- Department of Occupational Medicine and Environmental Toxicology, Nantong Key Laboratory of Environmental Toxicology, School of Public Health, Nantong University, Nantong, China
Plastic pollution, particularly in the form of nanoplastics, represents a growing global environmental crisis with profound impacts on ecosystems and human health. This review investigates the multifaceted interactions between fungi and nanoplastics, highlighting fungi’s dual role in both the degradation of plastics and their potential pathogenicity. Fungi possess specialized enzymatic pathways, which empower them to effectively break down a variety of plastic materials, leading to innovative bioremediation approaches. However, the omnipresence of nanoplastics in the environment poses significant challenges, as they can adversely affect fungal physiology, altering metabolic processes, enhancing virulence, and potentially contributing to antifungal resistance. This review examines the mechanisms through which different fungal species degrade specific plastics while emphasizing the influence of nanoplastics on fungal metabolic pathways and collective community dynamics. It explores the adaptations fungi may exhibit in response to nanoplastic exposure, including changes in enzymatic activity and resistance mechanisms. Additionally, the review addresses the implications of nanoplastic exposure for the pathogenicity of fungi, particularly concerning their interactions with human hosts and resistance to antifungal treatments. By providing a thorough analysis of the current understanding of nanoplastics and fungi, this review calls for urgent research into the ecological consequences of these interactions and the potential for increasing antifungal resistance. Ultimately, this work aims to inform effective strategies for mitigating the dual threats of plastic pollution and fungal-related health issues.
1 Introduction
Plastic pollution has emerged as a critical global environmental challenge, significantly affecting ecosystem health, human wellbeing, and long-term sustainability (Wilcox et al., 2015; Geyer et al., 2017; Hoseini and Bond, 2022; Cowger et al., 2024). The degradation of plastic materials occurs through various mechanisms, including UV irradiation, thermal degradation, mechanical stress, and biodegradation. These processes ultimately lead to the formation of microplastics and nanoplastics (Fotopoulou and Karapanagioti, 2019; Zhang et al., 2021). Microplastics, defined as plastic particles smaller than 5 mm, and nanoplastics, defined as particles smaller than 100 nm, have garnered significant attention in recent years (Kong et al., 2025; Sun et al., 2025). Nanoplastics, in particular, are concerning due to their ubiquitous presence in the environment and their potential harmful effects on living organisms. Their unique physicochemical properties enhance both their reactivity and bioavailability, raising severe environmental and health concerns (Gigault et al., 2021; Mitrano et al., 2021; Sui et al., 2023; Bu et al., 2025).
Nanoplastics are not only a pollutant but also an emerging factor influencing microbial communities in various ecosystems (Nath et al., 2020; Ren et al., 2022; Zhao et al., 2025). They interact with a diverse array of microorganisms, including bacteria, archaea, and fungi, potentially altering their physiological functions and ecological roles. For instance, studies have demonstrated that exposure to polystyrene nanoplastics leads to a decrease in both bacterial and fungal biomass within microfabricated soil models (Mafla-Endara et al., 2023). Interestingly, some bacterial species have demonstrated the ability to either degrade nanoplastics or utilize them as a substrate. This interaction can significantly affect microbial community dynamics and nutrient cycling in aquatic environments (Wang et al., 2023). Such changes are crucial, as they may influence the biogeochemical processes that underpin ecosystem services. Fungi, as essential microorganisms, play vital roles in nutrient cycling and overall ecosystem functioning. Certain fungal species have shown remarkable abilities to degrade various plastics, making them promising candidates for bioremediation strategies (Rodrigues et al., 2019; Ryu et al., 2020). Their significance in plastic biodegradation is attributed to their capacity to secrete a diverse array of degrading enzymes, which catalyze the conversion of complex plastic polymers into simpler, more manageable compounds. This enzymatic activity facilitates the oxidation or hydrolysis of plastics, resulting in the formation of functional groups that increase hydrophilicity. Consequently, high molecular weight plastics can be transformed into lower molecular weight compounds that fungi can readily assimilate (Temporiti et al., 2022; Ibrahim et al., 2024; Yang et al., 2024). Some fungi exhibit the ability to effectively degrade specific plastics within just a few days, underscoring their efficiency in addressing plastic pollution (Ibrahim et al., 2024).
Despite their beneficial potential, it is essential to recognize that fungi are not exclusively advantageous. Invasive fungal species contribute to an estimated 2.5 million human fatalities each year (Denning, 2024). Among these, Cryptococcus neoformans, Aspergillus fumigatus, Candida albicans, and Candida auris have been identified by the World Health Organization (WHO) as some of the most dangerous pathogenic fungi to humans (Kriegl et al., 2024). As research increasingly investigates the interactions between fungi and nanoplastics, it becomes crucial to evaluate how these plastic nanoparticles impact fungal physiology and pathogenicity. The adsorption of nanoplastics onto fungal cell surfaces may lead to alterations in physiological functions, potentially affecting ecological roles and interactions with other organisms. Furthermore, the presence of nanoplastics may influence the metabolic pathways of fungi, leading to changes in their enzymatic profiles and nutrient assimilation capabilities. This could result in both positive and negative outcomes, depending on the specific context and species involved. For example, while some fungi might enhance their plastic-degrading capabilities in response to nanoplastic exposure, others may experience detrimental effects that impair their metabolic functions and ecological interactions. The ability of fungi to adapt to the presence of nanoplastics raises important questions concerning their potential virulence and antifungal resistance, particularly in human pathogenic species. The alterations in fungal physiology due to nanoplastic interactions could lead to an increased expression of virulence factors or enhanced resistance mechanisms against antifungal agents. Understanding these dynamics is crucial for developing effective strategies to combat both plastic pollution and fungal infections.
This review aims to provide a comprehensive examination of the relationships between nanoplastics and fungi by systematically analyzing the mechanisms of plastic degradation by fungi, their metabolic pathways, and physiological adaptations. Additionally, it will address the potential implications for virulence and antifungal resistance in human pathogenic fungi. By elucidating these complex interactions, this review seeks to contribute to the broader understanding of how nanoplastics impact fungal communities and their ecological roles, as well as to inform strategies aimed at mitigating the dual threats of plastic pollution and fungal diseases.
2 The role of fungi in plastic degradation
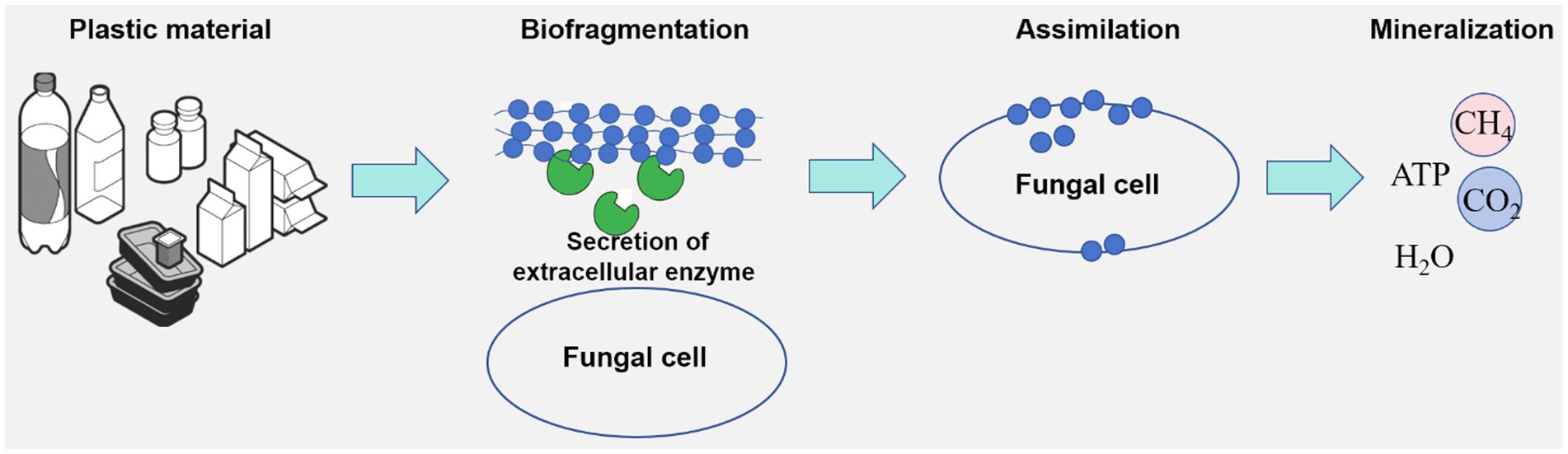
Fungi play a pivotal role in the degradation and assimilation of various plastic materials (Figure 1), including polyethylene, polystyrene, and polyvinyl chloride (Maity et al., 2021; Cowan et al., 2022; Vaksmaa et al., 2023). Certain fungal species have demonstrated the remarkable capacity to degrade specific plastics within a matter of days, highlighting their potential for addressing plastic pollution (Okal et al., 2023).
The advantages of fungal degradation can be summarized into three key points:
2.1 Enhanced substrate penetration
The apical growth pattern of filamentous fungal mycelia enables these organisms to penetrate soil substrates more effectively than bacteria. This capability is attributed to their secretion of extracellular enzymes and hydrophobic proteins, which enhance their adhesion to hydrophobic substrates (Sánchez, 2020). Such penetration is particularly pronounced in soil environments where saprotrophic fungi thrive (Dix et al., 1995).
2.2 Diverse enzymatic machinery
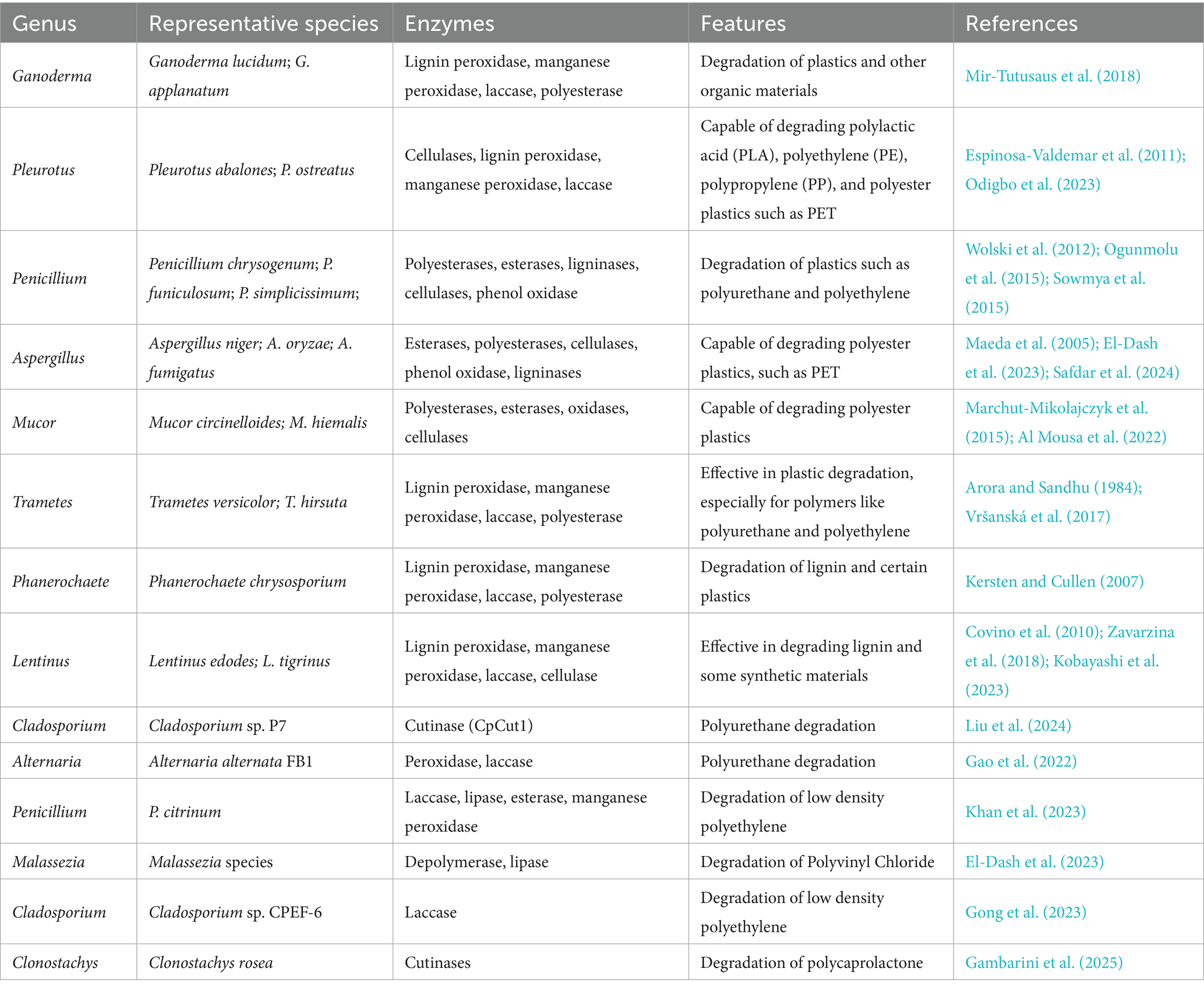
Fungi possess a wide range of non-specific enzyme cassettes (Aranda, 2016; Deshmukh et al., 2016), which equip them to degrade and utilize hydrocarbons and aromatic compounds found in plastics as carbon and energy sources. Secreted fungal enzymes, including lignin peroxidase, manganese peroxidase, laccase, and polyesterase (Table 1), facilitate the oxidation or hydrolysis of plastic polymers, leading to the formation of functional groups that increase hydrophilicity. This enhanced hydrophilicity promotes the breakdown of high molecular weight plastics into lower molecular weight compounds that fungi can readily assimilate. Additionally, various internal factors within fungal cells can induce pro-oxidant ions, which promote degradation through oxidative reactions, particularly through the generation of reactive oxygen species. Hydroxyl radicals, recognized for their high redox potential, are particularly effective oxidants in this context (Backa et al., 1993; Lawton and Robertson, 1999). This oxidative mechanism enables fungi to participate in the degradation of a variety of recalcitrant pollutants, including hydrocarbons, chlorophenols, and pesticides (Young et al., 2015; Deshmukh et al., 2016; Hasan, 2018).
2.3 Adaptability to diverse environment
Fungi exhibit remarkable adaptability, enabling them to utilize nearly any organic carbon source and thrive in conditions characterized by low humidity, nutrient scarcity, and acidic pH levels (Case et al., 2025). Some species can endure dry periods through a state of cryptobiosis (Magan, 2007; Mancera-López et al., 2008). The metabolic pathways within fungi are mediated by cytochrome P450 (CYP) monooxygenases (EC:1.14.13.12), which facilitate the oxidation of substrates in microsomes (Durairaj et al., 2015; Asemoloye et al., 2019). These enzymes belong to the heme-protein superfamily and are involved in various biological processes, including adaptation to environmental stress, toxin production, and the metabolism of both endogenous and xenobiotic compounds, thereby enhancing fungal fitness (Bernhardt, 2006; Van Beilen and Funhoff, 2007; Kelly et al., 2009; Moktali et al., 2012). CYP monooxygenases typically function as terminal oxidases in the electron transfer chain associated with NADPH reductase, facilitating the incorporation of an oxygen atom into the hydrocarbon chain of nanoplastics while reducing the other oxygen atom to water (Chen et al., 2014). The number of CYP genes varies according to the lifestyle of the fungal species; yeasts and yeast-like fungi possess relatively few CYPs (e.g., three in Saccharomyces cerevisiae, six in C. neoformans, and 10 in C. albicans), while filamentous fungi typically harbor a greater number of CYP genes, as exemplified by Aspergillus spp. with 79 genes and Agaricus bisporus with 109 (Doddapaneni et al., 2013; Dauda et al., 2022). As a result, plastic-degrading fungi are predominantly filamentous species (Table 1), including Ganoderma lucidum, Pleurotus abalone, Penicillium chrysogenum, and Aspergillus niger (Wolski et al., 2012; Mir-Tutusaus et al., 2018; Odigbo et al., 2023; Safdar et al., 2024). Ekanayaka et al. assessed 395 filamentous fungal strains from the Ascomycota and Basidiomycota phyla, identifying over 200 species capable of degrading various plastics under diverse environmental conditions (Ekanayaka et al., 2022). Their findings revealed that fungi such as Aspergillus tubingensis effectively disrupt the chemical bonds within plastic molecules and successfully colonize plastic surfaces (Ekanayaka et al., 2022). Numerous plastic-degrading fungi have been isolated from both terrestrial and marine environments (Viel et al., 2023), including Trichoderma sp., Clitocybe sp., Monascus sp., and Phanerochaete sp., which enhance the degradation of polyethylene (both LDPE and HDPE), polylactic acid, polyurethanes, polyethylene terephthalate, and bisphenol A polycarbonate (Artham and Doble, 2010; El-Morsy et al., 2017; Ojha et al., 2017; Satti et al., 2017; Janczak et al., 2018; Munir et al., 2018). Marine environments have yielded marine yeasts such as Rhodotorula mucilaginosa, Zalerion maritimum, Alternaria alternata, Penicillium spp., and Aspergillus sp., which significantly facilitate the degradation of polyethylene and polystyrene, contributing to healthier ecosystems by reducing plastic waste (Ameen et al., 2015; Sarkhel et al., 2020; Gao et al., 2022; Vaksmaa et al., 2023; do Paço, A.M.S., 2024). Overall, the capacity of fungi to degrade plastics presents a promising avenue for bioremediation strategies aimed at mitigating the environmental impacts of plastic waste. Advances in the exploration of fungal enzymes, along with genetic engineering techniques, could enhance biodegradation processes and contribute to sustainable waste management practices.
2.4 The impact of nanoplastics on fungal physiology and pathogenicity
Nanoplastics possess unique physical properties, including an increased surface area, specific transport characteristics, and distinctive interactions with light and natural colloids (Gigault et al., 2021). The larger surface area enhances the adsorption capacity of nanoplastics for natural organic matter in the environment (Liu et al., 2022). Adsorption predominantly occurs through chemical bonding on certain types of nanoplastics, facilitated by ligand exchange mechanisms with oxide nanoplastics. This interaction reduces surface hydrophobicity, increases interactions among plastic particles, and affects their aggregate size (Junaid and Wang, 2021). Furthermore, the presence of electron-attracting groups within the aromatic rings of nanoplastic polymers facilitates strong π–π interactions, contributing to their exceptional ion adsorption properties (Hüffer and Hofmann, 2016; Wang et al., 2020). The substances adsorbed onto nanoplastics can interact with extracellular polymers secreted by fungal cells, potentially enveloping the nanoplastics in a unique layered structure referred to as the eco-corona. This eco-corona can significantly alter the dynamics between nanoplastics and fungi (Liu et al., 2022). Fungal cell wall thickness typically ranges from 0.1 to 1.0 micrometers, and the formation of an ecological corona layer on these walls is contingent upon the abundance and physicochemical properties of biomolecules and plastic particles. The stability of this layer is influenced by hydrogen bonds, van der Waals forces, hydrophobic interactions, and other high-energy chemical or adhesive forces (Liu et al., 2022). Research indicates that the zeta potential of fungal cell walls is highly responsive to environmental conditions, generally fluctuating between −14 and −15 millivolts (Ramos et al., 2020). Changes in environmental pH, along with varying concentrations of ions and proteins, can promote heteroaggregation, which may consequently alter the zeta potential (Mikolajczyk et al., 2015). Exposure of fungal cells to nanoplastics may modulate the zeta potential of their cell walls, thereby affecting their functional integrity and potentially contributing to the toxicity of extracellularly secreted enzymes.
The fungal cell wall serves as the outermost layer, directly interacting with the external environment and playing a critical role in various physiological and ecological functions. It is a primary target for antimicrobial agents and the immune system, requiring a delicate balance of strength and flexibility to provide protection while facilitating nutrient uptake, membrane vesicle exchange, and external signal reception (Gow and Lenardon, 2023). Previous studies have shown that polymeric particles ranging from 100 nm to 300 nm do not penetrate the cell walls of pathogenic fungi, such as A. fumigatus and C. albicans (Orasch et al., 2023). Thus, it can be proposed that nanoplastics exceeding 100 nm primarily interact with the surfaces of fungal cell walls, impacting the outer wall polymers and glycoproteins associated with the chitin and β-glucan-based inner wall skeleton. This interaction could disrupt spatial organization and dynamic regulatory functions, impairing the fungal ability to effectively respond to changes in growth conditions and potentially leading to toxicity. Interestingly, certain filamentous fungi may induce a “dusting effect,” wherein high concentrations of nanoplastics allow initially colonizing hyphae to adsorb or internalize these particles into vacuoles, subsequently metabolizing them into less toxic forms. This adaptive response may mitigate toxicity to later-growing hyphae, thereby promoting favorable conditions for fungal proliferation (Mafla-Endara et al., 2023). Previous research in bacterial systems has demonstrated that nanoplastics with diameters of 60 nm can penetrate cells, accumulating internally and enhancing the generation of ROS, which impose stress on bacterial cells and significantly inhibit their growth (Dai et al., 2022). It is plausible that nanoplastics of similar sizes may also compromise pathogenic yeast cells, such as C. neoformans, given that ROS can modulate the expression of virulence factors, including capsule and melanin production (Zaragoza et al., 2008; Momin and Webb, 2021). The toxicological impact of nanoplastics on fungal cells is multifaceted, encompassing redox imbalances, membrane damage, immune responses, and genotoxic effects, which can induce various forms of cellular injury simultaneously. Collectively, nanoplastics have the potential to significantly alter the physiological states of fungi, highlighting the urgent need for further research to elucidate the complex interactions and effects of nanoplastics on fungal ecology.
2.5 Potential effects of nanoplastics on fungal drug resistance
The development of antifungal agents faces significant challenges due to the shared eukaryotic structures and metabolic pathways between humans and fungi, resulting in limited therapeutic options. Fungal infections are increasingly exhibiting resistance to conventional antifungal drugs, with the efficacy of existing treatments, such as azoles and polyenes, diminishing in clinical settings. According to data from the Centers for Disease Control and Prevention, drug-resistant fungal infections were responsible for at least 35,900 deaths in the United States in 2019 (Prevention, 2019). This resistance typically arises through natural selection, often driven by genetic mutations or gene transfer that confer additional resistant traits. Notably, fungi are sensitive to chemical toxicity and demonstrate rapid responses to environmental changes (Shruti et al., 2023). Recent studies suggest that nanoplastics may play a critical role in promoting antifungal resistance through several mechanisms. For instance, exposure to nanoplastics can trigger stress responses and adaptive mechanisms in fungi. In response to nanoplastic exposure, fungi may activate defense pathways, including oxidative stress responses and efflux pumps, which enhance their resistance to antifungal drugs. For example, Lactarius deliciosus exhibits oxidative stress in the presence of polystyrene, leading to increased secretion of organic acids and enhanced absorption of phosphorus and potassium, although growth is inhibited at high concentrations (Zhang and Gao, 2023). Moreover, exposure to nanoplastics has been shown to augment the secretion of extracellular enzymes in fungi, including β-glucosidase, glycine aminopeptidase, and phenol oxidase, thereby altering the community structure (Du et al., 2022). Similar observations in bacterial studies indicate that polystyrene exposure induces oxidative stress, leading to increased synthesis of glutathione and enhanced activity of the tricarboxylic acid (TCA) cycle, as well as of efflux pumps, which subsequently promote growth and resistance in Escherichia coli (Fang et al., 2023; Liu et al., 2023). Nanoplastics may also induce oxidative damage within fungal cells. For instance, low-density polystyrene has been shown to alter the membrane composition of Trichoderma harzianum, resulting in increased membrane permeability and enhanced activities of ROS, superoxide dismutase (SOD), and catalase (CAT) (Jasińska and Różalska, 2022). Recent findings from our research indicate that exposure to nanoplastics can induce ROS production in C. neoformans, disrupting normal cellular functions (unpublished data). Such oxidative damage may drive fungi to develop resistance through mutations or other adaptive changes that enhance their chances of survival. Moreover, fungal mitochondria play vital roles not only in cellular energy metabolism and oxidative stress responses but also in significantly influencing the activity and expression of drug efflux pumps (Black et al., 2021; Ma et al., 2025). Given that nanoplastics have been shown to induce mitochondrial damage in human cells (Lin et al., 2022), it is plausible to hypothesize that they could similarly affect mitochondrial function in fungi. Overall, the interaction of nanoplastics with fungi has the potential to significantly impact drug resistance (Figure 2). The multifaceted stress responses triggered by nanoplastics may not only enhance the ability of fungi to withstand antifungal agents but also promote the evolution of resistance mechanisms. These findings underscore the need for further research to elucidate the complexities of nanoplastic interactions and their implications for fungal pathogenicity and treatment strategies.
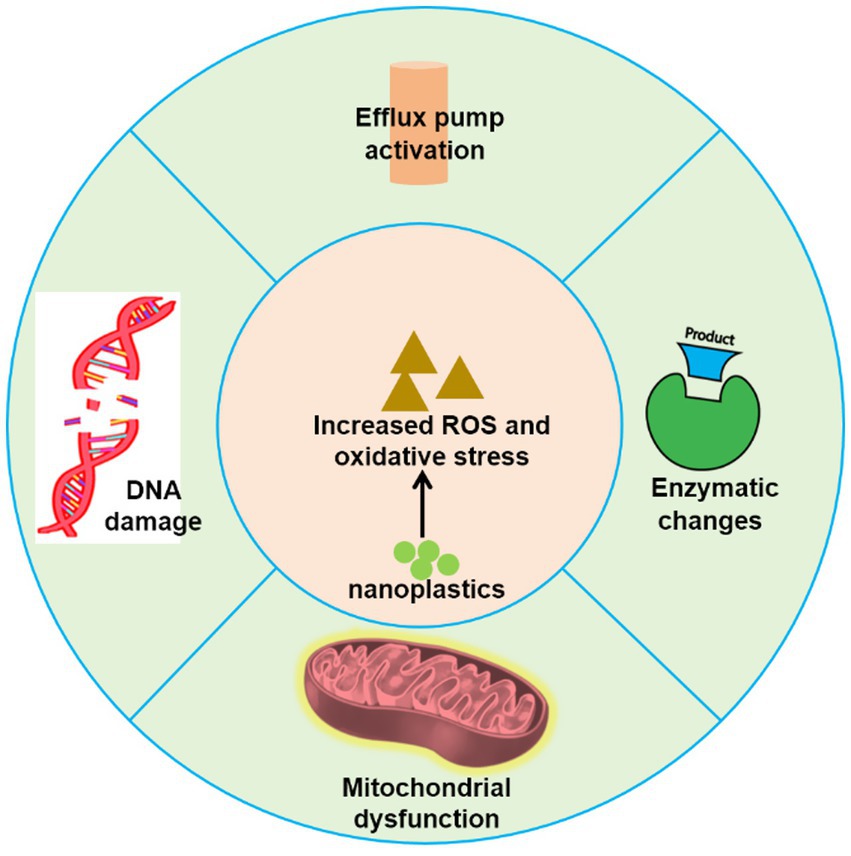
Figure 2. Schematic illustrating mechanisms of antifungal resistance potentially induced by nanoplastics.
3 Conclusions and future perspectives
The intricate interactions between nanoplastics and fungi present a dual-edged sword for environmental science and public health. As emerging pollutants, nanoplastics have demonstrated significant effects on fungal physiology, including alterations in metabolic pathways, physiological responses, and even virulence factors. The ability of certain fungal species to degrade plastics offers promising avenues for bioremediation, yet the presence of nanoplastics complicates these interactions by influencing fungal physiological functions and potentially enhancing antifungal resistance mechanisms. This review highlights the necessity for a deeper understanding of the multifaceted relationships between nanoplastics and fungi. The evidence indicates that while fungi have the ability to degrade plastic, exposure to these pollutants may concurrently promote adaptations that enhance their resistance to antifungal agents. This paradox emphasizes the urgency of investigating the mechanisms underlying these interactions, as they could have far-reaching implications for both ecological health and clinical outcomes in fungal infections. Future research should focus on several key areas to elucidate the complex dynamics of nanoplastic-fungi interactions. Firstly, studies should aim to identify the specific molecular pathways activated in fungi upon exposure to nanoplastics, particularly regarding oxidative stress responses and enzymatic adaptations. Recent advancements in image analysis demonstrate how end-to-end image analysis and data fusion can provide high throughput and objective phenotyping (Iqbal et al., 2020; Iqbal et al., 2025), and can be adapted to quantify subtle, exposure-dependent morphological shifts in hyphae, spores, and biofilms. Secondly, the ecological impact of these interactions on fungal communities in various environments, including terrestrial and aquatic ecosystems, warrants further exploration. Long-term studies are essential to assess the implications of chronic nanoplastic exposure on fungal diversity and function. Additionally, advancing our understanding of the link between nanoplastic exposure and antifungal resistance mechanisms is critical. Investigating the potential for genetic mutations and horizontal gene transfer in fungi exposed to nanoplastics could provide insights into the development of resistance traits. This knowledge is particularly vital given the rising incidence of drug-resistant fungal infections that pose substantial public health threats. In conclusion, while the potential of fungi in bioremediation strategies remains promising, the challenges posed by nanoplastics necessitate a comprehensive investigation into their effects on fungal physiology and ecology. By addressing these research gaps, we can develop more effective strategies for managing plastic pollution and mitigating the associated risks to human health and the environment. Continued interdisciplinary collaboration will be crucial in paving the way for innovative solutions to combat the dual challenges of plastic pollution and fungal diseases.
Author contributions
YM: Writing – original draft, Writing – review & editing. YZ: Writing – original draft, Writing – review & editing. DZ: Writing – original draft, Writing – review & editing. WB: Writing – review & editing. FW: Writing – review & editing. XZ: Writing – original draft, Writing – review & editing. PX: Writing – original draft, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was supported by the grants Natural Science Research of Jiangsu Higher Education Institutions of China (No. 24KJD430010), Natural Science Foundation of Jiangsu Province of China (No. BK20240948), Nantong Jiangsu Scientific Research Project of China (No. JC2023043), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_3537 and KYCX24_3538).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al Mousa, A. A., Hassane, A. M. A., Gomaa, A. E.-R. F., Aljuriss, J. A., Dahmash, N. D., and Abo-Dahab, N. F. (2022). Response-surface statistical optimization of submerged fermentation for pectinase and cellulase production by Mucor circinelloides and M. hiemalis. Fermentation 8:205. doi: 10.3390/fermentation8050205
Ameen, F., Moslem, M., Hadi, S., and Al-Sabri, A. E. (2015). Biodegradation of low density polyethylene (LDPE) by mangrove fungi from the red sea coast. Prog. Rubber Plastics Recycling Technol. 31, 125–143. doi: 10.1177/147776061503100204
Aranda, E. (2016). Promising approaches towards biotransformation of polycyclic aromatic hydrocarbons with Ascomycota fungi. Curr. Opin. Biotechnol. 38, 1–8. doi: 10.1016/j.copbio.2015.12.002
Arora, D. S., and Sandhu, D. K. (1984). Laccase production and wood degradation by Trametes hirsuta. Folia Microbiol. 29, 310–315. doi: 10.1007/BF02875963
Artham, T., and Doble, M. (2010). Biodegradation of physicochemically treated polycarbonate by fungi. Biomacromolecules 11, 20–28. doi: 10.1021/bm9008099
Asemoloye, M. D., Jonathan, S. G., and Ahmad, R. (2019). Synergistic plant-microbes interactions in the rhizosphere: a potential headway for the remediation of hydrocarbon polluted soils. Int. J. Phytoremediation 21, 71–83. doi: 10.1080/15226514.2018.1474437
Backa, S., Gierer, J., Reitberger, T., and Nilsson, T. (1993). Hydroxyl radical activity associated with the growth of white-rot fungi.
Bernhardt, R. (2006). Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 124, 128–145. doi: 10.1016/j.jbiotec.2006.01.026
Black, B., Lee, C., Horianopoulos, L. C., Jung, W. H., and Kronstad, J. W. (2021). Respiring to infect: emerging links between mitochondria, the electron transport chain, and fungal pathogenesis. PLoS Pathog. 17:e1009661. doi: 10.1371/journal.ppat.1009661
Bu, W., Yu, M., Ma, X., Shen, Z., Ruan, J., Qu, Y., et al. (2025). Gender-specific effects of prenatal polystyrene nanoparticle exposure on offspring lung development. Toxicol. Lett. 407, 1–16. doi: 10.1016/j.toxlet.2025.03.001
Case, N. T., Gurr, S. J., Fisher, M. C., Blehert, D. S., Boone, C., Casadevall, A., et al. (2025). Fungal impacts on earth’s ecosystems. Nature 638, 49–57. doi: 10.1038/s41586-024-08419-4
Chen, W., Lee, M.-K., Jefcoate, C., Kim, S.-C., Chen, F., and Yu, J.-H. (2014). Fungal cytochrome p450 monooxygenases: their distribution, structure, functions, family expansion, and evolutionary origin. Genome Biol. Evol. 6, 1620–1634. doi: 10.1093/gbe/evu132
Covino, S., Cvancarová, M., Muzikár, M., Svobodová, K., D'Annibale, A., Petruccioli, M., et al. (2010). An efficient PAH-degrading Lentinus (Panus) tigrinus strain: effect of inoculum formulation and pollutant bioavailability in solid matrices. J. Hazard. Mater. 183, 669–676. doi: 10.1016/j.jhazmat.2010.07.078
Cowan, A. R., Costanzo, C. M., Benham, R., Loveridge, E. J., and Moody, S. C. (2022). Fungal bioremediation of polyethylene: challenges and perspectives. J. Appl. Microbiol. 132, 78–89. doi: 10.1111/jam.15203
Cowger, W., Willis, K. A., Bullock, S., Conlon, K., Emmanuel, J., Erdle, L. M., et al. (2024). Global producer responsibility for plastic pollution. Sci. Adv. 10:eadj8275. doi: 10.1126/sciadv.adj8275
Dai, S., Ye, R., Huang, J., Wang, B., Xie, Z., Ou, X., et al. (2022). Distinct lipid membrane interaction and uptake of differentially charged nanoplastics in bacteria. J. Nanobiotechnol. 20:191. doi: 10.1186/s12951-022-01321-z
Dauda, W. P., Abraham, P., Glen, E., Adetunji, C. O., Ghazanfar, S., Ali, S., et al. (2022). Robust profiling of cytochrome P450s (P450ome) in notable Aspergillus spp. Life 12:451. doi: 10.3390/life12030451
Denning, D. W. (2024). Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 24, e428–e438. doi: 10.1016/S1473-3099(23)00692-8
Deshmukh, R., Khardenavis, A. A., and Purohit, H. J. (2016). Diverse metabolic capacities of fungi for bioremediation. Indian J. Microbiol. 56, 247–264. doi: 10.1007/s12088-016-0584-6
Dix, N. J., Webster, J., Dix, N. J., and Webster, J. (1995). Fungi of extreme environments. Fungal Ecol., 322–340. doi: 10.1007/978-94-011-0693-1_12
do Paço, A.M.S. (2024). Biotechnological tools for plastic waste remediation using marine Fungi. Universidade de Aveiro (Portugal).
Doddapaneni, H., Subramanian, V., Fu, B., and Cullen, D. (2013). A comparative genomic analysis of the oxidative enzymes potentially involved in lignin degradation by Agaricus bisporus. Fungal Genet. Biol. 55, 22–31. doi: 10.1016/j.fgb.2013.03.004
Du, J., Qv, W., Niu, Y., Qv, M., Jin, K., Xie, J., et al. (2022). Nanoplastic pollution inhibits stream leaf decomposition through modulating microbial metabolic activity and fungal community structure. J. Hazard. Mater. 424:127392. doi: 10.1016/j.jhazmat.2021.127392
Durairaj, P., Malla, S., Nadarajan, S. P., Lee, P.-G., Jung, E., Park, H. H., et al. (2015). Fungal cytochrome P450 monooxygenases of fusarium oxysporum for the synthesis of ω-hydroxy fatty acids in engineered Saccharomyces cerevisiae. Microb. Cell Factories 14, 1–16. doi: 10.1186/s12934-015-0228-2
Ekanayaka, A. H., Tibpromma, S., Dai, D., Xu, R., Suwannarach, N., Stephenson, S. L., et al. (2022). A review of the fungi that degrade plastic. J. Fungi 8:772. doi: 10.3390/jof8080772
El-Dash, H. A., Yousef, N. E., Aboelazm, A. A., and Awan, Z. A. (2023). Optimizing eco-friendly degradation of polyvinyl chloride (PVC) plastic using environmental strains of Malassezia species and Aspergillus fumigatus. Int. J. Mol. Sci. 24:15452. doi: 10.3390/ijms242015452
El-Morsy, E., Hassan, H., and Ahmed, E. (2017). Biodegradative activities of fungal isolates from plastic contaminated soils. Mycosphere 8, 1071–1087. doi: 10.5943/mycosphere/8/8/13
Espinosa-Valdemar, R. M., Turpin-Marion, S., Delfín-Alcalá, I., and Vázquez-Morillas, A. (2011). Disposable diapers biodegradation by the fungus Pleurotus ostreatus. Waste Manag. 31, 1683–1688. doi: 10.1016/j.wasman.2011.03.007
Fang, S., Huang, Y., Xiang, Z., Zeng, R., Zeng, S., and Liu, S. (2023). Polystyrene nanoplastics foster Escherichia coli O157:H7 growth and antibiotic resistance with a stimulating effect on metabolism. Environ. Sci. Nano 10, 1341–1351. doi: 10.1039/D2EN00982J
Fotopoulou, K. N., and Karapanagioti, H. K. (2019). “Degradation of various plastics in the environment.” The Handbook of Environmental Chemistry, (Eds.) Takada, H., Karapanagioti, H. K. 78:71–92. Springer, Cham. doi: 10.1007/698_2017_11
Gambarini, V., Pavlov, N., Young, P., Dawes, S., Auffret, A., Kingsbury, J. M., et al. (2025). Molecular mechanisms of plastic biodegradation by the fungus Clonostachys rosea. mBio, e00335–e00325. doi: 10.1128/mbio.00335-25
Gao, R., Liu, R., and Sun, C. (2022). A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene. J. Hazard. Mater. 431:128617. doi: 10.1016/j.jhazmat.2022.128617
Geyer, R., Jambeck, J. R., and Law, K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv. 3:e1700782. doi: 10.1126/sciadv.1700782
Gigault, J., El Hadri, H., Nguyen, B., Grassl, B., Rowenczyk, L., Tufenkji, N., et al. (2021). Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 16, 501–507. doi: 10.1038/s41565-021-00886-4
Gong, Z., Jin, L., Yu, X., Wang, B., Hu, S., Ruan, H., et al. (2023). Biodegradation of low density polyethylene by the fungus Cladosporium sp. recovered from a landfill site. J. Fungi 9:605. doi: 10.3390/jof9060605
Gow, N. A. R., and Lenardon, M. D. (2023). Architecture of the dynamic fungal cell wall. Nat. Rev. Microbiol. 21, 248–259. doi: 10.1038/s41579-022-00796-9
Hasan, I. F. (2018). Role of filamentous fungi to remove petroleum hydrocarbons from the environment. Microb. Action Hydrocarbons, 567–580. doi: 10.1007/978-981-13-1840-5_23
Hoseini, M., and Bond, T. (2022). Predicting the global environmental distribution of plastic polymers. Environ. Pollut. 300:118966. doi: 10.1016/j.envpol.2022.118966
Hüffer, T., and Hofmann, T. (2016). Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 214, 194–201. doi: 10.1016/j.envpol.2016.04.018
Ibrahim, S. S., Ionescu, D., and Grossart, H.-P. (2024). Tapping into fungal potential: biodegradation of plastic and rubber by potent fungi. Sci. Total Environ. 934:173188. doi: 10.1016/j.scitotenv.2024.173188
Iqbal, I., Mustafa, G., and Ma, J. (2020). Deep learning-based morphological classification of human sperm heads. Diagnostics 10:325. doi: 10.3390/diagnostics10050325
Iqbal, I., Ullah, I., Peng, T., Wang, W., and Ma, N. (2025). An end-to-end deep convolutional neural network-based data-driven fusion framework for identification of human induced pluripotent stem cell-derived endothelial cells in photomicrographs. Eng. Appl. Artif. Intell. 139:109573. doi: 10.1016/j.engappai.2024.109573
Janczak, K., Hrynkiewicz, K., Znajewska, Z., and Dąbrowska, G. (2018). Use of rhizosphere microorganisms in the biodegradation of PLA and PET polymers in compost soil. Int. Biodeterior. Biodegrad. 130, 65–75. doi: 10.1016/j.ibiod.2018.03.017
Jasińska, A., and Różalska, S. (2022). Microplastic-induced oxidative stress in metolachlor-degrading filamentous fungus Trichoderma harzianum. Int. J. Mol. Sci. 23:12978. doi: 10.3390/ijms232112978
Junaid, M., and Wang, J. (2021). Interaction of nanoplastics with extracellular polymeric substances (EPS) in the aquatic environment: a special reference to eco-corona formation and associated impacts. Water Res. 201:117319. doi: 10.1016/j.watres.2021.117319
Kelly, D. E., Kraševec, N., Mullins, J., and Nelson, D. R. (2009). The CYPome (cytochrome P450 complement) of Aspergillus nidulans. Fungal Genet. Biol. 46, S53–S61. doi: 10.1016/j.fgb.2008.08.010
Kersten, P., and Cullen, D. (2007). Extracellular oxidative systems of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Fungal Genet. Biol. 44, 77–87. doi: 10.1016/j.fgb.2006.07.007
Khan, S., Ali, S. A., and Ali, A. S. (2023). Biodegradation of low density polyethylene (LDPE) by mesophilic fungus ‘Penicillium citrinum’ isolated from soils of plastic waste dump yard, Bhopal, India. Environ. Technol. 44, 2300–2314. doi: 10.1080/09593330.2022.2027025
Kobayashi, N., Wada, N., Yokoyama, H., Tanaka, Y., Suzuki, T., Habu, N., et al. (2023). Extracellular enzymes secreted in the mycelial block of Lentinula edodes during hyphal growth. AMB Express 13:36. doi: 10.1186/s13568-023-01547-6
Kong, L., Li, S., Fu, Y., Cai, Q., Zhai, Z., Liang, J., et al. (2025). Microplastics/nanoplastics contribute to aging and age-related diseases: mitochondrial dysfunction as a crucial role. Food Chem. Toxicol. 199:115355. doi: 10.1016/j.fct.2025.115355
Kriegl, L., Egger, M., Boyer, J., Hoenigl, M., and Krause, R. (2024). New treatment options for critically important WHO fungal priority pathogens. Clin. Microbiol. Infect. doi: 10.1016/j.cmi.2024.03.006
Lawton, L. A., and Robertson, P. K. (1999). Physico-chemical treatment methods for the removal of microcystins (cyanobacterial hepatotoxins) from potable waters. Chem. Soc. Rev. 28, 217–224. doi: 10.1039/a805416i
Lin, S., Zhang, H., Wang, C., Su, X.-L., Song, Y., Wu, P., et al. (2022). Metabolomics reveal nanoplastic-induced mitochondrial damage in human liver and lung cells. Environ. Sci. Technol. 56, 12483–12493. doi: 10.1021/acs.est.2c03980
Liu, S., Junaid, M., Liao, H., Liu, X., Wu, Y., and Wang, J. (2022). Eco-corona formation and associated ecotoxicological impacts of nanoplastics in the environment. Sci. Total Environ. 836:155703. doi: 10.1016/j.scitotenv.2022.155703
Liu, J., Xin, K., Zhang, T., Wen, Y., Li, D., Wei, R., et al. (2024). Identification and characterization of a fungal cutinase-like enzyme CpCut1 from Cladosporium sp. P7 for polyurethane degradation. Appl. Environ. Microbiol. 90:e01477-01423. doi: 10.1128/aem.01477-23
Liu, X., Xu, R., Wu, H., Xu, K., Zhang, W., Wang, Z., et al. (2023). Nanoplastics promote the dissemination of antibiotic resistance through conjugative gene transfer: implications from oxidative stress and gene expression. Environ. Sci. Nano 10, 1329–1340. doi: 10.1039/D2EN01036D
Ma, Y., Zhou, Y., Jia, T., Zhuang, Z., Xue, P., and Yang, L. (2025). Deciphering the role of mitochondria in human fungal drug resistance. Mycology, 1–14.
Maeda, H., Yamagata, Y., Abe, K., Hasegawa, F., Machida, M., Ishioka, R., et al. (2005). Purification and characterization of a biodegradable plastic-degrading enzyme from Aspergillus oryzae. Appl. Microbiol. Biotechnol. 67, 778–788. doi: 10.1007/s00253-004-1853-6
Mafla-Endara, P. M., Meklesh, V., Beech, J. P., Ohlsson, P., Pucetaite, M., and Hammer, E. C. (2023). Exposure to polystyrene nanoplastics reduces bacterial and fungal biomass in microfabricated soil models. Sci. Total Environ. 904:166503. doi: 10.1016/j.scitotenv.2023.166503
Maity, S., Banerjee, S., Biswas, C., Guchhait, R., Chatterjee, A., and Pramanick, K. (2021). Functional interplay between plastic polymers and microbes: a comprehensive review. Biodegradation 32, 487–510. doi: 10.1007/s10532-021-09954-x
Mancera-López, M., Esparza-García, F., Chávez-Gómez, B., Rodríguez-Vázquez, R., Saucedo-Castañeda, G., and Barrera-Cortés, J. (2008). Bioremediation of an aged hydrocarbon-contaminated soil by a combined system of biostimulation–bioaugmentation with filamentous fungi. Int. Biodeterior. Biodegrad. 61, 151–160. doi: 10.1016/j.ibiod.2007.05.012
Marchut-Mikolajczyk, O., Kwapisz, E., Wieczorek, D., and Antczak, T. (2015). Biodegradation of diesel oil hydrocarbons enhanced with Mucor circinelloides enzyme preparation. Int. Biodeterior. Biodegrad. 104, 142–148. doi: 10.1016/j.ibiod.2015.05.008
Mikolajczyk, A., Gajewicz, A., Rasulev, B., Schaeublin, N., Maurer-Gardner, E., Hussain, S., et al. (2015). Zeta potential for metal oxide nanoparticles: a predictive model developed by a nano-quantitative structure–property relationship approach. Chem. Mater. 27, 2400–2407. doi: 10.1021/cm504406a
Mir-Tutusaus, J. A., Baccar, R., Caminal, G., and Sarrà, M. (2018). Can white-rot fungi be a real wastewater treatment alternative for organic micropollutants removal? A review. Water Res. 138, 137–151. doi: 10.1016/j.watres.2018.02.056
Mitrano, D. M., Wick, P., and Nowack, B. (2021). Placing nanoplastics in the context of global plastic pollution. Nat. Nanotechnol. 16, 491–500. doi: 10.1038/s41565-021-00888-2
Moktali, V., Park, J., Fedorova-Abrams, N. D., Park, B., Choi, J., Lee, Y.-H., et al. (2012). Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes. BMC Genomics 13, 1–13. doi: 10.1186/1471-2164-13-525
Momin, M., and Webb, G. (2021). The environmental effects on virulence factors and the antifungal susceptibility of Cryptococcus neoformans. Int. J. Mol. Sci. 22:6302. doi: 10.3390/ijms22126302
Munir, E., Harefa, R., Priyani, N., and Suryanto, D. (2018). Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. IOP Conf. Earth Environ. Sci. 126:012145. doi: 10.1088/1755-1315/126/1/012145
Nath, J., Dror, I., and Berkowitz, B. (2020). Effect of nanoplastics on the transport of platinum-based pharmaceuticals in water-saturated natural soil and their effect on a soil microbial community. Environ. Sci. Nano 7, 3178–3188. doi: 10.1039/D0EN00651C
Odigbo, C., Adenipekun, C., Oladosu, I., and Ogunjobi, A. (2023). Polyethylene terephthalate (PET) biodegradation by Pleurotus ostreatus and Pleurotus pulmonarius. Environ. Monit. Assess. 195:585. doi: 10.1007/s10661-023-11153-5
Ogunmolu, F. E., Kaur, I., Gupta, M., Bashir, Z., Pasari, N., and Yazdani, S. S. (2015). Proteomics insights into the biomass hydrolysis potentials of a hypercellulolytic fungus Penicillium funiculosum. J. Proteome Res. 14, 4342–4358. doi: 10.1021/acs.jproteome.5b00542
Ojha, N., Pradhan, N., Singh, S., Barla, A., Shrivastava, A., Khatua, P., et al. (2017). Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 7:39515. doi: 10.1038/srep39515
Okal, E. J., Heng, G., Magige, E. A., Khan, S., Wu, S., Ge, Z., et al. (2023). Insights into the mechanisms involved in the fungal degradation of plastics. Ecotoxicol. Environ. Saf. 262:115202. doi: 10.1016/j.ecoenv.2023.115202
Orasch, T., Gangapurwala, G., Vollrath, A., González, K., Alex, J., De San Luis, A., et al. (2023). Polymer-based particles against pathogenic fungi: a non-uptake delivery of compounds. Biomater Adv 146:213300. doi: 10.1016/j.bioadv.2023.213300
Ramos, M. M., Dos, S. M. E., da, S. S. I., Lima, A. L., de Oliveira, F. R., de Freitas, C. M., et al. (2020). Silver nanoparticle from whole cells of the fungi Trichoderma spp. isolated from Brazilian Amazon 42, 833–843. doi: 10.1007/s10529-020-02819-y
Ren, X., Wang, L., Tang, J., Sun, H., and Giesy, J. P. (2022). Combined effects of degradable film fragments and micro/nanoplastics on growth of wheat seedling and rhizosphere microbes. Environ. Pollut. 294:118516. doi: 10.1016/j.envpol.2021.118516
Rodrigues, M. O., Abrantes, N., Gonçalves, F. J. M., Nogueira, H., Marques, J. C., and Gonçalves, A. M. M. (2019). Impacts of plastic products used in daily life on the environment and human health: what is known? Environ. Toxicol. Pharmacol. 72:103239. doi: 10.1016/j.etap.2019.103239
Ryu, H. W., Kim, D. H., Jae, J., Lam, S. S., Park, E. D., and Park, Y. K. (2020). Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour. Technol. 310:123473. doi: 10.1016/j.biortech.2020.123473
Safdar, A., Ismail, F., and Imran, M. (2024). Biodegradation of synthetic plastics by the extracellular lipase of Aspergillus niger. Environ. Adv. 17:100563. doi: 10.1016/j.envadv.2024.100563
Sánchez, C. (2020). Fungal potential for the degradation of petroleum-based polymers: an overview of macro-and microplastics biodegradation. Biotechnol. Adv. 40:107501. doi: 10.1016/j.biotechadv.2019.107501
Sarkhel, R., Sengupta, S., Das, P., and Bhowal, A. (2020). Comparative biodegradation study of polymer from plastic bottle waste using novel isolated bacteria and fungi from marine source. J. Polym. Res. 27, 1–8. doi: 10.1007/s10965-019-1973-4
Satti, S. M., Shah, A. A., Auras, R., and Marsh, T. L. (2017). Isolation and characterization of bacteria capable of degrading poly (lactic acid) at ambient temperature. Polym. Degrad. Stab. 144, 392–400. doi: 10.1016/j.polymdegradstab.2017.08.023
Shruti, V. C., Kutralam-Muniasamy, G., and Pérez-Guevara, F. (2023). Do microbial decomposers find micro- and nanoplastics to be harmful stressors in the aquatic environment? A systematic review of in vitro toxicological research. Sci. Total Environ. 903:166561. doi: 10.1016/j.scitotenv.2023.166561
Sowmya, H. V., Ramalingappa,, Krishnappa, M., and Thippeswamy, B. (2015). Degradation of polyethylene by Penicillium simplicissimum isolated from local dumpsite of Shivamogga district. Environ. Dev. Sustain. 17, 731–745. doi: 10.1007/s10668-014-9571-4
Sui, A., Yao, C., Chen, Y., Li, Y., Yu, S., Qu, J., et al. (2023). Polystyrene nanoplastics inhibit StAR expression by activating HIF-1α via ERK1/2 MAPK and AKT pathways in TM3 Leydig cells and testicular tissues of mice. Food Chem. Toxicol. 173:113634. doi: 10.1016/j.fct.2023.113634
Sun, J., Peng, S., Yang, Q., Yang, J., Dai, Y., and Xing, L. (2025). Microplastics/nanoplastics and neurological health: an overview of neurological defects and mechanisms. Toxicology 511:154030. doi: 10.1016/j.tox.2024.154030
Temporiti, M. E. E., Nicola, L., Nielsen, E., and Tosi, S. (2022). Fungal enzymes involved in plastics biodegradation. Microorganisms 10:1180. doi: 10.3390/microorganisms10061180
Vaksmaa, A., Polerecky, L., Dombrowski, N., Kienhuis, M. V., Posthuma, I., Gerritse, J., et al. (2023). Polyethylene degradation and assimilation by the marine yeast Rhodotorula mucilaginosa. ISME Commun. 3:68. doi: 10.1038/s43705-023-00267-z
Van Beilen, J. B., and Funhoff, E. G. (2007). Alkane hydroxylases involved in microbial alkane degradation. Appl. Microbiol. Biotechnol. 74, 13–21. doi: 10.1007/s00253-006-0748-0
Viel, T., Manfra, L., Zupo, V., Libralato, G., Cocca, M., and Costantini, M. (2023). Biodegradation of plastics induced by marine organisms: future perspectives for bioremediation approaches. Polymers 15:2673. doi: 10.3390/polym15122673
Vršanská, M., Voběrková, S., Jiménez Jiménez, A. M., Strmiska, V., and Adam, V. (2017). Preparation and optimisation of cross-linked enzyme aggregates using native isolate white rot fungi Trametes versicolor and Fomes fomentarius for the decolourisation of synthetic dyes. Int. J. Environ. Res. Public Health 15:23. doi: 10.3390/ijerph15010023
Wang, R., Li, X., Li, J., Dai, W., and Luan, Y. (2023). Bacterial interactions with nanoplastics and the environmental effects they cause. Fermentation 9:939. doi: 10.3390/fermentation9110939
Wang, F., Zhang, M., Sha, W., and Wang, Y. (2020). Sorption behavior and mechanisms of organic contaminants to nano and microplastics. Molecules 25:1827. doi: 10.3390/molecules25081827
Wilcox, C., Van Sebille, E., and Hardesty, B. D. (2015). Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proc. Natl. Acad. Sci. USA 112, 11899–11904. doi: 10.1073/pnas.1502108112
Wolski, E. A., Durruty, I., Haure, P. M., and González, J. F. (2012). Penicillium chrysogenum: phenol degradation abilities and kinetic model. Water Air Soil Pollut. 223, 2323–2332. doi: 10.1007/s11270-011-1026-z
Yang, W.-K., Gong, Z., Wang, B.-T., Hu, S., Zhuo, Y., Jin, C.-Z., et al. (2024). Biodegradation of low-density polyethylene by mixed fungi composed of Alternaria sp. and Trametes sp. isolated from landfill sites. BMC Microbiol. 24:321. doi: 10.1186/s12866-024-03477-0
Young, D., Rice, J., Martin, R., Lindquist, E., Lipzen, A., Grigoriev, I., et al. (2015). Degradation of bunker C fuel oil by white-rot fungi in sawdust cultures suggests potential applications in bioremediation. PLoS One 10:e0130381. doi: 10.1371/journal.pone.0130381
Zaragoza, O., Chrisman, C. J., Castelli, M. V., Frases, S., Cuenca-Estrella, M., Rodríguez-Tudela, J. L., et al. (2008). Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 10, 2043–2057. doi: 10.1111/j.1462-5822.2008.01186.x
Zavarzina, A. G., Lisov, A. V., and Leontievsky, A. (2018). The role of ligninolytic enzymes laccase and a versatile peroxidase of the white-rot fungus Lentinus tigrinus in biotransformation of soil humic matter: comparative in vivo study. J. Geophys. Res. Biogeosci. 123, 2727–2742. doi: 10.1029/2017JG004309
Zhang, L., and Gao, B. (2023). Physiological response of ectomycorrhizal fungi (Lactarius delicious) to microplastics stress. Glob. Nest J. 25, 115–124. doi: 10.30955/gnj.004575
Zhang, K., Hamidian, A. H., Tubić, A., Zhang, Y., Fang, J. K., Wu, C., et al. (2021). Understanding plastic degradation and microplastic formation in the environment: a review. Environ. Pollut. 274:116554. doi: 10.1016/j.envpol.2021.116554
Keywords: nanoplastics, fungi, pathogenicity, antifungal resistance, plastic degradation
Citation: Ma Y, Zhou Y, Zheng D, Bu W, Wang F, Zhao X and Xue P (2025) Nanoplastics and fungi: exploring dual roles in degradation and pathogenicity. Front. Microbiol. 16:1679160. doi: 10.3389/fmicb.2025.1679160
Edited by:
Sikandar I. Mulla, REVA University, IndiaReviewed by:
Imran Iqbal, Helmholtz Association of German Research Centres (HZ), GermanyCopyright © 2025 Ma, Zhou, Zheng, Bu, Wang, Zhao and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Ma, bXl5Y3NkQG50dS5lZHUuY24=; Xinyuan Zhao, emhhb3hpbnl1YW5AbnR1LmVkdS5jbg==; Peng Xue, cGVuZ3h1ZUBudHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yuanyuan Ma*†
Yuanyuan Ma*† Fengxu Wang
Fengxu Wang Xinyuan Zhao
Xinyuan Zhao Peng Xue
Peng Xue