- 1Department of Medical Oncology, Dana-Farber Cancer Institute and Department of Medicine, Harvard Medical School, Boston, MA, United States
- 2Robert Wood Johnson Medical School, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, United States
- 3Replimune, Woburn, MA, United States
- 4Massachusetts General Hospital, Boston, MA, United States
- 5Earle a Chiles Research Institute, Providence Cancer Institute, Portland, OR, United States
- 6City of Hope, Duarte, CA, United States
- 7Beth Israel Deaconess Medical Center and Department of Medicine, Harvard Medical School, Boston, MA, United States
- 8Loyola University Medical Center, Maywood, IL, United States
- 9Biomedical Health Sciences, Rutgers University, New Brunswick, NJ, United States
- 10Department of Pediatrics, Child Health Institute of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ, United States
- 11Amgen, Thousand Oaks, CA, United States
- 12Bristol Myers-Squibb, Princeton, NJ, United States
- 13Department of Internal Medicine, Rush University Medical Center, Chicago, IL, United States
High-dose ipilimumab (IPI) and high-dose interleukin-2 (IL-2) are approved agents for metastatic melanoma, but the efficacy and safety of the combination are unknown. The objective of this study was to evaluate the feasibility, safety, and efficacy of combination high-dose IPI and high-dose IL-2 in patients with histologically confirmed advanced unresectable stage III and IV melanoma. This Phase II, multicenter, open-label, single-arm trial was conducted in nine patients enrolled between 12/2014 and 12/2015. Subjects were treated with high-dose IPI 10 mg/kg intravenous (IV) every 3 weeks for four doses starting at week 1 and high-dose IL-2 (600,000 IU/kg IV bolus every 8 h for up to 14 doses) concurrently with IPI at weeks 4 and 7. After the first 12 weeks of combination therapy, maintenance IPI (10 mg/kg IV) monotherapy was administered every 12 weeks for up to 1 year. No patient had received prior PD-1 blockade, and only one received prior vemurafenib. Confirmed partial response was achieved in one (11%), stable disease in four (44%), and progressive disease in four (44%) of nine patients. Two patients achieved durable disease control of 44+ and 50+ months at the most recent follow-up without subsequent therapy. The median overall survival was not reached after a minimum 24 months of follow-up time. One-year and 2-year survival rates were 89 and 67%, respectively. Seven patients (78%) experienced grade 3 or 4 adverse events related to the study therapy, three of which were attributed to both agents. One patient discontinued the treatment due to liver and kidney toxicity. While toxicity was significant, all events were reversible, and there was no treatment-related mortality. In peripheral blood of patients with decreasing tumor burden, the ratio of the non-classical MHC-II proteins HLA-DM to HLA-DO increased 2-fold, raising the possibility of the ratio of HLA-DM:HLA-DO as a novel biomarker of response to treatment. Although the sample size was limited, combination therapy with high-dose IPI and high-dose IL-2 was feasible and associated with clinical benefit. IL-2-based compounds in combination with CTLA-4 blockade should be studied in advanced melanoma patients who fail to benefit from first-line PD-1 blockade.
Clinical Trial Registration: ClinicalTrials.gov, NCT02203604. Registered 30 July 2014, https://clinicaltrials.gov/ct2/show/NCT02203604.
Introduction
The cytotoxic T lymphocyte antigen 4 (CTLA-4) checkpoint inhibitor, ipilimumab (IPI), which sustains T cell activity by blocking T cell response inhibition, is associated with a 15–20% response rate in melanoma patients (1) and with long-term progression-free survival of 21% (2). High-dose interleukin-2 (IL-2) administered as first-line therapy for patients with advanced melanoma results in a response rate of 15–20%, of which approximately one-third are durable complete responses (CRs) sometimes lasting for decades (3). High-grade but short-lived toxicities necessitate inpatient administration and have limited the widespread use of IL-2, but it can be delivered safely in experienced centers with appropriate supportive care (4–6). The role of high-dose IL-2 in subsequent lines of therapy for patients treated with first-line PD-1 antibody (pembrolizumab or nivolumab) is less clear, but retrospective, pooled datasets have suggested similar activity for high-dose IL-2 in this setting (7). CTLA-4 is expressed after T cell activation, and blocking this inhibitory signal while driving T cell proliferation, differentiation, and cytotoxicity with IL-2 is a rational treatment strategy. In fact, a phase I/II trial of IPI followed by IL-2 has previously been conducted, but with IPI doses only up to 2 mg/kg (8, 9). Responses were observed in eight of 36 (22%) patients (including 6 CRs). Five of 36 (14%) patients developed grade 3–4 IPI-related toxicities, but all were reversible. High-dose IPI (10 mg/kg) is significantly more effective than 3 mg/kg (HR for OS = 0.84, 95% CI 0.70–0.99, p = 0.04), but it is also more toxic, with grade 3–4 diarrhea in 10% and colitis in 5% of patients (10). Therefore, we conducted a trial to determine the feasibility, efficacy, and safety of combination high-dose IPI and high-dose IL-2 in patients with metastatic melanoma. We chose the sequence of IPI followed by IL-2 based on our hypothesis that IPI could prevent T cell exhaustion induced by IL-2-driven T cell proliferation.
Methods
Patient Selection
Adults with histologically confirmed unresectable stage III and IV melanoma and ECOG performance status 0–1 were enrolled at Rutgers Cancer Institute of New Jersey and Providence Cancer Institute. Main exclusion criteria were primary ocular, active brain metastases, active autoimmune disease, concurrent systemic immunosuppressive therapy, significant cardiopulmonary disease, and organ dysfunction. Patients with prior treatment with IPI or IL-2 were excluded. Prior PD-1-directed therapy and BRAF-directed therapy was allowed.
Design
This was a single-arm study with a primary endpoint of objective response rate in the first 24 weeks of treatment, reported with a 95% confidence interval (CI). The protocol (CINJ#091309) was approved by institutional IRBs and registered (NCT02203604). All patients gave written informed consent. Secondary endpoints included safety, feasibility, overall survival, 1- and 2-year survival, progression-free survival, and best overall response. The planned target sample size was up to 82 patients, but the sponsor stopped the trial early due to slow enrollment.
Treatment
All patients received induction with IPI (10 mg/kg IV every 3 weeks for four doses) starting at Week 1. At weeks 4 and 7, patients also received high-dose IL-2 (600,000 IU/kg IV bolus every 8 h for up to 14 doses, as tolerated) immediately following IPI. IL-2 dose was calculated using actual body weight, although adjustment to ideal body weight for obese patients was allowed. Following IPI induction, maintenance IPI (10 mg/kg IV) was administered every 12 weeks for four doses. Dose reductions were not permitted for either drug. Both drugs were held and/or discontinued for severe autoimmune toxicity. A physical examination and laboratory tests (including CBC with differential and comprehensive metabolic profile, including liver function and thyroid tests) were done at screening and every 3 weeks. Safety assessments were performed daily during hospitalization for IL-2 therapy. Imaging for tumor assessments was performed every 12 weeks. Response was assessed using WHO criteria modified for immune-related response (11). Adverse events (AEs) were evaluated and graded using NCI Common Toxicity Criteria v4.0.
Immune Studies and Statistical Analysis
Blood was collected at weeks 1, 4, 7, 12, and 24. Serum was analyzed for cytokines using the LEGENDPlex human CD8/NK panel (BioLegend), and peripheral blood mononuclear cells (PBMCs) were analyzed using flow cytometry. For intracellular measurement of HLA-DM and HLA-DO levels, samples were incubated with antibodies to identify peripheral B cells (CD45+ CD19+ MHC-II+), fixed and permeabilized with Cytofix/Cytoperm (BD PharMingen), and stained with antibodies specific for HLA-D (12) and HLA-DO (13). Ratios of the non-classical MHC-II proteins, HLA-DM, and HLA-DO, are a novel marker of antigen presentation potential and was obtained by dividing the mean fluorescent intensity levels obtained for HLA-DM by that obtained for HLA-DO (14, 15). Levels were compared in patients with decreasing vs. increasing tumor burden using two-way ANOVA with Bonferroni correction for multiple comparisons. Descriptive statistics were used for clinical outcome and safety reporting.
Results
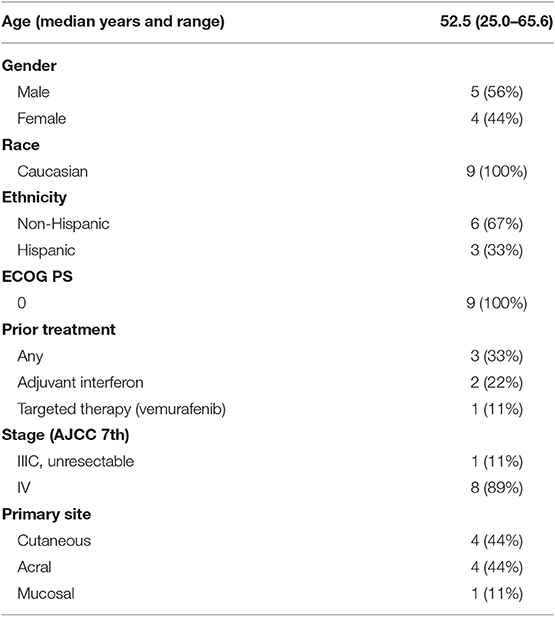
Patient Characteristics and Treatment Delivery
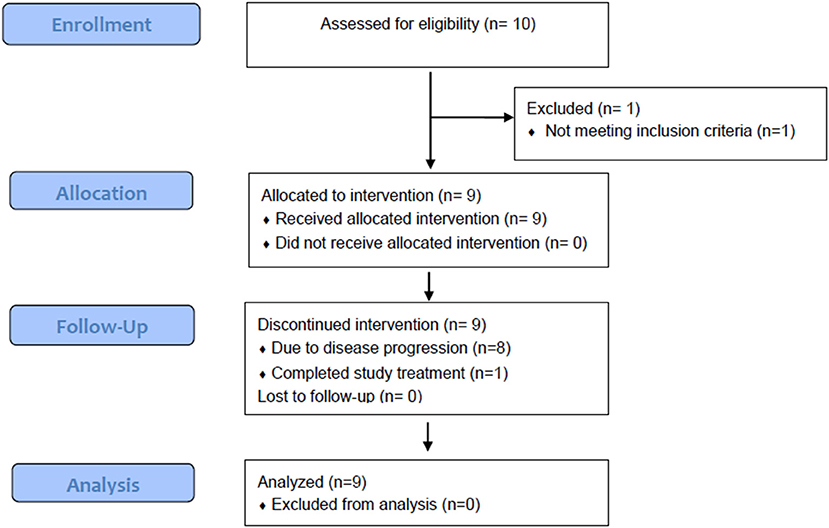
Although the trial was stopped in May 2016 due to discontinuation of funding, the safety and efficacy of treatment for nine patients enrolled between December 2014 and December 2015 are reported (Figure 1). Baseline patient characteristics are summarized in Table 1. Four patients with cutaneous, four with acral, and one with anal mucosal primary melanoma were enrolled. All patients had an ECOG performance status of 0. No patients received anti-PD-1 prior to enrolling. Two patients had received adjuvant interferon, and one received vemurafenib in the metastatic setting. Patients received a median of four IPI (range, 2–7) and 11 IL-2 (range, 3–18) doses. No patients received more than two cycles (one course) of IL-2.
Safety
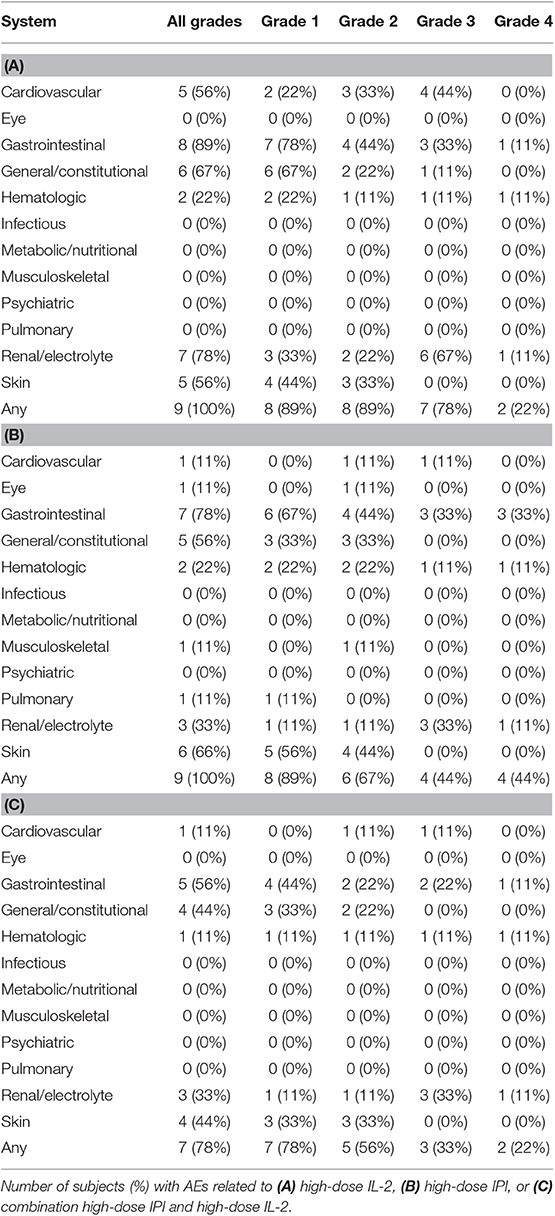
There were no treatment-related deaths. Seven of nine patients (78%) experienced a grade 3 or 4 treatment-related adverse event (AE), three of which were attributed to both agents (Table 2). The most frequent grade 3 and 4 AEs were liver and kidney injury, which occurred during the first 12 weeks of therapy in four patients. One patient experienced grade 4 hyperbilirubinemia, grade 3 transaminase elevation, and grade 4 acute kidney injury during the first cycle of combination high-dose IL-2 and high-dose IPI. The patient was given corticosteroids, and liver and kidney injury resolved 1 month later. The patient was removed from the study due to unacceptable toxicity. Another patient experienced grade 3 transaminase elevation, hyponatremia, and hypotension related to both agents. Approximately 2 weeks after discontinuation of high-dose IL-2, the transaminases rose to grade 4 and the patient required corticosteroid and mycophenolate mofetil for 2 months before the hepatitis resolved. A third patient had grade 4 hematologic toxicity, grade 3 anemia, grade 4 thrombocytopenia, and grade 3 acute kidney injury after the second cycle of combination high-dose IL-2 and high-dose IPI. A bone marrow biopsy showed adequate megakaryocytes, and the patient was treated with steroids for autoimmune thrombocytopenia for ~1 month with eventual resolution of platelet count. A fourth patient had grade 4 transaminase elevation during the first 12 weeks that was attributed only to IPI. This patient improved without steroids and went on to complete 1 year of study therapy with maintenance IPI. Colitis and serious diarrhea were not observed. Grade 1 and 2 diarrhea was noted in four and one patients, respectively.
Efficacy
Best overall response by week 24 was partial response in one patient (11%; 95% CI 0.57–43.5%) and stable disease in four patients (44%), with progressive disease in four patients (44%; Figure 2). Minimum follow-up time was 24 months. The median OS was not reached, with 1- and 2-year OS rates of 89 and 67%, respectively. Six patients received post-study therapy for progressive disease, most commonly PD-1-directed checkpoint blockade. The patient with the partial response was a 53-year-old woman with a cutaneous primary melanoma of the lower extremity, who experienced an 74% reduction in tumor burden at week 24. Her response was ongoing at 50.0 months of follow-up without subsequent therapy. A patient with stable disease had clear clinical benefit not meeting response criteria. This patient was a 50-year-old man with acral melanoma of the toe web, who experienced a 30% reduction in tumor burden at week 24 and continues without progression at 44.2 months of follow-up.
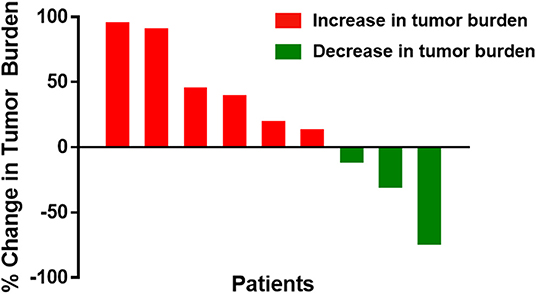
Figure 2. Waterfall plot of best overall response by week 24 for individual metastatic melanoma patients treated with high-dose IPI and high-dose IL-2. Bars represent the best overall response by week 24 as a percent change in tumor burden for each treated patient. Red bars denote patents with increased tumor burden, and green bars denote patients with decreased tumor burden.
Immune Responses
Patients were divided based on response, specifically increased vs. decreased tumor burden. Granzyme B, a marker of cytolytic potential, and HLA-DM:HLA-DO ratio, a marker of antigen presentation capability (14, 15), were significantly increased in patients with decreased tumor burden at weeks 12–24 (Figure 3). No differences were observed in CD8+ effector or CD4+ regulatory T cells (data not shown).
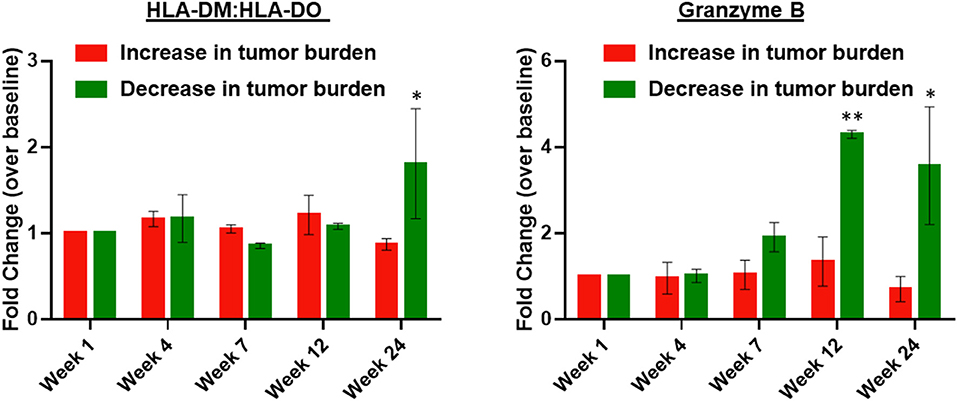
Figure 3. Peripheral blood HLA-DM:HLA-DO ratio and serum granzyme B are elevated in patients with decreased tumor burden following treatment with high-dose IPI and high-dose IL-2. The left panel shows the fold-change in HLA-DM:HLA-DO expression measured by flow cytometry within peripheral blood B cells (CD45+ CD19+ MHC-II+) of patients with an increase in tumor burden (n = 6) vs. patients with a decrease in tumor burden (n = 3). The right panel shows the fold-change in serum granzyme B over baseline (week 1) in patients with an increase in tumor burden (n = 6) vs. patients with a decrease in tumor burden (n = 3). Two-way ANOVA with Bonferroni correction; *P < 0.05 and **P < 0.01. Error bars represent S.E.M.
Discussion
The therapeutic landscape for melanoma has changed dramatically over the past decade, with 11 new agents approved since 2011 (16). This inlcudes multiple small molecule inhibitors of the MAPK pathway and three immune checkpoint inhibtiors. Yet, many patients do not respond to treatment or develop secondary resistance after exposure to individual agents and/or regimens. The potential to improve responses by combination treatment with two agents has emerged as a major area of clinical investigation, and has been largely supported by an improvement in clinical outcomes for combination IPI and nivolumab, although this regimen has been associated with significant toxicity (17). Since combination of approved melanoma agents is now a high priority, we sought to determine the feasibility, safety, and initial response rate of high-dose IPI and high-dose IL-2.
Combination high-dose IPI and high-dose IL-2 treatment was associated with significant AEs, including grade 3–4 AEs in seven of nine patients (78%). There were no new safety signals observed; however, expected IL-2-related side effects such as liver and kidney injury were more prolonged than usual, and systemic corticosteroids were required to treat immune related adverse events in three patients. There was no mortality and surprisingly, no serious diarrhea or colitis was observed. We did confirm the feasibility of combination high-doses IPI and high-dose IL-2, but our results are limited by the small sample size due to loss of funding and premature closure of the study. Of the nine patients who received treatment, there was only 1 responder. Two patients were alive without disease progression after 44+ and 50+ months without subsequent therapy. These durable responses consistent with previous experience with high-dose IL-2 therapy. These data suggest that the combination was tolerated, and further investigation is warranted to better define the response rate of this combination, but not with high dose IPI. Notably, in the previous study by Maker et al. (8), the response rate was higher, despite using very low doses of IPI, suggesting that high dose IPI (10 mg/kg) does not improve outcomes with IL-2 and should not be tested further.
Our data suggest that response to combination high-dose IPI and high-dose IL-2 is associated with markers of antigen presenting machinery regulated by the ratio of non-classical MHC-II proteins HLA-DM and HLA-DO. HLA-DM catalyzes the peptide loading of MHC-II molecules. HLA-DM is negatively regulated by HLA-DO; thus, a high HLA-DM:HLA-DO indicates that the antigen presentation machinery is optimally poised for peptide loading. Serial sampling of peripheral blood in patients treated with combination high-dose IPI and high-dose IL-2 identified that patients with decreased tumor burden had a higher ratio of the non-classical MHC-II HLA-DM:HLA-DO protein levels. HLA-DM promotes peptide loading into the MHC-II pocket by catalyzing the removal of CLIP, and HLA-DM is negatively regulated by HLA-DO. Thus, a high HLA-DM:HLA-DO ratio indicates that the antigen presentation machinery is poised for peptide loading (14, 15). The importance of MHC proteins as predictve biomarkers is demonstrated in a previous report in which MHC-I expression (although not MHC-II expression) was show to be associated with favorable response to ipilimumab (18); thus, our findings support the notions that effective antigen presentation is a key component of the mechanism of action of checkpoint blockade, and MHC protiens are emerging as predictive biomarkers. In addition, we observed that activated lymphocyes with increased cytolytic potential as demonstrated by enhanced granzyme B expression were associated with favorable response.
Our experience highlights potential opporunites and challenges in conducting such clinical trials in the contemporary period. According to a prospectively-collected registry study, high-dose IL-2 retains its efficacy in the post-anti-PD-1 monotherapy setting (7); therefore, safety and activity with this combination in the setting of disease progression following anti-PD-1 treatment may inform the development of novel formulations of IL-2 as salvage therapies. In particular, the use of modified IL-2 molecules designed to promote effector CD8+ T cell responses and limit CD4+ regulatory T cell responses may offer a better strategy for IL-2-based combinations (19). Future studies of IPI and IL-2 should utilize low doses of IPI and most likely will incorporate novel IL-2-based compounds.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Rutgers University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AS, HK, BC, JM, KM, DM, JC, WS, MK, and AZ contributed to the study concept, design, analysis and interpretation of data, and drafting of the manuscript. AS, HK, BC, JN, PB, LD, SN, AH, WS, and AZ analyzed and interpreted data, and helped draft the manuscript. AS, HK, BC, SN, AH, and AZ were involved in data acquisition. All authors contributed to the revisions of and approved the final manuscript.
Funding
The authors declare that this study received funding from Bristol Myers-Squibb. The funder had the following involvement with the study: approval of study design and approval of manuscript for publication. This research was supported by the Immune Monitoring, Biometrics, and Biospecimen Repository and Histopathology Service Shared Resources of Rutgers Cancer Institute of New Jersey (funded in part by NIH P30CA072720).
Conflict of Interest
AS reports receiving research funding from Merck and consulting fees from Bristol Myers-Squibb and Merck. HK and PB are employees of Replimune, Inc. DM reports receiving research funding from Prometheus Laboratories and Bristol Myers-Squibb, and consulting fees from Bristol Myers-Squibb, Pfizer, Merck, Novartis, Array BioPharma, Eli Lilly, EMD Serono, Jounce Therapeutics, Peloton, and Alkermes. JC reports receiving research funding from Bristol Myers-Squibb and from Prometheus, an unpaid advisory role for Prometheus, and speaking fees from Bristol Myers-Squibb. LD reports receiving research funding from Janssen. SN is an employee of Amgen. AH is an employee of Docs Global CRO and a consultant for Bristol-Myers Squibb. AZ reports receiving research funding from Merck and Fox Chase Chemical Diversity Center.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KZ declared a shared affiliation, with no collaboration, with the author KM to the handling editor at the time of review.
Acknowledgments
The authors acknowledge the members of the Cytokine Working Group for their input on the design and conduct of this study and the interpretation of the data.
Abbreviations
IPI, ipilimumab; IL-2, interleukin-2; IV, intravenous; CTLA-4, cytotoxic T lymphocyte antigen 4; CR, complete response; PBMC, peripheral blood mononuclear cell.
References
1. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
2. Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. (2015) 33:1889–94. doi: 10.1200/JCO.2014.56.2736
3. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. (1999) 17:2105–16. doi: 10.1200/JCO.1999.17.7.2105
4. Davar D, Ding F, Saul M, Sander C, Tarhini AA, Kirkwood JM, et al. High-dose interleukin-2 (HD IL-2) for advanced melanoma: a single center experience from the University of Pittsburgh Cancer Institute. J Immunother Cancer. (2017) 5:74. doi: 10.1186/s40425-017-0279-5
5. Payne R, Glenn L, Hoen H, Richards B, Smith JW, Lufkin R, et al. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a community hospital biotherapy program. J Immunother Cancer. (2014) 2:13. doi: 10.1186/2051-1426-2-13
6. Chow S, Galvis V, Pillai M, Leach R, Keene E, Spencer-Shaw A, et al. High-dose interleukin2 – a 10-year single-site experience in the treatment of metastatic renal cell carcinoma: careful selection of patients gives an excellent outcome. J Immunother Cancer. (2016) 4:67. doi: 10.1186/s40425-016-0174-5
7. Buchbinder EI, Dutcher JP, Daniels GA, Curti BD, Patel SP, Holtan SG, et al. Therapy with high-dose Interleukin-2 (HD IL-2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer. (2019) 7:49. doi: 10.1186/s40425-019-0522-3
8. Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. (2005) 12:1005–16. doi: 10.1245/ASO.2005.03.536
9. Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. (2012) 18:2039–47. doi: 10.1158/1078-0432.CCR-11-1823
10. Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2017) 18:611–22. doi: 10.1016/S1470-2045(17)30231-0
11. Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. (2009) 15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624
12. Hammond C, Denzin LK, Pan M, Griffith JM, Geuze HJ, Cresswell P. The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. J Immunol. (1998) 161:3282–91.
13. Glazier KS, Hake SB, Tobin HM, Chadburn A, Schattner EJ, Denzin LK. Germinal center B cells regulate their capability to present antigen by modulation of HLA-DO. J Exp Med. (2002) 195:1063–9. doi: 10.1084/jem.20012059
14. Denzin LK, Sant'Angelo DB, Hammond C, Surman MJ, Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. (1997) 278:106–9. doi: 10.1126/science.278.5335.106
15. Denzin LK. Inhibition of HLA-DM mediated MHC class II peptide loading by HLA-DO promotes self tolerance. Front Immunol. (2013) 4:465. doi: 10.3389/fimmu.2013.00465
16. Kaufman HL, Margolin K, Sullivan R. Management of metastatic melanoma in 2018. JAMA Oncol. (2018) 4:857–8. doi: 10.1001/jamaoncol.2018.0170
17. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19:1480–92. doi: 10.1016/S1470-2045(18)30700-9
18. Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med. (2018) 10:eaar3342. doi: 10.1126/scitranslmed.aar3342
19. Charych D, Khalili S, Dixit V, Kirk P, Chang T, Langowski J, et al. Modeling the receptor pharmacology, pharmacokinetics, and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy. PLoS ONE. (2017) 12:e0179431. doi: 10.1371/journal.pone.0179431
Keywords: melanoma, interleukin-2, cytokine, ipilimumab, combination immunotherapy, hepatitis, antigen presentation
Citation: Silk AW, Kaufman HL, Curti B, Mehnert JM, Margolin K, McDermott D, Clark J, Newman J, Bommareddy PK, Denzin L, Najmi S, Haider A, Shih W, Kane MP and Zloza A (2020) High-Dose Ipilimumab and High-Dose Interleukin-2 for Patients With Advanced Melanoma. Front. Oncol. 9:1483. doi: 10.3389/fonc.2019.01483
Received: 26 August 2019; Accepted: 10 December 2019;
Published: 10 January 2020.
Edited by:
Wafik S. El-Deiry, Alpert Medical School, Brown University, United StatesReviewed by:
Braden C. McFarland, University of Alabama at Birmingham, United StatesKeqiang Zhang, City of Hope National Medical Center, United States
Copyright © 2020 Silk, Kaufman, Curti, Mehnert, Margolin, McDermott, Clark, Newman, Bommareddy, Denzin, Najmi, Haider, Shih, Kane and Zloza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ann W. Silk, YW5uX3NpbGtAZGZjaS5oYXJ2YXJkLmVkdQ==
 Ann W. Silk
Ann W. Silk Howard L. Kaufman
Howard L. Kaufman Brendan Curti5
Brendan Curti5 Jenna Newman
Jenna Newman Lisa Denzin
Lisa Denzin Saltanat Najmi
Saltanat Najmi Michael P. Kane
Michael P. Kane Andrew Zloza
Andrew Zloza