- 1Department of Thoracic Surgery, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, China
- 2Medical Department, Nanjing Geneseeq Technology Inc., Nanjing, China
- 3Translational Medicine Research Institute, Geneseeq Technology Inc., Toronto, ON, Canada
Background: Kinase domain duplication of EGFR (EGFR-KDD) is a rare oncogenic driver alteration and serves as a potential therapeutic target. Its effect on EGFR–tyrosine kinase inhibitors (TKIs), especially the third-generation drug Osimertinib, and immune checkpoint inhibitors (ICIs) remains inconclusive.
Case Presentation: A 45-year old male with lung adenocarcinoma progressed with liver metastasis after receiving pemetrexed and cisplatin as adjuvant chemotherapy. Targeted next-generation sequencing (NGS) identified an EGFR-KDD in the resected left upper lung. Icotinib was used in the following treatment and the liver metastasis was found to shrink but the progression-free survival (PFS) only lasted for 4 months with the appearance of right hepatic metastasis. Meantime, the same EGFR-KDD was identified in the left hepatic re-biopsy. Afterward, the patient benefited from the third-line therapy of Osimertinib with a PFS as long as 21 months. Then he progressed with enlarged mediastinal lymph nodes, and targeted NGS consistently identified EGFR-KDD, as well as a new RELN p.G1774E mutation. Given the continually increasing tumor mutation burden (TMB, 3.4 mutation/Mb) and PD-L1 expression-based tumor proportion score (TPS, 1%), Nivolumab was used as the fourth-line salvage therapy, which lead to considerable efficacy, with decreased blood carcinoembryonic antigen (CEA), regressed mediastinal lymph nodes, and reduced liver metastases.
Conclusions: Our case provided direct evidence to support the role of Osimertinib in the treatment of EGFR-KDD, as well as added valuable insights into application of immune-based therapeutics in the specific subgroups bearing EGFR alteration(s).
Background
The discovery of oncogenic aberrations in epidermal growth factor receptor (EGFR), which commonly occur as 19 exon deletion or L858R mutation, boosts the treatment of targeted therapy in non-small cell lung cancer (NSCLC). As a rare EGFR alteration, kinase domain duplication (KDD), firstly identified as a driver aberration and therapeutic target in 2015, is an in-frame duplication in exons that encode the EGFR tyrosine kinase domain (1). The current reported prevalence of EGFR-KDD in NSCLCs is 0.04% (2) in European and American and 0.07% (3)~0.12% (4) in East Asian patients, respectively. When with this rare aberration, the response to EGFR-tyrosine kinase inhibitor (TKI) and immune checkpoint inhibitors (ICIs) remains inconclusive. Here we described a case with advanced lung adenocarcinoma harboring EGFR-KDD who achieved differentiate response to first and third generation EGFR-TKIs as well as programmed death receptor-1 (PD-1) inhibitor Nivolumab.
Case Presentation
A 45-year-old male underwent a left upper lobectomy and postoperative pathology revealed invasive stage IIIA lung adenocarcinoma (Figure 1). Targeted next- generation sequencing (NGS) with a customized panel (Geneseeq Prime panel) designed to target 425 cancer-specific genes was performed, and four somatic mutations and copy number alterations (CNAs) were identified, including EGFR-KDD of exon 18-25 [mutant allele frequency (MAF): 13.5%], EGFR amplification (4.5-fold), TP53 p.Y220C (MAF: 37.0%), and RB1 single copy loss (Figure 1). The tumor mutation burden (TMB) was estimated to be 1.1 mutation/Mb. The patient received pemetrexed and cisplatin as adjuvant chemotherapy. Four months later, he progressed with liver metastasis in left lobe (Figure 1).
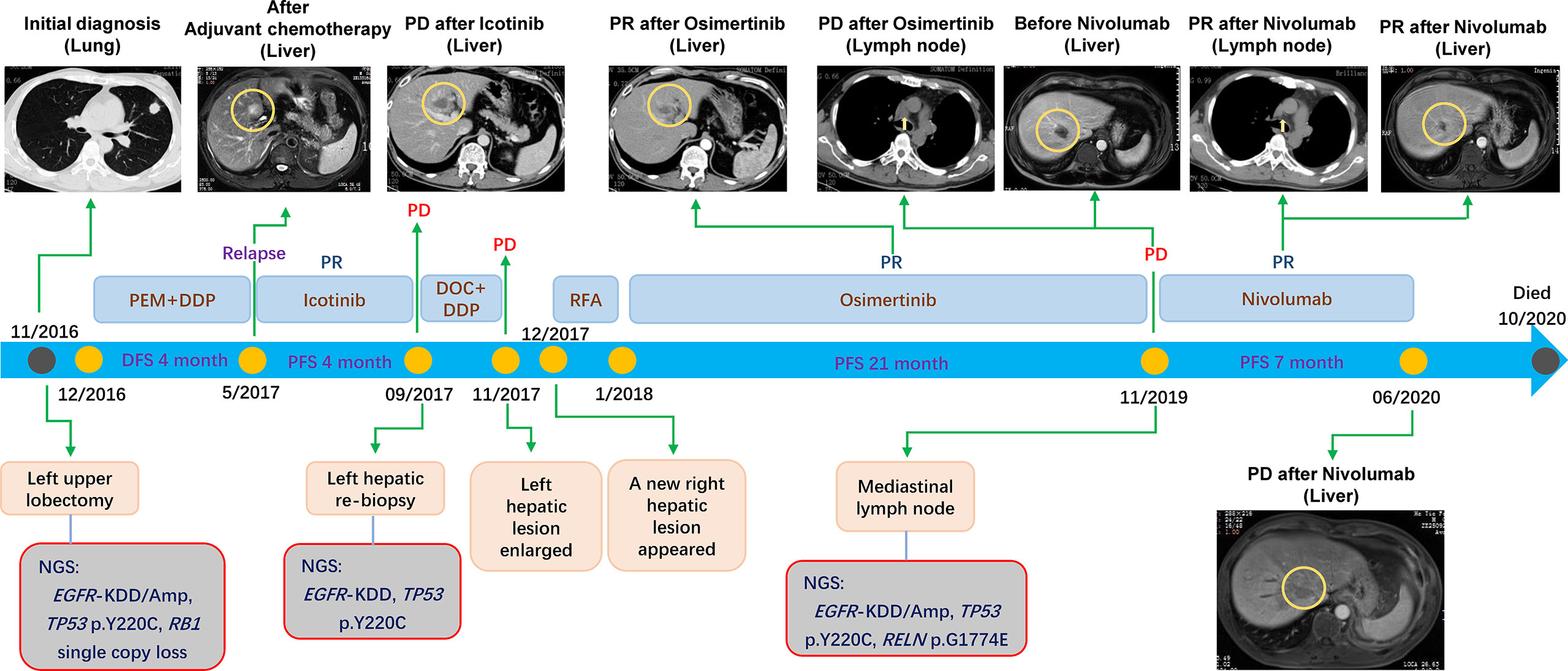
Figure 1 The timeline showing the history of treatment and examinations for the patient under current study.
Then, the patient was treated with Icotinib and the metastasis shrunk. Unfortunately, the drug resistance was observed only after 4 months, as evidenced by the fact that previously responsive liver lesion progressed. Left hepatic re-biopsy confirmed metastatic adenocarcinoma and target sequencing (Geneseeq Prime panel) detected the same EGFR-KDD (MAF: 4.9%) as well as mutation of TP53 p.Y220C (MAF: 0.5%) (Figure 1). The TMB was calculated as 2.2 mutation/Mb.
Docetaxel and cisplatin were initiated as the second-line therapy. However, the left hepatic metastasis enlarged rapidly after 2 cycles of chemotherapy. The blood carcinoembryonic antigen (CEA) level increased from 9.5 mg/ml (before chemotherapy) to 22.7 mg/ml. Even worse, a right hepatic metastasis appeared soon afterward. Radiofrequency ablation (RFA) of liver was conducted on both of the left and right hepatic metastases, but no reduction in liver lesions was observed, and the CEA level showed a slight increase from 7.3 to 10.3 mg/ml.
Afterward, the patient started taking Osimertinib (80 mg once daily). Encouragingly, both liver lesions showed significant regression (Figure 1). One month after initiation of Osimertinib, the CEA level decreased to 5.4 mg/ml, and remained at normal level for 18 months. Moreover, the progression-free survival (PFS) reached 21 months. However, the CEA level increased to 23.1 mg/ml at the 19th month after the initiation of Osimertinib treatment, and 2 months later, the patient progressed with enlarged mediastinal lymph nodes (Figure 1) with the CEA level of 73.9 mg/ml. Resampling and targeted sequencing (Geneseeq Prime panel) consistently identified EGFR-KDD (MAF: 33.9%), as well as EGFR amplification (6.6-fold), TP53 p.Y220C (MAF: 53.3%), and a new mutation of RELN p.G1774E (MAF: 45.4%) (Figure 1). The estimated TMB increased to 3.4 mutation/Mb. In addition, the assessment of PD-L1 expression using antibody 28-8 (pharm Dx, Dako’s Platform) showed tumor proportion score (TPS) of 1%.
On these bases, the fourth-line salvage therapy using Nivolumab was prescribed and the therapeutic efficacy was considerable, as evidenced by the decreased CEA, regressed mediastinal lymph nodes, reduced metastases in both left and right liver (Figure 1). Specifically, the CEA level decreased from 143.6 to 41.8 mg/ml one month later. The PFS reached 7 months and no obvious adverse effects were observed. The quality of life was in good status during the Nivolumab treatment. After that, the patient progressed with enlarged liver metastasis. Unfortunately, the patient was also infected with tuberculosis, and his condition took a sharp turn for the worse due to both tumor progression and tuberculosis. The families gave up further treatment and the patient died 4 months later.
Discussion
Classical EGFR alterations confer continual activation of protein kinase function and sensitivity to EGFR TKI (5). As a rare oncogenic variant, EGFR-KDD is able to form asymmetric homo-dimer and thus activate EGFR signaling pathway (1). Several pilot studies confirmed the effectiveness of EGFR-TKIs in NSCLCs harboring EGFR-KDD (1, 3, 4, 6–9) (Table 1). In our case, the patient bearing EGFR-KDD was sensitive to Icotinib and Osimertinib with PFS of 4 and 21 months, respectively. According to previous reports, there are greatly varying efficacies across the first-generation TKIs against EGFR-KDD, among which the longest PFS up to 6 years was achieved by Gefitinib (6). In our case, a PFS of only 4 months was observed on Icotinib treatment. In comparison, the third-generation TKI Osimertinib presented an encouraging PFS as long as 21 months. The mechanism underlying such difference in the clinical outcomes is worth investigation. Most recently, our group conducted a molecular dynamics simulation-guided study of EGFR-KDD effect on different TKIs (10). It was shown that Gefitinib, as the first-generation EGFR-TKI, suffered from more disturbances in the EGFR-KDD binding event than the third-generation EGFR-TKI, Osimertinib. Moreover, Osimertinib was found with higher binding affinity toward EGFR-KDD than Gefitinib. These results provide the structural basis of evidence that Osimertinib, compared to the first generation TKI, is able to bind and thus inhibit EGFR-KDD with more potency.
ICIs serve as a new standard of care for advanced NSCLCs with no EGFR mutation. However, the study concerning the therapeutic effect of ICIs on EGFR mutant lung cancer is sparse and the outcome seems not optimistic. Previous evidence showed that compared with chemotherapy, there was no superiority in terms of overall survival (OS) when ICIs were used as the second line treatment among EGFR-mutant subgroup (11). The Atlantic trial demonstrated the overall response rate (ORR) of ICIs was 9.8% with averaged PFS of only 1.9 months among EGFR+/ALK+ individuals (12). Cho et al. also suggested EGFR mutant NSCLC patients benefited less from ICIs treatment (13). Similar results were found in Italian Nivolumab expanded access program, which showed ORR of 8.8% among EGFR mutant subgroup (14). Consistently, a retrospective study by Hastings et al., concluded with an ORR of 9.9% for ICIs treatment in EGFR-mutant NSCLCs (15). Despite these, it is worth to mention that adding atezolizumab to standard-of-care Bevacizumab and chemotherapy increased PFS and OS benefit among the EGFR-mutant patients (16).
Of note, EGFR aberrations were found to be corelated with significantly increased rate of tumor growth after ICIs monotherapy (17). Pilot study suggested that EGFR pathway activation resulted in a signature of immuno-suppression, driving immune escape (18). Furthermore, certain EGFR aberrations, including EGFR 19Del and T790M, are considered to be related to ICIs-induced hyper-progressive disease (HPD). Recently, our group reported a patient with EGFR 20 exon insertion and MYC amplification who suffered from HPD after treatment of Nivolumab, resulting in rapid death in 2 months (19). Ex vivo study exhibited that PDX model carrying EGFR 21 exon L858R mutation also mirrored the clinical observation of HPD following Nivolumab treatment (20). Here, our patient significantly benefited from ICIs treatment in the presence of EGFR-KDD. Emerging evidence showed EGFR 20 exon insertion mutation tended to present higher PD-L1 expression than classic EGFR mutation, and in turn, was related with improved outcome in response to ICIs (21). Case series showed patients harboring EGFR G719X mutations along with high PD-L1 expression conferred sensitivity to ICIs-based treatment (22). The aforementioned Hastings’ study (15) further investigated the efficacy differences between various EGFR subtypes. Therapeutic efficacy was best for EGFR G719 but worst for EGFR L861Q. For common mutant subgroups, EGFR 19Del showed worse response than EGFR L858R. On contrary, negative association between EGFR alteration and HPD was observed from two independent cohorts (23, 24). These data suggest the responsiveness to ICIs in patients with EGFR aberrations may differ in terms of specific aberrant type. To overcome the low response rates to PD-1 pathway blockade, highly specific patient(s) with EGFR-driven tumor should be screened out for ICIs monotherapy and combinations.
There are several limitations in the present study. Owing to the coverage of currently used sequencing panel, it was not available to explore the molecular basis of mechanism underlying the drug resistance observed in the clinic, e.g., Icotinib and Osimertinib. According to previous studies, there exist varying conclusions as to the efficacies of the first-generation EGFR-TKIs in the treatment of EGFR-KDD, as well as the uncertain response to ICIs among EGFR mutant tumors. In this context, our current case report only provided an example but not guidance for the clinical intervention, which clearly demands more extensive investigations.
Collectively, our case provides direct evidence to support the role of Osimertinib in the treatment of EGFR-KDD, as well as added valuable insights into application of immune-based therapeutics in the specific subgroups bearing EGFR alteration(s).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Hwa Mei Hospital, University of Chinese Academy of Sciences. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
GZ designed the entire study. JL carried out patient clinical management and sample collection. JY, RC, and GD analyzed the data. JL and JY wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grant from Hwa Mei Research Foundation of 2016 (2016HMKY05).
Conflict of Interest
JY, RC, and GD were employed by the company Geneseeq Technology, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.575739/full#supplementary-material.
References
1. Gallant JN, Sheehan JH, Shaver TM, Bailey M, Lipson D, Chandramohan R, et al. EGFR Kinase Domain Duplication (EGFR-KDD) Is a Novel Oncogenic Driver in Lung Cancer That Is Clinically Responsive to Afatinib. Cancer Discov (2015) 5(11):1155–63. doi: 10.1158/2159-8290.CD-15-0654
2. Konduri K, Gallant JN, Chae YK, Giles FJ, Gitlitz BJ, Gowen K, et al. EGFR Fusions as Novel Therapeutic Targets in Lung Cancer. Cancer Discov (2016) 6(6):601–11. doi: 10.1158/2159-8290.CD-16-0075
3. Wang W, Xu C, Zhu Y, Fang M, Zhuang W, Chen Y, et al. An EGFR KDD Data in the Chinese NSCLC Population and the Response to EGFR-TKIs: A Multicenter Study. J Thoracic Oncol (2018) 13(12):S1081–1. doi: 10.1016/j.jtho.2018.10.102
4. Wang J, Li X, Xue X, Ou Q, Wu X, Liang Y, et al. Clinical outcomes of EGFR kinase domain duplication to targeted therapies in NSCLC. Int J Cancer (2019) 144(11):2677–82. doi: 10.1002/ijc.31895
5. Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, Ross S, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res (2006) 66(16):8163–71. doi: 10.1158/0008-5472.CAN-06-0453
6. Baik CS, Wu D, Smith C, Martins RG, Pritchard CC. Durable Response to Tyrosine Kinase Inhibitor Therapy in a Lung Cancer Patient Harboring Epidermal Growth Factor Receptor Tandem Kinase Domain Duplication. J Thorac Oncol (2015) 10(10):e97–9. doi: 10.1097/JTO.0000000000000586
7. Wiest G, Kohlhäufel M, Müller J, Lakis S, Wesseler C, Mariotti E, et al. Detection of an EGFR kinase domain duplication in a lung adenocarcinoma patient by liquid biopsy using hybrid capture based next generation sequencing. New Oncol (2016) Abstract #291. Available at:https://www.newoncology.com/fileadmin/content/Download_pdfs/Wiest_et_al_DGHO-2016-Abstract_final.pdf.
8. Zhu YC, Wang WX, Xu CW, Tan QH, Li JY, Zhuang W, et al. Lung adenocarcinoma patient with an EGFR kinase domain duplication (KDD) and the response to Icotinib. J Thorac Dis (2018) 10(5):E359–63. doi: 10.21037/jtd.2018.04.162
9. Xu C, Wang W, Zhang Q, Wu B, Zhuang W, Zhu Y, et al. A Novel Oncogenic Driver in a Lung Adenocarcinoma Patient Harboring an EGFR-KDD and Response to Afatinib. J Thoracic Oncol (2018) 13(10):S1032–3. doi: 10.1016/j.jtho.2018.08.1991
10. Jin R, Li J, Jin Z, Lu Y, Shao YW, Li W, et al. Osimertinib confers potent binding affinity to EGFR Kinase Domain Duplication. Int J Cancer (2019) 145(10):2884–5. doi: 10.1002/ijc.32617
11. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
12. Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol (2018) 19(4):521–36. doi: 10.1016/S1470-2045(18)30144-X
13. Cho JH, Jung HA, Lee SH, Ahn JS, Ahn MJ, Park K, et al. Impact of EGFR mutation on the clinical efficacy of PD-1 inhibitors in patients with pulmonary adenocarcinoma. J Cancer Res Clin (2019) 145(5):1341–9. doi: 10.1007/s00432-019-02889-0
14. Garassino MC, Gelibter AJ, Grossi F, Chiari R, Soto Parra H, Cascinu S, et al. Italian Nivolumab Expanded Access Program in Nonsquamous Non-Small Cell Lung Cancer Patients: Results in Never-Smokers and EGFR-Mutant Patients. J Thorac Oncol (2018) 13(8):1146–55. doi: 10.1016/j.jtho.2018.04.025
15. Hastings K, Yu H, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small cell lung cancer. Ann Oncol (2019) 30(8):1311–20. doi: 10.1093/annonc/mdz141
16. Mok TSK, Socinski MA, Reck M, Jotte RM, Lim DWT, Cappuzzo F, et al. IMpower150: An exploratory analysis of efficacy outcomes in patients with EGFR mutations. Ann Oncol (2018) 29(suppl_9):ix173–8. doi: 10.1093/annonc/mdy483.008
17. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res (2017) 23(15):4242–50. doi: 10.1158/1078-0432.CCR-16-3133
18. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov (2013) 3(12):1355–63. doi: 10.1158/1535-7163.TARG-13-B290
19. Huang X, Xia L, Lan F, Shao YW, Li W, Xia Y. Treatment of Nivolumab Results in Hyperprogressive Disease in a Patient Harboring EGFR Exon 20 Insertion and MYC Amplification. J Thorac Oncol (2019) 14(9):e189–91. doi: 10.1016/j.jtho.2019.04.009
20. Lo Russo G, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, et al. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin Cancer Res (2019) 25(3):989–99. doi: 10.1158/1078-0432.CCR-18-1390
21. Cardona AF, Rojas L, Zatarain-Barron ZL, Freitas HC, Granados ST, Castillo O, et al. CLICaP, EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2-CLICaP). Lung Cancer (2018) 125:265–72. doi: 10.1016/j.lungcan.2018.10.007
22. Taniguchi Y, Tamiya A, Ishii S, Atagi S. Effect of pembrolizumab on patients harboring uncommon epidermal growth factor receptor mutations. Ann Oncol (2018) 29(5):1331–3. doi: 10.1093/annonc/mdy087
23. Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol (2019) 30(7):1104–13. doi: 10.1093/annonc/mdz123
24. Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol (2018) 4(11):1543–52. doi: 10.1001/jamaoncol.2018.3676
Keywords: lung adenocarcinoma, targeted next-generation sequencing, EGFR-KDD, Osimertinib, Nivolumab
Citation: Li J, Yan J, Cao R, Du G and Zhao G (2020) Lung Adenocarcinoma Harboring EGFR Kinase Domain Duplication (EGFR-KDD) Confers Sensitivity to Osimertinib and Nivolumab: A Case Report. Front. Oncol. 10:575739. doi: 10.3389/fonc.2020.575739
Received: 08 July 2020; Accepted: 12 November 2020;
Published: 17 December 2020.
Edited by:
Yaxiong Zhang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Nicolas Marcoux, Centre Hospitalier Universitaire de Québec, CanadaJanaki Deepak, University of Maryland, Baltimore, United States
Copyright © 2020 Li, Yan, Cao, Du and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guofang Zhao, Z3VvZnpoYW9AaG90bWFpbC5jb20=
 Jie Li1
Jie Li1 Junrong Yan
Junrong Yan Ran Cao
Ran Cao