Abstract
Background:
Neuroimaging investigations reveal heterogeneous acupuncture-induced brain activation patterns. Integrating acupoint-specific activation patterns into a unified connectomic framework enables systematic elucidation of acupoint-specific therapeutic mechanisms through network-level neural decoding.
Methods:
This study integrated functional connectivity network mapping (FCNM) methodology, canonical brain networks, and neurotransmitter distributions to delineate the distinct cerebral activation profiles of ST36 (Zusanli) and GB34 (Yanglingquan), two acupoints with anatomical proximity but divergent therapeutic indications in healthy controls (HCs).
Results:
The neural networks activated by acupuncture at ST36 and GB34 are both composed of widely distributed brain regions. These two acupoints co-activated the somatomotor network, the ventral attention network, and the dorsal attention network. The activation pattern of ST36 additionally emphasizes the visual network, while the activation pattern of GB34 primarily involves subcortical regions. The spatial patterns of activation brain networks of ST36 showed exploratory spatial correlations with the distributions of 6-fluoro-(18F)-L-3,4-dihydroxyphenylalanine (FDOPA), noradrenaline transporter (NET) and vesicular acetylcholine transporter (VAChT) neurotransmitter, while the GB34 were correlated with dopamine D1, dopamine D2, dopamine transporter, FDOPA, NET, N-methyl-D-aspartic acid receptor (NMDA), serotonin transporter (SERT), and VAChT neurotransmitter.
Conclusion:
This study delineates the distinct physiological mechanisms of ST36 and GB34 from neuroimaging and molecular perspectives. This discovery not only elucidates acupoint effect specificity through brain network organization but also expands our understanding of acupoint therapeutic mechanisms within the framework of systems neuroscience, providing a scientific basis for the precise application of acupuncture in treating diseases.
Graphical Abstract

Localization of brain networks and spatial distribution of neurotransmitters in ST36 and GB34 following acupuncture in health participants.
1 Introduction
Acupuncture, an ancient technique in traditional Chinese medicine, has been widely accepted as a complementary therapy in modern medicine (Yu, 2020), yet the physiological mechanisms underlying different acupoints require further investigation. This study examines Zusanli (ST36) and Yanglingquan (GB34) to explore acupoint-specific brain networks. Although anatomically adjacent, their innervation differs: ST36 is innervated by the deep peroneal nerve (L4–L5), while GB34 is supplied by the common peroneal nerve (L5–S2) (Li S. H. et al., 2024; Yao et al., 2025) (Figure 1). Both acupoints alleviate lower limb dysfunction and pain (Chen et al., 2019; Tu et al., 2021), but their therapeutic profiles diverge. ST36 modulates gastrointestinal function and chronic fatigue, and is key in treating Yangming migraine (Allais et al., 2002; Cheng et al., 2017; Li et al., 2021; Pei et al., 2020), whereas GB34 focuses on post-stroke motor recovery and Shaoyang migraine (Jang et al., 2020; Jeun et al., 2005; Lu et al., 2023; Zhao et al., 2017). The specificity of these anatomically close yet therapeutically distinct acupoints remains unclear.
FIGURE 1

Location and neuro-segmental mapping of ST36 and GB34. ST36, Zusanli; GB34, Yanglingquan; L4, fourth lumbar vertebra; L5, fifth lumbar vertebra; S1, first sacral vertebra. Adapted from the “Human Anatomy Atlas 2018,” used under license purchased by Chengdu University of Traditional Chinese Medicine.
Advances in neuroimaging techniques have progressively elucidated the neural mechanisms underlying acupuncture’s multisystem integration, mediated through modulation of the somatomotor network (SMN), default mode network (DMN), limbic system, and cortico-basal ganglia circuitry (Kong et al., 2023; Wang et al., 2023; Zhou et al., 2023). Current neuroimaging evidence indicates that ST36 stimulation activates the anterior cingulate, insular, brainstem and enhances DMN connectivity (Wang et al., 2024; Zhou et al., 2023), while GB34 modulates the motor cortex, cerebellum, and basal ganglia (Lee et al., 2024; Ning et al., 2017). However, small sample sizes and methodological inconsistencies have led to heterogeneous findings and insufficient evidence for “acupoint specificity” (Huang et al., 2022), particularly in the context of their distinct clinical efficacy for conditions like migraine and stroke.
To address these gaps, the functional connectivity network mapping (FCNM) method integrates spatially heterogeneous brain regions with large-scale connectome data to establish robust brain–behavior correspondences. Recent studies employing this methodology have successfully delineated aberrant neural networks associated with symptom dimensions and illness stages of schizophrenia, major depressive disorder, and suicidality-related pathologies (Cheng et al., 2025; Li J. et al., 2024; Xu et al., 2024; Zhang et al., 2024). Therefore, the novel methodological framework simultaneously elucidating the distinct neuroimaging signatures of ST36 and GB34 acupoints has the potential to reveal the acupoint-specificity from the perspective of neuroimage. Previous studies have demonstrated that acupuncture can regulate cerebral neurotransmitter levels, such as alleviating pain through modulation of GABA and opioid receptors (Harris et al., 2009; Mawla et al., 2021), reducing drug addiction by modulating dopamine pathways (Lee et al., 2021). Dysregulation of these neurotransmitters is also a hallmark of both chronic headache pathologies and the stroke (Alves et al., 2025; Salinas-Abarca et al., 2025). The JuSpace toolbox1 provides a cross-modal assessment framework for neuroimaging and neurotransmitter mapping (Dukart et al., 2021). Therefore, this study proposes to investigate the physiological mechanisms underlying acupoint specificity from both neuroimaging and neurochemical perspectives.
In summary, this study integrates FCNM with systematic review to characterize neuroimaging signatures of ST36 and GB34 in healthy individuals. Combining canonical brain network and neurotransmitter analyses, we hypothesize that these anatomically adjacent but meridian-distinct acupoints exhibit shared and distinct network responses associated with their efficacy. A clear delineation of these networks is anticipated to provide a mechanistic explanation for their traditional use in managing headache and promoting recovery after cerebrovascular events, ultimately contributing to a more precise understanding of acupoint specificity.
2 Materials and methods
2.1 Registration
This study was registered on the PROSPERO2, with the registration number of CRD420251082684.
2.2 Search strategy
A systematic literature retrieval was conducted in the database of PubMed, Web of Science, Excerpta Medica Database (EMBASE), The Cochrane Library, China National Knowledge Infrastructure (CNKI), Wan fang Database, and Chongqing VIP, Chinese Biomedical Literature Database (CBM) from inception to March 2025, according to Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) (Page et al., 2021). The following four sets of search terms were adopted in English: (“acupuncture” or “acupuncture therapy” or “electroacupuncture” or “EA”) and (“zusanli” or “ST36” or “yanglinquan” or “GB34”) and “magnetic resonance imaging” or “neuro-imaging” or “fMRI” or “BOLD” or “FC” or “ALFF” or “ReHo” or “functional connectivity” or “amplitude Low-frequency fluctuation” or “regional homogeneity”). Relevant Chinese search terms were also searched. Then, we will manually screen the literature of the retrieved studies and reviews.
2.3 Inclusion and exclusion criteria
In this review, we included all studies that used fMRI to investigate the effect of acupuncture on the human brain. Studies meeting the following criteria were eligible for inclusion in this systematic review:
-
(1)
They had to include HCs only;
-
(2)
They needed to report findings related to ST36, GB34.
-
(3)
They had to report findings in 3D coordinates in either the Montreal Neurological Institute (MNI) space (Evans et al., 1992) or Talairach space (Talairach and Tournoux, 1988);
-
(4)
They had to incorporate whole-brain signaling analysis.
Studies were excluded if they:
-
(1)
Did not include a HCs group;
-
(2)
Investigated only region of interest (ROI) results or used analytical methods such as functional connectivity, independent component analysis, or graph theory;
-
(3)
Consisted of reviews, case reports, conference abstracts, or animal studies;
-
(4)
Did not report peak effect coordinates;
-
(5)
Were not available in full text;
-
(6)
Had a sample size of fewer than 10 participants were excluded in FCNM analysis.
2.4 Data extraction
The two authors (ZZ and OY) independently extracted data from the literature that met the inclusion criteria. The extracted contents included basic information of the literature (publication year, author, sample size, nation), basic characteristics of the study subjects (age, gender), and technical details of MRI (MRI scanner, method analysis, imaging results). The above information is shown in Supplementary Tables 1, 2. Any discrepancies were discussed with the third researcher until a consensus was reached.
3 Data analysis
3.1 Discovery and replication datasets
Our work employed Southwest University Longitudinal Imaging Multimodal (SLIM) as a discovery dataset3 and 1,000 Functional Connectomes Project (FCP) of Cambridge Buckner database4 as a cross-scanner replication dataset.
3.2 fMRI data acquisition and preprocessing
Resting-state functional magnetic resonance imaging (fMRI) data were acquired for SLIM and FCP participants using 3T Siemens Trio scanners. Preprocessing of the fMRI data was performed with DPABI version 4.35, following a standardized pipeline that included the following steps: (1) removal of the initial five time points to minimize the impact of scanner instability and facilitate participant adaptation to the scanning environment; (2) slice timing correction to account for inter-slice acquisition time differences; (3) realignment for head motion correction; (4) spatial normalization to Montreal Neurological Institute (MNI) space via deformation fields derived from structural image segmentation, with resampled voxel dimensions of 3 mm3; (5) spatial smoothing using a Gaussian kernel with a 6 mm full-width at half maximum; (6) linear detrending of the time series; (7) regression of nuisance covariates, including Friston-24 motion parameters, white matter signal, cerebrospinal fluid signal, and global signal; (8) band-pass filtering within the 0.01–0.08 Hz frequency range. Participants exhibiting excessive head motion—defined as translational displacement greater than 2.0 mm or rotational motion exceeding 2.0° were excluded from the analysis. Additional exclusions were applied in cases of incomplete brain coverage or suboptimal image quality. Detailed scanning parameters are provided in Supplementary Table 3.
3.3 Functional connectivity network mapping
Based on activation coordinates derived from healthy volunteers that reflect inter-individual differences, the FCNM approach was applied to construct central response network models for the ST36 and GB34 acupoints. The procedure involved the following steps: First, spherical regions with a 4-mm radius were created around each coordinate corresponding to a given contrast, and these were merged to form a combined seed mask specific to that contrast (referred to hereafter as the contrast seed). Second, using preprocessed resting-state fMRI data from the SLIM dataset, participant-level contrast seed-to-whole-brain functional connectivity (FC) maps were generated by computing Pearson’s correlation coefficients between the time series of the contrast seed and those of every voxel across the whole brain. The resulting correlation values were then subjected to Fisher’s z-transformation to approximate a normal distribution. Third, the 573 individual-level FC maps were submitted to a voxel-wise one-sample t-test to identify brain regions exhibiting significant functional connectivity with each contrast seed. It should be noted that only positive functional connectivity was considered, as the biological interpretation of negative correlations remains contentious. Fourth, the resulting group-level t-maps were thresholded at a significance level of P < 0.01, corrected for multiple comparisons using a voxel-wise false discovery rate (FDR) procedure, and subsequently binarized. Finally, the binarized maps were overlaid to create composite network probability maps. These were thresholded at a 60% probability level to produce the final central response network models for the ST36 and GB34 acupoints.
3.4 Relationship to canonical brain networks
For ease of interpretability, we investigated the spatial relations between one brain abnormality networks of healthy volunteers and 8 well-established canonical brain networks. The seven cortical networks were defined as the visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal control, and default networks according to the Yeo et al. (2011) study. The Brainnetome atlas (Fan et al., 2016) was adopted to defined the subcortical regions including the thalamus, caudate, putamen, pallidum, hippocampus, amygdala and nucleus accumbens. The proportion of overlapping voxels between each brain abnormality network and a canonical network to all voxels within the corresponding brain abnormality network was calculated to assess their spatial relation.
3.5 Spatial correlation with neurotransmitter density maps
The JuSpace toolbox (version 2.0, see text footnote 1) covering various neurotransmitter maps helped assess associations of spatial patterns of functional activation in HCs with specific neurotransmitter systems. The following neurotransmitter systems were considered: (Yu, 2020) serotonin transmission: serotonin 5-hydroxytryptamine receptor subtype 1a (5HT1a), serotonin 5-hydroxytryptamine receptor subtype 1b (5HT1b), serotonin 5-hydroxytryptamine receptor subtype 2a (5HT2a), and serotonin transporter (SERT); (2) dopamine transmission: dopamine D1 (D1), dopamine D2 (D2), dopamine transporter (DAT), and 6-fluoro-(18F)-L-3,4-dihydroxyphenylalanine (FDOPA); (3) gamma-aminobutyric acid type a (GABAa); (4) Opioid receptor: kappa opioid receptor (KappaOp), μ-opioid receptor (MU), (5) noradrenaline transporter (NAT); (6) cannabinoid 1 receptor (CB1); (7) N-methyl-D-aspartic acid receptor (NMDA); (8) vesicular acetylcholine transporter (VAChT), (9) metabotropic glutamate receptor 5 (mGluR5). The neurotransmitter/metabolic density maps selected were the most commonly used in the literature and were derived from the largest number of HCs.
Based on the FCNM maps of ST36 and GB34, the JuSpace toolbox (computing option 3) and the default Neuromorphometrics Atlas comprising 119 regions were employed to assess spatial correspondence with selected neurotransmitter receptor/transporter maps. Specifically, Fisher’z-transformed Spearman correlation coefficients were computed between each FCNM map and the spatial distributions of the respective receptors or transporters. To account for spatial autocorrelation, a gray matter probability map was incorporated as a covariate. Statistical significance of the correlation coefficients was evaluated using one-sample t-tests against zero, with exact P-values derived from 10,000 permutations. Multiple comparisons were controlled by applying FDR correction at a threshold of P < 0.05.
3.6 Validation analyses
To evaluate the robustness of our findings, several validation analyses were performed. First, the FCNM procedure was repeated using spherical seeds with radii of 1 and 7 mm to examine the influence of seed size. Second, the same analytical process was applied to an independent replication dataset (i.e., the FCP) to assess the impact of dataset selection. To quantitatively evaluate the spatial similarity between FCNM maps, the Dice coefficient—defined as 2 × (number of overlapping voxels)/(total voxels in network 1 + total voxels in network 2)—was computed, with higher values indicating greater spatial resemblance. In line with the established consensus that Dice coefficients between 0.5 and 1 reflect meaningful spatial overlap, a one-sample t-test was conducted to determine whether the obtained Dice coefficients were significantly lower than 0.5.
3.7 Acupoint specificity testing
To test the acupoint specificity of central response network, we selected ST36 and GB34 as contrasting acupoints based on their anatomical proximity yet distinct meridian affiliations.
4 Result
4.1 Literature selection process
A total of 6,841 studies were retrieved from the database for review. Initially, 1,722 duplicate studies were excluded. Subsequently, 4,781 studies were excluded after a preliminary screening of the remaining 5,118 studies [2,415 excluded after reading abstracts, 704 disease-related studies, 550 animal studies, 302 reviews and secondary analyses, 124 non-imaging studies, 78 non-acupuncture studies, 30 non-whole-brain studies, 4 non-meridian studies, and 573 others (studies not involving healthy participants)]. Following this, 302 studies were excluded after a full-text review (137 studies involving non-target acupoints, 119 studies with a study population of fewer than 10 participants, and 27 studies with unavailable coordinates). Ultimately, 35 studies (Bao et al., 2010; Bi et al., 2017; Cai et al., 2007; Chen and Liu, 2019; Chen and Liu, 2019; Cheng and Wang, 2014; Dong et al., 2013; Duan et al., 2012; Fang et al., 2006, 2012; Long et al., 2016; Lu et al., 2017; Na et al., 2009; Shang and Li, 2017; Tan et al., 2009, 2013; Tian et al., 2009; Wu et al., 2016; Xiao et al., 2008; Yeo et al., 2016; Yuan and Huang, 2015; Zhang et al., 2016; Zhang, 2011; Zhu et al., 2016) were included in this review, with 18 studies incorporated into FCNM analysis (including 14 focusing on ST36 and 4 on GB34), as illustrated in Figure 2, the PRISMA flow chart.
FIGURE 2

Flow chart describing the study selection process. CNKI, China National Knowledge Infrastructure; CBM, Chinese Biomedical Literature Database; VIP, the China Science and Technology Journal Database.
4.2 Study characteristics
This study included a total of 35 articles, all of which were incorporated into the systematic review and imaging analysis. Among these, 29 studies focused on imaging research related to acupuncture at the ST36 acupoint, while five studies investigated the GB34 acupoint, one study both investigated ST36 and GB34 acupoints. The total number of HCs included was 513 for ST36 and 112 for GB34. The age range of the HCs spanned from 19 to 36 years. The majority of the studies were conducted in China (33 studies), with one study each from South Korea and the United States. The primary acupuncture modalities employed were manual acupuncture (28 studies), electroacupuncture (6 studies), and moxibustion (1 study). Additionally, among the included studies, 20 utilized 1.5T MRI scanning, 13 utilized 3.0T MRI scanning, and two studies did not report the scanning method used. Furthermore, in the 14 included neuroimaging studies focusing on ST36, the top ten most frequently involved brain regions were ranked as follows: anterior cingulate cortex, thalamus, hippocampus, temporal lobe, postcentral gyrus, insula, middle frontal gyrus, parahippocampal gyrus, and amygdala. Among the 4 included studies investigating GB34, the most prominently engaged brain regions were identified in the frontal lobe, putamen, caudate and thalamus.
4.3 Activation network of ST36
The activated network of ST36 comprised a broadly distributed set of brain areas principally encompassing the precentral gyrus, postcentral gyrus, precuneus, cingulate gyrus, prefrontal lobe, temporal lobe, occipital lobe and thalamus (Figure 3). In terms of canonical brain networks, the activated network predominantly implicated the somatomotor network (SMN) (overlapping proportion: 38.45%), ventral attention network (VAN) (15.18%), visual (14.37%), dorsal attention network (DAN) (13.98%), default mode network (DMN) (6.97%), subcortical regions (6.77%), limbic (2.85%), frontoparietal control (1.44%) networks (Figure 5A). The ST36 activation networks were significantly correlated with exploratory spatial distribution of FDOPA (rho = 0.4425, P < 0.001), NAT (rho = 0.6226, P < 0.001), VAChT (rho = 0.2815, P = 0.0139) (Table 1 and Figure 5B).
FIGURE 3

Brain activated network of ST36 in healthy controls (HCs). The radius spheres of seed size with 4 mm (A), 1 mm (B), 7 mm (C), and another dataset of Functional Connectomes Project (FCP) (D) are shown as the network probability maps thresholded at 60%, indicating brain regions functionally connected to more than 60% of the contrast seeds.
TABLE 1
| Neurotransmitter activity maps | Fisher’s Z Spearman rho | P-value (FDR corrected) |
|---|---|---|
| ST36 | ||
| 5HT1a | 0.0282 | 0.8296 |
| 5HT1b | −0.2071 | 0.0765 |
| 5HT2a | −0.1678 | 0.3446 |
| CB1 | 0.1632 | 0.3509 |
| D1 | 0.0890 | 0.4176 |
| D2 | 0.1676 | 0.1308 |
| DAT | 0.2264 | 0.0544 |
| FDOPA | 0.4425 | 0.0008 |
| GABAa | 0.0417 | 0.8296 |
| KappaOp | 0.2629 | 0.1547 |
| MU | −0.0885 | 0.8296 |
| NET | 0.6226 | 0.0008 |
| NMDA | 0.2907 | 0.0765 |
| SERT | 0.1817 | 0.1207 |
| VAChT | 0.2815 | 0.0139 |
| mGluR5 | 0.2621 | 0.1308 |
| GB34 | ||
| 5HT1a | −0.1702 | 0.2202 |
| 5HT1b | 0.1217 | 0.2202 |
| 5HT2a | −0.1676 | 0.2338 |
| CB1 | 0.2231 | 0.1475 |
| D1 | 0.2982 | 0.0026 |
| D2 | 0.2244 | 0.0280 |
| DAT | 0.2963 | 0.0037 |
| FDOPA | 0.5792 | 0.0008 |
| GABAa | 0.0486 | 0.7501 |
| KappaOp | 0.3178 | 0.0645 |
| MU | 0.3713 | 0.1309 |
| NAT | 0.3698 | 0.0008 |
| NMDA | 0.4587 | 0.0008 |
| SERT | 0.2566 | 0.0119 |
| VAChT | 0.4208 | 0.0008 |
| mGluR5 | 0.2618 | 0.0938 |
Spatial correlation analyses between ST36 and GB34 brain activation and ne-urotransmitter/metabolism distribution.
ST36, Zusanli; GB34, Yanglingquan; 5HT1a, serotonin 5-hydroxytryptamine receptor subtype 1a; 5HT1b, serotonin 5-hydroxytryptamine receptor subtype 1b; 5HT2a, serotonin 5-hydroxytryptamine receptor subtype 2a; SERT, serotonin transporter; D1, dopamine D1; D2, dopamine D2; DAT, dopamine transporter; FDOPA, 6-fluoro-(18F)-L-3,4-dihydroxyphenylalanine; GABAa, gamma-aminobutyric acid type a; KappaOp, Opioid receptor, kappa opioid receptor; MU, μ-opioid receptor; NAT, noradrenaline transporter; CB1, cannabinoid 1 receptor; NMDA, N-methyl-D-aspartic acid receptor; VAChT, vesicular acetylcholine transporter; mGluR5, metabotropic glutamate receptor 5. The bold values indicated results corrected for false discovery rate P < 0.05.
4.4 Activation network of GB34
The activated network of GB34 comprised a broadly distributed set of brain areas principally encompassing the precentral gyrus, postcentral gyrus, prefrontal lobe, putamen, insula, caudate nucleus, thalamus, cingulate gyrus, supplementary motor area (Figure 4). In terms of canonical brain networks, the activated network predominantly implicated VAN (overlapping proportion: 25.78%), subcortical regions (25.75%), the SMN (24.45%), DAN (10.11%), DMN (5.99%), frontoparietal control (4.91%), visual (2.09%), and limbic (0.92%) networks (Figure 5C). The GB34 activation networks were significantly correlated with exploratory spatial distribution of D1 (rho = 0.2982, P = 0.0026), D2 (rho = 0.2244, P = 0.0280), DAT (rho = 0.2963, P = 0.0037), FDOPA (rho = 0.5792, P < 0.001), NAT (rho = 0.3698, P < 0.001), NMDA (rho = 0.4587, P < 0.001), SERT (rho = 0.2566, P = 0.0119), VAChT (rho = 0.4208, P < 0.001) (Figure 5D and Table 1).
FIGURE 4

Brain activated network of GB34 in healthy controls (HCs). The radius spheres of seed size with 4 mm (A), 1 mm (B), 7 mm (C), and another dataset of Functional Connectomes Project (FCP) (D) are shown as the network probability maps thresholded at 60%, indicating brain regions functionally connected to more than 60% of the contrast seeds.
FIGURE 5
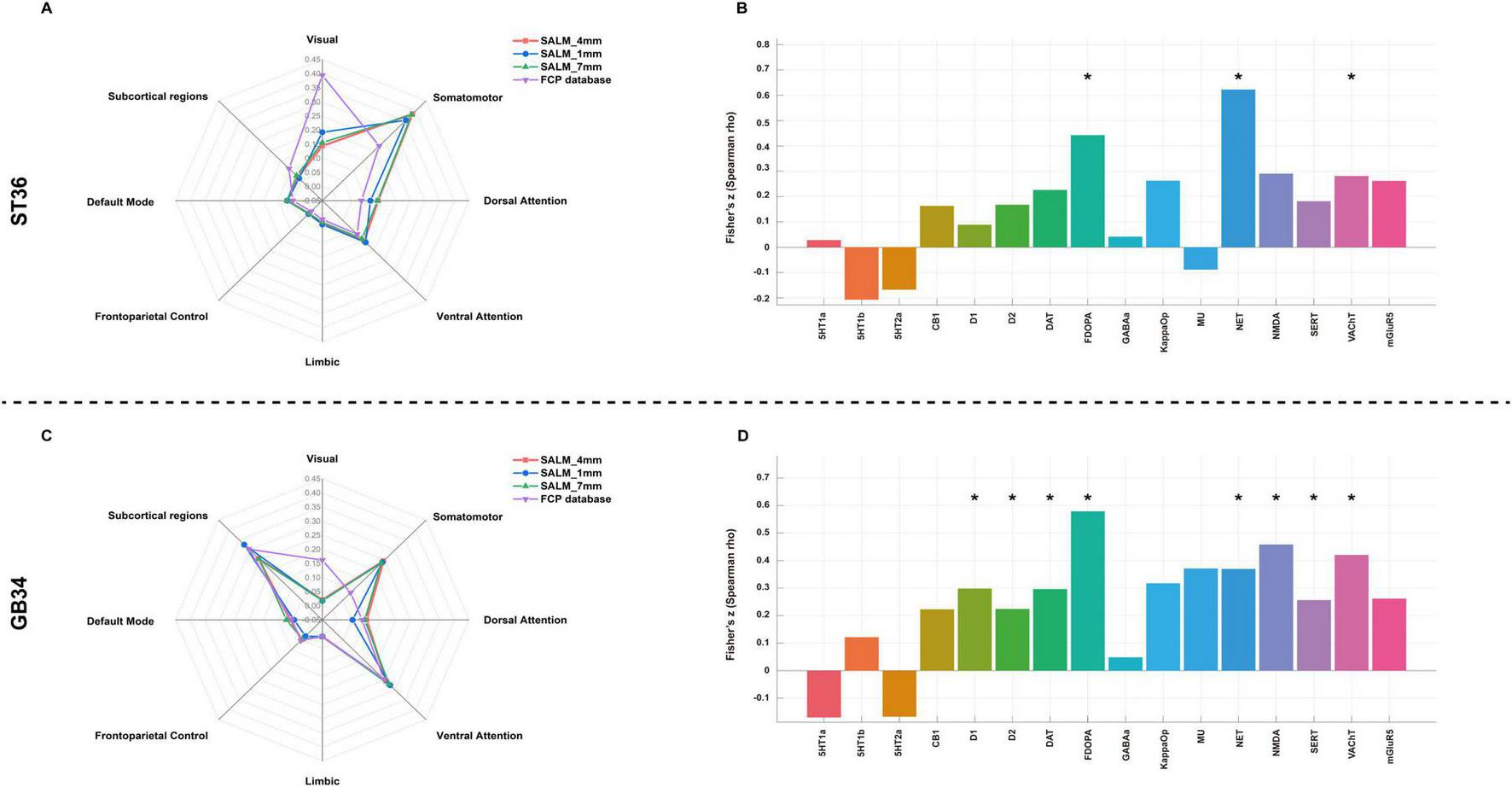
Brain activated networks of ST36 and GB34 in relation to canonical brain networks and Neurotransmitter system. (A) ST36 network. (B) The spatial correlation between ST36 network and neurotransmitter density maps. (C) GB34 network. (D) The spatial correlation between ST36 network and neurotransmitter density maps. Polar plots show the proportion of overlapping voxels between each brain abnormality network and a canonical network to all voxels within the corresponding brain abnormality network. *Indicate results corrected for false discovery rate (FDR) P < 0.05.
4.5 Validation analyses
First, when repeating the FCNM procedure with 1- and 7-mm radius spheres, we observed that the resulting brain networks of ST36 were nearly identical to those with the 4-mm-radius sphere (4- vs. 1-mm Dice coefficients: 0.83; 4- vs. 7-mm Dice coefficients: 0.90); and the result brain network of GB34 (4- vs. 1-mm Dice coefficients: 0.88; 4- vs. 7-mm Dice coefficients: 0.94). Second, we observed that the brain activation networks of ST36 and GB34 derived from the replication dataset (FCP) were consistent with those from the discovery dataset (SLIM), with minor differences possibly arising from variation in sample sizes (Dice coefficients: 0.56, 0.51). Furthermore, the sensitivity analysis of task-fMRI based study demonstrated high spatial consistency (Supplementary Figure 1).
5 Discussion
By integrating the novel FCNM method with large-scale human brain connectome data, this study systematically investigated the brain network localization and neurotransmitter distribution of ST36 and GB34 (two acupoints with similar anatomical positions but distinct therapeutic effects). The results demonstrated that although both acupoints activate widely distributed brain regions, including the VAN, DAN, and SMN, ST36 exhibits stronger modulation of the visual network, whereas GB34 primarily involves subcortical regions. Additionally, the spatial patterns of activated brain network of ST36 and GB34 were correlated with the spatial distribution of dopamine transmission, noradrenaline transporter, and acetylcholinergic neurotransmitters, the GB34 was also correlated with the spatial distribution of serotonin and glutamate receptors neurotransmitters.
5.1 Emerging paradigms in acupuncture neuroimaging research
With the advancement of neuroimaging technologies, fMRI has been widely used to explore the central response mechanisms of acupuncture. Previous studies have highlighted that factors such as sample size and research methodologies contribute to high heterogeneity in findings, raising concerns about scientific validity and reproducibility (Claunch et al., 2012; Zhang et al., 2023). Given the multidimensional regulatory characteristics of acupoint effects, recent research increasingly emphasizes moving beyond single brain regions to focus on interactions within brain networks (Cai et al., 2018; Jiang et al., 2024; Liu et al., 2022). The development of large-scale human brain connectome data and neuroimaging methods like FCNM (which maps lesions from diverse brain locations to a common neural network) offers potential solutions to address heterogeneity and low reproducibility. Since acupuncture effects are closely tied to central network dynamics, we applied this methodology to investigate the activated brain networks of ST36 (the most studied acupoint in current literature) and GB34 (an anatomically adjacent but meridian-distinct acupoint). The findings were further interpreted through alignment with canonical brain networks and neurotransmitter distribution.
5.2 Co-activated brain networks of ST36 and GB34 acupuncture points
As an invasive somatosensory stimulus, acupuncture has been documented to modulate sensorimotor networks (Cai et al., 2018; Claunch et al., 2012), a recent study based on neural tracing technology exploring the neural pathways of acupuncture has shown that acupoints exhibit common neural projections to regions such as the primary motor cortex and the secondary motor cortex (Liang et al., 2025), our FCNM-based functional mapping analysis corroborates these findings, demonstrating extensive SMN activation during stimulation of both ST36 and GB34. Clinical studies demonstrate that acupuncture can alleviate pain and improve functional impairments by modulating functional activities within the sensorimotor network (Chen et al., 2023; Qian et al., 2025). This shared SMN modulation provides a common neural substrate for their efficacy in both pain conditions like headache and motor rehabilitation post-stroke. However, the therapeutic emphasis diverges: the SMN activation by ST36 may be more linked to modulating the sensory-discriminative aspects of headache pain, whereas for GB34, SMN integration is paramount for restoring motor function, a core deficit in cerebrovascular diseases. These findings may partially elucidate the physiological mechanisms underlying the therapeutic effects of acupoint stimulation. However, whether similar co-activation patterns in sensorimotor networks exist with other acupoints requires further investigation through systematic comparative studies employing standardized neuroimaging paradigm.
Furthermore, our investigation identified the activation of the VAN and the DAN within the brain’s neural circuitry in response to stimulation of the ST36 and GB34 acupuncture points. The VAN is primarily associated with bottom-up attention allocation, while the DAN is involved in goal-directed, top-down attention regulation (Maillet et al., 2019). Additionally, the neurotransmitter primarily implicated in these two attention networks is norepinephrine (NET) (Shine, 2019). Our findings also suggest that the brain network activation associated with ST36 and GB34 correlates with a high distribution of NET. Previous studies have demonstrated that NET expression is downregulated in individuals with attention deficit disorder (Thiele and Bellgrove, 2018; Wiesman et al., 2025), establishing a link between NET and attention. Notably, the noradrenergic system is not only crucial for attention but also plays a significant role in post-stroke recovery and the pathophysiology of certain headaches (Wang et al., 2020; Zahrai et al., 2020). Consequently, the co-modulation of the attention networks and the noradrenergic system by both acupoints represents a convergent pathway for treating distinct disorders, primarily targeting headache pathology through sensory-attentional integration, while simultaneously supporting cognitive-attentional components of recovery in cerebrovascular patients.
5.3 ST36-specific modulation brain network
Our study demonstrated that acupuncture at ST36 elicits activation in the visual network. However, traditional Chinese medicine (TCM) does not describe ST36 as having therapeutic effects related to vision. While previous literature on the central responses to ST36 stimulation has occasionally reported activations in occipital lobe-related regions (Hu et al., 2025), no studies have specifically investigated the relationship between ST36 and the visual network. The functional connectivity map generated in our study primarily involved the visual network, whereas GB34 did not exhibit such an association. This finding suggests that ST36 may have a specific regulatory effect on the visual network, potentially expanding our understanding of its therapeutic mechanisms—though further clinical studies are needed for validation. Moreover, recent evidence indicates that the visual network plays a role in attentional modulation and allocation, forming part of the brain’s attention system alongside the VAN and DAN. Studies have shown that the attention system is functionally linked to autonomic nervous system (ANS) activity (Barber et al., 2020; Lin et al., 2020), chronic digestive disorders, such as irritable bowel syndrome and inflammatory bowel disease, are associated with altered ANS function (Rubio et al., 2015), high-quality randomized controlled trials (RCTs) have demonstrated that ST36, as a primary acupoint, can modulate gastrointestinal activity (Pei et al., 2025; Zhao et al., 2024). Therefore, we hypothesize that acupuncture at ST36 may regulate autonomic function by modulating the attentional network, thereby improving gastrointestinal symptoms. Furthermore, this ST36-specific visual-attention network integration provides a unique mechanistic explanation for its therapeutic profile. It strongly supports its use in treating headache disorders, particularly migraine with visual aura or gastrointestinal comorbidities (e.g., nausea) by modulating the neural circuits that process these specific symptoms. In contrast, its role in cerebrovascular diseases is more supportive and indirect, potentially aiding in the recovery of overall autonomic balance and well-being, rather than targeting core motor deficits.
5.4 GB34-specific modulation brain network
GB34 is primarily used to treat movement disorder-related diseases and pain such as post-stroke limb motor dysfunction, Parkinson’s disease and migraine (Chen et al., 2015; Zeng and Zhao, 2016; Zhao et al., 2017). Our findings demonstrated that GB34 specifically activates subcortical brain networks involving the basal ganglia, which are closely associated with movement disorders and pain signal transmission (Li et al., 2022; Motl et al., 2021). This specific subcortical targeting starkly differentiates its primary clinical role from ST36, positioning GB34 as a key acupoint for addressing the core motor impairments resulting from cerebrovascular diseases. Moreover, the activation of subcortical brain networks was found to correlate with high distributions of dopaminergic, NMDA, and SERT-related neurotransmitters. Parkinson’s disease and other movement disorders are linked to dysregulation of dopaminergic neurotransmission (Cramb et al., 2023), while NMDA, as an excitatory neurotransmitter, serves as a biomarker of neuronal integrity (Foerster et al., 2013), SERT agonists have been shown to alleviate involuntary movements in Parkinson’s patients (Pagano et al., 2017). Crucially, the serotonin (5-HT) system, implicated by the SERT correlation, is profoundly involved in the pathophysiology of headache disorders, particularly migraine (de Vries et al., 2020). Thus, we elucidate that GB34 possesses a unique therapeutic spectrum, primarily targeting motor deficits in cerebrovascular diseases with efficacy on migraine, achieved through the integrated modulation of subcortical networks and a broader neurochemical profile.
While our findings identify distinct brain network targets for ST36 and GB34 in healthy individuals, translating these into clinical practice and guide the optimization of stimulation parameter requires further exploration. Future research should validate whether modulating these specific networks correlates with symptom improvement in patients.
Current study has several limitations that warrant acknowledgment. Firstly, while resting-state data provide a stable framework for mapping inter-individual network architectures, they do not capture the immediate, dynamic neural responses elicited during acupuncture stimulation, future studies should incorporate task-fMRI paradigms to delineate the neural dynamics of acupuncture. Secondly, due to the lack of neuroimaging data, we were unable to perform validation analyses on acupuncture stimulation at ST36 and ST34. Future studies will incorporate post-acupuncture imaging data to assess the stability of our findings. Secondly, as JuSpace utilizes nuclear imaging-derived neurotransmitter profiles from healthy individuals and the results of the JuSpace can only indicate the spatial similarity between activation networks and neurotransmitter distribution, and cannot be directly reflected actual changes in neurotransmitter levels. Thirdly, our study was conducted in healthy participants and did not directly explore the pathological mechanisms of specific diseases such as migraine and stroke. Therefore, whether the acupoint-specific networks we identified are consistent with the impaired networks in the diseases they treat, and how they exert therapeutic effects by rectifying these specific pathological changes, remains to be validated in future clinical studies involving patient populations.
6 Conclusion
In conclusion, this study integrates the FCNM approach with large-scale human connectome data and neurotransmitter mapping to delineate the distinct physiological mechanisms underlying ST36 and GB34, acupoints with anatomical proximity but divergent therapeutic profiles from neuroimaging and molecular perspectives. This discovery not only elucidates acupoint effect specificity through brain network organization but also provides a scientific framework for precision acupoint selection in treating diseases, based on their shared and distinct central mechanisms.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The neuroimaging data used in this study were obtained from the 1000 Functional Connectomes Project (FCP) database (http://www.nitrc.org/projects/fcon_1000/). The FCP is an aggregated, fully anonymized dataset pooled from multiple independent sites worldwide. As clearly stated on the FCP portal, all contributing sites obtained local institutional review board (IRB) approval and informed consent from all participants prior to data collection and sharing. Since the data are fully preprocessed and de-identified, and our analysis involved only secondary use of this public dataset, this study did not require additional ethics approval from our institutional review board.
Author contributions
ZZ: Conceptualization, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. XO: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. CX: Formal analysis, Methodology, Writing – review & editing. LcZ: Methodology, Software, Writing – review & editing. QF: Data curation, Writing – review & editing. QY: Data curation, Writing – review & editing. TW: Data curation, Writing – review & editing. XW: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. LZ: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the National Natural Science Foundation of China (grant numbers: 82430124, 81973962, and 82204919) and Key Research and Development Program of Sichuan Province (grant number: 2024YFFK0168).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1740153/full#supplementary-material
Footnotes
1.^ https://github.com/juryxy/JuSpace
2.^ https://www.crd.york.ac.uk/PROSPERO/
3.^ https://fcon_1000.projects.nitrc.org/indi/retro/southwestuni_qiu_index.html
4.^ https://fcon_1000.projects.nitrc.org/fcpClassic/FcpTable.html
References
1
Allais G. De Lorenzo C. Quirico P. E. Airola G. Tolardo G. Mana O. et al (2002). Acupuncture in the prophylactic treatment of migraine without aura: A comparison with flunarizine.Headache42855–861. 10.1046/j.1526-4610.2002.02203.x
2
Alves P. N. Nozais V. Hansen J. Y. Corbetta M. Nachev P. Martins I. P. et al (2025). Neurotransmitters’ white matter mapping unveils the neurochemical fingerprints of stroke.Nat. Commun.16:2555. 10.1038/s41467-025-57680-2
3
Bao F. Li C. F. Xu C. S. Zhu Y. F. Xiang G. B. Huang J. J. et al (2010). Preliminary analysis of brain function imaging studies on acupuncture of Zusanli acupoint in healthy people.Neurol. Dis. Ment. Health10190–193. 10.3969/j.issn.1009-6574.2010.02.027
4
Barber A. D. John M. DeRosse P. Birnbaum M. L. Lencz T. Malhotra A. K. (2020). Parasympathetic arousal-related cortical activity is associated with attention during cognitive task performance.Neuroimage208:116469. 10.1016/j.neuroimage.2019.116469
5
Bi D. Y. Sun T. A. Liang H. Liu M. L. Liu H. R. Liu Q. et al (2017). Effects of gentle moxibustion at different distances on the Zusanli acupoint in normal subjects on brain functional imaging.Acupunct. Massage Med.15223–229.
6
Cai K. Chen M. Wang W. C. Zhou C. Zhou T. G. Li G. Z. (2007). The value of functional magnetic resonance imaging (fMRI) in determining the cerebral cortical response induced by acupuncture techniques.Med. Equip. Inf.2284–86. 10.3969/j.issn.1674-1633.2007.06.033
7
Cai R. L. Shen G. M. Wang H. Guan Y. Y. (2018). Brain functional connectivity network studies of acupuncture: A systematic review on resting-state fMRI.J. Integr. Med.1626–33. 10.1016/j.joim.2017.12.002
8
Chen B. Guo Q. Zhang Q. Di Z. Zhang Q. (2023). Revealing the central mechanism of acupuncture for primary dysmenorrhea based on neuroimaging: A narrative review.Pain Res. Manag.2023:8307249. 10.1155/2023/8307249
9
Chen J. Liu B. (2019). Dynamic brain functional network study on the follow-up effects of acupuncture at Zusanli acupoint.Chin. J. Integr. Tradit. West. Med. Imaging17111–114. 10.3969/j.issn.1672-0512.2019.02.001
10
Chen L. Deng H. Houle T. Zhang Y. Ahmed S. Zhang V. et al (2019). A randomized trial to assess the immediate impact of acupuncture on quantitative sensory testing, pain, and functional status.Pain1602456–2463. 10.1097/j.pain.0000000000001651
11
Chen X. Zhang H. Zou Y. (2015). A functional magnetic resonance imaging study on the effect of acupuncture at GB34 (Yanglingquan) on motor-related network in hemiplegic patients.Brain Res.160164–72. 10.1016/j.brainres.2015.01.011
12
Cheng C. S. Chen L. Y. Ning Z. Y. Zhang C. Y. Chen H. Chen Z. et al (2017). Acupuncture for cancer-related fatigue in lung cancer patients: A randomized, double blind, placebo-controlled pilot trial.Support Care Cancer253807–3814. 10.1007/s00520-017-3812-7
13
Cheng H. Wang B. G. (2014). Study on the analgesic specificity of acupuncture at Zusanli acupoint based on wavelet transform magnetoencephalography.Chin. J. Pain Med.20555–560. 10.3969/j.issn.1006-9852.2014.08.007
14
Cheng Y. Cai H. Liu S. Yang Y. Pan S. Zhang Y. et al (2025). Brain network localization of gray matter atrophy and neurocognitive and social cognitive dysfunction in schizophrenia.Biol. Psychiatry97148–156. 10.1016/j.biopsych.2024.07.021
15
Claunch J. D. Chan S. T. Nixon E. E. Qiu W. Q. Sporko T. Dunn J. P. et al (2012). Commonality and specificity of acupuncture action at three acupoints as evidenced by FMRI.Am. J. Chin. Med.40695–712. 10.1142/S0192415X12500528
16
Cramb K. M. L. Beccano-Kelly D. Cragg S. J. Wade-Martins R. (2023). Impaired dopamine release in Parkinson’s disease.Brain1463117–3132. 10.1093/brain/awad064
17
de Vries T. Villalón C. M. MaassenVanDenBrink A. (2020). Pharmacological treatment of migraine: Cgrp and 5-HT beyond the triptans.Pharmacol. Ther.211:107528. 10.1016/j.pharmthera.2020.107528
18
Dong Y. Dong Y. R. Jiang Y. Wang J. K. Han J. S. Wang H. et al (2013). Functional magnetic resonance imaging observation of manual acupuncture, electroacupuncture and transcutaneous electrical acupoint stimulation in regulating human brain function.Chin. J. Pain Med.1975–79. 10.3969/j.issn.1006-9852.2013.02.004
19
Duan X. H. Liu B. Chen J. Liu X. Long Y. Chen Z. G. et al (2012). Study on the posterior brain effect of acupuncture at Zusanli acupoint using the local consistency method.Chin. J. Integr. Tradit. West. Med. Imaging1010–12. 10.3969/j.issn.1672-0512.2012.01.003
20
Dukart J. Holiga S. Rullmann M. Lanzenberger R. Hawkins P. C. T. Mehta M. A. et al (2021). JuSpace: A tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps.Hum. Brain Mapp.42555–566. 10.1002/hbm.25244
21
Evans A. C. Marrett S. Neelin P. Collins L. Worsley K. Dai W. et al (1992). Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1:43–53. 10.1016/1053-8119(92)90006-9
22
Fan L. Li H. Zhuo J. Zhang Y. Wang J. Chen L. et al (2016). The human brainnetome atlas: A new brain atlas based on connectional architecture.Cereb. Cortex263508–3526. 10.1093/cercor/bhw157
23
Fang J. L. Wang X. L. Wang Y. Hong Y. Liu H. S. Liu J. et al (2012). [Comparison of brain effects of electroacupuncture at Zusanli (ST 36) and Guanyuan (CV 4) shown by fMRI in 21 healthy volunteers].Zhen Ci Yan Jiu3746–52.
24
Fang S. H. Zhang S. Z. Liu H. (2006). [Study on brain response to acupuncture by functional magnetic resonance imaging–observation on 14 healthy subjects].Zhongguo Zhong Xi Yi Jie He Za Zhi26965–968. 10.3321/j.issn:1003-5370.2006.11.001
25
Foerster B. R. Pomper M. G. Callaghan B. C. Petrou M. Edden R. A. Mohamed M. A. et al (2013). An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy.JAMA Neurol.701009–1016. 10.1001/jamaneurol.2013.234
26
Fu H. Y. Yin C. J. Zhang Z. Q. Sun J. H. Wang L. L. (2013). Study on the difference of fMRI imaging between acupuncture and grain-shaped moxibustion at Zusanli acupoint.Jiangsu J. Tradit. Chin. Med.45, 54–56.
27
Harris R. E. Zubieta J. K. Scott D. J. Napadow V. Gracely R. H. Clauw D. J. (2009). Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs).Neuroimage471077–1085. 10.1016/j.neuroimage.2009.05.083
28
He J. Z. Hou Z. C. Zhang H. Ma X. X. Wang C. (2013). A study on the repeatability of acupuncture brain functional magnetic resonance imaging.J. Shandong Univ.5145–49.
29
Hu J. W. Liu H. L. Wang W. Huang G. Y. (2012). Functional magnetic resonance imaging study on the effects of acupuncture at Zusanli point with different methods on brain function regulation.Chin. J. Phys. Med. Rehabil.34677–680. 10.3760/cma.j.issn.0254-1424.2012.09.009
30
Hu L. Zhang J. Wu X. Huang X. Li X. Liu X. et al (2025). Shared and distinct brain activation patterns of acupoints HT7, ST36, and KI4: A task-based fMRI study.Front. Neurol.16:1596306. 10.3389/fneur.2025.1596306
31
Huang H. Yue X. Huang X. Long W. Kang S. Rao Y. et al (2022). Brain activities responding to acupuncture at ST36 (zusanli) in healthy subjects: A systematic review and meta-analysis of task-based fMRI studies.Front. Neurol.13:930753. 10.3389/fneur.2022.930753
32
Huang X. J. Zhao C. H. Zhou Q. Gao P. (2010). fMRI study of electroacupuncture of Yanglingquan acupoint under visual stimulation.J. Med. Imaging201–4. 10.3969/j.issn.1006-9011.2010.01.001
33
Hui K. K. Liu J. Marina O. Napadow V. Haselgrove C. Kwong K. K. et al (2005). The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI.Neuroimage27479–496. 10.1016/j.neuroimage.2005.04.037
34
Jang J. H. Yeom M. J. Ahn S. Oh J. Y. Ji S. Kim T. H. et al (2020). Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson’s disease.Brain Behav. Immun.89641–655. 10.1016/j.bbi.2020.08.015
35
Jeun S. S. Kim J. S. Kim B. S. Park S. D. Lim E. C. Choi G. S. et al (2005). Acupuncture stimulation for motor cortex activities: A 3T fMRI study.Am. J. Chin. Med.33573–578. 10.1142/S0192415X0500317X
36
Jiang H. Zhao X. H. Wang G. L. Wang P. J. (2010). Study on the effect of acupuncture at Zusanli acupoint on the changes of immune function and brain function.Chin. J. Neurol.9697–701. 10.3760/cma.j.issn.1671-8925.2010.07.013
37
Jiang T. F. Chen Z. Y. Liu J. Yin X. J. Tan Z. J. Wang G. L. et al (2024). Acupuncture modulates emotional network resting-state functional connectivity in patients with insomnia disorder: A randomized controlled trial and fMRI study.BMC Complement. Med. Ther.24:311. 10.1186/s12906-024-04612-0
38
Kong Q. Sacca V. Walker K. Hodges S. Kong J. (2023). Thalamocortical mechanisms underlying real and imagined acupuncture.Biomedicines11:1830. 10.3390/biomedicines11071830
39
Lee H. M. Lee D. H. Lee H. G. Kwon S. Cho S. Y. Jung W. S. et al (2024). Functional neural substrates of Parkinson’s disease and potential underpinnings of acute responses to acupuncture stimulation.Neuroscience562148–159. 10.1016/j.neuroscience.2024.10.023
40
Lee M. Y. Lee B. H. Kim H. Y. Yang C. H. (2021). Bidirectional role of acupuncture in the treatment of drug addiction.Neurosci. Biobehav. Rev.126382–397. 10.1016/j.neubiorev.2021.04.004
41
Li J. Wang D. Xia J. Zhang C. Meng Y. Xu S. et al (2024). Divergent suicidal symptomatic activations converge on somato-cognitive action network in depression.Mol. Psychiatry291980–1989. 10.1038/s41380-024-02450-7
42
Li J. Zhang Y. Huang Z. Jiang Y. Ren Z. Liu D. et al (2022). Cortical and subcortical morphological alterations in motor subtypes of Parkinson’s disease.NPJ Parkinsons Dis.8:167. 10.1038/s41531-022-00435-3
43
Li L. M. Li X. Z. Lv F. J. Guo Z. J. Song Y. E. Xie H. W. et al (2013). Resting-state brain functional MRI observation of acupuncture analgesia in people with different sensitivity constitutions.J. Third Mil. Med. Univ.35547–552.
44
Li L. M. Lv F. J. Guo Z. J. Song Y. E. Xie H. W. Tang X. et al (2017). “Functional magnetic resonance imaging study on the difference in the effect of acupuncture on local consistency of the brain in normal subjects with different sensitivities,” in Proceedings of the 2017 Chongqing association of traditional Chinese medicine annual conference, Chong Qing, 22–33.
45
Li S. H. Liu S. Q. Wang Y. M. Yu S. G. Wu Q. F. (2024). Analysis of Yanglingquan’s main treatments and compatibility rules based on data mining.Chin. J. Tradit. Chin. Med. Inf.4885–92. 10.3969/j.issn.1008-8849.2024.19.020
46
Li W. J. Gao C. An L. X. Ji Y. W. Xue F. S. Du Y. (2021). Perioperative transcutaneous electrical acupoint stimulation for improving postoperative gastrointestinal function: A randomized controlled trial.J. Integr. Med.19211–218. 10.1016/j.joim.2021.01.005
47
Liang J. Sun W. Zhou Y. Zhang P. Chen Y. Li X. et al (2025). Study on the central neural pathways connecting the brain and peripheral acupoints using neural tracers.CNS Neurosci. Ther.31:e70554. 10.1111/cns.70554
48
Lin F. V. Tao Y. Chen Q. Anthony M. Zhang Z. Tadin D. et al (2020). Processing speed and attention training modifies autonomic flexibility: A mechanistic intervention study.Neuroimage213:116730. 10.1016/j.neuroimage.2020.116730
49
Liu B. Liu X. Long Y. Chen J. Chen Z. G. Shang X. J. et al (2009). MRI observation of the effect of acupuncture aftereffects on brain functional connectivity.Chin. J. Med. Imaging Technol.252186–2189. 10.3321/j.issn:1003-3289.2009.12.011
50
Liu L. Lyu T. L. Fu M. Y. Wang L. P. Chen Y. Hong J. H. et al (2022). Changes in brain connectivity linked to multisensory processing of pain modulation in migraine with acupuncture treatment.Neuroimage Clin.36:103168. 10.1016/j.nicl.2022.103168
51
Liu L. S. Liang C. H. Zeng D. H. Wu Y. F. Li H. G. Shi C. Z. et al (2016). fMRI local consistency study of acupuncture at Zusanli, Yanglingquan and the combination of the two acupoints.ShuZhou1.
52
Liu L. S. Zeng D. H. Meng Z. Z. Shi C. Z. Liang M. J. Li H. G. (2014). Study on the local consistency of resting brain function after acupuncture at Zusanli.Biomed. Eng. Clin. Med.18, 146–149.
53
Long Y. Liu B. Liu X. Yan C. G. Chen Z. G. Chen J. et al (2016). “Resting-state functional magnetic resonance imaging to evaluate the aftereffects of acupuncture at Zusanli acupoint,” in Proceedings of the 2016 Chinese medical doctor association integrated traditional Chinese and Western medicine physicians conference, Shanghai, 300.
54
Lu F. Gao J. Wang Y. Dai Q. Xin J. Zhao Y. et al (2017). Effects of three needling manipulations of the right-side Zusanli (ST 36) on brain using functional magnetic resonance imaging.J. Tradit. Chin. Med.37298–307.
55
Lu M. Du Z. Zhao J. Jiang L. Liu R. Zhang M. et al (2023). Neuroimaging mechanisms of acupuncture on functional reorganization for post-stroke motor improvement: A machine learning-based functional magnetic resonance imaging study.Front. Neurosci.17:1143239. 10.3389/fnins.2023.1143239
56
Maillet D. Beaty R. E. Kucyi A. Schacter D. L. (2019). Large-scale network interactions involved in dividing attention between the external environment and internal thoughts to pursue two distinct goals.Neuroimage19749–59. 10.1016/j.neuroimage.2019.04.054
57
Mawla I. Ichesco E. Zöllner H. J. Edden R. A. E. Chenevert T. Buchtel H. et al (2021). Greater somatosensory afference with acupuncture increases primary somatosensory connectivity and alleviates fibromyalgia pain via insular γ-aminobutyric acid: A randomized neuroimaging trial.Arthritis Rheumatol.731318–1328. 10.1002/art.41620
58
Motl R. W. Sandroff B. M. Benedict R. H. Hubbard E. A. Pilutti L. A. Sutton B. P. (2021). Do subcortical gray matter volumes and aerobic capacity account for cognitive-motor coupling in multiple sclerosis?Mult. Scler.27401–409. 10.1177/1352458520914822
59
Na B. J. Jahng G. H. Park S. U. Jung W. S. Moon S. K. Park J. M. et al (2009). An fMRI study of neuronal specificity of an acupoint: Electroacupuncture stimulation of Yanglingquan (GB34) and its sham point.Neurosci. Lett.4641–5. 10.1016/j.neulet.2009.08.009
60
Ning Y. Li K. Fu C. Ren Y. Zhang Y. Liu H. et al (2017). Enhanced functional connectivity between the bilateral primary motor cortices after acupuncture at Yanglingquan (GB34) in right-hemispheric subcortical stroke patients: A resting-state fMRI study.Front. Hum. Neurosci.11:178. 10.3389/fnhum.2017.00178
61
Pagano G. Niccolini F. Fusar-Poli P. Politis M. (2017). Serotonin transporter in Parkinson’s disease: A meta-analysis of positron emission tomography studies.Ann. Neurol.81171–180. 10.1002/ana.24859
62
Page M. J. McKenzie J. E. Bossuyt P. M. Boutron I. Hoffmann T. C. Mulrow C. D. et al (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.BMJ372:n71. 10.1136/bmj.n71
63
Pei L. Geng H. Guo J. Yang G. Wang L. Shen R. et al (2020). Effect of acupuncture in patients with irritable bowel syndrome: A randomized controlled trial.Mayo Clin. Proc.951671–1683. 10.1016/j.mayocp.2020.01.042
64
Pei L. Wang G. Yang S. Zhou S. Xu T. Zhou J. et al (2025). Electroacupuncture reduces duration of postoperative ileus after laparoscopic gastrectomy for gastric cancer: A multicenter randomized trial.Gastroenterology16973–84. 10.1053/j.gastro.2025.02.006
65
Qian X. Zhang H. Peng J. Song L. Lv Q. Jia W. et al (2025). Acupuncture modulates group neural activity in patients with post stroke sensory impairment: An fMRI study based on inter-subject correlation and inter-subject functional connectivity.Brain Res. Bull.222:111259. 10.1016/j.brainresbull.2025.111259
66
Rubio A. Van Oudenhove L. Pellissier S. Ly H. G. Dupont P. Lafaye de Micheaux H. et al (2015). Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system–a fMRI study in healthy volunteers.Neuroimage10710–22. 10.1016/j.neuroimage.2014.11.043
67
Salinas-Abarca A. B. Gamal-Eltrabily M. Romero-Reyes M. Akerman S. (2025). The role and interaction of hypothalamic-related neurotransmitters in migraine.J. Headache Pain26:110. 10.1186/s10194-025-02044-w
68
Shang H. C. Li F. (2017). Effect of acupuncture at Zusanli acupoint on functional magnetic resonance imaging of brain tissue.Chin. Prim. Med.242963–2967.
69
Shine J. M. (2019). Neuromodulatory influences on integration and segregation in the brain.Trends Cogn. Sci.23572–583. 10.1016/j.tics.2019.04.002
70
Talairach J. Tournoux P. (1988). Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system: An approach to cerebral imaging. New York, NY: Thieme Medical Publishers, Inc.
71
Tan H. B. Zhang W. Liu H. B. Tao F. D. Zhang D. Y. (2009). Exploring the action pathway of acupuncture at Zusanli acupoint by using brain function imaging and changes in serum gastrin levels.Chin. J. Integr. Tradit. West. Med. Imaging7241–250. 10.3969/j.issn.1672-0512.2009.04.001
72
Tan J. H. Wang Y. Y. Long X. J. Diao Y. J. (2013). Functional magnetic resonance imaging study on the persistent effects of manual acupuncture and electroacupuncture at Zusanli acupoint.J. Hunan Univ.4064–68. 10.3969/j.issn.1674-2974.2013.07.011
73
Thiele A. Bellgrove M. A. (2018). Neuromodulation of attention.Neuron97769–785. 10.1016/j.neuron.2018.01.008
74
Tian L. F. Zhou C. Chen M. Zou M. Z. Yang Z. H. (2009). [Study of the relationship between the acupoints of Zhongzhu (TE 3), Yanglingquan (GB 34) and their corresponding cortical areas with the functional MRI].Zhongguo Zhen Jiu29561–564.
75
Tu J. F. Yang J. W. Shi G. X. Yu Z. S. Li J. L. Lin L. L. et al (2021). Efficacy of intensive acupuncture versus sham acupuncture in knee osteoarthritis: A randomized controlled trial.Arthritis Rheumatol.73448–458. 10.1002/art.41584
76
Wang F. Wang J. Cao Y. Xu Z. (2020). Serotonin-norepinephrine reuptake inhibitors for the prevention of migraine and vestibular migraine: A systematic review and meta-analysis.Reg. Anesth. Pain Med.45323–330. 10.1136/rapm-2019-101207
77
Wang X. Luo P. Zhang L. Sun J. Cao J. Lei Z. et al (2024). Altered functional brain activity in first-episode major depressive disorder treated with electro-acupuncture: A resting-state functional magnetic resonance imaging study.Heliyon10:e29613. 10.1016/j.heliyon.2024.e29613
78
Wang Y. Lu M. Liu R. Wang L. Wang Y. Xu L. et al (2023). Acupuncture alters brain’s dynamic functional network connectivity in stroke patients with motor dysfunction: A randomised controlled neuroimaging trial.Neural Plast.2023:8510213. 10.1155/2023/8510213
79
Wiesman A. I. Madge V. Fon E. A. Dagher A. Collins D. L. Baillet S. et al (2025). Associations between neuromelanin depletion and cortical rhythmic activity in Parkinson’s disease.Brain148875–885. 10.1093/brain/awae295
80
Wu S. S. Chen J. Liu B. Shang X. J. Li X. F. (2016). “Brain functional imaging study on the follow-up effects of acupuncture at Zusanli acupoint,” in Proceedings of the 2016 Chinese medical doctor association integrated traditional Chinese and Western medicine physicians conference, Shanghai, 277.
81
Xiao Y. Y. Du L. Hong B. K. Chen X. K. Pei R. Q. Wu R. H. (2008). Functional magnetic resonance imaging study on the brain effects of manual acupuncture at Zusanli acupoint.Chin. J. Integr. Tradit. West. Med.28122–125.
82
Xu R. Zhang X. Zhou S. Guo L. Mo F. Ma H. et al (2024). Brain structural damage networks at different stages of schizophrenia.Psychol. Med.10.1017/S0033291724003088[Epub ahead of print].
83
Yao K. F. Liu Y. Y. Dou B. M. Chen Z. H. Zhang Y. Guo Y. et al (2025). Comparison and reflection on acupuncture treatment of Zusanli acupoint in clinical research and basic research.Liaoning J. Tradit. Chin. Med.11, 149–152.
84
Yeo B. T. Krienen F. M. Sepulcre J. Sabuncu M. R. Lashkari D. Hollinshead M. et al (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity.J. Neurophysiol.1061125–1165. 10.1152/jn.00338.2011
85
Yeo S. van den Noort M. Bosch P. Lim S. (2016). Ipsilateral putamen and insula activation by both left and right GB34 acupuncture stimulation: An fMRI study on healthy participants.Evid. Based Complement. Alternat. Med.2016:4173185. 10.1155/2016/4173185
86
Yu Z. (2020). Neuromechanism of acupuncture regulating gastrointestinal motility.World J. Gastroenterol.263182–3200. 10.3748/wjg.v26.i23.3182
87
Yuan X. L. Huang Y. J. (2015). Observation of activation changes in different brain regions after acupuncture at Zusanli acupoint by functional magnetic resonance imaging.Chin. Foreign Med. Res.13138–140.
88
Zahrai A. Vahid-Ansari F. Daigle M. Albert P. R. (2020). Fluoxetine-induced recovery of serotonin and norepinephrine projections in a mouse model of post-stroke depression.Transl. Psychiatry10:334. 10.1038/s41398-020-01008-9
89
Zeng B. Y. Zhao K. (2016). Effect of acupuncture on the motor and nonmotor symptoms in Parkinson’s disease–a review of clinical studies.CNS Neurosci. Ther.22333–341. 10.1111/cns.12507
90
Zhang J. Liu Y. Li Z. Hu Q. Huang X. Lv H. et al (2023). Functional magnetic resonance imaging studies of acupuncture at ST36: A coordinate-based meta-analysis.Front. Neurosci.17:1180434. 10.3389/fnins.2023.1180434
91
Zhang W. (2011). Comparative study on brain functional imaging of acupuncture at Zusanli acupoint to obtain qi and non-de-qi.Chin. Med. Equip.2638–41. 10.3969/j.issn.1674-1633.2011.12.010
92
Zhang W. Xu C. S. Li C. F. Liu J. P. Wu H. L. (2016). Effects of acupuncture duration and stimulation scheme on acupuncture brain functional imaging.J. Anhui Univ. Tradit. Chin. Med.3558–64. 10.3969/j.issn.2095-7246.2016.03.020
93
Zhang X. Xu R. Ma H. Qian Y. Zhu J. (2024). Brain structural and functional damage network localization of suicide.Biol. Psychiatry951091–1099. 10.1016/j.biopsych.2024.01.003
94
Zhao J. Zheng H. Wang X. Wang X. Shi Y. Xie C. et al (2024). Efficacy of acupuncture in refractory irritable bowel syndrome patients: A randomized controlled trial.Front. Med.18678–689. 10.1007/s11684-024-1073-7
95
Zhao L. Chen J. Li Y. Sun X. Chang X. Zheng H. et al (2017). The long-term effect of acupuncture for migraine prophylaxis: A randomized clinical trial.JAMA Intern. Med.177508–515. 10.1001/jamainternmed.2016.9378
96
Zhou Y. Dai A. Feng S. Zhu T. Liu M. Shi J. et al (2023). Immediate neural effects of acupuncture manipulation time for stroke with motor dysfunction: A fMRI pilot study.Front. Neurosci.17:1297149. 10.3389/fnins.2023.1297149
97
Zhu Y. F. Lu Q. Li G. Q. Xu C. S. Wang A. Q. Zhang C. Y. et al (2016). Feasibility analysis of arterial spin labeling technology in the study of acupuncture fMRI aftereffects.Chin. J. Integr. Tradit. West. Med. Imaging14373–389. 10.3969/j.issn.1672-0512.2016.04.002
Summary
Keywords
acupoint-specificity, functional connectivity network mapping, neurotransmitter, Yanglingquan, Zusanli
Citation
Zhang Z, Ouyang X, Xie C, Zeng L, Fu Q, Yan Q, Wang T, Wang X and Zhao L (2026) Localization of brain networks activated by acupuncture at anatomically adjacent acupoints in healthy participants: neuroimaging evidence and implications for migraine and stroke. Front. Neurosci. 19:1740153. doi: 10.3389/fnins.2025.1740153
Received
05 November 2025
Revised
11 December 2025
Accepted
17 December 2025
Published
12 January 2026
Volume
19 - 2025
Edited by
Wei Wang, Zhejiang University, China
Reviewed by
Shaoyuan Li, China Academy of Chinese Medical Sciences, China
Yubo Wang, The Scripps Research Institute, United States
Updates
Copyright
© 2026 Zhang, Ouyang, Xie, Zeng, Fu, Yan, Wang, Wang and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Zhao, zhaoling@cdutcm.edu.cnXiao Wang, wangxiao2@cdutcm.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.