- 1Department of Immunology, Zhuhai Campus of Zunyi Medical University, Zhuhai, China
- 2Department of Pharmaceutics, Guangdong Provincial People’s Hospital Zhuhai Hospital, Zhuhai, China
- 3College of Chemistry and Materials Science, Jinan University, Guangzhou, China
1 Introduction
Chronic wounds represent a major global health crisis, fundamentally characterized by the failure of the immune system to resolve inflammation and transition to a pro-reparative state (1, 2). Chronic wounds impose a staggering global burden, subjecting hundreds of millions of patients to persistent pain, social isolation, elevated rates of depression, and a markedly increased risk of mortality, thereby severely compromising their quality of life (3, 4). In healthy acute wounds, the immune response comprises a precisely regulated sequence of events, initiating with an inflammatory phase for the clearance of debris and pathogens, and transitioning to an active resolution phase that orchestrates tissue regeneration (2, 5). In chronic wounds, this coordinated process becomes dysregulated, resulting in a state of persistent, low-grade inflammation. This immunological dysregulation is the central biological lesion (2, 6).
The clinical management of chronic wounds is traditionally guided by principles of standard care, often summarized by the TIME framework (Tissue debridement, Inflammation and infection control, Moisture balance, and Epidermal edge advancement) (7, 8). While essential for preparing the wound bed, these measures are often primarily supportive in nature (9). They manage the wound’s condition but frequently fail to actively trigger the stalled healing cascade in a biologically non-permissive environment (10). This insufficiency of standard care to overcome the intrinsic biological barriers of non-healing wounds provides the fundamental rationale for shifting toward active therapeutic approaches (10, 11). Consequently, the field has increasingly focused on strategies in bioengineering and tissue engineering, which aim to directly intervene in and modulate the biological processes of the wound to break the cycle of healing futility.
Within this paradigm of active therapeutic intervention, several major technological branches have emerged. These include (1): Cell-based therapies, which involve the application of allogeneic or autologous cells such as fibroblasts, keratinocytes, and stem cells, sometimes delivered within living cellular constructs (e.g., Apligraf®, Dermagraft®) (2, 12, 13); Tissue-engineered scaffolds, utilizing materials like acellular dermal matrices (ADMs) to provide a structural template for cellular infiltration and tissue regrowth (3, 14, 15) Bioactive molecule delivery, which uses biomaterial carriers to release signaling molecules like growth factors (15, 16). Among these diverse strategies, the approach centered on growth factor delivery has attracted an immense volume of research.
Yet paradoxically, despite its compelling reparative potential and a substantial research foundation, its clinical translation has been profoundly disappointing (17, 18). This translational gap reveals a critical paradox: within a persistent, pro-inflammatory microenvironment, the biological efficacy of exogenously administered pro-regenerative factors is severely compromised. This failure suggests that current research strategies may suffer from a fundamental limitation: they focus primarily on optimizing the technical parameters of drug delivery (a pharmaceutical engineering problem), while overlooking the fact that the core etiology of impaired chronic wound healing is immunological dysregulation. To further elucidate and validate this point, we use basic fibroblast growth factor (bFGF) as a model for three reasons. First, as a potent mitogen for fibroblasts and endothelial cells, bFGF promotes granulation tissue formation and angiogenesis, addressing two key obstacles in chronic wound repair. Second, bFGF is among the most intensively studied growth factors in sustained-release and nanocarrier research (19–22). Third, its well-defined molecular pharmacology—requiring heparin/heparan sulfate–mediated receptor dimerization and downstream signaling—makes it an ideal candidate for both advanced materials-based manipulation and for systematically dissecting how inflammation attenuates growth factor efficacy at the receptor, signaling, and extracellular matrix (ECM) levels.
In this opinion article, we contend that the persistent failure of growth factor-based therapies stems from a biologically naive “container paradigm” that prioritizes delivery engineering over fundamental immunology. We posit that the true obstacle to chronic wound healing is the underlying immune dysregulation, which renders pro-regenerative signals futile. Therefore, we advocate for a paradigm shift toward “regenerative immuno-engineering,” an approach that first actively modulates the immune microenvironment to resolve inflammation, thereby creating a permissive biological context for subsequent, precisely controlled regenerative cues (Figure 1). This article will critically dissect the biological pillars of failure in current approaches and then outline a new blueprint for regenerative immuno-engineering, emphasizing the strategic integration of immunomodulation with spatiotemporal control of healing signals. It should be emphasized that while bFGF serves as our central model, the immuno-engineering paradigm we propose is equally applicable to other regenerative strategies mentioned prior.
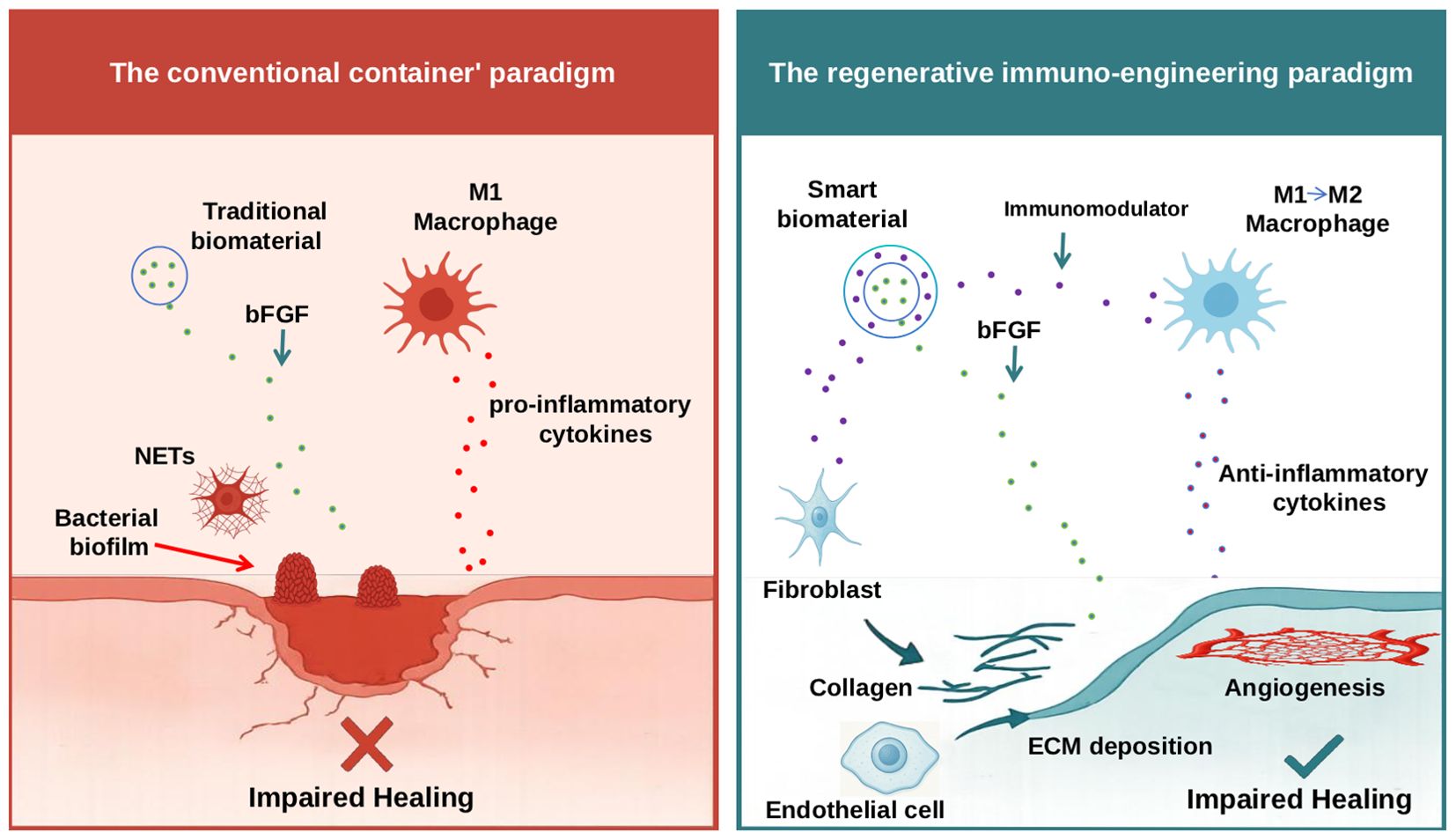
Figure 1. Schematic illustration of the conventional “container” paradigm versus the proposed regenerative immuno-engineering paradigm for chronic wound therapy. The left panel depicts the conventional “container” paradigm, where traditional biomaterials function as passive carriers, delivering bFGF into the chronic wound. However, this microenvironment is characterized by persistent, non-resolving inflammation, driven by pro-inflammatory M1-like macrophages secreting cytotoxic cytokines, excessive NETs, and the presence of bacterial biofilms. Despite bFGF delivery, this hostile inflammatory milieu largely renders the growth factor ineffective, leading to impaired healing. The right panel illustrates the regenerative immuno-engineering paradigm, a novel framework proposing the use of ‘smart biomaterials’ that first actively modulate the immune microenvironment. These smart biomaterials release immunomodulators to reprogram immune cells (e.g., promoting the phenotypic shift of M1 macrophages toward a pro-reparative M2 state, thereby facilitating the secretion of anti-inflammatory cytokines). Once immune homeostasis is restored and inflammation resolves, the biomaterial can then effectively deliver pro-regenerative factors like bFGF. In this permissive environment, bFGF can effectively stimulate fibroblasts to produce collagen and promote ECM deposition, and activate endothelial cells for angiogenesis, ultimately leading to enhanced tissue regeneration and successful wound healing.
2 The “container” paradigm: a biologically agnostic approach
The prevailing research framework for bFGF delivery can be described as the “container” paradigm. The primary goal of this framework has been to engineer a physicochemically perfect vessel, optimizing for key metrics, such as loading efficiency, stability, and sustained-release kinetics from various platforms like hydrogels, nanofibers, or microspheres (23, 24). A representative study within this paradigm might report a high bFGF encapsulation efficiency of > 90%, accompanied by elegant graphs depicting a smooth, linear, zero-order release over 21 days in vitro (25, 26). These results, however, would be typically obtained under sterile, acellular, and protease-free phosphate-buffered saline conditions that bear no resemblance to the chaotic, hostile milieu of a real chronic wound (25, 26). This engineering-first approach is intellectually attractive but biologically naive. Furthermore, this approach operates on the flawed assumption that the chronic wound is a passive void, and that a constant supply of a pro-regenerative factor is sufficient to trigger healing.
This approach, while technically sound from a pharmaceutical engineering standpoint, commits a dangerous reductionist fallacy in that it attempts to address a dynamic, multifactorial, and systemic biological problem with a single, constant, and context-independent input (i.e., local bFGF concentration). This perspective systematically overlooks the complex and dynamic immune microenvironment of chronic wounds, which is characterized by the predominance of pro-inflammatory M1-like macrophages, excessive neutrophil extracellular traps (NETs) that cause collateral tissue damage, and a milieu of cytotoxic cytokines and proteases (6, 27, 28). In this specific context, any attempt to promote regeneration is futile. This carrier-centric research paradigm has failed because the pro-regenerative signals it delivers cannot be effectively received and transduced by target cells that exist in a pro-inflammatory state with altered signaling pathways.
3 The biological pillars of failure: an immunological re-examination
The clinical failure of growth factor-based therapies does not stem from an insufficient potency of molecules like bFGF itself, but rather from a profound immunological veto that systematically dismantles their regenerative potential at every critical step (2, 14). The “container paradigm” is inherently flawed because the pro-regenerative “message” it delivers is intercepted, corrupted, and ultimately ignored by target cells trapped in a non-resolving inflammatory state. This failure is built upon three interconnected pillars of immunological destruction.
First, the delivered growth factor is rapidly neutralized before it can reach its target. The microenvironment of chronic wounds is characterized by the infiltration and dominance of pro-inflammatory immune cells, particularly M1-like macrophages and hyperactivated neutrophils (28–31). These cells release a complex cocktail of destructive enzymes, such as high levels of matrix metalloproteinases (MMPs) (27, 32). Any exogenously delivered protein, including bFGF, is highly susceptible to degradation in such a proteolytically rich milieu. Furthermore, excessive Neutrophil Extracellular Traps (NETs)—web-like structures composed of DNA and cytotoxic proteins—not only cause collateral tissue damage but may also indirectly impede the effective diffusion and utilization of growth factors (28, 29). Consequently, in an environment that is both proteolytically active and presents dual physical and chemical barriers, even the most sophisticated sustained-release carriers cannot guarantee the integrity and bioavailability of their payload.
Second, even if exogenous bFGF escapes degradation through protective strategies, such as conjugation with heparin analogs (26), the signaling responsiveness of target cells is already significantly blunted by inflammation. The biological function of bFGF is highly dependent on its specific binding to its cognate receptor complex. This process is critically mediated by cell-surface heparan sulfate proteoglycans (HSPGs) (33, 34), which in turn triggers downstream intracellular signaling cascades, most notably the mitogen-activated protein kinase/extracellular signal–regulated kinase (MAPK/ERK) and phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathways (35–37). Specifically, the MAPK/ERK pathway is a primary driver of cell proliferation and migration, stimulating the proliferation and motility of fibroblasts and keratinocytes, which are essential for granulation tissue formation and re-epithelialization (35, 36). Concurrently, the PI3K/AKT pathway plays a pivotal role in promoting cell survival by inhibiting apoptosis and regulating cell metabolism, while also fostering angiogenesis—all of which are indispensable processes for successful tissue regeneration (35, 37). The chronic wound microenvironment directly impairs or dismantles this signaling machinery. Activated immune cells, including M1 macrophages and neutrophils, secrete high levels of heparanase, and high expression of heparanase has been shown to degrade HSPGs in various models of chronic inflammation (38, 39). This creates a vicious cycle: inflammation destroys the very receptors required for a pro-regenerative response (40, 41). Concurrently, abundant pro-inflammatory cytokines in chronic wounds, such as tumor necrosis factor-α (TNF-α), can downregulate or interfere with several growth factor receptors and their downstream signaling, rendering cells refractory to proliferative/migratory stimuli (42, 43). Therefore, merely increasing the concentration of bFGF is a futile strategy when target cells have become insensitive or non-responsive to pro-regenerative stimuli due to persistent and strong inflammatory signal interference.
Finally, the entire cellular milieu is biologically misaligned and non-conducive to regeneration due to a profound failure in the process of “inflammation resolution.” Physiological healing is not merely the cessation of inflammation but a biochemically orchestrated program driven by specialized pro-resolving mediators (SPMs) that guides the cellular state from a pro-inflammatory phenotype to a pro-reparative phenotype (44–46). Chronic wounds are characterized by a severe deficit of these SPMs, leaving local cells trapped in a persistent, non-resolving state of inflammation (47–49). In this context, delivering a potent mitogen like bFGF via a passive carrier is biologically incoherent. It attempts to impose a proliferative signal upon an immune system and a stromal cell population that have not yet received the critical “stop inflammation” and “initiate resolution” commands. This mismatch does not lead to organized tissue regeneration but rather to aberrant, non-functional tissue deposition and potentially exacerbated fibrosis. In essence, the “container” approach fails because it attempts to initiate the tissue regeneration program before the critical biological prerequisite—the effective control and resolution of inflammation—has been met.
4 A new blueprint: principles of regenerative immuno-engineering
To break the translational stalemate, we must shift from the “container” paradigm to one of “Regenerative Immuno-engineering.” This approach views biomaterials not as carriers, but as active immunomodulatory platforms. The primary goal would be to re-establish immune homeostasis, thereby creating a permissive environment for endogenous and exogenous regenerative cues. This new framework is built on two core principles, as described below. Table 1 summarizes representative engineering approaches that operationalize this regenerative immuno-engineering paradigm, organized by therapeutic principle, engineering modality, mechanism, and examples.
4.1 Active immunomodulation as a therapeutic prerequisite
The primary objective of any advanced wound dressing must be the active modulation of the immune microenvironment. Pioneering studies have already demonstrated the feasibility of this approach (50). Biomaterials have been successfully engineered to drive macrophage reprogramming, shifting them from the M1 to the M2 phenotype, a critical step in resolving inflammation (51–53). Beyond macrophage reprogramming, future bioregulators could target other key immune players, for instance by designing materials that inhibit NETosis, deliver signals to promote regulatory T cell (Treg) expansion, or act as “cytokine sponges” to sequester excess TNF-α from the wound bed. Other “smart” materials could sense the pathological hallmarks of a chronic wound, such as high MMP levels or reactive oxygen species (ROS), and respond by releasing anti-inflammatory drugs or antioxidants, respectively (54, 55). Materials with intrinsic antibacterial properties, such as photodynamic hydrogels, can combat biofilms without inducing antibiotic resistance (56–58). Crucially, these functionalities can be synergistic; for example, disrupting a biofilm not only removes a bacterial source but also reduces the pathogen-associated molecular patterns (PAMPs) load that drives M1 macrophage activation. These studies exemplify the new role of this biomaterial as an “intelligent bioregulator” that first pacifies the battlefield.
4.2 Spatiotemporal control of immuno-regenerative signals
Once the immune environment is normalized, regenerative signals could be deployed effectively. This would require a sophisticated, “two-step” therapeutic strategy. The first wave of signals should be immunomodulatory, aimed at promoting the resolution of inflammation. Only after this is achieved should the second wave, featuring pro-regenerative factors such as bFGF and vascular endothelial growth factor (VEGF), be released. This necessitates a move away from simple and sustained release toward dynamic and sequential delivery (59, 60). This strategy could be achieved through a variety of sophisticated engineering strategies, such as multi-layered hydrogels with differential degradation rates, smart nano-valves that respond to specific pH or enzymatic cues, or on-demand systems triggered by external stimuli such as light or ultrasound (61–63). An idealized release profile might involve three stages: an initial burst of immunomodulators (e.g., IL-4 or SPMs) for the first 0–3 days; the subsequent release of pro-angiogenic and proliferative factors (e.g., bFGF/VEGF) from day 3 to 10; and a final phase delivering anti-fibrotic or tissue-remodeling agents to ensure high-quality tissue formation. The engineering of multi-compartment or layered systems, such as advanced microneedle arrays, provides a tangible platform for achieving this critical level of spatiotemporal control (64, 65). This approach, validated by the quantitative monitoring of downstream signaling pathways such as p-ERK and p-AKT would ensure that the right signal is delivered at the right time, in concert with the evolving immunological state of the wound (66, 67).
5 Conclusion and future perspectives
The long-standing failure to translate bFGF nanodelivery systems into clinical reality is not an indictment of nanotechnology, but of a research paradigm that has been divorced from fundamental immunology. The future research direction requires a fundamental shift in perspective: from designing materials as passive carriers to engineering intelligent biomaterials capable of specific molecular interactions with the immune system.
5.1 Bridging the bench-to-bedside gap: the path forward
We now call for the establishment of a truly integrated field of “regenerative immuno-engineering,” in which materials scientists and immunologists will collaborate from day one. This new field must be supported by a revolution in preclinical modeling. We must acknowledge the profound limitations of traditional murine models, whose rapid and robust healing capacity often masks the pathologies that prevent healing in humans (68, 69). The path forward requires embracing more physiologically relevant platforms such as three-dimensional (3D)-bioprinted and immune-competent skin equivalents that incorporate senescent cells and biofilms, organ-on-a-chip systems for high-throughput screening and the real-time monitoring of immune-material interactions, and large animal models of diabetes that better recapitulate systemic metabolic dysregulation (70–72).
Furthermore, the path to clinical translation is paved with regulatory challenges. These drug-device combination products present unique hurdles for agencies. Key questions arise regarding the characterization of the material’s mechanism of action (is it a device, a drug, or both)?, the establishment of relevant biomarkers to prove in vivo immunomodulation, and ensuring lot-to-lot consistency for complex, multi-functional materials (73, 74). A “design-for-translation” approach that incorporates regulatory science early in the development process is essential.
5.2 Technological convergence for personalized wound management
When positioned against the broader landscape of advanced therapies, the rationale for regenerative immuno-engineering becomes even more compelling. Compared to cell-based therapies, which introduce exogenous living cells into a hostile environment, our approach offers a more fundamental solution. Instead of delivering cells that may struggle to survive and function amidst chronic inflammation, regenerative immuno-engineering first focuses on “detoxifying” the wound bed, creating a permissive niche where the patient’s own endogenous cells can be activated to drive repair. This “host-centric” strategy may also offer significant advantages in terms of cost, scalability, and off-the-shelf availability over complex cell logistics. Specifically, the “off-the-shelf” nature of these advanced biomaterials circumvents the complex logistics, high costs, and patient-specific manufacturing challenges associated with cell-based therapies. This inherent scalability makes the approach more amenable to widespread clinical adoption. Furthermore, while navigating the regulatory pathway for drug-device combination products is not without its hurdles, the potential for standardized, large-scale manufacturing and stringent quality control may present a more straightforward route to approval compared to the highly personalized and variable nature of many cell-based products. Similarly, while tissue-engineered scaffolds provide a valuable physical framework, they do not inherently address the underlying immunological paralysis. Regenerative immuno-engineering acts as a functional complement, transforming these passive scaffolds into active immunomodulatory platforms. In essence, rather than simply replacing or supplementing tissue components, our paradigm seeks to restore the intrinsic regenerative capacity of the host by correcting the root immunological defect.
Looking ahead, the “intelligent bioregulator” will serve as a platform for converging technologies. Artificial intelligence (AI) and machine learning, for instance, can move beyond simple screening to perform inverse design, predicting material compositions and surface topographies that will elicit a desired immune response (75, 76). We envision systems integrated with biosensors for closed-loop and adaptive therapy, and platforms that combine protein delivery with gene-based approaches to provide both short-term signals and long-term cellular programming (77, 78). Ultimately, this culminates in the vision of personalized wound management. For example, a patient’s wound exudate could be rapidly analyzed via single-cell or proteomic profiling to identify their unique “inflammatory signature,” which could then inform the AI-driven fabrication of a bespoke bioregulator dressing with a personalized cocktail of therapeutic agents and a tailored release schedule.
Confronting the manufacturing challenges will be paramount. However, the first and most crucial step is conceptual. We must recognize that healing a chronic wound is fundamentally an immunological challenge. Future biomaterials must not only deliver bFGF but also be capable of actively modulating the patient’s immune response through the release of specific signaling molecules. Only by imparting specific immunomodulatory functions to materials, enabling them to precisely intervene in the immune response process, can we expect to ultimately achieve coordinated and effective tissue regeneration.
Author contributions
ZW: Writing – original draft. XZ: Writing – review & editing. WF: Writing – review & editing. YL: Writing – review & editing. PW: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The present research was supported by the Guizhou Province Science and Technology Foundation ((2022)609), and the Key Construction Discipline of Immunology and Pathogen biology in Zhuhai Campus of Zunyi Medical University (ZHGF2024-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Falanga V, Isseroff RR, Soulika AM, Romanelli M, Margolis D, Kapp S, et al. Chronic wounds. Nat Rev Dis Primers. (2022) 8:50. doi: 10.1038/s41572-022-00377-3
2. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, and Saparov A. Immunology of acute and chronic wound healing. Biomolecules. (2021) 11:700. doi: 10.3390/biom11050700
3. Sharma A, Shankar R, Yadav AK, Pratap A, Ansari MA, and Srivastava V. Burden of chronic nonhealing wounds: An overview of the worldwide humanistic and economic burden to the healthcare system. Int J Low Extrem Wounds. (2024) 24:15347346241246339. doi: 10.1177/15347346241246339
4. Carter M, DaVanzo J, Haught R, Nusgart M, Cartwright D, and Fife C. Chronic wound prevalence and the associated cost of treatment in Medicare beneficiaries: changes between 2014 and 2019. J Med Econ. (2023) 26:894–901. doi: 10.1080/13696998.2023.2232256
5. Larouche J, Sheoran S, Maruyama K, and Martino MM. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv Wound Care. (2018) 7:209–31. doi: 10.1089/wound.2017.0761
6. Li MR, Hou Q, Zhong LZ, Zhao YL, and Fu XB. Macrophage related chronic inflammation in non-healing wounds. Front Immunol. (2021) 12:681710. doi: 10.3389/fimmu.2021.681710
7. Atkin L. Chronic wounds: the challenges of appropriate management. Br J Community Nurs. (2019) 24:S26–32. doi: 10.12968/bjcn.2019.24.Sup9.S26
8. Sun H, Pulakat L, and Anderson DW. Challenges and new therapeutic approaches in the management of chronic wounds. Curr Drug Targets. (2020) 21:1264–75. doi: 10.2174/1389450121666200623131200
9. Frykberg RJ and Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. (2015) 4:560–82. doi: 10.1089/wound.2015.0635
10. Powers JG, Higham C, Broussard K, and Phillips TJ. Wound healing and treating wounds: Chronic wound care and management. J Am Acad Dermatol. (2016) 74:607–25. doi: 10.1016/j.jaad.2015.08.070
11. Popescu V, Cauni V, Petrutescu MS, Rustin MM, Bocai R, Turculet CR, et al. Chronic wound management: From gauze to homologous cellular matrix. Biomedicines. (2023) 11:2457. doi: 10.3390/biomedicines11092457
12. Boyce ST and Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burn Trauma. (2018) 6:4. doi: 10.1186/s41038-017-0103-y
13. Shevchenko RV, James SL, and James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. (2010) 7:229–58. doi: 10.1098/rsif.2009.0403
14. Barrientos S, Stojadinovic O, Golinko MS, Brem H, and Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regener. (2008) 16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x
15. MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. (2007) 445:874–80. doi: 10.1038/nature05664
16. Goh M, Du M, Peng WR, Saw PE, and Chen ZY. Advancing burn wound treatment: exploring hydrogel as a transdermal drug delivery system. Drug Delivery. (2024) 31:2300945. doi: 10.1080/10717544.2023.2300945
17. Yamakawa S and Hayashida K. Advances in surgical applications of growth factors for wound healing. Burns Trauma. (2019) 7:10. doi: 10.1186/s41038-019-0148-1
18. Mullin JA, Rahmani E, Kiick K, and Sullivan MO. Growth factors and growth factor gene therapies for treating chronic wounds. Bioeng Transl Med. (2024) 9:e10642. doi: 10.1002/btm2.10642
19. Son B, Kim M, Won H, Jung A, Kim J, Koo Y, et al. Secured delivery of basic fibroblast growth factor using human serum albumin-based protein nanoparticles for enhanced wound healing and regeneration. J Nanobiotechnology. (2023) 21:310. doi: 10.1186/s12951-023-02053-4
20. Zhang XY, Kang XN, Jin LJ, Bai J, Liu W, and Wang ZY. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int J Nanomedicine. (2018) 13:3897–906. doi: 10.2147/IJN.S168998
21. Zare R, Abdolsamadi H, Asl SS, Radi S, Bahrami H, and Jamshidi S. The bFGF can improve angiogenesis in oral mucosa and accelerate wound healing. Rep Biochem Mol Biol. (2023) 11:547–52. doi: 10.52547/rbmb.11.4.547
22. Chen A, Huang W, Wu L, An Y, Xuan TX, He HC, et al. Bioactive ECM mimic hyaluronic acid dressing via sustained releasing of bFGF for enhancing skin wound healing. ACS Appl Bio Mater. (2020) 3:3039–48. doi: 10.1021/acsabm.0c00096
23. Li HS, Li BY, Lv DL, Li WH, Lu YF, and Luo GX. Biomaterials releasing drug responsively to promote wound healing via regulation of pathological microenvironment. Adv Drug Delivery Rev. (2023) 196:114778. doi: 10.1016/j.addr.2023.114778
24. Tu Y, Li Y, Qu G, Ning Y, Li B, Li G, et al. A review of basic fibroblast growth factor delivery strategies and applications in regenerative medicine. J BioMed Mater Res A. (2025) 113:e37834. doi: 10.1002/jbm.a.37834
25. Huang Q, Liu B, and Wu W. Biomaterial-based bFGF delivery for nerve repair. Oxid Med Cell Longev. (2023) 2023:8003821. doi: 10.1155/2023/8003821
26. Lee J, Ban E, Park H, and Kim A. Stability enhancement of freeze-dried gelatin/alginate coacervates for bFGF delivery. Pharmaceutics. (2022) 14:2548. doi: 10.3390/pharmaceutics14122548
27. Boeringer T, Gould LJ, and Koria P. Protease-resistant growth factor formulations for the healing of chronic wounds. Adv Wound Care. (2020) 9:612–22. doi: 10.1089/wound.2019.1043
28. Zhu SN, Yu Y, Ren Y, Xu LY, Wang HL, Ling XM, et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. (2021) 12:984. doi: 10.1038/s41419-021-04294-3
29. Sabbatini M, Magnelli V, and Renò F. NETosis in wound healing: When enough is enough. Cells. (2021) 10:494. doi: 10.3390/cells10030494
30. Hassanshahi A, Moradzad M, Ghalamkari S, Fadaei M, Cowin AJ, and Hassanshahi M. Macrophage-mediated inflammation in skin wound healing. Cells. (2022) 11:2953. doi: 10.3390/cells11192953
31. Huang SM, Wu CS, Chiu MH, Wu CH, Chang YT, Chen GS, et al. High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-α: An important mechanism to delay the diabetic wound healing. J Dermatol Sci. (2019) 96:159–67. doi: 10.1016/j.jdermsci.2019.11.004
32. Tardáguila-García A, García-Morales E, García-Alamino JM, Álvaro−Afonso FJ, Molines−Barroso RJ, and Lázaro−Martínez JL. Metalloproteinases in chronic and acute wounds: A systematic review and meta−analysis. Wound Repair Regener. (2019) 27:415–20. doi: 10.1111/wrr.12717
33. Rapraeger AC, Krufka A, and Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. (1991) 252:1705–8. doi: 10.1126/science.1646484
34. Aviezer D, Hecht D, Safran M, Eisinger M, David G, and Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. (1994) 79:1005–13. doi: 10.1016/0092-8674(94)90031-0
35. Edirisinghe O, Ternier G, Alraawi Z, and Suresh Kumar TK. Decoding FGF/FGFR signaling: Insights into biological functions and disease relevance. Biomolecules. (2024) 14:1622. doi: 10.3390/biom14121622
36. Pandey S, Anshu T, Maharana KC, and Sinha S. Molecular insights into diabetic wound healing: Focus on Wnt/beta-catenin and MAPK/ERK signaling pathways. Cytokine. (2025) 191:156957. doi: 10.1016/j.cyto.2025.156957
37. Jere SW, Houreld NN, and Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3beta) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. (2019) 50:52–9. doi: 10.1016/j.cytogfr.2019.03.001
38. Meirovitz A, Goldberg R, Binder A, Rubinstein AM, Hermano E, and Elkin M. Heparanase in inflammation and inflammation-associated cancer. FEBS J. (2013) 280:2307–19. doi: 10.1111/febs.12184
39. Khanna M and Parish CR. Heparanase: Historical aspects and future perspectives. Adv Exp Med Biol. (2020) 1221:71–96. doi: 10.1007/978-3-030-34521-1_3
40. Nissen NN, Shankar R, Gamelli RL, Singh A, and DiPietro LA. Heparin and heparan sulphate protect basic fibroblast growth factor from non-enzymic glycosylation. Biochem J. (1999) 338:637–42. doi: 10.1042/bj3380637
41. Ishai-Michaeli R, Eldor A, and Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. (1990) 1:833–42. doi: 10.1091/MBC.1.11.833
42. Yamane K, Ihn H, Asano Y, Jinnin M, and Tamaki K. Antagonistic effects of TNF-alpha on TGF-beta signaling through down-regulation of TGF-beta receptor type II in human dermal fibroblasts. J Immunol. (2003) 171:3855–62. doi: 10.4049/jimmunol.171.7.3855
43. Hotamisligil GS, Murray DL, Choy LN, and Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA. (1994) 91:4854–8. doi: 10.1073/pnas.91.11.4854
44. Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. (2007) 21:325–32. doi: 10.1096/fj.06-7227rev
45. Buckley CD, Gilroy DW, and Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. (2014) 40:315–27. doi: 10.1016/j.immuni.2014.02.009
46. Serhan CN, Chiang N, and Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. (2015) 27:200–15. doi: 10.1016/j.smim.2015.03.004
47. Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. (2002) 196:1025–37. doi: 10.1084/jem.20020760
48. Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. (2009) 461:1287–91. doi: 10.1038/nature08541
49. Serhan CN and Levy BD. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J Clin Invest. (2018) 128:2657–69. doi: 10.1172/JCI97943
50. Badylak SF and Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. (2008) 20:109–16. doi: 10.1016/j.smim.2007.11.003
51. Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. (2015) 37:194–207. doi: 10.1016/j.biomaterials.2014.10.017
52. Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, and Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. (2019) 6:1900513. doi: 10.1002/advs.201900513
53. Álvarez MM, Liu JC, Trujillo-de Santiago G, Cha BH, Vishwakarma A, Ghaemmaghami A, et al. Delivery strategies to control inflammatory response: Modulating M1-M2 polarization in tissue engineering applications. J Control Release. (2016) 240:349–63. doi: 10.1016/j.jconrel.2016.01.026
54. Tu CX, Lu HD, Zhou T, Zhang WY, Deng LW, Cao WB, et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. (2022) 286:121597. doi: 10.1016/j.biomaterials.2022.121597
55. Deng PX, Shi ZH, Fang FY, Xu Y, Zhou LA, Liu Y, et al. Wireless matrix metalloproteinase-9 sensing by smart wound dressing with controlled antibacterial nanoparticles release toward chronic wound management. Biosens Bioelectron. (2025) 268:116860. doi: 10.1016/j.bios.2024.116860
56. Liu SY, Feng YH, Tan Y, Chen JY, Yang T, Wang XY, et al. Photosensitizer-loaded hydrogels: A new antibacterial dressing. Wound Repair Regener. (2024) 32:301–13. doi: 10.1111/wrr.13156
57. Du T, Xiao Z, Zhang G, Wei L, Cao J, Zhang Z, et al. An injectable multifunctional hydrogel for eradication of bacterial biofilms and wound healing. Acta Biomater. (2023) 161:112–33. doi: 10.1016/j.actbio.2023.03.008
58. Xie YX, Gan CC, Li ZX, Liu WF, Yang DJ, and Qiu XQ. Fabrication of a lignin-copper sulfide-incorporated PVA hydrogel with near-infrared-activated photothermal/photodynamic/peroxidase-like performance for combating bacteria and biofilms. ACS Biomater Sci Eng. (2022) 8:560–9. doi: 10.1021/acsbiomaterials.1c01406
59. Jimi S, Jaguparov A, Nurkesh A, Sultankulov B, and Saparov A. Sequential delivery of cryogel released growth factors and cytokines accelerates wound healing and improves tissue regeneration. Front Bioeng Biotechnol. (2020) 8:345. doi: 10.3389/fbioe.2020.00345
60. Li LM, Li Q, Gui L, Deng Y, Wang L, Jiao JL, et al. Sequential gastrodin release PU/n-HA composite scaffolds reprogram macrophages for improved osteogenesis and angiogenesis. Bioact Mater. (2022) 19:24–37. doi: 10.1016/j.bioactmat.2022.03.037
61. Badeau BA, Comerford MP, Arakawa CK, Shadish JA, and DeForest CA. Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat Chem. (2018) 10:251–8. doi: 10.1038/nchem.2917
62. Xing YH, Zeng BH, and Yang W. Light responsive hydrogels for controlled drug delivery. Front Bioeng Biotechnol. (2022) 10:1075670. doi: 10.3389/fbioe.2022.1075670
63. Yeingst TJ, Arrizabalaga JH, and Hayes DJ. Ultrasound-induced drug release from stimuli-responsive hydrogels. Gels. (2022) 8:554. doi: 10.3390/gels8090554
64. Zhang Y, Wang SQ, Yang YX, Zhao S, You JH, Wang JX, et al. Scarless wound healing programmed by core-shell microneedles. Nat Commun. (2023) 14:3413. doi: 10.1038/s41467-023-39129-6
65. Ma ZJ, Song W, He YH, and Li HY. A multilayer injectable hydrogel system sequentially delivers bioactive substances for each wound healing stage. ACS Appl Mater Interfaces. (2020) 12:29787–806. doi: 10.1021/acsami.0c06360
66. Ren S, Chen J, Duscher D, Liu YT, Guo GJ, Kang Y, et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res Ther. (2019) 10:47. doi: 10.1186/s13287-019-1152-x
67. Bonnici L, Suleiman S, Schembri-Wismayer P, and Cassar A. Targeting signalling pathways in chronic wound healing. Int J Mol Sci. (2023) 25:50. doi: 10.3390/ijms25010050
68. Zomer HD and Trentin AG. Skin wound healing in humans and mice: Challenges in translational research. J Dermatol Sci. (2018) 90:3–12. doi: 10.1016/j.jdermsci.2017.12.009
69. Barakat M, DiPietro LA, and Chen L. Limited treatment options for diabetic wounds: Barriers to clinical translation despite therapeutic success in murine models. Adv Wound Care. (2021) 10:436–60. doi: 10.1089/wound.2020.1254
70. Hofmann E, Fink J, Pignet A, Schwarz A, Schellnegger M, Nischwitz SP, et al. Human in vitro skin models for wound healing and wound healing disorders. Biomedicines. (2023) 11:1056. doi: 10.3390/biomedicines11041056
71. Deal HE, Brown AC, and Daniele MA. Microphysiological systems for the modeling of wound healing and evaluation of pro-healing therapies. J Mater Chem B. (2020) 8:7062–75. doi: 10.1039/d0tb00544d
72. Parnell LKS and Volk SW. The evolution of animal models in wound healing research: 1993-2017. Adv Wound Care. (2019) 8:692–702. doi: 10.1089/wound.2019.1098
73. Gupta DK, Tiwari A, Yadav Y, Soni P, and Joshi M. Ensuring safety and efficacy in combination products: Regulatory challenges and best practices. Front Med Technol. (2024) 6:1377443. doi: 10.3389/fmedt.2024.1377443
74. Reis ME, Bettencourt A, and Ribeiro HM. The regulatory challenges of innovative customized combination products. Front Med. (2022) 9:821094. doi: 10.3389/fmed.2022.821094
75. Suwardi A, Wang FK, Xue K, Han MY, Teo P, Wang P, et al. Machine learning-driven biomaterials evolution. Adv Mater. (2022) 34:e2102703. doi: 10.1002/adma.202102703
76. Ahmed E, Mulay P, Ramirez C, Tirado-Mansilla G, Cheong E, and Gormley AJ. Mapping biomaterial complexity by machine learning. Tissue Eng Part A. (2024) 30:662–80. doi: 10.1089/ten.TEA.2024.0067
77. Zheng ZY, Zhu RJ, Peng I, Xu ZT, and Jiang YW. Wearable and implantable biosensors: mechanisms and applications in closed-loop therapeutic systems. J Mater Chem B. (2024) 12:8577–604. doi: 10.1039/d4tb00782d
Keywords: chronic wounds, regenerative immuno-engineering, basic fibroblast growth factor, biomaterials, immunomodulation
Citation: Wang Z, Zeng X, Feng W, Lu Y and Wei P (2025) Reframing chronic wound therapy: from growth factor delivery to regenerative immuno-engineering. Front. Immunol. 16:1683591. doi: 10.3389/fimmu.2025.1683591
Received: 11 August 2025; Accepted: 13 October 2025;
Published: 22 October 2025.
Edited by:
Andrea Cavani, UOC Coordinamento Scientifico_National Institute for Health Migration and Poverty (NIHMP), ItalyReviewed by:
Archita Sharma, The University of Texas MD Anderson Cancer Center, United StatesCopyright © 2025 Wang, Zeng, Feng, Lu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei Wei, d2VpcGVpQHptdXpoLmVkdS5jbg==
 Zhiyong Wang
Zhiyong Wang Xuan Zeng
Xuan Zeng Wenzhao Feng3
Wenzhao Feng3 Yanxin Lu
Yanxin Lu Pei Wei
Pei Wei