- 1State Key Laboratory of Agricultural Microbiology, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China
- 2State Key Laboratory of Genetically Engineered Veterinary Vaccines, Qingdao, China
- 3Key Laboratory of Prevention and Control Agents for Animal Bacteriosis (Ministry of Agriculture), Institute of Animal Husbandry and Veterinary Sciences, Hubei Academy of Agricultural Sciences, Wuhan, China
- 4The Cooperative Innovation Center for Sustainable Pig Production, Huazhong Agricultural University, Wuhan, China
Actinobacillus pleuropneumoniae is the pathogen of porcine contagious pleuropneumonia. In A. pleuropneumoniae, the CpxAR two-component system is essential for fitness and growth. The O-antigen protrudes from the outer membrane to the exterior of the cell, and the outer membrane serves as a barrier that helps the bacteria to survive in harsh environments. WecA, a undecaprenyl phosphate GlcNAc-1-phosphate transferase, is involved in O-antigen repeating unit biosynthesis. In this study, we investigated the role of CpxAR in the expression of wecA in A. pleuropneumoniae. Our results revealed that CpxR positively regulates wecA expression by directly binding to the putative promoter region of wecA. Wild-type, ΔcpxAR, ΔwecA, and complemented strains were investigated under serum, oxidative, and osmotic stresses. The ΔcpxAR and ΔwecA strains were more susceptible to these stresses than the wild-type, but the complemented strains showed phenotypes similar to those of the wild-type. Mice infected with the ΔcpxAR and ΔwecA strains exhibited lower mortality and bacterial loads in the lung than those infected with the wild-type or complemented strains. This study reveals that the CpxAR two-component system contributes to A. pleuropneumoniae growth, stress resistance, and virulence, by upregulating expression of wecA. Our findings provide new insight into the pathogenesis of A. pleuropneumoniae.
Introduction
Porcine contagious pleuropneumonia is a highly contagious respiratory disease that causes considerable losses to the global swine industry (Sassu et al., 2018). Its causative agent, Actinobacillus pleuropneumoniae, is a Gram-negative bacterium that can be differentiated into 18 serovars (Bosse et al., 2018a, b). A. pleuropneumoniae is transmitted by direct contact or aerosol and can infect swine of all ages (Sheehan et al., 2003). Several virulence factors are involved in the pathogenesis of A. pleuropneumoniae, including capsule polysaccharide, lipopolysaccharide, proteases, Apx toxins, outer membrane proteins, and transferrin-binding proteins (Chiers et al., 2010).
Two-component systems (TCSs) are one of the major strategies of signal transduction used by bacteria to sense and respond to their changing environment (Jacob-Dubuisson et al., 2018). The Cpx TCS consists of the sensor kinase CpxA and the response regulator CpxR. CpxR can regulate the transcription of downstream genes after CpxA transfers a phosphate group to CpxR (Raivio and Silhavy, 1997; Vogt and Raivio, 2012). Previous studies have reported that the CpxAR TCS enables bacteria to respond to envelope stress and environmental stresses such as oxidative and osmotic stress (Bury-Mone et al., 2009; Srinivasan et al., 2012; Bontemps-Gallo et al., 2015; Cao et al., 2018; Lopez et al., 2018; Matter et al., 2018).
Gram-negative bacteria are surrounded by an outer membrane (OM). The OM serves as a barrier that helps them to survive in harsh environments (Nikaido, 2003). Lipopolysaccharide (LPS) is the central constituent of the OM, playing a vital role in interactions with the environment, including host organisms of pathogens. LPS is composed of O-antigen, lipid A, and core oligosaccharides (Raetz and Whitfield, 2002). The O-antigen is distal to the OM (Raetz and Whitfield, 2002). In Salmonella enterica serovar Typhimurium, the O-antigen helps cells to evade the complement cascade (Murray et al., 2006). In Erwinia amylovora, Bradyrhizobium japonicum, and Escherichia coli, the O-antigen protects cells against oxidative stress (Berry et al., 2009; Noh et al., 2015; Zheng et al., 2019). O-antigen was reported to protect B. japonicum and S. enterica serovar Typhi against osmotic stress (Bittner et al., 2002; Noh et al., 2015). In addition, some stress conditions influence O-antigen expression (Bittner et al., 2002; Silva-Valenzuela et al., 2016). In general, the genes involved in O-antigen biosynthesis are found in O-antigen gene clusters and close to each other (Skurnik et al., 1999; Yi et al., 2005; Liu et al., 2008, 2019). WecA (Rfe) initiates O-antigen repeating unit biosynthesis (Alexander and Valvano, 1994; Rick et al., 1994; Clarke et al., 1995; Lehrer et al., 2007).
Previous studies showed that the Cpx TCS is related to mediation of host environmental fitness (Ruiz and Silhavy, 2005; Yamamoto and Ishihama, 2006; Vogt and Raivio, 2012; Delhaye et al., 2016; Pezza et al., 2016), that the O-antigen helps bacteria to survive in harsh environments (Bittner et al., 2002; Noh et al., 2015; Silva-Valenzuela et al., 2016; Zheng et al., 2019), and that the CpxAR TCS is activated by overexpression of O-antigen (Bengoechea et al., 2002). However, it remained unclear whether the CpxAR system regulates O-antigen biosynthesis and affects stress resistance in A. pleuropneumoniae. This study reveals that CpxR can directly bind to the putative promoter region of wecA and upregulate wecA gene expression in A. pleuropneumoniae. ΔwecA and ΔcpxAR strains exhibited slower growth and were more sensitive to stressful conditions, including serum, oxidative, and osmotic stresses, than the wild-type strain and complemented mutants. Furthermore, in vivo evidence from a mouse infection model demonstrated that virulence of the ΔcpxAR and ΔwecA strains was attenuated compared with that of the wild-type. Our findings provide insight into the CpxAR system and WecA function in A. pleuropneumoniae.
Materials and Methods
Bacterial Strains and Culture Conditions
The bacterial strains, primers, and proteins used in this study are described in Table 1. Tryptic soy broth (TSB; Difco Laboratories, Detroit, MI, United States) containing 10 μg mL–1 nicotinamide adenine dinucleotide (NAD) and 10% (v/v) newborn bovine serum was used to grow A. pleuropneumoniae strains, and the solid medium was tryptic soy agar (TSA; Difco Laboratories). For antibiotic selection, 5 μg mL–1 chloramphenicol were added to the medium when necessary. E. coli strains were grown in Luria-Bertani broth or agar; appropriate antibiotics or 50 μg mL–1 diaminopimelic acid (Sigma-Aldrich, St. Louis, United States) were added when needed. Wild-type A. pleuropneumoniae in this study was strain 4074.
The mutant strain ΔwecA and the complemented strain CΔwecA were constructed as described previously (Frey, 1992; Li et al., 2018). Polymerase chain reaction and sequencing (data not shown) were used to verify the mutant strain and the complemented strain. Briefly, the upstream and downstream segments of wecA were amplified. These segments were combined by overlap PCR. Primers used in the PCR are listed in Table 2. The overlapped product was ligated into pEMOC2 to generate pEΔwecA. pEΔwecA was used to construct a ΔwecA mutant by double crossover recombination. The intact wecA gene was amplified and cloned into pJFF224-XN to obtain pJFF-wecA, which was electroporated into the ΔwecA strain. The resultant strain, CΔwecA, was selected on TSA containing chloramphenicol, bovine serum, and NAD.
RNA Extraction and Reverse Transcription Quantitative-PCR
The wild-type strain and ΔcpxAR were grown in TSB (containing inactivated bovine serum and NAD) overnight, and then diluted 1:100 into fresh medium and grown to an OD600 nm of 0.6. Bacteria were harvested and treated with serum, 1.5 M NaCl, or 0.5 mM H2O2. RNA was extracted using a Bacteria Total RNA Isolation Kit (Sangon Biotech, China), then used for cDNA synthesis using HiScript II Q RT SuperMix for qRT-PCR (+gDNA wiper) (Vazyme, China). Quantitative PCR was performed by a one-step reaction in a ViiATM 7 real-time PCR system (Walters et al., 2006). The reaction mixtures were prepared according to the manufacturer’s instructions for SYBR Green Master Mix (Vazyme). The 16S rRNA gene was used as an endogenous control. Primers used in the qRT-PCR analysis are listed in Table 2. The 2–ΔCt method was used to quantify and compare gene expression (Pfaffl, 2001). To determine whether wecA, APPSER1_RS08540, wecB, wecC, rffC, rffA, and wzxE lie in one operon, cDNA from the wild-type strain and intergenic region-spanning primers (Supplementary Table S1) were used for PCR analysis.
Electrophoretic Mobility Shift Assays (EMSAs)
A DNA probe containing the putative wecA promoter region (272 bp) was amplified from the wild-type strain by PCR with primers WecA-E-F/R (Table 2). A mutant probe for the putative wecA promoter region (with TTTAC in the putative CpxR-P binding box deleted, 267 bp) was synthesized, and was used to explore the specificity of CpxR-P binding to the putative wecA promoter region. These two DNA probes were labeled by using an EMSA Probe Biotin Labeling Kit (Beyotime, China). Recombinant CpxR protein was phosphorylated by acetyl phosphate (Sigma, United States) (Pogliano et al., 1997). EMSAs were performed as previously described with some modifications (Li et al., 2018).
Growth Characteristics of Strains
Overnight bacterial cultures of A. pleuropneumoniae 4074, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA were diluted 1:1000 in fresh medium, and were grown at 37°C. OD600 was measured using an Eppendorf Biophotometer (Eppendorf, Hamburg, Germany) every hour. When OD600 of the cultures reached 0.3, 0.6, 1.5, and 2.5, respectively, cultures were serially diluted tenfold in phosphate-buffered saline (PBS) and plated on TSA.
Serum Bactericidal Assays
Swine serum was collected from healthy piglets with no history of A. pleuropneumoniae infection and no clinical signs. All the collected sera were determined to be A. pleuropneumoniae-negative by ApxIV-enzyme-linked immunosorbent assays (Kqbio, China). Some sera were heat-inactivated at 56°C for 30 min. The wild-type strain, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA were grown to mid-log phase. Bacteria were collected and washed twice with PBS. Bacterial suspensions (50 μL) were mixed with 450 μL fresh serum or heat-inactivated serum. After 1 h of incubation at 37°C, samples were diluted serially and plated on TSA (containing bovine serum and NAD) for enumeration of colony-forming units (CFU). The percentage bacterial survival was calculated by comparing the number of colonies that survived in fresh serum to the number that survived in heat-treated serum.
Oxidative and Osmotic Stress Resistance Assays
Oxidative stress assays were performed as previously described with some modifications (Li et al., 2019). Osmotic stress assays were carried out at 1.5 M NaCl (Li et al., 2004; Linnes et al., 2013). Overnight cultures were diluted 1:100 in TSB containing 10% inactivated bovine serum and 10 μg/mL NAD, and the bacterial cultures were grown in TSB to mid-logarithmic phase. Bacteria were harvested by centrifugation, resuspended in TSB containing 0.5 mM H2O2 (for oxidative stress) or 1.5 M NaCl (for osmotic stress), and incubated for 30 min. Control samples were cultivated in TSB without any addition. After incubation, the samples were serially diluted, plated on TSA containing inactivated bovine serum and NAD, and incubated at 37°C for 12 h. Survival rate was calculated by dividing the number of CFU in the stressed sample by that in the control sample.
Bacterial Virulence in vivo
Female BALB/c mice (6-weeks-old) were purchased from the Center for Disease Control of Hubei Province (Hubei CDC, Wuhan, China). All animal experiments were performed in accordance with the guidelines of the Laboratory Animal Monitoring Committee of Huazhong Agricultural University.
The infection assay was performed as described previously with some modifications (Li et al., 2018). Mice were randomly divided into five groups (8 per group): wild-type, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA-treated. Briefly, overnight bacterial cultures were diluted 1:100 in fresh medium and then incubated again until an OD600 of 0.6 was reached. Bacteria were collected and washed twice with PBS. Each group of mice was injected via the abdominal cavity with PBS containing 1 × 107 CFU. Survival time was monitored for 5 days post-infection. At day five post-infection, surviving mice were euthanized for postmortem examination. When infected mice died, or were humanely sacrificed, their lungs were fixed in 10% neutral-buffered formalin. Thin sections were stained with hematoxylin/eosin.
Bacterial burden experiments were performed as previously described with some modifications (Li et al., 2018). Female BALB/c mice (six per group) were challenged intraperitoneally with 2 × 106 CFU of each strain. Bacteria in the lung were counted at hour six post-infection.
Statistical Analysis
Statistical analysis was conducted using GraphPad Prism software (San Diego, United States). The data obtained from the present study are expressed as mean ± SD. Student’s t-test was used to analyze differences between groups. The log-rank test was performed to present differences in survival rate. Statistically significant differences are presented at the level P < 0.05 and P < 0.01.
Results
Expression of wecA is Upregulated in A. pleuropneumoniae Under Environmental Stresses
To investigate whether WecA is involved in stress resistance, the wild-type strain A. pleuropneumoniae 4074 was treated with serum, NaCl, and H2O2, respectively. The transcription level of wecA was upregulated after exposure to serum, NaCl and H2O2 (Figure 1).
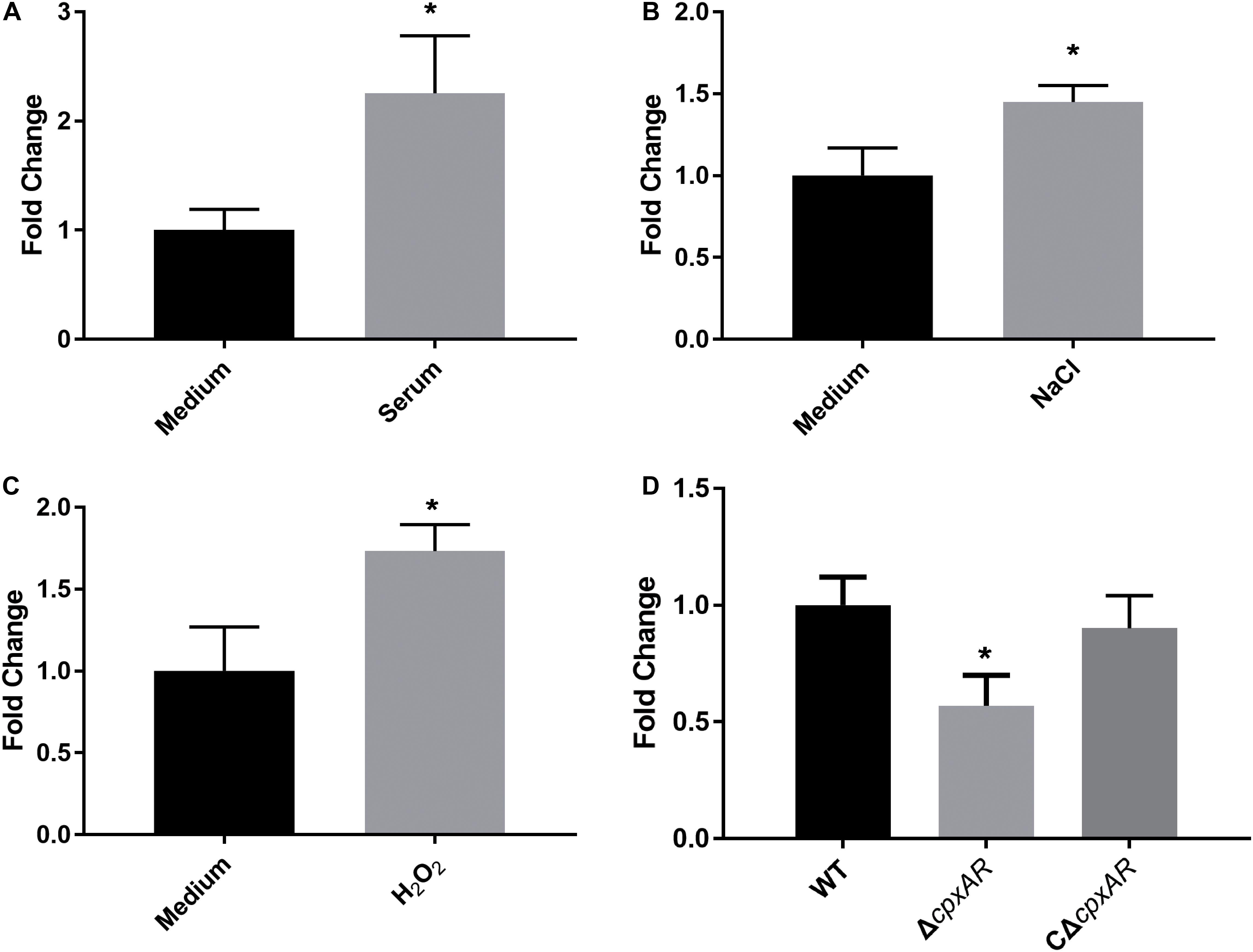
Figure 1. Upregulation of transcription level of wecA after exposure of wild-type Actinobacillus pleuropneumoniae (strain 4074) to serum (A), NaCl (B) and H2O2 (C). Quantitative real-time PCR analysis of expression of wecA in wild-type and ΔcpxAR strains (D),∗p < 0.05.
CpxAR TCS Influences Transcription of wecA
To investigate the effect of the CpxAR TCS on the transcription of wecA, the expression of wecA in ΔcpxAR and wild-type strains was determined by qRT-PCR. As shown in Figure 1D, the expression of wecA in the ΔcpxAR mutant decreased significantly (P < 0.05) compared with that in the wild-type strain. Complementation of the cpxAR mutation restored the expression level of wecA.
CpxR-P Directly Binds to the Putative wecA Promoter Region
EMSAs were performed with phosphorylated CpxR (CpxR-P) and a segment containing the putative wecA promoter region. CpxR-P slowed down the movement of the putative wecA promoter region in a dose-dependent manner, and the competition control demonstrated the specificity of CpxR-P binding (Figure 2A). CpxR-P was unable to bind to a mutant wecA putative promoter region in which TTTAC was deleted from the putative CpxR-P binding box (Figure 2B). The addition of mutant probe did not affect the binding of CpxR-P and the putative promoter region of wecA (Figure 2C). These results showed the specificity of the binding of CpxR-P and the putative wecA promoter region.
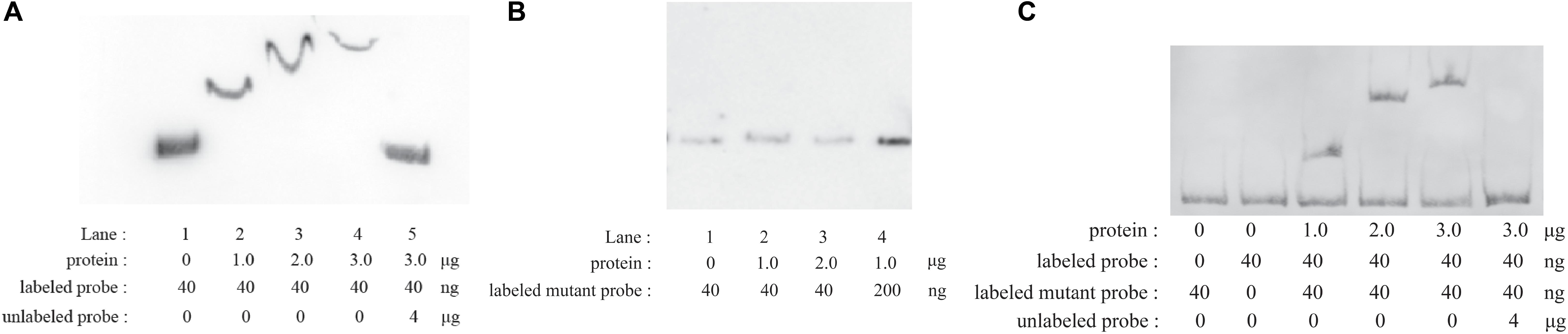
Figure 2. Electrophoretic mobility shift assays. Labeled wecA putative promoter region (A) or labeled mutant wecA putative promoter region (B) were incubated with various concentrations of CpxR-P. Labeled wecA putative promoter region and labeled mutant wecA putative promoter region were incubated with various concentrations of CpxR-P (C).
Growth Characteristics of A. pleuropneumoniae Strains
We constructed ΔwecA mutant and complemented strain (Supplementary Figure S1). The growth properties of the wild-type, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA A. pleuropneumoniae strains were investigated. When strains were grown in TSB, both ΔcpxAR and ΔwecA mutants exhibited growth defects, but complementation of the strains restored the growth properties. The trends of ΔcpxAR and ΔwecA growth curves in TSB were similar to those of the wild-type strain. However, as shown in Figure 3A, in the stationary phase, OD600 values of ΔcpxAR and ΔwecA were lower than those of the wild-type and complemented strains. When the OD600 value of the bacterial cultures reached 0.3, 0.6, 1.5, and 2.5, respectively, cultures were serially diluted and plated onto TSA. The number of viable bacteria of ΔcpxAR and ΔwecA strains was lower than that of wild-type strain at each OD600 value, whereas no significant difference was observed in the number of viable bacteria between CΔwecA or CΔcpxAR and the wild-type strain (Figures 3B–E).
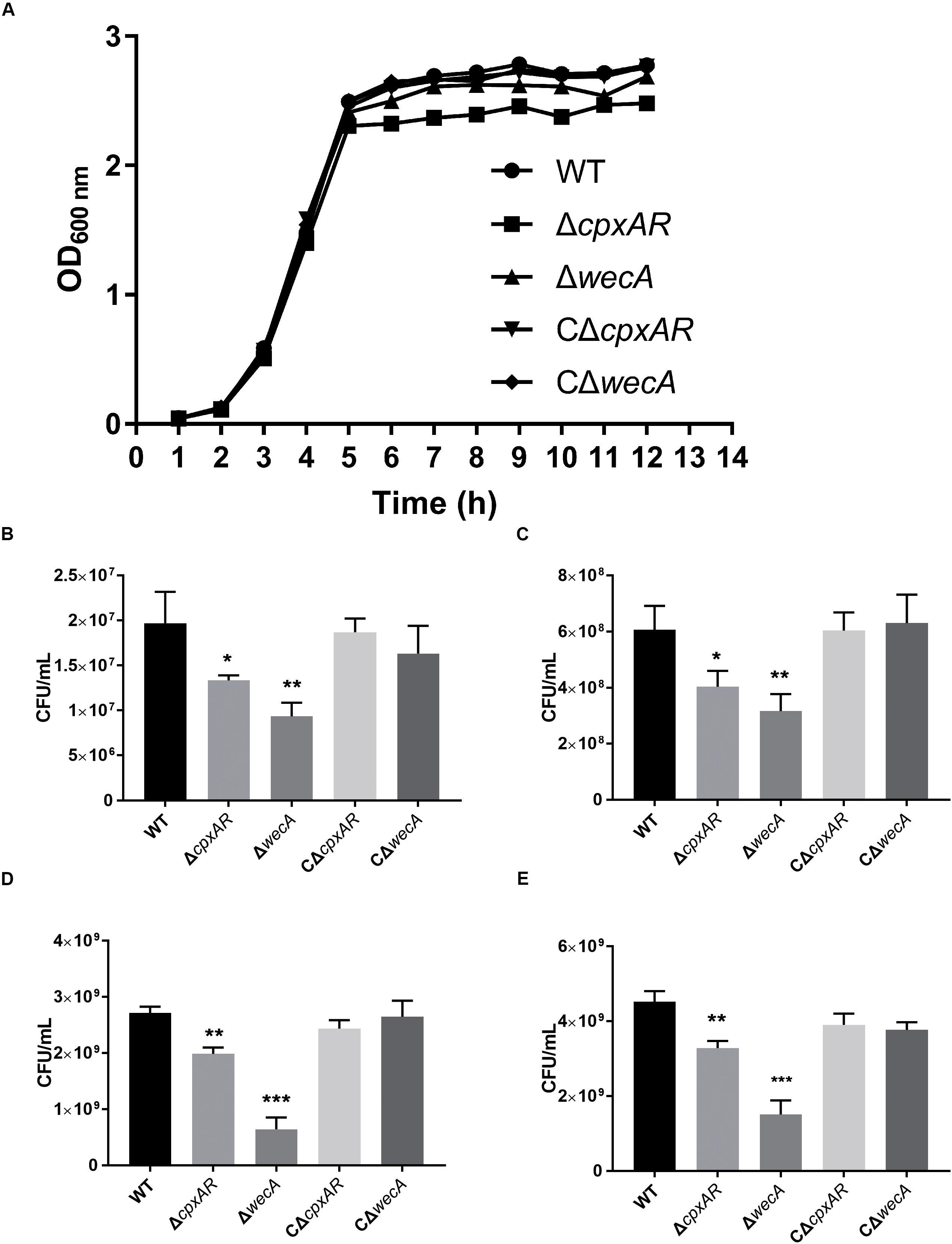
Figure 3. Growth characteristics of A. pleuropneumoniae strains. Growth curves of wild-type, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA strains (A). When the OD600 of the bacterial cultures reached 0.3 (B), 0.6 (C), 1.5 (D), and 2.5 (E), the cultures were serially diluted and plated onto tryptic soy agar. Data are presented as the mean ± SD. Significant differences are presented at the levels ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
ΔcpxAR and ΔwecA Strains Exhibit Reduced Resistance to Serum Killing
To examine whether the cpxAR and wecA genes are involved in serum resistance, the survival rates of wild-type, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA strains in serum were examined. The ΔcpxAR and ΔwecA mutants were significantly more sensitive to porcine serum than the wild-type, and the complemented strains were observed to have restored serum resistance (Figure 4A).
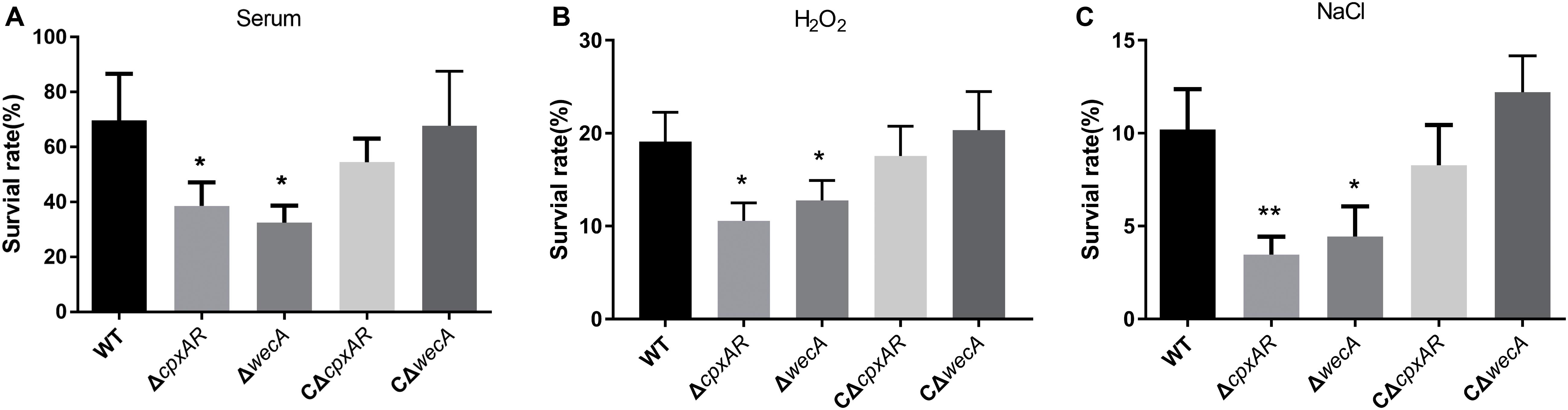
Figure 4. Serum and stress resistance assays. The strains were treated with serum (A), H2O2 (B), NaCl (C), or tryptic soy broth, then bacterial survival rates were calculated. Significant differences are presented at the levels ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
ΔcpxAR and ΔwecA Mutants Exhibit Reduced Tolerance to H2O2 and NaCl
To investigate whether cpxAR and wecA are involved in oxidative and osmotic stress tolerance, we tested the survival rates of wild-type, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA strains when they were exposed to 0.5 mM hydrogen peroxide or 1.5 M NaCl. On treatment with 0.5 mM hydrogen peroxide, the survival rates of the cpxAR and wecA mutant strains were significantly lower than that of the wild-type (Figure 4B). When the bacteria were exposed to NaCl-induced osmotic stress, the survival rates of the ΔcpxAR and ΔwecA strains were lower than that of the wild-type (Figure 4C). Complementation of the strains restored their original phenotypes. These results suggest that CpxAR and WecA are involved in oxidative and osmotic stress tolerance.
ΔcpxAR and ΔwecA Strains Display Attenuated Virulence and Colonization in a Mouse Model
To explore the roles of CpxAR and WecA in the virulence of A. pleuropneumoniae, a BALB/c mouse model was used. As shown in Figure 5A, the survival rates of mice at 5 days were 0, 50, 62.5, 12.5, and 0% in the wild-type, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA-treated groups, respectively.
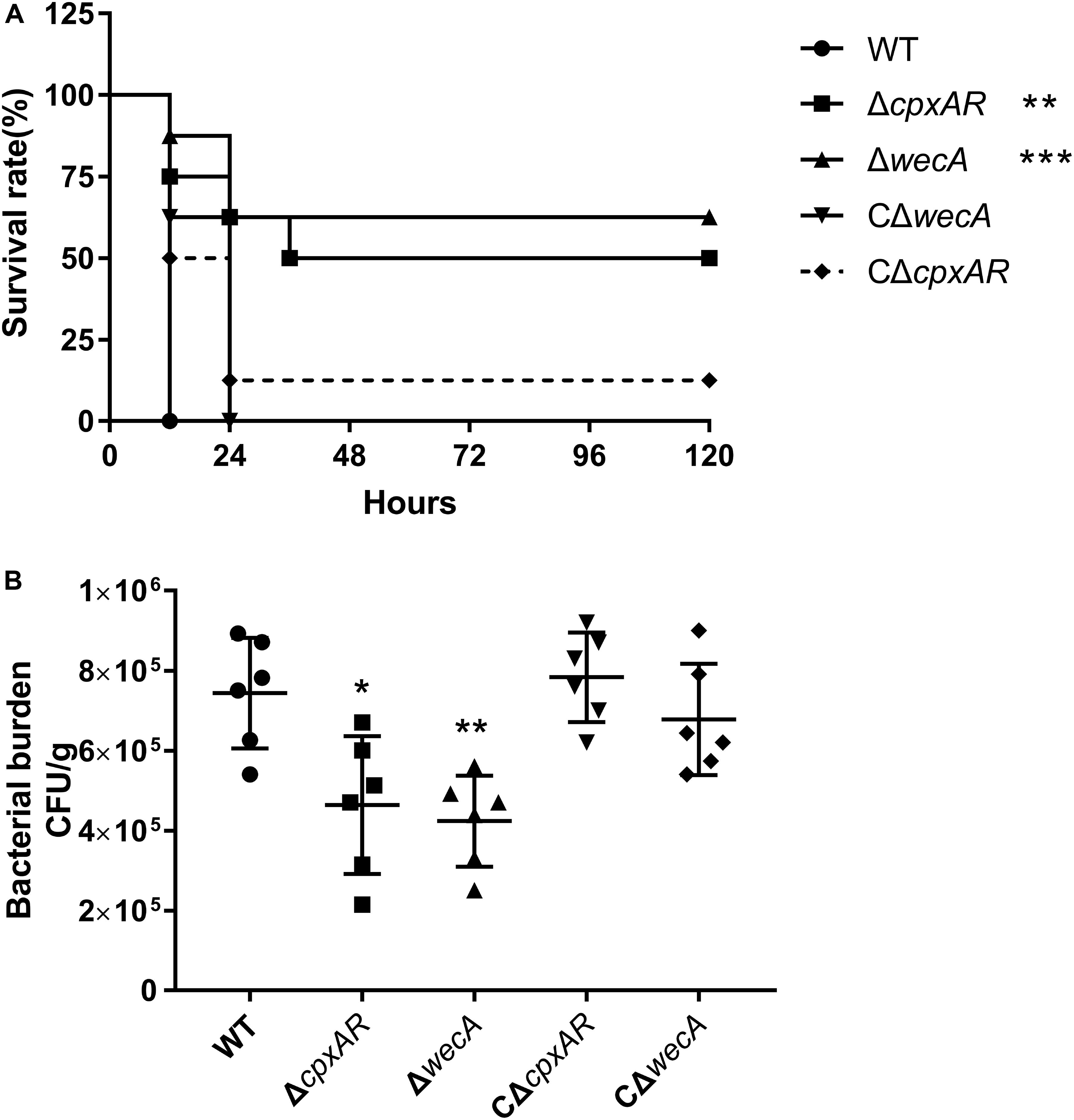
Figure 5. Attenuated virulence and colonization of A. pleuropneumoniae ΔcpxAR and ΔwecA strains in a BALB/c mouse model. (A) Survival curves of mice infected with A. pleuropneumoniae strains. Wild-type, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA-treated mice were monitored daily for 5 days post-infection. (B) Bacterial burden in lung. Mice were inoculated intraperitoneally with A. pleuropneumoniae strains. At hour six post-infection, lung samples were isolated to determine bacterial burdens. Significant differences are presented at the levels ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
As shown in Figure 5B, a significant difference (P < 0.05) in the number of bacteria recovered from lung was found between the wild-type and ΔcpxAR-treated groups, and there was a highly significant difference (P < 0.01) in the number of bacteria recovered from lung between the wild-type and ΔwecA-treated groups.
Severe acute hemorrhagic pneumonia symptoms were observed in mice in the wild-type, CΔcpxAR, and CΔwecA treatment groups, for example, thickened alveolar walls, extensive infiltration of erythrocytes, and more inflammatory cells in alveoli, whereas sections from ΔcpxAR- and ΔwecA-treated mice exhibited less severe pathological changes (Figure 6). These results revealed that the CpxAR TCS and WecA contribute to A. pleuropneumoniae virulence.
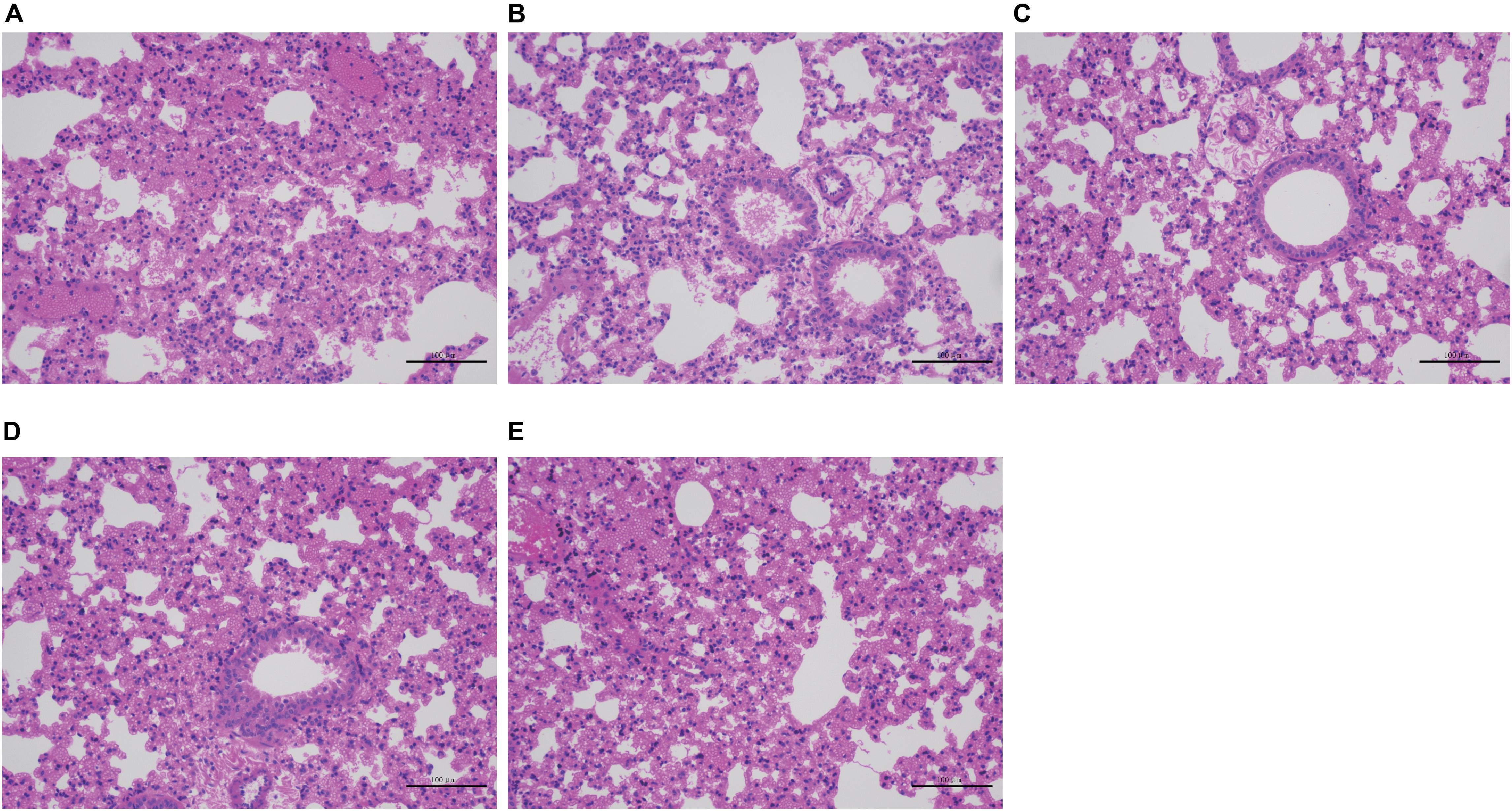
Figure 6. Histopathological assays. (A–E) Lung sections from wild-type, ΔcpxAR, ΔwecA, CΔcpxAR, and CΔwecA-treated mouse groups, respectively.
Discussion
In Gram-negative bacteria, the envelope has been reported to be essential for nutrient transport, adhesion, viability, stress resistance, and virulence (Bury-Mone et al., 2009). The envelope consists of the inner membrane and the OM. LPS is one of the central constituents of the OM. O-antigen, which is one constituent of LPS, is distal to the OM (Raetz and Whitfield, 2002). Previous studies have revealed that O-antigen plays a crucial role in stress tolerance and immune evasion in Gram-negative bacteria (Murray et al., 2006; Berry et al., 2009; Duerr et al., 2009; Wells et al., 2014; Noh et al., 2015; Zheng et al., 2019).
The Cpx system was identified as an important regulatory system, playing a major role in envelope stress resistance, cell wall integrity, stress tolerance, and virulence regulation in Gram-negative bacteria (Bengoechea et al., 2002; Ruiz and Silhavy, 2005; Rowley et al., 2006; Yamamoto and Ishihama, 2006; Vogt and Raivio, 2012; Raivio et al., 2013; Delhaye et al., 2016; Pezza et al., 2016). Since both O-antigen and the Cpx system are responsible for stress tolerance, it is necessary to examine their relationship in A. pleuropneumoniae.
Results here showed that the expression of wecA was upregulated after exposure of A. pleuropneumoniae to serum, NaCl, or H2O2. Activated CpxR enabled bacteria to adapt to the environmental stresses by regulating gene expression. The CpxR-binding consensus sequence was found to be GTAAA-(N)4–8-GTAAA, or TTTAC-(N)4–8-TTTAC (De Wulf et al., 2002; Yamamoto and Ishihama, 2006; Keilwagen et al., 2009; Srinivasan et al., 2012). Here, the putative CpxR binding sequence (TTTAC-N7-TTTAT) was located 46–30 bp upstream of the wecA start codon. Results showed that CpxR-P slowed down the movement of the putative wecA promoter region in EMSA, CpxR-P was unable to bind to a mutant wecA putative promoter region (in which TTTAC was deleted from the putative CpxR-P binding box), and the competition control demonstrated the specificity of CpxR-P binding. These results indicate that CpxR-P was able to bind to the putative CpxR-P binding box. The DNA–protein binding was not saturated. This may be because the concentration of DNA probe was too low, or the protein concentration was too high, for the DNA–protein reaction to be maximal. The promoter was predicted using Softberry1 (Supplementary Figure S3). As shown in Supplementary Figure S3, the CpxR-P binding site was located downstream of the putative wecA promoter. This finding was in accordance with another study, where the CpxR-binding site was located downstream of the cpxP promoter (Yamamoto and Ishihama, 2006). EMSA and qRT-PCR results revealed that CpxR-P directly bound to the putative wecA promoter region and positively regulated wecA gene expression. Previous studies revealed that the genes related to O-antigen biosynthesis were in one operon or several transcriptional units (Skurnik et al., 1999; Yi et al., 2005; Liu et al., 2008, 2019). The small intergenic space between wecA, APPSER1_RS08540, wecB, wecC, rffC, rffA, and wzxE in A. pleuropneumoniae suggested that these genes might be co-transcribed as one mRNA. Our RT-PCR results confirmed this speculation (Supplementary Figure S2). These results reveal that CpxAR is involved in O-antigen biosynthesis in A. pleuropneumoniae.
The CpxAR TCS upregulated the expression of genes related to O-antigen biosynthesis to resist environment stresses. Bengoechea et al. studied Yersinia enterocolitica and reported that overexpression of Wzz resulted in the generation of more O-antigen, thus activating the CpxA-CpxR TCS (Bengoechea et al., 2002). Both their study and our results indicate that bacteria should maintain a proper amount of O-antigen so as to be adaptable to environmental stresses.
One of our previous studies reported that the deletion of cpxAR from A. pleuropneumoniae affected growth of the bacterium (Li et al., 2018). This report was in accordance with the findings of a study in which deletion of the Cpx TCS affected the growth of E. coli (Delhaye et al., 2016). The present study revealed that viable bacterial numbers of ΔcpxAR and ΔwecA strains were lower than those of the wild-type strain at the OD600 values 0.3, 0.6, 1.5, and 2.5, but that, before the stationary phase, no significant difference in the OD600 values was observed between ΔcpxAR or ΔwecA and the wild-type strain (Figure 3). These two results might be due to the fact that the differences in the amount of O-antigen produced resulted in different surface structures of bacteria, thus leading to different absorbances.
The maximum number of CFU/mL for the mutants was less than that of the wild-type and complemented strains. The maximum number of CFU/mL for strain ΔwecA (4 × 109) was approximately equal to that of the wild-type strain in the late exponential phase, which might be explained by previous reports that the production of O-antigen was associated with the growth-phase; that in S. enterica serovar Typhi, O-antigen increased in the late exponential and stationary phases (Rojas et al., 2001; Bittner et al., 2004); and that bacteria modify LPS structures, promoting survival in various growth conditions (Bengoechea et al., 2003; Whitfield and Trent, 2014). Our study results indicated that CpxAR affected cell growth by regulating the expression of wecA.
Bacterial resistance to serum is vital for pathogenicity. Previous studies reported that in S. enterica serovar Typhimurium and Klebsiella pneumoniae, O-antigen was involved in resistance to serum bactericidal activity (Tomas et al., 1986; Ciurana and Tomas, 1987; Murray et al., 2006). The present study showed that when A. pleuropneumoniae strains were cultured in fresh serum, ΔcpxAR and ΔwecA mutants exhibited lower survival rates than the wild-type strain, while complementation of these strains restored bacterial resistance to serum, which was consistent with previous reports (Tomas et al., 1986; Ciurana and Tomas, 1987; Murray et al., 2006). Based on the above findings, the decreased resistance of the ΔcpxAR strain to the bactericidal activity of serum might be caused by lowered wecA expression.
Bacterial colonization of the respiratory tract requires the bacteria to overcome a variety of stresses, including oxidative and osmotic stresses, and LPS plays critical roles in this process. To investigate whether the CpxAR TCS and wecA were involved in tolerance to oxidative and osmotic stresses, we compared the survival rates of the wild-type, ΔcpxAR, CΔcpxAR, ΔwecA, and CΔwecA strains when they were exposed to NaCl or H2O2. The results indicated that the ΔcpxAR and ΔwecA mutants showed higher sensitivity to oxidative and osmotic stresses than the wild-type and complemented strains. These results suggested that the lack of the Cpx system or O-antigen affected the survival of A. pleuropneumoniae under H2O2 and osmotic stress. Our results are in line with a previous report that an O-antigen mutant exhibited increased sensitivity to H2O2 and osmotic stress (Berry et al., 2009; Noh et al., 2015; Zheng et al., 2019).
Bacterial burden experimental results showed that the colonization of mutant strains in mouse lung was decreased relative to that of the wild-type, indicating a higher bacterial clearance rate of the ΔcpxAR and ΔwecA strains. Survival rate assay in a mouse model and histopathological analysis of deceased mice revealed that CpxAR and WecA contributed to A. pleuropneumoniae virulence in vivo.
To the best of our knowledge, this is the first attempt to demonstrate that CpxAR positively regulates wecA expression, which contributes to the growth, stress resistance, and virulence of A. pleuropneumoniae. The CpxAR TCS and wecA are conserved in many bacteria. Therefore, it would be worthwhile to investigate the relationship between CpxAR and wecA in other bacteria. Our findings provide new insight into the pathogenesis of A. pleuropneumoniae.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by Laboratory Animal Monitoring Committee of Huazhong Agricultural University.
Author Contributions
WB, FY, KY, and HC conceived and designed the experiments. All authors contributed to the acquisition and analysis of the data and the writing of the manuscript.
Funding
This work was supported by grants from the Fundamental Research Funds for the Central Universities (No. 2662018PY042), State Key Laboratory of Genetically Engineered Veterinary Vaccines (No. AGVSK-ZD-201805) and the Technique Innovation Program of Hubei Province (No. 2018ABA108).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We greatly appreciate Dr. Gerald-F. Gerlach (Institute for Microbiology, Department of Infectious Diseases, University of Veterinary Medicine Hannover, Germany) for providing E. coli β2155 strain and vector pEMOC2. We thank Professor Ping Liu and Ms. Jia Tang for providing help. We thank James Allen, DPhil, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01026/full#supplementary-material
FIGURE S1 | Confirmation of A. pleuropneumoniae ΔwecA mutant and complemented strain. The ΔwecA mutant and complemented strains were verified by PCR with primers weca-exterior-F/R and weca-interior-F/R.
FIGURE S2 | Diagram of O-antigen gene cluster in A. pleuropneumoniae. (A) and co-expression results (B). Lanes 1–4 contain cDNA, RNA without reverse transcription, genomic DNA, and non-template control, respectively.
FIGURE S3 | Organization of wecA promoter DNA region. The promoter −10 and −35 sites are underlined. The promoter was predicted using Softberry (http://www.softberry.com/berry.phtml?topic=index&group=programs&subgroup=promoter). The CpxR-P binding box is indicated by a gray box.
TABLE S1 | Primers used in this study.
Footnotes
References
Alexander, D. C., and Valvano, M. A. (1994). Role of the rfe gene in the biosynthesis of the Escherichia coli O7-specific lipopolysaccharide and other O-specific polysaccharides containing N-acetylglucosamine. J. Bacteriol. 176, 7079–7084. doi: 10.1128/jb.176.22.7079-7084.1994
Bengoechea, J. A., Brandenburg, K., Arraiza, M. D., Seydel, U., Skurnik, M., and Moriyon, I. (2003). Pathogenic Yersinia enterocolitica strains increase the outer membrane permeability in response to environmental stimuli by modulating lipopolysaccharide fluidity and lipid A structure. Infect. Immun. 71, 2014–2021. doi: 10.1128/iai.71.4.2014-2021.2003
Bengoechea, J. A., Zhang, L., Toivanen, P., and Skurnik, M. (2002). Regulatory network of lipopolysaccharide O-antigen biosynthesis in Yersinia enterocolitica includes cell envelope-dependent signals. Mol. Microbiol. 44, 1045–1062. doi: 10.1046/j.1365-2958.2002.02940.x
Berry, M. C., McGhee, G. C., Zhao, Y., and Sundin, G. W. (2009). Effect of a waaL mutation on lipopolysaccharide composition, oxidative stress survival, and virulence in Erwinia amylovora. FEMS Microbiol. Lett. 291, 80–87. doi: 10.1111/j.1574-6968.2008.01438.x
Bittner, M., Saldias, S., Altamirano, F., Valvano, M. A., and Contreras, I. (2004). RpoS and RpoN are involved in the growth-dependent regulation of rfaH transcription and O antigen expression in Salmonella enterica serovar Typhi. Microb. Pathog. 36, 19–24. doi: 10.1016/j.micpath.2003.08.003
Bittner, M., Saldias, S., Estevez, C., Zaldivar, M., Marolda, C. L., Valvano, M. A., et al. (2002). O-antigen expression in Salmonella enterica serovar Typhi is regulated by nitrogen availability through RpoN-mediated transcriptional control of the rfaH gene. Microbiology 148, 3789–3799. doi: 10.1099/00221287-148-12-3789
Bontemps-Gallo, S., Madec, E., and Lacroix, J. M. (2015). The two-component system CpxAR is essential for virulence in the phytopathogen bacteria Dickeya dadantii EC3937. Environ. Microbiol. 17, 4415–4428. doi: 10.1111/1462-2920.12874
Bosse, J. T., Li, Y., Fernandez Crespo, R., Lacouture, S., Gottschalk, M., Sarkozi, R., et al. (2018a). Comparative sequence analysis of the capsular polysaccharide loci of Actinobacillus pleuropneumoniae serovars 1-18, and development of two multiplex PCRs for comprehensive capsule typing. Vet. Microbiol. 220, 83–89. doi: 10.1016/j.vetmic.2018.05.011
Bosse, J. T., Li, Y., Sarkozi, R., Fodor, L., Lacouture, S., Gottschalk, M., et al. (2018b). Proposal of serovars 17 and 18 of Actinobacillus pleuropneumoniae based on serological and genotypic analysis. Vet. Microbiol. 217, 1–6. doi: 10.1016/j.vetmic.2018.02.019
Bury-Mone, S., Nomane, Y., Reymond, N., Barbet, R., Jacquet, E., Imbeaud, S., et al. (2009). Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 5:e1000651. doi: 10.1371/journal.pgen.1000651
Cao, Q., Feng, F., Wang, H., Xu, X., Chen, H., Cai, X., et al. (2018). Haemophilus parasuis CpxRA two-component system confers bacterial tolerance to environmental stresses and macrolide resistance. Microbiol. Res. 206, 177–185. doi: 10.1016/j.micres.2017.10.010
Chiers, K., De Waele, T., Pasmans, F., Ducatelle, R., and Haesebrouck, F. (2010). Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet. Res. 41:65. doi: 10.1051/vetres/2010037
Ciurana, B., and Tomas, J. M. (1987). Role of lipopolysaccharide and complement in susceptibility of Klebsiella pneumoniae to nonimmune serum. Infect. Immun. 55, 2741–2746.
Clarke, B. R., Bronner, D., Keenleyside, W. J., Severn, W. B., Richards, J. C., and Whitfield, C. (1995). Role of Rfe and RfbF in the initiation of biosynthesis of D-galactan I, the lipopolysaccharide O antigen from Klebsiella pneumoniae serotype O1. J. Bacteriol. 177, 5411–5418. doi: 10.1128/jb.177.19.5411-5418.1995
De Wulf, P., McGuire, A. M., Liu, X., and Lin, E. C. (2002). Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277, 26652–26661. doi: 10.1074/jbc.M203487200
Delhaye, A., Collet, J. F., and Laloux, G. (2016). Fine-tuning of the Cpx envelope stress response is required for cell wall homeostasis in Escherichia coli. mBio 7:e0047-16. doi: 10.1128/mBio.00047-16
Duerr, C. U., Zenk, S. F., Chassin, C., Pott, J., Gutle, D., Hensel, M., et al. (2009). O-antigen delays lipopolysaccharide recognition and impairs antibacterial host defense in murine intestinal epithelial cells. PLoS Pathog. 5:e1000567. doi: 10.1371/journal.ppat.1000567
Frey, J. (1992). Construction of a broad host range shuttle vector for gene cloning and expression in Actinobacillus pleuropneumoniae and other Pasteurellaceae. Res. Microbiol. 143, 263–269. doi: 10.1016/0923-2508(92)90018-j
Jacob-Dubuisson, F., Mechaly, A., Betton, J. M., and Antoine, R. (2018). Structural insights into the signalling mechanisms of two-component systems. Nat. Rev. Microbiol. 16, 585–593. doi: 10.1038/s41579-018-0055-7
Keilwagen, J., Baumbach, J., Kohl, T. A., and Grosse, I. (2009). MotifAdjuster: a tool for computational reassessment of transcription factor binding site annotations. Genome Biol. 10:R46. doi: 10.1186/gb-2009-10-5-r46
Lehrer, J., Vigeant, K. A., Tatar, L. D., and Valvano, M. A. (2007). Functional characterization and membrane topology of Escherichia coli WecA, a sugar-phosphate transferase initiating the biosynthesis of enterobacterial common antigen and O-antigen lipopolysaccharide. J. Bacteriol. 189, 2618–2628. doi: 10.1128/JB.01905-06
Li, D., Gurkovska, V., Sheridan, M., Calderone, R., and Chauhan, N. (2004). Studies on the regulation of the two-component histidine kinase gene CHK1 in Candida albicans using the heterologous lacZ reporter gene. Microbiology 150, 3305–3313. doi: 10.1099/mic.0.27237-0
Li, H., Liu, F., Peng, W., Yan, K., Zhao, H., Liu, T., et al. (2018). The CpxA/CpxR Two-component system affects biofilm formation and virulence in Actinobacillus pleuropneumoniae. Front. Cell Infect. Microbiol. 8:72. doi: 10.3389/fcimb.2018.00072
Li, Y., Cao, S., Zhang, L., Yuan, J., Zhao, Q., Wen, Y., et al. (2019). A requirement of TolC1 for effective survival, colonization and pathogenicity of Actinobacillus pleuropneumoniae. Microb. Pathog. 134:103596. doi: 10.1016/j.micpath.2019.103596
Linnes, J. C., Ma, H., and Bryers, J. D. (2013). Giant extracellular matrix binding protein expression in Staphylococcus epidermidis is regulated by biofilm formation and osmotic pressure. Curr. Microbiol. 66, 627–633. doi: 10.1007/s00284-013-0316-7
Liu, B., Furevi, A., Perepelov, A. V., Guo, X., Cao, H., Wang, Q., et al. (2019). Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. doi: 10.1093/femsre/fuz028 [Epub ahead of print].
Liu, B., Knirel, Y. A., Feng, L., Perepelov, A. V., Senchenkova, S. N., Wang, Q., et al. (2008). Structure and genetics of Shigella O antigens. FEMS Microbiol. Rev. 32, 627–653. doi: 10.1111/j.1574-6976.2008.00114.x
Lopez, C., Checa, S. K., and Soncini, F. C. (2018). CpxR/CpxA Controls scsABCD transcription to counteract copper and oxidative stress in Salmonella enterica Serovar typhimurium. J. Bacteriol. 200:e00126-18. doi: 10.1128/JB.00126-18
Matter, L. B., Ares, M. A., Abundes-Gallegos, J., Cedillo, M. L., Yanez, J. A., Ygnacio, M. L., et al. (2018). The CpxRA stress response system regulates virulence features of avian pathogenic Escherichia coli. Environ. Microbiol. 20, 3363–3377. doi: 10.1111/1462-2920.14368
Murray, G. L., Attridge, S. R., and Morona, R. (2006). Altering the length of the lipopolysaccharide O antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J. Bacteriol. 188, 2735–2739. doi: 10.1128/JB.188.7.2735-2739.2006
Nikaido, H. (2003). Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656. doi: 10.1128/mmbr.67.4.593-656.2003
Noh, J. G., Jeon, H. E., So, J. S., and Chang, W. S. (2015). Effects of the Bradyrhizobium japonicum waaL (rfaL) gene on hydrophobicity. motility, stress tolerance, and symbiotic relationship with soybeans. Int. J. Mol. Sci. 16, 16778–16791. doi: 10.3390/ijms160816778
Pezza, A., Pontel, L. B., Lopez, C., and Soncini, F. C. (2016). Compartment and signal-specific codependence in the transcriptional control of Salmonella periplasmic copper homeostasis. Proc. Natl. Acad. Sci. U.S.A. 113, 11573–11578. doi: 10.1073/pnas.1603192113
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. doi: 10.1093/nar/29.9.e45
Pogliano, J., Lynch, A. S., Belin, D., Lin, E. C., and Beckwith, J. (1997). Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11, 1169–1182. doi: 10.1101/gad.11.9.1169
Raetz, C. R., and Whitfield, C. (2002). Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700.
Raivio, T. L., Leblanc, S. K., and Price, N. L. (2013). The Escherichia coli Cpx envelope stress response regulates genes of diverse function that impact antibiotic resistance and membrane integrity. J. Bacteriol. 195, 2755–2767. doi: 10.1128/JB.00105-13
Raivio, T. L., and Silhavy, T. J. (1997). Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179, 7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997
Rick, P. D., Hubbard, G. L., and Barr, K. (1994). Role of the rfe gene in the synthesis of the O8 antigen in Escherichia coli K-12. J. Bacteriol. 176, 2877–2884. doi: 10.1128/jb.176.10.2877-2884.1994
Rojas, G., Saldias, S., Bittner, M., Zaldivar, M., and Contreras, I. (2001). The rfaH gene, which affects lipopolysaccharide synthesis in Salmonella enterica serovar Typhi, is differentially expressed during the bacterial growth phase. FEMS Microbiol. Lett. 204, 123–128. doi: 10.1111/j.1574-6968.2001.tb10874.x
Rowley, G., Spector, M., Kormanec, J., and Roberts, M. (2006). Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4, 383–394. doi: 10.1038/nrmicro1394
Ruiz, N., and Silhavy, T. J. (2005). Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8, 122–126. doi: 10.1016/j.mib.2005.02.013
Sassu, E. L., Bosse, J. T., Tobias, T. J., Gottschalk, M., Langford, P. R., and Hennig-Pauka, I. (2018). Update on Actinobacillus pleuropneumoniae-knowledge, gaps and challenges. Transbound Emerg. Dis. 65(Suppl. 1), 72–90. doi: 10.1111/tbed.12739
Sheehan, B. J., Bosse, J. T., Beddek, A. J., Rycroft, A. N., Kroll, J. S., and Langford, P. R. (2003). Identification of Actinobacillus pleuropneumoniae genes important for survival during infection in its natural host. Infect. Immun. 71, 3960–3970. doi: 10.1128/iai.71.7.3960-3970.2003
Silva-Valenzuela, C. A., Velásquez, F., Peñailillo, J., Garcias-Papayani, H., Fernández, P., Tobar, P., et al. (2016). O-antigen chain-length distribution in Salmonella enterica serovar Enteritidis is regulated by oxygen availability. Biochem. Biophys. Res. Commun. 477, 563–567. doi: 10.1016/j.bbrc.2016.06.074
Skurnik, M., Venho, R., Bengoechea, J. A., and Moriyon, I. (1999). The lipopolysaccharide outer core of Yersinia enterocolitica serotype O:3 is required for virulence and plays a role in outer membrane integrity. Mol. Microbiol. 31, 1443–1462. doi: 10.1046/j.1365-2958.1999.01285.x
Srinivasan, V. B., Vaidyanathan, V., Mondal, A., and Rajamohan, G. (2012). Role of the two component signal transduction system CpxAR in conferring cefepime and chloramphenicol resistance in Klebsiella pneumoniae NTUH-K2044. PLoS One 7:e33777. doi: 10.1371/journal.pone.0033777
Subashchandrabose, S., Leveque, R. M., Kirkwood, R. N., Kiupel, M., and Mulks, M. H. (2013). The RNA chaperone Hfq promotes fitness of Actinobacillus pleuropneumoniae during porcine pleuropneumonia. Infect. Immun. 81, 2952–2961. doi: 10.1128/IAI.00392-13
Tomas, J. M., Benedi, V. J., Ciurana, B., and Jofre, J. (1986). Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect. Immun. 54, 85–89.
Vogt, S. L., and Raivio, T. L. (2012). Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol. Lett. 326, 2–11. doi: 10.1111/j.1574-6968.2011.02406.x
Walters, M., Sircili, M. P., and Sperandio, V. (2006). AI-3 synthesis is not dependent on luxS in Escherichia coli. J. Bacteriol. 188, 5668–5681. doi: 10.1128/JB.00648-06
Wells, T. J., Whitters, D., Sevastsyanovich, Y. R., Heath, J. N., Pravin, J., Goodall, M., et al. (2014). Increased severity of respiratory infections associated with elevated anti-LPS IgG2 which inhibits serum bactericidal killing. J. Exp. Med. 211, 1893–1904. doi: 10.1084/jem.20132444
Whitfield, C., and Trent, M. S. (2014). Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 83, 99–128. doi: 10.1146/annurev-biochem-060713-035600
Yamamoto, K., and Ishihama, A. (2006). Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci. Biotechnol. Biochem. 70, 1688–1695. doi: 10.1271/bbb.60024
Yi, W., Shao, J., Zhu, L., Li, M., Singh, M., Lu, Y., et al. (2005). Escherichia coli O86 O-antigen biosynthetic gene cluster and stepwise enzymatic synthesis of human blood group B antigen tetrasaccharide. J. Am. Chem. Soc. 127, 2040–2041. doi: 10.1021/ja045021y
Keywords: CpxAR, Growth, Stress resistance, Virulence, WecA
Citation: Yan K, Liu T, Duan B, Liu F, Cao M, Peng W, Dai Q, Chen H, Yuan F and Bei W (2020) The CpxAR Two-Component System Contributes to Growth, Stress Resistance, and Virulence of Actinobacillus pleuropneumoniae by Upregulating wecA Transcription. Front. Microbiol. 11:1026. doi: 10.3389/fmicb.2020.01026
Received: 14 November 2019; Accepted: 27 April 2020;
Published: 21 May 2020.
Edited by:
Thomas Keith Wood, Pennsylvania State University (PSU), United StatesReviewed by:
Sébastien Bontemps-Gallo, Institut Pasteur de Lille, FranceRicardo Oropeza, National Autonomous University of Mexico, Mexico
Copyright © 2020 Yan, Liu, Duan, Liu, Cao, Peng, Dai, Chen, Yuan and Bei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weicheng Bei, YmVpd2NAbWFpbC5oemF1LmVkdS5jbg==; Fangyan Yuan, ZmFuZ3lhbnl1YW4xMkAxNjMuY29t
 Kang Yan1,4
Kang Yan1,4 Huanchun Chen
Huanchun Chen Weicheng Bei
Weicheng Bei