Abstract
Backgrounds:
This study aimed to evaluate efficacy and safety of early radiotherapy combined with first-line chemo-immunotherapy in patients with extensive-stage small-cell lung cancer (ES-SCLC).
Methods:
ES-SCLC patients treated with first-line chemo-immunotherapy from August 2018 to January 2024 at five centers were included. Patients receiving early radiotherapy administered before disease progression were categorized into RT group, and were further separately stratified by the receipt of thoracic radiotherapy (TRT) and extra thoracic radiotherapy (eTRT). Propensity score matching (PSM) was performed to balance potential bias.
Results:
Totally, 771 patients were enrolled. RT group exhibited significantly better progression-free survival (PFS, p<0.001) and overall survival (OS, p<0.001) compared to Non-RT group in the whole and post-PSM cohort. The survival benefit was also observed in TRT group versus Non-TRT group, and eTRT group versus Non-eTRT group. Exploratory analyses were conducted within the TRT group. Consolidative TRT was associated with superior PFS (p<0.001) and OS (p=0.006) compared to the concurrent treatment. Additionally, survival outcomes were comparable between patients receiving a biological effective dose (BED) below or above the median dose of 60Gy and the salvage dose of 39Gy. No significance was observed in overall adverse events between RT group and Non-RT group, despite a higher rate of all-grade pneumonitis in RT group (9.9% versus 4.2%, p=0.002).
Conclusions:
Early radiotherapy, either thoracic or extra thoracic radiotherapy, combined with first-line chemo-immunotherapy constituted an effective and well-tolerated strategy for patients with ES-SCLC. These findings warrant investigation in prospective randomized trials.
Introduction
Small-cell lung cancer (SCLC), accounting for approximately 15% of all new lung cancer cases, is marked by its highly aggressive nature and poor prognosis (1). In the majority of cases, patients are initially diagnosed with metastatic extensive-stage disease (2). Etoposide-platinum chemotherapy has been the mainstay therapy for extensive-stage SCLC (ES-SCLC) over the past few decades, with an unfavorable median overall survival (OS) of approximately 10 months (3). Advent of immune checkpoint inhibitors (ICIs) has led to establishment of immunotherapy in conjunction with chemotherapy as the standard of care for ES-SCLC, as evidenced by landmark IMpower133, CASPIAN, CAPSTONG-1, ASTRUM-005, RATIONALE-312, as well as EXTENTORCH studies (4–9). This combination has been demonstrated to improve OS by 1–3 months compared to chemotherapy alone. Nevertheless, the enhancement was modest, and many patients would develop early resistance to systemic therapy within a few months (10). And a large proportion of patients with ES-SCLC would experience primary tumor and regional lymph nodes progression (11). Consequently, it’s eagerly anticipated for new regimens to address these issues.
Radiotherapy (RT) is a promising strategy for ES-SCLC. Before the emergence of ICIs, the phase III CREST study illuminated efficacy of radiotherapy in treatment of ES-SCLC. This study demonstrated that integration of thoracic radiotherapy with chemotherapy significantly improved 2-year OS rate (p=0.004) and induced an almost 50% reduction in intrathoracic recurrences compared to chemotherapy alone (12). In the era of immunotherapy, the value of radiotherapy has also been demonstrated with substantial evidence ranging from preclinical researches to real-world practices (13–16). Several retrospective studies have elucidated that addition of radiotherapy could improve survival outcomes of ES-SCLC patients receiving first-line chemo-immunotherapy (17–21). A recent prospective phase II study, which enrolled 67 ES-SCLC patients receiving first-line chemo-immunotherapy, has shown improvement of progression-free survival (PFS) and OS along with addition of consolidative thoracic radiotherapy (22). However, most current studies were presented with small sample sizes, and there is limited evidence on irradiated sites, optimal timing and dose of radiotherapy when combined with chemo-immunotherapy. This large, multi-center, real-world study aimed to evaluate the efficacy and safety of radiotherapy in ES-SCLC patients with first-line treatment of chemo-immunotherapy, with an attempt to identify optimal timing and dose of radiotherapy.
Materials and methods
Patients
In this cohort study, we reviewed medical records of patients who received first-line chemo-immunotherapy at five hospitals in China between August 2018 and January 2024. The inclusion criteria were: (I) aged 18 years or older, (II) cytologically or pathologically confirmed SCLC, (III) diagnosed with extensive stage according to Veterans Administration Lung Study Group [VALG] staging system, (IV) treated with chemo-immunotherapy as first-line therapy, and (V) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. The exclusion criteria were as follows: (I) a history of or current diagnosis of other malignancies, (II) received immunotherapy during limited-stage disease, and (III) lack of detailed treatment information. This study adhered to the principles of Declaration of Helsinki and was approved by the Ethical Review Boards and Institutional Review Boards of the participating facilities in this study. The informed consent process was aligned with standard clinical practice.
Treatment
The eligible patients received first-line platinum-based double-agent chemotherapy regimens combined with either anti-PD1 or anti-PD-L1 immunotherapy. According to the receipt of early radiotherapy, patients were grouped into RT group and Non-RT group. Stratified by administration of thoracic radiotherapy (TRT) and extra thoracic radiotherapy (eTRT) before disease progression, patients were further separately categorized into: TRT group vs Non-TRT group, and eTRT group vs Non-eTRT group. The treatment regimens and adjustments were determined by physicians in accordance with guidelines of the National Comprehensive Cancer Network (NCCN) or the Chinese Society of Clinical Oncology (CSCO). Irradiated sites and doses were determined by physicians based on comprehensive evaluation of each patient’s clinical data. Given the heterogeneous radiation fractionations, the biological effective dose (BED) formula was used: BED = nd × [1 + d/(α/β)] (23), where n represents the number of fractions, d is the dose per fraction, and α/β is set to 10. Moreover, according to the prescribed dose per fraction, radiotherapy was classified into conventional fractionated RT (1.8-2.2 Gy per fraction), hyperfractionated RT (≤1.5 Gy per fraction) and hypofractionated RT (≥2.5 Gy per fraction) (24). In order to further investigate the optimal timing of thoracic radiotherapy, the TRT group was subdivided into concurrent TRT subset (≤4 cycles of systemic therapy) and consolidative TRT subset (>4 cycles of systemic therapy) based on the interval from the initiation of chemo-immunotherapy to thoracic radiotherapy. For exploratory analysis of radiation dose, the TRT group was categorized into BED<60Gy group and BED≥60Gy group separated by median BED dose 60Gy, or BED ≤ 39Gy group and BED>39Gy group based on salvage prescription.
Assessment
PFS was defined as the interval from the date of initiation of immunotherapy to disease progression, death or the last-known follow-up date. OS was defined as the interval from the day of initiation of immunotherapy to the date of death due to any cause or the last follow-up date. The treatment-related adverse events (TRAEs) were evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 5.0).
Statistical analysis
Continuous variables were presented as median with interquartile range (IQR). Categorical variables were presented as frequency and percentage. The Student’s t test or Chi-square test was used for comparison as appropriate. PFS and OS were estimated using the Kaplan-Meier method and compared via the log-rank test. Univariate and multivariate analyses were performed using Cox proportional-hazards models. Subgroup analyses were conducted. Propensity score matching (PSM) was performed using baseline characteristics with p-value less than 0.05 to minimize bias, with a caliper of 0.02 between groups. All statistical analyses were conducted using R Studio (version 4.4.1, R Core Team, Austria), with two-sided p-values and a significance level of 0.05.
Results
Patients
From August 2018 to January 2024, a total of 771 patients with ES-SCLC receiving first-line chemo-immunotherapy were enrolled in this study. Among them, 292 patients received radiotherapy before disease progression and were categorized into RT group, and the other 479 patients in Non-RT group. The baseline characteristics of patients were listed in Table 1. A total of 343 (44.5%) patients aged more than 65 years and 670 (86.9%) patients were male. At baseline, 130 patients (16.9%) had brain metastases, 111 patients (14.4%) had lung metastases, 219 patients (28.4%) had bone metastases and 215 patients (27.9%) had liver metastases. 507 patients (65.8%) received anti-PDL1 immunotherapy, while the others received anti-PD1 treatment.
Table 1
| Characteristics | Total | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|---|
| RT(N = 292) | Non-RT(N = 479) | p | RT(N = 247) | Non-RT(N = 247) | p | ||
| Age, n(%) | |||||||
| <65 years | 428 (55.5) | 179 (61.3) | 249 (52.0) | 0.014 | 151 (61.1) | 149 (60.3) | 0.927 |
| ≥65 years | 343 (44.5) | 113 (38.7) | 230 (48.0) | 96 (38.9) | 98 (39.7) | ||
| Gender, n(%) | |||||||
| female | 101 (13.1) | 41 (14.0) | 60 (12.5) | 0.621 | 30 (12.1) | 33 (13.4) | 0.787 |
| male | 670 (86.9) | 251 (86.0) | 419 (87.5) | 217 (87.9) | 214 (86.6) | ||
| Smoker, n(%) | |||||||
| no | 288 (37.4) | 106 (36.3) | 182 (38.0) | 0.693 | 87 (35.2) | 96 (38.9) | 0.456 |
| yes | 483 (62.6) | 186 (63.7) | 297 (62.0) | 160 (64.8) | 151 (61.1) | ||
| Brain metastasis, n(%) | |||||||
| no | 641 (83.1) | 203 (69.5) | 438 (91.4) | <0.001 | 203 (82.2) | 206 (83.4) | 0.812 |
| yes | 130 (16.9) | 89 (30.5) | 41 (8.6) | 44 (17.8) | 41 (16.6) | ||
| Lung metastasis, n(%) | |||||||
| no | 660 (85.6) | 267 (91.4) | 393 (82.0) | <0.001 | 224 (90.7) | 229 (92.7) | 0.514 |
| yes | 111 (14.4) | 25 (8.6) | 86 (18.0) | 23 (9.3) | 18 (7.3) | ||
| Bone metastasis, n(%) | |||||||
| no | 552 (71.6) | 225 (77.1) | 327 (68.3) | 0.011 | 184 (74.5) | 183 (74.1) | 1.000 |
| yes | 219 (28.4) | 67 (22.9) | 152 (31.7) | 63 (25.5) | 64 (25.9) | ||
| Liver metastasis, n(%) | |||||||
| no | 556 (72.1) | 235 (80.5) | 321 (67.0) | <0.001 | 192 (77.7) | 189 (76.5) | 0.830 |
| yes | 215 (27.9) | 57 (19.5) | 158 (33.0) | 55 (22.3) | 58 (23.5) | ||
| Type of ICIs, n(%) | |||||||
| anti-PDL1 | 507 (65.8) | 199 (68.2) | 308 (64.3) | 0.310 | 173 (70.0) | 168 (68.0) | 0.697 |
| anti-PD1 | 264 (34.2) | 93 (31.8) | 171 (35.7) | 74 (30.0) | 79 (32.0) | ||
Baseline characteristics of RT group and Non-RT group before and after PSM.
RT, radiotherapy; ICIs, immune checkpoint inhibitors; PDL1, programmed cell death ligand 1; PD1, programmed cell death 1; PSM, propensity score matching.
To be more specific, 212 patients received early thoracic radiotherapy before disease progression in the TRT group, and the other 559 patients in the Non-TRT group. 203 patients had a documented TRT regimen. Among them, 97 patients (47.8%) received conventional fractionated TRT (40.0-61.6 Gy at 1.8-2.2 Gy per fraction, BED = 48-75.15 Gy), 33 patients (16.2%) received hyperfractionated RT (39.0-60.0Gy at 1.5Gy per fraction, BED = 44.85-68.0Gy) and 73 patients (36.0%) received hypofractionated TRT (15.0-84.0Gy at 2.5-15Gy per fraction, BED = 19.5-187.5Gy). The eTRT group consisted of 90 patients who underwent extra thoracic radiotherapy before disease progression. The predominantly irradiated metastatic site was brain (n=65), followed by bone (n=17). Totally, 12 patients received radiotherapy at more than one site.
Survival outcomes
At the data cutoff, the median follow-up period was 18.5 (IQR: 17.4-20.4) months. The median PFS was significantly longer in RT group compared to Non-RT group (12.1 months vs 6.0 months), with a hazard ratio (HR) of 0.44 (Figure 1A, 95% CI:0.38-0.52, p<0.001). The one-year PFS rate was 37.2% in RT group versus 13.0% in Non-RT group, while the two-year PFS rate was 9.7% versus 4.0%, respectively. After PSM, improved PFS was also observed, with median PFS of 11.0 months in RT group and 6.0 months in Non-RT group (Figure 1B, HR = 0.46, 95%CI:0.37-0.57, p<0.001). Univariate and multivariate cox analysis (Supplementary Table 1) for PFS revealed that male sex (HR = 1.59, 95%CI:1.22-2.08, p<0.001), and baseline brain (HR = 1.32, 95%CI:1.04-1.67, p=0.024), bone (HR = 1.40, 95%CI:1.16-1.68, p<0.001) or liver metastasis (HR = 1.25, 95%CI:1.04-1.50, p=0.018) were associated with poorer PFS, while early radiotherapy (HR = 0.41, 95%CI:0.34-0.50, p<0.001) was correlated with improved PFS. Subgroup analysis for PFS (Figure 1C) indicated that most subgroups could benefit from early radiotherapy.
Figure 1
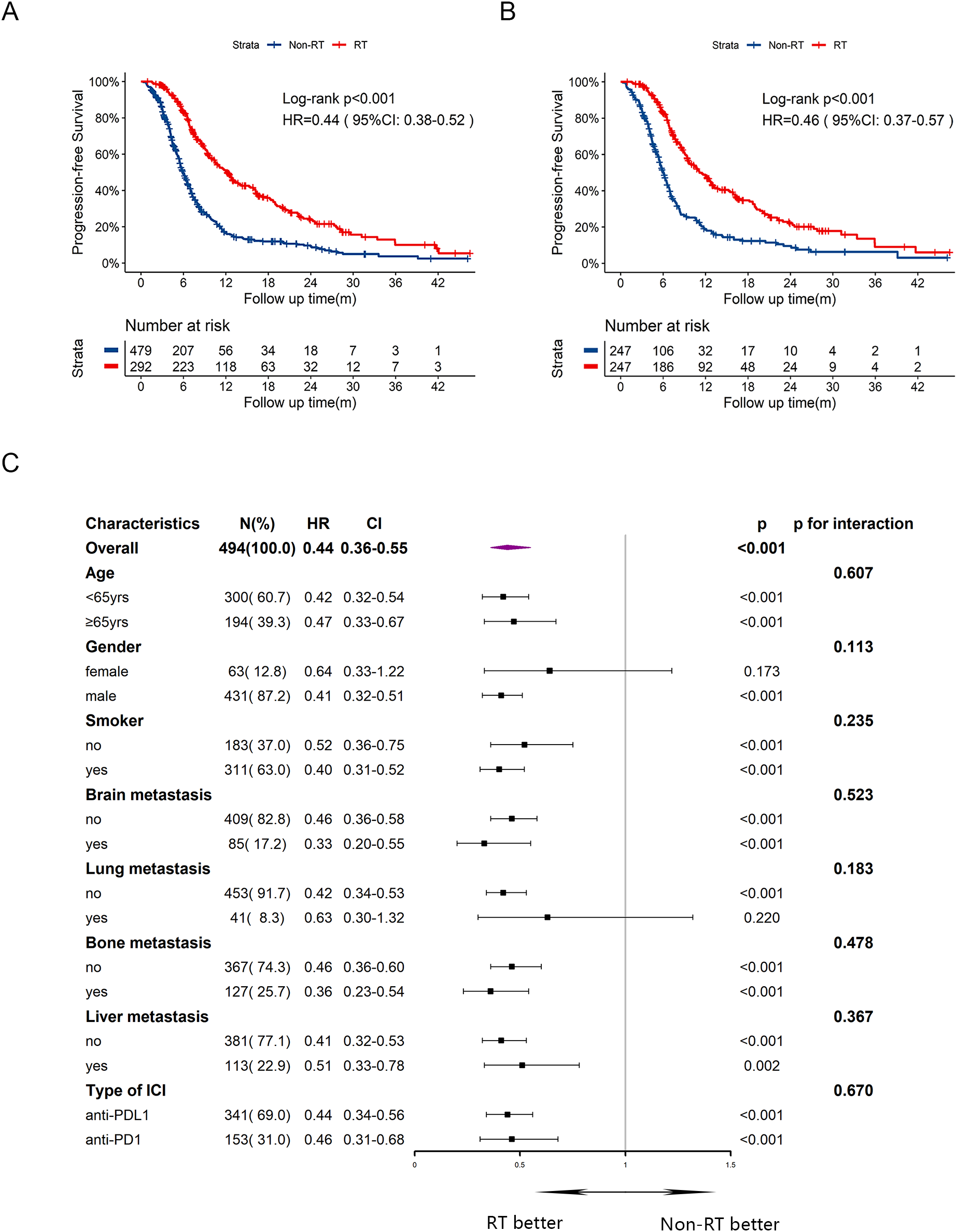
Kaplan-Meier survival curves of PFS in RT group and Non-RT group before and after PSM, and subgroup analysis of PFS in the post-PSM cohort. (A) Kaplan-Meier curve of PFS before PSM. (B) Kaplan-Meier curve of PFS after PSM. (C) Subgroup analysis of PFS after PSM. yrs, years; m, months; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; RT, radiotherapy.
In terms of OS, the median OS was 24.3 months in RT group and 16.0 months in Non-RT group, with a HR of 0.54 (Figure 2A, 95%CI:0.44-0.67, p<0.001). The one-year and two-year OS rates were 55.9% and 19.0%, respectively, in RT group, compared to 44.1% and 11.7%, respectively, in Non-RT group. After PSM, patients in RT group still had prolonged OS with median time of 26.8 months, compared to 16.3 months in Non-RT group (Figure 2B, HR = 0.53, 95%CI:0.40-0.70, p<0.001). In multivariate cox analysis (Supplementary Table 1) for OS, male sex (HR = 1.82, 95%CI:1.25-2.64, p=0.002) and liver metastasis at baseline (HR = 1.64, 95%CI:1.30-2.05, p<0.001) were independently risk factors for OS. Early radiotherapy was confirmed as an independently favorable predictor for OS (HR = 0.57, 95%CI: 0.45-0.72, p<0.001). Subgroup analysis for OS (Figure 2C) exhibited that early radiotherapy improved OS in most subgroups except for female and those with brain or lung metastasis at baseline, or receiving anti-PDL1 therapy. Notably, patients with bone or liver metastasis at baseline could also benefit from early radiotherapy.
Figure 2
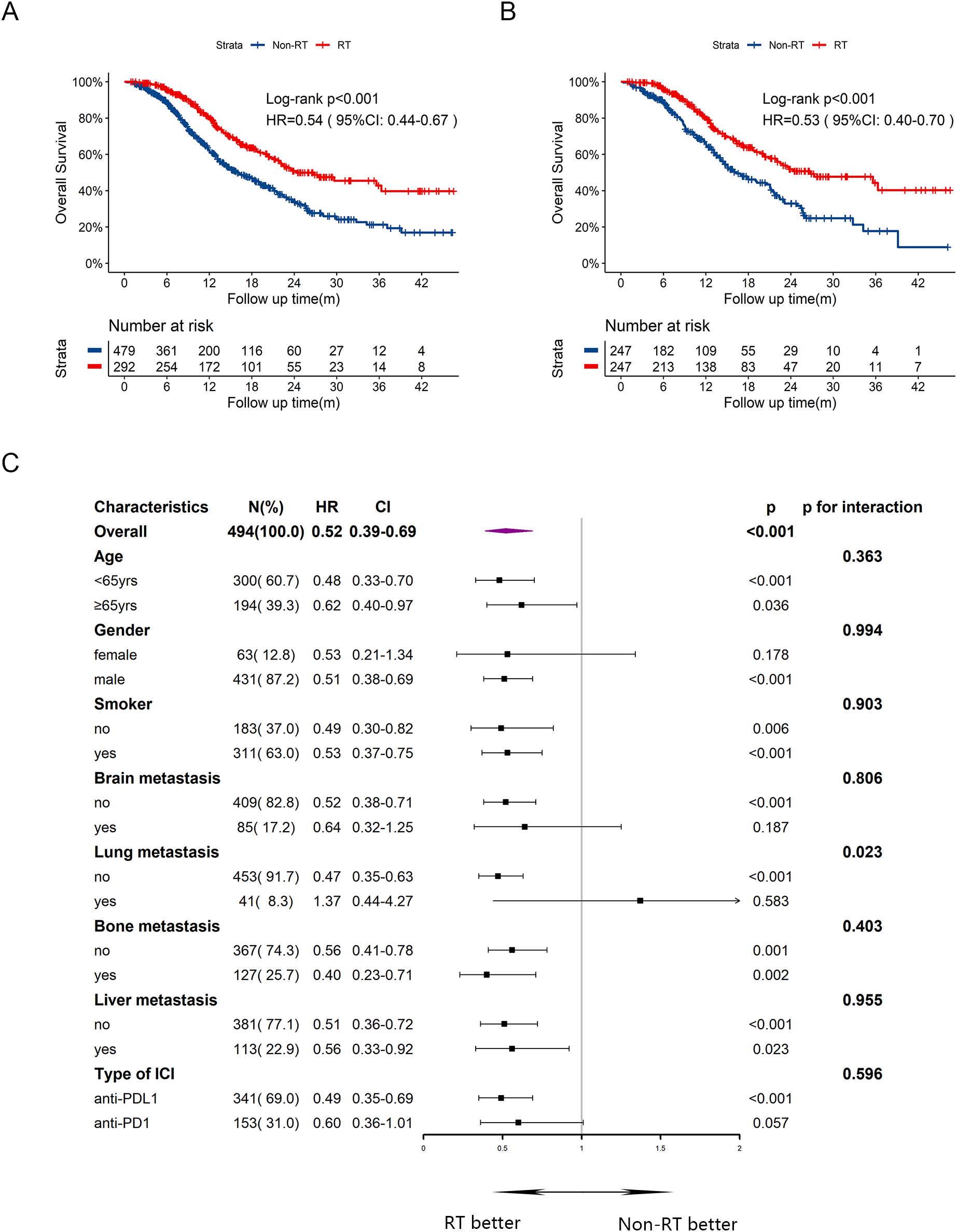
Kaplan-Meier survival curves of OS in RT group and Non-RT group before and after PSM, and subgroup analysis of OS in the post-PSM cohort. (A) Kaplan-Meier curve of OS before PSM. (B) Kaplan-Meier curve of OS after PSM. (C) Subgroup analysis of OS after PSM. yrs, years; m, months; OS, overall survival; HR, hazard ratio; CI, confidence interval; RT, radiotherapy.
With the aim to define the role of thoracic radiotherapy, comparisons between TRT group (N = 212) and Non-TRT group (N = 559) were performed (Supplementary Table 2). Similarly, TRT group exhibited significantly longer PFS (Figure 3A, 12.7 months vs 6.5 months, HR = 0.45, 95% CI:0.38-0.53, p<0.001) and OS (Figure 3B, 26.8 months vs 17.3 months, HR = 0.56, 95%CI:0.45-0.70, p<0.001) compared with those in the Non-TRT group. After PSM, significant improvements in PFS (Figure 3C, 26.8 months vs 6.5 months, HR = 0.47, 95%CI:0.39-0.56, p<0.001) and OS (Figure 3D, 26.8 months vs 17.1 months, HR = 0.56, 95%CI:0.44-0.71, p<0.001) persisted in TRT group.
Figure 3
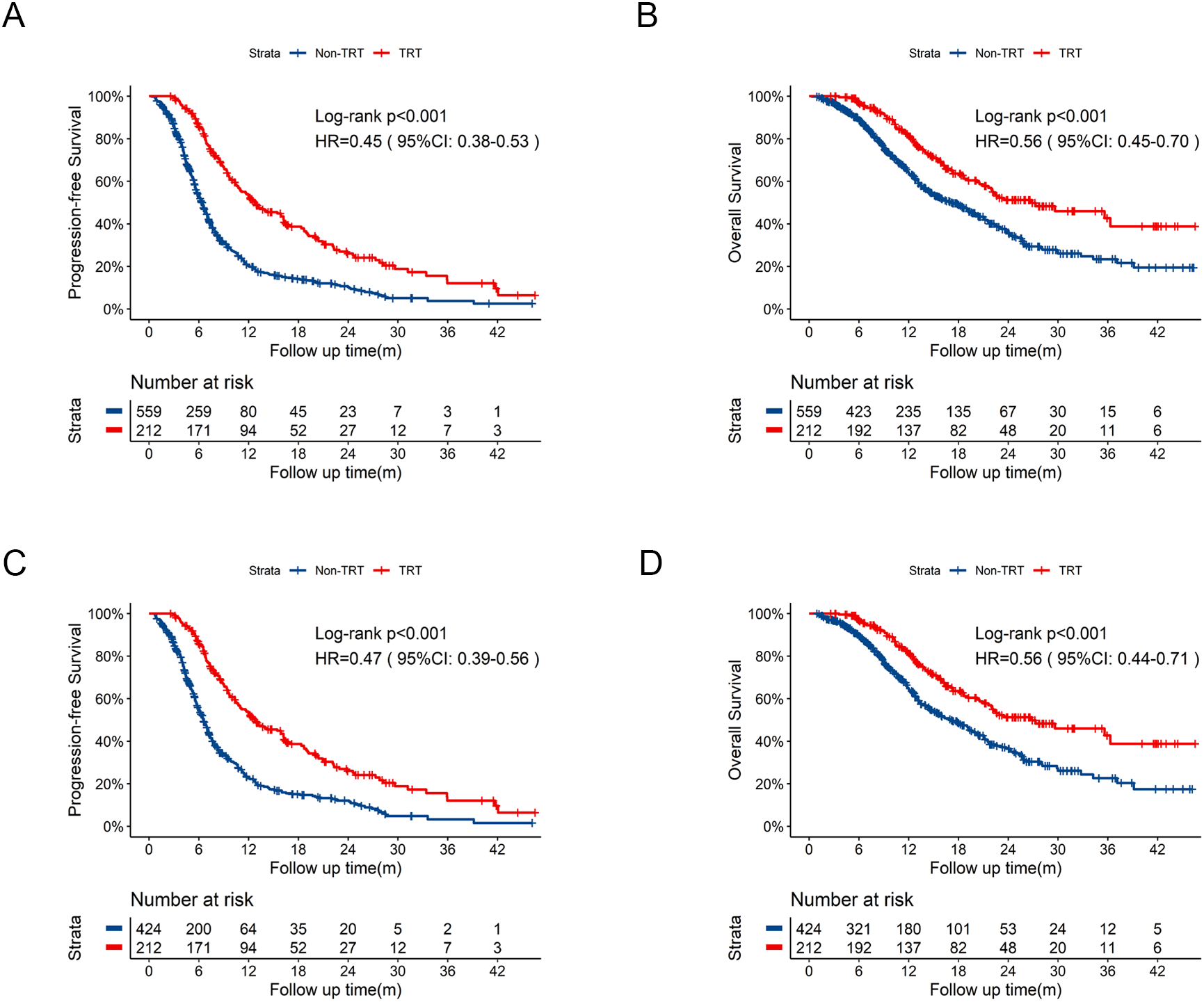
Kaplan-Meier survival curves of PFS and OS in TRT group compared to Non-TRT group before and after PSM. (A) Kaplan-Meier curve of PFS in TRT group compared to Non-TRT group before PSM. (B) Kaplan-Meier curve of OS in TRT group compared to Non-TRT group before PSM. (C) Kaplan-Meier curve of PFS in TRT group compared to Non-TRT group after PSM. (D) Kaplan-Meier curve of OS in TRT group compared to Non-TRT group after PSM. PSM, propensity score matching; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; TRT, thoracic radiotherapy; m, months.
Further comparisons between eTRT group (N = 90) and Non-eTRT group (N = 681) were conducted. eTRT group exhibited an extended PFS of 10.5 months vs 7.2 months (Figure 4A, HR = 0.71, 95%CI: 0.56-0.89, p=0.010) compared to Non-eTRT group, whereas OS was not statistically significant of 24.3 months vs 19.0 months (Figure 4B, HR = 0.70, 95%CI:0.49-0.98, p=0.073). With well-balanced baseline characteristics in Supplementary Table 3, significantly improved PFS (Figure 4C, HR = 0.52, 95%CI: 0.36-0.75, p<0.001) and OS (Figure 4D, HR = 0.56, 95%CI: 0.34-0.94, p=0.033) both were observed in eTRT group.
Figure 4
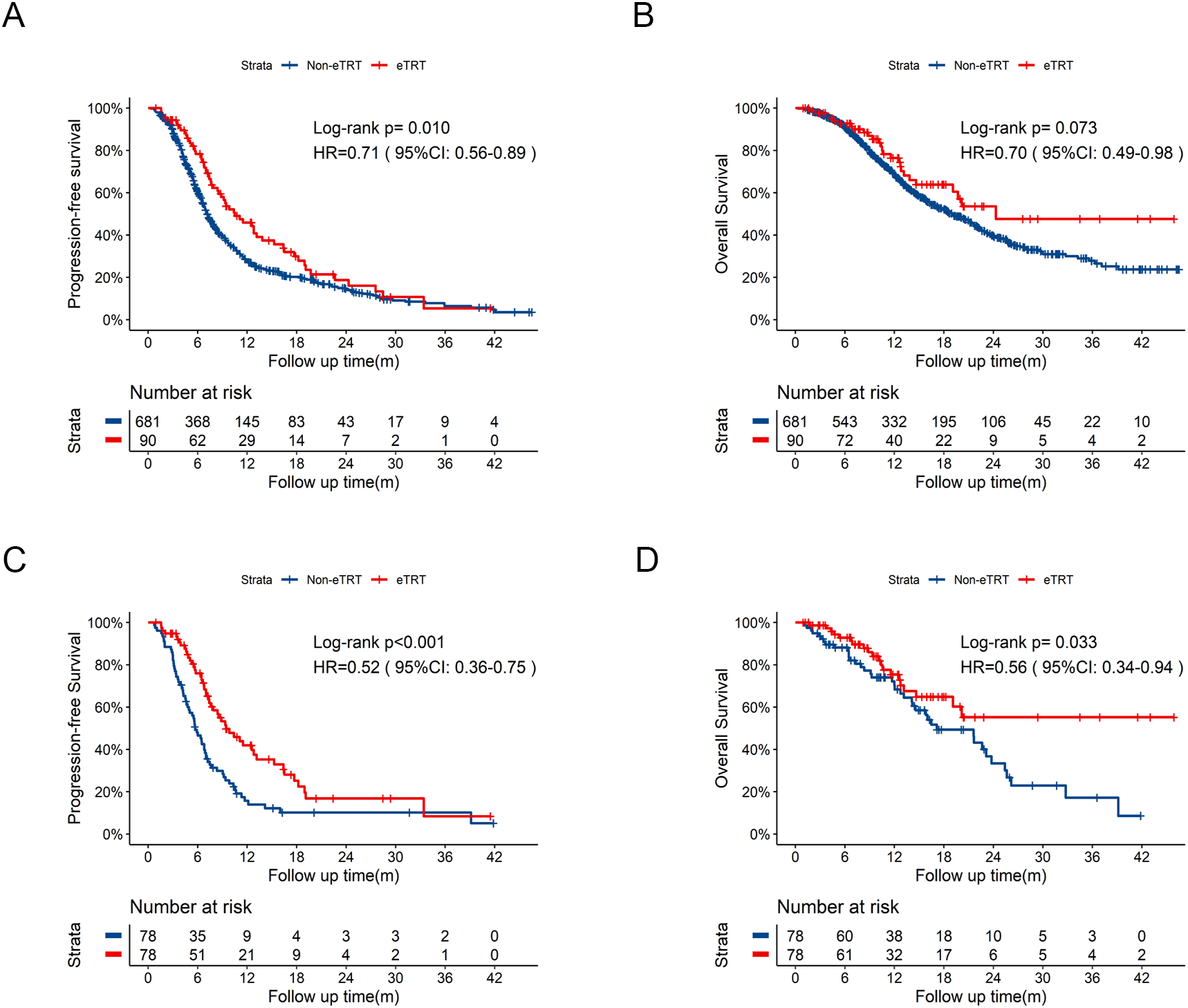
Kaplan-Meier survival curves of PFS and OS in eTRT group compared to Non-eTRT group before and after PSM. (A) Kaplan-Meier curve of PFS in eTRT group compared to Non-eTRT group before PSM. (B) Kaplan-Meier curve of OS in eTRT group compared to Non-eTRT group before PSM. (C) Kaplan-Meier curve of PFS in eTRT group compared to Non-eTRT group after PSM. (D) Kaplan-Meier curve of OS in eTRT group compared to Non-eTRT group after PSM. PSM, propensity score matching; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; eTRT, extra thoracic radiotherapy; m, months.
Exploratory analysis
To further explore optimal timing and dose of thoracic radiotherapy, we conducted additional analysis in subgroups of TRT group. TRT group was divided into two subsets based on the interval from the initiation of immunotherapy to radiotherapy: concurrent TRT subset (≤4 cycles of systemic therapy) and consolidative TRT subset (>4 cycles of systemic therapy). Also, PSM was performed and the baseline characteristics were listed in Supplementary Table 4. In the PSM cohort, consolidative TRT showed significantly prolonged median PFS (Supplementary Figure 1A) of 18.3 months vs 8.8 months (HR = 0.50, 95%CI:0.34-0.72, p<0.001). Consolidative TRT was associated with significantly longer OS (Supplementary Figure 1B, 35.6 vs 20.9 months; HR = 0.52, 95%CI:0.32-0.84, p=0.006) compared to the concurrent TRT subset.
A total of 203 patients in the TRT group with recorded BED data were included to assess the correlation between BED and survival outcomes. The median BED of TRT group was 60Gy. With comparable characteristics of those who received BED≥60Gy and BED<60Gy (Supplementary Table 5), no significant benefit was observed in PFS (Supplementary Figure 1C, p=0.832) and OS (Supplementary Figure 1D, p=0.210) associated with higher BED. Additionally, subsets with BED ≤ 39Gy or BED>39Gy in TRT group were compared, of which baseline characteristics were shown in Supplementary Table 6. After PSM, no significant difference was observed in PFS (Supplementary Figure 1E, p=0.413) and OS (Supplementary Figure 1F, p=0.447).
Safety
At the cut-off date, no treatment-related deaths were recorded. The toxicity profiles were shown in Table 2. Patients in Non-RT group experienced more treated-related adverse events compared to RT group (p<0.001). Grade 3 or higher adverse events between two groups were similar (p=0.198). The most common treatment-related adverse events were hematological toxicities, with 60 patients (20.5%) in RT group and 190 patients (39.7%) in Non-RT group. Additionally, pneumonitis occurred in 29 patients (9.9%) in RT group and 20 patients (4.2%) in Non-RT group, with a statistically significance (p=0.002). While the incidence of grade 3 or higher pneumonitis was not significant between the two groups (5.5% vs 2.5%, p=0.052).
Table 2
| Adverse events | RT (N = 292,%) | Non-RT (N = 479,%) | p |
|---|---|---|---|
| All grades | |||
| Adverse events | 94 (32.2) | 233 (48.6) | <0.001 |
| Hematological toxicities | 60 (20.5) | 190 (39.7) | <0.001 |
| Pneumonitis | 29 (9.9) | 20 (4.2) | 0.002 |
| Hepatic dysfunction | 6 (2.1) | 15 (3.1) | 0.507 |
| Rash | 5 (1.7) | 17 (3.5) | 0.207 |
| Esophagitis | 4 (1.4) | 2 (0.4) | 0.300 |
| Hypothyroidism | 5 (1.7) | 11 (2.3) | 0.771 |
| Diarrhea | 4 (1.4) | 1 (0.2) | 0.137 |
| Nausea or vomiting | 4 (1.4) | 12 (2.5) | 0.417 |
| Fatigue | 4 (1.4) | 9 (1.9) | 0.807 |
| Dizziness | 1 (0.3) | 4 (0.8) | 0.716 |
| Hyperthyroidism | 1 (0.3) | 1 (0.2) | 1.000 |
| Constipation | 1 (0.3) | 4 (0.8) | 0.716 |
| ≥Grade 3 | |||
| Adverse events≥Grade 3 | 48 (16.4) | 98 (20.5) | 0.198 |
| Hematological toxicities | 33 (11.3) | 86 (18.0) | 0.017 |
| Pneumonitis | 16 (5.5) | 12 (2.5) | 0.052 |
| Hepatic dysfunction | 2 (0.7) | 1 (0.2) | 0.664 |
| Rash | 2 (0.7) | 2 (0.4) | 1.000 |
| Diarrhea | 1 (0.3) | 1 (0.2) | 1.000 |
Treatment-related adverse events in RT and Non-RT group.
RT, radiotherapy.
Discussion
To our knowledge, this multi-center study is the largest real-world study to explore the efficacy and safety of early radiotherapy combined with first-line chemo-immunotherapy in ES-SCLC. The results demonstrated that early radiotherapy, whether thoracic or extra thoracic, conferred a superior survival advantage in patients with ES-SCLC receiving first-line chemo-immunotherapy with a tolerable safety profile.
ES-SCLC is an aggressive malignancy with poor prognosis. In the era of chemotherapy, Han et al. proposed that radiotherapy was essential for local disease control, especially administered within 6 cycles of chemotherapy (25). On the other hand, the phase III CREST trial demonstrated the efficacy of consolidative radiotherapy following chemotherapy, with great improvement in PFS, OS and intrathoracic disease control (12). With the emergence of immune checkpoint inhibitors (ICIs), chemo-immunotherapy has been established as the standard of care for ES-SCLC (4–8). However, the role of radiotherapy has not been well defined under the context of chemo-immunotherapy, mainly due to lack of permission in most phase III trials with concerns on potential increased risk of adverse events. Numerous preclinical researches have elucidated that radiotherapy could induce tumor-specific antigen exposure (26), reshape tumor microenvironment (27) and trigger systemic anti-tumor immune response (14, 17), laying the foundation for combination of radiotherapy and immunotherapy. In clinical settings, a recent meta-analysis, which encompassed 12 retrospective studies and 3 prospective studies, highlighted the efficacy of consolidative thoracic radiotherapy combined with first-line chemo-immunotherapy (28). Notably, in this large real-world study, our findings specifically emphasized that radiotherapy should be regarded as an early-intervention strategy, and reinforced the essential role of consolidative thoracic radiotherapy in treatment for ES-SCLC.
The optimal prescription of thoracic radiotherapy are still in suspense. Although a palliative dose of 30Gy in 10 fractions was suggested in the CREST trial, this was accompanied by an unsatisfactory intrathoracic recurrence rate of 41.7%, revealing the potential for dose escalation to improve local control (12). Under the context of chemo-immunotherapy, this real-word study showed lack of survival benefit from dose escalation (beyond BED of 60Gy or 39Gy), revealing the need for individualized dose selection. The phase II MATCH trial explored the feasibility of low-dose thoracic radiotherapy at a dosage of 15Gy in 5 fractions plus chemo-immunotherapy for patients with ES-SCLC (29). The ongoing phase III RAPTOR (NRG-LU007) study aimed to investigate different radiation dose adopted for different sites, specifically with definitive dose targeted at thoracic disease and palliative dose at metastatic lesions. The results are eagerly awaited (30).
Existing researches have emphasized the pivotal role of thoracic radiotherapy in ES-SCLC, while irradiation towards metastatic lesions is commonly considered as a symptomatic treatment. The phase II PEMBRO-RT study was conducted in patients with metastatic non-small-cell lung cancer. In this trial, those who received SBRT on a single, primarily metastatic site, followed by immunotherapy, had a doubling of the overall response rate, indicating the viability of early intervention of radiotherapy to metastatic sites (31). In our study, patients with ES-SCLC stratified by extra thoracic radiotherapy were directly compared. Align with our previous findings on thoracic radiotherapy, early extra thoracic radiotherapy exhibited improved survival outcomes. In summary, early radiotherapy, either thoracic or extra thoracic, combined with chemo-immunotherapy may be an ideal first-line treatment paradigm for ES-SCLC.
Generally, the safety profile is acceptable. In this study, the whole treated-related AEs were not significantly increased in patients receiving early radiotherapy, albeit with higher incidence of pneumonitis. However, there’s no significance in grade≥3 pneumonitis. Consistently, previous studies also exhibited manageable and primarily low-grade adverse events. Radiotherapy was reported with elevated risk of all-grade pneumonitis with incidence of about 13.8%-25.0%, and the grade 3 or higher pneumonitis was ranging from 2.6% to 6.5% (20–22, 32, 33). In summary, first-line radiotherapy combined with chemo-immunotherapy appears to be safe. Owing to limited evidence from prospective study, the safety of the combined regimen requires careful consideration.
This study has several limitations. First, although it was based on a large multi-center cohort, the non-randomized design may have introduced selection bias and unmeasured confounding factors. Second, heterogeneity existed in radiotherapy techniques, target volumes, and dosing regimens across centers, which might have influenced survival and toxicity outcomes. Additionally, the analysis was limited by missing clinical data, such as the application of prophylactic cranial irradiation, cycles of systemic therapy, and later-line treatments, which might have influenced the survival analyses. Finally, the limited follow-up duration precluded a definitive assessment of long-term survival and late toxicities. Due to the defects, the findings should be interpreted with caution.
In conclusion, early radiotherapy, either thoracic or extra thoracic radiotherapy, combined with first-line chemo-immunotherapy constituted an effective and well-tolerated strategy for patients with ES-SCLC. These findings warrant further investigation in large-scale prospective randomized trials in the future.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanghai Chest Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the informed consent process was aligned with standard clinical practice.
Author contributions
JJ: Data curation, Visualization, Conceptualization, Writing – review & editing, Investigation, Writing – original draft. YZ: Visualization, Writing – review & editing, Investigation, Writing – original draft, Conceptualization, Data curation. YJ: Conceptualization, Data curation, Writing – review & editing, Investigation, Writing – original draft, Visualization. HZ: Visualization, Writing – original draft, Investigation, Data curation, Conceptualization, Writing – review & editing. RM: Writing – review & editing, Data curation, Writing – original draft, Visualization, Investigation, Conceptualization. BX: Visualization, Writing – review & editing, Investigation, Writing – original draft, Data curation, Conceptualization. YW: Data curation, Visualization, Writing – review & editing. TS: Writing – review & editing, Data curation, Visualization. XS: Visualization, Data curation, Writing – review & editing. TZ: Visualization, Data curation, Writing – review & editing. YL: Writing – review & editing, Visualization, Data curation. LZ: Writing – review & editing, Visualization, Data curation. ZY: Visualization, Data curation, Writing – review & editing. XF: Visualization, Data curation, Writing – review & editing. XC: Supervision, Data curation, Writing – review & editing, Writing – original draft, Conceptualization, Funding acquisition.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the National Natural Science Foundation of China (No.82273575), Chinese Society of Clinical Oncology (No.Y-2019AZZD-0006), and Key Clinical Specialty Project of Shanghai (No.22Y31900102).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2026.1738352/full#supplementary-material
References
1
Nicholson AG Tsao MS Beasley MB Borczuk AC Brambilla E Cooper WA et al . The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003
2
Ganti AKP Loo BW Bassetti M Blakely C Chiang A D’Amico TA et al . Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1441–64. doi: 10.6004/jnccn.2021.0058
3
Farago AF Keane FK . Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. (2018) 7:69–79. doi: 10.21037/tlcr.2018.01.16
4
Horn L Mansfield AS Szczęsna A Havel L Krzakowski M Hochmair MJ et al . First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
5
Paz-Ares L Dvorkin M Chen Y Reinmuth N Hotta K Trukhin D et al . Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/s0140-6736(19)32222-6
6
Wang J Zhou C Yao W Wang Q Min X Chen G et al . Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:739–47. doi: 10.1016/s1470-2045(22)00224-8
7
Cheng Y Han L Wu L Chen J Sun H Wen G et al . Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. Jama. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
8
Cheng Y Fan Y Zhao Y Huang D Li X Zhang P et al . Tislelizumab plus platinum and etoposide versus placebo plus platinum and etoposide as first-line treatment for extensive-stage SCLC (RATIONALE-312): A multicenter, double-blind, placebo-controlled, randomized, phase 3 clinical trial. J Thorac Oncol. (2024) 19:1073–85. doi: 10.1016/j.jtho.2024.03.008
9
Cheng Y Zhang W Wu L Zhou C Wang D Xia B et al . Toripalimab plus chemotherapy as a first-line therapy for extensive-stage small cell lung cancer: the phase 3 EXTENTORCH randomized clinical trial. JAMA Oncol. (2025) 11:16–25. doi: 10.1001/jamaoncol.2024.5019
10
Sharma P Hu-Lieskovan S Wargo JA Ribas A . Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. doi: 10.1016/j.cell.2017.01.017
11
Kim D Kim HJ Wu HG Lee JH Kim S Kim TM et al . Intrathoracic progression is still the most dominant failure pattern after first-line chemo-immunotherapy in extensive-stage small-cell lung cancer: implications for thoracic radiotherapy. Cancer Res Treat. (2024) 56:430–41. doi: 10.4143/crt.2023.931
12
Slotman BJ van Tinteren H Praag JO Knegjens JL El Sharouni SY Hatton M et al . Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomized controlled trial. Lancet. (2015) 385:36–42. doi: 10.1016/s0140-6736(14)61085-0
13
Galluzzi L Aryankalayil MJ Coleman CN Formenti SC . Emerging evidence for adapting radiotherapy to immunotherapy. Nat Rev Clin Oncol. (2023) 20:543–57. doi: 10.1038/s41571-023-00782-x
14
Sharabi AB Nirschl CJ Kochel CM Nirschl TR Francica BJ Velarde E et al . Stereotactic radiation therapy augments antigen-specific PD-1–mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. (2015) 3:345–55. doi: 10.1158/2326-6066.Cir-14-0196
15
Deng L Liang H Burnette B Beckett M Darga T Weichselbaum R et al . Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. (2014) 124(2):687–95. doi: 10.1172/JCI67313
16
Herrera FG Ronet C Ochoa de Olza M Barras D Crespo I Andreatta M et al . Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. (2022) 12:108–33. doi: 10.1158/2159-8290.Cd-21-0003
17
Wu M Wu S Chen Y Sun L Zhou J . Immune activation effects at different irradiated sites and optimal timing of radioimmunotherapy in patients with extensive-stage small cell lung cancer: a real-world analysis. Biol Proced Online. (2023) 25:24. doi: 10.1186/s12575-023-00217-y
18
Diamond BH Verma N Shukla UC Park HS Koffer PP . Consolidative thoracic radiation therapy after first-line chemotherapy and immunotherapy in extensive-stage small cell lung cancer: A multi-institutional case series. Adv Radiat Oncol. (2022) 7:100883. doi: 10.1016/j.adro.2021.100883
19
Li L Yang D Min Y Liao A Zhao J Jiang L et al . First-line atezolizumab/durvalumab plus platinum-etoposide combined with radiotherapy in extensive-stage small-cell lung cancer. BMC Cancer. (2023) 23:318. doi: 10.1186/s12885-023-10784-8
20
Yao Y Li B Song R Yang L Zou B Wang L . Efficacy and safety of thoracic radiotherapy in extensive-stage small-cell lung cancer patients receiving first-line immunotherapy plus chemotherapy: a propensity score matched multicenter retrospective analysis. Radiat Oncol. (2024) 19:25. doi: 10.1186/s13014-024-02420-x
21
Cai Z Gu X Xie J Cheng D Chen J Cheng J et al . Safety and efficacy of thoracic radiotherapy combined with chemo-immunotherapy in patients with extensive-stage small cell lung cancer: a multicenter retrospective analysis. Transl Lung Cancer Res. (2023) 12:1987–2000. doi: 10.21037/tlcr-23-294
22
Chen D Zou B Li B Gao A Huang W Shao Q et al . Adebrelimab plus chemotherapy and sequential thoracic radiotherapy as first-line therapy for extensive-stage small-cell lung cancer (ES-SCLC): a phase II trial. EClinicalMedicine. (2024) 75:102795. doi: 10.1016/j.eclinm.2024.102795
23
Volpe S Piperno G Colombo F Biffi A Comi S Mastroleo F et al . Hypofractionated proton therapy for non-small cell lung cancer: Ready for prime time? A systematic review and meta-analysis. Cancer Treat Rev. (2022) 110:102464. doi: 10.1016/j.ctrv.2022.102464
24
Makinde AY Eke I Aryankalayil MJ Ahmed MM Coleman CN . Exploiting gene expression kinetics in conventional radiotherapy, hyperfractionation, and hypofractionation for targeted therapy. Semin Radiat Oncol. (2016) 26:254–60. doi: 10.1016/j.semradonc.2016.07.001
25
Han J Fu C Li B . Clinical outcomes of extensive-stage small cell lung cancer patients treated with thoracic radiotherapy at different times and fractionations. Radiat Oncol. (2021) 16:47. doi: 10.1186/s13014-021-01773-x
26
Guo S Yao Y Tang Y Xin Z Wu D Ni C et al . Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduction Targeted Ther. (2023) 8:205. doi: 10.1038/s41392-023-01462-z
27
Liu S Wang W Hu S Jia B Tuo B Sun H et al . Radiotherapy remodels the tumor microenvironment for enhancing immunotherapeutic sensitivity. Cell Death Disease. (2023) 14:679. doi: 10.1038/s41419-023-06211-2
28
Feng B Zheng Y Zhang J Tang M Na F . Chemoimmunotherapy combined with consolidative thoracic radiotherapy for extensive-stage small cell lung cancer: A systematic review and meta-analysis. Radiother Oncol. (2024) 190:110014. doi: 10.1016/j.radonc.2023.110014
29
Wang H Yao Z Kang K Zhou L Xiu W Sun J et al . Preclinical study and phase II trial of adapting low-dose radiotherapy to immunotherapy in small cell lung cancer. Med. (2024) 5(10):1237–54.e9. doi: 10.1016/j.medj.2024.06.002
30
RAndomized phase II/III trial of consolidation radiation + Immunotherapy for ES-SCLC: RAPTOR trial (2020). Available online at: https://clinicaltrials.gov/study/NCT04402788 (Accessed November 3, 2025).
31
Theelen WSME Peulen HMU Lalezari F van der Noort V de Vries JF Aerts JGJV et al . Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. (2019) 5:1276–82. doi: 10.1001/jamaoncol.2019.1478
32
Yao N Li S Hu L Pei Y Qin Z Li N et al . Consolidative thoracic radiotherapy improves the prognosis of extensive stage small-cell lung cancer patients in the chemoimmunotherapy era: a multicenter retrospective analysis. Ann Med. (2025) 57:2542434. doi: 10.1080/07853890.2025.2542434
33
Čakš M Janžič U Rutar T Unk M Demšar A Mohorčič K et al . Benefit of consolidation thoracic radiotherapy in extensive-stage small-cell lung cancer patients treated with immunotherapy: data from Slovenian cohort. Int J Mol Sci. (2025) 26(8):3631. doi: 10.3390/ijms26083631
Summary
Keywords
efficacy, extensive-stage small-cell lung cancer, first-line chemo-immunotherapy, immunotherapy, radiotherapy, safety
Citation
Jia J, Zeng Y, Ji Y, Zhu H, Meng R, Xia B, Wang Y, Shen T, Su X, Zhou T, Lu Y, Zhao L, Yang Z, Fu X and Cai X (2026) Efficacy and safety of early radiotherapy combined with first-line chemo-immunotherapy in extensive-stage small-cell lung cancer: a multi-center analysis. Front. Immunol. 17:1738352. doi: 10.3389/fimmu.2026.1738352
Received
03 November 2025
Revised
06 January 2026
Accepted
06 January 2026
Published
22 January 2026
Volume
17 - 2026
Edited by
Alessio Bruni, University of Modena and Reggio Emilia, Italy
Reviewed by
Valeria Dionisi, Integrated University Hospital Verona, Italy
Bishnu Joshi, Gel4Med, Inc. (A Harvard Innovation Labs Company), United States
Updates
Copyright
© 2026 Jia, Zeng, Ji, Zhu, Meng, Xia, Wang, Shen, Su, Zhou, Lu, Zhao, Yang, Fu and Cai.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuwei Cai, birdhome2000@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.