- 1Faculty of Pharmacy, Innovation Center for Holistic Health, Nutraceuticals, and Cosmeceuticals, Chiang Mai University, Chiang Mai, Thailand
- 2Office of Research Administration, Chiang Mai University, Chiang Mai, Thailand
- 3Department of Microbiology, Karpagam Academy of Higher Education, Coimbatore, India
- 4Department of Microbiology, PSG College of Arts and Science, Coimbatore, Tamil Nadu, India
Lactic acid bacteria (LAB) play a pivotal role in the food industry, particularly in the fermentation and preservation of meat products. These Gram-positive, non-spore-forming microorganisms contribute significantly to food safety, shelf-life extension, and sensory quality enhancement through the production of various bioactive compounds, including organic acids, bacteriocins, exopolysaccharides, and gamma-aminobutyric acid. Their antimicrobial and probiotic properties are attributed to inhibiting the growth of spoilage organisms and foodborne pathogens, thereby reducing the reliance on synthetic preservatives. This review discusses the general characteristics and selection criteria of LAB, with a focus on their biochemical contributions to the development of flavor, texture, and functional properties in meat-based products. LABs are increasingly being recognized for their potential as natural bio-preservatives, aligning with the growing consumer demand for clean-label and functional foods. However, several challenges persist, including strain-specific variability in functional properties, safety assessments, optimization of metabolite production, and consumer perception. Addressing these limitations through multidisciplinary research and technological innovation is essential to enhance the effective and sustainable application of LAB in the meat industry.
1 Introduction
Lactic acid bacteria (LAB) have long been recognized for their significant role in the food industry, particularly in fermentation, preservation, and quality enhancement. They are non-spore-forming bacteria belonging to genera including Lactiplantibacillus, Leuconostoc, and Streptococcus. They are classified as obligatory or relative anaerobes, which gives them the ability to withstand the acidic environmental conditions (Gupta et al., 2018). These microorganisms are primarily known for their ability to convert carbohydrates into lactic acid; this metabolic process allows them to produce organic compounds other than lactic acid, such as mannitol and dextran. These organic compounds play a crucial role in extending shelf life, enhancing safety, and improving the sensory properties of various food products (Mesele, 2018). To produce fermented dairy products, such as yogurt, cheese, butter, and sour cream, lactic fermentation is utilized to acidify milk. Furthermore, this method is utilized for cold cut maturation, and it is responsible for producing and stabilizing sourdough and vegetable silage (Muhialdin et al., 2020). As these LAB are widely used in the food industry, they are usually regarded as safe; additionally, they are an essential part of the natural microflora found in the human intestine (Agriopoulou et al., 2020). Among their many applications, their role in the meat industry is particularly significant. Meat and meat products are rich in nutrients like protein, fats, vitamins, and minerals; they have long been a staple of human diets. Meat, however, also serves as a significant source of nutrients for pathogenic bacteria, which can proliferate quickly, leading to increased food waste and financial losses for the meat industry (Woraprayote et al., 2016). Microbial contamination is therefore a significant concern regarding quality and safety in the meat industry (Pradhan et al., 2018). Thus, the meat industry employs a range of traditional techniques, including drying, freezing, packaging, canning, curing, and dehydration, as well as chemical treatment processes, to create safe food items with extended shelf life. Over the past several years, there has been a growing demand for high-quality meats and food products with high nutritional content, free from synthetic chemicals (Zhaxybayeva et al., 2020).
To address these issues, the food manufacturing sector is seeking innovative natural alternatives that serve as preservatives, ensure sufficient microbiological safety, and prolong the shelf life of products. According to numerous studies, a small number of microorganisms from the LAB can be added or utilized as bio-protective cultures or starters in meat-based products (Bintsis, 2018). LAB can stop the growth and other reactions of spoilage bacteria, possibly due to their metabolites, antimicrobial compounds, which help prevent meat degradation. LAB also has prospects as efficient and natural food preservatives, and a suitable alternative to chemicals. Additionally, LAB strains have been explored for their probiotic potential and ability to produce bioactive compounds, further increasing their value in functional meat products (Imade et al., 2021).
2 General characteristics and selection process of lactic acid bacteria
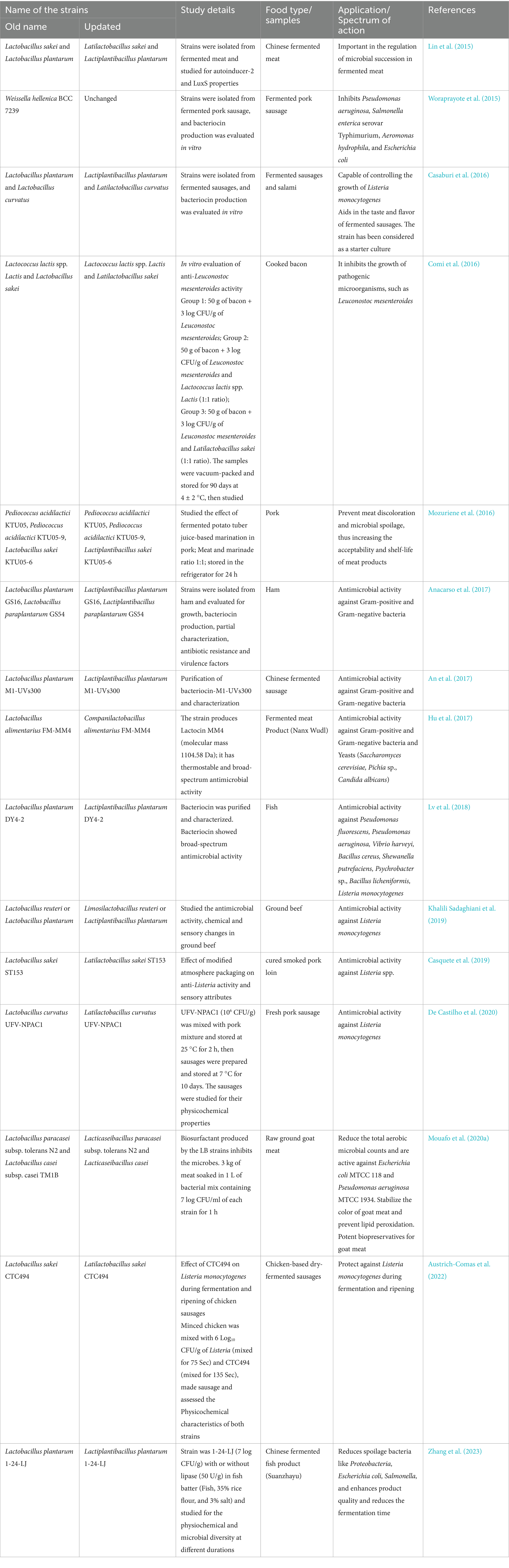
LAB are microaerophilic organisms and prefer anaerobic environments for their growth. Most of the LAB strains prefer an acidic pH (Strafella et al., 2020). More than 25 genera, including Schleiferilactobacillus, Lacticaseibacillus, Levilactobacillus, Agrilactobacillus, Furfurilactobacillus, Fructilactobacillus, Lactiplantibacillus, Ligilactibacillus, Paralactobacillus, Streptococcus, Carnobacterium, Enterococcus, Pediococcus, and Weissella, are considered as LAB (Da Costa et al., 2019) (Table 1). The genus Lactobacillus was split into 25 genera (e.g., Lacticaseibacillus, Lactiplantibacillus, Levilactobacillus) in 2020 due to its considerable diversity. To avoid confusion, new standard abbreviations have been proposed for scientific use, while also allowing references to the “former Lactobacillus” when discussing older studies (Zheng et al., 2020; Todorov et al., 2023). LAB plays an important role in food fermentations. Fermentation occurs with the participation of homo and hetero-fermentative LAB. Homo-fermentation is a mechanism by which certain LAB convert disaccharides into nearly pure lactic acid. Another slightly different process is called hetero-fermentation, wherein lactic acid is not the sole by-product of lactose breakdown, but also produces ethyl alcohol, carbon dioxide, hydrogen peroxide, diacetyl, acetoin, and acetic aldehyde (Mandha et al., 2021). LAB are crucial as they produce a variety of metabolites with antimicrobial activity during the growth and fermentation process, such as lactic acid, acetic acid, hydrogen peroxide, low molecular weight compounds (diacetyl, fatty acids, reuterin, reutericyclin), antifungal substances (phenyl lactate, propionate, hydroxyphenyl lactate), and bacteriocins (Castellano et al., 2017). LABs are primarily found in environments rich in nutrients. They are a significant component of the microbial communities present in dairy products, such as milk, cheeses, and kefir, as well as in fish, meat, and vegetables. They are also a part of the natural microbiota of the gastrointestinal tract and the vagina of both humans and animals (Bengoa et al., 2019). LAB have been utilized as starters, adjuncts, and protective microorganisms in the production of fermented meats, vegetables, dairy products (such as yogurt and cheese), and fish products (Ashaolu, 2020; Ashaolu and Reale, 2020; Peerajan et al., 2016; Woraharn et al., 2016) (Table 2).
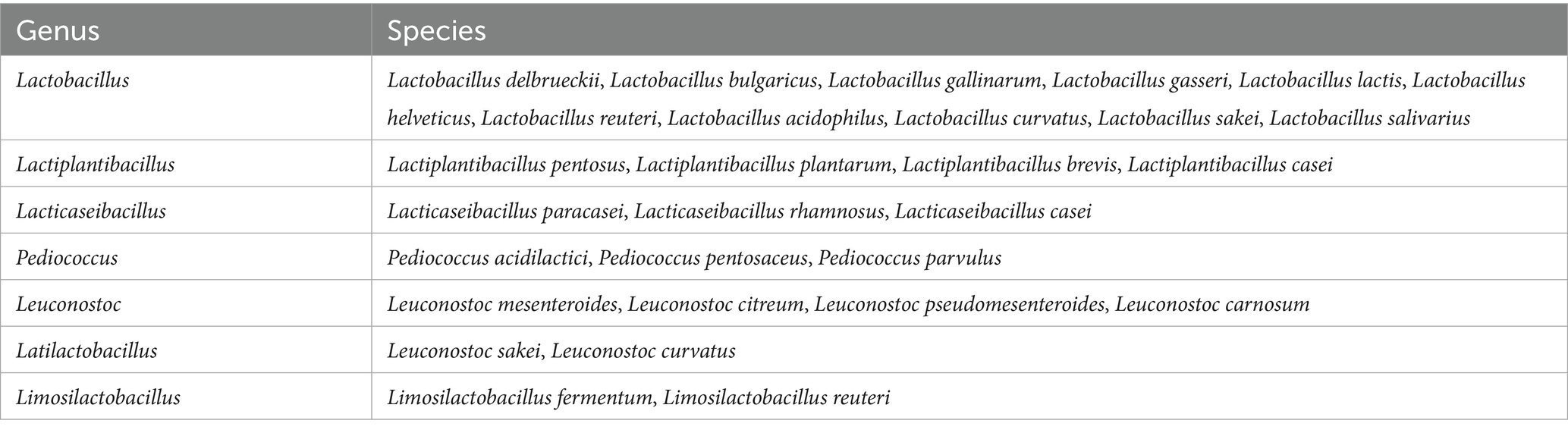
Table 1. Commonly used lactic acid bacteria in meat preservation (Kaveh et al., 2023).
According to the European Food Safety Authority and the Food and Drug Administration (FDA), LABs are considered Generally Recognized as Safe (GRAS), which means they are safe for consumption by humans and animals. LAB could be obtained from various sources, including decomposing sites, dairy and other fermented food products, animal and human gut, mouth cavities, and agroecosystems (Raman et al., 2022). The commonly used LABs are Lactiplantibacillus, Lacticaseibacillus, Latilactobacillus, Limosilactobacillus, Lactococcus, Leuconostoc, Pediococcus, Weissella, and Periweissella (Ayivi et al., 2020).
The initial screening and selection process of LAB involves several key factors, including immunogenicity, phenotype, and genotype stability (including plasmid stability), carbohydrate and protein utilization patterns, production of antimicrobial substances, and the capacity to inhibit known pathogens, spoilage organisms, or both. LAB can be isolated from various sources; however, to be used for human use, it must be safe and isolated from the human microflora system (Bikila, 2015).
The process of selecting LAB involves a comprehensive evaluation from four main perspectives: safety, technology, functionality, and benefits. The main goal of safety aspects is to identify and describe the bacterial strain’s species, genus, and place of origin. Assessing the strain’s pathogenicity and infectivity, as well as its virulence factors (including metabolic activity, toxicity, and inherent features such as antibiotic resistance), is essential for ensuring consumer safety. Technological aspects examine the strain’s stability and performance during production and storage. Genetic stability, excellent viability throughout processing, and the addition of desirable sensory attributes to the finished product are all characteristics of ideal strains. Functional characteristics assess a strain’s ability to endure difficult gastrointestinal conditions, including exposure to bile acids, low pH, and gastric and pancreatic secretions. Benefits revolve around the strain’s ability to suppress dangerous microorganisms and alter the immune system (Gupta et al., 2018).
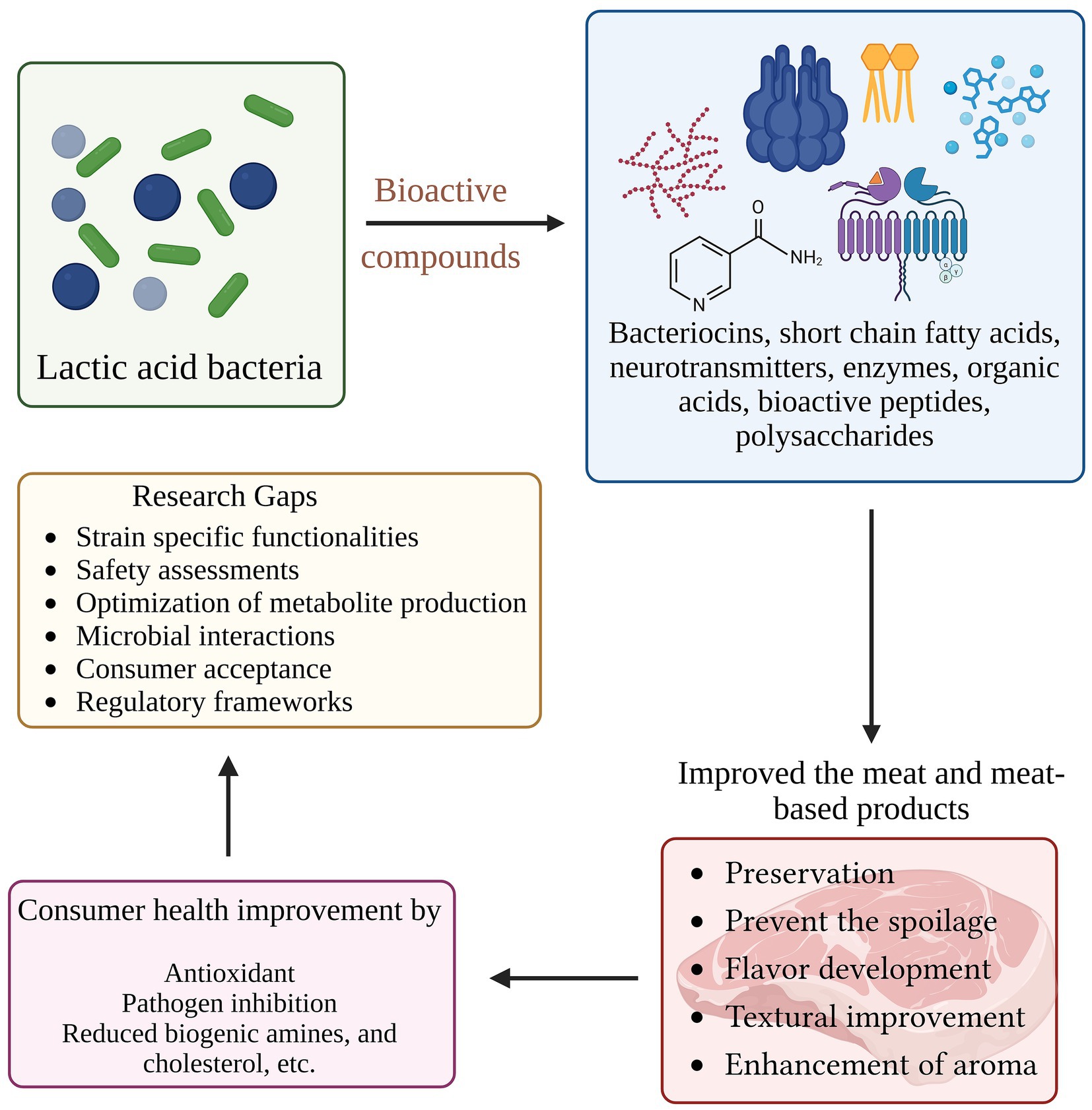
3 Bioactive compounds from lactic acid bacteria
A range of physical and chemical preservation techniques was employed to inhibit the pathogenic microbial growth and to increase the shelf life of meat products (Kaveh et al., 2023). However, most physical and chemical methods are associated with various drawbacks, including nutritional alterations and changes in the organoleptic properties of meat products. Moreover, the excessive consumption of these chemical preservatives causes carcinogenic effects in humans. Thus, the needs for bio-preservatives in the food industry possess significant importance and consumer interest to produce chemical-free food products (Kaveh et al., 2023; Gómez et al., 2020). Among the key LAB species involved in the processing of meat products are Latilactobacillus sakei, Latilactobacillus curvatus, and Lactiplantibacillus plantarum (Parlindungan et al., 2021). LAB plays a significant role in meat safety and protection through the production of various bioactive compounds. The capacity of lactic acid bacteria to generate significant quantities of bioactive compounds during fermentation is well established. The most significant bioactive substances produced by LAB during fermentation are peptides, EPS, bacteriocins, vitamins, gamma-aminobutyric acid, some amylases, proteases, lipase enzymes, and lactic acid (Fitsum et al., 2025; Anumudu et al., 2024). The health-promoting qualities of LAB make them useful microorganisms. Thus, LAB ensures the consumption of safe and nutritious food for all human beings (Perez and Ancuelo, 2023).
3.1 Bacteriocins
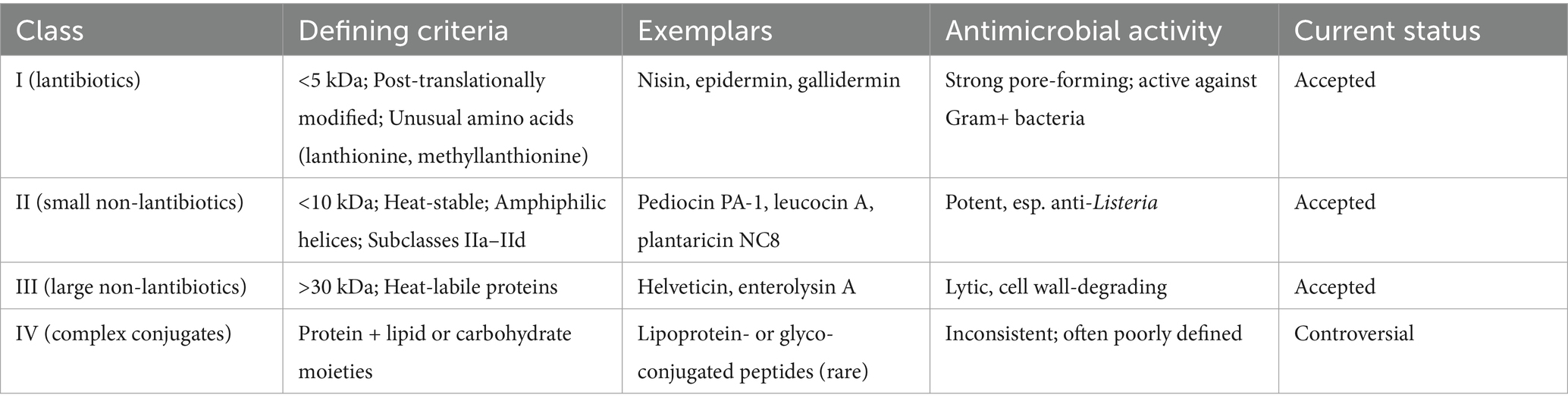
Active metabolic peptides known as bacteriocins are produced by the ribosome of specific LAB and non-lactic acid bacteria. Different LAB produce distinct bacteriocins, each with its own unique biochemical, structural, genetic, ecological, and metabolic properties (Balay et al., 2017; Choeisoongnern et al., 2020). The role of bacteriocins includes causing damage to the integrity of the target bacteria’s cells, impeding biological functions, and interfering with DNA or protein synthesis. Bacteriocin production is significantly influenced by several environmental parameters, including pH, incubation temperature, nutritional availability, and the composition of the growth medium (Kumariya et al., 2019). Generally, bacteriocins are positively charged molecules and are hydrophobic. Bacteriocins can interact with the negatively charged microbial membranes (phosphate groups) or the receptors present in the bacterial cell wall. Bacteriocins can target nucleic acid synthesis and protein synthesis in pathogenic bacteria and affect the balance of the cytoplasmic membrane. Furthermore, bacteriocins produce pores in the cell membranes of pathogenic bacteria, which ultimately affect the pH of the target cell and cause cellular material leakage (Kaveh et al., 2023). Bacteriocins typically display a narrow antimicrobial spectrum, often inhibiting microorganisms that are phylogenetically related (closely related species or genera) to the producing strain (Riley and Wertz, 2002; Cotter et al., 2005). In addition, Sakacin Q is a bacteriocin that was produced by Latilactobacillus curvatus ACU-1 (formerly Lactobacillus curvatus) isolated from artisanal dry sausages and can inhibit Listeria monocytogenes on cooked meat products (Rivas et al., 2014). Bacteriocins are classified into class I, class II, class III, and class IV bacteriocins (Table 3).
Bacteriocins exhibit strong activity against foodborne pathogens, such as Listeria monocytogenes, Clostridium spp., and Staphylococcus aureus, and have demonstrated stability across a range of pH values, temperatures, and storage conditions, thereby enhancing their technological applicability. Importantly, bacteriocinogenic LAB such as Latilactobacillus sakei (formerly Lactobacillus sakei) and Latilactobacillus curvatus (formerly Lactobacillus curvatus) have been widely isolated from fermented meat products and are recognized for producing sakacins and curvacins with potent anti-listerial activity, thus underscoring the potential of meat-derived strains in food biopreservation. Bacteriocins can be applied to meat products by adding them directly, incorporating them into antimicrobial packaging, or using bacteriocin-producing LAB as starter or protective cultures. Evidence suggests that bacteriocins not only inhibit spoilage and pathogenic microorganisms but also contribute positively to product shelf life and sensory quality. Nevertheless, limitations such as reduced efficacy against Gram-negative bacteria, possible inactivation by meat matrix components, and regulatory barriers must be addressed. It has been recognized that bacteriocins should be integrated into a hurdle technology framework, complementing other preservation strategies to ensure microbial safety and respond to consumer demand for minimally processed, natural, and safe meat products (Da Costa et al., 2019; Fernandes et al., 2024).
3.1.1 Class I bacteriocins (lantibiotics)
This type of bacteriocin consists of one or two small peptides (<5 kDa). Further, it is a post-translational modified bacteriocin. Hence, it consists of unusual amino acids like lanthionine, β-methyllanthionine, and dehydrated amino acids, which facilitate the structural stability for heat, pH, and proteolytic resistance. Furthermore, it commonly inhibits foodborne pathogens and gram-negative bacteria. Moreover, class I bacteriocins are again classified as Group Ia and Group Ib. Here, group Ia bacteriocins (nisin, epidermin, gallidermin) are positively charged peptides that form pores in the bacterial cell wall, thereby increasing the permeability in target bacteria, thus destroying the pathogens. Group Ia bacteriocins are screw-shaped, flexible, and amphipathic. Group Ib bacteriocins are negatively charged peptides that inhibit enzyme activity and kill the target bacteria. Examples of these bacteriocins are lacticin 481, cytolysin, and salivaricin, and they are globular-shaped and inflexible (Kaveh et al., 2023; Perez and Ancuelo, 2023; Lahiri et al., 2022a).
3.1.2 Class II bacteriocins (small non-lantibiotics)
Class II bacteriocins are small, stable peptides that are hydrophobic and resistant to heat. They typically consist of 30–60 amino acids and have a molecular weight of less than 10 kDa. These peptides exhibit an amphiphilic helical structure, which plays a crucial role in disrupting bacterial membranes, resulting in depolarization and ultimately leading to pathogen cell death. Since they lack lanthionine or methyllanthionine, they are classified as non-lantibiotics (Alvarez-Sieiro et al., 2016). The unusual amino acids that are present in class I bacteriocins are not present in class II bacteriocins. The post-translational modification causes bisulfide bridge formation in some bacteriocins, for example, pediocin PA-1 and pediocin AcH. Like class I bacteriocins, class II bacteriocins are also heat-stable, <10 kDa-sized peptides that cause larger pores on the bacterial surface, thus increasing their ease of entry into the bacterial cells, destroying them. Further, it was divided into four subclasses. Group IIa bacteriocins are linearly structured and consist of bisulfide bridges, which can inhibit or kill Listeria sp. Hence, it is called an anti-listerial bacteriocin, which includes leucocin A, acidocin A, and pediocin PA-1. Group IIb bacteriocins are called two-peptide bacteriocins, which have antimicrobial activity, and include lactococcin G, lactococcin Q, and plantaricin NC8. Group IIc bacteriocins are leader peptide sequences containing one or two cysteine residues, cystibiotics and thiolbiotics, respectively, which are known for their antimicrobial activity. Group IId bacteriocins are linear, non-pediocin-like, single-peptide bacteriocins which include epidermicin NI01 and lactococcin A (Yang et al., 2014; Abdul Hakim et al., 2023).
3.1.3 Class III bacteriocins (large non-lantibiotics)
Class III bacteriocins, which exceed 30 kDa in size, are produced by Lactobacillus helveticus and are heat-labile. These bacteriocins, like those synthesized by other bacteria, must be secreted to engage with target cells and exert their antimicrobial properties (Wang et al., 2021). Furthermore, this group was divided into two subgroups, designated as group IIIa and IIIb. Group IIIa is a lytic bacteriocin like Lysostaphin, which can lyse the cell membrane and thus destroy the bacterial cell. Moreover, the group IIIb bacteriocins, like Enterolysin A, disrupt the cell wall and reduce intercellular ATP concentration, resulting in the death of bacteria (Raman et al., 2022; Xu J. et al., 2025).
3.1.4 Class IV bacteriocins (complex conjugates)
A fourth group was traditionally proposed for complex bacteriocins with lipid or carbohydrate moieties (lipoprotein or glycosylated conjugates). However, these are controversial, as many lack a clear demonstration of ribosomal peptide origin and consistent antimicrobial activity. Modern consensus generally excludes Class IV from the bacteriocin framework, treating Classes I to III as the accepted groups. Class IV is best regarded as a deprecated or provisional category (Alvarez-Sieiro et al., 2016; Lahiri et al., 2022b; Solis-Balandra and Sanchez-Salas, 2024).
3.2 Enzymes
Enzymes are biocatalysts that are significantly involved in all anabolic and catabolic pathways, and LAB effectively produce some of these enzymes, including lactase, proteases, peptidases, fructanases, bile salt hydrolase, and phytases. Lactase, also called β-galactosidase enzyme, used in the milk industry, can degrade lactose molecules into glucose and galactose. Lack of this enzyme in human beings causes a health issue called lactose intolerance. The lactase produced by LAB is considered an excellent solution for lactose indigestion. Various species like Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus can produce the highest concentration of lactase (Ayivi and Ibrahim, 2022). The proteolytic enzymes produced by LAB include proteinases, peptidases, and transport proteins. Proteinases are involved in the degradation of casein in milk products into peptides. Further, peptidases cleave the peptides into amino acids and smaller peptides. The transport protein transfers amino acids and peptides across the cytoplasmic membrane (Kieliszek et al., 2021). It has been reported that some LABs produce fructanases, which break down fructan into fructose and add sugar to bread (Murniece et al., 2025). Similarly, Kusada et al. (2022) reported that bile salt hydrolase produced by LAB can hydrolyse glycine/taurine-conjugated bile salts produced by mammalian digestive tracts. The LAB-produced phytase breaks down phytate and releases myo-inositol, lesser forms of inositol phosphate, and solubilized forms of inorganic phosphate (Sharma et al., 2020). In addition to proteins, lipids, and glycogen in meat products, LAB degraded dietary compounds (Wang et al., 2021). Thus, LAB are involved in the production of various enzymes and play a crucial role in the digestion of various food products.
Enzymes play both endogenous and exogenous roles in meat processing. After slaughter, endogenous proteases such as calpains, cathepsins, and their regulators (e.g., calpastatin) gradually break down muscle proteins during aging or maturation, improving tenderness, juiciness, and flavor of the meat (Abril et al., 2023). To accelerate or control these changes, exogenous enzymes, particularly proteases from plant sources like papain, bromelain, ficin, actinidin, zingibain, and others, are applied to tougher or lower-quality meat cuts (Mohd Azmi et al., 2023; Abril et al., 2023; Fayaz et al., 2024). These proteolytic enzymes cleave structural proteins in myofibrils and connective tissue, reducing toughness and improving palatability (Mohd Azmi et al., 2023; Fayaz et al., 2024). Enzymes like transglutaminase are used to bind small meat pieces together, reducing waste and creating restructured products with better texture. Additionally, enzymatic control of glycolysis, lipolysis, and proteolysis during processing influences flavor development, color stability, and overall quality attributes. However, practical challenges such as enzyme stability, control of over-digestion (which can cause mushy texture), cost, and compatibility with other processing steps must be managed carefully (Mohd Azmi et al., 2023; Abril et al., 2023; Fayaz et al., 2024).
3.3 Gamma-aminobutyric acid
Gamma-aminobutyric acid (GABA), a neuroinhibitory amino acid, is naturally found in plants and mammals. GABA’s natural abundance in plants, foods, and mammalian tissues is generally low, necessitating chemical production or microorganism-based bioconversion (Wu and Li, 2018). LAB produce GABA via the glutamate decarboxylase (GAD) pathway, in which the enzyme GAD catalyzes the decarboxylation of L-glutamate (or its salt, e.g., monosodium glutamate) to yield GABA and CO₂. Many GABA-producing LAB strains carry one or more gad genes (e.g., gadA, gadB) and often a glutamate/GABA antiporter (gadC) to export GABA out of the cell (Yogeswara et al., 2020; Lyu et al., 2018; Diez-Gutiérrez et al., 2022; Cataldo et al., 2024). The process of converting glutamate to GABA is not irreversible. There is data in the literature demonstrating the use of GABA as an energy source by microorganisms. GABA can enter the tricarboxylic acid cycle and be converted to glucose. Furthermore, its production by LAB does not occur solely through the action of GAD. It can also be produced through the metabolism of putrescines (Cui et al., 2020; Diez-Gutiérrez et al., 2020).
The production of GABA by LAB is often associated with acid stress/acid tolerance mechanisms. Under low pH or acid challenge (such as during fermentation or in acidic environments), activation of the GAD system helps the cell consume intracellular protons (H+) through the decarboxylation reaction, thus contributing to maintaining intracellular pH homeostasis (Dhakal et al., 2012; Cui et al., 2020; Diez-Gutiérrez et al., 2022; Cataldo et al., 2024). In many LAB (e.g., Lactobacillus brevis), expression of the gad operon is upregulated at lower pH, linking GABA production to survival under acidic conditions (Lyu et al., 2018; Cataldo et al., 2024).
GABA is considered one of the bioactive compounds produced by LAB, which may be beneficial to the user’s health. By enhancing oxygen delivery and blood flow, GABA can improve the metabolism of brain cells that regulate blood pressure, protein synthesis, hormone production, and fat burning (Alizadeh Behbahani et al., 2020). Since the food industry prohibits the use of chemically manufactured GABA, bioconversion employing food-grade LAB has emerged as a crucial technique for producing GABA or GABA-rich foods (sprouted or germinated grains and legumes, and fermented foods like kimchi, yoghurt and cheese, especially when fermented with GABA-producing LAB) (Lee and Paik, 2017). LAB produces GABA by utilizing the enzyme glutamate decarboxylase to decarboxylate L-glutamate in an anaerobic environment, while also utilizing protons (Woraharn et al., 2014; Tang et al., 2023). Potential health benefits of GABA include lowering cholesterol, regulating blood pressure, having anti-carcinogenic qualities, and preventing depression by encouraging relaxation and lowering anxiety. Lactobacillus namurensis (Reclassified as Levilactobacillus namurensis), Lactobacillus paracasei (Reclassified as Lacticaseibacillus paracasei) and Lactobacillus brevis (Reclassified as Levilactobacillus brevis) are examples of LAB species that have demonstrated the ability to produce GABA through glutamate decarboxylase (Alizadeh Behbahani et al., 2020).
3.4 Short-chain fatty acid
The human intestine lacks some of the carbohydrate digestive enzymes, which affects gut health. Generally, cellulose, xylans, resistant starch, inulin, and dietary fibers often remain undigested by the human intestine due to a lack of digestive enzymes. These compounds are denoted as undigested carbohydrates. LAB can ferment this kind of undigested carbohydrate into short-chain fatty acids (SCFAs), including butyric acid, propionic acid, and acetic acid. These SCFAs have therapeutic applications and play a crucial role in maintaining gut health. Acetic acid is essential for controlling intestinal inflammation and plays a role in minimizing the spread of pathogens. Similarly, butyric and propionic acid can improve insulin responsiveness and decrease the risk of diet-induced obesity. Furthermore, butyric acid also exhibits anticancer activity against colon cancer (Pessione et al., 2015). Moreover, the formation of SCFAs in the gastrointestinal region creates an acidic environment, resulting in the depletion of growth of harmful bacteria and plays a vital role in diminishing the proliferation rate of harmful bacteria (LeBlanc et al., 2017). It has been reported that Lactobacillus plantarum (Reclassified as Lactiplantibacillus plantarum), Lactobacillus pentosus (Reclassified as Lactiplantibacillus pentosus), and Leuconostoc mesenteroides are the strains capable of effectively producing SCFAs with notable antibacterial properties (Pessione et al., 2015). Additionally, Lactiplantibacillus plantarum has been shown to degrade lipid compounds in meat, leading to the formation of SCFAs (Uppada et al., 2017).
3.5 Organic acids
LAB are known for their important role in fermentation, producing a variety of organic acids that are significant metabolic products (Von Wright and Axelsson, 2019; Chen et al., 2017). Depending on the metabolic pathway, some metabolisms, such as sugar metabolism, can produce different types of organic acids, including lactic acid, acetic acid, butyric acid, and propionic acid. The primary result of the metabolic pathway is lactic acid, which is then separated into l-lactic acid and d-lactic acid according to the various arrangements of the chiral atom. Lactic acid is produced due to anaerobic conditions throughout the glycolysis pathway, contributing to the sour taste of fermented foods (Chen et al., 2017). These organic acids influence the taste, consistency, and shelf life of fermented foods while also promoting food safety by preventing the growth of pathogens and spoilage organisms. The primary anti-bacterial actions of these acids result from their disruption of the bacterial cytoplasmic membrane, which impairs active transport pathways and disrupts the membrane potential, ultimately inhibiting the growth of harmful microorganisms (Bangar et al., 2022). Lactic acid is essential to the fermentation of foods, including cheese, yogurt, pickles, and sauerkraut (Ayivi and Ibrahim, 2022). LAB produces it, specifically Lactobacillus (Reclassified as Lacticaseibacillus, Levilactobacillus, Ligilactobacillus, and Lactiplantibacillus) and Streptococcus species, which break down carbohydrates, including lactose in milk, through metabolic processes (Bangar et al., 2022).
3.6 Vitamins
Vitamins are micronutrients that are involved in human metabolism, but humans are unable to synthesize them. Hence, food materials are considered the sole source of vitamins in humans. Many LABs can produce various vitamins, such as vitamin B and vitamin C. During lactic acid fermentation, vitamins are produced by LABs, which play a vital role in the production of nutrient-fortified food products. Folic acid or vitamin B9 is significant for the biosynthesis of nucleotides, DNA, RNA, and proteins. It has been demonstrated that Lactococcus lactis and Streptococcus thermophilus are capable of synthesizing folic acid in the human gut, which serves as a precursor for nucleotide and nucleic acid biosynthesis (Sybesma et al., 2003). Many LABs possess riboflavin (vitamin B2) synthase genes such as ribG, ribB, ribA, and ribH within their operon, which catalyze the production of riboflavin using guanosine triphosphate and ribulose-5-phosphate as substrates. Furthermore, it has been noted that Lactiplantibacillus plantarum CRL 725 can produce riboflavin (Juarez del Valle et al., 2014). Additionally, Li et al. (2017) indicated that vitamin B12 can be sourced from meat and meat-derived products (Bacon, sausage, ham, and other animal-source foods like milk and eggs).
Vitamin C is a water-soluble vitamin with high antioxidant potential, playing a crucial role in maintaining human health. During lactic acid fermentation, the LAB can produce Vitamin C (Quan et al., 2022). LABs like Lactiplantibacillus plantarum, Limosilactobacillus fermentum, Lactobacillus acidophilus, and Bifidobacterium longum can produce vitamin C. Moreover, LAB can also synthesize vitamin K2 (menaquinones) via the menaquinone-synthesis pathway. Lactococcus lactis has the potential to produce vitamin K2 using various carbon sources (fructose, trehalose, maltose, and mannitol). Considering the crucial role of LAB in the production of vitamins, it has also been used in recent years for therapeutic applications to reduce vitamin deficiency or inflammatory diseases (Liu et al., 2019).
3.7 Exopolysaccharides
Exopolysaccharides (EPS) are polysaccharides produced by microbes. They are expelled from the bacterial cell wall. LABs are the ones that create the most different types of EPS (Sanalibaba and Cakmak, 2016). For fermented foods to have their specific texture, viscosity, and probiotic qualities, EPS is essential. Due to their ability to retain water, these polymers are commonly used in the food industry as stabilizers and emulsifying agents (Singh and Saini, 2017). Conversely, EPS have been linked to the potential health advantages of their anti-inflammatory, antitumor, and anticancer properties. Numerous studies have demonstrated that EPS support gut health and encourage bacterial colonization by creating a protective matrix (Flemming, 2016). Weissella, Leuconostoc, Lactococcus, Fructilactobacillus, and Lactiplantibacillus plantarum are particularly capable of producing various types of EPS, depending on the strain. Environmental elements that affect EPS production include pH, temperature, time, and the LAB strain (Angelin and Kavitha, 2020).
Depending on the makeup of the sugar unit, these polymers can be divided into homopolysaccharides (HoPS) and heteropolysaccharides (HePS). HePS are made up of various kinds of monosaccharides, while HoPS are polysaccharides made up of a single type of monosaccharide. The species of lactic acid bacteria that contribute to the broad range of uses in the food industry determine the sugar composition and chain length of the EPS (Korcz and Varga, 2021). Numerous enzymes and regulatory proteins are involved in the intricate process of bacterial EPS biosynthesis. The biosynthesis of EPS can be broadly divided into three stages: First, the carbon substrate is taken up. Subsequently, the polysaccharides undergo intracellular synthesis before being excreted from the cell. Sugar transfer into the cytoplasm, sugar-1P synthesis, polymerization of repetitive unit precursors, and EPS transport outside the cell are the first four major processes in the biosynthesis of EPS in LAB (Becker, 2015). Among the key features of HoPS synthesis are the absence of active transportation phases in the synthetic process, the requirement for extracellular enzyme production, and the minimal energy expenditure. These extracellular enzymes are known as fructosyltransferases and glycosyltransferases. Glycansucrases are another name for glycosyltransferases. Glucose is used by this enzyme. Another name for fructosyltransferase is fructansucrase. Moreover, this enzyme uses fructose. When HoPS is being synthesized, these sugars serve as the glycosyl donor (Juvonen et al., 2015).
Furthermore, the manufacture and secretion of HePS include several proteins and/or enzymes. The production of HePS depends on the sugar nucleotides. The two main functions of these sugar nucleotides, which are produced from sugar-1-phosphates, are (1) sugar activation (monosaccharide polymerization requires sugar activation), and (2) sugar interconversions (epimerization, decarboxylation, dehydrogenation, and so on). The biosynthesis of HePS is an energy-intensive process. This process involves several energy-consuming steps: (1) the conversion of sugar-to-sugar phosphate requires one ATP, (2) each nucleotide requires another, and (3) the phosphorylation of the isoprenoid C55 lipid carrier requires an additional ATP (Madhuri and Prabhakar, 2014).
3.8 Bioactive peptides
The proteases and peptidases produced by humans can release bioactive peptides from encrypted proteins, which are then absorbed by the human gut and other peripheral organs. The enzymatic activity of LAB significantly influences the release of peptides from proteins and thus increases the digestion in humans. However, LAB possesses a limited genome length, and they have restricted capabilities in synthesizing amino acids. Hence, LAB adopted a complex and sophisticated proteolytic system to convert the external protein into amino acids and small peptides. Generally, bioactive peptides have the following beneficial effects in humans, including antimicrobials, hypocholesterolemia, opioid antagonists, angiotensin-converting enzyme inhibitors, anti-thrombotic, immunomodulators, cytomodulators, and antioxidants (Perez and Ancuelo, 2023; Mazorra-Manzano et al., 2020). As reported by Fan et al. (2019), Lactobacillus helveticus CICC6024 produces nearly 241 bioactive peptides under defined fermentation conditions. This corroborates the recent findings of Carneiro et al. (2024), indicating that LAB can synthesize bioactive peptides from meat and meat products.
4 LABs for the meat product preservation and safety
Human health and the economy were greatly affected by foodborne infections and intoxications. For the past several decades, various chemical preservatives have been employed in the food industry, which cause various toxic effects and diseases, including allergic reactions, heart disease, neurological problems, and cancer. Hence, to replace the chemical preservatives, biopreservatives like microorganism and their metabolites were used to make safe food for consumers. Further, worldwide consumers prefer products that do not contain chemical preservatives. Biopreservatives enhance the safety, quality, and shelf life of food items by inhibiting the growth of harmful microorganisms through the antagonistic activity of LAB, which is witnessed through the production of organic acids, hydrogen peroxide, diacetyl, bacteriocins, and other low-molecular-weight metabolites (Sharma et al., 2022).
Generally, LAB can eliminate various food spoilage-causing bacteria, such as Escherichia coli, Salmonella sp., and Listeria monocytogenes, which generally grow on the surface of meat products, thus spoiling their quality (Castellano and Vignolo, 2006). Specific spoilage organisms, such as Pseudomonas sp. Brochothrix thermosphacta, Enterobacteriaceae spp., Acinetobacter spp., Aeromonas spp., Alcaligenes spp., Moraxella spp., Flavobacterium spp., Staphylococcus spp., and Micrococcus spp. were found to grow predominantly on the meat surface. Thus, spoiling the quality of meat, including its color, texture, appearance, and flavor, makes the meat product undesirable or unfit for human consumption (Marcelli et al., 2024). However, the usage of LAB can promptly reduce the load of food spoilage organisms, thus enhancing its shelf life (Sharma et al., 2022).
It was evident from the previous study that LABs (Lactiplantibacillus plantarum, Levilactobacillus brevis, and Leuconostoc mesenteroides) isolated from poultry meat can produce lactic acid, hydrogen peroxide, and diacetyl, thereby inhibiting the growth of various pathogenic organisms (Adesokan et al., 2008). Lactiplantibacillus plantarum and Leuconostoc mesenteroides have antagonistic activity against Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli, thus helping to prevent meat spoilage. LAB produces lactic acid as a primary metabolite, which reduces the pH of the food product. Hence, in the acidic environment, the growth of foodborne microorganisms is inhibited by affecting their cell membrane and thus making the food product fit for human consumption (Lahiri et al., 2022a,b; García-Díez and Saraiva, 2021).
LAB produces hydrogen peroxide (H2O2) through the action of the enzyme flavoprotein oxidase in the presence of oxygen. Since LAB lack the catalase enzyme, H2O2 can accumulate in the environment, which can oxidize the lipid membranes and cellular proteins of pathogenic organisms, such as bacteria, yeasts, molds, and viruses (Sharma et al., 2022).
Diacetyl (2,3-butanodione) is a volatile organic compound produced by LAB through citrate fermentation and can inhibit the foodborne pathogenic organisms. Diacetyl produced by LAB can prevent the growth of gram-negative bacteria, yeasts, and molds than gram-positive bacteria by deactivating the key enzymes in the pathogenic microbes (Silva et al., 2023).
Lactococcus lactis subsp. lactis I23 and Lactococcus lactis subsp. lactis E91 resides in its ability to produce lactic acid and diacetyl and inhibit Brochothrix thermosphacta, a meat spoilage organism in fresh pork (Olaoye et al., 2015). Latilactobacillus curvatus CRL705 and its bacteriocin compounds, such as lactocin 705 and lactocin AL 705, when introduced into fresh meat, were found to inhibit the growth of Listeria innocua and Brochothrix thermosphacta in vacuum-packaged fresh meat at 2 °C (Castellano and Vignolo, 2006).
Meat and meat products are rich in protein, vital amino acids, minerals, and vitamin B groups, making them excellent sources of nutrients for people. Additionally, due to their optimal pH, nutritional elements, and high-water activity, they provide a suitable environment for the growth of a diverse range of microorganisms (Bohrer, 2017). The genera Brochothrix, Enterobacter, Acinetobacter, Moraxella, Pseudomonas, Leuconostoc, and Proteus are primary causes of meat deterioration; however, some of these bacteria, such as Enterobacter and Pseudomonas, also release biogenic amines (BAs) that may compromise food safety (Gao et al., 2022). Biogenic amines (BAs) are nitrogen-containing compounds that are mainly generated through the decarboxylation of amino acids. While BAs play a crucial role in various biological functions, elevated levels can pose risks to human health. Significant amounts of BAs are commonly present in fish sauces and fermented sausages. Various chromatography techniques and chemosensors are employed to identify BAs in food products. Preventive strategies include the application of starter cultures, control of physical and environmental conditions, and the incorporation of polyphenols. To ensure food safety, it is essential to conduct regular monitoring, adhere to hygienic production methods, and utilize effective starter cultures (Sivamaruthi et al., 2021). Additionally, harmful microbes such as Campylobacter jejuni, Salmonella spp., Yersinia enterocolitica, Bacillus cereus, Clostridium perfringens, Clostridium botulinum, Escherichia coli, and Listeria monocytogenes can contaminate meat and animal products (Favaro and Todorov, 2017). One of the primary issues facing the meat industry is the spoilage of fresh meat and meat products due to microbial contamination (Ashaolu et al., 2023). The meat industry employs several techniques to prevent microbiological growth and produce safe products with the desired quality and intended storage time. As a result, the most used methods include chemical approaches (such as the use of artificial preservatives) and physical methods (such as drying, freezing, heat treatment, packaging, and curing). However, chemical additives have several drawbacks, including altering the nutritional and organoleptic properties of food (Kaveh et al., 2022; Radi et al., 2023).
In this regard, LAB have garnered greater interest than other bio-preservative microorganisms for a variety of reasons, including their ability to encapsulate via extrusion during the creation of the antimicrobial film and their generally recognized as safe classification, which allows the FDA to approve them as a preservative in certain foods (Radosavljević et al., 2022). Therefore, LAB is essential to the development of fermented meat products, which increase texture and flavor while also preserving the product and, ultimately, extending its shelf life. Fresh meat’s high buffering capacity and low carbohydrate content result in mild fermentation, without altering the organoleptic qualities of the food. LAB produces a variety of bioactive substances, including biosurfactants and bacteriocins, which are utilized to preserve meat products. Bacteriocins may inhibit the growth of spoilage or pathogenic microorganisms. LAB-derived bacteriocins have demonstrated strong antimicrobial effects across a range of meat products, significantly enhancing preservation and safety. In ready-to-eat pork ham, bacteriocin-like inhibitory substances from Pediococcus pentosaceus inhibit Listeria seeligeri by 1.74 log CFU/g and reduce weight loss (de Azevedo et al., 2020). Similarly, Bacillus sonorensis-derived sonorensin effectively inhibited Listeria monocytogenes and Staphylococcus aureus in inoculated chicken meat (Chopra et al., 2015). Vacuum-packaged beef frankfurters treated with semi-purified bacteriocins from Latilactobacillus curvatus or Latilactobacillus sakei exhibited pathogen levels reduced to below the detectable limit (Castellano et al., 2018). In beef, bacteriocins from Lactobacillus crustorum MN047 (Reclassified as Companilactobacillus crustorum) significantly reduced the populations of Escherichia coli and Staphylococcus aureus by 4.3 and 4.5 log CFU/ml, respectively (Lu et al., 2020). Antimicrobial peptides, especially bacteriocins generated by probiotics, offer a promising therapeutic strategy for combating infectious diseases. LAB strains with probiotic potential were isolated from fermented foods and assessed for their ability to produce EPS, their susceptibility to antibiotics, tolerance to acid and bile, antibacterial properties, and their adhesion/cytotoxicity to gastric cell lines. Six LAB strains were chosen based on their high survival rates in the gastrointestinal tract, significant EPS production, low cytotoxicity, and strong adhesion to gastric cells. Notably, Weissella confusa CYLB30, Lactiplantibacillus plantarum CYLB47, and Limosilactobacillus fermentum CYLB55 demonstrated strong anti-bacterial effects against multidrug-resistant strains of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella enterica serovar choleraesuis, Enterococcus faecium, and Staphylococcus aureus (Thuy et al., 2024). These findings collectively demonstrate that bacteriocins from LAB offer potent, natural bio-preservatives that can significantly enhance the microbial safety and quality of various meat products.
Bio-surfactants are amphiphilic, biodegradable, and non-toxic substances produced as secondary metabolites by various microbes, including lactic acid bacteria (Jahan et al., 2020). Food products, including meat products, can be effectively preserved due to their antibacterial properties. Through a variety of mechanisms, they demonstrate their antibacterial properties, including: (Abdul Hakim et al., 2023) the inhibition of bio-film formation by lowering the bacterial interaction with the surface by changing the surface’s charge and wettability (Ashraf et al., 2019); (Abril et al., 2023) interference with the microorganisms’ regular function by inter-action with their intracellular components (Ines and Dhouha, 2015); (Adesokan et al., 2008) destruction of the microorganisms’ cell walls and membranes (Hippolyte et al., 2018). Biosurfactants derived from Lacticaseibacillus paracasei and Lacticaseibacillus casei have demonstrated notable antimicrobial activity in meat preservation. In raw ground goat meat, these biosurfactants led to a significant reduction in total aerobic counts, including Escherichia coli MTCC 118 and Pseudomonas aeruginosa MTCC 1934 (Mouafo et al., 2020a, b). Similarly, in fresh beef, biosurfactants produced by Lactobacillus paracasei demonstrated complete inhibition of multiple spoilage and pathogenic bacteria, including Bacillus sp. BC1, Staphylococcus aureus STP1, Staphylococcus xylosus STP2 (Mouafo et al., 2020a, b). These findings underscore the potential of LAB-derived biosurfactants as effective natural antimicrobial agents for enhancing meat safety and extending shelf life. Figure 1 summarizes the linkage between LAB metabolites and practical effects in the meat industry. The heatmap (Figure 2) clearly shows how LAB metabolites reduce pathogens and spoilage organisms in different meats.
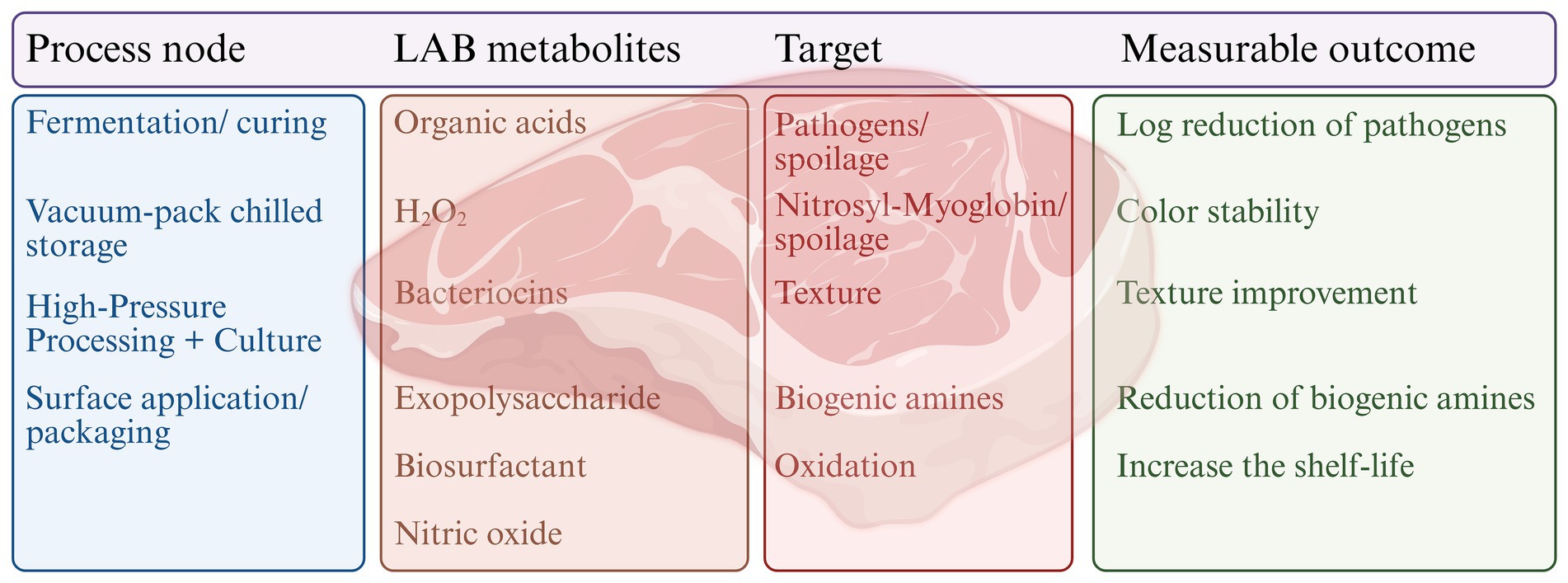
Figure 1. LAB metabolites with meat preservation processes and outcomes. Different process nodes influence the LAB metabolites, such as organic acids, hydrogen peroxide, bacteriocins, exopolysaccharides, biosurfactants, and nitric oxide. These metabolites target pathogens and spoilage organisms, nitrosyl-myoglobin stabilization, texture, biogenic amines, and oxidative reactions. Application of potent LAB could provide favorable outcomes, including pathogen reduction, color stability, texture improvement, decreased biogenic amine accumulation, and increased shelf-life of meat products.
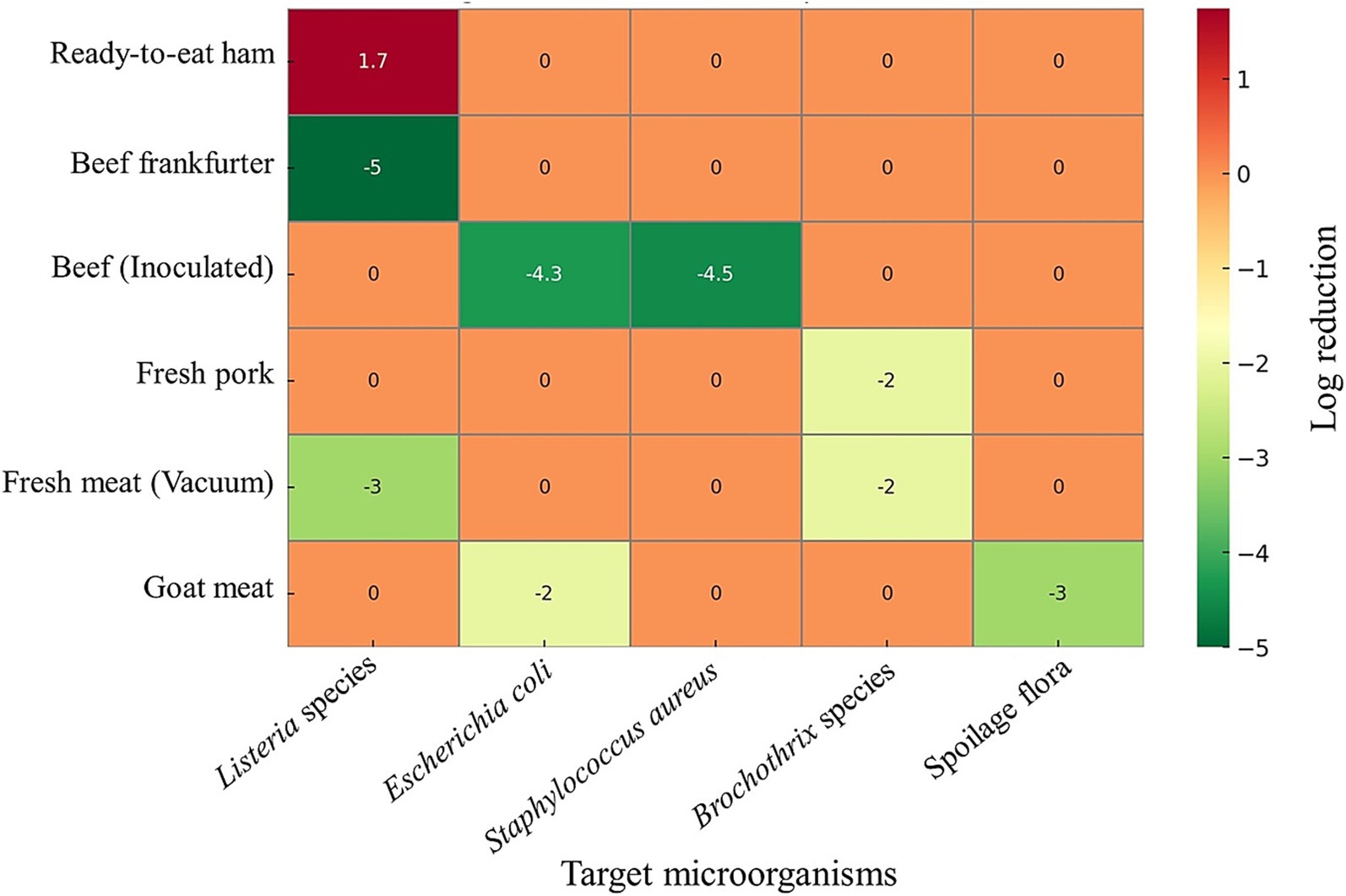
Figure 2. Log reduction of microorganisms in different meat matrices following LAB-associated interventions. The heatmap illustrates changes in microbial populations (log₁₀ CFU reduction or increase) across various meat products. Microorganisms tested were Listeria spp., Escherichia coli, Staphylococcus aureus, Brochothrix species, and general spoilage flora. Negative values (green) represent microbial reductions, positive values (red) indicate increases, and near-zero values (orange) denote negligible changes. The data has been entered in Microsoft Excel, and a heatmap was created using ChatGPT.
5 LAB as a quality enhancer of meat products
LABs have a significant advantage in the food fermentation and preservation process, enhancing the flavor, texture, aroma, digestible properties, and nutritional value of food products. The proteolytic and lipolytic effects of LAB convert protein and fat molecules into peptides, amino acids, and fatty acids, which enhance the flavor of food products. Hence, LAB paves a way for the development of preservation in the modern food biotechnology industries (Anumudu et al., 2024).
5.1 Flavor development
LAB strains, such as Lacticaseibacillus, Limosilactobacillus, Leuconostoc, and Pediococcus, can ferment various food compounds using their secreted enzymes to produce flavor precursors with complex sensory profiles. Carbohydrate fermentation, fatty acid metabolism, and amino acid catabolism are some of the significant metabolic processes carried out by LAB, which enhance the organoleptic properties of meat products by increasing tenderness and flavor (Anumudu et al., 2024). It has been reported that Pediococcus acidilactici BP2 enhanced the flavor of beef jerky (Wen et al., 2021). The raw meat contains skeletal muscles, which consist of myogenic fibrils, sarcoplasmic proteins, and matrix proteins. Further, LAB hydrolyses skeletal muscle proteins into oligopeptides. Subsequently, small peptides and amino acids are produced from oligopeptides and are further converted into α-keto acids and alcohols, which impart fruity flavors. Usually, aldehydes, alcohols, and aromatic substances are some of the flavor enhancement compounds that are produced via oxidative deamination and decarboxylation of proteins. Albano et al. (2009) stated that the flavor of a fermented meat sausage (Alheira) depends upon the LAB, quality of meat, and the ripening process.
The statistical analysis conducted by Xu B. et al. (2025) identified 47 volatile flavor compounds with sensory thresholds and 18 significant key flavor compounds with relative odor value activity values ranging from the relative odor activity (ROAV) value of 1 ≤ to ≤100 in sausage samples. These flavor compounds formed the distinctive flavor profile of Sichuan-style fermented sausages. The ROAV values for β-myrcene, caryophyllene, linalool, phenylethyl alcohol, 3-methyl-1-butanol, 1-octen-3-ol, 3-hydroxy-2-butanone, methyl isovalerate, methyl decanoate, 4-methoxy-6-(2-propenyl)-1,3-benzodioxole, anethole, and acetic acid were found to be higher in the five types of sausages that were inoculated with the combined starter cultures when compared to those in control group A. The contributions of β-myrcene, linalool, and anethole to the development of sausage flavor compounds were significant, suggesting that a greater number of flavor compounds were generated through microbial metabolism. Furthermore, the key flavor compounds such as acetic acid, caryophyllene, linalool, phenylethyl alcohol, 1,8-cineole, and 1-octen-3-ol in sausages inoculated with the combined starter culture F exhibited elevated ROAV values relative to the other compounds. It is hypothesized that Debaryomyces hansenii and Latilactobacillus curvatus present in the combined starter culture F facilitated the synthesis of key flavor compounds in Sichuan-style fermented sausages and enhanced the release of flavor compounds from spices.
5.2 Textural enhancement
LAB enhances the texture, sensory, and organoleptic qualities of meat products through various metabolic activities, including acidification, EPS production, and other enzymatic reactions. In meat and meat products, the protein and fatty acid compounds present in the muscle of meat undergo a gelation process due to the reduced pH caused by LAB, which enhances the disulfide bond formation in meat, thus increasing the chewiness of the meat (Anumudu et al., 2024). According to Smaoui et al. (2014), there was a reduction in hardness, springiness, and rigidity, increased adhesiveness, and chewiness in raw minced beef and chicken breast using BacTN635 (bacteriocin), extracted from Lactiplantibacillus plantarum sp. TN635. Similarly, Du et al. (2019) reported that LAB, such as Pediococcus pentosaceus and Staphylococcus xylosus, reduced the hardness of meat sausage.
5.3 Improvement of the color of the meat product
LABs are known to significantly enhance the coloration of meat products. For instance, Lactiplantibacillus plantarum has been demonstrated to reduce nitrite and nitrate to nitric oxide, which then reacts with myoglobin in sausages to form nitrosyl myoglobin, resulting in the characteristic pink color (Zhu et al., 2020). Similarly, Lactobacillus fermentum JCM1173 (Reclassified as Limosilactobacillus fermentum), Limosilactobacillus fermentum IFO3956, Lactiplantibacillus plantarum 8PA3, Lactiplantibacillus plantarum CMRC6, Latilactobacillus sakei CMRC15, and Lactiplantibacillus plantarum TN8 were identified as functionally active strains involved in the biochemical reduction of nitrate or nitrite to nitric oxide, thereby facilitating the formation of nitrosyl myoglobin and improving the color stability and appearance of meat products (Gou et al., 2019).
5.4 Enhancement of aroma
The amino acid and fatty acids were produced by proteolytic and lipolytic activity of LAB, which is further reduced to produce aroma-improving compounds such as alcohols, aldehydes, ketones, hydrocarbons, acids, aromatic compounds, esters, and sulfur-containing compounds. The free fatty acids are degraded into various compounds, including SCFAs (pungent and penetrating aroma) and secondary alcohols (fruity and fatty aroma). Whereas branched amino acids, including valine, isoleucine, and leucine, are decarboxylated to produce branched aldehydes, alcohols, and/or acids and cause malty and pungent aroma to meat. The aldehyde, alcohol, and acids from various amino acids, including phenylalanine, threonine, tryptophan, tyrosine, methionine, and cysteine, produce a fatty, tallow, malty, and fruity aroma to meat products (Flores, 2018).
6 Beneficial effects of lactic acid bacteria in meat products for consumer health
LABs are commonly used in food fermentation because they can preserve food. Nutritional and health advantages now influence consumer food preferences (Chaiyasut et al., 2018a), leading to decisions that increasingly favor the sustainable use of natural ingredients over chemicals as preservatives. This shift in consumer preferences has heightened the importance of utilizing LAB in food processing (Asioli et al., 2017).
Meat fermentation is a complicated process from the perspective of its microbial ecology, where coagulase-negative staphylococci and LAB both play a role in the development of the product’s typical sensory qualities and its bio-preservation (Fraqueza, 2015). LAB can be included in the non-starter microbiota in fermented products or employed as probiotics and/or meat starter cultures, interacting with the product’s natural microbes. In both situations, their existence may benefit the results. Using starter cultures, which include probiotic bacteria with potential health benefits (Chaiyasut et al., 2018b; Chaiyasut et al., 2018c; Kesika et al., 2022; Sivamaruthi et al., 2022), supports consumer acceptance and the stability and safety of the product. Several factors should be considered when selecting LAB to produce fermented meats. Since they prevent the growth of pathogenic and deteriorating microorganisms, facilitate maturation, ensure microbial stability during storage, stabilize the product’s color, and improve its texture, they increase the safety and shelf life of the finished products. For this reason, the ability to acidify and grow at low pH values is desirable for potential starter cultures in the meat industry and for preventing spoilage. Proteolytic activity is another desired quality that is crucial to the development of flavor during fermentation, as in the case of raw sausage fermentation. LAB can have a beneficial effect on the breakdown of proteins during meat fermentation. The flavor of the sausage depends on the ability to further transform the resultant peptides into volatile molecules (Todorov et al., 2017). Another advantageous feature of LAB is its antibacterial activity, as it inhibits the growth of microorganisms that cause spoilage and foodborne pathogens, which are crucial for maintaining product safety, shelf life, and quality (Zhang et al., 2021). Other advantageous characteristics to consider when screening LAB for use in fermented meat products are their capacity to break down biogenic amines, especially in smoked meat products, which contain amines, cholesterol, and carcinogens, as well as their ability to regulate lipid oxidation (Shao et al., 2021). The active players from LAB and their significant role in the improvement of meat products and their impact on consumers’ health have been showcased in Figure 3.
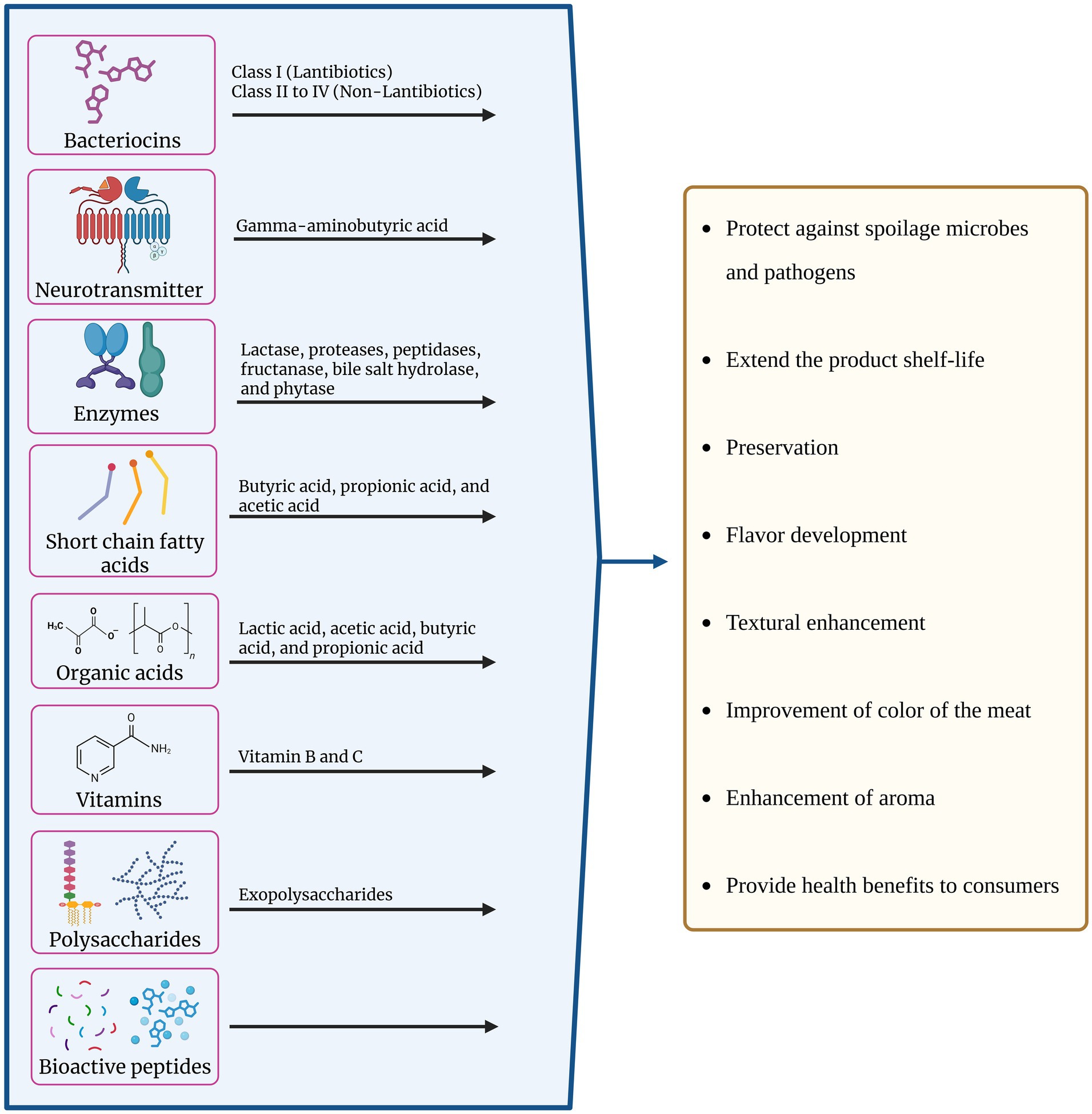
Figure 3. Bioactive metabolites produced by lactic acid bacteria (LAB) and their functional roles in meat products. LAB secrete a wide range of compounds including bacteriocins (Class I lantibiotics, Class II-IV non-lantibiotics), neurotransmitters (e.g., gamma-aminobutyric acid), enzymes (such as lactase, proteases, peptidases, fructanase, bile salt hydrolase, and phytase), short-chain fatty acids (butyric, propionic, and acetic acids), organic acids (lactic, acetic, butyric, and propionic acids), vitamins, polysaccharides (exopolysaccharides), and bioactive peptides. These metabolites collectively contribute to multiple technological and health-promoting effects in meat products, including protection against spoilage microbes and pathogens, extension of shelf-life, preservation, flavor and aroma development, textural enhancement, improvement of meat color, and provision of health benefits to consumers.
7 Safety and regulatory frameworks
Even when a species is generally regarded as safe, individual strains may acquire antimicrobial resistance (AMR) genes, virulence factors, mobile genetic elements, or decarboxylase pathways that raise safety concerns in fermented meats. A defensible workflow, therefore, evaluates each production strain at the strain level using whole-genome sequencing (WGS), phenotypic assays, and regulatory frameworks (QPS/GRAS) [EFSA Panel on Additives and Products or Substances used in Animal Feed et al., 2018].
High-quality WGS can be used to confirm strain identity and genome integrity, including assessment of assembly quality, contamination, and taxonomic assignment. Raw sequence data were deposited, and reporting followed European Food Safety Authority (EFSA) requirements, which mandate disclosure of assembly metrics, accession numbers, and database versions for microorganisms intended for use in the food chain (European Food Safety Authority, 2024).
AMR can be assessed using curated databases (CARD with RGI, ResFinder, and PointFinder), with all hits reported alongside cut-off values and database versions. The absence of acquired AMR determinants could be considered essential for acceptability in line with EFSA guidance. For any AMR-like signals, the genetic context can be examined, including neighboring elements such as integrases, transposases, and origin of transfer sites, to determine whether they were chromosomal or plasmid-associated [Alcock et al., 2023; Alcock et al., 2020; Florensa et al., 2022; EFSA Panel on Additives and Products or Substances used in Animal Feed et al., 2018; EFSA Panel on Additives and Products or Substances used in Animal Feed, 2012].
Virulence factors in the strain can be screened against the Virulence Factor Database (VFDB), with all hits reported by identity and coverage and subsequently evaluated for biological plausibility within the genus (Liu et al., 2022). Mobile genetic elements can be examined by identifying plasmid replicons and regions using PlasmidFinder and PLSDB, with annotation of integrative conjugative elements and prophages. Particular attention was given to co-localization of antimicrobial resistance or virulence genes on mobile elements, which was considered high risk. In cases of uncertainty, filter-mating assays were performed to verify the absence of horizontal transfer under food-relevant conditions (Carattoli et al., 2014).
The potential for biogenic amine (BA) formation can be assessed both genomically and phenotypically. Genomic screening targeted decarboxylase gene clusters, along with associated transporters and regulators. Phenotypically, strains would be tested to confirm the absence of BA production in meat matrices or defined media (EFSA Panel on Biological Hazards, 2011).
Antimicrobial susceptibility of the strain can be evaluated by determining minimum inhibitory concentrations (MICs) using standardized methods and comparing the results against EFSA-established cut-off values. Concordance between genotypic predictions and phenotypic outcomes can be expected, and any phenotypic resistance exceeding cut-off thresholds required genetic justification or led to exclusion of the strain [EFSA Panel on Additives and Products or Substances used in Animal Feed et al., 2018; EFSA Panel on Biological Hazards, 2011].
Additional safety and fitness characteristics relevant to meat applications can be evaluated. For instance, acceptable profiles included γ-hemolysis only, with strains required to be negative for gelatinase, DNase, and genus-specific toxin activities. Spoilage potential was assessed through measurements of gas and H₂S production, detection of amine and aldehyde off-odors, and evaluation of proteolysis and lipolysis under target pH-salt-temperature conditions, with only non-spoiling strains retained. Furthermore, phage susceptibility mapping and prophage induction assays were performed to minimize risks of fermentation failure and horizontal gene transfer [EFSA Panel on Additives and Products or Substances used in Animal Feed et al., 2018].
Genetic stability and batch consistency need to be monitored by periodically re-sequencing the master cell bank and production seed lots to confirm the absence of new mobile elements, AMR determinants, or virulence factor genes. Traceability was ensured by maintaining versioned database records for all comparative analyses over time (European Food Safety Authority, 2024).
The proper strain informative documentation aligned with regulatory frameworks, noting that EFSA’s Qualified Presumption of Safety (QPS) operates at the species or group level, with specific qualifications (e.g., for production purposes only or absence of toxigenic activity) are needed. However, QPS designation does not exempt strains from detailed safety evaluation, including assessments of AMR, toxigenic potential, and suitability for the intended use. Therefore, the most recent QPS updates need to be consulted when selecting candidate species (EFSA Panel on Biological Hazards et al., 2024a; EFSA Panel on Biological Hazards et al., 2024b). In the U. S., GRAS status has been established for a specific microbial strain and its intended use in a food matrix, rather than assumed by species identity. A GRAS report typically includes strain characterization, safety assessments, history of use or toxicological evidence, and exposure estimates under intended conditions.
8 Research gap in the field
LABs play a crucial role in the meat industry, contributing to fermentation, preservation, and the enhancement of sensory attributes. They significantly contribute to food safety by inhibiting the growth of spoilage and pathogenic microorganisms through the production of antimicrobial compounds, including organic acids, bacteriocins, and hydrogen peroxide. However, despite their widespread use, several research gaps remain that require further exploration.
One major gap involves the strain-specific functionalities of LAB in meat products. While different LAB strains can influence texture, flavor, and preservation, their specific mechanisms and impacts are not fully understood. Identifying the best-performing strains for food applications would improve product quality and consistency. Another critical gap is related to the safety of LAB strains. Although many LAB species are considered safe for consumption, some may exhibit potential virulence properties, raising concerns about their long-term safety in food formulations.
For example, horizontal gene transfer (HGT) lets bacteria swap genes outside of parent-to-offspring inheritance and is a major driver of traits that threaten food safety. Foods can carry resistant bacteria and resistance genes that originated in animals or processing environments and later reach people; multiple studies have reported this pathway and its risks for human infection and risk of hard-to-treat infections (Lyu et al., 2018; Founou et al., 2016). Biofilms on food-contact surfaces are hotspots where bacteria easily swap plasmids, so hard-to-clean areas in processing plants and slaughterhouses can become long-term reservoirs of harmful genes (Van Meervenne et al., 2014; Ban-Cucerzan et al., 2025).
Concrete harms include the rapid spread of plasmid-mediated colistin resistance (mcr-1) from food animals into retail meat and human infections (Liu et al., 2016; Kuo et al., 2016). In food processing, Listeria monocytogenes frequently carries mobile determinants that raise tolerance to sanitisers such as benzalkonium chloride, aiding long-term facility persistence and recurrent product contamination (Dutta et al., 2013; Minarovičová et al., 2018; Daeschel et al., 2022). HGT by Shiga toxin-encoding phages can convert naive Escherichia coli into Shiga toxin-producing Escherichia coli, elevating virulence potential within the food chain and in the human gut (Khalil et al., 2016; Herold et al., 2004; Zuppi et al., 2020). Together, these routes show how HGT amplifies antimicrobial resistance and virulence across food systems, increasing outbreak risk and narrowing therapeutic options. Thus, further studies are needed to assess potential health risks and establish regulatory guidelines that ensure consumer protection.
Additionally, optimizing the production of beneficial metabolites, such as bacteriocins, organic acids, and bioactive peptides, remains a challenge. While these compounds contribute to antimicrobial activity and improved product stability, their yield and effectiveness vary depending on environmental conditions and bacterial strain. More research is needed to enhance their production efficiency and stability in industrial applications. Understanding the interaction between LAB and other microorganisms in meat products is another research area that remains underexplored. The presence of LAB can influence the growth dynamics of other bacterial populations, impacting the overall microbial balance and safety of meat products. Investigating these interactions would enable manufacturers to control undesired microbial activity and enhance food quality. Despite the documented benefits of LAB in meat products, consumer acceptance remains a challenge, especially in regions unfamiliar with LAB-enhanced meat. Public perception, taste preferences, and concerns about food safety significantly influence purchasing decisions, necessitating targeted studies on consumer attitudes and educational initiatives to enhance acceptance. Regulatory frameworks surrounding the use of LAB in meat products also require deeper investigation. While LABs are widely accepted in fermented products such as yogurt and cheese, their application in meat is still evolving, and clear guidelines for their use, labeling, and health claims need to be established.
Addressing these research gaps through interdisciplinary studies that involve microbiology, food science, biotechnology, and consumer behavior will enhance the safe and effective use of LAB in the meat industry.
9 Conclusion
LABs have become essential players in modern food biotechnology, especially in meat processing and preservation. The broad application of LABs stems from their ability to enhance food safety, extend shelf life, improve sensory qualities, and support human health. As natural fermenters, LAB contribute to the production of fermented meat products by generating organic acids, peptides, and bacteriocins that inhibit the growth of spoilage and harmful microorganisms, thereby reducing reliance on synthetic preservatives. This aligns with the growing consumer demand for “clean label” and minimally processed foods.
In meat products, LAB provides both technological and nutritional benefits. From a technological standpoint, they promote product stability through acidification, preservation, and enzymatic activity, which collectively enhance flavor, texture, and color. Nutritionally, certain LAB strains offer probiotic benefits, including modulation of the gut microbiota, cholesterol reduction, and support for the immune system. Additionally, they produce functional compounds, such as GABA and EPS, further enhancing the health-promoting potential of LAB-fermented meat products. Despite their advantages, LABs face challenges that require further research. Their strain-specific behavior in different meat matrices, interactions with native microbiota, and adaptation to processing conditions need deeper exploration. While LABs are GRAS, some strains may carry undesirable traits, such as antibiotic resistance or virulence factors, making rigorous safety assessments crucial for their industrial use. Another hurdle is scaling up the production of LAB-derived bioactive compounds without compromising their effectiveness in industrial applications. Consumer awareness and regulatory clarity also play a significant role. Acceptance of LAB-based innovations in meat products varies across cultures and markets, influenced by concerns over microbial safety and a lack of familiarity with fermented meats. Clear labeling, well-supported health claims, and targeted educational efforts are necessary to improve market penetration and consumer trust.
In summary, LAB presents a sustainable and effective approach to meat preservation and enhancement. Through continued interdisciplinary research that addresses safety, functionality, and consumer perception, LABs have the potential to transform the meat industry by meeting technological demands and public health needs in a natural and environmentally friendly manner.
Author contributions
BS: Conceptualization, Formal analysis, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. PK: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing. SBSR: Data curation, Formal analysis, Writing – original draft. KS: Data curation, Formal analysis, Writing – original draft. SPR: Data curation, Formal analysis, Investigation, Writing – original draft. CC: Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing. PF: Data curation, Formal analysis, Resources, Writing – original draft. KA: Data curation, Formal analysis, Methodology, Project administration, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by Chiang Mai University, Chiang Mai, Thailand.
Acknowledgments
The authors (BS, PK, PF, and CC) gratefully acknowledge Chiang Mai University, Thailand, for its support. PF acknowledges the support of CMU Proactive Researcher, Chiang Mai University, Thailand [Grant Number: EX010096/2567].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdul Hakim, B. N., Xuan, N. J., and Oslan, S. N. H. (2023). A comprehensive review of bioactive compounds from lactic acid bacteria: potential functions as functional food in dietetics and the food industry. Foods 12:2850. doi: 10.3390/foods12152850
Abril, B., Bou, R., García-Pérez, J. V., and Benedito, J. (2023). Role of enzymatic reactions in meat processing and use of emerging Technologies for Process Intensification. Foods 12:1940. doi: 10.3390/foods12101940
Adesokan, I. A., Odetoyinbo, B. B., and Olubamiwa, A. O. (2008). Biopreservative activity of lactic acid bacteria on suya produced from poultry meat. Afr. J. Biotechnol. 7, 3570–3574. doi: 10.5897/AJB08.099
Agriopoulou, S., Stamatelopoulou, E., Sachadyn-Król, M., and Varzakas, T. (2020). Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: quality and safety aspects. Microorganisms 8:952. doi: 10.3390/microorganisms8060952
Albano, H., van Reenen, C. A., Todorov, S. D., Cruz, D., Fraga, L., Hogg, T., et al. (2009). Phenotypic and genetic heterogeneity of lactic acid bacteria isolated from “Alheira”, a traditional fermented sausage produced in Portugal. Meat Sci. 82, 389–398. doi: 10.1016/j.meatsci.2009.02.009
Alcock, B. P., Huynh, W., Chalil, R., Smith, K. W., Raphenya, A. R., Wlodarski, M. A., et al. (2023). CARD 2023: expanded curation, support for machine learning, and resistome prediction at the comprehensive antibiotic resistance database. Nucleic Acids Res. 51, D690–D699. doi: 10.1093/nar/gkac920
Alcock, B. P., Raphenya, A. R., Lau, T. T. Y., Tsang, K. K., Bouchard, M., Edalatmand, A., et al. (2020). CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 48, D517–D525. doi: 10.1093/nar/gkz935
Alizadeh Behbahani, B., Jooyandeh, H., Falah, F., and Vasiee, A. (2020). Gamma-aminobutyric acid production by Lactobacillus brevis A3: optimization of production, antioxidant potential, cell toxicity, and antimicrobial activity. Food Sci. Nutr. 8, 5330–5339. doi: 10.1002/fsn3.1838
Alvarez-Sieiro, P., Montalbán-López, M., Mu, D., and Kuipers, O. P. (2016). Bacteriocins of lactic acid bacteria: extending the family. Appl. Microbiol. Biotechnol. 100, 2939–2951. doi: 10.1007/s00253-016-7343-9
An, Y., Wang, Y., Liang, X., Yi, H., Zuo, Z., Xu, X., et al. (2017). Purification and partial characterization of M1-UVs300, a novel bacteriocin produced by Lactobacillus plantarum isolated from fermented sausage. Food Control 81, 211–217. doi: 10.1016/j.foodcont.2017.05.030
Anacarso, I., Gigli, L., Bondi, M., de Nierhausern, S., Stefani, S., Condò, C., et al. (2017). Isolation of two lactobacilli, producers of two new bacteriocin-like substances (BLS) for potential food-preservative use. Eur. Food Res. Technol. 243, 2127–2134. doi: 10.1007/s00217-017-2913-3
Angelin, J., and Kavitha, M. (2020). Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 162, 853–865. doi: 10.1016/j.ijbiomac.2020.06.190
Anumudu, C. K., Miri, T., and Onyeaka, H. (2024). Multifunctional applications of lactic acid bacteria: enhancing safety, quality, and nutritional value in foods and fermented beverages. Foods 13:3714. doi: 10.3390/foods13233714
Ashaolu, T. J. (2020). Safety and quality of bacterially fermented functional foods and beverages: a mini review. Food Qual. Saf. 4, 123–127. doi: 10.1093/fqsafe/fyaa003
Ashaolu, T. J., Khalifa, I., Mesak, M. A., Lorenzo, J. M., and Farag, M. A. (2023). A comprehensive review of the role of microorganisms on texture change, flavor and biogenic amines formation in fermented meat with their action mechanisms and safety. Crit. Rev. Food Sci. Nutr. 63, 3538–3555. doi: 10.1080/10408398.2021.1929059
Ashaolu, T. J., and Reale, A. (2020). A holistic review on euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms 8:1176. doi: 10.3390/microorganisms8081176
Ashraf, A., Ahmed, A. A., Fatma, I., and Zeinab, A. M. (2019). Characterization and bioactivities of Lactobacillus plantarum and Pediococcus acidilactici isolated from meat and meat products. Nat. Sci. 17, 187–193. doi: 10.7537/marsnsj171219.26
Asioli, D., Aschemann-Witzel, J., Caputo, V., Vecchio, R., Annunziata, A., Naes, T., et al. (2017). Making sense of the “clean label” trends: a review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 99, 58–71. doi: 10.1016/j.foodres.2017.07.022
Austrich-Comas, A., Serra-Castelló, C., Jofré, A., Gou, P., and Bover-Cid, S. (2022). Control of Listeria monocytogenes in chicken dry fermented sausages with bioprotective starter culture and high-pressure processing. Front. Microbiol. 13:983265. doi: 10.3389/fmicb.2022.983265
Ayivi, R. D., Gyawali, R., Krastanov, A., Aljaloud, S. O., Worku, M., Tahergorabi, R., et al. (2020). Lactic acid bacteria: food safety and human health applications. Dairy 1, 202–232. doi: 10.3390/dairy1030015
Ayivi, R. D., and Ibrahim, S. A. (2022). Lactic acid bacteria: an essential probiotic and starter culture for the production of yoghurt. Int. J. Food Sci. Technol. 57, 7008–7025. doi: 10.1111/ijfs.16076
Balay, D. R., Dangeti, R. V., Kaur, K., and McMullen, L. M. (2017). Purification of leucocin a for use on wieners to inhibit Listeria monocytogenes in the presence of spoilage organisms. Int. J. Food Microbiol. 255, 25–31. doi: 10.1016/j.ijfoodmicro.2017.05.016
Ban-Cucerzan, A., Imre, K., Morar, A., Marcu, A., Hotea, I., Popa, S. A., et al. (2025). Persistent threats: a comprehensive review of biofilm formation, control, and economic implications in food processing environments. Microorganisms 13:1805. doi: 10.3390/microorganisms13081805
Bangar, S. P., Suri, S., Trif, M., and Ozogul, F. (2022). Organic acids production from lactic acid bacteria: a preservation approach. Food Biosci. 46:101615. doi: 10.1016/j.fbio.2022.101615
Becker, A. (2015). Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front. Microbiol. 6:687. doi: 10.3389/fmicb.2015.00687
Bengoa, A. A., Iraporda, C., Garrote, G. L., and Abraham, A. G. (2019). Kefir micro-organisms: their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 126, 686–700. doi: 10.1111/jam.14107
Bikila, W. (2015). Lactic acid bacteria: benefits, selection criteria and probiotic potential in fermented food. J. Prob. Health 3:129. doi: 10.4172/2329-8901.1000129
Bintsis, T. (2018). Lactic acid bacteria as starter cultures: an update in their metabolism and genetics. AIMS Microbiol. 4, 665–684. doi: 10.3934/microbiol.2018.4.665
Bohrer, B. M. (2017). Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 65, 103–112. doi: 10.1016/j.tifs.2017.04.016
Carattoli, A., Zankari, E., García-Fernández, A., Voldby Larsen, M., Lund, O., Villa, L., et al. (2014). In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58, 3895–3903. doi: 10.1128/AAC.02412-14
Carneiro, K. O., Campos, G. Z., Scafuro Lima, J. M., Rocha, R. S., Vaz-Velho, M., and Todorov, S. D. (2024). The role of lactic acid bacteria in meat products, not just as starter cultures. Foods 13:3170. doi: 10.3390/foods13193170
Casaburi, A., Di Martino, V., Ferranti, P., Picariello, L., and Villani, F. (2016). Technological properties and bacteriocins production by Lactobacillus curvatus 54M16 and its use as starter culture for fermented sausage manufacture. Food Control 59, 31–45. doi: 10.1016/j.foodcont.2015.05.016
Casquete, R., Fonseca, S. C., Pinto, R., Castro, S. M., Todorov, S., Teixeira, P., et al. (2019). Evaluation of the microbiological safety and sensory quality of a sliced cured-smoked pork product with protective cultures addition and modified atmosphere packaging. Food Sci. Technol. Int. 25, 327–336. doi: 10.1177/1082013219825771
Castellano, P., Ibarreche, M. P., Massani, M. B., Fontana, C., and Vignolo, G. M. (2017). Strategies for pathogen biocontrol using lactic acid bacteria and their metabolites: a focus on meat ecosystems and industrial environments. Microorganisms 5:38. doi: 10.3390/microorganisms5030038
Castellano, P., Peña, N., Ibarreche, M. P., Carduza, F., Soteras, T., and Vignolo, G. (2018). Anti-listerial efficacy of Lactobacillus bacteriocins and organic acids on frankfurters: impact on sensory characteristics. J. Food Sci. Technol. 55, 689–697. doi: 10.1007/s13197-017-2979-8
Castellano, P., and Vignolo, G. (2006). Inhibition of Listeria innocua and Brochothrix thermosphacta in vacuum-packaged meat by addition of bacteriocinogenic Lactobacillus curvatus CRL705 and its bacteriocins. Lett. Appl. Microbiol. 43, 194–199. doi: 10.1111/j.1472-765X.2006.01933.x
Cataldo, P. G., Urquiza Martínez, M. P., Villena, J., Kitazawa, H., Saavedra, L., and Hebert, E. M. (2024). Comprehensive characterization of γ-aminobutyric acid (GABA) production by Levilactobacillus brevis CRL 2013: insights from physiology, genomics, and proteomics. Front. Microbiol. 15:1408624. doi: 10.3389/fmicb.2024.1408624
Chaiyasut, C., Kesika, P., Sirilun, S., Peerajan, S., and Sivamaruthi, B. S. (2018b). Formulation and evaluation of lactic acid bacteria fermented Brassica juncea (mustard greens) pickle with cholesterol lowering property. J. Appl. Pharm. Sci. 8, 33–42. doi: 10.7324/JAPS.2018.8405
Chaiyasut, C., Sivamaruthi, B. S., Makhamrueang, N., Peerajan, S., and Kesika, P. (2018a). A survey of consumer opinion about consumption and health benefits of fermented plant beverages in Thailand. Food Sci. Technol. (Braz.) 38, 299–309. doi: 10.1590/1678-457x.04917
Chaiyasut, C., Woraharn, S., Sivamaruthi, B. S., Kesika, P., Lailerd, N., and Peerajan, S. (2018c). Lactobacillus fermentum HP3 mediated fermented Hericium erinaceus juice as a health promoting food supplement to manage diabetes mellitus. J. Evid. Based Integr. Med. 23, 1–9. doi: 10.1177/2515690X18765699
Chen, C., Zhao, S., Hao, G., Yu, H., Tian, H., and Zhao, G. (2017). Role of lactic acid bacteria on the yogurt flavor: a review. Int. J. Food Prop. 20, S316–S330. doi: 10.1080/10942912.2017.1295988
Choeisoongnern, T., Sivamaruthi, B. S., Sirilun, S., Peerajan, S., Choiset, Y., Rabesona, H., et al. (2020). Screening and identification of bacteriocin-like inhibitory substances producing lactic acid bacteria from fermented products. Food Sci. Technol. 40, 571–579. doi: 10.1590/fst.13219
Chopra, L., Singh, G., Kumar Jena, K., and Sahoo, D. K. (2015). Sonorensin: a new bacteriocin with potential as an anti-biofilm agent and a food biopreservative. Sci. Rep. 5:13412. doi: 10.1038/srep13412
Comi, G., Andyanto, D., Manzano, M., and Iacumin, L. (2016). Lactococcus lactis and Lactobacillus sakei as bio-protective culture to eliminate Leuconostoc mesenteroides spoilage and improve the shelf life and sensorial characteristics of commercial cooked bacon. Food Microbiol. 58, 16–22. doi: 10.1016/j.fm.2016.03.001
Cotter, P. D., Hill, C., and Ross, R. P. (2005). Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3, 777–788. doi: 10.1038/nrmicro1273
Cui, Y., Miao, K., Niyaphorn, S., and Qu, X. (2020). Production of gamma-aminobutyric acid from lactic acid Bacteria: a systematic review. Int. J. Mol. Sci. 21:995. doi: 10.3390/ijms21030995
Da Costa, R. J., Voloski, F. L. S., Mondadori, R. G., Duval, E. H., and Fiorentini, Â. M. (2019). Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J. Food Qual. 2019, 1–12. doi: 10.1155/2019/4726510
Daeschel, D., Pettengill, J. B., Wang, Y., Chen, Y., Allard, M., and Snyder, A. B. (2022). Genomic analysis of Listeria monocytogenes from US food processing environments reveals a high prevalence of QAC efflux genes but limited evidence of their contribution to environmental persistence. BMC Genomics 23:488. doi: 10.1186/s12864-022-08695-2
De Azevedo, P. O. S., Mendonça, C. M. N., Seibert, L., Domínguez, J. M., Converti, A., Gierus, M., et al. (2020). Bacteriocin-like inhibitory substance of Pediococcus pentosaceus as a biopreservative for Listeria sp. control in ready-to-eat pork ham. Braz. J. Microbiol. 51, 949–956. doi: 10.1007/s42770-020-00245-w
De Castilho, N. P. A., Todorov, S. D., Oliveira, L. L., dos Santos Bersot, L., and Nero, L. A. (2020). Inhibition of Listeria monocytogenes in fresh sausage by bacteriocinogenic Lactobacillus curvatus UFV-NPAC1 and its semi-purified bacteriocin. LWT 118:108757. doi: 10.1016/j.lwt.2019.108757
Dhakal, R., Bajpai, V. K., and Baek, K. H. (2012). Production of GABA (γ - aminobutyric acid) by microorganisms: a review. Braz. J. Microbiol. 43, 1230–1241. doi: 10.1590/S1517-83822012000400001
Diez-Gutiérrez, L., Vicente, L. S., Barrón, L. J. R., del Carmen Villarán, M., and Chávarri, M. (2020). Gamma-aminobutyric acid and probiotics: multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 64:103669. doi: 10.1016/j.jff.2019.103669
Diez-Gutiérrez, L., Vicente, L. S., Sáenz, J., Esquivel, A., Barron, L. J. R., and Chávarri, M. (2022). Biosynthesis of gamma-aminobutyric acid by Lactiplantibacillus plantarum K16 as an alternative to revalue Agri-food by-products. Sci. Rep. 12:18904. doi: 10.1038/s41598-022-22875-w
Du, S., Cheng, H., Ma, J.-K., Li, Z., Wang, C., and Wang, Y.-L. (2019). Effect of starter culture on microbiological, physiochemical, and nutrition quality of Xiangxi sausage. J. Food Sci. Technol. 56, 811–823. doi: 10.1007/s13197-018-3541-z
Dutta, V., Elhanafi, D., and Kathariou, S. (2013). Conservation and distribution of the benzalkonium chloride resistance cassette bcrABC in Listeria monocytogenes. Appl. Environ. Microbiol. 79, 6067–6074. doi: 10.1128/AEM.01751-13
EFSA Panel on Additives and Products or Substances used in Animal Feed (2012). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10:2740. doi: 10.2903/j.efsa.2012.2740
EFSA Panel on Additives and Products or Substances used in Animal Feed Rychen, G., Aquilina, G., Azimonti, G., Bampidis, V., Bastos, M. L., et al. (2018). Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 16:e05206. doi: 10.2903/j.efsa.2018.5206
EFSA Panel on Biological Hazards (2011). Scientific opinion on scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 9:2393. doi: 10.2903/j.efsa.2011.2393
EFSA Panel on Biological Hazards Koutsoumanis, K., Allende, A., Alvarez-Ordóñez, A., Bolton, D., Bover-Cid, S., et al. (2024b). Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA 19: suitability of taxonomic units notified to EFSA until September 2023. EFSA J. 22:e8517. doi: 10.2903/j.efsa.2024.8517
EFSA Panel on Biological Hazards Koutsoumanis, K., Allende, A., Alvarez-Ordóñez, A., Bolton, D., Bover-Cid, S., et al. (2024a). Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA 20: suitability of taxonomic units notified to EFSA until march 2024. EFSA J. 22:e8882. doi: 10.2903/j.efsa.2024.8882
European Food Safety Authority (2024). EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA J. 22:e8912. doi: 10.2903/j.efsa.2024.8912
Fan, M., Guo, T., Li, W., Chen, J., Li, F., Wang, C., et al. (2019). Isolation and identification of novel casein-derived bioactive peptides and potential functions in fermented casein with Lactobacillus helveticus. Food Sci. Human Wellness 8, 156–176. doi: 10.1016/j.fshw.2019.03.010
Favaro, L., and Todorov, S. D. (2017). Bacteriocinogenic LAB strains for fermented meat preservation: perspectives, challenges, and limitations. Probiotics Antimicrob. Proteins 9, 444–458. doi: 10.1007/s12602-017-9330-6
Fayaz, H., Ahmad, S. R., Qureshi, A. I., Hussain, S. A., and Nazir, T. (2024). “Application of enzymes in processed meat products” in Handbook of processed functional meat products. eds. S. A. Rather and F. A. Masoodi (Cham: Springer). doi: 10.1007/978-3-031-69868-2_13
Fernandes, N., Achemchem, F., Gonzales-Barron, U., and Cadavez, V. (2024). Biopreservation strategies using bacteriocins to control meat spoilage and foodborne outbreaks. Ital. J. Food Saf. 13:12558. doi: 10.4081/ijfs.2024.12558
Fitsum, S., Gebreyohannes, G., and Sbhatu, D. B. (2025). Bioactive compounds in fermented foods: health benefits, safety, and future perspectives. Appl. Food Res. 5:101097. doi: 10.1016/j.afres.2025.101097
Florensa, A. F., Kaas, R. S., Clausen, P. T. L. C., Aytan-Aktug, D., and Aarestrup, F. M. (2022). ResFinder - an open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from genotypes. Microb. Genom. 8:000748. doi: 10.1099/mgen.0.000748
Flores, M. (2018). Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 144, 53–61. doi: 10.1016/j.meatsci.2018.04.016
Founou, L. L., Founou, R. C., and Essack, S. Y. (2016). Antibiotic resistance in the food Chain: a developing country-perspective. Front. Microbiol. 7:1881. doi: 10.3389/fmicb.2016.01881
Fraqueza, M. J. (2015). Antibiotic resistance of lactic acid bacteria isolated from dry fermented sausages. Int. J. Food Microbiol. 212, 76–88. doi: 10.1016/j.ijfoodmicro.2015.04.035
Gao, X., Li, C., He, R., Zhang, Y., Wang, B., Zhang, Z. H., et al. (2022). Research advances on biogenic amines in traditional fermented foods: emphasis on formation mechanism, detection and control methods. Food Chem. 405:134911. doi: 10.1016/j.foodchem.2022.134911
García-Díez, J., and Saraiva, C. (2021). Use of starter cultures in foods from animal origin to improve their safety. Int. J. Environ. Res. Public Health 18:2544. doi: 10.3390/ijerph18052544
Gómez, I., Janardhanan, R., Ibañez, F. C., and Beriain, M. J. (2020). The effects of processing and preservation technologies on meat quality: sensory and nutritional aspects. Foods 9:1416. doi: 10.3390/foods9101416
Gou, M., Liu, X., and Qu, H. (2019). The role of nitric oxide in the mechanism of lactic acid bacteria substituting for nitrite. CyTA 17, 593–602. doi: 10.1080/19476337.2019.1621949
Gupta, R., Jeevaratnam, K., and Fatima, A. (2018). Lactic acid bacteria: probiotic characteristic, selection criteria, and its role in human health. J. Emerg. Technol. Innov. Res. 5, 411–424.
Herold, S., Karch, H., and Schmidt, H. (2004). Shiga toxin-encoding bacteriophages-genomes in motion. Int. J. Med. Microbiol. 294, 115–121. doi: 10.1016/j.ijmm.2004.06.023
Hippolyte, M. T., Augustin, M., Hervé, T. M., Robert, N., and Devappa, S. (2018). Application of response surface methodology to improve the production of antimicrobial biosurfactants by Lactobacillus paracasei subsp. tolerans N2 using sugar cane molasses as substrate. Bioresour. Bioprocess. 5, 1–16. doi: 10.1186/s40643-018-0234-4
Hu, Y., Liu, X., Shan, C., Xia, X., Wang, Y., Dong, M., et al. (2017). Novel bacteriocin produced by Lactobacillus alimentarius FM-MM 4 from a traditional Chinese fermented meat Nanx Wudl: purification, identification and antimicrobial characteristics. Food Control 77, 290–297. doi: 10.1016/j.foodcont.2017.02.007
Imade, E. E., Omonigho, S. E., Babalola, O. O., and Enagbonma, B. J. (2021). Lactic acid bacterial bacteriocins and their bioactive properties against food-associated antibiotic-resistant bacteria. Ann. Microbiol. 71:44. doi: 10.1186/s13213-021-01652-6
Ines, M., and Dhouha, G. (2015). Lipopeptide surfactants: production, recovery and pore forming capacity. Peptides 71, 100–112. doi: 10.1016/j.peptides.2015.07.006
Jahan, R., Bodratti, A. M., Tsianou, M., and Alexandridis, P. (2020). Biosurfactants, natural alternatives to synthetic surfactants: physicochemical properties and applications. Adv. Colloid Interf. Sci. 275:102061. doi: 10.1016/j.cis.2019.102061
Juarez del Valle, M., Laiño, J. E., Savoy de Giori, G., and LeBlanc, J. G. (2014). Riboflavin producing lactic acid bacteria as a biotechnological strategy to obtain bio-enriched soymilk. Food Res. Int. 62, 1015–1019. doi: 10.1016/j.foodres.2014.05.029
Juvonen, R., Honkapää, K., Maina, N. H., Shi, Q., Viljanen, K., Maaheimo, H., et al. (2015). The impact of fermentation with exopolysaccharide producing lactic acid bacteria on rheological, chemical and sensory properties of pureed carrots (Daucus carota L.). Int. J. Food Microbiol. 207, 109–118. doi: 10.1016/j.ijfoodmicro.2015.04.031
Kaveh, S., Hashemi, S. M. B., Abedi, E., Amiri, M. J., and Conte, F. L. (2023). Bio-preservation of meat and fermented meat products by lactic acid bacteria strains and their antibacterial metabolites. Sustainability 15:10154. doi: 10.3390/su151310154
Kaveh, S., Mahoonak, A. S., Ghorbani, M., and Jafari, S. M. (2022). Fenugreek seed (Trigonella foenumgraecum) protein hydrolysate loaded in nanosized liposomes: characteristic, storage stability, controlled release and retention of antioxidant activity. Ind. Crop. Prod. 182:114908. doi: 10.1016/j.indcrop.2022.114908
Kesika, P., Sivamaruthi, B. S., and Chaiyasut, C. (2022). Health promoting effects of fermented foods against cancer: an updated concise review. Food Sci. Technol. 42:e18220. doi: 10.1590/fst.18220
Khalil, R. K., Skinner, C., Patfield, S., and He, X. (2016). Phage-mediated Shiga toxin (Stx) horizontal gene transfer and expression in non-Shiga toxigenic Enterobacter and Escherichia coli strains. Pathog. Dis. 74:ftw037. doi: 10.1093/femspd/ftw037
Khalili Sadaghiani, S., Aliakbarlu, J., Tajik, H., and Mahmoudian, A. (2019). Anti-Listeria activity and shelf-life extension effects of Lactobacillus along with garlic extract in ground beef. J. Food Saf. 39:e12709. doi: 10.1111/jfs.12709
Kieliszek, M., Pobiega, K., Piwowarek, K., and Kot, A. M. (2021). Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules 26:1858. doi: 10.3390/molecules26071858
Korcz, E., and Varga, L. (2021). Exopolysaccharides from lactic acid bacteria: techno-functional application in the food industry. Trends Food Sci. Technol. 110, 375–384. doi: 10.1016/j.tifs.2021.02.014
Kumariya, R., Garsa, A., Rajput, Y., Sood, S., Akhtar, N., and Patel, S. (2019). Bacteriocins: classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 128, 171–177. doi: 10.1016/j.micpath.2019.01.002
Kuo, S. C., Huang, W. C., Wang, H. Y., Shiau, Y. R., Cheng, M. F., and Lauderdale, T. L. (2016). Colistin resistance gene mcr-1 in Escherichia coli isolates from humans and retail meats, Taiwan. J. Antimicrob. Chemother. 71, 2327–2329. doi: 10.1093/jac/dkw122
Kusada, H., Arita, M., Tohno, M., and Tamaki, H. (2022). Bile salt hydrolase degrades β-lactam antibiotics and confers antibiotic resistance on Lactobacillus paragasseri. Front. Microbiol. 13:858263. doi: 10.3389/fmicb.2022.858263
Lahiri, D., Nag, M., Dutta, B., Sarkar, T., Pati, S., Basu, D., et al. (2022b). Bacteriocin: a natural approach for food safety and food security. Front. Bioeng. Biotechnol. 10:1005918. doi: 10.3389/fbioe.2022.1005918
Lahiri, D., Nag, M., Sarkar, T., Ray, R. R., Shariati, M. A., Rebezov, M., et al. (2022a). Lactic acid bacteria (LAB): autochthonous and probiotic microbes for meat preservation and fortification. Foods 11:2792. doi: 10.3390/foods11182792
LeBlanc, J. G., Chain, F., Martín, R., Bermúdez-Humarán, L. G., Courau, S., and Langella, P. (2017). Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 16:69. doi: 10.1186/s12934-017-0691-z
Lee, N. K., and Paik, H. D. (2017). Bioconversion using lactic acid bacteria: Ginsenosides, GABA, and phenolic compounds. J. Microbiol. Biotechnol. 27, 869–877. doi: 10.4014/jmb.1612.12005
Li, P., Gu, Q., Yang, L., Yu, Y., and Wang, Y. (2017). Characterization of extracellular vitamin B12 producing Lactobacillus plantarum strains and assessment of the probiotic potentials. Food Chem. 234, 494–501. doi: 10.1016/j.foodchem.2017.05.037
Lin, M., Zhou, G., Wang, Z., and Yun, B. (2015). Functional analysis of AI-2/LuxS from bacteria in Chinese fermented meat after high nitrate concentration shock. Eur. Food Res. Technol. 240, 119–127. doi: 10.1007/s00217-014-2313-x
Liu, Y., van Bennekom, E. O., Zhang, Y., Abee, T., and Smid, E. J. (2019). Long-chain vitamin K2 production in Lactococcus lactis is influenced by temperature, carbon source, aeration and mode of energy metabolism. Microb. Cell Factories 18:129. doi: 10.1186/s12934-019-1179-9
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7
Liu, B., Zheng, D., Zhou, S., Chen, L., and Yang, J. (2022). VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res. 50, D912–D917. doi: 10.1093/nar/gkab1107
Lu, Y., Aizhan, R., Yan, H., Li, X., Wang, X., Yi, Y., et al. (2020). Characterization, modes of action, and application of a novel broad-spectrum bacteriocin BM1300 produced by Lactobacillus crustorum MN047. Braz. J. Microbiol. 51, 2033–2048. doi: 10.1007/s42770-020-00311-3
Lv, X., Ma, H., Sun, M., Lin, Y., Bai, F., Li, J., et al. (2018). A novel bacteriocin DY4-2 produced by Lactobacillus plantarum from cutlassfish and its application as bio-preservative for the control of Pseudomonas fluorescens in fresh turbot (Scophthalmus maximus) fillets. Food Control 89, 22–31. doi: 10.1016/j.foodcont.2018.02.002
Lyu, C., Zhao, W., Peng, C., Hu, S., Fang, H., Hua, Y., et al. (2018). Exploring the contributions of two glutamate decarboxylase isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production. Microb. Cell Factories 17:180. doi: 10.1186/s12934-018-1029-1
Madhuri, K. V., and Prabhakar, K. V. (2014). Microbial exopolysaccharides: biosynthesis and potential applications. Orient. J. Chem. 30, 1401–1410. doi: 10.13005/ojc/300362
Mandha, J., Shumoy, H., Devaere, J., Schouteten, J. J., Gellynck, X., Winne, A., et al. (2021). Effect of lactic acid fermentation of watermelon juice on its sensory acceptability and volatile compounds. Food Chem. 358:129809. doi: 10.1016/j.foodchem.2021.129809
Marcelli, V., Osimani, A., and Aquilanti, L. (2024). Research progress in the use of lactic acid bacteria as natural biopreservatives against Pseudomonas spp. in meat and meat products: a review. Food Res. Int. 196:115129. doi: 10.1016/j.foodres.2024.115129
Mazorra-Manzano, M. A., Robles-Porchas, G. R., González-Velázquez, D. A., Torres-Llanez, M. J., Martínez-Porchas, M., García-Sifuentes, C. O., et al. (2020). Cheese whey fermentation by its native microbiota: proteolysis and bioactive peptides release with ACE-inhibitory activity. Fermentation 6:19. doi: 10.3390/fermentation6010019
Mesele, A. (2018). A review on food fermentation and the biotechnology of lactic acid bacteria. World J. Food Sci. Technol. 2, 19–24. doi: 10.11648/j.wjfst.20180201.13
Minarovičová, J., Véghová, A., Mikulášová, M., Chovanová, R., Šoltýs, K., Drahovská, H., et al. (2018). Benzalkonium chloride tolerance of Listeria monocytogenes strains isolated from a meat processing facility is related to presence of plasmid-borne bcrABC cassette. Antonie Van Leeuwenhoek 111, 1913–1923. doi: 10.1007/s10482-018-1082-0
Mohd Azmi, S. I., Kumar, P., Sharma, N., Sazili, A. Q., Lee, S. J., and Ismail-Fitry, M. R. (2023). Application of plant proteases in meat tenderization: recent trends and future prospects. Foods 12:1336. Erratum in: Foods 2024, 13(3), 379. doi: 10.3390/foods13030379
Mouafo, H. T., Baomog, A. M. B., Adjele, J. J. B., Sokamte, A. T., Mbawala, A., and Ndjouenkeu, R. (2020b). Microbial profile of fresh beef sold in the markets of Ngaoundéré, Cameroon, and antiadhesive activity of a biosurfactant against selected bacterial pathogens. J. Food Qual. 2020:5989428. doi: 10.1155/2020/5989428
Mouafo, H. T., Mbawala, A., Tanaji, K., Somashekar, D., and Ndjouenkeu, R. (2020a). Improvement of the shelf life of raw ground goat meat by using biosurfactants produced by lactobacilli strains as biopreservatives. LWT 133:110071. doi: 10.1016/j.lwt.2020.110071
Mozuriene, E., Bartkiene, E., Krungleviciute, V., Zadeike, D., Juodeikiene, G., Damasius, J., et al. (2016). Effect of natural marinade based on lactic acid bacteria on pork meat quality parameters and biogenic amine contents. LWT 69, 319–326. doi: 10.1016/j.lwt.2016.01.061
Muhialdin, B. J., Saari, N., and Hussin, A. S. M. (2020). Review on the biological detoxification of mycotoxins using lactic acid bacteria to enhance the sustainability of foods supply. Molecules 25:2655. doi: 10.3390/molecules25112655
Murniece, R., Reidzane, S., Radenkovs, V., Straumite, E., Keke, A., Kobrin, E. G., et al. (2025). Scald fermentation time as a factor determining the nutritional and sensory quality of rye bread. Foods 14:979. doi: 10.3390/foods14060979
Olaoye, O. A., Onilude, A. A., and Ubbor, S. C. (2015). Control of Brochothrix thermosphacta in pork meat using Lactococcus lactis subsp. lactis I23 isolated from beef. Appl. Food Biotechnol. 2, 49–55. doi: 10.22037/afb.v2i3.7993
Parlindungan, E., Lugli, G. A., Ventura, M., van Sinderen, D., and Mahony, J. (2021). Lactic acid bacteria diversity and characterization of probiotic candidates in fermented meats. Foods 10:1519. doi: 10.3390/foods10071519
Peerajan, S., Chaiyasut, C., Sirilun, S., Chaiyasut, K., Kesika, P., and Sivamaruthi, B. S. (2016). Enrichment of nutritional value of Phyllanthus emblica fruit juice using the probiotic bacterium, Lactobacillus paracasei HII01 mediated fermentation. Food Sci. Technol. (Braz.) 36, 116–123. doi: 10.1590/1678-457X.0064
Perez, R. H., and Ancuelo, E. A. (2023). “Diverse bioactive molecules from the genus Lactobacillus: Lactobacillus—a multifunctional genus” in Lactobacillus – A multifunctional genus. ed. M. Laranjo. 1st ed (London, UK: IntechOpen), 1–27. doi: 10.5772/intechopen.102747
Pessione, A., Lo Bianco, G., Mangiapane, E., Cirrincione, S., and Pessione, E. (2015). Characterization of potentially probiotic lactic acid bacteria isolated from olives: evaluation of short chain fatty acids production and analysis of the extracellular proteome. Food Res. Int. 67, 247–254. doi: 10.1016/j.foodres.2014.11.029
Pradhan, S. R., Patra, G., Nanda, P. K., Dandapat, P., Bandyopadhyay, S., and Das, A. K. (2018). Comparative microbial load assessment of meat, contact surfaces and water samples in retail chevon meat shops and abattoirs of Kolkata, W.B., India. Int. J. Curr. Microbiol. Appl. Sci. 7, 158–164. doi: 10.20546/ijcmas.2018.705.020
Quan, Q., Liu, W., Guo, J., Ye, M., and Zhang, J. (2022). Effect of six lactic acid bacteria strains on physicochemical characteristics, antioxidant activities and sensory properties of fermented orange juices. Foods 11:1920. doi: 10.3390/foods11131920
Radi, M., Shadikhah, S., Sayadi, M., Kaveh, S., Amiri, S., and Bagheri, F. (2023). Effect of Thymus vulgaris essential oil-loaded nanostructured lipid carriers in alginate-based edible coating on the postharvest quality of tangerine fruit. Food Bioprocess Technol. 16, 185–198. doi: 10.1007/s11947-022-02914-0
Radosavljević, M., Lević, S., Pejin, J., Mojović, L., and Nedović, V. (2022). “Encapsulation technology of lactic acid bacteria in food fermentation” in Lactic acid Bacteria in food biotechnology—Innovations and functional aspects. eds. C. R. Ray, S. Paramithiotis, V. A. de Carvalho Azevedo, and D. Montet (Amsterdam, The Netherlands: Elsevier), 319–347.
Raman, J., Kim, J. S., Choi, K. R., Eun, H., Yang, D., Ko, Y. J., et al. (2022). Application of lactic acid bacteria (LAB) in sustainable agriculture: advantages and limitations. Int. J. Mol. Sci. 23:7784. doi: 10.3390/ijms23147784
Riley, M. A., and Wertz, J. E. (2002). Bacteriocins: evolution, ecology, and application. Ann. Rev. Microbiol. 56, 117–137. doi: 10.1146/annurev.micro.56.012302.161024
Rivas, F. P., Castro, M. P., Vallejo, M., Marguet, E., and Campos, C. A. (2014). Sakacin Q produced by Lactobacillus curvatus ACU-1: functionality characterization and antilisterial activity on cooked meat surface. Meat Sci. 97, 475–479. doi: 10.1016/j.meatsci.2014.03.003
Sanalibaba, P., and Cakmak, G. A. (2016). Exopolysaccharides production by lactic acid bacteria. Appl. Microbiol. 2:1000115. doi: 10.4172/2471-9315.1000115
Shao, X., Xu, B., Chen, C., Li, P., and Luo, H. (2021). The function and mechanism of lactic acid bacteria in the reduction of toxic substances in food: a review. Crit. Rev. Food Sci. Nutr. 62, 5950–5963. doi: 10.1080/10408398.2021.1895059
Sharma, N., Angural, S., Rana, M., Puri, N., Kondepudi, K. K., and Gupta, N. (2020). Phytase producing lactic acid bacteria: cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci. Technol. 96, 1–12. doi: 10.1016/j.tifs.2019.12.001
Sharma, H., Fidan, H., Özogul, F., and Rocha, J. M. (2022). Recent development in the preservation effect of lactic acid bacteria and essential oils on chicken and seafood products. Front. Microbiol. 13:1092248. doi: 10.3389/fmicb.2022.1092248
Silva, B. N., Teixeira, J. A., Cadavez, V., and Gonzales-Barron, U. (2023). Mild heat treatment and biopreservatives for artisanal raw milk cheeses: reducing microbial spoilage and extending shelf life through thermisation, plant extracts and lactic acid bacteria. Foods 12:3206. doi: 10.3390/foods12173206
Singh, P., and Saini, P. (2017). Food and health potentials of exopolysaccharides derived from lactobacilli. Microbiol. Res. J. Int. 22, 1–14. doi: 10.9734/MRJI/2017/36935
Sivamaruthi, B. S., Alagarsamy, K., Suganthy, N., Thangaleela, S., Kesika, P., and Chaiyasut, C. (2022). The role and significance of Bacillus and Lactobacillus species in Thai fermented foods. Fermentation 8:635. doi: 10.3390/fermentation8110635
Sivamaruthi, B. S., Kesika, P., and Chaiyasut, C. (2021). A narrative review on biogenic amines in fermented fish and meat products. J. Food Sci. Technol. 58, 1623–1639. doi: 10.1007/s13197-020-04686-x
Smaoui, S., Elleuch, L., Ben Salah, R., Najah, S., Chakchouk Mtibaa, A., Sellem, I., et al. (2014). Efficient role of BacTN635 on the safety properties, sensory attributes, and texture profile of raw minced meat beef and chicken breast. Food Addit. Contam. Part A 31, 218–225. doi: 10.1080/19440049.2013.873144
Solis-Balandra, M. A., and Sanchez-Salas, J. L. (2024). Classification and multi-functional use of Bacteriocins in health, biotechnology, and food industry. Antibiotics 13:666. doi: 10.3390/antibiotics13070666
Strafella, S., Simpson, D. J., Yaghoubi Kha’nghahi, M., De Angelis, M., Gänzle, M., Minervini, F., et al. (2020). Comparative genomics and in vitro plant growth promotion and biocontrol traits of lactic acid bacteria from the wheat rhizosphere. Microorganisms 9:78. doi: 10.3390/microorganisms9010078
Sybesma, W., Starrenburg, M., Tijsseling, L., Hoefnagel, M. H. N., and Hugenholtz, J. (2003). Effects of cultivation conditions on folate production by lactic acid bacteria. Appl. Environ. Microbiol. 69, 4542–4548. doi: 10.1128/AEM.69.8.4542-4548.2003
Tang, H., Huang, W., and Yao, Y. F. (2023). The metabolites of lactic acid bacteria: classification, biosynthesis and modulation of gut microbiota. Microb. Cell 10, 49–62. doi: 10.15698/mic2023.03.792
Thuy, T. T. D., Lu, H. F., Bregente, C. J. B., Huang, F. A., Tu, P. C., and Kao, C. Y. (2024). Characterization of the broad-spectrum antibacterial activity of bacteriocin-like inhibitory substance producing probiotics isolated from fermented foods. BMC Microbiol. 24:85. doi: 10.1186/s12866-024-03245-0
Todorov, S. D., Baretto Penna, A. L., Venema, K., Holzapfel, W. H., and Chikindas, M. L. (2023). Recommendations for the use of standardized abbreviations for the former Lactobacillus genera, reclassified in 2020. Benef. Microbes 15, 1–4. doi: 10.1163/18762891-20230114
Todorov, S. D., Stojanovski, S., Iliev, I., Moncheva, P., Nero, L. A., and Ivanova, I. V. (2017). Technology and safety assessment for lactic acid bacteria isolated from traditional Bulgarian fermented meat product “Lukanka”. Braz. J. Microbiol. 48, 576–586. doi: 10.1016/j.bjm.2017.02.005
Uppada, S. R., Akula, M., Bhattacharya, A., and Dutta, J. R. (2017). Immobilized lipase from Lactobacillus plantarum in meat degradation and synthesis of flavor esters. J. Genet. Eng. Biotechnol. 15, 331–334. doi: 10.1016/j.jgeb.2017.07.008
Van Meervenne, E., De Weirdt, R., Van Coillie, E., Devlieghere, F., Herman, L., and Boon, N. (2014). Biofilm models for the food industry: hot spots for plasmid transfer? Pathog. Dis. 70, 332–338. doi: 10.1111/2049-632X.12134
Von Wright, A., and Axelsson, L. (2019). Lactic acid bacteria: An introduction. In Lactic acid Bacteria, 5. G. Vinderola, A. Ouwehand, S. Salminen, and A. Von Wright (Eds.). CRC Press: Boca Raton, FL, pp. 1–16.
Wang, Y., Wu, J., Lv, M., Shao, Z., Hungwe, M., Wang, J., et al. (2021). Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 9:612285. doi: 10.3389/fbioe.2021.612285
Wen, R., Sun, F., Wang, Y., Chen, Q., and Kong, B. (2021). Evaluation of the potential of lactic acid bacteria isolates from traditional beef jerky as starter cultures and their effects on flavor formation during fermentation. LWT-Food Sci. Technol. 142:110982. doi: 10.1016/j.lwt.2021.110982
Woraharn, S., Lailerd, N., Sivamaruthi, B. S., Wangcharoen, W., Sirisattha, S., and Chaiyasut, C. (2014). Screening and kinetics of glutaminase and glutamate decarboxylase producing lactic acid bacteria from fermented Thai foods. Food Sci. Technol. (Campinas) 34, 793–799. doi: 10.1590/1678-457X.6519
Woraharn, S., Lailerd, N., Sivamaruthi, B. S., Wangcharoen, W., Sirisattha, S., Peerajan, S., et al. (2016). Evaluation of factors that influence the L-glutamic and γ-aminobutyric acid production during Hericium erinaceus fermentation by lactic acid bacteria. CyTA 14, 47–54. doi: 10.1080/19476337.2015.1042525
Woraprayote, W., Malila, Y., Sorapukdee, S., Swetwiwathana, A., Benjakul, S., and Visessanguan, W. (2016). Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 120, 118–132. doi: 10.1016/j.meatsci.2016.04.004
Woraprayote, W., Pumpuang, L., Tosukhowong, A., Roytrakul, S., Perez, R. H., Zendo, T., et al. (2015). Two putatively novel bacteriocins active against gram-negative food borne pathogens produced by Weissella hellenica BCC 7293. Food Control 55, 176–184. doi: 10.1016/j.foodcont.2015.02.036
Wu, W., and Li, H. (2018). “Metabolites of lactic acid bacteria” in Lactic acid Bacteria in foodborne hazards reduction: Physiology to practice. eds. W. Chen and A. Narbad. 1st ed (Berlin/Heidelberg, Germany: Springer), 87–113.
Xu, B., Qiu, W., Liu, Y., Gong, F., Liu, Q., Chen, J., et al. (2025). Exploring the regulation of metabolic changes mediated by different combined starter cultures on the characteristic flavor compounds and quality of Sichuan-style fermented sausages. Food Res. Int. 208:116114. doi: 10.1016/j.foodres.2025.116114
Xu, J., Wang, Z., Shi, Z., Liu, M., Fan, X., Zhang, T., et al. (2025). Detection and development of lactic acid bacteria bacteriocins—a hint on the screening of bacteriocins of lactic acid bacteria. LWT 222:117627. doi: 10.1016/j.lwt.2025.117627
Yang, S. C., Lin, C. H., Sung, C. T., and Fang, J. Y. (2014). Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front. Microbiol. 5:241. doi: 10.3389/fmicb.2014.00241
Yogeswara, I. B. A., Maneerat, S., and Haltrich, D. (2020). Glutamate decarboxylase from lactic acid Bacteria-a key enzyme in GABA synthesis. Microorganisms 8:1923. doi: 10.3390/microorganisms8121923
Zhang, Q., Song, X., Sun, W., Wang, C., Li, C., He, L., et al. (2021). Evaluation and application of different cholesterol lowering lactic acid bacteria as potential meat starters. J. Food Prot. 84, 63–72. doi: 10.4315/JFP-20-225
Zhang, Z., Wu, R., Xu, W., Cocolin, L., Liang, H., Ji, C., et al. (2023). Combined effects of lipase and Lactiplantibacillus plantarum 1-24-LJ on physicochemical property, microbial succession and volatile compounds formation in fermented fish product. J. Sci. Food Agric. 103, 2304–2312. doi: 10.1002/jsfa.12445
Zhaxybayeva, E. Z., Dikhanbayeva, F., Dimitriev, Z. P., Imangalieva, Z., and Asenov, R. (2020). Development of a recipe and technology for the production of drinking yogurt from camel milk for gerodietetic nutrition based on the enzyme, probiotics and nutrient additive. EurAsian J. Biosci. 14, 355–363.
Zheng, J., Wittouck, S., Salvetti, E., Franz, C. M. A. P., Harris, H. M. B., Mattarelli, P., et al. (2020). A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 70, 2782–2858. doi: 10.1099/ijsem.0.004107
Zhu, Y., Guo, L., and Yang, Q. (2020). Partial replacement of nitrite with a novel probiotic Lactobacillus plantarum on nitrate, color, biogenic amines and gel properties of Chinese fermented sausages. Food Res. Int. 137:109351. doi: 10.1016/j.foodres.2020.109351
Keywords: meat products, lactic acid bacteria, food safety, bacteriocins, preservation
Citation: Sivamaruthi BS, Kesika P, Rafeek SBS, Sivakumar K, Ramasamy SP, Chaiaysut C, Fukngoen P and Alagarsamy K (2025) Lactic acid bacteria in the meat industry: flavor, function, and food safety. Front. Microbiol. 16:1703213. doi: 10.3389/fmicb.2025.1703213
Edited by:
Massimo Iorizzo, University of Molise, ItalyReviewed by:
Wei Jiang, Zhejiang Ocean University, ChinaKayque Ordonho Carneiro, Department of Health, Brazil
Copyright © 2025 Sivamaruthi, Kesika, Rafeek, Sivakumar, Ramasamy, Chaiaysut, Fukngoen and Alagarsamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pranom Fukngoen, cHJhbm9tLmZAY211LmFjLnRo; Karthikeyan Alagarsamy, a2FydGhpa2V5YW5hQHBzZ2Nhcy5hYy5pbg==
 Bhagavathi Sundaram Sivamaruthi
Bhagavathi Sundaram Sivamaruthi Periyanaina Kesika
Periyanaina Kesika Safreena Barwin Syed Rafeek
Safreena Barwin Syed Rafeek Kasthuri Sivakumar
Kasthuri Sivakumar Shanmuga Priya Ramasamy
Shanmuga Priya Ramasamy Chaiyavat Chaiaysut
Chaiyavat Chaiaysut Pranom Fukngoen
Pranom Fukngoen Karthikeyan Alagarsamy
Karthikeyan Alagarsamy