- 1Department of Engineering Technology and Industrial Distribution, Texas A&M University, College Station, TX, United States
- 2Department of Mechanical and Aerospace Engineering, University of Florida, Gainesville, FL, United States
- 3Department of Aerospace Engineering, Texas A&M University, College Station, TX, United States
- 4Department of Civil and Environmental Engineering, Texas A&M University, College Station, TX, United States
In the past decades, biomineralization-enabled structural composites with self-healing and self-growing properties have attracted significant amounts of attention from the research community. In this perspective article, we recapitulate the state of the art of biomineralization-enabled structural composites, its recent advances into 3D printing, and point out that nanomaterials may possess distinctive advantages to be included in the bioink, due to their remarkable effect on mechanical reinforcement and positive influence on microbial growth and biomineralization efficiency.
Introduction
Biomineralization is a well-regulated process where living organisms promote the precipitation of minerals, influencing their morphology, composition, and location (Lowenstam, 1981). In the past decades, biomineralization-enabled structural composites with self-healing and self-growing properties have attracted significant amounts of attention from the research community (Beatty et al., 2022). Various types of microorganisms have been investigated or engineered to produce bonding materials to glue fine granular particles into a cohesive structure.
A newly emerging and possibly revolutionary advancement of this research area is 3D printing, which utilizes the scheme of material addition to build 3D objects under automated control, creating geometrically complex structures with excellent resolution. Among various 3D printing techniques, direct ink writing stands out as a single-step method to print biomineralization-enabled structural composites. Using this technique, a bioink is dispensed through a nozzle programmed by the computer-aided design software to build 3D structures layer by layer (Baniasadi et al., 2024). The bioink is designed to include both microbial cells and granular particles, possess appropriate rheological properties to flow through the nozzle, and exhibit satisfactory structural integrity upon deposition. So far, this technology has achieved a significant level of advancement but still suffers from many limitations, such as insufficient mechanical strength.
Biomineralization-enabled structural composites
As early as 2012, Dosier (Author Anonymous, 2023) utilized bacterial biomineralization to consolidate sand particles into masonry units, as shown in Figure 1a. Dosier founded a company called bioMASON based on her patented process (Dosier). Samples are produced in a two-step process. Molds are first filled with sand and then inoculated with ureolytic bacteria, which are well-known for its capability of microbially induced calcite precipitation (Ma et al., 2020). To trigger biomineralization, the molds are then repeatedly flushed with a solution containing Ca2+ and urea.
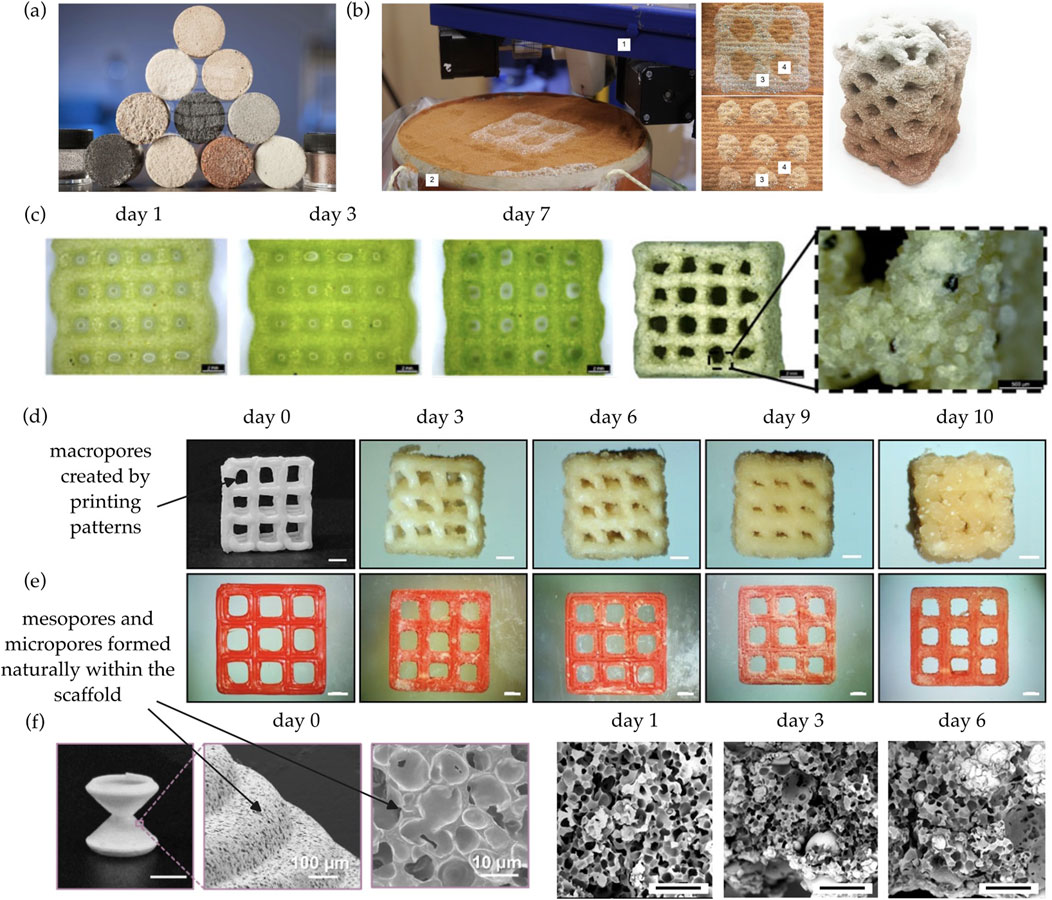
Figure 1. (a) Cylinders produced by bioMASON (Dosier). (b) Spatially patterned structures produced by powder bed-based 3D printing (Nething et al., 2020). One indicates the print head; two indicates the print bed; three indicates mixture of powder and sand; and four indicates pure sand. (c) Biomineralization-enabled structural composites printed via direct ink writing (Reinhardt et al., 2023). Scale bar is 2.0 mm. (d) Scaffolds with multi-scale pores demonstrate significant biomineralization, transforming into a load-bearing composite (Song et al., 2025). Scale bar is 1.0 mm. (e) In comparison, non-porous scaffolds printed by a traditional 3D printing technique called fused filament fabrication does not display significant biomineralization (Song et al., 2025). Scale bar is 1.0 mm. (f) The pores can be observed at different length scales (Song et al., 2025). Scale bar is 5.0 mm for the first image and 100 µm for the last three images.
In 2020, Nething et al. (Nething et al., 2020) fabricated a variety of structures using powder bed-based 3D printing, as shown in Figure 1b. Samples are produced in a three-step process. Pure sand and urease active powder containing ureolytic bacteria are selectively deposited in a mold. After printing, the mold is filled with a solution containing Ca2+ and urea. Powder-containing areas ureolytically produce CaCO3, which glues the sand particles into a cohesive structure. Pure sand does not solidify and is removed by rinsing with water. However, this method has many issues, such as dimensional deviations in the build-up direction and even avalanche-like collapses (Schmutzler et al., 2016). In addition, it is difficult to scale up, since the process requires multiple steps, including structure exposure to a mineralizing solution and removal of unsolidified material.
Utilizing recent advances in the development of cell-laden inks for 3D printing, some researchers (Reinhardt et al., 2023; Hirsch et al., 2023) developed microbe-loaded bioink to print structures in a single-step process via direct ink writing. The process is straightforward, i.e., microbial cells and granular particles are formulated into a bioink with controlled rheology and then extruded from a moving nozzle. After printing, the biominerals precipitated by the microbes within the printed scaffold convert the pliable scaffold into a load-bearing composite, as shown in Figure 1c. Note that any microbes that are capable of inducing biomineral precipitation can be used. For example, Reinhardt et al. used mineralizing cyanobacteria (Reinhardt et al., 2023) and Hirsch et al. used ureolytic bacteria (Hirsch et al., 2023).
It has been found that biomineralization efficiency is closely linked to the size, distribution, and interconnectivity of pores across multiple scales. To address this issue, some researchers (Zhao et al., 2023; Song et al., 2025) invented techniques to successfully print scaffolds with multi-scale pores. Using the porous scaffolds, much more significant biomineralization has been observed, as shown in Figure 1d, in comparison with non-porous scaffolds, as shown in Figure 1e. The presence of multi-scale pores, as shown in Figure 1f, is crucial to enable the biomineralization process. Each pore scale serves a different role, i.e., macropores enable efficient nutrient transport, mesopores facilitate cell accommodation and cell-cell interaction, and micropores promote biomineral precipitation and provide internal connectivity for the diffusion of metal ions. Macropores are typically created by printing patterns, whereas mesopores and micropores are formed naturally within the scaffold due to various physical or chemical mechanisms.
However, the mechanical strength of biomineralization-enabled structural composites obtained via direct ink writing is typically low compared with conventional building materials, because including too many granular particles into the bioink often renders the bioink stiff and brittle, not appropriate for extrusion-based printing. For example, Reinhardt et al. (Reinhardt et al., 2023) found that the highest sand percentage was 70 wt% in the cyanobacteria-loaded bioink, and when above 70 wt%, the bioink was no longer printable. To overcome this challenge of insufficient mechanical strength, the authors conducted extensive investigation and found that nanomaterials have distinctive advantages to be included in the bioink, due to their remarkable effect as mechanical reinforcement and positive influence on microbial growth and biomineralization efficiency.
Use of nanomaterials in 3D printing and bioprinting
Nanomaterials include materials with at least one dimension measuring between 1 nm and 100 nm and materials that contain nanoscale structures internally or on their surfaces (Mekuye and Abera, 2023). They can be composed of a variety of core materials, such as carbon, metals, and metal oxides, while taking on different morphologies, such as 0D nanoparticles, 1D nanofibers, 2D nanosheets, and 3D nanostructures. These materials exhibit unique properties due to their tiny size, differing remarkably from their bulk counterparts, and because of this, nanomaterials have been widely integrated into 3D printing to fabricate multifunctional materials for various applications (Zhang et al., 2022).
In particular, the incorporation of nanomaterials in 3D bioprinting of tissue and organ scaffolds is rapidly growing (Theus et al., 2021). It has been found that, besides the structural stability and shape fidelity enhancement during and after printing, the use of nanomaterials can trigger certain cellular activities, leading to improved cell growth, proliferation and extracellular matrix secretion (Bhattacharyya et al., 2021).
3D bioprinting of biomineralization-enabled structural composites distinguishes itself from conventional bioprinting of tissue and organ scaffolds, because the post-printing process is dominated by crystal nucleation and precipitation, and thus has its unique challenges. To the best of our knowledge, nanomaterials have never been incorporated into 3D bioprinting of biomineralization-enabled structural composites. The current practices (Reinhardt et al., 2023; Hirsch et al., 2023; Zhao et al., 2023; Song et al., 2025) did not involve the use of nanomaterials.
Use of nanomaterials in concrete
Similar to biomineral composites, concrete, the most widely employed construction material across the globe, is also a nano-structured composite material that contains multi-scale pores, from nano-sized to milli-sized. Concrete consists of calcium–silicate–hydrate gel, i.e., an amorphous phase produced during cement hydration, nano-sized and micro-sized crystals, and bound water. While strong in compression, concrete inherently suffers from insufficient tensile strength. To address this issue, a variety of nanomaterials, including 0D nanoparticles (such as SiO2, TiO2, Fe2O3, Cr2O3 and Al2O3), 1D nanomaterials (such as carbon nanotubes and carbon nanofibers), and 2D nanosheets (such as graphene, graphene oxide, reduced graphene oxide, and boron nitride) have been tested in concrete (Goel et al., 2022).
The enhanced mechanical performance due to addition of 0D nanoparticles can be mainly ascribed to two mechanisms (Sanchez and Sobolev, 2010). Firstly, they function as nucleation sites for hydration products, significantly promoting cement hydration thanks to their large specific surface area, large surface-to-volume ratio, and high reactivity. Secondly, by acting as nucleation sites, they provide the seeding of hydration products that can fill the available pore space in the cement matrix. As a result, the total porosity is reduced, the pore distribution is homogenized, and the pore shape is modified (Suh et al.).
In addition to increasing hydration nucleation and influencing pore structure, 1D nanofibers and 2D nanosheets also behave as reinforcing materials to bridge cracks. Reinforcements at the nanoscale in concrete are more powerful than conventional milli-sized reinforcements because they can control nano-sized cracks at the very initial stage of crack development (Konsta-Gdoutos et al., 2010). For example, Lv et al. (Lv et al., 2013) reported that incorporating 0.03 wt% graphene oxide into cement composites improves the tensile, flexural, and compressive strengths by 78.6%, 60.7%, and 38.9%, respectively. To bridge cracks, it is crucial for 1D and 2D nanomaterials to have high aspect ratios and sufficient intrinsic strength.
One of the most significant challenges associated with the use of 0D, 1D, and 2D nanomaterials in concrete is their dispersion in cement pore solution. Even low loadings often encounter aggregation and agglomeration issues. On the other hand, 3D nanomaterials, i.e., nanostructures with interconnected pores, do not have issues with aggregation and agglomeration, providing the largest accessible surface area among all the nanomaterials (Verma et al., 2023; Sun et al., 2020).
Similar to nanomaterials in concrete, 0D, 1D, 2D, and 3D nanomaterials can function as nucleation sites during biomineral precipitation and densify pore structure of the biomineral composites, whereas 1D, 2D, and 3D nanomaterials can also behave as reinforcing materials to bridge cracks. However, the effect of nanomaterials in biomineral composites will be more multifaceted due to the presence of microbes.
Effect of nanomaterials on mechanical reinforcement
Tanyildizi et al. (2024) studied the crack-healing of concrete with carbon nanotubes incorporated at different dosages. After 28 days of curing, the first group of samples was mechanically loaded to generate controlled cracks and then they were healed using ureolytic bacteria. After 20 days of healing, the tensile strength test was conducted on the healed samples. The second group of samples were used as control and did not go through the crack generation and bacterial healing processes. Due to the presence of carbon nanotubes, some healed samples displayed higher tensile strength than the control. The healed cracks remained intact after reloading even when some new cracks appeared close to the healed ones. In comparison, for concrete without carbon nanotubes, the recovery rate for tensile strengths after bacterial healing was 85% (Kan and Shi, 2012). It can be concluded that the presence of carbon nanotubes during biomineral precipitation creates an exceedingly strong structure, by providing additional nucleation sites on fracture surfaces (Siad et al., 2018) and acting as bridges across nano-sized cracks, preventing or delaying its propagation.
Effect of nanomaterials on microbial growth
In the past decades, studies on the interaction between nanomaterials and microbial cells appear conflicting (Zhang and Tremblay, 2020). Using graphene-based nanomaterials as an example, on one hand, many researchers suggested that they promote microbial colonization, because their large specific surface area can enhance microbial growth and metabolism (Ruiz et al., 2011). For example, graphene oxide sheets acted as a template for bacterial adhesion and biofilm formation (Ahamed et al., 2024). On the other hand, there are numerous studies that reported antimicrobial effects of graphene-based nanomaterials. Since the specific dosage thresholds are significantly varied depending on the application, we here instead focus on the mechanisms which could lead to the cytotoxic effects of nanomaterials. As summarized in Figure 2a, physical mechanisms include membrane disruption, wrapping effect, and phospholipids extraction (Perreault et al., 2015; Li et al., 2013; Chaudhary et al., 2024), whereas chemical mechanisms include reactive oxygen species (ROS)-dependent and ROS-independent oxidative stresses (Kumar et al., 2019). The sharp edges and nanoscale dimensions of nanomaterials can physically pierce or disrupt cell membranes, compromising their integrity and causing leakage of cellular contents (Li et al., 2013). The thin, flexible structure of graphene-based materials enables it to wrap around microbial cells, separating them from their growth medium and obstructing nutrient intake and waste elimination, eventually resulting in cell death (Chaudhary et al., 2024). In addition, phospholipids can be directly extracted from the lipid bilayer, facilitated by van der Waals forces between graphene planes and lipid tails (Perreault et al., 2015). Microbes can no longer proliferate upon damage of their lipids, proteins, and nucleic acids due to ROS produced by the graphene-based materials (Kumar et al., 2019). Some graphene-based materials can act as electron acceptors, drawing electrons from antioxidant biomolecules in cells. This electron transfer can disrupt cellular redox balance and damage cellular components, even without a significant increase in ROS (Kumar et al., 2019).
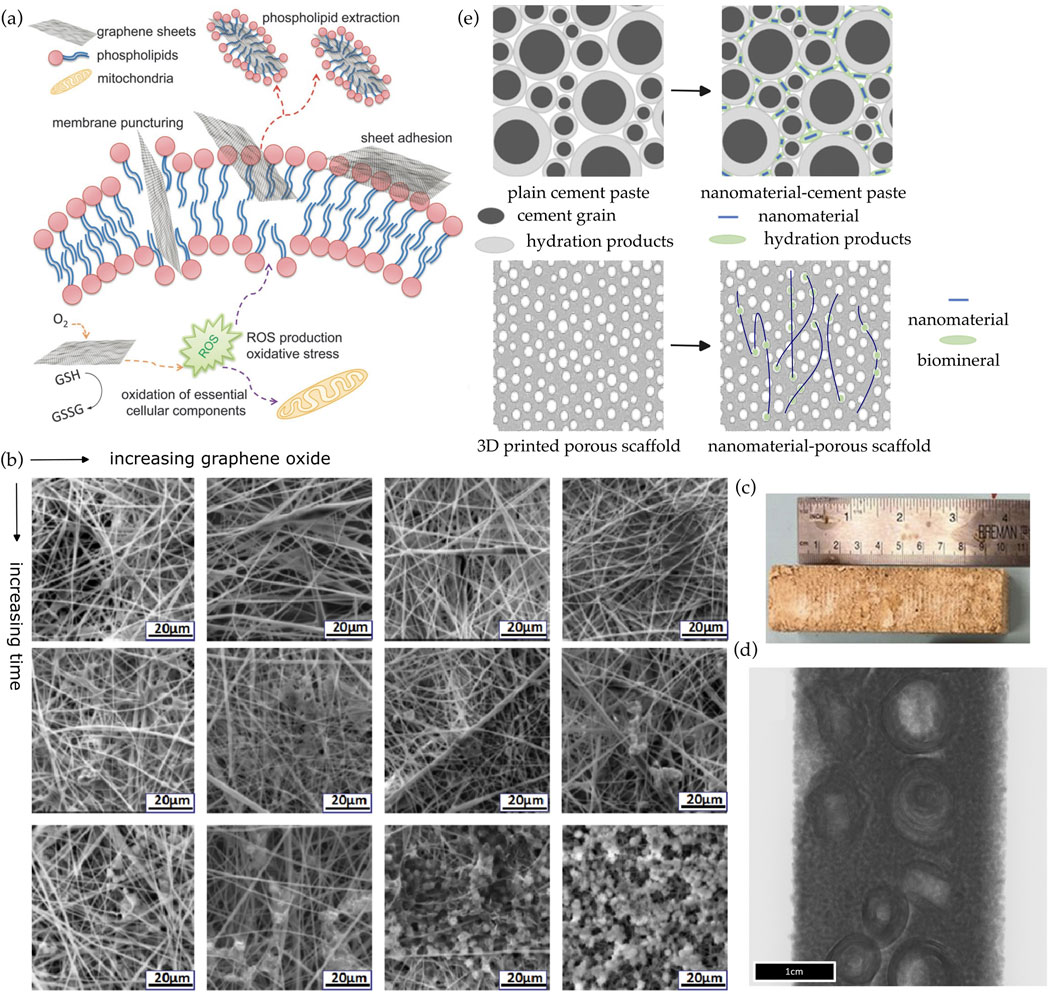
Figure 2. (a) The antimicrobial effects of graphene-based nanomaterials are due to differing mechanisms (Perreault et al., 2015). (b) The biomineralization process was accelerated with increasing amounts of graphene oxide, due to the additional nucleation sites provided by graphene oxide (Liu et al., 2017). (c,d) Biomineralized mycelium scaffolds with intentionally manipulated internal geometries were fabricated to mimic cortical bone (Viles et al., 2025). (e) Densification of the pore space in cement paste due to addition of nanomaterials (Ruan et al., 2021). Similarly, the addition of nanomaterials in biomineralizing composites provides the seeding of biomineral crystals which can fill the available pore space.
This contradiction suggests that specific conditions should be provided for nanomaterials to positively influence microbial survival and growth. The morphology of nanomaterials, such as size, shape, specific surface area, and surface roughness, can be adjusted to avoid physical damage to microbial cells and enhance microbial attachment and proliferation. The dosage and distribution of nanomaterials can be tailored to reduce harmful physical or chemical interaction with microbes. The surface chemistry of nanomaterials can be modified to eliminate or reduce the origins of nanomaterial-induced cytotoxicity by the addition of functional groups and defects, doping of heteroatoms, and coating of organic polymer (Luo et al., 2016). For example, Liu et al. (Liu et al., 2020) employed a one-step functionalization, i.e., a homogenous coating of carbon nanotubes, which remarkably enhanced cell adhesion, proliferation, and differentiation. Shi et al. (Shi et al., 2012) found that the surface chemistry of nanoparticles such as ligand type and surface oxidation can be manipulated to reduce ROS creation. These practices facilitate positive interactions between nanomaterials and microbes, which can significantly enhance the application potentials of nanomaterials in biomineral composites.
Effect of nanomaterials on microbial biomineralization
Similar to nanomaterials in concrete, 0D, 1D, 2D, and 3D nanomaterials can enhance biomineral precipitation by providing additional nucleation sites. In a previous study (Liu et al., 2017), the biomineralization process was accelerated with increasing amounts of graphene oxide in the nanofibers, due to the additional nucleation sites offered by graphene oxide, as shown in Figure 2b.
Since nanomaterials serve as nucleation sites, they can be spatially arranged into certain geometries as a means to spatially control over the biomineralization process and enhance the resulting composite’s mechanical properties. For example, Viles et al. (Viles et al., 2025) fabricated biomineralized beams inspired by the osteonal arrangement of cortical bone using intentionally manipulated internal geometries of mycelium scaffolds, as shown in Figures 2c,d. These cylindrical structures consist of concentric layers of bone around a Haversian canal, which hosts blood vessels and nerves. Osteons are interconnected by Volkmann’s canals, providing a network for nutrient and waste transport (Mishra and Knothe Tate, 2003). The osteonal structure is crucial for cortical bone to obtain its strength, ability to withstand stress, and capacity for nutrient and waste transport. Although fungal mycelium is not a nano-sized material, but the concept can be readily adapted and potentially applied to nanomaterials.
In addition to spatial distribution, surface chemistry of nanomaterials can also be manipulated to promote metal carbonate precipitation by attracting certain metal ions or by capturing CO2 from the air (which can be converted by the microbes into carbonate ions) (Fang and Achal, 2024). According to literature, the alteration of surface chemistry of the nanomaterials, including the addition of functional groups and defects, doping of heteroatoms, and coating of additional materials, often plays a crucial role in their ability to attract certain metal ions (Mensah et al., 2021) or CO2 from the air (Firdaus et al., 2021).
Synergistic effect of nanomaterial incorporation and pore generation
To print biomineral composites, a multi-scale pore structure needs to be created to enable the biomineralization process (Zhao et al., 2023; Song et al., 2025). For example, Song et al. (2025) invented a method to fabricate multi-scale pores with macropores created based on printing conditions such as the filament hatch distance for lattices and micropores generated through a phase separation process when a polymer solution is extruded into a non-solvent yield-stress support bath. Note that Song et al. did not include nanomaterials in the bioink.
If nanomaterials are added, there will be a synergistic effect of nanomaterial incorporation and pore generation. The final mechanical strength of the resulting composite may be significantly enhanced partly due to the densification of the pore space, i.e., similar to the commonly observed densification of the cement gel due to the addition of nanomaterials (de Souza et al., 2022; Ruan et al., 2021) as shown in Figure 2e. The addition of nanomaterials in biomineral composites provides the seeding of biomineral crystals which can fill the available pore space. Hence, it is expected that the total porosity is reduced, the pore distribution is homogenized, and the pore shape is modified.
Therefore, it is of high potential value to study the synergistic effect of nanomaterial incorporation and pore generation on cell viability, biomineralization efficiency, and mechanical properties of the final structure. A series of multi-scale pore structures can be fabricated to generate a variety of porosity, pore size, pore size distribution, pore shape, and pore interconnectivity. In parallel, a series of nanomaterials, including 0D, 1D, 2D, and 3D, each with a variety of surface chemistry, can be incorporated in the bioink at different dosages and with varying spatial distributions. Their synergistic effect can be studied using a combination of complementary material characterization techniques.
Material characterization and mechanical testing
To achieve a high level of control over the biomineralization process, it is essential to study the effect of nanomaterials and pore structures on the mineral precipitation at the microscopic level and directly under in situ conditions. A combination of in situ/operando techniques are needed to provide a complete picture of the reaction mechanisms involved. This suite includes scanning electron microscope (SEM), transmission electron microscope (TEM), cryo-scanning electron microscope (Cryo-SEM), cryo-transmission electron microscope (Cryo-TEM), liquid-cell TEM (LC-TEM), atomic force microscope (AFM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and X-ray computed tomography (micro-CT and nano-CT).
To monitor the structural and morphological changes over time, SEM and TEM will be performed at different stages of the biomineralization process. SEM can be used to characterize the morphological and composition evolution of the solid precipitates. TEM has an unparalleled ability to provide morphological, structural, and chemical information down to atomic and near atomic scales. However, SEM/TEM requires high vacuum to operate. Although environmental SEM (ESEM) operates in comparatively humid and low vacuum conditions, the pressure in an ESEM chamber is still too low for microbial samples except for dormant spores.
Cryo-SEM/-TEM is a form of SEM/TEM where the sample is studied at cryogenic temperatures, enabling imaging of wet samples that are flash-frozen. Such complementary tools will provide the much-needed insights into the fundamental mechanisms of biomineral precipitation in the environment of printed soft scaffolds, including the location and density for the heterogeneous nucleation of the biomineral phase, the nature of the microbe-biomineral and nanomaterial-biomineral interfaces, and the evolution of crystallinity and microstructure of the solid precipitates. However, Cryo-SEM/-TEM still cannot image the samples in fluid environment.
With development of nanofabrication techniques, imaging of samples in a liquid environment using TEM has only recently become possible. LCTEM typically involves a microchip with a thin membrane, typically made of silicon nitride or graphene, that creates a barrier between the liquid sample and the microscope’s vacuum. The electron beam from the TEM passes through the membrane and then through the liquid sample, directly imaging the sample in its liquid state. The development of nanofabrication techniques has enabled the creation of thin, robust, and electron-transparent membranes, which are essential for LCTEM. Taking advantage of this instrument, the microbe-biomineral and nanomaterial-biomineral interfaces can be studied in situ at the sub-nanometer scale, including the evolution of morphology, polymorphic type, and crystallographic orientation of the biomineral crystals.
AFM is highly versatile in monitoring the surface topography and mechanical properties of both microbial cells and nanomaterials during biomineral precipitation. AFM uses a sharp tip on a cantilever probe to scan the surface, creating a 3D map of the sample’s topography with high resolution. By tapping the surface, AFM can measure the mechanical properties of the material, such as elasticity, viscosity, and their spatial variation, based on the interaction between the tip and the surface. Lateral deflection of the cantilever indicates the strength of the interaction between the AFM tip and the surface. The phase lag between the tip and its driver provides additional information such as vibration damping of the surface, as the tip’s oscillation is affected by the surface’s characteristics. In addition, force spectroscopy, an approach-retract mode of AFM, can measure the adhesion strength between microbial precipitates and nanomaterials.
To complement the local information obtained by TEM, SEM, and AFM, mixtures of microbial cells, nanomaterials, and precipitated biominerals can be periodically collected for XRD and XPS analyses. XRD can help understand the phase transformation and associated strain evolution on the global scale. XPS can monitor the chemical composition evolution. In addition, micro-CT and nano-CT can be used for direct quantification of the total amount and distribution of the precipitates inside the printed scaffolds.
Finally, mechanical properties of the final structure at both mesoscale and macroscale, with or without going through further heat treatment, can be tested using soil column tests, tension tests, compression tests, flexural tests, round panel tests, biaxial tests, triaxial tests, cone penetration tests, etc.
Concluding remarks
For 3D bioprinting of biomineralization-enabled structural composites, the effect of incorporating nanomaterials to the bioink on both cell viability and biomineral precipitation is of high potential value to be researched. A variety of commercially available nanomaterials can be directly incorporated to the bioink, such as graphene oxide, carbon nanotubes, fullerenes, carbon dots, and their derivatives, etc.
Many critical questions need to be elucidated, such as the effect of varying dosage and distribution of nanomaterials on microbial survival, growth, and activities, the effect of the spatial arrangement and surface chemistry of nanomaterials on crystal nucleation and crystal growth kinetics, and the synergistic effect of both the incorporation of nanomaterials and the fabrication of multi-scale pores on the spatial and temporal control of the biomineralization process, etc.
With recent development of material characterization instruments, a combination of complementary in situ/operando techniques can be used to understand the effect of nanomaterials and pore structures on biomineral precipitation at the microscopic level and directly under in situ conditions. Such knowledge can be used to achieve biomineralization-enabled structural composites with optimized mechanical properties.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
CJ: Writing – review and editing, Writing – original draft, Investigation, Project administration, Methodology, Formal Analysis, Conceptualization. YH: Conceptualization, Investigation, Methodology, Writing – review and editing. MN: Writing – review and editing, Investigation, Methodology. CW: Formal Analysis, Methodology, Conceptualization, Project administration, Investigation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the reviewers for their helpful comments and feedback, which greatly strengthened the overall manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahamed, T., Li, C., Li, M., and Axe, L. (2024). Interactions of graphene oxide with the microbial community of biologically active filters from a water treatment plant. Water Res. 263, 122155. doi:10.1016/j.watres.2024.122155
Author Anonymous (2023). Available online at: https://inhabitat.com/biomasons-bricks-grown-with-sand-and-bacteria-to-hit-the-market-next-year.
Baniasadi, H., Abidnejad, R., Fazeli, M., Lipponen, J., Niskanen, J., Kontturi, E., et al. (2024). Innovations in hydrogel-based manufacturing: a comprehensive review of direct ink writing technique for biomedical applications. Adv. Colloid Interface Sci. 324, 103095. doi:10.1016/j.cis.2024.103095
Beatty, D. N., Williams, S. L., and Srubar, I. I. I. W. V. (2022). Biomineralized materials for sustainable and durable construction. Annu. Rev. Mater. Res. 52, 411–439. doi:10.1146/annurev-matsci-081720-105303
Bhattacharyya, A., Janarthanan, G., and Noh, I. (2021). Nano-biomaterials for designing functional bioinks towards complex tissue and organ regeneration in 3D bioprinting. Add. Manufact. 37, 101639. doi:10.1016/j.addma.2020.101639
Chaudhary, R., Singh, N. B., Nagpal, G., and Saah, F. K. (2024). Antibacterial activity of reduced graphene-silver oxide nanocomposite against gram-negative bacteria. Microbe 5, 100221. doi:10.1016/j.microb.2024.100221
de Souza, F. B., Shamsaei, E., Sagoe-Crentsil, K., and Duan, W. (2022). Proposed mechanism for the enhanced microstructure of graphene oxide–Portland cement composites. J. Build. Eng. 54, 104604. doi:10.1016/j.jobe.2022.104604
Dosier, G. K. (2021). Composition, tools, and methods for the manufacture of construction materials using enzymes. US8951786B1. Available online at: https://patents.google.com/patent/US8951786B1/en. (Accessed August 8, 2025).
Fang, C., and Achal, V. (2024). Enhancing carbon neutrality: a perspective on the role of microbially induced carbonate precipitation (MICP). Biogeotechnics 2, 100083. doi:10.1016/j.bgtech.2024.100083
Firdaus, R. M., Desforges, A., Rahman Mohamed, A., and Vigolo, B. (2021). Progress in adsorption capacity of nanomaterials for carbon dioxide capture: a comparative study. J. Clean. Prod. 328, 129553. doi:10.1016/j.jclepro.2021.129553
Goel, G., Sachdeva, P., Chaudhary, A. K., and Singh, Y. (2022). The use of nanomaterials in concrete: a review. Mater. Today Proc. 69, 365–371. doi:10.1016/j.matpr.2022.09.051
Hirsch, M., Lucherini, L., Zhao, R., Clarà Saracho, A., and Amstad, E. (2023). 3D printing of living structural biocomposites. Mater. Today 62, 21–32. doi:10.1016/j.mattod.2023.02.001
Kan, L. L., and Shi, H. S. (2012). Investigation of self-healing behavior of engineered cementitious composites (ECC) materials. Constr. Build. Mat. 29, 348–356. doi:10.1016/J.CONBUILDMAT.2011.10.051
Konsta-Gdoutos, M. S., Metaxa, Z. S., and Shah, S. P. (2010). Multi-scale mechanical and fracture characteristics and early-age strain capacity of high performance carbon nanotube/cement nanocomposites. Cem. Concr. Compos. 32, 110–115. doi:10.1016/j.cemconcomp.2009.10.007
Kumar, P., Huo, P., Zhang, R., and Liu, B. (2019). Antibacterial properties of graphene-based nanomaterials. Nanomaterials 9, 737. doi:10.3390/nano9050737
Li, Y., Yuan, H., von dem Bussche, A., Creighton, M., Hurt, R. H., Kane, A. B., et al. (2013). Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. U. S. A. 110, 12295–12300. doi:10.1073/pnas.1222276110
Liu, X., Shen, H., Song, S., Chen, W., and Zhang, Z. (2017). Accelerated biomineralization of graphene oxide – incorporated cellulose acetate nanofibrous scaffolds for mesenchymal stem cell osteogenesis. Colloids Surfaces B Biointerfaces 159, 251–258. doi:10.1016/j.colsurfb.2017.07.078
Liu, X., George, M. N., Park, S., Miller II, A. L., Gaihre, B., Li, L., et al. (2020). 3D-printed scaffolds with carbon nanotubes for bone tissue engineering: fast and homogeneous one-step functionalization. Acta Biomater. 111, 129–140. doi:10.1016/j.actbio.2020.04.047
Lowenstam, H. A. (1981). Minerals formed by organisms. Science 13, 1126–1131. doi:10.1126/science.7008198
Luo, Y., Yang, X., Tan, X., Xu, L., Liu, Z., Xiao, J., et al. (2016). Functionalized graphene oxide in microbial engineering: an effective stimulator for bacterial growth. Carbon 103, 172–180. doi:10.1016/j.carbon.2016.03.012
Lv, S., Ma, Y., Qiu, C., Sun, T., Liu, J., and Zhou, Q. (2013). Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mat. 49, 121–127. doi:10.1016/j.conbuildmat.2013.08.022
Ma, L., Pang, A. P., Luo, Y., Lu, X., and Lin, F. (2020). Beneficial factors for biomineralization by ureolytic bacterium Sporosarcina pasteurii. Microb. Cell Fact. 19, 12. doi:10.1186/s12934-020-1281-z
Mekuye, B., and Abera, B. (2023). Nanomaterials: an overview of synthesis, classification, characterization, and applications. Nano Sel. 4, 486–501. doi:10.1002/nano.202300038
Mensah, M. B., Lewis, D. J., Boadi, N. O., and Awudza, J. A. M. (2021). Heavy metal pollution and the role of inorganic nanomaterials in environmental remediation. R. Soc. Open Sci. 8, 201485. doi:10.1098/rsos.201485
Mishra, S., and Knothe Tate, M. L. (2003). Effect of lacunocanalicular architecture on hydraulic conductance in bone tissue: implications for bone health and evolution. Anat. Rec. 273A, 752–762. doi:10.1002/ar.a.10079
Nething, C., Smirnova, M., Gröning, J. A. D., Haase, W., Stolz, A., and Sobek, W. (2020). A method for 3D printing bio-cemented spatial structures using sand and urease active calcium carbonate powder. Mat. Des. 195, 109032. doi:10.1016/j.matdes.2020.109032
Perreault, F., Fonseca de Faria, A., and Elimelech, M. (2015). Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 44, 5861–5896. doi:10.1039/C5CS00021A
Reinhardt, O., Ihmann, S., Ahlhelm, M., and Gelinsky, M. (2023). 3D bioprinting of mineralizing cyanobacteria as novel approach for the fabrication of living building materials. Front. Bioeng. Biotechnol. 11, 1145177. doi:10.3389/fbioe.2023.1145177
Ruan, C., Lin, J., Chen, S., Sagoe-Crentsil, K., and Duan, W. (2021). Effect of graphene oxide on the pore structure of cement paste: implications for performance enhancement. ACS Appl. Nano Mater. 4, 10623–10633. doi:10.1021/acsanm.1c02090
Ruiz, O. N., Fernando, K. A. S., Wang, B. J., Brown, N. A., Luo, P. G., McNamara, N. D., et al. (2011). Graphene oxide: a nonspecific enhancer of cellular growth. ACS Nano 5, 8100–8107. doi:10.1021/nn202699t
Sanchez, F., and Sobolev, K. (2010). Nanotechnology in concrete – a review. Constr. Build. Mater. 24, 2060–2071. doi:10.1016/j.conbuildmat.2010.03.014
Schmutzler, C., Boeker, C., and Zaeh, M. F. (2016). Investigation of deviations caused by powder compaction during 3D printing. Procedia CIRP 57, 698–703. doi:10.1016/j.procir.2016.11.121
Shi, M., Kwon, H. S., Peng, Z., Elder, A., and Yang, H. (2012). Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 6, 2157–2164. doi:10.1021/nn300445d
Siad, H., Lachemi, M., Sahmaran, M., Mesbah, H. A., and Hossain, K. A. (2018). Advanced engineered cementitious composites with combined self-sensing and self-healing functionalities. Constr. Build. Mat. 176, 313–322. doi:10.1016/J.CONBUILDMAT.2018.05.026
Song, K., Wu, Q., Compaan, A. M., Shen, J., Zhou, C., Chen, M., et al. (2025). Solvent-rich pre-coagulation bath for tunable liquid-state fusion enables robust two-step polymer embedded printing. Adv. Sci., e08335. In Press. doi:10.1002/advs.202508335
Suh, H., Cho, S., Her, S., and Bae, S. (2023). Influence of multi-scale three-dimensional pore characteristics on the mechanical properties of graphene oxide and carbon nanotube incorporated cement paste. Cem. Concr. Res. 174, 107326. doi:10.1016/j.cemconres.2023.107326
Sun, Z., Fang, S., and Hu, Y. H. (2020). 3D graphene materials: from understanding to design and synthesis control. Chem. Rev. 120, 10336–10453. doi:10.1021/acs.chemrev.0c00083
Tanyildizi, H., Bulut, M., and Ziada, M. (2024). Bacteria-based crack healing of nanosilica and carbon nanotube modified engineered cementitious composites. J. Mat. Civ. Eng. 36, 04023515. doi:10.1061/JMCEE7.MTENG-15991
Theus, A. S., Ning, L., Jin, L., Roeder, R. K., Zhang, J., and Serpooshan, V. (2021). Nanomaterials for bioprinting: functionalization of tissue-specific bioinks. Essays Biochem. 65, 429–439. doi:10.1042/EBC20200095
Verma, C., Berdimurodov, E., Verma, D. K., Berdimuradov, K., Alfantazi, A., and Hussain, C. M. (2023). 3D Nanomaterials: the future of industrial, biological, and environmental applications. Inorg. Chem. Commun. 156, 111163. doi:10.1016/j.inoche.2023.111163
Viles, E., Heyneman, E., Lin, S., Montague, V., Darabi, A., Cox, L. M., et al. (2025). Mycelium as a scaffold for biomineralized engineered living materials. Cell Rep. Phys. Sci. 6, 102517. doi:10.1016/j.xcrp.2025.102517
Zhang, T., and Tremblay, P.-L. (2020). Graphene: an antibacterial agent or a promoter of bacterial proliferation? iScience 23, 101787. doi:10.1016/j.isci.2020.101787
Zhang, L., Forgham, H., Shen, A., Wang, J., Zhu, J., Huang, X., et al. (2022). Nanomaterial integrated 3D printing for biomedical applications. J. Mat. Chem. B 10, 7473–7490. doi:10.1039/d2tb00931e
Keywords: biomineralization, bioprinting, nanomaterial, bioink, porous scaffold
Citation: Jin C, Huang Y, Naraghi M and Wu C (2025) Nanomaterial: a neglected bioink ingredient for 3D printing of biomineralization-enabled structural composites. Front. Mech. Eng. 11:1655463. doi: 10.3389/fmech.2025.1655463
Received: 27 June 2025; Accepted: 12 August 2025;
Published: 01 September 2025.
Edited by:
Dinh Gia Ninh, Hanoi University of Science and Technology, VietnamReviewed by:
Shengbo Guo, George Washington University, United StatesQingqing He, San Diego State University, United States
Copyright © 2025 Jin, Huang, Naraghi and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Congrui Jin, amluY29uZ3J1aUB0YW11LmVkdQ==; Chenglin Wu, Y2hlbmdsaW53dUB0YW11LmVkdQ==
 Congrui Jin
Congrui Jin Yong Huang2
Yong Huang2 Chenglin Wu
Chenglin Wu