- 1Department of Oncology and Pathology, Karolinska Institutet, SciLifeLab, Solna, Sweden
- 2Dmitry Rogachev National Medical Center of Pediatric Hematology, Oncology, and Immunology, Moscow, Russia
- 3Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russia
A Correction on
CAR T cell therapy for central nervous system solid tumors: current progress and future directions
By Kaminskiy Y, Degtyarev V, Stepanov A and Maschan M (2025). Front. Immunol. 16:1600403. doi: 10.3389/fimmu.2025.1600403
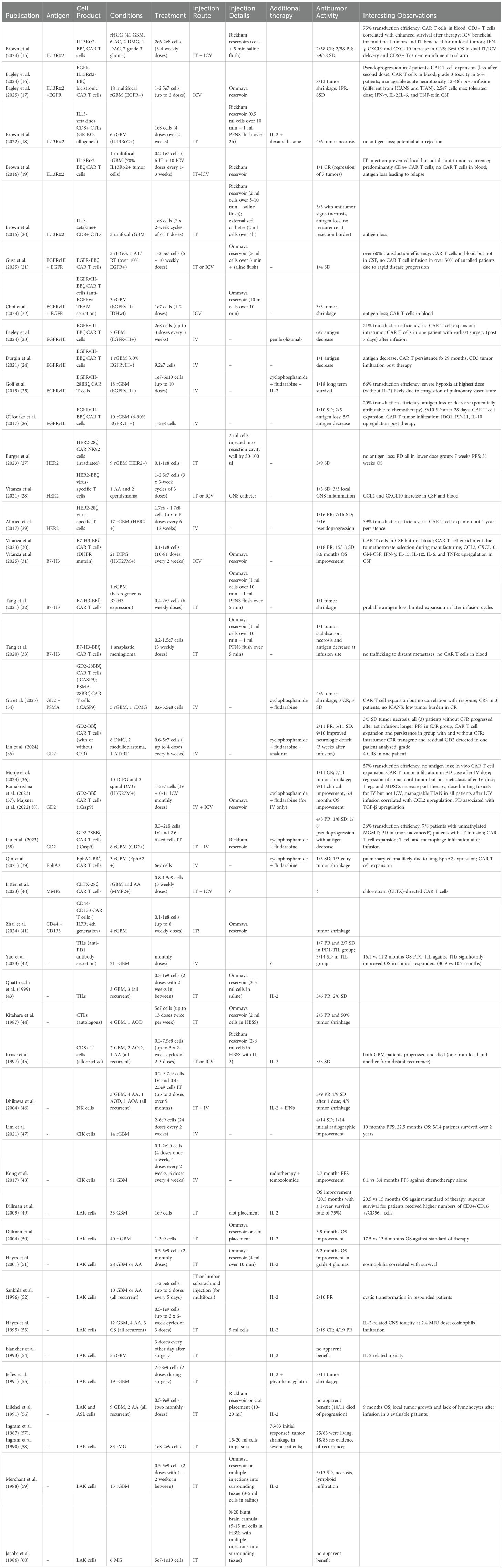
There was a mistake in Table 1 as published. “CAR T cells in blood; CD3+ T cells correlated with enhanced survival after therapy; ICV beneficial for multifocal tumors and IT beneficial for unifocal tumors; 75% transduction efficiency; IFN-γ, CXCL9 and CXCL10 increase in CNS; Best OS in dual IT/ICV delivery and CD62+ Tn/mem enrichment trial arm;”. The corrected text appears below.
“75% transduction efficiency; CAR T cells in blood; CD3+ T cells correlated with enhanced survival after therapy; ICV beneficial for multifocal tumors and IT beneficial for unifocal tumors; IFN-γ, CXCL9 and CXCL10 increase in CNS; Best OS in dual IT/ICV delivery and CD62+ Tn/mem enrichment trial arm”.
There was a mistake in Table 1 as published. “severe hypoxia at highest dose (without IL-2) likely due to congestion of pulmonary vasculature; 66% transduction efficiency”. The corrected text appears below.
“66% transduction efficiency; severe hypoxia at highest dose (without IL-2) likely due to congestion of pulmonary vasculature”.
There was a mistake in Table 1 as published. “antigen loss or decrease (potentially attributable to chemotherapy); 9/10 SD after 28 days; CAR T cell expansion; CAR T tumor infiltration; 20% transduction efficiency; IDO1, PD-L1, IL- 10 upregulation post therapy;”. The corrected text appears below.
“20% transduction efficiency; antigen loss or decrease (potentially attributable to chemotherapy); 9/10 SD after 28 days; CAR T cell expansion; CAR T tumor infiltration; IDO1, PD-L1, IL- 10 upregulation post therapy”
There was a mistake in Table 1 as published. “3/5 SD tumor necrosis; all (3) patients without C7R progressed after 1st infusion; longer PFS in C7R group; CAR T cell expansion and persistence in group with and without C7R; intratumor C7R transgene and residual GD2 detected in one patient analyzed; grade 4 CRS in one patient;”. The corrected text appears below.
“3/5 SD tumor necrosis; all patients without C7R progressed after 1st infusion; longer PFS in C7R group; CAR T cell expansion and persistence in group with and without C7R; intratumor C7R transgene and residual GD2 detected in one patient analyzed; grade 4 CRS in one patient”
There was a mistake in Table 1 as published. “no antigen loss; in vivo CAR T cell expansion; CAR T tumor infiltration in PD case after IV dose; regression of spinal cord tumor but not metastasis after IV dose; Tregs and MDSCs increase post therapy; dose limiting toxicity for IV but not ICV; manageable TIAN in all patients after ICV infusion correlated with CCL2 upregulation; PD associated with TGF-β upregulation; 57% transduction efficiency”. The corrected text appears below.
“57% transduction efficiency; no antigen loss; in vivo CAR T cell expansion; CAR T tumor infiltration in PD case after IV dose; regression of spinal cord tumor but not metastasis after IV dose; Tregs and MDSCs increase post therapy; dose limiting toxicity for IV but not ICV; manageable TIAN in all patients after ICV infusion correlated with CCL2 upregulation; PD associated with TGF-β upregulation”
There was a mistake in Table 1 as published. “8.1 vs 5.4 months PFS against chemotherapy alone;”. The corrected text appears below.
“8.1 vs 5.4 months PFS against chemotherapy alone”
There was a mistake in Table 1 as published. “17.5 vs 13.6 months OS against standard of therapy;”. The corrected text appears below.
“17.5 vs 13.6 months OS against standard of therapy”
There was a mistake in Table 1 as published. “IL-2-related CNS toxicity at 2.4 MIU dose; eosinophils infiltration;”. The corrected text appears below.
“IL-2-related CNS toxicity at 2.4 MIU dose; eosinophils infiltration”
The original version of this article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: CNS tumors, glioma, DMG, DIPG, glioblastoma, CAR T, CAR T solid tumors, brain cancer
Citation: Kaminskiy Y, Degtyarev V, Stepanov A and Maschan M (2025) Correction: CAR T cell therapy for central nervous system solid tumors: current progress and future directions. Front. Immunol. 16:1689398. doi: 10.3389/fimmu.2025.1689398
Received: 20 August 2025; Accepted: 15 September 2025;
Published: 23 September 2025.
Edited and reviewed by:
Philipp C. Rommel, University of Pennsylvania, United StatesCopyright © 2025 Kaminskiy, Degtyarev, Stepanov and Maschan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Maschan, bW1hc2NoYW5AeWFuZGV4LnJ1
 Yaroslav Kaminskiy
Yaroslav Kaminskiy Vitaly Degtyarev
Vitaly Degtyarev Alexey Stepanov3
Alexey Stepanov3 Michael Maschan
Michael Maschan