- Cancer Research Institute, Department of Neurosurgery, Xiangya Hospital, Central South University, Changsha, China
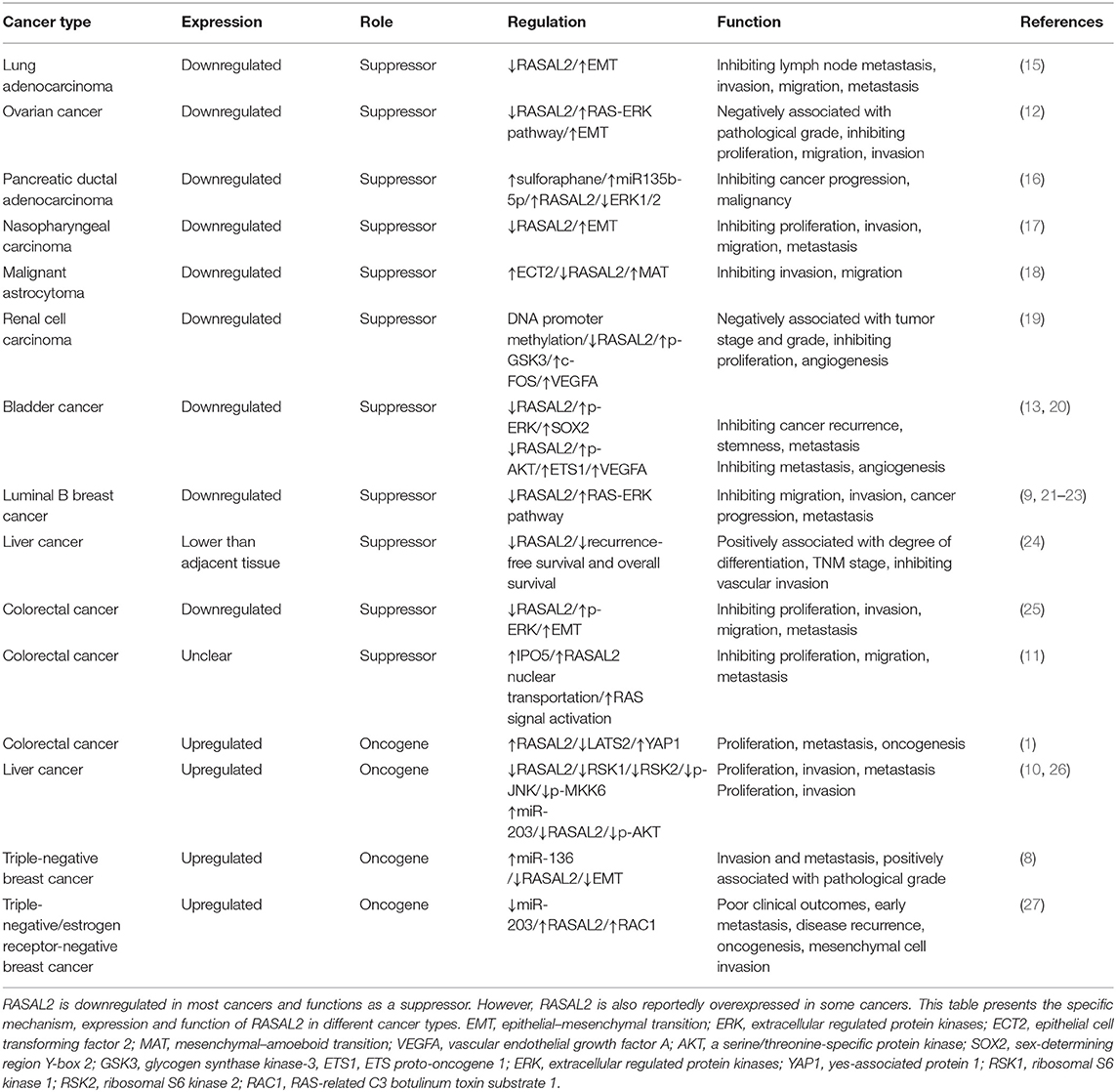
RAS protein activator like 2 (RASAL2) belongs to the RAS GTPase-activating protein family and plays an important role in several cancers, including ovarian cancer, nasopharyngeal carcinoma, malignant astrocytoma, renal cell carcinoma, bladder cancer, colorectal cancer, liver cancer, triple-negative breast cancer, lung adenocarcinoma, and pancreatic ductal adenocarcinoma. Traditionally, RASAL2 has been regarded as a tumor suppressor but recent studies have found that it is an oncogene in specific types of cancer, such as colorectal cancer, liver cancer, triple-negative breast cancer, triple-negative/estrogen receptor-negative breast cancer. In this review, we summarize the latest findings regarding RASAL2 in cancers, which may be important and useful in clinical practice. We discussed the specific functions and mechanisms of RASAL2 in different kinds of cancer cells (including its inhibition of invasion, metastasis and angiogenesis and its opposite effects), which may provide new directions for cancer research and treatments. RASAL2 exhibits different relationship with clinical cancer stage, histological grade, prognosis and overall survival in different kinds of tumor. RASAL2 is a potential prognostic factor and a new therapeutic target for diagnosis and treatment.
Introduction
RAS protein activator like 2 (RASAL2) is a member of the family of RAS GTPase-activating proteins (GAP), which negatively regulate the RAS signaling pathway by catalyzing the hydrolysis of RAS-GTP to RAS-GDP. RASAL2 is involved in many cellular activities and acts as a vital regulator of the RAS signaling pathway (1). The RASAL2 gene is located at chromosome 1q25.2 in humans. Early research reported that single-nucleotide polymorphisms (SNPs) located at or near RASAL2 were significantly associated with waist circumference and body mass index in Mexican-Mestizo children and adults (2). Furthermore, the SNP rs10913469 (Sec16B-Rasal2) was positively associated with body mass index in a genome-wide association study (3). RASAL2 promotes adipogenesis through extracellular regulated protein kinases (ERK)-independent repressing RAS activity, which plays a role in the obesity and related metabolic disorders. RASAL2 mutant mice had a drastic decrease in RASAL2 expression, impaired adipogenesis and lean phenotypes (4). Studies have also reported that RASAL2 may play a role in the isolation of cross-reacting antigen (5), creating a candidate vaccine against ticks (6) and lipopolysaccharide-induced activation of microglial cells (7). In addition, the function of RASAL2 can be regulated by microRNAs such as miR-136 (8).
It was reported that RASAL2 acts as a tumor suppressor when it was found that RASAL2 was downregulated in luminal-B breast cancer in one of the earliest studies (9). Recent research reported that RASAL2 suppressed cancer progression via the Hippo signaling pathway (1), WNT/β-catenin pathway (10), and RAS signaling pathway (11). However, RASAL2 also functions as an oncogene in various cancers via the RAS-ERK pathway (12), phosphoinositide 3-kinase (PI3K)/AKT/mechanistic target of rapamycin (mTOR) signaling pathway (9), nuclear factor (NF)-κB pathway (9), and ERK/mitogen activated protein kinase (MAPK) pathway (13). In human cancers, RASAL2 may exhibit pro- or anti-oncogenic behavior depending on the type of stimulus or cell context, which is quite interesting. Thus, what makes RASAL2 thought-provoking is its ability to exert opposite effects in different cancers, which is different from most regulators, which usually exhibit either pro- or anti-oncogenic behavior. The specific mechanisms deserve further study.
Inhibition of Invasion and Metastasis
The RAS pathway is one of the most commonly deregulated pathways in human cancer (14). RASAL2 plays an important role in the invasion and metastasis of some cancer cells, which regulates the action of the cancer cells (Table 1, Figure 1A). The RAS-GAP domain of RASAL2 is important for the activity of RASAL2. That RAS protein contribute to the oncogenesis caused by RASAL2 inactivation (9). In most cancers, RASAL2 is inhibited and downregulated so that the cancer cells can escape from its preventative effects.
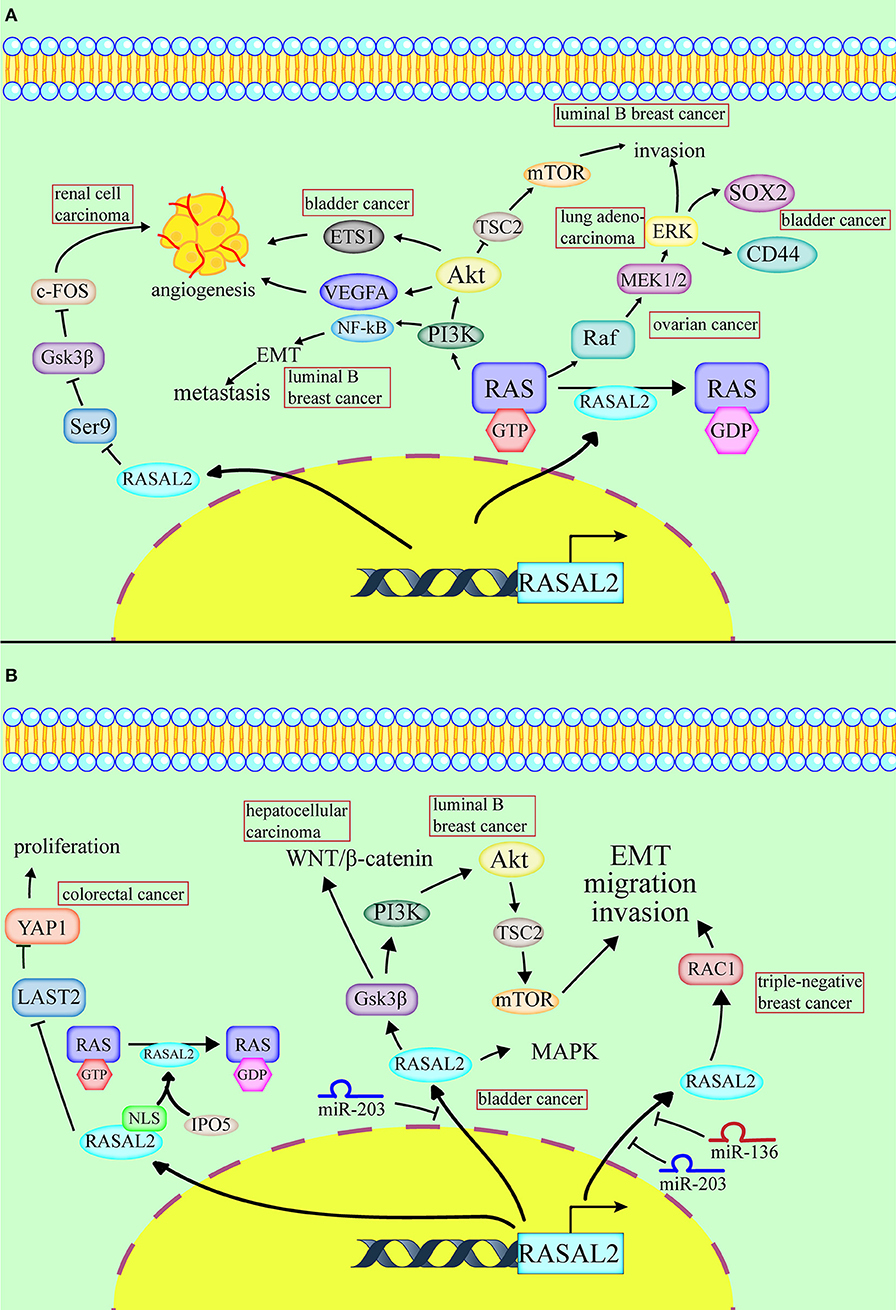
Figure 1. Conflicting roles of RASAL2 in different cancers. (A) RASAL2 inhibits angiogenesis, invasion and metastasis of several types of cancer cells. In luminal B breast cancer, the loss of RASAL2 activates wild-type RAS and then increases MEK (mitogen-activated protein kinase kinase)/ERK (extracellular regulated protein kinases) and PI3K (phosphoinositide 3-kinase)/AKT (a serine/threonine-specific protein kinase) signaling to promote invasion, and it induces NF-kB activation to promote epithelial–mesenchymal transition (EMT). In lung adenocarcinoma, the loss of RASAL2 promotes migration capability via EMT through ERK regulation. In ovarian cancer, the loss of RASAL2 activates the RAS-ERK pathway, leading to EMT. In renal cell carcinoma (RCC), RASAL2 reduces Serine9 (Ser9) phosphorylation to activate glycogen synthase kinase-3 β (GSK3β) and then decreases the expression of c-FOS and vascular endothelial growth factor A (VEGFA) to suppress RCC cells. In bladder cancer, RASAL2 depletion enhances the phosphorylation of AKT and then upregulates the expression of ETS proto-oncogene 1 (ETS1) and VEGFA, leading to angiogenesis. RASAL2 also downregulates sex-determining region Y-box 2 (SOX2) and CD44 expression by inhibiting the ERK/mitogen-activated protein kinases (MAPK) pathway to induce cancer stemness. (B) RASAL2 promotes invasion and metastasis of several types of cancer cells. In triple-negative breast cancer (TNBC), miR-136 and miR-203 downregulate RASAL2 to suppress cell migration, EMT and invasion. The activation of a RASAL2/ARHGAP24 (Rho GTPase activating protein 24)/RAC1 (RAS-related C3 botulinum toxin substrate 1) module contributes to TNBC tumorigenesis. RASAL2 is hypomethylated and promotes invasiveness in HCC. Downregulation of RASAL2 alters the phosphorylation of the effectors of the MAPK, WNT/β-catenin, and PI3K/AKT/mTOR signaling pathways and then impairs these pathways. RASAL2 is also the target of miR-203 in HCC cells. In colorectal cancer (CRC), importin-5 (IPO5) binds to the nuclear localization signal or sequence (NLS) sequence of RASAL2, which induces RAS signal activation. RASAL2 is also involved in the Hippo signaling pathway, which promotes tumorigenesis and metastasis by inhibiting the expression of large tumor suppressor kinase 2 (LATS2) and then increasing the expression of yes-associated protein 1 (YAP1) in CRC.
The most common cancer that is associated with RASAL2 is breast cancer, such as luminal B breast cancer. In luminal B breast cancer, due to promoter hypermethylation, disabled homolog 2-interacting protein (DAB2IP) is selectively suppressed (21). The combined loss of RASAL2 and DAB2IP in a subset of luminal B breast cancers results in epithelial–mesenchymal transition (EMT). In a luminal B mammary tumor mouse model, the loss of RASAL2 enhanced metastasis and, using this model, 60% of primary tumors spontaneously lost DAB2IP, which also increased metastasis. As for the mechanism, the loss of DAB2IP and RASAL2 increased MEK/ERK and PI3K/AKT signaling (which are quite important for invasion) and induced NF-κB activation to promote EMT (21).
RASAL2 negatively regulates EMT via the ERK pathway. For example, in lung adenocarcinoma, downregulation of RASAL2 promotes migration capability due to EMT via ERK regulation (15). Thus, RASAL2 may be important in lung adenocarcinoma treatment (15). In ovarian cancer, RASAL2 was downregulated, especially in patients with advanced stages and grades. Downregulation of RASAL2 activated the RAS-ERK pathway, leading to EMT, and inhibition of the pathway reversed the functional effects of RASAL2 depletion (12).
MiR135b-5p is induced by sulforaphane and then promotes the expression of RASAL2 to suppress pancreatic ductal adenocarcinoma (16). Low expression of miR-135b-5p and RASAL2 are indicators of pancreatic cancer malignancy (16). RASAL2 also inhibited the metastasis capability and proliferation of nasopharyngeal carcinoma (17). In astrocytoma cells, RASAL2 interacts with epithelial cell transforming factor 2 (ECT2), which activates Rho GTPases, in order to reduce Rho activity. When the ratio of ECT2 to RASAL2 activity is increased, ECT2 overcomes the RhoGAP activity of RASAL2 and results in mesenchymal–amoeboid transition (MAT), resulting in amoeboid cells (18).
Inhibition of Angiogenesis
RASAL2 plays a critical role in inhibiting tumor angiogenesis, which means that it can act as a tumor suppressor (Table 1, Figure 1A). In renal cell carcinoma (RCC), RASAL2 is usually epigenetically silenced and its loss is negatively associated with overall survival of RCC patients. RASAL2 targets tumor angiogenesis to suppress RCC cells. Mechanistically, RASAL2 reduces Ser9 phosphorylation to activate glycogen synthase kinase-3 β (GSK3β) and subsequently downregulates the expression of c-FOS and vascular endothelial growth factor A (VEGFA) (19).
In bladder cancer, RASAL2 is downregulated and negatively associated with clinical stage. RASAL2 inhibits angiogenesis of cancer cells via p-AKT/ETS proto-oncogene 1 (ETS1) signaling. Mechanistically, RASAL2 depletion enhances the phosphorylation of AKT and subsequently upregulates the expression of ETS1 (an important angiogenesis-related protein) and VEGFA. There is a negative association between RASAL2 and VEGFA expression, showing that RASAL2 inhibits angiogenesis via regulating VEGFA (20). RASAL2 also downregulates sex-determining region Y-box 2 (SOX2) and CD44, which are indicators of cancer stemness, by inhibiting the ERK/MAPK pathway (13).
Promotion of Invasion and Metastasis
RASAL2 plays inconsistent roles in different cancers, including promoting invasion and metastasis of some cancer cells and not others (Table 1, Figure 1B). RASAL2 is a target of anti-invasion miR-136 and miR-203 in triple-negative breast cancer (TNBC). RASAL2 drives mesenchymal invasion and metastasis and miR-136 and miR-203 downregulate RASAL2 to suppress cell migration, EMT and invasion (8). Furthermore, high RASAL2 expression is strongly associated with poor disease outcomes in TNBC patients. As for the mechanism, RASAL2 binds and antagonizes the RAS-related C3 botulinum toxin substrate 1 (RAC1)-specific Rho GTPase activating protein 24 (ARHGAP24) to promote signaling involving the small GTPase RAC1 and thereby promote mesenchymal invasion. To summarize, the activation of a RASAL2/ARHGAP24/RAC1 module contributes to TNBC tumorigenesis (27).
In hepatocellular carcinoma (HCC), RASAL2 is hypomethylated and RASAL2 is upregulated, thereby promoting invasiveness. Downregulation of RASAL2 alters the phosphorylation of the effectors of the MAPK, WNT/β-catenin and PI3K/AKT/mTOR signaling pathways and then impairs these pathways. For instance, RASAL2 depletion impairs phosphorylation of ribosomal S6 kinase 1 (RSK1) and ribosomal S6 kinase 2 (RSK2), which are downstream effectors of both PI3K and MAPK. Phosphorylation of GSK3β reduces its enzymatic activity and induces the WNT/β-catenin pathway (28). RASAL2 depletion attenuated GSK3β phosphorylation, which decreases AKT and RSK1/2 activities and downregulates WNT signal transduction (10). RASAL2 is also the target of miR-203 in HCC cells (26).
In colorectal cancer (CRC), RASAL2 is overexpressed and RASAL2 nuclear transportation is mediated by importin-5 (IPO5) to promote proliferation and tumorigenicity (25). IPO5 binds to the nuclear localization signal or sequence (NLS) of RASAL2, which induces RAS signal activation (11). RASAL2 is also involved in the Hippo signaling pathway, which promotes tumorigenesis and metastasis by inhibiting the expression of large tumor suppressor kinase 2 (LATS2), which increases the expression of yes-associated protein 1 (YAP1) in CRC. YAP1 is dephosphorylated and translocated to the cell nucleus, which promotes the expression of pro-proliferation genes and prevents YAP1 ubiquitination in the cytoplasm (1). The recent findings on RASAL2, which functions as a tumor suppressor or oncogene in different cancers, are presented in Table 1.
Conclusions and Future Perspectives
RASAL2 functions as a tumor suppressor and inhibits cancer progression in lung cancer (15), ovarian cancer (12), pancreatic ductal adenocarcinoma (16), nasopharyngeal carcinoma (17), malignant astrocytoma (18), RCC (19), bladder cancer (13, 20), and luminal B breast cancer (9, 21, 23). Thus, downregulation of RASAL2 often exhibits a positive relationship with clinical cancer stage and histological grade. In addition, low expression of RASAL2 often indicates a poor prognosis and is associated with overall survival. Following RASAL2 suppression, the RAS-ERK pathway is activated and functions as a promoting factor. Therefore, blockade of the RAS-ERK pathway using inhibitors may be effective in the treatment of cancers involving RAS pathway activation. Additionally, restoring RASAL2 expression or synthesizing a small molecule that can replace RASAL2 may be a novel approach to cancer treatment. Briefly, RASAL2 is a potential prognostic factor and a new therapeutic target for cancer diagnosis and treatment.
However, several studies have reported that RASAL2 was oncogenic in TNBC (8, 27), HCC (10, 26), and CRC (1). Overexpression of RASAL2 may be a predictive and prognostic marker in TNBC, as it is positively associated with clinical cancer stage and histological grade. These opposing conclusions may be the results of functional complexity, cancer microenvironments and the heterogeneity of molecular cancer pathways. The specific mechanisms deserve further study.
Author Contributions
BZ and WZ designed and wrote the manuscript. XJ and CR designed and revised the manuscript.
Funding
This present study was supported by the National Natural Science Foundation of China [Grant Nos. 81773179 and 81272972 (CR); Grant No. 81472355 (XJ)], the Hunan Provincial Science and Technology Department [Grant No. 2016JC2049 (CR); Grant No. 2014FJ6006 (XJ)], and the Undergraduate Training Programs for Innovation and Entrepreneurship [Grant Nos. 20181053368 and GS201910533474 (WZ); Grant No. GS201910533236 (BZ)].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Pan Y, Tong JHM, Lung RWM, Kang W, Kwan JSH, Chak WP, et al. RASAL2 promotes tumor progression through LATS2/YAP1 axis of hippo signaling pathway in colorectal cancer. Mol Cancer. (2018) 17:102. doi: 10.1186/s12943-018-0853-6
2. Leon-Mimila P, Villamil-Ramirez H, Villalobos-Comparan M, Villarreal-Molina T, Romero-Hidalgo S, Lopez-Contreras B, et al. Contribution of common genetic variants to obesity and obesity-related traits in mexican children and adults. PLoS ONE. (2013) 8:e70640. doi: 10.1371/journal.pone.0070640
3. Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. (2009) 41:18–24. doi: 10.1038/ng.274
4. Zhu X, Xie S, Xu T, Wu X, Han M. Rasal2 deficiency reduces adipogenesis and occurrence of obesity-related disorders. Mol Metab. (2017) 6:494–502. doi: 10.1016/j.molmet.2017.03.003
5. Shibui T, Kobayashi T, Shiratori M. Isolation of cross-reacting antigen candidates by mRNA-display using a mixed cDNA library. Biotechnol Lett. (2008) 30:2037–43. doi: 10.1007/s10529-008-9803-5
6. Shahein YE, Abouelella AM, Hussein NA, Hamed RR, El-Hakim AE, Abdel-Shafy S, et al. Identification of four novel Rhipicephalus annulatus upregulated salivary gland proteins as candidate vaccines. Protein J. (2013) 32:392–8. doi: 10.1007/s10930-013-9498-x
7. Sohn SH, Chung HS, Ko E, Jeong HJ, Kim SH, Jeong JH, et al. The genome-wide expression profile of Nelumbinis semen on lipopolysaccharide-stimulated BV-2 microglial cells. Biol Pharmaceut Bull. (2009) 32:1012–20. doi: 10.1248/bpb.32.1012
8. Yan M, Li X, Tong D, Han C, Zhao R, He Y, et al. miR-136 suppresses tumor invasion and metastasis by targeting RASAL2 in triple-negative breast cancer. Oncol Rep. (2016) 36:65–71. doi: 10.3892/or.2016.4767
9. McLaughlin SK, Olsen SN, Dake B, De Raedt T, Lim E, Bronson RT, et al. The RasGAP gene, RASAL2, is a tumor and metastasis suppressor. Cancer Cell. (2013) 24:365–78. doi: 10.1016/j.ccr.2013.08.004
10. Stefanska B, Cheishvili D, Suderman M, Arakelian A, Huang J, Hallett M, et al. Genome-wide study of hypomethylated and induced genes in patients with liver cancer unravels novel anticancer targets. Clin Cancer Res. (2014) 20:3118–32. doi: 10.1158/1078-0432.ccr-13-0283
11. Zhang W, Lu Y, Li X, Zhang J, Lin W, Zhang W, et al. IPO5 promotes the proliferation and tumourigenicity of colorectal cancer cells by mediating RASAL2 nuclear transportation. J Exp Clin Cancer Res. (2019) 38:296. doi: 10.1186/s13046-019-1290-0
12. Huang Y, Zhao M, Xu H, Wang K, Fu Z, Jiang Y, et al. RASAL2 down-regulation in ovarian cancer promotes epithelial-mesenchymal transition and metastasis. Oncotarget. (2014) 5:6734–45. doi: 10.18632/oncotarget.2244
13. Hui K, Gao Y, Huang J, Xu S, Wang B, Zeng J, et al. RASAL2, a RAS GTPase-activating protein, inhibits stemness and epithelial-mesenchymal transition via MAPK/SOX2 pathway in bladder cancer. Cell Death Dis. (2017) 8:e2600. doi: 10.1038/cddis.2017.9
14. Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. (2003) 3:11–22. doi: 10.1038/nrc969
15. Li N, Li S. RASAL2 promotes lung cancer metastasis through epithelial-mesenchymal transition. Biochem Biophys Res Commun. (2014) 455:358–62. doi: 10.1016/j.bbrc.2014.11.020
16. Yin L, Xiao X, Georgikou C, Luo Y, Liu L, Gladkich J, et al. Sulforaphane induces miR135b-5p and its target gene, RASAL2, thereby inhibiting the progression of pancreatic cancer. Mol Ther Oncolyt. (2019) 14:74–81. doi: 10.1016/j.omto.2019.03.011
17. Wang Z, Wang J, Su Y, Zeng Z. RASAL2 inhibited the proliferation and metastasis capability of nasopharyngeal carcinoma. Int J Clin Exp Med. (2015) 8:18765–71.
18. Weeks A, Okolowsky N, Golbourn B, Ivanchuk S, Smith C, Rutka JT. ECT2 and RASAL2 mediate mesenchymal-amoeboid transition in human astrocytoma cells. Am J Pathol. (2012) 181:662–74. doi: 10.1016/j.ajpath.2012.04.011
19. Hui K, Yue Y, Wu S, Gu Y, Guan B, Wang X, et al. The expression and function of RASAL2 in renal cell carcinoma angiogenesis. Cell Death Dis. (2018) 9:881. doi: 10.1038/s41419-018-0898-x
20. Hui K, Wu S, Yue Y, Gu Y, Guan B, Wang X, et al. RASAL2 inhibits tumor angiogenesis via p-AKT/ETS1 signaling in bladder cancer. Cell Signal. (2018) 48:38–44. doi: 10.1016/j.cellsig.2018.04.006
21. Sears R, Gray JW. Epigenomic inactivation of RasGAPs activates RAS signaling in a subset of luminal B breast cancers. Cancer Discov. (2017) 7:131–3. doi: 10.1158/2159-8290.cd-16-1423
22. Shen J, Wang Y, Hung MC. RASAL2: wrestling in the combat of Ras activation. Cancer Cell. (2013) 24:277–9. doi: 10.1016/j.ccr.2013.08.024
23. Olsen SN, Wronski A, Castano Z, Dake B, Malone C, De Raedt T, et al. Loss of RasGAP tumor suppressors underlies the aggressive nature of luminal B breast cancers. Cancer Discov. (2017) 7:202–17. doi: 10.1158/2159-8290.cd-16-0520
24. Shen H, Wu X, Zhang Y, Deng G, Ma J, Qu Y, et al. Expression of RASAL2 in hepatocellular carcinoma and the clinical significance. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2015) 40:250–5. doi: 10.11817/j.issn.1672-7347.2015.03.003
25. Jia Z, Liu W, Gong L, Xiao Z. Downregulation of RASAL2 promotes the proliferation, epithelial-mesenchymal transition and metastasis of colorectal cancer cells. Oncol Lett. (2017) 13:1379–85. doi: 10.3892/ol.2017.5581
26. Fang JF, Zhao HP, Wang ZF, Zheng SS. Upregulation of RASAL2 promotes proliferation and metastasis, and is targeted by miR-203 in hepatocellular carcinoma. Mol Med Rep. (2017) 15:2720–6. doi: 10.3892/mmr.2017.6320
27. Feng M, Bao Y, Li Z, Li J, Gong M, Lam S, et al. RASAL2 activates RAC1 to promote triple-negative breast cancer progression. J Clin Investig. (2014) 124:5291–304. doi: 10.1172/jci76711
Keywords: RASAL2, cancer, EMT, invasion, metastasis, RAS GTPase-activating protein
Citation: Zhou B, Zhu W, Jiang X and Ren C (2019) RASAL2 Plays Inconsistent Roles in Different Cancers. Front. Oncol. 9:1235. doi: 10.3389/fonc.2019.01235
Received: 20 August 2019; Accepted: 28 October 2019;
Published: 13 November 2019.
Edited by:
Luisa Lanfrancone, European Institute of Oncology (IEO), ItalyReviewed by:
Prasanna Ekambaram, University of Pittsburgh, United StatesXing Huang, Zhejiang University, China
Copyright © 2019 Zhou, Zhu, Jiang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caiping Ren, cmVuY2FpcGluZ0Bjc3UuZWR1LmNu
†These authors have contributed equally to this work
 Bolun Zhou
Bolun Zhou Wei Zhu
Wei Zhu Xingjun Jiang
Xingjun Jiang Caiping Ren
Caiping Ren