced opsonic phagocytosis may contribute to protection against SM (101). These functional differences may be influenced by antibody features including IgG subclass. IgG1 and IgG3 are effective in driving phagocytosis, but it is unknown how antibody subclass influences binding inhibition. IgG1 and IgG3 antibody titers against multiple ICAM1–binding DBLβ domains were associated with protection against severe disease in Beninese children (101). This suggests that individuals in Benin acquire functional antibodies that can bind to multiple DBLβ domains and may be protective.
3.3 Antibody responses that disrupt rosetting
Several studies have demonstrated that immune sera from malaria-exposed individuals can reverse rosetting in vitro, particularly in severe disease including CM (58, 67, 73). One study found that IgG to the SM-associated DBLα1.5 domain (selected based on its higher transcription in SM compared to UM) was significantly higher in UM compared to SM (95), suggesting that antibodies that disrupt rosetting may confer protection. The same study found that IgG to SM-associated CIDRγ12 was also significantly higher in UM compared to SM. Another study found that FcγRIIIb binding antibodies to CIDRγ12 were significantly higher in UM than CM and this was one of seven key features predictive of protection against CM (102). Though there is limited direct evidence linking the CIDRγ12 to rosetting, other CIDRγ domains have been linked to rosetting. Evidence suggests that CIDRγ2 can mediate rosetting. One study showed that the CIDRγ2 domain of 3D7A parasites binds glycophorin B on uninfected erythrocytes to mediate robust, plasma-independent rosetting, and anti-CIDRγ2 antibodies specifically inhibited rosetting in 3D7A parasites in a dose-dependent manner (9). Interestingly, anti-CIDRγ2 antibodies did not affect rosetting of another parasite line FCR3, whose rosetting involves IgM and α2-macroglobulin. In the FCR3 parasite, antibodies to DBL1α-CIDRβ blocked rosetting (103), highlighting that there are multiple, domain-specific mechanisms of rosette formation. These findings suggest a possible link between rosetting and the pathogenesis of CM, highlighting the need for further investigation into the underlying mechanisms and the potential for antibodies targeting rosetting domains to offer protection against CM.
Some rosetting parasites bind IgM and IgM facilitates the adhesion of the IEs to uninfected erythrocytes (104). IgM-positive rosetting parasites express distinct PfEMP1 variants with specific N-terminal and DBL domains, and polyclonal IgG antibodies raised against these variants could inhibit rosetting across different strains (73), indicating strain-transcending potential. In contrast, IgM-negative rosetting parasites exhibited strain-specific antibody activity (73). These strain-transcending antibodies represent the kind of broader protection that would be ideal for developing a preventative measure against CM. However, there is no data on which rosetting strains are expressed in CM or whether antibodies toward them provide protective immunity. These aspects require further investigation.
3.4 Functional features of antibodies protective against cerebral malaria
PfEMP1-specific antibody responses may offer protection against malaria by eliciting multiple effector functions, including blocking IE adhesion (73, 105) and promoting immune clearance via ADCP (76, 101, 105) and antibody-dependent cellular cytotoxicity (ADCC) (106–108).
Complement enhances antibody-mediated inhibition across multiple parasite stages, including sporozoites (CSP), merozoites (MSP1), and sexual forms (Pfs230) (104, 109), however the role of complement fixing antibodies against PfEMP1 and IEs is less clear (110). Recent findings emphasize the role of antibody-dependent effector functions in distinguishing CM from UM, identifying seven key antibody features predictive of malaria severity (102). Among these were antibodies that fix C1q (targeting the ICAM1 binding IT4VAR13_DBLβ) indicating that complement-mediated effector functions may be a protective mechanism. While these insights offer a valuable starting point, it is important to note that findings based on recombinant proteins may not fully capture native PfEMP1 conformation or function in vivo. Given that IgG1 and IgG3 dominate the PfEMP1-specific response in semi-immune individuals (111–113) and are potent complement activators, their ability to fix C1q may contribute to protection against CM. However, a study found that complement-mediated opsonization of IEs is limited in efficacy, potentially due to the patchy distribution of PfEMP1 on the IE surface (111), which could limit IgG hexamerization—an essential step for effective C1q activation (114). Although antibodies that fix C1q have been suggested to play a protective role in CM, it is important to confirm if complement deposition does occur on the IE and whether it enhances complement-mediated phagocytosis. Future studies should elucidate how C1q-fixing antibodies targeting PfEMP1 contribute to protection against CM, for better prognostic and therapeutic strategies.
Fc mediated functions may protect against CM; antibody features associated with protection from CM include FcγRIIIb-binding antibodies targeting ICAM1 binding DBLβ3 PfEMP1 domains (112). In the same study, Malawian children with UM had higher neutrophil-mediated phagocytosis of IEs expressing ICAM1 and EPCR-binding PfEMP1 (3D7VAR04) than children with CM (112), suggesting a protective role of FcγRIIIb-dependent phagocytosis. In contrast, children with CM had higher neutrophil-mediated phagocytosis of ICAM1 and CD36-binding IEs (IT4VAR13), indicating a difference in immune targeting of variant surface antigens. FcγRIIa can work synergistically with FcγRIIIb to enhance antibody-dependent neutrophil phagocytosis of merozoites (115) and sporozoites (116), it is currently unknown whether this is similar for PfEMP1. This suggests that exploring Fc-mediated immune functions, such as complement activation, ADCC, and FcγR engagement, could provide stronger predictive value for clinical outcomes than total neutralizing antibodies alone.
3.5 Fucose-free PfEMP1-specific antibodies
Antibody-dependent immune effector functions depend on IgG binding to FcγR on immune cells, a process influenced by glycosylation (the presence of sugar molecules on the antibody’s Fc region), which can significantly influence downstream immune functions. One key glycosylation modification is Fc-afucosylation, the absence of fucose on the biantennary glycan at asparagine 297 (N297) in the IgG Fc region. This enhances IgG binding to FcγRIIIa, greatly amplifying ADCC activity (117). Afucosylated IgG antibodies targeting enveloped viruses or alloantigens are highly effective at ADCC, as they can bind the FcγRIIIa receptor on immune cells up to 20 times more strongly (117–119).
A study on IgG afucosylation in pregnancy-associated malaria highlighted the potential importance of afucosylated antibodies in malaria immunity (107). Researchers found that naturally acquired IgG targeting pregnancy-associated PfEMP1 (VAR2CSA) was afucosylated and remained stable over time, whereas vaccination did not induce afucosylated antibodies (107). Notably, only afucosylated VAR2CSA-specific IgG could induce natural killer (NK) cell degranulation (107). In a more recent study, children with Fc-afucosylated IgG1 targeting the PfEMP1 variant HB3VAR06 were less likely to be anemic. Fc-afucosylation of these antibodies is acquired through repeated malaria exposure and persists over time. Protection from anemia was associated with immune cell activation via FcγRIIIa, rather than complement-mediated lysis, likely due to the uneven distribution of PfEMP1 on IEs (120), suggesting that Fc-afucosylation enhances antibody function by improving IgG binding to FcγRIIIa, which boosts NK cell activation and IE clearance. However, the role of NK cells in CM may be a double-edged sword, as children with CM have more activated NK cells than those with SM or UM (121).
Despite growing interest in antibody glycosylation, there is limited data on the role of afucosylated IgG antibodies in CM.
4 Where can we go from here?
Current evidence suggests that antibodies targeting PfEMP1 have the potential to protect against CM, but further research is needed to determine which PfEMP1 targets are most important for immunity. Longitudinal studies indicate that hyperimmune individuals in endemic areas develop antibodies against EPCR-binding CIDRα1 domains, which may limit clinically dense malaria (84, 86). Both EPCR-binding domains and those with dual binding to EPCR and ICAM1 have been associated with CM (38–40). It is likely that the determinants of protective immunity extend beyond the neutralizing capacity of PfEMP1-specific antibodies and understanding how adhesion inhibition and immune effector functions cooperate to eliminate infections will be important.
Our understanding of naturally acquired immunity to malaria, and CM in particular, remains incomplete. One key gap is our limited understanding of why some PfEMP1 variants trigger strong, protective antibody responses while others evade immunity, leaving young children—despite early exposure—more vulnerable to CM compared to older children and adults who develop sustained responses against EPCR-binding (86) and ICAM1-binding (79) domains. When considering a determinant of antibody function, such as afucosylation—acquired over repeated exposure and persisting over time—we recognize its potential to significantly enhance immune effector functions like ADCC. It would be interesting to explore how afucosylation evolves over time, whether it contributes to age-related differences in immunity, and how relevant it is for protection (Table 1). The gold standard for addressing some of these questions would be longitudinal cohort studies in malaria-endemic regions that follow individuals from infancy to adulthood. Such studies would provide comprehensive data on how antibody responses to PfEMP1 develop, persist, and change over time. However, realistically, these studies may not be feasible due to several limitations, including their high cost, the substantial manpower required for long-term follow-up, and the logistical challenges associated with maintaining consistent data collection over many years.
Post-translational modifications do not only occur on antibodies but can also occur on FcγRs located on immune effector cells and regulate antibody effector function. For example, ligand−induced ubiquitination of the FcγRIIIa ζ−chain targets the receptor for degradation, reducing its surface expression and dampening natural killer cell activation (122). In the context of anti−PfEMP1 antibodies, investigations into antibody Fc and FcγR modifications beyond glycosylation remain scarce. Mass spectrometry–based mapping of post-translational modifications (123) in anti-PfEMP1 antibodies from CM patients and in-silico structure-based predictions of potential modification sites (124) offer powerful strategies for identifying which post-translational modifications are important in CM.
EPCR-binding and dual-binding PfEMP1 variants are closely associated with CM, leading to the natural hypothesis that antibodies targeting these variants would provide protection. However, the data thus far on both DC8- and DC13-containing PfEMP1 variants, while suggestive of protection, is not conclusive. This presents a gap that needs further exploration: which isolates of these variants are most immunogenic, which peptides within them are immunogenic, and whether these responses are protective. Beyond antibodies to CIDRα1 and DBLβ domains, other regions of PfEMP1—such as DBLα and CIDRγ2 domains involved in rosetting, and CIDRγ6 and CIDRγ12 domains with unknown binding phenotypes—may also influence PfEMP1-mediated adhesion. It is imperative to assess how their presence in EPCR-binding PfEMP1 variants enhance CIDRα1’s interaction with EPCR and evaluate the protective potential of antibodies against each of these domains in CM.
Monoclonal antibodies like C7 and C74, which target the CIDRα1 domain of PfEMP1, show promise in preventing IE sequestration in CM by blocking adhesion to endothelial cells (125). Identifying post-translational modifications that are important for protection could help enhance the blocking capabilities of broadly inhibitory monoclonal antibodies, such as these.
How broad must the antibody repertoire be to confer effective protection, and what is the best way to measure immunity? Studies like Walker et al. (102) demonstrate the power of a systems serology approach in identifying targets and features of a possible protective antibody response. The study also illustrates potential shortcomings of recombinant protein-based multiplex immunoassays, which did not correlate well with assays using whole IEs (102). This discrepancy may be due to differences in antigen presentation and Fc receptor engagement, underscoring that assays based on individual recombinant PfEMP1 domains may not fully capture immunity to PfEMP1 in its native configuration or full-length proteins (126). PfEMP1 antigenic switching (127) remains a significant challenge for in vitro studies, and it will be critical to obtain antigenically stable parasite lines that accurately reflect the antigenic diversity in natural infections.
While this review has focused on neutrophils primarily in terms of antibody-mediated phagocytosis, the work by Zelter et al. (128) demonstrates that neutrophils can also directly recognize directly recognize and kill infected erythrocytes via ICAM-1, independently of antibodies. This suggests that, in addition to their antibody-dependent phagocytic function, neutrophils may exert selective pressure on the parasite population: IEs expressing ICAM1-binding PfEMP1 are preferentially eliminated, forcing surviving parasites to downregulate or switch away from these variants. This “filtering” mechanism may contribute to protection and raises an important question for protective immunity: are antibody-mediated opsonic phagocytosis and ICAM1–mediated killing both necessary for effective protection against severe disease?
Another gap is the lack of robust binding inhibition assays, and how these interactions influence immune effector functions remains largely unknown. Most binding assays to date have relied on 2D systems, such as flat layers of cultured cells or immobilized receptors, where parasites adhere under static conditions (129, 130). Recent work using engineered 3D human brain microvessels has shown IEs cytoadhere via PfEMP1–endothelial receptor interactions, mature, and rupture within a physiologically relevant microvascular environment (70, 131, 132). This binding triggers endothelial activation, including upregulation of ICAM1 and inflammatory signaling, promotes focal barrier disruptions and endothelial apoptosis, and elicits a stress response profile distinct from that differ from conventional 2D monolayers (132, 133). These 3D microvessel models are already proving valuable in investigating PfEMP1-mediated adhesion and offer a versatile platform for future research to assess how changes in the microenvironment influence PfEMP1 expression, transcription, and binding, as well as to evaluate the efficacy of broadly inhibitory monoclonal antibodies, both current and in development.
In conclusion, there is still much to learn about the antibody responses to PfEMP1 that provide protection from CM. Current research has laid a strong foundation for asking the crucial questions that will guide us toward those answers. There are many exciting future directions, including exploring monoclonal antibodies, post-translational modifications, and 3D vascular models, which may bring us closer to a deeper understanding of the immune mechanisms at play and potentially effective interventions.
Author contributions
JB: Conceptualization, Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. IW: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Investigation, Methodology. TN: Supervision, Writing – review & editing. EA: Supervision, Writing – review & editing, Investigation, Writing – original draft. SR: Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Visualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Institute of Health, United States, under grant number R01AI165595.
Acknowledgments
The authors would like to thank Karl Seydel (Blantyre Malaria Project/Michigan State University) for his on-going supervision of JB’s PhD, which helped motivate the development of this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. World Malaria Report 2024. Geneva: World Health Organization (2024). Available online at: https://www.who.int/publications/i/item/9789240104440 (Accessed October 14, 2025).
2. Chauhan VS, Chitnis CE, and Gaur D. Introduction. Recent Adv Malaria Advances in Malaria Research. (2016), 1–7. Hoboken, New Jersey: John Wiley & Sons Inc. doi: 10.1002/9781118493816.ch1
3. Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JG, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. (2004) 10:143–5. doi: 10.1038/nm986
4. Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. (2002) 419:498–511. doi: 10.1038/nature01097
5. Rask TS, Hansen DA, Theander TG, Pedersen AG, and Lavstsen T. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes – divide and conquer. PloS Comput Biol. (2010) 6:e1000933. doi: 10.1371/journal.pcbi.1000933
6. Smith JD, Rowe JA, Higgins MK, and Lavstsen T. Malaria’s deadly grip: Cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol. (2013) 15:1976–83. doi: 10.1111/cmi.12183
7. Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. (1995) 82:77–87. doi: 10.1016/0092-8674(95)900543
8. Smith JD, Subramanian G, Gamain B, Baruch DI, and Miller LH. Classification of adhesive domains in the Plasmodium falciparum Erythrocyte Membrane Protein 1 family. Mol Biochem Parasitol. (2000) 110:293–310. doi: 10.1016/s0166-6851(00)00279-6
9. Oleinikov AV, Amos E, Frye IT, Rossnagle E, Mutabingwa TK, Fried M, et al. High throughput functional assays of the variant antigen PfEMP1 reveal a single domain in the 3D7 Plasmodium falciparum genome that binds ICAM1 with high affinity and is targeted by naturally acquired neutralizing antibodies. PloS Pathog. (2009) 5:e1000386. doi: 10.1371/journal.ppat.1000386
10. Howell DP, Levin EA, Springer AL, Kraemer SM, Phippard DJ, Schief WR, et al. Mapping a common interaction site used by Plasmodium falciparum Duffy binding-like domains to bind diverse host receptors. Mol Microbiol. (2008) 67:78–87. doi: 10.1111/j.1365-2958.2007.06019
11. Hsieh FL, Turner L, Bolla JR, Robinson CV, Lavstsen T, and Higgins MK. The structural basis for CD36 binding by the malaria parasite. Nat Commun. (2016) 7:12837. doi: 10.1038/ncomms12837
12. Miller LH, Baruch DI, Marsh K, and Doumbo OK. The pathogenic basis of malaria. Nature. (2002) 415:673–9. doi: 10.1038/415673a
13. Quadt KA, Barfod L, Andersen D, Bruun J, Gyan B, Hassenkam T, et al. The Density of Knobs on Plasmodium falciparum-Infected Erythrocytes Depends on Developmental Age and Varies among Isolates. PloS One. (2012) 7(9):e45658. doi: 10.1371/journal.pone.0045658
14. Milner DA, Lee JJ, Frantzreb C, Whitten RO, Kamiza S, Carr RA, et al. Quantitative assessment of multiorgan sequestration of parasites in fatal pediatric cerebral malaria. J Infect Dis. (2015) 212:1317–21. doi: 10.1093/infdis/jiv205
15. Taylor TE and Molyneux ME. The pathogenesis of pediatric cerebral malaria: eye exams, autopsies, and neuroimaging. Ann N Y Acad Sci. (2015) 1342:44–52. doi: 10.1111/nyas.12690
16. Beare NA, Taylor TE, Harding SP, Lewallen S, and Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. (2006) 75:790–7. doi: 10.4269/ajtmh.2006.75.790
17. Jensen AR, Adams Y, and Hviid L. Cerebral Plasmodium falciparum malaria: The role of PfEMP1 in its pathogenesis and immunity, and PfEMP1-based vaccines to prevent it. Immunol Rev. (2020) 293:230–52. doi: 10.1111/imr.12807
18. Bujila I, Schwarzer E, Skorokhod O, Weidner JM, Troye-Blomberg M, and Östlund Farrants AK. Malaria-derived hemozoin exerts early modulatory effects on the phenotype and maturation of human dendritic cells. Cell Microbiol. (2016) 18:413–23. doi: 10.1111/cmi.12521
19. Eriksson EM, Sampaio NG, and Schofield L. Toll-like receptors and malaria – sensing and susceptibility. J Trop Dis. (2013) 2:126. doi: 10.4172/2329-891X.1000126
20. Dunst J, Kamena F, and Matuschewski K. Cytokines and chemokines in cerebral malaria pathogenesis. Front Cell Infect Microbiol. (2017) 7:324. doi: 10.3389/fcimb.2017.00324
21. Jambou R, Combes V, Jambou MJ, Weksler BB, Couraud PO, and Grau GE. Plasmodium falciparum adhesion on human brain microvascular endothelial cells involves transmigration-like cup formation and induces opening of intercellular junctions. PloS Pathog. (2010) 6:e1001021. doi: 10.1371/journal.ppat.1001021
22. Riggle BA, Manglani M, Maric D, Johnson KR, Lee MH, Abath Neto OL, et al. CD8+ T cells target cerebrovasculature in children with cerebral malaria. J Clin Invest. (2020) 130:1128–38. doi: 10.1172/JCI133474
23. Moxon CA, Heyderman RS, and Wassmer SC. Dysregulation of coagulation in cerebral malaria. Mol Biochem Parasitol. (2009) 166:99–108. doi: 10.1016/j.molbiopara.2009.03.006
24. Hollestelle MJ, Donkor C, Mantey EA, Chakravorty SJ, Craig A, Akoto AO, et al. von Willebrand factor propeptide in malaria: evidence of acute endothelial cell activation. Br J Haematol. (2006) 133:562–9. doi: 10.1111/j.1365-2141.2006.06067.x
25. Wassmer SC, Lépolard C, Traoré B, Pouvelle B, Gysin J, and Grau GE. Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. J Infect Dis. (2004) 189:180–9. doi: 10.1086/380761
26. Adams Y, Olsen RW, Bengtsson A, Dalgaard N, Zdioruk M, Satpathi S, et al. Plasmodium falciparum erythrocyte membrane protein 1 variants induce cell swelling and disrupt the blood–brain barrier in cerebral malaria. J Exp Med. (2021) 218:e20201266. doi: 10.1084/jem.20201266
27. Greiner J, Dorovini-Zis K, Taylor TE, Molyneux ME, Beare NAV, Kamiza S, et al. Correlation of hemorrhage, axonal damage, and blood-tissue barrier disruption in brain and retina of Malawian children with fatal cerebral malaria. Front Cell Infect Microbiol. (2015) 5:18. doi: 10.3389/fcimb.2015.00018
28. Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. (2015) 372:1126–37. doi: 10.1056/NEJMoa1400116
29. Sahu PK, Duffy FJ, Dankwa S, Vishnyakova M, Majhi M, Pirpamer L, et al. Determinants of brain swelling in pediatric and adult cerebral malaria. JCI Insight. (2021) 6:e145823. doi: 10.1172/jci.insight.145823
30. Kyriacou HM, Stone GN, Challis RJ, Raza A, Lyke KE, Thera MA, et al. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol Biochem Parasitol. (2006) 150:211–8. doi: 10.1016/j.molbiopara.2006.08.005
31. Barua P, Duffy MF, Manning L, Laman M, Davis TME, Mueller I, et al. Antibody to Plasmodium falciparum Variant Surface Antigens, var Gene Transcription, and ABO Blood Group in Children with Severe or Uncomplicated Malaria. J Infect Dis. (2023) 228:1099–107. doi: 10.1093/infdis/jiad217
32. Kalmbach Y, Rottmann M, Kombila M, Kremsner PG, Beck HP, and Kun JFJ. Differential var gene expression in children with malaria and antidromic effects on host gene expression. J Infect Dis. (2010) 202:313–7. doi: 10.1086/653586
33. Lavstsen T, Turner L, Saguti F, Magistrado P, Rask TS, Jespersen JS, et al. Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci U S A. (2012) 109:E1791–800. doi: 10.1073/pnas.1120455109
34. Jensen ATR, Magistrado P, Sharp S, Joergensen L, Lavstsen T, Chiucchiuini A, et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J Exp Med. (2004) 199:1179–90. doi: 10.1084/jem.20040274
35. Tembo DL, Nyoni B, Murikoli RV, Mukaka M, Milner DA, Berriman M, et al. Differential PfEMP1 expression is associated with cerebral malaria pathology. PloS Pathog. (2014) 10:e1004537. doi: 10.1371/journal.ppat.1004537
36. Warimwe GM, Keane TM, Fegan G, Musyoki JN, Newton CRJC, Pain A, et al. Plasmodium falciparum var gene expression is modified by host immunity. Proc Natl Acad Sci U S A. (2009) 106:21801–6. doi: 10.1073/pnas.0907590106
37. Lau CKY, Turner L, Jespersen JS, Lowe ED, Petersen B, Wang CW, et al. Structural conservation despite huge sequence diversity allows EPCR binding by the pfEMP1 family implicated in severe childhood malaria. Cell Host Microbe. (2015) 17:118–29. doi: 10.1016/j.chom.2014.11.007
38. Lennartz F, Adams Y, Bengtsson A, Olsen RW, Turner L, Ndam NT, et al. Structure-guided identification of a family of dual receptor-binding pfEMP1 that is associated with cerebral malaria. Cell Host Microbe. (2017) 21:403–14. doi: 10.1016/j.chom.2017.02.009
39. Duffy F, Bernabeu M, Babar PH, Kessler A, Wang CW, Vaz M, et al. Meta-analysis of Plasmodium falciparum var Signatures Contributing to Severe Malaria in African Children and Indian Adults. mBio. (2019) 10:e00217–19. doi: 10.1128/mBio.00217-19
40. Kessler A, Dankwa S, Bernabeu M, Harawa V, Danziger SA, Duffy F, et al. Linking EPCR-binding PfEMP1 to brain swelling in pediatric cerebral malaria. Cell Host Microbe. (2017) 22:601–14. doi: 10.1016/j.chom.2017.09.009
41. Storm J, Jespersen JS, Seydel KB, Szestak T, Mbewe M, Chisala NV, et al. Cerebral malaria is associated with differential cytoadherence to brain endothelial cells. EMBO Mol Med. (2019) 11:e9164. doi: 10.15252/emmm.201809164
42. Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JEV, Avril M, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. (2013) 498:502–5. doi: 10.1038/nature12216
43. Janes JH, Wang CP, Levin-Edens E, Vigan-Womas I, and Guillotte M. Investigating the host binding signature on the plasmodium falciparum pfEMP1 protein family. PloS Pathog. (2011) 7:e1002032. doi: 10.1371/journal.ppat.1002032
44. Smith JD, Craig AG, Kriek N, Hudson-Taylor D, Kyes S, Fagen T, et al. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc Natl Acad Sci U S A. (2000) 97:1766–71. doi: 10.1073/pnas.040545897
45. Avril M, Bernabeu M, Benjamin M, Brazier AJ, and Smith JD. Interaction between endothelial protein C receptor and intercellular adhesion molecule 1 to mediate binding of plasmodium falciparum-infected erythrocytes to endothelial cells. mBio. (2016) 7:e00615–16. doi: 10.1128/mBio.00615-16
46. Tuikue Ndam N, Moussiliou A, Lavstsen T, Kamaliddin C, Jensen ATR, Mama A, et al. Parasites causing cerebral falciparum malaria bind multiple endothelial receptors and express EPCR and ICAM1-binding pfEMP1. J Infect Dis. (2017) 215:1918–25. doi: 10.1093/infdis/jix230
47. Bernabeu M, Danziger SA, Avril M, Vaz M, Babar PH, Brazier AJ, et al. Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc Natl Acad Sci U S A. (2016) 113:E3270–9. doi: 10.1073/pnas.1524294113
48. Bertin GI, Lavstsen T, Guillonneau F, Doritchamou J, Wang CW, Jespersen JS, et al. Expression of the domain cassette 8 Plasmodium falciparum erythrocyte membrane protein 1 is associated with cerebral malaria in Benin. PloS One. (2013) 8:e68368. doi: 10.1371/journal.pone.0068368
49. Jespersen JS, Wang CW, Mkumbaye SI, Minja DT, Petersen B, Turner L, et al. Plasmodium falciparum var genes expressed in children with severe malaria encode CIDRα1 domains. EMBO Mol Med. (2016) 8:839–50. doi: 10.15252/emmm.201606188
50. Mkumbaye SI, Wang CW, Lyimo E, Jespersen JS, Manjurano A, Mosha J, et al. The Severity of Plasmodium falciparum Infection Is Associated with Transcript Levels of var Genes Encoding Endothelial Protein C Receptor-Binding P. falciparum Erythrocyte Membrane Protein 1. Infect Immun. (2017) 85:e00841–16. doi: 10.1128/IAI.00841-16
51. Abdi AI, Kariuki SM, Muthui MK, Kivisi CA, Fegan G, Gitau E, et al. Differential Plasmodium falciparum surface antigen expression among children with Malarial Retinopathy. Sci Rep. (2015) 5:18034. doi: 10.1038/srep18034
52. Shabani E, Hanisch B, Opoka RO, Lavstsen T, and John CC. Plasmodium falciparum EPCR-binding PfEMP1 expression increases with malaria disease severity and is elevated in retinopathy negative cerebral malaria. BMC Med. (2017) 15:183. doi: 10.1186/s12916-017-0945-y
53. Bull PC, Berriman M, Kyes S, Quail MA, Hall N, Kortok MM, et al. Plasmodium falciparum variant surface antigen expression patterns during malaria. PloS Pathog. (2005) 1:e26. doi: 10.1371/journal.ppat.0010026
54. Heddini A, Pettersson F, Kai O, Shafi J, Obiero J, Chen Q, et al. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect Immun. (2001) 69:5849–56. doi: 10.1128/IAI.69.9.5849-5856.2001
55. Mbagwu SI and Filgueira L. Differential expression of CD31 and von willebrand factor on endothelial cells in different regions of the human brain: potential implications for cerebral malaria pathogenesis. Brain Sci. (2020) 10:31. doi: 10.3390/brainsci10010031
56. Kikuchi M, Looareesuwan S, Ubalee R, Tasanor O, Suzuki F, Wattanagoon Y, et al. Association of adhesion molecule PECAM-1/CD31 polymorphism with susceptibility to cerebral malaria in Thais. Parasitol Int. (2001) 50:235–9. doi: 10.1016/s1383-5769(01)00082-4
57. Ohashi J, Naka I, Hananantachai H, and Patarapotikul J. Association of PECAM1/CD31 polymorphisms with cerebral malaria. Int J Mol Epidemiol Genet. (2016) 7:87–94.
58. Treutiger CJ, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, et al. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. (1992) 46:503–10. doi: 10.4269/ajtmh.1992.46.503
59. Rowe A, Obeiro J, Newbold CI, and Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. (1995) 63:2323–6. doi: 10.1128/iai.63.6.2323-2326.1995
60. Doumbo OK, Thera MA, Koné AK, Raza A, Tempest LJ, Lyke KE, et al. High levels of Plasmodium falciparum rosetting in all clinical forms of severe malaria in African children. Am J Trop Med Hyg. (2009) 81:987–93. doi: 10.4269/ajtmh.2009.09-0406
61. Carlson J, Helmby H, Wahlgren M, Carlson J, Helmby H, Wahlgren M, et al. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. (1990) 336:1457–60. doi: 10.1016/0140-6736(90)93174-n
62. Al-Yaman F, Genton B, Mokela D, Raiko A, Kati S, Rogerson S, et al. Human cerebral malaria: lack of significant association between erythrocyte rosetting and disease severity. Trans R Soc Trop Med Hyg. (1995) 89:55–8. doi: 10.1016/0035-9203(95)90658-4
63. Chotivanich K, Sritabal J, Udomsangpetch R, Newton P, Stepniewska KA, Ruangveerayuth R, et al. Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J Infect Dis. (2004) 189:1052–5. doi: 10.1086/381900
64. Lee WC, Russell B, Lau YL, Nosten F, and Enia LR. Rosetting responses of plasmodium-infected erythrocytes to antimalarials. Am J Trop Med Hyg. (2022) 106:1670–4. doi: 10.4269/ajtmh.21-1229
65. Lee WC, Russell B, Sobota RM, Ghaffar K, Howland SW, Wong ZX, et al. Plasmodium-infected erythrocytes induce secretion of IGFBP7 to form type II rosettes and escape phagocytosis. Elife. (2020) 9:e51546. doi: 10.7554/eLife.51546
66. Lee WC, Russell B, Lee B, Chu CS, Phyo AP, Sriprawat K, et al. Plasmodium falciparum rosetting protects schizonts against artemisinin. EBioMedicine. (2021) 73:103680. doi: 10.1016/j.ebiom.2021.103680
67. Lee WC, Russell B, and Rénia L. Evolving perspectives on rosetting in malaria. Trends Parasitol. (2022) 38:882–9. doi: 10.1016/j.pt.2022.08.001
68. Mcquaid F and Rowe JA. Parasitology Rosetting revisited: a critical look at the evidence for host erythrocyte receptors in Plasmodium falciparum rosetting. Plasmodium Falciparum Rosetting Parasitol. (2019) 147:1–11. doi: 10.1017/S0031182019001288
69. Vigan-Womas I, Guillotte M, Juillerat A, Hessel A, Raynal B, England P, et al. Structural basis for the ABO blood-group dependence of plasmodium falciparum rosetting. PloS Pathog. (2012) 8:e1002781. doi: 10.1371/journal.ppat.1002781
70. Bernabeu M, Howard C, Zheng Y, and Smith JD. Bioengineered 3D microvessels for investigating plasmodium falciparum pathogenesis. Trends Parasitol. (2021) 37:401–13. doi: 10.1016/j.pt.2020.12.008
71. Rowe JA, Newbold CI, and Miller LHP. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. (1997) 388:292–5. doi: 10.1038/40888
72. Albrecht L, Moll K, Blomqvist K, Normark J, Chen Q, and Wahlgren M. Var gene transcription and PfEMP1 expression in the rosetting and cytoadhesive Plasmodium falciparum clone FCR3S1.2. Malar J. (2011) 10:17. doi: 10.1186/1475-2875-10-17
73. Ghumra A, Semblat JP, Ataide R, Kifude C, Adams Y, Claessens A, et al. Induction of strain-transcending antibodies against group A pfEMP1 surface antigens from virulent malaria parasites. PloS Pathog. (2012) 8:e1002665. doi: 10.1371/journal.ppat.1002665
74. Vigan-Womas I, Guillotte M, Juillerat A, Vallieres C, and Lewit-Bentley A. Allelic diversity of the plasmodium falciparum erythrocyte membrane protein 1 entails variant-specific red cell surface epitopes. PloS One. (2011) 6:e16544. doi: 10.1371/journal.pone.0016544
75. McLean FE, Omondi BR, Diallo N, Otoboh S, Kifude C, Abdi A, et al. Identification of novel PfEMP1 variants containing domain cassettes 11, 15 and 8 that mediate the Plasmodium falciparum virulence-associated rosetting phenotype. PloS Pathog. (2025) 21:e1012434. doi: 10.1371/journal.ppat.1012434
76. Chan JA, Boyle MJ, Moore KA, Reiling L, Lin Z, Hasang W, et al. Antibody targets on the surface of plasmodium falciparum- infected erythrocytes that are associated with immunity to severe malaria in young children. J Infect Dis. (2019) 219:819–28. doi: 10.1093/infdis/jiy580
77. Bull PC, Lowe BS, Kortok M, and Marsh K. Antibody recognition of plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun. (1999) 67:733–9. doi: 10.1128/IAI.67.2.733-739.1999
78. Cham GK, Turner L, Kurtis JD, Mutabingwa T, Fried M, Jensen AT, et al. Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 in Tanzanian children. Infect Immun. (2010) 78:4653–9. doi: 10.1128/IAI.00593-10
79. Olsen RW, Ecklu-Mensah G, Bengtsson A, Ofori MF, Kusi KA, Koram KA, et al. Acquisition of IGG to ICAM1-Binding DBLβ Domains in the plasmodium falciparum erythrocyte membrane protein 1 antigen family varies between groups A, B and C. Infect Immun. (2019) 87:e00224-19. doi: 10.1128/iai.00224-19
80. Marsh K and Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. (2006) 28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x
81. Kinyua AW, Turner L, Kimingi HW, Mwai K, Mwikali K, Andisi C, et al. Antibodies to PfEMP1 and variant surface antigens: Protection after controlled human malaria infection in semi-immune Kenyan adults. J Infect. (2024) 89:106252. doi: 10.1016/j.jinf.2024.106252
82. Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, and van Eijk AM. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis. (2018) 18:e107–18. doi: 10.1016/S1473-3099(18)30066-5
83. Bull PC, Kortok M, Kai O, Ndungu F, Ross A, Lowe BS, et al. Plasmodium falciparum-infected erythrocytes: Agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J Infect Dis. (2000) 182:252–9. doi: 10.1086/315652
84. Tessema SK, Utama D, Chesnokov O, Hodder AN, Lin CS, Abby Harrison GL, et al. Antibodies to Intercellular Adhesion Molecule 1-Binding Plasmodium falciparum Erythrocyte Membrane Protein 1-DBL Are Biomarkers of Protective Immunity to Malaria in a Cohort of Young Children from Papua New Guinea. Infect Immun. (2018) 86(8):e00485-17. doi: 10.1128/IAI.00485-17
85. Moussiliou A, Turner L, Cottrell G, Doritchamou J, Gbédandé K, Fievet N, et al. The journal of infectious diseases dynamics of pfEMP1 antibody profile from birth to 12 months of age in Beninese infants. J Infect Dis. (2020) 221:2010–7. doi: 10.1093/infdis/jiaa043
86. Obeng-Adjei N, Larremore DB, Turner L, Ongoiba A, Li S, Doumbo S, et al. Longitudinal analysis of naturally acquired PfEMP1 CIDR domain variant antibodies identifies associations with malaria protection. JCI Insight. (2020) 5:e137262. doi: 10.1172/jci.insight.137262
87. Turner L, Lavstsen T, Mmbando BP, Wang CW, Magistrado PA, Vestergaard LS, et al. IgG antibodies to endothelial protein C receptor-binding cysteine-rich interdomain region domains of Plasmodium falciparum erythrocyte membrane protein 1 are acquired early in life in individuals exposed to malaria. Infect Immun. (2015) 83:3096–103. doi: 10.1128/IAI.00271-15
88. Tonkin-Hill GQ, Trianty L, Noviyanti R, Nguyen HHT, Sebayang BF, Lampah DA, et al. The Plasmodium falciparum transcriptome in severe malaria reveals altered expression of genes involved in important processes including surface antigen-encoding var genes. PloS Biol. (2018) 16:e2004328. doi: 10.1371/journal.pbio.2004328
89. Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, and Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. (1998) 4:358–60. doi: 10.1038/nm0398-358
90. Chan JA, Howell KB, Reiling L, Ataide R, Mackintosh CL, Fowkes FJ, et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest. (2012) 122:3227–38. doi: 10.1172/JCI62182
91. Travassos MA, Niangaly A, Bailey JA, Ouattara A, Coulibaly D, Lyke KE, et al. Children with cerebral malaria or severe malarial anaemia lack immunity to distinct variant surface antigen subsets. Sci Rep. (2018) 8:6281. doi: 10.1038/s41598-018-24462-4
92. Rambhatla JS, Turner L, Manning L, Laman M, Davis TME, Beeson JG, et al. Acquisition of antibodies against endothelial protein C receptor-binding domains of plasmodium falciparum erythrocyte membrane protein 1 in children with severe malaria. J Infect Dis. (2019) 219:808–18. doi: 10.1093/infdis/jiy564
93. Nunes-Silva S, Dechavanne S, Moussiliou A, Pstrąg N, Semblat JP, Gangnard S, et al. Beninese children with cerebral malaria do not develop humoral immunity against the IT4-VAR19-DC8 PfEMP1 variant linked to EPCR and brain endothelial binding. Malar J. (2015) 8:14:493. doi: 10.1186/s12936-015-1008-5
94. Opoka RO, Namazzi R, Datta D, Bangirana P, Conroy AL, Goings MJ, et al. Severe falciparum malaria in young children is associated with an increased risk of post-discharge readmission or death: A prospective cohort study. Malar J. (2024) 23:367. doi: 10.1186/s12936-024-05196-3
95. Rambhatla JS, Tonkin-Hill GQ, Takashima E, Tsuboi T, Noviyanti R, Trianty L, et al. Identifying Targets of Protective Antibodies against Severe Malaria in Papua, Indonesia, Using Locally Expressed Domains of Plasmodium falciparum Erythrocyte Membrane Protein 1. Infect Immun. (2022) 90:e0043521–. doi: 10.1128/iai.00435-21
96. Badaut C, Visitdesotrakul P, Chabry A, Bigey P, Tornyigah B, Roman J, et al. IgG acquisition against PfEMP1 PF11_0521 domain cassette DC13, DBLβ3_D4 domain, and peptides located within these constructs in children with cerebral malaria. Sci Rep. (2021) 11:3680. doi: 10.1038/s41598-021-82444-5
97. Kessler A, Campo JJ, Harawa V, Mandala. WL, Rogerson SJ, Mowrey WB, et al. Convalescent Plasmodium falciparum-specific seroreactivity does not correlate with paediatric malaria severity or Plasmodium antigen exposure. Malar J. (2018) 17:178. doi: 10.1186/s12936-018-2323-4
98. Joste V, Guillochon E, Fraering J, Vianou B, Watier L, Jafari-Guemouri S, et al. PfEMP1 A-type ICAM1-binding domains are not associated with cerebral malaria in Beninese children. mBio. (2020) 11:e02103–20. doi: 10.1128/mBio.02103-20
99. Olsen RW, Ecklu-Mensah G, Bengtsson A, Ofori MF, Lusingu JPA, Castberg FC, et al. Natural and vaccine-induced acquisition of cross-reactive igG-inhibiting ICAM1-specific binding of a plasmodium falciparum pfEMP1 subtype associated specifically with cerebral malaria. Infect Immun. (2018) 86:e00622-17. doi: 10.1128/IAI.00622-17
100. Tessema SK, Nakajima R, Jasinskas A, Monk SL, Lekieffre L, Lin E, et al. Protective immunity against severe malaria in children is associated with a limited repertoire of antibodies to conserved pfEMP1 variants. Cell Host Microbe. (2019) 26:579–590.e5. doi: 10.1016/j.chom.2019.10.012
101. Suurbaar J, Moussiliou A, Tahar R, Olsen RW, Adams Y, Dalgaard N, et al. ICAM1-binding Plasmodium falciparum erythrocyte membrane protein 1 variants elicits opsonic-phagocytosis IgG responses in Beninese children. Sci Rep. (2022) 12:12994. doi: 10.1038/s41598-022-16305-0
102. Walker IS, Dini S, Aitken EH, Damelang T, Hasang W, Alemu A, et al. A systems serology approach to identifying key antibody correlates of protection from cerebral malaria in Malawian children. BMC Med. (2024) 22:388. doi: 10.1186/s12916-024-03604-8
103. Deb B, Das A, Vilvadrinath R, Jangra A, Shukla MS, Akhouri RR, et al. Glycophorin B-PfEMP1 interaction mediates robust rosetting in Plasmodium falciparum. Int J Biol Macromol. (2024) 262(Pt 1):129868. doi: 10.1016/j.ijbiomac.2024.129868
104. Stevenson L, Huda P, Jeppesen A, Laursen E, Rowe JA, Craig A, et al. Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell Microbiol. (2015) 17:819–31. doi: 10.1111/cmi.12403
105. Oleinikov AV, Seidu Z, Oleinikov IV, Tetteh M, Lamptey H, Ofori MF, et al. Profiling the plasmodium falciparum erythrocyte membrane protein 1-specific immununoglobulin G response among Ghanaian children with hemoglobin S and C. J Infect Dis. (2024) 229:203–13. doi: 10.1093/infdis/jiad438
106. Arora G, Hart GT, Manzella-Lapeira J, Doritchamou JYA, Narum DL, Thomas LM, et al. NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. Elife. (2018) 7:e36806. doi: 10.7554/eLife.36806
107. Larsen MD, Lopez-Perez M, Dickson EK, Ampomah P, Tuikue Ndam N, Nouta J, et al. Afucosylated Plasmodium falciparum-specific IgG is induced by infection but not by subunit vaccination. Nat Commun. (2021) 12:5838. doi: 10.1038/s41467-021-26118-w
108. Ty M, Sun S, Callaway PC, Rek J, Press KD, van der Ploeg K, et al. Malaria-driven expansion of adaptive-like functional CD56-negative NK cells correlates with clinical immunity to malaria. Sci Transl Med. (2023) 15:eadd9012. doi: 10.1126/scitranslmed.add9012
109. Opi DH, Kurtovic L, Chan JA, Horton JL, Feng G, and Beeson JG. Multi-functional antibody profiling for malaria vaccine development and evaluation. Expert Rev Vaccines. (2021) 20:1257–72. doi: 10.1080/14760584.2021.1981864
110. Rathnayake D, Aitken EH, and Rogerson SJ. Beyond binding: the outcomes of antibody-dependent complement activation in human malaria. Front Immunol. (2021) 12:683404. doi: 10.3389/fimmu.2021.683404
111. Larsen MD, del Pilar Quintana M, Ditlev SB, Bayarri-Olmos R, Ofori MF, Hviid L, et al. Evasion of classical complement pathway activation on Plasmodium falciparum-infected erythrocytes opsonized by PfEMP1-Specific IgG. Front Immunol. (2019) 9:3088. doi: 10.3389/fimmu.2018.03088
112. Vigan-Womas I, Lokossou A, Guillotte M, Juillerat A, Bentley G, Garcia A, et al. The humoral response to Plasmodium falciparum VarO rosetting variant and its association with protection against malaria in Beninese children. Malar J. (2010) 9:267. doi: 10.1186/1475-2875-9-267
113. Elliott SR, Duffy MF, Byrne TJ, Beeson JG, Mann EJ, Wilson DW, et al. Cross-reactive surface epitopes on chondroitin sulfate A-adherent Plasmodium falciparum-infected erythrocytes are associated with transcription of var2csa. Infect Immun. (2005) 73:2848–56. doi: 10.1128/IAI.73.5.2848-2856.2005
114. Diebolder CA, Beurskens FJ, De Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. (2014) 343:1260–3. doi: 10.1126/science.1248943
115. Garcia-Senosiain A, Kana IH, Singh S, Das MK, Dziegiel MH, Hertegonne S, et al. Neutrophils dominate in opsonic phagocytosis of P. falciparum blood-stage merozoites and protect against febrile malaria. Commun Biol. (2021) 4:984. doi: 10.1038/s42003-021-02511-5
116. Feng G, Wines BD, Kurtovic L, Chan JA, Boeuf P, Mollard V, et al. Mechanisms and targets of Fcγ-receptor mediated immunity to malaria sporozoites. Nat Commun. (2021) 12:1742. doi: 10.1038/s41467-021-21998-4
117. Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Gloria Meng Y, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. (2002) 277:26733–40. doi: 10.1074/jbc.M202069200
118. Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of fc-receptor- and complement-mediated-effector activities. Front Immunol. (2017) 8:877. doi: 10.3389/fimmu.2017.00877
119. Oosterhoff JJ, Larsen MD, van der Schoot CE, and Vidarsson G. Afucosylated IgG responses in humans – structural clues to the regulation of humoral immunity. Trends Immunol. (2022) 43:800–14. doi: 10.1016/j.it.2022.08.001
120. Lopez-Perez M, Seidu Z, Larsen MD, Wang W, Nouta J, Wuhrer M, et al. Acquisition of Fc-afucosylation of PfEMP1-specific IgG is age-dependent and associated with clinical protection against malaria. Nat Commun. (2024) 16:1. doi: 10.21203/rs.3.rs-4165378/v1
121. Mandala WL, Msefula CL, Gondwe EN, Gilchrist JJ, Graham SM, Pensulo P, et al. Lymphocyte perturbations in Malawian children with severe and uncomplicated malaria. Clin Vaccine Immunol. (2015) 23:95–103. doi: 10.1128/CVI.00564-15
122. Molfetta R, Quatrini L, Gasparrini F, Zitti B, Santoni A, and Paolini R. Regulation of Fc receptor endocytic trafficking by ubiquitination. Front Immunol. (2014) 5:449. doi: 10.3389/fimmu.2014.00449
123. Virág D, Dalmadi-Kiss B, Vékey K, Drahos L, Klebovich I, Antal I, et al. Current trends in the analysis of post-translational modifications. Chromatographia. (2020) 83:1–10. doi: 10.1007/s10337-019-03796-9
124. Vatsa S. In silico prediction of post-translational modifications in therapeutic antibodies. MAbs. (2022) 14(1):2023938. doi: 10.1080/19420862.2021.2023938
125. Reyes RA, Raghavan SSR, Hurlburt NK, Introini V, Bol S, Kana IH, et al. Broadly inhibitory antibodies to severe malaria virulence proteins. Nature. (8041) 2024:636. doi: 10.1038/s41586-024-08220-3
126. Doritchamou JYA, Renn JP, Jenkins B, Fried M, and Duffy PE. A single full-length VAR2CSA ectodomain variant purifies broadly neutralizing antibodies against placental malaria isolates. eLife. (2022) 11:e76264. doi: 10.7554/eLife.76264
127. Roberts DJ, Craig AG, Berendt AR, Pinches R, Nash G, Marsh K, et al. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. (1992) 357:689–92. doi: 10.1038/357689a0
128. Zelter T, Strahilevitz J, Simantov K, Yajuk O, Adams Y, Ramstedt Jensen A, et al. Neutrophils impose strong immune pressure against PfEMP1 variants implicated in cerebral malaria. EMBO Rep. (2022) 23(6):e53641. doi: 10.15252/embr.202153641
129. Othman B, Zeef L, Szestak T, Rchiad Z, Storm J, Askonas C, et al. Different PfEMP1-expressing Plasmodium falciparum variants induce divergent endothelial transcriptional responses during co-culture. PloS One. (2023) 18:e0295053. doi: 10.1371/journal.pone.0295053
130. Olsen RW, Suurbaar J, and Jensen AR. Analysis of antibody inhibition of pfEMP1 binding by competition ELISA. Methods Mol Biol. (2022) 2470:485–91. doi: 10.1007/978-1-0716-2189-9_36
131. Piatti L, Howard CC, Zheng Y, and Bernabeu M. Binding of plasmodium falciparum-infected red blood cells to engineered 3D microvessels. Methods Mol Biol. (2022) 2470:557–85. doi: 10.1007/978-1-0716-2189-9_43
132. Bernabeu M, Gunnarsson C, Vishnyakova M, Howard CC, Nagao RJ, Avril M, et al. Binding heterogeneity of plasmodium falciparum to engineered 3D brain microvessels is mediated by EPCR and ICAM1. mBio. (2019) 10:e00420–19. doi: 10.1128/mBio.00420-19
Keywords: Plasmodium falciparum Erythrocyte Membrane Protein 1 (PfEMP1), cerebral malaria (CM), severe malaria (SM), antibodies, naturally acquired immunity
Citation: Banda JP, Walker IS, Nyirenda T, Aitken EH and Rogerson SJ (2025) Breaking the bind: PfEMP1-specific antibodies in cerebral malaria. Front. Immunol. 16:1681852. doi: 10.3389/fimmu.2025.1681852
Received: 08 August 2025; Accepted: 07 October 2025;
Published: 30 October 2025.
Edited by:
Wang Nguitragool, Mahidol University, ThailandReviewed by:
Krishanpal Karmodiya, Indian Institute of Science Education and Research, Pune, IndiaBhagyashree Deshmukh, Seattle Biomedical Research Institute, United States
Copyright © 2025 Banda, Walker, Nyirenda, Aitken and Rogerson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen J. Rogerson, c3JvZ2VyQHVuaW1lbGIuZWR1LmF1
 Josephine P. Banda1,2
Josephine P. Banda1,2 Elizabeth H. Aitken
Elizabeth H. Aitken Stephen J. Rogerson
Stephen J. Rogerson