- Department of Physics, Swiss Plasma Center (SPC), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Plasma-activated water (PAW) is an emerging antimicrobial agent with promising applications in bacterial inactivation. The PAW samples are generated by non-contact exposure of deionized water to a surface dielectric barrier discharge plasma and are characterized by a reactive nitrogen species-rich chemistry. In this work, fluorescence flow cytometry is applied to assess the viability of Escherichia coli treated with PAW. The results indicate that PAW exhibits a strong bactericidal effect, significantly increasing propidium iodide positive populations and leading to cell shrinkage. Comparative colony-forming unit counting confirmed these findings, showing agreement between both techniques and ruling out the possibility of a viable but non-culturable (VBNC) bacteria state. These results underscore the potential of the PAW samples produced with the present setup for safe decontamination applications, while also offering insights into the mechanisms of bacterial inactivation.
1 Introduction
Plasma-activated water (PAW) is a novel and promising technology in the decontamination sector, offering an alternative to traditional chemical and heat-based disinfectants (Wang and Salvi, 2021). PAW is produced by exposing water to low-temperature plasmas (LTPs), leading to the accumulation of reactive oxygen and nitrogen species (RONS) within the water (e.g., O3, H2O2, ). Exposure to LTPs also alters pH, electrical conductivity (EC), and oxidation reduction potential (ORP), inducing water acidification and an increase in EC and ORP (Tian et al., 2015; Zhou et al., 2018; Hadinoto et al., 2021). The synergistic effect of RONS and low pH is believed to be responsible for the antimicrobial effects of PAW, inactivating a broad spectrum of microorganisms, including bacteria, viruses, and fungi (Zhou et al., 2018, 2020).
Typically, the efficacy of antimicrobial agents is evaluated by measuring bacterial viability based on their proliferation. Various methods for bacterial counting have been developed, categorized into bulk methods or single-cell measurements. Bulk methods, which are relatively simple and fast, detect microbial growth by measuring changes in turbidity, gas pressure, or conductivity of the liquid medium (Nebe-Von-Caron et al., 2000). Alternatively, they rely on counting bacterial colonies on agar plates, assuming that each colony originates from a single microorganism (CFU counting) (Shapiro, 2000; Koch, 2007). Bulk methods are constrained by the necessity to cultivate bacteria in a controlled environment and do not take into account bacterial heterogeneity. Furthermore, the comparison between single-cell analysis and bulk methods can present discrepancies, as in cases of bacterial injury, presence of persister or dormant cells, or severe starvation, bacterial growth may become temporarily undetectable (Nebe-Von-Caron et al., 2000). The presence of viable but non-culturable (VBNC) cells within the culture might also be the cause of these discrepancies (Fakruddin et al., 2013; Ambriz-Aviña et al., 2014). The VBNC state is a physiological state in which bacteria are metabolically active but cannot duplicate on conventional culture media. Under favorable conditions, VBNC cells may resuscitate and regain their ability to duplicate. The VBNC state is an adaptive survival mechanism employed by many bacterial species, including numerous human pathogens, in response to environmental stressors. Although VBNC bacteria evade detection by conventional culturing methods, they remain viable and can retain virulence potential (Fakruddin et al., 2013; Liu et al., 2023). Environmental stressors such as UV radiation, exposure to toxic compounds, and nutrient deprivation have been shown to induce the VBNC state in bacteria (Trevors, 2011). Therefore, validating a decontamination method requires assessing the presence of the VBNC state. This verification can be achieved by single-cell methods such as flow cytometry (Fleischmann et al., 2021), which, combined with fluorescent staining, is increasingly recognized as a powerful tool for studying bacterial viability (Shapiro, 1985, 2000; Ambriz-Aviña et al., 2014).
A flow cytometer is a device that analyzes single cells by exposing them to a laser beam. The cell under investigation by the diagnostics scatters light in all directions. Forward scattering (FSC), defined as the light scattered at acute angles, is proportional to the particle size, while side scattering (SSC), produced at wide angles, contains information about the internal complexity of the particle (e.g., granularity). In addition to events enumeration, FSC and SSC, fluorescent dye staining allows bacteria species and biological activities to be distinguished, selectively targeting biochemical or structural properties of the cell (e.g., enzyme activities, nucleic acid sequences) (Veal et al., 2000). To select distinct subpopulations of cells within a heterogeneous sample, gating is performed based on the intensity of FSC, SSC, and fluorescence (Shapiro, 1985, 2000; Veal et al., 2000; Ambriz-Aviña et al., 2014).
Assessing bacterial viability with fluorescent flow cytometry is possible through the verification of metabolic activity, maintenance of membrane potential, or membrane impermeability to specific dyes (Shapiro, 2000). Membrane integrity and membrane potential can be measured through dye retention or exclusion methods, often based on multicolour labeling. One of the most common method for assessing membrane integrity is the exclusion of the red-fluorescent nucleic acid stain propidium iodide (PI), combined with the highly membrane-permeable DNA stain, SYTO9. Cells with an intact cytoplasmic membrane are impermeable to charged dyes such as PI. If membrane integrity is lost, PI binds to DNA, increasing the PI fluorescence 20- to 30-fold (Stiefel et al., 2015). Staining with PI is therefore a viability assessment test based on dye exclusion. On the contrary, the green-fluorescent nucleic acid stain SYTO9 permeates both live and dead bacterial cells. The SYTO9 fluorescent signal intensifies significantly upon binding to nucleic acid while displaying low intrinsic fluorescence signal when unbound. In the presence of both dyes, PI demonstrates a greater affinity for nucleic acids than SYTO9, leading to the displacement of SYTO9 by PI (Nebe-Von-Caron et al., 2000; Stiefel et al., 2015).
The live/dead BacLight staining kit (Invitrogen, 2004) utilizes SYTO9 and PI for viability determination and VBNC cell detection. This method has been widely used and extensively validated through microscopy and flow cytometry analyses (Lisle et al., 1998; Auty et al., 2001; Leuko et al., 2004; Berney et al., 2007; Taimur Khan et al., 2010; Ambriz-Aviña et al., 2014). It has been applied for microbial enumeration and detection (Leuko et al., 2004), as well as for antiseptic efficacy testing (Langsrud and Sundheim, 1996; Stocks, 2004). Additionally, its applications extend to water quality assessment (Endo et al., 1997; Boulos et al., 1999) and hygiene monitoring in processed meat (Duffy and Sheridan, 1998). These studies have shown a strong correlation between microscopic counts and direct viable counts, with discrepancies of ~ 0.1 log/mL (Boulos et al., 1999). This difference corresponds to about a 20% variation in percentage of inactivation.
It is important to acknowledge that PAW, as other decontamination methods, may not always induce complete bacterial cell death but could instead cause sublethal damage and resulting in VBNC state. Several studies have reported bacterial VBNC state following plasma treatment (Cooper et al., 2010; Kvam et al., 2012; Xu et al., 2018; Zhao et al., 2020). For instance, Dolezalova and Lukes (2015) observed a nearly 7-log reduction in E. coli populations following 15 min of plasma exposure when assessed by CFU counting. However, the same study reported a much smaller reduction (< 1 log, corresponding to 45% of inactivation) when viability was determined using the live/dead staining technique, suggesting that a significant proportion of bacteria had transitioned into the VBNC state (Dolezalova and Lukes, 2015). These findings underscore the need for complementary analytical techniques beyond traditional CFU counting to accurately assess bacterial viability following PAW treatment. Several microscope acquisitions of bacteria treated with PAW and stained with the live/dead BacLight kit, such as Staphylococcus aureus (Ma et al., 2015; Tian et al., 2015), Listeria monocytogenes (Handorf et al., 2021), and Escherichia coli biofilms (Xia et al., 2023), are present in the literature, but they were not associated with quantitative flow cytometry (Gan et al., 2022).
This study aims to address a critical gap in bacterial viability assessment by utilizing fluorescent flow cytometry to investigate the potential induction of the VBNC state in E. coli following treatment with PAW. Understanding whether PAW drives E. coli into a VBNC state is crucial for accurately evaluating bacterial inactivation beyond conventional culture-based methods and has significant implications for antimicrobial applications. Moreover, this study specifically tests VBNC induction of reactive nitrogen species (RNS)-rich PAW in contrast with existing research that predominantly focuses on PAW chemistries dominated by reactive oxygen species (ROS) (Ma et al., 2015; Gan et al., 2022; Xia et al., 2023).
2 Materials and methods
2.1 PAW reactor
The reactor used for the preparation of the PAW samples, illustrated in Figure 1, has been thoroughly described in previous publications (Agus et al., 2024a,b). The portable setup features a surface dielectric barrier discharge (SDBD) system, which consists of a copper back-plate electrode, a dielectric disk, and a stainless steel perforated disk electrode, as depicted in Figure 1a. The SDBD is positioned in contact with a glass spacer placed on top of a glass vessel. As shown in Figure 1b, the water and plasma do not come into direct contact, with a gap of ~ 4 mm between them, guaranteeing no changes in water temperature during the plasma exposure. The ultra-pure water can be recirculated within the glass vessel through a persistaltic pump with a constant flow rate of 200 mL/min. The blue arrows in Figure 1b indicate the direction of water flow when the recirculation system is activated. During the PAW reactor operation, 150 mL of ultra-pure water contained in the glass vessel, was exposed to the SDBD. The SDBD high voltage waveform featured a 8.8 kV peak-to-peak wave at 21 kHz, modulated at 100 Hz, resulting in a plasma discharge power of ~ 39 W, measured by Lissajous figures (Peeters and Butterworth, 2018; Agus et al., 2024a). This relatively high plasma discharge power ensured a nitrogen oxides-dominated chemistry, favoring the formation of nitrogen oxides over reactive oxygen species (Agus et al., 2024a).
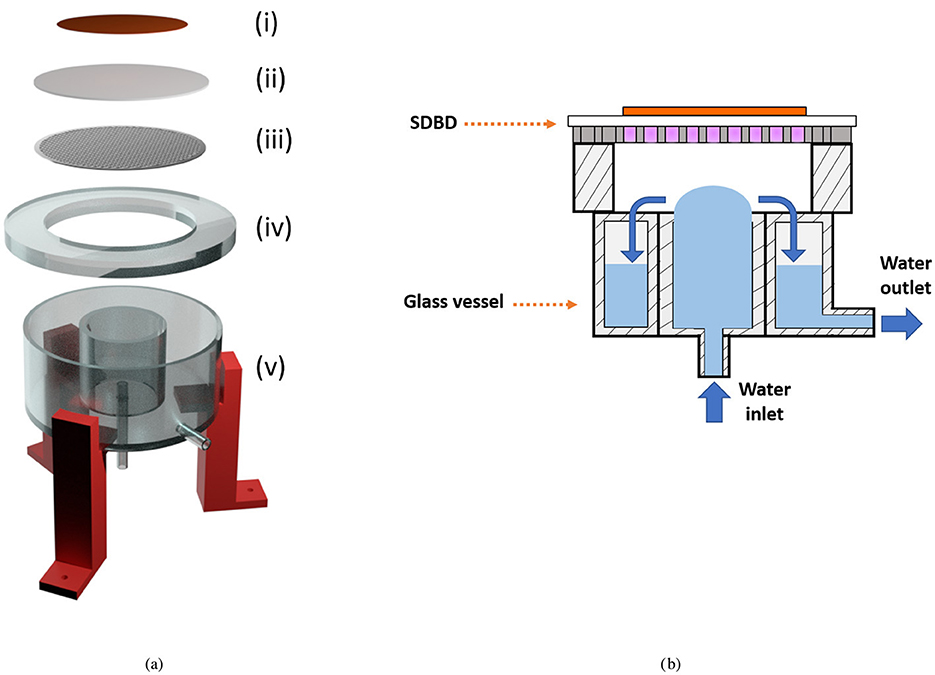
Figure 1. (a) Expanded view of the PAW reactor setup. The SDBD consists of a copper back-plate electrode (i), a dielectric disk (ii), and a stainless steel perforated disk electrode (iii). The SDBD is in contact with the glass spacer (iv), located on top of the glass vessel (v). A red 3D-printed structure supports the setup (Agus et al., 2024b). (b) Sketch of the assembled PAW reactor, illustrating the SDBD with the plasma on and the water configuration in the glass vessel. Blue arrows indicate the water flow direction.
The samples are labeled as dynamic PAW or static PAW, depending on whether the water recirculation is on or off, respectively. The chemical composition of PAW is influenced by both the presence of water recirculation and the duration of plasma exposure. To specify the plasma treatment duration, samples are labeled as PAW-XX, where XX denotes the exposure time in minutes.
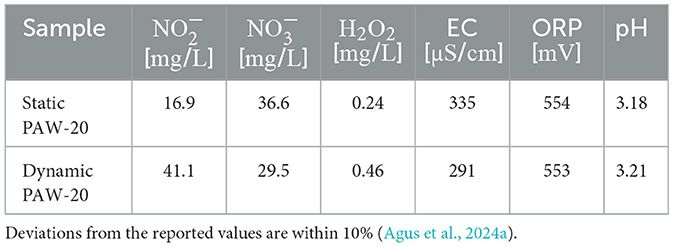
Two PAW samples were used for this investigation: static and dynamic PAW-20. These samples were chemically characterized in previous works (Agus et al., 2024a,b). The chemical properties of the PAW samples applied in this work are summarized in Table 1. The RONS concentrations reported have been measured by vis-spectrophotometry. The chemical analysis was conducted on PAW samples immediately following plasma exposure. The initial sample consisted of ultra-pure water, obtained from a Puranity TU 3UV/UF water dispenser, with a pH of ~ 6, an oxidation-reduction potential (ORP) of 320 mV, and an electrical conductivity (EC) of 0.055 μS cm-1 (Agus et al., 2024a). These chemical properties are representative of PAW throughout the entire bacterial treatment duration, as PAW samples were used for bacteria treatment immediately after plasma exposure and RONS variations during the first 10 min of storage remain within 10% deviations (Agus et al., 2024b). The setup exhibited remarkable bacteria inactivation capabilities, making it a promising candidate for real-world applications such as surface and instrument decontamination.
2.2 Microbiological protocol
To verify the accuracy of the live/dead BacLight staining under the applied protocol, positive and negative controls have been performed (Invitrogen, 2004). Not treated cells represent the negative control since they should exhibit minimal or no effect on cell viability and strong SYTO9 fluorescence, establishing a baseline for comparison. Positive controls are instead used to validate an expected response, such as a strong signal from the dead-staining component of the kit (PI). Cells treated for 1 hour with isopropanol have been used as positive controls. Studies have reported that isopropanol kills bacteria by enhancing the permeability of bacterial cell membranes and disrupting protein function by denaturing them (Stiefel et al., 2015). After isopropanol treatment, cells, although dead and with a deteriorated membrane, retain their structural integrity (Roth et al., 1997; Stiefel et al., 2015). Isopropanol-treated cells should predominantly exhibit PI fluorescence, indicative of cell death. The experiments were performed with exponentially growing E. coli cells with an optical density between 0.2 and 0.5; the cell population was adjusted to 1 × 106 CFU mL−1 as recommended by the live/dead BacLight staining protocol. To perform the PAW and isopropanol treatments, aliquots of 1 mL of E. coli cells were centrifuged at 4,000 rpm for 3 min. For PAW treatment, 960 μL of supernatant was removed, and the pellet was re-suspended in 960 μL of PAW immediately after plasma exposure. The negative control tubes underwent the same centrifugation cycles and were resuspended in fresh LB to assess any potential effect of the protocol. Tubes were incubated at 37 °C and 180 rpm during 10 min of PAW treatment. For the 1-hour-lasting isopropanol treatment, the cell pellet was re-suspended in 300 μL of pH 7 phosphate-buffered saline (PBS), and 100 μL of 70% of isopropanol were added (Invitrogen, 2004). After the treatment time, the tubes were centrifuged, and the PAW and isopropanol were removed. The pellet was then re-suspended in 1 mL of PBS (pH≈7.4) and the cell suspension was immediately stained. To perform the CFU counting, the cell culture was serially diluted in 10-fold and 100 μL were inoculated in Luria-Bertani agar and incubated at 37 °C overnight. The tubes used for bacterial centrifuging and PAW exposure were 1.5 mL microtubes (Thermo Fisher Scientific Inc.; cat. no 3641NK).
The staining mix (SYTO9 + PI) was prepared in a 1:1 ratio (Boulos et al., 1999; Invitrogen, 2004). The cell suspension was filtered into flow cytometry tubes, and 3 μL of the staining mix was added to each tube designated for staining. After mixing, the tubes were incubated for 15 min at room temperature, protected from light. Before flow cytometry measurements, 100 μL of each suspension was diluted in 700 μL of PBS.
All experiments have been performed in three biological replicates.
2.3 Flow cytometry settings
Flow cytometry measurements were performed on a LSR Fortessa, 5-laser and 18-detector analyzer flow cytometer, with 488 nm excitation from a blue solid-state laser operating at 100 mW. Red fluorescence (PI) was measured above 610 ± 10 nm, and green fluorescence (SYTO9) was measured at 530 ± 15 nm. For each measurement, at least 20,000 events were analyzed by flow cytometry, with a flow rate set at ~12 μL/min. The bacterial events were discriminated from debris using FSC-area and side scatter SSC-area. Single cells have been selected for analysis by FSC-height and FSC-area. The gates, applied for population discrimination, were set manually based on the negative and positive controls (Manoil and Bouillaguet, 2018). The percentages of PI and SYTO9-positive cells are then defined over the total stained population, calculated as the sum of cells positive to PI and SYTO9. Data were exported and analyzed with FlowJo software (FlowJo for Windows, Tree Star Inc., Ashland, Oregon, U.S.A.).
3 Results and discussion
Figure 2 presents four representative flow cytometry graphs displaying PI and SYTO9 fluorescence signal intensities for the negative control (a), positive control (b), and for cells treated with static (c) and dynamic (d) PAW-20. Rectangular gating was applied to distinguish PI-positive (non-viable) cells from SYTO9-positive (viable) cells, with numerical values indicating the percentage of single cells in each category.
In the negative control, the majority of cells were viable and therefore located at the bottom of the graph, exhibiting SYTO9 fluorescence. Conversely, in the positive control (Figure 2b), the cells treated with isopropanol were PI-positive, confirming their loss of viability and clustering on the top part of the graph. The flow cytometry profiles for static and dynamic PAW-20 (Figures 2c, d) revealed a stronger bactericidal effect for dynamic PAW-20, in agreement with previous findings (Agus et al., 2024a). After static PAW-20 treatment, approximately half of the population remained SYTO9-positive, while following dynamic PAW-20 treatment, the majority of cells were categorized as non-viable. Notably, in Figures 2b, c, PI-positive cells appear to segregate into two populations, both with strong PI fluorescence intensities (>103 arb. units). This suggests membrane permeability to PI, confirming non-viability, while still expressing residual SYTO9 fluorescence, indicating that SYTO9 has not been completely quenched by PI. As reported also by Berney et al. (2007), this configuration could be associated with the presence of cells in an unfinished state of division, containing a higher amount of nucleic acid, where part of the SYTO9 fluorescence could still be expressed. Another factor to consider is bacterial heterogeneity since bacteria can exhibit natural variations in physiological characteristics, including size, shape, and DNA content (Berney et al., 2007; Zacharias et al., 2015). Similarly, in Figure 2c, a portion of cells appears to be progressively transitioning from the SYTO9 to the PI gating. The majority of these cells already exhibit relatively high PI fluorescence intensities (of the order of 103 arb. units), indicative of compromised membranes and dying bacteria.
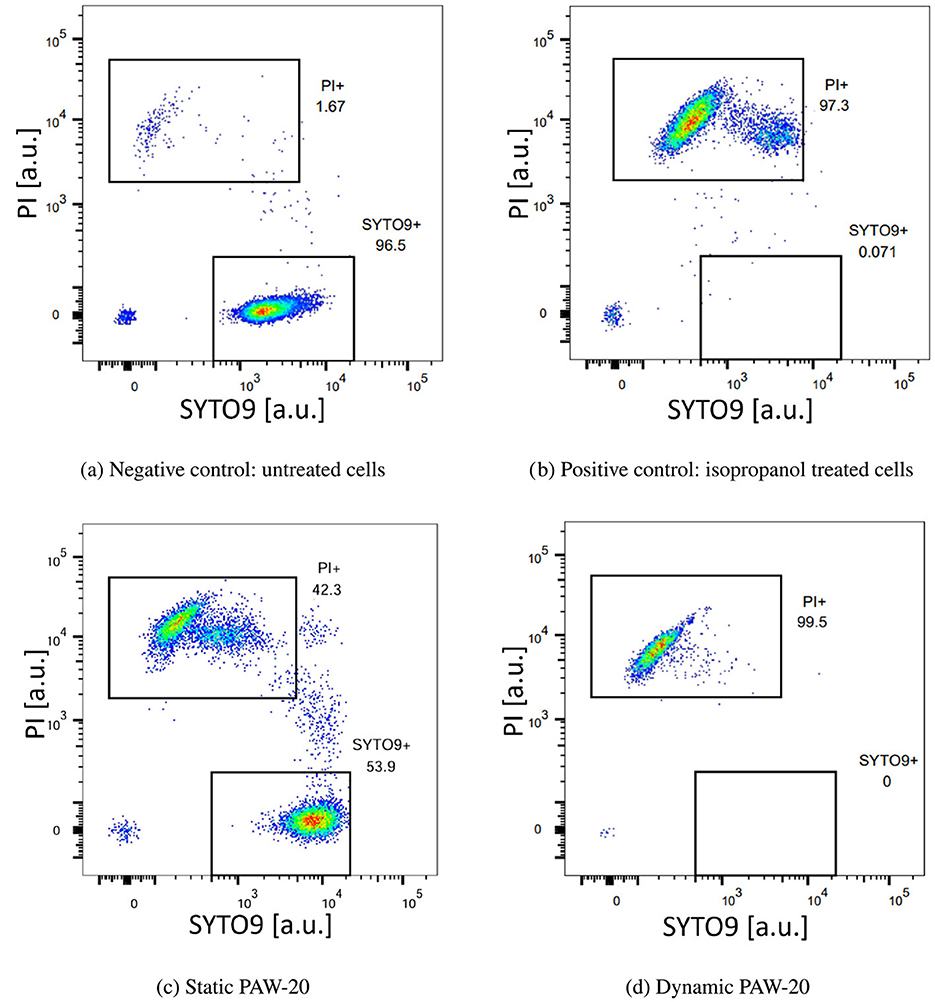
Figure 2. Representative density plots acquired by flow cytometry of E. coli negative control (a), positive control treated with isopropanol (b), static PAW-20 (c), and dynamic PAW-20 (d) treated E. coli stained with a mixture of PI (y-axes) and SYTO9 (x-axes). Plots represent the fluorescence intensity in arbitrary units.
The flow cytometry results, averaged over three biological replicates, are reported in Figure 3. The negative control, consisting of untreated bacteria, revealed that on average, 99.9% of single cells were stained with SYTO9. Conversely, in the positive control, where cells were treated with isopropanol, 98.9% of single cells tested positive for PI. Both positive and negative controls delivered the expected results, demonstrating a good performance of the staining. The error bars, not visible in the graph, accounted for a variability of < 1%. Static and dynamic PAW-20 treatments were performed for 10 min. Approximately half of the stained population (56.3%) tested positive for PI after static PAW-20, while dynamic PAW-20 accounted for 99.9% of single cells positive for PI. As shown in Table 1, dynamic PAW-20 exhibited higher and H2O2 concentrations, lower levels, and a similar pH compared to its static counterpart, resulting in enhanced antimicrobial properties and PI permeation.
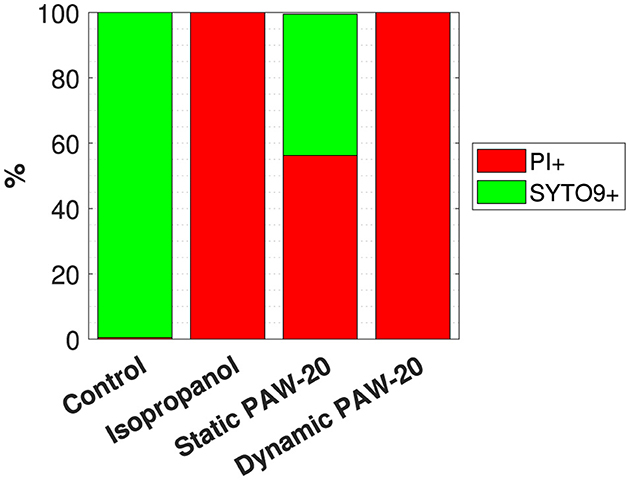
Figure 3. Flow cytometry results indicating the average % of single cells positive to PI and SYTO9 over three biological replicates of the negative control, the positive control treated with isopropanol and static and dynamic PAW-20-treated cells.
Figure 4 compares the inactivation percentage of E. coli cells treated with static (a) and dynamic (b) PAW-20, assessed by flow cytometry and CFU counting.
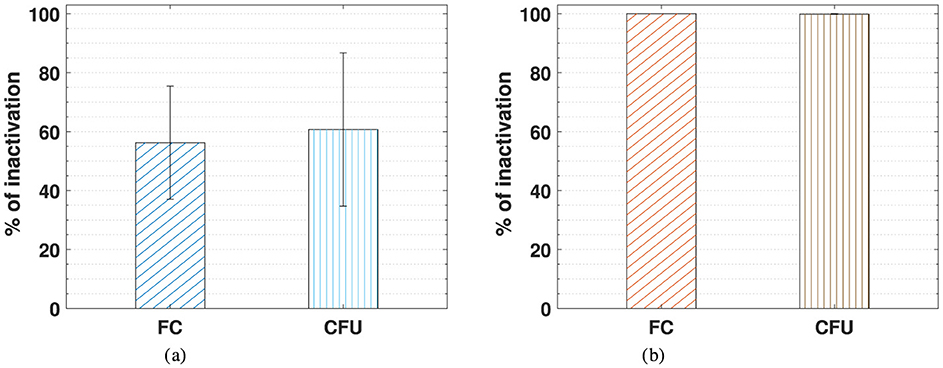
Figure 4. Comparison of the E. coli percentage of inactivation measured by flow cytometry (FC) and CFU counting following static PAW-20 (a) and dynamic PAW-20 treatment (b).
The two methods show good agreement, as indicated by the close average values and overlapping error bars. An inactivation of 56.3% was measured by flow cytometry and of 60.7% by CFU counting for static PAW-20. In the case of dynamic PAW the same percentage of inactivation of 99.9% was measured by both flow cytometry and CFU counting. Given the potential for biological variability, the technical challenges inherent in such experiments, and the possibility of instrument error, the discrepancy of about 4% between the two techniques in the case of PAW-20 static is deemed acceptable, especially if considering that the standard deviations associated with CFU counting can be an order of magnitude.
Critically, these findings suggest that bacterial inactivation is not associated with a VBNC state. For both static and dynamic PAW-20, the proportion of inactivated bacteria testing positive for PI closely matched CFU-based inactivation rates, ensuring that PAW treatment achieved reliable and safe bacterial inactivation. Additionally, these results suggest that the presence of PI-positive cells is unlikely to be solely due to temporary permeabilization of the membrane caused by PAW treatment. While such cells would typically stain positive for PI yet retain the ability to recover and proliferate on agar plates, the observed data do not support this as the dominant mechanism. The verification of bacteria inactivation by PAW through flow cytometry and the live/dead BacLight staining performed in this study is of fundamental importance for the validation of these PAW samples as reliable decontamination agents, excluding the possibility of the VBNC bacteria state. For food quality applications, for example, guaranteeing that bacteria are not viable and lack membrane integrity is an important step in safety assessment to avoid potential toxicity arising from metabolite accumulation by non-growing organisms. Metabolic activity and growth depend on the integrity of the cytoplasmic membrane, which shields the cell from its environment. Cells with intact membranes can perform metabolic functions, duplicate, and repair, unless confronted with irreparable DNA damage. Cells lacking an intact membrane are classified as non-viable since they are not capable of maintaining or establishing the negative membrane potential. Their internal structures become exposed to the environment, leading to eventual decomposition (Nebe-Von-Caron et al., 2000).
This flow cytometry study not only quantifies E. coli inactivation but also provides valuable insights into the bacterial morphological changes induced by PAW.
First, the presence of PI-positive bacteria indicates that the bacteria membrane has been compromised or damaged by the treatment (Rosenberg et al., 2019). Possibly, the extent of membrane disruption has to be sufficient to form pores large enough to allow PI molecules to penetrate (≈668.4 Da) (Johnson and Criss, 2013). Second, modifications of the cell size can be observed in the FSC and SSC plots of PAW-treated cells, as demonstrated by Figure 5.
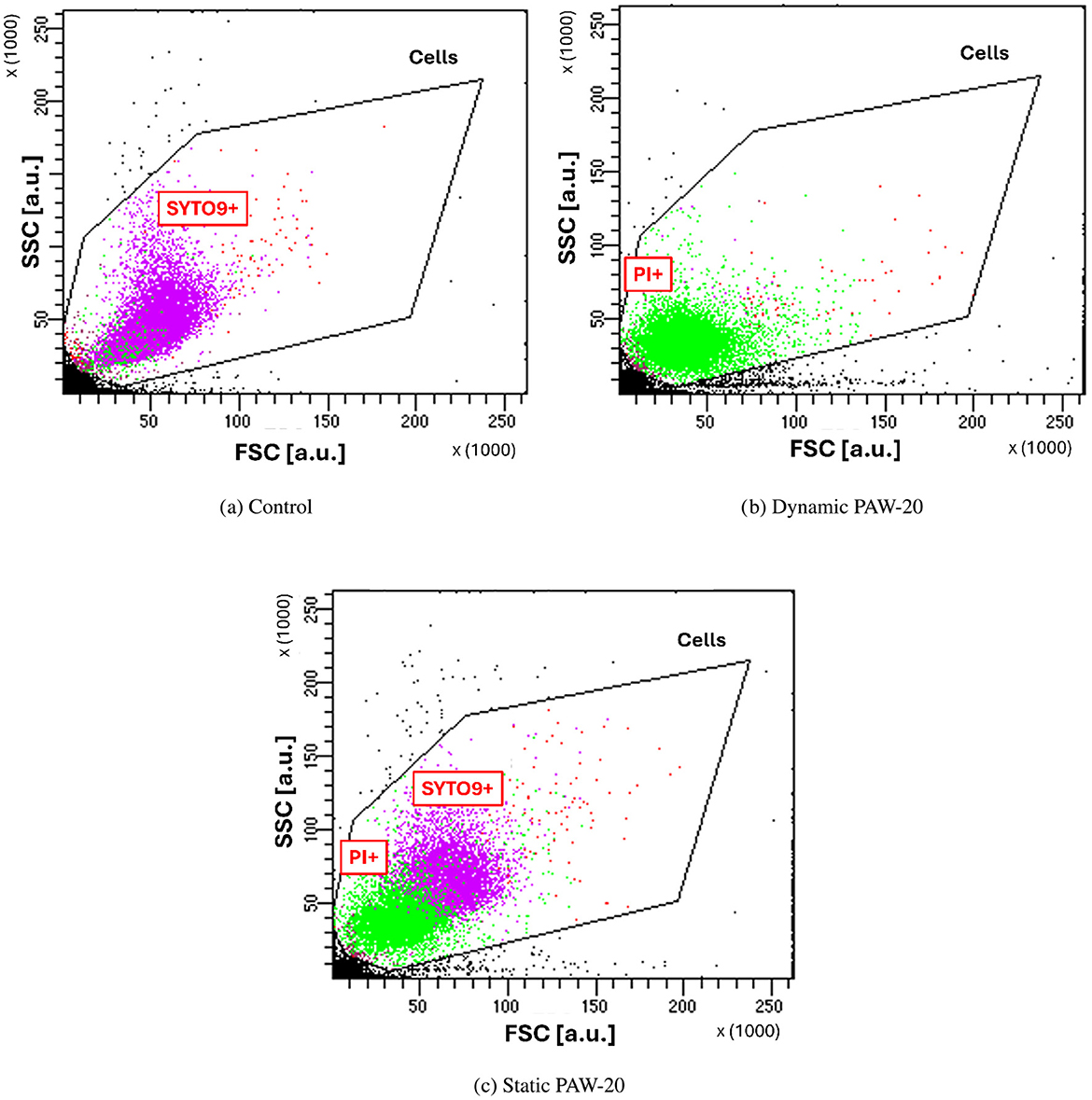
Figure 5. FSC and SSC measured for control cells (a), and dynamic (b) and static (c) PAW-20-treated E. coli. Noticeable cell shrinkage is visible for cells inactivated by the treatment and PI+.
Indeed, FSC intensity correlates with the cell diameter since it is associated with the light diffraction around the cell (FlowJo - Forward Scatter vs. Side Scatter, 2025), while SSC correlates with cell complexity (Vermes et al., 2000; Bortner and Cidlowski, 2007). Control cells, shown in Figure 5a, are almost entirely SYTO9-positive and cluster at higher SSC and FSC values compared to dynamic PAW-treated cells in Figure 5b. Following the sublethal static PAW treatment, a portion of cells remain alive and as shown in Figure 5c, PI-positive and SYTO9-positive populations form two distinct clusters, reflecting differences in cell size. Specifically, SYTO9-positive cells appear larger than PI-positive cells, suggesting that PAW inactivation induced cell shrinkage. The observed cell shrinkage is in agreement with previous findings, where cell shrinkage has been measured by TEM (Gan et al., 2022) and SEM (Zhao et al., 2021). This phenomenon is typically observed in bacteria in association with programmed cell death and membrane blebbing (Bayles, 2014), whereas the opposite, bacterial swelling, is more commonly induced by cell wall-targeting antibiotics, which inhibit cross-linking enzymes and lead to cell lysis (Wong and Amir, 2019).
As previously mentioned, the PAW samples analyzed in this study contain high concentrations of RNS, including nitrite () and nitrate (), whereas the hydrogen peroxide (H2O2) concentrations are two orders of magnitude lower than those of RNS. This suggests that the observed cell shrinkage and membrane damage, detected by flow cytometry, are most likely due to nitrosative stress (Agus, 2025). RNS are well-documented disruptors of essential cellular processes, including protein synthesis and DNA replication, while also compromising membrane integrity and function (Vine, 2011; Chautrand et al., 2022). Peroxynitrous acid is known to be capable of causing membrane poration (Balazinski et al., 2021); however, this hypothesis is excluded here, as both H2O2 and are required for its formation (Machala et al., 2018). Since the H2O2 concentrations are below 0.5 mg L-1 in each tested PAW sample and two orders of magnitude lower than the concentration, this reactive species is considered negligible (Agus et al., 2024b). Given that the average pH of the PAW samples is below 3.2, the majority of is expected to be in its protonated form as nitrous acid (HNO2), which has a pKa of 3.3 (Cai et al., 2001). Nitrous acid is known to undergo disproportionation, generating nitrogen oxide (NO), nitrogen dioxide (NO2), and nitric acid (HNO3) (Rayson et al., 2012; Agus et al., 2024b). In contrast, nearly all of the detected would remain in its ionic form due to the low acid constant (pKa = −1.37) (Lukes et al., 2012; Bradu et al., 2020). Notably, NO generated via nitrous acid disproportionation plays a crucial role in post-translational modifications of bacterial membrane proteins, particularly through S-nitrosylation of cysteine residues (Fang and Vázquez-Torres, 2019; Nguyen et al., 2023). At the same time, NO2 has been implicated in lipid peroxidation, leading to membrane damage, and it is also capable of oxidizing proteins at multiple sites in a non-specific manner (Halliwell, 1992; Ischiropoulos and Al-Mehdi, 1995; Shuhong, 2016).
4 Conclusions
Using florescence flow cytometry, this study demonstrates and quantifies E. coli inactivation by PAW generated through the non-contact exposure of ultra-pure deionized water to an SDBD plasma. Flow cytometry analysis confirmed that PAW treatment compromised bacterial membrane integrity, as evidenced by increased propidium iodide uptake in PAW-treated cells.
Comparing the flow cytometry results with CFU counting revealed consistent inactivation rates between the two methods. The agreement between these techniques suggests that PAW effectively inactivates bacteria rather than inducing a viable but non-culturable state, underscoring the reliability of this RNS-rich PAW as a decontamination agent.
Additionally, cell shrinkage was observed, highlighting significant physiological changes induced in E. coli by PAW exposure. The predominance of reactive nitrogen species in the PAW used here suggests that nitrosative stress plays a key role in bacterial inactivation, contributing to both membrane damage and cell shrinkage (Agus, 2025). Nitrosative stress is known to disrupt essential cellular processes, including protein synthesis and DNA replication (Vine, 2011; Chautrand et al., 2022).
Overall, these findings, excluding the presence of VBNC cell, reinforce the potential of PAW as an effective and robust bacterial decontamination strategy with promising applications in food safety, healthcare, and industrial sterilization. Other diagnostics, such as energy-dispersive X-ray spectroscopy, v-PCR, and EMA-PCR, could be performed to evaluate bacterial viability and support these findings (Truchado et al., 2020; Haddad et al., 2022; Chen et al., 2022). Additionally, scanning electron microscopy and atomic force microscopy could provide valuable insights into the outer membrane modifications induced by PAW (Eaton et al., 2008).
This study represents an initial step in elucidating the mechanisms underlying E. coli inactivation by RNS-rich PAW, suggesting cell shinkage and membrane damage. Future research should focus on molecular-level investigations such as proteomics or RNA sequencing to precisely characterize the pathways involved in PAW-induced bacterial inactivation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. FA: Writing – review & editing. AL: Writing – review & editing. BM: Writing – review & editing. IF: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Open access funding by Swiss Federal Institute of Technology in Lausanne (EPFL).
Acknowledgments
Flow cytometry analysis was performed at the EPFL Flow Cytometry Core Facility.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agus, R. (2025). Fundamentals in the mechanisms involved in plasma-activated water decontamination. [dissertation], EPFL École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland. [Thesis number 10885].
Agus, R., Avino, F., Ibba, L., Myers, B., Zampieri, L., Martines, E., et al. (2024a). Implementing water recirculation in a novel portable plasma-activated water reactor enhances antimicrobial effect against Escherichia coli. Chem. Eng. J. 486:149915. doi: 10.1016/j.cej.2024.149915
Agus, R., Pipoz, L., Avino, F., Lavrikova, A., Myers, B., and Furno, I. (2024b). Plasma-activated water retains antimicrobial properties against escherichia coli after 72 hours of storage. Plasma Phys. Control. Fusion. 67. doi: 10.1088/1361-6587/ad9950
Ambriz-Avi na, V., Contreras-Gardu no, J. A., and Pedraza-Reyes, M. (2014). Applications of flow cytometry to characterize bacterial physiological responses. BioMed Res. Int. 2014:461941. doi: 10.1155/2014/461941
Auty, M. A., Gardiner, G. E., McBrearty, S. J., O'Sullivan, E. O., Mulvihill, D. M., Collins, J. K., et al. (2001). Direct in situ viability assessment of bacteria in probiotic dairy products using viability staining in conjunction with confocal scanning laser microscopy. Appl. Environ. Microbiol. 67, 420–425. doi: 10.1128/AEM.67.1.420-425.2001
Balazinski, M., Schmidt-Bleker, A., Winter, J., and von Woedtke, T. (2021). Peroxynitrous acid generated in situ from acidified h2o2 and nano2. A suitable novel antimicrobial agent. Antibiotics. 10:1003. doi: 10.3390/antibiotics10081003
Bayles, K. W. (2014). Bacterial programmed cell death: making sense of a paradox. Nat. Rev. Microbiol. 12, 63–69. doi: 10.1038/nrmicro3136
Berney, M., Hammes, F., Bosshard, F., Weilenmann, H.-U., and Egli, T. (2007). Assessment and interpretation of bacterial viability by using the LIVE/DEAD baclight kit in combination with flow cytometry. Appl. Environ. Microbiol. 73, 3283–3290. doi: 10.1128/AEM.02750-06
Bortner, C. D., and Cidlowski, J. A. (2007). Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch. Biochem. Biophys. 462, 176–188. doi: 10.1016/j.abb.2007.01.020
Boulos, L., Prévost, M., Barbeau, B., Coallier, J., and Desjardins, R. (1999). LIVE/DEAD(®) baclight(TM): application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 37, 77–86. doi: 10.1016/S0167-7012(99)00048-2
Bradu, C., Kutasi, K., Magureanu, M., Puac, N., and Živkovic, S. (2020). Reactive nitrogen species in plasma-activated water: generation. J. Phys. D: Appl. Phys. 53:223001. doi: 10.1088/1361-6463/ab795a
Cai, Q., Zhang, W., and Yang, Z. (2001). Stability of nitrite in wastewater and its determination by ion chromatography. Anal. Sci. 17:917. doi: 10.14891/analscisp.17icas.0.i917.0
Chautrand, T., Souak, D., Chevalier, S., and Duclairoir-Poc, C. (2022). Gram-negative bacterial envelope homeostasis under oxidative and nitrosative stress. Microorganisms 10:924. doi: 10.3390/microorganisms10050924
Chen, M., Lan, X., Zhu, L., Ru, P., Xu, W., and Liu, H. (2022). PCR mediated nucleic acid molecular recognition technology for detection of viable and dead foodborne pathogens. Foods 11:2675. doi: 10.3390/foods11172675
Cooper, M., Fridman, G., Fridman, A., and Joshi, S. (2010). Biological responses of bacillus stratosphericus to floating electrode-dielectric barrier discharge plasma treatment. J. Appl. Microbiol. 109, 2039–2048. doi: 10.1111/j.1365-2672.2010.04834.x
Dolezalova, E., and Lukes, P. (2015). Membrane damage and active but nonculturable state in liquid cultures of escherichia coli treated with an atmospheric pressure plasma jet. Bioelectrochemistry 103:7–14. doi: 10.1016/j.bioelechem.2014.08.018
Duffy, G., and Sheridan, J. (1998). Viability staining in a direct count rapid method for the determination of total viable counts on processed meats. J. Microbiol. Methods 31, 167–174. doi: 10.1016/S0167-7012(97)00099-7
Eaton, P., Fernandes, J. C., Pereira, E., Pintado, M. E., and Malcata, F. X. (2008). Atomic force microscopy study of the antibacterial effects of chitosans on escherichia coli and staphylococcus aureus. Ultramicroscopy 108, 1128–1134. doi: 10.1016/j.ultramic.2008.04.015
Endo, H., Nakayama, J., Hayashi, T., and Watanabe, E. (1997). Application of flow cytometry for rapid determination of cell number of viable bacteria. Fish. Sci. 63, 1024–1029. doi: 10.2331/fishsci.63.1024
Fakruddin, M., Mannan, K. S. B., and Andrews, S. (2013). Viable but nonculturable bacteria: food safety and public health perspective. ISRN Microbiol. 2013, 1–6. doi: 10.1155/2013/703813
Fang, F. C., and Vázquez-Torres, A. (2019). Reactive nitrogen species in host-bacterial interactions. Curr. Opin. Immunol. 60, 96–102. doi: 10.1016/j.coi.2019.05.008
Fleischmann, S., Robben, C., Alter, T., Rossmanith, P., and Mester, P. (2021). How to evaluate non-growing cells–current strategies for determining antimicrobial resistance of vbnc bacteria. Antibiotics 10:115. doi: 10.3390/antibiotics10020115
FlowJo – Forward Scatter vs. Side Scatter (2025). Available online at: https://www.flowjo.com/learn/flowjo-university/flowjo/before-flowjo/58 (accessed Feburary 18, 2025).
Gan, Z., Zhang, Y., Gao, W., Wang, S., Liu, Y., Xiao, Y., et al. (2022). Effects of nonthermal plasma-activated water on the microbial sterilization and storage quality of blueberry. Food Biosci. 49:101857. doi: 10.1016/j.fbio.2022.101857
Haddad, G., Takakura, T., Bellali, S., Fontanini, A., Ominami, Y., Khalil, J. B., et al. (2022). A preliminary investigation into bacterial viability using scanning electron microscopy-energy-dispersive x-ray analysis: the case of antibiotics. Front. Microbiol. 13:967904. doi: 10.3389/fmicb.2022.967904
Hadinoto, K., Astorga, J. B., Masood, H., Zhou, R., Alam, D., Cullen, P. J., et al. (2021). Efficacy optimization of plasma-activated water for food sanitization through two reactor design configurations. Innov. Food Sci. Emerg. Technol. 74:102867. doi: 10.1016/j.ifset.2021.102867
Halliwell, B. (1992). Reactive oxygen species and the central nervous system. J. Neurochem. 59, 1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x
Handorf, O., Pauker, V. I., Weihe, T., Schäfer, J., Freund, E., Schnabel, U., et al. (2021). Plasma-treated water affects listeria monocytogenes vitality and biofilm structure. Front. Microbiol. 12, 1–16. doi: 10.3389/fmicb.2021.652481
Invitrogen (2004). LIVE/DEAD Baclight Kit. Available online at: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp07007.pdf (accessed April 5, 2024).
Ischiropoulos, H., and Al-Mehdi, A. B. (1995). Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 364, 279–282. doi: 10.1016/0014-5793(95)00307-U
Johnson, M. B., and Criss, A. K. (2013). Fluorescence microscopy methods for determining the viability of bacteria in association with mammalian cells. J. Vis. Exp. 79:50729. doi: 10.3791/50729
Koch, A. (1994). “Growth measurement,” in Methods for General and Molecular Bacteriology, eds. P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (Washington, DC: ASM Press), 248–277.
Kvam, E., Davis, B., Mondello, F., and Garner, A. L. (2012). Nonthermal atmospheric plasma rapidly disinfects multidrug-resistant microbes by inducing cell surface damage. Antimicrob. Agents Chemother. 56, 2028–2036. doi: 10.1128/AAC.05642-11
Langsrud, S., and Sundheim, G. (1996). Flow cytometry for rapid assessment of viability after exposure to a quaternary ammonium compound. J. Appl. Bacteriol. 81, 411–418. doi: 10.1111/j.1365-2672.1996.tb03527.x
Leuko, S., Legat, A., Fendrihan, S., and Stan-Lotter, H. (2004). Evaluation of the LIVE/DEAD BacLight kit for detection of extremophilic archaea and visualization of microorganisms in environmental hypersaline samples. Appl. Environ. Microbiol. 70, 6884–6886. doi: 10.1128/AEM.70.11.6884-6886.2004
Lisle, J. T., Broadaway, S. C., Prescott, A. M., Pyle, B. H., Fricker, C., and Mcfeters, G. A. (1998). Effects of starvation on physiological activity and chlorine disinfection resistance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 64, 4658–4662. doi: 10.1128/AEM.64.12.4658-4662.1998
Liu, J., Yang, L., Kjellerup, B. V., and Xu, Z. (2023). Viable but nonculturable (vbnc) state, an underestimated and controversial microbial survival strategy. Trends Microbiol. 31, 1013–1023. doi: 10.1016/j.tim.2023.04.009
Lukes, P., Locke, B. R., and Brisset, J.-L. (2012). Aqueous-Phase Chemistry of Electrical Discharge Plasma in Water and in Gas—Liquid Environments (John Wiley and Sons, Ltd) chapter 7, 243–308. doi: 10.1002/9783527649525.ch7
Ma, R., Wang, G., Tian, Y., Wang, K., Zhang, J., and Fang, J. (2015). Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 300, 643–651. doi: 10.1016/j.jhazmat.2015.07.061
Machala, Z., Tarabová, B., Sersenová, D., Janda, M., and Hensel, K. (2018). Chemical and antibacterial effects of plasma activated water: correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D: Appl. Phys. 52:34002. doi: 10.1088/1361-6463/aae807
Manoil, D., and Bouillaguet, S. (2018). Oxidative stress in bacteria measured by flow cytometry. Adv. Biotechnol. Microbiol. 8, 6–11. doi: 10.19080/AIBM.2018.08.555726
Nebe-Von-Caron, G., Stephens, P. J., Hewitt, C. J., Powell, J. R., and Badley, R. A. (2000). Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting. J. Microbiol. Methods 42, 97–114. doi: 10.1016/S0167-7012(00)00181-0
Nguyen, H. K., Picciotti, S. L., Duke, M. M., Broberg, C. A., and Schoenfisch, M. H. (2023). Nitric oxide-induced morphological changes to bacteria. ACS Infect. Dis. 9, 2316–2324. doi: 10.1021/acsinfecdis.3c00415
Peeters, F., and Butterworth, T. (2018). Electrical Diagnostics of Dielectric Barrier Discharges. IntechOpen: Rijeka. doi: 10.5772/intechopen.80433
Rayson, M. S., Mackie, J. C., Kennedy, E. M., and Dlugogorski, B. Z. (2012). Accurate rate constants for decomposition of aqueous nitrous acid. Inorg. Chem. 51, 2178–2185. doi: 10.1021/ic202081z
Rosenberg, M., Azevedo, N. F., and Ivask, A. (2019). Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 9, 1–12. doi: 10.1038/s41598-019-42906-3
Roth, B. L., Poot, M., Yue, S. T., and Millard, P. J. (1997). Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 63, 2421–2431. doi: 10.1128/aem.63.6.2421-2431.1997
Shapiro, H. M. (2000). Microbial analysis at the single-cell level: tasks and techniques. J. Microbiol. Methods 42, 3–16. doi: 10.1016/S0167-7012(00)00167-6
Shuhong, G. (2016). Bacteriostatic and Bactericidal Effects of Free Nitrous Acid on Model Microbes in Wastewater Treatment. [dissertation], The University of Queensland, [Australia].
Stiefel, P., Schmidt-Emrich, S., Maniura-Weber, K., and Ren, Q. (2015). Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 15, 1–9. doi: 10.1186/s12866-015-0376-x
Stocks, S. M. (2004). Mechanism and use of the commercially available viability stain, baclight. Cytometry A 61, 189–195. doi: 10.1002/cyto.a.20069
Taimur Khan, M. M., Pyle, B. H., and Camper, A. K. (2010). Specific and rapid enumeration of viable but nonculturable and viable-culturable gram-negative bacteria by using flow cytometry. Appl. Environ. Microbiol. 76, 5088–5096. doi: 10.1128/AEM.02932-09
Tian, Y., Ma, R., Zhang, Q., Feng, H., Liang, Y., Zhang, J., et al. (2015). Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process. Polym. 12, 439–449. doi: 10.1002/ppap.201400082
Trevors, J. (2011). Viable but non-culturable (vbnc) bacteria: gene expression in planktonic and biofilm cells. J. Microbiol. Methods 86, 266–273. doi: 10.1016/j.mimet.2011.04.018
Truchado, P., Gil, M. I., Larrosa, M., and Allende, A. (2020). Detection and quantification methods for viable but non-culturable (vbnc) cells in process wash water of fresh-cut produce: Industrial validation. Front. Microbiol. 11:673. doi: 10.3389/fmicb.2020.00673
Veal, D. A., Deere, D., Ferrari, B., Piper, J., and Attfield, P. V. (2000). Fluorescence staining and flow cytometry for monitoring microbial cells. J. Immunol. Methods 243, 191–210. doi: 10.1016/S0022-1759(00)00234-9
Vermes, I., Haanen, C., and Reutelingsperger, C. (2000). Flow cytometry of apoptotic cell death. J. Immunol. Methods. 243, 167–190. doi: 10.1016/S0022-1759(00)00233-7
Vine, C. E. (2011). Escherichia coli Response to Nitrosative Stress. [dissertation], University of Birmingham, [England].
Wang, Q., and Salvi, D. (2021). Recent progress in the application of plasma-activated water (PAW) for food decontamination. Curr. Opin. Food Sci. 42, 51–60. doi: 10.1016/j.cofs.2021.04.012
Wong, F., and Amir, A. (2019). Mechanics and dynamics of bacterial cell lysis. Biophys. J. 116, 2378–2389. doi: 10.1016/j.bpj.2019.04.040
Xia, B., Vyas, H. K. N., Zhou, R., Zhang, T., Hong, J., Rothwell, J. G., et al. (2023). The importance of superoxide anion for Escherichia coli biofilm removal using plasma-activated water. J. Environ. Chem. Eng. 11:109977. doi: 10.1016/j.jece.2023.109977
Xu, Z., Cheng, C., Shen, J., Lan, Y., Hu, S., Han, W., et al. (2018). In vitro antimicrobial effects and mechanisms of direct current air-liquid discharge plasma on planktonic staphylococcus aureus and escherichia coli in liquids. Bioelectrochemistry 121, 125–134. doi: 10.1016/j.bioelechem.2018.01.012
Zacharias, N., Kistemann, T., and Schreiber, C. (2015). Application of flow cytometry and PMA-qPCR to distinguish between membrane intact and membrane compromised bacteria cells in an aquatic milieu. Int. J. Hyg. Environ. Health. 218, 714–722. doi: 10.1016/j.ijheh.2015.04.001
Zhao, Y.-M., Ojha, S., Burgess, C. M., Sun, D.-W., and Tiwari, B. K. (2021). Inactivation efficacy of plasma-activated water: influence of plasma treatment time, exposure time and bacterial species. Int. J. Food Sci. Technol. 56, 721–732. doi: 10.1111/ijfs.14708
Zhao, Y.-M., Patange, A., Sun, D.-W., and Tiwari, B. (2020). Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Compreh. Rev. Food Sci. Food Saf. 19, 3951–3979. doi: 10.1111/1541-4337.12644
Zhou, R., Zhou, R., Prasad, K., Fang, Z., Speight, R., Bazaka, K., et al. (2018). Cold atmospheric plasma activated water as a prospective disinfectant: the crucial role of peroxynitrite. Green Chem. 20, 5276–5284. doi: 10.1039/C8GC02800A
Keywords: Plasma-activated water (PAW), low-temperature plasmas (LTPs), flow cytometry, live/dead BacLight PI/SYTO9 staining, viable but non-culturable bacteria
Citation: Agus R, Avino F, Lavrikova A, Myers B and Furno I (2025) Flow cytometry study of Escherichia coli treated with plasma-activated water: confirming the absence of the viable but non-culturable state in bacteria. Front. Microbiol. 16:1592471. doi: 10.3389/fmicb.2025.1592471
Received: 13 March 2025; Accepted: 12 May 2025;
Published: 04 June 2025.
Edited by:
Janja Trcek, University of Ljubljana, SloveniaReviewed by:
Marcia Nitschke, University of São Paulo, BrazilRobin Menthéour, UMR9018 Metabolic and Systemic Aspects of Oncogenesis (METSY), France
Thorsten Kuczius, University Hospital Münster, Germany
Copyright © 2025 Agus, Avino, Lavrikova, Myers and Furno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita Agus, cml0YS5hZ3VzQGVwZmwuY2g=
 Rita Agus
Rita Agus Fabio Avino
Fabio Avino Aleksandra Lavrikova
Aleksandra Lavrikova Brayden Myers
Brayden Myers Ivo Furno
Ivo Furno