- 1College of Forestry, Hebei Agricultural University, Baoding, China
- 2Saihanba Mechanical Forest Farm, Chengde, China
- 3Hebei Fertilizer Technology Innovation Centre, Institute of Agricultural Resources and Environment, Hebei Academy of Agriculture and Forestry Sciences, Shijiazhuang, China
Introduction: Clarifying the temporal dynamics of soil organic carbon (SOC) characteristics within aggregates and its underlying microbially mediated mechanisms is essential for long-term SOC sequestration in forest ecosystems; nevertheless, this information remains largely unknown during stand development.
Methods: Five Larix principis-rupprechtii plantations of different ages (7a, 18a, 25a, 34a, and 44a) at the Saihanba Mechanical Forest Farm were chosen to elucidate the temporal variations in SOC characteristics and microbial attributes within aggregates (>2 mm, 2−0.25 mm, and <0.25 mm) following reforestation, based on 13C NMR, phospholipid fatty acid (PLFAs) analysis, micro-plate enzyme technique, and amino sugar analysis, etc.
Results: Results demonstrated that as stand ages increased, aggregate stability as well as aggregate-associated SOC, microbial residue C (MRC), hydrolytic exo-enzymatic activities, and microbial biomass (as indicated by total PLFAs) initially increased and subsequently decreased, with most parameters peaking in the 18a stand, which indicated that long-term Larix principis-rupprechtii plantations (>25a) were not favorable for promoting microbial growth, hydrolytic functions, and microbial metabolism. Besides, regardless of the stand age, the above-mentioned indices were generally higher in larger aggregates (>2 mm and 2−0.25 mm) compared to smaller aggregates (<0.25 mm). Notably, the increased stand ages (i.e., 34a and 44a) or decreased aggregate sizes (<0.25 mm) enhanced SOC stability (as indicated by the recalcitrance index) and oxidative exo-enzymatic activities, as well as enlarged MRC (especially fungal residue C) contribution to SOC. The partial least squares path model highlighted that SOC stocks were primarily regulated by MRC, while the microbial community altered SOC stability by modulating exo-enzyme activities.
Discussion: These results offered novel insights into elucidating the coupling connections between microbial attributes and SOC sequestration during forest development in northern China.
1 Introduction
Soil organic C (SOC), the biggest terrestrial C reservoirs (ca. 2,344 Gt) (Sakschewski et al., 2016), is receiving increasing attention due to its potential role in alleviating global warming and maintaining terrestrial ecological stability (Stockmann et al., 2013; Liu Q. et al., 2024). Nevertheless, since the Industrial Revolution, a series of human activities, such as extensive deforestation, urbanization, and overgrazing, have resulted in large amounts of CO2 emissions and SOC loss (Wiesmeier et al., 2019). Afforestation (i.e., planted forests), an effective approach to mitigate global CO2 emission and promote SOC stocks, has been developed rapidly in recent decades (Liang et al., 2022). By 2023, the global area of planted forests had reached 360.22 million ha, accounting for ca. 7.35% of the world’s total forest area (Xu et al., 2024). Consequently, it is imperative to assess soil C sequestration capability of these newly established planted forests and to elucidate the underlying mechanisms influencing this capacity, in order to deeply evaluate the role of afforestation in mitigating global climate change (Wang et al., 2024).
Recent research revealed that the forest’s capability for SOC sequestration is associated with stand age, as the variations in forest structure and understory vegetation with stand age can affect soil microenvironment and litter input conditions (Mujuru et al., 2014; Dong et al., 2020). Nevertheless, studies examining changes in SOC stocks across stands of varying ages had yielded conflicting results, reporting positive (Abaker et al., 2016), negative (Liu et al., 2023), and no effects (Jing et al., 2022) on SOC storage. These inconsistent results are likely attributed to the variations in forest species, climate, and edaphic characteristics (Chen et al., 2020; Wang et al., 2024). To better elucidate SOC dynamics, it is essential to address this knowledge gap regarding the underlying mechanisms for SOC sequestration along stand development.
Researchers revealed that the processes involved SOC cycling, such as degradation and polymerization, etc., are primarily regulated by microbes and their secreted exo-enzymes (Shao et al., 2018; Sokol et al., 2022). Recently, the response of stand ages to microbial attributes has been widely attracting scholarly attention (Wang et al., 2021; Zhang et al., 2023). Studies have demonstrated that stand age, a crucial parameter for assessing the rewilding level of plantations post-afforestation, can strongly influence the dynamics of microbial communities and exo-enzymatic activities through the modulation of understory vegetation composition and the exogenous resources (e.g., litter debris and root depositions) inputs (Zhang et al., 2023; Liu X. et al., 2024). Notably, traditional perspectives emphasize that SOC is mainly derived from plant-originated C, with microbes accounting for <4% of SOC and playing a crucial role in SOC decomposition and mineralization (Wang et al., 2017). Nevertheless, increasing evidence suggests that microbial anabolic functions are important in mediating persistent soil C storage (Shao et al., 2018; Hu H. et al., 2024). Based on the “microbial C pump” theory, Liang et al. (2017) indicated that microbial residues (i.e., microbial-derived C), producted through microbial anabolism, are considered to be the important constituents of stable SOC pools and contribute approximately 50–80% to SOC storage. Taken together, the study of combining microbial communities, exo-enzymes, and microbial residues, can comprehensively provide several valuable insights for elucidating microbial-driven SOC dynamics with plantation development (Ma et al., 2023). Yet, to date, the integrated study of the above-mentioned microbial attributes during the development of plantations is lacking.
To further elucidate the temporal variations in the aforementioned microbial attributes during stand development, they should be investigated at different spatial scales (e.g., the aggregate scale) (Chen et al., 2014; Mao et al., 2023). Soil aggregates, which serve as the basic units of soil structure, are the primary “sites” for SOC storage and act as “hotspots” for microbial activity (Gupta and Germida, 2015; Kuzyakov and Blagodatskaya, 2015). Recent studies have identified distinct distribution patterns of microbial attributes within different aggregates, attributed to their spatial heterogeneity and diverse physicochemical characteristics (e.g., pore properties and nutritional conditions) (Yao Y. et al., 2019; Wang and Hu, 2023). However, the impacts of aggregate size on microbial attributes along stand development remains inconsistent, with studies reporting increases, decreases, or no changes (Liao et al., 2023; Mao et al., 2023). To address this knowledge gap, an integrated study of microbial attributes, i.e., microbial communities, exo-enzymes, and microbial residue dynamics, within aggregates, to some extent, will enhance our understanding of microbial-driven soil C dynamics during forest development.
To fulfill our research objectives, five distinct age classes of Larix principis-rupprechtii plantations, i.e., 7a, 18a, 25a, 34a, and 44a, were selected from the Saihanba Mechanized Forest Farm, which represents the biggest plantation base in China (Wang Z. et al., 2018). It is noteworthy that since 1962, afforestation efforts, primarily involving Larix principis-rupprechtii, have been systematically and continuously implemented in Saihanba to address the issue of land desertification resulting from previous indiscriminate logging practices (Zhang et al., 2022). Consequently, Larix principis-rupprechtii of various ages are prevalent throughout this region. In this study, based on a series of technologies (e.g., 13C NMR, phospholipid fatty acids, exo-enzyme, and microbial residue analysis, etc.), we attempt to clarify the temporal changes in SOC dynamics within aggregates and the underlying microbial-driven mechanisms resulting in these variations after afforestation. Specifically, we hypothesized that (i) the growth and metabolism of microbes would be progressively strengthen during the development of plantations due to increased nutrient availability from annual continuous litter inputs; (ii) the variations in aggregate-related nutrient and pore properties induced by stand ages would alter the contents of microbial biomass and residues within soil aggregates; and (iii) the storage and composition of SOC would change along stand development, which were closely linked to the temporal changes in microbial attributes (i.e., microbial community, exo-enzymes, and microbial residues). These results can offer several valuable information for the sustainable development of planted forests in northern China.
2 Materials and methods
2.1 Study sites description and experimental design
The study was conducted at the Saihanba Mechanical Forest Farm, located in Hebei Province, North China (42°10′–42°50′N, 117°12′–117°30′E) with an elevation between 1,100 and 1940 m (Figure 1). This region belongs to a typical forest-steppe eco-tone with a temperate continental monsoon climate. The mean annual temperature and precipitation are −1.4°C and 450.1 mm, respectively. The average annual frost-free period is 68 days. The soils in the study site belong to brown soil (FAO, 2014). Besides, The cold-temperate coniferous forests, e.g., Larix principis-rupprechtii (shorted as larch), are the major forest types in this region due to their extreme cold tolerance and strong adaptability to soil.
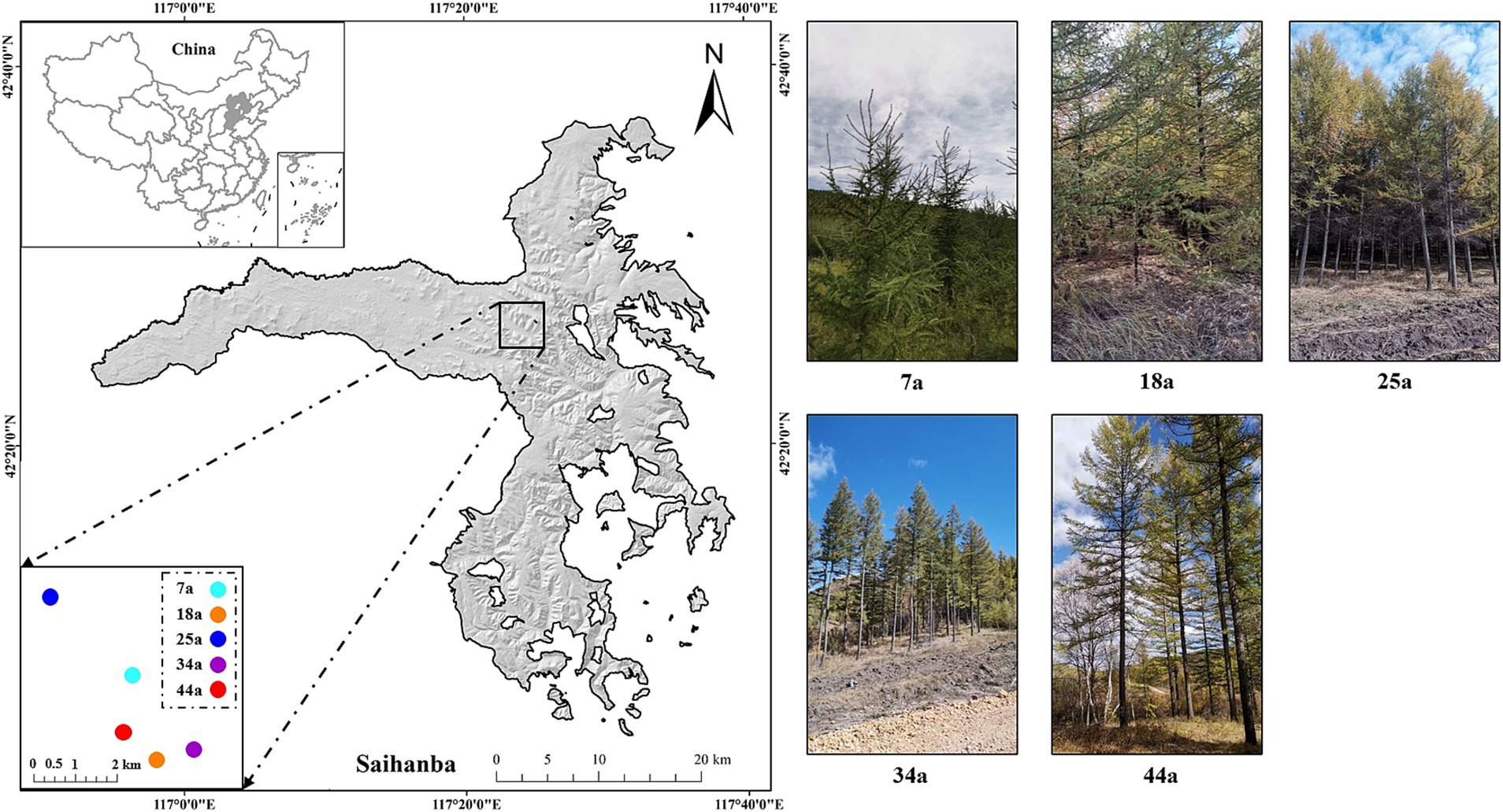
Figure 1. The study area and layout of plots for the five different-aged Larix principis-rupprechtii plantations.
The means of space-for-time substitution, a valuable method to survey soil temporal changes, was suitable for evaluating the temporal dynamics of forest’s soil evolution (Sparling et al., 2003). In the present study, five different-aged larch plantations, i.e., young (7a), middle-aged (18a and 25a), near mature (34a), and mature (41a) forests (see Figure 1), were selected to elucidate the temporal changes of soil characteristics that occur during the growth and development process of larch plantation. Three plots (20 m × 30 m) were randomly selected for investigation and sampling from each age of larch plantation. Besides, to be regarded as independent replications, each plot of the same stand age is separated by at least 0.2 km; meanwhile, all plots are within 10 km to ensure similar climatic and edaphic conditions. Other detailed information was shown in Table 1.
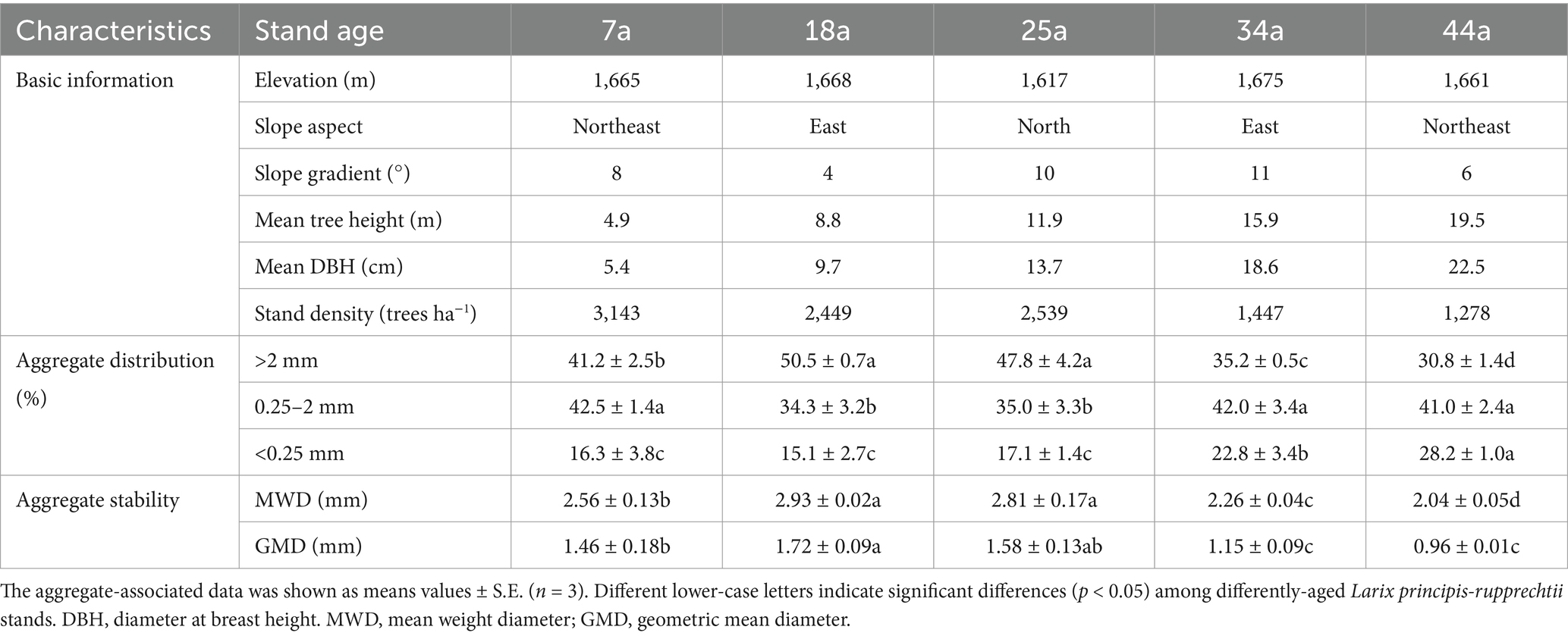
Table 1. The basic information, as well as the distribution characteristics of soil aggregates in the selected five aged stands of Larix principis-rupprechtii in the study sites.
2.2 Soil sampling and aggregate fractionation
In late August 2023, five undisturbed soil samples were randomly collected from the surface soil (0–20 cm depth) under the canopy of the larch stand in each plot and placed in a hard plastic box (20 cm × 12 cm × 6 cm) to ensure that they could sustain their original structure during transport to the laboratory. In total, 15 soil samples (3 replicates × 5 stand age) were stored in 4°C conditions and immediately transferred to the laboratory for further analysis. Then, after the removal of impurities (e.g., leaf debris and stone, etc.), these soil samples were gently broken along natural fracture planes and passed through an 8 mm sieve for determination of aggregate fractionation.
Based on the optimal-moisture sieving method, the above soil samples were manually fractionated through a nest of 2 sieves (2 mm and 0.25 mm) into three aggregate-size classes: >2 mm, 2–0.25 mm, and <0.25 mm aggregates. The specific procedure was described in Mao et al. (2021). Then, the aggregate samples were separated into two parts. One part was air-dried and passed through 0.15 mm sieve to determine aggregate-associated physicochemical properties and microbial residues; the other part was stored at −80°C for the determination of aggregate-associated microbial characteristics.
2.3 Determination of soil physicochemical properties
Soil pH was measured with a glass electrode at a water-to-soil ratio of 2.5:1 (v/w). Soil organic C (SOC, i.e., total C, due to soils being acidic) and total nitrogen (TN) were measured by the dry combustion method using an elemental analyzer (VELP EMA 502, Italy) (Vitti et al., 2016).
2.4 Soil microbial community (PLFAs) analysis
The aggregate-associated microbial community was classified and quantified by the phospholipid fatty acids (PLFAs) analysis, as described by Kramer et al. (2013). Briefly, the frozen-dried soil samples were treated with mild alkaline methanolysis to form fatty acid methyl esters (FAMEs), and then dissolved in hexane and estimated by gas chromatography (Agilent 6,890 GC, Santa Clara, CA, USA) with the MIDI peak identification 4.5 software (MIDI Inc., Newark, DE). Subsequently, 24 individual PLFAs were chosen and quantified based on the internal standard (C19:0), and classified into fungi, bacteria, and actinomycetes, etc. (Supplementary Table S1). The contents of PLFAs were expressed in units of nmol g−1.
2.5 Soil exo-enzyme activity analysis
Seven exo-enzyme activities (five hydrolytic exo-enzymes and two oxidative exo-enzymes; Supplementary Table S2) were determined to evaluate microbial functional attributes using the micro-plate enzyme method (Zhang et al., 2015). Five labeled fluorogenic substrates and L-DOPA substrate (Supplementary Table S2) were applied for the determination of hydrolytic and oxidative exo-enzymatic activities, respectively. The detailed procedure was referred to Luan et al. (2020). Then, the hydrolytic or oxidative exo-enzymatic activities were measured through a microplate reader (Synergy 2, Biotek, USA) with 365 nm excitation and 450 nm emission filters or 450 nm absorbance, respectively. The seven exo-enzyme activities were calculated as nmol g−1 soil h−1.
2.6 Soil microbial residue (amino sugar) analysis
The amino sugars (AS), valuable indicators widely used to describe microbial necromass, were quantified by the procedure described by Ding et al. (2010). The AS generally contains three constitutes [i.e., glucosamine (GluN), galactosamine (GalN), and muramic acid (MurA)], which were extracted (i.e., hydrolysis, filtration, nitrogen-evaporation) and derivatized into aldononitrile derivatives. Finally, they were determined by the internal standard myo-inositol, which was added before hydrolyzation. The sum of GluN, GalN, and MurA was used to evaluate the total microbial necromass pool.
2.7 Solid-state 13C NMR analysis
The SOC chemical composition was assessed by solid-state 13C NMR spectroscopy. Before NMR measurements, aggregate samples were repeatedly pre-treated using hydrofluoric acid (5%) to eliminate paramagnetic compounds (i.e., Fe3+ and Mn2+, etc.) following Zhuang et al. (2011). The 13C NMR spectra were obtained on a Bruker AVANCE 400 NMR spectrometer (Germany) equipped with a 4-mm probe. Besides, the spectra were divided into four major regions, i.e., alkyl C (0–45 ppm), O-alkyl C (45–110 ppm), aromatic C (110–160 ppm), and carbonyl C (160–190 ppm) (Simpson and Simpson, 2012), and calculated by integration with the MestReNova 8.0 software (Mestrelab Research, Santiago de Compostela, Spain).
2.8 Calculations
2.8.1 The indices of soil aggregate stability
By using the mass proportions of >2 mm, 2–0.25 mm, and <0.25 mm aggregates, mean weight diameter (MWD; mm) and geometric mean diameter (GMD; mm) were calculated to evaluate the stability of soil aggregates (Wang S. et al., 2018):
Where Xi is the average diameter of aggregates (mm); and Wi is the mass proportions of aggregates (%).
2.8.2 The geometric mean of the exo-enzyme activities
The geometric mean of the assayed hydrolytic and oxidative eco-enzymatic activities (GH and GOR) were calculated as follows:
Where the full names of αG, βG, CBH, XYL, NAG, PHOs, and PerX are shown in Supplementary Table S2.
2.8.3 Microbial residue C
Fungal and bacterial residue C (FRC and BRC, g kg−1) were calculated as follows (Cheng et al., 2024):
Where 179.2 and 251 are the molecular masses of GluN and MurA, respectively, and 9 is the conversion factor of fungal GluN to fungal C.
2.8.4 The indices of SOC stability
Three indices of SOC stability were calculated based on the relative abundance of SOC functional groups (Guo et al., 2019):
Where AI and RI are aromaticity index and recalcitrance index, respectively.
2.9 Statistical analysis
One-way analysis of variance (ANOVA) with Duncan tests was conducted to assess the effect of different stand ages (or soil aggregates) on the physicochemical and microbial variables. Two-way ANOVA based on 45 soil samples (5 stand ages × 3 soil aggregates × 3 replicates) was applied to compare the differences in these above-mentioned variables with the five stand ages and three soil aggregates as the main factors. These statistical analyses were conducted with SPSS 16.0 software (SPSS Inc. Chicago, IL, USA). By using CANOCO 4.5 software, principal component analysis (PCA) was applied to identify the differences of microbial community across five different-aged larch plantations within soil aggregates. Besides, the effects of microbial characteristics (i.e., microbial communities, exo-enzyme activities, and microbial residues) on SOC dynamics were explored by the partial least squares path model (PLS-PM; 1,000 bootstraps) with the packages “plspm” through the R software (version 3.6.1).
3 Results
3.1 Soil aggregates distribution and stability
The aggregates in the surface soils (0–20 cm) across five different-aged larch plantations were found to be dominated by >2 mm and 2–0.25 mm aggregates (30.8–50.5 and 34.3–42.5%, respectively), followed by the <0.25 mm aggregates (15.1–28.2%) (Table 1). The proportion of >2 mm aggregates were significantly (p < 0.05) higher by 16.0–64.0% in the 18a and 25a stands than those in other stands (7a, 34, and 44a). In contrast, the 18a and 25a stands contained lower proportions of 2–0.25 mm and <0.25 mm aggregates than other stands. Additionally, the values of MWD and GMD first increased and then decreased with increasing stand age, reaching the highest value at 18a stand (2.93 mm and 1.72 mm) (Table 1).
3.2 The physicochemical properties within aggregates
The physicochemical properties, e.g., soil organic C (SOC), total N (TN), SOC/TN, and pH within soil aggregates, are shown in Table 2. Overall, these physicochemical indices were significantly (p < 0.05) affected by stand ages (except for pH) and soil aggregates. The contents of SOC and TN as well as their ratios (SOC/TN) were observed highest in the 18a and 25a stands, followed by the 7a stand, and lowest in the 34a and 44a stands within aggregates; meanwhile, 0.25–2 mm aggregates contained higher values of SOC, TN, and SOC/TN by 15.2–26.8, 7.1–11.3, and 7.3–13.6%, respectively, than other aggregates (>2 mm and <0.25 mm). The soil pH was significantly (p < 0.05) higher in the 7a, 18a, and 25a stands than those in the 34 and 44a stands within aggregates; meanwhile, there were no significant differences in the values of pH across all aggregates (5.91–5.96) irrespective of the stand ages.
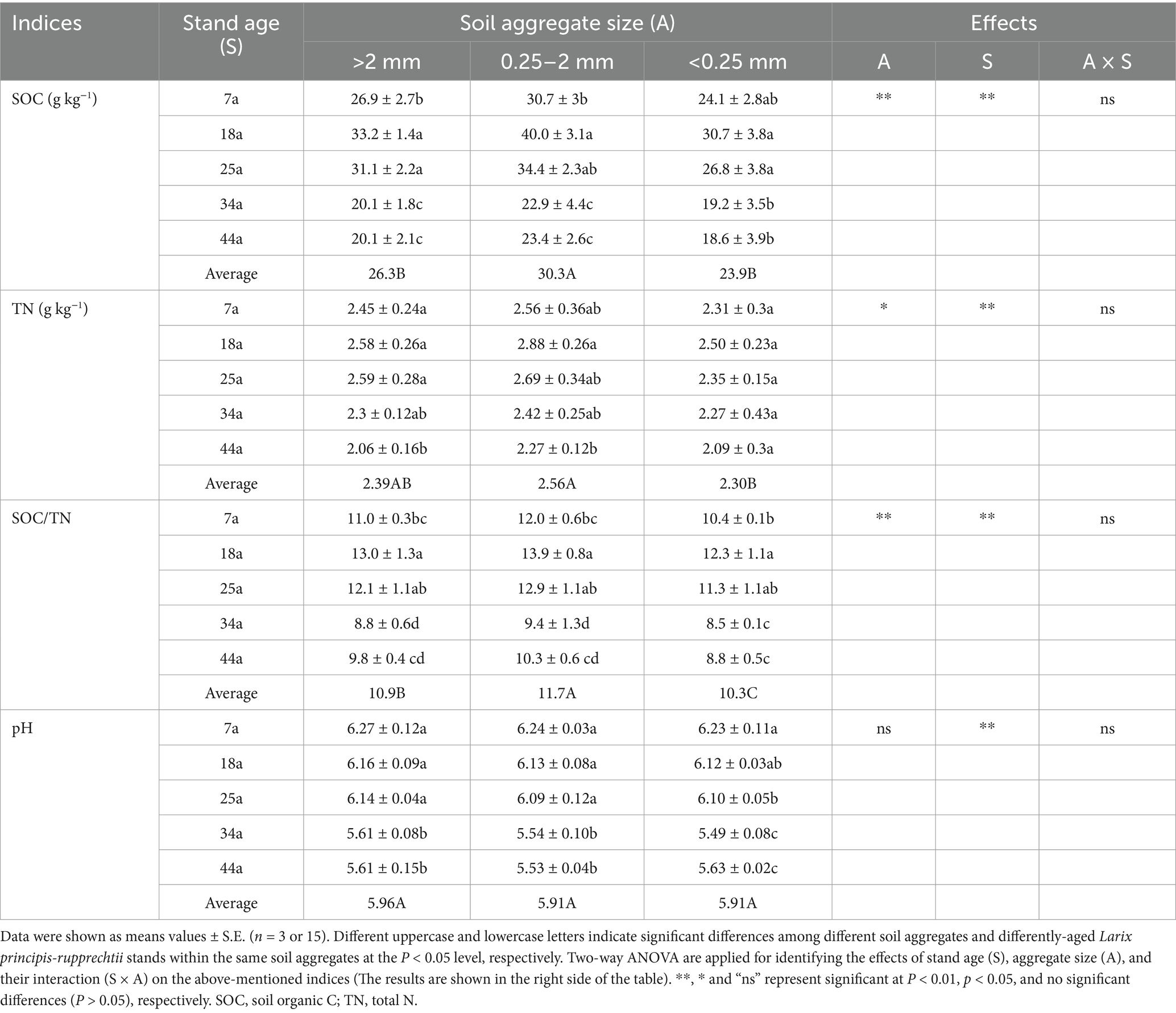
Table 2. The physicochemical properties within soil aggregates in differently-aged Larix principis-rupprechtii stands.
3.3 The microbial community composition within aggregates
Table 3 revealed that the contents of total microbes (i.e., total PLFAs) showed a unimodal trend with increasing stand ages within aggregates. Specifically, the values of total PLFAs were found higher by 21.5–53.4, 23.0–72.2, and 25.0–72.2%, respectively, in the 18a and 25a stands than other stands within >2 mm, 2–0.25 mm and <0.25 mm aggregates; meanwhile, >2 mm and 2–0.25 mm aggregates owned higher contents of total microbes by 27.4% on average than <0.25 mm aggregates. The relative abundance of fungi was found significantly (p < 0.05) lower in the 7a stand than that in the 18a, 25a, 34a, and 44a stands within aggregates. Besides, the relative abundance of bacteria showed ranked in the order: 18a and 25a > 7a, 34a, and 44a within aggregates. Notably, regardless of the stand age, the 2–0.25 mm aggregates contained lower relative abundance of fungi and higher relative abundance of bacteria than the >2 mm and <0.25 mm aggregates. These results induced the higher F/B ratios to be found in the 34a and 44a stands (or the <0.25 mm aggregates) than the 7a, 18a, and 25a stands (or the >2 mm and 2–0.25 mm aggregates) (Table 3). The PCA results (Figure 2) further identified stand age could alter the microbial community structure within aggregates. Specifically, the microbial profiles within >2 mm, 2–0.25 mm, and <0.25 mm aggregates among different-aged stands owned similar and obvious boundaries, which were categorized into four groups, i.e., 7a vs. 18a and 25a vs. 34a vs. 44a.
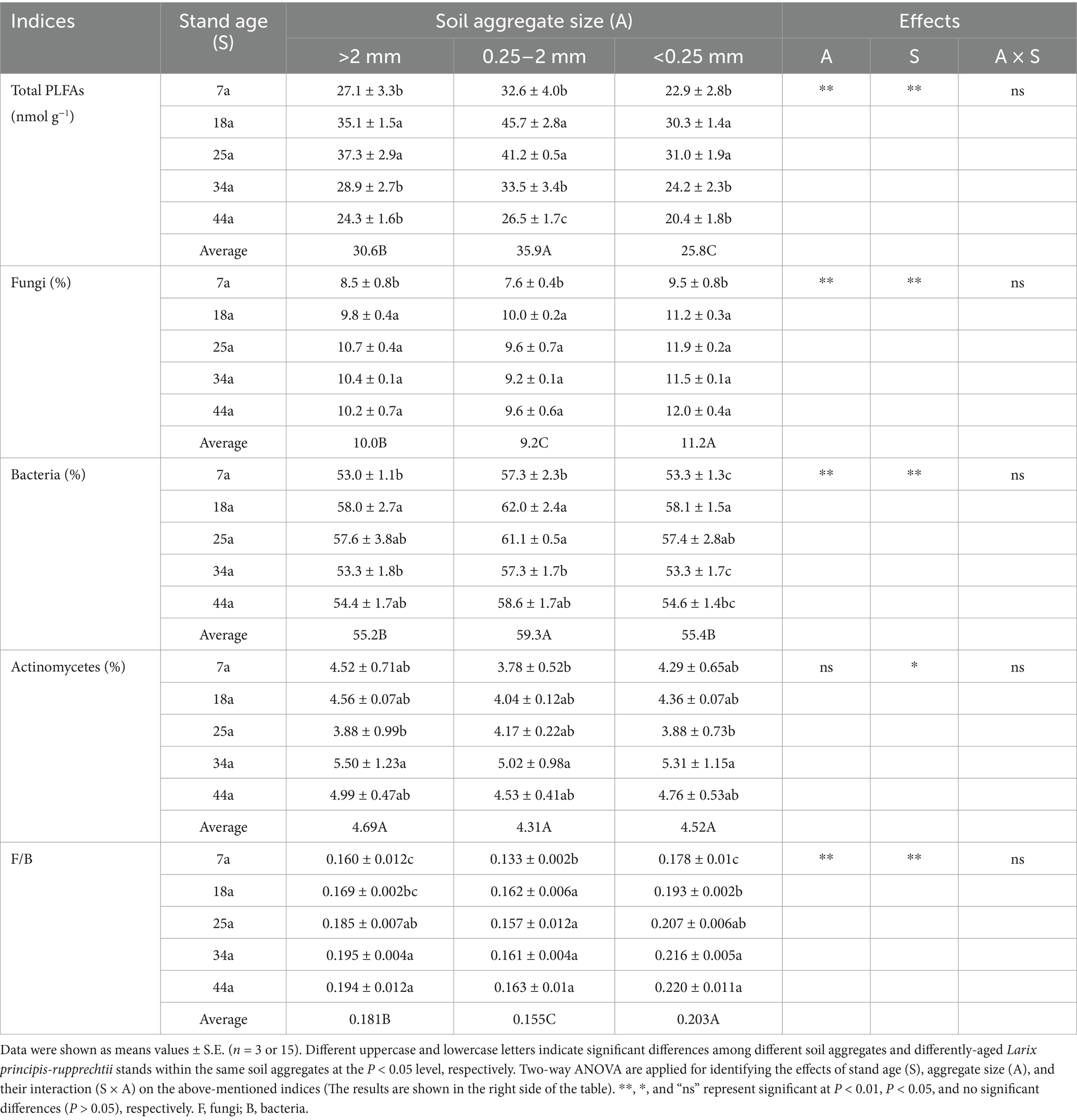
Table 3. The contents of total PLFAs, the relative abundance of microbial subgroups as well as associated ratios (F/B) within soil aggregates in differently-aged Larix principis-rupprechtii stands.
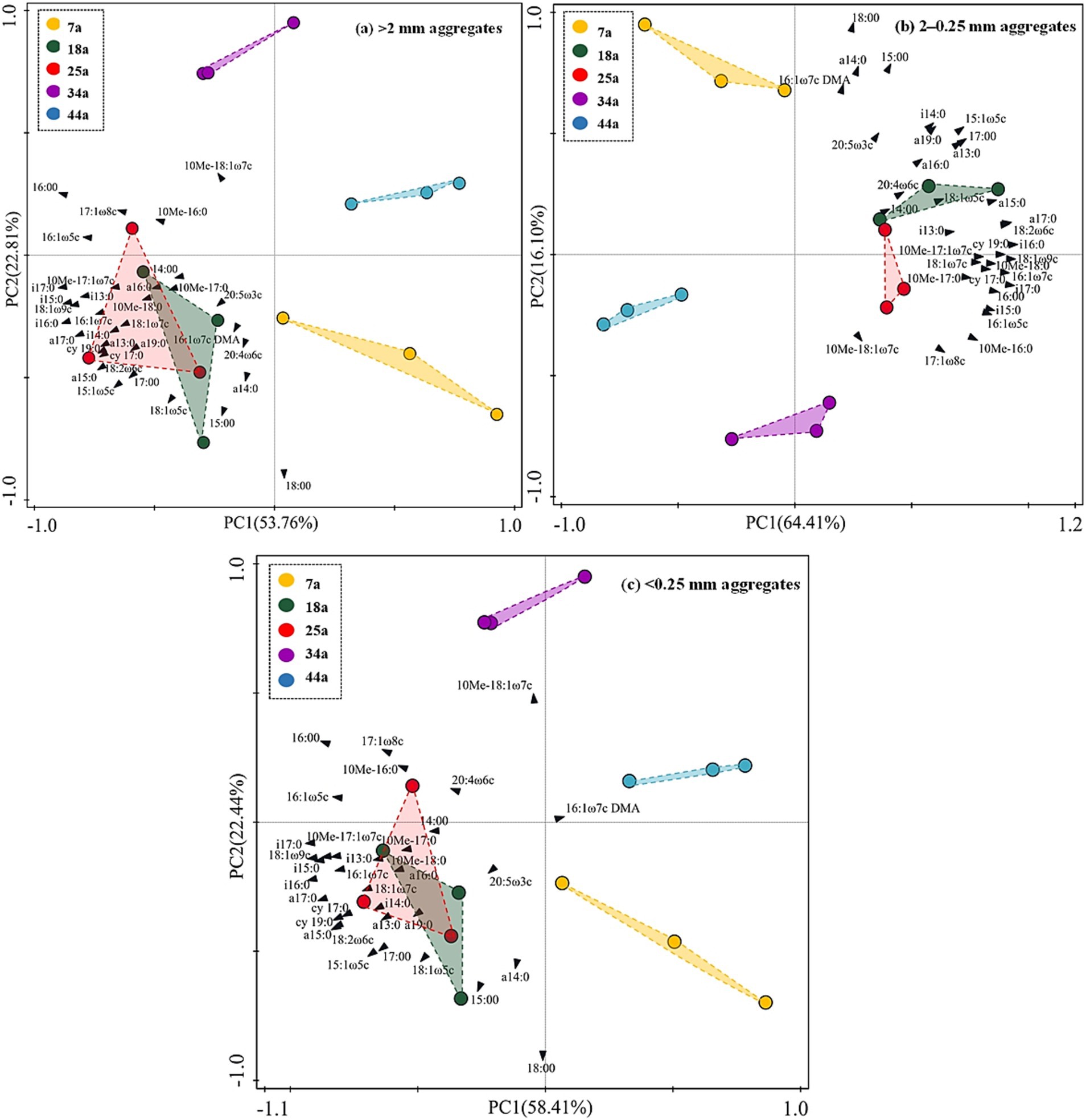
Figure 2. The principal component analysis (PCA) based on 24 individual PLFAs in the present study across differently-aged Larix principis-rupprechtii stands within different soil aggregates. The “10Me” in 10Me-18:1ω7c, 10Me-17:1ω7c, 10Me-16:0, 10Me-17:0, and 10Me-18:0 means “10-methyl”; the “i” in i13:0, i14:0, i15:0, i16:0, i17:0 means “iso”; the “a” in a14:0 a15:0, a16:0, a17:0 means “anteiso”; the “DMA” in 16:1ω7c DMA means “dimethyl acetal”; the “cy” in cy17:0 and cy19:0 means “cyclo”.
3.4 The exo-enzyme activities within aggregates
Table 4 and Figure 3 revealed that the activities of hydrolytic and oxidative exo-enzymes were significantly (p < 0.01) influenced by stand age and soil aggregates. For hydrolytic eco-enzymatic activities, these indices (e.g., GH, αG, βG, CBH, XYL, and NAG) within aggregates initially increased and subsequently decreased, with most parameters peaking in 18a or 25a stands. For oxidative exo-enzymatic activities, these indices (e.g., GOR, PHOs, and PerX) basically exhibited increasing trends as the stand age increased. Besides, regardless of the stand age, the >2 mm and 2–0.25 mm aggregates owned higher hydrolytic eco-enzymatic activities by 25.8–41.7% and lower oxidative exo-enzymatic activities by 21.8–26.7% than the <0.25 mm aggregates. Notably, the values of GH/GOR were consistently greater in the 18a stand (or >2 mm and 2–0.25 mm aggregates) than in the 7a, 25a, 34a, and 44a stands (or <0.25 mm aggregates) (Figure 3c).
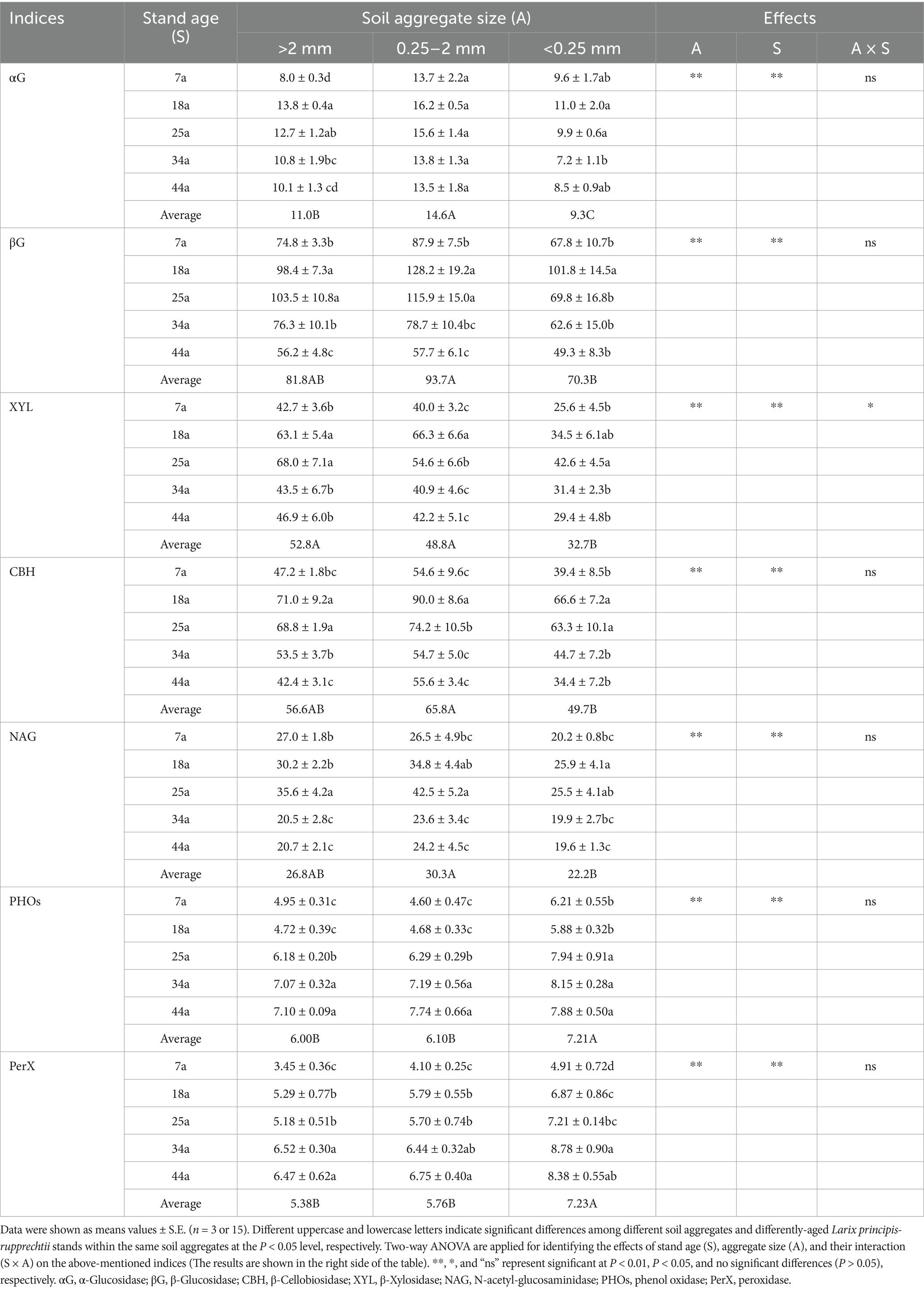
Table 4. The exo-enzyme activities (nmol g−1 soil h−1) within soil aggregates in differently-aged Larix principis-rupprechtii stands.
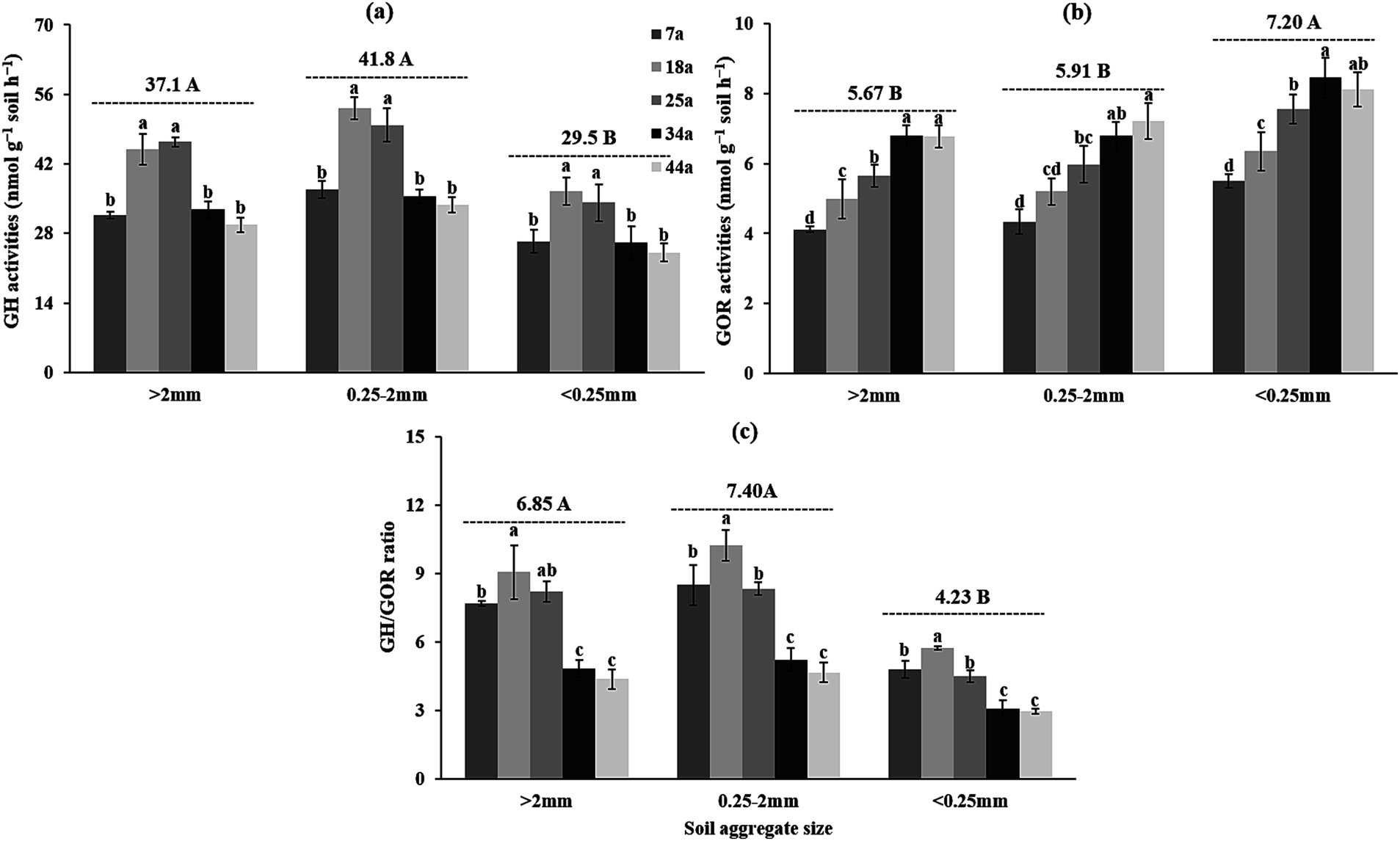
Figure 3. The geometric mean of the assayed exo-enzyme activities [(a) GH, (b) GOR and (c) GH/GOR] within soil aggregates across differently-aged Larix principis-rupprechtii stands. Different uppercase and lowercase letters indicate significant differences among different soil aggregates and differently-aged Larix principis-rupprechtii stands within the same soil aggregates at the p < 0.05 level, respectively. GH, the geometric mean of the hydrolytic exo-enzyme activities; GOR, the geometric mean of the oxidative exo-enzyme activities.
3.5 The microbial residue C within aggregates
The contents of amino sugars (total AS, GluN, GalN, and MurA; Table 5) and microbial residue C (FRC and BRC; Figure 4) basically and gradually increased from 7a to 14a stands but then declined in the 25a, 34a and 44a stands within aggregates; meanwhile, regardless of the stand age, the contents of FRC and BRC were higher by 5.3–15.6% and 20.6–40.9%, respectively, in the 2–0.25 mm aggregates than the >2 mm and <0.25 mm aggregates. Notably, the ratios of FRC/BRC and MRC/SOC were observed significantly (p < 0.05) higher in the 34a and 44a stands (3.50–5.18 and 29.2–33.5%) compared to the 7a, 14a, and 25a stands (2.61–4.06 and 24.6–31.1%) within aggregates; meanwhile, these indices were ranked as: [<0.25 mm aggregates (4.27 and 31.0%)] > [>2 mm and 2–0.25 mm aggregates (3.22–3.36 and 27.0–275%)].
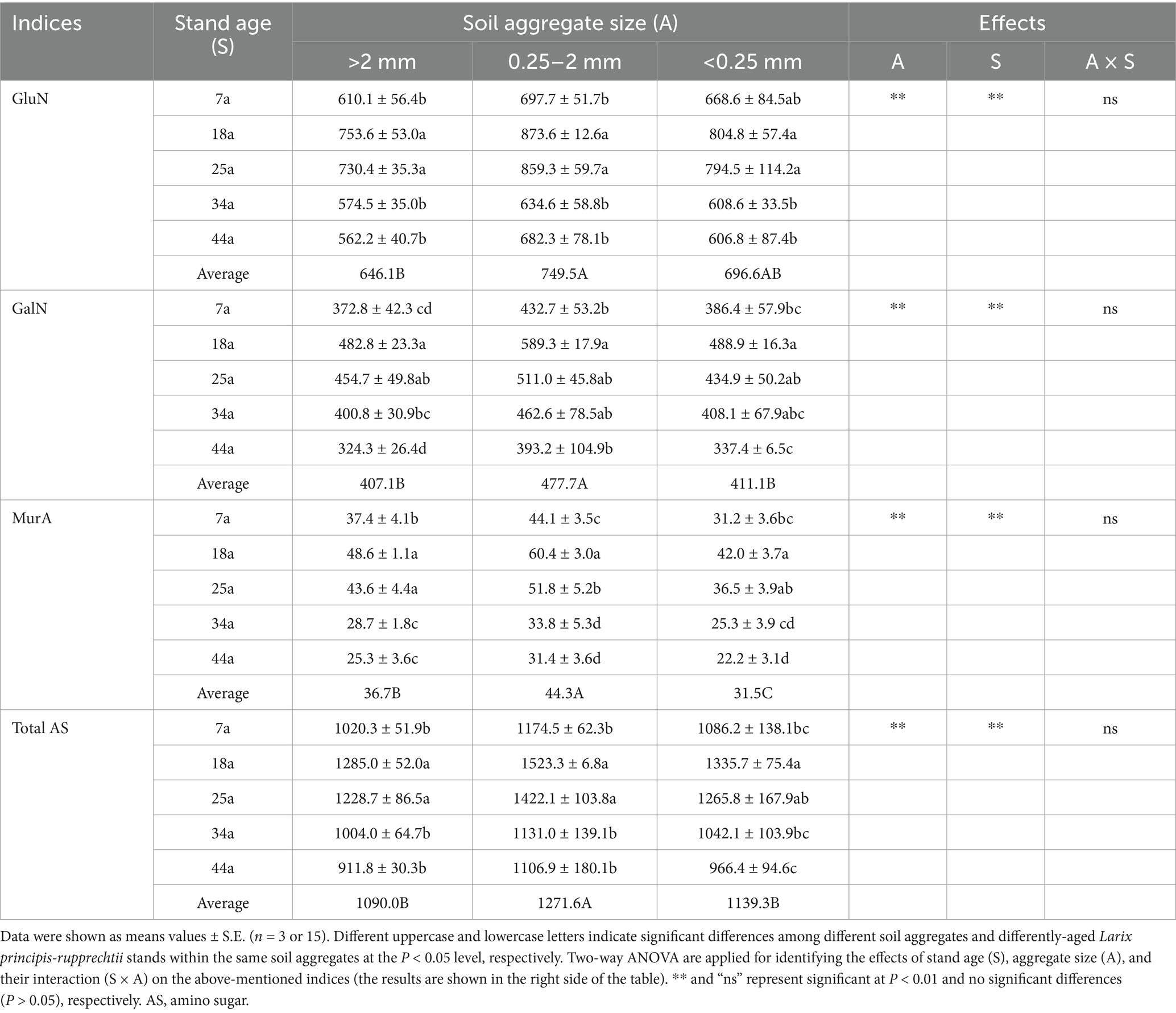
Table 5. The amino sugars (mg kg−1) within soil aggregates in differently-aged Larix principis-rupprechtii stands.
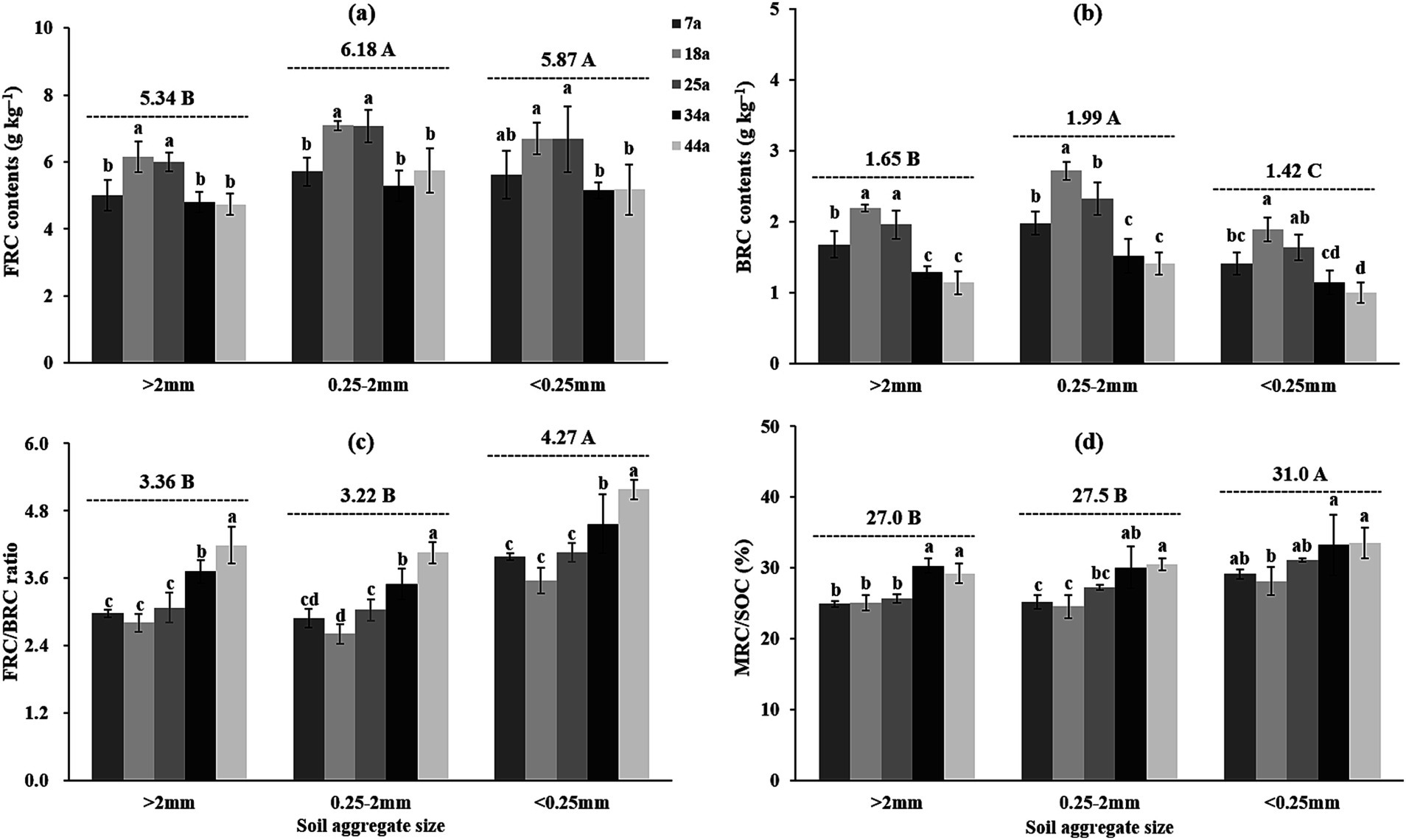
Figure 4. The contents of microbial residue C [(a) MRC, (b) FRC, and (c) BRC] as well as the associated ratio [(d) MRC/SOC] within soil aggregates across differently-aged Larix principis-rupprechtii stands. Different uppercase and lowercase letters indicate significant differences among different soil aggregates and differently-aged Larix principis-rupprechtii stands within the same soil aggregates at the p < 0.05 level, respectively. FRC, fungal residue C; BRC, bacterial residue C; MRC, microbial residue C; SOC, soil organic C.
3.6 The SOC chemical composition within aggregates
The results of 13C NMR analysis (Table 6) demonstrated that the effects of stand ages and aggregate sizes on SOC functional groups were primarily focused on aromatic C (p < 0.01) and O-alkyl C (p < 0.01), rather than carbonyl C and alkyl C (p > 0.05). Specifically, the relative abundance of aromatic C within aggregates increased with stand ages (except for 34a in 0.25–2 mm aggregates), whereas the relative abundance of O-alkyl C showed an opposite trend (7a, 18a, and 25a > 34a and 44a). Regardless of the stand ages, the SOC in the >2 mm and 0.25–2 mm aggregates exhibited greater relative abundance of O-alkyl C and lower relative abundance of aromatic C than those in the <0.25 mm aggregates; meanwhile, no significant differences (p > 0.05) were observed in the relative abundance of carbonyl C and alkyl C across different aggregates (Table 6). Notably, the values of RI, AI, and A/OA within aggregates were all ranked as 44a > 18a, 25a, and 34a > 7a (except for the RI and A/OA within >2 mm aggregates); meanwhile, the SOC in <0.25 mm aggregates owned significantly higher values of RI, AI, and A/OA than those in >2 mm and 0.25–2 mm aggregates irrespective of the stand ages (Table 6).
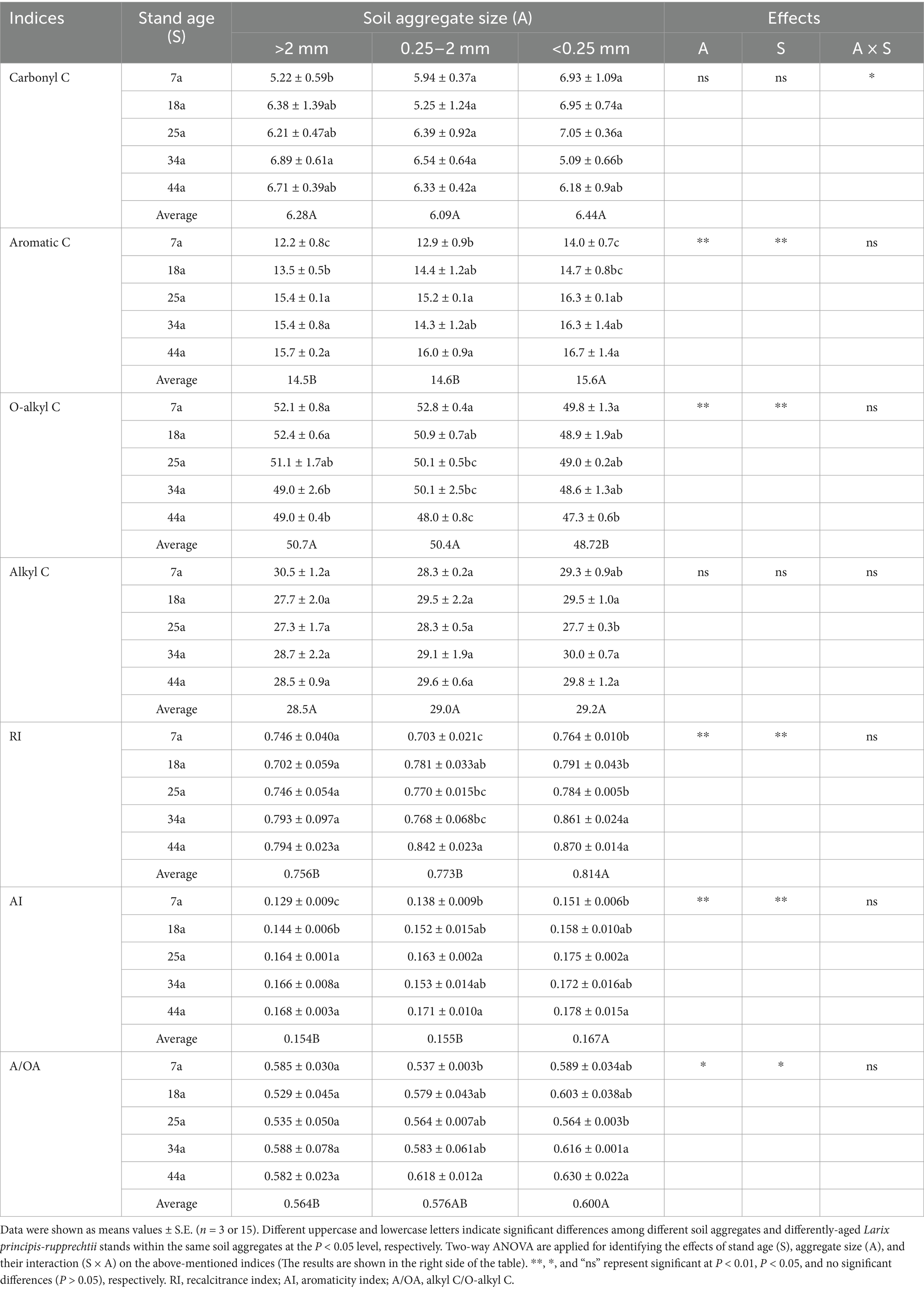
Table 6. The relative abundance (%) of different SOC functional groups and their spectroscopic indices within soil aggregates in differently-aged Larix principis-rupprechtii stands.
3.7 Interaction mechanisms between microbial attributes and SOC characteristics
The partial least squares path modeling (PLS-PM, GOF = 0.690; Figure 5) was conducted to investigate the correlation between microbial attributes (i.e., microbial community, exo-enzyme activities, and microbial residues) and SOC characteristics within soil aggregates of different-aged larch plantations and their potential mechanisms. The PLS-PM analysis validated the hypothesis (iii) and demonstrated that stand ages have significant and negative impacts on microbial communities (path coefficient = −0.354**) and exo-enzyme activities (−0.053**); concurrently, the aggregate sizes exhibited significantly positive and negative effects on microbial residue C (0.333**) and exo-enzyme activities (−0.284**), respectively. Besides, microbial community (0.348*) and exo-enzyme activities (0.670**) together positively regulated microbial residue C. Furthermore, microbial residue C (0.822**) and exo-enzyme activities (0.276**), rather than microbial community, were considered to be critical factors that positively affected SOC contents. Simultaneously, exo-enzyme activities (−0.960**) exhibited a significantly negative effect on SOC stability.

Figure 5. Partial least squares path model (PLS-PM) disentangling major pathways of microbial characteristics (i.e., microbial community, exo-enzyme activities, and microbial residue C) influences on SOC contents and their stability within aggregates across differently-aged Larix principis-rupprechtii stands. The arrows indicate the hypothesized direction of causation. The solid red and blue arrows indicate significant (p < 0.05) positive and negative relationships, respectively. Besides, the dashed arrows indicate non-significant effect (p > 0.05). The following variables were included: microbial community (total PLFAs, fungi, bacteria, and actinomycetes), exo-enzyme activities (α-Glucosidase, β-Glucosidase, β-Cellobiosidase, β-Xylosidase, N-acetyl-glucosaminidase, phenol oxidase, and peroxidase) and SOC stability (recalcitrance index, aromaticity index, and alkyl C/O-alkyl C). Asterisks indicate the statistical significance (**p < 0.01 and *p < 0.05).
4 Discussion
4.1 Changes in soil aggregate distribution and nutrient characteristics along stand development
Soil aggregate’s characteristics (i.e., aggregate distribution, MWD, and GMD), essential indicators for evaluating soil structure and quality, are governed by a series of factors, e.g., organic matter, Fe/Al oxides, and other biological attributes (Cheng et al., 2024). In this study, the proportion of > 2 mm aggregates and aggregate stability (as indicated by the values of MWD and GMD) increased along the stand ages, reaching the highest in the 18a stand (Table 1). This finding was consistent with previous study at the Beijiang River Forest Farm in Guangxi Zhuang Autonomous Region, China (He et al., 2021), which suggested with increasing stand ages, the proportions of > 2 mm aggregates and MWD initially increase and then decrease, and peak in the 17a stand. These findings could be ascribed to the temporal changes in organic C contents in soils during forest development (Ayoubi et al., 2012). Sun et al. (2024) also revealed that organic C in soils served as key “binding agents” for aggregate formation and enhancing aggregate stability. The similar temporal variation patterns between organic C and MWD in the present study also confirmed the above-mentioned opinions.
The stocks of SOC and nutrients (e.g., nitrogen) depends on the dynamic balance of exo-resources inputs (e.g., litters) vs. outputs (e.g., mineralization) (Luan et al., 2022). Generally, it is expected that SOC and nutrients would increase with stand age due to the annual input and accumulation of forest litters and root exudates in the subtropical regions in China (Chen et al., 2020; Hu J. et al., 2024). Nevertheless, our findings indicated that the contents of SOC and TN within aggregates initially increase, then decrease, peaking in the 18a stand (Table 2), which were contradicted the hypothesis (i). These inconsistent findings may be explained by the differences in climatic conditions across different study regions (i.e., subtropical region vs. temperate region in this study). Ma et al. (2022) highlighted that temperature, as one vital part of soil microclimates, may alter microbial growth and functional characteristics, thereby indirectly affecting litter degradation and SOC cycling processes. Donhauser and Frey (2018) also demonstrated that several adverse soil microenvironment, e.g., severely low temperature and desiccation, can impose significant stress on microbial communities, potentially inhibiting their activity and growth. Consequently, the climatic conditions characterized by low temperature (annual average temperature of −1.4°C) at the study sites impede microbial activity and litter decomposition (Tian et al., 2025). Meanwhile, the annual storage of litter detritus led to the development of thicker litter layers in the elder stands, which diminished soil aeration, thereby further inhibiting litter decomposition (Keiluweit et al., 2016). In other words, the continuous cold temperature and progressively worsening aeration conditions in soils were not beneficial for the conversion of litter into SOC, resulting in a reduction of SOC in the 25a, 34a and 44a stands in this study.
Yao Y. et al. (2019) demonstrated that SOC and nutrients were unevenly distributed across aggregates. In the present study, the contents of SOC and TN showed a similar distribution pattern within different aggregates, i.e., higher contents of SOC and TN were basically observed in the larger aggregates than those in the smaller aggregates irrespective of the stand ages (Table 2), which were verified the hypothesis (ii) and indicated that larger aggregates owned a higher capacity to retain these nutrients. This phenomenon could be explained by the “aggregate hierarchy model,” which posits that larger aggregates are constituted by smaller aggregates in conjunction with several “binding agents” (e.g., organic C constitutes); meanwhile, this process facilitated the storage of C within the larger aggregates (Ji et al., 2024). Wang et al. (2017) also reported that exogenous C and nutrient (e.g., forest litters) resources are difficult to reach the smaller aggregates due to the preferential “localization” of these resources within the larger aggregates, which further confirmed that the larger aggregates contained higher SOC and TN than the smaller aggregates in the present study.
4.2 Changes in microbial attributes within aggregates along stand development
Given the important role of microbes in the process of SOC cycling, clarifying the temporal–spatial changes in microbial attributes (e.g., microbial community, function, and metabolism) can provide valuable information for better understanding the mechanisms of SOC stabilization during forest development (Zhang et al., 2021). In the present study, it was observed that as stand age increased, microbial biomass (as indicated by the contents of total PLFAs; Table 3), hydrolytic exo-enzyme activities (Figure 3), and microbial residue C (Figure 4) within aggregates, initially increased and then decreased, and the highest values for these indices were observed in the 18a or 25a stands. This was partially aligned with the findings of Zhao et al. (2019), who reported microbial biomass showed a unimodal pattern with the progression of afforestation through meta-analysis. The likely reasons for these findings are as follows. Francini et al. (2018) revealed that appropriate soil microenvironment (e.g., adequate nutrients and O2) is the prerequisites for facilitating microbial growth and metabolism. In the present study, the better soil nutrient status (i.e., higher TN level) in the 18a and 25a stands, as presented in Table 2, created favorable soil conditions for microbial growth and metabolism, which caused higher contents of microbial biomass and microbial residue C in these stands. Sokol et al. (2022) indicated that the exo-enzymes, i.e., hydrolytic and oxidative exo-enzymes, are mainly secreted and produced by bacteria and fungi, respectively, with their activities being mainly regulated by soil properties (e.g., pH) and microbial community structure, etc. Therefore, the higher microbial biomass (Table 3) in the 18a and 25a stands were conducive to enhance hydrolytic exo-enzyme activities. Interestingly, we found that the variations in oxidative exo-enzymes, i.e., the gradually increasing oxidative exo-enzyme activities (as indicated by the values of GOR) during forest development (Figure 3b), were positively correlated with the values of F/B (Figure 6b), rather than with the contents of fungi (Figure 6a). Wang and Kuzyakov (2024) demonstrated that fungi can enhance their competitive ability for nutrients against bacteria by secreting more oxidative exo-enzymes under the premise of limited soil nutrient resources. Namely, although the restricted soil nutrient resources in the elder stands was not favorable for microbial growth (Table 3), they can stimulate fungi to secrete more oxidases to cope with this situation, which partially corroborates the results of Figure 6 and accounts for the observed variations in GOR across differently-aged stands in the present study.
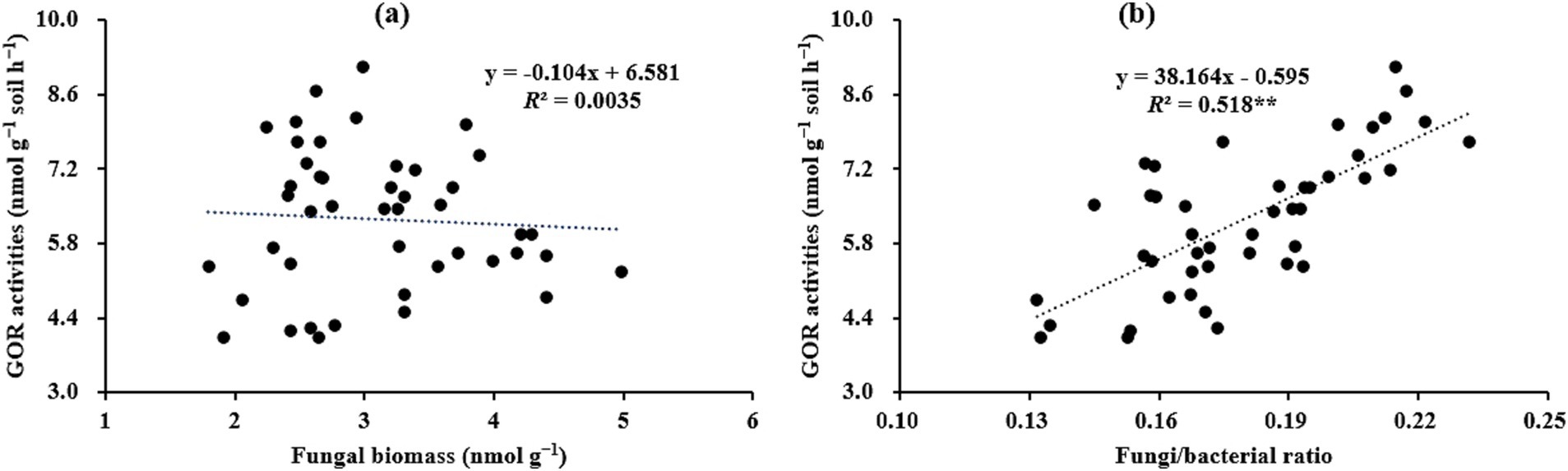
Figure 6. Relationships between the GOR activities and the fungal biomass (a) or the fungi/bacteria ratio (b) within soil aggregates across differently-aged Larix principis-rupprechtii stands (n = 45). GOR, the geometric mean of the oxidative exo-enzyme activities; F, fungi; B, bacteria. **Indicates significant at p < 0.01.
Nowadays, the distribution patterns of microbial attributes within aggregates have been widely reported, but these results remain controversial (Wang S. et al., 2018; Murugan et al., 2019). Our findings indicated that larger aggregates owned higher microbial biomass (Table 3) and hydrolytic exo-enzyme activities, alongside reduced oxidative exo-enzyme activities (Figure 3), compared to smaller aggregates, regardless of the stand ages. This could be explained by exo-enzyme function and aggregate’s characteristics (Yao S. et al., 2019; Sokol et al., 2022). The higher nutrient level (see Table 2) as well as more pore structure in the larger aggregates (Wang and Hu, 2023), rather than smaller aggregates, could create a better microenvironment for microbial growth. Furthermore, the nutrient deficiency in the smaller aggregates (see Table 2) may exacerbate bacterial–fungal competition for soil resources, thereby causing fungi to secrete more oxidative exo-enzymes to meet this competition (Wang and Kuzyakov, 2024). Notably, the increased FRC/BRC ratios observed in the smaller aggregates (Figure 4) suggested that FRC played a more dominant role than BRC in forming SOC in these aggregates. The possible explanation for these findings could be associated with differences in microbial necromass properties (Ding et al., 2010) and microbial community structure among different aggregates (Wang S. et al., 2018). He et al. (2022) indicated that fungal and bacterial biomass are the prerequisites for the formation of FRC and BRC, respectively. Hu J. et al. (2024) demonstrated BRC is relatively unstable and more susceptible to decomposition compared to FRC in soils characterized by “poor” nutrient levels. Based on the above-mentioned findings, the lower SOC and TN, along with higher F/B values observed in the smaller aggregates (Tables 5, 6), led to increased consumption of BRC and enhanced production of FRC, which explained the higher FRC/BRC in the smaller aggregates.
4.3 Changes in SOC characteristics within aggregates along stand development
Understanding the temporal dynamics in SOC chemical composition within aggregates can provide several new insights for better clarifying the mechanisms of SOC stabilization process during forest development (Wu et al., 2021). In the present study, the lower O-alkyl C abundance and higher aromatic C abundance were observed in the older stands (34a and 44a) compared to the younger stands (Table 6). This result was consistent with observations from Yang et al. (2021), in which the 64a stands contained higher aromatic C abundance and lower O-alkyl C abundance than the 19a and 37a stands in the Moso bamboo plantations. This could be mostly ascribed to the imbalance between soil resource vs. microbial nutrient demand (Gurmessa et al., 2024). The O-alkyl C (i.e., the labile C constitutes), rather than aromatic C (i.e., the stable C fractions), were preferentially consumed by microbes for their growth and metabolism under the “poor” soil nutrient status in the older stands (Table 2) (Yao S. et al., 2019). Notably, in accordance with previous studies (Luan et al., 2021; Wang et al., 2022), we observed that the SOC in the larger aggregates owned lower O-alkyl C abundance and higher aromatic C abundance than those in the smaller aggregates. The reduced nutrient levels observed in the smaller aggregates (see Table 2) could enhance the consumption of labile C (e.g., O-alkyl C) by microbes (Gurmessa et al., 2024), which could partially elucidate the above-mentioned findings. Besides, fungi are recognized as the key decomposers of stable C constitutes (e.g., aromatic C) and preferentially thrive in soils with high O2 levels (Liu et al., 2021). Consequently, the diminished fungal biomass and poor gas (e.g., O2) status in the smaller aggregates are not favorable for the decomposition of recalcitrant C (e.g., aromatic C). Taken together, the above-mentioned findings offer an explanation for the variations in SOC functional group composition across different aggregates.
The RI, AI, and A/OA were calculated by the four C functional groups, which could be applied to evaluate SOC stability, aromaticity, and degradation degree, respectively (Guo et al., 2019). In this study, as aggregate sizes decreased, these indices (i.e., RI, AI, and A/OA) showed increasing trends (Table 6), indicating that SOC in the smaller aggregates exhibited higher stability, greater aromaticity, and a higher degree of degradation. These rising patterns with decreasing aggregate sizes are likely attributed to enhanced consumption of O-alkyl C and diminished decomposition of aromatic C in the smaller aggregates (Table 6; Guo et al., 2019). The above-mentioned findings, in conjunction with our results (i.e., elevated RI, AI, and A/OA values in the 44a stand), also suggested an enhancement in SOC stability and aromaticity in the older stands.
5 Conclusion
The elucidation of the temporal dynamics of SOC characteristics and microbial attributes at the aggregate scale, employing a series of technologies (i.e., 13C NMR, PLFAs, exo-enzymatic activity, and microbial residue analysis, etc.), can offer novel insights into SOC stocks in relation to stand ages in Larix principis-rupprechtii plantations in Northern China. Firstly, with increasing stand age, the levels of SOC, microbial biomass, hydrolytic exo-enzymatic activities, and microbial residues, initially increase and then decrease, and generally peak in the 18a stand; meanwhile, these indices were generally higher in larger aggregates compared to smaller aggregates irrespective of the stand ages. Secondly, we observed that the increased stand ages and reduced aggregate size altered SOC chemical composition (more “labile” O-alkyl C and less “stable” aromatic C), enhanced SOC stability and oxidative exo-enzymatic activities, as well as enlarged the microbial contribution to SOC stocks. Finally, the PLS-PM model confirmed that SOC stocks were closely linked to microbial residues; meanwhile, the microbial community altered SOC stability by modulating exo-enzyme activities. These findings enhance our understanding of the microbial-driven mechanisms underlying SOC stabilization during forest development in Northern China.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: requests to access these datasets should be directed to bHVhbmhhb2FuQDE2My5jb20=.
Author contributions
MY: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. XH: Conceptualization, Investigation, Software, Writing – original draft. JL: Data curation, Investigation, Resources, Software, Writing – original draft. QL: Data curation, Investigation, Methodology, Software, Writing – review & editing. LF: Investigation, Methodology, Software, Writing – review & editing. XC: Data curation, Investigation, Methodology, Software, Writing – review & editing. SH: Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Writing – review & editing. HL: Conceptualization, Funding acquisition, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the National Key Research and Development Program of China (2021YFD1901001), the Natural Science Foundation of Hebei Province (C2024204108), the Scientific Research Projects for Talents Introduce in Hebei Agricultural University (YJ2020054 and YJ2020058), Hebei Province Forest and Grass Key Technology Innovation and Demonstration Project (2303087), Hebei Province Forest and Grass Technology Promotion Demonstration Project (JITG[2022]012), and College Student Innovation and Entrepreneurship Training Program Project (2025231).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1588030/full#supplementary-material
References
Abaker, W. E., Berninger, F., Saiz, G., Braojos, V., and Starr, M. (2016). Contribution of Acacia senegal to biomass and soil carbon in plantations of varying age in Sudan. For. Ecol. Manag. 368, 71–80. doi: 10.1016/j.foreco.2016.03.003
Ayoubi, S., Karchegani, P. M., Mosaddeghi, M. R., and Honarjoo, N. (2012). Soil aggregation and organic carbon as affected by topography and land use change in western Iran. Soil Till. Res. 121, 18–26. doi: 10.1016/j.still.2012.01.011
Chen, Y., Day, S. D., Wick, A. F., and McGuire, K. J. (2014). Influence of urban land development and subsequent soil rehabilitation on soil aggregates, carbon, and hydraulic conductivity. Sci. Total Environ. 495, 329–336. doi: 10.1016/j.scitotenv.2014.06.099
Chen, A., Wang, Z., Lin, Y., Wang, X., Li, Y., Zhang, Y., et al. (2020). Temporal variation of soil organic carbon pools along a chronosequence of reforested land in Southwest China. Catena 194:104650. doi: 10.1016/j.catena.2020.104650
Cheng, Z., Guo, J., Jin, W., Liu, Z., Wang, Q., Zha, L., et al. (2024). Responses of SOC, labile SOC fractions, and amino sugars to different organic amendments in a coastal saline-alkali soil. Soil Till. Res. 239:106051. doi: 10.1016/j.still.2024.106051
Ding, X., Zhang, X., He, H., and Xie, H. (2010). Dynamics of soil amino sugar pools during decomposition processes of corn residues as affected by inorganic N addition. J. Soils Sediments 10, 758–766. doi: 10.1007/s11368-009-0132-7
Dong, L., Lu, W., and Liu, Z. (2020). Determining the optimal rotations of larch plantations when multiple carbon pools and wood products are valued. For. Ecol. Manag. 474:118356. doi: 10.1016/j.foreco.2020.118356
Donhauser, J., and Frey, B. (2018). Alpine soil microbial ecology in a changing world. FEMS Microbiol. Ecol. 94:fiy099. doi: 10.1093/femsec/fiy099
FAO (2014). World Reference Base for soil resources. World soil resources reports no.106. Rome: FAO.
Francini, G., Hui, N., Jumpponen, A., Kotze, D. J., Romantschuk, M., Allen, J. A., et al. (2018). Soil biota in boreal urban greenspace: responses to plant type and age. Soil Biol. Biochem. 118, 145–155. doi: 10.1016/j.soilbio.2017.11.019
Guo, Z., Zhang, Z., Zhou, H., Wang, D., and Peng, X. (2019). The effect of 34-year continuous fertilization on the SOC physical fractions and its chemical composition in a vertisol. Sci. Rep. 9:2505. doi: 10.1038/s41598-019-38952-6
Gupta, V. V. S. R., and Germida, J. J. (2015). Soil aggregation: influence on microbial biomass and implications for biological processes. Soil Biol. Biochem. 80, A3–A9. doi: 10.1016/j.soilbio.2014.09.002
Gurmessa, B., Cocco, S., Ashworth, A. J., Udawatta, R. P., Cardelli, V., Serrani, D., et al. (2024). Enzyme activities and microbial nutrient limitations in response to digestate and compost additions in organic matter poor soils in the marches, Italy. Soil Till. Res. 242:106136. doi: 10.1016/j.still.2024.106136
He, M., Fang, K., Chen, L., Feng, X., Qin, S., Kou, D., et al. (2022). Depth-dependent drivers of soil microbial necromass carbon across Tibetan alpine grasslands. Glob. Chang. Biol. 28, 936–949. doi: 10.1111/gcb.15969
He, X., Huang, Y., Zhang, Q., Ye, S., and Wang, S. (2021). Distribution of organic carbon fractions in soil aggregates in Chinese fir plantations with different stand ages. Ecol. Process. 10:49. doi: 10.1186/s13717-021-00321-5
Hu, J., Liu, C., Gou, M., Lei, L., Chen, H., and Zhang, J. (2024). Contrasting change patterns of lignin and microbial necromass carbon and the determinants in a chronosequence of subtropical Pinus massoniana plantations. Appl. Soil Ecol. 198:105385. doi: 10.1016/j.apsoil.2024.105385
Hu, H., Qian, C., Xue, K., Jörgensen, R. G., Keiluweit, M., Liang, C., et al. (2024). Reducing the uncertainty in estimating soil microbial-derived carbon storage. P. Natl. Acad. Sci. USA. 121:e2401916121. doi: 10.1073/pnas.2401916121
Ji, L., Chen, X., Huang, C., and Tan, W. (2024). Arbuscular mycorrhizal hyphal networks and glomalin-related soil protein jointly promote soil aggregation and alter aggregate hierarchy in Calcaric Regosol. Geoderma 452:117096. doi: 10.1016/j.geoderma.2024.117096
Jing, Y., Zhao, X., Liu, S., Tian, P., Sun, Z., Chen, L., et al. (2022). Microbial residue distribution in microaggregates decreases with stand age in subtropical plantations. Forests 13:1145. doi: 10.3390/f13071145
Keiluweit, M., Nico, P. S., Kleber, M., and Fendorf, S. (2016). Are oxygen limitations under recognized regulators of organic carbon turnover in upland soils? Biogeochemistry 127, 157–171. doi: 10.1007/s10533-015-0180-6
Kramer, S., Marhan, S., Haslwimmer, H., Ruess, L., and Kandeler, E. (2013). Temporal variation in surface and subsoil abundance and function of the soil microbial community in an arable soil. Soil Biol. Biochem. 61, 76–85. doi: 10.1016/j.soilbio.2013.02.006
Kuzyakov, Y., and Blagodatskaya, E. (2015). Microbial hotspots and hot moments in soil: concept & review. Soil Biol. Biochem. 83, 184–199. doi: 10.1016/j.soilbio.2015.01.025
Liang, C., Schimel, J. P., and Jastrow, J. D. (2017). The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2:17105. doi: 10.1038/nmicrobiol.2017.105
Liang, B., Wang, J., Zhang, Z., Zhang, J., Zhang, J., Cressey, E. L., et al. (2022). Planted forest is catching up with natural forest in China in terms of carbon density and carbon storage. Fundamental Res. 2, 688–696. doi: 10.1016/j.fmre.2022.04.008
Liao, Z., Ye, S., and Wang, S. (2023). Soil bacterial community structure as affected by stand age in Chinese fir plantations: insights at the aggregate scale. Land Degrad. Dev. 34, 389–402. doi: 10.1002/ldr.4467
Liu, G., Deng, D., Yang, M., and Sun, Y. (2023). Root traits and soil bacterial composition explain the rhizosphere effects along a Chronosequence of rubber plantations. Forests 14:2209. doi: 10.3390/f14112209
Liu, X., Hou, L., Ding, C., Su, X., Zhang, W., and Peng, Z. (2024). Effects of stand age and soil microbial communities on soil respiration throughout the growth cycle of poplar plantations in northeastern China. Front. Microbiol. 15:1477571. doi: 10.3389/fmicb.2024.1477571
Liu, Z., Hou, L., Zhu, Y., and Xu, X. (2021). Vertical distribution and regulation of Olsen-phosphorus in 6-m soil profiles after farmland-to-apple orchard conversion on the Chinese loess plateau. Catena 202:105254. doi: 10.1016/j.catena.2021.105254
Liu, Q., Wang, S., Ma, R., Huang, F., Li, J., Ye, S., et al. (2024). Comparative analysis of forest soil carbon sink and source based on bibliometrics: development, hotspots, and trends. J. Clean. Prod. 480:144106. doi: 10.1016/j.jclepro.2024.144106
Luan, H., Gao, W., Tang, J., Li, R., Li, M., and Zhang, H. (2020). Aggregate-associated changes in nutrient properties, microbial community and functions in a greenhouse vegetable field based on an eight-year fertilization experiment of China. J. Integr. Agr. 19, 2530–2548. doi: 10.1016/S2095-3119(20)63269-5
Luan, H., Liu, Y., Huang, S., Qiao, W., Chen, J., and Guo, T. (2022). Successive walnut plantations alter soil carbon quantity and quality by modifying microbial communities and enzyme activities. Front. in Microbiol. 13:953552. doi: 10.3389/fmicb.2022.953552
Luan, H., Yuan, S., Gao, W., Tang, J., Li, R., and Zhang, H. (2021). Changes in organic C stability within soil aggregates under different fertilization patterns in a greenhouse vegetable field. J. Integr. Agr. 20, 2758–2771. doi: 10.1016/S2095-3119(21)63646-8
Ma, L., Liu, L., Lu, Y., Chen, L., Zhang, Z., Zhang, H., et al. (2022). When microclimates meet soil microbes: temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol. Biochem. 166:108566. doi: 10.1016/j.soilbio.2022.108566
Ma, T., Yang, Z., Shi, B., Gao, W., Li, Y., Zhu, J., et al. (2023). Phosphorus supply suppressed microbial necromass but stimulated plant lignin phenols accumulation in soils of alpine grassland on the Tibetan plateau. Geoderma 431:116376. doi: 10.1016/j.geoderma.2023.116376
Mao, L., He, X., Ye, S., and Wang, S. (2023). Soil aggregate-associated carbon-cycle and nitrogen-cycle enzyme activities as affected by stand age in Chinese fir plantations. J. Soil Sci. Plant Nut. 23, 4361–4372. doi: 10.1007/s42729-023-01355-8
Mao, L., Tang, L., Ye, S., and Wang, S. (2021). Soil organic C and total N as well as microbial biomass C and N affect aggregate stability in a chronosequence of Chinese fir plantations. Eur. J. Soil Biol. 106:103347. doi: 10.1016/j.ejsobi.2021.103347
Mujuru, L., Gotora, T., Velthorst, E. J., Nyamangara, J., and Hoosbeek, M. R. (2014). Soil carbon and nitrogen sequestration over an age sequence of Pinus patula plantations in Zimbabwean eastern highlands. For. Ecol. Manag. 313, 254–265. doi: 10.1016/j.foreco.2013.11.024
Murugan, R., Djukic, I., Keiblinger, K., Zehetner, F., Bierbaumer, M., Zechmeister-Bolternstern, S., et al. (2019). Spatial distribution of microbial biomass and residues across soil aggregate fractions at different elevations in the central Austrian Alps. Geoderma 339, 1–8. doi: 10.1016/j.geoderma.2018.12.018
Sakschewski, B., Bloh, W. V., Boit, A., Poorter, L., and Thonicke, K. (2016). Resilience of Amazon forests emerges from plant trait diversity. Nat. Clim. Chang. 6, 1032–1036. doi: 10.1038/nclimate3109
Shao, P., He, H., Zhang, X., Xie, H., Bao, X., and Liang, C. (2018). Responses of microbial residues to simulated climate change in a semiarid grassland. Sci. Total Environ. 644, 1286–1291. doi: 10.1016/j.scitotenv.2018.07.055
Simpson, M. J., and Simpson, A. J. (2012). The chemical ecology of soil organic matter molecular constituents. J. Chem. Ecol. 38, 768–784. doi: 10.1007/s10886-012-0122-x
Sokol, N. W., Slessarev, E., Marschmann, G. L., Nicolas, A., Blazewicz, S. J., Brodie, E. L., et al. (2022). Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 20, 415–430. doi: 10.1038/s41579-022-00695-z
Sparling, G., Ross, D., Trustrum, N., Arnold, G., West, A., Speir, T., et al. (2003). Recovery of topsoil characteristics after landslip erosion in dry hill country of New Zealand, and a test of the space-for-time hypothesis. Soil Biol. Biochem. 35, 1575–1586. doi: 10.1016/j.soilbio.2003.08.002
Stockmann, U., Adams, M. A., Crawford, J. W., Field, D. J., Henakaarchchi, N., Jenkins, M., et al. (2013). The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 164, 80–99. doi: 10.1016/j.agee.2012.10.001
Sun, X., Xing, Y., Yan, G., Liu, G., Wang, X., and Wang, Q. (2024). Dynamics of glomalin-related soil protein and soil aggregates during secondary succession in the temperate forest. Catena 234:107602. doi: 10.1016/j.catena.2023.107602
Tian, C., Cui, D., Cao, Y., Luo, S., Song, H., Yang, P., et al. (2025). Temperature-dependent soil storage: changes in microbial viability and respiration in semiarid grasslands. Soil Biol. Biochem. 202:109673. doi: 10.1016/j.soilbio.2024.109673
Vitti, C., Stellacci, A. M., Leogrande, R., Mastrangelo, M., Cazzato, E., and Ventrella, D. (2016). Assessment of organic carbon in soils: a comparison between the springer–Klee wet digestion and the dry combustion methods in Mediterranean soils (southern Italy). Catena 137, 113–119. doi: 10.1016/j.catena.2015.09.001
Wang, T., Dong, L., and Liu, Z. (2024). Factors driving carbon accumulation in forest biomass and soil organic carbon across natural forests and planted forests in China. Front. For. Glob. Change 6:1333868. doi: 10.3389/ffgc.2023.1333868
Wang, R., and Hu, X. (2023). Pore structure characteristics and organic carbon distribution of soil aggregates in alpine ecosystems in the Qinghai Lake basin on the Qinghai-Tibet plateau. Catena 231:107359. doi: 10.1016/j.catena.2023.107359
Wang, C., and Kuzyakov, Y. (2024). Rhizosphere engineering for soil carbon sequestration. Trends Plant Sci. 29, 447–468. doi: 10.1016/j.tplants.2023.09.015
Wang, S., Li, T., and Zheng, Z. (2017). Distribution of microbial biomass and activity within soil aggregates as affected by tea plantation age. Catena 153, 1–8. doi: 10.1016/j.catena.2017.01.029
Wang, S., Li, T., and Zheng, Z. (2018). Response of soil aggregate-associated microbial and nematode communities to tea plantation age. Catena 171, 475–484. doi: 10.1016/j.catena.2018.07.041
Wang, C., Xue, L., and Jiao, R. (2021). Soil organic carbon fractions, C-cycling associated hydrolytic enzymes, and microbial carbon metabolism vary with stand age in Cunninghamia lanceolate (lamb.) hook plantations. For. Ecol. Manag. 482:118887. doi: 10.1016/j.foreco.2020.118887
Wang, Z., Yao, X., and Wang, W. (2018). Variation of soil carbon pools in Pinus sylvestris plantations of different ages in North China. Acta Ecol. Sin. 38, 248–254. doi: 10.1016/j.chnaes.2017.08.004
Wang, X., Zhao, L., Comeau, L., Bian, Q., Jiang, Y., and Mao, J. (2022). Divergent carbon stabilization pathways in aggregates in Ultisols with and without organic amendments: implications from 13C natural abundance and NMR analysis. Geoderma 426:116088. doi: 10.1016/j.geoderma.2022.116088
Wiesmeier, M., Urbanski, L., Hobley, E., Lang, B., von Lützow, M., Marin-Spiotta, E., et al. (2019). Soil organic carbon storage as a key function of soils - a review of drivers and indicators at various scales. Geoderma 333, 149–162. doi: 10.1016/j.geoderma.2018.07.026
Wu, M., Pang, D., Chen, L., Li, X., Liu, L., Liu, B., et al. (2021). Chemical composition of soil organic carbon and aggregate stability along an elevation gradient in Helan Mountains, Northwest China. Ecol. Indic. 131:108228. doi: 10.1016/j.ecolind.2021.108228
Xu, H., He, B., Guo, L., Yan, X., Zeng, Y., Yuan, W., et al. (2024). Global forest plantations mapping and biomass carbon estimation. J. Geophys. Res-Biogeo. 129:e2023JG007441. doi: 10.1029/2023JG007441
Yang, C., Zhang, X., Ni, H., Gai, X., Huang, Z., du, X., et al. (2021). Soil carbon and associated bacterial community shifts driven by fine root traits along a chronosequence of Moso bamboo (Phyllostachys edulis) plantations in subtropical China. Sci. Total Environ. 752:142333. doi: 10.1016/j.scitotenv.2020.142333
Yao, Y., Ge, N., Yu, S., Wei, X., Wang, X., Jin, J., et al. (2019). Response of aggregate associated organic carbon, nitrogen and phosphorous to re-vegetation in agro-pastoral ecotone of northern China. Geoderma 341, 172–180. doi: 10.1016/j.geoderma.2019.01.036
Yao, S., Zhang, Y., Han, Y., Han, X., Mao, J., and Zhang, B. (2019). Labile and recalcitrant components of organic matter of a Mollisol changed with land use and plant litter management: an advanced 13C NMR study. Sci. Total Environ. 660, 1–10. doi: 10.1016/j.scitotenv.2018.12.403
Zhang, M., Bai, X., Wang, Y., Li, Y., Cui, Y., Hu, S., et al. (2023). Soil microbial trait-based strategies drive the storage and stability of the soil carbon pool in Robinia pseudoacacia plantations. Catena 222:106894. doi: 10.1016/j.catena.2022.106894
Zhang, K., Maltais-Landry, G., and Liao, H. (2021). How soil biota regulate C cycling and soil C pools in diversified crop rotations. Soil Biol. Biochem. 156:108219. doi: 10.1016/j.soilbio.2021.108219
Zhang, X., Yu, P., Wang, D., and Xu, Z. (2022). Density- and age- dependent influences of droughts and intrinsic water use efficiency on growth in temperate plantations. Agric. For. Meteorol. 325:109134. doi: 10.1016/j.agrformet.2022.109134
Zhang, Q., Zhou, W., Liang, G., Sun, J., Wang, X., and He, P. (2015). Distribution of soil nutrients, extracellular enzyme activities and microbial communities across particle size fractions in a long-term fertilizer experiment. Appl. Soil Ecol. 94, 59–71. doi: 10.1016/j.apsoil.2015.05.005
Zhao, F., Ren, C., Han, X., Yang, G., Wang, J., and Doughty, R. (2019). Trends in soil microbial communities in afforestation ecosystem modulated by aggradation phase. Forest. Ecol. Manag. 441, 167–175. doi: 10.1016/j.foreco.2019.03.036
Keywords: stand age, Larix principis-rupprechtii, soil aggregates, microbial characteristics, soil organic C dynamics
Citation: Yang M, He X, Liang J, Liu Q, Fu L, Cui X, Huang S and Luan H (2025) Linkage of living microbial biomass, function, and necromass to soil organic carbon storage along a chronosequence of Larix principis-rupprechtii plantation in North China. Front. Microbiol. 16:1588030. doi: 10.3389/fmicb.2025.1588030
Edited by:
Muhammad Zahid Mumtaz, Gansu Agricultural University, ChinaReviewed by:
Mohd Musheer Altaf, Institute of Information Management and Technology, Aligarh, IndiaAbubakar Dar, The Islamia University of Bahawalpur, Pakistan
Copyright © 2025 Yang, He, Liang, Liu, Fu, Cui, Huang and Luan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohui Huang, c2hhb2h1aTE5ODhAc2luYS5jb20=; Haoan Luan, bHVhbmhhb2FuQDE2My5jb20=
†These authors have contributed equally to this work
 Mengyun Yang1†
Mengyun Yang1† Shaohui Huang
Shaohui Huang Haoan Luan
Haoan Luan