- 1Department of Respiratory and Critical Care Medicine, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, China
- 2Medical Research Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 3Key Laboratory of Interventional Pulmonology of Zhejiang Province, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 4Department of Pulmonary and Critical Care Medicine, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, China
- 5Institute for Lung Health (ILH), Justus-Liebig University Giessen, Giessen, Germany
Pulmonary fibrosis (PF) is a progressive, fatal interstitial lung disease with a dire prognosis and limited therapeutic options. Current standard-of-care anti-fibrotic agents (e.g., nintedanib and pirfenidone) offer only modest efficacy in slowing disease progression. Mesenchymal stem cell-derived exosomes (MSC-Exos) have recently emerged as a promising cell-free therapeutic strategy, boasting superior biocompatibility, low immunogenicity, enhanced biodistribution, and an innate tropism for injured tissues. Their potent anti-fibrotic effects are mediated through multiple mechanisms: targeted homing to fibrotic niches; reprogramming of dysregulated immune responses, notably by shifting macrophage polarization from a pro-inflammatory (M1) to an anti-inflammatory/reparative (M2) phenotype; suppression of pathological extracellular matrix deposition via inhibition of core fibrogenic pathways; and alleviation of endoplasmic reticulum stress in alveolar epithelial cells. This review systematically delineates the biological functions and molecular mechanisms underpinning the therapeutic actions of MSC-Exos in PF. We further evaluate completed and ongoing clinical trials (2014–2024), appraise the current translational landscape, and identify persistent challenges in drug development. Ultimately, this integrative analysis aims to define the mechanistic basis of MSC-Exos' efficacy, evaluate their clinical trajectory, and provide a strategic roadmap for their development into precision nanotherapeutics for PF.
1 Introduction
Pulmonary fibrosis (PF), a progressive and fatal respiratory disorder characterized by irreversible interstitial scarring, imposes a significant global health burden with increasing morbidity and mortality. Idiopathic pulmonary fibrosis (IPF), the most severe form of PF, has a median survival duration of 2–3 years post-diagnosis, with mortality rates surpassing those of many cancers (1–3). Driven concurrently by population aging and the rising prevalence of established risk factors (e.g., smoking and environmental exposure), the global burden of IPF is projected to increase substantially (4). Despite significant advances in the understanding of the etiology, pathology, and diagnosis of IPF, therapeutic progress has remained limited since the U.S. Food and Drug Administration (FDA) approved pirfenidone and nintedanib over a decade ago (5). While both agents demonstrate efficacy in slowing lung function decline in IPF, critical limitations persist. Neither drug improves patient-reported symptoms and 20–30% of patients exhibit long-term intolerance due to adverse gastrointestinal effects (6, 7). These unmet needs underscore the urgency to develop transformative therapies targeting the multifactorial pathogenesis of the disease.
Exosomes are nanoscale extracellular vesicles (EVs) recognized as pivotal mediators of intercellular communication and immune regulation in addition to playing a role in disease diagnosis and prognosis. Exosome’s ability to directly facilitate the transfer of information and cargo between cells makes them significant carriers of circulating biomarkers (8), and their contents, including nucleic acids, proteins, and other molecular constituents, can profoundly influence the physiological state of recipient cells.
Substantial evidence implicates these vesicles as modulators of the pathogenesis of pulmonary diseases including PF. Exosomes exhibit considerable potential for regulating pulmonary inflammation and attenuating PF progression. They mitigate inflammatory lung injury and decelerate tissue fibrogenesis by conveying anti-inflammatory and anti-fibrotic signaling molecules, thereby suppressing myofibroblast proliferation and activation (9, 10). Additionally, exosomes can readily be obtained from accessible bodily fluids, such as blood, urine, and sputum, making them a promising noninvasive biomarker for PF (11, 12). Mesenchymal stem cell-derived exosomes (MSC-Exos) have demonstrated significant therapeutic efficacy against fibrotic diseases including PF (10, 13, 14). Notably, MSC-Exos possess a distinctive tissue-homing capacity, enabling specific targeting of inflamed or injured sites to promote lung tissue repair (15). Collectively, these findings provide a robust scientific foundation and rationale for developing novel MSC-Exo-based therapeutic agents for PF. This review summarizes the recent advances in understanding the composition, physiological functions, isolation methodologies, and mechanistic roles of MSC-Exos in PF pathogenesis. We further discuss the mechanistic insights into MSC-Exo-based PF therapy, and evaluates the clinical potential and challenges, as well as offering a roadmap for developing next-generation nanotherapeutics.
2 Biogenesis and characterization of MSC-Exos
2.1 MSC-Exos: biogenesis and isolation paradigms
MSCs originate from the embryonic mesoderm and are a population of multipotent stromal cells characterized by their self-renewal capacity and multilineage differentiation potential. They can be isolated from diverse tissue sources, including the bone marrow, adipose tissue, placental membranes, umbilical cord blood, and dental pulp, with each source conferring distinct biological properties (16). Bone marrow-derived MSCs (BM-MSCs) are particularly valuable because of their robust immunomodulatory and anti-inflammatory properties, whereas adipose tissue-derived MSCs exhibit enhanced differentiation plasticity and migratory capacity, making them advantageous for tissue regeneration applications (17). This source-dependent functional specialization necessitates strategic selection of MSC origins to align with specific therapeutic objectives.
Exosome isolation techniques leverage the unique biophysical and molecular characteristics of these nanovesicles, with methodologies ranging from differential centrifugation and size-exclusion chromatography to immunoaffinity capture, commercial kit-based purification, and microfluidic sorting. Ultracentrifugation remains the gold standard for obtaining high-purity exosome preparations, while emerging commercial kits offer accelerated processing times with comparable yields and purity profiles. Exosomes are characterized using a multimodal analytical approach combining flow cytometry, nanoparticle tracking analysis, transmission electron microscopy, and liquid chromatography-mass spectrometry (LC-MS). Western blotting is further employed to validate specific exosomal markers and cargo composition (18).
These nanoscale vesicles (30–150 nm), encapsulated by a lipid bilayer, constitute a dynamic “multimodal signaling hub” containing proteins, nucleic acids (miRNA, lncRNA, mRNA, circRNA), and lipids (Figure 1). Proteomic profiling reveals membrane enrichment of tetraspanin family proteins (CD63, CD81, and CD9), homing receptors (CXCR4 and integrin αvβ5), immunomodulatory molecules (HGF, and TGF-β1). The luminal core contains chaperones (HSP70, and HSP90) and RNA-binding proteins (Argonaute 2 and Y-box proteins, AGO2), which stabilize miRNA/mRNA complexes via electrostatic interactions (18).
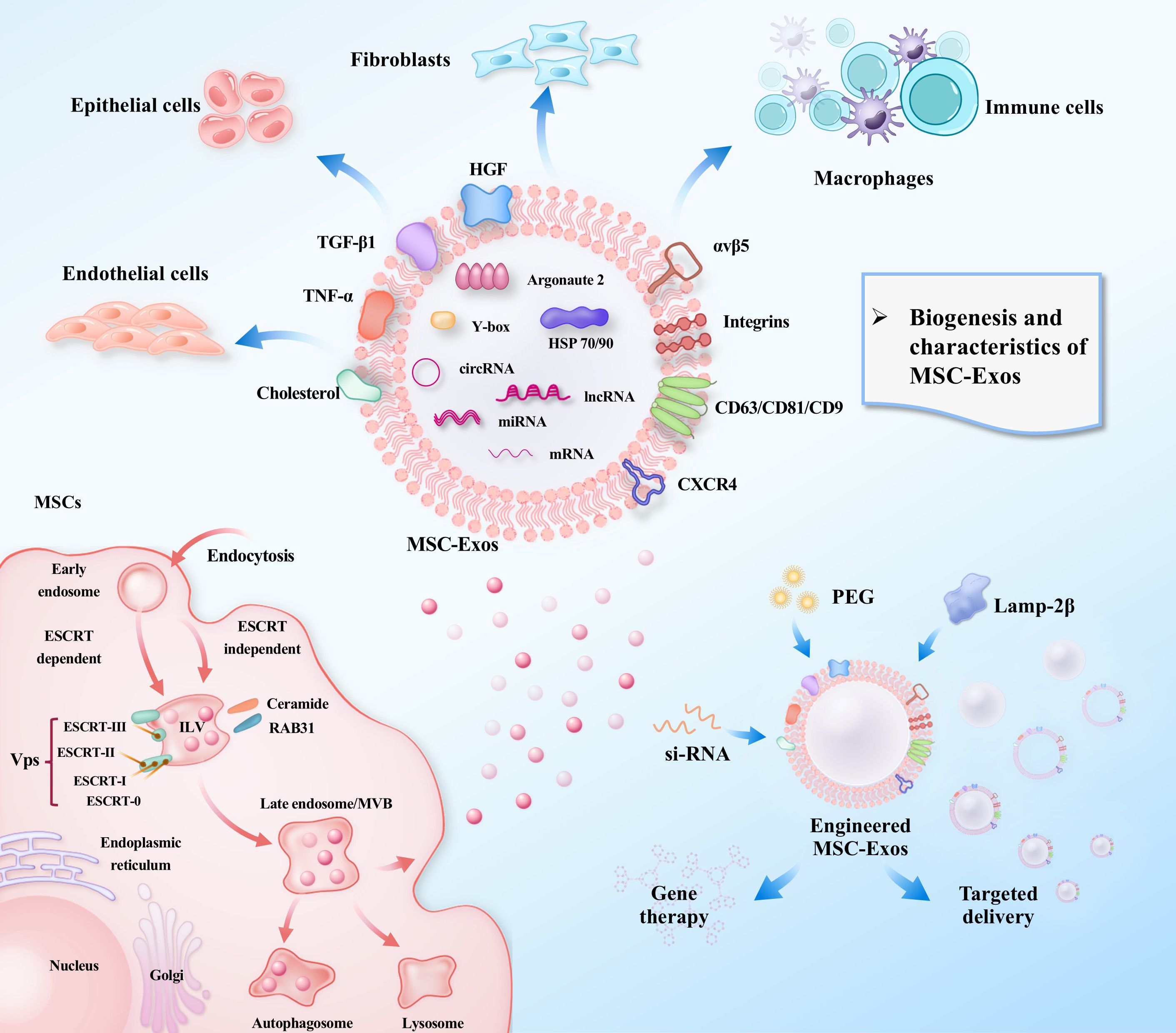
Figure 1. Biogenesis, cargo, and therapeutic targeting of MSC-Exos.This schematic illustrates the biogenesis of MSC-Exos, from intraluminal vesicle formation to secretion. It details their diverse molecular cargo (nucleic acids, proteins, lipids), their interaction with key target cells in the lung (e.g., macrophages, fibroblasts), and strategies for engineering exosomes to enhance their targeted therapeutic potential for treating pulmonary fibrosis.
MSC-Exos exhibit unique functional advantages over tumor- or immune cell-derived exomes. These vesicles are enriched in therapeutic biomolecules, including growth factors, cytokines, and non-coding RNAs, which orchestrate tissue repair while maintaining immune homeostasis. Unlike tumor-derived exosomes, which may promote oncogenic signaling, MSC-Exos demonstrate intrinsic anti-fibrotic and regenerative properties without eliciting pathological immune evasion (19). The evolving understanding of MSC-Exo biology continues to inform therapeutic optimization and standardization strategies for clinical translation.
2.2 MSC-Exos contents mediating intercellular communication
The therapeutic potential of MSCs is largely attributable to their robust paracrine activity, with exosomes representing the most extensively studied subclass of EVs. These nanovesicles serve as central carriers that mediate the intricate communication network between MSCs and target cells (Figure 1). Enriched in parent cell-derived proteins, lipids, nucleic acids and metabolites, they constitute a sophisticated molecular cargo delivery system. Thus, their roles in communication and therapeutic mechanisms are best understood through examination of their cargo components.
2.2.1 Nucleic acids
MSC-Exos are enriched with diverse cargo nucleic acids, particularly non-coding RNAs such as microRNAs (miRNAs). The selective loading of miRNAs into exosomes is a regulated process mediated by several mechanisms, including AGO2-dependent direct recognition, sphingomyelinase 2(nSMase2)-dependent sorting, microRNA-induced silencing complex (miRISC)-associated pathways, and recognition by SUMOylated heterogeneous nuclear ribonucleoproteins (hnRNPs; primarily hnRNPA2B1, hnRNPA1, and hnRNPC) (20).
The functional impact of exosomal miRNAs is highly context-dependent, varying significantly based on their cellular origin. For instance, miR-125a-5p—one of the most abundant miRNAs in MSC-Exos—is enriched in M2 macrophages and demonstrates cardioprotective effects in heart failure models (21). Similarly, hypoxia-primed MSC-Exos exhibit elevated levels of miR-612, which is associated with pro-angiogenic functions. Conversely, exosomes derived from gastric cancer cells deliver miR-23a to promote tumor angiogenesis (22), while BM-MSC-Exos carry miR-144, which suppresses non-small cell lung cancer proliferation by targeting cyclin E1 and E2.
Exosomal miRNAs also play established roles in fibrotic remodeling. In renal fibrosis models, exosomal miR-21 promotes fibroblast activation via PTEN downregulation (22). Similarly, in cancer microenvironments, tumor-derived exosomal miRNAs (e.g., miR-155, miR-21, and miR-124) induce fibroblast activation and extracellular matrix (ECM) remodeling through targeting factors such as FGF2, TGF-β, and α-SMA (23).
These findings underscore that exosomal miRNA profiles and functions are tightly linked to their cell of origin, ultimately dictating distinct—and often opposing—effects on recipient cells and tissue microenvironments.
2.2.2 Proteins
The MSC-Exos participates in pivotal biological processes, including intercellular communication, cellular structure maintenance, inflammation regulation, and exosome biogenesis. These proteins can modulate disease pathogenesis and facilitate tissue repair and regeneration, paralleling miRNA functionality (24). Anderson et al. (25) identified 1,927 proteins in human BM-MSC-Exos using LC-MS. Ischemic conditions exhibit elevated exosomal levels of angiogenesis-related growth factors, e.g., PDGFs, EGFs, and FGFs, but show constitutive levels of signaling mediators, TNF-α, TGF-β, Wnt5, β-catenin, and delta-like 4. Exosomal protein composition dynamically reflects the parental cell status and adapts to microenvironmental changes. Salomon et al. (26) demonstrated oxygen tension-dependent alterations, where hyperoxic exposure suppressed the exosomal expression of cytoskeletal signaling proteins and the clathrin-mediated endocytosis machinery. This microenvironment-responsive dynamism enables exosomes to actively remodel their extracellular environment.
2.2.3 Lipids
As essential components of exosomes, lipids contribute to membrane formation while critically regulating exosomal biogenesis and release. Exosome formation is governed by two primary mechanisms: endosomal sorting complex required for transport (ESCRT)-dependent and ESCRT-independent pathways (Figure 1). The ESCRT machinery, comprising four subcomplexes (ESCRT-0, -I, -II, and-III) assembled from class E vacuolar protein sorting (Vps) proteins, regulates exosomal biogenesis via distinct mechanisms (27). Specifically, phosphatidylinositol 3-phosphate binds to the FYVE domain of ESCRT-0 to recruit early ESCRT proteins (e.g., Vps27/Hrs), thereby promoting exosome formation. The ESCRT-associated protein, ALIX, plays a pivotal role in ESCRT-dependent exosome generation. Studies have demonstrated that ESCRT-III facilitates tetraspanin (CD9, CD63, and CD81) delivery to exosomes via the ESCRT-0–Bro1/ALIX–SNF7/CHMP4 alternative pathway in the presence of lysobisphosphatidic acid, which directly influences exosomal assembly (28). Exosomes also carry lipid-synthesizing enzymes that modulate recipient cell behavior by transferring lipids and lipid-metabolizing enzymes, thereby inducing lipid synthesis in recipient cells while serving as vehicles for the release of cell-synthesized lipids.
In essence, MSC-Exos achieve precise multi-target regulation through their unique molecular compositions and delivery mechanisms. Their tissue-specific cargo encompasses immunomodulatory proteins, selectively packaged non-coding RNAs, and biogenesis-governing lipids, enabling context-dependent signaling. This orchestrated molecular interplay facilitates targeted intercellular communication, positioning MSC-Exos as potent, tunable therapeutic vectors for complex pathologies, such as PF (Figure 1).
2.3 Exosomes-mediated pathological crosstalk in PF
Intricate intercellular communication is essential for maintaining pulmonary homeostasis and orchestrating responses to injury. This complex network—involving epithelial, mesenchymal, immune, and endothelial cells—becomes profoundly dysregulated in PF, driving a cycle of persistent injury and aberrant repair (29–33). Exosomes emerge as critical mediators of this pathological crosstalk, facilitating the exchange of pathogenic signals that drive disease progression (34, 35). Their role as biomarkers and therapeutic tools underscores their dual utility in both understanding and treating PF.
Through various delicate feedback mechanisms, lung mesenchymal cells reciprocally modulate epithelial function in a subtype-specific manner (36, 37). For instance, Lgr5+ mesenchymal cells support AT2 cell expansion via Wnt3a, while Lgr6+ cells stimulate the differentiation of airway epithelial progenitors through Wnt-FGF10 signaling. Furthermore, INK4a/P16+ mesenchymal cells drive the differentiation of airway progenitors into Club cells via SASP factors (32, 38). This cellular crosstalk becomes dysregulated within the pathological microenvironment of PF, where exosomes emerge as critical mediators of intercellular communication.
Chen et al. (39) demonstrated that in a BLM-induced PF model, exosomes derived from alveolar epithelial cells were enriched in the long non-coding RNA HOTAIRM1, which promotes myofibroblast activation and aberrant ECM accumulation via the miR-30d-3p/HSF1/YY1 axis. Conversely, Secreted frizzled-related protein 1 (Sfrp1), which is highly expressed in myofibroblasts (40, 41) and localizes to active fibrotic regions marked by α-SMA positivity, enhances exosome secretion from fibroblasts. These fibroblast-derived EVs impair alveolar epithelial cell differentiation, thereby exacerbating fibrosis (42). Further highlighting the role of fibroblast-derived EVs, Kadota et al. (43) reported that those carrying miR-23b-3p and miR-494-3p exacerbate mitochondrial DNA damage and senescence in alveolar epithelial cells by inducing mitochondrial dysfunction. In contrast, a protective paracrine mechanism was identified by Xie et al. (44). They found that GHR is highly expressed in mesenchymal cells, with its levels correlating positively with improved lung function in IPF patients. Notably, GHR-rich EVs derived from these cells promoted AT2 cell proliferation and attenuated PF in mice with mesenchymal-specific GHR deficiency. This finding illustrates that paracrine signaling is not exclusive to epithelial cells but is also a critical mechanism used by mesenchymal cells to modulate neighboring cellular responses.
Exosome-mediated intercellular communication significantly influences macrophage function in PF. A key mechanism involves YAP1 activation in fibroblasts, which upregulates CSF1 expression to promote macrophage recruitment and exacerbate fibrotic responses (45). The functional impact of exosomes on macrophages is further illustrated by Feng et al. (46), who demonstrated that AT2 cell-derived exosomes deliver a STIM-activating enhancer to tissue-resident alveolar macrophages. This enhances calcium ion influx and activates the PGC-1α–calcineurin signaling axis, stimulating mitochondrial biogenesis and oxidative phosphorylation. Such metabolic reprogramming alters the macrophage immunophenotype, ultimately attenuating IPF.
The exosomal miRNA cargo is a critical factor in this dialogue (47, 48). Guiot et al. (49) identified a robust increase in exosomal miR-142-3p in sputum and serum from IPF patients, with its levels positively correlating with macrophage abundance. Functionally, miR-142-3p was shown to delay IPF progression by suppressing TGF-β production in both airway epithelial cells and lung fibroblasts, while also reducing the expression of COL1A1 and COL3A1 to mitigate ECM deposition. Beyond epithelial-macrophage crosstalk, inflammatory monocytes regulate mesenchymal cell viability through EVs. This process was found to be mediated by GSDMD activation and pyroptosis (50), revealing another dimension of vesicle-mediated signaling in the fibrotic niche.
Collectively, a propagating cycle of exosome-mediated signaling underpins PF progression. Following epithelial injury, AT2 cells release TGF-β and exosomes that activate myofibroblasts, notably Sfrp1-positive cells. These activated myofibroblasts, in turn, secrete exosomes that further amplify fibrosis and macrophage activation, creating a self-sustaining feedback loop that drives disease exacerbation. This intricate cellular crosstalk is largely coordinated by exosome-derived miRNAs. However, the precise mechanisms by which exosomes orchestrate these intercellular communications following epithelial damage remain complex, tightly regulated, and necessitate further investigation.
3 Mechanism underlying MSC-Exos mediated therapy
3.1 Immune modulation
Immune cells are critically involved in the pathogenesis of PF (Figure 2) (51, 52). The fibrotic process is initiated when tissue-resident macrophages (TRMs) sense pathogens or damage-associated molecular patterns (DAMPs) via pattern recognition receptors (PRRs), triggering a rapid immune response. This activation prompts the secretion of cytokines and chemokines that recruit inflammatory monocytes, T cells, and fibroblasts, thereby establishing a profibrotic niche (53, 54). Immune modulation is a well-established cornerstone of the therapeutic efficacy of both MSCs and MSC-Exos. Although macrophages are considered primary cellular targets, the precise mechanisms by which MSC-Exos modulate macrophage function to counteract fibrogenesis require further elucidation. Furthermore, emerging evidence underscores the significant role of other immune cell types in mediating the therapeutic effects of MSC-Exos, as detailed below.
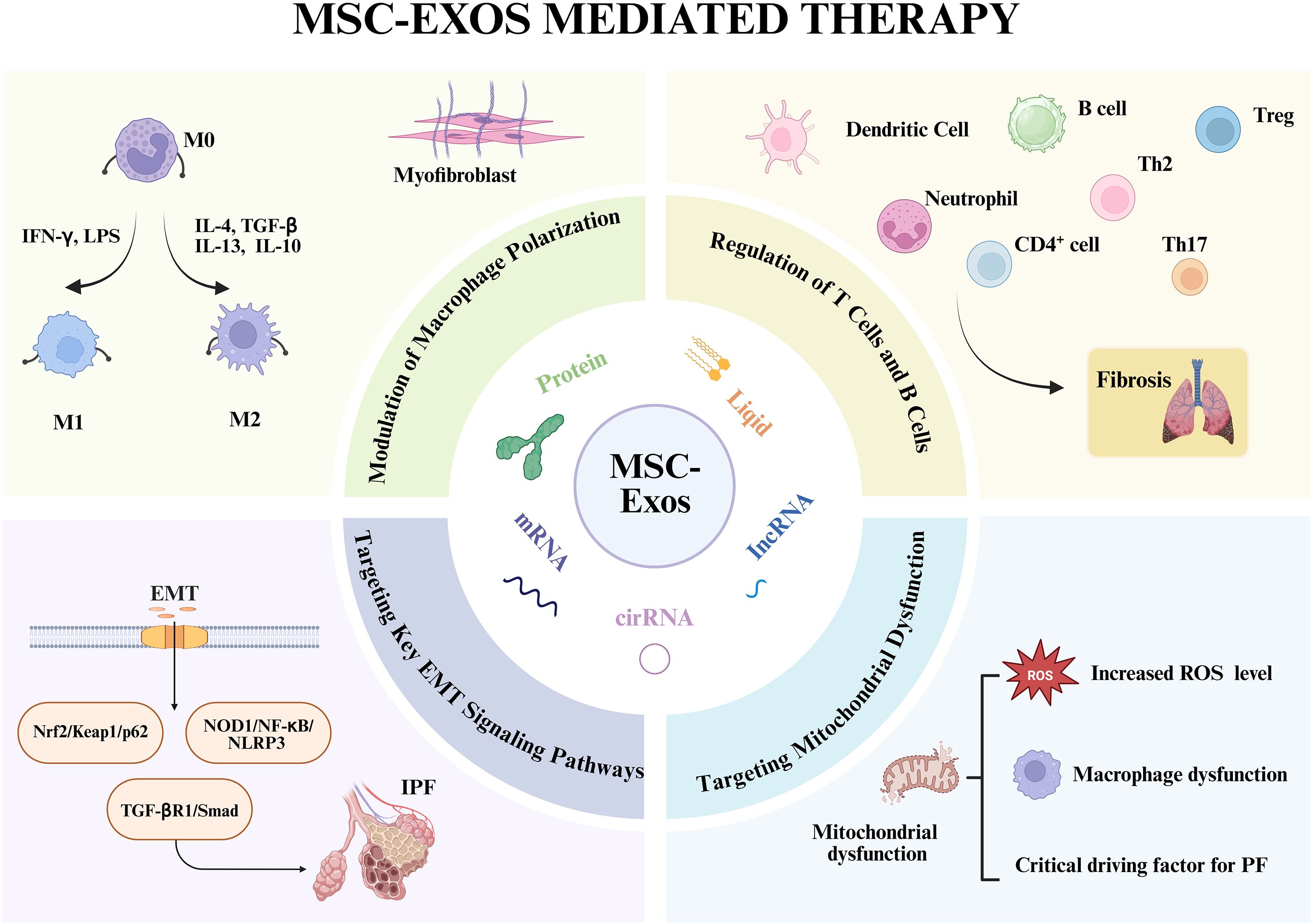
Figure 2. Mechanistic basis for the anti-fibrotic actions of MSC-Exos in PF. The central panel depicts the key molecular constituents of MSC-Exos that mediate their therapeutic effects. The surrounding panels detail the multifaceted mechanisms by which MSC-Exos attenuate PF phenotypes: (i) reprogramming macrophage polarization from a pro-inflammatory (M1) to an anti-inflammatory/reparative (M2) phenotype; (ii) modulating adaptive immune responses by regulating T, B and other cell activity; (iii) inhibiting EMT in alveolar epithelial cells; and (iv) restoring mitochondrial function and reducing ROS level in target cells. Together, these coordinated actions suppress myofibroblast activation and pathological ECM deposition. Created in Biorender. https://BioRender.com
3.1.1 Modulation of macrophage polarization
Macrophages represent the most extensively studied and centrally acting immune cells in PF, playing dual roles in both disease initiation and progression (55). During the initial phase of lung injury, classically activated macrophage predominates, secreting substantial quantities of pro-inflammatory cytokines that recruit additional immune cells to the site of damage. When this inflammatory response becomes excessive or fails to resolve appropriately, sustained M1 activation leads to severe tissue damage and epithelial cell death, establishing a foundation for fibrotic initiation. As the disease progresses to more stable stages, alternatively activated M2 macrophages become predominant, directly activating fibroblasts and promoting their differentiation into myofibroblasts through the secretion of pro-fibrotic mediators, including TGF-β and PDGF.
MSC-Exos demonstrate remarkable therapeutic potential by modulating this polarization dynamic, primarily through suppression of early-phase M1 macrophage activation, thereby mitigating inflammatory-mediated tissue damage in pulmonary fibrosis (56). Willis et al. (57) demonstrated that MSC-Exos ameliorate hyperoxia-induced lung injury by regulating pulmonary macrophage polarization, thereby attenuating fibrotic progression, enhancing lung development, and improving vascular remodeling. Dong et al. (58) further elucidated the underlying mechanism, revealing that MSC-Exos achieve these anti-inflammatory and polarization effects by targeting TRAF1, a critical downstream component of the NF-κB/PI3K/AKT signaling pathway. Supporting these findings, Mansouri et al. (59) observed that MSC-Exos treatment increases populations of alveolar macrophages and non-classical monocytes while simultaneously reducing pro-inflammatory monocyte recruitment.
At the functional level, MSC-Exos effectively reprogram myeloid cells toward an immunoregulatory phenotype, reducing the infiltration of pro-fibrotic monocytes into pulmonary tissues. This phenotypic shift translates to decreased collagen deposition, improved lung function, and overall attenuation of fibrotic progression. Molecular analyses reveal that MSC-Exos promote the M1-to-M2 transition through significant downregulation of iNOS coupled with concurrent upregulation of arginase-1 mRNA expression in alveolar macrophages (60, 61). These coordinated changes in key enzymatic markers confirm the polarization shift toward a reparative macrophage phenotype, positioning macrophage polarization targeting as a promising therapeutic strategy for PF intervention.
3.1.2 Regulation of Treg and Th17 cells
Beyond macrophages, MSC-Exos modulate lymphocyte populations central to PF progression (62). They upregulate Foxp3—a master transcription factor for regulatory T cell (Treg) differentiation—in an IDO-dependent manner. This suppresses T helper 17 (Th17) cell differentiation during pulmonary inflammation, thereby inhibiting T-cell-driven lung inflammation and fibrosis (63, 64). The cytokine IL-33 exacerbates PF by promoting myofibroblast activation and inducing the secretion of TGF-β and IL-13 from immune cells such as Th2 and Th17 lymphocytes (65). Xie et al. (66) demonstrated in a bleomycin (BLM)-induced murine PF model that MSC-Exos target IL-33, significantly reducing collagen expression and inhibiting epithelial-mesenchymal transition (EMT).
Tregs (CD4+CD25+FoxP3+ cells) are essential for maintaining immune homeostasis and exert potent anti-fibrotic effects. MSC-Exos can induce Treg production and an anti-inflammatory response by modulating cellular metabolic states (67). Notably, impaired Treg function is a recognized promoter of fibrotic progression in IPF (52). Recent studies have identified a specific MSC subtype characterized by low TNFSF4 expression that effectively induces Treg differentiation in both IPF patients and murine models. These TNFSF4-low MSCs regulate both proliferating (Ki67+) and activated (CD38+, HLA-DR+) Treg populations. The critical role of Tregs was confirmed by antibody-mediated depletion using anti-CD25 treatment, which effectively abolished their anti-fibrotic activity (68).
Although the direct mechanisms of MSC-Exo interactions with Tregs in PF remain to be fully characterized, current evidence indicates that MSC-Exo cargos—including proteins (e.g., IL-6, TSG-6, IDO) and miRNAs (e.g., miR-181c, miR-146a)—promote Treg expansion while simultaneously suppressing inflammatory pathways such as JAK/STAT and TLR/NF-κB signaling.
3.1.3 Effects on dendritic cells
MSC-Exos also modulate the function of dendritic cells (DCs), which are upregulated in the lungs of IPF patients and play a crucial role in antigen presentation (63, 64). Research indicates that MSC-Exos enhance the expression of immunosuppressive IL-10 and TGF-β, inhibit DC maturation, and reduce the expression of co-stimulatory molecules (CD40, CD80, and CD86) on immature DCs. This diminishes the activation of CD4+ T cells and Th2-driven fibrotic reactions (63), highlighting how MSC-Exos alleviate inflammatory lung diseases by modulating antigen-presenting cell phenotypes. Together, these immunomodulatory actions foster an immune-tolerant pulmonary microenvironment conducive to fibrosis resolution (69).
3.2 Targeting key EMT signaling pathways
EMT is a critical pathological event during the early stages of PF characterized by the loss of epithelial markers (e.g., E-cadherin) and acquisition of a mesenchymal phenotype (e.g., α-SMA, vimentin) in alveolar epithelial cells. MSC-Exos deliver molecular cargo that directly targets and inhibits fundamental EMT signaling pathways driving EMT in these epithelial cells (70). Another study showed that MSC-Exos mitigate BLM-induced PF by modulating the NOD1/NF-κB/NLRP3 pathway, thereby inhibiting EMT (71). These mechanisms are conserved across organs. Cheng et al. (72, 73) established that MSC-Exos attenuate EMT and ameliorate liver fibrosis through YAP signaling-mediated regulation of miR-27b-3p, resulting in downregulation of LOXL2 expression. In vitro evidence indicates that MSC-Exos ameliorate liver fibrosis by modulating the Nrf2/Keap1/p62 pathway, leading to the restoration of autophagy and Nrf2 levels, suppression of EMT, reduction of collagen deposition, and attenuation of apoptosis (74). In addition to directly targeting EMT-related pathways, MSC-Exos can also inhibit EMT by modulating cellular functions. Long et al. (75) demonstrated that targeting senescent AT2 cells with high CD38 expression could reverse EMT by restoring NAD+ levels and mitochondrial function, thereby mitigating age-related PF. Similarly, MSC-Exos carrying miR-29b-3p can target FZD6, reduce collagen I and α-SMA expression, and inhibit myofibroblast activity, consequently suppressing EMT formation (76). Notably, exosome-based targeting strategies represent a promising approach for EMT inhibition, which will be discussed in detail in the subsequent sections.
3.3 Targeting mitochondrial dysfunction
Mitochondrial dysfunction—characterized by impaired mitophagy, excessive oxidative stress, altered dynamics, and mitochondrial DNA (mtDNA) damage—is a key driver of PF (77, 78). This dysfunction disrupts core cellular functions, including energy production via oxidative phosphorylation (OXPHOS). Mitophagy, a selective form of autophagy for damaged mitochondria, is closely linked to the PINK1/Parkin signaling pathway. While early-stage mitophagy can prevent harmful ROS accumulation, late-stage impairment contributes to fibrotic progression (79, 80). Mitochondrial oxidative stress and mtDNA damage are particularly potent drivers of fibrosis. This dysfunction is closely associated with pulmonary macrophages. Mitochondria isolated from alveolar macrophages of IPF patients exhibit elevated PINK1 and Parkin expression. The protein Akt1 induces mitochondrial ROS (mtROS) production and enhances mitophagy in macrophages; impaired mitophagy in Akt1-knockout and Parkin-knockout mice delays PF progression, suggesting that Akt1 regulates macrophage function via mitophagy to promote fibrosis (81). Furthermore, NADPH oxidase 4 (NOX4) is upregulated in fibrotic lung macrophages, contributing to increased ROS levels that enhance mitophagy and exacerbate PF. PGC-1α is essential for this NOX4-mediated mitochondrial biogenesis (82, 83).
Metabolic reprogramming is fundamental to immune cell behavior. mtROS production is necessary for macrophage polarization, and elevated mtROS activity in the bronchoalveolar lavage of IPF patients correlates with disease severity (84, 85). Profibrotic alveolar macrophages exhibit enhanced glycolysis and tricarboxylic acid (TCA) cycle metabolism, and treatment with glycolysis inhibitors can reverse their phenotype and reduce fibrosis (86). MSC-Exos represent a powerful therapeutic strategy to reverse this mitochondrial dysfunction. Xia et al. (87) demonstrated that AdMSC-Exos can transfer functional mitochondria to alveolar macrophages, enhancing mitochondrial DNA content, membrane potential, OXPHOS activity, and ATP production. This restoration of mitochondrial function promotes a shift toward an anti-inflammatory macrophage phenotype, characterized by reduced pro-inflammatory mediators and increased production of IL-10 and Arg-1, protecting against lung injury. A recent study have demonstrated that FGF21-loaded M2-derived exosomes facilitate M1 to M2 transition by downregulating the expression of glycolysis-related enzymes such as PKM2, PFKFB3, HK2, PDK1, and LDH, and inflammation-related proteins (88). Significantly, MSC-Exos were found to restore alveolar-capillary barrier integrity and normalize oxidative phosphorylation levels via mitochondrial transfer (89).
In summary, MSC-Exos ameliorate fibrosis through multi-faceted mechanisms, including immunomodulation, regulation of EMT signaling pathways, and restoration of mitochondrial function. Importantly, these mechanisms are closely interconnected and strongly associated with immune cells, particularly macrophages. Given the pivotal role of macrophage as targets in mediating the therapeutic actions of MSC-Exos, future research should prioritize investigating the specific interactions between MSC-Exos and macrophages, focusing on: 1/ Molecular Cargo Specificity: Characterizing the key exosomal components (e.g., specific miRNAs, proteins, and lipids) responsible for modulating macrophage polarization and function. 2/ Metabolic Reprogramming: Elucidating the mechanisms and consequences of mitochondrial transfer and other metabolic interventions on macrophage physiology. 3/ Therapeutic Optimization: Engineering exosomes to enhance macrophage-specific targeting (e.g., by displaying specific surface motifs) and exploring synergistic combinations with existing macrophage-centric therapies.
4 Exosome engineering strategies for improved therapeutic benefit
Despite their significant therapeutic potential, the clinical application of native MSC-Exos in anti-fibrotic therapy faces considerable challenges (35). A primary limitation is the "off-target effect," largely attributable to rapid phagocytic clearance by mononuclear macrophages of the reticuloendothelial system, leading to unintended accumulation in the liver and spleen (90). To address this limitation and enhance drug bioavailability while minimizing adverse effects, exosome engineering has emerged as a promising strategy to improve targeting precision, drug-loading capacity, stability, and overall therapeutic efficacy.
4.1 Surface modification for enhanced targeting
4.1.1 Genetic engineering
A powerful approach involves genetically modifying parent cells to produce exosomes with engineered surfaces primarily aimed at enhancing their targeting specificity. The exosomal membrane, rich in transmembrane proteins such as LAMP-2B, GPI, CD63, CD9, and CD81, can be functionalized by fusing targeting moieties (e.g., peptides, aptamers) to these proteins (91, 92). For instance, the large N-terminal extracellular domain of lysosome-associated membrane protein 2B (LAMP-2B) serves as an excellent fusion scaffold. Long et al. (75) exemplified this by employing chimeric antigen receptor (CAR) technology to generate CD38-targeting EVs from umbilical cord MSCs. Transfection with a lentiviral vector encoding a CD38-specific antigen receptor fused to a CD8 transmembrane domain yielded exosomes that precisely targeted senescent AT2 cells, effectively mitigating age-related pulmonary fibrosis. Furthermore, Zhang et al. (93) have modified MSC-Exos with the SARS-CoV-2 spike protein receptor-binding domain (S-RBD) for treating radiation-induced pulmonary fibrosis. This engineered exosome formulation has been shown to exhibit prolonged retention in lung tissue and significantly improve survival rates while ameliorating pulmonary fibrosis in mouse models.
4.1.2 Peptide-based functionalization
Exosomes can be directly conjugated with tissue-specific peptides to achieve targeted accumulation. The RGD peptide (arginine-glycine-aspartic acid), which exhibits high affinity for integrins (e.g., αVβ3, αVβ5, α5β1) upregulated in fibrotic niches, enables precise delivery to activated fibroblasts and endothelial cells. Further demonstrating this versatility, the octapeptide CC8 (CNGQGEQC) specifically binds integrin α3β1—highly expressed on non-small cell lung cancer cells—making it a suitable ligand for oncology applications (94). Similarly, engineering exosomes with the CRV peptide (CRVLRSGSC), which targets tumor-associated macrophages (TAMs), has been used to create dual-targeting systems capable of homing to cancer cells and TAMs simultaneously, significantly improving anti-tumor efficacy (95). This strategy is readily adaptable to target pro-fibrotic immune cells in the lung.
4.2 Delivery of bioactive cargos
Due to their low immunogenicity, inherent biocompatibility, and the lipid bilayer membrane that protects the contents from degradation, engineered exosomes serve as ideal vectors for the targeted delivery of therapeutic molecules.
4.2.1 Delivery of miRNA and siRNA
MSC-Exos naturally carry anti-fibrotic miRNAs, and engineering them to overexpress specific miRNAs offers a potent therapeutic avenue. For instance, Zhang et al. (94) developed MSC-Exos overexpressing miR-486-5p, which combats PF by suppressing Smad2 and activating Akt phosphorylation. BMSC-Exos delivering miR-186 alleviate IPF by inhibiting SOX4 and downregulating DKK1 (96). Exosomes overexpressing miR-29b-3p downregulate Frizzled 6 (FZD6), inhibiting fibroblast activation, differentiation, and proliferation (76). Gu et al. (97) engineered endothelial cell-derived EVs to overexpress miR-125b-5p, enhancing barrier integrity and mitigating acute lung injury. Beyond miRNAs, exosomes can be engineered to deliver siRNA. Cationic lipid (DOTAP)-modified exosomal membranes loaded with Smad4-targeting siRNA (DOTAP/siSmad4@EM) specifically silence Smad4 in lung fibroblasts, exerting potent anti-fibrotic effects (98).
4.2.2 Advanced drug loading and delivery
Utilizing exosomes as drug carriers can enhance drug targeting and drug absorption rate. For example, recognizing the central role of macrophages in silicosis, Chen et al. (99) developed macrophage-derived exosomes loaded with pirfenidone. This system enhanced drug uptake by target cells while reducing systemic adverse effects. To improve drug-cell affinity in PF, fibroblast-derived exosomes co-loaded with bergenin and vitexin significantly reduced collagen deposition and improved lung function in a BLM-induced mouse model (100).
4.2.3 Macrophage-targeted precision therapy
Given the pivotal role of macrophages in orchestrating both the initial inflammatory injury and the subsequent progressive fibrotic cascade (101, 102), they represent a prime therapeutic target for intervention in pulmonary fibrosis. As central regulators of the immune microenvironment, specific macrophage subsets directly drive disease pathogenesis: pro-inflammatory M1 phenotypes mediate early tissue damage and epithelial cell death, while pro-fibrotic M2 and other specialized subsets (e.g., SPP1+) directly activate fibroblasts and promote pathological ECM deposition. The inherent capacity of MSC-Exos to reprogram macrophage polarization validates this cellular target and provides a robust foundation for further precision engineering (59, 101). Future strategies are therefore focused on moving beyond broad modulation to the precision targeting of specific pro-fibrotic macrophage subsets:
4.2.3.1 Targeting M2 macrophages
The macrophage mannose receptor (CD206) is a well-established surface marker for M2-polarized macrophages. Ghebremedhin et al. (103) demonstrated that targeting CD206 significantly attenuates BLM-induced PF. Exosomes engineered with CD206-specific ligands can enable precise delivery of anti-fibrotic cargo to this population.
4.2.3.2 Targeting SPP1+ macrophages
SPP1 (osteopontin)+ macrophages represent a distinct, highly pro-fibrotic subset. Engineering exosomes to anchor to SPP1+ macrophages offers another promising strategy for targeted intervention.
4.2.3.3 Targeting metabolic pathways
Metabolites like itaconate are critical regulators of macrophage function in fibrosis. Ogger et al. (104) showed itaconate directly influences human lung fibroblast phenotype in vitro, making it and its pathway components attractive targets for exosome-delivered therapeutics.
Collectively, exosome engineering represents a transformative approach overcoming the limitations of native vesicles. By enabling targeted delivery and potent regulation of core fibrotic pathways and the immune microenvironment, engineered exosomes substantially improve treatment precision and efficacy. Future work should focus on optimizing loading efficiency, scaling production, and conducting rigorous preclinical validation. While the methodological approaches are diverse, the overarching goal remains the translation of these advanced therapeutics into clinically effective and precise treatments for pulmonary fibrosis.
5 Preclinical and clinical translation
5.1 Validity of animal models
The BLM-induced rodent model remains the gold standard preclinical platform for evaluating MSC-Exos in IPF, as it recapitulates key pathological features of human disease, including fibroblast-to-myofibroblast transition, sustained alveolar inflammation, and progressive ECM remodeling. However, this model cannot fully capture the complexity and chronicity of human IPF. Notably, the therapeutic potential of MSC-Exos extends beyond the BLM model, demonstrating robust anti-fibrotic efficacy in silica-induced silicosis, radiation-induced pulmonary fibrosis, and other cross-species models (74, 87, 89, 105). This pan-fibrotic therapeutic effect, coupled with an excellent biosafety profile, positions MSC-Exos as a transformative therapeutic strategy for a spectrum of organ-specific fibrotic diseases that share common pathogenic pathways. These compelling preclinical data provide a critical scientific foundation for clinical translation.
5.2 Progress in clinical trials
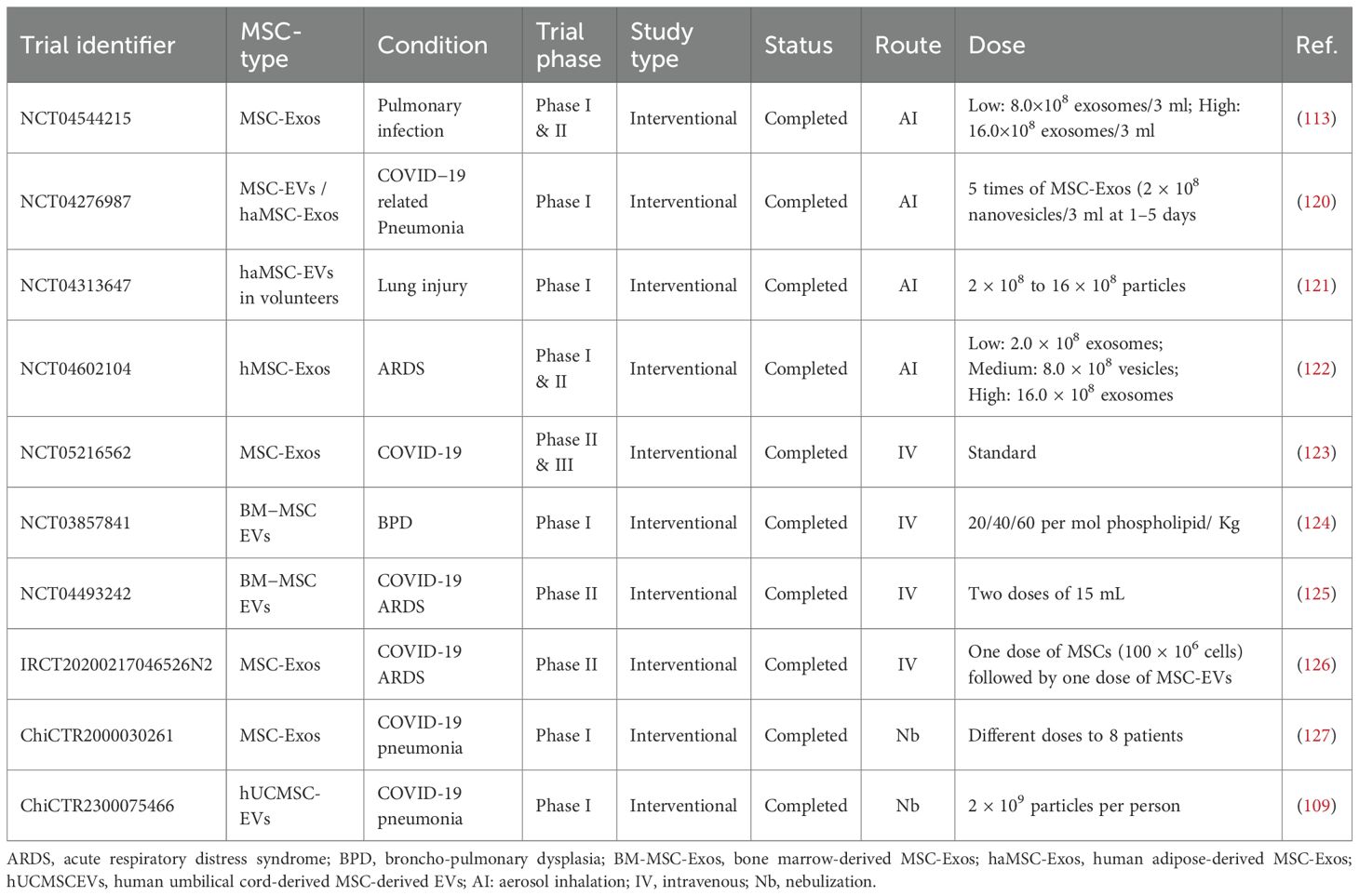
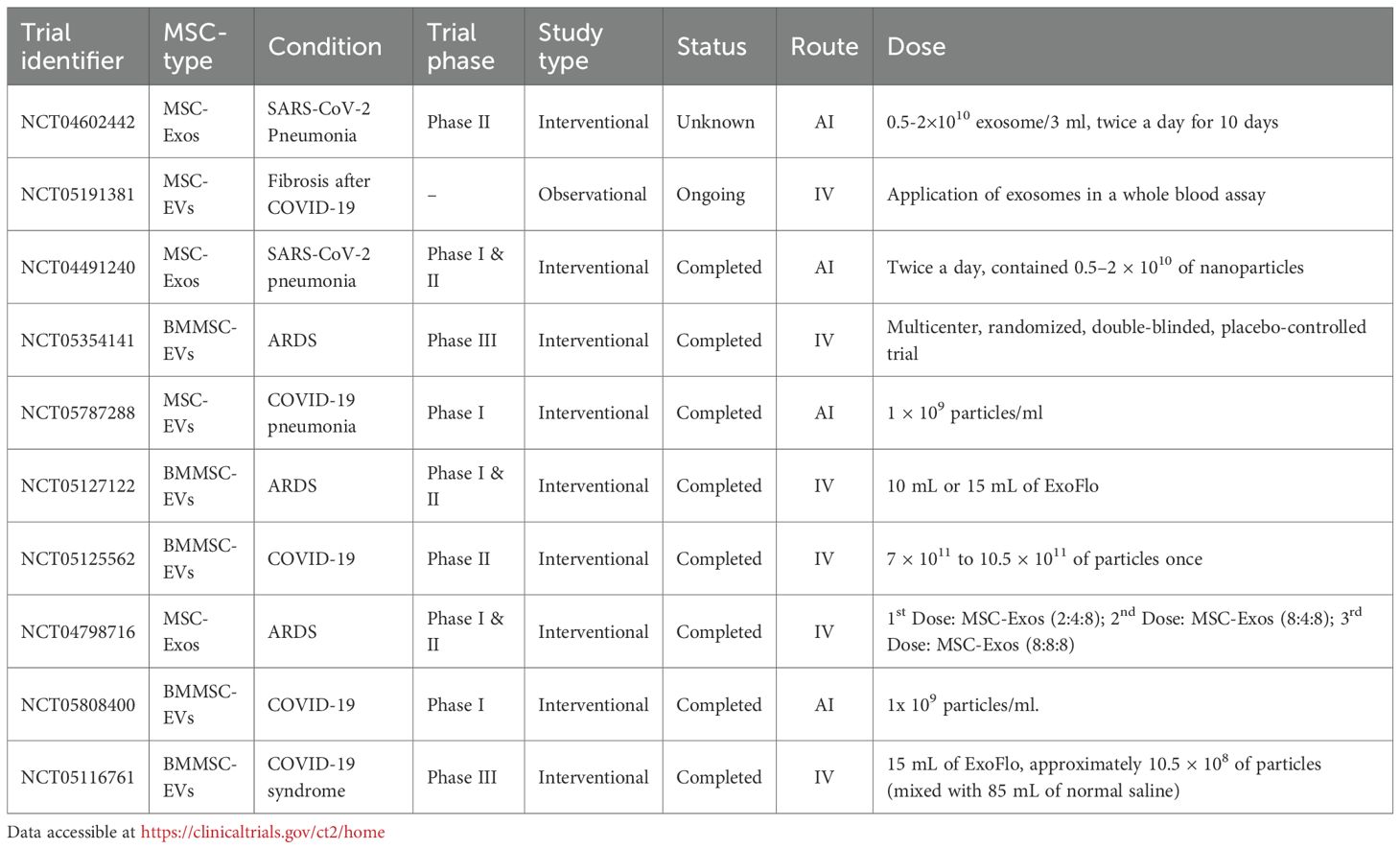
Although clinical trials investigating MSC-Exos for pulmonary diseases are still in early stages, they have demonstrated promising therapeutic potential. To date, most MSC-Exo-based clinical trials for pulmonary conditions focus on Acute Respiratory Distress Syndrome (ARDS) and COVID-19-related lung injury, where they have shown notable efficacy (Table 1). Several pioneering clinical trials using MSC-derived therapies for IPF have also been conducted, which have yielded valuable safety data and paved the way for future EV-based applications (Table 2).
5.2.1 NCT05191381
Up to the present, one registered clinical trial has been reported investigating MSC-EVs therapy for pulmonary fibrosis. The ongoing study initiated in 2021 includes participants aged 18–90 years for intravenous administration of MSC-EVs with pulmonary fibrosis after COVID-19 (106).
5.2.2 NCT01385644
This phase 1b, open-label, dose-escalation trial evaluated the safety of intravenous administration of placenta-derived MSCs in 8 patients with moderate to severe IPF. The intervention was found to be safe and well-tolerated at doses up to 2 × 106; cells/kg, with the majority of adverse events being mild and self-limiting. No significant changes in lung function, 6-minute walk distance, or CT fibrosis scores were observed over the 6-month follow-up (107).
5.2.3 ETHER (NCT02013700)
This single-center, non-randomized phase I study assessed the safety of a single intravenous infusion of allogeneic bone marrow-derived MSCs (20, 100, or 200 × 106; cells) in nine patients with mild-to-moderate IPF. The primary endpoint was the incidence of treatment-emergent serious adverse events (SAEs) within 4 weeks. No SAEs were reported, reinforcing the short-term safety of allogeneic MSC infusion and building confidence for future cell therapy trials in lung diseases (108).
5.2.4 The MR-46-22-004531 (ChiCTR2300075466)
This recent randomized, single-blind, placebo-controlled phase 1 trial involved 24 patients with PF of heterogeneous etiologies. Based on robust preclinical data in a BLM mouse model that identified miRNA-486-5p-mediated macrophage polarization as a key mechanism, the trial administered nebulized hUCMSC-EVs (2×109 particles/person). The treatment was well-tolerated with no severe adverse events. The experimental group showed significant improvements in pulmonary function (FEV1, FVC, MVV, DLCO), respiratory scores, and quality of life. Notably, marked radiological regression was observed in two patients with advanced post-inflammatory PF, while a control patient exhibited progression, suggesting a potential etiology-specific therapeutic benefit (109).
6 Clinical challenges and translation to therapy
Despite their significant promise as a next-generation therapeutic platform, several formidable challenges must be systematically addressed to facilitate the successful clinical translation of MSC-Exos for PF Therapy.
First, a critical challenge is the lack of standardized, scalable, and reproducible protocols for the isolation, purification, characterization, storage, and transport of MSC-Exos. Established isolation methods include ultracentrifugation, ultrafiltration, immunoaffinity capture, polymer-based precipitation via charge neutralization, size-exclusion chromatography, and microfluidic techniques. Each approach presents distinct advantages and limitations, as comprehensively reviewed by Nobendu Mukerjee et al. (110). Ultracentrifugation, the “gold standard,” yields high-purity exosomes but with lower overall output, whereas polymer-based precipitation provides higher yields at the cost of purity, reflected by its predominant use in published clinical trials. MSC-Exos characterization must adhere to the minimal experimental requirements for extracellular vesicles outlined in the MISEV2023 guidelines, encompassing specific marker expression and physical properties. Nearly all MSC-Exos express typical markers such as CD9, CD63, CD81, and TSG101, while lacking Calnexin and Cytochrome C (111). Techniques like electron microscopy and nanoparticle tracking analysis are most widely employed. Scaling from small preclinical batches to clinical-grade quantities requires advanced bioreactor systems while maintaining exosome integrity and bioactivity (112). Aggregation during production or storage can compromise therapeutic efficacy and pharmacokinetics. Variability in cell sources, culture conditions, purification, and isolation, affects the particle size, purity, and cargo composition to predominantly enhance the progression of clinical trials (110, 113). Hence, rigorous quality control metrics and standardized operating procedures (SOPs), despite European Medicines Agency (EMA), U.S. Food and Drug Administration (FDA) guideline for EVs is limited (114, 115). Furthermore, scaling up from laboratory research to GMP-compliant industrial manufacturing—while maintaining consistent therapeutic potency and purity—remains a pivotal obstacle in clinical translation and eventual commercialization (116).
Second, a comprehensive delineation of the complete pharmacological and safety profile of MSC-Exos is imperative. Critical aspects such as their potential toxicity, immunogenicity, biodistribution, clearance mechanisms, and long-term biosafety—particularly following repeated administration to diseased lung tissue—remain inadequately characterized. Essential pharmacological parameters, including the optimal therapeutic dosage, treatment frequency, pharmacokinetics, pharmacodynamics, and the most effective route of administration, must be rigorously established through systematic and well-designed preclinical and clinical studies (117, 118).
MSC-Exos are quantified by employing different metrics, some rely on protein concentration measurements (93, 119). However, that the highest tolerable dose does not necessarily equate to therapeutic efficacy. BCA assay method offer rapid and convenient protein quantification, directly reflecting the total protein content but is susceptible to inaccuracies. In contrast, particle number quantification, a more direct assessment of vesicle count, evaluate exosome yield and standardizing dosing regimens. Another crucial factor is the route of administration influencing the efficacy of MSC-Exos. Intravenous injection is the most commonly used method in preclinical studies for pulmonary diseases (113). For instance, nebulized inhalation represents the preferred route for targeted lung delivery, as it enables direct deposition of the therapeutic agent to the pulmonary region.
Furthermore, MSC-Exos therapeutic effects appear to involve a complex, multi-factorial "cocktail effect" resulting from the synergistic action of myriad molecular components (e.g., miRNAs, proteins, lipids). Key mechanistic questions remain unanswered: Are certain specific miRNAs (e.g., miR-21-5p, miR-let-7 family) playing dominant roles? How do specific cytokines or lipids contribute to the overall anti-fibrotic and immunomodulatory effect? The precise interaction networks between exosomes and various recipient cell types (e.g., fibroblasts, myofibroblasts, macrophages, epithelial cells) within the complex and dynamic PF microenvironment are still poorly characterized. Additionally, PF (including IPF and secondary PF) exhibits considerable patient-to-patient heterogeneity. Individuals with different disease etiologies, genetic backgrounds, and clinical stages likely have distinct molecular driving mechanisms, yet we have not identified which specific patient subsets are most responsive to MSC-Exos therapy. Predictive biomarkers for stratifying patients and forecasting treatment efficacy are currently lacking, hindering the development of personalized treatment approaches.
The path to clinical translation is undoubtedly complex; however, the encouraging safety and efficacy outcomes from early-phase clinical trials affirm the considerable clinical potential of MSC-Exos (109). A concerted effort to overcome the hurdles of production standardization, scalable manufacturing, rigorous quality control, and comprehensive safety assessment will be absolutely essential for the successful development and widespread clinical application.
7 Conclusion and perspective
In summary, MSC-Exos represent a transformative therapeutic paradigm for PF, capitalizing on their multifaceted mechanisms of action and favorable biosafety profile to address fundamental limitations of conventional treatments. Current research underscores that the pathophysiological status of parent cells critically determines exosomal cargo composition, emphasizing the necessity for stringent quality control protocols and standardized characterization of source materials. Despite all preclinical evidence in animal models, clinical translation of MSC-Exo therapy for PF remains limited. So far, no peer-reviewed publications mentioning completed clinical studies in this specific therapeutic domain have emerged.
Future investigations should prioritize several pivotal research directions (1): development of advanced biocompatible engineering strategies to enhance tissue-specific targeting and precision (2); implementing integrated multi-omics approaches to delineate mechanistic pathways and identify validated biomarkers for patient stratification (3); optimizing versatile loading methodologies to facilitate synergistic combination therapies with diverse therapeutic modalities (4). viable alternatives for pretreatment strategies to exhibit excellent biocompatibility and significantly potentiate regenerative outcomes. Addressing these challenges through rigorous mechanistic studies, harmonized quality-control frameworks, and well-designed clinical trials will be essential.
The clinical translation pathway for MSC-Exos is strengthened by their exceptional stability, minimal immunogenicity, modular and tunable delivery capabilities, effectively circumventing risks inherent to whole-cell therapies. As mechanistic insights deepen and manufacturing technologies advance, these exosome-based therapeutics are poised to transition from investigational agents to foundational elements of precision medicine for fibrotic lung disorders, potentially addressing critical unmet needs in treatment-refractory cases.
Author contributions
YX: Investigation, Software, Funding acquisition, Writing – original draft, Validation, Formal Analysis, Methodology, Data curation, Conceptualization. IH: Writing – original draft, Formal Analysis, Software, Validation, Methodology, Data curation, Investigation. LZ: Data curation, Validation, Writing – original draft, Investigation. SB: Resources, Validation, Writing – review & editing. XJ: Writing – review & editing, Supervision, Resources. HY: Writing – review & editing, Resources, Funding acquisition, Conceptualization. J-SZ: Conceptualization, Writing – review & editing, Investigation, Funding acquisition, Supervision, Formal Analysis, Visualization, Resources, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by grants from the Health Research Project of the Hunan Provincial Health Commission (No. D202303026282) and the Changsha Natural Science Foundation (Kq2403162). I.H. received CSC Scholarship (No. 2025SLJ017202). J-S.Z. is partially supported by the Discipline Cluster of Oncology at Wenzhou Medical University, China (No. Z1-2023004) and Zhejiang Province Public Welfare Fund Project (No. LY24H050003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iommi M, Faragalli A, Bonifazi M, Mei F, Latini LL, Pompili M, et al. Prognosis and survival in idiopathic pulmonary fibrosis in the era of antifibrotic therapy in Italy: evidence from a longitudinal population study based on healthcare utilization databases. Int J Environ Res Public Health. (2022) 19:16689. doi: 10.3390/ijerph192416689, PMID: 36554568
2. Honda K, Saraya T, and Ishii H. A real-world prognosis in idiopathic pulmonary fibrosis: a special reference to the role of Antifibrotic agents for the elderly. J Clin Med. (2023) 12:3564. doi: 10.3390/jcm12103564, PMID: 37240670
3. Khor YH, Ng Y, Barnes H, Goh NS, McDonald CF, and Holland AE. Prognosis of idiopathic pulmonary fibrosis without anti-fibrotic therapy: a systematic review. Eur Respir Review. (2020) 29:190158. doi: 10.1183/16000617.0158-2019, PMID: 32759374
4. Podolanczuk AJ, Thomson CC, Remy-Jardin M, Richeldi L, Martinez FJ, Kolb M, et al. Idiopathic pulmonary fibrosis: state of the art for 2023. Eur Respir J. (2023) 61:2200957. doi: 10.1183/13993003.00957-2022, PMID: 36702498
5. Saito S, Alkhatib A, Kolls JK, Kondoh Y, and Lasky JA. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac disease. (2019) 11:S1740. doi: 10.21037/jtd.2019.04.62, PMID: 31632751
6. Cottin V, Koschel D, Günther A, Albera C, Azuma A, Sköld CM, et al. Long-term safety of pirfenidone: results of the prospective, observational PASSPORT study. ERJ Open Res. (2018) 4(4):00084–2018. doi: 10.1183/23120541.00084-2018, PMID: 30364407
7. Crestani B, Huggins JT, Kaye M, Costabel U, Glaspole I, Ogura T, et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: results from the open-label extension study, INPULSIS-ON. Lancet Respir Med. (2019) 7:60–8. doi: 10.1016/S2213-2600(18)30339-4, PMID: 30224318
8. Aliakbari F, Marzookian K, Parsafar S, Hourfar H, Nayeri Z, Fattahi A, et al. The impact of hUC MSC–derived exosome-nanoliposome hybrids on α-synuclein fibrillation and neurotoxicity. Sci Advances. (2024) 10:eadl3406. doi: 10.1126/sciadv.adl3406, PMID: 38569030
9. Liu Q, Bi Y, Song S, Zhu K, Qiao X, Wang H, et al. Exosomal miR-17-5p from human embryonic stem cells prevents pulmonary fibrosis by targeting thrombospondin-2. Stem Cell Res Ther. (2023) 14:234. doi: 10.1186/s13287-023-03449-7, PMID: 37667335
10. Chen W, Lin F, Feng X, Yao Q, Yu Y, Gao F, et al. MSC-derived exosomes attenuate hepatic fibrosis in primary sclerosing cholangitis through inhibition of Th17 differentiation. Asian J Pharm Sci. (2024) 19:100889. doi: 10.1016/j.ajps.2024.100889, PMID: 38419761
11. Mayr CH, Simon LM, Leuschner G, Ansari M, Schniering J, Geyer PE, et al. Integrative analysis of cell state changes in lung fibrosis with peripheral protein biomarkers. EMBO Mol Med. (2021) 13:e12871. doi: 10.15252/emmm.202012871, PMID: 33650774
12. Enomoto T, Shirai Y, Takeda Y, Edahiro R, Shichino S, Nakayama M, et al. SFTPB in serum extracellular vesicles as a biomarker of progressive pulmonary fibrosis. JCI Insight. (2024) 9:e177937. doi: 10.1172/jci.insight.177937, PMID: 38855869
13. Periera-Simon S, Xia X, Catanuto P, Coronado R, Kurtzberg J, Bellio M, et al. Anti-fibrotic effects of different sources of MSC in bleomycin-induced lung fibrosis in C57BL6 male mice. Respirology. (2021) 26:161–70. doi: 10.1111/resp.13928, PMID: 32851725
14. Pan Y, Wu W, Jiang X, and Liu Y. Mesenchymal stem cell-derived exosomes in cardiovascular and cerebrovascular diseases: from mechanisms to therapy. Biomedicine Pharmacotherapy. (2023) 163:114817. doi: 10.1016/j.biopha.2023.114817, PMID: 37141733
15. Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, and Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. (2019) 76:3323–48. doi: 10.1007/s00018-019-03125-1, PMID: 31055643
16. de Brito K and Trentin AG. Role of mesenchymal stromal cell secretome on recovery from cellular senescence: an overview. Cytotherapy. (2024) 27:422–37., PMID: 39674933
17. Radermacher C, Craveiro RB, Jahnen-Dechent W, Beier JP, Bülow A, Wolf M, et al. Impact of compression forces on different mesenchymal stem cell types regarding orthodontic indication. Stem Cells Trans Med. (2024) 13:1028–39. doi: 10.1093/stcltm/szae057, PMID: 39181541
18. Zhang Y, Bi J, Huang J, Tang Y, Du S, and Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J nanomedicine. (2020) 6917-34:6917–34. doi: 10.2147/IJN.S264498, PMID: 33061359
19. Yan L, Li J, and Zhang C. The role of MSCs and CAR-MSCs in cellular immunotherapy. Cell Communication Signaling. (2023) 21:187. doi: 10.1186/s12964-023-01191-4, PMID: 37528472
20. Li W, Pang Y, He Q, Song Z, Xie X, Zeng J, et al. Exosome-derived microRNAs: emerging players in vitiligo. Front Immunol. (2024) 15:1419660. doi: 10.3389/fimmu.2024.1419660, PMID: 39040109
21. Gao L, Qiu F, Cao H, Li H, Dai G, Ma T, et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics. (2023) 13:685. doi: 10.7150/thno.73568, PMID: 36632217
22. Zhao S, Li W, Yu W, Rao T, Li H, Ruan Y, et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics. (2021) 11:8660. doi: 10.7150/thno.62820, PMID: 34522205
23. Tan S, Xia L, Yi P, Han Y, Tang L, Pan Q, et al. Exosomal miRNAs in tumor microenvironment. J Exp Clin Cancer Res. (2020) 39:67. doi: 10.1186/s13046-020-01570-6, PMID: 32299469
24. Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, De Kleijn DP, et al. Proteolytic potential of the MSC exosome proteome: Implications for an exosome-mediated delivery of therapeutic proteasome. Int J proteomics. (2012) 2012:971907. doi: 10.1155/2012/971907, PMID: 22852084
25. Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. (2016) 34:601–13. doi: 10.1002/stem.2298, PMID: 26782178
26. Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PloS One. (2013) 8:e68451. doi: 10.1371/journal.pone.0068451, PMID: 23861904
27. Levy-Myers R, Daudelin D, Na CH, and Sockanathan S. An independent regulator of global release pathways in astrocytes generates a subtype of extracellular vesicles required for postsynaptic function. Sci Advances. (2023) 9:eadg2067. doi: 10.1126/sciadv.adg2067, PMID: 37352348
28. Ju Y, Bai H, Ren L, and Zhang L. The role of exosome and the ESCRT pathway on enveloped virus infection. Int J Mol Sci. (2021) 22:9060. doi: 10.3390/ijms22169060, PMID: 34445766
29. Carraro G and Stripp BR. Insights gained in the pathology of lung disease through single-cell transcriptomics. J Pathology. (2022) 257:494–500. doi: 10.1002/path.5971, PMID: 35608561
30. Chong L, Ahmadvand N, Noori A, Lv Y, Chen C, Bellusci S, et al. Injury activated alveolar progenitors (IAAPs): the underdog of lung repair. Cell Mol Life Sci. (2023) 80:145. doi: 10.1007/s00018-023-04789-6, PMID: 37166489
31. Liu X, Zhang X, Yao C, Liang J, Noble PW, and Jiang D. A transcriptional cell atlas identifies the decline in the AT2 niche in aged human lungs. bioRxiv. (2023) 2023:06. 16.545378. doi: 10.1101/2023.06.16.545378, PMID: 37398304
32. Xie T, Liang J, Stripp B, and Noble PW. Cell-cell interactions and communication dynamics in lung fibrosis. Chin Med J Pulmonary Crit Care Med. (2024) 2:63–71. doi: 10.1016/j.pccm.2024.04.001, PMID: 39169931
33. Wisman M, Nizamoglu M, Noordhoek JA, Timens W, Burgess JK, and Heijink IH. Dysregulated cross-talk between alveolar epithelial cells and stromal cells in idiopathic pulmonary fibrosis reduces epithelial regenerative capacity. Front Med. (2023) 10:1182368. doi: 10.3389/fmed.2023.1182368, PMID: 37621459
34. Yang Y, Liu Y, Chai Y, Liu K, Hu W, Zhao K, et al. Exosomes in pathogenesis, diagnosis, and treatment of pulmonary fibrosis. Front Pharmacol. (2022) 13:927653. doi: 10.3389/fphar.2022.927653, PMID: 36091791
35. Nija R and Nithya T. Pulmonary fibrosis and exosomes: pathways to treatment. Mol Biol Rep. (2025) 52:749. doi: 10.1007/s11033-025-10855-y, PMID: 40699390
36. Zabihi M, Felordi MS, Lingampally A, Bellusci S, Chu X, and El Agha E. Understanding myofibroblast origin in the fibrotic lung. Chin Med J Pulmonary Crit Care Med. (2024) 2:142–50. doi: 10.1016/j.pccm.2024.08.003, PMID: 39403408
37. Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. (2017) 170:1134–48. e10. doi: 10.1016/j.cell.2017.07.034, PMID: 28886382
38. Yamada Z, Nishio J, Motomura K, Mizutani S, Yamada S, Mikami T, et al. Senescence of alveolar epithelial cells impacts initiation and chronic phases of murine fibrosing interstitial lung disease. Front Immunol. (2022) 13:935114. doi: 10.3389/fimmu.2022.935114, PMID: 36059455
39. Chen L, Yang Y, Yue R, Peng X, Yu H, and Huang X. Exosomes derived from hypoxia-induced alveolar epithelial cells stimulate interstitial pulmonary fibrosis through a HOTAIRM1-dependent mechanism. Lab Invest. (2022) 102:935–44. doi: 10.1038/s41374-022-00782-y, PMID: 35477975
40. Mayr CH, Sengupta A, Asgharpour S, Ansari M, Pestoni JC, Ogar P, et al. Sfrp1 inhibits lung fibroblast invasion during transition to injury-induced myofibroblasts. Eur Respir J. (2024) 63:2301326–2301326. doi: 10.1183/13993003.01326-2023, PMID: 38212077
41. Tsukui T, Sun K-H, Wetter JB, Wilson-Kanamori JR, Hazelwood LA, Henderson NC, et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. (2020) 11:1920. doi: 10.1038/s41467-020-15647-5, PMID: 32317643
42. Burgy O, Mayr CH, Schenesse D, Papakonstantinou EF, Ballester B, Sengupta A, et al. Fibroblast-derived extracellular vesicles contain SFRP1 and mediate pulmonary fibrosis. JCI Insight. (2024) 9:e168889. doi: 10.1172/jci.insight.168889, PMID: 39315549
43. Kadota T, Yoshioka Y, Fujita Y, Araya J, Minagawa S, Hara H, et al. Extracellular vesicles from fibroblasts induce epithelial-cell senescence in pulmonary fibrosis. Am J Respir Cell Mol Biol. (2020) 63:623–36. doi: 10.1165/rcmb.2020-0002OC, PMID: 32730709
44. Xie T, Kulur V, Liu N, Deng N, Wang Y, Rowan SC, et al. Mesenchymal growth hormone receptor deficiency leads to failure of alveolar progenitor cell function and severe pulmonary fibrosis. Sci Advances. (2021) 7:eabg6005. doi: 10.1164/ajrccm-conference.2021.TP107, PMID: 34108218
45. Zhou X, Franklin RA, Adler M, Carter TS, Condiff E, Adams TS, et al. Microenvironmental sensing by fibroblasts controls macrophage population size. Proc Natl Acad Sci. (2022) 119:e2205360119. doi: 10.1073/pnas.2205360119, PMID: 35930670
46. Feng Z, Jing Z, Li Q, Chu L, Jiang Y, Zhang X, et al. Exosomal STIMATE derived from type II alveolar epithelial cells controls metabolic reprogramming of tissue-resident alveolar macrophages. Theranostics. (2023) 13:991. doi: 10.7150/thno.82552, PMID: 36793853
47. Yang Y, Huang H, and Li Y. Roles of exosomes and exosome-derived miRNAs in pulmonary fibrosis. Front Pharmacol. (2022) 13:928933. doi: 10.3389/fphar.2022.928933, PMID: 36034858
48. Wei Y, Hong M, Zhu H, and Li F. Recent progress in exosomal non-coding RNAs research related to idiopathic pulmonary fibrosis. Front Genet. (2025) 16:1556495. doi: 10.3389/fgene.2025.1556495, PMID: 40212286
49. Guiot J, Cambier M, Boeckx A, Henket M, Nivelles O, Gester F, et al. Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p. Thorax. (2020) 75:870–81. doi: 10.1136/thoraxjnl-2019-214077, PMID: 32759383
50. Asghar S, Monkley S, Smith DJ, Hewitt RJ, Grime K, Murray LA, et al. Epithelial senescence in idiopathic pulmonary fibrosis is propagated by small extracellular vesicles. Respir Res. (2023) 24:51. doi: 10.1186/s12931-023-02333-5, PMID: 36788603
51. Pokhreal D, Crestani B, and Helou DG. Macrophage implication in IPF: updates on immune, epigenetic, and metabolic pathways. Cells. (2023) 12:2193. doi: 10.3390/cells12172193, PMID: 37681924
52. Deng L, Huang T, and Zhang L. T cells in idiopathic pulmonary fibrosis: crucial but controversial. Cell Death Discovery. (2023) 9:62. doi: 10.1038/s41420-023-01344-x, PMID: 36788232
53. Kamiya M, Carter H, Espindola MS, Doyle TJ, Lee JS, Merriam LT, et al. Immune mechanisms in fibrotic interstitial lung disease. Cell. (2024) 187:3506–30. doi: 10.1016/j.cell.2024.05.015, PMID: 38996486
54. Mutsaers SE, Miles T, Prêle CM, and Hoyne GF. Emerging role of immune cells as drivers of pulmonary fibrosis. Pharmacol Ther. (2023) 252:108562. doi: 10.1016/j.pharmthera.2023.108562, PMID: 37952904
55. Wang X, Zhou J, Li X, Liu C, Liu L, and Cui H. The role of macrophages in lung fibrosis and the signaling pathway. Cell Biochem Biophysics. (2024) 82:479–88. doi: 10.1007/s12013-024-01253-5, PMID: 38536578
56. Ogawa T, Shichino S, Ueha S, and Matsushima K. Macrophages in lung fibrosis. Int Immunol. (2021) 33:665–71. doi: 10.1093/intimm/dxab040, PMID: 34270737
57. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. (2018) 197:104–16. doi: 10.1164/rccm.201705-0925OC, PMID: 28853608
58. Dong B, Wang C, Zhang J, Zhang J, Gu Y, Guo X, et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res Ther. (2021) 12:204. doi: 10.1186/s13287-021-02244-6, PMID: 33761997
59. Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. (2019) 4:e128060. doi: 10.1183/23120541.lungscienceconference-2019.OP03, PMID: 31581150
60. wei Li J, Wei L, Han Z, and Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur J Pharmacol. (2019) 852:68–76. doi: 10.1016/j.ejphar.2019.01.022, PMID: 30682335
61. Grabiec AM and Hussell T. The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin immunopathology. (2016) 38:409–23. doi: 10.1007/s00281-016-0555-3, PMID: 26957481
62. Lei L, Zhao C, Qin F, He Z-Y, Wang X, and Zhong X-N. Th17 cells and IL-17 promote the skin and lung inflammation and fibrosis process in a bleomycin-induced murine model of systemic sclerosis. Clin Exp Rheumatol. (2016) 34:14–22. doi: No DOI provided (PMID listed instead: PM26750756), PMID: 26750756
63. Lai P, Chen X, Guo L, Wang Y, Liu X, Liu Y, et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol Oncol. (2018) 11:135. doi: 10.1186/s13045-018-0680-7, PMID: 30526632
64. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, and Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. (2019) 8:1605. doi: 10.3390/cells8121605, PMID: 31835680
65. Li D, Guabiraba R, Besnard A-G, Komai-Koma M, Jabir MS, Zhang L, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. (2014) 134:1422–32. e11. doi: 10.1016/j.jaci.2014.05.011, PMID: 24985397
66. Xie L, Long X, Mo M, Jiang J, Zhang Q, Long M, et al. Bone marrow mesenchymal stem cell-derived exosomes alleviate skin fibrosis in systemic sclerosis by inhibiting the IL-33/ST2 axis via the delivery of microRNA-214. Mol Immunol. (2023) 157:146–57. doi: 10.1016/j.molimm.2023.03.017, PMID: 37028129
67. Yang C, Sun J, Tian Y, Li H, Zhang L, Yang J, et al. Immunomodulatory effect of MSCs and MSCs-derived extracellular vesicles in systemic lupus erythematosus. Front Immunol. (2021) 12:714832. doi: 10.3389/fimmu.2021.714832, PMID: 34603289
68. Xie Y, Yi Q, Xu C, Wang Y, Jiang Y, Feng Y, et al. Identifying TNFSF4low-MSCs superiorly treating idiopathic pulmonary fibrosis through Tregs differentiation modulation. Stem Cell Res Ther. (2025) 16:194. doi: 10.1186/s13287-025-04313-6, PMID: 40254578
69. Harrell CR, Djonov V, Volarevic A, Arsenijevic A, and Volarevic V. Molecular mechanisms responsible for the therapeutic potential of mesenchymal stem cell-derived exosomes in the treatment of lung fibrosis. Int J Mol Sci. (2024) 25:4378. doi: 10.3390/ijms25084378, PMID: 38673961
70. Zhang E, Geng X, Shan S, Li P, Li S, Li W, et al. Exosomes derived from bone marrow mesenchymal stem cells reverse epithelial-mesenchymal transition potentially via attenuating Wnt/β-catenin signaling to alleviate silica-induced pulmonary fibrosis. Toxicol Mech Methods. (2021) 31:655–66. doi: 10.1080/15376516.2021.1950250, PMID: 34225584
71. Chen W, Peng J, Tang X, and Ouyang S. MSC-derived exosome ameliorates pulmonary fibrosis by modulating NOD 1/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Heliyon. (2025) 11:e41436. doi: 10.1016/j.heliyon.2024.e41436, PMID: 39872463
72. Cheng F, Yang F, Wang Y, Zhou J, Qian H, and Yan Y. Mesenchymal stem cell-derived exosomal miR-27b-3p alleviates liver fibrosis via downregulating YAP/LOXL2 pathway. J Nanobiotechnology. (2023) 21:195. doi: 10.1186/s12951-023-01942-y, PMID: 37328872
73. Tian S, Zhou X, Zhang M, Cui L, Li B, Liu Y, et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res Ther. (2022) 13:330. doi: 10.1186/s13287-022-03010-y, PMID: 35858897
74. Al Saihati HA, Badr OA, Dessouky AA, Mostafa O, Farid AS, Aborayah NH, et al. Exploring the cytoprotective role of mesenchymal stem Cell-Derived exosomes in chronic liver Fibrosis: Insights into the Nrf2/Keap1/p62 signaling pathway. Int Immunopharmacology. (2024) 141:112934. doi: 10.1016/j.intimp.2024.112934, PMID: 39178516
75. Long Y, Yang B, Lei Q, Gao F, Chen L, Chen W, et al. Targeting senescent alveolar epithelial cells using engineered mesenchymal stem cell-derived extracellular vesicles to treat pulmonary fibrosis. ACS nano. (2024) 18:7046–63. doi: 10.1021/acsnano.3c10547, PMID: 38381372
76. Wan X, Chen S, Fang Y, Zuo W, Cui J, and Xie S. Mesenchymal stem cell-derived extracellular vesicles suppress the fibroblast proliferation by downregulating FZD6 expression in fibroblasts via micrRNA-29b-3p in idiopathic pulmonary fibrosis. J Cell Physiol. (2020) 235:8613–25. doi: 10.1002/jcp.29706, PMID: 32557673
77. Tsitoura E, Vasarmidi E, Bibaki E, Trachalaki A, Koutoulaki C, Papastratigakis G, et al. Accumulation of damaged mitochondria in alveolar macrophages with reduced OXPHOS related gene expression in IPF. Respir Res. (2019) 20:264. doi: 10.1186/s12931-019-1196-6, PMID: 31775876
78. Bueno M, Calyeca J, Rojas M, and Mora AL. Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol. (2020) 33:101509. doi: 10.1016/j.redox.2020.101509, PMID: 32234292
79. Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. (2005) 8:3–5. doi: 10.1089/rej.2005.8.3, PMID: 15798367
80. Zhang X, Wang Y, Guo X, Xiao Y, Wan W, Zou H, et al. Mitochondrial dysfunction in fibrotic diseases: Research progress and MSC-exos therapy. Exp Mol Pathology. (2025) 143:104983. doi: 10.1016/j.yexmp.2025.104983, PMID: 40644771
81. Larson-Casey JL, Deshane JS, Ryan AJ, Thannickal VJ, and Carter AB. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity. (2016) 44:582–96. doi: 10.1016/j.immuni.2016.01.001, PMID: 26921108
82. He C, Larson-Casey JL, Davis D, Hanumanthu VS, Longhini ALF, Thannickal VJ, et al. NOX4 modulates macrophage phenotype and mitochondrial biogenesis in asbestosis. JCI Insight. (2019) 4:e126551. doi: 10.1172/jci.insight.126551, PMID: 31434799
83. Fernandez-Marcos PJ and Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. (2011) 93:884S–90S. doi: 10.3945/ajcn.110.001917, PMID: 21289221
84. Angajala A, Lim S, Phillips JB, Kim J-H, Yates C, You Z, et al. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front Immunol. (2018) 9:1605. doi: 10.3389/fimmu.2018.01605, PMID: 30050539
85. Osborn-Heaford HL, Ryan AJ, Murthy S, Racila A-M, He C, Sieren JC, et al. Mitochondrial Rac1 GTPase import and electron transfer from cytochrome c are required for pulmonary fibrosis. J Biol Chem. (2012) 287:3301–12. doi: 10.1074/jbc.M111.308387, PMID: 22157762
86. Xie N, Cui H, Ge J, Banerjee S, Guo S, Dubey S, et al. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am J Physiology-Lung Cell Mol Physiol. (2017) 313:L834–L44. doi: 10.1152/ajplung.00235.2017, PMID: 28798256
87. Xia L, Zhang C, Lv N, Liang Z, Ma T, Cheng H, et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. (2022) 12:2928. doi: 10.7150/thno.69533, PMID: 35401830
88. Wu F, Zhang Y, Tang Y, Kang Y, Li Y, Hu X, et al. FGF21-loaded M2 macrophage-derived exosomes attenuate sepsis-induced lung injury by regulating M2 macrophage polarization and glycolysis. Life Sci. (2025) 380:123932. doi: 10.1016/j.lfs.2025.123932, PMID: 40889696
89. Silva JD, Su Y, Calfee CS, Delucchi KL, Weiss D, McAuley DF, et al. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. (2021) 58:2002978. doi: 10.1183/13993003.02978-2020, PMID: 33334945
90. Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl materials interfaces. (2020) 12:36938–47. doi: 10.1021/acsami.0c10458, PMID: 32814390
91. Cao F, Gao Y, Chu Q, Wu Q, Zhao L, Lan T, et al. Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis. (2019) 40:3092–8. doi: 10.1002/elps.201900295, PMID: 31621929
92. Zou Y, Sun Y, Guo B, Wei Y, Xia Y, Huangfu Z, et al. α3β1 integrin-targeting polymersomal docetaxel as an advanced nanotherapeutic for nonsmall cell lung cancer treatment. ACS Appl Materials Interfaces. (2020) 12:14905–13. doi: 10.1021/acsami.0c01069, PMID: 32148016
93. Zhang W-Y, Wen L, Du L, Liu TT, Sun Y, Chen Y-Z, et al. S-RBD-modified and miR-486-5p-engineered exosomes derived from mesenchymal stem cells suppress ferroptosis and alleviate radiation-induced lung injury and long-term pulmonary fibrosis. J Nanobiotechnology. (2024) 22:662. doi: 10.1186/s12951-024-02830-9, PMID: 39462403
94. Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS nano. (2014) 8:483–94., PMID: 24383518
95. Lin Y, Yi M, Guan X, Chen E, Yang L, Li S, et al. Two birds with one stone” strategy for the lung cancer therapy with bioinspired AIE aggregates. J Nanobiotechnology. (2023) 21:49., PMID: 36759822
96. Zhou J, Lin Y, Kang X, Liu Z, Zhang W, and Xu F. microRNA-186 in extracellular vesicles from bone marrow mesenchymal stem cells alleviates idiopathic pulmonary fibrosis via interaction with SOX4 and DKK1. Stem Cell Res Ther. (2021) 12:96. doi: 10.1186/s13287-020-02083-x, PMID: 33536061
97. Gu Z, Sun M, Liu J, Huang Q, Wang Y, Liao J, et al. Endothelium-derived engineered extracellular vesicles protect the pulmonary endothelial barrier in acute lung injury. Advanced Science. (2024) 11:2306156., PMID: 38062916
98. Lu H, Liu X, Zhang M, Bera H, Xu W, Jiang H, et al. Pulmonary fibroblast-specific delivery of siRNA exploiting exosomes-based nanoscaffolds for IPF treatment. Asian J Pharm Sci. (2024) 19:100929. doi: 10.1016/j.ajps.2024.100929, PMID: 39258001
99. Chen Z, Yun X, Tian J, Li F, Zhang Z, Meng J, et al. Engineering macrophage-derived exosome to deliver pirfenidone: A novel approach to combat silicotic pulmonary fibrosis. Advanced Healthcare Materials. (2025) 14:2403227., PMID: 39382242
100. Sun Y, Cai S, Liu H, Zhao C, Wang X, Zhang J, et al. Bergenin and vitexin delivery platform using mouse lung fibroblasts-derived exosomes for bleomycin-induced pulmonary fibrosis therapy. Int J Pharmaceutics. (2025) 681:125750. doi: 10.1016/j.ijpharm.2025.125750, PMID: 40456424
101. Zhang W, Wan Z, Qu D, Sun W, Zhang L, Liang Y, et al. Profibrogenic macrophage-targeted delivery of mitochondrial protector via exosome formula for alleviating pulmonary fibrosis. Bioactive Materials. (2024) 32:488–501., PMID: 37965241
102. Chen S, Song X, and Lv C. Macrophages and pulmonary fibrosis. Curr Mol Med. (2025) 25:416–30. doi: 10.2174/0115665240286046240112112310, PMID: 39779550
103. Ghebremedhin A, Salam AB, Adu-Addai B, Noonan S, Stratton R, Ahmed MSU, et al. A novel CD206 targeting peptide inhibits bleomycin-induced pulmonary fibrosis in mice. Cells. (2023) 12:1254.
104. Ogger PP, Albers GJ, Hewitt RJ, O’Sullivan BJ, Powell JE, Calamita E, et al. Itaconate controls the severity of pulmonary fibrosis. Sci Immunol. (2020) 5:eabc1884. doi: 10.1126/sciimmunol.abc1884, PMID: 33097591
105. Rabelink TJ, Van Den Berg BM, Garsen M, Wang G, Elkin M, and van der Vlag J. Heparanase: roles in cell survival, extracellular matrix remodelling and the development of kidney disease. Nat Rev Nephrology. (2017) 13:201–12., PMID: 28163306
106. Zou Y, Zhou Y, Li G, Dong Y, and Hu S. Clinical applications of extracellular vesicles: recent advances and emerging trends. Front Bioengineering Biotechnol. (2025) 13:1671963. doi: 10.3389/fbioe.2025.1671963, PMID: 41220804
107. Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. (2014) 19:1013–8., PMID: 25039426
108. Glassberg MK, Minkiewicz J, Toonkel RL, Simonet ES, Rubio GA, DiFede D, et al. Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): a phase I safety clinical trial. Chest. (2017) 151:971–81., PMID: 27890713
109. Li M, Huang H, Wei X, Li H, Li J, Xie B, et al. Clinical investigation on nebulized human umbilical cord MSC-derived extracellular vesicles for pulmonary fibrosis treatment. Signal Transduction Targeted Ther. (2025) 10:179. doi: 10.1038/s41392-025-02262-3, PMID: 40461474
110. Mukerjee N, Bhattacharya A, Maitra S, Kaur M, Ganesan S, Mishra S, et al. Exosome isolation and characterization for advanced diagnostic and therapeutic applications. Materials Today Bio. (2025) 31:101613. doi: 10.1016/j.mtbio.2025.101613, PMID: 40161926
111. Welsh JA, Goberdhan DC, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J extracellular vesicles. (2024) 13:e12404. doi: 10.1002/jev2.12404, PMID: 38326288
112. Chowdhury R, Eslami S, Pham CV, Rai A, Lin J, Hou Y, et al. Role of aptamer technology in extracellular vesicle biology and therapeutic applications. Nanoscale. (2024) 16:11457–79., PMID: 38856692
113. Lotfy A, AboQuella NM, and Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. (2023) 14:66. doi: 10.1186/s13287-023-03287-7, PMID: 37024925
114. Berger T, Zeitlinger M, Popescu V, Magyari M, Airas L, Alkhawajah M, et al. Generics, biosimilars and follow-on non-biologic complex drugs for multiple sclerosis: A narrative review of the regulatory and clinical implications for european neurologists. Eur J Neurology. (2025) 32:e70140. doi: 10.1111/ene.70140, PMID: 40231751
115. Kirchlechner TM and Cohen HP. Global harmonization of biosimilar development by overcoming existing differences in regional regulatory requirements-outcomes of a descriptive review. Ther Innovation Regul Science. (2025) 59:245–55., PMID: 39821881
116. Du X, Chen S, Meng T, Liu L, Li L, Xiang R, et al. Extracellular vesicles as precision therapeutic vectors: charting the future of cell-targeted therapy. Precis Med Eng. (2025) 2:100031. doi: 10.1016/j.preme.2025.100031
117. Zhang F, Zhang L, and Yu H. Potential druggability of mesenchymal stem/stromal cell-derived exosomes. Curr Stem Cell Res Ther. (2024) 19:1195–209. doi: 10.2174/011574888X311270240319084835, PMID: 38523514
118. Feng Y, Guo K, Jiang J, and Lin S. Mesenchymal stem cell-derived exosomes as delivery vehicles for non-coding RNAs in lung diseases. Biomedicine Pharmacotherapy. (2024) 170:116008., PMID: 38071800
119. Charoenphannathon JS, Wong PD, Royce SG, Jaffar J, Westall GP, Wang C, et al. Human bone marrow mesenchymal stem cell-derived extracellular vesicles induce inverse dose-dependent anti-fibrotic effects in human myofibroblast cultures and bleomycin-injured mice with pulmonary fibrosis. Biomedicine Pharmacotherapy. (2025) 190:118370. doi: 10.1016/j.biopha.2025.118370, PMID: 40695046
120. Zhu Y-G, Shi M-m, Monsel A, Dai C-x, Dong X, Shen H, et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Res Ther. (2022) 13:220. doi: 10.1186/s13287-022-02900-5, PMID: 35619189
121. Shi M, Yang >Q, Monsel A, Yan J, Dai C, Zhao J, et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J Extracellular Vesicles. (2021) 10:e12134. doi: 10.1002/jev2.12134, PMID: 34429860
122. Wang J, Shi Y, Su Y, Pang C, Yang Y, and Wang W. Research advances of extracellular vesicles in lung diseases. Cell Transplantation. (2025) 34:09636897251362031.
123. Lv K, Wang Y, Lou P, Liu S, Zhou P, Yang L, et al. Extracellular vesicles as advanced therapeutics for the resolution of organ fibrosis: current progress and future perspectives. Front Immunol. (2022) 13:1042983. doi: 10.3389/fimmu.2022.1042983, PMID: 36341339
124. Guo H, Su Y, and Deng F. Effects of mesenchymal stromal cell-derived extracellular vesicles in lung diseases: current status and future perspectives. Stem Cell Rev Rep. (2021) 17:440–58. doi: 10.1007/s12015-020-10085-8, PMID: 33211245
125. Lightner AL, Sengupta V, Qian S, Ransom JT, Suzuki S, Park DJ, et al. Bone marrow mesenchymal stem cell-derived extracellular vesicle infusion for the treatment of respiratory failure from COVID-19: a randomized, placebo-controlled dosing clinical trial. Chest. (2023) 164:1444–53., PMID: 37356708
126. Chu M, Wang H, Bian L, Huang J, Wu D, Zhang R, et al. Nebulization therapy with umbilical cord mesenchymal stem cell-derived exosomes for COVID-19 pneumonia. Stem Cell Rev Rep. (2022) 18:2152–63. doi: 10.1007/s12015-022-10398-w, PMID: 35665467
Keywords: exosomes, mesenchymal stem cells, pulmonary fibrosis, immune modulation, extracellular matrix, drug delivery, targeted therapy
Citation: Xiao Y, Hoorain I, Zhang L, Bellusci S, Jin X, Yang H and Zhang J-S (2025) Unpacking the anti-fibrotic arsenal: molecular mechanisms and therapeutic translation of MSC-derived exosomes in pulmonary fibrosis. Front. Immunol. 16:1725041. doi: 10.3389/fimmu.2025.1725041
Received: 14 October 2025; Accepted: 24 November 2025; Revised: 20 November 2025;
Published: 12 December 2025.
Edited by:
Hector A. Cabrera-Fuentes, Imam Abdulrahman bin Faisal University, Saudi ArabiaReviewed by:
Noe Alvarado, National Institute of Respiratory Diseases (INER), MexicoJuan José Alpuche, Universidad Autónoma Benito Juárez de Oaxaca, Mexico
Efrén Emmanuel Jarquín González, Secretaria de Salud del Estado de Oaxaca, Mexico
Copyright © 2025 Xiao, Hoorain, Zhang, Bellusci, Jin, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongzhong Yang, aHpoeTgyQHNpbmEuY24=; Jin-San Zhang, WmhhbmdfSmluU2FuQHdtdS5lZHUuY24=
†These authors have contributed equally to this work
 Yijia Xiao
Yijia Xiao Iqra Hoorain2,3†
Iqra Hoorain2,3† Saverio Bellusci
Saverio Bellusci Jin-San Zhang
Jin-San Zhang