- 1Department of Respiratory and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Prevention of Organ Failure (PROOF) Centre of Excellence & Centre for Heart Lung Innovation, St Paul’s Hospital, University of British Columbia, Vancouver, BC, Canada
- 3Division of Respiratory Medicine, Department of Medicine, University of British Columbia, Vancouver, BC, Canada
- 4Systems Vaccinology, Center for Precision Health, Telethon Kids Institute, Nedlands, WA, Australia
- 5Toronto General Hospital Research Institute, University Health Network & Department of Immunology, University of Toronto, Toronto, ON, Canada
A Corrigendum on
Interferon-α2b Treatment for COVID-19
by Zhou Q, Chen V, Shannon CP, Wei X-S, Xiang X, Wang X, Wang Z-H, Tebbutt SJ, Kollmann TR and Fish EN (2020). Front. Immunol. 11:1061. doi: 10.3389/fimmu.2020.01061
Error in Figure/Table
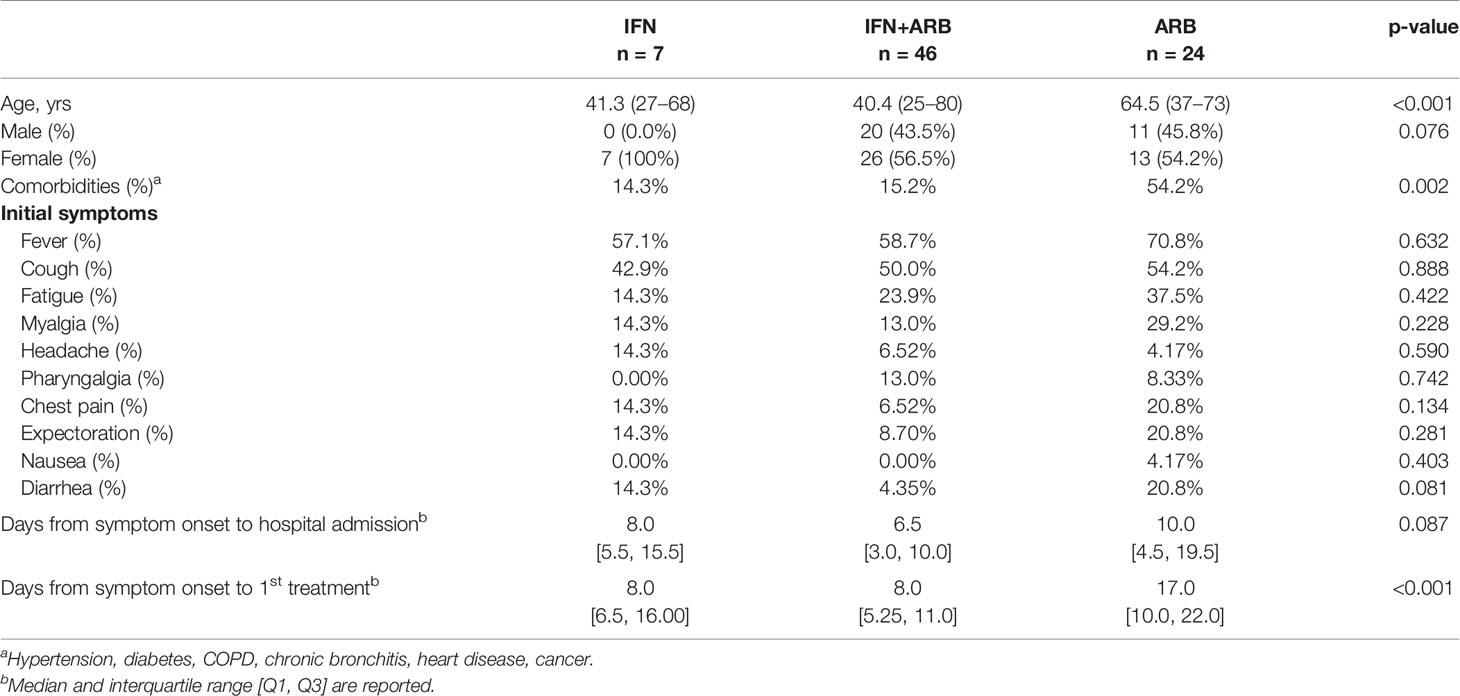
In the original article, there was a mistake in Table 1 as published. For ‘Days from symptom onset to treatment,’ the IFN+ARB and ARB values were inadvertently switched. IFN+ARB should be 8.0 [5.0,11.0] and ARB should be 17.0 [10.0, 22.0]. The corrected Table 1 appears below.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: interferon, COVID-19, viral shedding, IL-6, inflammation, ARDS
Citation: Zhou Q, Chen V, Shannon CP, Wei X-S, Xiang X, Wang X, Wang Z-H, Tebbutt SJ, Kollmann TR and Fish EN (2020) Corrigendum: Interferon-α2b Treatment for COVID-19. Front. Immunol. 11:615275. doi: 10.3389/fimmu.2020.615275
Received: 08 October 2020; Accepted: 09 October 2020;
Published: 27 October 2020.
Approved by: Frontiers Editorial Office, Frontiers Media SA, Switzerland
Copyright © 2020 Zhou, Chen, Shannon, Wei, Xiang, Wang, Wang, Tebbutt, Kollmann and Fish. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleanor N. Fish, ZW4uZmlzaEB1dG9yb250by5jYQ==
 Qiong Zhou1
Qiong Zhou1 Eleanor N. Fish
Eleanor N. Fish