- 1Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases, University of Cologne, Cologne, Germany
- 2Department I of Internal Medicine, ECMM Diamond Center of Excellence in Medical Mycology, German Centre for Infection Research (DZIF), University of Cologne, Cologne, Germany
- 3Clinical Trials Centre Cologne (ZKS Köln), University of Cologne, Cologne, Germany
- 4Labor Dr. Wisplinghoff, Cologne, Germany
- 5Institute for Medical Microbiology, Immunology and Hygiene, University of Cologne, Cologne, Germany
- 6Institute for Clinical Microbiology, University Witten/Herdecke, Witten, Germany
- 7Department of Internal Medicine III, University of Munich, Munich, Germany
- 8Department of Cellular Immunology, Clinic for Rheumatology and Clinical Immunology, Charité–University Medicine Berlin, Berlin, Germany
- 9German Rheumatism Research Centre (DRFZ) and Leibniz Association, Berlin, Germany
- 10Miltenyi Biotec GmbH, Bergisch Gladbach, Germany
Background: Blood or tissue culture or histology prove invasive Candida infection, but long time to result, limited feasibility and sensitivity call for new approaches. In this pilot project, we describe the diagnostic potential of quantitating Candida-reactive, CD4/CD69/CD154 positive lymphocytes in blood of patients with invasive Candida infection.
Methods: We used flow cytometry quantitating Candida-reactive, CD4/CD69/CD154 positive lymphocytes from peripheral blood of patients with invasive Candida infection, from patients at risk and healthy volunteers as controls.
Results: Elevated levels of Candida-reactive lymphocytes were measured in 13 patients with proven invasive Candida infection and in one patient with probable hepatosplenic candidiasis. Results of three candidemia patients were uninterpretable due to autofluorescence of samples. Twelve of 13 patients had Candida identified to species level by conventional methods, and T cell reactivity correctly identified Candida species in 10 of 12 patients. Nine hematological high-risk patients and 14 healthy donors had no elevated Candida-reactive T cell counts.
Conclusions: This Candida-reactive lymphocyte assay correctly identified the majority of patients with invasive Candida infection and the respective species. Our assay has the potential to support diagnosis of invasive Candida infection to species level and to facilitate tailored treatment even when biopsies are contraindicated or cultures remain negative.
Introduction
Invasive Candida Infection (ICI) is among the most common bloodstream infections and represents the most common invasive fungal infection (Wisplinghoff et al., 2004; Kullberg and Arendrup, 2015). Candida spp. are part of the mucosal mycobiota, and may translocate to the bloodstream. Candida spp. may also cause complicated deep tissue disease. Organs frequently involved are kidney, spleen, liver, central nervous system, eyes, bones and joints (Arendrup, 2010; Kullberg and Arendrup, 2015).
ICI is proven by direct tests, such as culture, histopathology or direct microscopy from biopsy samples (Cuenca-Estrella et al., 2012). Blood culture sensitivity is reported with 21–71% (Ness et al., 1989; Kami et al., 2002). Underlying medical conditions often prevent biopsies (Clancy and Nguyen, 2013). Indirect tests, such as Candida mannan antigen, anti-mannan antibodies, Candida albicans germ tube gem tube antibodies (CAGTA) and β-1,3-D-glucan (BDG) are not species specific (Ponton et al., 1994; Laín et al., 2007; Mikulska et al., 2010; Lamoth et al., 2012). Polymerase chain reaction (PCR) based assays are being evaluated, but lack validation and standardization in addition to variable sensitivity (Schelenz et al., 2015; Pappas et al., 2016).
Fungal antigens activate CD4+ T cells, which initiate inflammatory and antifungal immune response (Romani, 2011). These fungal-reactive T cells are specifically directed to individual pathogens and may promise species specificity (Bacher et al., 2013).
We used a recently developed flow cytometry assay quantitating Candida-reactive CD4+ T cells for ICI patients identifying Candida to species level (Bacher et al., 2015). The basic principle is the stimulation of patient derived CD4+ T cells with lysates from either C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, or C. krusei. Candida-spp.-reactive CD4+ T cells upregulate the activation markers CD154 (CD40L) and CD69, which are detected by flow cytometry (Frentsch et al., 2005; Bacher et al., 2013, 2014, 2015; Cossarizza et al., 2017). In a single case report of a Candida spondylodiscitis we previously demonstrated the benefits of the Candida-reactive lymphocyte assay detecting ICI and Candida spp. allowing tailored treatment (Koehler et al., 2017).
Material and Methods
Mechanical Lysis of candida spp.
C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei. were cultured for 5 days at 37°C in Sabouraud 2% Glucose Media (Carl Roth, Karlsruhe, Germany). Candida cells were recovered by centrifugation and washed with ultra-pure water (Biochrom GmbH, Berlin, Germany). For total mycelial lysate Candida cells were suspended in Dulbecco's PBS (PromoCell; Heidelberg, Germany) and lysed mechanically using gentleMACSTM M tubes and gentleMACSTM Dissociator (both Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Lysed mycelium of each Candida spp. was collected by centrifugation.
By use of PierceTM BCA Protein Assay Kit (Themo Fisher Scientific, Waltham, MA, USA) the concentration of Candida lysates was determined.
Lysates of each Candida spp. were stored separately in aliquots at −80°C.
Study Participants
Adult patients at risk were eligible to participate to the survey per protocol – Improving Diagnosis of Severe Infections of Immunocompromised Patients (ISI) (Identifier of the University of Cologne Ethics Committee: 08-160). Written informed consent was obtained from each patient or the legal guardian.
Buffy coats or peripheral ethylenediaminetetraacetic acid (EDTA) blood samples were obtained from the Institute for Transfusion Medicine, Klinikum Dortmund gGmbH, Germany, the DRK Dresden, Germany, the Charité blood bank, Charité Berlin, Germany or from in-house volunteers after informed consent (Identifiers of the Charité Berlin Ethics Committee: EA1/149/12; EA1/272/15).
Cell Preparation
Peripheral blood mononuclear cells (PBMC) were isolated from EDTA blood samples by density centrifugation with density gradient media (Axis-Shield, Oslo, Norway). PBMCs were washed with CliniMACS PBS/EDTA buffer and RPMI-1640 medium and were re-suspended with RPMI-1640 medium supplemented with 5% GemcellTM human AB serum (Gemini Bio Products, West Sacramento, CA, USA) and 1% 2 mM L-glutamin (Lonza Group, Basel, Switzerland).
Detection of Viable Cells and Culture of PBMCs
To quantify viable cell count, PBMCs were diluted 1:10 in RPMI-1640 medium and stained with 7-aminoactinomycin D (7AAD) (Miltenyi Biotec GmbH and eBioscience, San Diego, CA, USA). Data were measured with a MACSQuant® Analyzer 10 and MACSQuantifyTM software (Version 2.6, both Miltenyi Biotec GmbH,) was used for evaluation. 1 × 106 cells/100 μl and well were seeded in 100 μl RPMI-1640 medium supplemented with 5% GemcellTM human AB serum and 1% 2 mM L-Glutamin into 96 well flat-bottom plates. PBMCs were incubated overnight at 37°C with 5% CO2.
Stimulation of PBMCs
Cultured cells were stimulated with CD28 and CD40 pure antibodies (Miltenyi Biotec GmbH) and with lysate of C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei or Staphylococcal Enterotoxin B (SEB) (1 μg ml−1; Sigma-Aldrich GmbH, Munich, Germany) for 5 h at 37°C with 5% CO2. (Figure 1) Each Candida species was tested in separate stimulation. Missing challenge with fungal lysate served as negative control, addition of SEB as positive control.
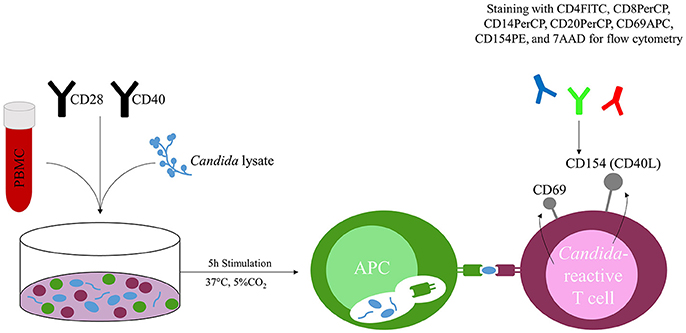
Figure 1. Concept of the Candida-reactive lymphocyte assay. Peripheral blood mononuclear cells (PBMC) are co-incubated with Candida spp. specific lysate (e.g., C. albicans) and stimulated with CD28 and CD40 antibodies. T cells react to the presented fungal peptides on the surface of antigen presenting cells (APC) and upregulate the activation markers CD69 and CD154 (CD40L), which are quantified by flow cytometry after 5 h stimulation at 37°C and 5% CO2.
Flow Cytometry
Stimulated PBMC were stained with CD4FITC (VIT4), CD8PerCP (BW135/80), CD14PerCP (TÜK4), CD20PerCP (LT20), CD69APC (FN50), CD154PE (5C8) (all Miltenyi Biotec GmbH) and 7AAD (Figure 1). Data were acquired on a MACSQuant® Analyzer 10 and MACSQuantifyTM software was used for analysis. Candida-reactive CD4+ T cells were detected based on the upregulation of CD69 and CD154 (CD40L) (Figure 1). Gating strategy is shown in Figure 2.
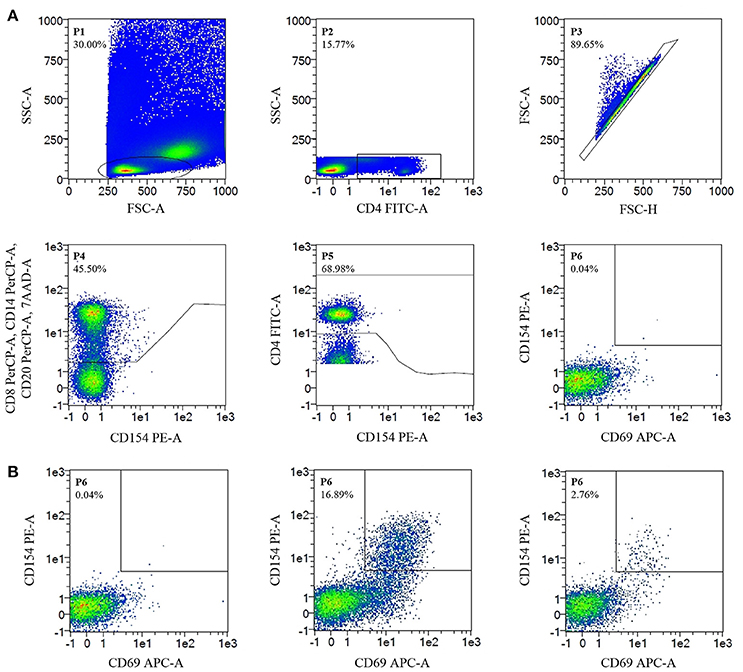
Figure 2. Flow Cytometry-Gating strategy and detection of C. glabrata-reactive T cells. Cell frequencies (%). (A) Gating strategy. Negative control, unstimulated CD4+ T cells, show no CD69/CD154 expression. (B) Detection of C. glabrata-reactive T cells. From left to right. Negative control; positive control (staphylococcal enterotoxin B stimulated CD4+ T cells show CD69/CD154 double-expression); antigen-stimulated probe (CD69/CD154 double expression of C. glabrata-reactive CD4+ T cells after antigen stimulation with C. glabrata lysate).
Fluorescence Microscopy
For fluorescence microscopy stimulated PBMCs were stained with CD4FITC (VIT4), CD69APC (FN50), and CD154PE (5C8) and microscopy was performed with a Zeiss LSM Meta710 confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany).
Conventional Diagnostics, Study Design and Definitions
Blood-cultures were processed using the BactAlert 3D (BioMerieux, Marcy l'etoile France) and the Bactec (BectonDickinson, Franklin Lakes, NJ, USA) systems. Identification (ID) of Candida spp. to species level and susceptibility testing (AST) was performed using VITEK2 (ID and AST) and API (ID, both, BioMerieux) and the MALDI Biotyper® (ID, Bruker Daltonics, Hilden, Germany), and E-Test (AST, BioMerieux). BDG measurements were done using the Fungitell ß-D-glucan ELISA assay (Associates of Cape Cod, MA, USA). Histology was stained with Grocott methenamine silver staining (Merck, Darmstadt, Germany and Diagonal GmbH, Münster, Germany).
To examine the performance of Candida-reactive T cells as diagnostic read-out for ICI, patients with proven and suspected ICI and disease control patients were enrolled in the web-based database of the European Confederation of Medical Mycology (ECMM) Candida Registry CandiReg (Identifier of the University of Cologne Ethics Committee: 17-485, ClinicalTrials.gov Identifier: NCT03450005) (ECMM, 2018). Risk factors, site of infection, symptoms, diagnosis, treatment and outcome were documented.
For clinical evaluation patients cohorts were grouped according to the 2008 European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria (De Pauw et al., 2008).
Statistical Analysis
Cut-off values discriminating between healthy donors, disease control and patients with proven ICI were calculated by receiver operating characteristic (ROC) analysis and p-values were determined by Fisher's exact test using IBM SPSS Statistics software (Version 23, IBM Corporation, Armonk, NY, USA). For the determination of the cut-off value of fold increase of antigen-stimulated T cells compared to unstimulated T cells, we excluded measurements of proven ICI patients in the presence of antifungal treatment without elevated frequencies of Candida-reactive T cells. To examine the cut-offs of levels of Candida-reactive T cells discriminating between individuals with and without ICI we excluded measurements of proven ICI patients in the presence of antifungal treatment without elevated frequencies of Candida-reactive T cells. Measurements of proven ICI patients with a fold frequencies smaller than the calculated 3.05-fold increase were excluded to exclude false-positive results and to find correct and sufficient stimulations.
Results
Patient Characteristics
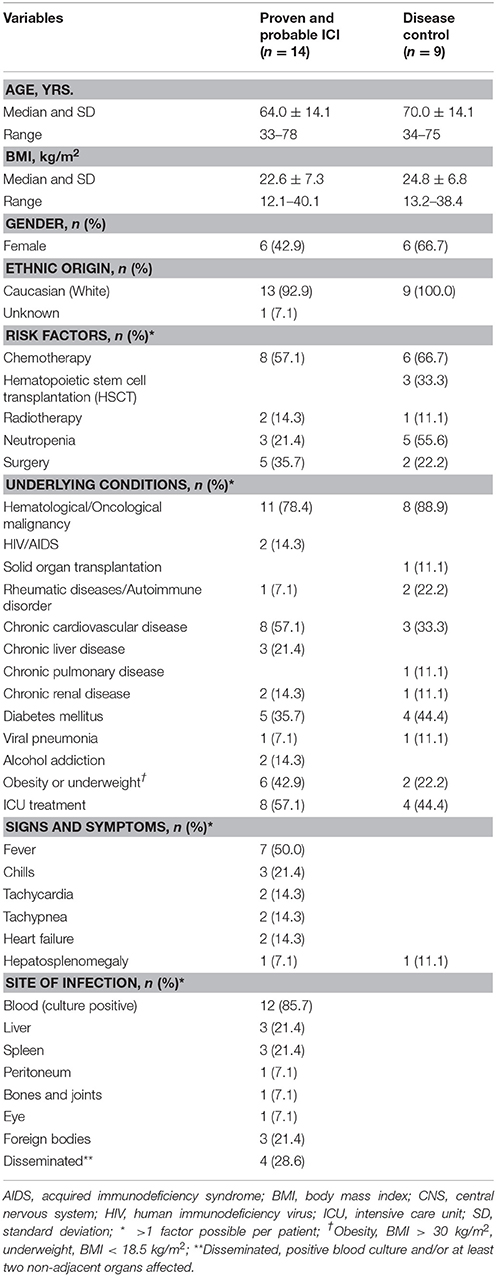
We determined the performance of the Candida-reactive lymphocyte assay in a cohort of 26 patients. Three (11.5%) patients were excluded from analysis due to autofluorescence of cells leading to elevated background expression of CD69+/CD154+ T cells. Thirteen patients (56.5%) had proven and one patient (4.3%) probable ICI, a culture negative hepatosplenic candidiasis (De Pauw et al., 2008). Nine hematological high-risk patients (39.1%) served as disease control cohort. To examine the mean frequency of C. albicans-reactive T cells in healthy individuals we included an additional cohort of 96 healthy blood donors.
Patient characteristics and demographic data are given in Table 1. Most common underlying conditions of patients with proven or probable ICI were hematological and oncological malignancies (n = 11, 78.4%). More than half of the patients were treated on intensive care unit (ICU) prior the diagnosis of ICI (n = 8, 57.1%). Eight patients (57.1%) received chemotherapy within 3 months prior the diagnosis of ICI and five patients (35.7%) had undergone major surgery recently. Most prevalent site of infection was the blood stream in 12 patients (85.7%). Hepatosplenic candidiasis was found in three patients (21.4%). Disseminated candidiasis, defined as positive blood culture and/or at least two non-contiguous sites affected, was diagnosed in four patients (28.6%).
Candida-Reactive T Cells in Healthy Donors and in Disease Control Patients
In the cohort of this prospective pilot study nine disease control patients and 14 healthy donors showed no elevated frequencies of Candida-reactive T cells. In the cohort of 96 blood donors, the mean frequency of C. albicans-reactive T cells was 0.19 ± 0.11%. Combined frequency of C. albicans-reactive T cells among 110 healthy donors was 0.17 ± 0.12%. Mean frequencies of C. albicans-, C. glabrata-, C. parapsilosis-, C. tropicalis-, and C. krusei-reactive T cells in the cohort of this prospective pilot study were 0.07 ± 0.15%, 0.07 ± 0.14%, 0.05 ± 0.16%, 0.03 ± 0.05%, and 0.08 ± 0.24%, respectively.
Candida-Reactive T Cells in Patients With Proven Invasive Candida Infection
We determined cut-off values to discriminate between healthy donors, disease control patients and patients with ICI regarding x-fold increase of Candida-reactive T cells and frequencies of Candida-reactive T cells using ROC-analysis. Measurements of healthy donors and disease controls were defined negative (combined cohort of blood donors, healthy donors and disease controls), proven invasive Candida infection was defined as a positive test. A cut-off value of 3.05-fold increase of antigen stimulated CD69+/CD154+ T cells compared to the unstimulated CD4+ T cells was calculated to discriminate best between individuals with and without ICI and to assure a sufficient stimulation (Figure S1). Test positivity considered elevated levels of Candida-reactive T cells with simultaneously 3.05-fold increase of antigen-stimulated T cells compared to unstimulated T cells to exclude false-positive results. Cut-off values for elevated levels of C. albicans-reactive T cells were 0.40% CD69+/CD154+ T cells among CD4+ T cells, for C. glabrata 0.22% and for C. parapsilosis 0.40%, respectively. As no patients with invasive C. tropicalis or C. krusei infections were in our cohort, we used the cut-off value of pooled Candida spp.-reactive T cells of 0.40% as cut-off value for C. tropicalis and C. krusei instead (Figure S1).
Sensitivity and Specificity of Candida-Reactive T Cells
Candida-reactive CD4+ T cells were detected based on the upregulation of the activation markers CD69 and CD154 (CD40L) (Figures 1, 3). We detected elevated levels of Candida-reactive T cells in 13/16 patients (81.3%) with proven ICI. In three patients (18.7%) with proven ICI no values could be determined due to autofluorescence and these were excluded from this study. In 23 individuals without ICI there were no elevated levels of Candida-reactive T cells. The sensitivity and specificity in our study were 81.3% and 100%, respectively (Table S1). This results in positive and negative predictive values of 100% and 88.5%. The correlation between elevated levels of Candida-reactive T cells and the clinical diagnosis of proven ICI was statistically significant with a p < 0.001 (Table S1). When excluding autofluorescent patients from analysis sensitivity increased to 100% with a specificity of 100% (Table S2).
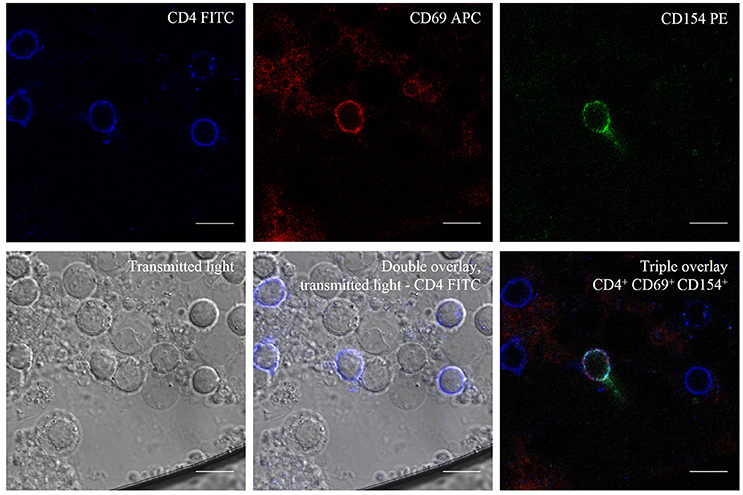
Figure 3. Transmitted Light—Differential Interference Contrast (DIC) and Fluorescence Microscopy of C. albicans-reactive T cells. CD4+ T cells after antigen stimulation with C. albicans lysate and show triple positivity, CD4+/CD69+/CD154+ after stimulation. Scale bar = 10 μm.
In 10/12 patients (83.3%) with proven ICI the Candida-reactive lymphocyte assay identified the same Candida spp. causing ICI as standard diagnostics (Table 2). Frequencies of C. albicans-reactive T cells and C. glabrata-reactive T cells in healthy donors, disease control and patients with probable or proven ICI are given in Figure 4. In one patient with a C. albicans candidemia and endophthalmitis we found elevated frequencies of C. glabrata-reactive T cells instead. In another patient with a C. albicans candidemia we measured C. albicans- and C. parapsilosis-reactive T cells. We determined elevated levels of C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis in a histologically proven case with hepatosplenic candidiasis without species identification and we excluded this patient from contingency analysis (Table 2). This leads to a sensitivity and a specificity of the Candida-reactive lymphocyte assay identifying ICI and the causing Candida spp. among evaluable patients with proven ICI of 83.3 and 100%, respectively. The positive and negative predictive values to detect ICI and the causing Candida spp. by species level were 100 and 92%. The p-value was < 0.001 calculated by Fisher's exact test (Table S3).
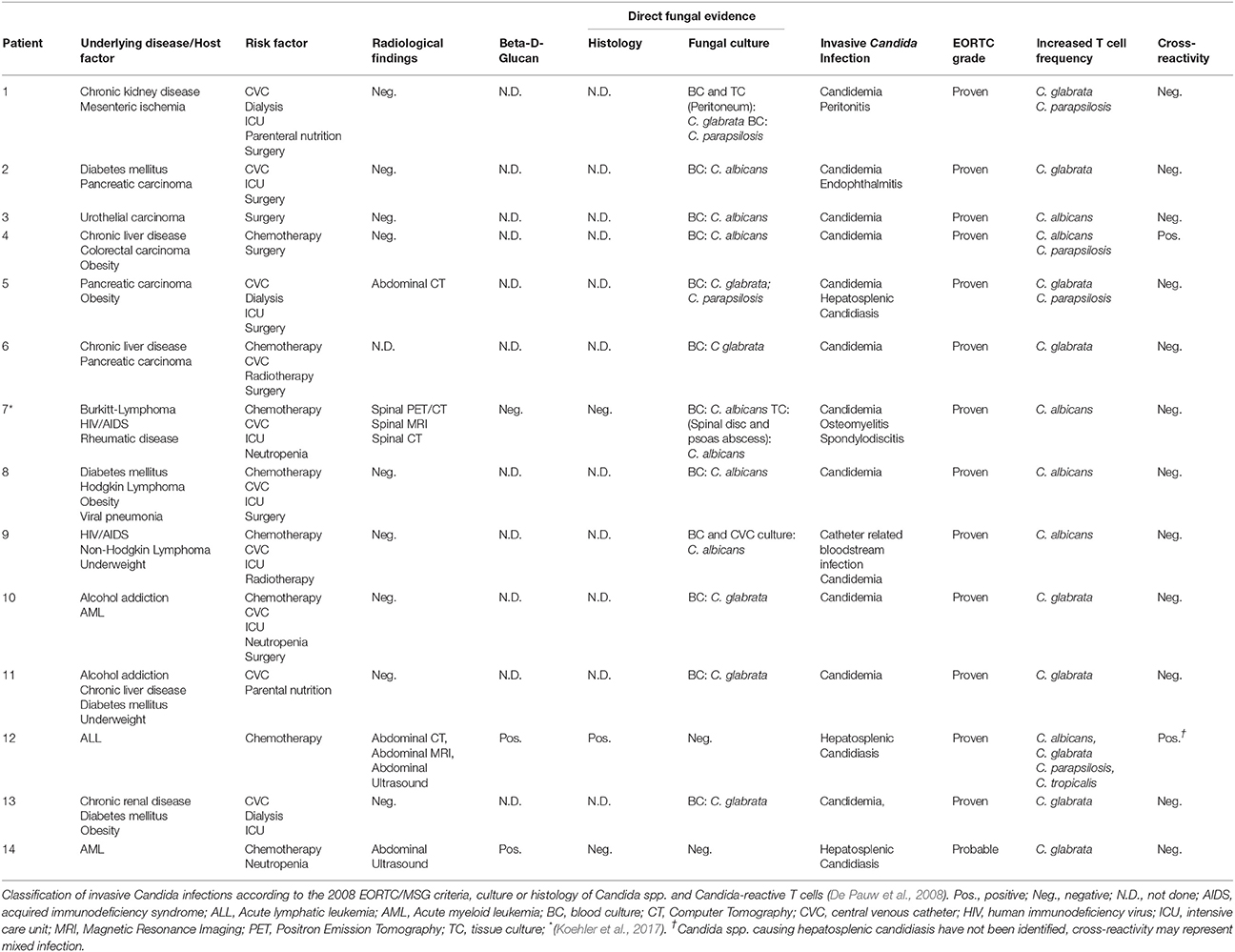
Table 2. Patients with proven and probable invasive Candida infections and corresponding frequencies of Candida-reactive T cells.
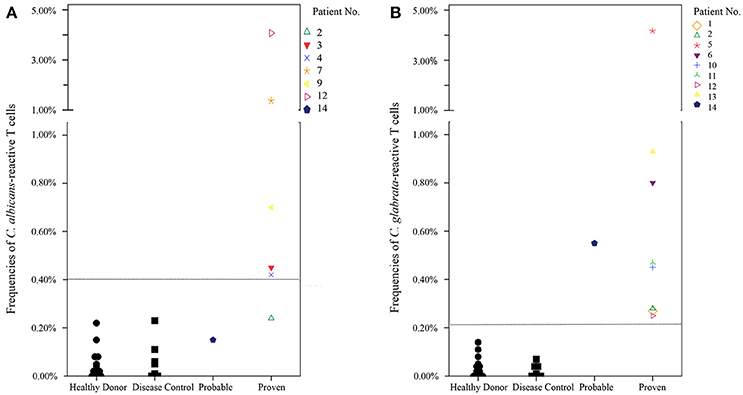
Figure 4. Frequencies of Candida-reactive T cells in healthy donors, disease control and patients with probable or proven invasive Candida infection. Patients with proven invasive Candida infection had a simultaneous 3.05-fold increase of antigen-stimulated T cells compared to unstimulated T cells to exclude false positive results and to determine quality of the stimulation. Dashed lines show cut-off values for C. albicans-reactive T cells (0.40%) and C. glabrata-reactive T cells (0.22%), respectively. Healthy donor n = 14, disease control n = 9. (A) Frequencies of C. albicans CD69+/CD154+ T cells among CD4+ T cells in donor/patient cohorts. Given is the highest frequency during the test series. (B) Frequencies of C. glabrata CD69+/CD154+ T cells among CD4+ T cells in donor/patient cohorts. Given is the highest frequency during the test series.
Discussion
In this pilot study, we evaluated the performance of a new Candida-reactive lymphocyte assay as diagnostic tool for ICI. Elevated levels of Candida-reactive CD4+ T cells were measured based on the upregulation of the activation markers CD69 and CD154 (CD40L) in patients with proven and probable ICI (Frentsch et al., 2005; Bacher et al., 2013, 2014, 2015; Cossarizza et al., 2017). Identification to species level was in agreement with conventional diagnostics in 83.3% of proven ICI. Disease controls and healthy controls had no elevated T cell count. In this cohort, there was negligible cross-reactivity between the different Candida spp. causing ICI.
The Candida-reactive lymphocyte assay compares favorable characteristics to current gold-standard diagnostic procedures. Volatile sensitivity ratios of blood culture are caused by the rapid elimination of viable Candida cells from blood circulation (Cuenca-Estrella et al., 2012; Nguyen et al., 2012; Kullberg and Arendrup, 2015; Pappas et al., 2016). The Candida-reactive lymphocyte assay may improve sensitivity of and time to the diagnosis of ICI including identification of the causative pathogen to species level and could therefore considerably improve the treatment of patients with ICI. However, both blood and tissue culture currently remain the only diagnostics allowing for susceptibility testing. Microscopy, histopathology, and culture of infectious tissue samples require surgery or other invasive procedures with significant risks for the patient, whereas the Candida-reactive lymphocyte assay is a non-invasive, peripheral blood test with little to no risks for the patient (Clancy and Nguyen, 2013).Candida mannan antigen and antimannan antibodies, CAGTA and BDG-measurements do not permit discrimination between different Candida spp. and therefore in contrast to the Candida-reactive lymphocyte assay they do not allow for definitive diagnosis and tailored treatment (Laín et al., 2007; Kullberg and Arendrup, 2015).
As reported previously, we were able to provide flow-cytometry based diagnostics with Candida-reactive T cells for invasive candidiasis in a case of a patient with a human immunodeficiency virus (HIV)-associated Burkitt lymphoma with a C. albicans spondylodiscitis (Koehler et al., 2017). We were able to diagnose ICI by species level, whereas in treatment course blood culture remained negative (Table 2) (Koehler et al., 2017). In this case, the Candida-reactive lymphocyte assay favored conventional diagnostics due to improved sensitivity and shortened time to diagnosis (Koehler et al., 2017).
Measuring antigen-reactive T cells by the upregulation of the activation markers CD69 and CD154 (CD40L) can be expanded to other pathogens with challenging diagnosis such as Aspergillus spp. and Mucorales spp. (Bacher et al., 2013, 2014, 2015; Potenza et al., 2016; Cossarizza et al., 2017). However, it remains unknown, how the Candida-reactive lymphocyte assay performs when dealing with other Candida spp. causing ICI than C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, or C. krusei.
Limitations of the Candida-reactive lymphocyte assay are insufficient cell count of T cells or lack of antigen-presenting cells, as well as autofluorescence of patient cells with elevated background levels of CD69 and CD154. Autofluorescence of cells is mainly caused by dead cells, which bind antibodies in a low-affinity and unspecific manner (Cossarizza et al., 2017). To avoid high yields of autofluorescent cells, dead cell staining, as well as processing and cultivation of PBMC without delay remain mandatory (Cossarizza et al., 2017; Wurster et al., 2017).
The performance of the Candida-reactive lymphocyte assay may be improved by the combined determination of activation markers and cytokines, which are typically secreted by reactive CD4+ T cells upon activation such as interleukin-17, interleukin-22 or interferon-γ (Potenza et al., 2016; Cossarizza et al., 2017).
With the reduction of fungal load by antimycotic treatment or even surgery in case of invasive mold infection, we expect the levels of fungus-reactive T cells to be decreasing over time, which may affect the performance of the assays in patients with extensive treatment in the past medical history, but it also provides a simple and sensitive opportunity to follow treatment courses of patients (Bacher et al., 2015). In our cohort, low number of proven and probable ICI patients may hamper the strength of the results of this pilot project so that further studies with larger patient numbers remain mandatory also analyzing the course of Candida-reactive T cells over the sequence of therapy.
In summary, the Candida-reactive lymphocyte assay may complement current diagnostics for ICI, especially when blood cultures remain negative, as well as in cases of underlying medical conditions with contraindication for biopsies or surgery. The Candida-reactive lymphocyte assay is a non-invasive, peripheral blood test and it allows the identification of Candida spp. for targeted treatment. As the Candida-reactive lymphocyte assay may establish the diagnosis of ICI within 36–48 h, it may enable earlier treatment in case of prolonged cultivation of blood cultures.
Author Contributions
FK performed experiments, contributed collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval. OC conceived the study idea, contributed collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval. HW contributed Candida strains for mechanical lysis, BDG-data, data analysis and interpretation, manuscript writing and final manuscript approval. ACS and JS-G contributed data analysis and interpretation, manuscript writing, and final manuscript approval. HO, PB, and AS contributed collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval. MZ, RA, and AR contributed data analysis and interpretation, manuscript writing and final manuscript approval. PK conceived the study idea, performed experiments, contributed collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval.
Funding
This study was supported by a sponsored research collaboration agreement with Miltenyi Biotec GmbH (Holder of European Patent - Number 11164382.1).
Conflict of Interest Statement
FK reports grants from the German Federal Ministry of Research and Education, non-financial support from Miltenyi Biotec GmbH during the conduct of the study. OC reports research grants from Actelion, Amplyx, Arsanis, Astellas, AstraZeneca, Basilea, Bayer, Cidara, Duke University, F2G, Gilead, GSK, Leeds University, Matinas, Medicines Company, MedPace, Melinta, Merck/MSD, Miltenyi Biotec GmbH, Pfizer, Rempex, Roche, Sanofi Pasteur, Scynexis, Seres, is a consultant to Amplyx, Actelion, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen, Matinas, Menarini, Merck/MSD, Paratek, PSI, Scynexis, Seres, Summit, Tetraphase, Vical, and received lecture honoraria from Astellas, Basilea, Gilead, Merck/MSD and Pfizer outside the submitted work. HW reports personal fees and non-financial support from BeckmanCoulter, non-financial support from Specific Technologies, Accelerate and Cepheid outside the submitted work. HO reports personal fees from Astra Zeneca, Bayer, BMS, Gilead, Leo, MSD, Orphan, and Pfizer outside the submitted work. AS reports other from Miltenyi Biotec GmbH outside the submitted work. RA and AR reports personal fees from Miltenyi Biotec GmbH during the cnduct of the study. PK reports indirect personal fees from the Cologne Cluster of Excellence—Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne as CECAD rotational “Gerok” position, non-financial support from Miltenyi Biotec GmbH during the conduct of the study; non-financial support from Merck/MSD and MedImmune and lecture honoraria from Astellas outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a non-financial, sponsored research collaboration agreement with Miltenyi Biotec GmbH. The authors thank Elisa Barth for technical assistance at the CECAD Translational Research Laboratory, Julia Milleck and Mario Assenmacher at Miltenyi Biotec GmbH, Denis Hoffmann at Labor Dr. Wisplinghoff, as well as study coordinators Larisa Idrizovic, Stefan Körfgen, and Tatjana Lammertz at the Infectious Diseases Clinical Trial Unit.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01381/full#supplementary-material
Abbreviations
ICI, Invasive Candida Infection; CAGTA, Candida albicans Germ Tube Antibody; BDG, β-1, 3-D-glucan; PCR, Polymerase Chain Reaction; ISI, Improving Diagnosis of Severe Infections of Immunocompromised Patients; EDTA, Ethylenediaminetetraacetic acid; 7AAD, 7-aminoactinomycin D; SEB, Staphylococcal Enterotoxin B; ID, Species identification; AST, Susceptibility testing; ECMM, European Confederation of Medical Mycology; EORTC/MSG, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group; ROC, Receiver operating characteristic analysis; ICU, Intensive care unit; HIV; Human immunodeficiency virus.
References
Arendrup, M. C. (2010). Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 16, 445–452. doi: 10.1097/MCC.0b013e32833e84d2
Bacher, P., Kniemeyer, O., Schonbrunn, A., Sawitzki, B., Assenmacher, M., Rietschel, E., et al. (2014). Antigen-specific expansion of human regulatory T cells as a major tolerance mechanism against mucosal fungi. Mucosal Immunol. 7, 916–928. doi: 10.1038/mi.2013.107
Bacher, P., Schink, C., Teutschbein, J., Kniemeyer, O., Assenmacher, M., Brakhage, A. A., et al. (2013). Antigen-reactive T cell enrichment for direct, high-resolution analysis of the human naive and memory Th cell repertoire. J. Immunol. 190, 3967–3976. doi: 10.4049/jimmunol.1202221
Bacher, P., Steinbach, A., Kniemeyer, O., Hamprecht, A., Assenmacher, M., Vehreschild, M. J., et al. (2015). Fungus-specific CD4(+) T cells for rapid identification of invasive pulmonary mold infection. Am. J. Respir. Crit. Care Med. 191, 348–352. doi: 10.1164/rccm.201407-1235LE
Clancy, C. J., and Nguyen, M. H. (2013). Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 56, 1284–1292. doi: 10.1093/cid/cit006
Cossarizza, A., Chang, H. D., Radbruch, A., Andrä, I., Annunziato, F., Bacher, P., et al. (2017). Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 47, 1584–1797. doi: 10.1002/eji.201646632
Cuenca-Estrella, M., Verweij, P. E., Arendrup, M. C., Arikan-Akdagli, S., Bille, J., Donnelly, J. P., et al. (2012). ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin. Microbiol. Infect. 18(Suppl. 7), 9–18. doi: 10.1111/1469-0691.12038
De Pauw, B., Walsh, T. J., Donnelly, J. P., Stevens, D. A., Edwards, J. E., Calandra, T., et al. (2008). Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821. doi: 10.1086/588660
ECMM (2018). Candida Registry CandiReg. University of Cologne. Availableonline at: http://www.clinicalsurveys.net/ (Accessed March 14, 2018).
Frentsch, M., Arbach, O., Kirchhoff, D., Moewes, B., Worm, M., Rothe, M., et al. (2005). Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 11, 1118–1124. doi: 10.1038/nm1292
Kami, M., Machida, U., Okuzumi, K., Matsumura, T., Mori Si, S., Hori, A., et al. (2002). Effect of fluconazole prophylaxis on fungal blood cultures: an autopsy-based study involving 720 patients with haematological malignancy. Br. J. Haematol. 117, 40–46. doi: 10.1046/j.1365-2141.2002.03414.x
Koehler, F. C., Cornely, O. A., Wisplinghoff, H., Chang, D. H., Richter, A., and Koehler, P. (2017). Candida-reactive T cells for the diagnosis of invasive Candida infection of the lumbar vertebral spine. Mycoses 61, 48–52. doi: 10.1111/myc.12696
Kullberg, B. J., and Arendrup, M. C. (2015). Invasive Candidiasis. N. Engl. J. Med. 373, 1445–1456. doi: 10.1056/NEJMra1315399
Laín, A., Elguezabal, N., Brena, S., Garcia-Ruiz, J. C., Del Palacio, A., Moragues, M. D., et al. (2007). Diagnosis of invasive candidiasis by enzyme-linked immunosorbent assay using the N-terminal fragment of Candida albicans hyphal wall protein 1. BMC Microbiol. 7:35. doi: 10.1186/1471-2180-7-35
Lamoth, F., Cruciani, M., Mengoli, C., Castagnola, E., Lortholary, O., Richardson, M., et al. (2012). beta-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin. Infect. Dis. 54, 633–643. doi: 10.1093/cid/cir897
Mikulska, M., Calandra, T., Sanguinetti, M., Poulain, D., and Viscoli, C. (2010). The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit. Care 14:R222. doi: 10.1186/cc9365
Ness, M. J., Vaughan, W. P., and Woods, G. L. (1989). Candida antigen latex test for detection of invasive candidiasis in immunocompromised patients. J. Infect. Dis. 159, 495–502. doi: 10.1093/infdis/159.3.495
Nguyen, M. H., Wissel, M. C., Shields, R. K., Salomoni, M. A., Hao, B., Press, E. G., et al. (2012). Performance of Candida real-time polymerase chain reaction, beta-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin. Infect. Dis. 54, 1240–1248. doi: 10.1093/cid/cis200
Pappas, P. G., Kauffman, C. A., Andes, D. R., Clancy, C. J., Marr, K. A., Ostrosky-Zeichner, L., et al. (2016). Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62, e1–e50. doi: 10.1093/cid/civ1194
Ponton, J., Quindos, G., Arilla, M. C., and Mackenzie, D. W. (1994). Simplified adsorption method for detection of antibodies to Candida albicans germ tubes. J. Clin. Microbiol. 32, 217–219.
Potenza, L., Vallerini, D., Barozzi, P., Riva, G., Gilioli, A., Forghieri, F., et al. (2016). Mucorales-specific T cells in patients with hematologic malignancies. PLoS ONE 11:e0149108. doi: 10.1371/journal.pone.0149108
Romani, L. (2011). Immunity to fungal infections. Nat. Rev. Immunol. 11, 275–288. doi: 10.1038/nri2939
Schelenz, S., Barnes, R. A., Barton, R. C., Cleverley, J. R., Lucas, S. B., Kibbler, C. C., et al. (2015). British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect. Dis. 15, 461–474. doi: 10.1016/S1473-3099(15)70006-X
Wisplinghoff, H., Bischoff, T., Tallent, S. M., Seifert, H., Wenzel, R. P., and Edmond, M. B. (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39, 309–317. doi: 10.1086/421946
Keywords: invasive candidiasis, candidemia, hepatosplenic candidiasis, flow cytometry, fungus-reactive T cells, CD154
Citation: Koehler FC, Cornely OA, Wisplinghoff H, Schauss AC, Salmanton-Garcia J, Ostermann H, Ziegler M, Bacher P, Scheffold A, Alex R, Richter A and Koehler P (2018) Candida-Reactive T Cells for the Diagnosis of Invasive Candida Infection—A Prospective Pilot Study. Front. Microbiol. 9:1381. doi: 10.3389/fmicb.2018.01381
Received: 18 April 2018; Accepted: 06 June 2018;
Published: 22 June 2018.
Edited by:
Agostinho Carvalho, University of Minho, PortugalReviewed by:
Eva Pericolini, Università degli Studi di Modena e Reggio Emilia, ItalyTeresa Zelante, University of Perugia, Italy
Copyright © 2018 Koehler, Cornely, Wisplinghoff, Schauss, Salmanton-Garcia, Ostermann, Ziegler, Bacher, Scheffold, Alex, Richter and Koehler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philipp Koehler, cGhpbGlwcC5rb2VobGVyQHVrLWtvZWxuLmRl
 Felix C. Koehler1
Felix C. Koehler1 Oliver A. Cornely
Oliver A. Cornely Jon Salmanton-Garcia
Jon Salmanton-Garcia Alexander Scheffold
Alexander Scheffold Anne Richter
Anne Richter Philipp Koehler
Philipp Koehler