- 1School of Pharmacy, Xianning Medical College, Hubei University of Science and Technology, Xianning, China
- 2Guangdong Provincial Key Laboratory for Plant Epigenetics, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen, China
- 3Department of Anesthesiology, People’s Hospital of Xinzhou, Wuhan, China
- 4Department of Anesthesiology, Yunxi People’s Hospital, Shiyan, China
- 5Department of Neurosurgery, Union Hospital, Huazhong University of Science and Technology, Wuhan, China
Immunotoxins (ITs), as targeted cancer therapies, confront limitations including off-target effects, immunogenicity, and inadequate tumor penetration, hindering clinical translation. Advances in tumor microenvironment (TME) understanding and genetic engineering have enabled engineered microorganisms such as attenuated Salmonella, E. coli Nissle 1917, and modified eukaryotic platforms (e.g., yeast, microalgae) to colonize tumors and act as efficient hosts for IT production. By integrating ITs into these microbes and employing precise circuits (e.g., phage lysis systems, signal peptide fusions), controlled secretion of recombinant immunotoxins (RITs) can be achieved. Balanced-lethal systems further enhance plasmid stability for sustained therapeutic delivery. This review highlights strategies leveraging engineered microbes to amplify IT efficacy, exemplified by preclinical successes like Salmonella-delivered TGFα-PE38 and E. coli-expressed anti-PD-L1-PE38. However, challenges persist, including dynamic TME interactions, systemic infection risks, manufacturing complexities and regulatory uncertainties demand resolution. By synergizing microbial targeting with RIT, this approach offers transformative potential for cancer therapy, yet requires multidisciplinary innovation to address technical, safety, and regulatory barriers for clinical adoption.
1 Introduction
Cancer remains a global threat, with nearly 20 million new cases and about 10 mil-lion deaths yearly (Bray et al., 2024). Despite various anticancer methods and drugs, minimizing damage to normal cells while maximizing cancer cell killing remains a constant pursuit (Anand et al., 2023; Feo et al., 2022; Katz et al., 2022; Labanieh and Mackall, 2023). Targeted therapy, which precisely identifies and targets cancer cell features while sparing normal tissues, has garnered significant attention (Lee et al., 2018). ITs and antibody-drug conjugates (ADCs) are both effective targeted therapy agents with similar structures. They share almost the same targeting components but differ in their cytotoxic payloads and conjugation methods. Targeting components, responsible for locating cancer-specific antigens, are usually composed of monoclonal antibodies or antibody fragments. ITs also used ligands binding to specific receptors, such as cytokines, chemokine receptor ligands and growth factors as targeting units (Babavalian et al., 2019; Janthur et al., 2012; Kreitman, 2006; Spiess et al., 2017). The cytotoxic payloads of ITs are typically protein toxins or their modified derivatives from bacteria (e.g., Pseudomonas aeruginosa exotoxin A, diphtheria toxin, or anthrax toxin), plants (e.g., ricin, saporin, or gelonin), humans (e.g., proapoptotic proteins and RNA enzymes), or other sources (e.g., chelona toxin) (Bachran and Leppla, 2016; Gill et al., 2024; Knödler and Buyel, 2021; Lu et al., 2021; Shafiee et al., 2019). In contrast, ADCs have a broader range of cytotoxic payloads, including microtubule inhibitors, DNA-damaging agents, RNA inhibitors, immunomodulators, proteasome inhibitors, small molecules, multi-drugs, phosphate prodrugs, and proteolysis-targeting chimeras (PROTACs) (Chen et al., 2020; Phuna et al., 2024; Tsuchikama et al., 2024; Wang et al., 2024; Xi M. et al., 2024). Due to the nature of their cytotoxic payloads, ADCs usually rely on chemical conjugation. ITs, have more flexible conjugation methods, as they can be either chemically conjugated or directly expressed as fusion proteins via amino acid linkers (Bacauanu et al., 2023; Khoshbakht et al., 2024; Oghalaie et al., 2024; You et al., 2021). Despite their similarities, ITs and ADCs have had different outcomes. Over 15 ADCs have been approved for clinical use, with hundreds more in clinical trials. In contrast, only a few ITs have been approved (Colombo et al., 2024; Khirehgesh et al., 2021; Kim et al., 2020). The main reason is that some key issues in IT design and development remain unresolved. These include off-target effects, where normal cells expressing the target are attacked, leading to systemic toxicity; immunogenicity caused by heterologous toxins and antibody molecules; the inability of IT molecules to efficiently permeate solid tumors to reach effective therapeutic concentrations; and the lack of efficient cytoplasmic delivery pathways after internalization (Balkhi et al., 2025; Dhillon, 2018; Markides et al., 2025). Recent studies have shown that some microorganisms can colonize cancers and tend to proliferate in the hypoxic and immunosuppressive TME, significantly influencing tumor progression (Kwon et al., 2024; Xu et al., 2018; Yu et al., 2020). Through bioengineering, these microbes can be utilized for cancer therapy in various ways, such as specifically infecting tumor tissue, activating innate and adaptive immunity, releasing toxins to kill cancer cells, competing with cancer cells for nutrients to impede tumor growth, or carrying therapeutic agents to treat cancer (Copland et al., 2024; Moon et al., 2020; Zhang et al., 2024). This presents a great opportunity for ITs, which allows directly expressed and processed in engineered hosts through recombinant gene construction (Figure 1). Many historical limitations of IT based cancer therapy can now be overcome using these microbial hosts. Here, we comprehensively review the key considerations for using microorganisms to express and deliver ITs for tumor treatment, as well as current research progress. We look forward to strengthening our cancer-fighting arsenal and expanding IT-based therapeutic strategies.
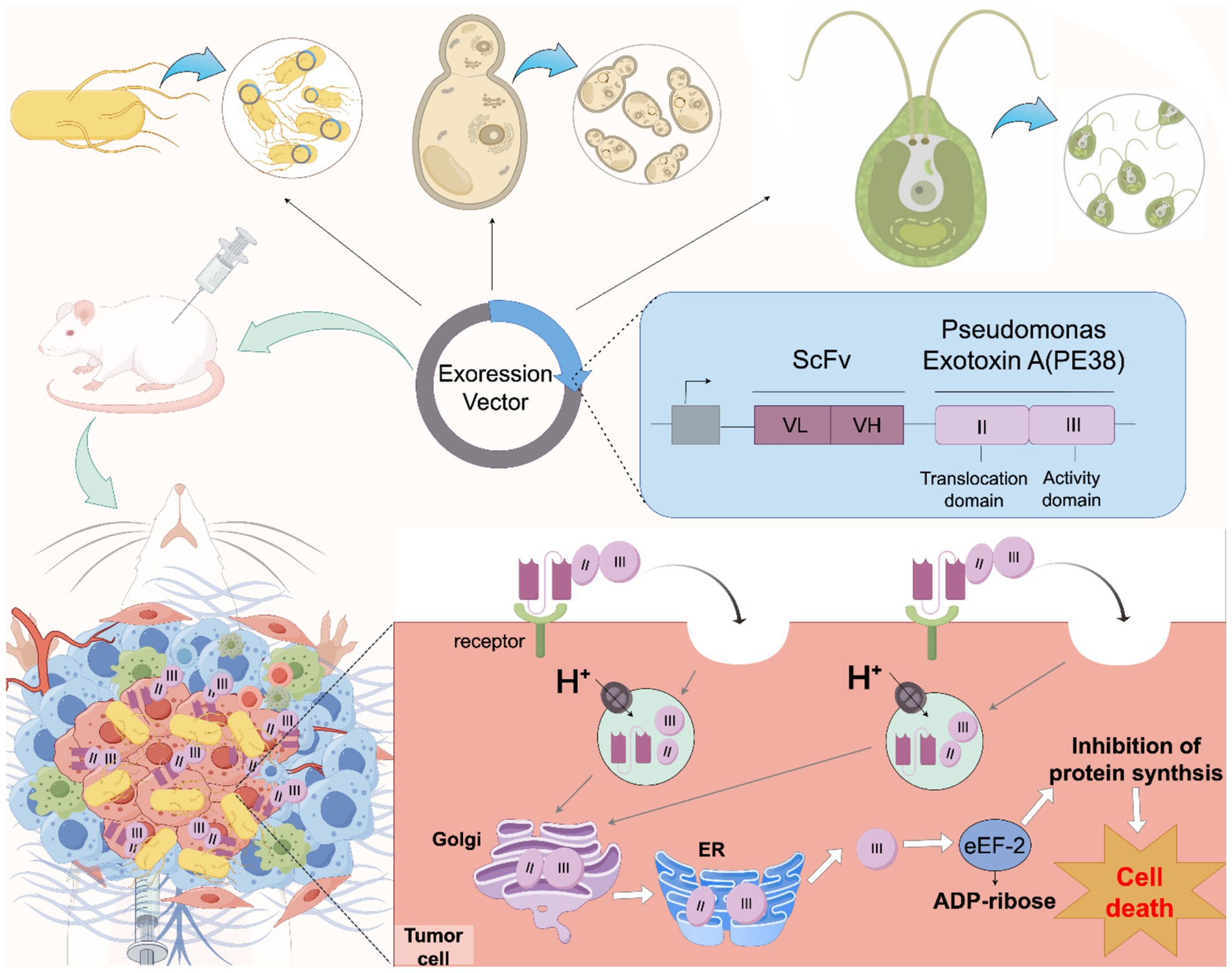
Figure 1. Schematic diagram of engineered microbes promoting IT cancer therapy. Engineered microorganisms (including bacteria and eukaryotes) with cancer colonization or targeting capabilities can serve as hosts for expressing RITs. Loading RITs into these microorganisms, combined with the design of specific release circuits, enables the microorganisms to release ITs within tumor tissues. Once released, the IT first binds to the target cell surface receptor in a targeted and cell-specific manner, then is internalized into the cell through receptor-mediated endocytosis, and directed to endosomes. Subsequently, in the endosomal lumen acidified by an ATPase proton pump, the toxic moiety is cleaved by an endosomal acidic pH-activated protease called furin. Taking Pseudomonas Exotixin A as an example, the cleaved PE38 active (catalytic) domain is transported to the trans-Golgi network and then the endoplasmic reticulum (ER) via retrograde trafficking before being released into the cytosol. Finally, the PE38’s active (catalytic) domain in the cytosol exerts its effector function by inactivating eukaryotic elongation factor 2 (eEF2) through catalyzing adenosine diphosphate (ADP) ribosylation, which inhibits protein synthesis and ultimately leads to cell death, thus achieving the killing of cancer cells.
2 Immunotoxin therapy for cancer: progress and issues
Although conjugation methods are not restricted, most immunotoxins currently depend on recombinant expression frameworks for direct production across various hosts (Zuppone et al., 2019). This approach is favored over chemical conjugation as it is more likely to yield a uniform product. Consequently, immunotoxins are now commonly designated as RITs. Since initial development by Thorpe in 1978, RITs have evolved through four iterations, resulting in four distinct generations of products (Thorpe et al., 1978). Researchers have endeavored to enhance this selective cancer-cell killing agent by modifying all of its structures, including the target units, payloads, and conjugation methods (Golichenari et al., 2025; Obozina et al., 2025; Wang et al., 2025; Wang et al., 2024). For instance, in the third and fourth-generation RITs, murine antibodies have been supplanted by humanized or fully human antibodies to reduce immunogenicity (Hauser et al., 2023; Waldmann, 2019). Furthermore, complete antibodies have been replaced with smaller fragments such as single chain antibody (scFv), and even nanobodies (VHH) to improve tumor penetration (Morgan et al., 2023; Naemi et al., 2023; Wang et al., 2024; Xi X. et al., 2024). Payload optimization has also been pursued, such as toxin structure optimization and epitope deletion (Golichenari et al., 2025; Hu et al., 2016; Mazor et al., 2015). Moreover, internalization efficiency, escape speed from vesicles to the cytoplasm, in vivo half-life, and administration routes are all optimized (Wang et al., 2025; Wei et al., 2018). However, these advancements do not always bring benefits and are sometimes accompanied by challenges. Using human toxin payloads can lower immunogenicity, but in vivo activity is often hindered by endogenous inhibitors. For example, Granzyme B (GrB) is inhibited by serine protease inhibitor B9, which greatly weakens its killing effectiveness (Hlongwane et al., 2018). Additionally, compared to plant and bacterial toxins, human toxins frequently lack a translocation domain, making them more susceptible to lysosomal degradation rather than migrating to the cytoplasm for therapeutic effect after cell internalization (Mungra et al., 2019). In summary, despite various improvement efforts, the clinical application prospects of RITs are still worrying. Some optimization measures beyond RITs themselves may solve this situation, such as the engineered microorganisms with unique abilities that we are currently focusing on.
3 Engineered microbes: potential vehicles for enhancing RIT cancer therapy
Research on the tumor microbiome has unveiled a complex ecosystem comprising tumor cells and intracellular microbes. This complexity is reflected in both the diversity of indigenous microbes, which includes various bacteria (e.g., Bacteroides, Enterococcus, Faecalibacterium, Ruminococcus, Clostridium, Lactobacillus, and Actinomyces), fungi (e.g., Yeast, Candida, Blastomyces, and Malassezia), and multiple viruses, and the intricate interrelationships within the system (Dohlman et al., 2022; Luca et al., 2021; Nejman et al., 2020; Sepich-Poore et al., 2021). On one hand, these microbes shield tumors by influencing their occurrence, development, metastasis, heterogeneity, and immune evasion. On the other hand, they compete with cancer cells for nutrients, activate innate and adaptive immunity to kill cancer cells, produce toxins to damage cancer cells, and modulate the tumor microenvironment to enhance treatment efficacy (Galeano Niño et al., 2022). These “double-edged” microbes, when genetically engineered, could maximize benefits and minimize drawbacks, holding great potential for future cancer treatment. This is why microbe-based cancer therapy (MCT) is gaining increasing attention recently (Zheng and Chen, 2024).
MCTs are actually not novel. Over 100 years ago, therapies using inactivated Streptococcus and Serratia marcescens (Coley’s toxins) injected into malignant tissues were employed and resulted in sarcoma regression (Coley, 1991). Another example is Bacillus Calmette-Guérin (BCG), a live attenuated strain of bovis Mycobacterium tuberculosis variant initially developed as a tuberculosis vaccine. It has been approved by the FDA for the treatment of bladder cancer (Boorjian et al., 2021). Unlike past methods using natural microbes or their toxins, employing microbes to create antitumor vaccines or deliver therapeutic agents shows much greater promise (Shende and Basarkar, 2019). Many recent studies substantiate this viewpoint. For example, using attenuated Salmonella typhimurium (SAM-FC) to deliver ClyA and FlaB significantly suppresses metastases and primary tumors, and VNP20009 to deliver Sgc8c (nucleic acid aptamer) targeting PTK7 in pancreatic cancer shows good effect, engineering Listeria monocytogenes secretes phospholipase (plcA, plcB) and hemolysin LLO to deliver tumor-specific antigen TAAs to alter TME and increase immune killing (Hassan et al., 2019; Nguyen et al., 2024; Xiao et al., 2024). Many cancer therapies and drugs, such as immune checkpoint blockades, antibodies, ADCs, and chemotherapy drugs, have an upper limit on their therapeutic effects and scope of application (Schuster et al., 2021). For example, the clinical benefit rate of immune checkpoint blockades is typically below 30% (Kalbasi and Ribas, 2020). However, combining these agents with cancer-colonizing microbes and genetic engineering techniques shows a high probability of breaking through such limitations. This also applies to RITs. In fact, attempts to use engineered microbes to express and deliver RITs have already begun.
A few pioneering research projects with ingenious design have already achieved promising initial results and are expected to yield broader applications in the near future (Table 1). For example, the engineered Salmonella typhimurium ΔppGpp strain can express and deliver the RIT composed of TGFα (transforming growth factor alpha, a ligand targeting epidermal growth factor receptor) and PE38 (Pseudomonas exotoxin A fragment), which can significantly inhibit mouse solid tumor growth (Lim et al., 2017). The RIT constructed from the non-neutralizing anti-TNF-α antibody Pigbak#2 and PE38, produced and delivered by E. coli BL21(DE3) Δlpp, have shown strong antitumor activity in mouse melanoma models (Hu et al., 2022). The construction of the anti-PD-L1 (programmed cell death ligand 1) antibodies and PE38 expression system in the Nissle 1917 strain has demonstrated superior suppression effects in mouse subcutaneous tumor models via intravenous injection (Li et al., 2025). Although “hitchhiking therapy” has shown promising results in mouse models, its potential risks, particularly in clinical settings, must be closely watched (Tang et al., 2022). Researchers have implemented many sophisticated regulatory circuit designs to avoid risks, enhance therapeutic effects, and increase controllability. These designs are worth highlighting and promoting. First, host selection is crucial. Many microbes preferentially infect tumor tissue, but only those that are naturally safe or engineered to be attenuated can be used to minimize infection and dissemination risks. For example, the Salmonella ΔppGpp strain with relA and spoT gene mutations is deficient in guanosine 5′-diphosphate-3′-diphosphate synthesis. This strain has almost lost its ability to invade mammalian cells and has good safety (Liu et al., 2022). The E. coli Nissle 1917 strain is not only sensitive to the immune system, does not produce pathogenic enterotoxins or cytotoxins, but can also antagonize pathogenic E. coli and has a good safety record for in vivo applications (Yu et al., 2020). To ensure the host can release RITs, advanced regulatory circuits have been introduced. Lim et al. successfully delivered immunotoxins using a Salmonella phage lysis system (pLYS) with three Salmonella phage genes. They also enabled efficient RIT secretion by fusing a soil cellulose-degrading bacterium’s cellulase (Psp) signal peptide to the RIT’s N terminus. Similarly, Li et al. fused yebF to the N terminus of αPD-L1-PE38 for secretion. In contrast, Hu et al. knocked out Braun’s lipoprotein-encoding gene to engineer a leaky strain that continuously releases Pigbak#2-PE38 extracellularly. Moreover, RIT recombinant genes need stable maintenance in the host without loss in the absence of antibiotic pressure. Lim et al. achieved this using a balanced-lethal host-vector system, which mutates the essential glmS gene and introduces a recombinant GlmS+ plasmid to ensure every surviving host carries the RIT and GlmS+ plasmid. In contrast, Hu et al. demonstrated that incorporating kanamycin resistance and a ColE1 origin into the recombinant plasmid ensures its stability for 8 days without antibiotic pressure. Finally, some other designs are also effective. For example, Li et al. demonstrated that extending the linker sequence between anti-PD-L1 and PE38 can reduce steric hindrance and enhance binding affinity. The use of inducible promoters can enhance controllable secretion, and adding the KEDL sequence to the RIT expression frame is believed to promote immunotoxin retention in the cytoplasm and boost toxin efficacy (Hu et al., 2022; Jeong et al., 2014; Li et al., 2025; Lim et al., 2017).
In summary, engineering microbes to express and deliver RITs for cancer therapy shows promise, especially with bacterial hosts. Engineered bacteria have successfully targeted and released RITs in solid tumors, inducing cancer cell apoptosis and showing good therapeutic effects (Shuwen et al., 2024; Tieu et al., 2024). However, RITs sometimes require post-translational modifications that bacteria cannot perform. Eukaryotic hosts can provide these modifications for fully functional RITs, yet there are no studies on using eukaryotic vehicles for RIT delivery in cancer treatment, despite their presence in tumor tissue (Zuppone et al., 2019). A recent study successfully engineered a yeast strain to express and secrete PD-1 high-affinity microantibodies. Oral administration targeted and alleviated cancer in a mouse intestinal tumor model (Rebeck et al., 2025). Additionally, a PDA-CV@PD-1 inhibitor delivery system, using microalgae coated with chemicals and loaded with immune checkpoint inhibitors, demonstrated the potential of microalgae to deliver drugs to tumors (Zeng et al., 2025). Both yeast and microalgae are promising eukaryotic platforms for expressing recombinant proteins like RITs, offering post-translational modifications for full functionality and potentially safer in vivo applications compared to bacterial hosts. Thus, engineered yeast and microalgae could become important vehicles for delivering RITs in cancer therapy.
4 Perspectives and challenges of IT-loaded microbes
In oncology, microbes have transitioned from being mere suppliers of essential anti-tumoral agents, including antibiotics such as doxorubicin and bleomycin, enzymes like L-asparaginase and arginine deaminase, and toxins such as Coley toxin and diphtheria toxin, to being recognized as live therapeutic entities. Their innate ability to target and proliferate within tumors significantly boosts their value in cancer therapy. This is because they cannot only activate immune responses against cancer cells but also serve as precise vehicles for delivering therapeutic agents to the TME. As of now, over 50 live microbial agents for treating various malignancies have completed clinical trials, and this number continues to grow (Nguyen et al., 2024). Immunotoxins, due to their facile incorporation into microorganisms, are anticipated to considerably augment their anticancer efficacy and broaden their applications through these “living agents.”
Despite its great prospects and several successful cases, the clinical application of live microbial agents loaded with IT faces the following major challenges: Firstly, in terms of the accuracy and controllability of drug delivery, although many studies have shown that microbes can proliferate rapidly in the TME and many MCTs have ultimately achieved significant intratumoral colonization effects in vivo, the main pathways and mechanisms by which bacteria reach tumors are still unclear. Further research on the main pathways and mechanisms of live microbes reaching tumors is of great significance for the clinical translation of IT-loaded microbes. In addition, due to the dynamic nature of the TME, when these IT-carrying microbes function in the tumor, both the TME and the tumor itself may change. This may expand bacterial colonies into normal tissues, thereby causing systemic infection. The dynamic TME also poses a huge challenge for precisely controlling IT release. This is because when tumor tissue declines due to treatment, “live microbial agents” and the IT cargo they produce may instead increase. More circuit design or additional antibiotic control is needed to balance this inconsistency. Moreover, this kind of live drug will apparently not follow the conventional pharmacokinetic characteristics, which also poses challenges to clinical drug monitoring and use. Secondly, regarding safety and patient individual differences, despite using attenuated or non-pathogenic microbes, the bacteria can still cause infection or excessive immune activation, posing safety risks. A patient’s immune status significantly impacts the effectiveness of live biotherapeutic products. Those with strong immune systems may quickly eliminate therapeutic bacteria, reducing treatment efficacy. Conversely, immunocompromised patients face higher infection risks and require careful management during treatment. Additionally, human microbiota varies between individuals, which can affect the performance of live biotherapeutic products. For example, a patient’s gut microbiota may interact with therapeutic bacteria, changing their growth, metabolism, and pharmacological effects, potentially leading to unstable treatment outcomes. Thirdly, in terms of production processes and quality control, unlike conventional drugs, the production process of these live microbial agents cannot rely on filtration or heat sterilization to eliminate other pathogenic bacteria, posing new challenges for production and quality control. Although additional resistance genes and antibiotics can be introduced during production to control other pathogenic bacteria, this approach carries the risk of resistance gene transfer within the body, potentially leading to antibiotic resistance. Finally, there is a lack of authoritative or official regulatory documents specifically targeting these live microbial agents.
5 Conclusion
In conclusion, engineered microbes present a promising and innovative approach to enhancing immunotoxin-based cancer therapy. Their unique capabilities to target and proliferate within tumors offer significant advantages, addressing several limitations of traditional immunotoxins. However, the clinical application of these live microbial agents faces substantial challenges, including ensuring the accuracy and controllability of drug delivery, managing safety concerns and patient individual differences, overcoming complexities in production processes and quality control, and navigating the lack of specific regulatory guidelines. Future research needs to focus on optimizing microbial delivery systems, improving our understanding of tumor-microbe interactions, and establishing appropriate regulatory frameworks. Despite these hurdles, the potential of engineered microbes to revolutionize cancer treatment and improve patient outcomes remains substantial, warranting continued exploration and development in this exciting field.
Author contributions
QW: Conceptualization, Data curation, Writing – original draft. RC: Funding acquisition, Supervision, Writing – review & editing. YX: Formal analysis, Visualization, Writing – review & editing. ZZ: Formal analysis, Visualization, Writing – review & editing. XL: Formal analysis, Writing – review & editing. YZ: Supervision, Writing – review & editing. HL: Formal analysis, Visualization, Writing – review & editing. HY: Formal analysis, Supervision, Writing – review & editing. PX: Funding acquisition, Supervision, Writing – review & editing. SN: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by National Natural Science Foundation of China (32273118), Hubei University of Science and Technology Doctoral Research Initiation Project (No. BK202406 and BK202325).
Acknowledgments
The authors would like to extend their sincere gratitude to the Guangdong Provincial Key Laboratory for Plant Epigenetics at the College of Life Sciences and Oceanography, Shenzhen University, as well as Dongguan Hanshuo Jianyuan Biotechnology Co., Ltd., for their generous support of this project. Additionally, we acknowledge with appreciation the developers of online tool Figdraw for providing invaluable tools that facilitated the creation of the figures in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anand, U., Dey, A., Chandel, A. K. S., Sanyal, R., Mishra, A., Pandey, D. K., et al. (2023). Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 10, 1367–1401. doi: 10.1016/j.gendis.2022.02.007
Babavalian, E., Zeinoddini, M., Saeedinia, A. R., Mohammadi, R., and Xodadadi, N. (2019). Design of a recombinant immunotoxin against the human granulocyte-colony stimulating factor receptor. Mol. Biol. Rep. 46, 1093–1097. doi: 10.1007/s11033-018-4567-z
Bacauanu, V., Merz, Z. N., Hua, Z. L., and Lang, S. B. (2023). Nickel-catalyzed antibody bioconjugation. J. Am. Chem. Soc. 145, 25842–25849. doi: 10.1021/jacs.3c10185
Bachran, C., and Leppla, S. H. (2016). Tumor targeting and drug delivery by anthrax toxin. Toxins 8:197. doi: 10.3390/toxins8070197
Balkhi, S., Bilato, G., De Lerma Barbaro, A., Orecchia, P., Poggi, A., and Mortara, L. (2025). Efficacy of anti-Cancer immune responses elicited using tumor-targeted IL-2 cytokine and its derivatives in combined preclinical therapies. Vaccine 13:69. doi: 10.3390/vaccines13010069
Boorjian, S. A., Alemozaffar, M., Konety, B. R., Shore, N. D., Gomella, L. G., Kamat, A. M., et al. (2021). Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 22, 107–117. doi: 10.1016/s1470-2045(20)30540-4
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. doi: 10.3322/caac.21834
Chen, L., Chen, G., Yang, Z., Wang, H., Liu, N., Liu, Y., et al. (2020). Enhanced cancer treatment by an acid-sensitive cytotoxic peptide-doxorubicin conjugate. J. Drug Deliv. Sci. Technol. 60:102048. doi: 10.1016/j.jddst.2020.102048
Coley, W. B. (1991). The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat. Res. 262, 3–11.
Colombo, R., Tarantino, P., Rich, J. R., LoRusso, P. M., and de Vries, E. G. E. (2024). The journey of antibody-drug conjugates: lessons learned from 40 years of development. Cancer Discov. 14, 2089–2108. doi: 10.1158/2159-8290.cd-24-0708
Copland, A., Mackie, G. M., Scarfe, L., Jinks, E., Lecky, D. A. J., Gudgeon, N., et al. (2024). Salmonella cancer therapy metabolically disrupts tumours at the collateral cost of T cell immunity. EMBO Mol. Med. 16, 3057–3088. doi: 10.1038/s44321-024-00159-2
Dhillon, S. (2018). Moxetumomab Pasudotox: First Global Approval. Drugs 78, 1763–1767. doi: 10.1007/s40265-018-1000-9
Dohlman, A. B., Klug, J., Mesko, M., Gao, I. H., Lipkin, S. M., Shen, X., et al. (2022). A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 185, 3807–3822.e12. doi: 10.1016/j.cell.2022.09.015
Feo, C. F., Ginesu, G. C., Fancellu, A., Perra, T., Ninniri, C., Deiana, G., et al. (2022). Current management of incidental gallbladder cancer: a review. Int. J. Surg. 98:106234. doi: 10.1016/j.ijsu.2022.106234
Galeano Niño, J. L., Wu, H., LaCourse, K. D., Kempchinsky, A. G., Baryiames, A., Barber, B., et al. (2022). Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817. doi: 10.1038/s41586-022-05435-0
Gill, S. K., Sugiman-Marangos, S. N., Beilhartz, G. L., Mei, E., Taipale, M., and Melnyk, R. A. (2024). An enhanced intracellular delivery platform based on a distant diphtheria toxin homolog that evades pre-existing antitoxin antibodies. EMBO Mol. Med. 16, 2638–2651. doi: 10.1038/s44321-024-00116-z
Golichenari, B., Heiat, M., Rezaei, E., Ramshini, A., Sahebkar, A., and Gholipour, N. (2025). Compromising the immunogenicity of diphtheria toxin-based immunotoxins through epitope engineering: an in silico approach. J. Pharmacol. Toxicol. Methods 131:107571. doi: 10.1016/j.vascn.2024.107571
Hassan, R., Alley, E., Kindler, H., Antonia, S., Jahan, T., Honarmand, S., et al. (2019). Clinical response of live-attenuated, Listeria monocytogenes expressing Mesothelin (CRS-207) with chemotherapy in patients with malignant pleural mesothelioma. Clin. Cancer Res. 25, 5787–5798. doi: 10.1158/1078-0432.ccr-19-0070
Hauser, S. L., Kappos, L., Bar-Or, A., Wiendl, H., Paling, D., Williams, M., et al. (2023). The development of Ofatumumab, a fully human anti-CD20 monoclonal antibody for practical use in relapsing multiple sclerosis treatment. Neurol. Ther. 12, 1491–1515. doi: 10.1007/s40120-023-00518-0
Hlongwane, P., Mungra, N., Madheswaran, S., Akinrinmade, O. A., Chetty, S., and Barth, S. (2018). Human Granzyme B based targeted Cytolytic fusion proteins. Biomedicines 6:72. doi: 10.3390/biomedicines6020072
Hu, C. W., Chang, Y. C., Liu, C. H., Yu, Y. A., and Mou, K. Y. (2022). Development of a TNF-α-mediated Trojan horse for bacteria-based cancer therapy. Mol. Ther. 30, 2522–2536. doi: 10.1016/j.ymthe.2022.04.008
Hu, X., Zhang, M., Zhang, C., Long, S., Wang, W., Yin, W., et al. (2016). Removal of B-cell epitopes for decreasing immunogenicity in recombinant immunotoxin against B-cell malignancies. J. BUON. 21, 1374–1378.
Janthur, W. D., Cantoni, N., and Mamot, C. (2012). Drug conjugates such as antibody drug conjugates (ADCs), immunotoxins and immunoliposomes challenge daily clinical practice. Int. J. Mol. Sci. 13, 16020–16045. doi: 10.3390/ijms131216020
Jeong, J. H., Kim, K., Lim, D., Jeong, K., Hong, Y., Nguyen, V. H., et al. (2014). Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PLoS One 9:e80050. doi: 10.1371/journal.pone.0080050
Kalbasi, A., and Ribas, A. (2020). Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39. doi: 10.1038/s41577-019-0218-4
Katz, M. H. G., Francescatti, A. B., and Hunt, K. K. (2022). Technical standards for cancer surgery: commission on Cancer standards 5.3-5.8. Ann. Surg. Oncol. 29, 6549–6558. doi: 10.1245/s10434-022-11375-w
Khirehgesh, M. R., Sharifi, J., Safari, F., and Akbari, B. (2021). Immunotoxins and nanobody-based immunotoxins: review and update. J. Drug Target. 29, 848–862. doi: 10.1080/1061186x.2021.1894435
Khoshbakht, M., Forghanifard, M. M., Aghamollaei, H., and Amani, J. (2024). Design and cytotoxicity evaluation of a Cancer-targeting immunotoxin based on a camelid Nanobody-PE fusion protein. Iran. J. Immunol. 21, 302–315. doi: 10.22034/iji.2024.104052.2878
Kim, J. S., Jun, S. Y., and Kim, Y. S. (2020). Critical issues in the development of immunotoxins for anticancer therapy. J. Pharm. Sci. 109, 104–115. doi: 10.1016/j.xphs.2019.10.037
Knödler, M., and Buyel, J. F. (2021). Plant-made immunotoxin building blocks: a roadmap for producing therapeutic antibody-toxin fusions. Biotechnol. Adv. 47:107683. doi: 10.1016/j.biotechadv.2020.107683
Kreitman, R. J. (2006). Immunotoxins for targeted cancer therapy. AAPS J. 8, E532–E551. doi: 10.1208/aapsj080363
Kwon, S. Y., Thi-Thu Ngo, H., Son, J., Hong, Y., and Min, J. J. (2024). Exploiting bacteria for cancer immunotherapy. Nat. Rev. Clin. Oncol. 21, 569–589. doi: 10.1038/s41571-024-00908-9
Labanieh, L., and Mackall, C. L. (2023). CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648. doi: 10.1038/s41586-023-05707-3
Lee, Y. T., Tan, Y. J., and Oon, C. E. (2018). Molecular targeted therapy: treating cancer with specificity. Eur. J. Pharmacol. 834, 188–196. doi: 10.1016/j.ejphar.2018.07.034
Li, X., Wang, Y., Wang, Y., Xie, H., Gong, R., Wu, X., et al. (2025). Anti-tumor activity of an αPD-L1-PE38 immunotoxin delivered by engineered Nissle 1917. Int. J. Biol. Macromol. 295:139537. doi: 10.1016/j.ijbiomac.2025.139537
Lim, D., Kim, K. S., Kim, H., Ko, K. C., Song, J. J., Choi, J. H., et al. (2017). Anti-tumor activity of an immunotoxin (TGFα-PE38) delivered by attenuated Salmonella typhimurium. Oncotarget 8, 37550–37560. doi: 10.18632/oncotarget.17197
Liu, X., Guo, Y., Sun, Y., Chen, Y., Tan, W., Min, J. J., et al. (2022). Comparison of anticancer activities and biosafety between Salmonella enterica Serovar typhimurium ΔppGpp and VNP20009 in a murine Cancer model. Front. Microbiol. 13:914575. doi: 10.3389/fmicb.2022.914575
Lu, D., Guo, Y., Hu, Y., Wang, M., Li, C., Gangrade, A., et al. (2021). Fusion of apoptosis-related protein cytochrome c with anti-HER-2 single-chain antibody targets the suppression of HER-2+ breast cancer. J. Cell. Mol. Med. 25, 10638–10649. doi: 10.1111/jcmm.17001
Luca, B. A., Steen, C. B., Matusiak, M., Azizi, A., Varma, S., Zhu, C., et al. (2021). Atlas of clinically distinct cell states and ecosystems across human solid tumors. Cell 184, 5482–5496.e28. doi: 10.1016/j.cell.2021.09.014
Markides, D. M., Hita, A. G., Merlin, J., Reyes-Gibby, C., and Yeung, S. J. (2025). Antibody-drug conjugates: the toxicities and adverse effects that emergency physicians must know. Ann. Emerg. Med. 85, 214–229. doi: 10.1016/j.annemergmed.2024.10.015
Mazor, R., Zhang, J., Xiang, L., Addissie, S., Awuah, P., Beers, R., et al. (2015). Recombinant immunotoxin with T-cell epitope mutations that greatly reduce immunogenicity for treatment of Mesothelin-expressing tumors. Mol. Cancer Ther. 14, 2789–2796. doi: 10.1158/1535-7163.mct-15-0532
Moon, C. M., Zheng, J. H., Min, J. J., Jeong, Y. Y., Heo, S. H., and Shin, S. S. (2020). In vivo bioluminescence imaging for targeting acute hypoxic/ischemic small intestine with engineered Salmonella typhimurium. Mol. Ther. 18, 484–492. doi: 10.1016/j.omtm.2020.06.021
Morgan, R. N., Saleh, S. E., Farrag, H. A., and Aboshanab, K. M. (2023). New insights on Pseudomonas aeruginosa exotoxin A-based immunotoxins in targeted cancer therapeutic delivery. Ther. Deliv. 14, 31–60. doi: 10.4155/tde-2022-0055
Mungra, N., Jordaan, S., Hlongwane, P., Naran, K., Chetty, S., and Barth, S. (2019). Targeted human cytolytic fusion proteins at the cutting edge: harnessing the apoptosis-inducing properties of human enzymes for the selective elimination of tumor cells. Oncotarget 10, 897–915. doi: 10.18632/oncotarget.26618
Naemi, A. A., Salmanian, A. H., Noormohammadi, Z., and Amani, J. (2023). A novel EGFR-specific recombinant ricin-panitumumab (scFv) immunotoxin against breast and colorectal cancer cell lines; in silico and in vitro analyses. Eur. J. Pharmacol. 955:175894. doi: 10.1016/j.ejphar.2023.175894
Nejman, D., Livyatan, I., Fuks, G., Gavert, N., Zwang, Y., Geller, L. T., et al. (2020). The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980. doi: 10.1126/science.aay9189
Nguyen, D. H., You, S. H., Ngo, H. T., Van Nguyen, K., Tran, K. V., Chu, T. H., et al. (2024). Reprogramming the tumor immune microenvironment using engineered dual-drug loaded Salmonella. Nat. Commun. 15:6680. doi: 10.1038/s41467-024-50950-5
Obozina, A. S., Pakhomov, A. A., Frolova, A. Y., Deyev, S. M., and Shipunova, V. O. (2025). Optimizing combination targeted immunotoxin therapy: insights from HER2 and EpCAM expression profiles. Biochem. Biophys. Res. Commun. 746:151218. doi: 10.1016/j.bbrc.2024.151218
Oghalaie, A., Hosseini, M. E., Hosseininejad-Chafi, M., Eftekhari, Z., Behdani, M., and Kazemi-Lomedasht, F. (2024). Advances in immunotoxin engineering: precision therapeutic strategies in modern oncology. Med. Oncol. 41:239. doi: 10.1007/s12032-024-02478-3
Phuna, Z. X., Kumar, P. A., Haroun, E., Dutta, D., and Lim, S. H. (2024). Antibody-drug conjugates: principles and opportunities. Life Sci. 347:122676. doi: 10.1016/j.lfs.2024.122676
Rebeck, O. N., Wallace, M. J., Prusa, J., Ning, J., Evbuomwan, E. M., Rengarajan, S., et al. (2025). A yeast-based oral therapeutic delivers immune checkpoint inhibitors to reduce intestinal tumor burden. Cell Chem. Biol. 32, 98–110.e7. doi: 10.1016/j.chembiol.2024.10.013
Schuster, S. J., Tam, C. S., Borchmann, P., Worel, N., McGuirk, J. P., Holte, H., et al. (2021). Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 22, 1403–1415. doi: 10.1016/s1470-2045(21)00375-2
Sepich-Poore, G. D., Zitvogel, L., Straussman, R., Hasty, J., Wargo, J. A., and Knight, R. (2021). The microbiome and human cancer. Science 371:eabc4552. doi: 10.1126/science.abc4552
Shafiee, F., Aucoin, M. G., and Jahanian-Najafabadi, A. (2019). Targeted diphtheria toxin-based therapy: a review article. Front. Microbiol. 10:2340. doi: 10.3389/fmicb.2019.02340
Shende, P., and Basarkar, V. (2019). Recent trends and advances in microbe-based drug delivery systems. Daru 27, 799–809. doi: 10.1007/s40199-019-00291-2
Shuwen, H., Yifei, S., Xinyue, W., Zhanbo, Q., Xiang, Y., and Xi, Y. (2024). Advances in bacteria-based drug delivery systems for anti-tumor therapy. Clin. Transl. Immunol. 13:e1518. doi: 10.1002/cti2.1518
Spiess, K., Jeppesen, M. G., Malmgaard-Clausen, M., Krzywkowski, K., Kledal, T. N., and Rosenkilde, M. M. (2017). Novel chemokine-based immunotoxins for potent and selective targeting of cytomegalovirus infected cells. J Immunol Res 2017:4069260. doi: 10.1155/2017/4069260
Tang, Q., Peng, X., Xu, B., Zhou, X., Chen, J., and Cheng, L. (2022). Current status and future directions of Bacteria-based immunotherapy. Front. Immunol. 13:911783. doi: 10.3389/fimmu.2022.911783
Thorpe, P. E., Ross, W. C., Cumber, A. J., Hinson, C. A., Edwards, D. C., and Davies, A. J. (1978). Toxicity of diphtheria toxin for lymphoblastoid cells is increased by conjugation to antilymphocytic globulin. Nature 271, 752–755. doi: 10.1038/271752a0
Tieu, M. V., Pham, D. T., and Cho, S. (2024). Bacteria-based cancer therapy: looking forward. Biochim. Biophys. Acta Rev. Cancer 1879:189112. doi: 10.1016/j.bbcan.2024.189112
Tsuchikama, K., Anami, Y., Ha, S. Y. Y., and Yamazaki, C. M. (2024). Exploring the next generation of antibody-drug conjugates. Nat. Rev. Clin. Oncol. 21, 203–223. doi: 10.1038/s41571-023-00850-2
Waldmann, H. (2019). Human monoclonal antibodies: the benefits of humanization. Methods Mol. Biol. 1904, 1–10. doi: 10.1007/978-1-4939-8958-4_1
Wang, X., Ding, Y., Li, S., Wang, F., Yang, L., Zhang, H., et al. (2025). Conditionally activated immunotoxins with prolonged half-life can enhance the anti-tumor activity. Int. J. Pharm. 669:125003. doi: 10.1016/j.ijpharm.2024.125003
Wang, T., Li, M., Wei, R., Wang, X., Lin, Z., Chen, J., et al. (2024). Small molecule-drug conjugates emerge as a new promising approach for Cancer treatment. Mol. Pharm. 21, 1038–1055. doi: 10.1021/acs.molpharmaceut.3c01049
Wei, J., Bera, T. K., Liu, X. F., Zhou, Q., Onda, M., Ho, M., et al. (2018). Recombinant immunotoxins with albumin-binding domains have long half-lives and high antitumor activity. Proc. Natl. Acad. Sci. USA 115, E3501–e3508. doi: 10.1073/pnas.1721780115
Xi, X., Wang, Y., An, G., Feng, S., Zhu, Q., Wu, Z., et al. (2024). A novel shark VNAR antibody-based immunotoxin targeting TROP-2 for cancer therapy. Acta Pharm. Sin. B 14, 4806–4818. doi: 10.1016/j.apsb.2024.08.023
Xi, M., Zhu, J., Zhang, F., Shen, H., Chen, J., Xiao, Z., et al. (2024). Antibody-drug conjugates for targeted cancer therapy: recent advances in potential payloads. Eur. J. Med. Chem. 276:116709. doi: 10.1016/j.ejmech.2024.116709
Xiao, Y., Pan, T., Da, W., Liu, Y., Chen, S., Chen, D., et al. (2024). Aptamer-drug conjugates-loaded bacteria for pancreatic cancer synergistic therapy. Signal Transduct. Target. Ther. 9:272. doi: 10.1038/s41392-024-01973-3
Xu, W., Zhou, T., Zhou, J., Qiang, Z., Zhang, J., and Hua, Z. (2018). Attenuated Salmonella VNP20009 mutant (ΔhtrA) is a promising candidate for bacteria-mediated tumour therapy in hosts with TNFR1 deficiency. Lett. Appl. Microbiol. 67, 97–103. doi: 10.1111/lam.12999
You, J., Zhang, J., Wang, J., and Jin, M. (2021). Cysteine-based coupling: challenges and solutions. Bioconjug. Chem. 32, 1525–1534. doi: 10.1021/acs.bioconjchem.1c00213
Yu, X., Lin, C., Yu, J., Qi, Q., and Wang, Q. (2020). Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy. Microb. Biotechnol. 13, 629–636. doi: 10.1111/1751-7915.13523
Zeng, C., Hua, S., Zhou, J., Zeng, T., Chen, J., Su, L., et al. (2025). Oral microalgae-based biosystem to enhance irreversible electroporation immunotherapy in hepatocellular carcinoma. Adv. Sci. 12:e2409381. doi: 10.1002/advs.202409381
Zhang, J., Liu, J., Yue, Y., Wang, L., He, Q., Xu, S., et al. (2024). The immunotoxin targeting PRLR increases tamoxifen sensitivity and enhances the efficacy of chemotherapy in breast cancer. J. Exp. Clin. Cancer Res. 43:173. doi: 10.1186/s13046-024-03099-4
Zheng, J., and Chen, H. (2024). Effects of intratumoral microbiota on tumorigenesis, anti-tumor immunity, and microbe-based cancer therapy. Front. Oncol. 14:1429722. doi: 10.3389/fonc.2024.1429722
Keywords: immunotoxin, cancer therapy, engineered microbes, circuits, tumor penetration
Citation: Wang Q, Cao R, Xie Y, Zhang Z, Li X, Zhang Y, Luo H, Yao H, Xue P and Ni S (2025) Unlocking the potential of engineered microbes in immunotoxin-based cancer therapy. Front. Microbiol. 16:1603671. doi: 10.3389/fmicb.2025.1603671
Edited by:
Karthik Loganathan, Salem Microbes Pvt. Ltd., IndiaReviewed by:
Gaurav Kumar, Amity University Jaipur, IndiaA. Sai Ramesh, Prathyusha Engineering College (PEC), India
Copyright © 2025 Wang, Cao, Xie, Zhang, Li, Zhang, Luo, Yao, Xue and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Xue, cGluZ3h1ZUBoYnVzdC5lZHUuY24=; Shuai Ni, bmlzaHVhaUBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Quan Wang
Quan Wang Rui Cao3†
Rui Cao3†