- 1Endoscopy Department, Digestive Disease Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Microbiology, School of Biology, University College of Sciences, University of Tehran, Tehran, Iran
- 3Gastrointestinal and Liver Diseases Research Center, Guilan University of Medical Sciences, Rasht, Iran
Background: The importance of coinfection of Helicobacter pylori (H.pylori) and Candida albicans (C. albicans) in the development of gastric diseases is not known. In this study, the frequency of concurrent infection of H. pylori and C. albicans in dyspeptic patients was assessed while considering age, gender, and PPI consumption of patients.
Methods: Gastric biopsies were taken from 74 yeast-positive dyspeptic patients and gastric disease, age, gender, and proton pump inhibitor (PPI) consumption of subjects were recorded. One antral biopsy was used for rapid urease test (RUT) and one for H. pylori and yeast cultivation and smear preparation. Bacterial isolates were identified according to spiral morphology and the biochemical characteristics. Yeast isolates were identified on Chromagar and by the Nested-PCR amplification of C. albicans-specific topoisomerase II gene. Twenty-seven biopsy smears were Gram-stained and examined by the light microscope for observing H. pylori and yeast cells.
Results: Fifty-four (73%) of patients were >40 year. Of 68 patients with PPI consumption record, 46 (67.6%) consumed PPI (p = 0). Comparison of patients in peptic ulcer group (12, 16.2%) with (6, 8.1%) or without (6, 8.1%) H. pylori or in gastritis group (62, 83.8%) with (25, 33.8%) or without (37, 50%) H. pylori showed no significant difference (p > 0.05). Of the 46 patients who consumed PPI, 13 (17.5%) were H. pylori-positive and 33 (44.6%) H. pylori-negative (p = 0). Ten out of twenty-seven smears showed the occurrence of H. pylori cells, including three with yeast cells. Of the 17 H. pylori-negative smears, three showed the occurrence of yeast cells only. Yeasts stained Gram-positive or Gram-negative and appeared as single or budding cells.
Conclusion: The older age and PPI consumption could favor fungal colonization in the human stomach. The occurrence of a considerable number of H. pylori-positive or H. pylori-negative patients with gastritis or peptic ulcer shows that co-infection of Candida and H. pylori or infection of yeast alone could be associated with dyspeptic diseases. The occurrence of yeast cells in gastric biopsies with different Gram's reactions indicates that fungi might change their cell wall components for establishing a persistent colonization in the stomach.
Introduction
Yeast colonies were first observed in our lab when gastric biopsies were cultured on selective Brucella blood agar containing antibacterials, for the isolation of Helicobacter pylori (H. pylori). However, the importance of yeasts, identified as Candida species, in the development of gastric diseases was not clear and indeed the isolated yeasts were regarded as the transient colonizers of the human stomach or secondary contaminants (Siavoshi, 1998). This conclusion was drawn from the known fact that an acidic environment of normal human stomach could kill the microbes which enter through oral cavity (Howden and Hunt, 1987; Martinsen et al., 2005; Delgado et al., 2013; Yang et al., 2013; Wang et al., 2014). Accordingly, some investigators described the fungal infection of the stomach as a rare phenomenon with no correlation with gastric diseases (Bearse and Pollock, 1936; Gorbach et al., 1967; Minoli et al., 1984; von Rosenvinge et al., 2013), although others presented the consensus of opinion that fungal infection could lead to gastritis and gastric ulcer and thus should be taken seriously and cured. Consistent with the latter, many investigators reported the isolation of Candida yeast from gastric ulcers (Bearse and Pollock, 1936; Kalogeropoulos and Whitehead, 1988; Zwolinska-Wcislo et al., 2001a; Sasaki, 2013) and gastritis cases (Zwolinska-Wcislo et al., 2001b; Kumamoto, 2011). Furthermore, the discovery of H. pylori (Marshall and Warren, 1984) and also the recognition of the bacterial genera such as Streptococcus, Lactobacillus, Propionibacterium, and staphylococcus in the stomach of healthy individuals (Delgado et al., 2013) revealed that the human stomach could serve as a specialized niche for certain microorganisms despite the occurrence of acidic pH and digestive enzymes (Bik et al., 2006; Li et al., 2009; Delgado et al., 2013).
Among published reports, some describe Candida yeast as a true microbiota of gastric environment (von Rosenvinge et al., 2013), others as a transient colonizer of gastric epithelium (Karczewska et al., 2009) and in both cases with the potential to become an opportunistic invader that could establish fungemia with severe clinical consequences (Miranda et al., 2009). Indeed, damaged gastric epithelium has been regarded as an important site for fungal spread to vital sites of the human body such as pleural cavity (Ishiguro et al., 2010) or kidney, liver, and spleen (Kennedy and Volz, 1985). Factors favoring fungal colonization in the stomach have been described as old age, malnutrition, intravascular, or bladder catheterization, H2-blocker therapy and the use of wide-spectrum antibiotics (Scott and Jenkins, 1982; Savino et al., 1994). Hypochlorhydria due to atrophic gastritis, gastric surgery or use of proton pump inhibitors (PPIs) could also increase the risk of fungal infection (Goenka et al., 1996; Martinsen et al., 2005). While the clinical significance of fungi in gastric diseases is controversial (Eras et al., 1972) and the need for antifungal therapy has not been reached a consensus (Sasaki, 2013; von Rosenvinge et al., 2013), the etiologic role of H. pylori infection in the development of gastritis, peptic ulcers, gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT) lymphoma has been widely accepted (Suerbaum and Michetti, 2002) and antimicrobial therapy measurements have been designed (Lam and Talley, 1998; Malfertheiner et al., 2012).
Several studies have reported Candida yeast and H. pylori as the inhabitants of the human stomach (Kalogeropoulos and Whitehead, 1988; Zwolinska-Wcislo et al., 2001a; Karczewska et al., 2009); however, the importance of their mutual interaction in the development of gastric diseases such as gastritis and peptic ulcers is not clearly understood. It is not known whether the concomitant infection of Candida albicans (C. albicans) and H. pylori in the stomach could exacerbate the clinical outcome compared with the infection of each microorganism alone. Indeed, a considerable number of studies have described the implication of yeast in gastric diseases through microscopic observations of the yeast cells in the stained histopathology slides of gastric biopsies, especially in H. pylori-negative patients (Sari et al., 2003; Jung et al., 2009; Sasaki, 2013). In this study, 74 dyspeptic patients with gastric biopsies positive for the growth of Candida yeast were recruited. Patients were classified into gastritis and peptic ulcer groups, with or without H. pylori infection. The frequency of the concurrence of yeast and H. pylori infection was assessed while considering PPI consumption and age and gender of patients.
Methods
Patients
From 530 dyspeptic referrals to the endoscopy unit of Shariati hospital, Tehran-Iran, within 2 years (2012–2014), 74 (14%) which were yeast-positive were recruited in this study. Patients included 36 (48.6%) males and 38 (51.4%) females with the mean age of 50 ± 15, 54 (73%) >40 year and 20 < 40 year. Patients were classified into gastritis and peptic ulcer groups according to endoscopic findings. The status of H. pylori infection and PPI consumption were recorded for each patient. All patients signed an informed consent, and the study was approved by the research ethics committee of Tehran University of Medical Sciences.
Rapid Urease Test (RUT), Isolation of H. pylori and Yeast and Smear Examination
Two antral biopsies were taken from each dyspeptic patient. One biopsy was used for RUT and the other for cultivation of H. pylori and yeast. Preparation of RUT solution and performance of the test was according to manufacturer's instruction (Bahar afshan Co., Iran). Briefly, gastric biopsies were put into urea solution (pH 6.8) and the color change from yellow to pink, within 24 h, was recorded as positive. For H. pylori and yeast cultivation, Brucella blood agar containing 7–10% defibrinated sheep blood and antibacterials; vancomycin (10 mg/L), trimethoprim (5 mg/L), and polymixin B (50 μg/L) was used for surface-inoculation of gastric biopsies. After culturing, the remaining of each biopsy was smeared on the glass slide, Gram-stained and examined by the light microscopy for observing H. pylori and yeast cells. Cultured plates were incubated in the CO2 incubator at 37°C and examined after 2–5 days for observing bacterial and yeast colonies. The identity of bacterial isolates as H. pylori was confirmed by spiral morphology and positive results of catalase, oxidase, and urease tests. Biopsies were determined as H. pylori-positive if the result of one test; RUT or culture showed H. pylori occurrence.
Identification of Yeast Isolates
All the 74 yeast isolates were identified as C. albicans according to their interaction on Chromagar (Chromagar, France) and production of green colonies. The identity of yeasts colonies was confirmed by the Nested-PCR amplification of C. albicans—specific topoisomerase II gene according to Kanbe et al. study (Kanbe et al., 2002). The first-step PCR was performed using a set of degenerate primers (F: 5′-GGTGGWMGDAAYGGDTWYGGYGC-3′ and R: 5′-CRTCNTGATCYTGATCBGYCAT-3′). The amplified product was then used for the second PCR amplification using the primers F: 5′-TTGAACATCTCCAGTTTCAAAGGT-3′ and R: 5′-AGCTAAATTCATAGCAGAAAGC-3′. Electrophoresis was performed for observing the amplicons with the size of 665 bp.
Statistical Analysis
Statistical analysis was performed to find the correlation between age, gender or PPI consumption and H. pylori or fungal colonization in the stomach. Further analysis was performed to determine the probability of the consistency of positive or negative results of culture and RUT. Non-parametric methods used for data analysis were Chi-square and Fisher's Exact tests with the level of significance p < 0.05. Data were collected and analyzed using SPSS version 19.
Results
Among the 74 yeast-positive patients, 36 (48.6%) were males and 38 (51.4%) females with no significant difference (p > 0.05). Fifty-four (73%) were >40 year of age and 20 < 40 year of age, with a significant difference (p = 0). Among 36 males, the number of patients with age >40 year (30, 83.3%) was higher than those with age < 40 year (6, 16.7%), showing a significant difference (p = 0). However, among 38 females, 24 (63.2%) were >40 year of age and 14 (36.8%) < 40 year of age, with a considerable difference but not significant (p > 0.05). Out of 68 patients whose PPI consumption was recorded, 46 (62.1%) consumed PPI and the remaining 22 (29.8%) did not, showing a significant difference (P = 0). Thirty-one out of the 74 patients (41.9%) were H. pylori-positive (15 males and 16 females). Twenty (27%) were >40 year of age and 11 (14.9%) < 40 year of age (p > 0.05). The remaining 43 (58.1%) were H. pylori-negative (21 males and 22 females); 34 (45.9%) >40 year of age and 9 (12.2%) < 40 year of age (p = 0). Patients were classified into two groups; 12 (16.2%) with ulcer and 62 (83.8%) with gastritis. Out of the 12 ulcer patients; 6 (8.1%) were H. pylori-positive and 6 (8.1%) H. pylori-negative (p > 0.05). Among the 62 gastritis patients, 25 (33.8%) were H. pylori-positive and 37(50%) H. pylori-negative (p > 0.05). Out of the 46 patients who consumed PPI, 13 (17.5%) were H. pylori-positive and 33 (44.6%) H. pylori-negative (p = 0). The remaining 22 (29.8%) patients without PPI consumption included 15 (20.3%) H. pylori-positive and 7 (9.5%) H. pylori-negative (p > 0.05; Table 1).
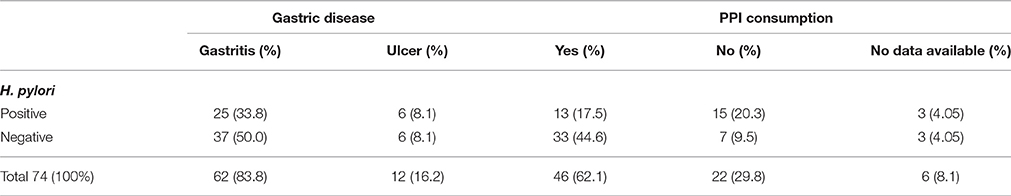
Table 1. Classification of 74 yeast-positive patients according to gastric disease, the occurrence of H. pylori infection and PPI consumption.
Seventy-four biopsies were classified according to the results of culture and RUT and 27 according to the results of the microscopic examination of gastric biopsies smears (Table 2). Statistical analyses showed that the frequency of biopsies with consistency in the results of RUT and culture (A: 57, 77%), including both positive (19, 25.7%) and both negative (38, 51.3%) was higher than those without consistency in the results of two tests (B: 7, 9.5%), including 3 (4.1%) RUT-positive and 4 (5.4%) culture -positive (p = 0). The remaining biopsies (C:10, 13.5%) included 2 (2.7%) culture- positive, 4 (5.4%) culture- negative, 3(4.1%) RUT- positive and 1(1.3%) RUT-negative (Table 2, Figure 1).
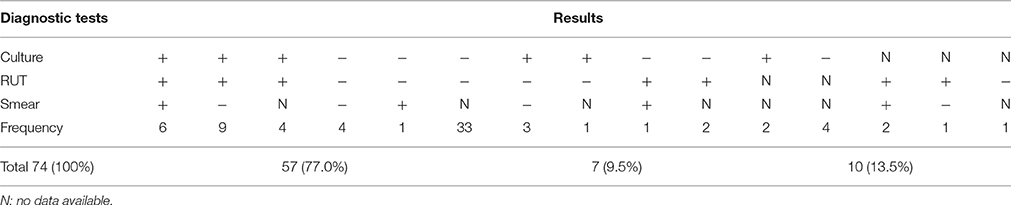
Table 2. The frequency of positive or negative results of culture and RUT of 74 gastric biopsies and microscopic observation of 27 gastric biopsies.
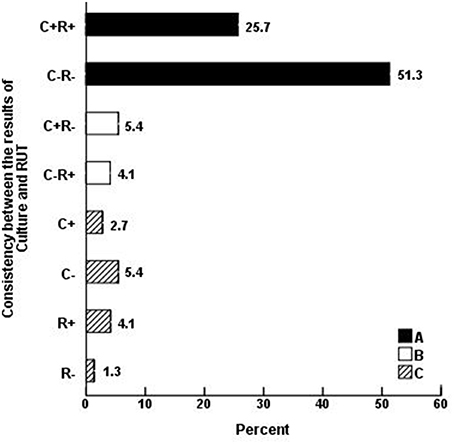
Figure 1. Consistency between the results of H. pylori culture (C) and RUT (R) in 74 gastric biopsies. The frequency of biopsies with consistency in the results of RUT and culture (A: 57, 77%), including both positive (19, 25.7%) and both negative (38, 51.3%) was higher than those without consistency in the results of two tests (B: 7, 9.5%), including 4 (5.4%) culture-positive and 3 (4.1%) RUT-positive (p = 0). The remaining (C: 10, 13.5%) biopsies included 2 (2.7%) culture-positive, 4 (5.4%) culture-negative, 3 (4.1%) RUT-positive, and 1(1.3%) RUT-negative.
Examination of 27 Gram-stained biopsy smears by light microscopy revealed the presence of H. pylori cells in 10, including three with the concomitant occurrence of H. pylori and yeast cells. The three biopsies were culture- and RUT-positive and belonged to two patients with gastritis and one with peptic ulcer. The remaining 17 biopsy smears were negative for the presence of H. pylori, but three showed the presence of yeast cells. The three biopsies were culture- and RUT-negative and belonged to the patients with gastritis. The six yeast-positive biopsies were taken from patients with no record of PPI consumption (Tables 1, 2). In microscopic observations, yeast cells appeared as single cells, some in the stage of budding. Moreover, some yeast cells appeared Gram-positive and some Gram-negative (Figure 2).
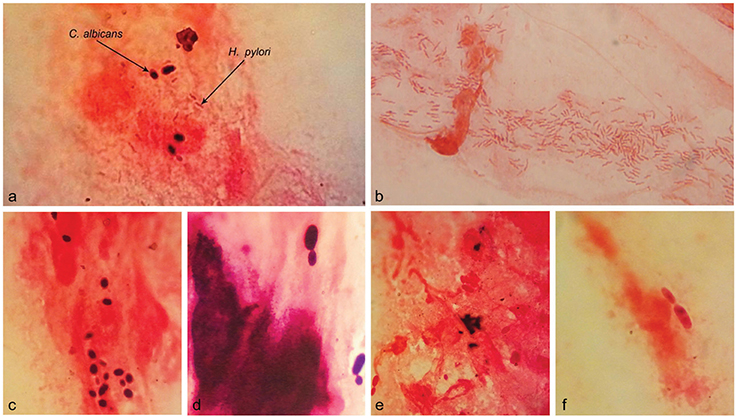
Figure 2. Microscopic observation of H. pylori and Candida yeast in the Gram-stained smears of gastric biopsies from patients with gastritis. The Concurrence of H. pylori and Candida yeast cells (A). The spiral cells of H. pylori (B). Yeast cells; Gram positive (C) or Gram-negative (E) and some in the stage of budding (D,F). Original magnification: X 1250.
Discussion
Candida yeast (Whyte et al., 1982; Sharp et al., 1992) and H. pylori (Webb et al., 1994; Malaty et al., 2002) colonize the human gastrointestinal tract early in life. It appears that both microorganisms are well-adapted to establish in the specialized environment of the human stomach (Kalogeropoulos and Whitehead, 1988; Zwolinska-Wcislo et al., 2001a; Loster et al., 2006; Karczewska et al., 2009). Detection of either H. pylori or Candida yeast in gastric biopsies of dyspeptic patients indicates the implication of these two microorganisms in gastric diseases. However, the relationship between their concomitant occurrence and gastric diseases has not been thoroughly investigated.
Out of the 74 yeast-positive patients, 36 (48.6%) were males and 38 (51.4%) females with the mean age of 50 ± 15, 54 (73%) were >40 year of age and 20 < 40 year of age, with a significant difference (p = 0). Forty six out of 68 patients (67.6%) consumed PPI and 22 (32.4%) did not, showing a significant difference (p = 0). These results indicate that both the older age and PPI consumption could be important risk factors in favor of fungal colonization. Candida spp. have been isolated from gastric biopsies, the brush samples of gastric mucosa and peritoneal fluid and the frequency of fungal isolation was higher in older patients (Morishita et al., 1993; Goenka et al., 1996) or in those with hypochlorhydria (Goenka et al., 1996). Furthermore, among the 178 patients with benign gastric ulcer, there was a significant difference between the number of older patients at a mean age of 64.2 year with yeast in their histopathology preparation (36, 20.2%) and those with the mean age of 56.2 year without yeast (142, 79.8%; p < 0.01). It was concluded that the older age was the only risk factor for fungal infection, and gender, cigarette smoking and tea or coffee consumption were not implicated in the development of gastric ulcer (Wu et al., 1995). It appears that immunosuppression due to aging could facilitate fungal invasion of the gastric ulcer (Neeman et al., 1981; Loffeld et al., 1988). Reports also indicate that hypochlorhydria provides favorable conditions for the growth of fungi (Goenka et al., 1996; Martinsen et al., 2005) and bacteria (Thorens et al., 1996) in the stomach. Statistical analysis showed a high consistency between the positive or negative results of H. pylori culture and RUT. When comparing the H. pylori-positive (31, 41.9%) with H. pylori-negative (43, 58.1%) patients, it was found that age, gender, and PPI consumption are not the important risk factors for H. pylori infection.
Among the 74 yeast-positive patients, 12 (16.2%) had peptic ulcer and 62 (83.8%) chronic gastritis. While reports indicate the fungal colonization in the gastric mucosa of 7–33% asymptomatic patients (Minoli et al., 1982; Loffeld et al., 1988), the fungal infection in the gastric ulcers has been estimated as 6.9% (Di Febo et al., 1985) to 33.3% (Katzenstein and Maksem, 1979) and 36% (Gotlieb-Jensen and Andersen, 1983). In ulcer group, the number of H. pylori-positive patients (6, 8.1%) and H. pylori-negative patients (6, 8.1%) was similar (p > 0.05). The same results were observed in gastritis group; 26 (35.1%) H. pylori-positive and 36 (48.7%) H. pylori-negative, with no significant difference (p > 0.05). These results demonstrate that a considerable number of H. pylori-negative patients also suffered from gastric ulcer or gastritis. Accordingly, the concurrence of Candida yeast and H. pylori or yeast infection alone could increase the risk of development of chronic gastritis or peptic ulcer. In a study on 293 biopsies of dyspeptic patients RUT, culture and microscopy were used to demonstrate the concurrence of fungal cells with H. pylori in 14% of gastric ulcer patients, 4% of those with chronic gastritis and 2% of control subjects (Zwolinska-Wcislo et al., 2001a). In another study on 158 patients, the co-existence of H. pylori and Candida yeast was found in 18 (11%) and was more frequent in patients with gastric ulcer (Karczewska et al., 2009). It has been proposed that the establishment of H. pylori in gastric epithelium might play an important role in the fungal colonization (Zwolińska-Wcisło et al., 2003). Furthermore, the coexistence of yeast and H. pylori in the stomach might exacerbate the inflammatory response and tissue damage (Diebel et al., 1999; Zwolinska-Wcislo et al., 2001a; Karczewska et al., 2009).
Microscopic examination of the 27 Gram-stained smears of gastric biopsies showed the presence of yeast cells in six (8.1%) specimens, three concomitant with H. pylori. Yeasts appeared as single cells which stained Gram-positive or Gram-negative and some in the stage of budding. A report showed microscopic observation of both Candida yeast and Campylobacter-like organisms in 12% of gastric biopsies from patients with gastric ulcer (Kalogeropoulos and Whitehead, 1988). Previous studies, dating back to 1936 or earlier, demonstrated that microscopic observation of fungi in biopsy specimens from gastric ulcer is an important indication of disease. It was proposed that fungi typical oval morphology might change to hyphae when colonizing the tissues (Bearse and Pollock, 1936). However, more recent reports proposed that most dimorphic fungi that are human pathogens, invade tissues by budding and form filamentous hyphae in the external environment (Gow et al., 2002). Investigators believe that fungi have learned to establish on the mucosal surfaces of the human body by minimizing the induction of host immune response (Hube, 2009). They do this by changing the proteins and carbohydrates of their cell wall which determine the fungal morphology as yeast or hyphae. Accordingly, components of the cell wall and thus morphology play an important role in the fungal establishment in the host (Gow et al., 2002; Gow and Hube, 2012). In the present study, the occurrence of yeast cells in the biopsy smears, with different reactions to Gram's stain, might reflect changes in the cell wall components that help the persistent colonization of yeast cells in the stomach.
Results of this study demonstrate that both the older age and PPI consumption are among the risk factors for fungal colonization in the stomach. Furthermore, the concurrence of Candida yeast and H. pylori or yeast infection alone could be involved in the development of peptic diseases. Considering the well-known role of H. pylori and less-recognized importance of Candida yeast in gastric diseases, the possible role of the concurrent infection of these two microorganisms in the development of chronic gastritis and peptic ulcer warrants in depth investigations (Zwolinska-Wcislo et al., 2001a). Furthermore, the widely accepted role of H. pylori in gastric cancer (Malfertheiner et al., 2012) and the frequent detection of fungal infection in patients with gastric cancer (Sasaki, 2013) indicate that the mutual interaction of Candida yeast with H. pylori might go much further, leading to the development of gastric malignancies (Wang et al., 2014). Concomitant colonization of H. pylori and Candida yeast in the human gastric niche might refer to their evolutionary association which begun long time ago and has led to the endosymbiotic relationship of H. pylori with Candida yeast (Siavoshi et al., 2005; Siavoshi and Saniee, 2014). Future studies will elucidate the crucial and determinant role of these two microorganisms in human health and disease.
Author Contributions
SM, PS, and FS designed the experiment. FM provided the gastric biopsies. RM did part of the research work. FS and PS did part of the lab work. SK did the graphics and tables. FS and PS wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors wish to thank Dr. Saeid Latifi-Navid for kindly editing the manuscript. This research was funded by the research councils of University of Tehran and Tehran University of Medical Sciences.
References
Bearse, C., and Pollock, L. H. (1936). Mycotic infection of the stomach: report of a case with perforation. Ann. Surg. 104, 167–174. doi: 10.1097/00000658-193608000-00002
Bik, E. M., Eckburg, P. B., Gill, S. R., Nelson, K. E., Purdom, E. A., Francois, F., et al. (2006). Molecular analysis of the bacterial microbiota in the human stomach. Proc. Natl. Acad. Sci. U.S.A. 103, 732–737. doi: 10.1073/pnas.0506655103
Delgado, S., Cabrera-Rubio, R., Mira, A., Suarez, A., and Mayo, B. (2013). Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb. Ecol. 65, 763–772. doi: 10.1007/s00248-013-0192-5
Diebel, L. N., Liberati, D. M., Diglio, C. A., Dulchavsky, S. A., and Brown, W. J. (1999). Synergistic effects of Candida and Escherichia coli on gut barrier function. J. Trauma 47, 1045–1050. discussion: 1050–1041. doi: 10.1097/00005373-199912000-00009
Di Febo, G., Miglioli, M., Calo, G., Biasco, G., Luzza, F., Gizzi, G., et al. (1985). Candida albicans infection of gastric ulcer frequency and correlation with medical treatment. Results of a multicenter study. Dig. Dis. Sci. 30, 178–181. doi: 10.1007/BF01308206
Eras, P., Goldstein, M. J., and Sherlock, P. (1972). Candida infection of the gastrointestinal tract. Medicine (Baltimore) 51, 367–379. doi: 10.1097/00005792-197209000-00002
Goenka, M. K., Kochhar, R., Chakrabarti, A., Kumar, A., Gupta, O., Talwar, P., et al. (1996). Candida overgrowth after treatment of duodenal ulcer. A comparison of cimetidine, famotidine, and omeprazole. J. Clin. Gastroenterol. 23, 7–10. doi: 10.1097/00004836-199607000-00003
Gorbach, S. L., Nahas, L., Lerner, P. I., and Weinstein, L. (1967). Studies of intestinal microflora. I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology 53, 845–855.
Gotlieb-Jensen, K., and Andersen, J. (1983). Occurrence of Candida in gastric ulcers. Significance for the healing process. Gastroenterology 85, 535–537.
Gow, N. A., Brown, A. J., and Odds, F. C. (2002). Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5, 366–371. doi: 10.1016/S1369-5274(02)00338-7
Gow, N. A., and Hube, B. (2012). Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 15, 406–412. doi: 10.1016/j.mib.2012.04.005
Howden, C. W., and Hunt, R. H. (1987). Relationship between gastric secretion and infection. Gut 28, 96–107. doi: 10.1136/gut.28.1.96
Hube, B. (2009). Fungal adaptation to the host environment. Curr. Opin. Microbiol. 12, 347–349. doi: 10.1016/j.mib.2009.06.009
Ishiguro, T., Takayanagi, N., Ikeya, T., Yoshioka, H., Yanagisawa, T., Hoshi, E., et al. (2010). Isolation of Candida species is an important clue for suspecting gastrointestinal tract perforation as a cause of empyema. Intern. Med. 49, 1957–1964. doi: 10.2169/internalmedicine.49.3667
Jung, M. K., Jeon, S. W., Cho, C. M., Tak, W. Y., Kweon, Y. O., Kim, S. K., et al. (2009). Treatment of gastric candidiasis in patients with gastric ulcer disease: are antifungal agents necessary? Gut Liver 3, 31–34. doi: 10.5009/gnl.2009.3.1.31
Kalogeropoulos, N. K., and Whitehead, R. (1988). Campylobacter-like organisms and Candida in peptic ulcers and similar lesions of the upper gastrointestinal tract: a study of 247 cases. J. Clin. Pathol. 41, 1093–1098. doi: 10.1136/jcp.41.10.1093
Kanbe, T., Horii, T., Arishima, T., Ozeki, M., and Kikuchi, A. (2002). PCR-based identification of pathogenic Candida species using primer mixes specific to Candida DNA topoisomerase II genes. Yeast 19, 973–989. doi: 10.1002/yea.892
Karczewska, E., Wojtas, I., Sito, E., Trojanowska, D., Budak, A., Zwolinska-Wcislo, M., et al. (2009). Assessment of co-existence of Helicobacter pylori and Candida fungi in diseases of the upper gastrointestinal tract. J. Physiol. Pharmacol. 60(Suppl. 6), 33–39.
Katzenstein, A. L., and Maksem, J. (1979). Candidal infection of gastric ulcers. Histology, incidence, and clinical significance. Am. J. Clin. Pathol. 71, 137–141. doi: 10.1093/ajcp/71.2.137
Kennedy, M. J., and Volz, P. A. (1985). Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect. Immun. 49, 654–663.
Kumamoto, C. A. (2011). Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 14, 386–391. doi: 10.1016/j.mib.2011.07.015
Lam, S. K., and Talley, N. J. (1998). Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J. Gastroenterol. Hepatol. 13, 1–12. doi: 10.1111/j.1440-1746.1998.tb00537.x
Li, X. X., Wong, G. L., To, K. F., Wong, V. W., Lai, L. H., Chow, D. K., et al. (2009). Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PLoS ONE 4:e7985. doi: 10.1371/journal.pone.0007985
Loffeld, R. J., Loffeld, B. C., Arends, J. W., Flendrig, J. A., and van Spreeuwel, J. P. (1988). Fungal colonization of gastric ulcers. Am. J. Gastroenterol. 83, 730–733.
Loster, B. W., Majewski, S. W., Czesnikiewicz-Guzik, M., Bielanski, W., Pierzchalski, P., and Konturek, S. J. (2006). The relationship between the presence of Helicobacter pylori in the oral cavity and gastric in the stomach. J. Physiol. Pharmacol. 57(Suppl. 3), 91–100.
Malaty, H. M., El-Kasabany, A., Graham, D. Y., Miller, C. C., Reddy, S. G., Srinivasan, S. R., et al. (2002). Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet 359, 931–935. doi: 10.1016/S0140-6736(02)08025-X
Malfertheiner, P., Megraud, F., O'Morain, C. A., Atherton, J., Axon, A. T., Bazzoli, F., et al. (2012). Management of Helicobacter pylori infection–the Maastricht IV/ Florence Consensus Report. Gut 61, 646–664. doi: 10.1136/gutjnl-2012-302084
Marshall, B. J., and Warren, J. R. (1984). Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315. doi: 10.1016/S0140-6736(84)91816-6
Martinsen, T. C., Bergh, K., and Waldum, H. L. (2005). Gastric juice: a barrier against infectious diseases. Basic Clin. Pharmacol. Toxicol. 96, 94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x
Minoli, G., Terruzzi, V., Butti, G., Frigerio, G., and Rossini, A. (1982). Gastric candidiasis: an endoscopic and histological study in 26 patients. Gastrointest. Endosc. 28, 59–61. doi: 10.1016/S0016-5107(82)72998-0
Minoli, G., Terruzzi, V., Ferrara, A., Casiraghi, A., Rocca, F., Rainer, H., et al. (1984). A prospective study of relationships between benign gastric ulcer, Candida, and medical treatment. Am. J. Gastroenterol. 79, 95–97.
Miranda, L. N., van der Heijden, I. M., Costa, S. F., Sousa, A. P., Sienra, R. A., Gobara, S., et al. (2009). Candida colonisation as a source for candidaemia. J. Hosp. Infect. 72, 9–16. doi: 10.1016/j.jhin.2009.02.009
Morishita, T., Kamiya, T., Munakata, Y., and Tsuchiya, M. (1993). Radiologic and endoscopic studies of gastric ulcers associated with Candida infection. Acta Gastroenterol. Latinoam. 23, 223–229.
Neeman, A., Avidor, I., and Kadish, U. (1981). Candidal infection of benign gastric ulcers in aged patients. Am. J. Gastroenterol. 75, 211–213.
Sari, R., Altunbas, H., Mahsereci, E., Meric, M., Gelen, T., and Karayalcin, U. (2003). Multiple gastric ulcers caused by gastric candidiasis in a diabetic patient: a rare cause of upper GI bleeding. Gastrointest. Endosc. 58, 309–311. doi: 10.1067/mge.2003.330
Sasaki, K. (2013). Candida-associated gastric ulcer relapsing in a different position with a different appearance. World J. Gastroenterol. 18, 4450–4453. doi: 10.3748/wjg.v18.i32.4450
Savino, J. A., Agarwal, N., Wry, P., Policastro, A., Cerabona, T., and Austria, L. (1994). Routine prophylactic antifungal agents (clotrimazole, ketoconazole, and nystatin) in nontransplant/nonburned critically ill surgical and trauma patients. J. Trauma 36, 20–25. discussion: 25–26. doi: 10.1097/00005373-199401000-00004
Scott, B. B., and Jenkins, D. (1982). Gastro-oesophageal candidiasis. Gut 23, 137–139. doi: 10.1136/gut.23.2.137
Sharp, A. M., Odds, F. C., and Evans, E. G. (1992). Candida strains from neonates in a special care baby unit. Arch. Dis. Child. 67(1 Spec. No.), 48–52. doi: 10.1136/adc.67.1_Spec_No.48
Siavoshi, F., Salmanian, A. H., Kbari, F. A., Malekzadeh, R., and Massarrat, S. (2005). Detection of Helicobacter pylori-specific genes in the oral yeast. Helicobacter 10, 318–322. doi: 10.1111/j.1523-5378.2005.00319.x
Siavoshi, F., and Saniee, P. (2014). Vacuoles of Candida yeast as a specialized niche for Helicobacter pylori. World J. Gastroenterol. 20, 5263–5273. doi: 10.3748/wjg.v20.i18.5263
Suerbaum, S., and Michetti, P. (2002). Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186. doi: 10.1056/NEJMra020542
Thorens, J., Froehlich, F., Schwizer, W., Saraga, E., Bille, J., Gyr, K., et al. (1996). Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut 39, 54–59. doi: 10.1136/gut.39.1.54
von Rosenvinge, E. C., Song, Y., White, J. R., Maddox, C., Blanchard, T., and Fricke, W. F. (2013). Immune status, antibiotic medication and pH are associated with changes in the stomach fluid microbiota. ISME J. 7, 1354–1366. doi: 10.1038/ismej.2013.33
Wang, Z. K., Yang, Y. S., Stefka, A. T., Sun, G., and Peng, L. H. (2014). Review article: fungal microbiota and digestive diseases. Aliment. Pharmacol. Ther. 39, 751–766. doi: 10.1111/apt.12665
Webb, P. M., Knight, T., Greaves, S., Wilson, A., Newell, D. G., Elder, J., et al. (1994). Relation between infection with Helicobacter pylori and living conditions in childhood: evidence for person to person transmission in early life. BMJ 308, 750–753. doi: 10.1136/bmj.308.6931.750
Whyte, R. K., Hussain, Z., and deSa, D. (1982). Antenatal infections with Candida species. Arch. Dis. Child. 57, 528–535. doi: 10.1136/adc.57.7.528
Wu, C. S., Wu, S. S., and Chen, P. C. (1995). A prospective study of fungal infection of gastric ulcers: clinical significance and correlation with medical treatment. Gastrointest. Endosc. 42, 56–58. doi: 10.1016/S0016-5107(95)70244-X
Yang, I., Nell, S., and Suerbaum, S. (2013). Survival in hostile territory: the microbiota of the stomach. FEMS Microbiol. Rev. 37, 736–761. doi: 10.1111/1574-6976.12027
Zwolińska-Wcisło, M., Brzozowski, T., Kwiecień, S., Drozdowicz, D., Bogdał, J., Budak, A., et al. (2003). Kolonizacja grzybicza przewodu pokarmowego w badaniach klinicznych i doÅŻwiadczalnych. Przew Lek 6, 81–89.
Zwolinska-Wcislo, M., Budak, A., Bogdal, J., Trojanowska, D., and Stachura, J. (2001a). Effect of fungal colonization of gastric mucosa on the course of gastric ulcers healing. Med. Sci. Monit. 7, 266–275.
Keywords: H. pylori, C. albicans, coinfection, gastric diseases
Citation: Massarrat S, Saniee P, Siavoshi F, Mokhtari R, Mansour-Ghanaei F and Khalili-Samani S (2016) The Effect of Helicobacter pylori Infection, Aging, and Consumption of Proton Pump Inhibitor on Fungal Colonization in the Stomach of Dyspeptic Patients Front. Microbiol. 7:801. doi: 10.3389/fmicb.2016.00801
Received: 20 February 2016; Accepted: 11 May 2016;
Published: 25 May 2016.
Edited by:
Mohammad H. Derakhshan, University of Glasgow, UKReviewed by:
Amedeo Amedei, University of Florence, ItalyHridayesh Prakash, University of Hyderabad, India
Copyright © 2016 Massarrat, Saniee, Siavoshi, Mokhtari, Mansour-Ghanaei and Khalili-Samani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farideh Siavoshi, c2lhdm9zaGlAa2hheWFtLnV0LmFjLmly
 Sadegh Massarrat1
Sadegh Massarrat1 Farideh Siavoshi
Farideh Siavoshi Saman Khalili-Samani
Saman Khalili-Samani