- 1Department of Clinical Laboratory, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Institute for Bacterial Diseases, Jinan Center for Disease Control and Prevention Institute for Bacterial Diseases, Jinan, China
- 3Emergency Department, Qilu Hospital of Shandong University, Jinan, China
Metabolic (dysfunction)-associated fatty liver disease (MAFLD) has emerged as a leading cause of chronic liver disease worldwide. Its pathogenesis is closely associated with gut microbiota dysbiosis and metabolic disturbances. In recent years, numerous studies have demonstrated that bioactive compounds produced by gut microbial metabolism—such as short-chain fatty acids, secondary bile acids, tryptophan derivatives, and bacterial extracellular vesicles—play critical roles in the development and progression of MAFLD by modulating hepatic lipid metabolism, inflammatory responses, and epigenetic regulation. The characteristic expression patterns of these gut microbiota-derived bioactive compounds provide novel options for differential diagnosis of the disease. Moreover, elucidation of the underlying pathological mechanisms has paved novel avenues for MAFLD treatment. Strategies including dietary interventions, prebiotics, probiotics, and other microbiota-targeted therapies are considered potential approaches to modulate MAFLD progression. This review systematically summarizes the molecular mechanisms underlying the development of MAFLD influenced by gut microbiota-derived bioactive compounds. It also explores the feasibility of utilizing specific gut microbial metabolite profiles for MAFLD diagnosis and highlights potential therapeutic strategies targeting microbiota-host metabolic interactions, including the use of engineered bacteria to produce specific metabolites, probiotic/prebiotic interventions, and the clinical prospects of fecal microbiota transplantation.
1 Introduction
Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) is a metabolic disorder characterized primarily by hepatic fat accumulation resulting from metabolic dysfunction (1). It has become the most prevalent chronic liver disease globally (2). With the widespread adoption of nutrient-rich dietary patterns, the global burden of MAFLD continues to rise, with its reported prevalence increasing from 21.9% in 1990 to 32.4% in 2021 (3, 4). Current diagnostic methods for MAFLD include non-invasive assessments and liver biopsy. Among the non-invasive methods, imaging-based detection of hepatic steatosis is common, whereas the abdominal ultrasound alone has a sensitivity of only 20%. Controlled attenuation parameter (CAP) derived from vibration-controlled transient elastography (VCTE) and liver stiffness measurement (LSM) is primarily used for qualitative assessment, while its correlation with disease severity is limited. Although the magnetic resonance imaging (MRI) remains the gold standard for quantifying hepatic fat, it is not ideal for screening due to its high cost, time consumption, and limited accessibility. Ultrasound-guided liver biopsy, though useful for confirming atypical cases, is limited by sampling errors, cost, and potential complications (5, 6). Beyond the diagnostic challenges, therapeutic options for MAFLD remain limited. The first-line treatment still relies on lifestyle modifications such as dietary control, weight management, and physical activity. Although several drugs targeting hepatic fibrosis and inflammation have entered clinical trials, none have been approved for routine clinical use (7).
The gut microbiota (GM), a complex community of microorganisms—including bacteria, archaea, fungi, and viruses—that colonize the human gastrointestinal tract, plays a key role in host nutrient metabolism, intestinal barrier maintenance, immune system development, and metabolic homeostasis (8). In various metabolic pathways, the gut microbiota ferments dietary fibers and polysaccharides indigestible by the host, producing short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate (9). These metabolites serve as major energy sources for intestinal epithelial cells and regulate immune responses and energy metabolism. Dysbiosis, commonly observed in MAFLD patients, is marked by reduced microbial diversity and imbalanced composition, which in turn alters the types and quantities of gut microbiota-derived bioactive compounds and contributes to MAFLD pathogenesis (10).
In recent years, therapeutic agents and strategies for MAFLD targeting gut microbiota-derived bioactive compounds have emerged. However, existing reviews predominantly focus on broader microbiota compositional changes or generic “microbiota-targeted” therapeutics (11, 12), do not adequately cover the diagnostic/therapeutic potential of defined microbial metabolites. Accordingly, this review aims to synthesize current knowledge by elucidating the pathogenic mechanisms of gut microbiota-derived bioactive compounds in MAFLD, summarizing their diagnostic applications, and exploring targeted therapeutic strategies, thereby providing a comprehensive perspective toward clinical translation.
2 Major bioactive compounds derived from gut microbiota
2.1 Short-chain fatty acids
2.1.1 Metabolism, functions, and targets of short-chain fatty acids
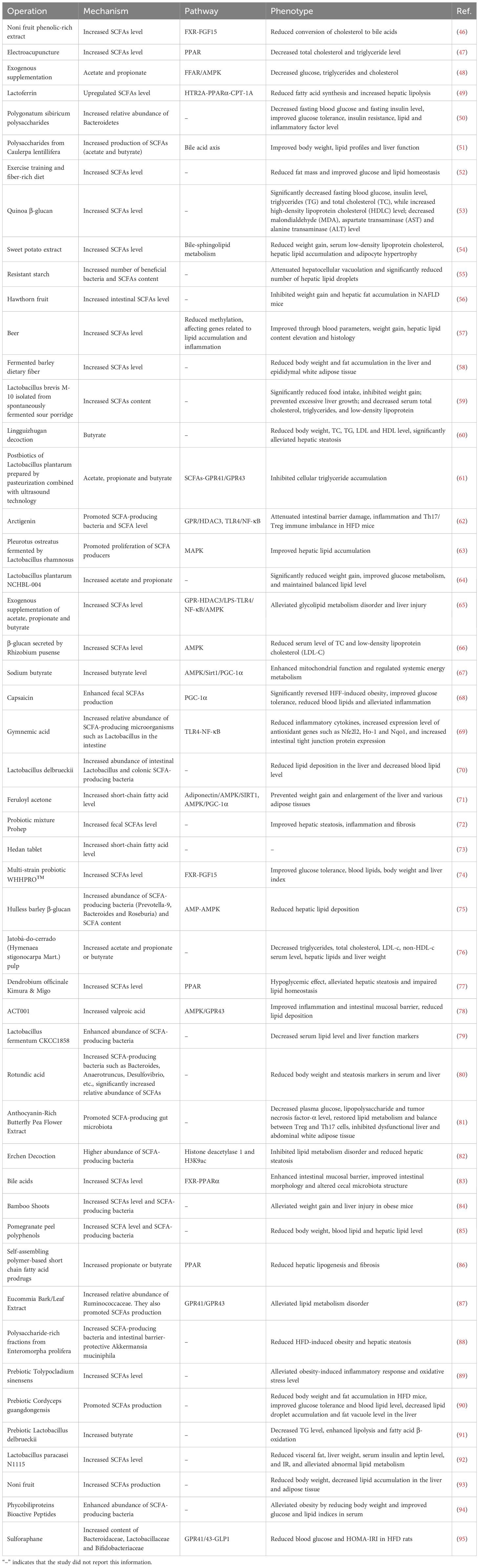
SCFAs are primarily produced via the fermentation of dietary fibers by anaerobic bacteria in the colon. These bacteria first break down dietary fiber into monosaccharides, which are then metabolized into acetate, propionate, as well as butyrate through various fermentation pathways. In addition to dietary fibers, host-derived mucins and certain amino acids and organic acids can also serve as substrates for SCFA production (13, 14). Acetate is produced by most gut bacteria, whereas propionate and butyrate are synthesized via species- and pathway-specific mechanisms—propionate mainly by Bacteroides via the succinate pathway, and butyrate by various bacteria within the Firmicutes phylum. SCFAs play central roles in energy metabolism and immune regulations (15). In host tissues, SCFAs can be converted into acetyl-CoA (based on acetate and β-oxidized butyrate) or succinyl-CoA (from propionate) to enter the tricarboxylic acid cycle (TCA) for energy production, or used for lipogenesis and gluconeogenesis (16). Each SCFA has its distinct biological roles (Table 1). Acetate primarily participates in energy metabolism and circulates through the portal vein, ultimately utilized by the liver and peripheral tissues. In the liver, acetate can support gluconeogenesis or fatty acid synthesis (17). At optimal concentrations, acetate also activates G-protein-coupled receptors (GPCRs), particularly GPR43, on immune cells in the portal system. Propionate is mainly metabolized in the liver to activate GPCRs, such as GPR41 and GPR43,and exert immune-regulatory functions (18). Butyrate is mainly metabolized in the colon and serves as the preferred energy source for colonocytes. Although butyrate can enter the portal circulation, its concentration in the portal vein (approximately 0.29 mM in pigs) is significantly lower than that of acetate (3.8 mM) and propionate (1.1 mM) (19). Butyrate also acts as a histone deacetylase (HDAC) inhibitor, promoting tight junction formation and mucin secretion to maintain gut barrier integrity and prevent translocation of toxins and microbial metabolites (20, 21). It further activates the AMPK pathway (22), enhancing insulin sensitivity in both hepatocytes and adipocytes, improving glucose metabolism, and reducing hepatic lipid accumulation (23). Additionally, butyrate increases the levels of anti-inflammatory cytokines (e.g., IL-10) (24) and suppresses pro-inflammatory mediators (e.g., TNF-α and IL-6) (25).
SCFAs primarily exert their effects via GPCRs that regulate physiological functions, such as hormone secretion, glucose and lipid metabolism, and immune responses (30). GPR43 has high affinity for acetate and propionate, while GPR41 has high affinity for propionate and butyrate (18). Recent studies have shown that SCFAs can reduce levels of gut pH, activating the anti-inflammatory Gαs-coupled receptor GPR65 on intestinal epithelial and immune cells (31), while other receptors, including the butyrate-specific GPR109A (32) and OLFR78 (33), are activated by acetate and propionate. However, SCFA-based treatment approaches should consider the dose effect. Some reports indicate a positive correlation between pro-inflammatory biomarkers and butyrate and propionate. Therefore, more research is needed to elucidate the mechanisms and dose effects before SCFAs supplementation is widely approved for MAFLD (34).
2.1.2 Short-chain fatty acids involved in MAFLD
In MAFLD, disruptions in microbial metabolic functions lead to significant changes in the levels, proportions, and distributions of SCFAs (35). The total quantity of SCFAs is reduced due to insufficient fiber intake and microbial dysbiosis (36, 37). Particularly, the levels of protective SCFAs—propionate and butyrate—are markedly decreased, as evidenced in portal vein, peripheral blood, and fecal samples (38). The reduction in butyrate is especially critical, as it compromises energy supply to colonocytes, damages the intestinal barrier (i.e., “leaky gut”), and facilitates translocation of endotoxins (e.g., lipopolysaccharides), aggravating hepatic inflammation and accelerating the progression from simple steatosis to nonalcoholic steatohepatitis (NASH) and fibrosis (39). Furthermore, lower butyrate levels weaken the AMPK/PPARα signaling, reducing fatty acid oxidation and insulin sensitivity, thereby exacerbating gluconeogenesis and lipogenesis (40–43). Importantly, hepatic steatosis, inflammation, and insulin resistance in MAFLD further hinder SCFA transport and utilization, forming a positive feedback loop. Numerous studies have confirmed that restoring the levels of total SCFAs—especially propionate and butyrate—can exert therapeutic effects on the treatment of MAFLD, underscoring their upstream regulatory roles (Table 2).
Given the well-established positive feedback effect of SCFAs in MAFLD, targeting SCFAs for therapeutic intervention has become an attractive strategy, with the potential to serve as an ideal causal treatment by modulating SCFA levels. Although numerous preclinical studies have demonstrated the benefits of SCFA supplementation, clinical trials primarily aimed at increasing SCFAs remain limited and have yet to yield strongly positive endpoint conclusions. Several ongoing clinical strategies focus on restoring SCFA levels through dietary fiber interventions or prebiotic/probiotic supplementation. A clinical trial investigating cellulose supplementation has not reported results (NCT04520724). Similarly, two clinical trials evaluating SCFA-producing probiotics have not yet published outcomes (NCT06491342, NCT05402449) (44). Another study on a synbiotic formulation designed to boost SCFAs is still ongoing (NCT05821010). Therefore, the clinical value of SCFAs requires further validation through more clinical studies rather than additional preclinical research. Important future directions include developing precise delivery systems for SCFAs or their analogs to enhance colonic bioavailability, and defining optimal SCFA intervention strategies for different stages of MAFLD. Since SCFA levels can be influenced by multiple metabolic factors, constructing composite diagnostic models that incorporate SCFAs represents a feasible approach for using SCFAs as non-invasive biomarkers for MAFLD. For instance, Lin et al. developed an integrated model including physical indicators, laboratory parameters, and gut metabolite SCFAs, which demonstrated strong diagnostic performance (AUC=0.938) (45). However, the clinical applicability of such models depends on validation in multicenter studies and their ability to differentiate MAFLD from other conditions.
2.2 Bile acids
2.2.1 Metabolism, functions, and targets of bile acids
Primary bile acids (BAs) are synthesized in the liver and metabolized by gut microbiota (primarily including Bacteroides, Clostridium, Lactobacillus, Bifidobacterium, etc.) into secondary BAs (96). The ratio of primary to secondary BAs plays a key role in regulating lipid absorption efficiency and metabolic signaling transduction (97, 98). As signaling molecules, both primary and secondary BAs can act as ligands for the farnesoid X receptor (FXR) (99). Upon activation of intestinal FXR, it stimulates enteroendocrine cells to secrete fibroblast growth factor 15/19 (FGF15/19) (100). Once the hormone reaches the liver, it binds to specific receptors on the liver cell membrane (a complex formed by FGFR4 and the co-receptor β-Klotho), which subsequently inhibits the BA synthesis enzyme CYP7A1 (101, 102). This creates a negative feedback loop regulating the size of the BA pool, while simultaneously improving glucose and lipid metabolism, i.e., inhibiting lipogenesis, promoting fatty acid oxidation, and enhancing insulin sensitivity (99). Additionally, BAs can activate the G protein-coupled bile acid receptor 1 (TGR5), exerting effects on enteroendocrine cells, macrophages, hepatocytes (103), and other cell types, for example, promoting GLP-1 release to improve glucose metabolism and satiety and inhibiting macrophage inflammatory responses to reduce inflammation (104, 105).
BAs also possess antimicrobial activity. Changes in their composition and concentration directly impact the structure of the gut microbiota (106). Conversely, dysregulation of BA metabolism can lead to dysbiosis, e.g., an increase in the relative proportion of lipopolysaccharide (LPS)-producing bacteria, forming an interacting cycle (107).
2.2.2 Bile acid metabolism in MAFLD
BA metabolism is under tight control through a complex feedback loop involving the liver, gut, and gut microbiota. In MAFLD patients, abnormalities often occur in BA synthesis, metabolism, and signaling (108, 109). Specifically, signaling through the receptors FXR and TGR5 is frequently reduced, which links to problems like insulin resistance, fat accumulation in the liver (steatosis), and inflammation (98).
Studies have shown that the levels and types of BAs are altered in MAFLD patients, detected in their liver, blood, and stool (110). Typically, in the blood, the levels of primary BAs, such as cholic acid (CA) and chenodeoxycholic acid (CDCA), are increased. A key enzyme responsible for making 12α-hydroxylated (12-OH) BAs, called CYP8B1, is also activiated (111). This leads to a significant increasd in the levels of 12-OH BAs like CA and deoxycholic acid (DCA). Importantly, the ratio of these 12-OH BAs to non-12-OH BAs serves as a key marker for metabolic performance in MAFLD, correlating strongly with the levels of liver fat and inflammation (108, 112). Conversely, the levels of non-12-OH BAs, such as ursodeoxycholic acid (UDCA) and lithocholic acid (LCA), tend to be lower in MAFLD patients. Since these acids often have protective anti-inflammatory effects, the reduction in the levels of these acids could worsen the disease (98). Excretion of secondary bile acids like DCA and LCA is also reduced. This happens because imbalances in gut bacteria impair the conversion of primary to secondary BAs. Consequently, this limits the ability of these secondary acids to activate beneficial receptors like FXR and TGR5, which normally regulate fat metabolism and reduce inflammation. Therefore, finding ways to restore a healthy balance (homeostasis) in BA metabolism is considered a promising potential treatment for MAFLD (113).
Therapeutic strategies targeting BAs for MAFLD warrant extensive exploration. Bile acid signaling pathways, particularly those involving FXR and TGR5, are emerging as key pharmacological targets for metabolic diseases. Obeticholic acid (6α-ethyl-chenodeoxycholic acid, an FXR agonist) has been shown to improve histological features of NASH and demonstrated favorable outcomes in a Phase III clinical trial (NCT01265498) (114–116). Two novel FXR agonists, cilofexor and TQA352, have successfully passed clinical safety assessments (NCT02781584, ChiCTR1800019570) (117). The development of TGR5 agonists aims to leverage their ability to promote GLP-1 secretion and suppress inflammation, thereby improving glycemic control and mitigating hepatic inflammation. One clinical study observed increased TGR5 expression levels in peripheral blood mononuclear cells and reduced liver fat content in patients following curcumin supplementation (ChiCTR2200058052) (118).
It is important to note that targeting these pathways requires precise balance. For instance, obeticholic acid has been associated with side effects such as an increased risk of drug-induced liver injury, unfavorable changes in lipid levels, and severe pruritus (119–121), leading the FDA to deny its conditional approval for NASH. This underscores the importance of developing tissue-specific agonists/antagonists. Beyond directly targeting signaling pathways, other BA-focused therapeutic strategies should be considered. For example, modifying the bile acid pool composition using non-12-OH bile acids like UDCA and its derivatives has shown promise. An 18-week treatment with berberine ursodeoxycholate resulted in histological improvement in most MAFLD patients, with dose-dependent improvements observed across various biomarkers (NCT03656744) (122). Furthermore, a clinical study demonstrated that aerobic exercise increased total bile acid and ursodeoxycholic acid levels in MAFLD patients, significantly improving body composition and liver function while also reducing blood lipid and glucose levels (NCT06338449). Additionally, microbiome intervention strategies—using probiotics or prebiotics to restore microbial function and promote the production of secondary bile acids (e.g., LCA)—should be explored to achieve natural and mild activation of the FXR/TGR5 pathways. On the diagnostic front, analyzing the bile acid pool represents a potential non-invasive strategy, but it should be combined with other diagnostic approaches to avoid confounding factors from gallbladder diseases.
2.3 Tryptophan derivatives
2.3.1 Metabolism, functions, and targets of tryptophan derivatives
Gut microbiota uses tryptophan as a precursor to generate various derivatives through specific enzyme systems (123). Bacteria like Clostridium, Bacteroides, and Bifidobacterium are involved, producing compounds such as indole, indole-3-propionic acid (IPA), tryptamine, and indole-3-acetic acid (IAA) (124). For example, Enterobacteriaceae produce indole by deaminating tryptophan via tryptophanase (125); Lactobacillus sp. generates tryptamine catalyzed by aromatic amino acid decarboxylase (126); Clostridium creates IPA through hydroxylation (127); and Bacteroides synthesizes IAA (128–130). Once absorbed by the host, these metabolites undergo further processing, i.e., indole is oxidized by hepatic CYP2E1 (131) or SULT1A1 (132) into indoxyl, then sulfated or glucuronidated for excretion; IPA acts freely after sulfation; and tryptamine is degraded by host monoamine oxidase (MAO) into indoleacetic acid.
Tryptophan derivatives support gut barrier integrity, immune regulation, and metabolic control (133). Indole and IPA activate the aryl hydrocarbon receptor (AhR), boosting expression of tight junction proteins (occludin and claudin-1) and mucins (MUC2), strengthening the gut barrier (134). IAA accelerates mucosal repair by regulating intestinal stem cell proliferation. Through AhR activation, indole promotes regulatory T cell (Treg) differentiation and IL-10 secretion, curbing Th17-driven gut inflammation (127), while IPA inhibits pro-inflammatory cytokines like TNF-α and IL-6 from macrophages (135), exerting its anti-inflammatory effects by regulating AhR-NLRP3 axis (136).
2.3.2 Tryptophan derivatives in MAFLD
Studies have shown an inverse link between indole levels and liver fat content—obese individuals typically have lower indole and higher hepatic fat (137). The beneficial roles of tryptophan derivatives in MAFLD are well-documented (138, 139). Indole and its derivatives exhibit anti-inflammatory properties by increasing the levels of IL-10 andinhibiting TNF-α–driven NF-κB activation and pro-inflammatory chemokine IL-8 expression. This helps maintain gut barrier functions and has been demonstrated in both cellular and animal models to reduce liver steatosis and fibrosis (140, 141). In addition, targeting the AhR pathway has been shown to inhibit lipid accumulation in the liver, reduce the level of triglycerides and total cholesterol, and alleviate oxidative stress (142). Research by Ding et al. demonstrated that exogenous administration of indole-3-acetate (I3A) improved hepatic pathology without altering the gut microbiota state, suggesting its direct effect on hepatic metabolic function (143). Indoleamine 2,3-dioxygenase (IDO), a key rate-limiting enzyme in gut tryptophan metabolism, catalyzes the conversion from tryptophan to kynurenine. Research in high-fat-diet-fed IDO-knockout (IDO-/-) mice revealed less inflammatory macrophage infiltration and reduced susceptibility to obesity-linked fatty liver and insulin resistance (144) suggesting the beneficial roles of indole and its derivatives in MAFLD.
In MAFLD, tryptophan metabolism shifts towards the detrimental kynurenine pathway, while the beneficial microbial pathways (such as the production of AhR agonists) are suppressed. This imbalance directly contributes to intestinal barrier disruption, systemic inflammation, and hepatic steatosis (145). Therefore, restoring tryptophan metabolic balance represents an etiology-targeting strategy. Potential therapeutic approaches include using specific probiotics to remodel gut microbiota function and enhance the production of endogenous beneficial metabolites, as well as exploring drugs like IDO1 inhibitors to reduce the generation of pro-inflammatory kynurenines. From a diagnostic perspective, blood levels of indole/IPA or the indole/kynurenine ratio show promise as non-invasive biomarkers for assessing gut ecological function and liver disease severity. However, further research is needed to clarify the discriminatory power of such models.
2.4 Trimethylamine N-oxide
Gut microbes convert precursor substances such as choline, L-carnitine, and phosphatidylcholine ingested by the host into trimethylamine (TMA). The produced TMA enters the liver via the bloodstream, where it is oxidized by flavin-containing monooxygenases (FMOs) into trimethylamine N-oxide (TMAO) (146). In recent years, multiple studies have reported the correlation between TMAO levels and the pathogenesis of MAFLD. A clinical investigation by Ma et al. found that higher blood TMAO concentrations were associated with an increased risk of MAFLD (147). Additionally, fecal TMAO levels have been shown to correlate with the severity of MAFLD (148). Subsequently, numerous experimental studies have demonstrated that TMAO disrupts lipid metabolism and promotes the occurrence and progression of MAFLD (149, 150).
The promotion of MAFLD by TMAO involves multiple phenotypes and pathways. Some studies have reported that TMAO exacerbates hepatic steatosis by inhibiting bile acid (BA)-mediated hepatic FXR signaling (151). Furthermore, TMAO exhibits pro-inflammatory properties by activating the TLR4/MyD88/NF-κB signaling pathway, upregulating the expression of various inflammation-related genes, and simultaneously inducing the polarization of liver macrophages toward the pro-inflammatory M1 phenotype, thereby triggering liver inflammation (148). Novel mechanisms of TMAO-mediated MAFLD have also been discovered. For example, TMAO inhibits OTUB1-mediated SLC7A11 stability, leading to hepatocyte ferroptosis and accelerating MAFLD progression (152). TMAO upregulates the expression of HULC, followed by P38MAPK overexpression, thereby mediating hepatocyte apoptosis and promoting MAFLD development (148). Yang et al. found that TMAO can activate the PERK signaling pathway, subsequently inducing MAFLD (153).
However, it is important to note that conflicting research conclusions exist. Miyata et al. reported that after feeding FXR-null mice a diet containing 0.3% TMAO for 13 weeks, markers of liver injury were significantly reduced, suggesting that TMAO may improve liver function through pathways independent of bile acid metabolism (154). Therefore, more studies with variables such as dosage and administration duration are needed to clarify the precise role of TMAO in MAFLD.
The aforementioned findings establish TMAO as a significant risk biomarker and potential pathogenic factor in the onset and progression of MAFLD, warranting consideration for incorporating TMAO levels into diagnostic models aimed at assessing MAFLD risk and disease severity. It should be noted that the association of TMAO with conditions such as cardiovascular disease and cancer has been extensively reported (155, 156), suggesting the potential of developing composite biomarker diagnostic models based on TMAO. On the therapeutic front, evaluating dietary interventions that reduce the intake of precursor substances rich in choline and L-carnitine becomes important. Furthermore, the development of FMO enzyme inhibitors to block the conversion of TMA to TMAO, along with preclinical and clinical trials to validate their therapeutic value, represents a viable strategy.
2.5 Endotoxins
Endotoxins (i.e., LPS), major components of Gram-negative bacterial cell walls in Enterobacteriaceae, strongly activate the host innate immune system (157). In MAFLD, LPS acts as a key mediator of “leaky gut”—translocating into the liver via the portal vein when the intestinal barrier is compromised (158–160). As the primary ligand for Toll-like receptor 4 (TLR4), which is expressed in hepatocytes, Kupffer cells (liver macrophages), and hepatic stellate cells, LPS binding triggers downstream pathways like MyD88-dependent signaling (161). This activates transcription factors such as NF-κB, driving production of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and chemokines (162)—key mechanisms regulating liver inflammation, cell damage, and progression to NASH. TLR4 signaling also induces insulin resistance by disrupting insulin receptor substrate pathways (163). Sustained inflammation activates hepatic stellate cells, promoting extracellular matrix deposition and fibrosis. Besides TLR4, LPS recognition involves LPS-binding protein (LBP) and CD14 (164). Clinically, elevated LBP levels correlate with insulin resistance and dyslipidemia in non-alcoholic fatty liver disease (NAFLD) or NASH patients (165). In high-fat-diet MAFLD models, LBP-knockout mice show improved lipid metabolism and milder pathology (166). LPS can cleave membrane-bound CD14 (mCD14), releasing presepsin into circulation (167), while CD14 depletion reduces liver lipids and macrophage content, ultimately alleviating steatosis (168). Both MAFLD patients and animal models exhibit increased serum LPS (169). Studies have demonstrated that antibiotic treatment (e.g., polymyxin B targeting Gram-negative bacteria) effectively lowers TNF production and plasma LPS levels, reversing hepatic steatosis (170).
Therapeutic strategies targeting LPS hold promising potential for clinical exploration. As a central mediator linking “leaky gut” to hepatic inflammation, LPS acts as an accelerator in the pathogenesis of MAFLD. Its multi-faceted mechanisms—driving liver inflammation, insulin resistance, and fibrosis through the TLR4 signaling pathway—make it a valuable therapeutic target. From a diagnostic perspective, the levels of serum LPS, LBP, or CD14 should be considered as potential non-invasive biomarkers for evaluating intestinal barrier function and systemic inflammatory status.
2.6 Bacterial extracellular vesicles
Both Gram-positive and Gram-negative bacteria produce bacterial extracellular vesicles (BEVs) (171). Under normal physiological conditions, the liver manages the physiological stress caused by BEVs. However, in MAFLD, dysbiotic gut microbiota releases excessive BEVs loaded with bioactive bacterial components (e.g., LPS, bacterial DNA, and proteins) (172). These vesicles cross the compromised gut barrier (“leaky gut”) and enter the liver via the portal circulation. BEVs from different bacteria exert harmful effects through distinct molecular activations, i.e., outer membrane vesicles (OMVs) from Gram-negative bacteria are LPS-rich, with lipid A specifically recognized by TLR4 (173); cytoplasmic membrane vesicles from Gram-positive bacteria express lipoteichoic acid, activating TLR2 (174); and BEVs from pathogens like Porphyromonas gingivalis (with high LPS levels) induce M1 macrophage polarization and amplify pro-inflammatory responses (175). By delivering virulence factors directly to host cells, BEVs significantly contribute to inflammation and disease progression. Fizann et al.’s research reinforces the detrimental role of BEVs in disease states, demonstrating that administration of fecal-derived extracellular vesicles (fEVs) from NASH patients upregulates pro-fibrotic and pro-inflammatory protein expression in hepatic stellate cells and increases intestinal permeability in wild-type mice. Their study specifically highlighted the pathogenic contributions of nmMLCK and LPS carried within BEV cargo (176). Critically, additional evidence implicates BEV-carried DNA in pathology: Luo et al. demonstrated that Vsig4+ macrophage deficiency in disease states facilitates translocation of microbiota-containing extracellular vesicles (mEVs), leading to accumulation of microbial DNA in hepatocytes and hematopoietic stem cells. This subsequently activates the cGAS/STING signaling pathway, mediating inflammatory responses (177).
It should be noted that previous studies have also confirmed the beneficial effects of beneficial intestinal bacteria on disease phenotypes, for instance, BEVs from lactic acid bacteria demonstrate efficacy in reducing oxidative damage (178), and studies confirm the protective effects of Enterococcus faecium-derived EVs against ethanol-induced hepatic injury in rats (179). Therefore, the source of gut bacteria and the type of cargo content may constitute the pivotal determinant underlying the double-edged effects of BEVs. There is an urgent need for in-depth research to differentiate the roles of BEV origin and cargo composition in MAFLD (Figure 1).
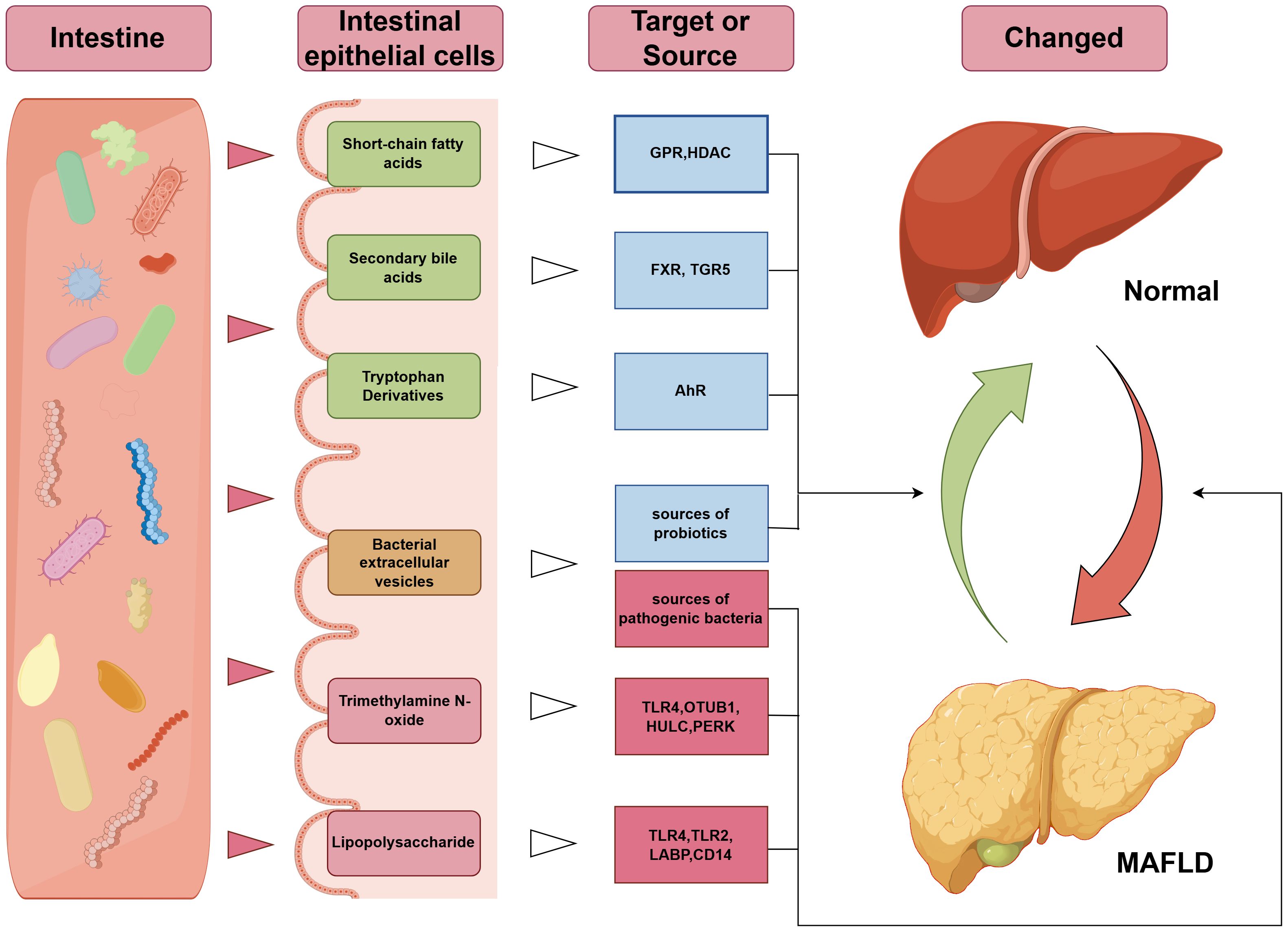
Figure 1. Mechanisms underlying the association between gut microbiota-related bioactive substances and MAFLD. The green terms in the “intestinal epithelial cells” column indicate beneficial components, yellow represents neutral components, and red indicates harmful components; the blue terms in the “Target or Source” column denote beneficial targets, while red denotes harmful targets. GPR, G-Protein Coupled Receptor; HDAC, Histone Deacetylase; FXR, Farnesoid X Receptor; TGR5, Takeda G-protein-coupled Receptor 5; AhR, Aryl Hydrocarbon Receptor; TLR, Toll-Like Receptor; OTUB1, OTU deubiquitinase, binary 1; HULC, Highly Upregulated in Liver Cancer; PERK, PKR-like Endoplasmic Reticulum Kinase; LBP, Lipopolysaccharide-Binding Protein; CD14, Cluster of Differentiation 14.
BEVs hold broad potential for exploration, primarily encompassing applications as non-invasive diagnostic biomarkers and targeted therapeutic vehicles. For instance, specific BEVs in the blood or their cargo—such as microbial DNA and proteins—could serve as novel non-invasive biomarkers for assessing gut microbiota status and MAFLD disease activity. Additionally, engineered exosomes derived from intestinal probiotics are being investigated for their ability to precisely deliver anti-inflammatory or metabolic regulatory factors, combining favorable biocompatibility with therapeutic efficacy.
2.7 Crosstalk among bioactive substances
It is important to note that gut microbiota-derived bioactive substances do not exert their biological functions independently but rather regulate each other’s metabolic homeostasis through various crosstalk mechanisms, thereby achieving integrated regulation of hepatic lipid metabolism. Studies have reported the mechanisms by which SCFAs influence bile acid metabolism. Tolhurst et al. discovered that short-chain fatty acids can trigger the secretion of glucagon-like peptide-1 (GLP-1) (180). In a clinical study, GLP-1 agonists demonstrated even better efficacy in treating bile acid diarrhea than the standard-of-care bile acid sequestrant colesevelam (181), suggesting that short-chain fatty acids play a positive role in stabilizing bile acid metabolism. Lu et al. indicated that short-chain fatty acids activate the FXR-FGF15-CYP7A1 pathway, reducing bile acid synthesis and improving bile acid metabolism (182). Crosstalk between tryptophan and bile acid metabolism has also been reported. Chen et al. found through exogenous supplementation in mice that tryptophan inhibits intestinal FXR signaling and promotes hepatic bile acid synthesis and excretion, accompanied by elevated levels of conjugated bile acids and an increased ratio of non-12-OH to 12-OH bile acids in hepatic and fecal bile acid profiles (183). IDO-1 is the rate-limiting enzyme in tryptophan degradation. However, Qiao et al. demonstrated that inhibition of IDO-1 expression leads to a decrease in SCFA levels (184), suggesting a crosstalk effect between tryptophan or its derivatives and SCFAs, though further research is needed to elucidate the underlying mechanisms. In summary, crosstalk exists among gut bioactive substances such as SCFAs, bile acids, and tryptophan metabolites. Abnormal levels of any of these gut microbiota-derived bioactive compounds may impact the levels of others, ultimately leading to changes in the overall metabolic network. More research is needed to achieve a deeper understanding of this network, which could aid in the formulation of postbiotic combination therapies.
3 Diagnostic potential of gut microbiota-derived bioactive compounds in MAFLD
The dysregulation of the gut microbiome and its bioactive compounds is closely linked to MAFLD progression, offering a promising diagnostic tool for MAFLD (36). Advances in multi-omics technologies have made it feasible to leverage gut microbiota-derived bioactive compounds for MAFLD diagnosis (12). For instance, Zhang et al. studied 60 MAFLD patients and developed a metabolomics model centered on propionate and butyrate analogues as key differentially expressed markers. This model achieved an AUC of 0.94, outperforming phenomics (AUC=0.91) and gut metagenomics (AUC=0.78), and combined metabolomics-phenomics model further improved diagnostic accuracy (AUC=0.97) (185). Beyond distinguishing MAFLD from healthy individuals, these compounds can also identify MAFLD comorbidities. For example, Li et al. used a random forest (RF) machine learning algorithm integrating gut metagenomics and plasma metabolomics to recognize MAFLD patients at risk of cardiovascular disease (186). Furthermore, monitoring disease progression using gut microbiota-derived bioactive compounds has also been reported. Luo et al. demonstrated through targeted metabolomics that nine metabolites are involved in the metabolic reprogramming of MAFLD-related inflammation. They constructed a machine learning model using seven of these inflammation-related metabolites to assess MAFLD disease progression (187). Lin et al. established a comprehensive model incorporating short-chain fatty acid/tryptophan metabolites and clinical variables such as arm circumference, which showed good predictive power for severe liver steatosis (45). In addition to building diagnostic models, some studies have reported independent risk metabolites for MAFLD. Barrea et al. found that Trimethylamine N-oxide (TMAO) can serve as an early biomarker for adipose tissue dysfunction and NAFLD even in the absence of overt metabolic syndrome, suggesting that a TMAO-based threshold could help identify the NAFLD population (188).
BEVs deserve special attention as diagnostic tools due to their accessibility and non-invasive nature. Their stronger immunogenicity compared to host-derived vesicles enhances diagnostic specificity (189). Studies have shown significantly elevated LPS-positive BEVs in plasma from patients with gut barrier dysfunction, correlating positively with plasma ZO-1 levels, indicating reduced mucosal integrity and increased permeability. This positions LPS-positive BEVs as promising biomarkers for intestinal barrier damage (172, 190). However, diagnostic potential varies based on the sources of BEVs source, i.e., fecal BEVs are abundant but prone to environmental contamination during collection, limiting their accuracy for systemic conditions, while blood-derived BEVs reflect more accurately the whole-body status but face technical challenges in isolation and characterization due to low biomass, typically yielding less material than fecal samples (191).
Despite these encouraging findings, the current research landscape has limitations. Many studies are preliminary, with small sample sizes, and lack validation in large, independent cohorts. The diagnostic models often require further refinement for clinical application. For BEVs, standardized protocols for isolation and characterization are urgently needed. Therefore, while existing evidence robustly confirms the principle that microbial products can serve as biomarkers, future research must focus on translational validation, standardization of assays, and determining the incremental value of these biomarkers over established clinical parameters.
4 Therapeutic strategies targeting gut microbiota-derived bioactive compounds
Modulating levels of SCFAs, BAs, and tryptophan derivatives has proven effective therapeutic treatment against MAFLD. For instance, Yan et al. demonstrated that fecal microbiota transplantation from mice treated with Morinda citrifolia polyphenol extract elevated SCFA-producing bacteria. The resulting increased levels in SCFAs activated the intestinal FXR-FGF15 pathway, subsequently triggering hepatic FXR to suppress CYP7A1 expression—thereby regulating cholesterol-to-BA conversion and maintaining lipid homeostasis (46). Liu et al. found electroacupuncture (EA) improved adipose tissue pathology and reduced the levels of total cholesterol/triglycerides by modulating lipid metabolism-associated gut microbiota, increasing the levels of SCFAs, and activating PPAR signaling (47). Wu et al. showed that Akkermansia muciniphila supplementation reshaped BA profiles by regulating the gut FXR-FGF15 axis (192). Nie et al. identified multiple microbially modified BAs, including the previously uncharacterized 3-succinylated cholic acid (3-sucCA), that inversely correlated with liver injury in biopsy-confirmed MAFLD patients. Furthermore, 3-sucCA alleviated MASH by promoting A. muciniphila (193). Moreover, oral administration of indole-3-acetate has been demonstrated in preclinical studies to suppress the expression of several enzymes involved in hepatic lipogenesis and beta-oxidation, while concurrently mediating anti-inflammatory effects in macrophages through the AMPK signaling pathway (143).
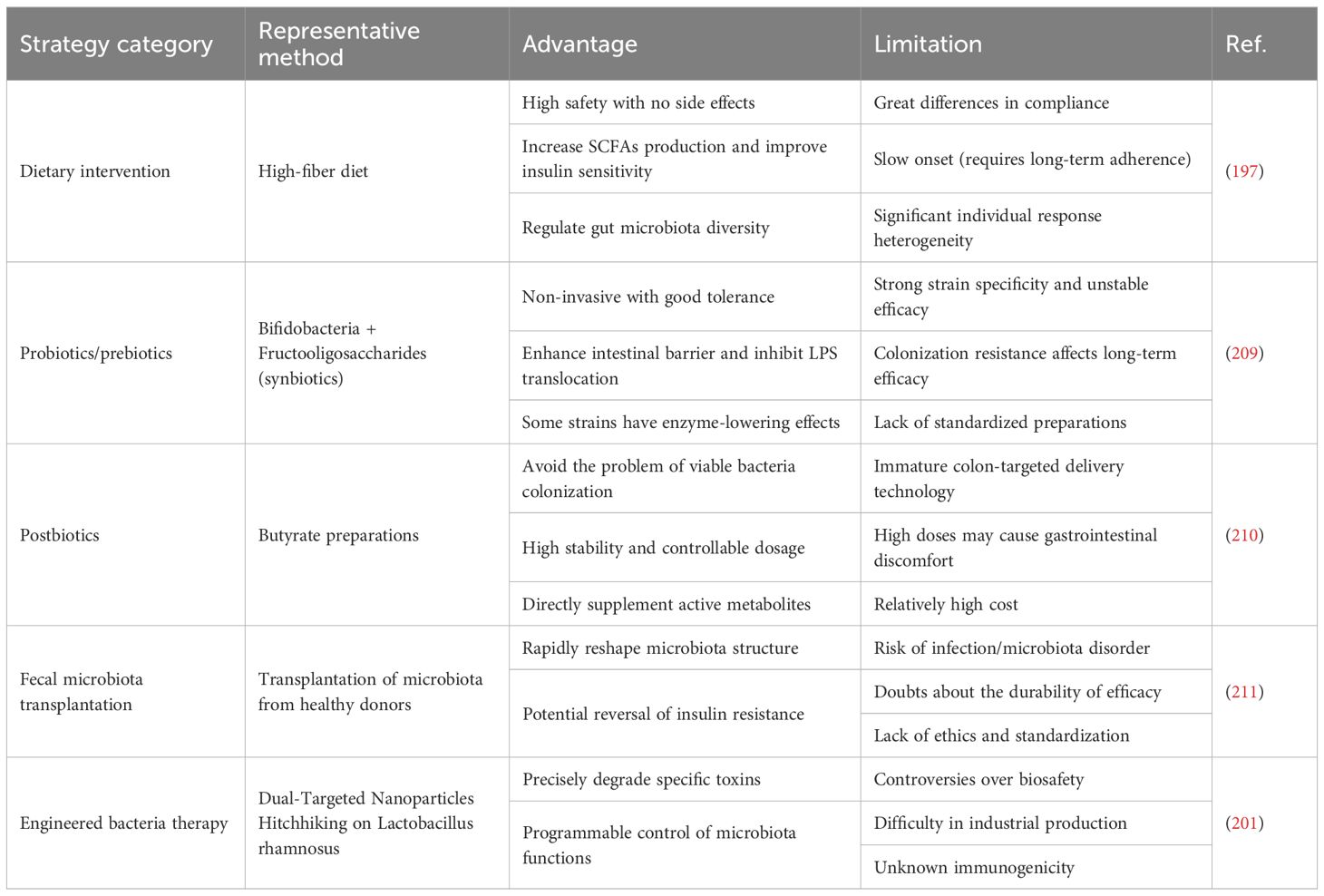
Dietary interventions, probiotics/prebiotics, microbiota transplantation, engineered bacteria, and postbiotics all show therapeutic promise. However, each has its own advantages and disadvantages (Table 3). Dietary intervention is a relatively safe treatment strategy. High-fiber diets provide abundant SCFA precursors (194), while Mediterranean diets—rich in polyphenols, polyunsaturated fatty acids (PUFAs), oleic acid, carotenoids, and fiber—exert antioxidant, anti-inflammatory, and antimicrobial effects (195). Both types of diets are recognized as MAFLD mitigators. A meta-analysis integrating 11 studies assessing Mediterranean diet adherence scores demonstrated that this dietary strategy significantly reduced body weight and alanine aminotransferase (ALT) levels, suggesting its efficacy in supporting weight loss and improving liver health in patients with MASLD/MASH (196). Clinical observations indicate that a daily intake of 24 grams of fiber reduces hepatic steatosis and significantly lowers aspartate aminotransferase (AST) and total cholesterol levels (197). However, patient compliance, individual variability, and delayed efficacy necessitate positioning dietary therapy as a foundational approach. Meanwhile, Probiotic/prebiotic supplements also demonstrate benefits. A clinical study demonstrated that short-term probiotic supplementation can improve ALT, AST, and BMI (NCT06074094) (198). Supplementing with prebiotics alone has also yielded positive clinical results. For example, Lycium barbarum polysaccharide (LBP) supplementation reduced ALT levels in MAFLD patients (ChiCTR2000034740) (199). It should be noted that most prebiotic research remains at the preclinical stage. For instance, Ma et al. revealed that Tricholoma mongolicum polysaccharide (TMP) significantly enhanced gut microbial α-diversity in MAFLD models, restructured community composition, lowered Firmicutes/Bacteroidetes ratios, and enriched SCFA-producing microbial genera. Proteomics confirmed that TMP suppressed hepatic immune inflammation and ferroptosis while enhancing metabolic homeostasis pathways (200). Whether these agents have clinical translational value requires further validation. Engineered bacteria represent another group of frontiers. Zhang et al. used dual-targeted nanoparticles hitchhiking on Lactobacillus rhamnosus to enhance gut accumulation and liver-targeted delivery of anthocyanins, improving MAFLD treatment (201). However, it must be noted that safety concerns, bioethical concerns and industrial-scale production hurdles remain. Fecal microbiota transplantation (FMT) was previously considered a potential therapeutic approach and demonstrated success in preclinical studies (202). However, conflicting results in clinical trials have introduced uncertainty regarding this strategy. The study by Xue et al. reported that allogeneic FMT administered three times within three days reduced hepatic fat accumulation (203). However, Groenewegen et al. found no significant effects of FMT on liver steatosis, glucose tolerance, hepatic biochemistry, or gut microbiota composition in their clinical trial (NCT04465032) (204). Craven et al. reached an intermediate conclusion, demonstrating that FMT did not improve hepatic proton density fat fraction (PDFF) but might reduce intestinal permeability in patients with MAFLD (NCT02496390) (205). Moreover, FMT still faces significant challenges before it can be widely adopted in clinical practice, including infection risks, potential dysbiosis, and ethical dilemmas (206). The clarified roles of gut microbiota-derived bioactive compounds have paved the way for postbiotics. These overcome limitations of live bacteria (i.e., colonization issues, stability challenges, and dosing precision) and stand as a promising MAFLD treatment strategy (207). Clinical trials by Fogacci et al. have demonstrated that a butyrate-based therapeutic strategy reduces hepatic steatosis scores and improves key lipid profile indicators (208).
5 Conclusion
MAFLD, the most prevalent chronic liver condition worldwide, is increasingly linked to gut dysbiosis and metabolic disruption. This review systematically details how gut microbiota-derived bioactive compounds—SCFAs, BAs, tryptophan derivatives, MTAO, endotoxins, and bacterial extracellular vesicles—orchestrate MAFLD progression from steatosis to steatohepatitis and fibrosis. These gut microbiota-derived bioactive compounds directly or indirectly modulate hepatic lipid metabolism, gut barrier integrity, immune responses, and signaling pathways (e.g., FXR, TLR4, and AMPK). Clinically, characteristic microbial metabolite profiles offer novel diagnostic biomarkers (e.g., propionate/butyrate ratios for SCFAs and 12-OH/non-12-OH BA ratio) to enhance diagnostic accuracy. BEVs, with their high specificity and accessibility, show promise as non-invasive indicators for MAFLD progression. Therapeutically, strategies targeting gut microbiota-host metabolic linkages—high-fiber diets, probiotics/prebiotics, FMT, engineered bacteria, and postbiotics—hold significant potential for the treatment of MAFLD. By restoring microbial balance and metabolite homeostasis (e.g., boosting the levels of SCFAs, and normalizing BAs), they effectively combat hepatic lipid accumulation and inflammation. Currently, elucidating the role of gut microbiota-related bioactive substances in MAFLD remains challenging. Firstly, while most studies suggest that SCFA supplementation is beneficial for individuals, there are also reports of ineffective or even harmful outcomes. Similarly, conflicting research results exist for BAs and TMAO, necessitating more precise variable control to clarify the effective and harmful ranges of these bioactive substances, thereby unlocking their potential for clinical applications. Secondly, the heterogeneity of individual microbiomes affects diagnostic consistency. Relatively few studies focus on the diagnostic utility of gut-derived bioactive substances, highlighting the need for larger sample sizes and stricter enrollment criteria to develop diagnostic models with clinical applicability. Additionally, the mechanisms of certain bioactive compounds (e.g., BEVs) in MAFLD progression are not yet fully understood. Greater efforts to clarify the roles of these bioactive compounds will facilitate drug target design and translation. In terms of clinical translation, challenges such as probiotic colonization efficiency, the safety of FMT, and ethical concerns regarding engineered bacteria remain significant obstacles. Further discussions are needed to determine the most suitable therapeutic strategies for MAFLD.
Author contributions
CM: Investigation, Writing – original draft. JW: Writing – original draft, Investigation. XS: Investigation, Writing – review & editing. XW: Writing – review & editing, Investigation. SZ: Supervision, Writing – review & editing, Project administration, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Shandong Natural Science Foundation Youth Fund (ZR2023QH258).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eslam M, Sanyal AJ, and George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
2. Raj SV, Ismail M, Chan WK, and Majid HA. A systematic review on factors associated with non-alcoholic fatty liver disease (NAFLD) among adolescents. Clin Nutr ESPEN. (2023) 57:131–7. doi: 10.1016/j.clnesp.2023.06.014
3. Zhang H, Zhou XD, Shapiro MD, Lip GYH, Tilg H, Valenti L, et al. Global burden of metabolic diseases, 1990–2021. Metabolism: Clin Exp. (2024) 160:155999. doi: 10.1016/j.metabol.2024.155999
4. Guo Z, Wu D, Mao R, Yao Z, Wu Q, and Lv W. Global burden of MAFLD, MAFLD related cirrhosis and MASH related liver cancer from 1990 to 2021. Sci Rep. (2025) 15:7083. doi: 10.1038/s41598-025-91312-5
5. Fouad Y, Alboraie M, and Shiha G. Epidemiology and diagnosis of metabolic dysfunction-associated fatty liver disease. Hepatol Int. (2024) 18:827–33. doi: 10.1007/s12072-024-10704-3
6. Wong VW, Petta S, Hiriart JB, Cammà C, Wong GL, Marra F, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. (2017) 67:577–84. doi: 10.1016/j.jhep.2017.05.005
7. Khaznadar F, Khaznadar O, Petrovic A, Hefer M, Gjoni F, Gjoni S, et al. MAFLD pandemic: updates in pharmacotherapeutic approach development. Curr Issues Mol Biol. (2024) 46:6300–14. doi: 10.3390/cimb46070376
8. Garcia-Bonete MJ, Rajan A, Suriano F, and Layunta E. The underrated gut microbiota helminths, bacteriophages, fungi, and archaea. Life (Basel Switzerland). (2023) 13. doi: 10.3390/life13081765
9. Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, and Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. (2015) 7:2839–49. doi: 10.3390/nu7042839
10. Sharma S, Tiwari N, and Tanwar SS. The current findings on the gut-liver axis and the molecular basis of NAFLD/NASH associated with gut microbiome dysbiosis. Naunyn-Schmiedeberg’s Arch Pharmacol. (2025). doi: 10.1007/s00210-025-04069-z
11. Shen S, Liu Y, Wang N, Huang Z, and Deng G. The role of microbiota in nonalcoholic fatty liver disease: mechanism of action and treatment strategy. Front Microbiol. (2025) 16:1621583. doi: 10.3389/fmicb.2025.1621583
12. Dongoran RA, Tu FC, and Liu CH. Current insights into the interplay between gut microbiota-derived metabolites and metabolic-associated fatty liver disease. Tzu chi Med J. (2023) 35:290–9. doi: 10.4103/tcmj.tcmj_122_23
13. Mukhopadhya I and Louis P. Gut microbiota-derived short-chain fatty acids and their role in human health and disease. Nat Rev Microbiol. (2025). doi: 10.1038/s41579-025-01183-w
14. Glover JS, Ticer TD, and Engevik MA. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci Rep. (2022) 12:8456. doi: 10.1038/s41598-022-11819-z
15. Kimura I, Ichimura A, Ohue-Kitano R, and Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev. (2020) 100:171–210. doi: 10.1152/physrev.00041.2018
16. Pettinato E, Steiner TM, Cassens EA, Geisberger T, Seitz C, König S, et al. Propionate metabolism in Desulfurella acetivorans. Front Microbiol. (2025) 16:1545849. doi: 10.3389/fmicb.2025.1545849
17. Canfora EE, Jocken JW, and Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. (2015) 11:577–91. doi: 10.1038/nrendo.2015.128
18. Ang Z and Ding JL. GPR41 and GPR43 in obesity and inflammation - Protective or causative? Front Immunol. (2016) 7:28.
20. Kalkan AE, BinMowyna MN, Raposo A, Ahmad MF, Ahmed F, Otayf AY, et al. Beyond the gut: unveiling butyrate’s global health impact through gut health and dysbiosis-related conditions: A narrative review. Nutrients. (2025) 17. doi: 10.3390/nu17081305
21. Korsten S, Vromans H, Garssen J, and Willemsen LEM. Butyrate protects barrier integrity and suppresses immune activation in a caco-2/PBMC co-culture model while HDAC inhibition mimics butyrate in restoring cytokine-induced barrier disruption. Nutrients. (2023) 15. doi: 10.3390/nu15122760
22. Yang CJ, Chang HC, Sung PC, Ge MC, Tang HY, Cheng ML, et al. Oral fecal transplantation enriches Lachnospiraceae and butyrate to mitigate acute liver injury. Cell Rep. (2024) 43:113591. doi: 10.1016/j.celrep.2023.113591
23. Mayorga-Ramos A, Barba-Ostria C, Simancas-Racines D, and Guamán LP. Protective role of butyrate in obesity and diabetes: New insights. Front Nutr. (2022) 9:1067647. doi: 10.3389/fnut.2022.1067647
24. Yang W, Yu T, Liu X, Yao S, Khanipov K, Golovko G, et al. Microbial metabolite butyrate modulates granzyme B in tolerogenic IL-10 producing Th1 cells to regulate intestinal inflammation. Gut Microbes. (2024) 16:2363020. doi: 10.1080/19490976.2024.2363020
25. Chen Y, Liu Y, Wang Y, Chen X, Wang C, Chen X, et al. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages. J Exp Clin Cancer research: CR. (2022) 41:1. doi: 10.1186/s13046-021-02201-4
26. Hu L, Liu Q, Ke X, Zhao P, Fang W, and Ren Y. Correlation of the intestinal flora and its metabolites with the colonic transport function in functional constipation. Front Microbiol. (2025) 16:1591697. doi: 10.3389/fmicb.2025.1591697
27. Ozturk O, Celebi G, Duman UG, Kupcuk E, Uyanik M, and Sertoglu E. Short-chain fatty acid levels in stools of patients with inflammatory bowel disease are lower than those in healthy subjects. Eur J Gastroenterol Hepatol. (2024) 36:890–6. doi: 10.1097/MEG.0000000000002789
28. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, and Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. (2014) 4:e121. doi: 10.1038/nutd.2014.23
29. Ruppin H, Bar-Meir S, Soergel KH, Wood CM, and Schmitt MG Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology. (1980) 78:1500–7. doi: 10.1016/S0016-5085(19)30508-6
30. Luo P, Lednovich K, Xu K, Nnyamah C, Layden BT, and Xu P. Central and peripheral regulations mediated by short-chain fatty acids on energy homeostasis. Trans Res. (2022) 248:128–50. doi: 10.1016/j.trsl.2022.06.003
31. Xie L, Alam MJ, Marques FZ, and Mackay CR. A major mechanism for immunomodulation: Dietary fibres and acid metabolites. Semin Immunol. (2023) 66:101737. doi: 10.1016/j.smim.2023.101737
32. Geng HW, Yin FY, Zhang ZF, Gong X, and Yang Y. Butyrate suppresses glucose metabolism of colorectal cancer cells via GPR109a-AKT signaling pathway and enhances chemotherapy. Front Mol Biosci. (2021) 8:634874. doi: 10.3389/fmolb.2021.634874
33. Nishida A, Miyamoto J, Shimizu H, and Kimura I. Gut microbial short-chain fatty acids-mediated olfactory receptor 78 stimulation promotes anorexigenic gut hormone peptide YY secretion in mice. Biochem Biophys Res Commun. (2021) 557:48–54. doi: 10.1016/j.bbrc.2021.03.167
34. Olsson A, Gustavsen S, Nguyen TD, Nyman M, Langkilde AR, Hansen TH, et al. Serum short-chain fatty acids and associations with inflammation in newly diagnosed patients with multiple sclerosis and healthy controls. Front Immunol. (2021) 12:661493. doi: 10.3389/fimmu.2021.661493
35. Niu C, Tu Y, Jin Q, Chen Z, Yuan K, Wang M, et al. Mapping the human oral and gut fungal microbiota in patients with metabolic dysfunction-associated fatty liver disease. Front Cell infection Microbiol. (2023) 13:1157368. doi: 10.3389/fcimb.2023.1157368
36. Buchynskyi M, Kamyshna I, Halabitska I, Petakh P, Kunduzova O, Oksenych V, et al. Unlocking the gut-liver axis: microbial contributions to the pathogenesis of metabolic-associated fatty liver disease. Front Microbiol. (2025) 16:1577724. doi: 10.3389/fmicb.2025.1577724
37. Ganesan R, Gupta H, Jeong JJ, Sharma SP, Won SM, Oh KK, et al. A metabolomics approach to the validation of predictive metabolites and phenotypic expression in non-alcoholic fatty liver disease. Life Sci. (2023) 322:121626. doi: 10.1016/j.lfs.2023.121626
38. Dardi P, Dos Santos-Eichler RA, de Oliveira S, Vinolo MAR, Câmara NOS, and Rossoni LV. Reduced intestinal butyrate availability is associated with the vascular remodeling in resistance arteries of hypertensive rats. Front Physiol. (2022) 13:998362. doi: 10.3389/fphys.2022.998362
39. Kessoku T, Kobayashi T, Tanaka K, Yamamoto A, Takahashi K, Iwaki M, et al. The role of leaky gut in nonalcoholic fatty liver disease: A novel therapeutic target. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22158161
40. Rios-Morales M, Vieira-Lara MA, Homan E, Langelaar-Makkinje M, Gerding A, Li Z, et al. Butyrate oxidation attenuates the butyrate-induced improvement of insulin sensitivity in myotubes. Biochim Biophys Acta Mol basis Dis. (2022) 1868:166476. doi: 10.1016/j.bbadis.2022.166476
41. Li X, Wang C, Zhu J, Lin Q, Yu M, Wen J, et al. Sodium butyrate ameliorates oxidative stress-induced intestinal epithelium barrier injury and mitochondrial damage through AMPK-mitophagy pathway. Oxid Med Cell Longevity. (2022) 2022:3745135. doi: 10.1155/2022/3745135
42. Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. (2011) 13:517–26. doi: 10.1016/j.cmet.2011.02.018
43. Ding J, Liu J, Chen J, Cheng X, Cao H, Guo X, et al. Sodium butyrate alleviates free fatty acid-induced steatosis in primary chicken hepatocytes via the AMPK/PPARα pathway. Poultry Sci. (2024) 103:103482. doi: 10.1016/j.psj.2024.103482
44. Augustijn QJJ, Grefhorst A, de Groen P, Wortelboer K, Seegers JFM, Gül IS, et al. Randomised double-blind placebo-controlled trial protocol to evaluate the therapeutic efficacy of lyophilised faecal microbiota capsules amended with next-generation beneficial bacteria in individuals with metabolic dysfunction-associated steatohepatitis. BMJ Open. (2025) 15:e088290. doi: 10.1136/bmjopen-2024-088290
45. Lin YH, Wang CH, Huang YH, Shen HC, Wu WK, Yeh HY, et al. Models incorporating physical, laboratory and gut metabolite markers can be used to predict severe hepatic steatosis in MAFLD patients. Kaohsiung J Med Sci. (2024) 40:1095–105. doi: 10.1002/kjm2.12904
46. Yang D, Yang X, Zhou Y, Wang H, and Wang R. Fecal microbiota transplantation from noni fruit phenolic-rich extract intervention mouse donors ameliorates lipid metabolism disorder by regulating the FXR-FGF15 pathway in a gut microbiota-dependent manner. J Agric Food Chem. (2025) 73:17672–84. doi: 10.1021/acs.jafc.5c05184
47. Liu X, She C, Li X, Yang M, Zhang Y, Yu P, et al. Electroacupuncture improves lipid metabolism via proteome and gut microbiota profiling in obese rats. Am J Trans Res. (2025) 17:4008–22. doi: 10.62347/ZQZS9458
48. Gao J, Mang Q, Sun Y, and Xu G. Short-Chain Fatty Acids (SCFAs) Modulate the Hepatic Glucose and Lipid Metabolism of Coilia nasus via the FFAR/AMPK Signaling Pathway In Vitro. Int J Mol Sci. (2025) 26. doi: 10.3390/ijms26083654
49. Ding L, Chen JS, Xing YF, Li DM, Fu AQ, Tong X, et al. Effects of lactoferrin on high-fat and high-cholesterol diet-induced non-alcoholic fatty liver disease in mice. J Nutr Biochem. (2025) 143:109938. doi: 10.1016/j.jnutbio.2025.109938
50. Li Q, Cheng J, Sun Y, He L, and Li R. Protective effects of polygonatum sibiricum polysaccharides against type 2 diabetic mice induced by high-fat diet and low-dose streptozotocin. Toxics. (2025) 13. doi: 10.3390/toxics13040255
51. Long H, Huang R, Zhu S, Wang Z, Liu X, and Zhu Z. Polysaccharide from Caulerpa lentillifera alleviates hyperlipidaemia through altering bile acid metabolism mediated by gut microbiota. Int J Biol macromolecules. (2025) 306:141663. doi: 10.1016/j.ijbiomac.2025.141663
52. Kovynev A, Charchuta MM, Begtašević A, Ducarmon QR, Rensen PCN, and Schönke M. Combination of dietary fiber and exercise training improves fat loss in mice but does not ameliorate MASLD more than exercise alone. Am J Physiol Gastrointestinal liver Physiol. (2025) 328:G399–g410. doi: 10.1152/ajpgi.00317.2024
53. Ma N, Li R, Zhang GF, Gao RH, and Zhang DJ. Fermentation-enriched quinoa β-glucan ameliorates disturbed gut microbiota and metabolism in type 2 diabetes mellitus mice. Int J Biol macromolecules. (2025) 306:141666. doi: 10.1016/j.ijbiomac.2025.141666
54. Liu T, Zhang M, Xie Q, Gu J, Zeng S, and Huang D. Unveiling the antiobesity mechanism of sweet potato extract by microbiome, transcriptome, and metabolome analyses in mice. J Agric Food Chem. (2025) 73:7807–21. doi: 10.1021/acs.jafc.4c13173
55. Zhang X, Jiang A, An S, Guo C, You F, Huang Z, et al. Dietary resistant starch supplementation improves the fish growth, lipid metabolism and intestinal barrier in largemouth bass (Micropterus salmoides) fed high-fat diets. Int J Biol macromolecules. (2025) 306:141356. doi: 10.1016/j.ijbiomac.2025.141356
56. Zhou Y, Wang M, Wang Z, Qiu J, Wang Y, Li J, et al. Polysaccharides from hawthorn fruit alleviate high-fat diet-induced NAFLD in mice by improving gut microbiota dysbiosis and hepatic metabolic disorder. Phytomedicine. (2025) 139:156458. doi: 10.1016/j.phymed.2025.156458
57. Vornoli A, Souid A, Lazzari B, Turri F, Pizzi F, Bramanti E, et al. A moderate intake of beer improves metabolic dysfunction-associated steatotic liver disease (MASLD) in a high-fat diet (HFD)-induced mouse model. Molecules (Basel Switzerland). (2024) 29. doi: 10.3390/molecules29245954
58. Zhu Y, Ba K, Li X, He Y, Zhang Y, Ai L, et al. Comparative analysis of barley dietary fiber fermented with and without Lactiplantibacillus plantarum dy-1 in promoting gut health and regulating hepatic energy metabolism in high-fat diet-induced obese mice. Food Funct. (2025) 16:219–31. doi: 10.1039/D4FO04776A
59. Wang Q, Peng J, Tian Y, Li J, Cai J, Qin W, et al. Evaluation of the decreased cholesterol potential of levilactobacillus brevis M-10 isolated from spontaneously fermented sour porridge in mice with high-cholesterol levels. Curr Microbiol. (2024) 82:24. doi: 10.1007/s00284-024-03974-5
60. Huang W, Wang J, Xiao Z, Lin J, Tan Z, and Sun G. Lingguizhugan decoction alleviates obesity in rats on a high-fat diet through the regulation of lipid metabolism and intestinal microbiota. Front Microbiol. (2024) 15:1462173. doi: 10.3389/fmicb.2024.1462173
61. Miao C, Wang L, Wang H, Shen Y, Man C, Zhang W, et al. Lacticaseibacillus plantarum postbiotics prepared by the combined technique of pasteurization and ultrasound: effective measures to alleviate obesity based on the SCFAs-GPR41/GPR43 signaling pathway. Food Funct. (2024) 15:11005–19. doi: 10.1039/D4FO03591G
62. Wang N, Li C, and Zhang Z. Arctigenin ameliorates high-fat diet-induced metabolic disorders by reshaping gut microbiota and modulating GPR/HDAC3 and TLR4/NF-κB pathways. Phytomedicine. (2024) 135:156123. doi: 10.1016/j.phymed.2024.156123
63. Dai Z, Lin Y, Chen G, Yu P, Wu H, Ning M, et al. Novel approach for ameliorating high-fat diet-induced syndromes via probiotic-fermented oyster mushroom: from metabolites and microbiota to regulation mechanisms. Food Funct. (2024) 15:10472–89. doi: 10.1039/D4FO02142H
64. Jang AR, Jung DH, Lee TS, Kim JK, Lee YB, Lee JY, et al. Lactobacillus plantarum NCHBL-004 modulates high-fat diet-induced weight gain and enhances GLP-1 production for blood glucose regulation. Nutr (Burbank Los Angeles County Calif). (2024) 128:112565. doi: 10.1016/j.nut.2024.112565
65. Wang N, Dilixiati Y, Xiao L, Yang H, and Zhang Z. Different short-chain fatty acids unequally modulate intestinal homeostasis and reverse obesity-related symptoms in lead-exposed high-fat diet mice. J Agric Food Chem. (2024) 72:18971–85. doi: 10.1021/acs.jafc.4c04193
66. Zhang B, Zhao W, Song D, and Lyu X. Regulatory effect of β-glucan secreted by Rhizobium pusense on triglyceride metabolism and their relationships with the modulation of intestinal microbiota in mice fed a high-fat diet. Food Funct. (2024) 15:8759–74. doi: 10.1039/D4FO01123F
67. Ye K, Zhao Y, Huang W, and Zhu Y. Sodium butyrate improves renal injury in diabetic nephropathy through AMPK/SIRT1/PGC-1α signaling pathway. Sci Rep. (2024) 14:17867. doi: 10.1038/s41598-024-68227-8
68. Chen Z, Liu J, Ding H, Yan C, Zhu H, Huang S, et al. Dietary supplementation with capsaicinoids alleviates obesity in mice fed a high-fat-high-fructose diet. Food Funct. (2024) 15:8572–85. doi: 10.1039/D4FO02102A
69. Li Y, Sun M, Tian X, Bao T, Yu Q, Ma NL, et al. Gymnemic acid alleviates gut barrier disruption and lipid dysmetabolism via regulating gut microbiota in HFD hamsters. J Nutr Biochem. (2024) 133:109709. doi: 10.1016/j.jnutbio.2024.109709
70. Hou G, Wei L, Li R, Chen F, Yin J, Huang X, et al. Lactobacillus delbrueckii Ameliorated Blood Lipids via Intestinal Microbiota Modulation and Fecal Bile Acid Excretion in a Ningxiang Pig Model. Animals: an Open Access J MDPI. (2024) 14. doi: 10.3390/ani14121801
71. Koh YC, Hsu HW, Ho PY, Hsu KY, Lin WS, Nagabhushanam K, et al. Structural variances in curcumin degradants: impact on obesity in mice. J Agric Food Chem. (2024) 72:14786–98. doi: 10.1021/acs.jafc.4c03768
72. Zhang F, Lo EKK, Chen J, Wang K, Felicianna, Ismaiah MJ, et al. Probiotic mixture ameliorates a diet-induced MASLD/MASH murine model through the regulation of hepatic lipid metabolism and the gut microbiome. J Agric Food Chem. (2024) 72:8536–49. doi: 10.1021/acs.jafc.3c08910
73. Pan X, Zhang Y, Qiao Y, Cao Q, Wei L, and Zhao M. Investigation of the therapeutic effect of Hedan tablets on high-fat diet-induced obesity in rats by GC-MS technology and 16S ribosomal RNA gene sequencing. Biomed chromatography: BMC. (2024) 38:e5848. doi: 10.1002/bmc.5848
74. Chen C, Gao K, Chen Z, Zhang Q, Ke X, Mao B, et al. The supplementation of the multi-strain probiotics WHHPRO™ alleviates high-fat diet-induced metabolic symptoms in rats via gut-liver axis. Front Nutr. (2023) 10:1324691.
75. Liu H, Nie C, Hu X, and Li J. Highland barley β-glucan supplementation attenuated hepatic lipid accumulation in Western diet-induced non-alcoholic fatty liver disease mice by modulating gut microbiota. Food Funct. (2024) 15:1250–64. doi: 10.1039/D3FO03386D
76. Mattos Rocha Olivieri C, Aparecida Manólio Soares Freitas R, and Alfredo Gomes Arêas J. Jatobá-do-cerrado (Hymenaea stigonocarpa Mart.) pulp positively affects plasma and hepatic lipids and increases short-chain fatty acid production in hamsters fed a hypercholesterolemic diet. Food Res Int (Ottawa Ont). (2024) 175:113766. doi: 10.1016/j.foodres.2023.113766
77. Song Q, Cheng SW, Zou J, Li KSL, Cheng H, Wai Lau DT, et al. Role of gut microbiota on regulation potential of Dendrobium officinale Kimura & Migo in metabolic syndrome: In-vitro fermentation screening and in-vivo verification in db/db mice. J ethnopharmacology. (2024) 321:117437. doi: 10.1016/j.jep.2023.117437
78. Zhou Y, Chen Z, Zhou H, Niu B, Liu J, Li Y, et al. ACT001 Alleviates chronic kidney injury induced by a high-fat diet in mice through the GPR43/AMPK pathway. Lipids Health Dis. (2023) 22:198. doi: 10.1186/s12944-023-01949-2
79. Wang J, Liu A, Li A, Song H, Luo P, Zhan M, et al. Lactobacillus fermentum CKCC1858 alleviates hyperlipidemia in golden hamsters on a high-fat diet via modulating gut microbiota. Food Funct. (2023) 14:9580–90. doi: 10.1039/D3FO02618C
80. Zeng W, Yang B, Wang Y, Sun M, Yang W, Cui H, et al. Rotundic acid alleviates hyperlipidemia in rats by regulating lipid metabolism and gut microbiota. Phytotherapy research: PTR. (2023) 37:5958–73. doi: 10.1002/ptr.8008
81. Yu Q, Yu F, Li Q, Zhang J, Peng Y, Wang X, et al. Anthocyanin-rich butterfly pea flower extract ameliorating low-grade inflammation in a high-fat-diet and lipopolysaccharide-induced mouse model. J Agric Food Chem. (2023) 71:11941–56. doi: 10.1021/acs.jafc.3c02696
82. Zhang L, Chen N, Zhan L, Bi T, Zhou W, Zhang L, et al. Erchen Decoction alleviates obesity-related hepatic steatosis via modulating gut microbiota-drived butyric acid contents and promoting fatty acid β-oxidation. J ethnopharmacology. (2023) 317:116811. doi: 10.1016/j.jep.2023.116811
83. Li G, Wang X, Liu Y, Gong S, Yang Y, Wang C, et al. Bile acids supplementation modulates lipid metabolism, intestinal function, and cecal microbiota in geese. Front Microbiol. (2023) 14:1185218. doi: 10.3389/fmicb.2023.1185218
84. Zhou X, Pak S, Li D, Dong L, Chen F, Hu X, et al. Bamboo shoots modulate gut microbiota, eliminate obesity in high-fat-diet-fed mice and improve lipid metabolism. Foods (Basel Switzerland). (2023) 12. doi: 10.3390/foods12071380
85. Shi H, Li X, Hou C, Chen L, Zhang Y, and Li J. Effects of pomegranate peel polyphenols combined with inulin on gut microbiota and serum metabolites of high-fat-induced obesity rats. J Agric Food Chem. (2023) 71:5733–44. doi: 10.1021/acs.jafc.3c01014
86. Shashni B, Tajika Y, Ikeda Y, Nishikawa Y, and Nagasaki Y. Self-assembling polymer-based short chain fatty acid prodrugs ameliorate non-alcoholic steatohepatitis and liver fibrosis. Biomaterials. (2023) 295:122047. doi: 10.1016/j.biomaterials.2023.122047
87. Wang Z, Yao W, Sun Y, Han Y, Chen X, Gong P, et al. Eucommia bark/leaf extract improves lipid metabolism disorders by affecting intestinal microbiota and microbiome-host interaction in HFD mice. J Agric Food Chem. (2023). doi: 10.1021/acs.jafc.2c07239
88. Zou T, Xie F, Liang P, Chen J, Wang Z, Du M, et al. Polysaccharide-rich fractions from Enteromorpha prolifera improve hepatic steatosis and gut barrier integrity in high-fat diet-induced obese mice linking to modulation of gut microbiota. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2023) 157:114034. doi: 10.1016/j.biopha.2022.114034
89. Wang X, Li L, Bai M, Zhao J, Sun X, Gao Y, et al. Dietary supplementation with Tolypocladium sinense mycelium prevents dyslipidemia inflammation in high fat diet mice by modulation of gut microbiota in mice. Front Immunol. (2022) 13:977528. doi: 10.3389/fimmu.2022.977528
90. Wang G, Sun C, Xie B, Wang T, Liu H, Chen X, et al. Cordyceps guangdongensis lipid-lowering formula alleviates fat and lipid accumulation by modulating gut microbiota and short-chain fatty acids in high-fat diet mice. Front Nutr. (2022) 9:1038740. doi: 10.3389/fnut.2022.1038740
91. Hou G, Yin J, Wei L, Li R, Peng W, Yuan Y, et al. Lactobacillus delbrueckii might lower serum triglyceride levels via colonic microbiota modulation and SCFA-mediated fat metabolism in parenteral tissues of growing-finishing pigs. Front veterinary Sci. (2022) 9:982349. doi: 10.3389/fvets.2022.982349
92. Miao ZH, Wang JN, Shen X, Zhou QQ, Luo YT, Liang HJ, et al. Long-term use of Lacticaseibacillus paracasei N1115 from early life alleviates high-fat-diet-induced obesity and dysmetabolism in mice. Beneficial Microbes. (2022) 13:407–16. doi: 10.3920/BM2021.0171
93. Wang R, Wang L, Wang S, Wang J, Su C, Zhang L, et al. Phenolics from noni (Morinda citrifolia L.) fruit alleviate obesity in high fat diet-fed mice via modulating the gut microbiota and mitigating intestinal damage. Food Chem. (2023) 402:134232. doi: 10.1016/j.foodchem.2022.134232
94. Liu J, Zhen D, Hu C, Liu Y, Shen X, Fu P, et al. Reconfiguration of gut microbiota and reprogramming of liver metabolism with phycobiliproteins bioactive peptides to rehabilitate obese rats. Nutrients. (2022) 14. doi: 10.3390/nu14173635
95. Tian S, Lei Y, Zhao F, Che J, Wu Y, Lei P, et al. Improving insulin resistance by sulforaphane via activating the Bacteroides and Lactobacillus SCFAs-GPR-GLP1 signal axis. Food Funct. (2024) 15:8644–60. doi: 10.1039/D4FO01059K
96. Tyagi A and Kumar V. The gut microbiota-bile acid axis: a crucial regulator of immune function and metabolic health. World J Microbiol Biotechnol. (2025) 41:215. doi: 10.1007/s11274-025-04395-7
97. Kiriyama Y and Nochi H. Physiological role of bile acids modified by the gut microbiome. Microorganisms. (2021) 10. doi: 10.3390/microorganisms10010068
98. Gillard J, Clerbaux LA, Nachit M, Sempoux C, Staels B, Bindels LB, et al. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice. JHEP reports: Innovation Hepatol. (2022) 4:100387. doi: 10.1016/j.jhepr.2021.100387
99. Chiang JYL and Ferrell JM. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol Cell Endocrinol. (2022) 548:111618. doi: 10.1016/j.mce.2022.111618
100. Katafuchi T and Makishima M. Molecular basis of bile acid-FXR-FGF15/19 signaling axis. Int J Mol Sci. (2022) 23(11). doi: 10.3390/ijms23116046
101. Wang W, Li L, Li X, Chen J, Wang R, Yang Q, et al. FXR overexpression alleviates cholestasis via NLRC4 inflammasome suppression and bile acid homeostasis regulation. Free Radical Biol Med. (2025) 238:152–68. doi: 10.1016/j.freeradbiomed.2025.06.039
102. Di Ciaula A, Bonfrate L, Baj J, Khalil M, Garruti G, Stellaard F, et al. Recent advances in the digestive, metabolic and therapeutic effects of farnesoid X receptor and fibroblast growth factor 19: from cholesterol to bile acid signaling. Nutrients. (2022) 14. doi: 10.3390/nu14234950
103. Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatol (Baltimore Md). (2018) 68:1574–88. doi: 10.1002/hep.29857
104. Kim H and Fang S. Crosstalk between FXR and TGR5 controls glucagon-like peptide 1 secretion to maintain glycemic homeostasis. Lab Anim Res. (2018) 34:140–6. doi: 10.5625/lar.2018.34.4.140
105. Pi Y, Wu Y, Zhang X, Lu D, Han D, Zhao J, et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome. (2023) 11:19. doi: 10.1186/s40168-022-01458-x
106. Lin C, Wang Y, Le M, Chen KF, and Jia YG. Recent progress in bile acid-based antimicrobials. Bioconjugate Chem. (2021) 32:395–410. doi: 10.1021/acs.bioconjchem.0c00642
107. Cai J, Rimal B, Jiang C, Chiang JYL, and Patterson AD. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. (2022) 237:108238. doi: 10.1016/j.pharmthera.2022.108238
108. Wu D, Liu J, Guo Z, Wang L, Yao Z, Wu Q, et al. Natural bioactive compounds reprogram bile acid metabolism in MAFLD: Multi-target mechanisms and therapeutic implications. Int Immunopharmacol. (2025) 157:114708. doi: 10.1016/j.intimp.2025.114708
109. Lyu S, Yang J, Xin X, Sun Q, Cai B, Wang X, et al. Characteristics of serum bile acid profiles among individuals with metabolic dysfunction-associated steatotic liver disease. BMC Gastroenterol. (2025) 25:334. doi: 10.1186/s12876-025-03903-1
110. Chen H, Yu X, Wang X, Huang J, Wu J, Zhou X, et al. Longitudinal serum total bile acid trajectories and risk of metabolic dysfunction-associated fatty liver disease: a retrospective cohort study. Eur J Med Res. (2025) 30:685. doi: 10.1186/s40001-025-02837-4
111. Wahlström A, Sayin SI, Marschall HU, and Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. (2016) 24:41–50. doi: 10.1016/j.cmet.2016.05.005
112. Bertaggia E, Jensen KK, Castro-Perez J, Xu Y, Di Paolo G, Chan RB, et al. Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption. Am J Physiol Endocrinol Metab. (2017) 313:E121–e33.
113. Li T and Chiang JYL. Bile acid-based therapies for non-alcoholic steatohepatitis and alcoholic liver disease. Hepatobiliary Surg Nutr. (2020) 9:152–69. doi: 10.21037/hbsn.2019.09.03
114. Zhao J, Li B, Zhang K, and Zhu Z. The effect and safety of obeticholic acid for patients with nonalcoholic steatohepatitis: A systematic review and meta-analysis of randomized controlled trials. Medicine. (2024) 103:e37271. doi: 10.1097/MD.0000000000037271
115. Shen W, Middleton MS, Cunha GM, Delgado TI, Wolfson T, Gamst A, et al. Changes in abdominal adipose tissue depots assessed by MRI correlate with hepatic histologic improvement in non-alcoholic steatohepatitis. J Hepatol. (2023) 78:238–46. doi: 10.1016/j.jhep.2022.10.027
116. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet (London England). (2019) 394:2184–96. doi: 10.1016/S0140-6736(19)33041-7
117. Younis IR, Kirby BJ, Billin AN, Xiao D, Song Q, Watkins TR, et al. Pharmacokinetics, pharmacodynamics, safety and tolerability of cilofexor, a novel nonsteroidal Farnesoid X receptor agonist, in healthy volunteers. Clin Trans Sci. (2023) 16:536–47. doi: 10.1111/cts.13469
118. He Y, Chen X, Li Y, Liang Y, Hong T, Yang J, et al. Curcumin supplementation alleviates hepatic fat content associated with modulation of gut microbiota-dependent bile acid metabolism in patients with nonalcoholic simple fatty liver disease: a randomized controlled trial. Am J Clin Nutr. (2024) 120:66–79. doi: 10.1016/j.ajcnut.2024.05.017
119. Alkhouri N, LaCerte C, Edwards J, Poordad F, Lawitz E, Lee L, et al. Safety, pharmacokinetics and pharmacodynamics of obeticholic acid in subjects with fibrosis or cirrhosis from NASH. Liver Int. (2024) 44:966–78. doi: 10.1111/liv.15816
120. He Q, Jiang M, Wang Y, and Zheng T. Pharmacovigilance of obeticholic acid: An analysis of the Food and Drug Administration Adverse Event Reporting System database. Br J Clin Pharmacol. (2025). doi: 10.1002/bcp.70186
121. Zhang X, Lau HC, and Yu J. Pharmacological treatment for metabolic dysfunction-associated steatotic liver disease and related disorders: Current and emerging therapeutic options. Pharmacol Rev. (2025) 77:100018. doi: 10.1016/j.pharmr.2024.100018
122. Wong VW, Neff GW, Di Bisceglie AM, Bai R, Cheng J, Yu M, et al. HTD1801 demonstrates promising potential for histologic improvements in metabolic dysfunction-associated steatohepatitis in both a preclinical and phase 2 study. Clin Mol Hepatol. (2025) 31:1071–83. doi: 10.3350/cmh.2025.0145
123. Bardhan P, Mei X, Lai NK, Mell B, Tummala R, Aryal S, et al. Salt-responsive gut microbiota induces sex-specific blood pressure changes. Circ Res. (2024) 135:1122–37. doi: 10.1161/CIRCRESAHA.124.325056
124. Wang G, Fan Y, Zhang G, Cai S, Ma Y, Yang L, et al. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome. (2024) 12:59. doi: 10.1186/s40168-024-01750-y
125. Rety S, Deschamps P, and Leullio TN. Structure of Escherichia coli tryptophanase purified from an alkaline-stressed bacterial culture. Acta crystallographica Section F Struct Biol Commun. (2015) 71:1378–83. doi: 10.1107/S2053230X15017549
126. Irmler S, Bavan T, Binz E, and Portmann R. Ability of Latilactobacillus curvatus FAM25164 to produce tryptamine: Identification of a novel tryptophan decarboxylase. Food Microbiol. (2023) 116:104343. doi: 10.1016/j.fm.2023.104343
127. Gao H, Sun M, Li A, Gu Q, Kang D, Feng Z, et al. Microbiota-derived IPA alleviates intestinal mucosal inflammation through upregulating Th1/Th17 cell apoptosis in inflammatory bowel disease. Gut Microbes. (2025) 17:2467235. doi: 10.1080/19490976.2025.2467235
128. Sun Y, Liu X, Zhao L, Li D, Guan K, Ma Y, et al. Lacticaseibacillus rhamnosus HF01 postbiotics reprogram gut microbial tryptophan metabolism to coordinate enterohepatic barrier-insulin signaling axis. Curr Res Food Sci. (2025) 11:101111. doi: 10.1016/j.crfs.2025.101111
129. Wang N, Sun C, Yang Y, Zhang D, Huang L, Xu C, et al. Gut microbiota-derived indoleacetic acid attenuates neuroinflammation and neurodegeneration in glaucoma through ahr/rage pathway. J Neuroinflamm. (2025) 22:179. doi: 10.1186/s12974-025-03505-4
130. Liu W, Wang J, Yang H, Li C, Lan W, Chen T, et al. The metabolite indole-3-acetic acid of bacteroides ovatus improves atherosclerosis by restoring the polarisation balance of M1/M2 macrophages and inhibiting inflammation. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2025) 12:e2413010. doi: 10.1002/advs.202413010
131. Haduch A, Bromek E, Kuban W, and Daniel WA. The engagement of cytochrome P450 enzymes in tryptophan metabolism. Metabolites. (2023) 13. doi: 10.3390/metabo13050629
132. Lin TY, Wu WK, and Hung SC. High interindividual variability of indoxyl sulfate production identified by an oral tryptophan challenge test. NPJ biofilms microbiomes. (2025) 11:15. doi: 10.1038/s41522-025-00651-8
133. Niu B, Pan T, Xiao Y, Wang H, Zhu J, Tian F, et al. The therapeutic potential of dietary intervention: based on the mechanism of a tryptophan derivative-indole propionic acid on metabolic disorders. Crit Rev Food Sci Nutr. (2025) 65:1729–48. doi: 10.1080/10408398.2023.2299744
134. Li J, Zhang L, Wu T, Li Y, Zhou X, and Ruan Z. Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier. J Agric Food Chem. (2021) 69:1487–95. doi: 10.1021/acs.jafc.0c05205
135. Chen Y, Li Y, Li X, Fang Q, Li F, Chen S, et al. Indole−3−propionic acid alleviates intestinal epithelial cell injury via regulation of the TLR4/NF−κB pathway to improve intestinal barrier function. Mol Med Rep. (2024) 30. doi: 10.3892/mmr.2024.13313
136. Li Z, Geng H, Ye C, Cao L, Qin R, Chen K, et al. Gut microbial metabolite indole-3-propionic acid alleviates polycystic ovary syndrome in mice by regulating the AhR-NLRP3 axis. Int Immunopharmacol. (2025) 148:114038. doi: 10.1016/j.intimp.2025.114038
137. Sehgal R, Ilha M, Vaittinen M, Kaminska D, Männistö V, Kärjä V, et al. Indole-3-propionic acid, a gut-derived tryptophan metabolite, associates with hepatic fibrosis. Nutrients. (2021) 13. doi: 10.3390/nu13103509
138. Guo Z, Yang S, Qi L, Ma X, Wang Y, Li B, et al. Lactobacillus acidophilus KLDS1.0901 ameliorates non-alcoholic fatty liver disease by modulating the tryptophan metabolite indole-3-aldehyde and acting on its receptor AhR. Food Funct. (2025) 16:4939–57. doi: 10.1039/D4FO05280C
139. Luo Z, Liu Y, Wang X, Fan F, Yang Z, and Luo D. Exploring tryptophan metabolism: The transition from disturbed balance to diagnostic and therapeutic potential in metabolic diseases. Biochem Pharmacol. (2024) 230:116554. doi: 10.1016/j.bcp.2024.116554
140. Cherayil BJ. Indoleamine 2,3-dioxygenase in intestinal immunity and inflammation. Inflamm Bowel Dis. (2009) 15:1391–6. doi: 10.1002/ibd.20910
141. Bansal T, Alaniz RC, Wood TK, and Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. (2010) 107:228–33. doi: 10.1073/pnas.0906112107
142. Tian C, Deng S, Zhang Z, Zheng K, and Wei L. Bifidobacterium bifidum 1007478 derived indole-3-lactic acid alleviates NASH via an aromatic hydrocarbon receptor-dependent pathway in zebrafish. Life Sci. (2025) 369:123557. doi: 10.1016/j.lfs.2025.123557
143. Ding Y, Yanagi K, Yang F, Callaway E, Cheng C, Hensel ME, et al. Oral supplementation of gut microbial metabolite indole-3-acetate alleviates diet-induced steatosis and inflammation in mice. eLife. (2024) 12. doi: 10.7554/eLife.87458.3.sa2
144. Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. (2018) 24:1113–20. doi: 10.1038/s41591-018-0060-4
145. Tan Q, Deng S, and Xiong L. Role of kynurenine and its derivatives in liver diseases: recent advances and future clinical perspectives. Int J Mol Sci. (2025) 26(3). doi: 10.3390/ijms26030968
146. Shi C, Pei M, Wang Y, Chen Q, Cao P, Zhang L, et al. Changes of flavin-containing monooxygenases and trimethylamine-N-oxide may be involved in the promotion of non-alcoholic fatty liver disease by intestinal microbiota metabolite trimethylamine. Biochem Biophys Res Commun. (2022) 594:1–7. doi: 10.1016/j.bbrc.2022.01.060
147. Ma R, Shi G, Li Y, and Shi H. Trimethylamine N-oxide, choline and its metabolites are associated with the risk of non-alcoholic fatty liver disease. Br J Nutr. (2024) 131:1915–23. doi: 10.1017/S0007114524000631
148. Nian F, Chen Y, Xia Q, Zhu C, Wu L, and Lu X. Gut microbiota metabolite trimethylamine N-oxide promoted NAFLD progression by exacerbating intestinal barrier disruption and intrahepatic cellular imbalance. Int Immunopharmacol. (2024) 142:113173. doi: 10.1016/j.intimp.2024.113173
149. Bahramirad Z, Moloudi MR, Moradzad M, Abdollahi A, and Vahabzadeh Z. Trimethylamine-N-oxide, a New Risk Factor for Non-alcoholic Fatty Liver Disease Changes the Expression of miRNA-34a, and miRNA-122 in the Fatty Liver Cell Model. Biochem Genet. (2025) 63:1298–309. doi: 10.1007/s10528-024-10754-0
150. Vallianou NG, Kounatidis D, Psallida S, Panagopoulos F, Stratigou T, Geladari E, et al. The interplay between dietary choline and cardiometabolic disorders: A review of current evidence. Curr Nutr Rep. (2024) 13:152–65. doi: 10.1007/s13668-024-00521-3
151. Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, et al. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol Nutr Food Res. (2019) 63:e1900257. doi: 10.1002/mnfr.201900257
152. Zhong B, Zhu Q, Wei P, and Wang X. TMAO regulates OTUB1-mediated SLC7A11 stability to promote NAFLD progression. Biochem Biophys Res Commun. (2025) 778:152352. doi: 10.1016/j.bbrc.2025.152352
153. Yang B, Tang G, Wang M, Ni Y, Tong J, Hu C, et al. Trimethylamine N-oxide induces non-alcoholic fatty liver disease by activating the PERK. Toxicol Lett. (2024) 400:93–103. doi: 10.1016/j.toxlet.2024.08.009
154. Miyata M, Takeda K, Nagira S, and Sugiura Y. Trimethylamine N-oxide ameliorates hepatic damage including reduction of hepatic bile acids and cholesterol in Fxr-null mice. Int J Food Sci Nutr. (2024) 75:385–95. doi: 10.1080/09637486.2024.2346765
155. Mohsenzadeh A, Pourasgar S, Mohammadi A, Nazari M, Nematollahi S, Karimi Y, et al. The gut microbiota and cardiovascular disease: Exploring the role of microbial dysbiosis and metabolites in pathogenesis and therapeutics. Life Sci. (2025) 123981. doi: 10.1016/j.lfs.2025.123981
156. Saha B, Banerjee A, Pathak R, Duttaroy AK, and Pathak S. Trimethylamine N-Oxide (TMAO) and cancer risk: Insights into a possible link. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2025) 192:118592. doi: 10.1016/j.biopha.2025.118592
157. Putker F, Bos MP, and Tommassen J. Transport of lipopolysaccharide to the Gram-negative bacterial cell surface. FEMS Microbiol Rev. (2015) 39:985–1002. doi: 10.1093/femsre/fuv026
158. Akiba Y, Maruta K, Takajo T, Narimatsu K, Said H, Kato I, et al. Lipopolysaccharides transport during fat absorption in rodent small intestine. Am J Physiol Gastrointestinal liver Physiol. (2020) 318:G1070–g87. doi: 10.1152/ajpgi.00079.2020
159. Kong J, Han X, and Wei C. Causal relationship between metabolic dysfunction-associated fatty liver disease and endotoxin biomarkers: A Mendelian randomization study. Medicine. (2025) 104:e42311. doi: 10.1097/MD.0000000000042311
160. Barchetta I, Cimini FA, Sentinelli F, Chiappetta C, Di Cristofano C, Silecchia G, et al. Reduced lipopolysaccharide-binding protein (LBP) levels are associated with non-alcoholic fatty liver disease (NAFLD) and adipose inflammation in human obesity. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms242417174
161. Lee YS, Kim YH, Jung YS, Kim KS, Kim DK, Na SY, et al. Hepatocyte toll-like receptor 4 mediates lipopolysaccharide-induced hepcidin expression. Exp Mol Med. (2017) 49:e408. doi: 10.1038/emm.2017.207
162. Wang YF, Zhang WL, Li ZX, Liu Y, Tan J, Yin HZ, et al. METTL14 downregulation drives S100A4(+) monocyte-derived macrophages via MyD88/NF-κB pathway to promote MAFLD progression. Signal transduction targeted Ther. (2024) 9:91. doi: 10.1038/s41392-024-01797-1
163. Yaribeygi H, Farrokhi FR, Butler AE, and Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. (2019) 234:8152–61. doi: 10.1002/jcp.27603
164. Ryu JK, Kim SJ, Rah SH, Kang JI, Jung HE, Lee D, et al. Reconstruction of LPS transfer cascade reveals structural determinants within LBP, CD14, and TLR4-MD2 for efficient LPS recognition and transfer. Immunity. (2017) 46:38–50. doi: 10.1016/j.immuni.2016.11.007
165. Pang J, Xu W, Zhang X, Wong GL, Chan AW, Chan HY, et al. Significant positive association of endotoxemia with histological severity in 237 patients with non-alcoholic fatty liver disease. Alimentary Pharmacol Ther. (2017) 46:175–82. doi: 10.1111/apt.14119
166. Jin CJ, Engstler AJ, Ziegenhardt D, Bischoff SC, Trautwein C, and Bergheim I. Loss of lipopolysaccharide-binding protein attenuates the development of diet-induced non-alcoholic fatty liver disease in mice. J Gastroenterol Hepatol. (2017) 32:708–15. doi: 10.1111/jgh.13488
167. Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, and Claessens YE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clinica chimica acta; Int J Clin Chem. (2015) 450:97–103. doi: 10.1016/j.cca.2015.06.026
168. Roncon-Albuquerque R Jr., Moreira-Rodrigues M, Faria B, Ferreira AP, Cerqueira C, Lourenço AP, et al. Attenuation of the cardiovascular and metabolic complications of obesity in CD14 knockout mice. Life Sci. (2008) 83:502–10. doi: 10.1016/j.lfs.2008.07.021
169. Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, and Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Internal Emergency Med. (2024) 19:275–93. doi: 10.1007/s11739-023-03374-w
170. Brandt A, Jin CJ, Nolte K, Sellmann C, Engstler AJ, and Bergheim I. Short-term intake of a fructose-, fat- and cholesterol-rich diet causes hepatic steatosis in mice: effect of antibiotic treatment. Nutrients. (2017) 9. doi: 10.3390/nu9091013
171. Dai K, Liao B, Huang X, and Liu Q. Consistency in bacterial extracellular vesicle production: key to their application in human health. Extracellular vesicles circulating Nucleic Acids. (2025) 6:1–20. doi: 10.20517/evcna.2024.76
172. Zhou Y, Sun Y, Yin P, Zuo S, Li H, and Cao K. Bacterial extracellular vesicles: emerging mediators of gut-liver axis crosstalk in hepatic diseases. Front Cell infection Microbiol. (2025) 15:1620829. doi: 10.3389/fcimb.2025.1620829
173. Kaparakis-Liaskos M and Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. (2015) 15:375–87. doi: 10.1038/nri3837
174. Brown L, Wolf JM, Prados-Rosales R, and Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. (2015) 13:620–30. doi: 10.1038/nrmicro3480
175. Choi Y, Kwon Y, Kim DK, Jeon J, Jang SC, Wang T, et al. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci Rep. (2015) 5:15878. doi: 10.1038/srep15878
176. Fizanne L, Villard A, Benabbou N, Recoquillon S, Soleti R, Delage E, et al. Faeces-derived extracellular vesicles participate in the onset of barrier dysfunction leading to liver diseases. J extracellular vesicles. (2023) 12:e12303. doi: 10.1002/jev2.12303
177. Luo Z, Ji Y, Zhang D, Gao H, Jin Z, Yang M, et al. Microbial DNA enrichment promotes liver steatosis and fibrosis in the course of non-alcoholic steatohepatitis. Acta physiologica (Oxford England). (2022) 235:e13827. doi: 10.1111/apha.13827
178. Pirolli NH, Levy D, Schledwitz A, Moura NS, Solomon TJ, Powsner EH, et al. Genetically-programmed hypervesiculation of lactiplantibacillus plantarum increases production of bacterial extracellular vesicles with therapeutic efficacy in a preclinical inflammatory bowel disease model. bioRxiv: preprint server Biol. (2025). doi: 10.1101/2025.06.20.660770
179. Zhu Y, Zhang X, Huang W, Luo M, Feng X, Zhang H, et al. Protective effect of enterococcus faecium against alcohol-induced acute liver injury via extracellular vesicles in rats. Foodborne Pathog Dis. (2025). doi: 10.1089/fpd.2025.0005
180. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. (2012) 61:364–71. doi: 10.2337/db11-1019
181. Ellegaard AM, Kårhus ML, Winther-Jensen M, Lund AB, and Knop FK. Treatment of bile acid diarrhea with glucagon-like peptide 1 receptor agonists: A promising yet understudied approach. Clin Trans Gastroenterol. (2025) 16:e00815. doi: 10.14309/ctg.0000000000000815
182. Lu H, Zhang M, Hu Y, Sun X, Zhang R, Zhang X, et al. Short-chain fatty acids alleviate cholestatic liver injury by improving gut microbiota and bile acid metabolism. Int Immunopharmacol. (2025) 154:114564. doi: 10.1016/j.intimp.2025.114564
183. Chen J, Yang H, Qin Y, Zhou X, and Ma Q. Tryptophan ameliorates metabolic syndrome by inhibiting intestinal farnesoid X receptor signaling: the role of gut microbiota-bile acid crosstalk. Res (Washington DC). (2024) 7:0515. doi: 10.34133/research.0515
184. Qiao CM, Ma XY, Tan LL, Xia YM, Li T, Wu J, et al. Indoleamine 2, 3-dioxygenase 1 inhibition mediates the therapeutic effects in Parkinson’s disease mice by modulating inflammation and neurogenesis in a gut microbiota dependent manner. Exp Neurol. (2025) 385:115142. doi: 10.1016/j.expneurol.2025.115142
185. Zhang D, Wang Q, Li D, Chen S, Chen J, Zhu X, et al. Gut microbiome composition and metabolic activity in metabolic-associated fatty liver disease. Virulence. (2025) 16:2482158. doi: 10.1080/21505594.2025.2482158
186. Li Z, Gong R, Chu H, Zeng J, Chen C, Xu S, et al. A universal plasma metabolites-derived signature predicts cardiovascular disease risk in MAFLD. Atherosclerosis. (2024) 392:117526. doi: 10.1016/j.atherosclerosis.2024.117526
187. Luo M, Luo J, Kaminga AC, Wei J, Dai W, Zhong Y, et al. Targeted metabolomics reveals bioactive inflammatory mediators from gut into blood circulation in children with NAFLD. NPJ biofilms microbiomes. (2025) 11:119. doi: 10.1038/s41522-025-00706-w
188. Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, et al. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients. (2018) 10. doi: 10.3390/nu10121971
189. Su KY, Koh Kok JY, Chua YW, Ong SD, Ser HL, Pusparajah P, et al. Bacterial extracellular vesicles in biofluids as potential diagnostic biomarkers. Expert Rev Mol diagnostics. (2022) 22:1057–62. doi: 10.1080/14737159.2022.2166403
190. Jones E, Stentz R, Telatin A, Savva GM, Booth C, Baker D, et al. The origin of plasma-derived bacterial extracellular vesicles in healthy individuals and patients with inflammatory bowel disease: A pilot study. Genes. (2021) 12. doi: 10.3390/genes12101636
191. De Langhe N, Van Dorpe S, Guilbert N, Vander Cruyssen A, Roux Q, Deville S, et al. Mapping bacterial extracellular vesicle research: insights, best practices and knowledge gaps. Nat Commun. (2024) 15:9410. doi: 10.1038/s41467-024-53279-1
192. Wu W, Kaicen W, Bian X, Yang L, Ding S, Li Y, et al. Akkermansia muciniphila alleviates high-fat-diet-related metabolic-associated fatty liver disease by modulating gut microbiota and bile acids. Microbial Biotechnol. (2023) 16:1924–39. doi: 10.1111/1751-7915.14293
193. Nie Q, Luo X, Wang K, Ding Y, Jia S, Zhao Q, et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway. Cell. (2024) 187:2717–34.e33. doi: 10.1016/j.cell.2024.03.034
194. Becerril-Campos AA, Ramos-Gómez M, De Los Ríos-Arellano EA, Ocampo-Anguiano PV, González-Gallardo A, Macotela Y, et al. Bean leaves ameliorate lipotoxicity in fatty liver disease. Nutrients. (2023) 15. doi: 10.3390/nu15132928
195. Xiao Y, Zhang X, Yi D, Qiu F, Wu L, Tang Y, et al. Mediterranean diet affects the metabolic outcome of metabolic dysfunction-associated fatty liver disease. Front Nutr. (2023) 10:1225946. doi: 10.3389/fnut.2023.1225946
196. Arita VA, Cabezas MC, Hernández Vargas JA, Trujillo-Cáceres SJ, Mendez Pernicone N, Bridge LA, et al. Effects of Mediterranean diet, exercise, and their combination on body composition and liver outcomes in metabolic dysfunction-associated steatotic liver disease: a systematic review and meta-analysis of randomized controlled trials. BMC Med. (2025) 23:502. doi: 10.1186/s12916-025-04320-7
197. Maciejewska-Markiewicz D, Drozd A, Palma J, Ryterska K, Hawryłkowicz V, Załęska P, et al. Fatty acids and eicosanoids change during high-fiber diet in NAFLD patients-randomized control trials (RCT). Nutrients. (2022) 14. doi: 10.3390/nu14204310
198. Abd El Hamid AA, Mohamed AE, Mohamed MS, Amin GEE, Elessawy HAA, and Allam MF. The effect of probiotic supplementation on non-alcoholic fatty liver disease (NAFLD) fibrosis score in patients attending a tertiary hospital clinic in Cairo, Egypt. BMC Gastroenterol. (2024) 24:354. doi: 10.1186/s12876-024-03424-3
199. Gao LL, Li YX, Ma JM, Guo YQ, Li L, Gao QH, et al. Effect of Lycium barbarum polysaccharide supplementation in non-alcoholic fatty liver disease patients: study protocol for a randomized controlled trial. Trials. (2021) 22:566. doi: 10.1186/s13063-021-05529-6
200. Ma C, Bao Y, Hereid S, Zhang H, Bai X, Bai Q, et al. Mechanistic Elucidation of Tricholoma mongolicum Polysaccharides in Treating MAFLD via Regulation of the Gut Microbiota-Metabolite-Ferroptosis Axis: A Multi-Omics Perspective. J Agric Food Chem. (2025) 73:17040–56. doi: 10.1021/acs.jafc.5c05877
201. Zhang X, Liu R, Chen Y, Wang H, Su W, Song Y, et al. Dual-targeted nanoparticles hitchhiking on lactobacillus rhamnosus bacterial ghosts to alleviate nonalcoholic steatohepatitis. ACS nano. (2025) 19:14010–27. doi: 10.1021/acsnano.4c18280
202. Qiu XX, Cheng SL, Liu YH, Li Y, Zhang R, Li NN, et al. Fecal microbiota transplantation for treatment of non-alcoholic fatty liver disease: Mechanism, clinical evidence, and prospect. World J Gastroenterol. (2024) 30:833–42. doi: 10.3748/wjg.v30.i8.833
203. Xue L, Deng Z, Luo W, He X, and Chen Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: A randomized clinical trial. Front Cell infection Microbiol. (2022) 12:759306. doi: 10.3389/fcimb.2022.759306
204. Groenewegen B, Ruissen MM, Crossette E, Menon R, Prince AL, Norman JM, et al. Consecutive fecal microbiota transplantation for metabolic dysfunction-associated steatotic liver disease: a randomized controlled trial. Gut Microbes. (2025) 17:2541035. doi: 10.1080/19490976.2025.2541035
205. Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: A randomized control trial. Am J Gastroenterol. (2020) 115:1055–65. doi: 10.14309/ajg.0000000000000661
206. Maestri M, Santopaolo F, Pompili M, Gasbarrini A, and Ponziani FR. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: Effects of current treatments and future strategies. Front Nutr. (2023) 10:1110536. doi: 10.3389/fnut.2023.1110536
207. Arellano-García LI, Portillo MP, Martínez JA, Courtois A, and Milton-Laskibar I. Postbiotics for the management of obesity, insulin resistance/type 2 diabetes and NAFLD. Beyond microbial viability. Crit Rev Food Sci Nutr. (2024) 1–24.
208. Fogacci F, Giovannini M, Di Micoli V, Grandi E, Borghi C, and Cicero AFG. Effect of supplementation of a butyrate-based formula in individuals with liver steatosis and metabolic syndrome: A randomized double-blind placebo-controlled clinical trial. Nutrients. (2024) 16. doi: 10.3390/nu16152454
209. Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, et al. Design and rationale of the INSYTE study: A randomised, placebo controlled study to test the efficacy of a synbiotic on liver fat, disease biomarkers and intestinal microbiota in non-alcoholic fatty liver disease. Contemp Clin trials. (2018) 71:113–23. doi: 10.1016/j.cct.2018.05.010
210. Mattace Raso G, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O, et al. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One. (2013) 8:e68626.
Keywords: metabolic dysfunction-associated fatty liver disease, gut microbiota-derived bioactive compound, microbiota-host crosstalk, pathogenesis, clinical application
Citation: Ma C, Wang J, Song X, Wang X and Zong S (2025) Molecular mechanisms and clinical applications of gut microbiota-derived bioactive compounds in metabolic dysfunction-associated fatty liver disease. Front. Immunol. 16:1682755. doi: 10.3389/fimmu.2025.1682755
Received: 09 August 2025; Accepted: 29 September 2025;
Published: 20 October 2025.
Edited by:
Fengjie Sun, Georgia Gwinnett College, United StatesReviewed by:
Zhonghai Yan, Columbia University, United StatesYongbing Qian, Shanghai Jiao Tong University, China
Linshan Shang, University of Minnesota Twin Cities, United States
Copyright © 2025 Ma, Wang, Song, Wang and Zong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuai Zong, em9uZ3pvbmdfMTExNUAxNjMuY29t
†These authors have contributed equally to this work
 Chengyun Ma1†
Chengyun Ma1† Shuai Zong
Shuai Zong