- 1College of Horticulture, Ludong University, Yantai, Shandong Province, China
- 2Key Laboratory of Coastal Biology and Biological Resources Utilization, Yantai Institute of Coastal Zone Research Chinese Academy of Sciences, Yantai, China
Marine endophytes are prolific sources of structurally diverse secondary metabolites with significant pharmaceutical potential, including anticancer, antimicrobial, and antioxidant agents. However, their commercial utilization is hindered by genomic instability in axenic cultures and inconsistent metabolite yields. While current studies focus on symbiotic interactions and compound discover, critical gaps persist in harnessing their biosynthetic capabilities. This review synthesizes knowledge on marine fungal metabolites and proposes a paradigm shift toward resource-driven research. It addresses strain improvement limitations and suggests strategies like mutagenesis, protoplast fusion, and metabolic engineering to bolster production stability and efficiency. The paper also discusses biological process optimization, including fermentation tuning, inducer and precursor addition, and adsorbent use, to enhance natural product synthesis. By identifying these research gaps and proposing a strategic roadmap, the review advances the stable and scalable production of bioactive metabolites, unlocking the commercial and therapeutic potential of marine endophytic fungi.
1 Introduction
Marine endophytes, which live within the tissues of their hosts, are ecologically significant in the oceanic ecosystem. They engage in a symbiotic relationship with their hosts, influencing growth and evolution through complex signal transduction pathways and providing protective substances that enhance the host’s survival value (Handayani et al., 2019; Rodriguez et al., 2009; Dastogeer et al., 2018). Building upon the xenohormesis hypothesis—which proposes that heterotrophs sense stress-induced chemical cues from other species to mount preemptive defenses—marine endophytes may utilize analogous mechanisms to perceive host-derived stress signals. This signaling interplay could trigger adaptive responses in endophytes, including the production of bioactive metabolites that synergistically enhance host defense (Howitz and Sinclair, 2008). Analogous to the biosynthesis of mycosporine-like amino acids (MAAs) in cyanobacteria—stress-induced molecules generated via conserved biosynthetic gene clusters that accumulate in marine consumers—metabolite induction in marine endophytes likely originates from fungal stress-responsive pathways (Jain et al., 2017). These pathways may function independently of host metabolite replication, consistent with observations that marine endophytic fungi represent a rich source of structurally unique bioactive compounds. Indeed, marine endophytic fungi have been demonstrated to be a rich source of biologically active natural products with unique structures and potent medicinal properties (Strobel and Daisy, 2003; Bugni and Ireland, 2004).
Marine endophytic fungi are known to produce a plethora of bioactive secondary metabolites, such as steroids, alkaloids, terpenoids, and peptides, many of which possess biological activities including anti-inflammatory, antioxidant, antimicrobial, and antitumor properties (Mohamed El-Bondkly et al., 2020; El-Bondkly et al., 2021; Santos et al., 2019; Tan and Zou, 2001). These include potential anticancer drugs, antimicrobial agents, antifungal compounds, antiviral substances, and more (Mm et al., 2015; El-Gendy et al., 2016; Strobel, 2018; El-Gendy et al., 2018; El-Gendy et al., 2008a; El-Gendy et al., 2000; El-Gendy et al., 2008b; El-Bondkly et al., 2012; El-Gendy and El-Bondkly, 2010; El-Gendy and El-Bondkly, 2011; El-Gendy et al., 2017). The prospect of utilizing endophytic fungi for the sustainable production of life-saving drugs is highly promising. Despite the identification of a multitude of bioactive molecules from marine endophytic fungi over the past two decades, the commercial exploitation of these organisms as a source of biologically active secondary metabolites has yet to see substantial breakthroughs. The primary constraint on the commercialization is believed to be the reduction in product yield following the subculturing of endophytic fungi under sterile conditions, which may be due to the loss of biosynthetic pathways or changes in regulatory mechanisms (Sandrawati et al., 2020; El-Bondkly and El-Gendy, 2010).
This review synthesizes literature evidence supporting the presence of host-independent biosynthetic machinery within endophytic fungi. It then explores the spectrum of marine endophytes and their secondary metabolites and highlights the need for a deeper understanding of the intricate interactions between marine endophytic fungi and their hosts. Advances in genetic engineering, such as CRISPR-Cas9 technology, offer new avenues for strain improvement, potentially enhancing the production of desired metabolites (Bary, 1866; Hardoim et al., 2015; Wilson, 1995). Furthermore, cutting-edge fermentation optimization techniques, including systems biology approaches and synthetic biology, are discussed to create an optimal culture environment for the sustainable and high-yield production of valuable secondary metabolites (Hallmann et al., 1997; Petrini, 1991; Muralikrishnan, 2013). By integrating these strategies, the review aims to provide a roadmap for harnessing the full potential of marine endophytic fungi in the biotechnological and pharmaceutical industries.
2 Definition, status and diversity of marine endophytic fungi
Endophytes, first identified by Bary (1866), are defined as organisms that can colonize the interior of plants without causing harm, a definition that has evolved over time (Hardoim et al., 2015; Wilson, 1995; Hallmann et al., 1997; Petrini, 1991) definition encompasses a broad range of organisms, including bacteria, fungi, mycoplasmas, and archaea (Muralikrishnan, 2013; Zhao et al., 2011; Stone et al., 2000; Hollants et al., 2011). Endophytic fungi are particularly notable for their potential to produce a diverse array of bioactive compounds. Estimates suggest that there may be over a million species of endophytic fungi, with only a fraction described (Fau et al., 1997; Dreyfuss and Chapela, 1994). These fungi are found in a variety of marine organisms, from plants to invertebrates and vertebrates (Sandrawati et al., 2020; Wu and Morris, 1973), and they play crucial roles in promoting growth, enhancing disease resistance, and improving environmental stress tolerance in their hosts (Cheng et al., 2020). The symbiotic relationship between endophytic fungi and their hosts often results in the production of secondary metabolites with potential applications in medicine, agriculture, and industry (Cheng et al., 2020; Strobel, 2002).
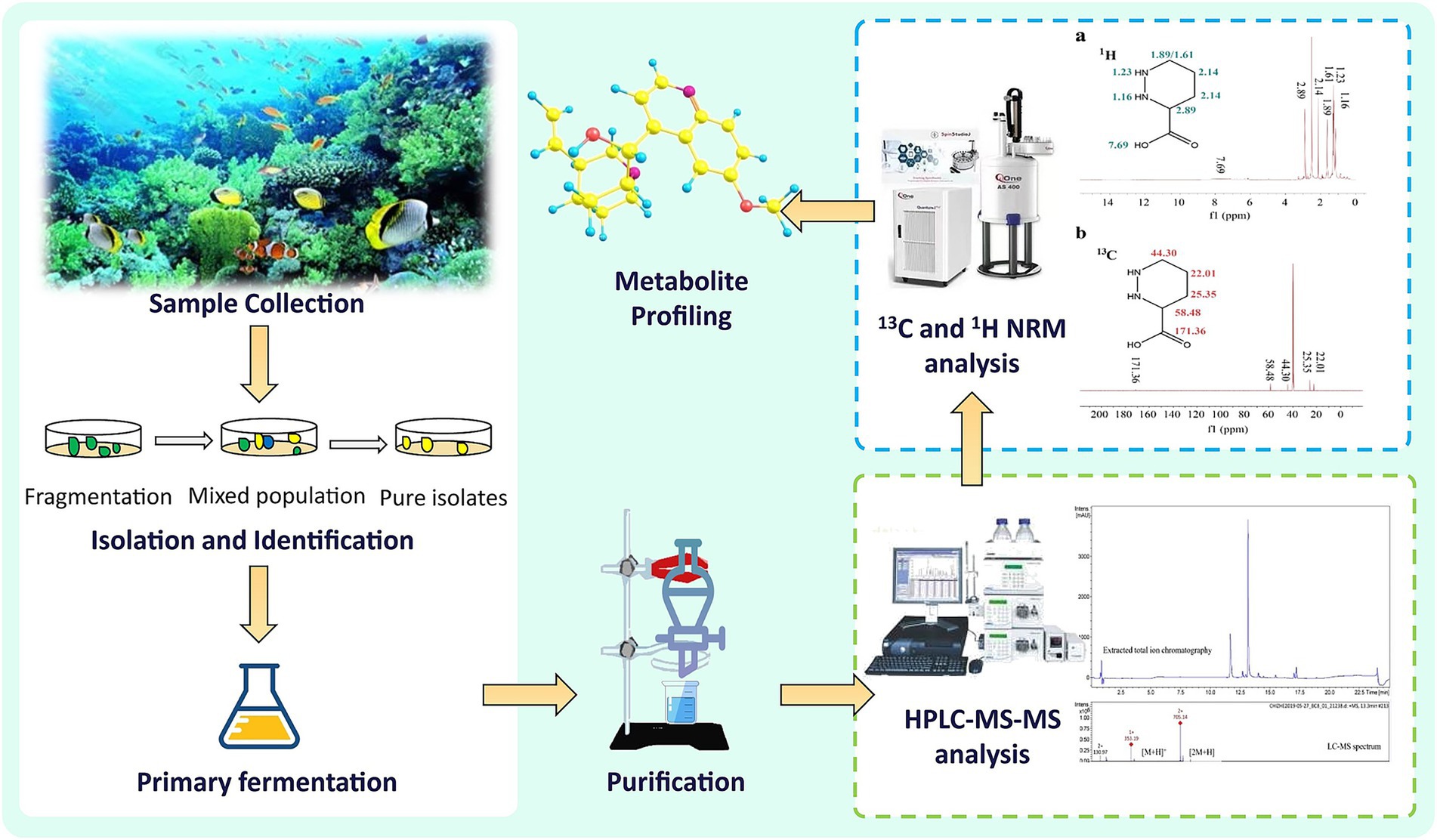
The identification of marine endophytic fungi has been advanced by molecular methods, which overcome the limitations of traditional culture methods (El-Gendy et al., 2008a; El-Gendy et al., 2010; Song et al., 2021; Fadiji and Babalola, 2020; Higgins et al., 2007). Endophytic fungi exhibit a range of host specificities, from narrow to broad, and their composition is influenced by factors such as geography and host age (Gao et al., 2018; González-Menéndez et al., 2014). Gao et al. (2018) found that even within the same geographical location, different sponge species harbor distinct endophytic fungal communities, highlighting the specificity and diversity of these associations. Marine endophytic fungi are a rich source of bioactive compounds, with marine algae and corals being particularly prolific sources (El-Demerdash et al., 2020; Couttolenc et al., 2015). These fungi produce compounds with anticancer, antioxidant, antimicrobial, antiviral, and other properties (El-Bondkly et al., 2021; Burragoni and Jeon, 2021; Kamat et al., 2020). For instance, the endophytic algal fungus Paecilomyces variotii produces indole derivatives with cytotoxic effects on cancer cell lines, while the red algal endophytic fungus Microsporum sp. produces compounds that induce apoptosis in HeLa cells. The potential of marine endophytic fungi as a source of novel bioactive compounds is vast and largely unexplored (Drake et al., 2018). Figure 1 lists marine endophyte host organisms and their associated bioactive compound categories. As research progresses, these fungi are poised to become increasingly significant in the development of new pharmaceuticals and agricultural products (El-Demerdash et al., 2020; Couttolenc et al., 2015). Their unique ecological niches, characterized by conditions such as high salinity and pressure, drive the production of specialized metabolites with potential for novel pharmaceutical applications (Sharma et al., 2020; Tidke et al., 2019).
Figure 1. Marine endophyte host organisms and their associated bioactive compound categories. This figure systematically categorizes the major marine host organisms (Corals, Mangrove, Algae, Sponge) and their corresponding bioactive compound classes (Peptide, Terpenoid, Flavonoid, Alkaloid, Steroid, Xanthone, Benzopyranone, Polyketide) produced by symbiotic endophytic fungi.
3 High value compounds found in marine endophyte fungi
Marine fungal endophytes are known to establish symbiotic relationships with marine organisms such as sponges, corals, algae, and mangroves, producing a variety of bioactive metabolites with potential applications in agriculture, pharmaceuticals, food, and cosmetics (Cheng et al., 2020; Gao et al., 2018; Gouda et al., 2016). These metabolites, which include ayamycin, benzopyrone derivatives, and iso-coumarin derivatives, are often the basis for the development of drugs to treat various diseases (Cheng et al., 2020; Gao et al., 2018; Gouda et al., 2016). The secondary metabolites produced by marine endophytic fungi are diverse, encompassing alkaloids, benzopyranones, chinones, flavonoids, phenolic acids, quinones, steroids, saponins, tannins, terpenoids, tetra ketones, xanthones, and more (El-Gendy et al., 2016; Strobel, 2018; El-Gendy et al., 2008a; El-Gendy et al., 2000; El-Gendy et al., 2008b; El-Gendy et al., 2016; El-Gendy et al., 2003). These compounds are not only chemically diverse but also biologically active, making them valuable for various industries.
The symbiotic relationship between endophytic fungi and their hosts is mutually beneficial, with the fungi obtaining nutrients while also enhancing the host’s environmental adaptability through the production of secondary metabolites (Bamisile et al., 2018). For instance, fungal endophytes from algae promote algal growth and are isolated through careful culturing and surface disinfection to remove epiphytes (Wu and Morris, 1973; Teixeira et al., 2019; Zhang et al., 2016). The metabolites produced by these associations are often peptides, polyketones, lactones, alkaloids, and terpenes, which are listed in Table 1. Sponges, as primitive metazoans, have been a source of a wide array of secondary metabolites and are considered a rich source of new drug candidates (Love et al., 2009; Faulkner, 2000). The fungi within sponge tissues, which can constitute a significant portion of the biomass, are believed to be the true producers of some sponge natural products (Cheng et al., 2020; Friedrich et al., 2001). Researchers have identified a range of compounds from sponge-derived endophytic fungi, including alkaloids, terpenoids, amino acids, nucleosides, cyclic peptides, polyethers, macrolides, peroxides, polyenes, polyalkynes, and steroids, many of which exhibit antiangiogenic, antimicrobial, antiparasitic, antitumor, antiviral, hemolytic, and cytotoxic activities (Elsebai et al., 2021; Sun et al., 2017). Corals, a class of marine invertebrates, is also a significant source of medicinal value, particularly due to the metabolic products of the symbiotic microorganisms (El-Demerdash et al., 2020; Liu et al., 2019). The study of the secondary metabolites of coral-associated fungi is an important field, with recent research focusing on their roles in antitumor, antibacterial, antifouling, and osteoclast differentiation inhibition (El-Demerdash et al., 2020; Liu et al., 2019). Galkiewicz’s work marks the first report of fungi extracted from deep-sea corals, providing insight into the microbial community’s constituent members and their potential functions (Galkiewicz et al., 2012). Mangrove ecosystems, characterized by their unique saline environment and rich mineral resources, are home to a diverse array of endophytic fungi that form the second largest group of marine microorganisms (Glaser and Mayer, 2009; Xing and Guo, 2011). The endophytic fungi in mangroves, including Aspergillus, Penicillium, Trichoderma, Pestalotiopsis, and Streptomyces, produce a wide range of metabolites such as coumarin, chromone, terpenoids, alkaloids, peptides, quinones, and esters (Deshmukh et al., 2018; Ananda and Sridhar, 2002). These compounds represent a vast natural pharmacy with novel structures and significant biological activities (Deshmukh et al., 2018; Xin et al., 2013; Li et al., 2010).
4 Using endophytic fungi as production platforms for marine natural products
The necessity of employing marine endophytic fungi as production platforms for secondary metabolites is underscored by several critical factors. Firstly, the natural abundance of marine organisms that produce valuable secondary metabolites is often insufficient for large-scale pharmaceutical applications, particularly for species such as sponges and soft corals. The limited biomass of these organisms poses a significant constraint for the sustainable extraction of bioactive compounds in quantities sufficient for drug development and commercial production (El-Bondkly and El-Gendy, 2010; El-Bondkly and El-Gendy, 2012).
Marine endophytic fungi, which coexist with marine flora and fauna in a symbiotic relationship, have demonstrated the capacity to evolve unique biosynthetic pathways. This evolutionary adaptation suggests that these fungi may be the actual producers of the secondary metabolites traditionally attributed to their host organisms (Cheng et al., 2020; Gao et al., 2018). The ability of marine endophytic fungi to synthesize a range of bioactive compounds, including those with antibacterial, antifungal, and anticancer properties, positions them as promising candidates for the production platforms of these valuable metabolites (Gouda et al., 2016; Hyde, 2019).
Numerous valuable compounds have been isolated from endophytic fungi associated with marine organisms. These include antibacterial agents such as benzopyrone and isocoumarin derivatives (El-Gendy et al., 2008a; El-Gendy et al., 2000; El-Gendy et al., 2008b; El-Gendy and El-Bondkly, 2010), antifungal compounds like mycopane and sardamycin (El-Bondkly et al., 2012; El-Gendy and El-Bondkly, 2010), and anticancer agents including lovastatin (Nurunnabi et al., 2020; Sopalun and Iamtham, 2020). Additionally, bioactive compounds such as antifungal and cytotoxic polyoxygenated steroids (Penicisteroids A and B), anthraquinone, cyclopentanone, and naphthoquinone derivatives have been isolated from algae endophytes (Gao et al., 2018). Furthermore, isobenzofuranone derivatives, marilones A-C, stachylines A-D, and marilines A-C with antioxidant properties have been extracted from algicolous fungi and sponge-derived fungi (Almeida et al., 2011a; Almeida et al., 2011b; Kamat et al., 2020).
Despite the potential of endophytic fungi to produce high-value pharmaceuticals, commercial production of these fungi for drug synthesis has not yet been realized. The primary obstacle to commercialization is the reduction in target product yield following the subculturing of endophytes, which some researchers attribute to the loss of biosynthetic capabilities in vitro (Heinig et al., 2013). However, studies such as those by Yang et al., who conducted whole-genome sequencing and multiple sequence alignment of the paclitaxel-producing endophyte Penicillium aurantiogriseum NRRL 62431, have refuted this notion (Yang et al., 2014). Genomic analysis by Yang et al. revealed that Penicillium aurantiogriseum NRRL 62431 possesses evolutionarily distinct biosynthetic pathways for paclitaxel synthesis, with key enzymes (e.g., taxadiene synthase homologs) sharing <30% amino acid identity to those in Taxus hosts. This supports the capacity for autonomous production of secondary metabolites in axenic culture across multiple generations, though natural symbiotic metabolite exchange remains possible.
5 Strategies for enhancing secondary metabolite production in marine endophytic fungi
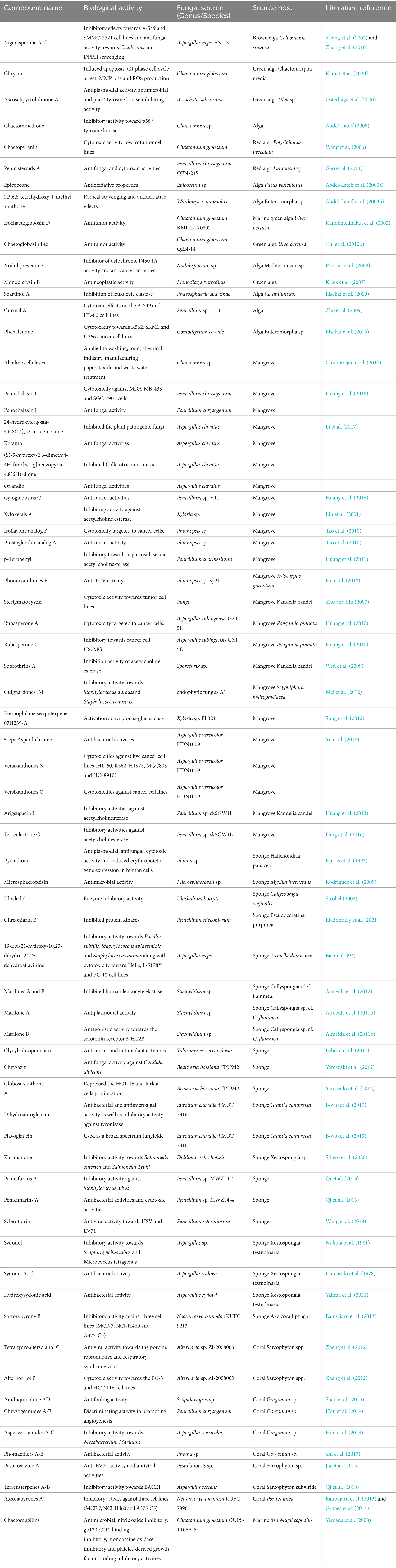
Despite their promise as a source of natural therapeutics, marine endophytic fungi produce secondary metabolites at levels that are typically too low for commercial viability. To overcome this, strain enhancement techniques are crucial for increasing the yield and efficiency of metabolite production to a scale suitable for industrial applications. Advanced strains can then be subjected to fermentation medium optimization, which is a key to further boosting the output and productivity of these valuable compounds. Figure 2 outlines a strategic approach for the industrial-scale production of secondary metabolites derived from marine endophytic fungi.
5.1 Isolation and culture of marine endophytic fungi
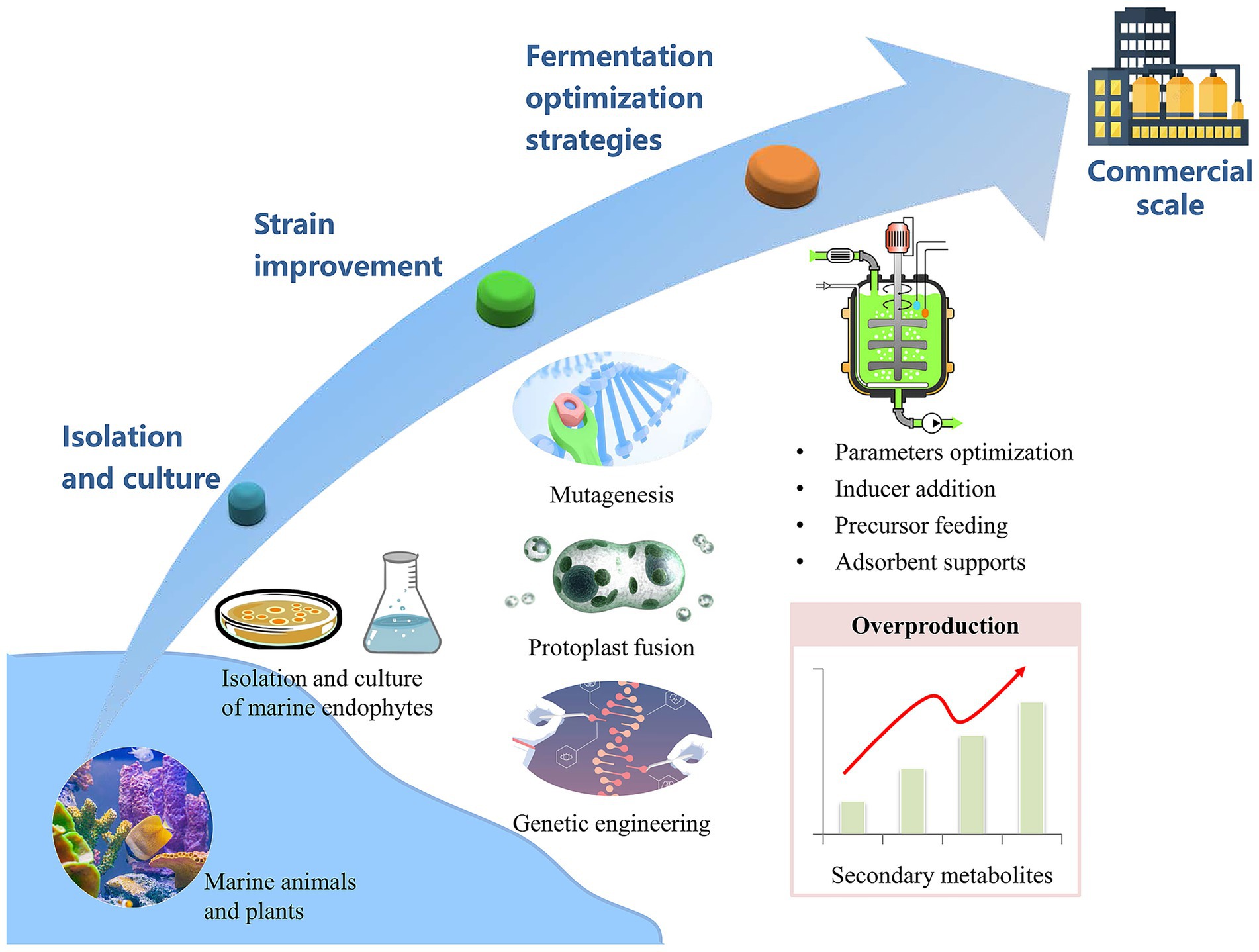
Isolating and culturing marine endophytic fungi is essential for harnessing their secondary metabolites. It involves extracting these fungi from a variety of marine habitats while meticulously excluding epiphytic microorganisms to ensure the purity of endophytic isolates. Selecting healthy, disease-free samples is crucial to prevent the isolation of pathogenic species and to focus on endophytes with beneficial traits (Strobel and Daisy, 2003; Strobel, 2003). To minimize contamination risks, samples should be processed promptly or kept at 4 °C in temporary storage (Strobel et al., 1996; Bacon and White, 2018).
The surface sterilization of samples, tailored to the host’s species and tissue type, is a critical step to guarantee the isolation of true endophytes (Bissegger and Sieber, 1994). This process commonly employs mechanical, enzymatic, or chemical methods (Hollants et al., 2010). For delicate organisms like algae, sterilization must be carefully adapted to their specific characteristics (Schulz and Boyle, 2005). Typically, this involves rinsing with sterile water, followed by treatment with 70% ethanol and sodium hypochlorite (1–4%), and finally rinsing with sterile distilled water to eliminate residual NaOCl (Stone et al., 2000; Strobel, 2002; Arnold et al., 2000). The appropriate concentration and duration of sterilization are determined based on the host and tissue type, with successful sterilization confirmed by the lack of microbial growth on the growth medium (Schulz and Boyle, 2005). Post-sterilization, samples are aseptically dissected and transferred to culture media, often supplemented with antibiotics like chloramphenicol, streptomycin, tetracycline, or penicillin to curb bacterial contamination (Tupac Otero et al., 2002). After incubation at 26 °C, fungal hyphal tips are isolated for subculturing, and the strains are archived. Through repeated transfers, endophytes are purified from the interior tissues (Suryanarayanan et al., 2003). Figure 3 provides a visual overview of the marine endophyte isolation process and metabolite profiling.
5.2 Strain improvement of marine endophytic fungi
The inherent activity of marine endophytic fungal strains found in nature is often insufficient for industrial-scale production of secondary metabolites. To bridge this gap, strain improvement techniques are imperative to enhance their productivity. With the advancement of biotechnology, methods such as mutagenesis and genetic engineering have become central to boosting the metabolite yield of these fungi (Demain and Adrio, 2008; Kong et al., 2022; Kong et al., 2021). Mutagenesis, both physical and chemical, is a traditional approach to induce genetic changes in microorganisms. Physical mutagens like ultraviolet radiation and chemical agents including alkylating compounds are used to increase the genetic variation, which can lead to strains with improved metabolite production (Shima et al., 1996; Hu et al., 2002). Resistance screening, leveraging antibiotic resistance as a selection tool, is a straightforward and effective method for isolating strains with desirable traits (Partridge et al., 2018). However, mutagenesis breeding suffers from inherent limitations including uncontrollable mutation sites and phenotypic instability. As evidenced by Khoshbakht et al.’s study, only 5 novel chalaniline derivatives were successfully generated from 23 precursor modifications (Khoshbakht et al., 2021). The low positive mutation rate significantly escalates both time investment and operational costs.
Protoplast fusion, a technique that merges cells by fusing their protoplasts, has been instrumental in developing high-yielding strains. This method was employed to develop a recombinant strain of Streptomyces pristinaespiralis with enhanced pristinamycin production capability. Through four rounds of protoplast fusion and screening, the obtained recombinant strain G4-17 achieved a pristinamycin yield of 0.89 g/L, representing a 145.9% increase compared to the original strain, while demonstrating excellent genetic stability (Xu et al., 2008). These results confirm the potential of protoplast fusion methodology for microbial strain improvement. While protoplast fusion offers significant potential, it faces considerable challenges in overcoming species barriers and may compromise the integrity of secondary metabolic gene clusters.
Metabolic engineering offers a targeted strategy for enhancing the biosynthesis of specific metabolites. By understanding the metabolic pathways and identifying rate-limiting steps, metabolic engineers can redirect the flow of metabolites towards the desired products (Kong et al., 2021). This can be achieved by overexpressing key genes or introducing synthetic gene clusters into the fungi. The endophytic paclitaxel-producing fungus Ozonium sp. EFY-21 represents a successful case of metabolic engineering for enhancing the production of high-value compounds (Wei et al., 2010). Studies demonstrated that by introducing the rate-limiting enzyme gene taxadiene synthase (ts) to modify the paclitaxel biosynthetic pathway, the paclitaxel yield in engineered transformant T4 significantly increased from 87.4 ± 6.3 μg/L in the wild-type strain to 417.1 ± 22.3 μg/L, achieving a 3.77-fold enhancement (Wei et al., 2012). However, metabolic engineering faces limitations in pathway elucidation, with the vast majority of biosynthetic gene clusters (BGCs) in marine fungi remaining functionally uncharacterized (Kumar et al., 2018). Moreover, heterologous expression may reduce enzymatic activity in certain cases, significantly diminishing the synthesis yield of target metabolites.
In summary, technological integration represents a breakthrough strategy. The synergistic combination of mutagenesis, protoplast fusion, and metabolic engineering significantly enhances the robustness of industrial microbial strains, thereby enabling sustainable and efficient production of high-value secondary metabolites. This approach is crucial for meeting industrial-scale metabolite production demands and facilitates the discovery of novel compounds with therapeutic potential.
5.3 Metabolic pathway engineering strategies for marine endophytic fungi
Beyond strain optimization, fermentation conditions significantly impact the production of target metabolites and their precursors in marine endophytic fungi. A well-designed fermentation process is essential to realize the full potential of engineered strains for natural product synthesis (Lau et al., 2002; Singh et al., 2017). Target product yields can be enhanced through precursor feeding, fermentation medium optimization, and the strategic use of inducers and adsorbent resins (Parekh et al., 2000). Fermentation conditions, including medium composition, pH, temperature, and stirring speed, are critical for improving secondary metabolite yields. The OSMAC (One Strain Many Compounds) strategy, pioneered by Bode et al. in 2002(Bode et al., 2002), systematically modulates culture parameters (e.g., medium composition, salinity, physical state) to activate silent biosynthetic gene clusters, thereby greatly expanding the metabolic diversity of a single strain (Wang et al., 2014). For instance, the endophytic fungus Hypomontagnella monticulosa cultivated in Wickerham medium produced 23 metabolites including antibacterial and anticancer briarane-type diterpenes (Lutfia et al., 2024). Comparative cultivation of Fucus vesiculosus symbionts in liquid vs. solid media resulted in 40% condition-exclusive metabolic nodes, with specific media inducing anticancer activity (Fan et al., 2019). Addition of NaI to rice medium triggered the production of unprecedented sulfur-containing alkaloids (aplospojaveedins A–C) in Aplosporella javeedii (Gao et al., 2024). These cases demonstrate OSMAC’s power to unlock novel chemical scaffolds and diversify metabolite profiles, proving essential for discovering antimicrobial and anticancer lead compounds. Inducer selection and timing are crucial for maximizing the yield of microbial secondary metabolites. By considering the physiological state and growth capacity of engineered strains, the appropriate induction conditions can be determined to enhance the expression of exogenous pathway proteins and target product yield (Sassi et al., 2016). Precursors feeding is another effective strategy, as demonstrated by the significant increase in argenocarcin production through proline and glucose supplementation (Dhakal et al., 2016).
In situ product removal (ISPR) is a valuable technique for managing self-toxic metabolites, ensuring high product levels and preventing their detrimental effects on microbial growth (Singh et al., 2010; Schügerl and Hubbuch, 2005). Solid adsorbents, such as polymer resins, are preferred over liquid solvents due to their lower toxicity risk and are widely applicable in endophytic fungal fermentation (Xu et al., 2009). The use of inert solid carriers has also been shown to enhance metabolite production and discovery (Bigelis et al., 2006). Environmental stimuli in liquid media can influence fungal development and metabolism, affecting metabolite production. This strategy has been effectively utilized in the fermentation of Phomopsis sp., where the use of adsorbent materials increased mycoepoxydiene production (Thammajaruk et al., 2011). These metabolic engineering strategies are pivotal for planning and executing the efficient production of secondary metabolites in marine endophytic fungi.
In conclusion, marine endophytic fungi represent not only integral components supporting the health and function of marine ecosystems but also constitute a treasure trove of high-value bioactive substances due to their unique metabolic capabilities and adaptation to diverse ecological niches (El-Bondkly et al., 2021; Sahoo et al., 2021; Tan et al., 2023). Their ability to produce a wide array of specialized metabolites in response to environmental stressors positions them as a promising and sustainable source for discovering new drugs and advancing biotechnological applications (Sahoo et al., 2021; Tan et al., 2023). As essential synthesis factories for secondary metabolites, they offer a scalable solution to current bottlenecks in drug discovery and development within the marine biotechnology sector, underscoring the critical importance of continued research and exploration in this field.
6 Discussion
Marine endophytic fungi inhabit diverse marine ecosystems, constituting an underexplored reservoir of biodiversity. These symbiotic microorganisms serve as crucial sources of structurally diverse and biologically significant secondary metabolites (e.g., anticancer, antimicrobial, and antioxidant compounds), further highlighting their potential as a valuable resource for biotechnological innovation (El-Bondkly et al., 2021; Wang et al., 2025). However, industrial applications currently face bottlenecks such as genomic instability and metabolic yield fluctuations under pure culture conditions (Shabana et al., 2021). Prevailing research predominantly focuses on strain isolation and preliminary activity screening, suffering from methodological homogeneity and insufficient quantitative production data, which severely hinders the translation from basic research to industrial applications.
To achieve efficient resource utilization, a transition from the conventional “species–compound–activity” model to a resource-driven research paradigm is imperative. This paradigm emphasizes dual-track advancement through strain improvement and process optimization: Strain enhancement: Integrating mutagenesis, protoplast fusion, and metabolic engineering to boost strain stability and biosynthetic efficiency; Process innovation: Implementing dynamic fermentation control, precision addition of elicitors/precursors, and targeted adsorption techniques to enhance metabolite production. For instance, our earlier work significantly increased the production of L-piperazic acid and putrescine in Aureobasidium melanogenum by employing metabolic engineering and optimized culture conditions, thereby validating the pivotal role of process engineering (Kong et al., 2022; Kong et al., 2021).
The integration of synthetic biology and systems biology heralds a transformative era in gene cluster mining. CRISPR-Cas9-mediated activation of silent biosynthetic gene clusters (BGCs) will enable the discovery of novel molecular scaffolds (e.g., isocoumarins, aminofulvenes). For instance, CRISPR-Cas9-mediated disruption of the Fusarium graminearumC16 BGC (targeting polyketide synthase PKS15 and terpene synthase TS genes) confirmed its products as decalin-containing diterpenoid pyrones FDDP-D and FDDP-E (Noor et al., 2020). Future efforts should combine bioinformatics-driven BGC prediction (e.g., antiSMASH analysis) with optimized heterologous expression platforms (e.g., yeast artificial chromosome systems) to reconstruct complex pathways directionally.
Advancements in PDB technology represent a pivotal breakthrough. Khoshbakht et al. successfully generated five novel chalaniline derivatives via 23 precursor modifications, demonstrating the enzymatic flexibility of fungal systems (Khoshbakht et al., 2021). Future strategies should integrate machine learning-assisted precursor design with enzyme engineering (e.g., P450 enzyme specificity modulation) for customized production of bioactive molecules. Concurrently, developing bionic fermentation systems (e.g., algal-fungal co-culture mimicking host microenvironments) could resolve metabolic instability in pure cultures, facilitating scaled-up production of algal-derived metabolites.
To establish a fully-integrated development system that bridges the pathway from strain to product, a “Strain-Process-Product” trinity framework serves as the ultimate solution. This includes: 1. Strain improvement: Integrating mutagenesis and genomic reprogramming to enhance the robustness of industrial strains; 2. Intelligent fermentation: Coupling real-time metabolic sensing with adaptive control (e.g., gradient elicitor release technology) to increase product titers; 3. Green separation: Developing biomimetic adsorption materials (e.g., functionalized XAD-7 resins) to reduce downstream purification costs. Through interdisciplinary technological integration, marine endophytic fungi are poised to become highly efficient “cell factories,” providing a sustainable repository of high-value natural products for drug development.
7 Future perspective
To fully unlock the potential of marine endophytic fungi as sustainable sources of high-value natural products, future research must adopt an integrated and interdisciplinary strategy. Moving beyond traditional isolation and screening approaches, efforts should prioritize the development of robust, industrially applicable systems through synergistic advances in strain engineering, process control, and pathway discovery. Key directions will include: leveraging CRISPR-Cas9 and synthetic biology tools to activate silent biosynthetic gene clusters and enable heterologous production of novel compounds; employing machine learning and enzyme engineering to optimize precursor-directed biosynthesis and metabolic flux; and designing bionic co-culture systems to mimic native host microenvironments and stabilize metabolic output. Ultimately, the implementation of a holistic “Strain–Process–Product” framework—combining genetically enhanced strains, intelligently controlled fermentation, and eco-friendly downstream purification—will transform these fungi into efficient cell factories, bridging the gap between laboratory discovery and industrial-scale production of pharmaceuticals and other bioactive compounds.
Author contributions
C-CK: Conceptualization, Funding acquisition, Software, Writing – review & editing. JW: Writing – review & editing, Data curation, Formal analysis, Software. BS: Writing – review & editing, Data curation, Methodology, Resources. H-XZ: Project administration, Resources, Writing – original draft. SQ: Methodology, Supervision, Validation, Writing – original draft. C-GR: Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work has been supported by Natural Science Foundation of Shandong Province (grant numbers ZR2023QC034 and ZR2024MH247).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Lateff, A. (2008). Chaetominedione, a new tyrosine kinase inhibitor isolated from the algicolous marine fungus Chaetomium sp. Tetrahedron Lett. 49, 6398–6400. doi: 10.1016/j.tetlet.2008.08.064
Abdel-Lateff, A., Fisch, K. M., Wright, A. D., and König, G. M. (2003a). A new antioxidant isobenzofuranone derivative from the algicolous marine fungus Epicoccum sp. Planta Med. 69, 831–834.
Abdel-Lateff, A., Klemke, C., König, G. M., and Wright, A. D. (2003b). Two new xanthone derivatives from the algicolous marine fungus Wardomyces anomalus. J. Nat. Prod. 66, 706–708. doi: 10.1021/np020518b
Almeida, C., Hemberger, Y., Schmitt, S. M., Bouhired, S., Natesan, L., Kehraus, S., et al. (2012). Marilines A-C: novel phthalimidines from the sponge-derived fungus Stachylidium sp. Chemistry 18, 8827–8834. doi: 10.1002/chem.201103278
Almeida, C., Kehraus, S., Prudêncio, M., and König, G. M. (2011b). Marilones A-C, phthalides from the sponge-derived fungus Stachylidium sp. Beilstein J. Org. Chem. 7, 1636–1642. doi: 10.3762/bjoc.7.192
Almeida, C., Part, N., Bouhired, S., and Kehraus, S. (2011a). Stachylines A− D from the Sponge-derived fungus Stachylidium sp. J. Nat. Products 74, 21–25.
Ananda, K., and Sridhar, K. R. J. C. J. M. (2002). Diversity of endophytic fungi in the roots of mangrove species on the west coast of India. Can. J. Microbiol. 48, 871–878. doi: 10.1139/w02-080
Arnold, A. E., Maynard, Z., Gilbert, G. S., Coley, P. D., and Kursar, T. A. J. E. (2000). Are tropical fungal endophytes hyperdiverse? Ecol. Lett. 3, 267–274. doi: 10.1046/j.1461-0248.2000.00159.x
Bacon, W. J. (1994). Stains, media, and procedures for Analyzing endophytes. London: CRC Press, 47–56.
Bacon, C. W., and White, J. F. (2018). “Stains, media, and procedures for analyzing endophytes” in Biotechnology of endophytic fungi of grasses. ed. C. W. Bacon (London: CRC Press), 47–56.
Bamisile, B. S., Dash, C. K., Akutse, K. S., Keppanan, R., and Wang, L. (2018). Fungal endophytes: beyond herbivore management. Front. Microbiol. 9:544.
Bary, A. (1866). Morphologie und Physiologie der Pilze, Flechten und Myxomyceten. Leipzig: W. Engelmann.
Bigelis, R., He, H., Yang, H. Y., Chang, L. P., and Greenstein, M. (2006). Production of fungal antibiotics using polymeric solid supports in solid-state and liquid fermentation. J. Ind. Microbiol. Biotechnol. 33, 815–826.
Bissegger, M., and Sieber, T. N. J. M. (1994). Assemblages of endophytic fungi in coppice shoots of Castanea sativa. Mycologia 86, 648–655. doi: 10.1080/00275514.1994.12026464
Bode, H. B., Bethe, B., Höfs, R., and Zeeck, A. (2002). Big effects from small changes: possible ways to explore nature's chemical diversity. ChemBioChem 3, 619–627.
Bovio, E., Garzoli, L., Poli, A., Luganini, A., Villa, P., Musumeci, R., et al. (2019). Marine Fungi from the sponge Grantia compressa: biodiversity, Chemodiversity, and biotechnological potential. Mar. Drugs 17:220. doi: 10.3390/md17040220
Bugni, T. S., and Ireland, C. M. (2004). Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 21, 143–163. doi: 10.1039/b301926h
Burragoni, S. G., and Jeon, J. (2021). Applications of endophytic microbes in agriculture, biotechnology, medicine, and beyond. Microbiol. Res. 245:126691. doi: 10.1016/j.micres.2020.126691
Cheng, M. M., Tang, X. L., Sun, Y. T., Song, D. Y., Cheng, Y. J., Liu, H., et al. (2020). Biological and chemical diversity of marine sponge-derived microorganisms over the last two decades from 1998 to 2017. Molecules 25:853.
Chinnarajan, R., Naveenan, T., and Varatharajan, G. R. (2010). Optimization of alkaline cellulase production by the marine-derived fungus Chaetomium sp. using agricultural and industrial wastes as substrates. Bot. Mar. 53, 275–282.
Couttolenc, A., Espinoza, C., Fernández, J. J., Norte, M., Plata, G. B., Padrón, J. M., et al. (2015). Antiproliferative effect of extract from endophytic fungus Curvularia trifolii isolated from the "Veracruz reef system" in Mexico. Pharm. Biol. 54, 1392–1397. doi: 10.3109/13880209.2015.1081254
Cui, C. M., Li, X. M., Li, C. S., Proksch, P., and Wang, B. G. (2010b). Cytoglobosins A-G, cytochalasans from a marine-derived endophytic fungus, Chaetomium globosum QEN-14. J. Nat. Prod. 73, 729–733. doi: 10.1021/np900569t
Dastogeer, K. M. G., Li, H., Sivasithamparam, K., Jones, M. G. K., and Wylie, S. J. (2018). Host specificity of endophytic mycobiota of wild Nicotiana plants from arid regions of northern Australia. Microb. Ecol. 75, 74–87. doi: 10.1007/s00248-017-1020-0
Demain, A. L., and Adrio, J. L. (2008). Strain improvement for production of pharmaceuticals and other microbial metabolites by fermentation. Nat. Compounds Drugs 1, 251–289.
Deshmukh, S. A.-O., Gupta, M. A.-O., Prakash, V., and Saxena, S. A.-O. (2018). Endophytic fungi: A source of potential antifungal compounds. J. Fungi 4:77.
Dhakal, D., Chaudhary, A. K., Yi, J. S., Pokhrel, A. R., Shrestha, B., Parajuli, P., et al. (2016). Enhanced production of nargenicin A1 and creation of a novel derivative using a synthetic biology platform. Appl. Microbiol. Biotechnol. 100, 9917–9931. doi: 10.1007/s00253-016-7705-3
Ding, B., Wang, Z., Huang, X., Liu, Y., Chen, W., and She, Z. (2016). Bioactive α-pyrone meroterpenoids from mangrove endophytic fungus penicillium sp. Nat. Prod. Res. 30, 2805–2812. doi: 10.1080/14786419.2016.1164702
Drake, I., White, J. F. Jr., and Belanger, F. C. (2018). Identification of the fungal endophyte of Ammophila breviligulata (American beachgrass) as Epichloë amarillans. PeerJ 6:4300. doi: 10.7717/peerj.4300
Dreyfuss, M., and Chapela, I. H. J. (1994). Potential of fungi in the discovery of novel, low-molecular weight pharmaceuticals. Disc. Novel Nat. Products Therapeutic Potential 22, 49–80.
Eamvijarn, A., Gomes, N. M., Dethoup, T., Buaruang, J., Manoch, L., Silva, A., et al. (2013). Bioactive meroditerpenes and indole alkaloids from the soil fungus Neosartorya fischeri (KUFC 6344), and the marine-derived fungi Neosartorya laciniosa (KUFC 7896) and Neosartorya tsunodae (KUFC 9213). Tetrahedron 69, 8583–8591. doi: 10.1016/j.tet.2013.07.078
El-Bondkly, E. A. M., El-Bondkly, A. A. M., and El-Bondkly, A. A. M. (2021). Marine endophytic fungal metabolites: A whole new world of pharmaceutical therapy exploration. Heliyon 7:3.
El-Bondkly, A. M., and El-Gendy, M. M. (2012). Cellulase production from agricultural residues by recombinant fusant strain of a fungal endophyte of the marine sponge Latrunculia corticata for production of ethanol. Antonie Van Leeuwenhoek 101, 331–346.
El-Bondkly, A. M., and El-Gendy, M. M. (2010). Keratinolytic activity from new recombinant fusant AYA2000, derived from endophytic Micromonospora strains. Can. J. Microbiol. 56, 748–760. doi: 10.1139/w10-058
El-Bondkly, A. M., El-Gendy, M., Bassyouni, R. H., and Bassyouni, R. H. (2012). Overproduction and biological activity of prodigiosin-like pigments from recombinant fusant of endophytic marine Streptomyces species. Antonie Van Leeuwenhoek 102, 719–734.
El-Bondkly, A. A. M., El-Gendy, M. M. A. A., and El-Bondkly, A. M. A. (2021). Construction of efficient recombinant strain through genome shuffling in marine endophytic fusarium sp. ALAA-20 for improvement lovastatin production using agro-industrial wastes. Arab. J. Sci. Eng. 46, 175–190. doi: 10.1007/s13369-020-04925-5
El-Demerdash, A., Kumla, D., and Kijjoa, A. (2020). Chemical diversity and biological activities of meroterpenoids from marine derived-fungi: A comprehensive update. Marine Drugs 18:317.
El-Gendy, M. M., Al-Zahrani, H. A., and El-Bondkly, A. M. (2016). Genome shuffling of mangrove endophytic Aspergillus luchuensis MERV10 for improving the cholesterol-lowering agent lovastatin under solid state fermentation. Mycobiology 44, 171–179.
El-Gendy, M., Al-Zahrani, S. H. M., and El-Bondkly, A. M. A. (2003). Construction of potent recombinant strain through intergeneric protoplast fusion in endophytic fungi for anticancerous enzymes production using rice straw. Appl. Biochem. Biotechnol. 183, 30–50. doi: 10.1007/s12010-017-2429-0
El-Gendy, M. M., and El-Bondkly, A. M. (2010). Production and genetic improvement of a novel antimycotic agent, saadamycin, against dermatophytes and other clinical fungi from endophytic Streptomyces sp. Hedaya48. J. Ind. Microbiol. Biotechnol. 37, 831–841. doi: 10.1007/s10295-010-0729-2
El-Gendy, M. M., and El-Bondkly, A. M. (2011). Genome shuffling of marine derived bacterium Nocardia sp. ALAA 2000 for improved ayamycin production. Antonie Van Leeuwenhoek 99, 773–780. doi: 10.1007/s10482-011-9551-8
El-Gendy, M. M., Fau, H. U., and Jaspars, M. (2000). Novel bioactive metabolites from a marine derived bacterium Nocardia sp. ALAA 61, 379–386.
El-Gendy, M., Hassanein, N. M., Abd El-Hay Ibrahim, H., and Abd El-Baky, D. H. (2017). Heavy metals biosorption from aqueous solution by endophytic Drechslera hawaiiensis of Morus alba L. derived from heavy metals habitats. Mycobiology 45, 73–83. doi: 10.5941/MYCO.2017.45.2.73
El-Gendy, M. M. A. A., Mohamed, Z. K., Hekal, N. Z., Ali, F. M., and Yousef, A. E. M. (2018). Production of bioactive metabolites from different marine endophytic Streptomyces species and testing them against methicillin-resistant Staphylococcus aureus (MRSA) and cancer cell lines. Biotechnologia 99, 13–35. doi: 10.5114/bta.2018.73559
El-Gendy, M. M., Shaaban, M., El-Bondkly, A. M., El-Bondkly, A., Shaaban, K. A., and Shaaban, K. A. (2008a). Bioactive benzopyrone derivatives from new recombinant fusant of marine Streptomyces. Appl. Biochem. Biotechnology 150, 85–96.
El-Gendy, M. M., Shaaban, M., Shaaban, K. A., Shaaban, K., El-Bondkly, A. M., El-Bondkly, A., et al. (2008b). Essramycin: a first triazolopyrimidine antibiotic isolated from nature. J. Antibiotics 61, 149–157.
El-Gendy, M. M. A. A., Taha, T., Abo-Dahab, N., and Hassan, F. S. M. (2016). Process optimization of L-glutaminase production; a tumour inhibitor from marine endophytic isolate aspergillus sp. ALAA-2000. J. Microb. Biochem. Technol. 9, 256–267.
El-Gendy, M., Yahya, S. M. M., Hamed, A. R., Soltan, M. M., and El-Bondkly, A. M. A. (2010). Phylogenetic analysis and biological evaluation of marine endophytic fungi derived from Red Sea sponge Hyrtios erectus. Appl. Biochem. Biotechnol. 185, 755–777. doi: 10.1007/s12010-017-2679-x
Elsebai, M. F., Ghabbour, H. A., and Mehiri, M. (2016). Unusual nitrogenous Phenalenone derivatives from the marine-derived fungus Coniothyrium cereale. Molecules 21:178. doi: 10.3390/molecules21020178
Elsebai, M. F., Kehraus, S., Gütschow, M., and König, G. M. (2009). New polyketides from the marine-derived fungus Phaeosphaeria spartinae. Nat. Prod. Commun. 4, 1463–1468.
Elsebai, M. A.-O., Schoeder, C. T., and Müller, C. A.-O. (2021). Fintiamin: A diketopiperazine from the marine sponge‐derived fungus Eurotium sp. Archiv der Pharmazie 354:2100206.
Fadiji, A. E., and Babalola, O. O. (2020). Metagenomics methods for the study of plant-associated microbial communities: a review. J. Microbiol. Methods 170:105860. doi: 10.1016/j.mimet.2020.105860
Fan, B., Parrot, D., Blümel, M., Labes, A., and Tasdemir, D. (2019). Influence of OSMAC-based cultivation in metabolome and anticancer activity of Fungi associated with the Brown alga Fucus vesiculosus. Mar. Drugs 17:67. doi: 10.3390/md17010067
Fau, H. D., Rossman, A. Y., and Rossman, A. Y. (1997). Where are all the undescribed fungi? Phytopathology 87, 888–891.
Faulkner, D. J. (2000). Highlights of marine natural products chemistry (1972–1999). Nat. Product Rep. 17, 1–d.
Friedrich, A. B., Fischer, I., Proksch, P., Hacker, J., and Hentschel, U. J. F. M. E. (2001). Temporal variation of the microbial community associated with the Mediterranean sponge Aplysina aerophoba 38, 105–113.
Galkiewicz, J. P., Stellick, S. H., Gray, M. A., and Kellogg, C. A. (2012). Cultured fungal associates from the deep-sea coral Lophelia pertusa. Deep Sea Res. Part I Oceanogr. Res. Pap. 67, 12–20. doi: 10.1016/j.dsr.2012.05.001
Gao, Y., Frank, M., Teusch, N., Woschko, D., Janiak, C., Mándi, A., et al. (2024). Aplospojaveedins A-C, unusual sulfur-containing alkaloids produced by the endophytic fungus Aplosporella javeedii using OSMAC strategy. Front. Microbiol. 15:1458622. doi: 10.3389/fmicb.2024.1458622
Gao, S. S., Li, X. M., Li, C. S., Proksch, P., and Wang, B. G. (2011). Penicisteroids a and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus penicillium chrysogenum QEN-24S. Bioorg. Med. Chem. Lett. 21, 2894–2897. doi: 10.1016/j.bmcl.2011.03.076
Gao, H., Li, G. A.-O., and Lou, H. X. (2018). Structural diversity and biological activities of novel secondary metabolites from endophytes. Molecules 23:646.
Glaser, K. B., and Mayer, A. M. J. B. (2009). A renaissance in marine pharmacology: from preclinical curiosity to clinical reality. Biochem. Pharmacol. 78, 440–448. doi: 10.1016/j.bcp.2009.04.015
Gomes, N. M., Bessa, L. J., Buttachon, S., Costa, P. M., Buaruang, J., Dethoup, T., et al. (2014). Antibacterial and antibiofilm activities of Tryptoquivalines and Meroditerpenes isolated from the marine-derived Fungi Neosartorya paulistensis, N. Laciniosa, N. Tsunodae, and the soil Fungi N. Fischeri and N. siamensis. Mar. Drugs 12, 822–839. doi: 10.3390/md12020822
González-Menéndez, V., Asensio, F., Moreno, C., de Pedro, N., Monteiro, M. C., de la Cruz, M., et al. (2014). Assessing the effects of adsorptive polymeric resin additions on fungal secondary metabolite chemical diversity. Mycology 5, 179–191. doi: 10.1080/21501203.2014.942406
Gouda, S., Das, G., Sen, S. K., Shin, H. S., and Patra, J. K. (2016). Endophytes: a treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 7:1538.
Hallmann, J., Quadt-Hallmann, A., Mahaffee, W. F., and Kloepper, J. W. (1997). Bacterial endophytes in agricultural crops. Can. J. Microbiol. 43, 895–914. doi: 10.1139/m97-131
Hamasaki, T., Nagayama, K., and Hatsuda, Y. (1978). Two new metabolites, Sydonic acid and hydroxysydonic acid, from aspergillus sydowi. Agric. Biol. Chem. 42, 37–40. doi: 10.1271/bbb1961.42.37
Handayani, D., Ananda, N., Artasasta, M., Ruslan, R., Fadriyanti, O., and Tallei, T. (2019). Antimicrobial activity screening of endophytic fungi extracts isolated from brown algae Padina sp. J. Appl. Pharm. Sci. 9, 9–13. doi: 10.7324/JAPS.2019.90302
Hardoim, P. R., van Overbeek, L. S., Berg, G., Pirttilä, A. M., Compant, S., Campisano, A., et al. (2015). The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 79, 293–320. doi: 10.1128/MMBR.00050-14
Harris, G. H., Hoogsteen, K., Silverman, K. C., Raghoobar, S. L., Bills, G. F., Lingham, R. B., et al. (1993). Isolation and structure determination of pycnidione, a novel bistropolone stromelysin inhibitor from a Phoma sp. Tetrahedron 49, 2139–2144. doi: 10.1016/S0040-4020(01)80357-4
Heinig, U., Scholz, S., and Jennewein, S. J. F. (2013). Getting to the bottom of Taxol biosynthesis by fungi. Fungal Diversity 60, 161–170.
Higgins, K. L., Arnold, A., Miadlikowska, J., Miadlikowska, J., Sarvate, S. D., Sarvate, S., et al. (2007). Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol. Phylogenetics Evol. 42, 543–555.
Hollants, J., Leliaert, F., De Clerck, O., and Willems, A. J. S. (2010). How endo-is endo-? Surface sterilization of delicate samples: a Bryopsis (Bryopsidales, Chlorophyta) case study 51, 131–138.
Hollants, J., Leroux, O., Leliaert, F., Decleyre, H., De Clerck, O., and Willems, A. (2011). Who is in there? Exploration of endophytic bacteria within the siphonous green seaweed Bryopsis (Bryopsidales, Chlorophyta). PLoS One 6:e26458.
Hou, X. M., Hai, Y., Gu, Y. C., Wang, C. Y., and Shao, C. L. (2019). Chemical and bioactive marine natural products of coral-derived microorganisms (2015-2017). Curr. Med. Chem. 26, 6930–6941. doi: 10.2174/0929867326666190626153819
Howitz, K. T., and Sinclair, D. A. (2008). Xenohormesis: sensing the chemical cues of other species. Cell 133, 387–391. doi: 10.1016/j.cell.2008.04.019
Hu, H.-B., Luo, Y.-F., Wang, P., Wang, W.-J., and Wu, J. (2018). Xanthone-derived polyketides from the Thai mangrove endophytic fungus Phomopsis sp. xy21. Fitoterapia 131, 265–271. doi: 10.1016/j.fitote.2018.11.004
Hu, H., Zhang, Q., FauOchi, K., and Ochi, K. (2002). Activation of antibiotic biosynthesis by specified mutations in the rpoB gene (encoding the RNA polymerase beta subunit) of Streptomyces lividans. J. Bacteriol. 184, 3984–3991. doi: 10.1128/JB.184.14.3984-3991.2002
Huang, S., Chen, H., Li, W., Zhu, X., Ding, W., and Li, C. (2016). Bioactive Chaetoglobosins from the mangrove endophytic fungus penicillium chrysogenum. Mar. Drugs 14:172. doi: 10.3390/md14100172
Huang, H. B., Feng, X. J., Liu, L., Chen, B., Lu, Y. J., Ma, L., et al. (2010). Three dimeric naphtho-γ-pyrones from the mangrove endophytic fungus aspergillus tubingensis isolated from Pongamia pinnata. Planta Med. 76, 1888–1891. doi: 10.1055/s-0030-1249955
Huang, H., Feng, X., Xiao, Z., Liu, L., Li, H., Ma, L., et al. (2011). Azaphilones and p-terphenyls from the mangrove endophytic fungus penicillium chermesinum (ZH4-E2) isolated from the South China Sea. J. Nat. Prod. 74, 997–1002. doi: 10.1021/np100889v
Huang, X., Sun, X., Ding, B., Lin, M., Liu, L., Huang, H., et al. (2013). A new anti-acetylcholinesterase α-pyrone meroterpene, arigsugacin I, from mangrove endophytic fungus penicillium sp. sk5GW1L of Kandelia candel. Planta Med. 79, 1572–1575. doi: 10.1055/s-0033-1350896
Jain, S., Prajapat, G., Abrar, M., Ledwani, L., Singh, A., and Agrawal, A. (2017). Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 57, 715–727. doi: 10.1002/jobm.201700044
Jia, Y.-L., Guan, F.-F., Ma, J., Wang, C.-Y., and Shao, C.-L. (2015). Pestalotiolide a, a new antiviral phthalide derivative from a soft coral-derived fungus Pestalotiopsis sp. Nat. Prod. Sci. 21:227. doi: 10.20307/nps.2015.21.4.227
Kamat, S., Kumari, M., Sajna, K. V., and Jayabaskaran, C. (2020). Endophytic fungus, Chaetomium globosum, associated with marine green alga, a new source of chrysin. Sci. Rep. 10:18726. doi: 10.1038/s41598-020-72497-3
Kamat, S., Kumari, M., Taritla, S., and Jayabaskaran, C. (2020). Endophytic fungi of marine alga from Konkan coast, India—a rich source of bioactive material. Front. Mar. Sci. 7:31. doi: 10.3389/fmars.2020.00031
Kanokmedhakul, S., Kanokmedhakul, K., Phonkerd, N., Soytong, K., Kongsaeree, P., and Suksamrarn, A. (2002). Antimycobacterial anthraquinone-chromanone compound and diketopiperazine alkaloid from the fungus Chaetomium globosum KMITL-N0802. Planta Med. 68, 834–836. doi: 10.1055/s-2002-34415
Khoshbakht, M., Srey, J., Adpressa, D. A., Jagels, A., and Loesgen, S. (2021). Precursor-directed biosynthesis of aminofulvenes: new chalanilines from endophytic fungus Chalara sp. Molecules 26:4418. doi: 10.3390/molecules26154418
Kong, C., Wang, Z., Liu, G., Chi, Z., Ledesma-Amaro, R., and Chi, Z. J. M. B. (2021). Bioproduction of L‐piperazic acid in gram scale usingAureobasidium melanogenum. Microbial. Biotechnol. 14, 1722–1729. doi: 10.1111/1751-7915.13838
Kong, C. C., Wei, X., Liu, G. L., Chi, Z. M., and Chi, Z. (2022). Metabolic engineering of Aureobasidium melanogenum for the overproduction of putrescine by improved L-ornithine biosynthesis. Microbiol. Res. 260:127041. doi: 10.1016/j.micres.2022.127041
Krick, A., Kehraus, S., Gerhäuser, C., Klimo, K., Nieger, M., Maier, A., et al. (2007). Potential cancer chemopreventive in vitro activities of monomeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 70, 353–360. doi: 10.1021/np060505o
Kumar, A., Sørensen, J. L., Hansen, F. T., Arvas, M., Syed, M. F., Hassan, L., et al. (2018). Genome sequencing and analyses of two marine fungi from the North Sea unraveled a plethora of novel biosynthetic gene clusters. Sci. Rep. 8:10187. doi: 10.1038/s41598-018-28473-z
Lau, J., Frykman, S., Regentin, R., Ou, S., Tsuruta, H., and Licari, P. (2002). Optimizing the heterologous production of epothilone D in Myxococcus xanthus. Biotechnol. Bioeng. 78, 280–288.
Lebeau, J., Venkatachalam, M., Fouillaud, M., Petit, T., Vinale, F., Dufossé, L., et al. (2017). Production and new extraction method of polyketide red pigments produced by ascomycetous fungi from terrestrial and marine habitats. J. Fungi(Basel, Switzerland) 3:3.
Li, K. K., Lu, Y. J., Song, X. H., She, Z. G., Wu, X. W., An, L. K., et al. (2010). The metabolites of mangrove endophytic fungus Zh6-B1 from the South China Sea. Bioorg. Med. Chem. Lett. 20, 3326–3328. doi: 10.1016/j.bmcl.2010.04.036
Li, W., Xiong, P., Zheng, W., Zhu, X., She, Z., Ding, W., et al. (2017). Identification and antifungal activity of compounds from the mangrove endophytic fungus aspergillus clavatus R7. Mar. Drugs 15:259. doi: 10.3390/md15080259
Lin, Y., Wu, X., Feng, S., Jiang, G., Luo, J., Zhou, S., et al. (2001). Five unique compounds: xyloketals from mangrove fungus Xylaria sp. from the South China Sea coast. J. Org. Chem. 66, 6252–6256. doi: 10.1021/jo015522r
Liu, L., Zheng, Y.-Y., Shao, C.-L., and Wang, C.-Y. (2019). Metabolites from marine invertebrates and their symbiotic microorganisms: molecular diversity discovery, mining, and application. Mar. Life Sci. Technol. 1, 60–94. doi: 10.1007/s42995-019-00021-2
Love, G. D., Grosjean, E., Stalvies, C., Stalvies, C., Fike, D. A., Fike, D., et al. (2009). Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457, 718–721.
Lutfia, A., Munir, E., Yurnaliza, Y., Basyuni, M., and Oku, H. (2024). Chemical profiling of fungal metabolites via the OSMAC approach: novel identification of Brianthein W from an endophytic fungus, Hypomontagnella monticulosa Zg15SU. Curr. Res. Microbial Sci. 7:100288. doi: 10.1016/j.crmicr.2024.100288
Mei, W. L., Zheng, B., Zhao, Y. X., Zhong, H. M., Chen, X. L., Zeng, Y. B., et al. (2012). Meroterpenes from endophytic fungus A1 of mangrove plant Scyphiphora hydrophyllacea. Mar. Drugs 10, 1993–2001.
Mm, A., Abo-Dahab, N., Taha, T., and Sm, F. (2015). Production, purification and characterization of L-asparaginase from marine endophytic aspergillus sp. ALAA-2000 under submerged and solid state fermentation. J. Microb. Biochem. Technol. 7, 165–172.
Mohamed El-Bondkly, A. A., El-Gendy, M. M. A. A., El-Bondkly, E. A. M., and Ahmed, A. M. (2020). Biodiversity and biological activity of the fungal microbiota derived from the medicinal plants Salvia aegyptiaca L. and Balanties aegyptiaca L. Biocatal. Agric. Biotechnol. 28:101720. doi: 10.1016/j.bcab.2020.101720
Muralikrishnan, V. (2013). Isolation and characterization of endophytic actinomycetes from mangrove plant for antimicrobial activity. Int. J. Curr. Microbiol. Appl. Sci 2, 78–89.
Noor, A. O., Almasri, D. M., Bagalagel, A. A., Abdallah, H. M., Mohamed, S. G. A., Mohamed, G. A., et al. (2020). Naturally occurring isocoumarins derivatives from endophytic fungi: sources, isolation, structural characterization, biosynthesis, and biological activities. Molecules 25:395. doi: 10.3390/molecules25020395
Nukina, M., Sato, Y., Ikeda, M., and Sassa, T. (1981). Sydonol, a new fungal morphogenic substance produced by an unidentified aspergillus sp. Agric. Biol. Chem. 45, 789–790. doi: 10.1080/00021369.1981.10864605
Nurunnabi, T. R., Sabrin, F., Sharif, D. I., Nahar, L., Sohrab, M. H., Sarker, S. D., et al. (2020). Antimicrobial activity of endophytic fungi isolated from the mangrove plant Sonneratia apetala (Buch.-ham) from the Sundarbans mangrove forest. Adv. Tradit. Med. 20, 419–425. doi: 10.1007/s13596-019-00422-9
Osterhage, C., Kaminsky, R., König, G. M., and Wright, A. D. (2000). Ascosalipyrrolidinone a, an antimicrobial alkaloid, from the obligate marine fungus Ascochyta salicorniae. J. Org. Chem. 65, 6412–6417. doi: 10.1021/jo000307g
Parekh, S., Vinci, V., Strobel, R. J., and Strobel, R. J. (2000). Improvement of microbial strains and fermentation processes. Appl. Microbiol. Biotechnol. 54, 287–301.
Partridge, S. R., Kwong, S. M., Firth, N., and Jensen, S. O. (2018). Mobile genetic elements associated with antimicrobial resistance. Clinical Microbiol. Rev. 31, 10–1128.
Pontius, A., Krick, A., Kehraus, S., Foegen, S. E., Müller, M., Klimo, K., et al. (2008). Noduliprevenone: a novel heterodimeric chromanone with cancer chemopreventive potential. Chemistry 14, 9860–9863. doi: 10.1002/chem.200801574
Qi, C., Qiao, Y., Gao, W., Liu, M., Zhou, Q., Chen, C., et al. (2018). New 3,5-dimethylorsellinic acid-based meroterpenoids with BACE1 and AchE inhibitory activities from aspergillus terreus. Org. Biomol. Chem. 16, 9046–9052. doi: 10.1039/C8OB02741B
Qi, J., Shao, C. L., Li, Z. Y., Gan, L. S., Fu, X. M., Bian, W. T., et al. (2013). Isocoumarin derivatives and benzofurans from a sponge-derived penicillium sp. fungus. J. Nat. Prod. 76, 571–579. doi: 10.1021/np3007556
Rodriguez, R. J., White, J. F. Jr., Arnold, A. E., and Redman, R. S. (2009). Fungal endophytes: diversity and functional roles. New Phytol. 182, 314–330. doi: 10.1111/j.1469-8137.2009.02773.x
Sahoo, S., Subban, K., and Chelliah, J. (2021). Diversity of marine macro-Algicolous endophytic Fungi and cytotoxic potential of Biscogniauxia petrensis metabolites against Cancer cell lines. Front. Microbiol. 12:650177. doi: 10.3389/fmicb.2021.650177
Sandrawati, N., Hati, S., Yunita, F., Putra, A., Ismed, F., Tallei, T., et al. (2020). Antimicrobial and cytotoxic activities of marine sponge-derived fungal extracts isolated from Dactylospongia sp. J. Appl. Pharm. Sci. 10:28-033.
Santos, C. M. D., Ribeiro, A. D. S., Garcia, A., Polli, A. D., Polonio, J. C., Azevedo, J. L., et al. (2019). Enzymatic and antagonist activity of endophytic fungi from Sapindus saponaria L. J Acta Biológica Colombiana. 24, 322–330. doi: 10.15446/abc.v24n2.74717
Sassi, H., Delvigne, F., Kar, T., Nicaud, J.-M., Coq, A.-M. C.-L., Steels, S., et al. (2016). Deciphering how LIP2 and POX2 promoters can optimally regulate recombinant protein production in the yeast Yarrowia lipolytica 15, 1–11.
Schulz, B., and Boyle, C. (2005). The endophytic continuum. Mycol. Res. 109, 661–686. doi: 10.1017/s095375620500273x
Shabana, S., Lakshmi, K. R., and Satya, A. K. (2021). An updated review of secondary metabolites from marine Fungi. Mini Rev. Med. Chem. 21, 602–642. doi: 10.2174/1389557520666200925142514
Shao, C. L., Xu, R. F., Wang, C. Y., Qian, P. Y., Wang, K. L., and Wei, M. Y. (2015). Potent antifouling marine dihydroquinolin-2(1H)-one-containing alkaloids from the gorgonian coral-derived fungus Scopulariopsis sp. Mar. Biotechnol. (N.Y.) 17, 408–415. doi: 10.1007/s10126-015-9628-x
Sharma, A., Malhotra, B., Kharkwal, H., Kulkarni, G. T., and Kaushik, N. (2020). Therapeutic agents from endophytes harbored in Asian medicinal plants. Phytochem. Rev. 19, 691–720. doi: 10.1007/s11101-020-09683-8
Shi, T., Qi, J., Shao, C. L., Zhao, D. L., Hou, X. M., and Wang, C. Y. (2017). Bioactive diphenyl ethers and isocoumarin derivatives from a gorgonian-derived fungus Phoma sp. (TA07-1). Mar. Drugs 15:146. doi: 10.3390/md15060146
Shima, J., Hesketh, A., Okamoto, S., Kawamoto, S., and Ochi, K. (1996). Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). J. Bacteriol. 178, 7276–7284.
Sibero, M., Zhou, T., Igarashi, Y., Radjasa, o., Sabdono, A., Trianto, A., et al. (2020). Chromanone-type compounds from marine sponge-derived Daldinia eschscholtzii KJMT FP 4.1. J. Appl. Pharm. Sci. 10, 1–7.
Singh, V., Haque, S., Niwas, R., Srivastava, A., Pasupuleti, M., and Tripathi, C. K. (2017). Strategies for fermentation medium optimization: an in-depth review. Front. Microbiol. 7:2087.
Singh, M. P., Leighton, M., Barbieri, L. R., Barbieri, L., Roll, D. M., Roll, D., et al. (2010). Fermentative production of self-toxic fungal secondary metabolites. J. Industrial Microbiol. Biotechnol. 37, 335–340.
Song, Y., Wang, J., Huang, H., Ma, L., Wang, J., Gu, Y., et al. (2012). Four eremophilane sesquiterpenes from the mangrove endophytic fungus Xylaria sp. BL321. Mar. Drugs 10, 340–348. doi: 10.3390/md10020340
Song, C., Yang, J., Zhang, M., Ding, G., Jia, C., Qin, J. A.-O., et al. (2021). Marine natural products: The important resource of biological insecticide. Chem. Biodiv. 18:e2001020.
Sopalun, K., and Iamtham, S. (2020). Isolation and screening of extracellular enzymatic activity of endophytic fungi isolated from Thai orchids. S. Afr. J. Bot. 134, 273–279. doi: 10.1016/j.sajb.2020.02.005
Stone, J. K., Bacon, C. W., and White, J. F. J. M. Jr. (2000). An overview of endophytic microbes: Endophytism defined. Microbial Endophytes 25, 17–44.
Strobel, G. A. (2002). Rainforest endophytes and bioactive products. Critical Reviews Biotechnol. 22, 315–333.
Strobel, G. (2018). The emergence of endophytic microbes and their biological promise. J. Fungi 4:57.
Strobel, G., and Daisy, B. (2003). Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 67, 491–502. doi: 10.1128/MMBR.67.4.491-502.2003
Strobel, G., Yang, X., Sears, J., Kramer, R., Sidhu, R. S., and Hess, W. J. M. (1996). Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana 142, 435–440.
Sun, Y., Liu, J., Li, L., Gong, C., Wang, S., Yang, F., et al. (2017). New butenolide derivatives from the marine sponge-derived fungus aspergillus terreus. Bioorg. Med. Chem. Lett. 28, 315–318. doi: 10.1016/j.bmcl.2017.12.049
Suryanarayanan, T., Venkatesan, G., and Murali, T. J. C. S. (2003). Endophytic fungal communities in leaves of tropical forest trees: diversity and distribution patterns 25, 489–493.
Tan, M., Xu, X., Zhang, W., Wu, F., Bo, X., Qin, F., et al. (2023). Isolation and insecticidal activities of new cyclic peptides from mangrove endophytic fungus aspergillus sp. GXNU-4QQY1a. Fitoterapia 171:105693. doi: 10.1016/j.fitote.2023.105693
Tan, R. X., and Zou, W. X. (2001). Endophytes: a rich source of functional metabolites. Nat. Prod. Rep. 18, 448–459. doi: 10.1039/b100918o
Tao, L. Y., Zhang, J. Y., Liang, Y. J., Chen, L. M., Zhen, L. S., Wang, F., et al. (2010). Anticancer effect and structure-activity analysis of marine products isolated from metabolites of mangrove fungi in the South China Sea. Mar. Drugs 8, 1094–1105. doi: 10.3390/md8041094
Teixeira, T. A.-O., Santos, G. A.-O., Armstrong, L. A.-O., Colepicolo, P., and Debonsi, H. A.-O. (2019). Antitumor potential of seaweed derived-endophytic fungi. Antibiotics 8:205.
Thammajaruk, N., Sriubolmas, N., Israngkul, D., Meevootisom, V., and Wiyakrutta, S. (2011). Optimization of culture conditions for mycoepoxydiene production by Phomopsis sp. Hant25. J. Ind. Microbiol. Biotechnol. 38, 679–685. doi: 10.1007/s10295-010-0813-7
Tidke, S. A., Kiran, S., Giridhar, P., and Gokare, R. A. J. R. S. P. (2019). Current understanding and future perspectives of endophytic microbes Vis-a-Vis production of secondary metabolites. Cham: Springer.
Tupac Otero, J., Ackerman, J., Bayman, P., and Bayman, P. (2002). Diversity and host specificity of endophytic Rhizoctonia‐like fungi from tropical orchids. American J. Botany 89, 1852–1858.
Wang, C. Y., Hao, J. D., Ning, X. Y., Wu, J. S., Zhao, D. L., and Kong, C. J. (2018). Penicilazaphilones, two new azaphilones from a spongederived strain of the fungus penicillium sclerotiorum. RSC Adv. 8, 4348–4353.
Wang, W.-J., Li, D.-Y., Li, Y.-C., Hua, H.-M., and Ma, E.-L.Li, Z-LJJoNP (2014). Caryophyllene sesquiterpenes from the marine-derived fungus Ascotricha sp. ZJ-M-5 by the one strain–many compounds strategy 77, 1367–1371.
Wang, S., Li, X. M., Teuscher, F., Li, D. L., Diesel, A., Ebel, R., et al. (2006). Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 69, 1622–1625. doi: 10.1021/np060248n
Wang, Z., Zhao, M., Yu, Y., Kong, F., Lin, N., and Wang, Q. (2025). Marine fungal metabolites as potential antidiabetic agents: a comprehensive review of their structures and enzyme inhibitory activities. Mar. Drugs 23:142. doi: 10.3390/md23040142
Wei, Y., Liu, L., Zhou, X., Lin, J., Sun, X., and Tang, K. (2012). Engineering taxol biosynthetic pathway for improving taxol yield in taxol-producing endophytic fungus EFY-21 (Ozonium sp.). Afr. J. Biotechnol. 11, 9094–9101.
Wei, Y., Zhou, X., Liu, L., Lu, J., Wang, Z., Yu, G., et al. (2010). An efficient transformation system of taxol-producing endophytic fungus EFY-21 (Ozonium sp.). Afr. J. Biotechnol. 9, 1726–1733. doi: 10.5897/AJB2010.000-3019
Wen, L., Cai, X., Xu, F., She, Z., Chan, W. L., Vrijmoed, L. L. P., et al. (2009). Three metabolites from the mangrove endophytic fungus Sporothrix sp. (#4335) from the South China Sea. J. Org. Chem. 74, 1093–1098. doi: 10.1021/jo802096q
Wilson, D. (1995). Endophyte: the evolution of a term, and clarification of its use and definition. Oikos 73, 274–276. doi: 10.2307/3545919
Wu, W. H., and Morris, D. R. (1973). Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J. Biol. Chem. 248, 1687–1695.
Xin, W., M, Z.-G., S, B.-B., C, C.-H., X, W.-W., H, B., et al. (2013). Advances in the study of the structures and bioactivities of metabolites isolated from mangrove-derived fungi in the South China Sea 11, 3601–3616.
Xing, X., and Guo, S. J. E. R. (2011). Fungal endophyte communities in four Rhizophoraceae mangrove species on the south coast of China. Ecol. Res. 26, 403–409. doi: 10.1007/s11284-010-0795-y
Xu, B., Jin, Z., Wang, H., Jin, Q., Jin, X., and Cen, P. (2008). Evolution of Streptomyces pristinaespiralis for resistance and production of pristinamycin by genome shuffling. Appl. Microbiol. Biotechnol. 80, 261–267.
Xu, L.-J., Liu, Y.-S., Zhou, L.-G., and Wu, J.-Y. J. P. B. (2009). Enhanced beauvericin production with in situ adsorption in mycelial liquid culture of fusarium redolens Dzf2. Process Biochem. 44, 1063–1067. doi: 10.1016/j.procbio.2009.05.004
Yajima, A., Shirakawa, I., Shiotani, N., Ueda, K., Murakawa, H., Saito, T., et al. (2021). Practical synthesis of aromatic bisabolanes: synthesis of 1,3,5-bisabolatrien-7-ol, peniciaculin a and B, and hydroxysydonic acid. Tetrahedron 92:132253. doi: 10.1016/j.tet.2021.132253
Yamada, T., Doi, M., Shigeta, H., Muroga, Y., Hosoe, S., Numata, A., et al. (2008). Absolute stereostructures of cytotoxic metabolites, chaetomugilins A–C, produced by a Chaetomium species separated from a marine fish. Tetrahedron Lett. 49, 4192–4195. doi: 10.1016/j.tetlet.2008.04.060
Yamazaki, H., Rotinsulu, H., Kaneko, T., Murakami, K., Fujiwara, H., Ukai, K., et al. (2012). A new dibenz[b,e]oxepine derivative, 1-hydroxy-10-methoxy-dibenz[b,e]oxepin-6,11-dione, from a marine-derived fungus, Beauveria bassiana TPU942. Mar. Drugs 10, 2691–2697. doi: 10.3390/md10122691
Yang, Y., Zhao, H., Barrero, R. A., Zhang, B., Sun, G., Wilson, I. W., et al. (2014). Genome sequencing and analysis of the paclitaxel-producing endophytic fungus penicillium aurantiogriseum NRRL 62431 15, 1–14.
Yu, G., Wu, G., Sun, Z., Zhang, X., Che, Q., Gu, Q., et al. (2018). Cytotoxic Tetrahydroxanthone dimers from the mangrove-associated fungus aspergillus versicolor HDN1009. Mar. Drugs 16:335. doi: 10.3390/md16090335
Zhang, Y., Li, X. M., Feng, Y., and Wang, B. G. (2010). Phenethyl-alpha-pyrone derivatives and cyclodipeptides from a marine algous endophytic fungus Aspergillus niger EN-13. Nat. Prod. Res. 24, 1036–1043. doi: 10.1080/14786410902940875
Zhang, Y., Li, X. M., and Wang, B. G. (2007). Nigerasperones a approximately C, new monomeric and dimeric naphtho-gamma-pyrones from a marine alga-derived endophytic fungus Aspergillus niger EN-13. J. Antibiot. 60, 204–210. doi: 10.1038/ja.2007.24
Zhang, P., Li, X., and Wang, B. G. (2016). Secondary metabolites from the marine algal-derived endophytic fungi: Chemical diversity and biological activity. Planta Medica 82, 832–842.
Zhao, K., Penttinen, P., Guan, T., Xiao, J., Chen, Q., Xu, J., et al. (2011). The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Current Microbiol. 62, 182–190.
Zheng, C. J., Shao, C. L., Guo, Z. Y., Chen, J. F., Deng, D. S., Yang, K. L., et al. (2012). Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 75, 189–197. doi: 10.1021/np200766d
Zhu, T. J., Du, L., Hao, P. F., Lin, Z. J., and Gu, Q. Q. (2009). Citrinal a, a novel tricyclic derivative of citrinin, from an algicolous fungus penicillium sp. i-1-1. Chin. Chem. Lett. 20, 917–920. doi: 10.1016/j.cclet.2009.03.009
Keywords: marine endophytic fungi, bioactive metabolites, strain improvement, fermentation optimization, biosynthetic potential
Citation: Kong C-C, Wang J, Shan B, Zhang H-X, Qin S and Ren C-G (2025) Marine endophytes: biosynthetic engines for novel bioactive metabolites. Front. Microbiol. 16:1684777. doi: 10.3389/fmicb.2025.1684777
Edited by:
Mugesh Sankaranarayanan, Vel Tech Rangarajan Dr. Sagunthala R&D Institute of Science and Technology, IndiaReviewed by:
Nirmala Nithya Raju, Vel Tech Rangarajan Dr. Sagunthala R&D Institute of Science and Technology, IndiaMahavir Joshi, Chandigarh University, India
Copyright © 2025 Kong, Wang, Shan, Zhang, Qin and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cun-Cui Kong, Y2dyZW5AeWljLmFjLmNu
 Cun-Cui Kong
Cun-Cui Kong Jiayi Wang1
Jiayi Wang1