- 1Microbiome-X, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Jinan Institute of Child Health Care, Children’s Hospital Affiliated to Shandong University (Jinan Children’s Hospital), Jinan, China
- 3School of Biological Science and Technology, University of Jinan, Jinan, China
Precocious puberty (PP) is the second most common pediatric endocrine disorder globally and poses a growing public health concern, particularly among girls. While the exact biological mechanisms underlying PP remain unclear, unhealthy dietary patterns, particularly the consumption of a high-fat diet (HFD), are recognized as significant modifiable risk factors. The gut microbiota (GM) is an environmental factor that is disrupted by HFD and may modulate the onset and progression of PP. This review explored the intricate relationship between HFD, GM, and PP, and elucidated the potential mechanisms by which HFD may promote PP development by summarizing evidence from preclinical to clinical research, focusing on the role of GM and its derived metabolites, including short-chain fatty acids, bile acids, lipopolysaccharides, and neurotransmitters. Mechanistic exploration provides novel insights for developing microbiota-targeted therapeutic strategies, such as dietary and lifestyle interventions, fecal microbiota transplantation, probiotics, and traditional Chinese medicine, paving the way for promising approaches to prevent and manage PP.
1 Introduction
Precocious puberty (PP) is characterized by the premature emergence of secondary sexual characteristics—before age 8 in girls and 9 in boys (Alghamdi and Alghamdi, 2023). PP can be classified into two main categories considering the underlying pathogenetic mechanism: central PP (CPP) and peripheral PP (PPP). CPP is caused by early activation of the hypothalamic–pituitary–gonadal (HPG) axis, leading to the development of both primary and secondary sexual characteristics. This form accounts for approximately 80% of PP cases. In contrast, PPP results from factors that elevate steroid hormone levels to those typical of puberty, independent of gonadotropin secretion (For detailed discussions on the etiology, diagnosis, and treatment of both subtypes, see the review; Cheuiche et al., 2021). Notably, the incidence of PP in girls is markedly higher than in boys, with a rate 15 to 20 times greater, and its global prevalence continues to rise (Shim et al., 2021; Park et al., 2021). The onset of PP results from a complex interaction of genetic, dietary, environmental, and lifestyle factors. Among these, a high-fat diet (HFD) has emerged as a modifiable risk factor that has garnered significant attention.
With advancements in microbiomics, the gut microbiota (GM) and its derived metabolites have been increasingly recognized as important molecules in the gut-organ crosstalk, exerting either beneficial or detrimental effects on various extra-intestinal organs. Mounting evidence indicates various perturbations in GM associated with PP. Notably, excessive fat consumption in HFD results in consequences such as gut dysbiosis, gut barrier dysfunction, emphasizing GM’s potential mediating role in the pathogenesis of PP induced by HFD. In this review, we summarized studies exploring the relationship between HFD, GM, and PP and specifically discussed the direct effects of HFD on PP and its indirect effects mediated by GM and its metabolites. Finally, we reviewed the potential of GM regulation as a strategy to mitigate or manage PP, offering valuable insights for future research and therapeutic interventions.
2 HFD promotes PP
HFD significantly disrupts neuroendocrine and metabolic homeostasis, serving as a critical environmental determinant in the pathogenesis of PP. Robust epidemiological studies have revealed differential effects of HFD components on pubertal timing (Supplementary Table 1; Chen et al., 2024; Gu et al., 2024; Günther et al., 2010; Xiong et al., 2022; Du et al., 2024; Cheng et al., 2021b; Xu et al., 2022; Cheng et al., 2021a; Shahatah et al., 2021; Nguyen et al., 2020; Jansen et al., 2016; Mueller et al., 2015; Carwile et al., 2015; Mervish et al., 2013; Wiley, 2011; Rogers et al., 2010; Koo et al., 2002; Berkey et al., 2000; Maclure et al., 1991; Merzenich et al., 1993; Szamreta et al., 2020). Animal protein intake is consistently associated with accelerated pubertal development, manifesting as premature onset of thelarche, enhanced growth velocity, and earlier menarche (Gu et al., 2024; Günther et al., 2010; Cheng et al., 2021b; Rogers et al., 2010; Berkey et al., 2000). Conversely, milk’s effects remain controversial, with studies reporting negative (Du et al., 2024; Wiley, 2011), positive (Wiley, 2011), or no significant associations (Carwile et al., 2015) with early pubertal onset, potentially due to genetic backgrounds, study design, dietary intake assessment, and temporal context. These inconsistencies highlight the need for future studies to control confounding variables more rigorously to derive precise conclusions.
Regarding dietary lipids, polyunsaturated fatty acids (PUFAs)—essential steroidogenesis precursors—exhibited a dose-dependent positive correlation with earlier pubertal onset and menarche in girls (Xu et al., 2022; Cheng et al., 2021a; Nguyen et al., 2020; Rogers et al., 2010), whereas monounsaturated fatty acids (MUFAs) demonstrated inhibitory effects (Nguyen et al., 2020). Additionally, plant-derived components such as flavonoids, soy, and dietary fiber were associated with delayed pubertal timing (Xiong et al., 2022; Nguyen et al., 2020; Mervish et al., 2013), suggesting potential preventive strategies through reduced animal protein/dietary fat consumption and increased plant-based diet intake.
Human studies primarily provided observational evidence, but preclinical research established direct causality, demonstrating that maternal or female offspring exposure to HFD significantly accelerated vaginal opening (VO), a key marker of puberty onset, and gonadal maturation (Bo et al., 2022; Wang et al., 2020). The underlying mechanisms are illustrated in Figure 1.
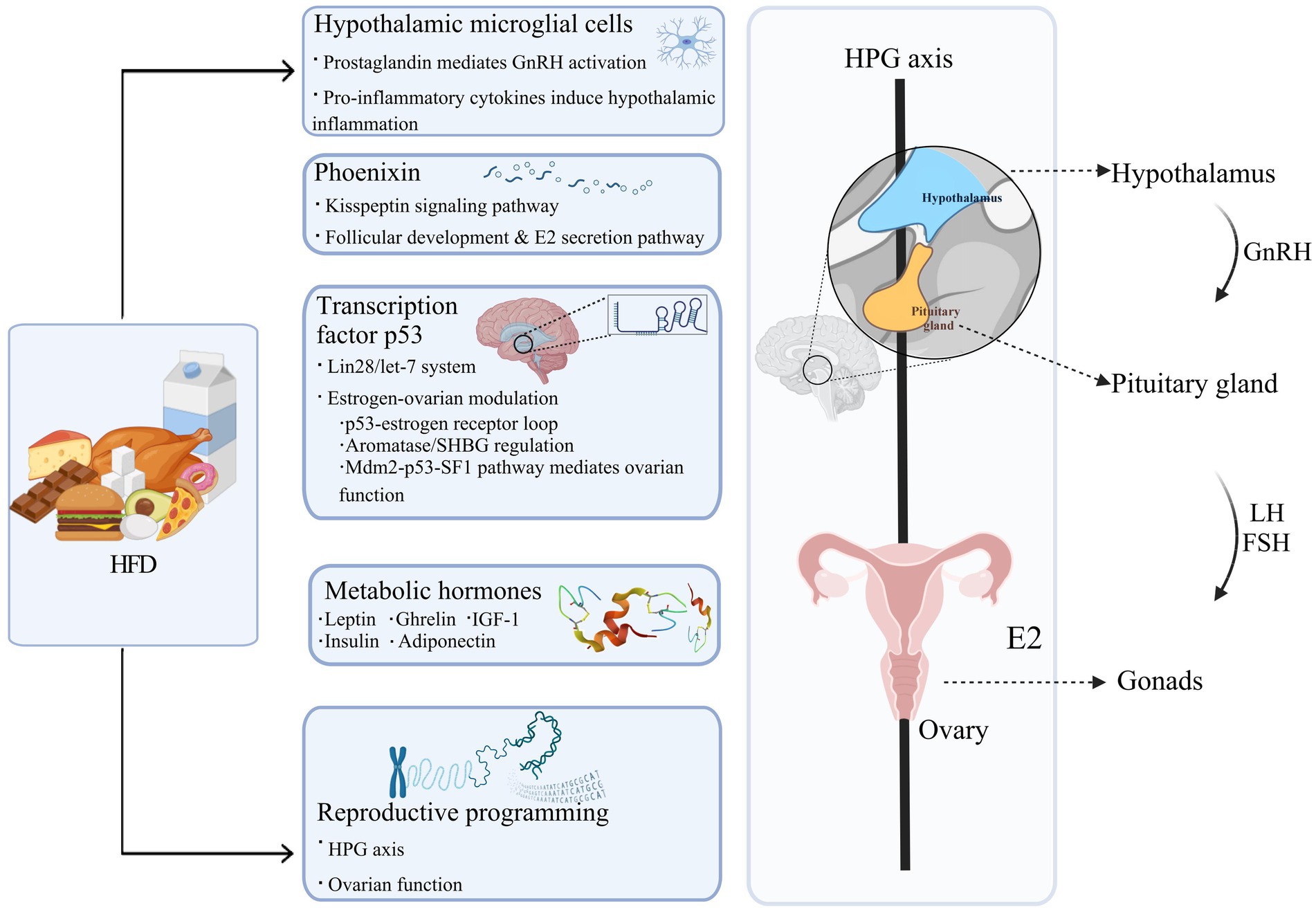
Figure 1. The pathways through which HFD contributes to PP. HFD, a high-fat diet; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; HPG axis, hypothalamic–pituitary-gonadal axis; E2, estradiol; SHBG, sex hormone-binding globulin; Mdm2, mouse double minute 2 homolog; SF1, steroidogenic factor 1; IGF-1, insulin-like growth factor-1. Figure created with BioRender.com.
2.1 Hypothalamic microglial cells
Microglial cells play a critical role in the context of PP and exhibit heightened sensitivity to fatty acids, particularly long-chain saturated fatty acids, which further enhance their activation (Wang et al., 2012; Gupta et al., 2012). Activated hypothalamic microglia release prostaglandins, acting as neurotrophic factors that stimulate GnRH neurons and subsequently hasten puberty (Sheremeta et al., 2024; Fujioka et al., 2013). Additionally, sustained HFD exposure further activates microglial cells to secrete pro-inflammatory cytokines, including interleukin-1β, interleukin-6, nitric oxide (NO), tumor necrosis factor-α, and reactive oxygen species, which collectively induce hypothalamic inflammation and exacerbate the aberrant activation of GnRH neurons (Tzounakou et al., 2024; Chen et al., 2022). This mechanism has been thoroughly reviewed by Stathori et al., whose comprehensive analysis elucidates the potential pathways through which HFD-induced neuroinflammation affects the activity of HPG axis (Stathori et al., 2025). Intriguingly, evidence suggests that HFD Short-term HFD induces triggers microglial activation beyond the effects of obesity, suggesting that HFD introduces unique stressors to the central nervous system, amplifying microglial activation (Gao et al., 2014).
2.2 Phoenixin
Phoenixin, a reproductive peptide crucial for regulating the estrous cycle, is elevated in response to fatty acids such as palmitate and oleate (Mcilwraith et al., 2018), thereby accelerating puberty through GnRH activation mediated by kisspeptin (Stein et al., 2016; Gozukara et al., 2016; Dhillo et al., 2005; Clarke and Dhillo, 2016). In addition, research studies have demonstrated that Phoenixin not only impairs the HPG axis but also adversely affects the follicular development and function. Nguyen et al. were the first to identify Phoenixin, along with its receptor G-protein coupled receptor (GPR) 173, in the ovarian follicles of women post-cervical cancer surgery. In vitro studies further verified Phoenixin’s capacity to promote follicular growth and increase ovulated oocyte numbers, as well as enhance estradiol (E2) secretion in a dose-responsive manner (Nguyen et al., 2019; Clarke and Dhillo, 2019). Overall, these findings establish Phoenixin serves as a key mediator of HFD-induced PP by integrating metabolic signals with the neuroendocrine system and ovarian development.
2.3 Transcription factor p53
The transcription factor p53, a well-known tumor suppressor, has also been found to regulate the genetic pathways governing puberty onset (Chen et al., 2020). In the HFD-induced PP model, hypothalamic p53 expression positively correlated with pubertal onset timing, where p53 overexpression significantly accelerated puberty progression, providing causal evidence for p53 in the development of PP (Chen et al., 2021). Mechanistically, p53 exerts dual regulatory effects through the Lin28/let-7 system: centrally, it activates the Kiss1/GPR54 and phosphatidylinositol 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) signaling pathways to stimulate GnRH secretion (Chen et al., 2021; Xie et al., 2025); gonadally, it directly promotes ovarian granulosa cells proliferation and E2 synthesis (Sui et al., 2023). In addition, p53 orchestrates estrogen signaling and ovarian function through multiple mechanisms: forming a bidirectional regulatory loop with estrogen receptor α (ERα) that enables mutual transcriptional control through promoter binding and estrogen response element-mediated transactivation (Shirley et al., 2009; Berger et al., 2012); modulating estrogen levels and bioactivity by regulating the expression of aromatase (the rate-limiting enzyme in estrogen synthesis) and sex hormone-binding globulin (SHBG; Charni-Natan et al., 2019); and participating in ovarian function regulation via the mouse double minute 2 homolog (Mdm2)-p53-steroidogenic factor 1(SF1) signaling pathway in granulosa cells (Zanjirband et al., 2023). While our understanding of the mechanisms and regulation of p53 in HFD-induced PP is gradually improving, the upstream mechanisms by which HFD upregulates p53 expression are not completely understood. However, emerging evidence suggests that ketone bodies, particularly β-hydroxybutyrate, may play a potential role in this regulatory process (Roberts et al., 2017).
2.4 Metabolic hormones
Long-term HFD consumption disrupts the regulation of key metabolic hormones, which regulate both energy homeostasis and the pathogenesis of PP. Clinical observations reveal that girls with PP exhibit characteristic endocrine alterations, including elevated leptin (Zurita-Cruz et al., 2021), insulin (Zhang et al., 2025), and insulin-like growth factor-1 (IGF-1; Zhang et al., 2025) levels alongside reduced adiponectin (APN; Zurita-Cruz et al., 2021; Sitticharoon et al., 2017), while ghrelin changes remain inconsistent (Tarçin et al., 2024; Zhu et al., 2008). Mechanistic investigations have elucidated distinct neuroendocrine pathways through which metabolic hormones coordinate pubertal timing. Leptin accelerates sexual maturation through dual mechanisms, including central activation of hypothalamic Kisspeptin-GnRH neurons and direct stimulation of ovarian granulosa cell function (Childs et al., 2021). APN, conversely, exerts inhibitory control by suppressing GnRH neuronal activity through adenosine-monophosphate-activated protein kinase (AMPK)-mediated pathway (Mathew et al., 2018). Ghrelin activates GnRH neurons via growth hormone secretagogue receptors (GHSR) or kisspeptin-dependent pathways, and potentially modulates the HPG axis through adrenocorticotropic hormone (ACTH; Farkas et al., 2013; Shi et al., 2022). Insulin enhances leptin synthesis to exert synergistic effects, while IGF-1 likely regulates pubertal progression through multiple mechanisms involving IGF-binding proteins, GnRH neurons, Kisspeptin neurons, and gonadotropins (Salvi et al., 2006).
Collectively, these hormones act on both the hypothalamus and ovarian tissues to regulate pubertal progression, with leptin, insulin, and IGF-1 primarily exerting stimulatory effects while APN and ghrelin mainly demonstrate inhibitory actions.
2.5 Reproductive programming
Reproductive system development begins around the fifth week of gestation and continues until after birth, during which newborns experience a temporary surge in gonadotropin levels, triggering a phenomenon known as “mini-puberty.” Following this transient activation, the HPG axis becomes dormant through a combination of gonadal and non-gonadal inhibitory mechanisms (Yao et al., 2021). These physiological changes highlight how adverse environmental conditions during pregnancy and the early postnatal period can significantly affect the development and function of the reproductive system.
Maternal overnutrition or obesity during pregnancy and lactation substantially accelerates reproductive maturation in offspring have been demonstrated. A large-scale cohort study found that daughters of mothers who gained more than 40 pounds during pregnancy were 30% more likely to experience menarche before age 11 (Boynton-Jarrett et al., 2011). Similarly, female offspring of maternal mice fed HFD during pregnancy and lactation also exhibited earlier puberty onset, with increased E2 levels and decreased luteinizing hormone (LH) levels. Interestingly, supplementation with conjugated linoleic acid during these stages effectively reversed early-onset puberty in offspring (Reynolds et al., 2015). Collectively, maternal HFD during pregnancy and lactation promotes early puberty and reproductive system abnormalities in offspring by disrupting the developmental programming of the HPG axis and ovarian function, with dietary fatty acids potentially influencing this process.
3 HFD disrupts the balance of the GM
The GM exhibits high sensitivity to dietary shifts and changes in the physiological state of the digestive system, with effects observable within 24 h (Qin et al., 2020). Short-term HFD exposure induces dysbiosis characterized by an elevated Firmicutes/Bacteroidetes ratio (Shang et al., 2017), concomitant with increased abundance of bile-resistant genera (e.g., Alistipes, Bacteroides) and decreased populations of plant polysaccharide-degrading Firmicutes (e.g., Roseburia, Ruminococcus bromii; Bisanz et al., 2019; David et al., 2014). Importantly, while short-term HFD induces transient microbial shifts, long-term dietary patterns exert more profound effects: European children consuming a long-term HFD, rich in animal proteins and fats, exhibited a gut microbiome predominantly composed of Bacteroides enterotypes, contrasting sharply with the microbial profiles of Burkinabe children maintained on traditional carbohydrate-rich, low-protein diets (De Filippo et al., 2010; Daniel et al., 2014; Wu et al., 2011).
The types of fatty acids prevalent in HFD also have important consequences for both GM and health. Elevated n-6 PUFA levels promote the proliferation of pro-inflammatory species, such as Mucispirillum schaedleri and Lactobacillus murinus (Selmin et al., 2021), establishing a pro-inflammatory intestinal milieu that exacerbates metabolic endotoxemia and harms health. Furthermore, increased intake of saturated fatty acids, another key component of HFD, facilitates the entry of Gram-negative bacterial lipopolysaccharides (LPS) into the bloodstream, triggering the release of pro-inflammatory cytokines and activating the toll-like receptor 4 signaling pathway, contributing to insulin resistance and inflammation (Hu et al., 2022).
4 Gut microbial imbalance promotes PP
Evidence that antibiotic exposure may elevate the risk of PP in children points toward a role of gut microbiome dysbiosis in PP pathogenesis (Hu et al., 2022). Multiple 16S rRNA gene sequencing studies have revealed global perturbations in the GM of girls with PP compared to healthy controls (Supplementary Table 2; Bo et al., 2022; Wang et al., 2020; Wang Y. et al., 2024; Wang L. et al., 2024; Huang et al., 2023; Huang et al., 2022; Li et al., 2021b; Dong et al., 2020; Qian et al., 2024; Yi et al., 2024; Nguyen et al., 2024; Yuan et al., 2023; Wang et al., 2022), characterized by altered abundances of key taxa (such as Ruminococcus; Wang Y. et al., 2024; Huang et al., 2022; Dong et al., 2020), Bifidobacterium (Wang L. et al., 2024; Huang et al., 2023; Qian et al., 2024), and Bacteroides (Wang Y. et al., 2024; Wang L. et al., 2024; Huang et al., 2022; Qian et al., 2024), along with increased butyrate-producing bacteria (Wang Y. et al., 2024; Huang et al., 2022), and enhanced metabolic potential for neuroendocrine and oxidative stress-related pathways (Wang Y. et al., 2024; Huang et al., 2023; Li et al., 2021b). These findings were further validated in PP animal models (Bo et al., 2022; Yi et al., 2024). Integrating evidence on microbiota-derived metabolites such as short-chain fatty acids (SCFAs) and bile acids (BAs; detailed in Section 5.2), these observations suggest a role for GM and their metabolites in PP development.
However, cross-sectional studies cannot distinguish whether microbial alterations represent causative “driver” microorganisms or secondary “passenger” species proliferating in the PP microenvironment. To address this limitation, recent animal experiments utilizing fecal microbiota transplantation (FMT) from PP donors (either human patients or PP model rats) to recipient female rats demonstrated accelerated puberty onset, accompanied by elevated serum levels of LH, follicle-stimulating hormone (FSH), and E2, as well as upregulated hypothalamic Kiss1 and Gnrh gene expression (Bo et al., 2022; Qian et al., 2024). These findings provided compelling evidence that GM can promote PP through modulation of the HPG axis, although the precise molecular mechanisms by which specific microbial taxa and their metabolic products regulate pubertal timing remain to be elucidated.
5 HFD promotes PP development through changes in GM
From a physiological standpoint, a pivotal connection between a HFD and PP lies in the GM, which acts as a “virtual endocrine organ” with endocrine function, and the bioactive metabolites it produces influence the host’s physiological processes. Figure 2 illustrates the interaction mechanisms through which HFD promotes PP by the modulation of GM and its metabolic products, including SCFAs, BAs, LPS, and neurotransmitters such as γ-aminobutyric acid (GABA), dopamine (DA), and serotonin (5-HT), which are further detailed in subsequent sections.
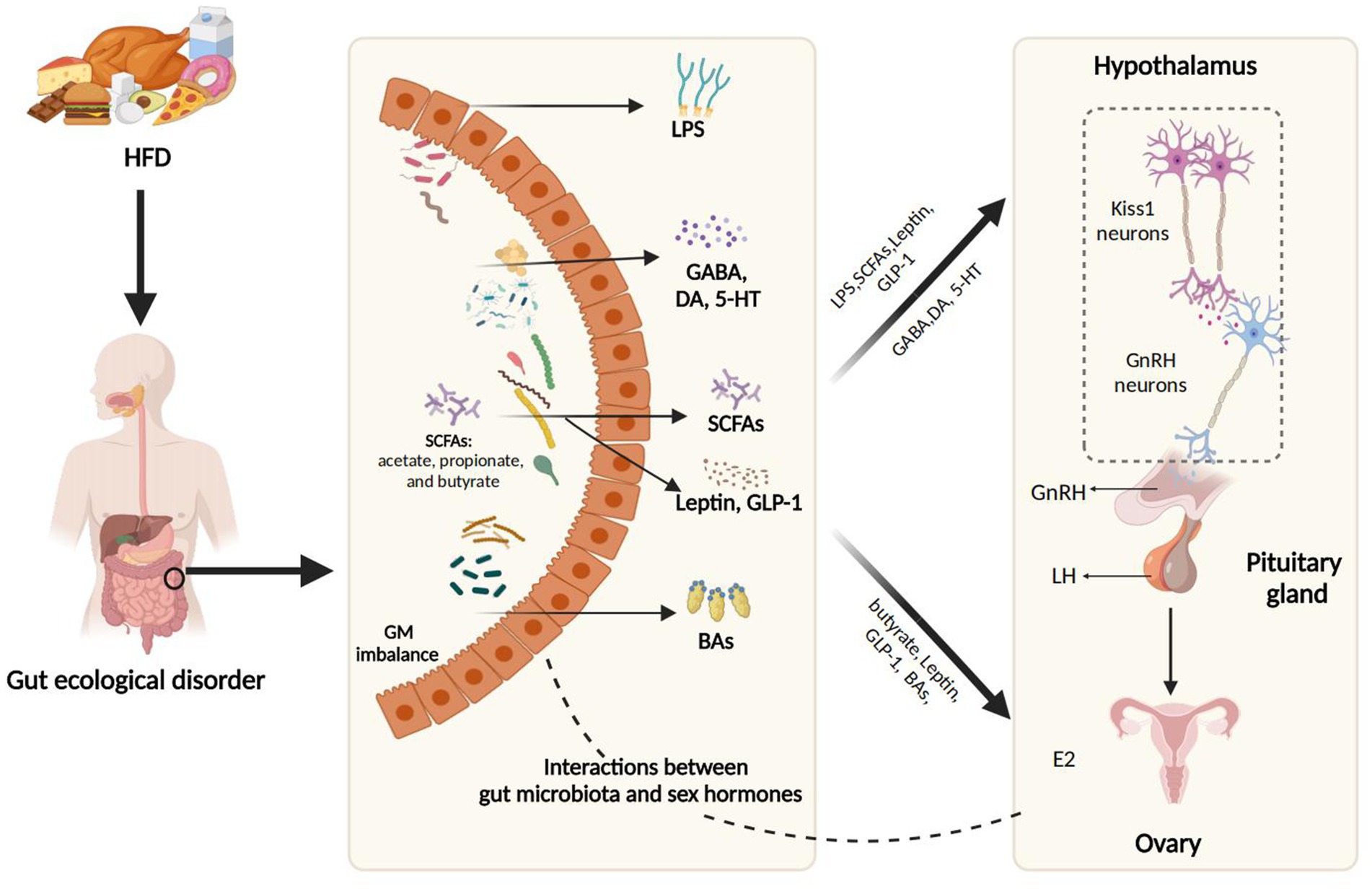
Figure 2. The interaction mechanisms through which HFD promotes PP by modulation of GM and its metabolic products. HFD, a high-fat diet; SCFAs, short-chain fatty acids; LPS, lipopolysaccharide; GABA, Gamma-aminobutyric acid; DA, dopamine; 5-HT, 5-hydroxytryptamine; GLP-1, glucagon-like peptide-1; BAs, bile acids; E2, estradiol; iNOS, inducible nitric oxide synthase; NO, nitric oxide; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone. Figure created with BioRender.com.
5.1 Interaction between GM and sex hormones in HFD
Mutual interactions between GM and sex hormones have been reported, particularly in the context of HFD. The GM can directly modulate sex hormone levels. For example, β-glucuronidase, an enzyme secreted by Clostridium species prevalent in HFD, activates estrogen by deconjugation, thereby enhancing its bioavailability and absorption in the gut and peripheral tissues (Zengul, 2019). Conversely, sex hormone levels can also shape GM composition and diversity. Mueller et al. noted that premenopausal healthy women had lower Bacteroides and Prevotella abundances than age-matched men, a difference absent in postmenopausal women (Mueller et al., 2006). A parallel sexual dimorphism in the composition of rodent GM emerged concomitantly with pubertal onset and was subsequently abolished after castration (Org et al., 2016). Additionally, direct evidence of the influence of sex hormones on the GM was provided in E2-induced PP mice, where an increase in Dubosiella, Faecalibaculum, and Bifidobacterium was noted in response to E2 (Bo et al., 2022; For a more detailed discussion on the bidirectional interactions between GM and sex hormones, refer to the review; Calcaterra et al., 2022)
A deeper exploration of this complex interplay between GM and sex hormones will advance both mechanistic understanding of PP pathogenesis and development of microbiota-targeted therapies.
5.2 HFD promotes PP through metabolic intermediates
5.2.1 SCFAs
SCFAs are fatty acids with fewer than six carbon atoms, primarily produced in the colon through the fermentation of dietary fibers by GM. The most common SCFAs, including acetate, propionate, and butyrate, play crucial roles in gut health and overall metabolism. HFD typically suppresses the proliferation of SCFA-producing gut bacteria while fostering pathogenic bacteria growth (Onishi et al., 2017). The link between SCFAs and PP is long established, with a metabolomic study revealing negative correlations between fecal butyrate, isovalerate, and caproate levels and early puberty onset (Yuan et al., 2023). Notably, a 2022 animal study provided the first experimental evidence that SCFAs exert protective effects against HFD-induced PP by reversing pubertal symptoms through the Kiss1-GPR54-protein kinase C (PKC)-extracellular-regulated kinase 1/2 (ERK1/2) signaling pathway (Wang et al., 2022). This suggests a promising non-invasive therapeutic strategy for preventing and ameliorating PP.
Despite these promising results, the role of SCFAs in PP is incompletely understood as 16S rRNA gene sequencing studies paradoxically revealed increased SCFA-producing bacteria in PP girls (Wang Y. et al., 2024; Huang et al., 2022; Dong et al., 2020). This discrepancy may reflect fundamental differences between experimental and physiological conditions. First, animal studies utilizing supraphysiological SCFA doses through direct administration may not accurately mirror the complex gut-brain communication mediated by microbial metabolites in humans. Second, cross-sectional human studies are unable to distinguish whether increased SCFA producers are drivers or passengers in PP. Third, physiological barriers—including intestinal absorption, hepatic metabolism, and blood–brain barrier permeability—may weaken SCFAs’ effects (Parker et al., 2020), explaining why higher SCFA-producing bacterial abundance does not necessarily prevent PP. Consequently, the dysbiosis induced by HFD, leading to altered SCFA profiles, necessitates further research within the PP context.
5.2.2 BAs
BAs are vital for fat metabolism, aiding in the emulsification and absorption of dietary fats and fat-soluble vitamins in the intestine. HFD significantly boosts bile acid (BA) synthesis and secretion—particularly of hydrophobic secondary BAs—with certain gut bacteria (such as Clostridium and Ruminococcaceae families; Araki et al., 2005; Stenman et al., 2013; Chen et al., 2023; Jiao et al., 2017) catalyzing this process via 7α-dehydroxylation reactions (Li et al., 2021a). The relationship between BAs and puberty was first noted by Bergmann et al. in 1986, who found elevated BA saturation during female puberty, positively correlating with estrogen levels (Bergmann et al., 1986). Recent integrated 16S rRNA gene sequencing and metabolomic analyses revealed notably decreased metabolites involved in primary BA biosynthesis (such as glycocholate, cholic acid, and taurochenodeoxycholic acid) in CPP, with glycocholate potentially enhancing sex hormone absorption (Huang et al., 2023). Mechanistically, HFD-induced female PP rats showed significantly reduced GDCA levels, with glycodeoxycholic acid (GDCA) supplementation ameliorating PP symptoms through the modulation of the hypothalamic Sirt1/Kiss1 signaling pathway (Wu et al., 2025). BAs may also exert diverse biological effects via receptors, such as takeda G-protein-coupled receptor 5 (TGR5) and farnesoid X receptor (FXR). As Vanden Brink et al. showed, hypothalamic TGR5 receptor activation accelerated puberty in normal female rats through kisspeptin receptor-dependent GnRH secretion (Vanden Brink et al., 2024). This ‘paradox’ may be attributable to factors such as genetic background variations, context-dependent effects (HFD versus normal diet), and non-native forms of GDCA that mediate hypothalamic signaling. Importantly, significant species differences in BA metabolism—particularly the abundance of rodent-specific hydrophilic and 6α-hydroxylated BAs—underscore the need for clinical validation when translating these animal findings to human applications (Straniero et al., 2020).
5.2.3 LPS
LPS, a major component of the outer membrane of Gram-negative bacteria, acts as an endotoxin that triggers strong inflammatory responses in host organisms. Mounting evidence indicates that HFD increases the prevalence of LPS-producing bacteria (such as those in the S24-7 family, Enterobacteriaceae, and Desulfovibrionaceae), and exacerbate intestinal permeability, leading to elevated intestinal LPS levels entering the bloodstream (Stenman et al., 2013; Moreira et al., 2012; Kang et al., 2017). LPS activates toll-like receptor 4 receptors on glial cells in the brain, inducing the release of inflammatory mediators and promoting potentiates oxidative stress, which may be accentuated during puberty (Iwasa et al., 2015; Zhao et al., 2019; Schoeler and Caesar, 2019).
Additionally, LPS stimulates the expression of inducible NO synthase in various cells, increasing NO levels that are involved in regulating female reproduction. A research group found that the NO synthesis pathway is more active in girls with CPP compared to healthy controls. Further mechanistic studies showed that NO not only stimulates GnRH secretion (Araki et al., 2005; Zhao et al., 2019), but may also promote follicular development through the PI3K/protein kinase B (AKT)/FoxO3a pathway, thereby accelerating puberty onset (Han G. et al., 2022; Luo et al., 2021; Li et al., 2020).
5.2.4 Neurotransmitters
Most neurotransmitters closely associated with PP are produced by the GM. HFD disrupts the intestinal microbial balance, diminishing populations of Lactobacillus and Bifidobacterium, both known to produce GABA through glutamate decarboxylase activity (Chen et al., 2023; Strandwitz, 2018). Genome-wide association studies have identified several alleles associated with the GABA signaling pathway that correlate with the age of menarche (Perry et al., 2014). GABA acts as a critical inhibitory modulator of hypothalamic GnRH neurons by engaging GABA receptors, thereby suppressing GnRH secretion (Watanabe et al., 2014). Further experiments in prepubertal rhesus monkeys demonstrated that GABA inhibits the upstream gene Kiss1 and its expression, contributing to the delay in puberty onset (Kurian et al., 2012). Additionally, GABA reduces NO production in the hypothalamus, diminishing the stimulation of the HPG axis and the promotion of follicular development (Yang et al., 2020).
In parallel, HFD also augments gut bacteria linked to intestinal inflammation (e.g., Bilophila, Desulfovibrio, and Escherichia), which synthesize neurotransmitters like dopamine and 5-HT, both of which are critical regulators during puberty (Chen et al., 2023; Wang M. et al., 2024). DA activates GnRH through a cAMP-mediated mechanism, enhancing the amplitude and duration of GnRH pulses to promote the onset of puberty (Winters et al., 2014). In contrast, 5-HT exerts a biphasic effect on GnRH neurons: rapid inhibition via 5-HT1A receptors, followed by slow excitation through 5-HT2A receptors (Bhattarai et al., 2014). Despite these insights, the exact mechanisms and causal relationships between neurotransmitters and PP require more detailed exploration.
6 Targeting GM modulation for PP
6.1 Dietary and lifestyle interventions
Recent advancements in dietary interventions have demonstrated their effectiveness as a supplemental strategy for managing PP by influencing GM composition and biological activity, thereby modulating the host’s sexual development. Diets rich in vegetables and proteins have shown effectiveness in mitigating the onset and progression of PP (Gu et al., 2024). Specifically, plant-based high-fiber diets may diminish estrogen levels by inhibiting the dissociation of estrogen-binding proteins and facilitating increased estrogen excretion via feces. This reduction in circulating estrogen may delay puberty onset and slow sexual maturation (Cheng et al., 2012).
In addition to dietary modifications, clinical trials have highlighted the efficacy of certain supplements in delaying sexual development. For instance, a combination of GnRHa and pomegranate extract has proven more effective than GnRHa alone in managing idiopathic CPP (Liu and Tang, 2017). Treatment with decaffeinated green tea polyphenol in prepubertal obese girls not only improved obesity but also delayed puberty onset (Xie et al., 2021). Lifestyle interventions like intermittent fasting have yielded positive outcomes in PP management (Yu et al., 2021). Taken together, while these dietary and lifestyle interventions show promise in alleviating symptoms of PP, further research is required to identify and establish the most effective and evidence-based approaches for managing this condition.
6.2 Probiotics
Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts (Sanders et al., 2019). While direct clinical studies on the effects of probiotics in children with PP are lacking, emerging animal researches evaluate potential advantages. Specific gut microbial combinations, such as a regimen predominantly containing Bifidobacterium longum or a blend of Lactobacillus rhamnosus and Lactobacillus helveticus, have demonstrated the potential in delaying puberty onset in female mice with PP (Yuan et al., 2023; Cowan and Richardson, 2019). Future research should prioritize large-scale, randomized controlled trials to confirm the efficacy and safety of these interventions.
6.3 FMT
FMT is an innovative therapeutic option in treating conditions such as Clostridium difficile infection (Cheng Y. W. et al., 2021), diabetes (De Groot et al., 2021), metabolic syndrome (Li et al., 2016), and autism (Kang et al., 2020). This procedure involves transplanting fecal material from a healthy donor into the patient’s gastrointestinal tract to reestablish microbial balance (Hamamah et al., 2022). There are currently only two animal experimental studies that have been reported FMT could play a critical role in puberty regulation. In both studies, researchers transplanted fecal microbiota from HFD-induced PP rats and from PP girls, and observed an accelerated puberty onset in healthy rats (Bo et al., 2022). In summary, FMT could serve as a viable future treatment for PP, although more studies are needed to fully elucidate the underlying mechanisms and safety in children (Yue and Zhang, 2024).
6.4 Traditional Chinese medicine (TCM)
TCM has been effectively utilized for centuries to treat various health issues, including Alzheimer’s disease (Ding et al., 2022), Parkinson’s disease (Muhammad et al., 2022), rheumatoid arthritis (Jakobsson et al., 2022), and PP (Han X. X. et al., 2022). Several TCM herbs, such as Anemarrhena (Yan et al., 2021), Phellodendron (Su et al., 2021), Berberine (Yang et al., 2022), and Poria (Shan-Shan et al., 2019), show efficacy in modulating the dysregulation of the gut microbial ecosystem. Currently, commonly prescribed formulations for PP in clinical practice include Zhibai Dihuang Wan, Dabu Yin Wan, and Shugan Zhi Yin Jiao Huo Fang. A meta-analysis also revealed integrating TCM with GnRHa therapy improved treatment outcomes compared to GnRHa alone (Lee et al., 2020). Despite these advantages, integrated treatment approaches should be approached cautiously, with careful consideration to ensure that TCM complements rather than interferes with conventional therapies.
7 Discussion
Given the contribution already made by the study described above, we believe that future research should focus on the following key areas:
7.1 The relationship between HFD, GM, and PP
Extensive observational studies have established that HFD (particularly PUFAs and animal proteins) represents a significant risk factor for PP in girls (Chen et al., 2024; Gu et al., 2024; Günther et al., 2010; Xiong et al., 2022; Du et al., 2024; Cheng et al., 2021b; Xu et al., 2022; Cheng et al., 2021a; Shahatah et al., 2021; Nguyen et al., 2020; Jansen et al., 2016; Mueller et al., 2015; Carwile et al., 2015; Mervish et al., 2013; Wiley, 2011; Rogers et al., 2010; Koo et al., 2002; Berkey et al., 2000; Maclure et al., 1991; Merzenich et al., 1993; Szamreta et al., 2020). Preclinical investigations further revealed that maternal or prepubertal HFD exposure induces PP in rodent models (Bo et al., 2022; Wang et al., 2020), with the reported potential mechanisms involving two distinct yet interconnected pathways: microbiota-independent mechanisms (detailed in Section 2), including hypothalamic microglial activation, p53 upregulation, metabolic hormone dysregulation, and others, all of which have been elucidated in multiple reviews as contributing to the activation of the HPG axis (Stathori et al., 2025; Calcaterra et al., 2023; Valsamakis et al., 2021); and microbiota-dependent pathways, whereby HFD-induced gut dysbiosis directly interferes with sex hormone metabolism while modulating key metabolic mediators (such as SCFAs, BAs, and LPS) through microbial enzymatic activities. The latter pathway highlights the mediating role of gut microbiota and their metabolites in HFD-induced PP.
Although 16S rRNA gene sequencing and metabolomics from clinical and preclinical studies have identified characteristic microbial and metabolic signatures in PP, these cross-sectional approaches cannot establish causal relationships with HFD-induced PP (Bo et al., 2022; Wang et al., 2020; Wang Y. et al., 2024; Wang L. et al., 2024; Huang et al., 2023; Huang et al., 2022; Li et al., 2021b; Dong et al., 2020; Qian et al., 2024; Yi et al., 2024; Nguyen et al., 2024; Yuan et al., 2023; Wang et al., 2022). Intervention studies employing FMT and targeted metabolite administration have confirmed that GM influences HFD-induced PP by regulating the hypothalamic Kiss1-GnRH system, with specific metabolites like SCFAs and GDCA exerting ameliorative effects through this pathway (Bo et al., 2022; Wang et al., 2022; Wu et al., 2025). GDCA upregulated Sirt1 expression to suppress the Kiss1 gene. Intriguingly, SCFAs (Jiao et al., 2020; Caetano-Silva et al., 2023; Lin et al., 2022) and specific BAs (Shihabudeen et al., 2015; Yanguas-Casás et al., 2017; Paluschinski et al., 2019; such as tauroursodeoxycholic acid, glycochenodeoxycholic acid, chenodeoxycholic acid, and others) may inhibit multiple key nodes in microbiota-independent pathways, including attenuating p53 expression, reducing microglial activation, and modulating metabolic hormone dysregulation. Leptin, in turn, has been reported to modulate gut microbial metabolic function by enhancing duodenal sympathetic tone and to regulate systemic BA metabolism (Wen et al., 2021; Toledo et al., 2025). However, critical issues still remain: first, the discordance between increased SCFA-producing bacteria and their biological effects in PP may stem from multiple factors, such as pharmacologic versus physiologic concentration disparities, cross-sectional study limitations, and distinct mechanisms of direct interventions versus microbial-mediated actions; second, species-specific variations in BA metabolism constrain clinical translation to humans (Straniero et al., 2020); third, it remains unclear the specific microbial sources of bioactive metabolites and how GM and metabolites regulate hypothalamic gene expression via the microbiota-gut-brain (MGB), potentially through vagal or circulatory pathways (detailed in Section 7.2).
Additionally, key neuromodulators (GABA, DA, 5-HT) and LPS are promising metabolites warranting further investigation, though their precise roles and concentration changes in HFD-induced PP remain to be elucidated. Collectively, HFD impacts pubertal timing through both microbiota-dependent and microbiota-independent pathways that exhibit cross-regulation, providing a conceptual framework for understanding PP pathogenesis and developing targeted interventions.
7.2 Evidence between the gut-brain axis and PP
Mounting evidence linking the GM to hypothalamic dysfunction in the context of PP has underscored the bidirectional communication between the GM and the brain via the MGB axis. MGB axis is theorized to occur through various systems, including the autonomic and enteric nervous systems, neuroendocrine pathways, and the immune system (Cryan et al., 2019). Nowadays, understanding of the mechanisms of PP through the MGB axis focuses on several pathways.
First, GM may modulate brain function through vagal nerve activation, the most extensively studied pathway. Clinical evidence shows cardiac autonomic dysfunction in girls with idiopathic PP, mainly manifested as a decrease in vagal nerve tension (Yi et al., 2017). Animal experiments further demonstrate that prepubertal vagotomy could lead to ovarian dysfunction, confirming its crucial role in the development of puberty (Morales-Ledesma et al., 2004). Mechanistically, as the main conduction pathway connecting the enteric nervous system and the central nervous system, the vagus nerve expresses receptors for a variety of bioactive compounds on its surface (such as GLP-1 receptor, cholecystokinin 1 receptor, neuropeptide Y receptor Y2, GPR35, and GPR119), enabling direct recognition of signaling molecules from GM and their metabolites, such as LPS-producing bacteria (Ma et al., 2024), acetate-producing bacteria and acetate (Zheng et al., 2021), kynurenic acid (Fan et al., 2023b), indole (Jaglin et al., 2018) and others. While cross-disease studies provide experimental support for vagus nerve–mediated microbiota-brain communication, critical gaps persist in understanding its specific regulatory mechanisms in PP pathogenesis—particularly how distinct microbial metabolites modulate HPG axis activity via the vagus nerve.
Second, under pathological conditions, harmful gut microbes and their metabolites in the bloodstream can also disrupt hypothalamus function. Given its anatomical proximity to the blood–brain barrier (BBB), the hypothalamus is particularly vulnerable to the detrimental effects of BBB dysfunction and leakage (Thaler et al., 2012). Developmental studies have shown that sex hormones in the neonatal period can significantly regulate the development of cerebral blood vessels and the integrity of the BBB. This critical period coincides with the early activation peak of the HPG axis, providing important clues for understanding the association between PP and abnormal BBB function (Collignon et al., 2024). In addition, a study demonstrated a causal relationship between microbial alterations and gut barrier dysfunction, as alterations were observed in gut barrier permeability and in tight junction protein occludin in both the frontal cortex and hippocampus in germ-free adult mice, and these alterations were shown to recover following treatment with SCFA-producing bacterial strains or sodium butyrate (Braniste et al., 2014). These findings provide direct evidence for the regulation of neuroendocrine function by the MGB axis, suggesting that the GM may play a crucial role in the pathogenesis of PP by affecting the integrity of the BBB. However, direct animal and clinical data on the specific regulatory mechanisms of the MGB axis in PP are remain lacking, underscoring the urgent need for future research in this field.
Although there is growing discussion about the role of the MGB axis in regulating neurological and metabolic disorders, direct animal and clinical evidence in the context of PP remains lacking. Future research in this area is urgently needed.
7.3 The role of HFD and obesity
The relationship between HFD, obesity, and PP has been a subject of ongoing debate. Emerging evidence confirms that HFD can indeed trigger PP through obesity-independent pathways: first, animal studies demonstrate that both HFD-induced and kisspeptin-induced PP occur independently of weight changes, with short-term HFD exposure sufficient to elevate gonadotropins without altering body fat or leptin levels (Ullah et al., 2019; Frisch et al., 1977; Sahin et al., 2015); second, gut microbes specifically enriched in female rats with PP (such as Bilophila; Kimura et al., 2013; Kimura et al., 2011), Lachnoclostridium (Zhao et al., 2021), Lactobacillus (Henning et al., 2018), Lactobacillus murinus (Li et al., 2024), Lactobacillus reuteri (Zhang et al., 2022) are negatively correlated with obesity, indicating differences between the microbial communities regulating pubertal development and the microbiota associated with obesity; third, the conventional paradigm recognizes obesity as a significant risk factor for PP through its induction of metabolic hormone dysregulation, a well-established mechanism extensively documented in reviews (Shi et al., 2022; Calcaterra et al., 2023). However, HFD serves as the primary instigator of both metabolic hormone imbalance and subsequent obesity development (Chakraborty et al., 2016; Fan et al., 2023a). Under HFD conditions, metabolic hormone dysregulation and obesity exhibit complex bidirectional interactions, with obesity serving as an exacerbating factor rather than a prerequisite for metabolic dysfunction. This is evidenced by findings that long-term HFD-fed mice exhibit increased GLP-1 secretion and insulin resistance independent of obesity and body weight (Wang et al., 2015; Clegg et al., 2011), and girls with CPP show high serum leptin levels uncorrelated with body mass index (BMI; Zurita-Cruz et al., 2021; Kang et al., 2018). Collectively, these findings confirm that HFD induces PP not only through the traditional “obesity-metabolic hormone” pathway but also via obesity-independent mechanisms.
7.4 Integrated omics technologies and population research
Intestinal microbes and their metabolic byproducts provide new biological insights into the link between HFD and PP. However, existing research still has significant limitations. Firstly, the current evidence primarily relies on cross-sectional designs and the low-resolution 16S rRNA gene sequencing technology, which limits the comprehensive revelation of dynamic evolution of GM and lacks precision at the species level. To break through this bottleneck, future research should prioritize conducting longitudinal cohort studies combined with high-throughput technologies such as metagenomic sequencing for multi-omics integration analysis (including metabolomics, epigenomics, and proteomics). Such research can dynamically capture the key biological processes in the occurrence and development of PP, identify reliable biomarker prediction models through machine learning algorithms, and deeply analyze the “microorganisms-metabolites-hosts” interaction network. The feasibility of this methodological approach has been well established in prior research. For example, Huang et al. developed a diagnostic classifier to differentiate CPP from healthy controls using integrated microbiome-metabolome analysis, identifying potential therapeutic biomarkers (Huang et al., 2023). In addition, complementary animal studies using combined transcriptomic-epigenomic profiling elucidated critical regulatory genes and pathway networks in PP pathogenesis (Mohamed et al., 2022). Secondly, most mechanistic data on PP is largely based on animal experiments and lacks sufficient clinical evidence to definitively support its role in humans. Given the complexity and specificity of the disease, large-scale clinical studies are likely to provide more valuable insights into its pathophysiology than animal models.
In conclusion, HFD-induced PP arises from the overlapping effects of GM dysbiosis and high-fat intake, involving multiple mechanisms. Fortunately, advances in omics research and breakthroughs in mechanistic studies using animal models are rapidly enhancing our understanding of the causal mechanisms between HFD, GM, and PP. These advancements also reveal the potential therapeutic effects of key bacterial species or taxa, paving the way for optimizing microbiota-based therapeutic strategies and developing innovative, non-invasive treatment options for children with PP.
Author contributions
NW: Visualization, Writing – original draft. KN: Visualization, Writing – original draft. YL: Visualization, Writing – original draft. QW: Conceptualization, Funding acquisition, Writing – review & editing. NL: Conceptualization, Funding acquisition, Writing – review & editing. LZ: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (82370785 and 82172320); Shandong Provincial Natural Science Foundation (ZR2024MH220); Shandong University Outstanding Young Scholars Program, and National Center for Women and Children’s Health, China CDC “Maternal and Infants Nutrition and Health Research Programs” (2022FYH008); Key R&D Program (Innovation Capacity Improvement Project for Science and Technology-based Small and Medium-sized Enterprises) of Shandong Province, China (2024TSGC0372).
Acknowledgments
Thanks for the support of Children’s Hospital Affiliated to Shandong University (Jinan Children’s Hospital). Thanks to all the participants for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1564902/full#supplementary-material
References
Alghamdi, A., and Alghamdi, A. H. (2023). Precocious puberty: types, pathogenesis and updated management. Cureus 15:e47485. doi: 10.7759/cureus.47485
Araki, Y., Katoh, T., Ogawa, A., Bamba, S., Andoh, A., Koyama, S., et al. (2005). Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the Caco-2 cell line. Free Radic. Biol. Med. 39, 769–780. doi: 10.1016/j.freeradbiomed.2005.04.026
Berger, C. E., Qian, Y., Liu, G., Chen, H., and Chen, X. (2012). p53, a target of estrogen receptor (Er) α, modulates Dna damage-induced growth suppression in Er-positive breast cancer cells. J. Biol. Chem. 287, 30117–30127. doi: 10.1074/jbc.M112.367326
Bergmann, K. V., Becker, M., and Leiss, O. (1986). Biliary cholesterol saturation in non-obese women and non-obese men before and after puberty. Eur. J. Clin. Investig. 16, 531–535.
Berkey, C. S., Gardner, J. D., and Lindsay Frazier, A. (2000). Relation of childhood diet and body size to menarche and adolescent growth in girls. Am. J. Epidemiol. 152, 446–452.
Bhattarai, J. P., Roa, J., Herbison, A. E., and Han, S. K. (2014). Serotonin acts through 5-Ht1 and 5-Ht2 receptors to exert biphasic actions on Gnrh neuron excitability in the mouse. Endocrinology 155, 513–524. doi: 10.1210/en.2013-1692
Bisanz, J. E., Upadhyay, V., and Turnbaugh, J. A. (2019). Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe 26, 265–272. doi: 10.1016/j.chom.2019.06.013
Bo, T., Liu, M., Tang, L., Lv, J., Wen, J., and Wang, D. (2022). Effects of high-fat diet during childhood on precocious puberty and gut microbiota in mice. Front. Microbiol. 13:930747. doi: 10.3389/fmicb.2022.930747
Boynton-Jarrett, R., Rich-Edwards, J., Fredman, L., Hibert, E. L., Michels, K. B., Forman, M. R., et al. (2011). Gestational weight gain and daughter’s age at menarche. J. Women’s Health 20, 1193–1200. doi: 10.1089/jwh.2010.2517
Braniste, V., Al-Asmakh, M., and Kowal, C. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6:263ra158. doi: 10.1126/scitranslmed.3009759
Caetano-Silva, M. E., Rund, L., Hutchinson, N. T., Woods, J. A., Steelman, A. J., and Johnson, R. W. (2023). Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 13:2819. doi: 10.1038/s41598-022-27086-x
Calcaterra, V., Magenes, V. C., Hruby, C., Siccardo, F., Mari, A., Cordaro, E., et al. (2023). Links between childhood obesity, high-fat diet, and central precocious puberty. Child. Aust. 10:241. doi: 10.3390/children10020241
Calcaterra, V., Rossi, V., Massini, G., Regalbuto, C., Hruby, C., Panelli, S., et al. (2022). Precocious puberty and microbiota: the role of the sex hormone–gut microbiome axis. Front. Endocrinol. 13:1000919. doi: 10.3389/fendo.2022.1000919
Carwile, J. L., Willett, W. C., Wang, M., Rich-Edwards, J., Frazier, A. L., and Michels, K. B. (2015). Milk consumption after age 9 years does not predict age at menarche. J. Nutr. 145, 1900–1908. doi: 10.3945/jn.115.214270
Chakraborty, T. R., Donthireddy, L., Adhikary, D., and Chakraborty, S. (2016). Long-term high fat diet has a profound effect on body weight, hormone levels, and estrous cycle in mice. Medical Sci. Monitor: Int. Medical J. Experimental Clin. Res. 22, 1601–1608. doi: 10.12659/MSM.897628
Charni-Natan, M., Aloni-Grinstein, R., Osher, E., and Rotter, V. (2019). Liver and steroid hormones—can a touch of p53 make a difference? Front. Endocrinol. 10:374. doi: 10.3389/fendo.2019.00374
Chen, T., Chen, C., Wu, H., Chen, X., Xie, R., Wang, F., et al. (2021). Overexpression of p53 accelerates puberty in high-fat diet–fed mice through Lin28/let-7 system. Exp. Biol. Med. 246, 66–71. doi: 10.1177/1535370220961320
Chen, X., Fu, S., Chen, C., Yuan, Y., Dai, Z., Chen, A., et al. (2024). Association of Traditional dietary pattern with early and precocious puberty: a population-based cross-sectional study. Pediatr. Res. 96, 245–252. doi: 10.1038/s41390-024-03110-w
Chen, X., Huang, L., Cui, L., Xiao, Z., Xiong, X., and Chen, C. (2022). Sodium–glucose cotransporter 2 inhibitor ameliorates high fat diet-induced hypothalamic–pituitary–ovarian axis disorders. J. Physiol. 600, 4549–4568. doi: 10.1113/JP283259
Chen, T., Wu, H., Chen, X., Xie, R., Wang, F., Sun, H., et al. (2020). p53 mediates Gnrh secretion via Lin28/let-7 system in Gt1-7 cells. Diabetes, Metabolic Syndrome Obesity 13, 4681–4688. doi: 10.2147/DMSO.S279901
Chen, J., Xiao, Y., Li, D., Zhang, S., Wu, Y., Zhang, Q., et al. (2023). New insights into the mechanisms of high-fat diet mediated gut microbiota in chronic diseases. iMeta 2:e69. doi: 10.1002/imt2.69
Cheng, Y.-W., Alhaffar, D., Saha, S., Khanna, S., Bohm, M., Phelps, E., et al. (2021). Fecal microbiota transplantation is safe and effective in patients with Clostridioides difficile infection and cirrhosis. Clin. Gastroenterol. Hepatol. 19, 1627–1634. doi: 10.1016/j.cgh.2020.06.051
Cheng, G., Buyken, A. E., Shi, L., Karaolis-Danckert, N., Kroke, A., Wudy, S. A., et al. (2012). Beyond overweight: nutrition as an important lifestyle factor influencing timing of puberty. Nutr. Rev. 70, 133–152. doi: 10.1111/j.1753-4887.2011.00461.x
Cheng, T. S., Day, F. R., and Perry, J. R. (2021a). Prepubertal dietary and plasma phospholipid fatty acids related to puberty timing: longitudinal cohort and Mendelian randomization analyses. Nutrients 13:1868. doi: 10.3390/nu13061868
Cheng, T. S., Sharp, S. J., and Brage, S. (2021b). Longitudinal associations between prepubertal childhood total energy and macronutrient intakes and subsequent puberty timing in UK boys and girls. Eur. J. Nutr. 61, 1–11. doi: 10.1007/s00394-021-02629-6
Cheuiche, A. V., Da Silveira, L. G., and De Paula, L. C. P. (2021). Diagnosis and management of precocious sexual maturation: an updated review. Eur. J. Pediatr. 180, 3073–3087. doi: 10.1007/s00431-021-04022-1
Childs, G. V., Odle, A. K., and Macnicol, M. C. (2021). The importance of leptin to reproduction. Endocrinology 162:bqaa204. doi: 10.1210/endocr/bqaa204
Clarke, S. A., and Dhillo, W. S. (2016). Kisspeptin across the human lifespan: evidence from animal studies and beyond. J. Endocrinol. 229, R83–R98. doi: 10.1530/JOE-15-0538
Clarke, S A, and Dhillo, W S. “Phoenixin and its role in reproductive hormone release”; Proceedings of the seminars in reproductive medicine, (2019). Thieme Medical Publishers.
Clegg, D. J., Gotoh, K., Kemp, C., Wortman, M. D., Benoit, S. C., Brown, L. M., et al. (2011). Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol. Behav. 103, 10–16. doi: 10.1016/j.physbeh.2011.01.010
Collignon, A., Dion-Albert, L., Ménard, C., and Coelho-Santos, V. (2024). Sex, hormones and cerebrovascular function: from development to disorder. Fluids Barriers CNS 21:2. doi: 10.1186/s12987-023-00496-3
Cowan, C. S., and Richardson, R. (2019). Early-life stress leads to sex-dependent changes in pubertal timing in rats that are reversed by a probiotic formulation. Dev. Psychobiol. 61, 679–687. doi: 10.1002/dev.21765
Cryan, J. F., O’Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The Microbiota-Gut-Brain Axis. Physiol Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
Daniel, H., Gholami, A. M., Berry, D., Desmarchelier, C., Hahne, H., Loh, G., et al. (2014). High-fat diet alters gut microbiota physiology in mice. ISME J. 8, 295–308. doi: 10.1038/ismej.2013.155
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
De Filippo, C., Cavalieri, D., and Di Paola, M. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. 107, 14691–14696. doi: 10.1073/pnas.1005963107
De Groot, P., Nikolic, T., and Pellegrini, S. (2021). Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 70, 92–105. doi: 10.1136/gutjnl-2020-322630
Dhillo, W. S., Chaudhri, O. B., Patterson, M., Thompson, E. L., Murphy, K. G., Badman, M. K., et al. (2005). Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. Clin. Endocrinol. Metab. 90, 6609–6615. doi: 10.1210/jc.2005-1468
Ding, M.-R., Qu, Y.-J., Hu, B., and An, H. M. (2022). Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed. Pharmacother. 152:113208. doi: 10.1016/j.biopha.2022.113208
Dong, G., Zhang, J., Yang, Z., Feng, X., Li, J., Li, D., et al. (2020). The association of gut microbiota with idiopathic central precocious puberty in girls. Front. Endocrinol. 10:941. doi: 10.3389/fendo.2019.00941
Du, Y., Yan, W., Bigambo, F. M., Zhou, Q., Ma, C., Gu, W., et al. (2024). Association between dietary behavior and puberty in girls. BMC Pediatr. 24:349. doi: 10.1186/s12887-024-04840-w
Fan, S., Chen, S., and Lin, L. (2023a). Research progress of gut microbiota and obesity caused by high-fat diet. Front. Cell. Infect. Microbiol. 13:1139800. doi: 10.3389/fcimb.2023.1139800
Fan, S., Guo, W., and Xiao, D. (2023b). Microbiota-gut-brain axis drives overeating disorders. Cell Metab. 35, 2011–2027. doi: 10.1016/j.cmet.2023.09.005
Farkas, I., Vastagh, C., Sárvári, M., and Liposits, Z. (2013). Ghrelin decreases firing activity of gonadotropin-releasing hormone (Gnrh) neurons in an estrous cycle and endocannabinoid signaling dependent manner. PLoS One 8:e78178. doi: 10.1371/journal.pone.0078178
Frisch, R., Hegsted, D., and Yoshinaga, K. (1977). Carcass components at first estrus of rats on high-fat and low-fat diets: body water, protein, and fat. Proc. Natl. Acad. Sci. USA 74, 379–383.
Fujioka, H., Kakehashi, C., Funabashi, T., and Akema, T. (2013). Immunohistochemical evidence for the relationship between microglia and Gnrh neurons in the preoptic area of ovariectomized rats with and without steroid replacement. Endocr. J. 60, 191–196. doi: 10.1507/endocrj.EJ12-0280
Gao, Y., Ottaway, N., Schriever, S. C., Legutko, B., García-Cáceres, C., de la Fuente, E., et al. (2014). Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62, 17–25. doi: 10.1002/glia.22580
Gozukara, I., Dokuyucu, R., Özgür, T., Özcan, O., Pınar, N., Kurt, R. K., et al. (2016). Histopathologic and metabolic effect of ursodeoxycholic acid treatment on Pcos rat model. Gynecol. Endocrinol. 32, 492–497. doi: 10.3109/09513590.2015.1134478
Gu, Q., Wu, Y., Feng, Z., Chai, Y., Hou, S., Yu, Z., et al. (2024). Dietary pattern and precocious puberty risk in Chinese girls: a case-control study. Nutr. J. 23:14. doi: 10.1186/s12937-024-00916-6
Günther, A. L., Karaolis-Danckert, N., Kroke, A., Remer, T., and Buyken, A. E. (2010). Dietary protein intake throughout childhood is associated with the timing of puberty. J. Nutr. 140, 565–571. doi: 10.3945/jn.109.114934
Gupta, S., Knight, A. G., Gupta, S., Keller, J. N., and Bruce-Keller, A. J. (2012). Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J. Neurochem. 120, 1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x
Hamamah, S., Gheorghita, R., Lobiuc, A., Sirbu, I. O., and Covasa, M. (2022). Fecal microbiota transplantation in non-communicable diseases: recent advances and protocols. Front. Med. 9:1060581. doi: 10.3389/fmed.2022.1060581
Han, G., Xuanyuan, S., and Zhang, B. (2022). Activation of Pi3K/Akt prevents hypoxia/reoxygenation-induced Gnrh decline via Foxo3a. Physiol. Res. 71:509. doi: 10.33549/physiolres.934861
Han, X. X., Zhao, F.-Y., Gu, K.-R., Wang, G. P., Zhang, J., Tao, R., et al. (2022). Development of precocious puberty in children: surmised medicinal plant treatment. Biomed. Pharmacother. 156:113907. doi: 10.1016/j.biopha.2022.113907
Henning, S. M., Yang, J., Hsu, M., Lee, R.-P., Grojean, E. M., Ly, A., et al. (2018). Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 57, 2759–2769. doi: 10.1007/s00394-017-1542-8
Hu, Y., Li, J., Yuan, T., Yu, T., Chen, Y., Kong, H., et al. (2022). Exposure to antibiotics and precocious puberty in children: a school-based cross-sectional study in China. Environ. Res. 212:113365. doi: 10.1016/j.envres.2022.113365
Huang, X., Chen, J., Zou, H., Huang, P., Luo, H., Li, H., et al. (2023). Gut microbiome combined with metabolomics reveals biomarkers and pathways in central precocious puberty. J. Transl. Med. 21:316. doi: 10.1186/s12967-023-04169-5
Huang, C., Liu, H., Yang, W., Li, Y., Wu, B., Chen, J., et al. (2022). Distinct gut microbiota structure and function of children with idiopathic central and peripheral precocious puberty. Int. J. Endocrinol. 2022, 1–11. doi: 10.1155/2022/7175250
Iwasa, T., Matsuzaki, T., Tungalagsuvd, A., Munkhzaya, M., Kawami, T., Yamasaki, M., et al. (2015). Developmental changes in hypothalamic toll-like-receptor 4 mrna expression and the effects of lipopolysaccharide on such changes in female rats. Int. J. Dev. Neurosci. 40, 12–14. doi: 10.1016/j.ijdevneu.2014.10.002
Jaglin, M., Rhimi, M., Philippe, C., Pons, N., Bruneau, A., Goustard, B., et al. (2018). Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front. Neurosci. 12:216. doi: 10.3389/fnins.2018.00216
Jakobsson, P. J., Robertson, L., Welzel, J., Zhang, M., Zhihua, Y., Kaixin, G., et al. (2022). Where traditional Chinese medicine meets Western medicine in the prevention of rheumatoid arthritis. J. Intern. Med. 292, 745–763. doi: 10.1111/joim.13537
Jansen, E. C., Marín, C., Mora-Plazas, M., and Villamor, E. (2016). Higher childhood red meat intake frequency is associated with earlier age at menarche. J. Nutr. 146, 792–798. doi: 10.3945/jn.115.226456
Jiao, S., Cao, H., Dai, Y., Wu, J., Lv, J., du, R., et al. (2017). Effect of high-fat diet and growth stage on the diversity and composition of intestinal microbiota in healthy bovine livestock. J. Sci. Food Agric. 97, 5004–5013. doi: 10.1002/jsfa.8380
Jiao, A., Yu, B., He, J., Yu, J., Zheng, P., Luo, Y., et al. (2020). Short chain fatty acids could prevent fat deposition in pigs via regulating related hormones and genes. Food Funct. 11, 1845–1855. doi: 10.1039/C9FO02585E
Kang, D.-W., Adams, J. B., Vargason, T., Santiago, M., Hahn, J., and Krajmalnik-Brown, R. (2020). Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. Msphere 5:e00314-20. doi: 10.1128/msphere.00314-20
Kang, M. J., Oh, Y. J., Shim, Y. S., Baek, J. W., Yang, S., and Hwang, I. T. (2018). The usefulness of circulating levels of leptin, kisspeptin, and neurokinin B in obese girls with precocious puberty. Gynecol. Endocrinol. 34, 627–630. doi: 10.1080/09513590.2017.1423467
Kang, C., Wang, B., Kaliannan, K., Wang, X., Lang, H., Hui, S., et al. (2017). Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. MBio 8:e00470-17. doi: 10.1128/mbio.00470-17
Kimura, I., Inoue, D., Maeda, T., Hara, T., Ichimura, A., Miyauchi, S., et al. (2011). Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (Gpr41). Proc. Natl. Acad. Sci. USA 108, 8030–8035. doi: 10.1073/pnas.1016088108
Kimura, I., Ozawa, K., Inoue, D., Imamura, T., Kimura, K., Maeda, T., et al. (2013). The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor Gpr43. Nat. Commun. 4:1829. doi: 10.1038/ncomms2852
Koo, M. M., Rohan, T. E., Jain, M., McLaughlin, J. R., and Corey, P. N. (2002). A cohort study of dietary fibre intake and menarche. Public Health Nutr. 5, 353–360. doi: 10.1079/PHN2002261
Kurian, J. R., Keen, K. L., Guerriero, K. A., and Terasawa, E. (2012). Tonic control of kisspeptin release in prepubertal monkeys: implications to the mechanism of puberty onset. Endocrinology 153, 3331–3336. doi: 10.1210/en.2012-1221
Lee, Y. B., Lee, J. A., and Lee, H. L. (2020). Herbal medicine for idiopathic central precocious puberty: a systematic review and meta-analysis. J. Altern. Complement. Med. 26, 976–999. doi: 10.1089/acm.2019.0312
Li, Y., Hou, H., Wang, X., Dai, X., Zhang, W., Tang, Q., et al. (2021a). Diammonium glycyrrhizinate ameliorates obesity through modulation of gut microbiota-conjugated bas-Fxr signaling. Front. Pharmacol. 12:796590. doi: 10.3389/fphar.2021.796590
Li, Y., Shen, L., Huang, C., Li, X., Chen, J., Li, S. C., et al. (2021b). Altered nitric oxide induced by gut microbiota reveals the connection between central precocious puberty and obesity. Clin. Transl. Med. 11:e299. doi: 10.1002/ctm2.299
Li, X., Yang, J., Zhou, X., Dai, C., Kong, M., Xie, L., et al. (2024). Ketogenic diet-induced bile acids protect against obesity through reduced calorie absorption. Nat. Metab. 6, 1397–1414. doi: 10.1038/s42255-024-01072-1
Li, J., Zhang, W., Zhu, S., and Shi, F. (2020). Nitric oxide synthase is involved in follicular development via the Pi3K/Akt/FoxO3a pathway in neonatal and immature rats. Animals 10:248. doi: 10.3390/ani10020248
Li, S. S., Zhu, A., Benes, V., Costea, P. I., Hercog, R., Hildebrand, F., et al. (2016). Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 352, 586–589. doi: 10.1126/science.aad8852
Lin, T., Yang, W.-Q., Luo, W.-W., Zhang, L. L., Mai, Y. Q., Li, Z. Q., et al. (2022). Disturbance of fatty acid metabolism promoted vascular endothelial cell senescence via acetyl-CoA-induced protein acetylation modification. Oxidative Med. Cell. Longev. 2022, 1–24. doi: 10.1155/2022/1198607
Liu, J., and Tang, J. (2017). Effects of pomegranate extract in supplementing gonadotropin-releasing hormone therapy on idiopathic central precocious puberty in Chinese girls: a randomized, placebo-controlled, double-blind clinical trial. Food Funct. 8, 695–700. doi: 10.1039/C6FO01616B
Luo, Y., Zhu, Y., Basang, W., Wang, X., Li, C., and Zhou, X. (2021). Roles of nitric oxide in the regulation of reproduction: a review. Front. Endocrinol. 12:752410. doi: 10.3389/fendo.2021.752410
Ma, X., Kim, J.-K., Shin, Y.-J., Park, H. S., Lee, D. Y., Yim, S. V., et al. (2024). Lipopolysaccharide-producing Veillonella infantium and Escherichia fergusonii cause vagus nerve-mediated cognitive impairment in mice. Brain Behav. Immun. 118, 136–148. doi: 10.1016/j.bbi.2024.02.031
Maclure, M., Travis, L. B., Willett, W., and MacMahon, B. (1991). A prospective cohort study of nutrient intake and age at menarche. Am. J. Clin. Nutr. 54, 649–656.
Mathew, H., Castracane, V. D., and Mantzoros, C. (2018). Adipose tissue and reproductive health. Metab. Clin. Exp. 86, 18–32. doi: 10.1016/j.metabol.2017.11.006
Mcilwraith, E. K., Loganathan, N., and Belsham, D. D. (2018). Phoenixin expression is regulated by the fatty acids palmitate, docosahexaenoic acid and oleate, and the endocrine disrupting chemical bisphenol a in immortalized hypothalamic neurons. Front. Neurosci. 12:838. doi: 10.3389/fnins.2018.00838
Mervish, N. A., Gardiner, E. W., Galvez, M. P., Kushi, L. H., Windham, G. C., Biro, F. M., et al. (2013). Dietary flavonol intake is associated with age of puberty in a longitudinal cohort of girls. Nutr. Res. 33, 534–542. doi: 10.1016/j.nutres.2013.04.005
Merzenich, H., Boeing, H., and Wahrendorf, J. (1993). Dietary fat and sports activity as determinants for age at menarche. Am. J. Epidemiol. 138, 217–224.
Mohamed, A. R., Naval-Sanchez, M., Menzies, M., Evans, B., King, H., Reverter, A., et al. (2022). Leveraging transcriptome and epigenome landscapes to infer regulatory networks during the onset of sexual maturation. BMC Genomics 23:413. doi: 10.1186/s12864-022-08514-8
Morales-Ledesma, L., Betanzos-Garcı́a, R. O., and Domı́nguez-Casalá, R. (2004). Unilateral or bilateral vagotomy performed on prepubertal rats at puberty onset of female rat deregulates ovarian function. Arch. Med. Res. 35, 279–283. doi: 10.1016/j.arcmed.2004.03.007
Moreira, A. P. B., Texeira, T. F. S., Ferreira, A. B., do Carmo Gouveia Peluzio, M., and de Cássia Gonçalves Alfenas, R. (2012). Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 108, 801–809. doi: 10.1017/S0007114512001213
Mueller, N. T., Jacobs, D. R. Jr., and Maclehose, R. F. (2015). Consumption of caffeinated and artificially sweetened soft drinks is associated with risk of early menarche. Am. J. Clin. Nutr. 102, 648–654. doi: 10.3945/ajcn.114.100958
Mueller, S., Saunier, K., Hanisch, C., Norin, E., Alm, L., Midtvedt, T., et al. (2006). Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 72, 1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006
Muhammad, F., Liu, Y., Zhou, Y., Yang, H., and Li, H. (2022). Antioxidative role of traditional Chinese medicine in Parkinson’s disease. J. Ethnopharmacol. 285:114821. doi: 10.1016/j.jep.2021.114821
Nguyen, N. T. K., Fan, H.-Y., Tsai, M.-C., Tung, T. H., Huynh, Q. T. V., Huang, S. Y., et al. (2020). Nutrient intake through childhood and early menarche onset in girls: systematic review and meta-analysis. Nutrients 12:2544. doi: 10.3390/nu12092544
Nguyen, N. N., Lin, C.-Y., Tsai, W.-L., Huang, H. Y., Chen, C. M., Tung, Y. T., et al. (2024). Natural sweetener glycyrrhizin protects against precocious puberty by modulating the gut microbiome. Life Sci. 350:122789. doi: 10.1016/j.lfs.2024.122789
Nguyen, X. P., Nakamura, T., Osuka, S., Bayasula, B., Nakanishi, N., Kasahara, Y., et al. (2019). Effect of the neuropeptide phoenixin and its receptor Gpr173 during folliculogenesis. Reproduction 158, 25–34. doi: 10.1530/REP-19-0025
Onishi, J. C., Campbell, S., Moreau, M., Patel, F., Brooks, A. I., Zhou, Y. X., et al. (2017). Bacterial communities in the small intestine respond differently to those in the caecum and colon in mice fed low-and high-fat diets. Microbiology 163, 1189–1197. doi: 10.1099/mic.0.000496
Org, E., Mehrabian, M., Parks, B. W., Shipkova, P., Liu, X., Drake, T. A., et al. (2016). Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7, 313–322. doi: 10.1080/19490976.2016.1203502
Paluschinski, M., Castoldi, M., Schöler, D., Bardeck, N., Oenarto, J., Görg, B., et al. (2019). Tauroursodeoxycholate protects from glycochenodeoxycholate-induced gene expression changes in perfused rat liver. Biol. Chem. 400, 1551–1565. doi: 10.1515/hsz-2019-0204
Park, S., Lee, S., Kim, Y., Lee, Y., Kang, M. W., Kim, K., et al. (2021). Causal effects of relative fat, protein, and carbohydrate intake on chronic kidney disease: a Mendelian randomization study. Am. J. Clin. Nutr. 113, 1023–1031. doi: 10.1093/ajcn/nqaa379
Parker, A., Fonseca, S., and Carding, S. R. (2020). Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 11, 135–157. doi: 10.1080/19490976.2019.1638722
Perry, J. R., Day, F., Elks, C. E., Sulem, P., Thompson, D. J., Ferreira, T., et al. (2014). Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 514, 92–97. doi: 10.1038/nature13545
Qian, Y., Fang, X., Chen, Y., Ding, M., and Gong, M. (2024). Gut flora influences the hypothalamic-gonadal axis to regulate the pathogenesis of obesity-associated precocious puberty. Sci. Rep. 14:28844. doi: 10.1038/s41598-024-80140-8
Qin, N., Song, G., Ren, X., Zhang, L., Gao, J., Xia, X., et al. (2020). Fish oil extracted from Coregonus peled improves obese phenotype and changes gut microbiota in a high-fat diet-induced mouse model of recurrent obesity. Food Funct. 11, 6158–6169. doi: 10.1039/d0fo00911c
Reynolds, C. M., Segovia, S. A., and Zhang, X. D. (2015). Conjugated linoleic acid supplementation during pregnancy and lactation reduces maternal high-fat-diet-induced programming of early-onset puberty and hyperlipidemia in female rat offspring. Biol. Reprod. 92, 1–10. doi: 10.1095/biolreprod.114.125047
Roberts, M. N., Wallace, M. A., Tomilov, A. A., Zhou, Z., Marcotte, G. R., Tran, D., et al. (2017). A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 26, 539–546.e5. doi: 10.1016/j.cmet.2017.08.005
Rogers, I. S., Northstone, K., Dunger, D. B., Cooper, A. R., Ness, A. R., and Emmett, P. M. (2010). Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr. 13, 2052–2063. doi: 10.1017/S1368980010001461
Sahin, Z., Canpolat, S., Ozcan, M., Ozgocer, T., and Kelestimur, H. (2015). Kisspeptin antagonist prevents Rf9-induced reproductive changes in female rats. Reproduction 149, 465–473. doi: 10.1530/REP-14-0683
Salvi, R., Castillo, E., Voirol, M.-J., Glauser, M., Rey, J. P., Gaillard, R. C., et al. (2006). Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology 147, 816–826. doi: 10.1210/en.2005-0728
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R., and Rastall, R. A. (2019). Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 605–616. doi: 10.1038/s41575-019-0173-3
Schoeler, M., and Caesar, R. (2019). Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 20, 461–472. doi: 10.1007/s11154-019-09512-0
Selmin, O. I., Papoutsis, A. J., Hazan, S., Smith, C., Greenfield, N., Donovan, M. G., et al. (2021). N-6 high fat diet induces gut microbiome dysbiosis and colonic inflammation. Int. J. Mol. Sci. 22:6919. doi: 10.3390/ijms22136919
Shahatah, M. A., Jadkarim, A. M., Banjar, R. Z., Kabli, Y. O., Milyani, A. A., and al-Agha, A. E. (2021). The relationship between body weight and dietary habits with respect to the timing of puberty among Saudi children and adolescents. Ann. Afr. Med. 20, 193–197. doi: 10.4103/aam.aam_41_20
Shang, Y., Khafipour, E., Derakhshani, H., Sarna, L. K., Woo, C. W., Siow, Y. L., et al. (2017). Short term high fat diet induces obesity-enhancing changes in mouse gut microbiota that are partially reversed by cessation of the high fat diet. Lipids 52, 499–511. doi: 10.1007/s11745-017-4253-2
Shan-Shan, S., Kai, W., Ke, M., Bao, L., and Liu, H.-W. (2019). An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in Ob/Ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 17, 3–14. doi: 10.1016/S1875-53641930003-2
Sheremeta, C.-L., Yarlagadda, S., Smythe, M. L., and Noakes, P. G. (2024). Prostaglandins in the inflamed central nervous system: potential therapeutic targets. Curr. Drug Targets 25, 885–908. doi: 10.2174/0113894501323980240815113851
Shi, L., Jiang, Z., and Zhang, L. (2022). Childhood obesity and central precocious puberty. Front. Endocrinol. 13:1056871. doi: 10.3389/fendo.2022.1056871
Shihabudeen, M. S., Roy, D., James, J., and Thirumurugan, K. (2015). Chenodeoxycholic acid, an endogenous Fxr ligand alters adipokines and reverses insulin resistance. Mol. Cell. Endocrinol. 414, 19–28. doi: 10.1016/j.mce.2015.07.012
Shim, Y. S., Lee, H. S., and Hwang, J. S. (2021). Genetic factors in precocious puberty. Clin. Exp. Pediatr. 65:172. doi: 10.3345/cep.2021.00521
Shirley, S. H., Rundhaug, J. E., Tian, J., Cullinan-Ammann, N., Lambertz, I., Conti, C. J., et al. (2009). Transcriptional regulation of estrogen receptor-α by p53 in human breast cancer cells. Cancer Res. 69, 3405–3414. doi: 10.1158/0008-5472.CAN-08-3628
Sitticharoon, C., Sukharomana, M., Likitmaskul, S., Churintaraphan, M., and Maikaew, P. (2017). Increased high molecular weight adiponectin, but decreased total adiponectin and kisspeptin, in central precocious puberty compared with aged-matched prepubertal girls. Reprod. Fertil. Dev. 29, 2466–2478. doi: 10.1071/RD16282
Stathori, G., Tzounakou, A.-M., Vlahos, N. F., Charmandari, E., and Valsamakis, G. (2025). Unveiling the link: obesity, diet, hypothalamic inflammation and central precocious puberty-recent insights and implications. Horm. Res. Paediatr., 1–17. doi: 10.1159/000544837
Stein, L. M., Tullock, C. W., Mathews, S. K., Garcia-Galiano, D., Elias, C. F., Samson, W. K., et al. (2016). Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am. J. Phys. Regul. Integr. Comp. Phys. 311, R489–R496. doi: 10.1152/ajpregu.00191.2016
Stenman, L. K., Holma, R., Eggert, A., and Korpela, R. (2013). A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. American J. Physiol. Gastrointestinal Liver Physiol. 304, G227–G234. doi: 10.1152/ajpgi.00267.2012
Strandwitz, P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Res. 1693, 128–133. doi: 10.1016/j.brainres.2018.03.015
Straniero, S., Laskar, A., Savva, C., Härdfeldt, J., Angelin, B., and Rudling, M. (2020). Of mice and men: murine bile acids explain species differences in the regulation of bile acid and cholesterol metabolism. J. Lipid Res. 61, 480–491. doi: 10.1194/jlr.RA119000307
Su, S., Wang, X., Xi, X., Zhu, L., Chen, Q., Zhang, H., et al. (2021). Phellodendrine promotes autophagy by regulating the Ampk/mtor pathway and treats ulcerative colitis. J. Cell. Mol. Med. 25, 5707–5720. doi: 10.1111/jcmm.16587
Sui, Z., Zhang, Y., Zhang, Z., Wang, C., Li, X., and Xing, F. (2023). Lin28B overexpression decreases let-7b and let-7g levels and increases proliferation and estrogen secretion in Dolang sheep ovarian granulosa cells. Archives Animal Breed. 66, 217–224. doi: 10.5194/aab-66-217-2023
Szamreta, E. A., Qin, B., Rivera-Núñez, Z., Parekh, N., Barrett, E. S., Ferrante, J., et al. (2020). Greater adherence to a Mediterranean-like diet is associated with later breast development and menarche in peripubertal girls. Public Health Nutr. 23, 1020–1030. doi: 10.1017/S1368980019002349
Tarçin, G., Bayramoğlu, E., and Güneş Kaya, D. (2024). The role of body composition and appetite-regulating hormones in idiopathic central precocious puberty and their changes during Gnrh analog therapy. J. Endocrinol. Investig. 48, 1–8. doi: 10.1007/s40618-024-02413-3
Thaler, J. P., Yi, C.-X., Schur, E. A., Guyenet, S. J., Hwang, B. H., Dietrich, M. O., et al. (2012). Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162. doi: 10.1172/JCI59660
Toledo, M., Martínez-Martínez, S., and Van Hul, M. (2025). Rapid modulation of gut microbiota composition by hypothalamic circuits in mice. Nat. Metab., 1–13. doi: 10.1038/s42255-025-01280-3
Tzounakou, A.-M., Stathori, G., Paltoglou, G., Valsamakis, G., Mastorakos, G., Vlahos, N. F., et al. (2024). Childhood obesity, hypothalamic inflammation, and the onset of puberty: a narrative review. Nutrients 16:1720. doi: 10.3390/nu16111720
Ullah, R., Raza, A., Rauf, N., Shen, Y., Zhou, Y. D., and Fu, J. (2019). Postnatal feeding with a fat rich diet induces precocious puberty independent of body weight, body fat, and leptin levels in female mice. Front. Endocrinol. 10:758. doi: 10.3389/fendo.2019.00758
Valsamakis, G., Arapaki, A., Balafoutas, D., Charmandari, E., and Vlahos, N. F. (2021). Diet-induced hypothalamic inflammation, phoenixin, and subsequent precocious puberty. Nutrients 13:3460. doi: 10.3390/nu13103460
Vanden Brink, H., Vandeputte, D., and Brito, I. L. (2024). Changes in the bile acid pool and timing of female puberty: potential novel role of hypothalamic Tgr5. Endocrinology 165:bqae098. doi: 10.1210/endocr/bqae098
Wang, Y., Jin, C., Li, H., Liang, X., Zhao, C., Wu, N., et al. (2024). Gut microbiota-metabolite interactions mediate the effect of dietary patterns on precocious puberty. Iscience 27:109887. doi: 10.1016/j.isci.2024.109887
Wang, Z., Liu, D., Wang, F., Liu, S., Zhao, S., Ling, E. A., et al. (2012). Saturated fatty acids activate microglia via toll-like receptor 4/Nf-κB signalling. Br. J. Nutr. 107, 229–241. doi: 10.1017/S0007114511002868
Wang, M., Sun, P., Chai, X., Liu, Y. X., Li, L., Zheng, W., et al. (2024). Reconstituting gut microbiota-colonocyte interactions reverses diet-induced cognitive deficits: the beneficial of eucommiae cortex polysaccharides. Theranostics 14, 4622–4642. doi: 10.7150/thno.99468
Wang, L., Xu, H., Tan, B., Yi, Q., Liu, H., Deng, H., et al. (2022). Gut microbiota and its derived Scfas regulate the Hpga to reverse obesity-induced precocious puberty in female rats. Front. Endocrinol. 13:1051797. doi: 10.3389/fendo.2022.1051797
Wang, L., Yi, Q., Xu, H., Liu, H., Tan, B., Deng, H., et al. (2024). Alterations in the gut microbiota community are associated with childhood obesity and precocious puberty. BMC Microbiol. 24:311. doi: 10.1186/s12866-024-03461-8
Wang, F., Yoder, S. M., Yang, Q., Kohan, A. B., Kindel, T. L., Wang, J., et al. (2015). Chronic high-fat feeding increases Gip and Glp-1 secretion without altering body weight. American J. Physiol. Gastrointestinal Liver Physiol. 309, G807–G815. doi: 10.1152/ajpgi.00351.2013
Wang, M., Zhang, Y., Miller, D., Rehman, N. O., Cheng, X., Yeo, J. Y., et al. (2020). Microbial reconstitution reverses early female puberty induced by maternal high-fat diet during lactation. Endocrinology 161:bqz041. doi: 10.1210/endocr/bqz041
Watanabe, M., Fukuda, A., and Nabekura, J. (2014). The role of GABA in the regulation of Gnrh neurons. Front. Neurosci. 8:387. doi: 10.3389/fnins.2014.00387
Wen, J., Jiang, Y., Lei, Z., He, J., Ye, M., and Fu, W. (2021). Leptin influence cholelithiasis formation by regulating bile acid metabolism. Turk J Gastroenterol 32, 97–105. doi: 10.5152/tjg.2020.19594
Wiley, A. S. (2011). Milk intake and total dairy consumption: associations with early menarche in Nhanes 1999-2004. PLoS One 6:e14685. doi: 10.1371/journal.pone.0014685
Winters, S. J., Ghooray, D. T., Yang, R. Q., Holmes, J. B., O’Brien, A. R. W., Morgan, J., et al. (2014). Dopamine-2 receptor activation suppresses Pacap expression in gonadotrophs. Endocrinology 155, 2647–2657. doi: 10.1210/en.2013-2147
Wu, G. D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y. Y., Keilbaugh, S. A., et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. doi: 10.1126/science.1208344
Wu, N., Jiang, X., Liu, Y., Zhang, M., Yue, M., Chen, F., et al. (2025). Glycodeoxycholic acid alleviates central precocious puberty by modulating gut microbiota and metabolites in high-fat diet-fed female rats. Cell. Mol. Life Sci. 82, 1–14. doi: 10.1007/s00018-025-05680-2
Xie, Y., Li, X., Wang, M., Chu, M., and Cao, G. (2025). Lin28b-let-7 modulates mrna expression of Gnrh1 through multiple signaling pathways related to glycolysis in Gt1-7 cells. Animals 15:120. doi: 10.3390/ani15020120
Xie, L., Tang, Q., Yao, D., Gu, Q., Zheng, H., Wang, X., et al. (2021). Effect of decaffeinated green tea polyphenols on body fat and precocious puberty in obese girls: a randomized controlled trial. Front. Endocrinol. 12:736724. doi: 10.3389/fendo.2021.736724
Xiong, J., Xu, Y., Liu, X., Wang, X., Shan, S., Crabbe, M. J. C., et al. (2022). Prospective association of dietary soy and fibre intake with puberty timing: a cohort study among Chinese children. BMC Med. 20:145. doi: 10.1186/s12916-022-02320-5
Xu, Y., Xiong, J., Gao, W., Wang, X., Shan, S., Zhao, L., et al. (2022). Dietary fat and polyunsaturated fatty acid intakes during childhood are prospectively associated with puberty timing independent of dietary protein. Nutrients 14:275. doi: 10.3390/nu14020275
Yan, D., Fan, P., Sun, W., Ding, Q., Zheng, W., Xiao, W., et al. (2021). Anemarrhena asphodeloides modulates gut microbiota and restores pancreatic function in diabetic rats. Biomed. Pharmacother. 133:110954. doi: 10.1016/j.biopha.2020.110954
Yang, Y., Cao, S., Xu, W., Zang, C., Zhang, F., Xie, Y., et al. (2022). Dual modulation of gut bacteria and fungi manifests the gut-based anti-hyperlipidemic effect of Coptidis Rhizoma. Biomed. Pharmacother. 153:113542. doi: 10.1016/j.biopha.2022.113542
Yang, X., Tan, J., Xu, X., Yang, H., Wu, F., Xu, B., et al. (2020). Prepubertal overexposure to manganese induce precocious puberty through Gabaa receptor/nitric oxide pathway in immature female rats. Ecotoxicol. Environ. Saf. 188:109898. doi: 10.1016/j.ecoenv.2019.109898
Yanguas-Casás, N., Barreda-Manso, M. A., Nieto-Sampedro, M., and Romero-Ramírez, L. (2017). Tudca: An agonist of the bile acid receptor Gpbar1/Tgr5 with anti-inflammatory effects in microglial cells. J. Cell. Physiol. 232, 2231–2245. doi: 10.1002/jcp.25742
Yao, S., Lopez-Tello, J., and Sferruzzi-Perri, A. N. (2021). Developmental programming of the female reproductive system—a review. Biol. Reprod. 104, 745–770. doi: 10.1093/biolre/ioaa232
Yi, L.-F., Wen, H.-X., and Qiu, M. (2017). An analysis of cardiac autonomic nerve function in girls with idiopathic central precocious puberty. Zhongguo Dang Dai Er Ke Za Zhi = Chin. J. Contemp. Pediatr. 19, 1239–1242. doi: 10.7499/j.issn.1008-8830.2017.12.003
Yi, C., Zou, H., Lin, X., Liu, S., Wang, J., Tian, Y., et al. (2024). Zhibai dihuang pill (Zbdh) exhibits therapeutic effects on idiopathic central sexual precocity in rats by modulating the gut microflora. Heliyon 10:e29723. doi: 10.1016/j.heliyon.2024.e29723
Yu, Z., Zhan, Q., Chen, A., Han, J., Zheng, Y., Gong, Y., et al. (2021). Intermittent fasting ameliorates di-(2-ethylhexyl) phthalate-induced precocious puberty in female rats: a study of the hypothalamic–pituitary–gonadal axis. Reprod. Biol. 21:100513. doi: 10.1016/j.repbio.2021.100513
Yuan, X., Shangguan, H., Zhang, Y., Lin, X., and Chen, R. (2023). Intervention effect of probiotics on the early onset of puberty induced by Daidzein in female mice. Mol. Nutr. Food Res. 67:e2200501. doi: 10.1002/mnfr.202200501
Yue, M., and Zhang, L. (2024). Exploring the mechanistic interplay between gut microbiota and precocious puberty: a narrative review. Microorganisms 12:323. doi: 10.3390/microorganisms12020323
Zanjirband, M., Hodayi, R., Safaeinejad, Z., Nasr-Esfahani, M. H., and Ghaedi-Heydari, R. (2023). Evaluation of the p53 pathway in polycystic ovarian syndrome pathogenesis and apoptosis enhancement in human granulosa cells through transcriptome data analysis. Sci. Rep. 13:11648. doi: 10.1038/s41598-023-38340-1
Zengul, A. G. (2019). Exploring the link between dietary fiber, the gut microbiota and estrogen metabolism among women with breast cancer. Birmingham: the University of Alabama.
Zhang, C., Fang, R., Lu, X., Zhang, Y., Yang, M., Su, Y., et al. (2022). Lactobacillus reuteri J1 prevents obesity by altering the gut microbiota and regulating bile acid metabolism in obese mice. Food Funct. 13, 6688–6701. doi: 10.1039/D1FO04387K
Zhang, H.-R., Xiao, Y., and Jiang, S.-Q. (2025). Diagnostic value of fasting insulin and insulin-like growth factor-1 levels in girls with central precocious puberty. J. Pediatr. Endocrinol. Metab. 38, 240–247. doi: 10.1515/jpem-2024-0479
Zhao, J., Bi, W., Xiao, S., Lan, X., Cheng, X., Zhang, J., et al. (2019). Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 9:5790. doi: 10.1038/s41598-019-42286-8
Zhao, Q., Hou, D., Fu, Y., Xue, Y., Guan, X., and Shen, Q. (2021). Adzuki bean alleviates obesity and insulin resistance induced by a high-fat diet and modulates gut microbiota in mice. Nutrients 13:3240. doi: 10.3390/nu13093240
Zheng, H., Xu, P., Jiang, Q., Xu, Q., Zheng, Y., Yan, J., et al. (2021). Depletion of acetate-producing bacteria from the gut microbiota facilitates cognitive impairment through the gut-brain neural mechanism in diabetic mice. Microbiome 9:145. doi: 10.1186/s40168-021-01088-9
Zhu, H., Chen, L., and Jiang, Y.-J. (2008). Relationship of plasma ghrelin and adenohypophyseal hormone levels in female precocious puberty. Zhejiang Da Xue Xue Bao Yi Xue Ban = J. Zhejiang Univ. Med. Sci. 37, 506–510. doi: 10.3785/j.issn.1008-9292.2008.05.015
Zurita-Cruz, J. N., Villasís-Keever, M. A., Manuel-Apolinar, L., Damasio-Santana, L., Gutierrez-Gonzalez, A., Wakida-Kusunoki, G., et al. (2021). Altered cardiometabolic profile in girls with central precocious puberty and adipokines: a propensity score matching analysis. Cytokine 148:155660. doi: 10.1016/j.cyto.2021.155660
Glossary
PP - precocious puberty
HFD - a high-fat diet
GM - gut microbiota
CPP - central precocious puberty
PPP - peripheral precocious puberty
HPG - hypothalamic–pituitary-gonadal
NO - nitric oxide
E2 - estradiol
LPS - lipopolysaccharides
SCFAs - short-chain fatty acids
SCFA - short-chain fatty acid
BA - bile acid
BAs - bile acids
GABA - γ-aminobutyric acid
5-HT - serotonin
DA - dopamine
GLP-1 - glucagon-like peptide-1
GPR - G-protein coupled receptor
FMT - Fecal Microbiota Transplantation
TCM - Traditional Chinese medicine
MGB - microbiota-gut-brain
BBB - blood–brain barrier
GDCA - glycodeoxycholic acid
BMI - body mass index
PKC - protein kinase C
ERK1/2 - extracellular signal-regulated kinase1/2
LH - luteinizing hormone
FSH - follicle-stimulating hormone
APN - adiponectin
IGF-1 - insulin-like growth factor-1
PI3K - phosphatidylinositol-3-kinase
MUFAs - monounsaturated fatty acids
PUFAs - polyunsaturated fatty acids
TGR5 - takeda G-protein-coupled receptor 5
FXR - farnesoid X receptor
mTOR - mammalian target of rapamycin
SF1 - steroidogenic factor 1
Mdm2 - mouse double minute 2 homolog
AMPK - adenosine-monophosphate-activated protein kinase
ACTH - adrenocorticotropic hormone
ERα - estrogen receptor α
GHSR - growth hormone secretagogue receptors
AKT - protein kinase B
Keywords: high-fat diet, gut microbiota, precocious puberty, probiotics, gut metabolites
Citation: Wu N, Ning K, Liu Y, Wang Q, Li N and Zhang L (2025) Relationship between high-fat diet, gut microbiota, and precocious puberty: mechanisms and implications. Front. Microbiol. 16:1564902. doi: 10.3389/fmicb.2025.1564902
Edited by:
Mohammad Altamimi, An-Najah National University, PalestineReviewed by:
Doureradjou Peroumal, University of Pittsburgh, United StatesHuoxiang Zhou, Zhengzhou University, China
Copyright © 2025 Wu, Ning, Liu, Wang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, emhhbmdsZWk3QHNkdS5lZHUuY24=; Ning Li, NzU0NTIwNjIzQHFxLmNvbQ==; Qinghua Wang, Y2htX3dhbmdxaEB1am4uZWR1LmNu
†These authors have contributed equally to this work
 Nan Wu1†
Nan Wu1† Ke Ning
Ke Ning Lei Zhang
Lei Zhang