- 1Center for Cybernics Research, University of Tsukuba, Tsukuba, Japan
- 2Department of Neurosurgery, Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba, Japan
- 3Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 4Department of Rehabilitation Medicine, University of Tsukuba Hospital, Tsukuba, Japan
- 5Department of Orthopaedic Surgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 6Department of Neurosurgery, Kennan Hospital, Tsuchiura, Japan
- 7Department of Neurosurgery, Kobari General Hospital, Noda, Japan
- 8Department of Biostatistics, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
We hypothesized that a single-leg version of the Hybrid Assistive Limb (HAL) system could improve the gait and physical function of patients with hemiparesis following a stroke. In this pilot study, we therefore compared the efficacy of HAL-based gait training with that of conventional gait training (CGT) in patients with acute stroke. Patients admitted to the participating university hospital were assigned to the HAL group, whereas those admitted to outside teaching hospitals under the same rehabilitation program who did not use the HAL were assigned to the control group. Over 3 weeks, all participants completed nine 20 min sessions of gait training, using either HAL (i.e., the single-leg version of HAL on the paretic side) or conventional methods (i.e., walking aids and gait orthoses). Outcome measures were evaluated before and after the nine training sessions. The Functional Ambulation Category (FAC) was the primary outcome measure, but the following secondary outcome measures were also assessed: National Institutes of Health Stroke Scale, Fugl–Meyer Assessment (Lower Extremity), comfortable walking speed, step length, cadence, 6-min walk distance, Barthel Index, and Functional Independence Measure. In total, 22 post-stroke participants completed the clinical trial: 12 in the HAL group and 10 in the CGT group. No serious adverse events occurred in either group. The HAL group showed significant improvement in FAC after nine sessions when compared with the CGT group (P = 0.014). However, secondary outcomes did not differ significantly between the groups. Our results demonstrate that HAL-based gait therapy may improve independent walking in patients with acute stroke hemiplegia who are dependent on ambulatory assistance. A larger-scale randomized controlled trial is needed to clarify the effectiveness of single-leg HAL therapy.
Clinical Trial Registration: UMIN Clinical Trials Registry, identifier UMIN000022410.
Introduction
Stroke is a serious and disabling health problem that is common worldwide (Langhorne et al., 2011). Approximately 90% of patients who suffer a stroke will have persistent neurological motor deficits that cause disability and handicap (Hesse and Werner, 2003). In Japan, stroke is the third leading cause of death and the second leading cause of social care needs (Ministry of Health, Labour and Welfare, 2016, 2018). Given that one-third of survivors will only achieve a poor functional outcome 5 years after a stroke (Barker-Collo et al., 2010; Takashima et al., 2017), it is unsurprising that stroke-related problems place a serious burden on both patients and their families. However, effective early treatment and rehabilitation can significantly improve outcomes (Rigby et al., 2009).
The recovery of gait and independent walking are common goals following a major stroke (Dobkin, 2005; Peurala et al., 2009), and the intensity of rehabilitation therapy for the arms and legs is known to be positively correlated with motor outcomes after stroke (Kwakkel et al., 1999). Given that the recovery of walking function mainly occurs within the first 11 weeks after a stroke (Jørgensen et al., 1995), active and intensive gait treatment should start from the acute stage. Since the 1990s, automated or robot-assisted motor rehabilitation has emerged as a viable aid in this process (Hesse et al., 2003). Indeed, a recent systematic review of the Cochrane database suggested that electromechanical-assisted gait training in combination with physiotherapy was more likely to produce independent walking after a stroke than gait training without these devices. Moreover, most benefit was seen in the first 3 months after stroke and in those unable to walk (Mehrholz et al., 2017).
The Hybrid Assistive Limb (HAL) robotic suit is the world’s first cyborg-type wearable device for supporting, improving, and expanding a wearer’s physical functions based on the detection of bio-electrical signals (BES) detected on the skin surface when a wearer tries to generate muscle force (Lee and Sankai, 2005; Suzuki et al., 2007; Sankai et al., 2014). HAL treatment is based on the principles of the interactive biofeedback hypothesis. It uses a hybrid control system composed of two cybernic control modes (voluntary and autonomous) (Kawamoto et al., 2009). Once activated by the wearer’s BES, the voluntary control mode provides physical support and actions through voluntary intention (Kawamoto and Sankai, 2005). For example, the wearer’s desire to move his or her legs causes the transmission of a signal from the brain to the peripheral nerves, muscles, and skin. However, patients with stroke-induced hemiparesis cannot move their extremities, and in such patients, HAL can support movement by utilizing very weak BES on the skin surface to activate HAL autonomously (Sankai and Sakurai, 2018). This movement and exercise may enhance recovery of the impaired neuronal network, while interactive biofeedback may further promote appropriate reorganization of the neuronal network (Morishita and Inoue, 2016; Sankai and Sakurai, 2018). The single-leg version of the HAL is a new wearable robotic device that has been designed to be used by people with hemiplegia.
To date, there has been no study comparing HAL and conventional therapies on patient outcomes after acute stroke. We therefore wanted to compare the effects of gait treatment with a single-leg version of HAL against that of conventional gait training (CGT) in patients with acute stroke.
Materials and Methods
Objective
The purpose of this pilot clinical study was to investigate whether motion assistance during gait treatment, using the single-leg version of HAL, could produce early improvements in independent walking after acute stroke compared to CGT.
Design and Setting
This is a non-randomized, non-blinded clinical trial. Patients hospitalized at the University of Tsukuba Hospital were assigned to the HAL group. Patients hospitalized at Kennan Hospital, Kobari General Hospital, Tsukuba Memorial Hospital, or Ibaraki Seinan Medical Center Hospital were assigned to the CGT group. Patients in the HAL group received gait training with HAL, whereas those in the CGT group received gait training without HAL. Training was provided thrice weekly for 20 min per day over a 3-week period. This led to nine sessions each during the study.
Types of Participants
Patients admitted to one of five hospitals (University of Tsukuba Hospital, Kennan Hospital, Kobari General Hospital, Tsukuba Memorial Hospital, and Ibaraki Seinan Medical Centre Hospital) with acute-onset stroke between September 2016 and March 2019 were invited to participate in this study. The targeted numbers of cases were 20 and 30 patients in the HAL and CGT groups, respectively.
Inclusion Criteria
All of the following inclusion criteria needed to be met:
• Hemiparesis due to unilateral ischemic or hemorrhagic acute stroke.
• Age 40–80 years.
• A time since stroke onset within 7–14 days.
• A score of 1 or 2 on the Functional Ambulation Category (FAC).
• Likely to survive or persist with the intervention for at least 3 weeks.
• A score of >4 on FAC before stroke.
• Ability to consent (using a surrogate to sign a form if handwriting is difficult).
• Suitable for HAL (only in the HAL group).
Exclusion Criteria
Patients meeting any of the following criteria were excluded:
• Difficulty performing voluntary limb movement due to altered consciousness.
• Patient had another condition, such as severe cardiac disease or musculoskeletal problems, that could limit treatment using HAL.
• Investigator or sub-investigator deemed the patient inappropriate for inclusion in the clinical trial (i.e., patients with malignant tumors that have not been completely cured, bleeding tendencies, and/or severe mental illness).
• Patient received magnetic stimulation, electrical stimulation, or neuromodulation therapy before inclusion in the study.
Treatments
Gait Treatment Using HAL and CGT
The HAL group received gait treatment using a single-leg version of HAL. To prevent falls, all patients were harnessed in a mobile suspension system (All-In-One Walking Trainer, Ropox A/S, Denmark). Distance and walking speed depended on the patient’s tolerance. The physical therapist gradually increased the training intensity by changing the speed and duration of walking based on patient fatigue and endurance. The physical therapist provided verbal instructions to achieve a symmetric gait according to the ability of each patient. Specifically, the treatment aimed to swing the paretic leg outward during the swing phase and extend the hip joint during the stance phase. The physical therapist provided real-time feedback to the patient based on the BES and floor reaction force data during gait treatment. Gait orthoses were allowed. The heart rate, blood pressure, and ratings of subjective exercise intensity (Modified Borg scale) were monitored during walking and after each 20-min treatment. The duration of each gait treatment session, including the rest time, was 20 min. Patients participated in three sessions per week with a maximum of one session per day for a total of nine sessions over a 3-week period. We measured the actual walking time (i.e., non-rest time) and distance in each 20-min session. The intervention goal was to improve independent walking, as characterized by improved walking speed, endurance, postural stability, and symmetry (Watanabe et al., 2014). The cybernic voluntary control mode was mainly used; however, for patients who could not achieve motion with this mode, the cybernic autonomous control mode was used until they became familiar with the voluntary control (Kawamoto and Sankai, 2005; Kawamoto et al., 2009). CGT in the control group, was carried out without the HAL system by the physical therapist in charge of the patient, but otherwise using the same methodology.
Single-Leg HAL Therapy
The single-leg version of HAL (HAL-ML05) has been described in detail in previous reports (Kawamoto et al., 2009). The device is composed of an exoskeletal frame, power units, a battery, a controller, BES sensors, and floor reaction force sensors, together with belts to secure the waist, thigh, and lower leg (Figure 1). The weight of this HAL is approximately 9 kg. Electrodes are attached on the surface of the wearer’s skin over the rectus femoris, gluteus maximus, vastus lateralis, and biceps femoris muscles to detect the nerve and muscle action potentials as BES (Figure 2). The single-leg HAL system assists hip and knee joints with flexion and extension by a hybrid control system consisting of cybernic voluntary and autonomous control (Lee and Sankai, 2005). The voluntary control system provides harmonious support based on BES, reflecting the wearer’s voluntary intentions (Kawamoto and Sankai, 2002), with the gain in assistive torque at each joint in response to the BES being controlled by a physical therapist. The autonomous control system provides torque autonomously according to the walking pattern based on input from the floor reaction force (Figure 3; Kawamoto and Sankai, 2005). These mechanisms enable the HAL to coordinate an appropriate level and timing of torque to assist with hip and knee joint motion.
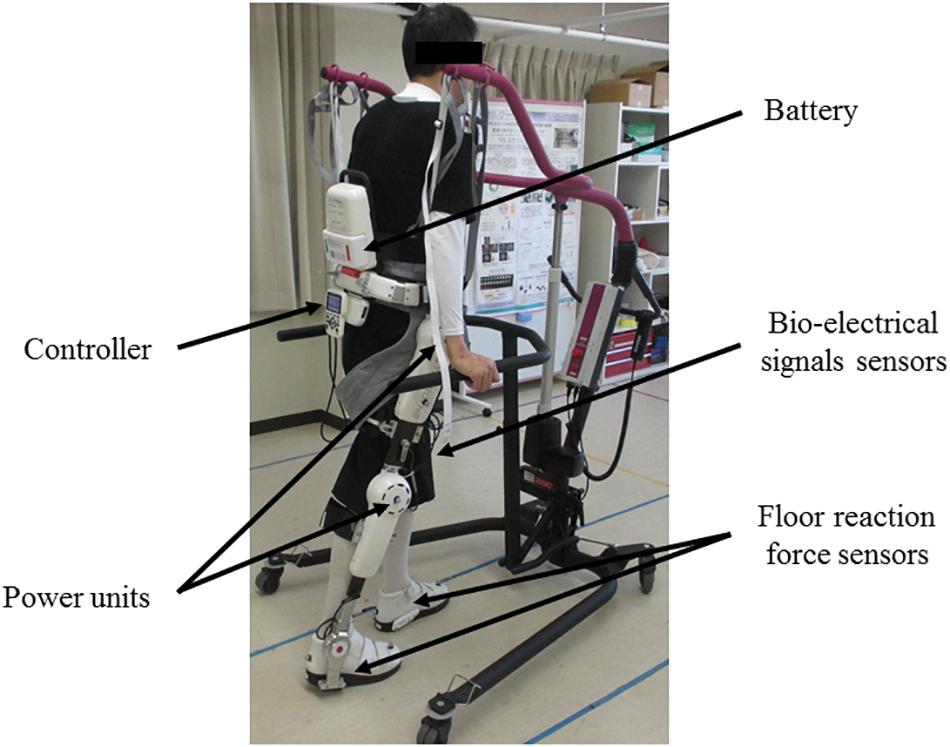
Figure 1. Gait treatment using single-leg version of HAL on the paretic side. The HAL is a battery-powered exoskeleton type robot. The physical therapist adjusts the belt and brings the wearer in close contact with the HAL. The controller of the HAL drives the power units based on the information of the bio-electrical signals sensors and the floor reaction force sensors to support the walking motion.
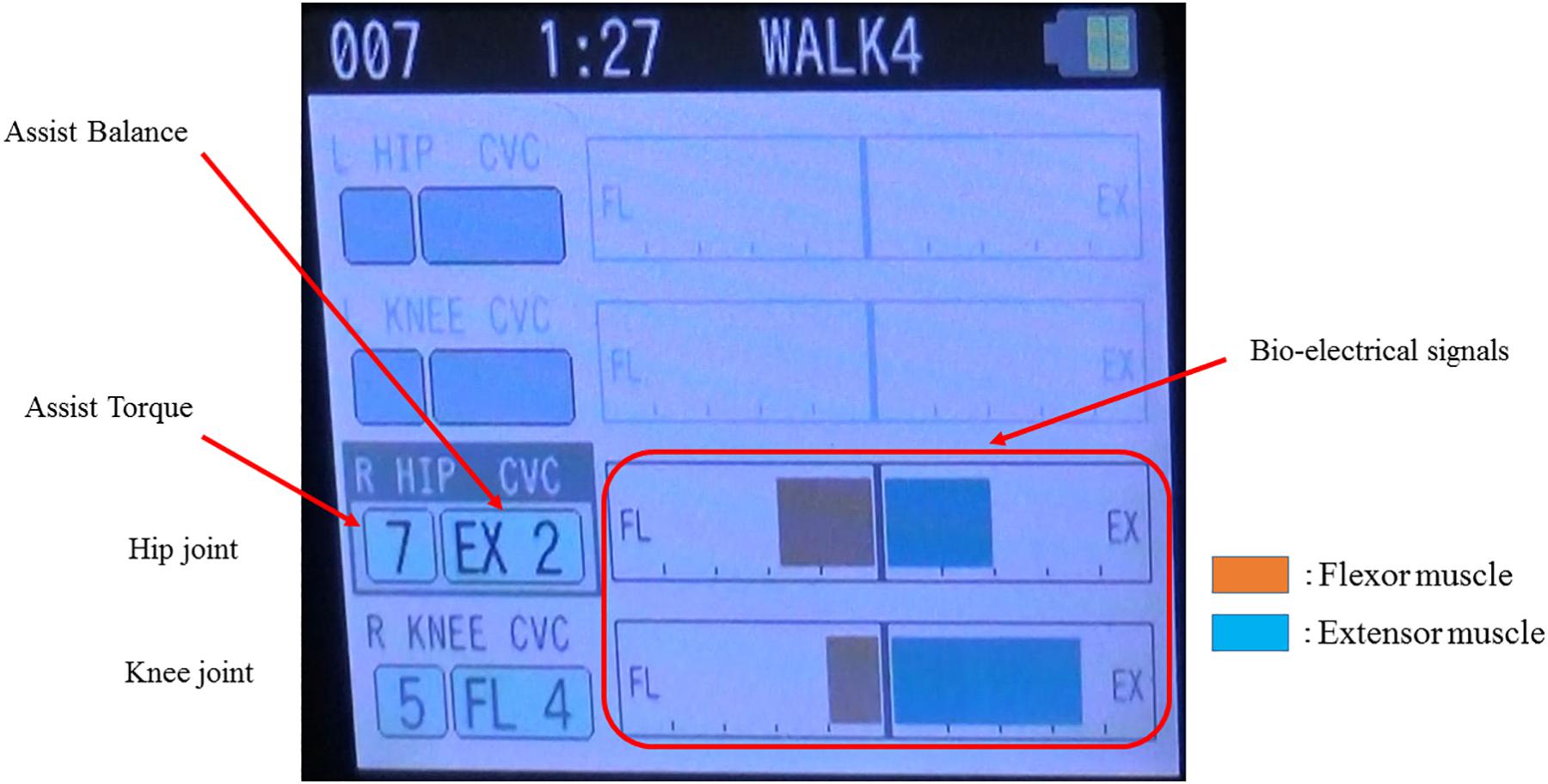
Figure 2. Example of the bio-electrical signals on the paralyzed side during gait treatment with HAL. The physical therapist adjusts the assist torque and assist balance based on the information of the bio-electrical signals. In this case, the assist torque of the hip joint is 7, knee joint is 5, and walking assistance is performed with the hip extension and knee flexion being dominant.
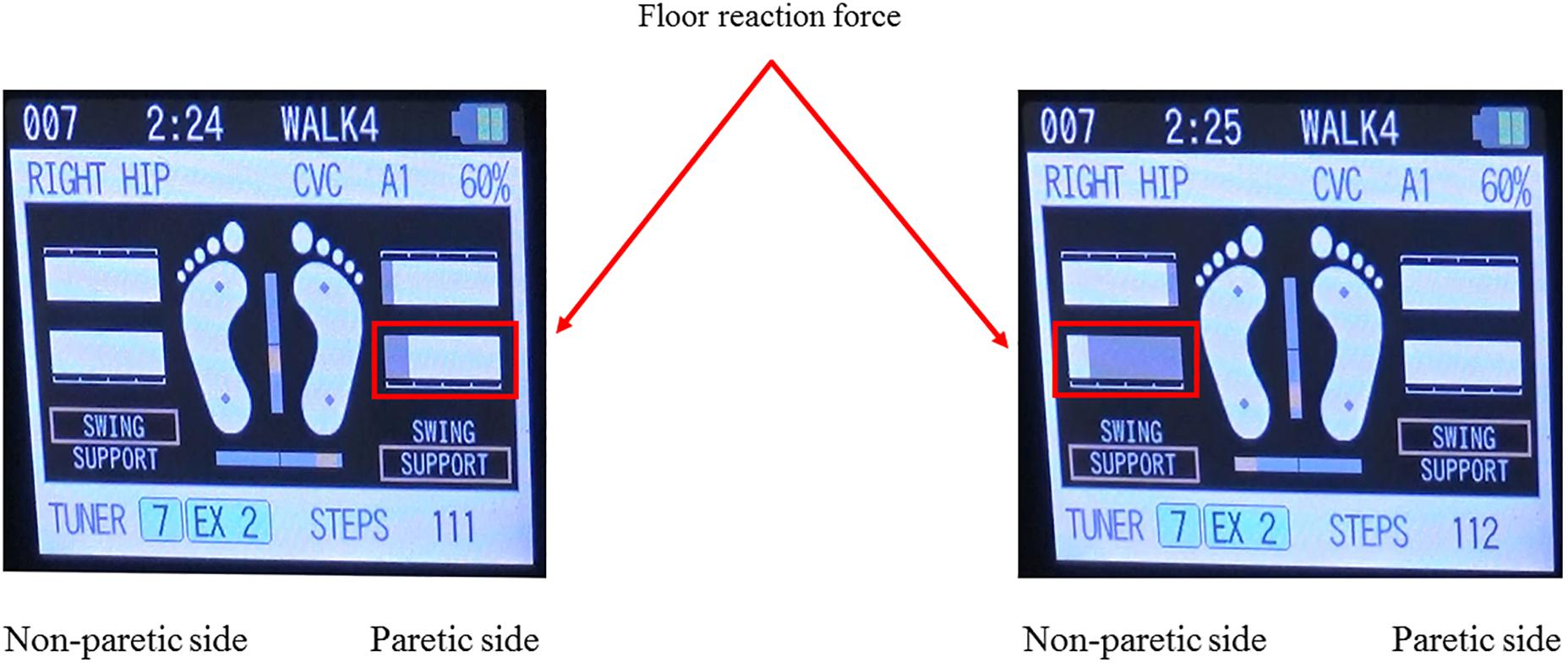
Figure 3. Example of the floor reaction force on the both leg during gait treatment with HAL. The floor reaction force during gait treatment with HAL can be calculated from the floor reaction force sensors built into the HAL shoes, and visual feedback can be provided to the physical therapist and the patient in real time on the controller screen as shown. This figure shows an example of the floor reaction force in the paretic and non-paretic stance phase, and it can be seen that the floor reaction force on the paralyzed side is smaller than that on the non-paralyzed side.
Permitted and Prohibited Concomitant Treatments
Patients in both the groups were prohibited from participating in robot-assisted training before and during the study period. HAL-based gait treatment was considered a component of physical therapy. The content and amount of physical therapy received before study participation were not specified. Patients in both the groups participated in conventional physical therapy, including muscle and endurance strengthening and conventional over-ground gait training. No patient in either group participated in robot-assisted gait training before or during the study period. The total duration of physical therapy during the 3-week intervention protocol was limited to 16 h. However, other routine aspects of physical therapy were permitted. In addition to normal exercise therapy (i.e., range of motion, muscle and endurance strengthening, and cooperative exercise), we permitted voluntary promotion exercises, lower limb stretches, basic motion exercises, and conventional over-ground gait training. No limitations were placed on the amount or content of intervention permitted by occupational and speech therapists.
Outcomes
Primary Outcome Measure
The primary outcome measure was FAC. This primary outcome was selected because a feasibility and safety study has reported the most significant improvement in this variable when compared with other outcome measures (unpublished data). FAC was evaluated by two physical therapists. Additionally, a physical therapist evaluated the appropriateness of the environment and evaluation measurement method and ensured implementation according to the manual during each gait assessment conducted at an external hospital. The gait were filmed, and assessed by a third party who was not involved in the study to confirm the correct implementation.
Secondary Outcome Measures
The following secondary outcomes measures were also assessed: National Institutes of Health Stroke Scale score, Fugl–Meyer Assessment of Lower Extremity, comfortable walking speed, step length, cadence, 6-min walk distance, Barthel Index, and Functional Independence Measure (total, motor scores, and cognitive scores).
Safety Outcome Measurement
Safety was examined through adverse events, which were defined as any event clearly related to HAL or CGT. If an adverse event occurred, the investigator responded appropriately and immediately, taking care to describe the episode consistently in the medical record and a case report. Researchers recorded any unfavorable symptoms as adverse events using a case report form during the study period. A serious adverse event was considered when it led to death or threatened life, required hospitalization or extension of hospitalization for treatment, and resulted in persistent or noticeable dysfunction or treatment failure.
Statistical Analysis
We tested differences in the baseline variables between the HAL and CGT groups by the Fisher exact test for categorical data and by the Mann–Whitney U test used for continuous data. The outcome measures in each group compared before and after gait training by the Wilcoxon signed-rank test; the Mann–Whitney U test was also used to compare the amount of change between both groups. All statistical analyses will be conducted using IBM SPSS version 24.0 (IBM Corp., Armonk, NY, United States). Statistical significance was set at p < 0.05.
Ethics Approval and Consent to Participate
The study protocol was designed according to tenets of the Declaration of Helsinki and relevant ethical guidance for clinical research. All patients recruited from the four participating hospitals provided written informed consent after learning about the study aims and design. The ethics committee of the University of Tsukuba Hospital approved this study (H27-257), and the protocol has been registered with the UMIN Clinical Trials Registry (UMIN000022410).
Results
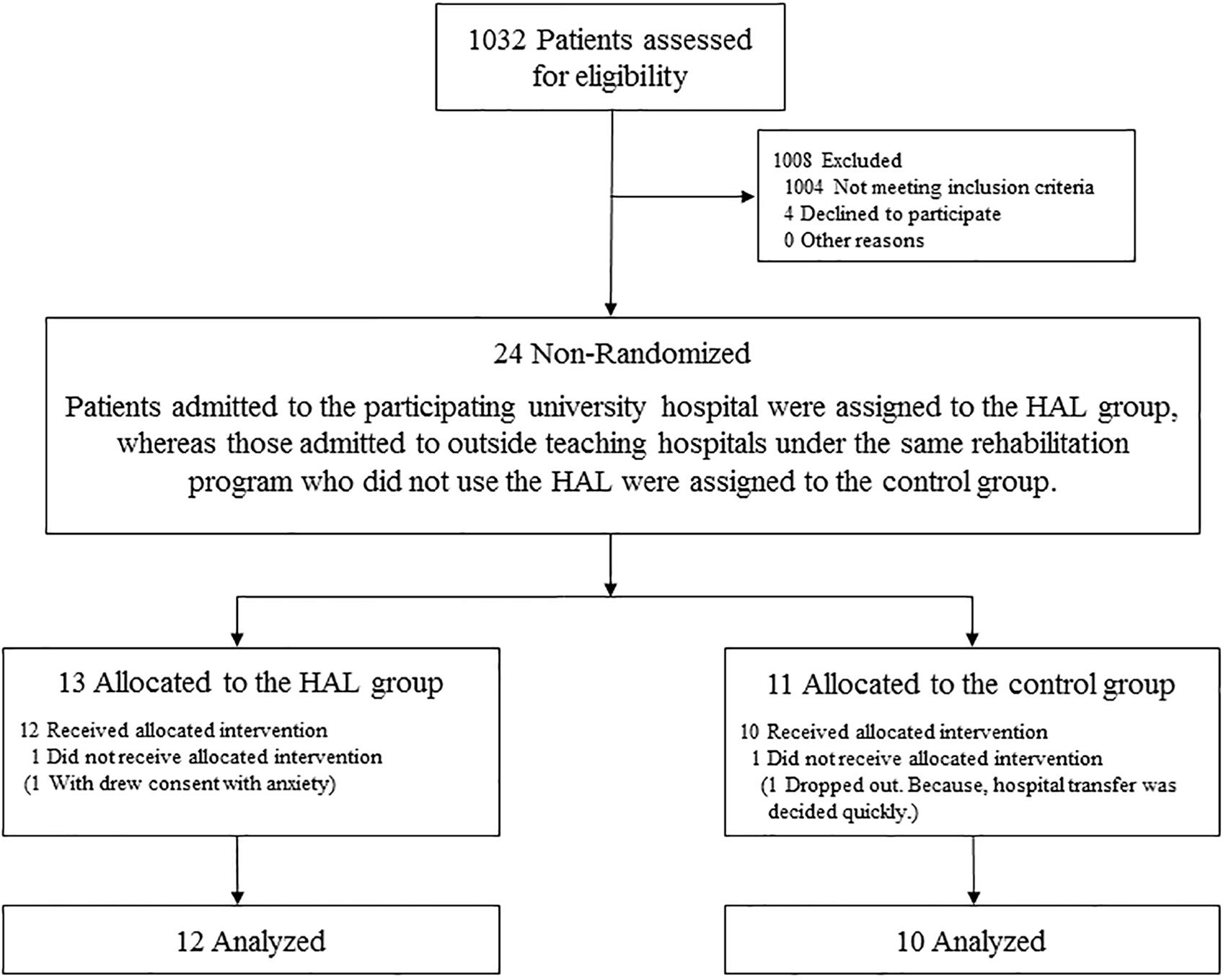
In total, 24 patients suffering from acute stroke participated in this study (Figure 4). Among the 13 patients allocated to the HAL group, one withdrew consent because of a high degree of stooped back in contact with the lumbar frame of HAL that caused anxiety. All remaining patients were ultimately able to use the cybernic voluntary control (one patient required cybernic autonomous control at the start of the intervention only). Among the 11 patients allocated to the CGT group, one dropped out because hospital transfer was decided quickly and the principal investigator agreed that it was clinically appropriate. Finally, 22 patients completed this study (Table 1). This study was terminated at the end of the indicated period before the target number of cases was reached. No serious adverse events occurred in either group. One patient in the HAL group reported pain in the contralateral (i.e., non-paralyzed) upper and lower limbs. This patient did not develop fever or inflammatory symptoms, and the pain disappeared immediately after follow-up. No patient in either group experienced orthostatic hypotension, scratches, or falls. These findings suggest that this protocol can be safely implemented.
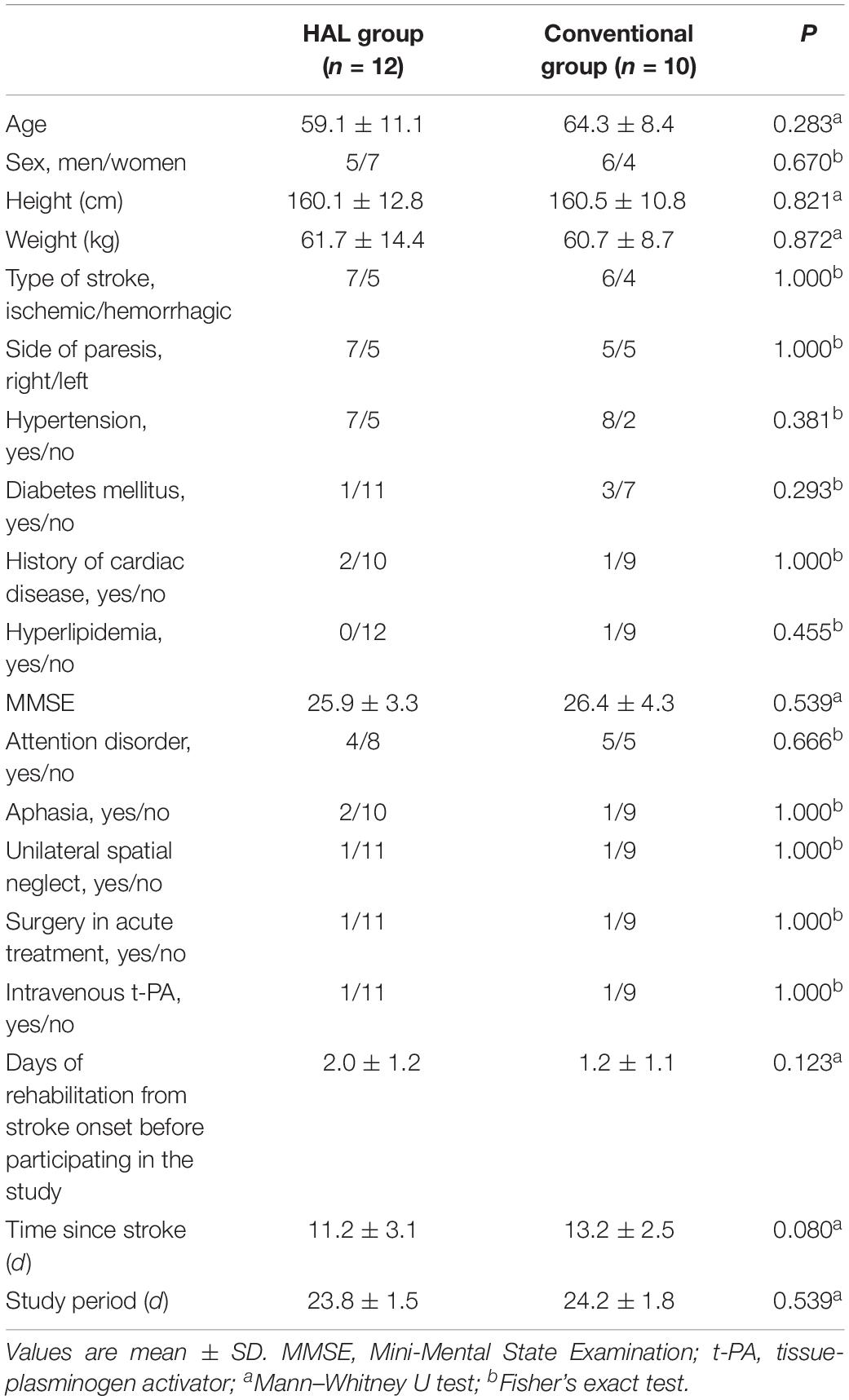
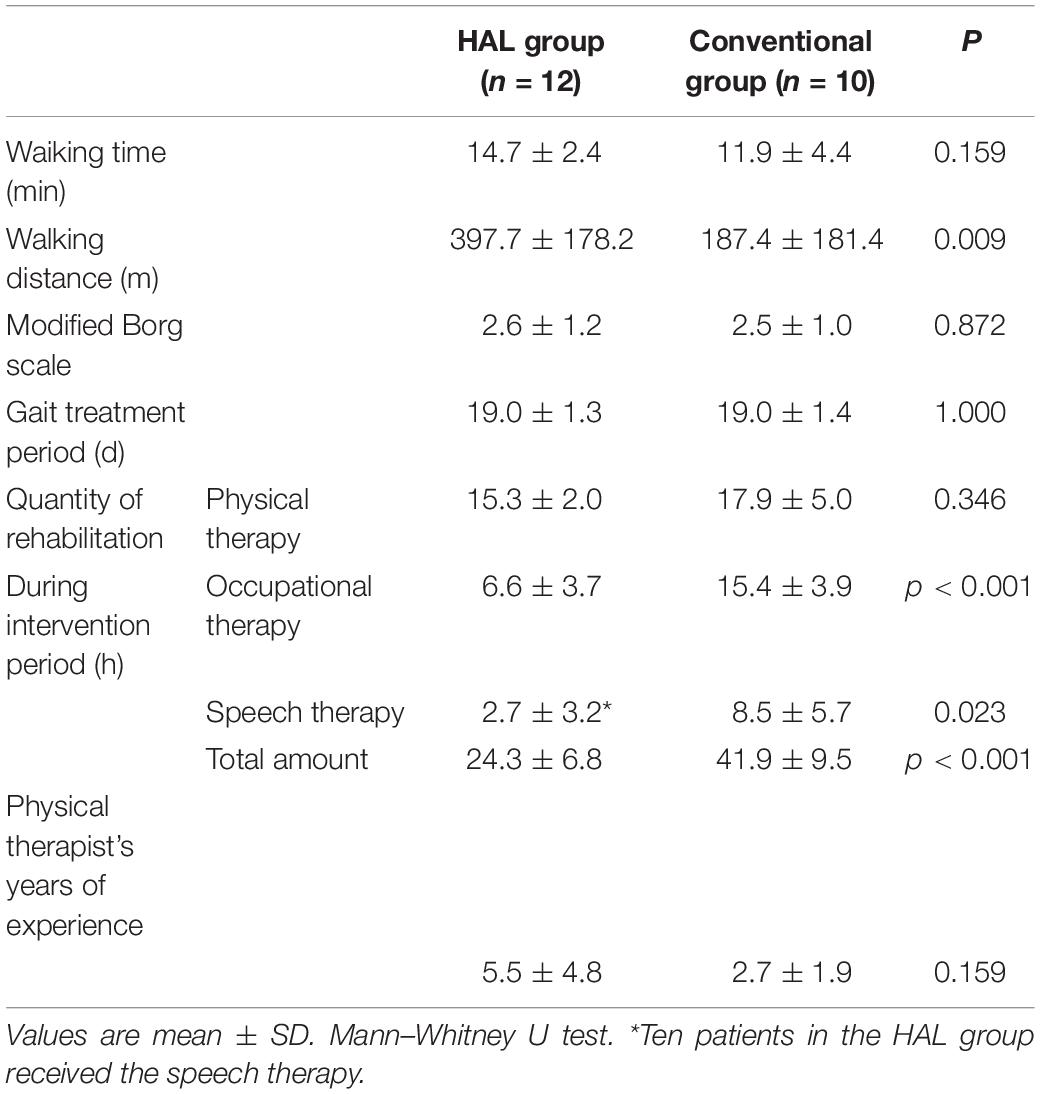
Patients in both the groups who did not undergo surgery during the acute phase received conservative treatment (e.g., antiplatelet therapy, brain protection therapy, antihypertensive therapy) according to individual conditions. No differences were observed between the two groups in terms of their characteristics and baseline clinical data (Table 1). The quantity of physical therapy during the intervention period, including hours spent on gait training, did not differ between the two groups (Table 2). Although the HAL group had significantly less occupational therapy and speech therapy than the CGT group (p < 0.05), it had a significantly higher walking distance (397.7 ± 178.2 vs. 187.7 ± 181.4, p < 0.05, Table 2). However, the actual walking time and the Modified Borg scale were not significantly different between the HAL and CGT groups at the end of the intervention.
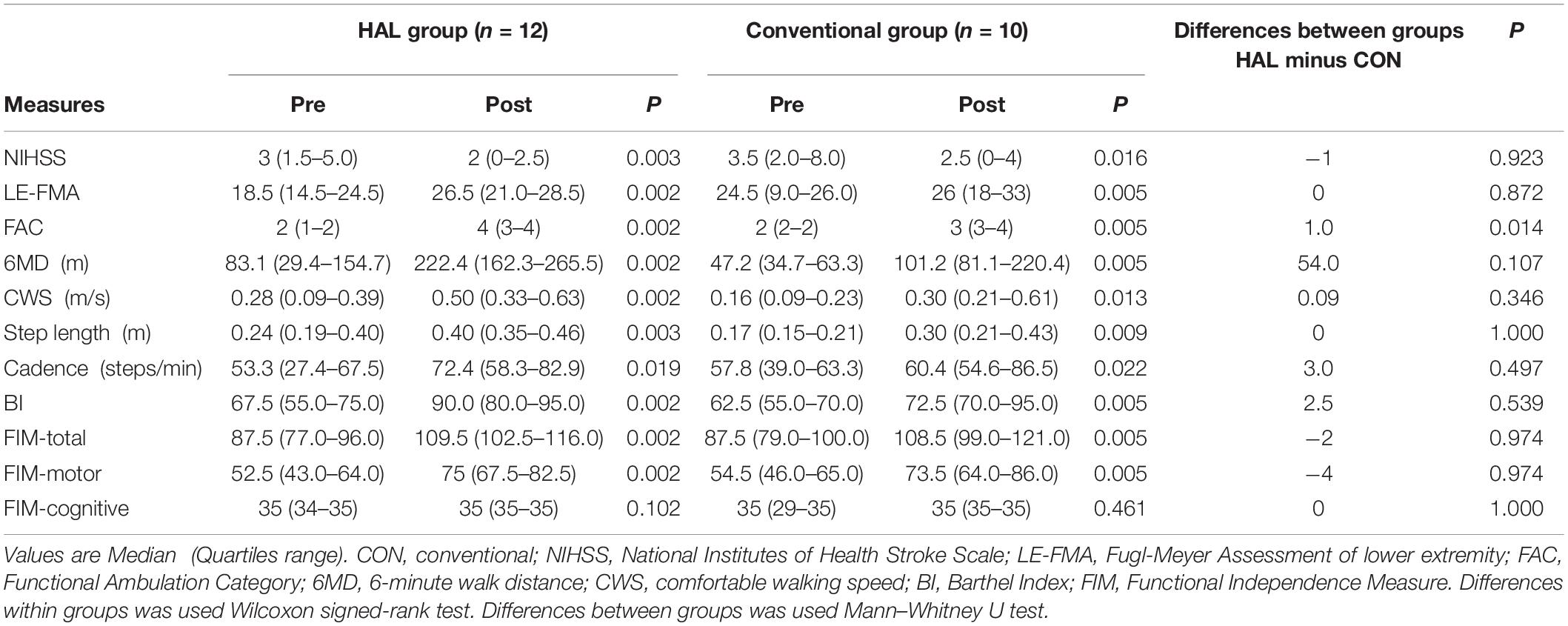
Significantly greater improvements in FAC scores were observed in the HAL group than in the CGT group, with differences between both the group (HAL-CON) 1.0 of median FAC score (p < 0.05, Table 3). However, the amount of change in the secondary outcome measures did not differ significantly between the two groups, with both groups experiencing significant improvements in each of the assessed variables (p < 0.05, Table 3).
Discussion
To the best of our knowledge, this is the first non-randomized clinical trial to examine the feasibility and efficacy of a single-leg version of HAL in patients with acute stroke. We aimed to investigate whether treatment using the motion assistance provided by a single-leg version of HAL could produce early improvements in independent walking compared to CGT in patients after acute stroke. Our preliminary data indicate that participants who received HAL treatment had a significantly greater improvement in their gait than those who received CGT based on FAC outcome measures.
Previous studies have established the safety and feasibility of HAL treatment in the early stages of stroke (Ueba et al., 2013; Nilsson et al., 2014; Ogata et al., 2015). A cohort study suggested that patients with intracerebral hemorrhage and a low Brunnstrom stage should be carefully monitored for orthostatic hypotension (Ueba et al., 2013), whereas others demonstrated that intensive gait training with HAL early after stroke is safe when delivered by experienced physical therapists as part of an inpatient rehabilitation program (Nilsson et al., 2014; Wall et al., 2015). Another retrospective study showed that there were no differences in outcomes between groups with and without HAL treatment (Ogata et al., 2015). In previous research, our group found that gait treatment with HAL improved independent walking more efficiently than CGT in patients with sub-acute stroke (Watanabe et al., 2014), and that independent walking improved significantly in the HAL group at 2 months (Watanabe et al., 2017). However, no study to date had compared the effect of HAL treatment with that of conventional treatment after acute stroke. To resolve this issue, we therefore compared gait treatment between HAL and CGT cohorts in patients after acute strokes.
A recent systematic review of the Cochrane database previously reported that patients who received electromechanical-assisted gait training and physiotherapy after a stroke had a significantly increased odds of walking independently than those who received unassisted gait training (odds ratio, 1.94; 95% confidence interval, 1.39 to 2.71; P < 0.001). However, there was no significant increase in the walking velocity or capacity in 6 min, with mean differences of 0.04 m/s (95% confidence interval 0.00–0.09; P = 0.08) and 5.84 m (95% confidence interval -16.73 to 28.40; P = 0.61), respectively (Mehrholz et al., 2017). We produced similar results in this study. FAC is a commonly used evaluation index in the treatment of gait acquisition after stroke. Many stroke patients experience a reduced gait capacity; therefore, this outcome measure is effective for evaluating independent walking (Holden et al., 1986; Geroin et al., 2013).
A previous study has investigated the effectiveness of robot-assisted gait training with a Lokomat vs. conventional physical therapy in patients with subacute stroke (Chang et al., 2012). This highly attractive study evaluated objective parameters such as oxygen consumption. Our research differed from this study in some major aspects. The Lokomat is a robotic-driven orthotic gait device used for posture control, body-weight support, and treadmill walking (Colombo et al., 2001). Therefore, the operating principle of this device differs from that of the HAL. In addition, the assistance provided by the Lokomat tends to become passive over time, and it is unclear how much the patient is trying to move (Hidler et al., 2009; Hornby et al., 2012; Krishnan et al., 2013). In general, repetition of motion and feedback are important factors for enhancing motor learning. Motor learning is a process of reducing differences between desired and actual behavior through performance and proactive responses to feedback (Schmidt, 1991). The lack of variability in kinematic trajectories of the lower limbs during walking in the Lokomat may limit the amount of error experienced during training, which is thought to be critical for successful motor adaptation as investigated in human studies (Emken et al., 2007; Hidler et al., 2009; Marchal-Crespo and Reinkensmeyer, 2009). In contrast, the HAL provides gait support by detecting bioelectrical signals according to the wearer’s voluntary movements (Lee and Sankai, 2005; Suzuki et al., 2007). This type of movement may enhance the recovery of the impaired neuronal network, and the interactive biofeedback approach may promote the appropriate reorganization of the neuronal network (Morishita and Inoue, 2016; Sankai and Sakurai, 2018). These benefits may facilitate the achievement of gait independence. Further study verifying the effects and mechanisms of the gait treatment with a single-leg HAL system after acute stroke is required.
A voluntary drive is essential to achieve a desired behavior in robot-assisted motor learning (Lotze, 2003). The HAL system provides motion support tailored to the wearer’s voluntary drive (Kawamoto et al., 2013), and it has been shown that the walking performance in patients wearing HAL approximates that of healthy people using HAL (Puentes et al., 2018; Tan et al., 2018). A single-leg version of HAL can give active and intensive support to the gait pattern when rehabilitating a patient after acute stroke (Nilsson et al., 2014). By assisting the knee and hip joints on the paretic side, HAL can increase the frequency or duration of gait treatment a patient can undergo in a single treatment session and over a fixed period (Sczesny-Kaiser et al., 2017). In the present study, the actual walking distance was significantly higher in the HAL group than in the CGT group, implying that more repetitions of the walking motion were possible. The HAL system allows the paralyzed side to be used without excessive fatigue and may lead to a more efficient motor learning. With these benefits, a patient could make greater gains in exercise performance over a given period compared with a patient undergoing conventional treatment. HAL also makes it possible for stroke patients with neurological disorders to repeat gait training with appropriate walking performance.
In an exploratory study, Yoshikawa et al. (2016) suggested that HAL treatment in cases of sub-acute stroke may enhance the walking speed by changing the walking pattern and asymmetry ratio in the late recovery stage. Mizukami et al. (2017) also argued that, if gait treatment with HAL can be started early, walking with an appropriate pattern can be improved and patients can avoid acquiring an incorrect or compensatory walking pattern. Early intensive HAL treatment may contribute not only to improved independent walking but also to improved walking patterns. Moreover, a recent systematic review showed that asymmetry measures provide additional information regarding the coordinative requirements for gait and can potentially be used to indicate recovery (Wonsetler and Bowden, 2017). In the future, we plan to elucidate the effects of HAL treatment on spatiotemporal parameters and asymmetry ratios.
The mechanism by which independent walking improved significantly in our HAL group is unclear. Our previous research suggests that HAL treatment may improve coordination in the lower limbs and change the pattern of muscle synergy (Puentes et al., 2018; Tan et al., 2018). Although these factors may have contributed to the outcomes observed in this pilot study, the lack of randomization will have led to the inclusion of many biases. In addition to the clinical evaluation indices, such as independent walking and walking speed, there is a need to establish new evaluation methods for motion analysis and muscle activity patterns. Improving the degree of independence of stroke patients may contribute to improvements in activities of daily living and quality of life, as well as a shortening of inpatient stays and an increase in returns to independent living. In turn, these improvements may contribute to reduced burdens on caregivers and healthcare costs, and indirectly help to solve problems with social care in Japan. Improving independent walking (i.e., reducing the amount of walking assistance) is not only important for patients, assistants, and medical staff but also for wider society (Rigby et al., 2009).
We conclude that HAL-based gait treatment may improve the independent walking ability of patients with acute stroke hemiplegia who are dependent on ambulators. However, this pilot study has several limitations that necessitate a large-scale randomized controlled trial to confirm the likely effectiveness. A major limitation was the lack of randomization, with treatment type only divided by facility. However, we did standardize both the amount of physical therapy and the breadth and type of any additional content. It was reassuring that there were no significant differences between the two groups in these factors. Another limitation was the low statistical power due to the small sample. Moreover, we could not exclude the possibility of observer bias because the same therapists implemented training and assessment without blinding to treatment allocation. In the near future, we plan to confirm our results by conducting a larger-scale randomized controlled trial with a blinded protocol and third-party evaluator.
Data Availability Statement
The datasets for this manuscript are not publicly available because secondary use of the data is not permitted by the Clinical Research Ethics Review Board. Requests to access the datasets should be directed to AMar,YWlraS5tYXJ1c2hpbWFAbWQudHN1a3ViYS5hYy5qcA==.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the University of Tsukuba Hospital (H27-257). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HW and AMar: conceptualization and writing of the original draft. MY and AMat: funding acquisition. HW, AMar, HKad, TU, YSh, SK, TH, MS, YI, MH, TT, HF, NS, and KM: methodology and project administration. AMar: review and editing. AMar, HKad, TU, YShi, SK, TH, MS, YI, MH, HT, TT, AT, HF, NS, KM, HKaw, YH, MY, YSa, EI, YM, and AMat: confirming the final manuscript.
Funding
This study was supported by the Industrial Disease Clinical Research Grants of the Ministry of Health Labour and Welfare, Japan (14060101-01) and JSPS KAKENHI Grant Number JP19K19788.
Conflict of Interest
HKaw is a stockholder of Cyberdyne, Inc. (Tsukuba, Japan). YSa is a stockholder and the CEO of the company, the manufacturer of the robot suit HAL. YSa’s conflict of interest is managed by board of directors of Cyberdyne and managed by the University of Tsukuba according to national university rules. The patent royalty is paid to the University of Tsukuba from Cyberdyne and a part of the royalty is paid to YSa from the University of Tsukuba according to the national university rules. This study was proposed by the authors, and Cyberdyne was not involved in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the manuscript for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mayuko Sakamaki and Yumiko Ito, Center for Innovative Medicine and Engineering (CIME), University of Tsukuba Hospital, for their excellent technical assistance. We are grateful to the rehabilitation staff at University of Tsukuba Hospital, Kennan Hospital, Kobari General Hospital, Tsukuba Memorial Hospital, and Ibaraki Seinan Medical Center Hospital for their support during the study.
References
Barker-Collo, S., Feigin, V. L., Parag, V., Lawes, C. M., and Senior, H. (2010). Auckland stroke outcomes study. part 2: cognition and functional outcomes 5 years poststroke. Neurology 75, 1608–1616. doi: 10.1212/WNL.0b013e3181fb44c8
Chang, W. H., Kim, M. S., Huh, J. P., Lee, P. K., and Kim, Y. H. (2012). Effects of robot-assisted gait training on cardiopulmonary fitness in subacute stroke patients: a randomized controlled study. Neurorehabil. Neural. Repair. 26, 318–324. doi: 10.1177/1545968311408916
Colombo, G., Wirz, M., and Dietz, V. (2001). Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal. Cord. 39, 252–255. doi: 10.1038/sj.sc.3101154
Dobkin, B. H. (2005). Clinical practice. Rehabilitation after stroke. N. Engl. J. Med. 352, 1677–1684. doi: 10.1056/NEJMcp043511
Emken, J. L., Benitez, R., Sideris, A., Bobrow, J. E., and Reinkensmeyer, D. J. (2007). Motor adaptation as a greedy optimization of error and effort. J. Neurophysiol. 97, 3997–4006. doi: 10.1152/jn.01095.2006
Geroin, C., Mazzoleni, S., Smania, N., Grandlfi, M., Bonaiuti, D., Gasperini, G., et al. (2013). Systematic review of outcome measures of walking training using electromechanical and robotic devices in patients with stroke. J. Rehabil. Med. 45, 987–996. doi: 10.2340/16501977-1234
Hesse, S., Schmidt, H., Werner, C., and Bardeleben, A. (2003). Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr. Opin. Neurol. 16, 705–710. doi: 10.1097/01.wco.0000102630.16692.38
Hesse, S., and Werner, C. (2003). Poststroke motor dysfunction and spasticity: novel pharmacological and physical treatment strategies. CNS Drugs 17, 1093–1107. doi: 10.2165/00023210-200317150-00004
Hidler, J., Nichols, D., Pelliccio, M., Brady, K., Campbell, D. D., Kahn, J. H., et al. (2009). Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil. Neural. Repair. 23, 5–13. doi: 10.1177/1545968308326632
Holden, M. K., Gill, K. M., and Magliozzi, M. R. (1986). Gait assessment for neurologically impaired patients. Standards for outcome assessment. Phys. Ther. 66, 1530–1539. doi: 10.1093/ptj/66.10.1530
Hornby, T. G., Kinnaird, C. R., Holleran, C. L., Rafferty, M. R., Rodriguez, K. S., and Cain, J. B. (2012). Kinematic, muscular, and metabolic responses during exoskeletal-, elliptical-, or therapist-assisted stepping in people with incomplete spinal cord injury. Phys. Ther. 92, 1278–1291. doi: 10.2522/ptj.20110310
Jørgensen, H. S., Nakayama, H., Raaschou, H. O., and Olsen, T. S. (1995). Recovery of walking function in stroke patients: the Copenhagen stroke study. Arch. Phys. Med. Rehabil. 76, 27–32. doi: 10.1016/s0003-9993(95)80038-7
Kawamoto, H., Hayashi, T., Sakurai, T., Eguchi, K., and Sankai, Y. (2009). Development of single leg version of HAL for hemiplegia. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 5038–5043. doi: 10.1109/IEMBS.2009.5333698
Kawamoto, H., Kamibayashi, K., Nakata, Y., Yamawaki, K., Ariyasu, R., Sankai, Y., et al. (2013). Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol. 13:141. doi: 10.1186/1471-2377-13-141
Kawamoto, H., and Sankai, Y. (2002). “Power assist system HAL-3 for gait disorder person,” in Proceedings of the 8th International Conference on Computers Helping People with Special Needs (ICCHP 2002), Linz, 196–203. doi: 10.1007/3-540-45491-8_43
Kawamoto, H., and Sankai, Y. (2005). Power assist method based on phase sequence and muscle force condition for HAL. Adv.Robot. 19, 717–734. doi: 10.1163/1568553054455103
Krishnan, C., Ranganathan, R., Dhaher, Y. Y., and Rymer, W. Z. (2013). A pilot study on the feasibility of robot-aided leg motor training to facilitate active participation. PLoS One 8:e77370. doi: 10.1371/journal.pone.0077370
Kwakkel, G., Wagenaar, R. C., Twisk, J. W., Lankhorst, G. J., and Koetsier, J. C. (1999). Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet 354, 191–196. doi: 10.1016/S0140-6736(98)09477-X
Langhorne, P., Bernhardt, J., and Kwakkel, G. (2011). Stroke rehabilitation. Lancet 377, 1693–1702. doi: 10.1016/S0140-6736(11)60325-5
Lee, S., and Sankai, Y. (2005). Virtual impedance adjustment in unconstrained motion for an exoskeletal robot assisting the lower limb. Adv. Robot. 19, 773–795. doi: 10.1163/1568553054455095
Lotze, M. (2003). Motor learning elicited by voluntary drive. Brain 126, 866–872. doi: 10.1093/brain/awg079
Marchal-Crespo, L., and Reinkensmeyer, D. J. (2009). Review of control strategies for robotic movement training after neurologic injury. J. Neuroeng. Rehabil. 6:20. doi: 10.1186/1743-0003-6-20
Mehrholz, J., Thomas, S., Werner, C., Kugler, J., Pohl, M., and Elsner, B. (2017). Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 5:CD006185. doi: 10.1002/14651858.CD006185.pub4
Ministry of Health, Labour and Welfare, (2016). The results of Comprehensive Survey of Living Conditions, 2016. Available at: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa16/dl/05.pdf (accessed August 5, 2019).
Ministry of Health, Labour and Welfare, (2018). Results of Vital Statistics, 2018. Available at: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai18/dl/gaikyou30-190626.pdf (accessed August 5, 2019).
Mizukami, M., Yoshikawa, K., Kawamoto, H., Sano, A., Koseki, K., Asakwa, Y., et al. (2017). Gait training of subacute stroke patients using a hybrid assistive limb: a pilot study. Disabil. Rehabil. Assist. Technol. 2017, 197–204. doi: 10.3109/17483107.2015.1129455
Morishita, T., and Inoue, T. (2016). Interactive bio-feedback therapy using hybrid assistive limbs for motor recovery after stroke: current practice and future perspectives. Neurol. Med. Chir. 56, 605–612. doi: 10.2176/nmc.st.2016-0094
Nilsson, A., Vreede, K. S., Häglund, V., Kawamoto, H., Sankai, Y., and Borg, J. (2014). Gait training early after stroke with a new exoskeleton–the hybrid assistive limb: a study of safety and feasibility. J. Neuroeng. Rehabil. 11:92. doi: 10.1186/1743-0003-11-92
Ogata, T., Abe, H., Samura, K., Hamada, O., Nonaka, M., Iwaasa, M., et al. (2015). Hybrid Assistive Limb (HAL) rehabilitation in patients with acute hemorrhagic stroke. Neurol. Med. Chir. 55, 901–906. doi: 10.2176/nmc.oa.2015-0209
Peurala, S. H., Airaksinen, O., Huuskonen, P., Jäkälä, P., Juhakoski, M., Sandell, K., et al. (2009). Effects of intensive therapy using gait trainer or floor walking exercises early after stroke. J. Rehabil. Med. 41, 166–173. doi: 10.2340/16501977-0304
Puentes, S., Kadone, H., Watanabe, H., Ueno, T., Yamazaki, M., Sankai, Y., et al. (2018). Reshaping of bilateral gait coordination in hemiparetic stroke patients after early robotic intervention. Front. Neurosci. 12:719. doi: 10.3389/fnins.2018.00719
Rigby, H., Gubitz, G., and Phillips, S. (2009). A systematic review of caregiver burden following stroke. Int. J. Stroke. 4, 285–292. doi: 10.1111/j.1747-4949.2009.00289.x
Sankai, Y., and Sakurai, T. (2018). Exoskeletal cyborg-type robot. Sci. Robot. 3:eaat3912. doi: 10.1126/scirobotics.aat3912
Sankai, Y., Suzuki, K., and Hasegawa, Y. (2014). Cybernics: Fusion of Human, Machine and Information Systems. Tokyo: Springer Japan, 3–18. doi: 10.1007/978-4-431-54159-2
Schmidt, R. A. (1991). Motor Learning & Performance: From Principles to Practice. Champaign, IL: Human Kinetics Books.
Sczesny-Kaiser, M., Kowalewski, R., Schildhauer, T. A., Aach, M., Jansen, O., Grasmücke, D., et al. (2017). Treadmill training with HAL exoskeleton-A novel approach for symptomatic therapy in patients with limb-girdle muscular dystrophy-preliminary study. Front. Neurosci. 11:449. doi: 10.3389/fnins.2017.00449
Suzuki, K., Mito, G., Kawamoto, H., Hasegawa, Y., and Sankai, Y. (2007). Intention-based walking support for paraplegia patients with robot suit HAL. Adv. Robot. 21, 1441–1469.
Takashima, N., Arima, H., Kita, Y., Fujii, T., Miyamatsu, N., Komori, M., et al. (2017). Incidence, management and short-term outcome of stroke in a general population of 1.4 million japanese -shiga stroke registry. Circ. J. 81, 1636–1646. doi: 10.1253/circj.CJ-17-0177
Tan, C. K., Kadone, H., Watanabe, H., Marushima, A., Yamazaki, M., Sankai, Y., et al. (2018). Lateral symmetry of synergies in lower limb muscles of acute post-stroke patients after robotic intervention. Front. Neurosci. 12:276. doi: 10.3389/fnins.2018.00276
Ueba, T., Hamada, O., Ogata, T., Inoue, T., Shiota, E., and Sankai, Y. (2013). Feasibility and safety of acute phase rehabilitation after stroke using the hybrid assistive limb robot suit. Neurol. Med. Chir. 53, 287–290. doi: 10.2176/nmc.53.287
Wall, A., Borg, J., and Palmcrantz, S. (2015). Clinical application of the Hybrid Assistive Limb (HAL) for gait training-a systematic review. Front. Syst. Neurosci. 9:48. doi: 10.3389/fnsys.2015.00048
Watanabe, H., Goto, R., Tanaka, N., Matsumura, A., and Yanagi, H. (2017). Effects of gait training using the Hybrid Assistive Limb® in recovery-phase stroke patients: a 2-month follow-up, randomized, controlled study. NeuroRehabilitation 40, 363–367. doi: 10.3233/NRE-161424
Watanabe, H., Tanaka, N., Inuta, T., Saitou, H., and Yanagi, H. (2014). Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients: a randomized controlled pilot study. Arch. Phys. Med. Rehabil. 95, 2006–2012. doi: 10.1016/j.apmr.2014.07.002
Wonsetler, E. C., and Bowden, M. G. (2017). A systematic review of mechanisms of gait speed change post-stroke. Part 1: spatiotemporal parameters and asymmetry ratios. Top. Stroke Rehabil. 24, 435–446. doi: 10.1080/10749357.2017.1285746
Keywords: Hybrid Assistive Limb, acute stroke, independent walking, Functional Ambulation Category, gait treatment
Citation: Watanabe H, Marushima A, Kadone H, Ueno T, Shimizu Y, Kubota S, Hino T, Sato M, Ito Y, Hayakawa M, Tsurushima H, Takada T, Tsukada A, Fujimori H, Sato N, Maruo K, Kawamoto H, Hada Y, Yamazaki M, Sankai Y, Ishikawa E, Matsumaru Y and Matsumura A (2020) Effects of Gait Treatment With a Single-Leg Hybrid Assistive Limb System After Acute Stroke: A Non-randomized Clinical Trial. Front. Neurosci. 13:1389. doi: 10.3389/fnins.2019.01389
Received: 31 August 2019; Accepted: 10 December 2019;
Published: 22 January 2020.
Edited by:
Reinhold Scherer, University of Essex, United KingdomReviewed by:
Markus Kneihsl, Medical University of Graz, AustriaYongtian He, University of Houston, United States
Copyright © 2020 Watanabe, Marushima, Kadone, Ueno, Shimizu, Kubota, Hino, Sato, Ito, Hayakawa, Tsurushima, Takada, Tsukada, Fujimori, Sato, Maruo, Kawamoto, Hada, Yamazaki, Sankai, Ishikawa, Matsumaru and Matsumura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aiki Marushima, YWlraS5tYXJ1c2hpbWFAbWQudHN1a3ViYS5hYy5qcA==
 Hiroki Watanabe
Hiroki Watanabe Aiki Marushima
Aiki Marushima Hideki Kadone
Hideki Kadone Tomoyuki Ueno4
Tomoyuki Ueno4 Yukiyo Shimizu
Yukiyo Shimizu Mikito Hayakawa
Mikito Hayakawa Naoaki Sato
Naoaki Sato Eiichi Ishikawa
Eiichi Ishikawa