- 1Department of Cell Biology, Zunyi Medical University, Zunyi, China
- 2Zunyi Academy of Agricultural Sciences, Zunyi, China
- 3Department of Medical Instrumental Analysis, Zunyi Medical University, Zunyi, China
Endophytes in medicinal plants possess significant biological value since they have in them the ability to provide elicitors crucial in regulating plant growth as also different secondary metabolites. This review emphasizes the effective ability of endophytic fungi to induce their hosts, explains the biological mechanisms of using endophytic fungi to enhance the accumulation of secondary metabolites in medicinal plant cultures, and summarizes the extensive application of endophytic fungi elicitors in medicinal plant cultivation. The main goal of this article is to clarify the mechanism and important role of endophytic elicitors in the production of natural drugs. This information will be helpful for scientific researchers in controlling the quality of medicinal materials, prioritizing endophytic resources, and achieving a circular and sustainable development and production of natural medicine.
Highlights
• Endophytic elicitors significantly boost secondary metabolite levels in medicinal plants.
• Elucidates molecular pathways involving JA, SA, and ROS in plant-endophyte interactions.
• Advances in metagenomics and bioinformatics reveal diverse endophyte communities.
• Enhances sustainable cultivation of medicinal plants through microbial symbiosis.
• Highlights potential biotechnological applications of endophytic fungi elicitors.
1 Introduction
The use of natural drugs for treating or preventing diseases has been the primary means of safeguarding human health for thousands of years. The further exploitation of medicinal components found in plants is currently a focal point in drug development. However, due to prolonged land and forest degradation caused by human activity, the availability of natural medicinal resources has dwindled. Rare medicinal plants often exhibit characteristics such as limited natural distribution, weak regenerative capabilities, extended growth cycles, and challenging chemical synthesis of active ingredients. As a result, the pharmaceutical industry reliant on plant-derived materials has become resource intensive, leading to a significant surge in the price of traditional Chinese medicinal materials plant in the market. The challenge, therefore, lies in striking a balance between resource protection and sustainable utilization within the pharmaceutical industry. Extracting more effective secondary metabolites from endophytic fungi and host plants' symbiotic systems has gradually become a new method for conserving medicinal natural resources and reducing resource wastage.
Digging out endophytes from medicinal plants to synthesize medicinal ingredients or their precursors provides a new approach for the protection of medicinal plants. Previous studies have shown that the interaction between endophytic fungi and hosts is different from the process of pathogenic fungal infection. The interaction between endophytic fungi and hosts is a balance and long-term coevolution between virulence factors of endophytic fungi and host defense responses, gradually forming a relatively stable equilibrium for long-term coexistence (Wang et al., 2021). However, in reality, the mechanisms maintaining this equilibrium are far more complex and intricate than the balance itself (Cheng et al., 2024). In the symbiotic system of endophytes and host plants in a stable equilibrium, endophytes can regulate host plants through three ways. These include direct synthesis of metabolites (Lv et al., 2024), selective catalytic synthesis of metabolites (bioconversion) (Tian et al., 2014), and induction of a secondary metabolite effect or horizontal gene transfer (Ku et al., 2024; Ambrose et al., 2014). Among them, relevant studies have shown that endophytic fungi isolated from Camptotheca acuminata can directly biosynthesize camptothecin. This finding demonstrates that endophytic fungi can maintain a stable symbiotic system through direct synthesis of metabolites (Kusari et al., 2011). In addition, researchers monitored changes in leaf compounds of Cephalotaxus harringtonia caused by the endophytic fungus Paraconiothyrium variabile using metabolomics methods, and then characterized the altered products. They observed the specific biotransformation of glycosylated flavonoids by the endophytic fungus (Tian et al., 2014). These studies provide strong evidence for the important role of endophytic fungi in regulating the stability of symbiotic systems. Based on genomics, metabolomics, and microbiomics, we can discover that endophytic fungi act as special “inducers” to regulate the growth metabolism of plants in the plant-microbe interaction system (Ding et al., 2022), In addition, endophytic fungi enter plant tissues and alleviate biotic and abiotic stress by producing a large number of secondary metabolites (Li et al., 2022). They are dedicated to de novo synthesis of structural compounds and stimulate plant immunity (Yipare et al., 2022). They also participate in the process of excluding pathogens through niche competition and actively participate in the phenylpropanoid metabolism process. In addition, we also pay attention to the fact that endophyte metabolites have efficient growth-promoting and regulatory effects on host cells in vitro (Wang et al., 2022; Tran et al., 2022; Agarwal et al., 2020). Therefore, the development of efficient inducers in plant tissue culture technology, elucidation of the mechanisms by which endophytic fungi directly synthesize or induce plants to synthesize effective components, are of special significance in solving the current dilemma of medicinal plant production and resource conservation, and also promotes the sustainable production of secondary metabolites from medicinal plants.
2 Endophytic elicitors possess the power to effortlessly coax the synthesis of valuable medicinal components
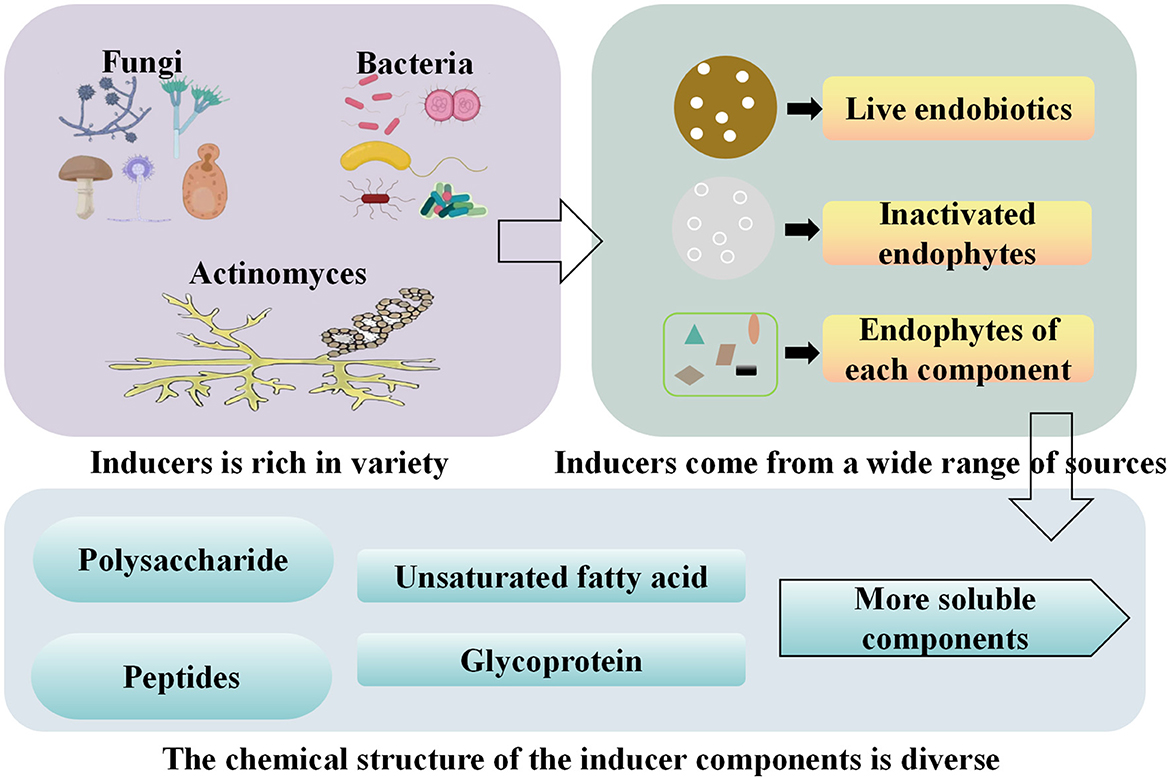
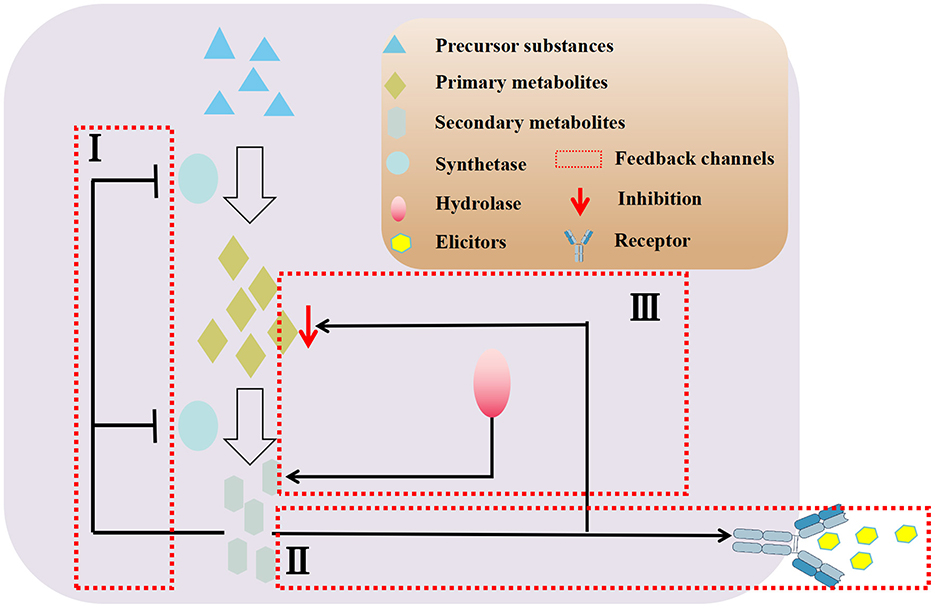
Endophytic elicitors are external biological elicitors, whose use to control the production of active components in medicinal plants has gained popularity in research. By considering factors such as the unique characteristics of different inducers, the conditions required for induction, and their impact on the physiological state of host cells (Figure 1), The rational selection and effective implementation of inducers can promote the accumulation of secondary metabolites in medicinal plants (Zhang, 2022).
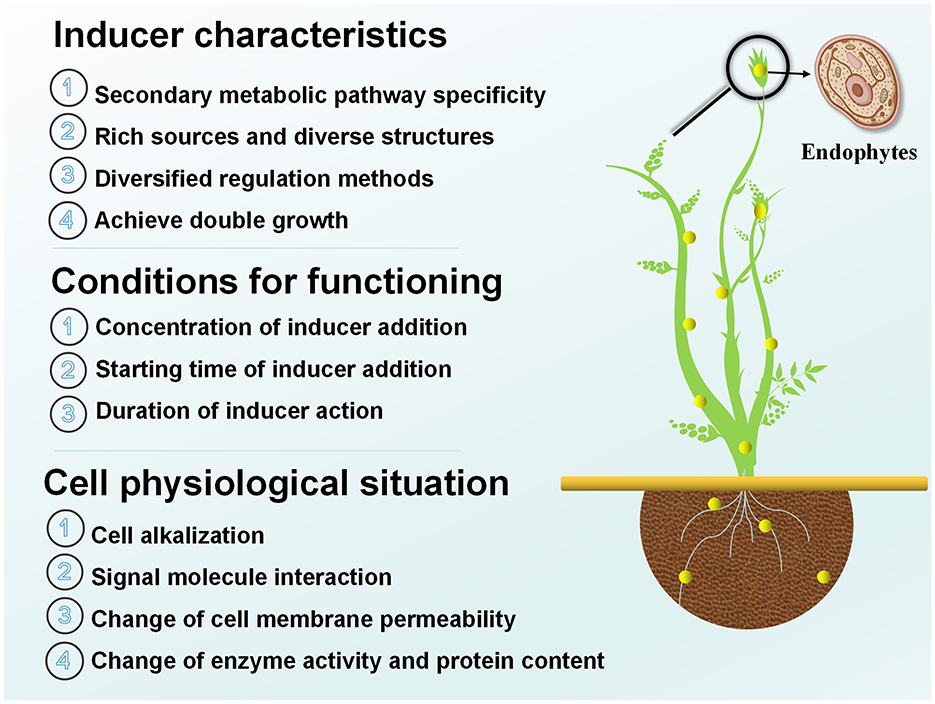
Figure 1. Characterization, conditions, and effects of efficient induction of endophytes on the physiological posture of host cells.
2.1 The fascinating characteristics of endophytic inducers
Endophytic elicitors have significant advantages over abiotic elicitors and pathogens in regulating the synthesis of secondary metabolites in the host due to their unique properties.
These inducers have a diverse range of sources and structures (Figure 2). The endophytes within the symbiotic system exhibit a rich variety of species, including endophytic bacteria, fungi, actinomycetes, and yeasts. The key components of the induction mechanism are mainly derived from endophytic viable cells, endophytic inactivated cells, and components of endophytic bacteria. These chemical structures include polysaccharides, polypeptides, glycoproteins, and unsaturated fatty acids.
There are various methods to regulate inducers. Current research has discovered that endophytic fungi can specifically regulate the production of secondary metabolites by inducing the synthesis of new enzymes and increasing the content of key enzymes in primary metabolic pathways (Cao et al., 2024).
This leads to high induction efficiency and dual growth, meaning that gentle stimulation from endophytic elicitors can avoid plant destruction caused by pathogenic elicitors and promotes the simultaneous growth of host cells and secondary metabolites (Liu et al., 2021).
The activation of secondary metabolic pathways by elicitors in medicinal plants is specific to species and growth stages. Specific metabolic pathways contribute to the efficient accumulation of particular secondary metabolites (Singh et al., 2023). Therefore, it is necessary to establish an optimal induction culture system and strictly control induction conditions based on the characteristics of the inducer.
2.2 Action conditions of endophytic elicitors
The effectiveness of endophytic inducers is closely related to various factors, including the concentration, of the inducer, the timing of its addition, and the duration of its action. The concentration effects of endophytic elicitors can be classified into two types: reactive saturation type and optimal concentration type. In general, excessive inducers can cause plants to have an excessive immune response, resulting in the production of toxin substances that damage the host plant cells and cause localized cell death. Suitable concentrations of fungal inducers can induce the synthesis and accumulation of active substances that stabilize products in the interaction between plants and microorganisms when the HR resistance reaction occurs (Zhu et al., 2024). In most medicinal plant cell cultures, the accumulation of secondary metabolites reaches its peak after the exponential growth period, so it is important to select an appropriate time for the addition of inducers.
Furthermore, adding the correct amount of inducers at the optimal time does not guarantee the maximum amount of secondary metabolites (Wang et al., 2004). Over time, some plant culture systems may experience a decline in the production of secondary metabolites. This can be attributed to factors such as the excess of synthesized secondary metabolites inhibiting the synthesis pathway through feedback, competition between secondary metabolites and endophytic inducers for binding sites in plant cells (Figure 3), and a lack of raw materials for primary metabolites (Pang et al., 2021).
2.3 Effects of endophytic elicitors on the physiological situation of medicinal plant cells
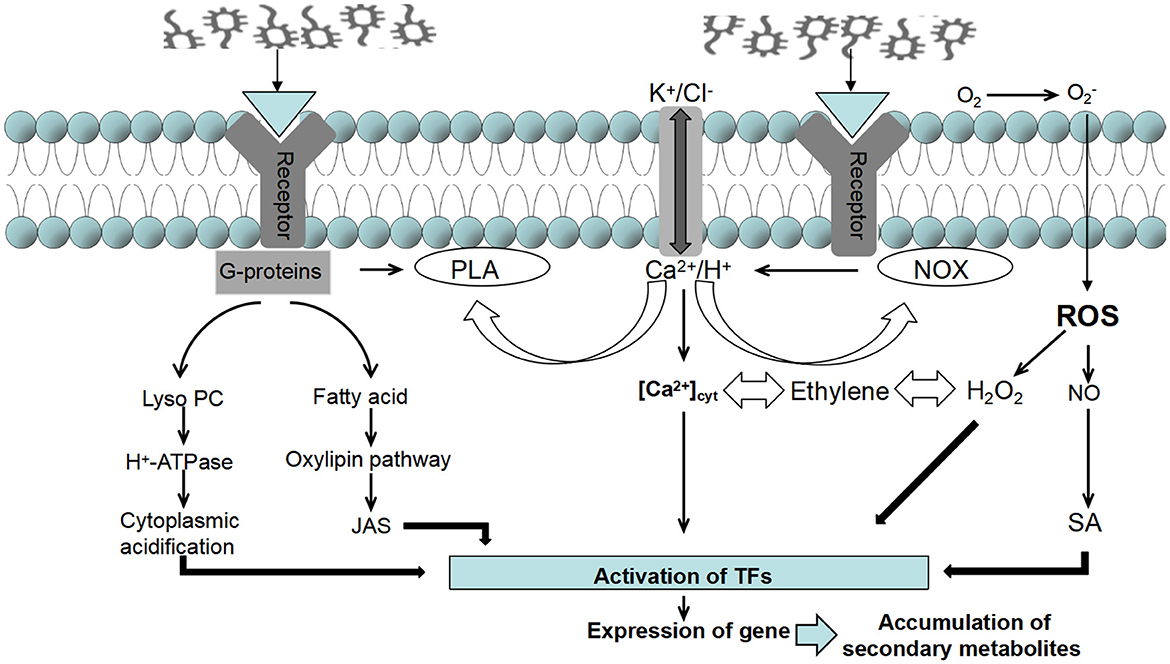
When endophytic fungi induce derivative to join the plant cell suspension culture medium, the plant undergoes changes in cell membrane permeability, enzyme activity and protein content, cell alkalization, and signaling molecule interaction in order to protect itself, thereby affecting the accumulation process of secondary metabolites (Wróbel-Kwiatkowska et al., 2024). After the endophytic inducer binds to receptors on the cell membrane, it causes an increase in membrane permeability and changes in fluidity, leading to cell infiltration. At the same time, it can cause metabolic changes in cells, enabling them to actively synthesize phytoalexins, lignin, etc., required for plant defense, strengthening specific secondary metabolic pathways (Li et al., 2024; Liu Y. H. et al., 2024). The occurrence of these processes is mainly achieved by regulating the amount and activity of enzymes, and changes in protein content reflect the quantitative change process of these enzymes. For example, after adding Aspergillus oryzae inducer on the 8th day of cell culture of Arnebia yunnanensis, the content of intracellular soluble protein increases rapidly (Ning et al., 1996).
In addition, endophytic inducers also induce the alkalization of the cell culture system. The researchers found that before induction, the Taxus cells were in a relatively stable growth state, with intact cells and well-developed organelles. The redox potential was low, and the enzyme system related to reactive oxygen species (ROS) was relatively stable. The primary metabolism of the cells was vigorous, but the synthesis rate of paclitaxel was low. After induction, the Taxus cells shifted toward product synthesis. The activity of superoxide dismutase (SOD) increased rapidly, while the activities of catalase (CAT) and peroxidase (POD) were strongly inhibited. ROS burst occurred in the cells, and the redox potential increased. Ion channels on the cell membrane were activated, leading to the influx of H+ and Ca2+ and the efflux of Cl−. The vacuoles showed a large number of high-electron-density areas containing Ca2+ ions, exhibiting regular changes. The culture environment became alkaline, inhibiting cell growth but increasing the synthesis rate and yield of paclitaxel (Zhang et al., 2001). The synergistic activation of the intracellular phospholipase C dual messenger pathway and Ca2+ channel can accelerate the accumulation of inducers for paclitaxel synthesis, while Ca2+ influx significantly enhances the signal of intracellular oxygen burst (Li et al., 2002). Oxygen bursts and nitric oxide (NO) induced by endophytic elicitors have become key components in the signal transduction network of secondary metabolism in medicinal plant cells. These elements can work in conjunction with signal molecules like reactive oxygen species (ROS), jasmonic acid (JA), and salicylic acid (SA) to jointly regulate the biosynthesis of secondary metabolites in plant cells (Lv et al., 2021).
2.4 Screening of endophytic inducers
The effective selection of inducers is based on the advantage that endophytic inducers can fully utilize their highly effective induction effects, by selectively inducing specific secondary metabolites through the selection of suitable endophytic bacteria or endophytic components. For instance, fungal elicitors can rapidly activate phenylalanine ammonia lyases in the hairy roots of Loiuscor niculatus to synthesize isoflavones, without significantly affecting other unrelated enzymes. Jian and colleagues conducted a screening of various elicitors from 12 fungal substances to treat suspension-cultured cells of Catharanthus roseus, and the results showed that different mycelium homogenates could stimulate production of diverse indole alkaloids (Zhao et al., 2001). A large number of studies have demonstrated the efficient inducing effect of endophytic fungi. However, in order to achieve effective screening and fully utilize the induction effect, it is necessary to must first understand the synthesis pathway of specific secondary metabolites, the structure and characteristics of key enzymes, and the relationship between the structure and function of inducers and secondary metabolites. Therefore, elucidating the mechanism of endophyte inducers is not only essential for optimizing screening strategies but also crucial for uncovering the co-evolutionary relationships between plants and microbes. Effective screening of endophyte inducers will significantly improve the efficiency of targeted synthesis of secondary metabolites in medicinal plant cells and offer a new paradigm for the development of natural drugs.
3 Delving into the mechanism of endophytic elicitors
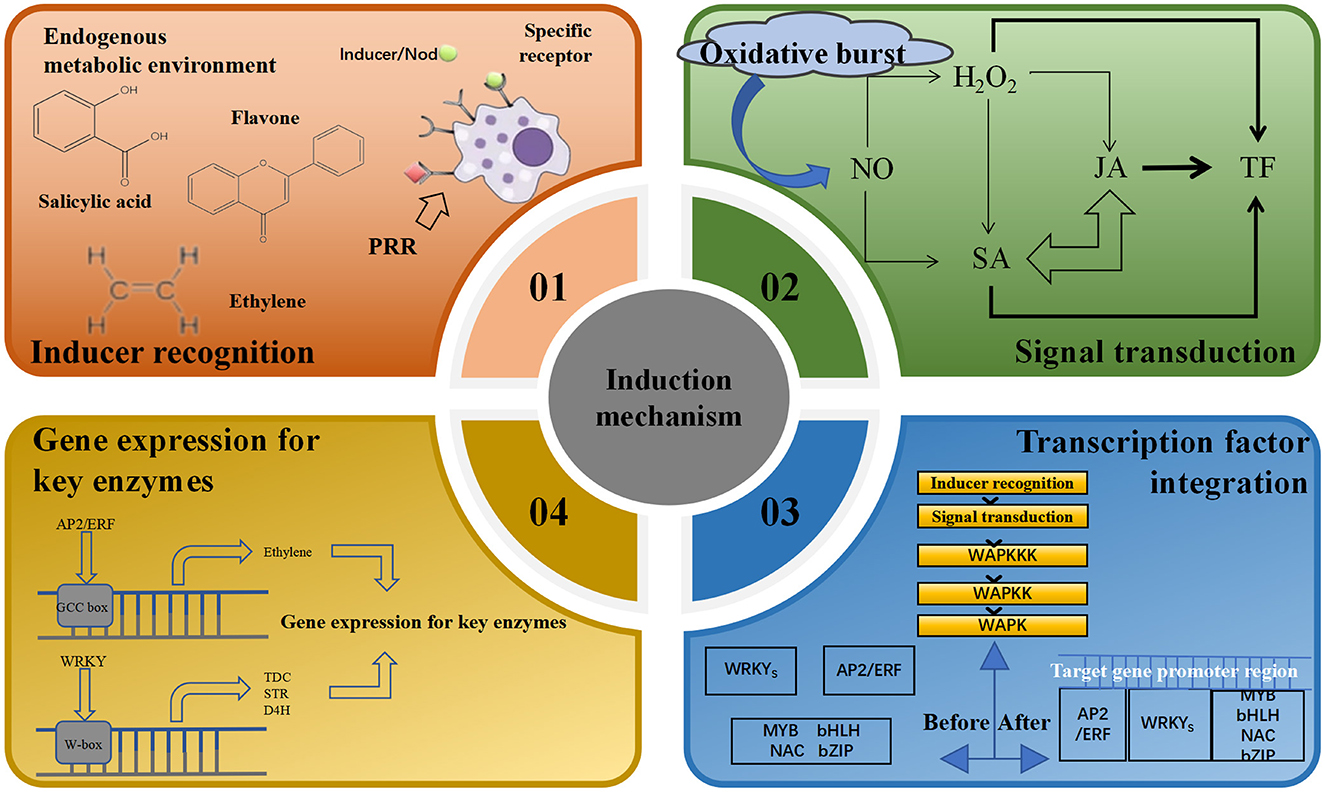
Endogenous inducers can affect physiological and biochemical levels, gene expression and metabolic pathways in host cells as they induce the accumulation of secondary metabolites in plants. Over time, the collaborative interaction between the two results in a comprehensive and complex induction mechanism (Cao L. S. et al., 2022; Bauer et al., 2001). Recognition of the source of endophyte inducer stimulation by the host plant body is central to the plant's ability to respond to cellular changes such as activation of kinases, production of reactive oxygen species, ion fluxes and cytoplasmic acidification. Receptors on the surface of the plasma membrane of plant cells can recognize different structures of specific inducers, trigger the plant to generate a signal transduction network, elicit a plant response, and transmit signals to transcription factors under stress (Wu et al., 2024). Changes in the mRNA transcript levels of the relevant genes trigger changes in the expression of key enzymes involved in secondary metabolites, regulating cellular metabolism and leading to the accumulation of certain secondary metabolites (Zhang et al., 2022; Vasconsuelo and Boland, 2007; Yu and Facchini, 2000), thus regulating the immune defense system of the host plant (Bu et al., 2023). For example, treatment of poppy cell suspension cultures with fungal triggers induces glutathione s-transferase (GST) synthesis, which catalyzes a GSH-coupled reaction and regulates the levels of secondary metabolites. Thus, the complete molecular mechanism of action of elicitors on secondary metabolism consists of four levels: inducer recognition, signal transduction, transcription factor integration, gene expression and activation of key enzymes (Figure 4).
3.1 Inducer identification
In numerous studies on the effects of endophytes on the accumulation of effective components in plants, it has been discovered that the initial step in establishing symbiotic relationships (Qu et al., 2021; Tian and Nan, 2017) and inducing the synthesis of secondary metabolites is the recognition of endophytic elicitors by host cells. The characteristics and genetic composition of microbial communities constructed by different plants in the same habitat gradually change over long-term evolution (Ehinger et al., 2009). Therefore, symbiotic systems jointly determine whether to initiate induction programs based on the metabolic environment and multiple receptors in the habitat (Limpens et al., 2003; Thoms et al., 2021). In complex metabolic environments, some substances play particularly important roles. These include nutrients that are absorbed and utilized by endogenous bacteria, antibacterial substances that can selectively eliminate toxic pathogens, and specific compounds that attract specific endogenous bacteria (Ancheeva et al., 2020). For example, the roots of Arabidopsis thaliana secrete organic acids that serve as important nutrients for recruiting Bacillus subtilis (Rudrappa et al., 2008). The inoculation of Pseudomonas fluorescens improved the plant nutrient cycle and enhanced the adaptation of the interleaf microbes to the host environmental conditions, suggesting that the ability to utilize host nutrients may be a key factor in symbiotic association (Li et al., 2025; Choi and Klessig, 2016; Liu et al., 2018).
Similarly, plants may create a wide spectrum of antimicrobial chemicals, but the regulation mechanisms of how these molecules resist pathogenic bacteria while allowing endophytes to grow have not been fully explained. However, experiments have fully demonstrated that plants can use metabolites to selectively choose specific endophytes while excluding other microorganisms. For example, coumarins derived from plants have antibacterial activity against some pathogenic bacteria, but do not exhibit antibacterial activity against endophytic bacteria (Voges et al., 2019).
Moreover, metabolic components such as flavonoids, benzoxazines, and ethylene can all act as specific attractants for specific endophytes (Ran et al., 2024; Harbort et al., 2020). The current research on these metabolic compounds provides a basis for plants to selectively utilize advantageous endophytes in complex microbial communities. The specific metabolic environment of the symbiotic system has the potential to become the primary determining factor in the formation of specific symbioses between host plants and endophytes.
When under the attack from host antibacterial metabolites, endophytic actively compete for nutrients and establish symbiotic relationship with host plants when they successfully survive and reproduce. During this process, endophytes engage the induction mechanism; nevertheless, activation of the induction mechanism requires many receptors to sense and integrate complex and diverse signals. Although some inducer affinity proteins encoded by the pattern recognition receptor (PRR) in plant genomes have been successfully isolated (Zhang et al., 2010), further research is needed to study the structure and function of related receptor proteins on the cell membrane of medicinal plants. In addition, the endogenous fungal effector NOd can also act as a ligand receptor to participate in the recognition of inducers (Sun et al., 2022; Miyata et al., 2016). For example, there are two assumed Nod factor receptors, NFP and LYK3, that can recognize inducers of Nod factors in the case of clover and alfalfa. Among them, MtNFP acts as the signal receptor for Nod factors, and MtLYK3 acts as the entry receptor for Nod factors. In the dual receptor model, both of them jointly regulate the downstream signaling pathway (Liu et al., 2023; Jones et al., 2007). Additionally, the uptake of endophyte metabolites and hormones by host cells also creates conditions to promote the occurrence of endophyte-induced secondary metabolism and the establishment of symbiotic relationships (Etalo et al., 2018; Furtado et al., 2019; Xia et al., 2022; Chen et al., 2023).
Endosymbiotic microorganisms that establish a symbiotic relationship with the host plant exhibit gene expression that is largely distinct from that of other microorganisms and possess the potential to produce novel secondary metabolites (Wang L. et al., 2024). Metabolites produced by endophytic microorganisms may also regulate the synthesis of more diverse metabolites by the host plant (Raza et al., 2020; Schenkel et al., 2019), favoring host plant growth while influencing microbial colonization (Shen et al., 2015; Johri et al., 2015; Hong et al., 2024). Therefore, different receptor molecules located on the plasma membrane of the host plant cell can recognize and bind specific endogenous enzymes. This process has certain selectivity. Subsequently, symbiotic signals are transmitted to downstream intracellular signaling molecules (Mengistu, 2020; Santoyo, 2022).
3.2 Signal transduction
Intracellular signal transduction is a crucial link between endophytic elicitors and the secondary metabolites of medicinal plants. Once the host plant detects the endophytic inducer, the cell membrane depolarizes, resulting in the opening and closing of ion channels and the coupling of G proteins. This activation triggers various messenger systems that enhance the induction signal (Zhao et al., 2023). Research and summaries of different medicinal plants such as Artemisia annua, Atractylodes macrocephala, Ginkgo biloba, Pueraria lobata, Astragalus membranaceus, and Taxus chinensis, have revealed that NO, ROS, JAs, and SA are key signaling molecules that regulate the growth, development, expression of resistance genes, and accumulation of pharmacologically active substances in host plant cells induced by endophytic bacteria in medicinal plants (Zhang et al., 2015; Qi et al., 2021).
NO serves as an important upstream signaling molecule in the regulatory network. It can mediate the process of endophytic elicitors inducing the synthesis of secondary metabolites in plants through two different pathways, partially depending on oxidative burst and partially independent of oxidative burst. For instance, the exogenous addition of NO can improve PEPC enzyme activity and promote the generation of cyclopheneether terpenoids in gentian (Song et al., 2023). Additionally, applying NO alone can promote the production of ROS in Taxus chinensis cells (Xu and Dong, 2006a). This suggests that the accumulation of ROS synthesis in the endophytic induction mechanism is a downstream signal transduction event of the NO pathway. Among different host plant cells, the occurrence of H2O2 is the most probable in oxidative bursts (Jedelská et al., 2021; Zheng et al., 2016). For example, H2O2 appears in the induction of β-eucalyptol synthesis in Atractylodes lancea cells (Cao L. S. et al., 2022).
However, NO enhances the synthesis of secondary metabolites while inhibiting the accumulation of most ROS in the cell. Studies have shown that specific NO specific scavengers (cPTIO) and lipoxygenase inhibitors (NDGA) can hinder the promotion of NO and JA on the synthesis of plant secondary metabolites in the cell wall inducer pathway of Aspergillus niger (Ren, 2014). Furthermore, experimental results demonstrate that increasing intracellular JA content in hypericum cells treated with NO alone indicates that NO is located upstream of JA and has a promoting effect on JA accumulation (Song et al., 2023).
JAs also plays a significant role in signal transduction pathways that promote plant growth, enhance plant resistance (Chen et al., 2020; Mao et al., 2021), and stimulate the synthesis of plant secondary metabolites (Wang X. Y. et al., 2024). Yan et al. (2019) discovered that the content of MeJA and volatile oil increases when the inducer of Penicillium endophytic fungus 2J1 induces Cinnamomum longepaniculatum (Gamble) N. Chao suspension cells. Other studies have confirmed that both JA and SA are signal molecules involved in PB90 (a protein elicitor from Phytophthora boehmeriae) induced flavonol glycoside production, and there is a specific interaction between the two (Ullah et al., 2022; Xu and Dong, 2006b). SA can bind to various SABP binding proteins, suggesting that it can mediate different pathways of induced responses in host cells. For example, SA can effectively counteract the damaging effect of excessive ROS on functional molecules (Liu A. et al., 2024), and it can also directly mediate the accumulation of pilocarpine in pilocarpine (Chen et al., 2021b). The endophytic Acinetobacter sp. ALEB16 induces and stimulates medicinal plant Atractylodes lanceolata to synthesize SA, accelerating the accumulation of volatile oils from Atractylodes lanceolata (Wang et al., 2015).
This confirms the molecular mechanism of endophytic elicitors directly inducing the synthesis of volatile oil from Atractylodes macrocephala through the SA signal pathway. In summary, the signal molecules that induce the synthesis of plant secondary metabolites intersect, restrict, and coordinate with each other. This interplay occurs through complex signal networks and influences the expression of related genes, alters the activity of related enzymes, and mediates the synthesis and accumulation process of plant secondary metabolites (Figure 5).
3.3 Transcription factor integration and key gene expression
Transcription factors regulate the co-expression of multiple genes involved in production of secondary metabolites in medicinal plants by activating or inhibiting the transcription process. In the induction mechanism of most endophytic elicitors, signal molecules play a significant role in the secondary metabolism of host plants by bridging the indirect regulation of transcription factors by elicitors (Du et al., 2023). For example, Yang et al. reported that the rapid accumulation of MEJA in Catharanthus roseus cells after induction led to the integration of CrWEKY1, a member of the WRKY family in the metabolic pathway of terpene indole alkaloid precursors. This integration resulted in the overexpression of TDC, STR, and D4H genes, ultimately promoting the synthesis of alkaloid secondary metabolites (Yang et al., 2014). Similarly, under the influence of endogenous fungal elicitors, endophytic fungus NDZKDF13 enhances the gene expression of key enzyme LDC in the corresponding metabolic pathway, ultimately promoting the synthesis and accumulation of OMA in Sophora flavescens (Sun et al., 2018; Namdeo et al., 2002).
In addition, endogenous inducers can directly lead to the rapid accumulation of transcription factors in the AP2/ERF family within the cell, binding cis-elements, and thereby activating gene expression involved in the synthesis of alkaloids. This process ultimately promotes the accumulation of alkaloids (Yue et al., 2022). The relationship between transcription factors and various signaling molecules in the induction of plant secondary metabolites by medicinal plant endophytes remains poorly understood. Further research is necessary to enhance our understanding of the induction mechanisms of endophytes and to explore their potential for secondary metabolite production.
4 Application of endophytic elicitors in accumulation of secondary metabolites in medicinal plants
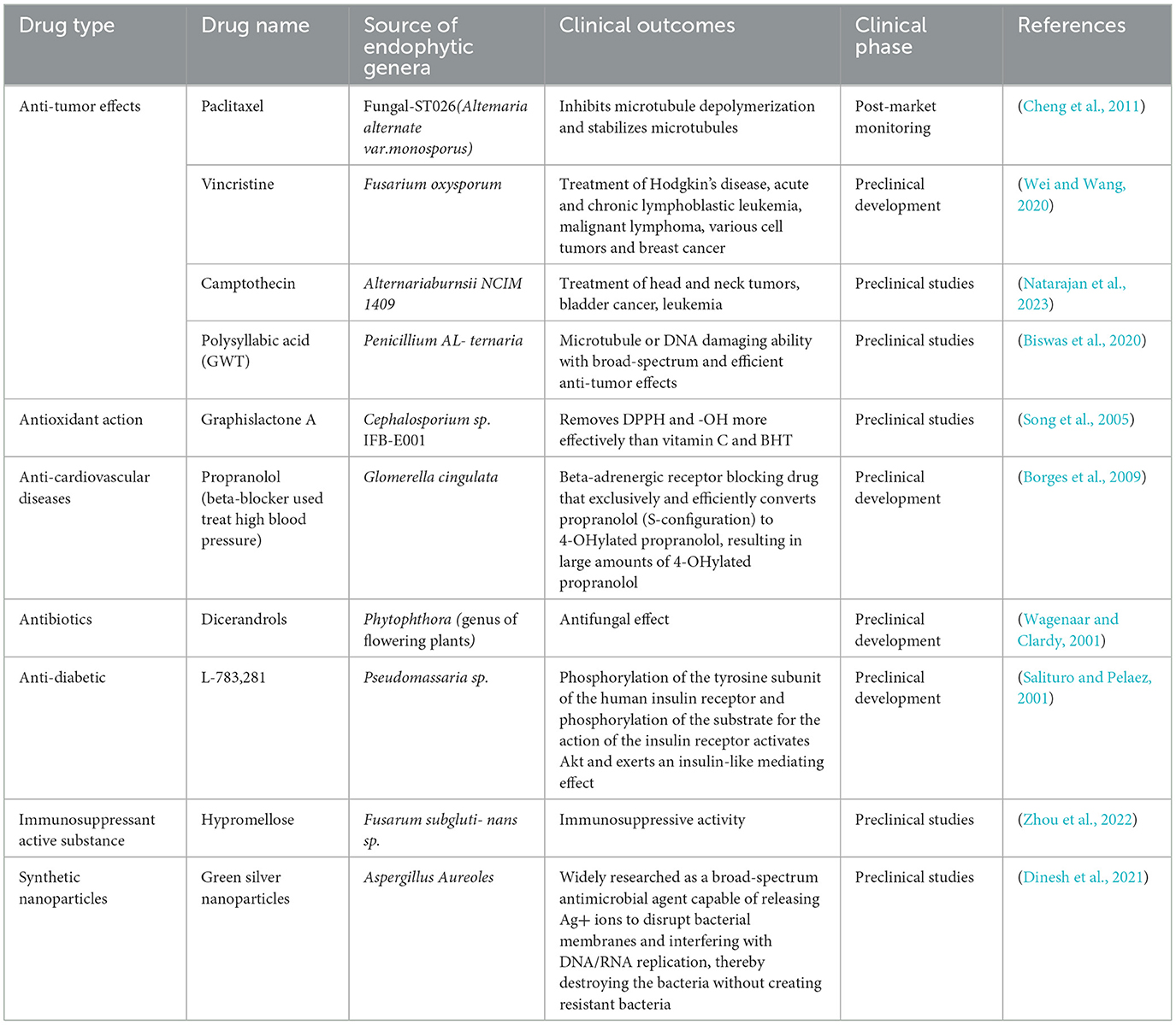
Medical plants are widely used as the main source of natural medicine in various fields. In the field of traditional medicine, such as Chinese medicine, Tibetan medicine, and Indian Buddhist medicine, medicinal plants provide primary healthcare services for approximately 80% of the population in developing countries worldwide (Aye et al., 2019). Furthermore, despite the rapid development of modern medicine a significant number of clinical drugs are still derived from natural products found in medicinal plants (Feng et al., 2024). Notably, some drug metabolites can now be directly obtained from their endophytic microbes and exhibit distinct pharmacological effects. Although endophyte-derived drugs are increasingly being applied at various clinical stages, their development and clinical adoption remain limited (Table 1). Accordingly, it is crucial to regulate the synthesis and accumulation of effective and active components in medicinal plants. In recent years, research on plant microbiology has deepened, leading to the gradual adoption of endophytic elicitors as an important method to enhance the production of secondary metabolites from medicinal plants (Cao X. et al., 2022). This mechanism has been successfully applied to the accumulation of effective active ingredients in various valuable traditional Chinese medicines. The newly synthesized pool of biologically active metabolites includes characteristic metabolites with medical and pharmaceutical potential, such as alkaloids, terpenes, flavonoids, and saponins (Table 2).
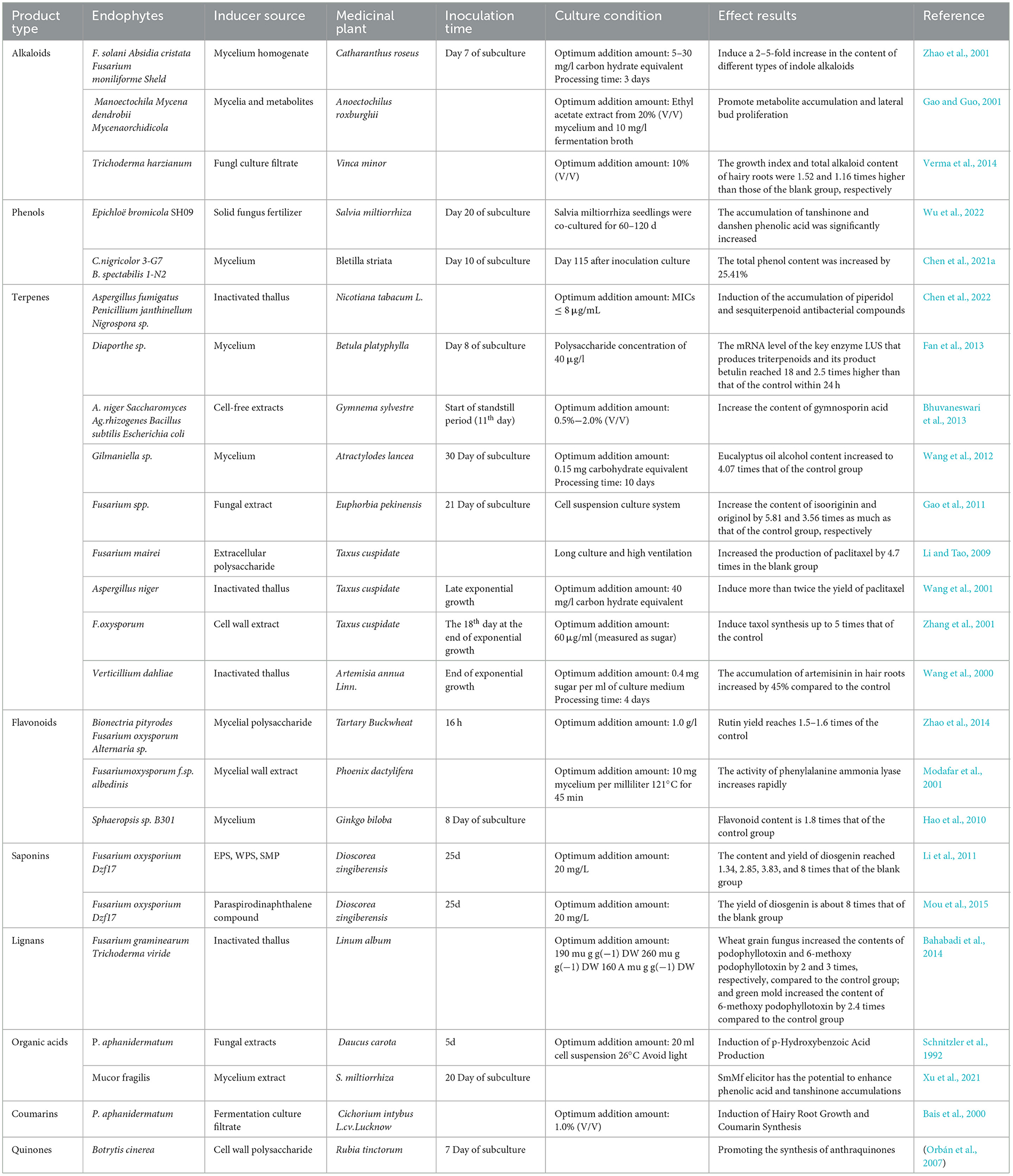
Table 2. Effect of endophytic elicitors on the accumulation of secondary metabolites in medicinal plants.
Endophytic elicitors have become an effective application tool in traditional Chinese medicine research and plant cell culture. For instance, endophytic bacteria obtained from the medicinal plant Euphorbia geniculate can produce alkaloids and terpenes with antibacterial activity (Ikram et al., 2020). Therefore, utilizing an endogenous inducer mechanism to regulate the synthesis of secondary metabolites not only enhances our understanding of the molecular mechanisms underlying plant-microbe interactions but also offers a sustainable solution to the shortage of natural drug resources. Additionally, it modernizes traditional Chinese medicine by establishing a model of green innovation.
5 Prospects
Endophytic fungi, an important biological resource, play a role in both plant growth and enhancing plant resistance in an environmentally friendly manner. However, the widespread application of industrial production of natural drugs using endophytes has not been fully realized yet, and there are still many problems to be solved in the development and application of endophytes. While the application prospects of endophytic bacteria producing secondary metabolites through improved strain methods are promising, there is still a gap between practical applications in large-scale industrial production. Therefore, future research should focus on further exploring the abundant resources of endophytics and their complex relationships with plants to establish them as a high-quality source of new plant metabolites.
Endophytic elicitors induce the synthesis of secondary metabolites in medicinal plants through various complex and diverse mechanisms. These mechanisms are strictly regulated by a range of factors within and outside the cell. Each link represents a specific expression pattern in regulation and metabolism that ultimately translates into a noticeable physiological response, resulting in an increase in secondary metabolites. When studying the regulatory mechanism of endophytic bacteria systematically, it is necessary to fully consider the influencing factors. These factors include the type, concentration, addition time, induction time, and culture system of endophytic elicitors. Additionally, it is necessary to analyze the functional characteristics and interrelationships of each essential element in the interaction process. These elements include specific receptors, signal molecules, transcription factors, key enzymes, and expression genes. Although current research on endophytics as inducers to increase the content of effective components in medicinal plants is still limited, it has been revealed that endophytics are the best source of stimulation for promoting the synthesis and accumulation of effective components in medicinal plants.
The complex interaction between endophytics and host plants means that plants are likely to trigger an innate immune response when recognizing endophytic elicitors (Hacquard et al., 2017). During an immune response, plants respond to this stress stimulus by producing antioxidants and inhibiting their own growth. However, currently, the key factors that enable host plants to successfully activate the induction mechanism during an immune response, the co-evolution mechanism that progressively formed during continuous co-symbiosis between endophytes and host plants, and the repayment mechanism in the evolutionary relationship still need to be explained. Numerous recent studies have confirmed that specific metabolites associated with endophytic genes can participate in plant immune processes through the production of plant hormones such as indoleacetic acid and gibberellin. This discovery provides a reasonable explanation for the coordinated relationship between the induction mechanism and immune response.
Most current research focuses on the interaction between a single endophyte and a plant. However, it is important recognize that in natural ecosystems, the regulation of host plants is commonly achieved through the microbiota of endophytes (Ku et al., 2024). Therefore, more research is needed to explain the assembly process of microbial communities and the impact of microbial communities on host plants. Understanding the interactions among various microbial groups, as well as within them, is a prerequisite for investigating the regulation of endophytic microbial communities. The composition and effectiveness of endophyte communities are controlled and determined by a number of drivers, including the host immune system, host genotype, environmental factors, microbial interactions, and soil and nutrient types. Furthermore, by creating artificially synthetic microbial communities that closely resemble the structural patterns of plant-microbe interactions, it may be possible to explore the intricate relationships between plant-microbe-environment modules through the gradual reconstruction and manipulation of microbial community members and environmental parameters (Chesneau et al., 2025). In order to create more effective synthetic microbial communities, the metabolic modeling of microflora can also be utilized to determine the metabolic activities of various combinations of microorganisms and validate their internal gene expression (Ruan et al., 2024). Therefore, based on the above strategies for constructing synthetic microbial communities, further in-depth investigation of the relationship between endophyte microbiota and host plants will help to accelerate the development and utilization of abundant endophyte resources.
The advancement and maturation of various technologies and disciplines, such as cell biology, molecular genetic methods, metagenomics, and bioinformatics, may provide a more comprehensive explanation of the diverse composition of endophytes in symbiotic environments, their life history, and their interactions with plants. We can extensively screen the diversity and community structure of endophytic microbiomes, design and use a new dual genome-based symbiosis chip tool to study the symbiosis between endophytic bacteria and their host plants (Tai et al., 2023), reveal and explore the physiological and ecological functions of endophytic elicitors in medicinal plants, and fully dissect the complete pathway for inducing events. This will continuously enrich the content of the human drug treasure house, bring strategic improvements, and strengthen the circular and sustainable development of medicinal plant active ingredients with medical and industrial value in medicine, food, agriculture, and forestry.
Author contributions
YW: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. NC: Data curation, Investigation, Resources, Software, Validation, Writing – review & editing. KD: Funding acquisition, Project administration, Writing – review & editing. XZ: Formal analysis, Investigation, Methodology, Resources, Software, Writing – review & editing. ZL: Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. LL: Formal analysis, Investigation, Project administration, Supervision, Writing – original draft. DX: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was financially supported by the National Natural Science Foundation of China (32260089), the Science and Technology Department Foundation of Guizhou Province (QKHJC-MS[2025]371, QKPTRC[2019]-027, and QKHJC-MS[2025]359), the Future Outstanding Teachers Training Program of Zunyi Medical University (XJ2023-JX-01-06), the Undergraduate Education and Teaching Reform Projects of Zunyi Medical University (XJKCSZ2023-9 and XJJG2024-09), and the inaugural Class Advisor Studios at Zunyi Medical University (2024BZR-01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
cPTIO, Carboxy-PTIO; DNA, Deoxyribonucleic acid; D4H, desacetoxyvindoline-4-hydroxylase; EPS, Extracellular Polysaccharide; H2O2, hydrogen peroxide; JAS, jasmonic acids; LDC, lysine decarboxylase; MeJA, Methyl Jasmonate; mRNA, Messenger RNA; NDGA, Nordihydroguaiaretic acid; NO, nitric oxide; Nod, nucleotide-binding oligomerization domain; OMA, oxymatrine; PEPC, Phosphoenolpyruvate carboxykinase; pH, potential of hydrogen; PRR, pattern recognition receptor; PTIO, 2-phenyl-4,4,5,5-tetramethylimidazolineoxyl-1-oxyl-3-oxide; ROS, reactive oxygen species; SA, salicylic acid; SABP, SA-binding protein; SMP, Sodium Hydroxide Extracted Mycelial Polysaccharide; STR, strictosidine synthase; TDC, tryptophan decarboxylase; WPS, Water Extracted Mycelial Polysaccharide; WRKY, WRKY transcription factor.
References
Agarwal, H., Dowarah, B., Baruah, P. M., Bordoloi, K. S., Krishnatreya, D. B., and Agarwala, N. (2020). Endophytes from Gnetum gnemon L. can protect seedlings against the infection of phytopathogenic bacterium Ralstonia solanacearum as well as promote plant growth in tomato. Microbiol. Res. 238:126503. doi: 10.1016/j.micres.2020.126503
Ambrose, K. V., Koppenhöfer, A. M., and Belanger, F. C. (2014). Horizontal gene transfer of a bacterial insect toxin gene into the Epichloë fungal symbionts of grasses. Sci. Rep. 4:5562. doi: 10.1038/srep05562
Ancheeva, E., Daletos, G., and Proksch, P. (2020). Bioactive secondary metabolites from endophytic fungi. Curr. Med. Chem. 27, 1836–1854. doi: 10.2174/0929867326666190916144709
Aye, M. M., Aung, H. T., Sein, M. M., and Armijos, C. (2019). A review on the phytochemistry, medicinal properties and pharmacological activities of 15 selected Myanmar medicinal plants. Molecules 24:293. doi: 10.3390/molecules24020293
Bahabadi, S. E., Sharifi, M., Chashmi, N. A., Murata, J., and Satake, H. (2014). Significant enhancement of lignan accumulation in hairy root cultures of Linum album using biotic elicitors. Acta Physiol. Plant 36:3325. doi: 10.1007/s11738-014-1700-z
Bais, H. P., Govindaswamy, S., and Ravishankar, G. A. (2000). Enhancement of growth and coumarin production in hairy root cultures of witloof chicory (Cichorium intybus L.cv. Lucknow local) under the influence of fungal elicitors. J. Biosci. Bioeng. 90, 648–653. doi: 10.1263/jbb.90.648
Bauer, Z., Gómez-Gómez, L., Boller, T., and Felix, G. (2001). Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. J. Biol. Chem. 276, 45669–45676. doi: 10.1074/jbc.M102390200
Bhuvaneswari, C., Kiranmayee, R., Suryakala, G., and Archana, G. (2013). Improved gymnemic acid production in the suspension cultures of Gymnema sylvestre through biotic elicitation. Plant Biotechnol. Rep. 7:519. doi: 10.1007/s11816-013-0290-3
Biswas, D., Biswas, P., Nandy, S., Mukherjee, A., Pandey, D. K., and Dey, A. (2020). Endophytes producing podophyllotoxin from Podophyllum sp. and other plants: A review on isolation, extraction and bottlenecks. S. Afr. J. Bot. 134, 303–313. doi: 10.1016/j.sajb.2020.02.038
Borges, K. B., Pupo, M. T., and Bonato, P. S. (2009). Enantioselective analysis of propranolol and4-hydroxypropranolol by CE with application to biotransformation studies employing endophytic fungi. Electrophoresis 30, 3910–3917. doi: 10.1002/elps.200900216
Bu, Z., Li, W., Liu, X., Liu, Y., Gao, Y., Pei, G., et al. (2023). The rice endophyte-derived α-Mannosidase ShAM1 degrades host cell walls to activate DAMP-triggered immunity against disease. Microbiol. Spectr. 11:e0482422. doi: 10.1128/spectrum.04824-22
Cao, J. Z., Du, X. W., Li, Q., Guo, L. D., and Yu, D. (2024). Progress in biosynthesis of endophytes in medicinal plants. Zhongnan Phar. 22, 445–452. doi: 10.7539/j.issn.1672-2981.2024.02.026
Cao, L. S., Chen, F., and Dai, C. C. (2022). The effect of grass manipulation and endophyte interaction signals on its active components. J. Agric. Environ. Sci. 41, 2831–2839.
Cao, X., Xu, L., Wang, J., Dong, M., Xu, C., Kai, G., et al. (2022). Endophytic fungus Pseudodidymocyrtis lobariellae KL27 promotes taxol biosynthesis and accumulation in Taxus chinensis. BMC Plant Biol. 22:12. doi: 10.1186/s12870-021-03396-6
Chen, J., Tian, Y., Aijia, L., and Xia, X. (2020). Jasmonic acid signaling and its research progress in woody plants. Sci. Sin. Vitae. 50, 215–226. doi: 10.1360/ssv-2019-0203
Chen, J. H., Li, L. L., Tian, P. W., et al. (2021a). Fungal endophytes from medicinal plant Bletilla striata (Thunb.) Reichb. F. promote the host plant growth and phenolic accumulation. South African J. Botany 143, 25–32. doi: 10.1016/j.sajb.2021.07.041
Chen, J. X., Xia, D. D., Yang, X. Q., Yang, Y. B., and Ding, Z. T. (2022). The antifeedant and antifungal cryptic metabolites isolated from tobacco endophytes induced by host medium and coculture. Fitoterapia 163:105335. doi: 10.1016/j.fitote.2022.105335
Chen, X., Wang, W., Cai, P., et al. (2021b). The role of the MAP kinase-kinase protein StMKK1 in potato immunity to different pathogens. Hortic Res. 8:117. doi: 10.1038/s41438-021-00556-5
Chen, Z. R., Liu, X. Y., Zhao, X. D., Ma, H. Z., and Liang, H. C. (2023). Progress in plant endophyte community composition and their functions. Life Sci. 35, 132–139. doi: 10.13376/j.cbls/2023019
Cheng, L. Y., Zhang, G. H., Sun, Y., Zhao, J. L., Ni, C. Y., and Sun, Y. (2024). Research progress in medicinal plants-endophytes-rhizosphere microbial interactions. Chinese Herbal Med. 55, 5264–5273. doi: 10.7501/j.issn.0253-2670.2024.15.026
Cheng, S. X., Wang, R. H., Chen, J. P., Duan, L. L., and Qiu, X. L. (2011). Isolation purification, and identification of paclitaxel from the fermented extracts of fungus ST026 (Altemaria alternate var. Monosporus). J. China Pharmac. Univ. 42, 570–572. doi: 10.11665/j.issn.1000-5048.20110617
Chesneau, G., Herpell, J., Garrido-Oter, R., and Hacquard, S. (2025). From synthetic communities to synthetic ecosystems: exploring causalities in plant-microbe-environment interactions. New Phytol. 245, 496–502. doi: 10.1111/nph.20250
Choi, H. W., and Klessig, D. F. (2016). DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 16:232. doi: 10.1186/s12870-016-0921-2
Dinesh, B., Chethan, M. U., Pratap, G. K., Poyya, J., Shantaram, M., Hampapura, J. S., et al. (2021). Biogenic synthesis of silver nanoparticles using Aspergillus aureoles (endophyte) and demonstration of their anti-microbial activity. Anal. Chem. Lett. 11, 899–910. doi: 10.1080/22297928.2021.2007789
Ding, C., Wang, S., Li, J., and Wang, Z. (2022). Transcriptomic analysis reveals the mechanism of host growth promotion by endophytic fungus of Rumex gmelinii Turcz. Arch. Microbiol. 204:443. doi: 10.1007/s00203-022-03072-9
Du, Z., Sun, J., Yan, Z., Chen, X., Wang, H., and Zhou, T. (2023). Research progress and application prospects on the mechanism of regulating the synthesis of medicinal components from hairy roots of medicinal plants. J. Chinese Med. Mater. 1, 259–265. doi: 10.13863/j.issn1001-4454.2023.01.045
Ehinger, M., Koch, A. M., and Sanders, I. R. (2009). Changes in arbuscular mycorrhizal fungal phenotypes and genotypes in response to plant species identity and phosphorus concentration. New Phytol. 184, 412–423. doi: 10.1111/j.1469-8137.2009.02983.x
Etalo, D. W., Jeon, J. S., and Raaijmakers, J. M. (2018). Modulation of plant chemistry by beneficial root microbiota. Nat. Prod. Rep. 35, 398–409. doi: 10.1039/C7NP00057J
Fan, G., Zhai, Q., Li, X., and Zhan, Y. (2013). Compounds of Betula platyphylla cell suspension cultures in response to fungal elicitor. Biotechnol. Biotec. Eq. 27:3569. doi: 10.5504/BBEQ.2012.0114
Feng, J., Pan, H. X., and Tang, G. L. (2024). Research advances in biosynthesis of natural product drugs within the past decade. Synthetic Biol. J. 5, 408–446.
Furtado, B. U., Gołebiewski, M., Skorupa, M., Hulisz, P., and Hrynkiewicz, K. (2019). Bacterial and fungal endophytic microbiomes of salicornia europaea. Appl. Environ. Microbiol. 85, e305–e319. doi: 10.1128/AEM.00305-19
Gao, F., Yong, Y., and Dai, C. (2011). Effects of endophytic fungal elicitor on two kinds of terpenoids production and physiological indexes in Euphorbia pekinensis suspension cells. J. Med. Plants Res. 5:4418.
Gao, W., and Guo, S. (2001). Effects of endophytic fungal hyphae and their metabolites on the growth of Dendrobium candidum and Anoectochilus roxburghii. Acta Acad. Med. Sini. 23, 556–569. doi: 10.1007/s11769-001-0027-z
Hacquard, S., Spaepen, S., Garrido-Oter, R., and Schulze-Lefert, P. (2017). Interplay between innate immunity and the plant microbiota. Annu. Rev. Phytopathol. 55, 565–589. doi: 10.1146/annurev-phyto-080516-035623
Hao, G., Du, X., Zhao, F., and Ji, H. (2010). Fungal endophytes-induced abscisic acid is required for flavonoid accumulation in suspension cells of Ginkgo biloba. Biotechnol. Lett. 32, 305–314. doi: 10.1007/s10529-009-0139-6
Harbort, C. J., Hashimoto, M., Inoue, H., Niu, Y., Guan, R., Rombolà, A. D., et al. (2020). Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis. Cell Host Microbe 28, 825–837. doi: 10.1016/j.chom.2020.09.006
Hong, L., Wang, Q., Zhang, J., Chen, X., Liu, Y., Asiegbu, F. O., et al. (2024). Advances in the beneficial endophytic fungi for the growth and health of woody plants. For. Res. 20:e028. doi: 10.48130/forres-0024-0025
Ikram, M., Ali, N., Jan, G., Jan, F. G., and Khan, N. (2020). Endophytic fungal diversity and their interaction with plants for agriculture sustainability under stressful condition. Recent Pat. Food Nutr. Agric. 11, 115–123. doi: 10.2174/2212798410666190612130139
Jedelská, T., Luhová, L., and Petrivalský, M. (2021). Nitric oxide signalling in plant interactions with pathogenic fungi and oomycetes. J. Exp. Bot. 72, 848–863. doi: 10.1093/jxb/eraa596
Johri, A. K., Oelmüller, R., Dua, M., Yadav, V., Kumar, M., Tuteja, N., et al. (2015). Fungal association and utilization of phosphate by plants: success, limitations, and future prospects. Front. Microbiol. 6:984. doi: 10.3389/fmicb.2015.00984
Jones, K. M., Kobayashi, H., Davies, B. W., Taga, M. E., and Walker, G. C. (2007). How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat. Rev. Microbiol. 5, 619–633. doi: 10.1038/nrmicro1705
Ku, Y. S., Liao, Y. J., Chiou, S. P., Lam, H. M., and Chan, C. (2024). From trade-off to synergy: microbial insights into enhancing plant growth and immunity. Plant Biotechnol. J. 22, 2461–2471. doi: 10.1111/pbi.14360
Kusari, S., Zühlke, S., and Spiteller, M. (2011). Effect of artificial reconstitution of the interaction between the plant Camptotheca acuminata and the fungal endophyte Fusarium solani on camptothecin biosynthesis. J. Nat. Prod. 74, 764–775. doi: 10.1021/np1008398
Li, C., Yuan, Y., Ma, Z., and Hu, Z. (2002). Changes of physiological state of suspension cultures of taxus chenensis var. mairei induced by oligosaccharide. Huagong Xuebao 53, 1133–1138. doi: 10.3321/j.issn:0438-1157.2002.11.007
Li, M., Liu, X., Wu, F., Shi, X., Kong, D., Li, X., et al. (2024). Fermentation broth of a novel endophytic fungus enhanced maize salt tolerance by regulating sugar metabolism and phytohormone biosynthesis or signaling. Plant Physiol. Biochem. 216:109125. doi: 10.1016/j.plaphy.2024.109125
Li, P., Mou, Y., Shan, T., Xu, J., Li, Y., Lu, S., et al. (2011). Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgenin production in cell suspension culture of Dioscorea zingiberensis. Molecules 16, 9003–9016. doi: 10.3390/molecules16119003
Li, Q., He, Y., Feng, J., He, Y., and Zhang, S. (2025). Pseudomonas fluorescens inoculation enhances salix matsudana growth by modifying phyllosphere microbiomes, surpassing nitrogen fertilization. Plant Cell Environ. 48, 599–614. doi: 10.1111/pce.15162
Li, Y., and Tao, W. (2009). Effects of paclitaxel-producing fungal endophytes on growth and paclitaxel formation of Taxus cuspidata cells. Plant Growth Regul. 58:97. doi: 10.1007/s10725-008-9355-7
Li, Z., Wen, W., Qin, M., He, Y., Xu, D., and Li, L. (2022). Biosynthetic mechanisms of secondary metabolites promoted by the interaction between endophytes and plant hosts. Front. Microbiol. 13:928967. doi: 10.3389/fmicb.2022.928967
Limpens, E., Franken, C., Smit, P., Willemse, J., Bisseling, T., and Geurts, R. (2003). LysM domain receptor kinases regulating rhizobial Nod factorinduced infection. Science. 302, 630–633. doi: 10.1126/science.1090074
Liu, A., Wang, M., Dong, J., Yan, Z., Wang, X., Li, J., et al. (2024). Foliar application of exogenous salicylic acid mitigates the detrimental effects caused by salt stress in sunflower seedlings. Ind. Crops Prod. 222, 119854–119854. doi: 10.1016/j.indcrop.2024.119854
Liu, S., Wang, Y., He, Y., Li, L., Fang, S., and Zhang, H. (2021). Research progress on the effect of endophytic fungi on the growths and secondary metabolites of host plants. J. Tianjin Univ. Tradit. Chinese Med. 40, 128–136. doi: 10.11656/j.issn.1673-9043.2021.01.24
Liu, Y. H., Nie, J. L., and Pei, Y. (2024). A review on the role of inducers on the accumulation of secondary metabolites in medicinal plants. Tianjin Agric. Forest. Sci. Technol. 20, 39–42. doi: 10.3969/j.issn.1002-0659.2024.05.012
Liu, Z., Beskrovnaya, P., Melnyk, R. A., Hossain, S. S., Khorasani, S., O'Sullivan, L. R., et al. (2018). A genome-wide screen identifies genes in rhizosphere-associated Pseudomonas required to evade plant defenses. MBio 9, e00433–e00418. doi: 10.1128/mBio.00433-18
Liu, Z., Yang, J., Long, Y., Zhang, C., Wang, D., Zhang, X., et al. (2023). Single-nucleus transcriptomes reveal spatiotemporal symbiotic perception and early response in Medicago. Nat Plants 9, 1734–1748. doi: 10.1038/s41477-023-01524-8
Lv, J., Yang, S., Zhou, W., Liu, Z., Tan, J., and Wei, M. (2024). Microbial regulation of plant secondary metabolites: impact, mechanisms and prospects. Microbiol. Res. 283:127688. doi: 10.1016/j.micres.2024.127688
Lv, Z. Y., Sun, W. J., Jiang, R., Chen, J. F., Ying, X., Chen, W. S., et al. (2021). Phytohormones jasmonic acid, salicylic acid, gibberellins, and abscisic acid are key mediators of plant secondary metabolites. World J. Tradit. Chinese Med. 7:307. doi: 10.4103/wjtcm.wjtcm_20_21
Mao, J., Xiong, X., and Lu, Y. (2021). Advances in the regulation of plant stress response by jasmonic acid. Chinese J. Bioproc. Eng. 19:8. doi: 10.3969/j.issn.1672-3678.2021.04.008
Mengistu, A. A. (2020). Endophytes: colonization, behaviour, and their role in defense mechanism. Int. J. Microbiol. 2020:6927219. doi: 10.1155/2020/6927219
Miyata, K., Hayafune, M., Kobae, Y., Kaku, H., Nishizawa, Y., Masuda, Y., et al. (2016). Evaluation of the role of the LysM receptor-like kinase, OsNFR5/OsRLK2 for AM symbiosis in Rice. Plant Cell Physiol. 57, 2283–2290. doi: 10.1093/pcp/pcw144
Modafar, C. E., Tantaoui, A., and Boustani, E. S. (2001). Differential induction of phenylalanine ammonia-lyase activity in date palm roots in response to inoculation with Fusarium oxysporum f. sp. albedinis and elicitation with fungal wall elicitor. J. Plant Physiol. 158, 715–722. doi: 10.1078/0176-1617-00258
Mou, Y., Zhou, K., Xu, D., Yu, R., Li, J., Yin, C., et al. (2015). Enhancement of diosgenin production in plantlet and cell cultures of Dioscorea zingiberensis by palmarumycin C13 from the endophytic fungus, Berkleasmium sp. Dzf12. Trop. J. Pharm. Res. 14:241. doi: 10.4314/tjpr.v14i2.8
Namdeo, A., Patil, S., and Fulzele, D. P. (2002). Influence of fungal elicitors on production of ajmalicine by cell cultures of Catharanthus roseus. Biotechnol. Prog. 18, 159–162. doi: 10.1021/bp0101280
Natarajan, S., Pucker, B., and Srivastava, S. (2023). Genomic and transcriptomic analysis of camptothecin producing novel fungal endophyte: Alternaria burnsii NCIM 1409. Sci. Rep. 13:14614. doi: 10.1038/s41598-023-41738-6
Ning, W., Tao, Y., and Cao, R. (1996). Effect of comfrey pigment inducers from Aspergillus oryzae on the cellular metabolism of Compositae dianthus. Plant Physiol. J. 22, 74–80.
Orbán, N., Boldizsár, I., Szűcs, Z., and Dános, B. (2007). Influence of different elicitors on the synthesis of anthraquinone derivatives in Rubia tinctorum L. cell suspension cultures. Dyes Pigments 77, 249–257. doi: 10.1016/j.dyepig.2007.03.015
Pang, Z., Chen, J., Wang, T., Gao, C., Li, Z., Guo, L., et al. (2021). Linking plant secondary metabolites and plant microbiomes: a review. Front. Plant Sci. 2:621276. doi: 10.3389/fpls.2021.621276
Qi, W., Sun, W., and Ma, L. (2021). Research progress of reactive oxygen speies involved in regulating plant growth and development and the mechanisms of stress response. Agric. Res. Arid Areas 39:14. doi: 10.7606/j.issn.1000-7601.2021.03.09
Qu, Z., Zhang, H., Wang, Q., Zhao, H., Liu, X., Fu, Y., et al. (2021). Exploring the symbiotic mechanism of a virus mediated endophytic fungus in its host by dual unique molecular identifier–RNA sequencing. mSystems 6, e00814–e00821. doi: 10.1128/msystems.00814-21
Ran, Q., Dong, C., Zhang, Q., Shao, Q., Zhang, Y., Long, X., et al. (2024). Seed endophytes reshape rhizosphere microbiota to promote the growth of Eucommia ulmoides seedlings. Appl, Soil Ecol, 201, 105487–105487. doi: 10.1016/j.apsoil.2024.105487
Raza, W., Wang, J., Jousset, A., Friman, V. P., Mei, X., Wang, S., et al. (2020). Bacterial community richness shifts the balance between volatile organic compound-mediated microbe–pathogen and microbe–plant interactions. Proc. Biol. Sci. 287:20200403. doi: 10.1098/rspb.2020.0403
Ren, C. (2014). Study on the signaling mechanism of endophytic bacteria promoting volatile oil accumulation in the medicinal plant Atractylodes macrocephala. Nanjing Normal Univ. 1, 1–119.
Ruan, Z., Chen, K., Cao, W., Meng, L., Yang, B., Xu, M., et al. (2024). Engineering natural microbiomes toward enhanced bioremediation by microbiome modeling. Nat. Commun. 15:4694. doi: 10.1038/s41467-024-49098-z
Rudrappa, T., Czymmek, K. J., Paré, P. W, and Bais, H. P. (2008). Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 148, 1547–1556. doi: 10.1104/pp.108.127613
Salituro, C. M., and Pelaez, F. (2001). Discovery of a small molecule insulin neceptor activator. Recent Prog. Horm. Res. 56, 107–126. doi: 10.1210/rp.56.1.107
Santoyo, G. (2022). How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 40, 45–58. doi: 10.1016/j.jare.2021.11.020
Schenkel, D., Deveau, A., Niimi, J., Mariotte, P., Vitra, A., Meisser, M., et al. (2019). Linking soil's volatilome to microbes and plant roots highlights the importance of microbes as emitters of belowground volatile signals. Environ. Microbiol. 21, 3313–3327. doi: 10.1111/1462-2920.14599
Schnitzler, J. P., Madlung, J., Rose, A., and Ulrich Seitz, H. (1992). Biosynthesis of p-hydroxybenzoic acid in elicitor-treated carrot cell cultures. Planta 188, 594–600. doi: 10.1007/BF00197054
Shen, L., Li, L. Y., Zhang, X. J., Li, M., and Song, Y. C. (2015). A new indole derivative from endophyte Myrothecium roridum IFB-E091 in Artemisia annua. Acta Pharm Sin 50, 1305–1308.
Singh, S., Apoorva, n., Saha, P., Rai, N., Kumari, S., and Pandey-Rai, S. (2023). Unravelling triterpenoid biosynthesis in plants for applications in bioengineering and large-scale sustainable production. Ind. Crops Prod. 199:116789. doi: 10.1016/j.indcrop.2023.116789
Song, X., Liu, S. J., He, L. W., and Meng, X. C. (2023). Study on the regulation of secondary metabolism and mass formation of gentian caused by exogenous NO. Chinese Herbal Med. 54, 5716–5724. doi: 10.7501/j.issn.0253-2670.2023.17.024
Song, Y. C., Huang, W. Y., Sun, C., Wang, F. W., and Tan, R. X. (2005). Characterization of graphislactone a as the antioxidant and free radical-scavenging substance from the culture of Cephalosporium sp. 1FB-E001, an endophytic fungus in Trachelospermum jasminoides. Biol. Pharm. Bull. 28, 506–509. doi: 10.1248/bpb.28.506
Sun, M., Zhang, Q., Hu, L., Li, W. X., Yan, S. Y., Lv, M., et al. (2018). Fluorescence quantitative PCR assay of endophytic fungal inducers of bitter bean seeds to promote gene expression of key enzymes for host alkaloid synthesis. Chinese Tradit. Herbal Drugs 49, 4621–4627. doi: 10.7501/j.issn.0253-2670.2018.19.024
Sun, Y., Wang, Y., Zhang, X., Chen, Z., Xia, Y., Wang, L., et al. (2022). Plant receptor-like protein activation by a microbial glycoside hydrolase. Nature 610, 335–342. doi: 10.1038/s41586-022-05214-x
Tai, C., Zhang, W., Xu, J., Ou, Y. X., and Luo, Q. (2023). Simultaneous rapid identification of blaKPC-coding carbapenemase- producing Klebsiella pneumoniae by duplex chip digital PCR. Microbiol. China 50, 3058–3072. doi: 10.13344/j.microbiol.china.220935
Thoms, D., Liang, Y., and Haney, C. H. (2021). Maintaining symbiotic homeostasis: how do plants engage with beneficial microorganisms while at the same time restricting pathogens? Mol. Plant Microbe Interact. 34, 462–469. doi: 10.1094/MPMI-11-20-0318-FI
Tian, P., and Nan, Z. B. (2017). Signaling in the mutualistic symbiotic interaction between endophytes and their hosts. Acta Pratac. Sinica 26, 196–210. doi: 10.11686/cyxb2016176
Tian, Y., Amand, S., Buisson, D., Kunz, C., Hachette, F., Dupont, J., et al. (2014). The fungal leaf endophyte Paraconiothyrium variabile specifically metabolizes the host-plant metabolome for its own benefit. Phytochemistry 108, 95–101. doi: 10.1016/j.phytochem.2014.09.021
Tran, T., French, E., and Iyer-Pascuzzi, A. S. (2022). In vitro functional characterization predicts the impact of bacterial root endophytes on plant growth. J. Exp. Bot. 73, 5758–5772. doi: 10.1093/jxb/erac228
Ullah, C., Schmidt, A., Reichelt, M., Tsai, C. J., and Gershenzon, J. (2022). Lack of antagonism between salicylic acid and jasmonate signalling pathways in poplar. New Phytol. 235, 701–717. doi: 10.1111/nph.18148
Vasconsuelo, A., and Boland, R. (2007). Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 172, 861–875. doi: 10.1016/j.plantsci.2007.01.006
Verma, P., Khan, S. A., Mathur, A. K., Shanker, K., and Kalra, A. (2014). Fungal endophytes enhanced the growth and production kinetics of Vinca minor hairy roots and cell suspensions grown in bioreactor. Plant Cell Tiss Org. 118:257. doi: 10.1007/s11240-014-0478-4
Voges, M. J. E. E.E., Bai, Y., Schulze-Lefert, P., and Sattely, E. S. (2019). Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc. Natl. Acad. Sci. USA. 116, 12558–12565. doi: 10.1073/pnas.1820691116
Wagenaar, M. M., and Clardy, J. (2001). Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint. J. Nat. Prod. 64, 1006–1009. doi: 10.1021/np010020u
Wang, C., Wu, J., and Mei, X. (2001). Enhancement of Taxol production and excretion in Taxus chinensis cell culture by fungal elicitation and medium renewal. Appl. Microbiol. Biotechnol. 55, 404–410. doi: 10.1007/s002530000567
Wang, H., Luo, H., and Sun, M. (2004). Application of elicitor to cell culture of medicinal plants. Chinese Tradit. Herbal Drugs 35, 3–7. doi: 10.7501/j.issn.0253-2670.2004.8.448
Wang, H., Ye, H., Li, G., and Liu, B. Y. (2000). Effects of fungal elicitors on cell growth and artemisinin accumulation in hairy root cultures of Artemisia annua. Bull. Botany 42, 905–909. Available online at: https://www.jipb.net/EN/Y2000/V42/I9/905
Wang, L., Xia, Y., and Hou, Y. (2024). Candy or poison: plant metabolites as swing factors against microbes. Mol. Plant 17, 1341–1343. doi: 10.1016/j.molp.2024.08.005
Wang, L., Yu, Z., Guo, X., Huang, J. P., Yan, Y., Huang, S. X., et al. (2021). Bisaspochalasins D and E: two heterocycle-fused cytochalasan homodimers from an endophytic Aspergillus flavipes. J. Org. Chem. 86, 11198–11205. doi: 10.1021/acs.joc.1c00425
Wang, S., Bi, Y., Quan, W., and Christie, P. (2022). Growth and metabolism of dark septate endophytes and their stimulatory effects on plant growth. Fungal Biol. 126, 674–686. doi: 10.1016/j.funbio.2022.08.006
Wang, X. M., Yang, B., Ren, C. G., Wang, H. W., Wang, J. Y., and Dai, C. C. (2015). Involvement of abscisic acid and salicylic acid in signal cascade regulating bacterial endophyte-induced volatile oil biosynthesis in plantlets of Atractylodes lancea. Physiol. Plant. 153, 30–42. doi: 10.1111/ppl.12236
Wang, X. Y., Zhu, N. N., Yang, J. S., Zhou, D., Yuan, S. T., Pan, X. J., et al. (2024). CwJAZ4/9 negatively regulates jasmonate-mediated biosynthesis of terpenoids through interacting with CwMYC2 and confers salt tolerance in Curcuma wenyujin. Plant Cell Environ. 47, 3090–3110. doi: 10.1111/pce.14930
Wang, Y., Dai, C., Cao, J., and Xu, D. (2012). Comparison of the effects of fungal endophyte Gilmaniella sp. and its elicitor on Atractylodes lancea plantlets. World J. Microbiol. Biotechnol. 28, 575–584. doi: 10.1007/s11274-011-0850-z
Wei, X., and Wang, X. L. (2020). Optimization in fermentation conditions of vincristine-producing Fusarium oxysporum. Chem. Bioeng. 37, 40–45.
Wróbel-Kwiatkowska, M., Osika, A., Liszka, J., Lipiński, M., Dymińska, L., Piegza, M., et al. (2024). The impact of a non-pathogenic strain of Fusarium oxysporum on structural and biochemical properties of flax suspension cultures. Int. J. Mol. Sci. 25:9616. doi: 10.3390/ijms25179616
Wu, H., Wan, L., Liu, Z., Jian, Y., Zhang, C., Mao, X., et al. (2024). Author Correction: Mechanistic study of SCOOPs recognition by MIK2-BAK1 complex reveals the role of N-glycans in plant ligand-receptor-coreceptor complex formation. Nat. Plants 10:2079. doi: 10.1038/s41477-024-01879-6
Wu, S. J., Xie, X. G., Yang, Y., Zhen, C. J., and Han, T. (2022). Effects of endophytic fungus SH09 on plant growth and accumulation of active components in Salvia miltiorrhiza. J. Pharmac. Pract. Serv. 40, 213–217. doi: 10.12206/j.issn.1006-0111.202108055
Xia, Y., Liu, J., Chen, C., Mo, X., Tan, Q., He, Y., et al. (2022). The multifunctions and future prospects of endophytes and their metabolites in plant disease management. Microorganisms 10:1072. doi: 10.3390/microorganisms10051072
Xu, M., and Dong, J. (2006a). Nitric oxide mediates the fungal elicitor-induced taxol biosynthesis of Taxus chinensis suspension cells through the reactive oxygen species-dependent and -independent signal pathways. Chine. Sci. Bull. 51, 1675–1682. doi: 10.1007/s11434-006-2081-5
Xu, M., and Dong, J. (2006b). NO can mediate the promotion of puerarin biosynthesis in powdered kudzu suspension cells by fungal inducers through salicylic acid or jasmonic acid signaling pathways. Sci. Sin. 36, 66–75. doi: 10.3969/j.issn.1674-7232.2006.01.009
Xu, W., Jin, X., Yang, M., Xue, S., Luo, L., Cao, X., et al. (2021). Primary and secondary metabolites produced in Salvia miltiorrhiza hairy roots by an endophytic fungal elicitor from Mucor fragilis. Plant Physiol. Biochem. 160, 404–412. doi: 10.1016/j.plaphy.2021.01.023
Yan, K., Liu, J., Wei, Q., and Xie, H. (2019). Study on the accumulation of volatile oil in Cinnamomum camphora cells mediated by endophytic fungal elicitors via MeJA. Tea In Fujian 41, 6–7.
Yang, Z., Chen, Z., and Li, R. (2014). Jasmonates-induced transcriptional machineries of alkanoid metabolism in Catharanthus roseus. Chinese J. Biochem. Molec. Biol. 30, 533–542. doi: 10.13865/j.cnki.cjbmb.2014.06.003
Yipare, P., Zulihumaer, R., Tian, Y., Zhu, Y. L., Li, Y. T., and Ma, X. L. (2022). Research progress in diversity of endophytes microbial communities isolated from desert plants and their strengthening effects on drought and salt tolerance in crops. Biotechnol. Bull. 38, 88–99. doi: 10.13560/j.cnki.biotech.bull.1985.2021-1525
Yu, M., and Facchini, J. P. (2000). Molecular cloning and characterization of a type III glutathione S-transferase from cell suspension cultures of opium poppy treated with a fungal elicitor. Physiol. Plant. 108, 101–109. doi: 10.1034/j.1399-3054.2000.108001101.x
Yue, M. F., Zhang, C., and Wu, Z. Y. (2022). Research progress in the structural and functional analysis of plant transcription factor AP2/ERF protein family. Biotechnol. Bull. 38, 11–26. doi: 10.13560/j.cnki.biotech.bull.1985.2022-0432
Zhang, C., Li, C., Yuan, Y., Sun, A. C., and Hu, C. (2001). Effects of fungal elicitor on cell status and taxol production in cells suspension cultures of TaxUs chinesis var.mairei. Chin. J. Biotechnol. 17, 436–440. doi: 10.3321/j.issn:1000-3061.2001.04.018
Zhang, H. (2022). Screening and bioinformatics analysis of Huperzine A produced endophytic fungi from Huperzia serrata. Northwest Univ. 2, 1–87.
Zhang, H., Liu, M., Liu, X, Li, Z. Y., Zhao, L. L., and Yang, Q. X. (2022). Impact of endophytic microorganisms on the pharmaco-active compounds production in medicinal plants: a review. Biotechnol. Bull. 38, 41–51. doi: 10.13560/j.cnki.biotech.bull.1985.2021-1487
Zhang, L., Zhang, C., He, F., Zhao, X., Qi, H., Wan, B., et al. (2015). Research progress on the interaction effects and its neural mechanisms between physical fatigue and mental fatigue. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 32, 1135–1140.
Zhang, Y., Yang, X., Liu, Q., Qiu, D., Zhang, Y., Zeng, H., et al. (2010). Purification of novel protein elicitor from Botrytis cinerea that induces disease resistance and drought tolerance in plants. Microbiol. Res. 165, 142–151. doi: 10.1016/j.micres.2009.03.004
Zhao, J., Zhu, W., and Hu, Q. (2001). Selection of fungal elicitors to increase indole alkaloid accumulation in catharanthus roseus suspension cell culture. Enzyme Microb. Technol. 28, 666–672. doi: 10.1016/S0141-0229(01)00309-X
Zhao, J., Zou, L., Zhang, C., Peng, L., Xiao, W., Zhao, G., et al. (2014). Efficient promotion of the sprout growth and rutin production of tartary buckwheat by associated fungal endophytes. Cereal Res. Commun. 42:401. doi: 10.1556/CRC.2013.0068
Zhao, Y., Liu, G., Yang, F., Liang, Y., Gao, Q., Xiang, C., et al. (2023). Multilayered regulation of secondary metabolism in medicinal plants. Mol. Hortic 3:11. doi: 10.1186/s43897-023-00059-y
Zheng, L. P., Tian, H., Yuan, Y. F., and Wang, J. W. (2016). The influence of endophytic Penicillium oxalicum B4 on growth and artemisinin biosynthesis of in vitro propagated plantlets of Artemisia annua L. Plant Growth Regul. 80, 93–102. doi: 10.1007/s10725-016-0162-2
Zhou, Y. B., Mai, M. Q., and Huang, Z. W. (2022). Research progress of medicinal plant endophytes in natural drug development. Xiandai Horticulture 45, 170–172. doi: 10.3969/j.issn.1006-4958.2022.18.060
Keywords: endophytic, secondary metabolite, biosynthesis, biological mechanism, medicinal plant
Citation: Wang Y, Chen N, Deng K, Zhong X, Li Z, Li L and Xu D (2025) Effects and molecular mechanism of endophytic elicitors on the accumulation of secondary metabolites in medicinal plants. Front. Microbiol. 16:1558567. doi: 10.3389/fmicb.2025.1558567
Received: 10 January 2025; Accepted: 24 April 2025;
Published: 02 June 2025.
Edited by:
Marcelino Gutiérrez, Instituto de Investigaciones Científicas y Servicios de Alta Tecnología, PanamaReviewed by:
Siamak Farhadi, Seed and Plant Improvement Institute, IranAsha Chaubey, Indian Institute of Integrative Medicine (CSIR), India
Ping Zhu, Institute of Materia Medica, China
Copyright © 2025 Wang, Chen, Deng, Zhong, Li, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delin Xu, eHVkZWxpbjIwMDBAMTYzLmNvbQ==; Lin Li, bGlsaW56bWMyMDE1QDE2My5jb20=; Kuanping Deng, NDA4MzY3NDYyQHFxLmNvbQ==
†These authors share first authorship
 Yaxuan Wang1†
Yaxuan Wang1† Nana Chen
Nana Chen Zhaogao Li
Zhaogao Li Lin Li
Lin Li Delin Xu
Delin Xu