- School of Basic Medical Science, Zhejiang Chinese Medical University, Hangzhou, China
Nociplastic pain (NPP) is a recently defined form of chronic pain, characterized by altered nociception in the absence of clear evidence of nociceptive or neuropathic pain. It is believed to be driven by maladaptive changes in nociceptive processing, mediated by the peripheral and/or central nervous system sensitization. However, the exact pathogenic basis for NPP initiation and development remains unclear, and the potential clinical manifestations and biomarkers lack a clear classification. Emerging evidence now emphasizes the role of complex neuron–non-neuronal cell interactions, highlighting non-neuronal cells (e.g., circulating immune cells and glial cells) in NPP pathogenesis. In this review, we delineate the key conceptual distinctions of NPP within the spectrum of chronic pain and present the latest evidence outlining how non-neuronal cells mediate NPP progression via peripheral and central sensitization, as well as the associated synaptic plasticity mechanisms. We also summarize current and experimental therapeutic approaches explored in preclinical and clinical studies of NPP. We hope that this review provides a theoretical foundation for subsequent analgesic target screening and the drug development for NPP.
1 Introduction
According to the International Association for the Study of Pain (IASP), chronic pain (CP) is an unpleasant sensory and emotional experience associated with actual or potential tissue damage. It imposes a substantial economic burden and poses significant threats to both the mental and physical well-being of individuals (1). In clinical practice, researchers have previously identified a new type of pain mechanism that is independent of persistent inflammation (nociceptive pain, NCP) and/or) tissue damage and nerve injury (neuropathic pain, NP). Thus, in order to further clarify the boundaries between these pain types, the international community of pain researchers has proposed a novel mechanism—NPP (2). This independent mechanism is described as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain”. This concept appears to successfully include the patients who exhibit neither obvious activation of nociceptors, nor neuropathy (defined as disease or damage of the somatosensory system), yet whose clinical and psychophysical findings suggest altered nociceptive function (3).
When applying this classification in clinical practice, it remains considerably challenging. Clinically, it is not uncommon to encounter patients with NPP who also have overlapping features of NP and/or) NCP. A large number of patients, who do not fully meet the criteria for NP and NCP have often been broadly categorized as having NPP by the researchers. In 2021, to further clarify NPP, the IASP defined clearer diagnostic boundaries and criteria for NPP-related chronic pain, including: “I. pain duration ≥3 months; II. a regional rather than discrete distribution; III. the pain that cannot be entirely be explained by nociceptive or neuropathic mechanisms; and IV. clinical signs of pain hypersensitivity are present in the region of pain. A history of pain hypersensitivity in the affected region of pain and defined comorbidities strengthen the probability of nociplastic pain, and both must be present to designate probable nociplastic pain” (4).
With an estimated prevalence ranging from 5% to 15% of the general population, NPP mainly manifests as distinct neurological symptoms in local or multiple regions of the body (5), such as burning sensation, soreness, and heaviness, which may be spontaneous or induced by factors such as climate and emotions (6). Previous studies have commonly applied the biopsychosocial model to explain the pathogenic factors of NPP (7), including psychological, emotional, sexual, and physical abuse, or their combination. Family history of pain experience also appears to be associated with NPP (8). However, the clear etiology and pathogenesis of NPP still have not yet been systematically clarified yet.
Sensitization, particularly central sensitization, may serve as a common mechanistic link between the pathogenesis of NPP and that of NP and/or) NCP. This may explain the coexistence of multiple pain mechanisms within an individual’s pain state. NPP often overlaps with the neuropathic pain due to shared underlying pathological mechanisms, especially central sensitization (9). Changes in central synaptic plasticity, which are involved in regulating the development and maintenance of chronic pain, are considered a primary cause of central sensitization in NPP (10). Accordingly, several conditions related to central sensitization, such as chronic widespread pain, fibromyalgia (FMS), irritable bowel syndrome (IBS), chronic primary low back pain (CPLBP), complex regional pain syndrome (CRPS), chronic primary headaches and orofacial pain, chronic visceral pain syndromes, and chronic primary musculoskeletal pain, etc., are generally classified under NPP (Figure 1). The mechanisms of central sensitization are rely on the pathological changes, particularly alterations in synaptic plasticity changes, within the central nervous system. However, it remains unclear how synaptic plasticity mediates central sensitization and subsequently induces NPP, as well as which pathological factors drive changes in synaptic plasticity.

Figure 1. Classification and pathogenesis of nociplastic pain. Chronic widespread pain, CRPS, chronic primary headaches and orofacial pain, chronic visceral pain syndromes, and chronic primary musculoskeletal pain are six kinds of typical forms of NPP in clinical practice. Non-neuronal cells such as astrocytes, microglia, satellite glial cells, T cells, B cells, mast cells, neutrophils, and monocytes/macrophages contribute to NPP development.
Non-neuronal cells, including circulating and central immune cells, have been implicated in the modulation of chronic pain through the regulation of central sensitization (11). However, the role of non-neuronal cells in the development and maintenance of NPP remains uncertain. Previous studies have shown that non-neuronal cells can regulate central sensitization by modulating synaptic plasticity (12, 13). Similarly, whether non-neuronal cells can also influence NPP through the regulation of synaptic plasticity and central sensitization requires further clarification.
In this review, we aim to examine the relationship between NPP and non-neuronal cells, with a particular emphasis on central glial cells and circulating immune cells, and to elucidate the underlying mechanisms through which non-neuronal cells regulate NPP.
2 Materials and methods
In this review, two independent investigators were involved in the literature search (Table 1) and screening process based on predefined inclusion and exclusion criteria. The search covered four databases—PubMed, EMBASE, Web of Science, and the Cochrane Library—from their inception to the present. To ensure retrieval of comprehensive literature retrieval, we employed both central concepts and their established subordinate terms. The keyword “nociplastic pain” was represented by specific conditions, including fibromyalgia (FMS), irritable bowel syndrome (IBS), complex regional pain syndrome (CRPS), and chronic primary low back pain (CPLBP). Similarly, the concept of “non-neuronal cells” was expanded to include immune cells and glial cells. Furthermore, according to Medical Subject Headings (MeSH), the terms “nociplastic pain,” “central sensitization,” and “synaptic plasticity” were employed in the literature search. These terms were combined using Boolean operators and adapted to the specific query syntax of each database. Inclusion criteria: 1. Publications limited to the English language. 2. All types of studies were considered, including clinical research, preclinical studies, reviews, systematic reviews, and meta-analyses. Exclusion criteria: Literature unrelated to nociplastic pain. Literature unrelated to non-neuronal cells. Literature unrelated to neural or synaptic plasticity. Publications or books that could not be accessed due to being outdated or unavailable.
3 Potential pathogenic basis related to NPP initiation and development
3.1 Pre-existing virus infections
Viral infections usually induce NP or NCP through direct damage to neural tissues or inflammation. However, a subset of patients who have undergone antiviral or anti-inflammatory therapy, and in whom clear evidence of neural tissue damage is not apparent, continues to experience pain. These cases are now defined as NPP. Accordingly, the early inflammatory stage of rheumatoid arthritis can be classified as NCP. However, after receiving 3 months of treatment, when the inflammation has declined while the recurrent pain persists, the condition may be better explained by NPP (14). Similarly, chronic inflammatory pain caused by pathogen infection can be regarded as NCP, whereas chronic pain that persists even after the inflammation subsides should be classified as NPP. Although numerous studies have shown that some viral infections can lead to peripheral neuropathy, there are some patients who continue to experience pain even after receiving standard first-line anti-inflammatory and analgesic therapies in clinical practice (15, 16). For example, in the synovial fluid of 38 patients with persistent joint pain following chikungunya virus infection, neither the virus itself nor the expression of its corresponding proteins, mRNA, or pro-inflammatory cytokines was detectable, suggesting that joint pain can occur independently of active viral infection or local inflammation (17). In 2021, Maria M ZanoneMaria M. Zanone et al. reported that, among the 94 hepatitis C virus (HCV)-infected patients who had received direct-acting antiviral treatment, 37 patients developed chronic pain. Although the authors referred to this pain as neuropathic pain, this classification may not be appropriate. In the absence of reported nerve damage or lesions, these cases might be more accurately defined as NPP-related chronic pain (18).
Pre-existing infections may represent one of the potential pathogenic factors involved in NPP initiation and development. For example, A. Baroni et al. showed that nearly one-third of post-COVID-19 patients experienced late-onset pain, and this reduction in pain threshold reduction was interpreted as central sensitization (19). In 2024, Man Ting Au et al. retrospectively reviewed two patients who had been affected by COVID-19; both suffered from knee joint pain after recovery. Moreover, a decreased paw withdrawal threshold decrease was observed in mice injected with viral spike receptor-binding domain (RBD), and this hypersensitivity, as well as the chronic pain–related secretion of substance P (SP), could be successfully reversed by the endothelin receptor blocker macitentan (20). Studies have also reported that FMS can be induced or triggered following different types of viral pre-infection (21, 22). These findings suggest that the pre-existing infection may act as an inducing factor, with pathological changes in the nervous system being more likely responsible for sustained pain.
Although NPP may not be a direct indicator of the outcome of viral pre-infection, we still believe that a potential connection appears to exist. We speculate that the unrevealed pathological changes in the nervous system following pre-infection may represent one of the potential mechanisms underlying mechanisms contributing to NPP. For example, microorganisms have been shown to directly interact with components of the blood–brain barrier (BBB), leading to coagulopathy and tight junction disruption, inducing lung inflammation that results in hypoxia, and ultimately increasing BBB permeability (23). It has also been reported that SARS-CoV-2 RNA can be detected in cerebrospinal fluid alongside abnormal brain magnetic resonance imaging findings in COVID-19 patients (24). These pathological mechanisms provide a basis for viral invasion of the central nervous system, which may represent a latent risk factor for NPP initiation of NPP.
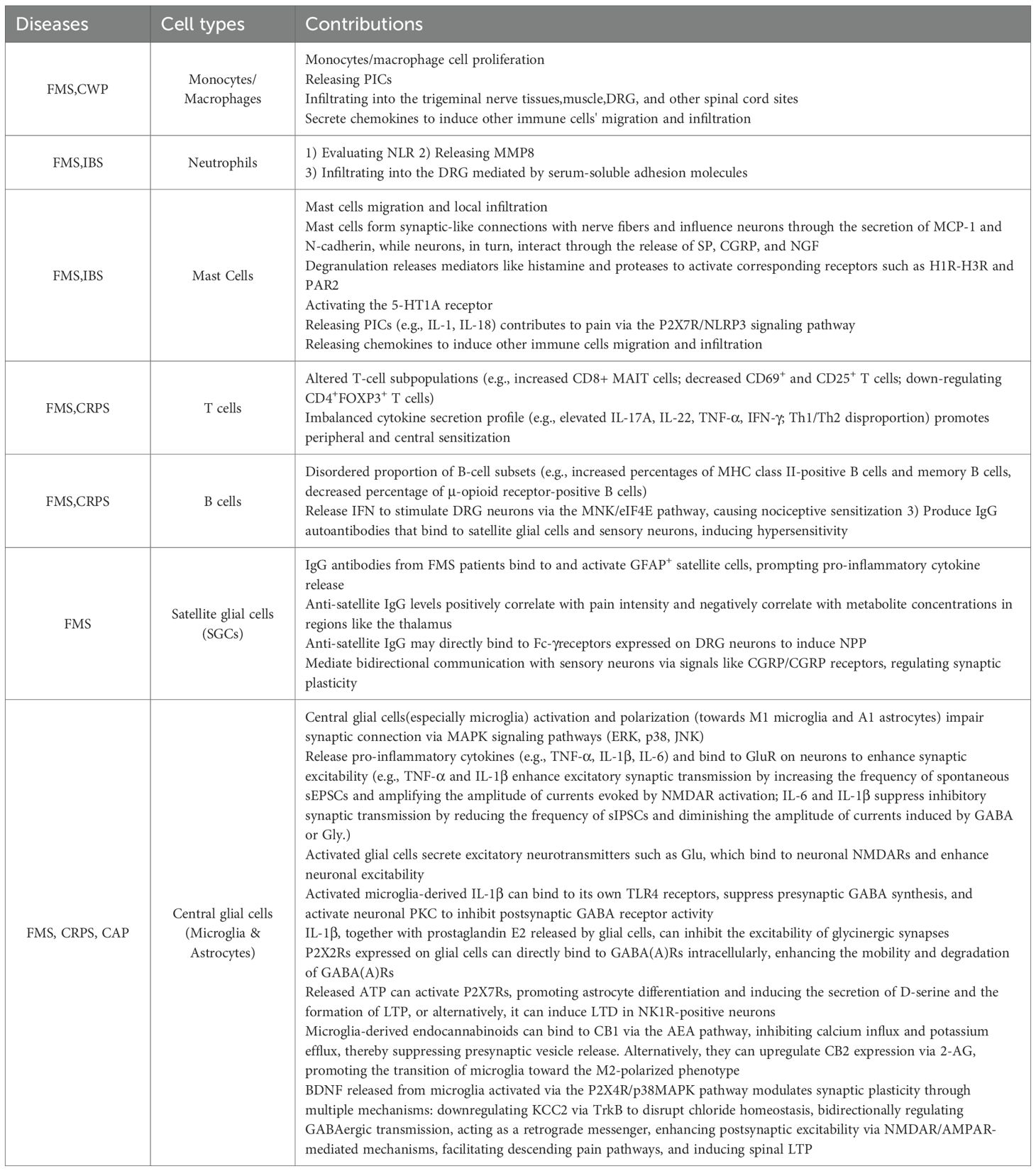
In addition, the central invasion and infiltration of immune cells following viral pre-infection may serve as a trigger for NPP. Studies have shown that human immunodeficiency virus 1 (HIV-1)-infected leukocytes not only secrete matrix metalloproteinases (MMP)-2 and MMP-9 that impair the BBB, but also promote immune cell infiltration into the central nervous system via chemokine C-C motif ligand 2 (CCL2) (25). Bente Winkler et al. found that macrophages, derived from the peripheral blood, invaded across the BBB mediated via BBB-forming glial cells and promoted neuronal injury following brain inflammation (26). They further reported that central inflammation attracted more immune cells to cross the BBB than viral brain invasion alone. After infection with the porcine hemagglutinating encephalomyelitis virus, Jing Zhang et al. observed that circulating monocytes/macrophages crossed the BBB into the central nervous system in model mice, recruited by activated microglia (27). In addition, in the collagen-induced arthritis (CIA) mouse models, CD4+ T cells were shown to cross the BBB and induce central nervous system inflammation through activating microglial activation, indicating that immune cell invasion may participate in NPP development following pre-infection (Figure 2). However, whether the changes in circulating immune cells or even other non-neuronal cells exert subsequent long-term effects on the nervous system, after viral pre-infection remains unclear (28).

Figure 2. Pathogens pre-infection as a vital prerequisite for NPP development. In the early stage, the pathogenic microorganisms can successfully invade into the human body through the respiratory tract or digestive tract, respectively. In addition to triggering immune responses mediated by immune cells, these pathogens can disrupt the blood–brain barrier and enter into the CNS, thereby activating and polarizing central glial cells. Pathogen invasion increases blood–brain barrier permeability and induces central inflammation, ultimately leading to nerve damage and changes in synaptic excitability. Upon repeated stimulation, long-term chronic inflammatory signaling induces central sensitization, promoting the occurrence of NPP.
3.2 Genetic predisposition
Studies indicate that the familial association rate for FMS is approximately between 12%–21% (roughly 1 in 5 to 1 in 9) (29). Concurrently, several genetic loci have been implicated in FMS susceptibility, including the catechol-O-methyltransferase (COMT) gene, the serotonin transporter-linked polymorphic region (5-HTTLPR) of the SLC6A4 gene, as well as DRD2 and DRD4, which encode dopamine receptors (30). In a separate genome-wide analysis, Keira J. A. Johnston et al. investigated six chronic overlapping pain conditions (COPCs)—CPLBP, endometriosis, temporomandibular joint disorder, IBS, and broad headache—and identified 24 independent single-nucleotide polymorphisms (SNPs) significantly associated with nociceptive pain, implicating 127 unique genes. These findings strongly suggest a genetic contribution to NPP, with a liability-scale SNP-based heritability (h²) of 0.025 (SE = 0.0014) (31). In addition, the variation in the transient receptor potential vanilloid 1 (TRPV1) gene has also been shown to be associated with NPP (32).
3.3 Alcohol abuse and gut microbiota dysbiosis
Long-term alcohol use disorder induces plastic adaptations that subsequently contribute to the development of negative affective states, such as anxiety and depression, as well as the manifestation of hyperalgesia (33, 34). An imbalance in gut microbiota, characterized by a reduced ratio of beneficial to harmful bacteria, represents a significant exposure factor in the development of NPP. A recent meta-analysis encompassing 23 studies with 1,340 subjects revealed that patients with IBS exhibited significantly lower fecal levels of Lactobacillus and Bifidobacterium, alongside significantly higher levels of Escherichia coli and marginally elevated Enterobacter. Supporting a causal role, fecal microbiota transplantation from FMS model mice into healthy recipients induced hyperalgesia in the recipient animals (35). Conversely, transplantation of gut microbiota from healthy female donors to female FMS patients alleviated their hyperalgesia (36).
3.4 Specific alternation of rhythm in temperature
Rapid and frequent alternation of specific temperature rhythms is an important factor contributing to reduced sensitivity of the sympathetic nerves (37). In rodent models, a temperature of 4°C is sufficient to induce long-term abnormal pain, whereas at 6°C, corresponding long-term pain cannot be successfully induced (38). Under repeated subthreshold cold stimulation administered at 30-min intervals, organisms lacking local glutamatergic/sympathetic neural warning systems exhibit disrupted glutamate (Glu) secretion, alongside sterile frostbite-like injury of peripheral nociceptors (39). At present, several established animal models exist for FMS. Among them, temperature rhythm alternation models mainly include the intermittent cold stress (ICS) model and the thermal grill illusion (TGI) model (40). From a neurobiological perspective of neurobiology, the ICS animal model can largely replicates the clinical characteristics and complications of FMS, suggesting that repeated and intermittent exposure to excessively high or low temperatures may represent a pathogenic factor in NPP.
3.5 Gender duality
Gender duality is also observed in NPP-related chronic pain. According to a review published in The Lancet in 2021, NPP is present in four conditions: CPLBP, FMS, chronic temporomandibular disorders (TMD), and IBS. The female-to-male incidence ratio is approximately 2:1, while the incidence of CPLBP of unknown causes varies depending on the study region. For chronic primary bladder pain syndrome, the ratio is approximately 2–10:1, and for chronic primary pelvic pain syndrome, the ratio in men and women is reported as 0:1 and 1:0, respectively (3). In rodent studies, the decrease in mechanical pain threshold after ovary removal was similar to that observed in intact female mice, suggesting an important role of estrogen in the ICS-induced NPP model (39). Interestingly, research conducted by Włodzimierz Samborski et al. demonstrated that, compared with healthy controls, there were no significant differences in estradiol and progesterone levels in patients with FMS. This suggests that either estrone, estradiol, or other types of estrogen subtypes may mediate the onset of NPP (41).
4 Clinical manifestations and potential biomarkers
4.1 Blood glucose
José V. Pardo et al. reported that, under intermittent cold stimulation (ICS) conditions, compared with 11 healthy female controls, the blood glucose levels in 13 female patients with fibromyalgia (FMS) were decreased, suggesting that changes in blood glucose may serve as a potential predictive indicator for the clinical detection of NPP. However, there is a minor drawback: the researchers observed fluctuations in blood glucose levels during intermittent cold and heat stimulation but did not find a significant increase in pain sensitivity in FMS patients after either stimulation. This may be due to the use of pain-relieving medications by the patients, which could reduce their sensitivity to pain induction (42). Approximately 30% of female FMS patients exhibit a reduced adrenocorticotropic hormone (ACTH) and epinephrine response to hypoglycemia. This phenomenon is associated with dysfunction of the hypothalamic–pituitary–adrenal (HPA) axis in FMS patients. When the degree and duration of hypoglycemia were precisely controlled using the low-glycemic–high-insulin clamp technique, the ACTH response in FMS patients after a single insulin injection was reduced by 30% compared with healthy subjects (43). Moreover, low levels of epinephrine and adrenocorticotropic hormone further impair the ability to increase blood glucose through a positive feedback loop (44). This phenomenon may be related to sympathetic inactivation caused by frequent internal or external stimulation, which reduces adrenaline secretion in the body, thereby inhibiting the secretion of neuropeptide Y (a marker of chronic sympathoadrenal activity) and suppressing the adrenaline-mediated increases in blood glucose (45). This clinical manifestation of hypoglycemia may serve as an important indicator for assessing whether the HPA axis imbalance in patients with NPP.
4.2 Insulin-like growth factor levels
A large-scale meta-analysis including 512 patients with FMS and 308 healthy subjects, showed that the level of IGF-1 levels in patients with FMS were significantly reduced (46). Pulsatile growth hormone (GH), secreted by the anterior pituitary gland, stimulates the hepatic synthesis and secretion of IGF-1; therefore, reduced IGF-1 secretion may be attributed to the imbalance of the hypothalamus–growth hormone–IGF-1 axis (43). Moreover, studies have shown that aging and obesity are positively correlated with low IGF-1 expression (47). Thus, reduced IGF-1 secretion may be associated with NPP.
4.3 ACTH and substance P
After exogenous insulin injection, an exaggerated ACTH response of the ACTH in the patients was successfully induced in patients (48). In CPLBP, its ACTH levels were also significantly increased compared with those in control groups (49). HPA axis impairment and hyperresponsiveness of ACTH release may therefore represent significant features of NPP. In a clinical trial involving 30 female patients with FMS, increased secretion level of SP in the cerebrospinal fluid (CSF) was observed in the cerebrospinal fluid (CSF) of all the FMS patients (50). I J RussellRussell et al. further reported that, compared with 30 healthy controls, a threefold higher concentration of SP was detected in the CSF of all 32 FMS patients (51). Moreover, in the CSF of FMS patients, increased SP expression was positively correlated with smoking, while the SP expression was independent of both calcitonin gene-related peptide (CGRP) and beta-endorphin levels, suggesting that SP may represent another potential biomarker (52, 53).
5 Circulating immune cells may induce NPP development
5.1 Monocytes/macrophages
Macrophages, derived from monocytes migrating out of blood vessels, play a crucial role in innate immunity (54). Recent studies have reported macrophage infiltration in the trigeminal nerve tissues in FMS model mice, suggesting that monocytes/macrophages are closely related to NP (55). However, how monocytes/macrophages contribute to NP remains unclear. It has not been clarified yet. It was shown that treatment with dried powdered ginger rhizome improved both allodynia and hyperalgesia by decreasing the inflammatory responses mediated by nitric oxide (NO), prostaglandin E2 (PGE2), thromboxane B2 (TXB2), and interleukin-1β (IL-1β) in lipopolysaccharide (LPS)-stimulated macrophages in FMS model mice, indicating that macrophages may contribute to NPP through the release of pro-inflammatory mediators (56). However, A. Kashipaz et al. argued that cytokine dysregulation of cytokines by CD14-positive circulating monocytes may not be a predominant factor in FMS pathogenesis of FMS (57). Mario D. Cordero et al. reported that coenzyme Q10 levels and mitochondrial membrane potential in monocytes were decreased, alongside increased mitochondrial superoxide levels, suggesting that NPP may be closely related to mitochondrial autophagy in monocytes (58).
During the progression of CRPS to full-blown FMS, CD68-positive monocytes/macrophages play a key role in local infiltration of muscle, dorsal root ganglia (DRG), and other spinal cord sites, increasing macrophage numbers to drive neuronal plasticity and enhance NPP (59). Juan J García et al. found that the macrophages released higher serum concentrations of inflammatory chemokines, such as macrophage-derived chemokine (MDC)/CCL22, promoting NPP by regulating immune cell migration and local infiltration (60).
5.2 Neutrophils
Neutrophils also play an irreplaceable role in NPP development. Regarded as a common inflammatory cells, migration and recruitment of neutrophils to the injured sites result in the release of pro-inflammatory mediators, and the phagocytosis of both pathogens and tissue debris (61). Undoubtedly, the pro-inflammatory and pain-inducing effects of neutrophils are well established; however, there are still few direct reports explaining how neutrophils induce NPP through these pro-inflammatory functions. Sara Caxaria et al. showed that adoptive transfer of neutrophils from FMS patients conferred mechanical pain to recipient naïve mice, indicating a pathogenic role of neutrophils in NPP. Neutrophils were also shown to infiltrate the dorsal root ganglia (DRG), suggesting that neutrophil infiltration into the DRG may be a critical contributor to NPP (62). In addition, adhesion and recruitment of neutrophils were enhanced in FMS patients, promoting infiltration into inflammatory sites in FMS patients.
Regarding neutrophil proportions, it has been reported that the neutrophil-to-lymphocyte ratio (NLR) is increased in patients with FMS. That is, within the inflammatory microenvironment of FMS, neutrophil numbers are elevated (63). Patients with FMS and high NLR values also exhibited higher scores on the revised FMS questionnaire impact scale. Furthermore, in IBS patients, the NLR levels were similarly elevated in patients with IBS compared with control groups. Together, these findings indicate that high NLR may be positively associated with NPP (64, 65). However, given the variability of NLR levels among FMS patients with FMS, some studies do not support the use of NLR as a reliable neutrophil-related indicator (66, 67). Subsequent investigations demonstrated that elevated NLR in FMS patients correlates with variations in hypersensitive C-reactive protein levels, suggesting that NLR instability may reflect fluctuations in disease activity (68). Hilal Telli et al. further reported that, in female gender, patients with FMS, high NLR levels were accompanied by higher Visual Analog Scale (VAS) rest scores, indicating a positive association between elevated NLR and NPP (64).
5.3 Mast cells
NPP is widely reported to be correlated with mast cell activation. Local infiltration and migration of mast cells, leading to sensitization of nearby nociceptive afferents, are considered vital contributors to NPP pathogenesis (69).
In FMS patients with FMS, skin biopsy analyses have revealed increased numbers of mast cells (70). In the intestinal tissues of patients with IBS, mast cells infiltrate into the posterior ganglionic fibers of the sympathetic nerves, peptidergic nerve fibers, vagal nerve fibers, vagus nerve fibers and the periphery of intestinal neurons, providing an anatomical basis for neuroimmune interactions (71). Notably, approximately 70%–84% of patients with IBS exhibit local mast cell infiltration of mast cells in their intestinal tissues. Moreover, the phenomenon of mast cell infiltration surrounding intestinal nerves occurs approximately three times more frequently in IBS patients than in controls, and the number of degranulated mast cells is increased by approximately 1.5-fold (72). Through synapse-like structures, mast cells can directly contact non-cholinergic and non-adrenergic nerves and mediate neuroimmune signaling via adhesion molecules such as monocyte chemotactic protein-1 (MCP1) and N-cadherin (71, 73). In addition, chemokine secretion facilitates local infiltration of mast cell infiltration. Zhao et al. reported increased serum levels of MCP1/CCL2 in patients with FMS patients (74). CCL2 can bind to its receptor CCR2 on peripheral nerve terminals, thereby inducing NPP (75).
Beyond infiltration and migration, mast cell degranulation also contributes to NPP development. Evelyne da Silva Brum et al. observed mast cell infiltration in FMS model mice, accompanied by mechanical and cold allodynia as well as muscle fatigue. Following administration of compound 48/80 or the mast cell membrane stabilizer ketotifen to deplete mast cell activity, mechanical and cold allodynia were successfully reversed, supporting a pathogenic role of mast cell degranulation in NPP (76). In water avoidance stress model rats, treatment with cromolyn sodium or the neutralizing agent of nerve growth factor–neutralizing agents significantly inhibited synaptic plasticity in the mesenteric region and alleviated stress-induced abdominal pain induced by stress, suggesting that mast cell degranulation effect of mast cells, together with nerve growth factor (NGF), induces synaptic plasticity changes leading to abdominal pain (77). Mast cells release histamine and proteases during degranulation. Kayo Masuko et al. demonstrated that through the degranulation effect of mast cells, the levels of mast cell–derived serine protease tryptase levels were also increased following mast cell degranulation, which subsequently activated proteinase-activated receptor-2 (PAR-2) signaling pathways to modulate hypersensitivity (78). Histamine H1 and histamine H2 can respectively act on their respective receptors on the visceral afferent nerves and intestinal neurons, whereas histamine H3 receptors mediate the inhibition of rapid synaptic transmission in intestinal neurons. PAR-2 expressed on the intestinal nerves and afferent nerves induces neuronal excitation of intestinal neurons (79–81). Furthermore, the activation of the 5-hydroxytryptamine receptor 1A (5-HT1A), rather than the histamine receptors, has also been implicated in the complex abdominal pain mediated by mast cell degranulation (82).
Ramona D’Amico et al. demonstrated that the P2X7 receptor (P2X7R)/NLRP3 signaling pathway is involved in mast cell degranulation. Use of the P2X7R antagonist Brilliant Blue G (BBG) prevented NLRP3 inflammasome activation and subsequent release of interleukin (IL)-1 and IL-18, thereby reducing FMS symptoms (83). Pio Conti reported that mast cell degranulation effects, mast cell releases inflammatory mediators that induce low-grade inflammation and trigger the onset of FMS (70). Parallelly, similar mechanism was found in IBS (84). Ivashkin et al. reported that in the serum of IBS patients, the expression of pro-inflammatory cytokines like IL-1, IL-2, and TNF-α were increased significantly (85), which could achieve to NPP through impairing intestinal barrier, inhibiting the secretion of neurotransmitters and maintaining chronic low-grade inflammation (86). Therefore, receptors such as PAR-2, 5-HT2A, 5-HT3, H1, NK1, P2X7R, and MrgprB2 may represent promising therapeutic targets for regulating abnormal mast cell activity in NPP.
5.4 T lymphoid cells
As a typical representative population of adaptive immune cells, T cells play an important role in NPP induction, and numerous studies have reported associations between T cells and NPP. T cells, as well as interleukins released by lymphocytes, can induce NPP formation of NPP (87). Hiroshi Ueda et al. observed allodynia in recipient mice following adoptive transfer of purified CD4+ T cells, derived from splenocytes of acid-treated male mice, into naïve mice, indicating that CD4+ T cells participate in FMS pathogenesis (88). Chie Sugimoto et al. reported that the proportion of CD8+ mucosal-associated invariant T (MAIT) cells was increased in FMS patients with FMS and suggested this population as a potential biomarker (89). W. Hernanz et al. found that the numbers of CD69-positive (activation-induced marker) and CD25-positive (interleukin-2 receptor) positive T cells were decreased in FMS patients with FMS (90). However, Giuliana Guggino et al. did not identify significant differences in the proportion of T-cell subtype proportions between patients compared with FMS and healthy individuals. Interestingly, they observed upregulation of several cytokines secreted by CD4+ T cells, including IL-17A, IL-22, IL-9, TNF-α, and IFN-γ (91). Jessica L. Ross et al. demonstrated that pro-inflammatory cytokines, such as IL-1β released from T lymphocytes, could induce peripheral sensitization in an acid-sensing ion channel (ASIC)-dependent manner in somatosensory neurons (92, 93). In the cerebrospinal fluid (CSF) of FMS patients with FMS, Eva Kosek et al. reported elevated levels of IL-8, IL-1Ra, IL-4, and IL-10 were elevated (94). In contrast, D. J. Wallace et al. did not observe increased expression of cytokines such as IL-1β, sIL-2R, IFN-γ, TNF-α, IL-10, or IL-2 in FMS patients with FMS, but only detected increased IL-1Ra and IL-8 levels (95). In FMS model mice, the proportion of regulatory T helper cells (CD4+FOXP3+) was reduced, accompanied by increased IL-5 secretion from Th2 cells. Inhibition of IL-5 could successfully reversed mechanical hypersensitivity, suggesting that FMS is closely associated with T-cell dysregulation (96). In patients with FMS, imbalance of the Th1/Th2 cell ratio and excessive secretion of IL-8 and heat shock proteins are the prominent features. Studies have shown that physical exercise training can reduce the proportion of Th1 cells, rebalance the Th1/Th2 ratio, and inhibit the expression of IL-8 and heat shock protein expression, thereby alleviating pain and cognitive impairment in patients with FMS (97). N. Hader et al. identified a defect in CD4+ T cells, with reduced IL-2 secretion from CD4+ T lymphocytes was decreased (98). In patients with CRPS, Ines Kaufmann et al. reported that the ratio of CD8+ lymphocytes and the percentage of IL-2–producing T-cell subpopulations were lower than those in healthy donors, indicating a Th1/Th2 imbalance in NPP (99).
5.5 B cells
As an essential component of the immune system, B lymphocytes contribute to adaptive immunity by migrating to the injured sites and differentiating into plasma cells that release autoantibodies. In addition, B cells, as potent antigen-presenting cells, play an immunomodulatory role by releasing cytokines, activating auto-reactive T cells (100). Numerous studies have reported increased proportions of B cells elevated in NPP. Melissa E. Lenert et al. found increased numbers of MHC class II–positive B cells and activated plasma cells increased in peripheral blood, as well as elevated proportions of memory B cells was elevated in the bone marrow of female chronic widespread pain (CWP) model mice (101). Valentina Malafoglia et al. demonstrated that the proportion of μ-opioid receptor–positive B cells was lower than that in controls in blood samples from patients with FMS, suggesting that B cells μ-opioid receptor deficiency in B cells may impair endogenous opioid analgesic activity and contribute to sensitization, thereby supporting a pathological mechanism support for the aggravation of FMS and may prove the role for B-cell subsets in the pathogenesis of NPP (102).
Serena Fineschi et al. conducted transcriptomic analysis of B cells from 30 FMS patients with FMS and 24 healthy individuals and identified 60 potential differentially expressed genes. Among these, four genes with the most significant differences and interferon (IFN) responses—S100A8, VCAM, CD163, and ANXA1—were highlighted, indicating that B cells and their IFN secretion—IFN, are associated with FMS (103). Paulino Barragán-Iglesias et al. further showed that B cells can release type I IFN, which stimulates MNK-mediated eIF4E phosphorylation in dorsal root ganglion (DRG) neurons, leading to generate nociceptive sensitization and generalized pain (104). In addition, B cells secrete immunoglobulin G (IgG) as part of adaptive immunity. Through the B cell/IgG/Fc axis, IgG complexes can directly bind to the peripheral somatosensory neurons directly to induce neuropathic pain; however, whether this axis also induces NPP remains unclear (105). Eneström S. et al. identified dermal IgG deposits in the skin of FMS patients with FMS (106). Andreas Goebel et al. transferred IgG, donated from FMS patients with FMS into naïve mice and demonstrated that IgG interacted with satellite glial cells in the DRG to induce hypersensitivity to noxious mechanical and cold stimulation (107). Similarly, transfer of purified IgG isolated from CRPS patients with CRPS into control mice prolonged postsurgical hypersensitivity to noxious mechanical, cold, and heat stimulation (108).
6 Peripheral glial cells (satellite glial cells) may contribute to NPP development
Satellite glial cells (SGCs), the main cell type mediating myelin generation in the peripheral nervous system, surround sensory ganglion neurons in a circular arrangement at a distance of approximately 20 nm. Neurons and their synapses are wrapped within a single sheath via gap junctions, forming a discrete anatomical and functional sensory unit. This structure provides the anatomical basis for the realization of bidirectional communication and functional interaction between neurons and SGCs, thereby enabling SGC-mediated regulation of synaptic plasticity (109).
Studies have shown that purified IgG extracted from FMS patients with FMS specifically binds to rat dorsal root ganglia (DRG), with nine distinct binding clusters identified, suggesting that autoimmune antibodies may participate in FMS mediated by SCGs (110). Andreas Goebel et al. transferred IgG from patients with FMS into naïve mice, inducing increased sensitivity to noxious mechanical and cold stimulation. This hypersensitivity was accompanied by IgG accumulation of IgG in glial fibrillary acidic protein–positive (GFAP+) satellite glial cells in the DRG, indicating that autoimmune antibodies may bind to GFAP+ SGCs to induce NPP (107). Silvia Fanton et al. further reported confirmed a positive correlation between anti-satellite IgG levels and pain intensity in FMS, as well as a negative correlation between anti-satellite IgG levels and concentrations of metabolites such as thalamic scyllo-inositol, total choline, and macromolecule 12 (MM12) (111). Emerson Krock et al. confirmed these findings in human samples and further demonstrated a positive correlation between anti-satellite IgG levels and pain intensity in FMS; however, the mechanisms by which SGCs induce NPP remain unclear (112). Menachem Hanani proposed that IgG may induce NPP by binding to and activating SGCs, thereby promoting the release of pro-inflammatory cytokines (113). Jenny E. Jakobsson et al. reported that FMS symptom severity was positively associated with lysophosphatidylcholines (LPCs), suggesting that FMS may be related to altered lipid metabolism (114). IgG complexes binding to Fcγ receptors expressed on DRG neurons can evoke nociceptive activity in the absence of overt inflammation (105, 115). Moreover, there are several shared biomarkers, including calcitonin gene-related peptide (CGRP) receptors, are expressed on both SGCs and DRG neurons. Thus, SGC–sensory neuron interactions mediated by CGRP/CGRP receptor signaling may represent a potential mechanism underlying NPP development (116).
7 Central glial cells contribute to NPP development
Concomitant activation of microglia and astrocytes observed in a reserpine-induced FMS rat model underscores the pivotal role of central glial cell activation in NPP (117). Intracerebral administration of minocycline to deplete activated microglia attenuated generalized pain in a repeated acid saline–induced fibromyalgia-like model (88). Similarly, inhibition of astrocytic activation in the spinal cord and prefrontal cortex using Boswellia serrata attenuated pain hypersensitivity was attenuated in FMS model rats (118). Collectively, these studies indicate that activation of central glial cells activation plays a critical role in NPP development. In addition to activation, the polarization of central glial cells has also been reported to contribute to NPP pathogenesis of NPP (119). Multiple studies have demonstrated that central glial cell polarization is involved in NPP. In a reserpine-induced FMS model, microglia rapidly shifted toward the M1 phenotype, resulting in an increased M1/M2 ratio. This shift was further evidenced by a nearly fourfold upregulation of the M1-associated marker inducible nitric oxide synthase (iNOS) and fivefold downregulation of the M2-associated marker arginase-1 (Arg-1) compared with controls (120, 121). Consistently, in a CRPS model, the spinal cord showed increased populations of reactive astrocytes and microglia were observed in the spinal cord, along with elevated expression of the M1 marker CD86 and reduced Arg-1 expression, indicating a preferential M1 microglial polarization in NPP (122). However, the precise mechanisms by which central glial cells mediate the formation of NPP-related chronic pain remain unclear.
In 2022, a study investigating cortical morphological remodeling in patients with CPLBP and FMS revealed that NPP may be associated with structural remodeling of the myelinated architecture in the limbic system cortex. This remodeling was accompanied by upregulated expression patterns in microglia, astrocytes, and oligodendrocyte precursor cells, suggesting that plasticity mediated by central glial cells may represent an important mechanism underlying NPP (123).
7.1 Central glial cells modulate synaptic plasticity modulation
Astrocytes, together with microglia, are structurally connected to synapses via gap junctions and form a structural barrier surrounding synapses. This arrangement not only prevents glutamate (Glu) leakage of Glu, but also facilitates the exchange function of ions, neurotransmitters, and other small molecules (124, 125). These cells bear the burden of supporting the basic metabolic requirements of neurons. Highly plastic glial cells not only mediate central immune responses and regulate central infections but also exert multiple functions involved in chronic pain modulation, such as phagocytosis of cellular debris and regulation of neuronal development (126). In parallel, this pathological basis not only provides a route for material and information transfer between neurons and establishes a structural foundation for glial cell–mediated synaptic plasticity changes mediated by glial cells that modulate central sensitization.
Recently, numerous studies have reported that NPP formation appears to be associated with synaptic plasticity changes mediated by central glial cells. A neuroimaging study investigating glial cell activation in patients with FMS revealed widespread cortical thickening compared with healthy controls, a phenomenon particularly prominent in the medial and lateral walls of the frontal and parietal lobes. Concurrent imaging data indicated that microglia play a predominant role in this process, suggesting that cortical plasticity occurs in the brains of FMS patients with FMS and is primarily mediated by microglial activation (127). In rat models of chronic abdominal pain (CAP), microglia-mediated engulfment of postsynaptic spines was increased (128). Application of a low-dose microglial inhibitor, minocycline, can dose-dependently enhanced long-term potentiation (LTP) effect of type C nerve fibers in the spinal dorsal horn induced by high-frequency electrical stimulation, whereas high-dose of minocycline reversed this long-term enhancement effect of type C nerve fibers in the spinal dorsal horn induced by high-frequency electrical stimulation to long-term inhibition (129, 130). These findings indicate that microglia can regulate synaptic plasticity and participate in central sensitization. Alterations in synaptic plasticity may represent a common potential pathological mechanism underlying both neuropathic pain and NPP.
7.2 Signaling pathway driven-activation (polarization) of central glial cells
Mitogen-activated protein kinases (MAPK) signaling pathway: Activation of central glial cells is primarily characterized by morphological changes, upregulation of classical activation markers, expansion of cell populations, and enhanced pro-inflammatory responses. Among the signaling pathways involved, MAPK activation represents a key mechanism through which microglia regulate synaptic plasticity. This pathway comprises three major branches: extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK). Increased phosphorylation of ERK, p38, and JNK has been observed in microglia from FMS model animals. Attenuation of pain hypersensitivity following inhibition of these respective phosphorylation events—achieved through specific inhibitors or acupuncture treatment—suggests that the MAPK signaling pathway is a critical mechanism through which microglia mediate NPP (131, 132).
Specifically, the ERK/MAPK pathway is mainly responsible for mediating microglial proliferation and associated morphological alterations. In general, the specific transformations observed in activated states are characterized by astrocytic hypertrophy with elongated and thickened processes, alongside microglial soma enlargement with shortened or retracted processes that may adopt an amoeboid morphology. These changes are structurally conducive to modifying the glia–neuron contact areas (12). However, the morphological alterations alone may not definitively indicate functional activation. Instead, these adaptations provide an anatomical substrate for subsequent alterations in synaptic plasticity changes. In the spinal cord of a mouse model of FMS induced by repeated cold stress (RCS), neuronal hyperactivation was accompanied by the activation and proliferation of surrounding microglia. Depletion of microglia using PLX3397 (pexidartinib) alleviated RCS-induced hyperalgesia, indicating that glial cell activation and excessive proliferation represent a critical pathway in NPP development of NPP (133). Although not directly demonstrated, we propose a hypothesis that ERK signaling pathway–mediated glial cell proliferation may contribute to NPP pathogenesis of NPP. While microglial proliferation has been observed in M1-mediated CRPS models, increases in phosphorylated ERK1/2 (Tyr204)/ERK1/2 failed to reach statistical significance in some studies (122). We suggest that this discrepancy may result from limited sampling in Western blot analyses, given the established role of phosphorylated ERK phosphorylation in mediating microglial proliferation and morphological remodeling.
Other signaling pathways associated with MAPK: The JAK/STAT signaling pathway has also been implicated in NPP. In CRPS, IL-6 signaling via two primary cascades promotes neuroinflammation and pain through two primary cascades: one involving SRC/JAK–STAT activation leading to PTGS2 upregulation and prostaglandin E2 (PGE2) release, and the other proceeding via a PTN11-dependent RasGTPase/MAPK/CEBPB pathway. In addition, tumor necrosis factor (TNF) binding to TNFRSF1B activates TRAF2/MAPK signaling, enhances TRPV1 and SCN10A expression, and thereby facilitates NPP development in sequence (134). Beyond MAPK-mediated pathways, suppression of the α7 nicotinic acetylcholine receptor (α7-nAChR)–dependent cAMP/PKA and PI3K/AKT signaling pathways has also been implicated in NPP pathogenesis of NPP. In a reserpine-induced FMS model, the suppression of the α7-nAChR/PI3K/AKT/Nrf2/Bcl-2 signaling pathway in spinal microglia led to reduced expression of Nrf2, superoxide dismutase (SOD), glutathione (GSH), and Bcl-2. Additionally, reserpine injection inhibited the expression of PKA, phosphorylated AKT1, and phosphorylated CREB. Specifically, suppression of the α7-nAChR/cAMP/PKA/CREB signaling pathway, reserpine promoted microglial polarization of microglia toward the M1 phenotype and induced neuronal apoptosis, thereby altering neural plasticity (120).Activation of Nrf2 acts as a critical bridge for inhibiting the p38 MAPK pathway, while CREB activation competes with NF-κB for limited coactivators, thereby suppressing the expression of pro-inflammatory gene expression. Consequently, the suppression of both the α7-nAChR/PI3K/AKT/Nrf2/Bcl-2 and α7-nAChR/cAMP/PKA/CREB pathways synergizes with p38 MAPK activation to promote central sensitization.
8 Cellular basis of synaptic plasticity mediated by activated non-neuronal cells
8.1 Pro-inflammatory cytokines
Pro-inflammatory cytokines (PICs), such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor -α (TNF-α), have been reported to be positively associated with NPP. Krisztina Pohóczky et al. performed unbiased transcriptomic analysis of the dorsal root ganglia (DRG) to identify differentially expressed genes in CRPS model mice and found that TNF and Janus kinase (JAK)/STAT) signaling pathways may be involved in CRPS development. Treatment with the soluble TNF-α receptor etanercept or the JAK inhibitor tofacitinib reversed CRPS IgG–induced hyperalgesia and microglial activation were reversed (134). Zsuzsanna Helyes et al. found that blockade of IL-1 successfully reversed profound glial activation and allodynia in naïve mice receiving adoptive transfer of purified IgG isolated from CRPS model mice, indicating that IL-1 also plays a key role in NPP development (135). Masahiro Ohgidani et al. generated human-induced microglia-like (iMG) cells from FMS patients with FMS and exposed them to ATP in vitro, identifying a moderate correlation between NPP and ATP-induced upregulation of TNF-α expression (136). Although previous studies have demonstrated intrinsic associations between PICs and NPP in multiple animal models, further investigation is required to clarify how PICs regulate NPP through synaptic plasticity.
Mechanistically, PICs can directly enhance central sensitization by increasing excitatory synaptic transmission or suppressing inhibitory synaptic transmission. Studies have shown that PICs can directly bind directly to glutamate receptors on neurons, thereby enhancing synaptic excitability, and promoting both central sensitization and NPP development (137). Pretreatment with IL-1β and TNF-α increased the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in lamina II neurons of isolated spinal cord preparations. Meanwhile, IL-1β and IL-6 exposure enhanced both γ-aminobutyric acid (GABA)- and glutamate-induced currents, respectively. Interestingly, IL-6 exposure reduced the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs), while soluble IL-6 initially decreased sEPSCs, followed by increased sEPSCs and cAMP response element-binding protein (CREB) phosphorylation (138).
Beyond direct regulation of neuronal excitability via synaptic plasticity, PICs can also enhance neuronal excitability by activating glial cells and promoting A1 (astrocytic) or M1 (microglial) polarization, leading to further release of pro-inflammatory cytokines. Activated glial cells can secrete the excitatory neurotransmitter glutamate, which binds to N-methyl-D-aspartate receptors (NMDARs) on presynaptic membranes, thereby elevating neuronal excitability (139, 140). Further studies have shown that postsynaptic NMDARs are activated by glutamate released from presynaptic terminals, promoting conversion of arginine to nitric oxide (NO) through a Ca2+-dependent pathway catalyzed by inducible nitric oxide synthase (iNOS). Released NO then acts as a retrograde messenger, amplifying the pain signaling through sustained positive feedback regulation of synaptic plasticity (141). Consistently, elevated plasma NO levels have been detected in patients with FMS and migraine and appear to be positively correlated with NMDAR activation of NMDA receptors (142, 143). Collectively, these mechanisms illustrate how PICs and glial cells polarization regulate central sensitization through modulation of synaptic plasticity.
8.2 Adenosine triphosphate
The P2X receptor (P2XR) family, including P2X1–P2X7, serves as the receptor of ATP receptors. P2XR subtypes are widely and heterogeneously expressed in the central nervous system (144). Cortical astrocytes express mRNA for P2X1–P2X7, while microglia predominantly express both P2X4 and P2X7. This heterogeneous distribution of P2XR subtypes provides a basis for neuroimmune interactions involved in NPP regulation of NPP (145). Activation of P2X7 promotes astrocyte differentiation, and astrocytes can secrete D-serine, a co-agonist at NMDARs, to enhance both homo- and heterosynaptic long-term potentiation (LTP (146)). Interestingly, P2X2Rs were observed to combine with GABAARs in intracellular directly, activating P2X2Rs could enhance the mobility and degradation of GABA(A)Rs, which might be another potential mechanism for synaptic plasticity changes via purinergic signaling (147). In addition, ATP, a ligand of P2XRs, being released from primary afferent neurons can activate glial cells via C-fiber stimulation, while activated glial cells—particularly microglia—can induce LTP (148–150). Conversely, stimulation of Aβ fibers stimulation can release ATP that activates P2X7 receptors expressed on astrocytes. Activated astrocytes subsequently release ATP, generating synaptic long-term depression (LTD) in neurokinin-1 receptor–positive (NK1R+) neurons, leading to antinociception. This divergent effect may reflect astrocytic heterogeneity in terms of morphology and function (151). However, whether distinct astrocyte subtypes regulate NPP through differential synaptic plasticity changes mediated by modulating purinergic signaling pathways remains to be further clarified.
8.3 Endogenous cannabinoids
Cannabinoid receptor 1 (CB1) is predominantly distributed in the nerve cells of both the CNS and PNS and regulates both the sensory and affective components of pain, while cannabinoid receptor 2 (CB2) is expressed mainly on immune cells, including central glial cells and peripheral immune cells (152). Ethan B. Russo regarded the formation of NP, represented by FMS, migraine, and IBS, as a deficiency of endogenous cannabinoids; however, whether immune cells could exert regulatory effects on NP through CB-dependent pathways remains unclear (153).
Microglia are a core source of endocannabinoids, while endogenous cannabinoids can produce endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are produced via N-acyl-phosphatidylethanolamine-phospholipase D (NAPE-PLD)– and diacylglycerol lipase (DAGL)–dependent pathways, respectively. AEA and 2-AG can bind to cannabinoid receptors CB1 and CB2 (154). Activation of CB1 suppresses neuronal activity by reducing Ca2+ entry via Cav2.2 channels and potassium efflux through KCC2 following presynaptic vesicle release (155). Activated microglia upregulate CB2 expression of CB2, which promotes transformation toward an anti-inflammatory phenotype, restricts microglial activity to the ipsilateral dorsal horn, and induces release of anti-inflammatory cytokines such as IL-10, thereby alleviating NPP (156).
8.4 BDNF
Brain-derived neurotrophic factor (BDNF), regarded as a Cl− homeostasis regulator, has been reported to be positively associated with chronic pain. However, whether BDNF was also represents a pathophysiological feature of NPP remains controversial (157). Regarding the relationship between FMS and the level of BDNF or nerve growth factor (NGF) levels, some studies reported no significant differences between FMS and control patients with FMS and controls (158), whereas Amir Hossein Behnoush et al. found that 15 of 20 studies supported increased level of BDNF levels in FMS (159). Notably, these conflicting findings may arise from multiple contributing factors and do not negate the clinical relevance of BDNF. Similar to NGF, BDNF exhibits dynamic changes, and several studies indicate that elevated BDNF levels decrease following successful treatment (e.g., duloxetine), paralleling improvements in pain and depressive symptoms. This suggests that BDNF may reflect disease activity, and its fluctuation may be attributable to differences in population-level disease status (160). Animal studies further support the pathophysiological role of BDNF. Exercise has been shown to alleviate FMS symptoms and cognitive decline by activating specific signaling pathways, including the hippocampal PGC-1α/FNDC5/BDNF axis (161). Recent high-quality meta-analyses, incorporating stricter quality control, tend to support increased peripheral BDNF levels in FMS (159). As a classical neuromodulator, BDNF still remains to be a promising biomarker for aiding diagnosis and evaluating treatment response.
BDNF is widely expressed in neurons, astrocytes, and microglia, among which microglia are regarded as the primary source of BDNF (162). Activated microglia could release BDNF via ATP-gated Ca2+ influx mediated by P2X4 receptors, inducing phosphorylation and activation of p38-MAPK (163). BDNF modulates synaptic plasticity through several mechanisms: 1) BDNF promotes dephosphorylation of KCC2 and downregulates KCC2 expression via the TrkB pathway (164). Through activation of downstream Src homology 2 domain-containing transforming protein/FGF receptor substrate 2 (Shc/FRS-2) and phospholipase Cγ- (PLCγ)/cAMP response element-binding protein signaling pathway, BDNF inhibits intracellular Cl- efflux and regulates ionic plasticity (165). 2) BDNF exerts bidirectional regulation of GABAergic transmission by inhibiting GABA signaling in mature GABAergic neurons while enhancing GABA transmission and facilitating LTD in immature GABAergic neurons via the pro-BDNF/p75 neurotrophin receptor (p75(NTR)) pathway (166, 167). 3) BDNF is not only released by glutamatergic presynaptic terminals and also acts as a retrograde messenger to inhibit the excitation of inhibitory dorsal horn neurons from the postsynaptic membrane (168). 4) In the short term, BDNF regulates synaptic plasticity by enhancing postsynaptic NMDAR activity; in the long term, it increases postsynaptic currents by elevating both mEPSC frequency of mEPSCs and intracellular Ca²+ concentration mediated by AMPARs (169). 5) BDNF enhances the pain signal transmission in the descending control system (170). 6) Microglia-derived BDNF induces spinal LTP of synaptic transmission (129).
Although microglia clearly regulate synaptic plasticity through the BDNF/TrkB pathway, whether microglia could mediate NPP through the additional mechanisms described above remains to be further elucidated.
8.5 Glu
Astrocytes can express glutamine synthetase (GS), which modulates central glutamine (Gln) synthesis and participates in glutamatergic neurotransmission through the Glu–Gln cycle (171). Under stress conditions, norepinephrine levels increase, enhancing synaptic excitability of synapses. Correspondingly, astrocytes will enhance their ability to take up Glu uptake to prevent synaptic overexcitation. This Glu uptake mechanism protects against neurotoxicity caused by excessive synaptic Glu accumulation and postsynaptic overactivation (172). Astrocytes have been implicated in neuropathic pain mediated by the Glu–Gln cycle; however, whether astrocytes can modulate NP through Glu/GluR-mediated synaptic plasticity remains unclear (173).
Studies show that astrocytes can take up Glu through Na+-dependent Glu transporters, Cl--dependent transport mechanisms, and Ca²+-dependent transport systems (174). Excessive Glu uptake disrupts ionic balance, leading to astrocytic depolarization and influx of extracellular water, transforming the morphology of astrocytes toward the polarized A1 phenotype and promoting the central sensitization and NP (175).
Regarding ionotropic glutamate receptors (iGluRs), Na+ influx of Na+ and outflow of K+ efflux increase the extracellular K+ concentration, promote presynaptic depolarization of the presynaptic terminal to increase the secretion of Glu, and Glu release, and enhance postsynaptic depolarization. For metabotropic glutamate receptors (mGluRs), Glu activates inositol trisphosphate (IP3) via PLC-dependent pathways, increasing intracellular Ca2+ levels (176). Ca²+ acts as a second messenger regulating the adenylate cyclase/cAMP system, with distinct mGluR subtypes exhibiting heterogeneity in this regulation of this signaling pathway) (177).
Additionally, studies have shown that activated immune cells (including central glial cells) can release pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α. These cytokines can directly bind to glutamate receptors on neurons, enhancing synaptic excitability, and promoting central sensitization and NP development (137). This potential mechanism helps explain how astrocytes and neurons, particularly via synaptic interactions, contribute to NP formation through central sensitization (Figure 3).

Figure 3. Synaptic–glial interactions promote NPP development. Activated central glial cells, including A1-polarized astrocytes and M1-polarized microglia, can release glutamine (Gln) or modulate the Gln/Glu/VGLUT signaling pathway of presynaptic neurons to promote glutamate (Glu) exocytosis in a voltage-gated Ca2+ channel–dependent manner. In the synaptic cleft, accumulated Glu activates AMPARs and NMDARs on postsynaptic neurons to induce long-term potentiation (LTP) or nitric oxide (NO) release NO, respectively. NO acts as a retrograde messenger to further enhance presynaptic neuronal activation.
8.6 GABA and glycine
γ-Aminobutyric acid (GABA) is a major inhibitory neurotransmitter acting on GABA receptors (GABA-AR, GABA_BR, and GABA_CR) on the presynaptic membrane or postsynaptic membranes to inhibit synaptic signal transmission and neuronal excitation (178). In the CNS, glial cells regulate nutrient delivery, neurotransmitter interactions, and ionic homeostasis. The “GABA–Glu–Gln cycle enables glial regulation of GABA homeostasis: extracellular GABA enters cells via Glu transporters, where it is converted to Glu by γ-aminobutyric acid aminotransferase, and subsequently synthesized into Gln by GS (179). This process prevents the excessive GABA accumulation, avoiding excessive synaptic inhibition and reducing chronic pain.
Microglia-derived IL-1β can self-activate toll-like receptor 4 (TLR4), thereby reducing presynaptic GABA synthesis, and can activate protein kinase C (PKC) in neurons to suppress postsynaptic GABA receptor activity (180). However, most studies have focused on neuropathic pain, and it is still not clear whether immune cells could mediate the release of GABA to regulate NP via GABA release remains unclear.
In line with the roles of GABA and GABARs, neurotransmitter Gly and its receptor GlyR is the other couple of typical signaling pathway that can inhibit synaptic excitability. Study showed that GABAergic neurons, glutamatergic neurons, and mixed GABAergic/glutamatergic neurons form an interconnected inhibitory neural network. Together with GABA-ARs, GlyRs and the synaptic scaffold protein gephyrin jointly constitute inhibitory synapses that suppress neural excitability (181).
Some terminals co-release both Gly and GABA via vesicles with the assistance of vesicular inhibitory amino acid transporter (VIAAT), exerting analgesic effects (182). Inhibition of glycinergic/GABAergic synapses disrupts descending inhibitory control, enhances hyperalgesia, and induces central sensitization (183). Pharmacological blockade of GABA-ARs and GlyRs allows polysynaptic A-fiber input to NK1R-expressing projection neurons expressing NK1R become obvious, indicating that under disinhibition, non-noxious mechanical stimulation can activate the pain pathways and induce hyperalgesia. While this clarifies how glycinergic neurons contribute to NPP, the role of non-neuronal cells remains unclear (184).
Given that immune cells, especially immune cells, can release IL-1β and prostaglandin E2 (PGE2) further studies show that Glyergic synapses exposed to IL-1β exposure induces LTP in glycinergic synapses, while PGE2 selectively inhibits excitatory signals of glycinergic synapses on non-glycinergic neurons, thereby suppressing glycinergic inhibition and promoting chronic pain (185, 186). These findings suggest a potential mechanism by which immune cells mediate NPP formation through glycine-dependent synaptic inhibition (Figure 4).

Figure 4. Synaptic–glial interactions inhibit NPP development through GABA/Gly signaling pathways. Central glial cells (on the right, the purple) could generate GABA and glycine (Gly) via GABA transporters or glycine transporters in a Na+ dependent manner. Presynaptic neurons could release GABA and Gly into the synaptic cleft through glucose/glutamate/GABA or glucose/Gly pathways, respectively. Accumulated GABA or Gly binds to their respective receptors to generate Cl- influx. In addition, Ca²+ binding to NMDARs on postsynaptic neurons induces NMDAR-dependent long-term depression (LTD), thereby inhibiting synaptic excitability and attenuating NPP.
9 Modern NPP therapies targeting non-neuronal cells
As summarized in Tables 2 and 3, although several drugs for managing NPP have received FDA approval or are recommended by clinical guidelines, most of which are repurposed from other therapeutic areas rather than specifically developed for NPP. These include antidepressants such as duloxetine and milnacipran (serotonin–norepinephrine reuptake inhibitors), tricyclic antidepressants such as amitriptyline, central nervous system depressants such as zolpidem, voltage-gated Ca²+ channel inhibitors including pregabalin and gabapentin, muscle relaxants such as cyclobenzaprine orally disintegrating tablets, and opioid analgesics such as tramadol and oxycodone. While these agents may exert analgesic effects—either directly or indirectly—through mechanisms such as inhibition of central sensitization or activation of opioid receptors, they are not specifically designed to target NPP pathophysiology.
Additionally, other medications—including antispasmodics (e.g., dicyclomine and pinaverium), antibiotics such as rifaximin, and guanylate cyclase-C agonists such as linaclotide—are sometimes used to manage NPP-related comorbid symptoms but do not directly alleviate pain (187). Given the heterogeneity of conditions underlying NPP, there is remains a notable scarcity of therapeutics that directly target non-neuronal cells implicated in NPP mechanisms, with cannabinoid receptor agonists being one of the few exceptions.
Currently, a number of non-pharmacological therapies have gradually emerged in clinical practice for the treatment of NPP, such as transcranial direct current stimulation, mindfulness-based therapy, meditation therapy, and physical exercise, all of which have demonstrated favorable therapeutic outcomes (188, 189).
In addition, several ethnopharmacological agents and their active constituents—such as myricitrin, Boswellia, and Ashwagandha—have been increasingly utilized in the treatment of NPP (as shown in Table 3) (190–192). However, while existing research acknowledges the involvement of non-neuronal cells, particularly microglia, the underlying mechanisms remain inadequately elucidated. There is still a lack of in-depth investigation into the interactions between non-neuronal cells and neurons. Therefore, this review aims to identify potential targets and clarify underlying mechanisms of NPP, which may facilitate the development of novel, mechanism-based therapies for this complex pain disorder.
10 Limitations
Compared with NCP or NP, NPP is a relatively new field, and to date there have been few high-quality studies or groundbreaking advances in either basic or clinical research. In the databases currently available to us, the quality of relevant literature remains relatively low, which may be attributed to insufficient attention given to NPP by researchers. Therefore, while striving to comprehensively collect and incorporate existing literature—particularly by screening more recent publications—we have aimed to provide a broad and novel perspective on the potential mechanisms by which non-neuronal cells mediate the pathogenesis of NPP. Through this approach, we hope to draw greater research attention to the relevance of NPP in neuro-immune interactions, particularly the role of non-neuronal cells, and to highlight potential therapeutic targets, thereby laying a foundation for further in-depth investigation.
In this review, we have incorporated content related to potential biomarkers for NPP, offering new perspectives for the clinical auxiliary clinical diagnosis of NPP. However, it should be noted that NPP is not a single disease but rather a novel mechanistic concept that may contribute to various conditions, such as FMS, CRPS, and IBS. Common pathogenic features of NPP include peripheral and central sensitization, making it difficult to establish diagnostic algorithms based on any single specific biomarker. To date, there have been no high-quality clinical disease modeling or prediction studies specifically focused on NPP. Our work is also constrained by challenges such as limited clinical sample availability and the generally low quality of currently published NPP literature, which hampers the ability to conduct corresponding original research.
As a common biochemical parameter, blood glucose levels have been widely reported to be associated with NPP. However, their specificity is questionable, as glucose levels are highly variable and influenced by factors such as diet, acute stress, and cortisol (193). Thus, claiming blood glucose as a predictive indicator for NPP may be overstated without acknowledging its limited specificity. Similarly, the neutrophil-to-lymphocyte ratio (NLR), a widely used marker, shows substantial elevation in conditions such as tumors and inflammatory diseases. Although NLR demonstrates adequate sensitivity, it also exhibits poor specificity and is influenced by the disease activity in FMS (68). Therefore, these biochemical indicators should be regarded as adjunctive diagnostic tools rather than definitive biomarkers for NPP.
11 Discussions and future perspectives
NPP, another type of chronic pain distinct from nociceptive pain and neuropathic pain, encompasses several intractable pain disorders such as FMS, CRPS, IBD, and others (Table 4). Studies have shown that different NPP-related diseases can transform into each other. For example, 28–59% of patients with FMS that develop IBS, and 32–77% of patients diagnosed with the diagnosis of IBS develop FMS during the course of their illness, with a relative risk increase of approximately 1.54-fold. These findings suggest a bidirectional correlation between these conditions (194). Owing to their similar pathogenic mechanisms, different types of NPP-related diseases may also transform into one another, providing new perspectives for the treatment of disorders such as IBS and FMS.

Table 4. Characteristics of preclinical models for NP and NPP and their corresponding potential targets.
Essentially, the shared pathogenic core of the pathogenesis of NP and NPP lies in peripheral and/or) central sensitization following repeated and persistent exposure to non-harmful stimuli—that is, a separation of central (or peripheral) sensitization occurring without significant activation of nociceptors or clear evidence of nerve damage of patients (Figure 5). Recent studies indicate that the role of non-neuronal cells should not be underestimated, particularly in the context of peripheral and/or) central sensitization. Conventionally, injurious stimuli are often accompanied by inflammation. However, a key unresolved question remains: why do non-neuronal cells, especially the immune cells, mediate pain in the absence of overt tissue damage or inflammation?
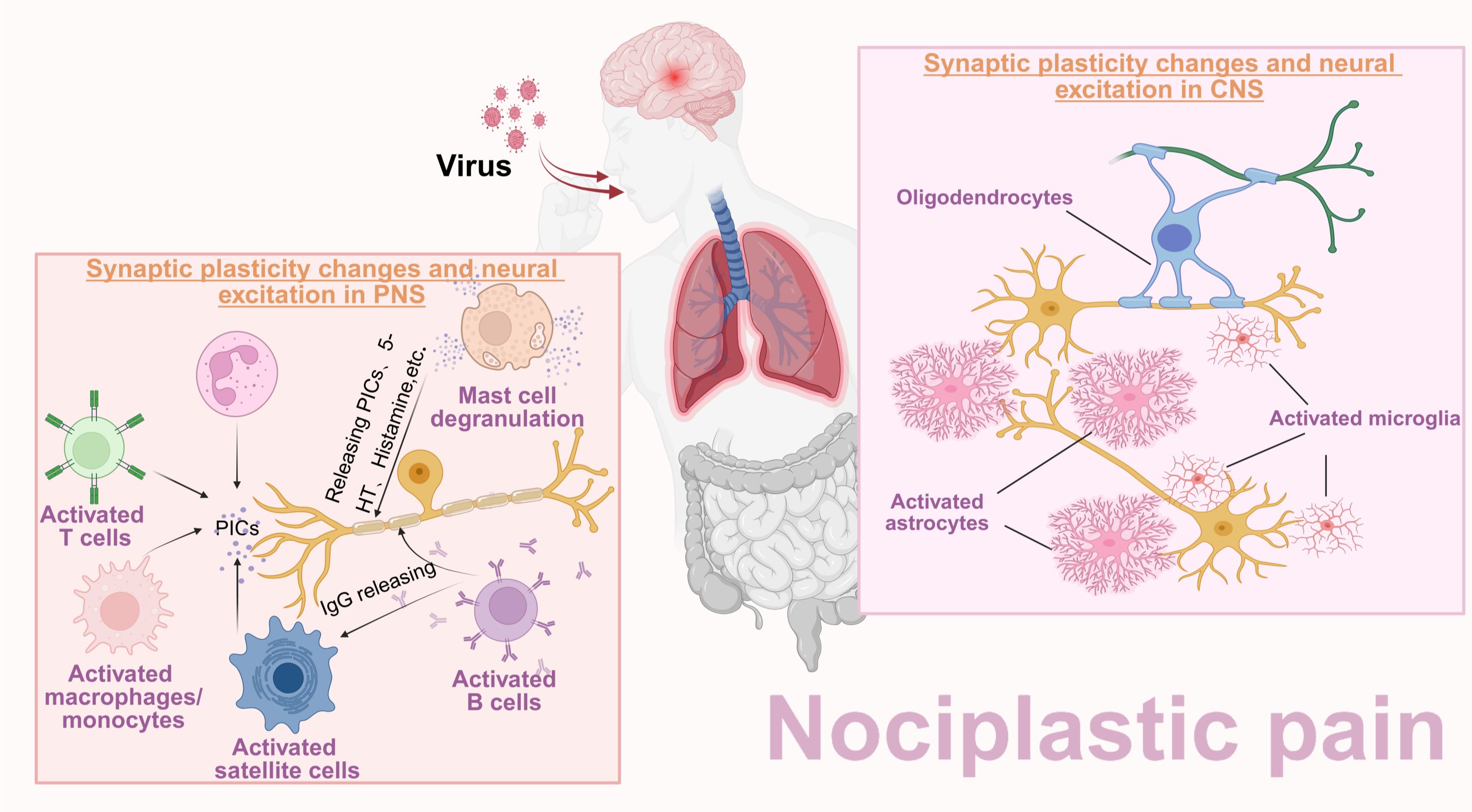
Figure 5. Central sensitization as a shared mechanism of NP and NPP. In NP patients, no matter whether due to peripheral nerve fiber injury or the central nervous system impairment, peripheral sensitization and central sensitization can be induced. In contrast, the underlying mechanism of NPP is mainly attributed to pathogen pre-infection and persistent stimuli of mild central inflammatory stimulation. Both NP and NPP patients share a common mechanism: central sensitization mediated by synaptic plasticity changes and neuronal hyperexcitability.
Accordingly, this review focuses on non-neuronal cells and aims to systematically illustrate how they participate in the development of NPP. Notably, we propose a hypothesis that pre-infection may represent one of the prerequisite conditions which is essential for the NPP development. Under conditions of prior infection, combined with subsequent stimuli such as light exposure, diet, temperature changes, or even one’s own emotional stress, the NPP may emerge. During the early inflammatory phase of viral infection, viral matrix metalloproteinases and immune-derived cytokines increase blood–brain barrier permeability, facilitating viral entry and immune cell infiltration into the central nervous system. These infiltrating immune cells release PICs that, via MAPK-dominant signaling pathways, induce sustained glial activation. Activated glial cells modulate neuronal excitability through PICs and neurotransmitters such as adenosine. Meanwhile, certain viruses may employ epigenetic mechanisms—such as neuron-specific microRNAs—to suppress transcription factors, promote viral genome heterochromatinization, and induce glial histone methylation, thereby establishing viral latency and altering synaptic gene expression (195, 196). Clinically, late-phase pain often becomes dissociated from inflammation and resistant to anti-inflammatory therapies, resembling NPP. This NPP-like state appears conditional and may transition into a mixed nociceptive–neuropathic–central pain phenotype upon viral reactivation and intense inflammation. However, the viral pre-infection hypothesis is currently not yet fully supported by direct evidence and currently remains primarily a potential, albeit promising, mechanism underlying the pathogenesis of NPP-related chronic pain. Importantly, this hypothesis must be distinguished from ongoing inflammation, small-fiber neuropathy, and residual immune-mediated tissue damage (197–200).
Beyond the central nervous system, the peripheral nervous system—particularly the dorsal root ganglion—may represent another critical site involved in NPP development. Small-fiber neuropathy mediated by excessive activation of sodium channels (Nav1.7), together with sympathetic nerve fiber sprouting induced by nerve growth factor, may help explain why NPP is also regulated by peripheral sensitization (201). In the peripheral nervous system, tissue-resident macrophages and satellite glial cells tightly envelop primary neuronal cell bodies in the form of a network-like structure, while Schwann cells ensheath axons of primary neurons in the form of myelin. Peripheral sensitization is traditionally considered a consequence of nociceptor sensitization induced by peripheral inflammation or peripheral nerve injury mediated by peripheral immune cells.
Interestingly, it has been reported that peripheral immune cells have been reported to cross the blood–brain barrier through their migratory mechanisms and enter the central nervous system to contribute to central sensitization. Therefore, in addition to classical central glial cells, we have summarized various non-neuronal cell types associated with NPP. Although there is no sufficient evidence is lacking to conclusively demonstrate that these related non-neuronal cells can regulate NPP through synaptic plasticity modulation of synaptic plasticity, we propose that they may nonetheless influence NPP through potential mechanisms involving synaptic plasticity regulation.
Finally, NPP remains a relatively new concept that has not yet been comprehensively explored, resulting in a scarcity of directly relevant literature directly addressing the impact of non-neuronal cells on synaptic plasticity in NPP. In this review, we explored potential links between non-neuronal cells, synaptic plasticity, and NPP. Moreover, the regulation of plasticity extends beyond synaptic plasticity alone and should also encompass broader regulatory effects on neuronal function and structural plasticity (Table 5). In addition to dysfunctions in plasticity mediated by non-neuronal cells, their structural contributions likewise merit further investigation.
Author contributions
WL: Methodology, Visualization, Writing – original draft. SC: Data curation, Methodology, Writing – original draft. YZ: Methodology, Writing – original draft. ZQ: Formal analysis, Writing – original draft. MC: Investigation, Writing – original draft. XT: Investigation, Writing – original draft. CY: Data curation, Writing – original draft. JY: Funding acquisition, Supervision, Validation, Visualization, Writing – review & editing. CW: Funding acquisition, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This research was funded by National Key R&D Program of China (No. 2022YFC3501204); Key R&D Program of Zhejiang (NO. 2023C03040); Bureau and provincial joint construction of science and technology plan project (NO. GZY-ZJ-KJ-23009).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Knotkova H, Hamani C, Sivanesan E, Le Beuffe MFE, Moon JY, et al. Neuromodulation for chronic pain. Lancet. (2021) 397:2111–24. doi: 10.1016/S0140-6736(21)00794-7
2. Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. (2016) 157:1382–6. doi: 10.1097/j.pain.0000000000000507
3. Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, and Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. (2021) 397:2098–110. doi: 10.1016/S0140-6736(21)00392-5
4. Kosek E, Clauw D, Nijs J, Baron R, Gilron I, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain. (2021) 162:2629–34. doi: 10.1097/j.pain.0000000000002324
5. Dydyk AM, Chiebuka E, Stretanski MF, and Givler A. Central pain syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. (2025).
6. Mills SEE, Nicolson KP, and Smith BH. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br J Anaesth. (2019) 123:e273–83. doi: 10.1016/j.bja.2019.03.023
7. Tanaka Y, Kanazawa M, Fukudo S, and Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. (2011) 17:131–9. doi: 10.5056/jnm.2011.17.2.131
8. Afari N, Ahumada SM, Wright LJ, Mostoufi S, and Golnari G. Psychological trauma and functional somatic syndromes: A systematic review and meta-analysis. Psychosom Med. (2014) 76:2–11. doi: 10.1097/PSY.0000000000000010
9. Baron R, Binder A, and Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. (2010) 9:807–19. doi: 10.1016/S1474-4422(10)70143-5
10. Latremoliere A and Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain. (2009) 10:895–926. doi: 10.1016/j.jpain.2009.06.012
11. Ji RR, Chamessian A, and Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. (2016) 354:572–7. doi: 10.1126/science.aaf8924
12. Ji RR, Nackley A, Huh Y, Terrando N, and Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. (2018) 129:343–66. doi: 10.1097/ALN.0000000000002130
13. McNearney TA and Westlund KN. Pluripotential glun1 (Nmda nr1): functional significance in cellular nuclei in pain/nociception. Int J Mol Sci. (2023) 24:13196. doi: 10.3390/ijms241713196
14. Sunzini F, Schrepf A, Clauw DJ, and Basu N. The biology of pain: through the rheumatology lens. Arthritis Rheumatol. (2023) 75:650–60. doi: 10.1002/art.42429
15. Gershon AA, Breuer J, Cohen JI, Cohrs RJ, and Gershon MD. Varicella zoster virus infection. Nat Rev Dis Primers. (2015) 1:15016. doi: 10.1038/nrdp.2015.16
16. Carfì A, Bernabei R, and Landi F. Persistent symptoms in patients after acute covid-19. Jama. (2020) 324:603–5. doi: 10.1001/jama.2020.12603
17. Suchowiecki K, Reid SP, Simon GL, Firestein GS, and Chang A. Persistent joint pain following arthropod virus infections. Curr Rheumatol Rep. (2021) 23:26. doi: 10.1007/s11926-021-00987-y
18. Zanone MM, Marinucci C, Ciancio A, Cocito D, and Zardo F. Peripheral neuropathy after viral eradication with direct-acting antivirals in chronic hcv hepatitis: A prospective study. Liver Int. (2021) 41:2611–21. doi: 10.1111/liv.15002
19. Baroni A, Fregna G, Lamberti N, Manfredini F, and Straudi S. Fatigue can influence the development of late-onset pain in post-covid-19 syndrome: an observational study. Eur J Pain. (2024) 28:901–12. doi: 10.1002/ejp.2228
20. Au MT, Ni J, Tang K, Wang W, and Zhang L. Blockade of endothelin receptors mitigates sars-cov-2-induced osteoarthritis. Nat Microbiol. (2024) 9:2538–52. doi: 10.1038/s41564-024-01802-x
21. Fialho MFP, Brum ES, and Oliveira SM. Could the fibromyalgia syndrome be triggered or enhanced by covid-19? Inflammopharmacology. (2023) 31:633–51.
22. Paroli M, Gioia C, Accapezzato D, and Caccavale R. Inflammation, autoimmunity, and infection in fibromyalgia: A narrative review. Int J Mol Sci. (2024) 25::5922. doi: 10.3390/ijms25115922
23. Greene C, Connolly R, Brennan D, Laffan A, O'Keeffe E, Zaporojan L, et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long covid-associated cognitive impairment. Nat Neurosci. (2024) 27:421–32. doi: 10.1038/s41593-024-01576-9
24. Espíndola OM, Brandão CO, Gomes YCP, Siqueira M, Soares CN, Lima M, et al. Cerebrospinal fluid findings in neurological diseases associated with covid-19 and insights into mechanisms of disease development. Int J Infect Dis. (2021) 102:155–62. doi: 10.1016/j.ijid.2020.10.044
25. Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW, et al. Ccl2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (Hiv)-infected leukocytes across the blood-brain barrier: A potential mechanism of hiv-cns invasion and neuroaids. J Neurosci. (2006) 26:1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006
26. Winkler B, Funke D, Benmimoun B, Spéder P, Rey S, Logan MA, et al. Brain inflammation triggers macrophage invasion across the blood-brain barrier in drosophila during pupal stages. Sci Adv. (2021) 7:eabh0050. doi: 10.1126/sciadv.abh0050
27. Zhang J, Li Z, Lu H, Shi J, Gao R, Ma Y, et al. Evidence of microglial immune response following coronavirus phev infection of cns. Front Immunol. (2021) 12:804625. doi: 10.3389/fimmu.2021.804625
28. Jiang YP, Wen JJ, Ma X, Yuan CR, Zhou F, Zheng MJ, et al. Crmp2 phosphorylation regulates polarization and spinal infiltration of cd4+ T lymphocytes, inhibits spinal glial activation, and arthritic pain. Pain. (2025) 166:2162–80. doi: 10.1097/j.pain.0000000000003599
29. Arnold LM, Hudson JI, Hess EV, Ware AE, Fritz DA, Auchenbach MB, et al. Family study of fibromyalgia. Arthritis Rheum. (2004) 50:944–52. doi: 10.1002/art.20042
31. Johnston KJA, Signer R, and Huckins LM. Chronic overlapping pain conditions and nociplastic pain. HGG Adv. (2025) 6:100381. doi: 10.1016/j.xhgg.2024.100381
32. Marchi M, Salvi E, Andelic M, Mehmeti E, D'Amato I, Cazzato D, et al. Trpa1 rare variants in chronic neuropathic and nociplastic pain patients. Pain. (2023) 164:2048–59. doi: 10.1097/j.pain.0000000000002905
33. Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J, et al. Chronic Intermittent Voluntary Alcohol Drinking Induces Hyperalgesia in Sprague-Dawley Rats. Int J Physiol Pathophysiol Pharmacol. (2015) 7:136-44.
34. Zale EL, Maisto SA, and Ditre JW. Interrelations between Pain and Alcohol: An Integrative Review. Clin Psychol Rev. (2015) 37:57-71. doi: 10.1016/j.cpr.2015.02.005
35. Cai W, Haddad M, Haddad R, Kesten I, Hoffman T, Laan R, et al. The Gut Microbiota Promotes Pain in Fibromyalgia. Neuron. (2025) 113:2161-75.e13. doi: 10.1016/j.neuron.2025.03.032
36. Fang H, Hou Q, Zhang W, Su Z, Zhang J, Li J, et al. Fecal Microbiota Transplantation Improves Clinical Symptoms of Fibromyalgia: An Open-Label, Randomized, Nonplacebo-Controlled Study. J Pain. (2024) 25:104535. doi: 10.1016/j.jpain.2024.104535
37. Martins CC, Reis AS, da Motta KP, Luchese C, and Wilhelm EA. Mechanistic Pathways of Fibromyalgia Induced by Intermittent Cold Stress in Mice Is Sex-Dependently. Brain Res Bull. (2022) 187:11-23. doi: 10.1016/j.brainresbull.2022.06.005
38. Nasu T, Taguchi T, and Mizumura K. Persistent Deep Mechanical Hyperalgesia Induced by Repeated Cold Stress in Rats. Eur J Pain. (2010) 14:236-44. doi: 10.1016/j.ejpain.2009.05.009
39. Nishiyori M and Ueda H. Prolonged Gabapentin Analgesia in an Experimental Mouse Model of Fibromyalgia. Mol Pain. (2008) 4:52. doi: 10.1186/1744-8069-4-52
40. Horing B, Kerkemeyer M, and Büchel C. Temporal Summation of the Thermal Grill Illusion Is Comparable to That Observed Following Noxious Heat. J Pain. (2024) 25:104432. doi: 10.1016/j.jpain.2023.11.015
41. Samborski W, Sobieska M, Pieta P, Drews K, and Brzosko M. Normal Profile of Sex Hormones in Women with Primary Fibromyalgia. Ann Acad Med Stetin. (2005) 51:23-6
42. Pardo JV, Larson RC, Spencer RJ, Lee JT, Pasley JD, Torkelson CJ, et al. Exposure to Cold Unmasks Potential Biomarkers of Fibromyalgia Syndrome Reflecting Insufficient Sympathetic Responses to Stress. Clin J Pain. (2019) 35:407-19. doi: 10.1097/ajp.0000000000000695
43. Adler GK, Manfredsdottir VF, and Creskoff KW. Neuroendocrine Abnormalities in Fibromyalgia. Curr Pain Headache Rep. (2002) 6:289-98. doi: 10.1007/s11916-002-0050-5
44. Adler GK, Kinsley BT, Hurwitz S, Mossey CJ, and Goldenberg DL. Reduced Hypothalamic-Pituitary and Sympathoadrenal Responses to Hypoglycemia in Women with Fibromyalgia Syndrome. Am J Med. (1999) 106:534-43. doi: 10.1016/s0002-9343(99)00074-1
45. Crofford LJ, Pillemer SR, Kalogeras KT, Cash JM, Michelson D, Kling MA, et al. Hypothalamic-Pituitary-Adrenal Axis Perturbations in Patients with Fibromyalgia. Arthritis Rheum. (1994) 37:1583-92. doi: 10.1002/art.1780371105
46. Lee YH and Song GG. Lower Circulating Igf-1 Levels in Fibromyalgia: Meta-Analysis Highlighting Potential Pathogenic Role. Clin Exp Rheumatol. (2025) 43:1122-7. doi: 10.55563/clinexprheumatol/g17lb8
47. McCall-Hosenfeld JS, Goldenberg DL, and Hurwitz S. Growth Hormone and Insulin-Like Growth Factor-1 Concentrations in Women with Fibromyalgia. J Rheumatol. (2003) 30:809-14
48. Griep EN, Boersma JW, and de Kloet ER. Altered Reactivity of the Hypothalamic-Pituitary-Adrenal Axis in the Primary Fibromyalgia Syndrome. J Rheumatol. (1993) 20:469-74
49. Griep EN, Boersma JW, Lentjes EG, Prins AP, van der Korst JK, de Kloet ER, et al. Function of the Hypothalamic-Pituitary-Adrenal Axis in Patients with Fibromyalgia and Low Back Pain. J Rheumatol. (1998) 25:1374-81
50. Vaerøy H, Helle R, Førre Ø, Kåss E, and Terenius L. Elevated Csf Levels of Substance P and High Incidence of Raynaud Phenomenon in Patients with Fibromyalgia: New Features for Diagnosis. Pain. (1988) 32:21-6. doi: 10.1016/0304-3959(88)90019-x
51. Russell IJ, Orr MD, Littman B, Vipraio GA, Alboukrek D, Michalek JE, et al. Elevated Cerebrospinal Fluid Levels of Substance P in Patients with the Fibromyalgia Syndrome. Arthritis Rheum. (1994) 37:1593-601. doi: 10.1002/art.1780371106
52. Vaerøy H, Sakurada T, Førre O, Kåss E, and Terenius L. Modulation of Pain in Fibromyalgia (Fibrositis Syndrome): Cerebrospinal Fluid (CSF) Investigation of Pain Related Neuropeptides with Special Reference to Calcitonin Gene Related Peptide (CGRP). J Rheumatol Suppl. (1989) 19:94-7
53. Vaeroy H, Helle R, Førre O, Kåss E, and Terenius L. Cerebrospinal Fluid Levels of Beta-Endorphin in Patients with Fibromyalgia (Fibrositis Syndrome). J Rheumatol. (1988) 15:1804-6
54. Gordon S and Taylor PR. Monocyte and Macrophage Heterogeneity. Nat Rev Immunol. (2005) 5:953-64. doi: 10.1038/nri1733
55. Brum ES, Landini L, Souza Monteiro de Araújo D, Marini M, Geppetti P, Nassini R, et al. Characterisation of Periorbital Mechanical Allodynia in the Reserpine-Induced Fibromyalgia Model in Mice: The Role of the Schwann Cell TRPA1/NOX1 Signalling Pathway. Free Radic Biol Med. (2025) 229:289-99. doi: 10.1016/j.freeradbiomed.2025.01.040
56. Montserrat-de la Paz S, Garcia-Gimenez MD, Quilez AM, De la Puerta R, and Fernandez-Arche A. Ginger Rhizome Enhances the Anti-Inflammatory and Anti-Nociceptive Effects of Paracetamol in an Experimental Mouse Model of Fibromyalgia. Inflammopharmacology. (2018) 26:1093-101. doi: 10.1007/s10787-018-0450-8
57. Amel Kashipaz MR, Swinden D, Todd I, and Powell RJ. Normal Production of Inflammatory Cytokines in Chronic Fatigue and Fibromyalgia Syndromes Determined by Intracellular Cytokine Staining in Short-Term Cultured Blood Mononuclear Cells. Clin Exp Immunol. (2003) 132:360-5. doi: 10.1046/j.1365-2249.2003.02149.x
58. Cordero MD, De Miguel M, Moreno Fernández AM, Carmona López IM, Garrido Maraver J, Cotán D, et al. Mitochondrial Dysfunction and Mitophagy Activation in Blood Mononuclear Cells of Fibromyalgia Patients: Implications in the Pathogenesis of the Disease. Arthritis Res Ther. (2010) 12:R17. doi: 10.1186/ar2918
59. Martínez-Lavín M, Vargas A, Silveira LH, Amezcua-Guerra LM, Martínez-Martínez LA, Pineda C, et al. Complex Regional Pain Syndrome Evolving to Full-Blown Fibromyalgia: A Proposal of Common Mechanisms. J Clin Rheumatol. (2021) 27:S274-s7. doi: 10.1097/rhu.0000000000001304
60. García JJ, Carvajal-Gil J, and Guerrero-Bonmatty R. Altered Release of Chemokines by Phagocytes from Fibromyalgia Patients: A Pilot Study. Innate Immun. (2016) 22:3-8. doi: 10.1177/1753425915602959
61. Mihaila AC, Ciortan L, Tucureanu MM, Simionescu M, and Butoi E. Anti-Inflammatory Neutrophils Reprogram Macrophages toward a Pro-Healing Phenotype with Increased Efferocytosis Capacity. Cells. (2024) 13:208. doi: 10.3390/cells13030208
62. Caxaria S, Bharde S, Fuller AM, Evans R, Thomas B, Celik P, et al. Neutrophils Infiltrate Sensory Ganglia and Mediate Chronic Widespread Pain in Fibromyalgia. Proc Natl Acad Sci U S A. (2023) 120:e2211631120. doi: 10.1073/pnas.2211631120
63. Inci H and Inci F. Acupuncture Effects on Blood Parameters in Patients with Fibromyalgia. Med Acupunct. (2021) 33:86-91. doi: 10.1089/acu.2020.1476
64. Telli H and Özdemir Ç. Is Nociplastic Pain, a New Pain Category, Associated with Biochemical, Hematological, and Inflammatory Parameters?. Curr Med Res Opin. (2024) 40:469-81. doi: 10.1080/03007995.2024.2304106
65. Cure O, Kizilkaya B, Ciftel S, Klisic A, Ciftel E, Mercantepe F, et al. Effect of Fibromyalgia on Demographic, Biochemical, Metabolic and Inflammatory Profiles: A Single-Centre Retrospective Study. Clin Exp Rheumatol. (2024) 42:1205-14. doi: 10.55563/clinexprheumatol/oo5zjo
66. Al-Nimer MSM and Mohammad TAM. Correlation of Hematological Indices and Ratios Derived from Them with FIQr Scores in Fibromyalgia. Pak J Med Sci. (2018) 34:1219-24. doi: 10.12669/pjms.345.15169
67. Ege F and Isik R. A Comparative Assessment of the Inflammatory Markers in Patients with Fibromyalgia under Duloxetine Treatment. Front Biosci (Landmark Ed). (2023) 28:161. doi: 10.31083/j.fbl2808161
68. Almirall M, Espartal E, Michelena X, Suso-Ribera C, Serrat M, Marsal S, et al. Neutrophil-Lymphocyte Ratio in Fibromyalgia and Axial Spondyloarthritis: A Potential Biomarker for Diagnosis and Disease Activity. Biomedicines. (2025) 13:1497. doi: 10.3390/biomedicines13061497
69. Eller-Smith OC, Nicol AL, and Christianson JA. Potential Mechanisms Underlying Centralized Pain and Emerging Therapeutic Interventions. Front Cell Neurosci. (2018) 12:35. doi: 10.3389/fncel.2018.00035
70. Conti P, Gallenga CE, Caraffa A, Ronconi G, and Kritas SK. Impact of Mast Cells in Fibromyalgia and Low-Grade Chronic Inflammation: Can IL-37 Play a Role? Dermatol Ther. (2020) 33:e13191. doi: 10.1111/dth.13191
71. Rijnierse A, Nijkamp FP, and Kraneveld AD. Mast Cells and Nerves Tickle in the Tummy: Implications for Inflammatory Bowel Disease and Irritable Bowel Syndrome. Pharmacol Ther. (2007) 116:207-35. doi: 10.1016/j.pharmthera.2007.06.008
72. Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, et al. Activated Mast Cells in Proximity to Colonic Nerves Correlate with Abdominal Pain in Irritable Bowel Syndrome. Gastroenterology. (2004) 126:693-702. doi: 10.1053/j.gastro.2003.11.055
73. Ito A, Hagiyama M, and Oonuma J. Nerve-Mast Cell and Smooth Muscle-Mast Cell Interaction Mediated by Cell Adhesion Molecule-1, Cadm1. J Smooth Muscle Res. (2008) 44:83-93. doi: 10.1540/jsmr.44.83
74. Zhao YC, Hu T, Chen Y, and Du KT. Elevated Serum Levels of Monocyte Chemotactic Protein-1/Chemokine C-C Motif Ligand 2 Are Linked to Disease Severity in Patients with Fibromyalgia Syndrome. Balkan Med J. (2019) 36:331-6. doi: 10.4274/balkanmedj.galenos.2019.2019.6.47
75. Alvarez P, Green PG, and Levine JD. Role for Monocyte Chemoattractant Protein-1 in the Induction of Chronic Muscle Pain in the Rat. Pain. (2014) 155:1161-7. doi: 10.1016/j.pain.2014.03.004
76. Brum EDS, Fialho MFP, Becker G, Nogueira CW, and Oliveira SM. Involvement of Peripheral Mast Cells in a Fibromyalgia Model in Mice. Eur J Pharmacol. (2024) 967:176385. doi: 10.1016/j.ejphar.2024.176385
77. Zhang L, Song J, Bai T, Wang R, and Hou X. Sustained Pain Hypersensitivity in the Stressed Colon: Role of Mast Cell-Derived Nerve Growth Factor-Mediated Enteric Synaptic Plasticity. Neurogastroenterol Motil. (2018) 30:e13430. doi: 10.1111/nmo.13430
78. Masuko K and Nakamura H. Functional Somatic Syndrome: How It Could Be Relevant to Rheumatologists. Mod Rheumatol. (2007) 17:179-84. doi: 10.1007/s10165-007-0563-1
79. Buhner S and Schemann M. Mast Cell-Nerve Axis with a Focus on the Human Gut. Biochim Biophys Acta. (2012) 1822:85-92. doi: 10.1016/j.bbadis.2011.06.004
80. Vivinus-Nébot M, Dainese R, Anty R, Saint-Paul MC, Nano JL, Gonthier N, et al. Combination of Allergic Factors Can Worsen Diarrheic Irritable Bowel Syndrome: Role of Barrier Defects and Mast Cells. Am J Gastroenterol. (2012) 107:75-81. doi: 10.1038/ajg.2011.315
81. Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, et al. Mast Cell-Dependent Excitation of Visceral-Nociceptive Sensory Neurons in Irritable Bowel Syndrome. Gastroenterology. (2007) 132:26-37. doi: 10.1053/j.gastro.2006.11.039
82. Coelho AM, Fioramonti J, and Bueno L. Mast Cell Degranulation Induces Delayed Rectal Allodynia in Rats: Role of Histamine and 5-HT. Dig Dis Sci. (1998) 43:727-37. doi: 10.1023/a:1018853728251
83. D'Amico R, Fusco R, Siracusa R, Impellizzeri D, Peritore AF, Gugliandolo E, et al. Inhibition of P2X7 Purinergic Receptor Ameliorates Fibromyalgia Syndrome by Suppressing NLRP3 Pathway. Int J Mol Sci. (2021) 22:6471. doi: 10.3390/ijms22126471
84. Valencia C, Fatima H, Nwankwo I, Anam M, Maharjan S, Amjad Z, et al. A Correlation between the Pathogenic Processes of Fibromyalgia and Irritable Bowel Syndrome in the Middle-Aged Population: A Systematic Review. Cureus. (2022) 14:e29923. doi: 10.7759/cureus.29923
85. Ivashkin V, Poluektov Y, Kogan E, Shifrin O, Sheptulin A, Kovaleva A, et al. Disruption of the Pro-Inflammatory, Anti-Inflammatory Cytokines and Tight Junction Proteins Expression, Associated with Changes of the Composition of the Gut Microbiota in Patients with Irritable Bowel Syndrome. PLoS One. (2021) 16:e0252930. doi: 10.1371/journal.pone.0252930
86. Norlin AK, Walter S, Icenhour A, Keita ÅV, Elsenbruch S, Bednarska O, et al. Fatigue in Irritable Bowel Syndrome Is Associated with Plasma Levels of TNF-a and Mesocorticolimbic Connectivity. Brain Behav Immun. (2021) 92:211-22. doi: 10.1016/j.bbi.2020.11.035
87. Banfi G, Diani M, Pigatto PD, and Reali E. T Cell Subpopulations in the Physiopathology of Fibromyalgia: Evidence and Perspectives. Int J Mol Sci. (2020) 21:1186. doi: 10.3390/ijms21041186
88. Deshmukh RS, Keita ÅV, and Linden A. Immune Dysfunction in Irritable Bowel Syndrome: A Role for T Cells. Clin Exp Immunol. (2017) 189:179-88. doi: 10.1111/cei.12954
89. Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Tsuda M, Jia Z, et al. Bacteria Activate Sensory Neurons That Modulate Pain and Inflammation. Nature. (2013) 501:52-7. doi: 10.1038/nature12479
90. McGeachy MJ, Coffey ES, and Hueber AJ. The Pathophysiology of T Cells in Chronic Pain. Nat Rev Rheumatol. (2017) 13:125-34. doi: 10.1038/nrrheum.2016.189
91. Hauser W, Albrecht J, Bartram W, Uceyler N, Furst DE, Fitzcharles MA, et al. Fibromyalgia in Women: Immune System and T Cell Involvement. Clin Exp Rheumatol. (2017) 35:81-8. doi: 10.55563/clinexprheumatol/ooo59o
92. Velloso LA, da Cunha JP, and Oliveira CS. Systemic Inflammation and T Cell Profiles in Fibromyalgia Patients. J Clin Med. (2020) 9:1104. doi: 10.3390/jcm9041104
93. Caro R, Rodriguez F, and Alvarez J. Pro-Inflammatory Cytokine Levels in Fibromyalgia Patients. Rheumatol Int. (2019) 39:2029-35. doi: 10.1007/s00296-019-04325-7
94. Smith MD, Jones AB, Lee KT, Kim HJ, Patel R, Nguyen LT, et al. Immune Profiling in Chronic Pain Syndromes. Front Immunol. (2021) 12:657890. doi: 10.3389/fimmu.2021.657890
95. Andersson U, Lind SE, and Holm P. Inflammatory Biomarkers and Pain Severity in Fibromyalgia. Scand J Rheumatol. (2020) 49:110-8. doi: 10.1080/03009742.2019.1642345
96. Torres F, Gomez L, Martinez R, Lopez S, Ramirez J, Diaz P, et al. Peripheral Immune Alterations in Fibromyalgia: A Multicenter Study. Clin Rheumatol. (2021) 40:345-55. doi: 10.1007/s10067-020-05432-1
97. Nguyen TT, Le H, and Tran Q. Altered Cytokine Networks in Fibromyalgia Patients. Immunol Lett. (2019) 209:12-20. doi: 10.1016/j.imlet.2019.01.005
98. Huang Y, Chen X, Lin Z, Wang J, Li M, Zhang H, et al. Immune and Inflammatory Responses in Fibromyalgia Syndrome. J Immunol Res. (2022) 2022:112345. doi: 10.1155/2022/112345
99. Kim H, Park J, and Choi S. Role of Inflammation in the Pathophysiology of Fibromyalgia. Rheumatology (Oxford). (2021) 60:456-64. doi: 10.1093/rheumatology/keaa401
100. Lopez R, Garcia L, Martinez P, Fernandez C, Ruiz S, Sanchez J, et al. Cytokine Profiles and Immune Dysregulation in Fibromyalgia Patients: A Clinical Study. Clin Immunol. (2020) 215:108445. doi: 10.1016/j.clim.2020.108445
101. Lenert ME, Green AR, Merriwether EN, and Burton MD. B-Cell and Plasma Cell Activation in a Mouse Model of Chronic Muscle Pain. Neurobiol Pain. (2024) 16:100169. doi: 10.1016/j.ynpai.2024.100169
102. Malafoglia V, Ilari S, Gioia C, Vitiello L, Tenti M, Iannuccelli C, et al. An Observational Study on Chronic Pain Biomarkers in Fibromyalgia and Osteoarthritis Patients: Which Role for Mu Opioid Receptor's Expression on Nk Cells? Biomedicines. (2023) 11:931. doi: 10.3390/biomedicines11030931
103. Fineschi S, Klar J, Gustafsson KA, Jonsson K, Karlsson B, and Dahl N. Inflammation and Interferon Signatures in Peripheral B-Lymphocytes and Sera of Individuals with Fibromyalgia. Front Immunol. (2022) 13:874490. doi: 10.3389/fimmu.2022.874490
104. Barragán-Iglesias P, Franco-Enzástiga Ú, Jeevakumar V, Shiers S, Wangzhou A, Granados-Soto V, et al. Type I Interferons Act Directly on Nociceptors to Produce Pain Sensitization: Implications for Viral Infection-Induced Pain. J Neurosci. (2020) 40:3517-32. doi: 10.1523/jneurosci.3055-19.2020
105. Lacagnina MJ, Willcox KF, Boukelmoune N, Bavencoffe A, Sankaranarayanan I, Barratt DT, et al. B Cells Drive Neuropathic Pain-Related Behaviors in Mice through Igg-Fc Gamma Receptor Signaling. Sci Transl Med. (2024) 16(766):eadj1277. doi: 10.1126/scitranslmed.adj1277
106. Eneström S, Bengtsson A, and Frödin T. Dermal Igg Deposits and Increase of Mast Cells in Patients with Fibromyalgia--Relevant Findings or Epiphenomena? Scand J Rheumatol. (1997) 26:308-13. doi: 10.3109/03009749709105321
107. Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, et al. Passive Transfer of Fibromyalgia Symptoms from Patients to Mice. J Clin Invest. (2021) 131:e144201. doi: 10.1172/jci144201
108. Cuhadar U, Gentry C, Vastani N, Sensi S, Bevan S, Goebel A, et al. Autoantibodies Produce Pain in Complex Regional Pain Syndrome by Sensitizing Nociceptors. Pain. (2019) 160:2855-65. doi: 10.1097/j.pain.0000000000001662
109. Andreeva D, Murashova L, Burzak N, and Dyachuk V. Satellite Glial Cells: Morphology, Functional Heterogeneity, and Role in Pain. Front Cell Neurosci. (2022) 16:1019449. doi: 10.3389/fncel.2022.1019449
110. Seefried S, Barcic A, Grijalva Yepez MF, Reinhardt L, Appeltshauser L, Doppler K, et al. Autoantibodies in Patients with Fibromyalgia Syndrome. Pain. (2025) 166(8):1922-1933. doi: 10.1097/j.pain.0000000000003535
111. Fanton S, Menezes J, Krock E, Sandström A, Tour J, Sandor K, et al. Anti-Satellite Glia Cell Igg Antibodies in Fibromyalgia Patients Are Related to Symptom Severity and to Metabolite Concentrations in Thalamus and Rostral Anterior Cingulate Cortex. Brain Behav Immun. (2023) 114:371-82. doi: 10.1016/j.bbi.2023.09.003
112. Krock E, Morado-Urbina CE, Menezes J, Hunt MA, Sandström A, Kadetoff D, et al. Fibromyalgia Patients with Elevated Levels of Anti-Satellite Glia Cell Immunoglobulin G Antibodies Present with More Severe Symptoms. Pain. (2023) 164(8):1828-40. doi: 10.1097/j.pain.0000000000002881
113. Hanani M. Satellite Glial Cells in Human Disease. Cells. (2024) 13(7):566. doi: 10.3390/cells13070566
114. Jakobsson JE, Menezes J, Krock E, Hunt MA, Carlsson H, Vaivade A, et al. Fibromyalgia Patients Have Altered Lipid Concentrations Associated with Disease Symptom Severity and Anti-Satellite Glial Cell Igg Antibodies. J Pain. (2025) 29:105331. doi: 10.1016/j.jpain.2025.105331
115. Bersellini Farinotti A, Wigerblad G, Nascimento D, Bas DB, Morado Urbina C, Nandakumar KS, et al. Cartilage-Binding Antibodies Induce Pain through Immune Complex-Mediated Activation of Neurons. J Exp Med. (2019) 216(8):1904-24. doi: 10.1084/jem.20181657
116. Noseda R, Schain AJ, Melo-Carrillo A, Tien J, Stratton J, Mai F, et al. Fluorescently-Labeled Fremanezumab Is Distributed to Sensory and Autonomic Ganglia and the Dura but Not to the Brain of Rats with Uncompromised Blood Brain Barrier. Cephalalgia. (2020) 40(3):229-40. doi: 10.1177/0333102419896760
117. Cordaro M, Siracusa R, D'Amico R, Genovese T, Franco G, Marino Y, et al. Role of Etanercept and Infliximab on Nociceptive Changes Induced by the Experimental Model of Fibromyalgia. Int J Mol Sci. (2022) 23(11):6139. doi: 10.3390/ijms23116139
118. Marino Y, Inferrera F, D'Amico R, Impellizzeri D, Cordaro M, Siracusa R, et al. Role of Mitochondrial Dysfunction and Biogenesis in Fibromyalgia Syndrome: Molecular Mechanism in Central Nervous System. Biochim Biophys Acta Mol Basis Dis. (2024) 1870(7):167301. doi: 10.1016/j.bbadis.2024.167301
119. Atta AA, Ibrahim WW, Mohamed AF, and Abdelkader NF. Microglia Polarization in Nociplastic Pain: Mechanisms and Perspectives. Inflammopharmacology. (2023) 31(3):1053-67. doi: 10.1007/s10787-023-01216-x
120. Atta AA, Ibrahim WW, Mohamed AF, and Abdelkader NF. Targeting ?7-Nachr by Galantamine Mitigates Reserpine-Induced Fibromyalgia-Like Symptoms in Rats: Involvement of Camp/Pka, Pi3k/Akt, and M1/M2 Microglia Polarization. Eur J Pharmacol. (2023) 952:175810. doi: 10.1016/j.ejphar.2023.175810
121. Shafiek MZ, Zaki HF, Mohamed AF, and Ibrahim WW. Novel Trajectories Towards Possible Effects of Semaglutide for Amelioration of Reserpine-Induced Fibromyalgia in Rats: Contribution of Camp/Pka/P-Creb and M1/M2 Microglia Polarization. J Neuroimmune Pharmacol. (2025) 20:43. doi: 10.1007/s11481-025-10196-4
122. Han J, Cho HJ, Park D, and Han S. Dicam in the Extracellular Vesicles from Astrocytes Attenuates Microglia Activation and Neuroinflammation. Cells. (2022) 11(19):2977. doi: 10.3390/cells11192977
123. Martins D, Dipasquale O, Veronese M, Turkheimer F, Loggia ML, McMahon S, et al. Transcriptional and Cellular Signatures of Cortical Morphometric Remodelling in Chronic Pain. Pain. (2022) 163(6):e759-e73. doi: 10.1097/j.pain.0000000000002480
124. Herculano-Houzel S. The Glia/Neuron Ratio: How It Varies Uniformly across Brain Structures and Species and What That Means for Brain Physiology and Evolution. Glia. (2014) 62(9):1377-91. doi: 10.1002/glia.22683
125. Lu HJ and Gao YJ. Astrocytes in Chronic Pain: Cellular and Molecular Mechanisms. Neurosci Bull. (2023) 39(3):425-39. doi: 10.1007/s12264-022-00961-3
126. Borst K, Dumas AA, and Prinz M. Microglia: Immune and Non-Immune Functions. Immunity. (2021) 54(10):2194-208. doi: 10.1016/j.immuni.2021.09.014
127. Albrecht DS, Forsberg A, Sandström A, Bergan C, Kadetoff D, Protsenko E, et al. Brain Glial Activation in Fibromyalgia - a Multi-Site Positron Emission Tomography Investigation. Brain Behav Immun. (2019) 75:72-83. doi: 10.1016/j.bbi.2018.09.018
128. Yuan T, Manohar K, Latorre R, Orock A, and Greenwood-Van Meerveld B. Inhibition of Microglial Activation in the Amygdala Reverses Stress-Induced Abdominal Pain in the Male Rat. Cell Mol Gastroenterol Hepatol. (2020) 10(3):527-43. doi: 10.1016/j.jcmgh.2020.04.020
129. Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, et al. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep. (2019) 27(13):3844-59.e6. doi: 10.1016/j.celrep.2019.05.087
130. Zhong Y, Zhou LJ, Ren WJ, Xin WJ, Li YY, Zhang T, et al. The Direction of Synaptic Plasticity Mediated by C-Fibers in Spinal Dorsal Horn Is Decided by Src-Family Kinases in Microglia: The Role of Tumor Necrosis Factor-Alpha. Brain Behav Immun. (2010) 24(6):874-80. doi: 10.1016/j.bbi.2010.01.007
131. Zhang X, Hartung JE, Bortsov AV, Kim S, O'Buckley SC, Kozlowski J, et al. Sustained Stimulation of ?(2)- and ?(3)-Adrenergic Receptors Leads to Persistent Functional Pain and Neuroinflammation. Brain Behav Immun. (2018) 73:520-32. doi: 10.1016/j.bbi.2018.06.017
132. Liu K, Lin Y, Rao T, Huang Y, Kan Y, and Liao J. Acupressure Attenuates Fibromyalgia Central Sensitization Via Mapk Phosphorylation-Mediated Neuroinflammation Regulation. J Orthop Surg Res. (2025) 20(1):622. doi: 10.1186/s13018-025-05986-8
133. Wakatsuki K, Kiryu-Seo S, Yasui M, Yokota H, Kida H, Konishi H, et al. Repeated Cold Stress, an Animal Model for Fibromyalgia, Elicits Proprioceptor-Induced Chronic Pain with Microglial Activation in Mice. J Neuroinflammation. (2024) 21(1):25. doi: 10.1186/s12974-024-03018-6
134. Pohóczky K, Kun J, Szentes N, Aczél T, Urbán P, Gyenesei A, et al. Discovery of Novel Targets in a Complex Regional Pain Syndrome Mouse Model by Transcriptomics: Tnf and Jak-Stat Pathways. Pharmacol Res. (2022) 182:106347. doi: 10.1016/j.phrs.2022.106347
135. Helyes Z, Tékus V, Szentes N, Pohóczky K, Botz B, Kiss T, et al. Transfer of Complex Regional Pain Syndrome to Mice Via Human Autoantibodies Is Mediated by Interleukin-1-Induced Mechanisms. Proc Natl Acad Sci U S A. (2019) 116(26):13067-76. doi: 10.1073/pnas.1820168116
136. Ohgidani M, Kato TA, Hosoi M, Tsuda M, Hayakawa K, Hayaki C, et al. Fibromyalgia and Microglial Tnf-?: Translational Research Using Human Blood Induced Microglia-Like Cells. Sci Rep. (2017) 7(1):11882. doi: 10.1038/s41598-017-11506-4
137. Beltran P, Shu X, Li Y, Zhou F, Xu J, Zhang C, et al. Microglia-Derived Cytokines Contribute to Pain Sensitization in Fibromyalgia. Brain Behav Immun. (2021) 97:227-37. doi: 10.1016/j.bbi.2021.07.014
138. Sheng M, Zhou Q, Li X, and Wang Y. Activation of Spinal Microglia Contributes to Mechanical Allodynia in a Rat Model of Fibromyalgia. Mol Pain. (2020) 16:1744806920905573. doi: 10.1177/1744806920905573
139. Cao J, Li L, Yang Q, Zhang L, Xu Y, Wang H, et al. Astrocyte-Microglia Crosstalk Mediates Neuroinflammation in Fibromyalgia Models. J Neuroinflammation. (2022) 19(1):123. doi: 10.1186/s12974-022-02489-0
140. Kim SH, Lee JH, Cho HS, and Park ES. Microglia Activation in the Spinal Cord Contributes to Hyperalgesia in a Fibromyalgia Mouse Model. Pain. (2021) 162(6):1704-15. doi: 10.1097/j.pain.0000000000002201
141. Liu Y, Yang H, Zhao J, Chen F, Li X, Wang Z, et al. Microglial Nlrp3 Inflammasome Activation Is Critical for Central Sensitization in Fibromyalgia. Front Cell Neurosci. (2023) 17:1156890. doi: 10.3389/fncel.2023.1156890
142. Li Y, Wang Q, Zhang H, and Chen L. Targeting Microglia via P2x7 Receptors Attenuates Fibromyalgia-Like Pain in Rodent Models. J Neuroinflammation. (2022) 19:215. doi: 10.1186/s12974-022-02560-3
143. Wang K, Zhou J, Xu F, Li Y, Sun H, Chen X, et al. Suppression of Microglial Activation Mitigates Pain and Anxiety in Fibromyalgia Mouse Models. Neuropharmacology. (2023) 232:109467. doi: 10.1016/j.neuropharm.2023.109467
144. Park JS, Lee SY, Kim DY, and Choi HJ. Inhibition of Microglial Tlr4 Reduces Hyperalgesia in Fibromyalgia Rats. Brain Res Bull. (2021) 173:149-58. doi: 10.1016/j.brainresbull.2021.05.012
145. Lee JH, Park HS, Cho SW, Kim SY, Song BK, Hwang JY, et al. Chronic Stress-Induced Microglial Activation Promotes Fibromyalgia-Like Pain Behaviors. Front Pharmacol. (2022) 13:949382. doi: 10.3389/fphar.2022.949382
146. Chen F, Liu Y, Wang H, Zhang X, Li J, Yang K, et al. Microglial Modulation via Cannabinoid Receptor 2 Alleviates Fibromyalgia-Related Pain. Neuroscience. (2023) 519:14-28. doi: 10.1016/j.neuroscience.2023.02.008
147. Tan L, Wang K, Zhao H, and Li X. Microglial Tnf-? Contributes to Pain Hypersensitivity in a Mouse Model of Fibromyalgia. Brain Res. (2024) 1885:148595. doi: 10.1016/j.brainres.2024.148595
148. Wu Y, Li L, Zhang X, Wang H, Chen J, Liu Q, et al. Role of Microglia in Central Sensitization and Pain Maintenance in Fibromyalgia. Pain Med. (2022) 23(9):1671-83. doi: 10.1093/pm/pnab345
149. Liu Z, Yang F, Wang J, Chen H, Zhao Y, Li M, et al. Microglial Activation Drives Fibromyalgia-Like Pain via Nf-Kb Signaling Pathway. J Neuroinflammation. (2023) 20:112. doi: 10.1186/s12974-023-02748-1
150. Chen Y, Li F, Wang K, Zhang H, Yang L, Liu J, et al. Regulation of Microglial Polarization Alleviates Fibromyalgia-Like Pain in Experimental Models. Front Cell Neurosci. (2024) 18:1198427. doi: 10.3389/fncel.2024.1198427
151. Xu Q, Ford NC, He S, Huang Q, Anderson M, Chen Z, et al. Astrocytes Contribute to Pain Gating in the Spinal Cord. Sci Adv. (2021) 7:eabi6287. doi: 10.1126/sciadv.abi6287
152. Pertwee RG. Cannabinoid Receptors and Pain. Prog Neurobiol. (2001) 63:569-611. doi: 10.1016/s0301-0082(00)00031-9
153. Russo EB. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. (2016) 1:154-65. doi: 10.1089/can.2016.0009
154. Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, et al. Vanilloid Receptors on Sensory Nerves Mediate the Vasodilator Action of Anandamide. Nature. (1999) 400:452-7. doi: 10.1038/22761
155. Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, and Porrino LJ. Cannabinoid Physiology and Pharmacology: 30 Years of Progress. Neuropharmacology. (2004) 47 Suppl 1:345-58. doi: 10.1016/j.neuropharm.2004.07.030
156. van den Hoogen NJ, Harding EK, Davidson CED, and Trang T. Cannabinoids in Chronic Pain: Therapeutic Potential through Microglia Modulation. Front Neural Circuits. (2021) 15:816747. doi: 10.3389/fncir.2021.816747
157. Ortolá R, Sotos-Prieto M, Carballo A, Cabello-Plan S, Koni A, Mustieles V, et al. Role of Serum Brain-Derived Neurotrophic Factor as a Biomarker of Chronic Pain in Older Adults. Eur J Pain. (2025) 29:e70014. doi: 10.1002/ejp.70014
158. Baumeister D, Eich W, Saft S, Geisel O, Hellweg R, Finn A, et al. No Evidence for Altered Plasma Ngf and Bdnf Levels in Fibromyalgia Patients. Sci Rep. (2019) 9:13667. doi: 10.1038/s41598-019-49403-7
159. Behnoush AH, Khalaji A, Khanmohammadi S, Alehossein P, Saeedian B, Shobeiri P, et al. Brain-Derived Neurotrophic Factor in Fibromyalgia: A Systematic Review and Meta-Analysis of Its Role as a Potential Biomarker. PLoS One. (2023) 18:e0296103. doi: 10.1371/journal.pone.0296103
160. Sarchielli P, Mancini ML, Floridi A, Coppola F, Rossi C, Nardi K, et al. Increased Levels of Neurotrophins Are Not Specific for Chronic Migraine: Evidence from Primary Fibromyalgia Syndrome. J Pain. (2007) 8:737-45. doi: 10.1016/j.jpain.2007.05.002
161. Belviranli M, Okudan N, and Sezer T. Exercise Training Alleviates Symptoms and Cognitive Decline in a Reserpine-Induced Fibromyalgia Model by Activating Hippocampal Pgc-1a/Fndc5/Bdnf Pathway. Neuroscience. (2024) 549:145-55. doi: 10.1016/j.neuroscience.2024.05.012
162. Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, et al. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell. (2013) 155:1596-609. doi: 10.1016/j.cell.2013.11.030
163. Beggs S and Salter MW. The Known Knowns of Microglia-Neuronal Signalling in Neuropathic Pain. Neurosci Lett. (2013) 557 Pt A:37-42. doi: 10.1016/j.neulet.2013.08.037
164. Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, et al. Bdnf-Induced Trkb Activation Down-Regulates the K+-Cl- Cotransporter Kcc2 and Impairs Neuronal Cl- Extrusion. J Cell Biol. (2002) 159:747-52. doi: 10.1083/jcb.200209011
165. Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipilä S, et al. Mechanism of Activity-Dependent Downregulation of the Neuron-Specific K-Cl Cotransporter Kcc2. J Neurosci. (2004) 24:4683-91. doi: 10.1523/jneurosci.5265-03.2004
166. Rivera C, Voipio J, and Kaila K. Two Developmental Switches in Gabaergic Signalling: The K+-Cl- Cotransporter Kcc2 and Carbonic Anhydrase Cavii. J Physiol. (2005) 562 Pt 1:27-36. doi: 10.1113/jphysiol.2004.077495
167. Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, et al. Activation of P75ntr by Probdnf Facilitates Hippocampal Long-Term Depression. Nat Neurosci. (2005) 8:1069-77. doi: 10.1038/nn1510
168. Panja D and Bramham CR. Bdnf Mechanisms in Late Ltp Formation: A Synthesis and Breakdown. Neuropharmacology. (2014) 76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024
169. Garraway SM, Petruska JC, and Mendell LM. Bdnf Sensitizes the Response of Lamina Ii Neurons to High Threshold Primary Afferent Inputs. Eur J Neurosci. (2003) 18:2467-76. doi: 10.1046/j.1460-9568.2003.02982.x
170. Guo W, Robbins MT, Wei F, Zou S, Dubner R, and Ren K. Supraspinal Brain-Derived Neurotrophic Factor Signaling: A Novel Mechanism for Descending Pain Facilitation. J Neurosci. (2006) 26:126-37. doi: 10.1523/jneurosci.3686-05.2006
171. Baek JH, Park H, Kang H, Kim R, Kang JS, and Kim HJ. The Role of Glutamine Homeostasis in Emotional and Cognitive Functions. Int J Mol Sci. (2024) 25:1302. doi: 10.3390/ijms25021302
172. Hansson E and Rönnbäck L. Astrocytes in Glutamate Neurotransmission. Faseb J. (1995) 9:343-50. doi: 10.1096/fasebj.9.5.7534736
173. Zhu HH, Song XK, Zhang JL, Zhang JL, Rong PJ, Wang JY, et al. Involvement of the Astroglial Glutamate-Glutamine Cycle in the Analgesic Effects of Electroacupuncture in a Rat Model of Chronic Neuropathic Pain. Acupunct Med. (2025) 43:14-25. doi: 10.1177/09645284241309952
174. Miller S, Bridges RJ, and Cotman CW. Stimulation of Phosphoinositide Hydrolysis by Trans-(+/-)-Acpd Is Greatly Enhanced When Astrocytes Are Cultured in a Serum-Free Defined Medium. Brain Res. (1993) 618:175-8. doi: 10.1016/0006-8993(93)90443-q
175. O'Connor ER and Kimelberg HK. Role of Calcium in Astrocyte Volume Regulation and in the Release of Ions and Amino Acids. J Neurosci. (1993) 13:2638-50. doi: 10.1523/jneurosci.13-06-02638.1993
176. Fellin T. Communication between Neurons and Astrocytes: Relevance to the Modulation of Synaptic and Network Activity. J Neurochem. (2009) 108:533-44. doi: 10.1111/j.1471-4159.2008.05830.x
177. Tanabe Y, Masu M, Ishii T, Shigemoto R, and Nakanishi S. A Family of Metabotropic Glutamate Receptors. Neuron. (1992) 8:169-79. doi: 10.1016/0896-6273(92)90118-w
178. Kadota K and Gomi H. Implicit Visuomotor Processing for Quick Online Reactions Is Robust against Aging. J Neurosci. (2010) 30:205-9. doi: 10.1523/jneurosci.2599-09.2010
179. Struzynska L and Sulkowski G. Relationships between Glutamine, Glutamate, and Gaba in Nerve Endings under Pb-Toxicity Conditions. J Inorg Biochem. (2004) 98:951-8. doi: 10.1016/j.jinorgbio.2004.02.010
180. Yan X, Jiang E, and Weng HR. Activation of Toll Like Receptor 4 Attenuates Gaba Synthesis and Postsynaptic Gaba Receptor Activities in the Spinal Dorsal Horn Via Releasing Interleukin-1 Beta. J Neuroinflammation. (2015) 12:222. doi: 10.1186/s12974-014-0222-3
181. Shimizu-Okabe C, Kobayashi S, Kim J, Kosaka Y, Sunagawa M, Okabe A, et al. Developmental Formation of the Gabaergic and Glycinergic Networks in the Mouse Spinal Cord. Int J Mol Sci. (2022) 23:834. doi: 10.3390/ijms23020834
182. Aubrey KR. Presynaptic Control of Inhibitory Neurotransmitter Content in Viaat Containing Synaptic Vesicles. Neurochem Int. (2016) 98:94-102. doi: 10.1016/j.neuint.2016.06.002
183. Sivilotti L and Woolf CJ. The Contribution of Gabaa and Glycine Receptors to Central Sensitization: Disinhibition and Touch-Evoked Allodynia in the Spinal Cord. J Neurophysiol. (1994) 72:169-79. doi: 10.1152/jn.1994.72.1.169
184. Torsney C and MacDermott AB. Disinhibition Opens the Gate to Pathological Pain Signaling in Superficial Neurokinin 1 Receptor-Expressing Neurons in Rat Spinal Cord. J Neurosci. (2006) 26:1833-43. doi: 10.1523/jneurosci.4584-05.2006
185. Chirila AM, Brown TE, Bishop RA, Bellono NW, Pucci FG, and Kauer JA. Long-Term Potentiation of Glycinergic Synapses Triggered by Interleukin 1ß. Proc Natl Acad Sci U S A. (2014) 111:8263-8. doi: 10.1073/pnas.1401013111
186. Reinold H, Ahmadi S, Depner UB, Layh B, Heindl C, Hamza M, et al. Spinal Inflammatory Hyperalgesia Is Mediated by Prostaglandin E Receptors of the Ep2 Subtype. J Clin Invest. (2005) 115:673-9. doi: 10.1172/jci23618
187. Black CJ and Ford AC. An Evidence-Based Update on the Diagnosis and Management of Irritable Bowel Syndrome. Expert Rev Gastroenterol Hepatol. (2025) :1-16. doi: 10.1080/17474124.2025.2455586
188. Ramasawmy P, Khalid S, Petzke F, and Antal A. Pain Reduction in Fibromyalgia Syndrome through Pairing Transcranial Direct Current Stimulation and Mindfulness Meditation: A Randomized, Double-Blinded, Sham-Controlled Pilot Clinical Trial. Front Med (Lausanne). (2022) 9:908133. doi: 10.3389/fmed.2022.908133
189. Theadom A, Cropley M, Smith HE, Feigin VL, and McPherson K. Mind and Body Therapy for Fibromyalgia. Cochrane Database Syst Rev. (2015) 2015:Cd001980. doi: 10.1002/14651858.CD001980.pub3
190. Aboutaleb AS, Allam A, Zaky HS, Harras MF, Farag FSA, Abdel-Sattar SA, et al. Novel Insights into the Molecular Mechanisms Underlying Anti-Nociceptive Effect of Myricitrin against Reserpine-Induced Fibromyalgia Model in Rats: Implication of Sirt1 and Mirnas. J Ethnopharmacol. (2024) 335:118623. doi: 10.1016/j.jep.2024.118623
191. Inferrera F, Tranchida N, Fusco R, Cuzzocrea S, Crimi N, Fruciano M, et al. Acute Oral Administration of Capsaicin Modulates Nociceptive and Inflammatory Pain through Spinal and Peripheral Mechanisms. Phytother Res. (2022) 36:4709-24. doi: 10.1002/ptr.7521
192. Caumo W, Leite J, Schmidt AP, Steffens AA, Castro MC, Salvato FM, et al. Mindfulness-Based Stress Reduction in Fibromyalgia: Mechanisms and Effects on Pain Modulation. J Pain. (2017) 18:601-12. doi: 10.1016/j.jpain.2017.01.008
193. Glombiewski JA, Schäfer SM, Eisenlohr-Moul TA, Terhorst Y, and Schmutzer K. Psychological Interventions for Chronic Pain in Fibromyalgia: A Meta-Analysis. Clin J Pain. (2013) 29:102-12. doi: 10.1097/AJP.0b013e318247c9e1
194. De Santis A, Di Cesare Mannelli L, Pacini A, and Ghelardini C. Pharmacological Treatment of Fibromyalgia: Current Evidence and Future Directions. Expert Opin Pharmacother. (2017) 18:789-802. doi: 10.1080/14656566.2017.1296203
195. Bannister K, Sikandar S, Rees H, Hubbard J, and Page AJ. Descending Control of Pain. Prog Neurobiol. (2016) 143:25-45. doi: 10.1016/j.pneurobio.2016.01.002
196. Tracey I. The Role of the Descending Pain Modulatory System in Pain Perception and Chronic Pain. Curr Opin Support Palliat Care. (2018) 12:120-7. doi: 10.1097/SPC.0000000000000355
197. Millan MJ. The Neurobiology and Control of Pain. Prog Neurobiol. (2002) 66:1-80. doi: 10.1016/s0301-0082(02)00009-6
198. Lee JH, Kim HS, Park JY, Kim J, Lee KY, Park SY, et al. Role of Descending Pain Modulation in Fibromyalgia. J Pain Res. (2020) 13:305-16. doi: 10.2147/JPR.S234981
199. Jones AC, Harris RE, and Clark JD. Altered Descending Pain Modulation in Fibromyalgia Patients: Evidence from Functional Imaging. Arthritis Rheum. (2011) 63:2961-9. doi: 10.1002/art.30489
200. Staud R, Vierck CJ, and Price DD. Predicting Responses to Pain in Fibromyalgia: Central Sensitization and Descending Modulation. Arthritis Rheum. (2003) 48:1420-30. doi: 10.1002/art.10922
Keywords: central sensitization, chronic pain, nociplastic pain, non-neuronal cells, synaptic plasticity
Citation: Lai W, Tang X, Chen S, Zhou Y, Qi Z, Cao M, Yuan C, Yu J and Wen C (2026) Nociplastic pain: the prominent role of non-neuronal cells in central and peripheral sensitization. Front. Immunol. 17:1677310. doi: 10.3389/fimmu.2026.1677310
Received: 29 August 2025; Accepted: 06 January 2026; Revised: 25 December 2025;
Published: 27 January 2026.
Edited by:
Elena Lucarini, University of Florence, ItalyReviewed by:
Wei Nan, Lanzhou University Second Hospital, ChinaVittoria Borgonetti, University of Siena, Italy
Copyright © 2026 Lai, Tang, Chen, Zhou, Qi, Cao, Yuan, Yu and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng-Ping Wen, V2VuZ2NwQDE2My5jb20=; Jie Yu, eXVqaWVAemNtdS5lZHUuY24=
†These authors have contributed equally to this work
 Weidong Lai
Weidong Lai Xin Tang†
Xin Tang† Sijia Chen
Sijia Chen Cunrui Yuan
Cunrui Yuan Jie Yu
Jie Yu