- 1Division of Imaging & Oncology, University Medical Center Utrecht, Utrecht, Netherlands
- 2Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, Netherlands
- 3Copernicus Institute of Sustainable Development, Utrecht University, Utrecht, Netherlands
The new radiotherapy high field, 1.5 Tesla MRI-guided linear accelerator (MR-Linac) is being clinically introduced. Sensing and evaluating opportunities and barriers at an early stage will facilitate its eventual scale-up. This study investigates the opportunities and barriers to the implementation of MR-Linac into prostate cancer care based on 43 semi-structured interviews with Dutch oncology care professionals, hospital and division directors, patients, payers and industry. The analysis was guided by the Non-adoption, Abandonment, Scale-up, Spread, and Sustainability framework of new medical technologies and services. Opportunities included: the acquirement of (1) advanced MRI-guided radiotherapy technology with (2) the potential for improved patient outcomes and (3) economic benefits, as well as (4) professional development and (5) a higher hospital quality profile. Barriers included: (1) technical complexities, (2) substantial staffing and structural investments, (3) the current lack of empirical evidence of clinical benefits, (4) professional silos, and (5) the presence of patient referral patterns. While our study confirms the expected technical and clinical prospects from the literature, it also reveals economic, organizational, and socio-political challenges.
Introduction
The implementation of medical technology and services usually involves individual, organizational and environmental factors (1–4). All three are relevant to the introduction of MRI-guided linear accelerator (MR-Linac) systems: the 0.35 Tesla ViewRay MRIdian system and the 1.5 Tesla Elekta Unity system (5, 6). Yet, their introduction into routine oncology care has mainly been reported from a technical and clinical perspective (7–10). In this study we will focus on the Unity MR-Linac, recently developed by the University Medical Center Utrecht (Utrecht, The Netherlands) in collaboration with Elekta AB (Stockholm, Sweden) and Philips (Best, The Netherlands). This technology integrates a 1.5 Tesla MR-imaging scanner with a radiotherapy linear accelerator (9, 11–15). This enables online adaptive radiotherapy delivery and diagnostic quality imaging simultaneously that allows the visualization of tumor and surrounding organs before, during and after treatment (9, 12, 16–19), with potentially higher treatment accuracy, the sparing of healthy tissue and the possibility of hypofractionation (providing the total dose in fewer treatment sessions). These features are expected to deliver real health benefits for patients including better tumor control, fewer side effects, and a shorter treatment course (17, 20–22). Since MR-Linac’s CE approval in June 2018 and FDA approval in December 2018, the technology has been installed in institutions worldwide (23, 24).
Despite the promising clinical and technological prospects, challenges remain. The use of MR-Linac requires high capital investments in equipment, logistics, quality assurance and complementary training (7), and evidence of superior patient outcomes (8). Implementing technical developments in cancer treatment may disrupt standard treatment practices, which call for financial considerations, collective learning, and organizational renewal (25, 26). Collective learning and organizational renewal can be hampered by hospital autonomy and by cultures of secrecy within specialties (2, 3, 27, 28). These are potential bottlenecks that are seldom investigated in radiotherapy centers (29). While some attention has been given to potential implementation challenges, these aspects need untangling and a clearer understanding in order to maximize benefits and to avoid setbacks for patients and care givers (8, 17, 19, 25, 30).
This study aims to identify the opportunities and barriers for successful implementation of MR-Linac into prostate cancer care. The choice for focusing on prostate cancer is based on the following reasons. First, online adaptive radiotherapy is most likely to be of benefit for tumors that move between and during treatment (13, 31), as is the case in prostate cancer (18, 32). Prostate cancer, the most common cancer in men worldwide, does have high survival rates (33, 34). However, current treatments may interfere with quality of life: external beam radiotherapy, brachytherapy, and even minimally invasive robotic procedures, cause adverse effects such as erectile dysfunction and urinary incontinence (33–35). MR-Linac minimizes uncertainties about the actual tumor’s location, shape and the surrounding organs at risk, which may reduce adverse effects and in turn improve a patient’s quality of life (9, 11). Second, clinical interest in MRI-guided radiotherapy in prostate cancer management has been increasing in recent years (10, 17, 36), and understanding the dynamics of its implementation is timely.
Materials and Methods
Design
We conducted a qualitative study including semi-structured interviews, an approach most appropriated to make sensitive issues and attitudes, opinions and experiences of individuals explicit (37). We used the Non-adoption, Abandonment, Scale-up, Spread and Sustainability (NASSS) framework of new healthcare technologies and services which is designed to explore determinants of success and failure of technology adoption in healthcare organizations. The NASSS framework considers seven domains: the condition or clinical indication, the technology to be implemented, the value proposition, the adopter system (patient, technology user and other staff), the organization, the wider institutional and social context, and organizational resilience and technology development over time (38).
Recruitment
Respondents were recruited through purposive and snowball sampling using recommendations from initial respondents. We wanted to obtain a convenience sample of compelling roles among the populations of interest. Because implementing medical technologies and services requires a comprehensive multilevel consideration of individual, organizational and environmental influences (1–4), we attempt to select respondents at each of these levels of influence and based on their expertise. Therefore, we adopted a number of selection criteria:
1. Working at a hospital offering MR-Linac treatment; or
2. Providing other prostate cancer treatments (e.g., external beam radiotherapy, low- or high-dose-rate brachytherapy, proton beam therapy, robotic surgery, radiosurgery); or
3. Management experience relevant to the implementation and insurance coverage of new medical technologies or services; or
4. Stakeholders outside the hospital (e.g., patients, care insurers, manufacturing industry).
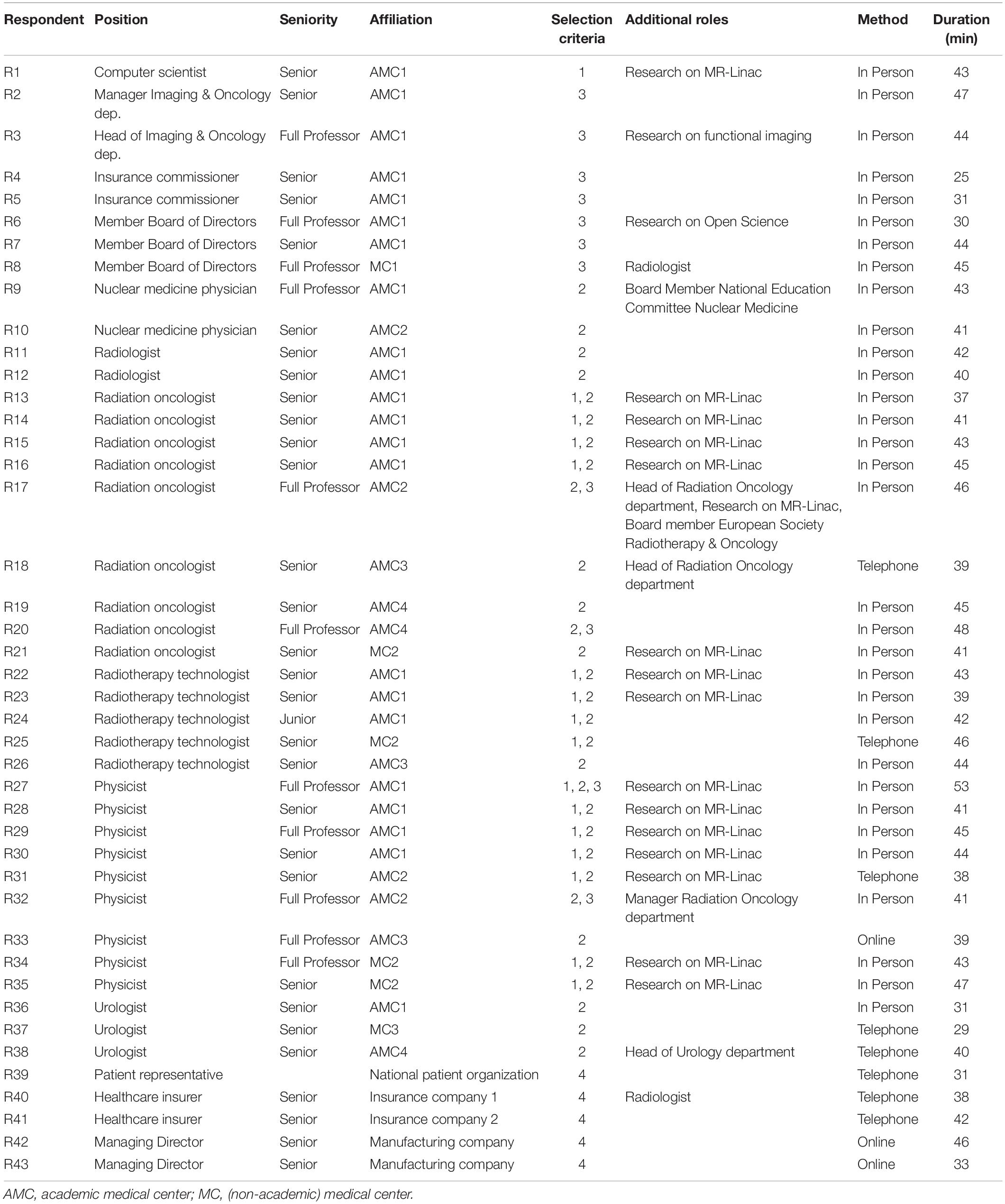
We included physicists, radiation oncologists, radiotherapy technologists and ICT staff, currently practicing MR-Linac (9). We also interviewed urologists (the referring physician in the Netherlands), radiologists and nuclear medicine physicians. Further, we included hospital directors, division and insurance managers. We included respondents from different hospitals, to limit selection bias. At the time of writing this article, only two hospitals offer Unity MR-Linac treatment in the Netherlands. This country is a suitable context, considering that it has been the first nation in which this technology has been introduced. At the contextual level, we included the perspectives of patients, care insurers and the executives of industries that hold MR-Linac’s intellectual property rights. Our respondents, except those in industries, are located in Netherlands.
Data Collection
The research objective was explained in the invitation and at the start of each interview. The questionnaire is based on the interview questions of the NASSS framework and the first interviews (see Supplementary Appendix A). It included open-ended questions to explore each respondent’s experience with and views on MR-Linac for prostate cancer treatment, including implementation opportunities and barriers. All interviews were conducted by one trained researcher and lasted approximately 45 minutes until saturation occurred and no new information appeared in the data. Interviews were conducted in person (N = 35), by phone (N = 5), or by Skype (N = 3). All interviews were audio-recorded with the consent of the respondents and transcribed. Each respondent validated their transcript. Audio recordings and transcript of interviews are confidential and therefore not publicly available.
Data Analysis
Interview transcripts were analyzed in NVivo software. We first applied open coding based on the research objective. We then applied axial coding, systematically identifying areas of interest based on the NASSS framework. This iterative step involved repetitions aimed at revising primary codes. We triangulated responses across different respondents and subsequently identified the opportunities and barriers. Resulted codes were validated by a second reviewer. We regularly discussed whether the empirical data matched the NASSS framework, ensuring that results were correctly classified within their domain. To include variation in findings and increase construct validity, we interviewed more than one person per profession and also considered perspectives from various hospitals.
Results
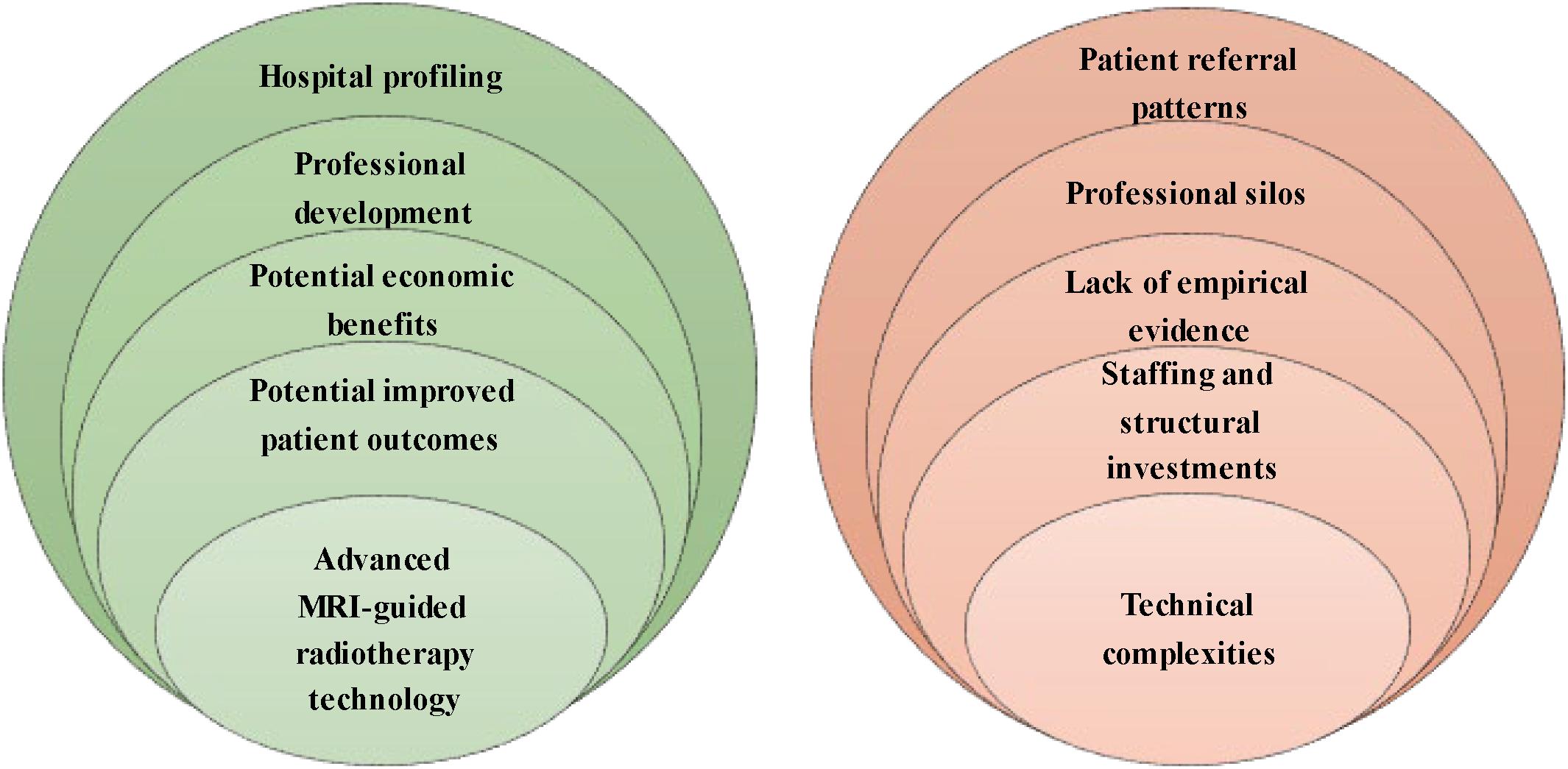
We conducted 43 interviews with professionals in MRI-guided radiotherapy as well as other prostate cancer treatments, hospital and department directors, insurance commissioners, and external stakeholders between November 2018 and March 2019 (see Table 1). Hospital respondents work in four academic and three non-academic Dutch hospitals, of which two hospitals installed Unity MR-Linac and one hospital ViewRay MRIdian. Five opportunities and five barriers to the implementation of MR-Linac have been identified (see Figure 1). We first present the opportunities, followed by the barriers according to the frequency stated by the respondents.
Opportunities
Our respondents revealed five opportunities to MR-Linac implementation for prostate cancer: (1) advanced MRI-guided radiotherapy technology, (2) potential improvement in patient outcomes, (3) potential economic benefits, (4) professional development, and (5) hospital profiling. Figure 2 shows the percentages of the interview cohort who discussed the opportunities, by main theme and subtheme. Supplementary Appendix B provides an overview of the respondents referencing opportunities.
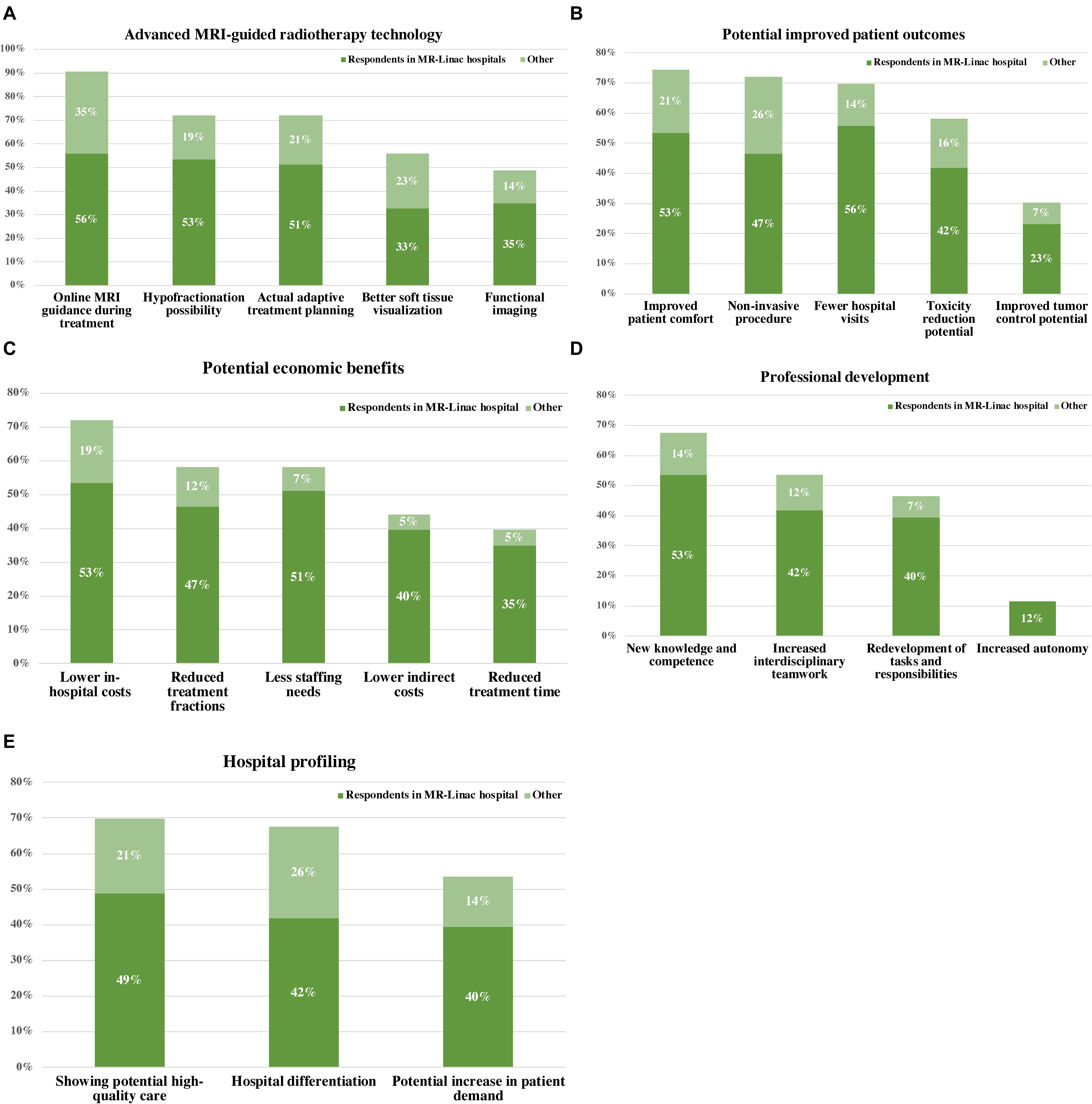
Figure 2. Percentages of the interview cohort who discussed opportunities to the implementation of MR-Linac into prostate cancer care, by main theme and subtheme. (A) Advanced MRI-guided radiotherapy technology, (B) potential improved patient outcomes, (C) potential economic benefits, (D) professional development, and (E) hospital profiling.
Advanced MRI-Guided Radiotherapy Technology
Given the increasing demand in radiotherapy for advanced image-guidance and adaptive treatments subsequently, the use of MRI during radiotherapy is perceived as an inevitable follow-on advancement in this field. The opportunity of real-time diagnostic-strength 1.5 Tesla MRI-imaging that enables better soft tissue visualization; daily on-table adaptation to anatomical changes; actual adaptive treatment planning; hypofractionation and evaluation of tumor response during the course of radiotherapy. Further, actual anatomical and functional information of the prostate tumor and greater confidence in avoiding organs at risk during treatment is perceived as very promising, allowing more accurate, targeted treatment and avoiding radiation of healthy tissue. According to current technology users, these prospects promise new treatment avenues in radiation oncology as well as in related medical disciplines.
Potential Improved Patient Outcomes
Prostate cancer is a well-characterized disease with effective treatment modalities. However, the potential adverse effects of present treatments are substantial and can interfere with the patient’s quality of life; this remains a key target in present treatment development. Radiotherapy practitioners and members of hospital management expect MR-Linac to solve this issue and to yield improved patient outcomes. The majority of respondents mentioned improved patient comfort as main benefit resulting from: (1) possibly fewer adverse effects, (2) possibly improved tumor control, (3) the non-invasive procedure; the implantation of gold fiducial markers within the prostate is no longer necessary for position verification, and (4) hypofractionation allows prostate cancer treatment in fewer hospital visits and may shorten waiting lists. For example, the current standard is to give prostate radiotherapy in 20 fractions and hypofractionation has the potential to perform to allow completion of the entire treatment in only 2–5 times.
Potential Economic Benefits
In view of the current, unsustainable growth in medical expenditures, the present-day value of new treatments will require an improvement in both treatment quality and cost reduction. According several radiotherapy professionals MR-Linac may offer quality and efficiency gains. First, both the preparation of the treatment plan as well as the execution takes place on the same device. Second, digital developments, such as deep learning, may allow operational benefits: automation processes during treatment (e.g., automatic contouring of tumor and organs at risk) to reduce staffing needs and waiting times. Ultimately, improved efficiency, together with fewer treatment sessions, fewer hospital visits, potentially fewer adverse effects and lower direct in-hospital costs such as anesthesia provision or indirect care costs (e.g., treatment of adverse effects and transport costs) can reduce overall costs.
Professional Development
Implementing MR-Linac allows room for professional development and multidisciplinary learning. First, users experience an increased communication and collaboration across radiation oncology and imaging specialties (e.g., for the development of scanning protocols on MR-Linac). In most hospitals diagnostics and treatment are performed by different groups and the interaction between them is therefore limited. Second, the required knowledge of both MR-imaging and radiotherapy integrates different competences and expertise. As consequence, MR-Linac users may be attracted by the development and use of new knowledge and competences and the redevelopment of tasks and responsibilities. Third, radiotherapy technologists also reported their potential increased autonomy and involvement in decisions. They would have more responsibility like the maintenance of MRI protocols and active safeguarding of radiation requirements for target volume and organs at risk. The empowerment of employees fosters a better workplace culture.
Hospital Profiling
The implementation of MR-Linac also offers hospitals a way to profile themselves as innovative; providing potentially high-quality care. They also expect that hospitals implement MR-Linac to keep up with recent developments in radiation oncology and attract patients accordingly. According to the patient representative and several professionals, the target population is generally aware of the treatment modalities and prefers MRI-guided treatment. This could increase patient referral to the radiotherapy department and related medical specialties. Implementing MR-Linac could therefore provide hospitals a competitive advantage.
Barriers
Our respondents revealed five main barriers to MR-Linac implementation for prostate cancer: (1) technical complexities, (2) staffing and structural investments, (3) the lack of empirical evidence of clinical benefits, (4) professional silos, and (5) the presence of patient referral patterns. Figure 3 shows the percentages of the interview cohort who discussed the barriers, by main theme and subtheme. Supplementary Appendix C provides an overview of the respondents referencing barriers.
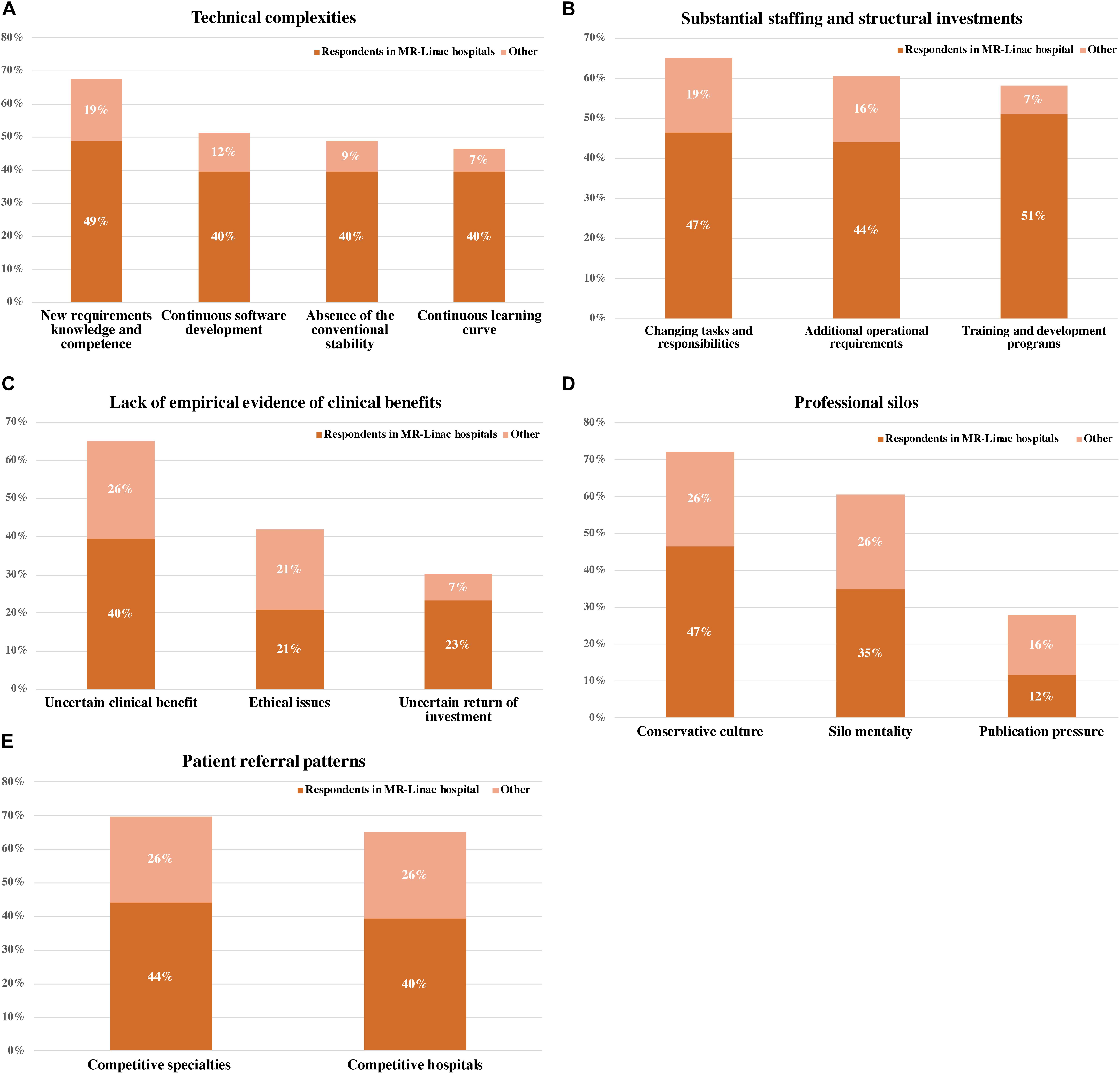
Figure 3. Percentages of the interview cohort who discussed barriers to the implementation of MR-Linac into prostate cancer care, by main theme and subtheme. (A) Technical complexities, (B) substantial staffing and structural investments, (C) Lack of empirical evidence of clinical benefits, (D) professional silos, and (E) patient referral patterns.
Technical Complexities
The involvement of MRI in radiotherapy is expected to transform current radiation oncology practice in terms of target identification, tumor response assessment, treatment planning and delivery, quality assurance and staffing. MR-Linac’s ultimate impact on the current radiation oncology development is not yet known considering its continuous development, which largely depends on software upgrades rather than hardware upgrades. The technology’s output is vulnerable to the interpretations of individual practitioners and may associate with inter- and intra-variability in treatment procedure, which in turn could affect clinical outcomes. Hence, the absence of the conventional security of the traditional linear accelerator necessitates the presence of experienced staff. This, together with continuing software developments, requires users to anticipate an ongoing learning curve.
In practice, MR-Linac’s value is limited by software challenges in real-time tumor tracking during radiation. One treatment session is relatively also longer compared to conventional external beam radiotherapy, and this longer treatment duration could be a potential barrier for the patient. Each treatment lasts approximately 45 min, which is three to four times longer than conventional external beam radiotherapy (22).
Substantial Staffing and Structural Investments
The required MRI competence, knowledge and the need for on-the-spot decision making were at the same time also seen as a challenge. For example, a radiation oncologist reported that brachytherapy practitioners are more used in making decisions on the spot than those involved in conventional external beam radiotherapy only. Adequate training programs are therefore a prerequisite to ensure that MR-Linac is used effectively and that MRI is safe for both patients and users. Further, several respondents also mentioned the need to expand the responsibilities of radiotherapy technologists to reduce the presence of the radiation oncologist and physicist during treatment and staffing costs subsequently. Although radiation technologists could bear more responsibility, other concerns are their limited availability and that existing Dutch policy does not allow therapists to approve treatment plans.
Another perceived barrier is the substantial structural investments required: today’s radiotherapy centers often lack the needed combination of MR-imaging and radiation facilities. To illustrate, a single MR-Linac costs 10 million euros without the requisite infrastructure, such as MRI compatibility, MRI safety, clinical workflow and its accompanying software development, quality assurance and the development of protocols, roles and responsibilities. Early adopters are therefore well financed medical research centers with MR-imaging expertise and facilities. Accordingly, MR-Linac reflects the trend that cancer care is increasingly centralized (39).
Lack of Empirical Evidence of Clinical Benefits
Despite promising theoretical benefits, clinical value remains undocumented and the patient categories that will most benefit remain unclear. For present prostate radiotherapy, there is some room for improvement in terms of adverse effects and patient comfort. However, some respondents doubted the actual reduction in toxicity and clinical added-value. Also, respondents doubted whether hypofractionation would actually compensate for the increased cost because of more expensive technology, increased treatment time per fraction and organizational investments (e.g. the requirement of more highly trained staff). Further, few respondents questioned the clinical added-value of MR-Linac compared to ViewRay MRIdian as well as other potential emerging techniques in prostate cancer treatment (e.g., CT-based adaptive radiotherapy).
The present lack of empirical evidence also explains MR-Linac’s lack of insurance coverage. Consequently, this can hamper real savings for hospitals and care insurers, as the potential reduction in treatment costs cannot be achieved. Further, the provision of treatment with unproven efficacy and safety to the patient may also lead to ethical discussions. High-quality randomized control trials are imperative to compare the value of MR-Linac with alternative treatments: preferably with comparable outcomes across different centers. A multi-center clinical evaluation would also hasten the recruitment of patients needed. Paradoxically, our respondents reported the lack of clinical evidence hindering successful implementation, while also mentioning the need to install the technology in a clinical environment for empirical evaluation.
Professional Silos
Amongst the redevelopment of tasks and responsibilities, practicing MR-Linac can threaten users’ professional identity. Several radiotherapy professionals reported the potential conservative behavior and resistance as response to delegate tasks and change daily practice. Another perceived barrier is the publicity pressure exerted upon medical research centers which may hamper knowledge exchange and open communication about MR-Linac between hospitals. The political climate can hinder effective multicenter collaboration within and across hospitals, and the technology’s further development. These challenges relate to the silo mentality and conservative culture that often prevails in hospitals.
Patient Referral Patterns
Finally, introducing MR-Linac into routine care could raise patient referral discussions among specialties where patient demand may be compromised. The relationship between radiation oncology and surgery can be complementary, but also be competitive (26). In the Dutch prostate cancer care, urologists play an important role in patient access to MR-Linac as they discuss the treatment modalities with the patient. Likewise, radiotherapy centers offering MR-Linac may also be a perceived threat to hospitals that do not offer this technology, and hence would resist patient referral to this treatment.
Discussion and Implications for Practice and Future Research
Our findings help radiation oncology departments determining focus areas in their strategy for successful MR-Linac implementation into prostate cancer care. Consistent with prior research, MR-Linac users expect to benefit from advanced MRI-guided radiation technology with online adaptive treatment and response assessment that may potentially improve patient outcomes and identify new treatment opportunities (7, 8, 10, 19, 40). The possibility of prostate hypofractionation promises improved treatment and economic benefits (17, 20, 41–43). Users boost their hospital profile and professional development, irrespective of radiation oncologists, technologists and physicists (7, 8, 25, 44, 45). Our study also confirms the need to generate clinical evidence, while dealing with technical complexities and substantial staffing and structural investments (7). However, simply addressing these barriers is not enough: successful implementation also raises economic, organizational and socio-political concerns embedded in the presence of patient referral patterns and professional silos. These concerns are understudied in the current efforts on MR-Linac implementation into routine prostate cancer care.
Many respondents perceive MR-Linac as a complex innovation with a high implementation burden: its multidisciplinary nature disrupts the traditional barrier between radiation oncology and diagnostic radiology (7, 10, 26) which practically justifies all barriers. The involvement of MRI in radiation oncology transforms current practices either within and outside the radiation oncology department (7). Users are clearly concerned with substantial structural and staffing investments, established determinants in new technology and service implementation in healthcare (21, 46). The substantial investments are also explained by the technical complexities inherent in MR-Linac. Further, our respondents have identified concerns about software deficiencies and the relative longer treatment fractions. Technological development should focus on improving workflow and the automation of both imaging and treatment (12, 36, 47).
MR-Linac’s technical character has a major impact on staffing roles, which can lead to both efficiency improvements and professional identity threats. The ongoing technology development together with the acquirement of new skills (e.g., MRI competence, on-the-spot decision-making) illustrate the need for users to anticipate new learnings and responsibilities. However, the transformation of existing staff roles is not easy and is perceived as more than just learning how to use a new technology. This would require acceptance of changes in professional identity and autonomy as well as increased communication across disciplinary boundaries. Moreover, current staffing policies in radiation oncology impede the reallocation of responsibilities for radiotherapy technologists. Technology users should therefore invest in workplace training and development with supporting staffing policies. Radiotherapy education will have to change in order to prepare physicists, radiation oncologists and technologists on the technical developments of MR-Linac. Further impact studies can focus on the professional development of users and the right staff policy to ensure a sustainable use of MR-Linac.
The reallocation of staffing is made more difficult by the presence of professional silos. Interestingly, in prostate cancer and cancer treatment in general, communication and cooperation between different disciplines tangled have already been proposed as prerequisites in effective cancer care (48–51), however, these features are still being raised as potential hindrances in MR-Linac implementation. Professional silos can be expressed by the presence of specialisms and related conservative behavior, a common challenging determinant in changing existing practices in hospitals (27), which also applies here. This also impedes the smooth collaboration and integration of diagnostic imaging and radiotherapy.
Another barrier is the likely presence of patient referral patterns. Safeguarding patients’ access to MR-Linac requires participation of radiotherapy professionals as well as referring physicians (the urologist in the context of Netherlands). Ultimately, successful implementation would therefore require active support and participation from hospital executives, and alignment between departments (radiotherapy and urology. The required communication and collaboration strengthen horizontal connections between different disciplines (e.g., radiation oncology and imaging), but also vertical connections inside (e.g., between radiotherapy technologist and radiation oncologist) and outside the radiotherapy department.
Future efforts should generate clinical evidence to prove expectations and justify return on investment concerns, an indispensable determinant in technology implementation which has been given greater emphasis in the new European Medical Device Regulation (46, 52). Evaluation of an evolving technology such as MR-Linac is very difficult (53). Therefore, the international Unity MR-Linac consortium (9) has set up a prospective registry to include patients treated on MR-Linac in seven large institutions (MOMENTUM registry). Here, patients provide informed consent for the use of their technical (imaging) and clinical data for academic and clinical research as well as response assessment. Costs and quality of life data will be collected as well, to identify cost-effective MR-Linac treatment strategies compared to alternative treatments. This is particularly useful in the field of prostate cancer, where many treatment modalities with comparable outcomes, but with different costs are available (54). Further MR-Linac impact studies can also provide insights into its effects on prostate cancer treatment allocation and hospital infrastructure.
The lack of clinical evidence also causes gaps in insurance coverage. This, together with the substantial investments, creates a high implementation burden and uncertainty for potential MR-Linac users and payers. Interestingly, this has not prevented radiation oncology departments from implementing the technology. The increasing belief in image-guided technologies without proven results to profile users with state-of-the-art treatments and high quality care also applies to MR-Linac (8, 25). Despite the mutual skepticism among fellow professionals and health insurers about the clinical added-value of MR-Linac, collaboration between them facilitates technology users to meet requirements in treatment evaluation for insurance coverage.
Our study provides the first multifaceted assessment of opportunities and barriers in MR-Linac implementation for prostate cancer including perspectives from professionals, hospital and division directors, patients, payers and industry. The value of qualitative research is to explore phenomena in-depth and to question respondents about their relevant knowledge, opinions and experience. While a more extensive and systematic sampling method would limit selection bias, this study likely captures a significant proportion of the relevant qualitative information. Interviews with early adopters revealed hitherto unanticipated implementation challenges (29). Typical feasibility or cost-effectiveness studies would overlook the potential effects of potential resistance to patient referral, changing practice habits and silo mentality. Whereas this study focuses on prostate cancer, the operational and organizational prospects discussed by respondents are likely to be valid for the implementation of MR-Linac for other tumor indications as well. A comprehensive comparison between MR-Linac systems [e.g., MRIdian of ViewRay (6)] as well as with emerging radiotherapy techniques and present prostate cancer treatments goes beyond the scope of this paper. Finally, generalizability of our findings to other contexts has to be carefully considered. Future efforts can determine how country-specific therapeutic standards, political and social contexts influence MR-Linac’s implementation activities.
Conclusion
Given the rapid development of MR-Linac, research into factors that stimulate or hamper its local implementation, is needed, as the first step to understand its long-term impact. Our findings define the main opportunities and barriers for successful MR-Linac implementation into routine care. We raise issues that are known in the field but largely overlooked in the current literature on MR-Linac implementation. The discussion of the topics that emerge from the interviews leads to reflection and learning, but also to new connections in MR-Linac implementation and the organization of care. Four fundamental conclusions can be given:
• Successful implementation of MR-Linac not only considers technical and clinical aspects, but also economic, organizational and socio-political challenges.
• MR-Linac implementation is expected to affect present prostate cancer care within and outside the radiation oncology department as well as hospital culture and identity of professionals.
• Involvement of the referring physician is crucial in successfully implementing MR-Linac into routine prostate cancer care.
• Clinical evaluation supported by patients, radiation oncology professionals, referring physicians and payers has to justify MR-Linac’s perceived benefits and substantial investments.
Data Availability Statement
The datasets presented in this article are not readily available because interview recordings and transcripts are confidential. Requests to access the datasets should be directed to CM,Yy5oZWhha2F5YUB1bWN1dHJlY2h0Lm5s.
Ethics Statement
This study was exempted from ethical approval according to the criteria of Dutch Medical Research involving Human Subjects Act. At the beginning of an interview, each participant was verbally informed about the research objective and interview purpose. All participants provided explicit verbal consent for participation in the interviews and publication of the results.
Author Contributions
CH conducted the interviews and completed the initial coding of the transcripts, prepared the first draft of this manuscript, and had access to all of the data and final responsibility for the decision to submit for publication. JV, JL, DG, HV, and EM contributed and commented on the draft. All authors contributed to the study design, read and approved the final manuscript, and involved in the analysis of the interview data.
Funding
This project is part of the project “Clinical introduction online and real-time MRI-guided prostate cancer radiotherapy” (project number 104006004) funded by the Netherlands Organization for Health Research and Development (ZonMw) via Innovative Medical Devices Initiative for Technology for Sustainable Healthcare. The funding body has had no part in the study design, data collection and analysis, and writing of this manuscript.
Conflict of Interest
Several other MR-Linac scientific projects at the Division of Imaging and Oncology of University Medical Center Utrecht have been partly funded Elekta AB (Stockholm, Sweden) and Philips Medical Systems (Best, Netherlands).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the respondents for sharing their opinions and personal experiences in our interviews.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01741/full#supplementary-material
APPENDIX A | Questionnaire based on NASSS framework and initial interviews.
APPENDIX B | Frequency of respondents who discussed opportunities to the implementation of MR-Linac into prostate cancer care, by main theme and subtheme.
APPENDIX C | Frequency of respondents who discussed barriers to the implementation of MR-Linac into prostate cancer care, by main theme and subtheme.
Abbreviations
MR-Linac, MRI-guided linear accelerator.
References
1. John RK, Evanisko MJ. Organizational innovation?: the influence of individual, and contextual a doption factors on hospital of technological and andministrative. Acad Manag J. (2014) 24:689–713. doi: 10.5465/256170
2. Länsisalmi H, Kivimäki M, Aalto P, Ruoranen R. Innovation in healthcare: a systematic review of recent research. Nurs Sci Q. (2006) 19:66–72. doi: 10.1177/0894318405284129
3. Thune T, Mina A. Hospitals as innovators in the health-care system: a literature review and research agenda. Res Policy. (2016) 45:1545–57. doi: 10.1016/j.respol.2016.03.010
4. Teplensky JD, Pauly MV, Kimberly JR, Hillman AL, Schwartz JS. Hospital adoption of medical technology: an empirical test of alternative models. Health Serv Res. (1995) 30:437–65.
5. Raaymakers BW, Lagendijk JJW, Overweg J, Kok JGM, Raaijmakers AJE, Kerkhof EM, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. (2009) 54:N229–37. doi: 10.1088/0031-9155/54/12/N01
6. Mutic S, Dempsey JF. The viewray system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. (2014) 24:196–9. doi: 10.1016/j.semradonc.2014.02.008
7. Hall WA, Paulson ES, van der Heide UA, Fuller CD, Raaymakers BW, Lagendijk JJW, et al. The transformation of radiation oncology using real-time magnetic resonance guidance: a review. Eur J Cancer. (2019) 122:42–52. doi: 10.1016/j.ejca.2019.07.021
8. van Herk M, McWilliam A, Dubec M, Faivre-Finn C, Choudhury A. Magnetic resonance imaging–guided radiation therapy: a short strengths weaknesses, opportunities, and threats analysis. Int J Radiat Oncol. (2018) 101:1057–60. doi: 10.1016/j.ijrobp.2017.11.009
9. Kerkmeijer LGW, Fuller CD, Verkooijen HM, Verheij M, Choudhury A, Harrington KJ, et al. The MRI-Linear accelerator consortium: evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front Oncol. (2016) 6:1–6. doi: 10.3389/fonc.2016.00215
10. Murray J, Tree AC. Prostate cancer – Advantages and disadvantages of MR-guided RT. Clin Transl Radiat Oncol. (2019) 18:68–73. doi: 10.1016/j.ctro.2019.03.006
11. Lagendijk JJW, Raaymakers BW, and van Vulpen M. The magnetic resonance imaging–linac system. Semin Radiat Oncol. (2014) 24:207–9. doi: 10.1016/j.semradonc.2014.02.009
12. Winkel D, Bol GH, Kroon PS, van Asselen B, Hackett SS, Werensteijn-Honingh AM, et al. Adaptive radiotherapy: the elekta unity MR-linac concept. Clin Transl Radiat Oncol. (2019) 18:54–9. doi: 10.1016/j.ctro.2019.04.001
13. Kontaxis C, Bol GH, Lagendijk JJW, Raaymakers BW. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol. (2015) 60:7485–97. doi: 10.1088/0031-9155/60/19/7485
14. Bol GH, Hissoiny S, Lagendijk JJW, Raaymakers BW. Fast online Monte Carlo-based IMRT planning for the MRI linear accelerator. Phys Med Biol. (2012) 57:1375–85. doi: 10.1088/0031-9155/57/5/1375
15. Lagendijk JJW, van Vulpen M, Raaymakers BW. The development of the MRI linac system for online MRI-guided radiotherapy: a clinical update. J Intern Med. (2016) 280:203–8. doi: 10.1111/joim.12516
16. Raaymakers BW, Jürgenliemk-Schulz IM, Bol GH, Glitzner M, Kotte ANTJ, Van Asselen B, et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. (2017) 62:L41–50. doi: 10.1088/1361-6560/aa9517
17. Pathmanathan AU, van As NJ, Kerkmeijer LGW, Christodouleas J, Lawton CAF, Vesprini D, et al. Magnetic resonance imaging-guided adaptive radiation therapy: a “game changer” for prostate treatment? Int J Radiat Oncol Biol Phys. (2018) 100:361–73. doi: 10.1016/j.ijrobp.2017.10.020
18. Kontaxis C, Bol GH, Kerkmeijer LGW, Lagendijk JJW, Raaymakers BW. Fast online replanning for interfraction rotation correction in prostate radiotherapy. Med Phys. (2017) 44:5034–42. doi: 10.1002/mp.12467
19. Corradini S, Alongi F, Andratschke N, Belka C, Boldrini L, Cellini F, et al. MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiat Oncol. (2019) 14:92. doi: 10.1186/s13014-019-1308-y
20. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. (2016) 17:1047–60. doi: 10.1016/S1470-2045(16)30102-4
21. Gunnlaugsson A, Kjellén E, Hagberg O, Thellenberg-Karlsson C, Widmark A, Nilsson P. Change in prostate volume during extreme hypo-fractionation analysed with MRI. Radiat Oncol. (2014) 9:1–6. doi: 10.1186/1748-717X-9-22
22. Pathmanathan A, Bower L, Creasey H, Dunlop A, Hall E, Hanson I, et al. EP-1566 MR-guided online adaptive radiotherapy: first experience in the UK. Radiother Oncol. (2019) 133:S845. doi: 10.1016/s0167-8140(19)31986-3
23. Werensteijn-Honingh AM, Kroon PS, Winkel D, Aalbers EM, van Asselen B, Bol GH, et al. Feasibility of stereotactic radiotherapy using a 1.5?T MR-linac: Multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol. (2019) 134:50–4. doi: 10.1016/j.radonc.2019.01.024
24. Liney GP, Whelan B, Oborn B, Barton M, Keall P. MRI-Linear Accelerator Radiotherapy Systems. Clin Oncol. (2018) 30:686–91. doi: 10.1016/j.clon.2018.08.003
25. Rai R, Kumar S, Batumalai V, Elwadia D, Ohanessian L, Juresic E, et al. The integration of MRI in radiation therapy: collaboration of radiographers and radiation therapists. J Med Radiat Sci. (2017) 64:61–8. doi: 10.1002/jmrs.225
26. Adam A, Kenny LM. Interventional oncology in multidisciplinary cancer treatment in the 21st century. Nat Rev Clin Oncol. (2015) 12:105–13. doi: 10.1038/nrclinonc.2014.211
27. Caccia-Bava MDC, Guimaraes T, Harrington SJ. Hospital organization culture, capacity to innovate and success in technology adoption. J Heal Organ Manag. (2006) 20:194–217. doi: 10.1108/14777260610662735
28. Edmondson AC, Bohmer RM, Pisano GP. Disrupted routines: team learning and new technology implementation in hospitals. Adm Sci Q. (2001) 46:685. doi: 10.2307/3094828
29. Maria J, Liesbeth B, Andre D, Rachelle S, Rachelle S, Philippe L. What is the impact of innovation on output in healthcare with a special focus on treatment innovations in radiotherapy? A literature review. Br J Radiol. (2017) 90:20170251. doi: 10.1259/bjr.20170251
30. Fischer-Valuck BW, Henke L, Green O, Kashani R, Acharya S, Bradley JD, et al. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Adv Radiat Oncol. (2017) 2:485–93. doi: 10.1016/j.adro.2017.05.006
31. Keall P, Poulsen P, Booth JT. See think, and act: real-time adaptive radiotherapy. Semin Radiat Oncol. (2019) 29:228–35. doi: 10.1016/j.semradonc.2019.02.005
32. Soete G, Verellen D, Storme G. Image guided radiotherapy for prostate cancer. Bull Cancer. (2008) 95:374–80. doi: 10.1684/bdc.2008.0599
33. Bashir MN. Epidemiology of prostate cancer. Asian Pacific J Cancer Prev. (2015) 16:5137–41. doi: 10.7314/APJCP.2015.16.13.5137
34. Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European Cancer Observatory. Eur J Cancer. (2015) 51:1164–87. doi: 10.1016/j.ejca.2013.09.002
35. Lardas M, Liew M, van den Bergh RC, De Santis M, Bellmunt J, Van den Broeck T, et al. Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur Urol. (2017) 72:869–85. doi: 10.1016/j.eururo.2017.06.035
36. Bertelsen AS, Schytte T, Møller PK, Mahmood F, Riis HL, Gottlieb KL, et al. First clinical experiences with a high field 1.5 T MR linac. Acta Oncol (Madr). (2019) 58:1352–7. doi: 10.1080/0284186X.2019.1627417
38. Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption abandonment, and challenges to the scale-up spread, and sustainability of health and care technologies. J Med Internet Res. (2017) 19:e367. doi: 10.2196/jmir.8775
39. Dutch Institute National Health Care (Zorginstituut Nederland). ZorgCijfers Monitor Regeling ziekenvervoer?: Gebruik en Kosten. (2019). Available online at: https://www.zorginstituutnederland.nl/publicaties/rapport/2019/03/12/zorgcijfers-monitor—regeling-ziekenvervoer-gebruik-en-kosten (accessed March 12, 2019).
40. Tetar SU, Bruynzeel AME, Lagerwaard FJ, Slotman BJ, Bohoudi O, Palacios MA. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imaging Radiat Oncol. (2019) 9:69–76. doi: 10.1016/j.phro.2019.02.002
41. Kalbasi A, Li J, Berman AT, Swisher-McClure S, Smaldone M, Uzzo RG, et al. Dose-escalated irradiation and overall survival in men with nonmetastatic prostate cancer. JAMA Oncol. (2015) 1:897–906. doi: 10.1001/jamaoncol.2015.2316
42. Alongi F, Rigo M, Figlia V, Cuccia F, Giaj-levra N, Nicosia L, et al. 1.5T MR-guided and daily adapted SBRT for prostate cancer: feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat Oncol. (2020) 15:69. doi: 10.1186/s13014-020-01510-w
43. Nicosia L, Mazzola R, Rigo M, Figlia V, Giaj-Levra N, Napoli G, et al. Moderate versus extreme hypofractionated radiotherapy: a toxicity comparative analysis in low- and favorable intermediate-risk prostate cancer patients. J Cancer Res Clin Oncol. (2019) 145:2547–54. doi: 10.1007/s00432-019-02983-3
44. Singer L, Rosenberg SA. The impact of MRI on radiation oncology graduate medical education. J Am Coll Radiol. (2019) 16:859–63. doi: 10.1016/j.jacr.2018.11.030
45. Botman R, Tetar SU, Palacios MA, Slotman BJ, Lagerwaard FJ, Bruynzeel AME. The clinical introduction of MR-guided radiation therapy from a RTT perspective. Clin Transl Radiat Oncol. (2019) 18:19. doi: 10.1016/j.ctro.2019.04.019
46. De EC, Prognon P, Chatellier G, Sapoval M. New european regulation for medical devices: what is changing?? Cardiovasc Intervent Radiol. (2019) 42:1272–8. doi: 10.1007/s00270-019-02247-0
47. De Muinck KDM, Pathmanathan AU, Andreychenko A, Kerkmeijer LGW, Van Der Voort Van Zyp JRN, Tree AC, et al. Fiducial marker based intra-fraction motion assessment on cine-MR for MR-linac treatment of prostate cancer. Phys Med Biol. (2019) 64:07NT02. doi: 10.1088/1361-6560/ab09a6
48. Engler J, Güthlin C, Dahlhaus A, Kojima E, Müller-Nordhorn J, Weißbach L, et al. Physician cooperation in outpatient cancer care. an amplified secondary analysis of qualitative interview data. Eur J Cancer Care (Engl). (2017) 26:1–8. doi: 10.1111/ecc.12675
49. Strebel RT, Sulser T, Schmid HP, Gillessen S, Fehr M, Huber U, et al. Multidisciplinary care in patients with prostate cancer: room for improvement. Support Care Cancer. (2013) 21:2327–33. doi: 10.1007/s00520-013-1791-x
50. Eylert MF, Persad R. Management of prostate cancer. Br J Hosp Med (Lond). (2012) 73:95–9. doi: 10.12968/hmed.2012.73.2.95
51. Fennell ML, Das IP, Clauser S, Petrelli N, Salner A. The organization of multidisciplinary care teams: modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr. (2010) 10:72–80. doi: 10.1093/jncimonographs/lgq010
52. Turner S, D’Lima D, Hudson E, Morris S, Sheringham J, Swart N, et al. Evidence use in decision-making on introducing innovations: a systematic scoping review with stakeholder feedback. Implement Sci. (2017) 12:1–12. doi: 10.1186/s13012-017-0669-6
53. Van Loon J, Grutters J, Macbeth F. Evaluation of novel radiotherapy technologies: what evidence is needed to assess their clinical and cost effectiveness, and how should we get it? Lancet Oncol. (2012) 13:169–77. doi: 10.1016/S1470-2045(11)70379-5
Keywords: cancer care, healthcare management, image-guided radiotherapy, implementation, MRI-guided radiotherapy, MR-Linac, prostate cancer, qualitative research
Citation: Hehakaya C, Van der Voort van Zyp JR, Lagendijk JJW, Grobbee DE, Verkooijen HM and Moors EHM (2020) Problems and Promises of Introducing the Magnetic Resonance Imaging Linear Accelerator Into Routine Care: The Case of Prostate Cancer. Front. Oncol. 10:1741. doi: 10.3389/fonc.2020.01741
Received: 17 April 2020; Accepted: 04 August 2020;
Published: 02 September 2020.
Edited by:
Christopher Schultz, Medical College of Wisconsin, United StatesReviewed by:
Seong Ki Mun, Virginia Tech, United StatesPeter B. Schiff, New York University, United States
Copyright © 2020 Hehakaya, Van der Voort van Zyp, Lagendijk, Grobbee, Verkooijen and Moors. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charisma Hehakaya, Yy5oZWhha2F5YUB1bWN1dHJlY2h0Lm5s
 Charisma Hehakaya
Charisma Hehakaya Jochem R. Van der Voort van Zyp
Jochem R. Van der Voort van Zyp Jan J. W. Lagendijk1
Jan J. W. Lagendijk1 Helena M. Verkooijen
Helena M. Verkooijen