- 1Department of Obstetrics and Gynaecology, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 2Department of Biosciences, Faculty of Health and Life Sciences, Medical Research Council Centre for Medical Mycology at the University of Exeter, Exeter, United Kingdom
Lower genital tract infections, particularly vulvovaginal candidiasis (VVC) and bacterial vaginosis (BV), are among the most prevalent infections in women worldwide, especially those of reproductive age. These conditions not only cause significant clinical symptoms but also severely impact women’s quality of life and mental health. Despite extensive research on the pathogens involved, substantial gaps remain in understanding the vaginal immune response and the complexity of the vaginal ecosystem, which is largely shaped by a Lactobacillus-dominated microbiota and the high concentration of lactic acid, contributing to the vagina’s unique acidic pH. This review explores the underlying pathophysiology of VVC, BV, and fungal-bacteria co-infections, as well as conventional and emerging treatments, including zinc, Lactobacillus spp., and lactic acid. The challenges of antifungal drug resistance are also discussed, in parallel with immune cell dysfunction and its potential link to the vaginal microbiota and ecosystem. Personalized treatments and approaches tailored to the individual vaginal environment are essential for maintaining eubiosis and preventing recurrent infections. Future research should prioritize modulating host and environmental factors rather than targeting pathogens alone, to develop targeted therapies that prevent reinfection, minimize side effects, reduce development of drug-resistance, and ultimately improve women’s health outcomes.
1 Introduction
Lower genital tract infections and sexually transmitted infections (STIs) are among the most common primary reasons for women to visit the gynecology clinic. These conditions include a wide range of clinical manifestations, including bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), aerobic vaginitis (AV), and STIs caused by Chlamydia, Neisseria gonorrhoea, Trichomonas, and human immunodeficiency virus (HIV) (Pramanick et al., 2019). BV and VVC are the two most prevalent vaginal infections globally and are particularly common among women of reproductive age (Arastehfar et al., 2021; Denning et al., 2018; Sobel and Vempati, 2024). Understanding the complexity of these infections is crucial to addressing the widespread health challenges they pose. These infections significantly affect women’s quality of life and mental health (Neal and Martens, 2022), underscoring the need for a deeper exploration into their underlying mechanisms to improve the management of these infections. While substantial research has focused on pathogens, there remains a notable gap in our understanding of the human vagina’s physiological immune response. The unique acidic environment and diverse microbiota of the vagina further contribute to this complexity, with additional influences from factors such as ethnicity, socioeconomic status, and lifespan. To improve the treatment of VVC, in both its acute and recurrent forms, and its co-infections with anaerobic bacteria associated with BV, it is essential to investigate beyond the pathogens themselves. This requires examining the role of vaginal pH, lactic acid, and neutrophil (PMN) recruitment and dysfunction, as well as understanding how these factors influence the host’s immune response. Further research into how these elements interact in in vitro and ex vivo systems that mimic the human vaginal environment, along with studies involving human vaginal samples and patient-centered research, may lead to the development of novel, effective personalized therapies. Such advancements could ultimately improve women’s health outcomes by targeting individual vaginal ecosystems, preventing dysbiosis, and reducing infections.
2 Vulvovaginal candidiasis
Vulvovaginal candidiasis affects approximately 75% of women at least once in their lifetime, with 5–10% developing recurrent VVC (RVVC), defined as four or more symptomatic episodes per year, potentially persisting for decades (Donders et al., 2022; Denning et al., 2018; Neal and Martens, 2022; Rosati et al., 2020). Together, these infections account for half a billion cases annually (Roselletti et al., 2023). Predisposing factors for VVC include diabetes mellitus, contraceptive use, broad-spectrum antibiotics, pregnancy, and host genetics (Denning et al., 2018; Tsega and Mekonnen, 2019). Genetic mutations, such as polymorphisms in TLR2 and mannose-binding lectin 2, have been linked to RVVC, possibly increasing susceptibility to infection and leading to a hyperinflammatory response to Candida colonization and invasion (Rosati et al., 2020). However, no clear predisposing factors are identified in 20–30% of women with acute or recurrent infections (Rosati et al., 2020). Symptoms of VVC include irritation, itching, burning, redness, pain, dyspareunia, and vaginal discharge, which interfere with normal daily activities, and vary in severity among patients (Denning et al., 2018; Neal and Martens, 2022). Beyond physical discomfort, VVC and RVVC significantly reduce quality of life, leading to psychosocial issues such as increased stress, anxiety, depression, and social isolation (Neal and Martens, 2022). Acute and recurrent forms of VVC also globally contribute to a significant economic burden, affecting both healthcare systems and individuals. An annual treatment cost of at least $368 million has been estimated (Nyirjesy et al., 2022), with global losses amounting to approximately $14 billion due to a decline in productivity (Neal and Martens, 2022). The most common pathogen responsible for VVC is Candida albicans, but infections caused by non-albicans Candida (NAC) species are rising, particularly in developing countries where these infections range from 21 to 72%. The primary NAC species associated with VVC include Candida glabrata, Candida krusei, Candida parapsilosis, Candida tropicalis, Candida dubliniensis, Candida lusitaniae, and Candida africana (Pramanick et al., 2019; Arastehfar et al., 2021; Tsega and Mekonnen, 2019; Dunaiski et al., 2022; Monroy-Perez et al., 2016; Bitew and Abebaw, 2018; Mukasa et al., 2015; Brandolt et al., 2017; Waikhom et al., 2020; Khan et al., 2018; Fakhim et al., 2020). VVC infections caused by C. albicans, and NAC species have also increased in postmenopausal women, and are primarily due to an imbalance in the vaginal microbiome, rather than being estrogen related. During the childbearing age, estrogen promotes the microbial balance (eubiosis) and a low vaginal pH. In postmenopausal women, decreased or absent estrogen secretion leads to thinner, less elastic vaginal tissues, depletion of lactobacilli, and an increase in pH, resulting in higher susceptibility to infections (Donders et al., 2022; Al Halteet et al., 2020; Kim and Park, 2017). Postmenopausal women with diabetes or those undergoing hormone replacement therapy have the higher risk for complicated VVC (Kim and Park, 2017). In symptomatic C. albicans-induced VVC, the pathogen activates inflammasome receptors in vaginal epithelial cells through the production of virulence and immuno-inflammatory factors, triggering the release of cytokines and a neutrophilic inflammatory response, which often fails to resolve the infection (Roselletti et al., 2017; Roselletti et al., 2019b; Roselletti et al., 2019a; Roselletti et al., 2023; Cheng et al., 2024). The pathology of NAC species-induced VVC is currently poorly understood, however by contrast, tends to provoke less inflammation and PMN recruitment in in vitro and mouse models (Nash et al., 2016; Willems et al., 2018). Absence or significant reduction of PMN recruitment is also observed in vaginal samples from women with acute VVC caused by C. glabrata VVC (Roselletti et al., 2023).
3 Bacterial vaginosis and VVC co-infections
Bacterial vaginosis is a common vaginal disorder more than an infection, and it is characterized by a shift from protective, hydrogen peroxide-producing Lactobacillus species to a polymicrobial community dominated by anaerobic bacteria, including Gardnerella vaginalis, Atopobium vaginae, Prevotella, and Mobiluncus species (Abou Chacra et al., 2021). The pathogenesis of BV remains incompletely understood, but key factors involve the disruption of the vaginal microbiome, and the subsequent loss of the acidic environment maintained by Lactobacilli (Chen et al., 2021). Gardnerella vaginalis plays a pivotal role in this process by forming biofilms on the vaginal epithelium, which provide a scaffold for the colonization of other anaerobes, and which are resistant to host immune responses and antibiotic treatments, contributing to the chronic and recurrent nature of BV (Chen et al., 2021; Onderdonk et al., 2016). Recent studies suggest that the host immune system plays a limited role in actively combating the microbial imbalance, contributing to the high recurrence rate following antibiotic treatment (Machado et al., 2015; Amabebe and Anumba, 2022). BV is often associated with specific symptoms such as fishy vaginal odor, thin grayish-white discharge, itching and irritation, although many women may be completely asymptomatic (Kairys et al., 2024). BV is also associated with an increased risk of STIs, preterm birth, and pelvic inflammatory disease, impacting women’s physical and mental health (Kairys et al., 2024). Addressing BV effectively requires considering both its physical and psychological effects. VVC and BV induce distinct clinical manifestations and pathologies, with VVC characterized by an aberrant inflammatory response and PMN accumulation and BV by a complete absence of PMN infiltration and epithelial apoptosis (Roselletti et al., 2023; Roselletti et al., 2019b; Roselletti et al., 2020). Despite these differences, 30% of VVC cases involve bacterial co-infections, most commonly with G. vaginalis, which increases clinical severity and complicates diagnosis and treatment (Sobel et al., 2013) (Figure 1). Interestingly, the predominant Candida species isolated during bacterial vaginal co-infection are C. albicans and C. glabrata (Pramanick et al., 2019). The co-occurrence of vaginal infections characterized by the overgrowth of C. albicans and G. vaginalis at the same time is completely understudied but poses significant therapeutic challenges, as antifungal treatments for VVC do not address the bacterial imbalance in BV, and vice versa (Sobel and Vempati, 2024). To find targets for intervention, it is important to fully characterize the underlying pathologies.
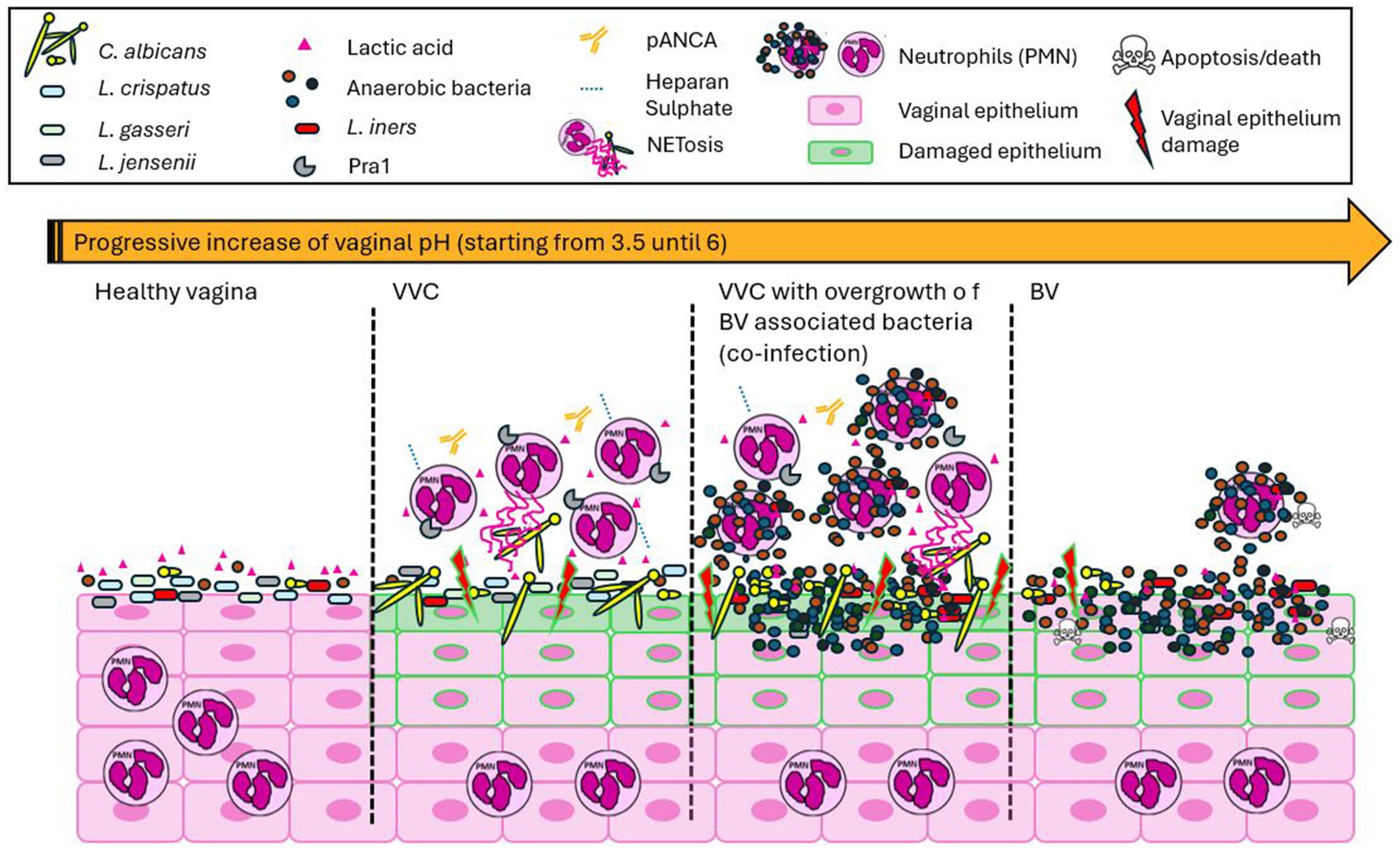
Figure 1. Distinct manifestations of vaginal infections. The healthy human vagina is characterized by a microbiota predominantly composed of Lactobacillus spp., which produce lactic acid and maintain an acidic environment, with absent host immune cell presence. Vaginal candidiasis (VVC) is characterized by a slight or no increase in acidic pH, an overgrowth of Candida, and a heightened host inflammatory response, primarily involving the recruitment of polymorphonuclear leukocytes (PMNs) that are unable to resolve the infection. This is mainly due to the involvement of proteins such as Pra1, pANCA antibodies, and heparan sulfate. During VVC, Lactobacillus spp. are still present, and no significant correlation with microbiome imbalance is observed. A decrease or complete absence of Lactobacillus spp. is associated with co-infections of Candida and anaerobic bacteria, as well as bacterial infections alone, inducing an increase in the vaginal pH. VVC, whether as a mono- or co-infection, is marked by an aberrant inflammatory response due to PMN accumulation, significant epithelial damage, and impaired infection resolution. In contrast, BV is characterized by the absence of PMN infiltration and the apoptosis of host cells.
4 VVC treatment and antifungal drug resistance
Treatment of VVC and co-infections is critical, especially in pregnant women, as Candida colonization is linked to preterm birth, infant mortality, and neonatal invasive candidiasis (Tsega and Mekonnen, 2019). Co-infections with anaerobic bacteria, such as G. vaginalis, further increase the risk of pregnancy complications and STIs (Kairys et al., 2024). Treatment in pregnant women remains challenging due to the risks associated with long-term fluconazole use and frequent recurrences after discontinuation, highlighting the need for safer, more effective, therapies (Neal and Martens, 2022). Current treatment guidelines for acute VVC recommend various prescription and over-the-counter (OTC) agents, typically azoles, administered topically or orally. However, treatment of RVVC is more complex, and differ from country to country, depending on regulatory factors. The standard approach generally involves 7–14 days of induction therapy with an oral azole, followed by weekly maintenance treatment for 6 months (Donders et al., 2022). For NAC infections, boric acid is often recommended for the same duration (at least 7–14 consecutive days) (Neal and Martens, 2022; Centers for Disease Control and Prevention, 2021; Sobel et al., 2004). However, it is estimated that 50% of women with NAC infections are completely asymptomatic (Kennedy and Sobel, 2010). For this reason, the most recent guidelines recommend that long-term treatment be offered only to symptomatic women with no other identifiable cause, using not only boric acid but also nystatin or amphotericin B (Vieira-Baptista and Sobel, 2023). Despite these treatments, pharmacological control of VVC, RVVC, and co-infections, remains challenging due to antimicrobial therapy not eliminating the risk of re-infections and emergent antimicrobial resistance developing due to prolonged or repeated use of a single agent, mainly azoles (Agrawal et al., 2023; Marchaim et al., 2012; File et al., 2023). Symptom resolution is achieved in about 90% of women receiving RVVC maintenance therapy (Sobel et al., 2004), but more than 50% experience recurrence and re-infections after stopping treatment (Collins et al., 2020; Neal and Martens, 2022; Crouss et al., 2018). Vaginal co-infections show further significant therapeutic implications and complicate treatments. Up to 74% of women with recurrent BV have Candida colonization, leading to symptomatic co-infection in around 30% of cases (Sobel et al., 2013; Redondo-Lopez et al., 1990). Currently, it is not standard practice to treat both fungal and bacterial infections simultaneously (Sobel et al., 2013; Balkus et al., 2011), leading to a cycle of recurrent VVC following BV treatment (Sobel and Vempati, 2024). This phenomenon, although not officially recognized in all medical guidelines, is well-known among patients and health care practitioners. As a result, many women with recurrent BV often self-use oral fluconazole for prophylaxis during symptomatic BV episodes (Sobel and Vempati, 2024). This unofficial practice contributes to the development of fluconazole resistance in C. albicans isolates, leading to refractory VVC episodes (Sobel and Vempati, 2024). Fluconazole-resistant C. albicans and azole-resistant NAC species are becoming increasingly problematic due to the indiscriminate use of antifungals. Despite progress in understanding azole resistance, effective solutions remain limited. Approximately 7.3% of RVVC cases are associated with clinically defined fluconazole-resistant C. albicans (File et al., 2023). Personalized therapy, taken into account both the role of fungal and bacterial pathogens, tailored to each woman’s medical history and resident Candida species, could help prevent reinfection, minimize side effects, and reduce the risk of drug-resistant pathogens. Drug resistance is particularly prevalent in developing countries, where the diagnosis of vaginal infections is often made on clinical symptoms rather than laboratory testing, and NAC species are a more common cause of VVC (Tsega and Mekonnen, 2019; Bitew and Abebaw, 2018; Mukasa et al., 2015; Arastehfar et al., 2021; Brandolt et al., 2017; Waikhom et al., 2020; Khan et al., 2018; Guzel et al., 2013). C. glabrata and C. krusei, the two most isolated NAC species, naturally resistant to fluconazole, are increasingly replacing C. albicans in these regions (Bitew and Abebaw, 2018). Treating azole-resistant vaginitis remains a clinical challenge. Boric acid, nystatin, amphotericin B, and flucytosine are possible alternatives for azole-resistant RVVC, but NAC infections are more difficult to manage due to their inherent or acquired resistance to common antifungals (Neal and Martens, 2022). Amphotericin B, although effective, is not routinely prescribed due to its high cost, difficulty of administration, and significant kidney toxicity (Tsega and Mekonnen, 2019). Similarly, flucytosine remains effective against many Candida species, but it is also prohibitively expensive in many developing countries, and monotherapy is associated with rapid resistance development (Paavonen and Robert, 2020).
5 Preventive measures
Several strategies to develop a vaccine against Candida infections were tested, including inactivated yeast and protein-based formulations, trying to consider what type of immune response is protective under natural conditions (Vecchiarelli et al., 2012). Despite limited early success, recent advancements have renewed interest, with several peptide-based and DNA vaccines targeting specific Candida antigens in development. Among these, the immunotherapeutic NDV-3A vaccine has shown promise. It contains a recombinant C. albicans adhesin/invasin protein and utilizes an innovative delivery system to enhance immunogenicity. In an exploratory phase 2, randomized, double-blind, placebo-controlled trial conducted on 188 women with recurrent vulvovaginal candidiasis (RVVC), aged 18–55 years, who were using an approved method of birth control and presented with a clinically diagnosed active episode of VVC, NDV-3A was demonstrated to elicit a targeted immune response, significantly reducing the incidence and recurrence of VVC (Edwards et al., 2018). Specifically, the study showed that a single intramuscular dose of NDV-3A was safe, generated rapid and robust B- and T-cell immune responses, and reduced the incidence and frequency of symptomatic RVVC episodes for up to 12 months in women under 40 years of age (Edwards et al., 2018). This vaccine represents a transformative step toward a preventative strategy that could greatly improve the quality of life for those prone to VVC. Moreover, it also serves as an important tool for the prevention of disseminated candidiasis in neonates (Singh et al., 2022).
6 The vaginal microbiota
The human vagina is a unique environment, characterized by a microbiota predominantly composed of Lactobacillus spp., accounting for approximately 80% of the total microbial population (Pramanick et al., 2019). The vaginal pH is acidic, ranging between 3.5 and 5, a unique feature among mammals (Miller et al., 2016; Pramanick et al., 2019) (Figure 2). Five major groups of vaginal microbial communities have been identified, each varying in the type and number of Lactobacillus spp., bacteria, archaea, viruses, and fungi. Groups I, II, III, and V are characterized by lower microbial diversity, and are dominated by Lactobacillus spp.: Lactobacillus crispatus (Group I), L. gasseri (Group II), L. iners (Group III), and L. jensenii (Group V). Group IV, however, is characterized by greater diversity, with a lack of Lactobacillus dominance and a higher prevalence of anaerobic bacteria, increasing the risk of infections (Sun et al., 2023; Ottinger et al., 2024; Ravel et al., 2011; Wei et al., 2024). Groups I, II, III, and V are more common in white and Asian women, while Group IV is predominantly found in Black and Hispanic women (Petrova et al., 2015) (Figure 2). Despite the identification of these five main groups, the vaginal microbiome is highly variable across individuals due to factors such as sexual habits, menstrual hygiene, stress, geography, and socioeconomic status (Sun et al., 2023). Additionally, the microbiome composition changes throughout different life stages, including puberty, pregnancy, postpartum, and menopause (Ottinger et al., 2024).
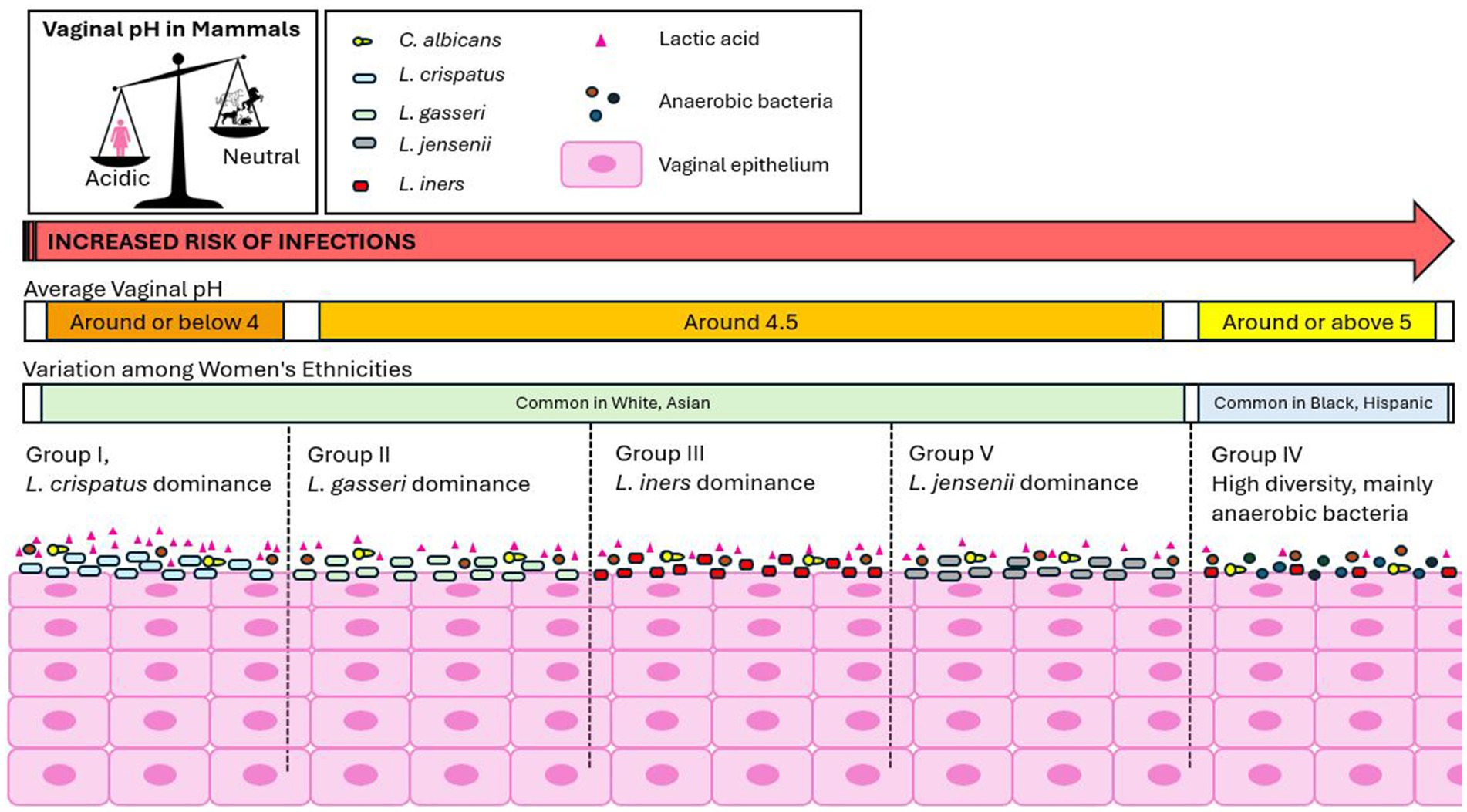
Figure 2. The unique human vaginal environment. The human vagina is characterized by a microbiota predominantly composed of Lactobacillus species, which helps maintain an acidic vaginal pH, a unique feature among mammals. Five major groups of vaginal microbial communities have been identified: Groups I, II, III, and V: These groups are characterized by lower microbial diversity and are predominantly dominated by Lactobacillus spp. Group IV: This group exhibits greater microbial diversity, with a lack of Lactobacillus dominance and a higher prevalence of anaerobic bacteria, which increases the risk of infections. Groups I, II, III, and V are more commonly found in white and Asian women, while Group IV is predominantly observed in Black and Hispanic women. The physiological vaginal pH varies slightly depending on the microbial community group. Women in Group I tend to have the lowest pH (around or below 4.0), while Group IV has the highest pH (around 5.4). The pH values for Groups II, III, and V fall in between.
7 Vaginal pH and lactic acid
The physiological vaginal pH varies slightly depending on the microbial community group. Women in Group I have the lowest pH (around or below 4.0), while Group IV has the highest (around 5.4). Groups II, III, and V fall in between, with pH values of around 4.7, 4.2, and 4.8, respectively (Ravel et al., 2011; Aldunate et al., 2013). In Group IV, the higher pH is due to the decreased presence of Lactobacillus spp., with L. iners being the only exception (Aldunate et al., 2013) (Figure 2). The acidic vaginal pH is primarily due to lactobacilli metabolites, with lactic acid being one of the most significant. Lactic acid, a lipid-soluble, membrane-permeable carboxylic acid, exists in two forms based on the environmental pH: a neutral protonated form under acidic conditions and a charged, unprotonated lactate anion under neutral conditions (Aldunate et al., 2013). It is the protonated form that provides protective activity for the host (Aldunate et al., 2013). Physiological vaginal lactic acid concentrations range from 55 to 111 mM, and it is constituted by racemic DL-lactic acid isoforms, with higher concentrations as the pH becomes more acidic. Human metabolism produces only the L-isomer, with less than 15% of the total vaginal lactic acid coming from vaginal epithelial metabolism (Aldunate et al., 2013), while Lactobacillus spp. produce different isomers: L. crispatus and L. gasseri produce both D- and L-lactic acids, L. iners produces only the L-isomer, and L. jensenii produces only the D-isomer (Tachedjian et al., 2017). The benefits of Lactobacillus spp. extend beyond lactic acid production and pH regulation, including the production of hydrogen peroxide, bacteriocins, competitive adherence to the vaginal epithelium, and immunomodulation (Mejia et al., 2023). In contrast to VVC, which typically involves a single pathogen inducing infection, BV represents a severe polymicrobial dysbiosis with the loss of protective Lactobacillus species and an overgrowth of anaerobic bacteria (Sobel and Vempati, 2024). In VVC, changes in the vaginal microbiota are associated with an increased pro-inflammatory response in vaginal epithelial cells due to surface exposure and invasion by the fungus. In cases of VVC co-infections, this response is compounded by the severe polymicrobial dysbiosis of BV, leading to increased inflammation and symptom severity (Sobel and Vempati, 2024). Vaginal microbial perturbations are associated with adverse outcomes such as preterm birth, pelvic inflammatory disease, urinary tract infections, and STIs (Mejia et al., 2023), similar to the complications seen in VVC and BV. This suggests a connection between various vaginal infections and microbiome composition. Changes in the microbiome not only affect pH but may also influence the progression to more serious conditions and infections. Lactic acid, with an acid dissociation constant of solution (pKa) of 3.9, is significantly more concentrated in the vagina than in other parts of the body (Foucher and Tubben, 2023; Aldunate et al., 2013). The vaginal distinct acidity raises concerns about the limitations of current cell line and mouse models for studying vaginal infections, as these models typically have a neutral vaginal pH (Roselletti et al., 2019a). To more accurately reflect the human vaginal environment, adjustments to model systems are needed. Further research is needed to understand how lactic acid concentrations and isomers vary across different microbial communities over the lifespan, and whether targeting its modulation could help maintain a balanced vaginal microbiome, preventing the progression to immunopathology, inflammatory diseases, or infections.
8 The dual role of lactic acid
Lactic acid has well-documented protective effects against pathogens. At vaginal physiological concentrations, it shows potent virucidal activity against HIV (Aldunate et al., 2013; Tachedjian et al., 2017) and inhibitory effects against Herpes Simplex virus types 1 and 2 (HSV-1 and HSV-2) (Tachedjian et al., 2017), and Chlamydia trachomatis (Edwards et al., 2019). It also protects against BV-associated bacteria such as Escherichia coli, G. vaginalis, Neisseria gonorrhoeae, and Group B Streptococcus (Plummer et al., 2021; Tachedjian et al., 2017). Notably, lactic acid’s protective effects are distinct from its acidity alone and surpass those of acetic acid (Tachedjian et al., 2017; Aldunate et al., 2013). However, several studies have not found a correlation between a protective effect of lactic acid or Lactobacilli against Candida species in VVC (Tachedjian et al., 2017). This may be explained by the fact that yeast are highly tolerant to low pH levels (Lourenco et al., 2018). The impact of lactic acid on the host remains controversial, and still not fully understood. In the vagina, Lactobacilli are associated with a non-inflammatory environment (Tachedjian et al., 2017; Sakai et al., 2004; Nikolaitchouk et al., 2008; Kyongo et al., 2012), while dysbiosis and infections are associated with inflammation and a higher risk of STIs (Tachedjian et al., 2017). Some studies suggest lactic acid may have pro-inflammatory effects on immune and vaginal epithelial cells (Witkin et al., 2011; Mossop et al., 2011). Additionally, acidic pH has been shown to reduce the motility and viability of immune cells like monocytes, macrophages, and lymphocytes potentially compromising their role in preventing HIV transmission (Olmsted et al., 2005). Although this study did not specifically examine lactic acid, it raises important questions about its effects, particularly in combination with acidic pH, on immune cell function in the vaginal environment, suggesting that its role may not always be protective. Further research is needed to clarify the role of lactic acid in the host and explore its therapeutic potential in combination with other molecules or its involvement in vaginal infections.
9 Lactobacillus spp. and lactic acid as treatments for vaginal infections
Lactobacillus spp. and lactic acid play vital roles in maintaining vaginal health and have been explored as treatments for infections like BV and VVC (Abavisani et al., 2024). Lactic acid’s key strength lies in its ability to restore and maintain the acidic vaginal pH and inhibiting pathogen growth (Barrientos-Duran et al., 2020). Research shows that lactic acid, in gel or suppository form, is particularly effective in treating BV when used with antibiotics, helping to restore the microbiome and reduce symptoms (Plummer et al., 2021; Abbasi et al., 2022). It has also shown promise in prophylactic use, helping to prevent BV and VVC recurrences by maintaining a stable vaginal environment (Werner et al., 2022). However, lactic acid alone is often insufficient and may require combination with other treatments for optimal efficacy (Werner et al., 2022). Lactobacillus spp., particularly L. crispatus and L. jensenii, dominate a healthy vaginal microbiome and are crucial for lactic acid production. Probiotics containing Lactobacillus strains can help re-establish a balanced vaginal microbiome and reduce harmful pathogen overgrowth (Liu et al., 2023; Cribby et al., 2009). Probiotics are effective in treating BV and may reduce recurrences when used with antibiotics (Chen et al., 2022). For VVC, they can be, but are not always, associated with antifungal effects, stabilizing the microbiome and preventing Candida overgrowth, although the evidence is less robust (Akinosoglou et al., 2024; Gaziano et al., 2020; van de Wijgert and Verwijs, 2020). However, the use of Lactobacillus probiotics has several limitations. While generally beneficial, probiotics can sometimes disrupt the vaginal microbiome, leading to imbalances or even irritation. Introducing external bacteria, such as L. crispatus, may disturb the natural balance of the vaginal ecosystem, potentially causing dysbiosis or overgrowth of beneficial bacteria, which can outcompete other essential microbes. The vaginal microbiome varies significantly between individuals, meaning what works as a beneficial probiotic for one woman might have adverse effects for another (Pagar et al., 2024; Chee et al., 2020). Moreover, the probiotic market is poorly regulated, leading to inconsistencies in product quality, concentration, and viability (van de Wijgert and Verwijs, 2020) and may result in side effects such as irritation or allergic reactions. Probiotic formulations also often contain additional components, like adjuvants, that can cause sensitivities or discomfort in some women. Additionally, the effects of probiotics are typically temporary (van de Wijgert and Verwijs, 2020), necessitating continuous use for sustained benefits, which may not always be practical or beneficial (Liu et al., 2023). Considering these aspects, both lactic acid and Lactobacillus probiotics could be promising treatments, but only when used as part of a broader, personalized strategy carefully tailored to each woman’s unique vaginal microbiome and specific infection.
10 The immunomodulatory role of zinc
Our own research has introduced zinc supplementation as a promising therapeutic strategy for C. albicans-induced VVC (Roselletti et al., 2023). During infection, C. albicans expresses a zinc-binding protein, Pra1, which helps the yeast capture zinc from its environment, particularly when the nutrient is limited (Citiulo et al., 2012), as it is in the vaginal mucosa (Roselletti et al., 2023). Pra1 expression is linked to local inflammation and PMN recruitment, which drives the immunopathology of VVC. Because Pra1 is used by the yeast to capture zinc, its expression is negatively regulated by this micronutrient. Zinc supplementation effectively inhibits Pra1 expression, reducing PMN infiltration and inflammation in both in vitro and in vivo models at neutral and acidic pH (Roselletti et al., 2023) (Figure 3). In a pilot study, five out of six women who applied a zinc-containing vaginal gel for 3 months did not experience recurrence (Roselletti et al., 2023). From a therapeutic perspective, Pra1 is an attractive target for treating C. albicans-induced VVC, offering significant improvements in quality of life for affected women. However, many NAC species, such as C. glabrata and C. krusei, have lost the PRA1 gene during evolution (Roselletti et al., 2023) (Figure 3), limiting the efficacy of zinc treatment in such cases and posing a significant challenge for future treatment. Moreover, zinc may not be a viable option for fungal-bacterial vaginal co-infections due to its ineffectiveness against bacteria (Roselletti et al., 2023), as PRA1 gene, is unique to the fungal kingdom. These observations underline the critical need to find new targets for intervention in VVC infections, suggesting the possibility of directly targeting the host inflammatory response to treat VVC while reducing antifungal usage.
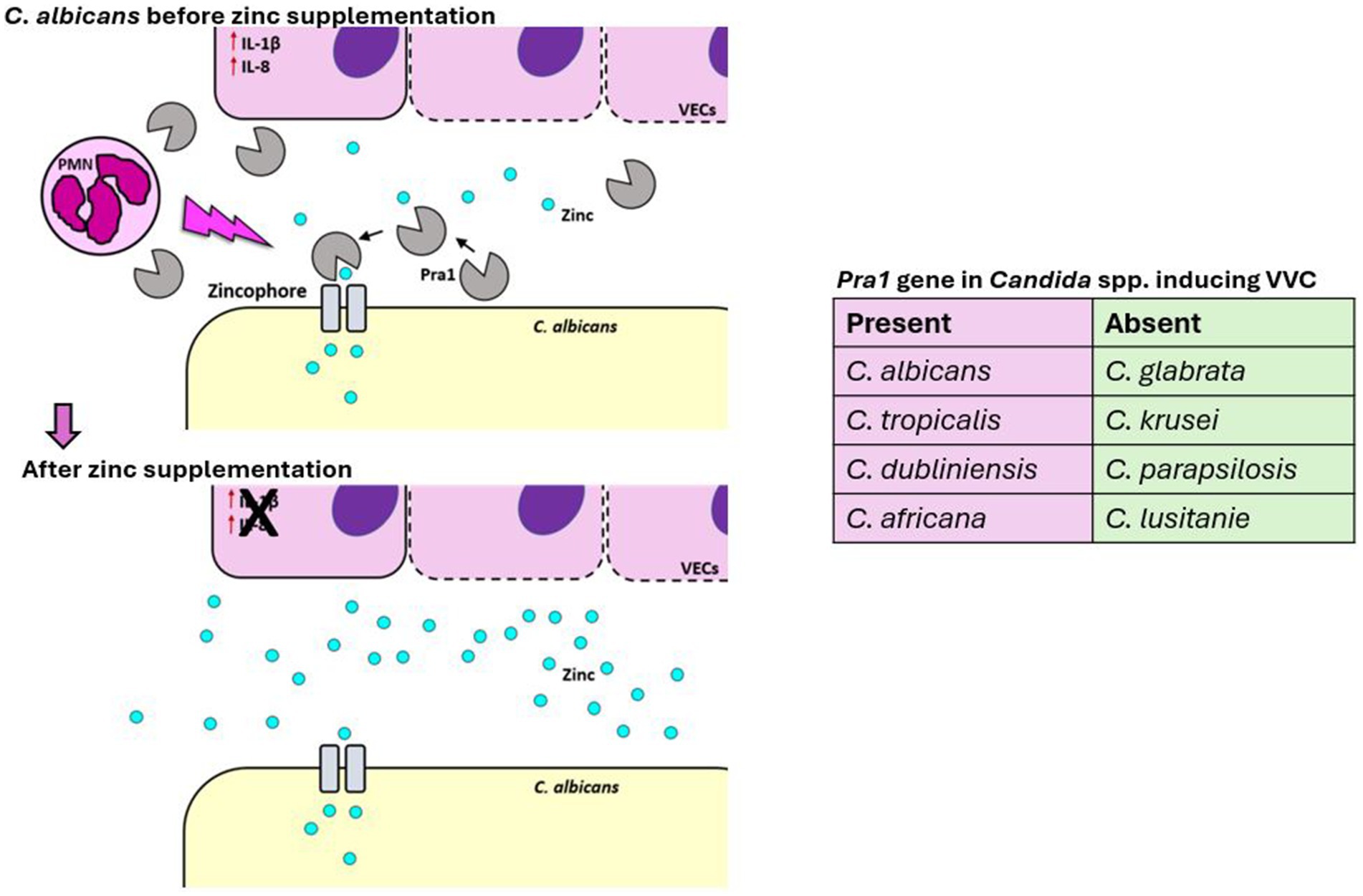
Figure 3. Effect of zinc supplementation on C. albicans during VVC. During VVC infection, C. albicans expresses and releases a zinc-binding protein, Pra1, which is linked to local inflammation and PMN recruitment, driving the VVC immunopathology. Since Pra1 facilitates zinc capture by the yeast, its expression is negatively regulated by zinc availability. Zinc supplementation effectively inhibits Pra1 expression, reducing PMN infiltration, inflammation, and reinfection in humans. From a therapeutic perspective, Pra1 is an attractive target for treating C. albicans-induced VVC, potentially improving the quality of life for affected women.
11 Immune cell dysfunction in VVC
While it is known that Pra1 and other immuno-inflammatory molecules play a role in PMN recruitment during VVC (Roselletti et al., 2023; Gabrielli et al., 2016; Richardson et al., 2018), why the PMNs are not able to clear the infection is unclear (Cheng et al., 2024; Kalia et al., 2019; Ardizzoni et al., 2021). One hypothesis is that the vaginal environment in VVC induces neutrophil dysfunction (Cheng et al., 2024). Heparan sulfate, a proteoglycan found on mammalian cell surfaces (Sarrazin et al., 2011), has been identified as a key contributor to this dysfunction, binding to CD11b on PMNs and impairing reactive oxygen species (ROS) production and NETosis, thereby reducing C. albicans clearance (Yano et al., 2017; Yano et al., 2014). estrogen may exacerbate this process by increasing heparan sulfate expression, and it has been shown that heparinase III can restore neutrophil activity in VVC-susceptible mice (Yano et al., 2017). Additionally, neutrophil hyperactivation, triggered by fungal virulence factors such as Pra1, candidalysin, and Secreted aspartyl proteinases (SAPs), can cause tissue damage through excessive NET formation, protease release, and ROS production (Roselletti et al., 2019a; Roselletti et al., 2023; Cheng et al., 2024). While these responses are meant to neutralize C. albicans, their premature release may result in tissue injury rather than effective pathogen clearance. This hyperactivation, compounded by the presence of markers like perinuclear anti-neutrophil cytoplasmic antibodies (pANCA), contributes to the inflammatory damage observed in VVC, suggesting a misalignment in the timing and location of neutrophil responses, worsening the infection (Cheng et al., 2024; Ardizzoni et al., 2020). Despite these insights, more research is needed to fully understand the role of neutrophil dysfunction in the pathophysiology of human VVC. Much of the current knowledge on VVC pathology is based on studies using cell lines and mouse models at neutral pH. While informative, these models do not accurately reflect the acidic vaginal environment characteristic of human VVC, which is shaped by a unique concentration of lactic acid (Roselletti et al., 2019a; Roselletti et al., 2023). PMNs are essential and effective immune cells in systemic candidiasis and other mucosal infections, such as oral candidiasis. However, they fail to provide protection in the vaginal environment, suggesting that this unique and specific setting plays a primary role in their impaired function.
12 Discussion and future implications
Acute and recurrent VVC infections are the most common vaginal infections globally, particularly among reproductive-age women. These conditions severely impact women’s quality of life, contributing to significant psychosocial and economic burdens across all income regions. Despite extensive research into the pathogens involved, the physiological immune response of the human vagina remains poorly understood and underexplored. This gap in knowledge can be attributed, in part, to the unique acidic vaginal environment, the extremely high physiological levels of lactic acid, and its highly diverse microbiome, influenced by factors such as ethnicity, socioeconomic status, and age. To advance our understanding, it is essential to study the effects of the acidic vaginal environment on host immune cells using models that closely mimic the human vaginal environment. These studies should include not only Lactobacilli and lactic acid but also local factors like estrogen, bacteria, mucus, and released metabolites. A deeper understanding of these elements could clarify critical targets for developing personalized therapies. Such strategies would be tailored to each woman’s unique vaginal microbiome and specific infection profiles, aiming to support individual vaginal ecosystems, prevent dysbiosis, and improve health outcomes by addressing imbalances before they result in infection.
Author contributions
FS: Writing – review & editing. AW: Writing – review & editing. ER: Writing – review & editing, Project administration, Data curation, Writing – original draft, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We acknowledge funding from the MRC Centre for Medical Mycology at the University of Exeter (MR/N006364/2 and MR/V033417/1) and the NIHR Exeter Biomedical Research Centre (NIHR203320).
Acknowledgments
We would like to thank all members of the MRC Centre for Medical Mycology at the University of Exeter for their support and stimulating discussions of our research, particularly Duncan Wilson.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.
References
Abavisani, M., Sahebi, S., Dadgar, F., Peikfalak, F., and Keikha, M. (2024). The role of probiotics as adjunct treatment in the prevention and management of gynecological infections: an updated meta-analysis of 35 RCT studies. Taiwan J. Obstet. Gynecol. 63, 357–368. doi: 10.1016/j.tjog.2024.03.004
Abbasi, A., Aghebati-Maleki, L., and Homayouni-Rad, A. (2022). The promising biological role of postbiotics derived from probiotic Lactobacillus species in reproductive health. Crit. Rev. Food Sci. Nutr. 62, 8829–8841. doi: 10.1080/10408398.2021.1935701
Abou Chacra, L., Fenollar, F., and Diop, K. (2021). Bacterial vaginosis: what do we currently know? Front. Cell. Infect. Microbiol. 11:672429. doi: 10.3389/fcimb.2021.672429
Agrawal, P., Yazdy, G., Ghanem, K. G., Handa, V. L., Schumacher, C. M., Sobel, J. D., et al. (2023). Vaginal Candida albicans: high frequency of in vitro fluconazole resistance in a select population-a brief note. Sex. Transm. Dis. 50, 121–123. doi: 10.1097/OLQ.0000000000001730
Akinosoglou, K., Schinas, G., Polyzou, E., Tsiakalos, A., and Donders, G. G. G. (2024). Probiotics in the management of Vulvovaginal Candidosis. J. Clin. Med. 13:5163. doi: 10.3390/jcm13175163
Al Halteet, S., Abdel-Hadi, A., Hassan, M., and Awad, M. (2020). Prevalence and antifungal susceptibility profile of clinically relevant Candida species in postmenopausal women with diabetes. Biomed. Res. Int. 2020:7042490. doi: 10.1155/2020/7042490
Aldunate, M., Tyssen, D., Johnson, A., Zakir, T., Sonza, S., Moench, T., et al. (2013). Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 68, 2015–2025. doi: 10.1093/jac/dkt156
Amabebe, E., and Anumba, D. O. C. (2022). Mechanistic insights into immune suppression and evasion in bacterial vaginosis. Curr. Microbiol. 79:84. doi: 10.1007/s00284-022-02771-2
Arastehfar, A., Kargar, M. L., Mohammadi, S. R., Roudbary, M., Ghods, N., Haghighi, L., et al. (2021). A high rate of recurrent vulvovaginal candidiasis and therapeutic failure of azole derivatives among Iranian women. Front. Microbiol. 12:655069. doi: 10.3389/fmicb.2021.655069
Ardizzoni, A., Sala, A., Colombari, B., Giva, L. B., Cermelli, C., Peppoloni, S., et al. (2020). Perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) impair neutrophil Candidacidal activity and are increased in the cellular fraction of vaginal samples from women with vulvovaginal candidiasis. J. Fungi 6:225. doi: 10.3390/jof6040225
Ardizzoni, A., Wheeler, R. T., and Pericolini, E. (2021). It takes two to tango: how a dysregulation of the innate immunity, coupled with Candida virulence, triggers VVC onset. Front. Microbiol. 12:692491. doi: 10.3389/fmicb.2021.692491
Balkus, J. E., Richardson, B. A., Mandaliya, K., Kiarie, J., Jaoko, W., Ndinya-Achola, J. O., et al. (2011). Establishing and sustaining a healthy vaginal environment: analysis of data from a randomized trial of periodic presumptive treatment for vaginal infections. J. Infect. Dis. 204, 323–326. doi: 10.1093/infdis/jir241
Barrientos-Duran, A., Fuentes-Lopez, A., De Salazar, A., Plaza-Diaz, J., and Garcia, F. (2020). Reviewing the composition of vaginal microbiota: inclusion of nutrition and probiotic factors in the maintenance of Eubiosis. Nutrients 12:419. doi: 10.3390/nu12020419
Bitew, A., and Abebaw, Y. (2018). Vulvovaginal candidiasis: species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health 18:94. doi: 10.1186/s12905-018-0607-z
Brandolt, T. M., Klafke, G. B., Goncalves, C. V., Bitencourt, L. R., Martinez, A. M., Mendes, J. F., et al. (2017). Prevalence of Candida spp. in cervical-vaginal samples and the in vitro susceptibility of isolates. Braz. J. Microbiol. 48, 145–150. doi: 10.1016/j.bjm.2016.09.006
Centers for Disease Control and Prevention (2021) Vulvovaginal Candidiasis (Vvc). Sexually transmitted infections treatment guidelines.
Chee, W. J. Y., Chew, S. Y., and Than, L. T. L. (2020). Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 19:203. doi: 10.1186/s12934-020-01464-4
Chen, R., Li, R., Qing, W., Zhang, Y., Zhou, Z., Hou, Y., et al. (2022). Probiotics are a good choice for the treatment of bacterial vaginosis: a meta-analysis of randomized controlled trial. Reprod. Health 19:137. doi: 10.1186/s12978-022-01449-z
Chen, X., Lu, Y., Chen, T., and Li, R. (2021). The female vaginal microbiome in health and bacterial vaginosis. Front. Cell. Infect. Microbiol. 11:631972. doi: 10.3389/fcimb.2021.631972
Cheng, K. O., Montano, D. E., Zelante, T., Dietschmann, A., and Gresnigt, M. S. (2024). Inflammatory cytokine signalling in vulvovaginal candidiasis: a hot mess driving immunopathology. Oxf. Open Immunol. 5:iqae010. doi: 10.1093/oxfimm/iqae010
Citiulo, F., Jacobsen, I. D., Miramon, P., Schild, L., Brunke, S., Zipfel, P., et al. (2012). Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 8:e1002777. doi: 10.1371/journal.ppat.1002777
Collins, L. M., Moore, R., and Sobel, J. D. (2020). Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J. Low. Genit. Tract Dis. 24, 48–52. doi: 10.1097/LGT.0000000000000496
Cribby, S., Taylor, M., and Reid, G. (2009). Vaginal microbiota and the use of probiotics. Interdiscip. Perspect. Infect. Dis 2008, 1–9. doi: 10.1155/2008/256490
Crouss, T., Sobel, J. D., Smith, K., and Nyirjesy, P. (2018). Long-term outcomes of women with recurrent vulvovaginal candidiasis after a course of maintenance antifungal therapy. J. Low. Genit. Tract Dis. 22, 382–386. doi: 10.1097/LGT.0000000000000413
Denning, D. W., Kneale, M., Sobel, J. D., and Rautemaa-Richardson, R. (2018). Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect. Dis. 18, e339–e347. doi: 10.1016/S1473-3099(18)30103-8
Donders, G., Sziller, I. O., Paavonen, J., Hay, P., De Seta, F., Bohbot, J. M., et al. (2022). Management of recurrent vulvovaginal candidosis: narrative review of the literature and European expert panel opinion. Front. Cell. Infect. Microbiol. 12:934353. doi: 10.3389/fcimb.2022.934353
Dunaiski, C. M., Kock, M. M., Jung, H., and Peters, R. P. H. (2022). Importance of Candida infection and fluconazole resistance in women with vaginal discharge syndrome in Namibia. Antimicrob. Resist. Infect. Control 11:104. doi: 10.1186/s13756-022-01143-6
Edwards, J. E., Schwartz, M. M., Schmidt, C. S., Sobel, J. D., Nyirjesy, P., Schodel, F., et al. (2018). A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis-a phase 2 randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 66, 1928–1936. doi: 10.1093/cid/ciy185
Edwards, V. L., Smith, S. B., Mccomb, E. J., Tamarelle, J., Ma, B., Humphrys, M. S., et al. (2019). The Cervicovaginal microbiota-host interaction modulates Chlamydia trachomatis infection. MBio 10, e01548–e01519. doi: 10.1128/mBio.01548-19
Fakhim, H., Vaezi, A., Javidnia, J., Nasri, E., Mahdi, D., Diba, K., et al. (2020). Candida africana vulvovaginitis: prevalence and geographical distribution. J. Mycol. Med. 30:100966. doi: 10.1016/j.mycmed.2020.100966
File, B., Sobel, R., Becker, M., and Nyirjesy, P. (2023). Fluconazole-resistant Candida albicans vaginal infections at a referral center and treated with boric acid. J. Low. Genit. Tract Dis. 27, 262–265. doi: 10.1097/LGT.0000000000000733
Foucher, C. D., and Tubben, R. E. (2023). Lactic Acidosis (Nursing). eds. S. Baddam, R. E. Tubben, and S. Bryan-Irving (StatPearls).
Gabrielli, E., Sabbatini, S., Roselletti, E., Kasper, L., Perito, S., Hube, B., et al. (2016). In vivo induction of neutrophil chemotaxis by secretory aspartyl proteinases of Candida albicans. Virulence 7, 819–825. doi: 10.1080/21505594.2016.1184385
Gaziano, R., Sabbatini, S., Roselletti, E., Perito, S., and Monari, C. (2020). Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front. Microbiol. 11:718. doi: 10.3389/fmicb.2020.00718
Guzel, A. B., Aydin, M., Meral, M., Kalkanci, A., and Ilkit, M. (2013). Clinical characteristics of Turkish women with Candida krusei vaginitis and antifungal susceptibility of the C. krusei isolates. Infect. Dis. Obstet. Gynecol. 2013:698736. doi: 10.1155/2013/698736
Kairys, N., Carlson, K., and Garg, M. (eds.) (2024). Bacterial Vaginosis. Treasure Island (FL): StatPearls Publishing.
Kalia, N., Singh, J., and Kaur, M. (2019). Immunopathology of recurrent vulvovaginal infections: new aspects and research directions. Front. Immunol. 10:2034. doi: 10.3389/fimmu.2019.02034
Kennedy, M. A., and Sobel, J. D. (2010). Vulvovaginal candidiasis caused by non-albicans Candida species: new insights. Curr. Infect. Dis. Rep. 12, 465–470. doi: 10.1007/s11908-010-0137-9
Khan, M., Ahmed, J., Gul, A., Ikram, A., and Lalani, F. K. (2018). Antifungal susceptibility testing of vulvovaginal Candida species among women attending antenatal clinic in tertiary care hospitals of Peshawar. Infect. Drug Resist. 11, 447–456. doi: 10.2147/IDR.S153116
Kim, J. M., and Park, Y. J. (2017). Probiotics in the prevention and treatment of postmenopausal vaginal infections: review article. J. Menopausal Med. 23, 139–145. doi: 10.6118/jmm.2017.23.3.139
Kyongo, J. K., Jespers, V., Goovaerts, O., Michiels, J., Menten, J., Fichorova, R. N., et al. (2012). Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLoS One 7:e43951. doi: 10.1371/journal.pone.0043951
Liu, P., Lu, Y., Li, R., and Chen, X. (2023). Use of probiotic lactobacilli in the treatment of vaginal infections: in vitro and in vivo investigations. Front. Cell. Infect. Microbiol. 13:1153894. doi: 10.3389/fcimb.2023.1153894
Lourenco, A., Pedro, N. A., Salazar, S. B., and Mira, N. P. (2018). Effect of acetic acid and lactic acid at low pH in growth and azole resistance of Candida albicans and Candida glabrata. Front. Microbiol. 9:3265. doi: 10.3389/fmicb.2018.03265
Machado, D., Castro, J., Palmeira-De-Oliveira, A., Martinez-De-Oliveira, J., and Cerca, N. (2015). Bacterial vaginosis biofilms: challenges to current therapies and emerging solutions. Front. Microbiol. 6:1528. doi: 10.3389/fmicb.2015.01528
Marchaim, D., Lemanek, L., Bheemreddy, S., Kaye, K. S., and Sobel, J. D. (2012). Fluconazole-resistant Candida albicans vulvovaginitis. Obstet. Gynecol. 120, 1407–1414. doi: 10.1097/AOG.0b013e31827307b2
Mejia, M. E., Mercado-Evans, V., Zulk, J. J., Ottinger, S., Ruiz, K., Ballard, M. B., et al. (2023). Vaginal microbial dynamics and pathogen colonization in a humanized microbiota mouse model. Npj Biofilms Microbi. 9:87. doi: 10.1038/s41522-023-00454-9
Miller, E. A., Beasley, D. E., Dunn, R. R., and Archie, E. A. (2016). Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front. Microbiol. 7:1936. doi: 10.3389/fmicb.2016.01936
Monroy-Perez, E., Paniagua-Contreras, G. L., Rodriguez-Purata, P., Vaca-Paniagua, F., Vazquez-Villasenor, M., Diaz-Velasquez, C., et al. (2016). High virulence and antifungal resistance in clinical strains of Candida albicans. Can. J. Infect. Dis. Med. Microbiol. 2016:5930489. doi: 10.1155/2016/5930489
Mossop, H., Linhares, I. M., Bongiovanni, A. M., Ledger, W. J., and Witkin, S. S. (2011). Influence of lactic acid on endogenous and viral RNA-induced immune mediator production by vaginal epithelial cells. Obstet. Gynecol. 118, 840–846. doi: 10.1097/AOG.0b013e31822da9e9
Mukasa, K. J., Herbert, I., Daniel, A., Sserunkuma, K. L., Joel, B., and Frederick, B. (2015). Antifungal susceptibility patterns of vulvovaginal Candida species among women attending antenatal Clinic at Mbarara Regional Referral Hospital, South Western Uganda. Br. Microbiol. Res. J. 5, 322–331. doi: 10.9734/BMRJ/2015/13804
Nash, E. E., Peters, B. M., Lilly, E. A., Noverr, M. C., and Fidel, P. L. Jr. (2016). A murine model of Candida glabrata vaginitis shows no evidence of an inflammatory Immunopathogenic response. PLoS One 11:e0147969. doi: 10.1371/journal.pone.0147969
Neal, C. M., and Martens, M. G. (2022). Clinical challenges in diagnosis and treatment of recurrent vulvovaginal candidiasis. Sage Open Med. 10:20503121221115201. doi: 10.1177/20503121221115201
Nikolaitchouk, N., Andersch, B., Falsen, E., Strombeck, L., and Mattsby-Baltzer, I. (2008). The lower genital tract microbiota in relation to cytokine-, SLPI- and endotoxin levels: application of checkerboard DNA-DNA hybridization (CDH). APMIS 116, 263–277. doi: 10.1111/j.1600-0463.2008.00808.x
Nyirjesy, P., Brookhart, C., Lazenby, G., Schwebke, J., and Sobel, J. D. (2022). Vulvovaginal candidiasis: a review of the evidence for the 2021 Centers for Disease Control and Prevention of sexually transmitted infections treatment guidelines. Clin. Infect. Dis. 74, S162–S168. doi: 10.1093/cid/ciab1057
Olmsted, S. S., Khanna, K. V., Ng, E. M., Whitten, S. T., Johnson, O. N., Markham, R. B., et al. (2005). Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect. Dis. 5:79. doi: 10.1186/1471-2334-5-79
Onderdonk, A. B., Delaney, M. L., and Fichorova, R. N. (2016). The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 29, 223–238. doi: 10.1128/CMR.00075-15
Ottinger, S., Robertson, C. M., Branthoover, H., and Patras, K. A. (2024). The human vaginal microbiota: from clinical medicine to models to mechanisms. Curr. Opin. Microbiol. 77:102422. doi: 10.1016/j.mib.2023.102422
Paavonen, J. A. B., and Robert, C. (2020). Vaginitis in nonpregnant patients: ACOG practice bulletin number 215. Obstet. Gynecol. 135, 1229–1230. doi: 10.1097/AOG.0000000000003857
Pagar, R., Deshkar, S., Mahore, J., Patole, V., Deshpande, H., Gandham, N., et al. (2024). The microbial revolution: unveiling the benefits of vaginal probiotics and prebiotics. Microbiol. Res. 286:127787. doi: 10.1016/j.micres.2024.127787
Petrova, M. I., Lievens, E., Malik, S., Imholz, N., and Lebeer, S. (2015). Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 6:81. doi: 10.3389/fphys.2015.00081
Plummer, E. L., Bradshaw, C. S., Doyle, M., Fairley, C. K., Murray, G. L., Bateson, D., et al. (2021). Lactic acid-containing products for bacterial vaginosis and their impact on the vaginal microbiota: a systematic review. PLoS One 16:e0246953. doi: 10.1371/journal.pone.0246953
Pramanick, R., Mayadeo, N., Warke, H., Begum, S., Aich, P., and Aranha, C. (2019). Vaginal microbiota of asymptomatic bacterial vaginosis and vulvovaginal candidiasis: are they different from normal microbiota? Microb. Pathog. 134:103599. doi: 10.1016/j.micpath.2019.103599
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., Mcculle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 108, 4680–4687. doi: 10.1073/pnas.1002611107
Redondo-Lopez, V., Meriwether, C., Schmitt, C., Opitz, M., Cook, R., and Sobel, J. D. (1990). Vulvovaginal candidiasis complicating recurrent bacterial vaginosis. Sex. Transm. Dis. 17, 51–53. doi: 10.1097/00007435-199001000-00011
Richardson, J. P., Willems, H. M. E., Moyes, D. L., Shoaie, S., Barker, K. S., Tan, S. L., et al. (2018). Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect. Immun. 86, e00645–e00617. doi: 10.1128/IAI.00645-17
Rosati, D., Bruno, M., Jaeger, M., Ten Oever, J., and Netea, M. G. (2020). Recurrent vulvovaginal candidiasis: an immunological perspective. Microorganisms 8:144. doi: 10.3390/microorganisms8020144
Roselletti, E., Monari, C., Sabbatini, S., Perito, S., Vecchiarelli, A., Sobel, J. D., et al. (2019a). A role for yeast/pseudohyphal cells of Candida albicans in the correlated expression of NLRP3 inflammasome inducers in women with acute vulvovaginal candidiasis. Front. Microbiol. 10:2669. doi: 10.3389/fmicb.2019.02669
Roselletti, E., Pericolini, E., Nore, A., Takacs, P., Kozma, B., Sala, A., et al. (2023). Zinc prevents vaginal candidiasis by inhibiting expression of an inflammatory fungal protein. Sci. Transl. Med. 15:eadi3363. doi: 10.1126/scitranslmed.adi3363
Roselletti, E., Perito, S., Gabrielli, E., Mencacci, A., Pericolini, E., Sabbatini, S., et al. (2017). NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans. Sci. Rep. 7:17877. doi: 10.1038/s41598-017-17649-8
Roselletti, E., Perito, S., Sabbatini, S., Monari, C., and Vecchiarelli, A. (2019b). Vaginal epithelial cells discriminate between yeast and hyphae of Candida albicans in women who are colonized or have vaginal candidiasis. J. Infect. Dis. 220, 1645–1654. doi: 10.1093/infdis/jiz365
Roselletti, E., Sabbatini, S., Perito, S., Mencacci, A., Vecchiarelli, A., and Monari, C. (2020). Apoptosis of vaginal epithelial cells in clinical samples from women with diagnosed bacterial vaginosis. Sci. Rep. 10:1978. doi: 10.1038/s41598-020-58862-2
Sakai, M., Ishiyama, A., Tabata, M., Sasaki, Y., Yoneda, S., Shiozaki, A., et al. (2004). Relationship between cervical mucus interleukin-8 concentrations and vaginal bacteria in pregnancy. Am. J. Reprod. Immunol. 52, 106–112. doi: 10.1111/j.1600-0897.2004.00203.x
Sarrazin, S., Lamanna, W. C., and Esko, J. D. (2011). Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3:a004952. doi: 10.1101/cshperspect.a004952
Singh, S., Nabeela, S., Barbarino, A., Ibrahim, A. S., and Uppuluri, P. (2022). Antibodies targeting Candida albicans Als3 and Hyr1 antigens protect neonatal mice from candidiasis. Front. Immunol. 13:925821. doi: 10.3389/fimmu.2022.925821
Sobel, J. D., Subramanian, C., Foxman, B., Fairfax, M., and Gygax, S. E. (2013). Mixed vaginitis-more than coinfection and with therapeutic implications. Curr. Infect. Dis. Rep. 15, 104–108. doi: 10.1007/s11908-013-0325-5
Sobel, J. D., and Vempati, Y. S. (2024). Bacterial vaginosis and vulvovaginal candidiasis pathophysiologic interrelationship. Microorganisms 12:108. doi: 10.3390/microorganisms12010108
Sobel, J. D., Wiesenfeld, H. C., Martens, M., Danna, P., Hooton, T. M., Rompalo, A., et al. (2004). Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 351, 876–883. doi: 10.1056/NEJMoa033114
Sun, Z., Ge, X., Qiu, B., Xiang, Z., Jiang, C., Wu, J., et al. (2023). Vulvovaginal candidiasis and vaginal microflora interaction: microflora changes and probiotic therapy. Front. Cell. Infect. Microbiol. 13:1123026. doi: 10.3389/fcimb.2023.1123026
Tachedjian, G., Aldunate, M., Bradshaw, C. S., and Cone, R. A. (2017). The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 168, 782–792. doi: 10.1016/j.resmic.2017.04.001
Tsega, A., and Mekonnen, F. (2019). Prevalence, risk factors and antifungal susceptibility pattern of Candida species among pregnant women at Debre Markos referral hospital, Northwest Ethiopia. BMC Pregnancy Childbirth 19:527. doi: 10.1186/s12884-019-2494-1
Van De Wijgert, J., and Verwijs, M. C. (2020). Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG 127, 287–299. doi: 10.1111/1471-0528.15870
Vecchiarelli, A., Pericolini, E., Gabrielli, E., and Pietrella, D. (2012). New approaches in the development of a vaccine for mucosal candidiasis: progress and challenges. Front. Microbiol. 3:294. doi: 10.3389/fmicb.2012.00294
Vieira-Baptista, P. S., and Sobel, J. D. (2023). “International society for the study of vulvovaginal disease,” in Recommendations for the diagnosis and treatment of vaginitis. eds. P. Vieira-Baptista, C. K. Stockdale, and J. Sobel.
Waikhom, S. D., Afeke, I., Kwawu, G. S., Mbroh, H. K., Osei, G. Y., Louis, B., et al. (2020). Prevalence of vulvovaginal candidiasis among pregnant women in the ho municipality, Ghana: species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth 20:266. doi: 10.1186/s12884-020-02963-3
Wei, X., Tsai, M. S., Liang, L., Jiang, L., Hung, C. J., Jelliffe-Pawlowski, L., et al. (2024). Vaginal microbiomes show ethnic evolutionary dynamics and positive selection of Lactobacillus adhesins driven by a long-term niche-specific process. Cell Rep. 43:114078. doi: 10.1016/j.celrep.2024.114078
Werner, M., Maged Atef El, S., and Lei, Z. (2022). The role of lactic acid in the Management of Bacterial Vaginosis: a systematic literature review. Future Pharmacol. 2, 198–213. doi: 10.3390/futurepharmacol2030014
Willems, H. M. E., Lowes, D. J., Barker, K. S., Palmer, G. E., and Peters, B. M. (2018). Comparative analysis of the capacity of the Candida species to elicit vaginal immunopathology. Infect. Immun. 86, e00527–e00518. doi: 10.1128/IAI.00527-18
Witkin, S. S., Alvi, S., Bongiovanni, A. M., Linhares, I. M., and Ledger, W. J. (2011). Lactic acid stimulates interleukin-23 production by peripheral blood mononuclear cells exposed to bacterial lipopolysaccharide. FEMS Immunol. Med. Microbiol. 61, 153–158. doi: 10.1111/j.1574-695X.2010.00757.x
Yano, J., Noverr, M. C., and Fidel, P. L. (2017). Vaginal Heparan sulfate linked to neutrophil dysfunction in the acute inflammatory response associated with experimental vulvovaginal candidiasis. MBio 8, e00211–e00217. doi: 10.1128/mBio.00211-17
Yano, J., Palmer, G. E., Eberle, K. E., Peters, B. M., Vogl, T., Mckenzie, A. N., et al. (2014). Vaginal epithelial cell-derived S100 alarmins induced by Candida albicans via pattern recognition receptor interactions are sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect. Immun. 82, 783–792. doi: 10.1128/IAI.00861-13
Keywords: vulvovaginal candidiasis, vaginal co-infections, microbiota, lactic acid, immune cell dysfunction
Citation: De Seta F, Warris A and Roselletti E (2025) New insights toward personalized therapies for vulvovaginal candidiasis and vaginal co-infections. Front. Microbiol. 16:1625952. doi: 10.3389/fmicb.2025.1625952
Edited by:
Svetlana Khaiboullina, University of Nevada, United StatesReviewed by:
Megan L. Falsetta, University of Rochester, United StatesAngel Gonzalez, University of Antioquia, Colombia
Copyright © 2025 De Seta, Warris and Roselletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Roselletti, ZS5yb3NlbGxldHRpQGV4ZXRlci5hYy51aw==
 Francesco De Seta
Francesco De Seta Adilia Warris
Adilia Warris Elena Roselletti
Elena Roselletti