- Food and Feed Safety Research Unit, USDA-ARS Southern Regional Research Center, New Orleans, LA, United States
Background: Mycotoxigenic fungi pose significant threats to food safety and marketability. Crop-specific differences in susceptibility to these fungi can influence contamination levels.
Objectives: The resistance or susceptibility of protein-rich pulse crops—chickpeas (Cicer arietinum L. cv. CDC Frontier), lentils (Lens culinaris Medik cv. Eston), peas (Pisum sativum L. cv. LeRoy), and corn (Zea mays L. cv. H97C) to infection by Aspergillus flavus were evaluated using a kernel screening assay (KSA).
Methodology: A. flavus strain 70 (AF-70) expressing green-fluorescent protein (GFP) was used to quantify fungal spread and mycotoxin production. Fungal infection and toxin levels, including aflatoxins (AFB1, AFB2), cyclopiazonic acid (CPA), and α-aflatrem, were monitored at 2-day intervals over a 10-day period post inoculation.
Results: Although all seeds were infected by A. flavus, corn produced significantly higher levels of AFB1 and AFB2 compared to pulses. However, pulses accumulated relatively higher levels of CPA and α‑aflatrem.
Conclusion: While pulses may be less susceptible to aflatoxin contamination than corn, the elevated concentrations of CPA and α‑aflatrem underscore the need for further toxicological evaluation and mechanistic studies. Future research should explore the underlying resistance mechanisms from field to storage to better ensure crop safety.
1 Introduction
Increasing human population and depletion of natural resources in recent decades have become critical issues that need to be addressed globally (Quintieri et al., 2023). The global population is projected to reach 9.7 billion by 2050, up from the current 8.0 billion, thus adding approximately 1.7 billion people over the next 26 years (United Nations Department of Economic and Social Affairs, 2022). Consequently, the demand for healthy, nutritious, and sustainable food and feed products is paramount. Current food and feed systems, particularly livestock-based meat production, would benefit from incorporating plant-rich protein sources like pulses (i.e., beans, chickpeas, lentils, etc.). This would create a synergistic balance to feed the growing population and reduce environmental impacts through decreased water and land usage (Heinke et al., 2020; Godfray et al., 2018). The plant-based protein industry is rapidly expanding and is projected to become a $27 billion industry by 2030 (Singh et al., 2024). Including pulses would aid in diversifying cropping systems, providing a sustainable nutrition source, and developing a resilient food and feed supply chain for the growing population (Singh et al., 2024).
Chickpeas (Cicer arietinum L.), lentils (Lens culinaris Medik), and peas (Pisum sativum L.) are cultivated pulse crops grown worldwide, belonging to the Fabaceae (Leguminosae) family (Henchion et al., 2017; Quintieri et al., 2023). Currently, these pulses offer a good balance between sustainability and nutritional value, providing high dietary protein, fiber, and energy content (Acuña-Gutiérrez et al., 2022). The Dietary Guidelines Advisory Committee recommends a plant-based diet to reduce cholesterol, improve muscle mass, maintain bone health, address obesity, and meet protein requirements more effectively than animal-based proteins (Gavrilova et al., 2020; George et al., 2020; Singh et al., 2024). Protein concentrations for chickpeas, lentils, and peas range from 12.6–30.6%, 20.6–31.4%, and 21.2–32.9%, respectively (Dahl et al., 2012; Wood and Grusak, 2007; Yadav et al., 2007). In comparison, the average protein content of animal-based proteins, excluding fish and insects, is 22.0% (Day et al., 2022). Introducing pulses into diets may prevent various diseases, enhance global food and feed security, and provide high protein content alongside traditional animal-meat products or in formulations for meat alternatives (Hertzler et al., 2020; Massawe et al., 2016; Quintieri et al., 2023). Despite being labeled as “underutilized” legume crops, pulses are widely used in many countries. These plant-rich protein sources need to be evaluated for food and feed safety and quality due to their inclusion in human and livestock diets. Pulses are excellent candidates for developing novel food and feed products, including meat-based alternatives (Acuña-Gutiérrez et al., 2022; Gräfenhahn and Beyrer, 2024; Rebello et al., 2014).
Contamination of grains and pulses by fungi, and subsequently mycotoxins, can occur at any stage of the supply chain: in the field, at harvest, during transportation, and storage (Begum and Samajpati, 2000). Aflatoxins, secondary metabolites produced by Aspergillus spp., contaminate a variety of food and feed crops globally, including well-studied crops such as corn, cottonseeds, peanuts, and tree nuts pre- and post-harvest (CAST, 2003). Aflatoxin B1 (AFB1), a potent mutagenic and carcinogenic mycotoxin produced by Aspergillus flavus and A. parasiticus, has been extensively studied in various food and feed sources (Kumar et al., 2021). However, there is limited literature on the susceptibility of pulses to Aspergillus spp. and aflatoxin production (including strains B1 and B2) (Acuña-Gutiérrez et al., 2022). Currently, there are no specific regulatory limits for aflatoxin in pulses; however, in the US, corn has a 20-ppb (parts per billion) limit for general commerce (FDA, 2025). Chickpeas have been documented as susceptible to fungal pathogens, including Aspergillus and Fusarium species, primarily due to poor field conditions, mechanical damage during harvest, inadequate transportation, processing issues, and poor storage conditions (Donato et al., 2022; Ramirez et al., 2018). While aflatoxin has been reported in lentils in Iran and Egypt, the prevalence was low, with mean levels below the limit of detection (LOD) (Ahmadi et al., 2022; El-Maraghy, 1988). Conversely, in Bangladesh, aflatoxin levels in lentils exceeded the U.S. maximum regulatory limit of 20 ppb for human food consumption (Roy et al., 2013). Peas (Pisum sativum L.) have shown high resistance to aflatoxin formation, with studies indicating no detectable toxin from pulses infected with A. flavus (El-Kady et al., 1996).
With limited data on the accumulation of aflatoxin in pulses such as chickpeas, lentils, and peas, more studies are needed to understand the effects of infection, given the increasing demand for protein-rich plant sources for dietary consumption and the need to address public health concerns and sustainability of food supply systems. This study utilized Aspergillus flavus AF-70, a toxin-producing strain expressing a green fluorescent protein (GFP), to assess fungal growth and the accumulation rates of AFB1, AFB2, cyclopiazonic acid (CPA), and α-aflatrem in chickpeas, lentils, peas, and corn over a 10-day period with 2-day interval sampling post-inoculation. The objective was to understand fungal infection patterns, colonization, growth, and aflatoxin accumulation in protein-rich pulses using a kernel screening assay (KSA). The hypothesis was that under identical infection pressure by Aspergillus flavus, protein-rich pulse crops, specifically chickpeas, lentils, and peas, accumulate lower levels of aflatoxins compared to corn.
2 Materials and methods
2.1 Fungal strains and growth conditions
Aflatoxigenic A. flavus 70-GFP (AF-70 GFP) was grown in the dark at 31°C on a 2X V8 medium (10% V8 juice, 2% agar, pH 5.2) (Rajasekaran et al., 2008). Spores from 7-d old cultures were suspended in 0.02% Triton X-100, and the conidial concentration was determined with a hemocytometer and adjusted to 3.0 × 106 conidia/mL. In AF-70 GFP, GFP is produced under control of the constitutively expressed A. nidulans glyceraldehyde phosphate dehydrogenase (gpdA) gene promoter inserted into niaD (Rajasekaran et al., 2008).
2.2 Plant-rich protein sources
Chickpeas [Cicer arietinum L. cv. CDC Frontier], lentils [Lens culinaris Medik cv. Eston], and peas [Pisum sativum L. cv. LeRoy] were provided by USDA-ARS laboratories (Dr. Clarice J. Coyne and Dr. Marilyn Warburton, Western Regional Plant Introduction Station, Washington State University, Pullman, WA). The seed source location and year for chickpeas, lentils, and peas were Central Ferry, WA, and Richland, MT, in years 2022, 2019, and 2023, respectively. These varieties were selected due to germplasm resources provided by USDA-ARS. A non-GMO, yellow, dent-corn hybrid [Zea mays], H97C (Hybrid85, Omaha, NE, United States) was purchased from corn produced for growers seed in 2022. Corn was included as a positive control in this study. All botanical terms in this manuscript are according to Kiesselbach (1999) and Allaire and Brady (2008).
2.3 Kernel screening assay
Undamaged lentil, corn, pea, and chickpea seeds were surface sterilized with 70% ethanol and subjected to a KSA (Brown et al., 1993; Brown et al., 1995; Rajasekaran et al., 2013). The same procedures developed for corn were used for pulses to determine A. flavus infection, as there was a lack of published methods specifically for pulses. Surface sterilized seeds were briefly immersed in a 3.0 × 106 conidial inoculum and placed in plastic vial caps (20 mm diameter, 6 mm height). The same procedure was applied for day 0 seeds but were not treated with A. flavus suspension. Four caps were placed in an open-tissue culture dish (60 × 15 mm; Becton Dickinson, CO., Oxnard, CA, United States), representing one experimental unit. Culture dishes were placed side by side in a clear tray (243 × 243 × 18 mm, Nunc bioassay dish; Thomas Scientific, Swedesboro, NJ, USA) lined with 3 mm chromatography paper (Whatman International, Maidstone, UK) (Figure 1). The lid was placed on top of the tray but was not sealed. High humidity (>95% RH) was maintained by adding 25 mL of sterile deionized water to the trays. Seeds were incubated in the dark at 31°C and sampled at day 0, 2, 4, 6, 8, and 10 after inoculation with A. flavus.
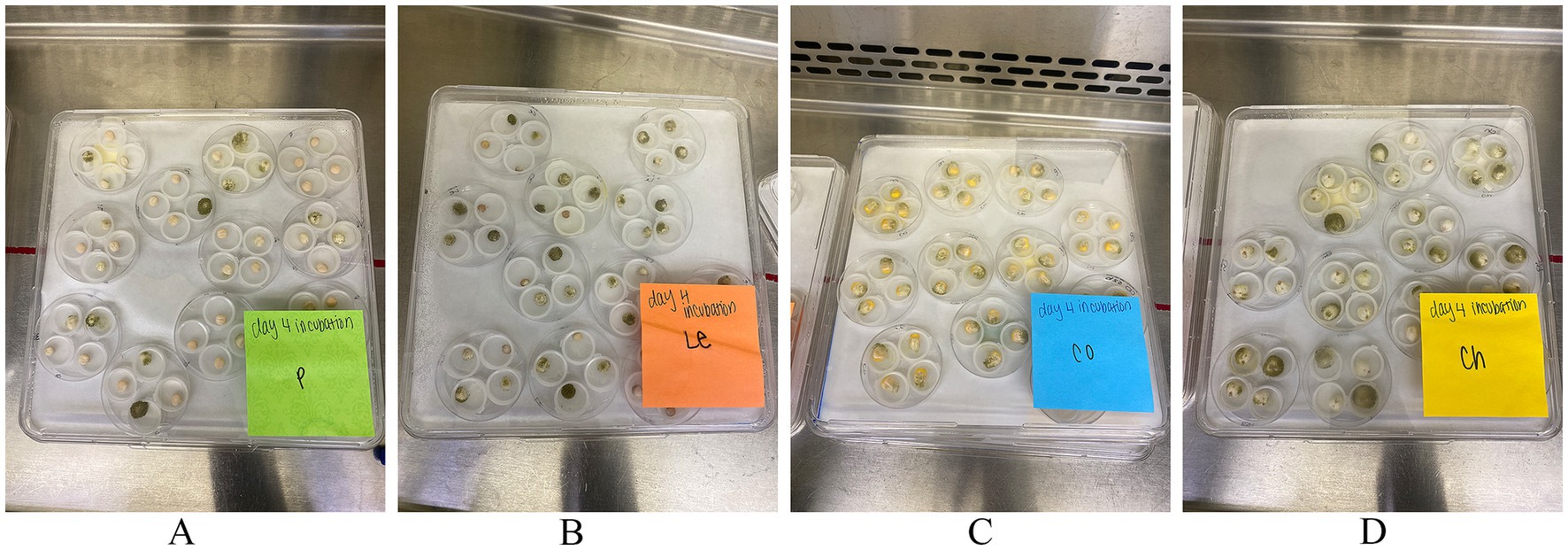
Figure 1. A typical kernel screening assay (KSA) set up. Each culture dish contains 4 seeds, constituting a replication, with 9 total replicates for analysis and 2 replicates for imaging. Seeds showcase Aspergillus flavus infection on day 4 of (A) peas, (B) lentils, (C) corn, and (D) chickpeas.
2.4 GFP quantitation
Chickpeas, lentils, peas, and corn were ground and homogenized using a SPEX SamplePrep Geno/Grinder 2010 (1740 rpm, 1.5 min; Cole-Parmer, Vernon Hills, IL, United States), with 3/8″ stainless steel balls. The samples were stored at −80°C until analysis, where seeds were diluted in 1.0 mL Sorenson’s phosphate buffer (pH 7.0) and centrifuged at 10,000 rpm for 15 min. Supernatants were then analyzed for GFP using a BioTek Synergy Neo2 (Agilent, Santa Clara, CA) with excitation at 485 nm and emission at 535 nm. Relative fluorescence units were used for statistical analyses and normalized as % values relative to the highest data point.
2.5 Aflatoxin, cyclopiazonic acid, and α-alfatrem extraction and analysis
Following the growth of fungal strains on seeds, AFB1, AFB2, CPA, and α-aflatrem were extracted for analysis. The materials were ground in 15 mL polycarbonate vials (OPS Diagnostics, Lebanon, NJ, United States) with a SPEX SamplePrep Geno/Grinder 2010 (1,740 rpm, 1.5 min; Cole-Parmer, Vernon Hills, IL, United States), and 25 mg of sample material was weighed with 500 mL of 100% methanol (MeOH). Samples were shaken overnight on a shaker table at room temperature (22°C) and 210 RPM. The extracts were filtered through cotton plugs, and the filtrates were concentrated using a Savant speedvac (Thermo Scientific). Each extract was redissolved in methanol (1 mL), particulates were removed via centrifuge, and the supernatant was analyzed using a Waters (Milford, MA, United States) Acquity Ultra Performance Liquid Chromatography (UPLC) system (40% methanol in water, BEH C18 1.7 μm, 2.1 mm × 50 mm column) using fluorescence detection (excitation: λ = 365 nm, emission: λ = 440 nm). Samples were diluted to 10-fold if the aflatoxin signal saturated the detector. An analytical standard (Sigma-Aldrich) was used to identify and quantify AFB1 and AFB2. Aflatoxin content was expressed in ng AFB1/g and AFB2/g seed sample (ppb).
To assess the presence of CPA and α-aflatrem, the re-dissolved, centrifuged extracts were diluted 10-fold and analyzed on a Waters Acquity UPLC and Xevo G2 XS QTOF mass spectrometer (MS) as previously reported (Moore et al., 2022), briefly: the MS was equipped with a Z-spray ionization source running in ESI + mode using Waters MassLynx 4.2 software Separation was achieved with a gradient solvent system (A: 0.1% formic acid in water; B: 0.1% formic acid in acetonitrile) on a Waters BEH C18 1.7 μm, 2.1 × 50 mm column: 5% B (0–1.25 min.), to 25% B (1.25–1.5 min.), to 100% B (1.5–5.0 min.), then 100% B (5.0–7.5 min.), followed by column equilibration at 5% B (7.6–10.1 min.). Data were analyzed on Waters UNIFI 1.9.4 software using the “Quantify Assay Tof 2D” analysis method with lock mass corrected by UNIFI. CPA and α-aflatrem was purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, United States) and used for quantification. CPA and α-aflatrem content were expressed in ng CPA/g per sample (ppb) and ng α-aflatrem/g per sample (ppb).
2.6 Microscopy
At each sampling time frame, eight seeds were randomly chosen and photographed using a Nikon-SMZ25 research stereomicroscope (Nikon Instruments, Melville, NY, United States) equipped with an Andor Zyla 4.2 sCMOS Digital Camera (Nikon Instruments, Melville, NY, United States) including fluorescence and bright field images of AF-70 GFP. The seeds were then divided evenly for use in aflatoxin analysis and GFP quantitation. A minimum of 9 replicates (4 seeds each) were used for each day of sampling. The seed exteriors were cleaned with 9 mL of deionized water and vortexed for 15 s to remove spores on the seed pericarps for counting and stored at 4.0°C. All seeds were photographed both externally and internally with a horizontal cross-section following methods published by Rajasekaran et al. (2013).
2.7 Spore counts on seed coats
The seeds were placed in 15 mL centrifuge tubes with a plug seal cap (Corning Inc., Corning, NY, United States) with 9 mL of deionized water, vortexed for 15 s, and removed to collect external mycelia for spore counts. The samples were stored at 4.44°C until analysis, where samples were vortexed for 5 s, 10 μL of liquid was pipetted into disposable hemocytometer slides containing 4 replicates per sample and analyzed using an Olympus Cell Counter model R1 (Olympus Life Sciences, Waltham, MA, United States). The seeds were externally cleaned with Kimtech Wipes (Kimtech Science, Vaughan, Ontario), deionized water, and placed in 15 mL polycarbonate vials (OPS Diagnostics, Lebanon, NJ, United States) stored at −80°C until further analysis.
2.8 Statistical analysis
Average AF-70 GFP fluorescence, AFB1 and AFB2 values, external spore counts, CPA, and α-aflatrem from a minimum of nine replicates per days 0, 2, 4, 6, 8, and 10 were subjected to two-way ANOVA with Geisser–Greenhouse correction, with Dunnett’s multiple comparison to test for simple effects within rows in GraphPad Prism (version 10.2.0) software (GraphPad Software Inc., La Jolla, CA, USA). The fixed effects were time and plant type, and random-effects were aflatoxin levels. Corn kernels were used as control for comparison of infection and contamination versus pulses. To investigate the relationship between aflatoxin and AF-70 GFP expression, a correlation analysis was performed using Pearson correlation coefficients. Statistical significance for treatment effects were declared at p ≤ 0.05. Trends are discussed at p ≤ 0.10. All data is presented as means ± the standard error of the mean (SEM) unless stated otherwise.
3 Results
3.1 Fungal entry, infection, and colonization in seeds
Undamaged seeds from four protein-rich plant sources (chickpeas, lentils, peas, and corn) were inoculated with AF-70 GFP conidial suspension, and the fungus was allowed to colonize the seeds under high humidity conditions (Figure 1). Infected seeds were observed under a stereo light microscope and photographed at regular intervals for each day (0, 2, 4, 6, 8, and 10), as shown in Figure 2. Mycelia were observed on the seed coats 2 days after inoculation in all seeds. The first visible infection for corn kernels was at the pedicel, whereas pulses had visible infection around the entire seed coat on day 2 (Figure 2). By day 4, lentils exhibited visible swelling and on day 6, peas and chickpeas swelled due to internal fungal growth (Figure 2). Sclerotia development was noted only on lentils by day 4 and was present on all seeds and kernels by day 6. On day 10, the entire endosperm was colonized to the fungus across all seeds. Future studies will separate out the endosperm and embryo to determine where fungal growth is localized.
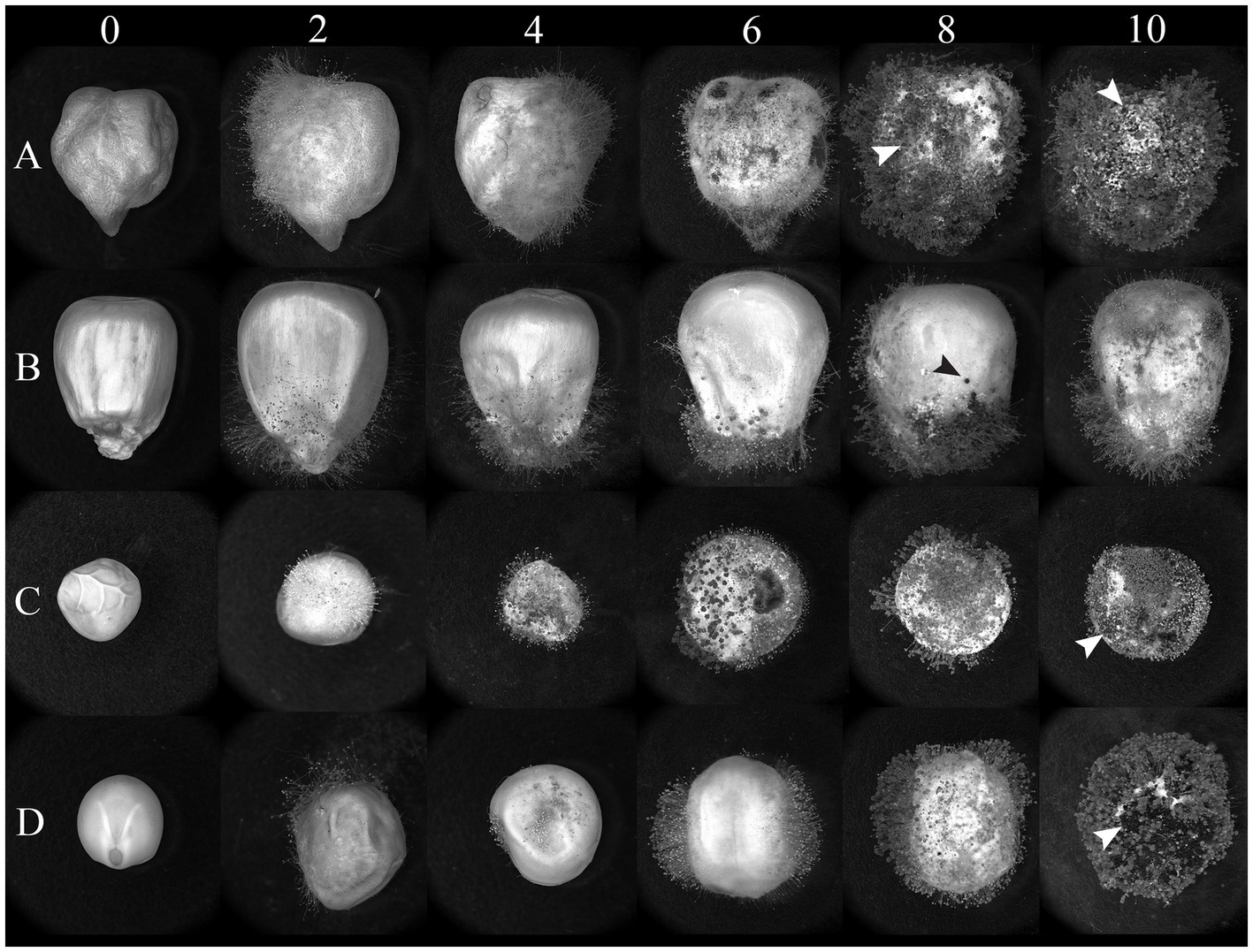
Figure 2. Light micrographs of (A) chickpeas, (B) corn, (C) peas, and (D) lentils over time (days) after inoculation with AF 70-GFP. Visual photographs are taken on a 2-day interval from day 0 to day 10. The arrows represent sclerotia formation across seeds.
Fluorescence due to AF-70 GFP growth inside the seeds of chickpeas, peas, lentils, and corn was detected in longitudinal sections (Figure 3). A typical progression of AF-70 GFP fluorescence was observed in corn kernels, where entry was first observed by near the corn pedicel (base), which spread throughout the endosperm and embryo by day 10. Fluorescence in chickpea micropyles was also observed. Production of sclerotia was observed inside all seeds, including corn kernels, by day 8; however, the sclerotia did not fluoresce. The path of fungal spread was more evident in corn than the other pulses (Figure 3).
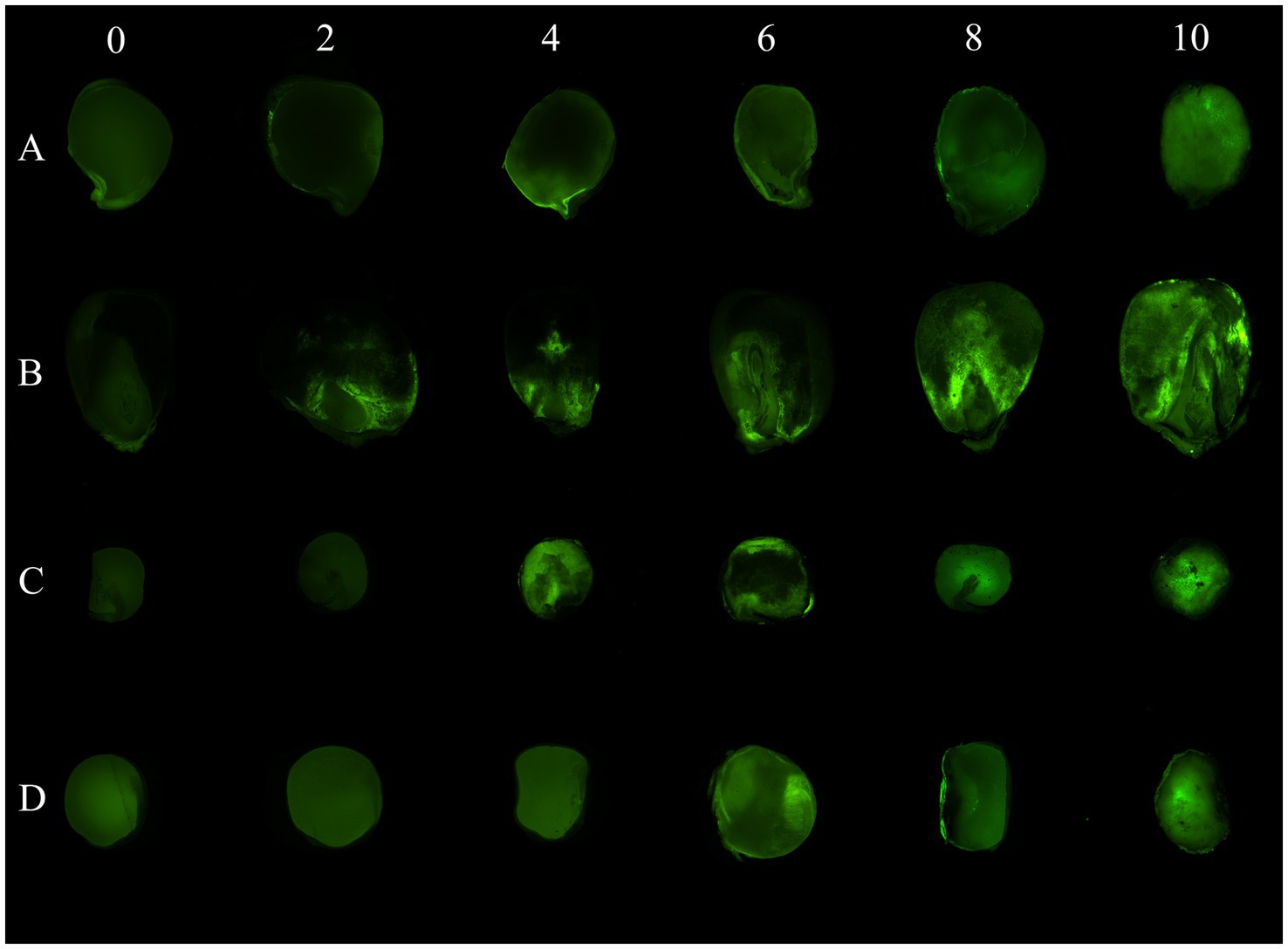
Figure 3. AF 70-GFP fluorescence in longitudinal sections of (A) chickpeas, (B) corn, (C) peas, and (D) lentils over time (in days) after inoculation. Visual photographs are taken on a 2-day interval from day 0 to day 10.
3.2 External Aspergillus flavus spore counts
Spores were collected from seed coats of chickpeas, lentils, peas, and corn for the respective day-intervals of 2, 4, 6, 8, and 10 (Figure 4); day 0 was not measured due to surface seed sterilization. Chickpeas resulted in the greatest conidial production by A. flavus spores (3.76 × 106 ± 1.38 × 105 conidia mL−1), whereas lentils supported the least conidial production (1.60 × 106 ± 3.04 × 105 conidia mL−1) (p = 0.24; Supplementary Table 1). Aspergillus flavus spore production (conidia mL−1) increased each day on chickpeas, corn, and peas with maximum spore production the final day (10); however, maximum spore production on lentils occurred on day 6 (2.28 × 106 ± 2.78 × 105 conidia mL−1) followed by a decrease in conidia, which accompanied a noticeable reduction in lentil size on day 8 and 10. Differences of pulse seeds compared to corn over day intervals for spore production (conidia mL−1) was significant for chickpeas on day 8 and 10 (p ≤ 0.01; Supplementary Table 1).
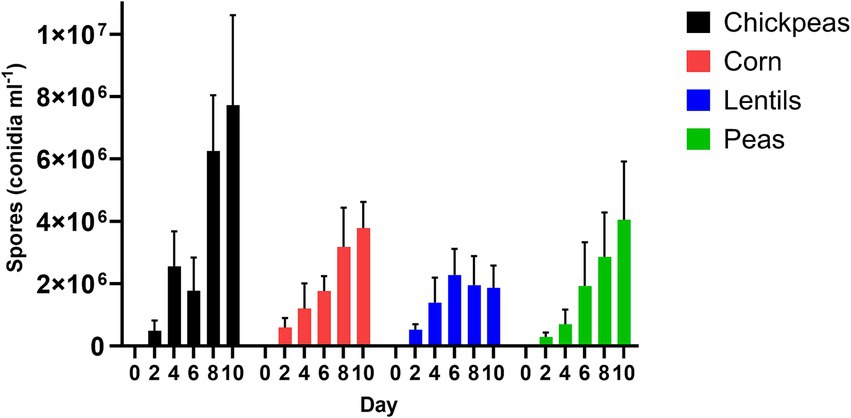
Figure 4. Aspergillus flavus spores (conidia ml−1) on seed coats of chickpeas, corn, lentils, and peas over a 10-day interval period.
3.3 Aflatoxin content and GFP fluorescence
The amount of A. flavus fungal colonization by chickpeas, lentils, peas, and corn, as estimated by GFP fluorescence, was measured at the day intervals 0, 2, 4, 6, 8, and 10 after inoculation (Figure 5). Corn had the highest average GFP relative fluorescence and fungal colonization (30.93 ± 12.02%), with a maximum value of 74.04 ± 12.02% compared to the pulses (p = 0.63). Peas had the lowest average relative fluorescence (25.05 ± 9.26%) compared to corn (p = 0.35). Lentils had an average GFP relative fluorescence of 30.43 ± 8.36%, with a maximum value of 49.06 ± 8.36%; whereas chickpeas had an average GFP relative fluorescence of 29.18 ± 11.18%, with a maximum value of 63.92 ± 11.18%. Maximum GFP fluorescence was recorded for chickpeas, corn, and peas on day 10; however, lentils had the highest maximum value on day 8 (Figure 5; Supplementary Table 1).
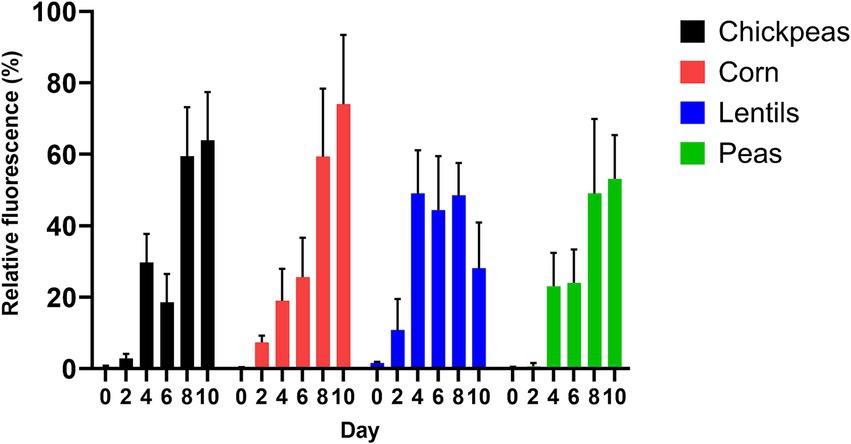
Figure 5. Production of AF 70-GFP on 2-day intervals after inoculation (as indicated by relative fluorescence %) on chickpeas, corn, lentils, and peas.
AFB1 and AFB2 production in the four plant-rich protein sources was determined by UPLC (Figure 6). Corn was contaminated by the most AFB1 (15658.0 ± 7975.0 ng/g−1 or ppb), with a maximum value of 44428.0 ng/g−1 (Figure 6; Supplementary Table 1). Lentils were the least contaminated with average AFB1 (1325.0 ± 416.4 ng/g−1), with a maximum value of 2390.0 ng/g−1. On day 8 and 10, all pulses had statistically significant differences compared to corn for AFB1 and AFB2 (p < 0.001; Supplementary Table 1). Similarly, for AFB2, corn had the highest quantity (907.6 ± 449.6 ng/g−1), with a maximum value of 2540.0 ng/g−1, and lentils had the lowest average AFB2 (63.6 ± 19.6 ng/g−1), with a maximum value of 103.7 ng/g−1. Differences of pulses compared to corn over day intervals for AFB1 and AFB2 is in Supplementary Table 1.
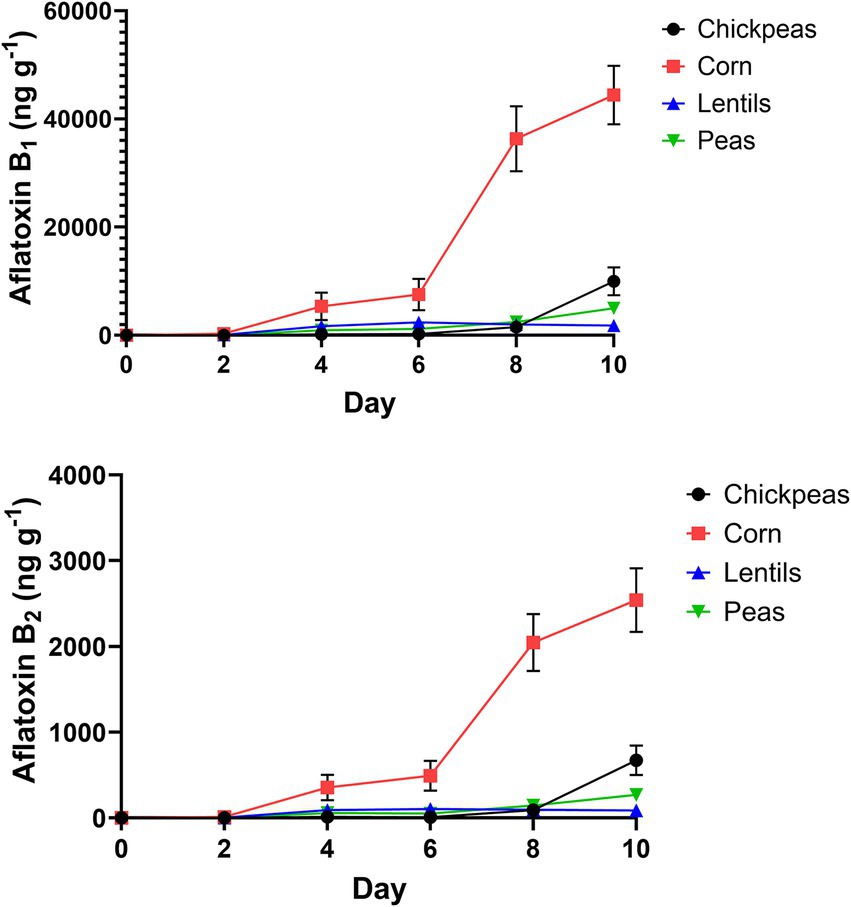
Figure 6. Growth of AFB1 and AFB2 (ng/g−1) on 2-day intervals after inoculation of chickpeas, corn, lentils, and peas.
A correlation analysis between relative fluorescence and AFB1 production by AF-70 GFP was compared to determine the connection between fungal growth and aflatoxin production (Figure 7). There was a very strong positive correlation (r = 0.92) between relative fluorescence and AFB1 in corn. There was a strong positive correlation (r = 0.71) between relative fluorescence and AFB1 in peas. Additionally, there was a moderate positive correlation (r = 0.55 and r = 0.57) between relative fluorescence and AFB1 in chickpeas and lentils, respectively. Aspergillus flavus in corn (measured by AF-70 GFP) is a good indication of aflatoxin contamination, whereas infection of peas, and to a lesser extent chickpea and lentils infection, is not always a good indication of aflatoxin contamination.
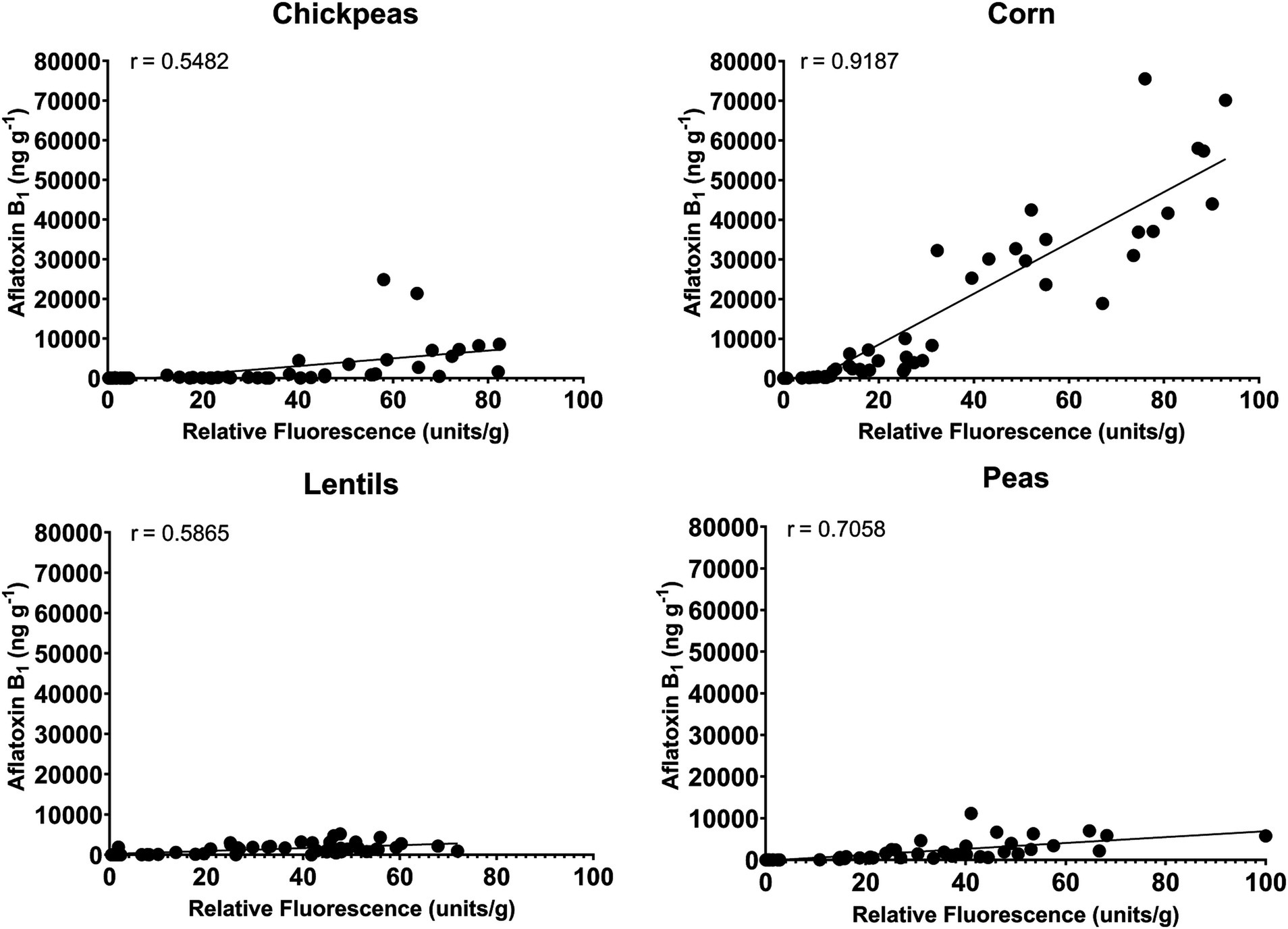
Figure 7. Correlation between relative fluorescence and AFB1 produced from AF 70-GFP for chickpeas, corn, lentils, and peas.
Cyclopiazonic acid (CPA), a phytotoxin with potential virulence factor, was measured across the four plant-rich protein sources determined by UPLC–MS. CPA production in corn had an average of 851.0 ± 621.30 ng/g−1. Chickpeas had the highest overall average at 5548.0 ± 3661.0 ng/g−1. Lentils averaged 3208.0 ± 1492.0 ng/g−1 for CPA, whereas, peas averaged 2768.0 ± 2074.0 ng/g−1, respectively. Additionally, α-aflatrem, a tremorgenic mycotoxin produced from A. flavus, was measured across the four plant-rich protein sources determined by UPLC–MS (Figure 8). Corn had the lowest average at 1.4 ± 0.9 ng/g−1 of α-aflatrem production. Lentils had the highest average with 2381.0 ± 1420.0 ng/g−1 of α-aflatrem production. Similarly, chickpeas averaged 1620.0 ± 1487.0 ng/g−1 whereas peas averaged 1926.0 ± 18550.0 ng/g−1 across all day intervals. Differences of pulses compared to corn over day intervals for α-aflatrem is in Supplementary Table 1.
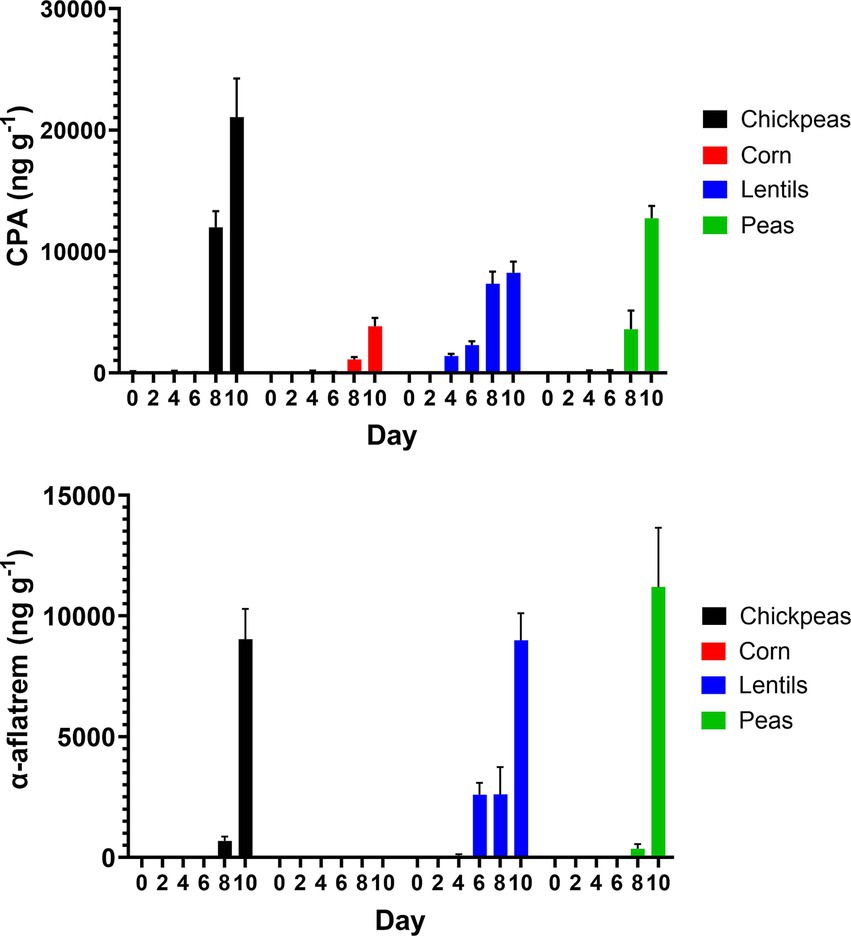
Figure 8. Production of CPA (ng/g−1) and α-aflatrem (ng/g−1) on 2-day intervals after inoculation on chickpeas, corn, lentils, and peas.
4 Discussion
Pulses serve as a protein alternative or compliment to animal-based products (Henchion et al., 2017; Kunz et al., 2020; Quintieri et al., 2023). Increasingly, global citizens are incorporating pulses into their diet, driven by health interests and environmental sustainability awareness (Singh et al., 2024). The Food and Agriculture Organization of the United Nations (FAO) announced in 2016 it was the “Year of Pulses” to highlight their global importance, with consumption steadily increasing in recent decades, projecting to reach a $27 billion industry by 2030 (Singh et al., 2024). The benefits of using pulses in human diets are multifaceted, including improvement of cardiovascular health, enhanced metabolism, obesity prevention, and overall gut microbiome improvement due to their rich nutrient profile, including proteins, fiber, vitamin, and minerals (Curran, 2012; Mudryj et al., 2014; Patterson et al., 2009; Singh et al., 2024). Additionally, pulses are crucial for crop diversification, contributing to a resilient food chain. Ensuring the safety of pulses in food and feed is essential due to their global health relevance (Kunz et al., 2020). Therefore, in this study, growth of A. flavus, and production of aflatoxins, CPA, and α-aflatrem were examined using a KSA procedure over a 10-day timeframe to determine infection patterns, fungal growth, and colonization of undamaged pulse seeds (chickpeas, lentils, and peas) under a controlled environment, with cereal grain corn as a positive control.
Using an A. flavus strain expressing GFP genes to track aflatoxin contamination in pulses is a new method that has previously been applied to monitor fungal spread and aflatoxin levels in corn and cottonseed (Rajasekaran et al., 2008; Rajasekaran et al., 2013; Rajasekaran et al., 2017). This method is easy, sensitive, and rapid, allowing the evaluation of resistance or susceptibility of undamaged seeds based on GFP fluorescence. In corn, a strong correlation exists between GFP fluorescence and aflatoxin levels (Rajasekaran et al., 2013; Figure 6), with our study showing an exceptionally high correlation (r = 0.92). However, in pulses such as chickpeas, lentils, and peas, the direct relationship between A. flavus infection (Figure 7) and aflatoxin accumulation (Figure 6) was less robust, with correlations of r = 0.71 in peas, and lower correlations of r = 0.55 and 0.57 in chickpeas and lentils, respectively. This discrepancy is possibly due to the presence of antifungal factors in pulses, for example, interference from seed coat compounds like flavonoids, polyphenols, or differences in lipid, lipoxygenase, and oxylipin content (Doehlert et al., 1993; Burow et al., 1997; Xue et al., 2003). Minimal AFB1 and AFB2 production was observed in pulses compared to corn under identical conditions (Acuña-Gutiérrez et al., 2022; Davidson et al., 2012; Figures 4, 5). Moreover, in our study, the degradation of lentils, peas, and chickpeas during the cleaning process on days 8 and 10, likely due to complete internal tissue rot, may partly explain the reduced aflatoxin production despite high external spore counts and GFP fluorescence. Finally, it is important to note that GFP fluorescence primarily emanates from young A. flavus mycelia and spore structures, while older fungal structures such as sclerotia and spores exhibit diminished fluorescence. This characteristic, along with inherent differences in seed coat composition among pulses, contributes to the limitations of using GFP as a universal marker for fungal colonization in these diverse seed types.
Pulses have been shown to resist aflatoxin accumulation despite high fungal infection rates with Aspergillus spp. (Acuña-Gutiérrez et al., 2022; Figures 5–7). Specific characteristics of pulses, including thick and hard seed coats, antifungal proteins, high phenolic compounds, and oxylipins, contribute to their ability to regulate aflatoxin accumulation by protecting against phytopathogenic fungal attacks and reducing spore germination and/or mycelial growth (Acuña-Gutiérrez et al., 2022; Kralova et al., 2006; Makun et al., 2010; Martínez et al., 2017; Zabka and Pavela, 2013). The antifungal protein pisumin, identified in Pisum sativum varieties, has demonstrated efficacy against fungal pathogens such as Alternaria, potentially explaining the absence of its toxins in pea samples (Kralova et al., 2006). While direct research on P. sativum and A. flavus involving pisumin remains limited, other antifungal proteins, such as defensins, serve critical roles in plant immunity by disrupting fungal cell membranes and reducing infection severity. Seed coat structure further supports aflatoxin suppression, functioning as both a physical and biochemical barrier against fungal invasion. Thick seed coats, particularly in lentils, may contribute to reduced conidial production (1.60 × 106 ± 3.04 × 105 conidia mL−1), compared to chickpeas, which supported the highest levels of A. flavus conidial production (3.76 × 106 ± 1.38 × 105 conidia mL−1) (Supplementary Table 1). The delayed peak of conidia production in lentils (day 6) followed by a reduction on days 8 and 10 suggests a potential structural or biochemical factor influencing fungal development.
Phenolic compounds, secondary metabolites involved in plant defense, play a significant role in reducing aflatoxin accumulation. Flavonoids, tannins, and phenolic acids have been reported to exhibit antimycotoxigenic properties (Ahmed et al., 2022; Castano-Duque et al., 2022; Loi et al., 2020). While in vitro studies have demonstrated the effectiveness of phenolic extracts in reducing mycotoxin content across cereals, fruits, algae, and other plant products (Hua et al., 1999), research specifically on pulses remains limited. In this study, lentils, which exhibited lower A. flavus conidial production and fungal colonization (relative GFP fluorescence of 30.43 ± 8.36%), also had the lowest levels of aflatoxin contamination (1325.0 ± 416.4 ng/g−1 AFB1), highlighting the potential contribution of phenolic and structural defense mechanisms (Figure 6). Additionally, lipid-derived oxylipins, including oleic and linoleic acids, are key regulators of aflatoxin biosynthesis (Doehlert et al., 1993). The differential response of pulse crops to fungal colonization and aflatoxin accumulation suggests that variability in oxylipin content may be an underlying factor. For instance, lentils exhibited peak fungal colonization on day 8, followed by a reduction in conidia and seed shrinkage, which coincided with significantly lower aflatoxin levels compared to corn (p < 0.001; Supplementary Table 1). Further investigation into oxylipin profiles among protein-rich pulses is warranted to assess their role in aflatoxin suppression (Acuña-Gutiérrez et al., 2022; Cardador-Martinez et al., 2002; Singh, 2017).
AFB1 and AFB2 levels were inversely related to the production of CPA and α-aflatrem, other toxic A. flavus secondary metabolites, with higher levels observed in pulses compared to corn (Figure 8). CPA, produced by certain species of Aspergillus and Penicillium fungi, is found in various food sources including cereals, legumes, milk, meat, and cheese (Burdock and Flamm, 2000). The CPA levels measured in this study, particularly in chickpeas (5548.0 ± 3661.0 ng/g−1) and lentils (3208.0 ± 1492.0 ng/g−1), are significantly higher compared to corn (851.0 ± 621.30 ng/g−1) and align with previous studies reporting increased CPA production in legume-rich environments (Chang et al., 2009). CPA is a tremorgenic mycotoxin that causes symptoms such as weight loss, fever, diarrhea, dehydration, ataxia, immobility, and muscle spasms, primarily affecting the gastrointestinal tract, liver, spleen, and muscle tissues. Although CPA mycotoxicosis is considered less harmful compared to aflatoxin toxicity, its relatively high presence in pulses raises concerns regarding its potential health implications. Despite its toxicological significance, the FDA has not established regulatory guidelines for CPA, highlighting a gap in food safety assessments. However, the tolerable daily intake for CPA of 0.1 μg/kg body weight has been proposed by extrapolating toxicity data from test animals to humans (De Waal, 2002; Ostry et al., 2018). α-Aflatrem, another tremorgenic mycotoxin produced by A. flavus (Gallagher and Wilson, 1978), is a potent neurotoxin known to cause tremors and neurological disorders, such as mental confusion, seizures, and hyperexcitability, in rats and cattle (Valdes et al., 1985). Similarly, CPA in this study, pulses exhibited higher α-aflatrem concentrations compared to corn, with lentils having the highest levels (2381.0 ± 1420.0 ng/g−1) followed by peas (1926.0 ± 1855.0 ng/g−1) and chickpeas (1620.0 ± 1487.0 ng/g−1), while corn contained the lowest levels (1.4 ± 0.9 ng/g−1). α-Aflatrem is part of a diverse class of indole diterpene metabolites produced by A. flavus that concentrate in hardened fungal mycelia (i.e., sclerotia) (Gloer, 1995). These compounds function as antiinsectans, protecting sclerotia from predation. The production of α-aflatrem is regulated by the veA gene, which is also involved in the biosynthesis of aflatoxin and CPA (Duran et al., 2009). α-Aflatrem remains largely unregulated despite its neurotoxic effects. The observed inverse relationship between aflatoxin production and CPA/α-aflatrem levels suggests a potential shift in secondary metabolite biosynthesis regulation within A. flavus. This phenomenon may be linked to metabolic competition for biosynthetic precursors and regulatory pathways influenced by global transcription factors such as veA and laeA (Calvo et al., 2004; Cary et al., 2015). These genes are known to coordinate secondary metabolite production and may play a role in prioritizing the synthesis of CPA and α-aflatrem in certain environmental conditions. Understanding this regulatory interplay could provide insight into fungal secondary metabolism and help evaluate whether pulses, despite their lower aflatoxin contamination, may still pose significant food safety risks due to elevated CPA and α-aflatrem levels.
Addressing the issue of aflatoxin in pulses requires a thorough understanding of their growth conditions, storage stability, and post-harvest handling techniques to implement preventative measures globally. Evaluating aflatoxin infection in chickpeas, lentils, peas, and potentially other protein-rich pulses like beans is essential for making informed decisions regarding health and safety risks (Singh et al., 2024). Understanding the growth of aflatoxins in pulses is crucial for developing effective risk analysis techniques that can be translated into political decisions and regulatory compliance worldwide. Employing good agricultural practices through a holistic approach with regular monitoring can significantly reduce the risk of aflatoxin in pulses, through improved analysis and measurement (Nyangi and Runyogote, 2024). Awareness of these factors across the entire supply chain is vital for maintaining food and feed safety in the coming years, given the projected population increase and the need for sustainable food sources to reduce the carbon footprint (Gräfenhahn and Beyrer, 2024). Future research will focus on evaluating the impact of A. flavus growth in both fresh and powdered plant products, as well as assessing the stability of proteins during growth and storage. Moreover, a standardized methodology for A. flavus growth in pulses will be developed to enhance consistency in research and safety protocols. By adopting a comprehensive strategy that includes good agricultural practices, continuous monitoring, and effective risk management, the risk of aflatoxin contamination in pulses can be greatly reduced, ensuring food safety and security on a global scale.
5 Conclusion
Pulses, including chickpeas [Cicer arietinum L.], lentils [Lens culinaris Medik], and peas [Pisum sativum L.], are highly nutritious and protein-dense food products that are anticipated to gain popularity in the coming decades due to global demand. Despite increased usage in human diets, limited data has been published regarding A. flavus and aflatoxin infection in these pulses from a food and feed safety standpoint. This study presents a novel methodology to determine aflatoxin accumulation using a GFP-expressing A. flavus (AF-70 GFP) strain to assess fungal growth, and consequently aflatoxin infection rates, CPA, and α-aflatrem in chickpeas, lentils, and peas. Pulses did not exhibit higher amounts of aflatoxins compared to corn and other grains; however, CPA and α-aflatrem production was higher in pulses. Considering the health relevance of pulses for human and livestock diets in present day and in the future for sustainable aspects, more attention should be drawn to understanding growth conditions of aflatoxin in pulses in field, including growing conditions, to storage. Similarly, the mechanism of aflatoxin accumulation and resistance to fungal attacks, needs to be further explored. Aflatoxin contamination of pulses in planta and in stored, powdered commercial products will be examined in a subsequent study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
EB-S: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. CS: Conceptualization, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. ML: Data curation, Methodology, Writing – original draft, Writing – review & editing. CC-W: Data curation, Methodology, Writing – original draft, Writing – review & editing. KR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was provided by USDA-ARS CRIS project #6054-42000-027-000D.
Acknowledgments
This research was supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by ORAU under DOE contract number DE-SC0014664. The pulse seeds were provided by Dr. Clarice J. Coyne and Dr. Marilyn Warburton from the Western Regional Plant Introduction Station (USDA-ARS), Washington State University, Pullman, WA and corn kernels were provided by Dr. Rebecca Sweany from the Southern Regional Research Center (USDA-ARS), New Orleans, LA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. In the preparation of this manuscript, generative AI tools were employed solely for the purposes of reference management and grammatical editing. The AI tools assisted in organizing and formatting references according to the required citation style, as well as in identifying and correcting grammatical errors to ensure clarity and coherence. All scientific content, ideas, and conclusions presented in the manuscript are the original work of the authors.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1587035/full#supplementary-material
Abbreviations
AFB1, Aflatoxin B1; AFB2, Aflatoxin B2; CPA, Cyclopiazonic acid; AF-70, A. flavus strain 70; GFP, Green fluorescent protein; LOD, Limit of detection; KSA, Kernel screening assay; FAO, Food and Agriculture Organization of the United Nations; FDA, Food and Drug Administration.
References
Acuña-Gutiérrez, C., Jiménez, V. M., and Müller, J. (2022). Occurrence of mycotoxins in pulses. Compr. Rev. Food Sci. Food Saf. 21, 4002–4017. doi: 10.1111/1541-4337.13008
Ahmadi, M., Jahed Khaniki, G., Shariatifar, N., and Molaee-Aghaee, E. (2022). Investigation of aflatoxins level in some packaged and bulk legumes collected from Tehran market of Iran. Int. J. Environ. Anal. Chem. 102, 4804–4813. doi: 10.1080/03067319.2020.1789614
Ahmed, O. S., Tardif, C., Rouger, C., Atanasova, V., Richard-Forget, F., and Waffo-Téguo, P. (2022). Naturally occurring phenolic compounds as promising antimycotoxin agents: where are we now? Compr. Rev. Food Sci. Food Saf. 21, 1161–1197. doi: 10.1111/1541-4337.12891
Allaire, H., and Brady, T. (2008). Classification and botanical description of legumes : Academics Hamilton Available at: https://academics.hamilton.edu/foodforthought/our_research_files/beans_peas.pdf.
Begum, F., and Samajpati, N. (2000). Mycotoxin production on rice, pulses and oilseeds. Naturwissenschaften 87, 275–277. doi: 10.1007/s001140050720
Brown, R. L., Cleveland, T. E., Payne, G. A., Woloshuk, C. P., Campbell, K. W., and White, D. G. (1995). Determination of resistance to aflatoxin production in maize kernels and detection of fungal colonization using an aspergillus flavus transformant expressing Escherichia coli β-glucuronidase. Phytopathology 85, 983–989. doi: 10.1094/Phyto-85-983
Brown, R. L., Cotty, P. J., Cleveland, T. E., and Widstrom, N. W. (1993). Living maize embryo influences accumulation of aflatoxin in maize kernels. J. Food Prot. 56, 967–971. doi: 10.4315/0362-028X-56.11.967
Burdock, G. A., and Flamm, W. G. (2000). Safety assessment of the mycotoxin cyclopiazonic acid. Int. J. Toxicol. 19, 195–218. doi: 10.1080/10915810050074964
Burow, G. B., Nesbitt, T. C., Dunlap, J., and Keller, N. P. (1997). Seed lipoxygenase products modulate aspergillus mycotoxin biosynthesis. Mol. Plant-Microbe Interact. 10, 380–387. doi: 10.1094/MPMI.1997.10.3.380
Calvo, A. M., Cary, J. W., and Rajasekaran, K. (2004). Role of the fungal gene veA in the regulation of secondary metabolism. Appl. Microbiol. Biotechnol. 67, 367–372. doi: 10.1007/s00253-004-1777-2
Cardador-Martinez, A., Castano-Tostado, E., and Loarca-Pina, G. (2002). Antimutagenic activity of natural phenolic compounds present in the common bean (Phaseolus vulgaris) against aflatoxin B1. Food Addit. Contam. 19, 62–69. doi: 10.1080/02652030110062110
Cary, J. W., Harris-Coward, P. Y., Ehrlich, K. C., and Mack, B. M. (2015). Regulation of aflatoxin and CPA production in aspergillus flavus. Toxins 7, 3268–3282. doi: 10.3390/toxins7083268
Castano-Duque, L., Lebar, M. D., Carter-Wientjes, C., Ambrogio, D., and Rajasekaran, K. (2022). Flavonoids modulate aspergillus flavus proliferation and aflatoxin production. J. Fungi 8:1211. doi: 10.3390/jof8111211
Chang, P. K., Horn, B. W., and Dorner, J. W. (2009). Genetic transformation of aspergillus flavus to disrupt the production of cyclopiazonic acid. Appl. Environ. Microbiol. 75, 284–290. doi: 10.1128/AEM.01575-08
Curran, J. (2012). The nutritional value and health benefits of pulses in relation to obesity, diabetes, heart disease and cancer. Br. J. Nutr. 108, S1–S2. doi: 10.1017/S0007114512003534
Dahl, W. J., Foster, L. M., and Tyler, R. T. (2012). Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 108, S3–S10. doi: 10.1017/S0007114512000852
Davidson, J. A., McMurray, L. S., Wilmshurst, C. J., Sherriff, S. A., and Pointon, A. M. (2012). Tests for field mould and associated mycotoxins in south Australian lentil (Lens culinaris) grain. Aust Plant Dis Notes 7, 79–83. doi: 10.1007/s13314-012-0054-x
Day, L., Cakebread, J. A., and Loveday, S. M. (2022). Food proteins from animals and plants: differences in the nutritional and functional properties. Trends Food Sci. Technol. 119, 428–442. doi: 10.1016/j.tifs.2021.12.020
De Waal, E. J. (2002). Safety assessment of cyclopiazonic acid. Int. J. Toxicol. 21, 425–427. doi: 10.1080/10915810290096658
Doehlert, D. C., Wicklow, D. T., and Gardner, H. W. (1993). Evidence implicating the lipoxygenase pathway in providing resistance to soybeans against aspergillus flavus. Phytopathology 83, 1473–1477. doi: 10.1094/Phyto-83-1473
Donato, C. J. R., Cendoya, E., Demonte, L. D., Repetti, M. R., Chulze, S. N., and Ramirez, M. L. (2022). Influence of abiotic factors (water activity and temperature) on growth and aflatoxin production by aspergillus flavus in a chickpea-based medium. Int. J. Food Microbiol. 379:109841. doi: 10.1016/j.ijfoodmicro.2022.109841
Duran, R. M., Cary, J. W., and Calvo, A. M. (2009). The role of veA in aspergillus flavus infection of peanut, corn and cotton. Open Mycol. J. 3, 27–36. doi: 10.2174/1874437000903010027
El-Kady, I. A., Mohamed El-Maraghy, S. S., and Zohri, A. A. (1996). Aflatoxin formation and varietal difference of cow pea (Vigna unguiculata (L.) Walp.) and garden pea (Pisum sativum L.) cultivars. Mycopathologia 133, 185–188. doi: 10.1007/BF02373026
El-Maraghy, S. M. (1988). Aflatoxins and fungal flora in lentil (Lens esculenta L.). Mycopathologia 102, 31–35. doi: 10.1007/BF00436249
FDA (2025) Mycotoxins. Available online at: https://www.fda.gov/food/natural-toxins-food/mycotoxins (Accessed April 25, 2025).
Gallagher, R. T., and Wilson, B. J. (1978). Aflatrem, the tremorgenic mycotoxin from aspergillus flavus. Mycopathologia 66, 183–185. doi: 10.1007/BF00683969
Gavrilova, N., Chernopolskaya, N., Rebezov, M., Schetinina, E., Suyazova, I., Safronov, S., et al. (2020). Development of specialized food products for nutrition of sportsmen. J. Crit. Rev. 7, 233–236. doi: 10.31838/jcr.07.04.43
George, K. S., Muñoz, J., Akhavan, N. S., Foley, E. M., Siebert, S. C., Tenenbaum, G., et al. (2020). Is soy protein effective in reducing cholesterol and improving bone health? Food Funct. 11, 544–551. doi: 10.1039/C9FO01081E
Gloer, J. B. (1995). Antiinsectan natural products from fungal sclerotia. Acc. Chem. Res. 28, 343–350. doi: 10.1021/ar00056a004
Godfray, H. C. J., Aveyard, P., Garnett, T., Hall, J. W., Key, T. J., Lorimer, J., et al. (2018). Meat consumption, health, and the environment. Science 361:eaam5324. doi: 10.1126/science.aam5324
Gräfenhahn, M., and Beyrer, M. (2024). Plant-based meat analogues in the human diet: what are the hazards? Food Secur. 13:1541. doi: 10.3390/foods13101541
Heinke, J., Lannerstad, M., Gerten, D., Havlík, P., Herrero, M., Notenbaert, A. M. O., et al. (2020). Water use in global livestock production—opportunities and constraints for increasing water productivity. Water Resour. Res. 56:e2019WR026995. doi: 10.1029/2019WR026995
Henchion, M., Hayes, M., Mullen, A. M., Fenelon, M., and Tiwari, B. (2017). Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Food Secur. 6:53. doi: 10.3390/foods6070053
Hertzler, S. R., Lieblein-Boff, J. C., Weiler, M., and Allgeier, C. (2020). Plant proteins: assessing their nutritional quality and effects on health and physical function. Nutrients 12:3704. doi: 10.3390/nu12123704
Hua, S. S., Grosjean, O. K., and Baker, J. L. (1999). Inhibition of aflatoxin biosynthesis by phenolic compounds. Lett. Appl. Microbiol. 29, 289–291. doi: 10.1046/j.1472-765X.1999.00635.x
Kiesselbach, T. A. (1999). The structure and reproduction of corn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Kralova, J. A. N. A., Hajslova, J., Poustka, J., Hochman, M., Bjelková, M., and Odstrcilova, L. (2006). Occurrence of Alternaria toxins in fibre flax, linseed, and peas grown in organic and conventional farms: monitoring pilot study. Czech J. Food Sci. 24, 288–296. doi: 10.17221/3327-CJFS
Kumar, A., Pathak, H., Bhadauria, S., and Sudan, J. (2021). Aflatoxin contamination in food crops: causes, detection, and management: a review. Food Prod. Process. Nutr. 3, 1–9. doi: 10.1186/s43014-021-00064-y
Kunz, B. M., Wanko, F., Kemmlein, S., Bahlmann, A., Rohn, S., and Maul, R. (2020). Development of a rapid multi-mycotoxin LC-MS/MS stable isotope dilution analysis for grain legumes and its application on 66 market samples. Food Control 109:106949. doi: 10.1016/j.foodcont.2019.106949
Loi, M., Paciolla, C., Logrieco, A. F., and Mulè, G. (2020). Plant bioactive compounds in pre-and postharvest management for aflatoxins reduction. Front. Microbiol. 11:243. doi: 10.3389/fmicb.2020.00243
Makun, H. A., Anjorin, S. T., Moronfoye, B., Adejo, F. O., Afolabi, O. A., Fagbayibo, G., et al. (2010). Fungal and aflatoxin contamination of some human food commodities in Nigeria. Afr. J. Food Sci. 4, 127–135. doi: 10.5897/AJFS2025.2342
Martínez, G., Regente, M., Jacobi, S., Del Rio, M., Pinedo, M., and de la Canal, L. (2017). Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 140, 30–35. doi: 10.1016/j.pestbp.2017.05.012
Massawe, F., Mayes, S., and Cheng, A. (2016). Crop diversity: an unexploited treasure trove for food security. Trends Plant Sci. 21, 365–368. doi: 10.1016/j.tplants.2016.02.006
Moore, G. G., Lebar, M. D., and Carter-Wientjes, C. H. (2022). Cumulative effects of non-aflatoxigenic aspergillus flavus volatile organic compounds to abate toxin production by mycotoxigenic aspergilli. Toxins 14:340. doi: 10.3390/toxins14050340
Mudryj, A. N., Yu, N., and Aukema, H. M. (2014). Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 39, 1197–1204. doi: 10.1139/apnm-2013-0557
Nyangi, C., and Runyogote, J. (2024). Quantitative risk assessment for aflatoxins in beans from northern Tanzania. World Mycotoxin J. 1, 1–11. doi: 10.1163/18750796-bja10009
Ostry, V., Toman, J., Grosse, Y., and Malir, F. (2018). Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 11, 135–148. doi: 10.3920/WMJ2017.2243
Patterson, C. A., Maskus, H., and Dupasquier, C. (2009). Pulse crops for health. Cereal Foods World 54:108. doi: 10.1094/CFW-54-3-0108
Quintieri, L., Nitride, C., De Angelis, E., Lamonaca, A., Pilolli, R., Russo, F., et al. (2023). Alternative protein sources and novel foods: benefits, food applications and safety issues. Nutrients 15:1509. doi: 10.3390/nu15061509
Rajasekaran, K., Cary, J. W., Cotty, P. J., and Cleveland, T. E. (2008). Development of a GFP-expressing aspergillus flavus strain to study fungal invasion, colonization, and resistance in cottonseed. Mycopathologia 165, 89–97. doi: 10.1007/s11046-007-9085-9
Rajasekaran, K., Ford, G., Sethumadhavan, K., Carter-Wientjes, C., Bland, J., Cao, H., et al. (2017). Aspergillus flavus growth and aflatoxin production as influenced by total lipid content during growth and development of cottonseed. J. Crop Improv. 31, 91–99. doi: 10.1080/15427528.2016.1263811
Rajasekaran, K., Sickler, C. M., Brown, R. L., Cary, J. W., and Bhatnagar, D. (2013). Evaluation of resistance to aflatoxin contamination in kernels of maize genotypes using a GFP-expressing aspergillus flavus strain. World Mycotoxin J. 6, 151–158. doi: 10.3920/WMJ2012.1497
Ramirez, M. L., Cendoya, E., Nichea, M. J., Zachetti, V. G. L., and Chulze, S. N. (2018). Impact of toxigenic fungi and mycotoxins in chickpea: a review. Curr. Opin. Food Sci. 23, 32–37. doi: 10.1016/j.cofs.2018.05.003
Rebello, C. J., Greenway, F. L., and Finley, J. W. (2014). A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 15, 392–407. doi: 10.1111/obr.12144
Roy, M., Harris, J., Afreen, S., Deak, E., Gade, L., Balajee, S. A., et al. (2013). Aflatoxin contamination in food commodities in Bangladesh. Food Addit. Contam. Part B Surveill. 6, 17–23. doi: 10.1080/19393210.2012.720617
Singh, N. (2017). Pulses: an overview. J. Food Sci. Technol. 54, 853–857. doi: 10.1007/s13197-017-2537-4
Singh, A. K., Elango, D., Raigne, J., Van der Laan, L., Rairdin, A., Soregaon, C., et al. (2024). Plant-based protein crops and their improvement: current status and future perspectives. Crop Sci. 65:e21389. doi: 10.1002/csc2.21389
United Nations Department of Economic and Social Affairs. (2022). World population prospects 2022. Available online at: https://population.un.org/wpp/ (Accessed June 11, 2024).
Valdes, J. J., Cameron, J. E., and Cole, R. J. (1985). Aflatrem: a tremorgenic mycotoxin with acute neurotoxic effects. Environ. Health Perspect. 62, 459–463. doi: 10.1289/ehp.8562459
Wood, J. A., and Grusak, M. A. (2007). Nutritional value of chickpea. Chickpea Breed. Manag. 101:142. doi: 10.1079/9781845932138.005
Xue, H. Q., Isleib, T. G., Payne, G. A., Wilson, R. F., Novitzky, W. P., and O'Brian, G. (2003). Comparison of aflatoxin production in normal-and high-oleic backcross-derived peanut lines. Plant Dis. 87, 1360–1365. doi: 10.1094/PDIS.2003.87.11.1360
Yadav, S. S., McNeil, D., and Stevenson, P. C. (Eds.) (2007). Lentil: An ancient crop for modern times. Dorderecht, The Netherlands: Springer Science & Business Media.
Keywords: aflatoxin contamination, cyclopiazonic acid, kernel screening assay, green fluorescent protein, pulses
Citation: Branstad-Spates EH, Sickler CM, Lebar MD, Carter-Wientjes C and Rajasekaran K (2025) Evaluation of aflatoxin contamination in protein-rich pulses using a GFP-expressing Aspergillus flavus strain. Front. Microbiol. 16:1587035. doi: 10.3389/fmicb.2025.1587035
Edited by:
Alexa Elena Alexandra, Technological University Dublin, IrelandReviewed by:
Theophilus Kwabla Tengey, CSIR-Savanna Agricultural Research Institute, GhanaAnindya Chanda, Mycologics LLC, United States
Copyright © 2025 Branstad-Spates, Sickler, Lebar, Carter-Wientjes and Rajasekaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanniah Rajasekaran, S2FubmlhaC5yYWphc2VrYXJhbkB1c2RhLmdvdg==
 Emily H. Branstad-Spates
Emily H. Branstad-Spates Christine M. Sickler
Christine M. Sickler Matthew D. Lebar
Matthew D. Lebar Carol Carter-Wientjes
Carol Carter-Wientjes Kanniah Rajasekaran
Kanniah Rajasekaran