- 1Department of Ophthalmology, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Ophthalmology, ShangjinNanfu Hospital, Chengdu, China
Choroidal melanomas are the most common ocular malignant tumors worldwide. The onset of such tumors is insidious, such that affected patients often have no pain or obvious discomfort during early stages. Notably, enucleation is required for patients with a severe choroidal melanoma, which can seriously impact their quality of life. Moreover, choroidal melanomas metastasize early, often to the liver; this eventually causes affected patients to die of liver failure. Therefore, early diagnosis of choroidal melanomas is extremely important. Unfortunately, an early choroidal melanoma is easily confused with a choroidal nevus, which is the most common benign tumor of the eye and does not often require surgical treatment. This review discusses recent advances in the use of multimodal and molecular imaging to identify choroidal melanomas and choroidal nevi, detect early metastasis, and diagnose patients with choroidal melanomas.
Introduction
Choroidal melanomas are the most common intraocular malignant tumors worldwide, as well as the second most common type of malignant melanoma. However, the current consensus is that choroidal melanomas and cutaneous melanomas are different types of tumors (1). Thus, their causes, pathogenesis, diagnosis, treatment, and prognosis are quite different (2). Choroidal melanomas can originate from choroidal nevi, which are the most common benign ocular tumors and typically do not require surgical treatment. Notably, 6–10% of patients with a choroidal melanoma have a second primary tumor (3). Furthermore, patients with an advanced unilateral retinoblastoma may have isolated choroidal melanocytosis in the contralateral eye, which can progress to a choroidal melanoma (4, 5).
Choroidal melanomas have an insidious onset and often do not result in pain or obvious discomfort, until they cause inflammation, neovascular glaucoma, or ocular extension (3). Nevertheless, patients with a severe choroidal melanoma require enucleation, which can seriously impact their quality of life. In the past decade, radiotherapy has gradually become the first-line treatment for such patients (6); this treatment modality includes proton beam and plaque brachytherapy (7). However, compared with enucleation, the 5-year survival rate of radiotherapy has not significantly improved (8).
The prognosis of a choroidal melanoma is closely linked with its cytogenetic type and histological grade, which is a distinguishing feature from other cancers (9). The haplotype of chromosome 3 and amplification of chromosome 8 are significantly associated with tumor metastasis, consistent with the American Joint Committee on Cancer (AJCC) staging of the tumor. In addition, a combination of AJCC staging and cytogenetic status can provide greater accuracy than separate assessment methods in predicting patient prognosis (10). At the genetic level, mutations in BAP1, EIF1AX, SF3B1 and other genes have been shown to affect patient prognosis; specifically, patients with EIF1AX mutations have relatively more positive prognoses, while patients with SF3B1 mutations are more prone to have advanced metastasis. Furthermore, absence of the tumor suppressor gene BAP1 will lead to tumor metastasis, thus significantly reducing the survival rate among affected patients (11–13). Choroidal melanomas have been classified into two types on the basis of the gene expression profile: I (low-risk) and II (high-risk) (14). For patients with type II uveal melanoma, the prognosis is not influenced by interventions, but has a strong relationship with the largest basal diameter (LBD); LBD < 12 mm is associated with better prognosis (14, 15). Many prognostic prediction models have been developed, including the Liverpool Uveal Melanoma Prognosticator Online; this model combines tumor histology (e.g., LBD), genetic status (e.g., chromosome 3 monosomy), and other factors to predict all-cause mortality and support personalized treatment (16–18).
Metastasis reportedly affects 50% of patients with uveal melanomas (1). The most common route of choroidal melanoma metastasis is through blood to the liver (19); this event occurs early in the progression of disease. Some results have implied that early metastasis occurs 5 years before the diagnosis and treatment of a choroidal melanoma (3). Other studies have suggested that metastasis is inevitable for patients with choroidal melanomas (20); there remains no optimal scheme or evidence for the treatment of metastatic uveal melanomas (1).
Because of the insidious onset and poor prognosis of choroidal melanomas, early diagnosis of affected patients is extremely important (14). The gold standard for tumor diagnosis is a biopsy and subsequent pathological examination. However, fine-needle aspiration biopsy of the vitreous body in an eye with a choroidal melanoma can lead to seeding of the ciliary body and sclera (21). Therefore, fine needle aspiration biopsy is not an ideal diagnostic method for patients with a suspected choroidal melanoma. Furthermore, early non-invasive diagnosis is important for timely detection of choroidal melanomas and prediction of patient prognosis.
This review focuses on the important roles of multimodal and molecular imaging (e.g., positron emission tomography/computed tomography [PET/CT]) in the identification and monitoring of choroidal nevi, early detection of choroidal melanomas, and recognition of metastasis.
Identification and Monitoring of Choroid Nevus
According to the Collaborative Ocular Melanoma Study classification, a choroidal nevus constitutes a choroidal melanocytic lesion with LBD ≤ 5 mm and thickness ≤ 1 mm (22). Most instances of choroidal nevus (91%) occur in the posterior portion of the eye (22). Histologically, a nevus is composed of benign cells (i.e., atypical melanocytes). A choroidal nevus is not a congenital condition and most often is acquired (23). Some studies indicate that a high estrogen level and high body mass index are important risk factors for choroidal nevi (24). Furthermore, Singh et al. suggested that systematic resistance to estrogen leads to obesity and increased body mass index; they presumed that this mechanism contributes to choroidal nevus onset. Studies of postmenopausal women also revealed that the incidence of choroidal nevi was twofold greater in overweight women than in normal-weight women. Vitamin C has been reported to reduce the incidence of choroidal nevi (22).
However, when the mass thickness is > 2 mm, as measured by ultrasound, the hazard ratio significantly increases for transformation from a choroidal nevus to a choroidal melanoma (25). Additional risk factors include worse Snellen visual acuity, empty echo findings on ultrasound, LBD > 5 mm on fundus examination, subretinal fluid on optical coherence tomography (OCT) examination, and orange pigment deposition (i.e., lipochrome) on autofluorescence examination (26–29). Traditional angiography methods (e.g., fluorescein angiography) are important for differentiating a choroidal nevus from a choroidal melanoma on the basis of subretinal vessel morphology. Current OCT technology is suitable for observation of vascular morphology information. When a choroidal nevus exhibits long-term exudative subretinal fluid and multiple punctures, OCT angiography (OCTA) shows choroidal neovascularization, consistent with fluorescein angiography findings (24, 30). Swept-source OCT can show narrow border vessels significantly related to the subretinal fluid (31); enhanced depth imaging (EDI)-OCT has demonstrated thin blood vessels covering 94% of choroidal nevi (32). Francis et al. (31) proposed that the tumor diameter is closely related to secondary retinopathy, while OCT can distinguish a choroidal nevus by identifying fine retinal structure. OCTA can reveal that the Bruch’s membrane–retinal pigment epithelium–Bruch’s membrane complex is complete and regular in a choroidal nevus, while the Bruch’s membrane–retinal pigment epithelium–Bruch’s membrane complex and the shape of the outer retinal layer are fuzzy in a choroidal melanoma (33). Notably, EDI-OCT has shown that photoreceptor cells exposed to subretinal fluid in a choroidal nevus exhibit atrophy (i.e., “stalactites” or “cracks”), while photoreceptor cells in a small melanoma became loose and exhibit “furry” morphology, with more irregular retinal layers accompanied by considerable structural damage (22, 26, 32). OCTA can distinguish the macular characteristics of choroidal nevi and choroidal melanomas; Valverde et al. suggested that the mass thickness is closely related to the difference of macular characteristics. The superimposed macular microvascular changes are smaller in choroidal nevi (34); the central macular thickness, fovea avascular area, and choroidal capillary thickness are similar to those parameters in healthy fellow eyes. In choroidal melanomas, central macular thickness increases, fovea avascular area increases, and choroidal capillary thickness decreases; moreover, the blood flow rate is considerably lower than normal (11.2%) (33).
Diagnosis of Choroid Melanoma
For large choroidal melanomas, the diagnosis is mainly performed by slit lamp examination, indirect ophthalmoscopy, fluorescein angiography, and ultrasound (35). For early small choroidal melanomas, there is controversy regarding the most suitable diagnostic approach. Kivela et al. reported that biopsy should be performed to distinguish this type of tumor from a choroidal nevus (35), whereas Singh et al. stated that fine needle aspiration cytology should not be used due to the risk of tumor spread (3). Therefore, imaging is particularly important for the diagnosis of choroidal melanomas. Currently available imaging methods for choroidal melanomas include ultrasound (A-mode, B-mode, and color Doppler), magnetic resonance imaging (MRI), and OCT (i.e., swept-source OCT, spectral domain OCT, EDI-OCT, and OCTA). Each approach has unique advantages and disadvantages in various situations.
Since choroidal melanomas usually appear as low reflectivity, A-mode ultrasound can provide the best imaging effect (26). In A-mode ultrasound, choroidal melanomas exhibit low echo, smooth attenuation, and vascular pulsation (3). In B-mode ultrasound, choroidal melanomas can appear to be bulging. If the tumor penetrates Bruch’s membrane, it will assume a collar button or mushroom-like appearance; such tumors have no echo and contain a cavity in the posterior wall. These tumors also contain a choroid gap and orbital shadow (3, 36). Real-time high-resolution ultrasound (i.e., fusion ultrasound) can demonstrate choroidal melanoma and optic nerve structures in real time. Additionally, MRI combined with real-time color Doppler ultrasound can observe the tumor structure and evaluate the retrobulbar vascular system (37). Ultrasound is considered the most important method for evaluation of choroidal melanoma progression; ultrasound is superior to MRI in the detection of extrascleral extension (38, 39). When a choroidal melanoma involves the optic nerve, the fundus may have a similar manifestation of optic neuritis; MRI may show the tumor as a mass adjacent to the optic nerve. However, ultrasound evaluation can show the absence of an echo and reveal intraocular components, thus aiding in diagnosis of the mass (40). Nevertheless, for small choroidal melanomas, the specificity of ultrasound involving subretinal fluid is low, because such tumors cannot be distinguished from retinal thickening, cystic changes, and pigment epithelial detachment (41). EDI-OCT can partially compensate for this deficiency (36, 42).
MRI has limited diagnostic value for choroidal melanomas (3). For large tumors or those with poorly reflective structures in ultrasound examination, MRI can be used to detect whether the mass has penetrated through the sclera (36). However, the presence of nonspecific inflammation, angiogenesis, and motion artifacts can lead to false positive results (38).
OCT can reveal extensive details of choroidal melanomas. In particular, this method can show whether the choroidal melanoma surface is regular and lobular (43), whether it exhibits normal retinal thickness and a complete photoreceptor cell layer, and whether extensive retinal detachment and “debris” are present in the dorsal retina (19). Swept-source OCT can show fine details of the choroid near the fovea and posterior pole (44). Furthermore spectral domain OCT can help to observe lesions in the pigment epithelium (45). The combined use of OCT and fundus fluorescein angiography can help to identify subretinal fluid and orange pigment (35). Previous studies have shown that 60% of the subretinal fluid in a choroidal melanoma is patchy, while 40% is diffuse (46). EDI-OCT is an effective tool to identify structural changes in the retina in patients with small choroidal melanomas (47). This method can show retinal edema, “fluffy” or lost photoreceptor cells, an irregular ganglion cell layer, broken connections between internal and external segments, an irregular inner plexus layer, the loss of external membrane, and other structural damage (26). It is also more accurate for measurement of tumor thickness, compared with ultrasound. Specifically, the tumor thickness is 55% greater when measured by ultrasound than when measured by EDI-OCT, which reveals the most actual thickness. Additionally, EDI-OCT can identify a subclinical level of peripheral subretinal fluid that is not yet visible via ophthalmoscopy (36). Therefore, EDI-OCT is important in the early diagnosis and evaluation of choroidal melanomas.
In addition to the retinal structure, advancements in OCT technology have improved the visibility of the tumor vasculature. Large choroidal melanomas have a double cycle; extensive and progressive fluorescence are evident under fundus fluorescein angiography (3). Furthermore, swept-source OCT can clearly display the intrinsic tumor vasculature, with an effect similar to that of indocyanine green angiography (48). With the exception of hemangiomas, most choroidal tumors show internal compression of the vasculature (45). Therefore, OCTA shows that choroidal melanomas have a dense and irregular vascular network in the outer retinal layer and choroidal layer, while choroidal nevi show reduced blood flow in the corresponding area (49).
PET/CT to Predict Recurrence or Metastasis of Choroidal Melanoma
As mentioned above, early melanocyte aggressiveness is closely related to tumor prognosis. Therefore, evaluation of the risks of choroidal melanoma recurrence and metastasis on the basis of early cytological behavior can provide timely prognostic information; this can be combined with histological evaluation of the tumor to provide more guidance for medical decision-making. PET/CT is appropriate for this application; it can aid in early intervention before morphological recurrence and tumor metastasis, thus greatly improving patient survival.
PET/CT is typically used to evaluate tumor activity and the risks of recurrence or metastasis by means of cell metabolism assessment. Metabolic activity is negatively correlated with metastasis duration (50). Parameters related to cell metabolism include maximum standardized uptake value (SUVmax) and metabolic rate of glucose. SUVmax is defined as the ratio between the radiation concentration and the injection dose in the region with the highest uptake signals of 18F-fluorodeoxyglucose (18F-FDG, the analog of glucose) and other tracers; this parameter can be used to semi-quantitatively evaluate cellular metabolic rates (51). The metabolic rate of glucose is the product of 18F-FDG clearance rate and blood glucose concentration (i.e., glucose uptake rate per unit of tracer distribution) (52). This parameter can accurately quantify the degree of metabolic activity; it also helps to distinguish among tumor cell types, thereby indirectly evaluating the risks of recurrence and metastasis. Notably, epithelioid cell melanomas have worse prognoses than spindle cell melanomas (53). Similarly, SUVmax and LBD are significantly associated with metastatic death. Higher SUVmax is reportedly indicative of greater tumor diameter or thickness (54). Among patients with choroidal melanoma who underwent 6 months of treatment, choroidal thickness was significantly reduced, compared with baseline (55). Therefore, the magnitude of SUVmax may influence the therapeutic effect. Furthermore, the magnitude of SUVmax is reportedly related to the pathological classification of melanoma. Higher SUVmax often indicated a nodular tumor, while lower SUVmax implied diffuse infiltration (54). Chromosome 3 monosomy and chromosome 8 amplification are strongly correlated with choroidal melanoma metastasis. The metastasis and prognosis of choroidal melanoma are mainly related to the above cytogenetic changes, rather than the AJCC stage. Notably, some studies have suggested that SUVmax is related to chromosome 3 monosomy in choroidal melanomas. Among those tumors, 92% have SUVmax > 2.5, while 67% have SUVmax > 4. Therefore, SUVmax > 4 may be an indirect indicator of chromosome 3 monosomy in choroidal melanomas (56).
PET can qualitatively assess tumor development by monitoring variations in cell metabolism. Surveillance with 18F-FDG PET/CT may exclude metastasis or suggest necrotic choroidal melanoma if cell metabolism is obviously low. Clinically significant changes in the metabolic activity of a lesion may indicate local recurrence (57, 58).
Combined Multimodal and Molecular Imaging to Assess Metastasis of Choroidal Melanoma
Routine Detection of Liver Metastasis
The liver is the most common metastatic site of choroidal melanomas (59), such that approximately half of affected patients have liver metastasis (60). Moreover, liver metastases are often the first non-ocular locations affected (61). The median disease-free survival time of patients with choroidal melanoma is 26 months, while the median survival time is only 8 months after initial metastasis detection (62). Patients often die of liver failure, manifested by ascites and hepatomegaly. Biopsy of metastases revealed a maximum size of 100 cm2 (63). Presumably, the most important factors influencing survival rate are the levels of liver enzymes, especially lactate dehydrogenase and alkaline phosphatase (64). Mariani et al. have found that if LDH is 1.5 times higher than normal, it indicates poor prognosis of the patient (65). If these levels have been normal and the disease-free interval is > 36 months, the overall survival period is expected to be considerably longer (62). Therefore, early treatment of metastasis and management of liver enzyme levels are important considerations for patient survival.
Serial hepatic ultrasound and confirmatory scans (e.g., CT) can reveal asymptomatic metastasis (66). However, Mariani et al. have suggested that USG can only detect lesions on the liver surface, which indicated the limited effect (65). For newly diagnosed uveal melanoma, MRI staging can accurately recognize early liver metastasis (61, 67). Mariani et al. believe that the number and maximum surface area (>800mm2) of metastasis under MRI can be used as important indicators to predict the survival rate of patients (65). Because of the vascular richness in uveal melanoma, a metastasis on MRI appears as multiple enhanced solid liver lesions with the high T1 signal characteristic of melanomas (68). Diffuse-weighted images (DWI) can be used to detect more metastasis, which appearances as the high signal, and the lesion can still exist when the dispersion sensitivity coefficient is adjusted to the highest, thus more metastasis < 5mm can be detected, regardless of the location of the metastasis in the liver (65). Regarding the frequency of MRI detection, intervals of 3 months (60) and 6 months (69) have been suggested.
In CT scans, liver metastases often constitute multiple, heterogeneous, hypodense, and enhanced lesions, with an average size of 46.8 cm2 (63). The detection efficiency of dual-energy CT with low kVp is superior to that of virtual 120 kVp CT with digital subtraction angiography images (70). Positive CT or MRI findings were compared with positive ultrasound findings; the results showed that 53% of the CT/MRI findings were completely consistent with ultrasound findings, 11% were completely inconsistent, 29% were negative findings on ultrasound, and 7% could not detect the positive findings on ultrasound. It has been suggested that CT scans of the chest, abdomen, and pelvis should be performed before the assessment of the patient’s prognosis (71). Thus, CT/MRI is presumed to constitute a more efficient approach than ultrasound; in particular, confirmatory MRI scanning may be useful in patients with abnormal liver enzyme levels (72).
Usefulness of PET Staging for Choroidal Melanomas
According to the AJCC, choroidal melanomas are divided into the following four stages: T1, LBD < 10 mm and thickness < 2.5 mm; T2, LBD 10–16 mm and thickness 2.5–10 mm; T3, LBD > 16 mm and thickness > 10 mm, without extraocular invasion; T4, LBD > 16 mm and thickness > 10 mm, with extraocular invasion. T1 and T2 stages are each divided into three clinical sub-stages on the basis of the external invasion status: none, microscopically visible, and visible with the unaided eye. Thus, extraocular invasion status (i.e., extraocular extension and metastasis) is important for the staging of choroidal melanomas.
Although PET/CT is not currently used as a routine method for detection of metastasis, it can be used to detect metastasis in patients with normal CT findings and normal liver enzyme levels (73, 74). Thus, this approach is helpful for metastasis screening and tumor staging; it can also be used as a supplementary alternative for patients with contraindications to MRI (75). Most liver metastases can be qualitatively detected through PET/CT, in combination with 18F-FDG to detect local uptake and MRI for confirmatory diagnosis (76, 77). Furthermore, PET/CT is suitable for detecting extrahepatic metastases of T4 stage tumors (78). Second primary cancers in 10% of patients with choroidal melanomas can be detected by PET/CT (77). However, the initial staging value of PET/CT for choroidal melanoma is not superior to MRI (76, 79), because PET/CT can only detect 33% of T2 stage tumors and 75% of T3 stage tumors (80). Small liver lesions cannot be detected by PET/CT (59, 79), while MRI of the abdomen and chest can detect nearly all metastases of uveal melanomas (81). In addition to lung and liver metastases, PET/CT can detect suspected local uptake of 18F-FDG in lymph nodes, which can then be confirmed by ultrasound and fine needle aspiration cytology (58). Therefore, ultrasound combined with PET/CT has been proposed for the initial staging of choroidal melanomas; chest radiography combined with analysis of liver enzyme levels may be appropriate for long-term staging (77).
Because PET/CT can assess cell metabolism and anatomical changes throughout the body, it is applicable for use in all patients with choroidal melanoma who require evaluation and/or restaging of extrahepatic metastases (82). Figure 1 accounts for the current multimodal and molecular imaging techniques assessing the primary tumor and metastasis of choroidal melanomas.
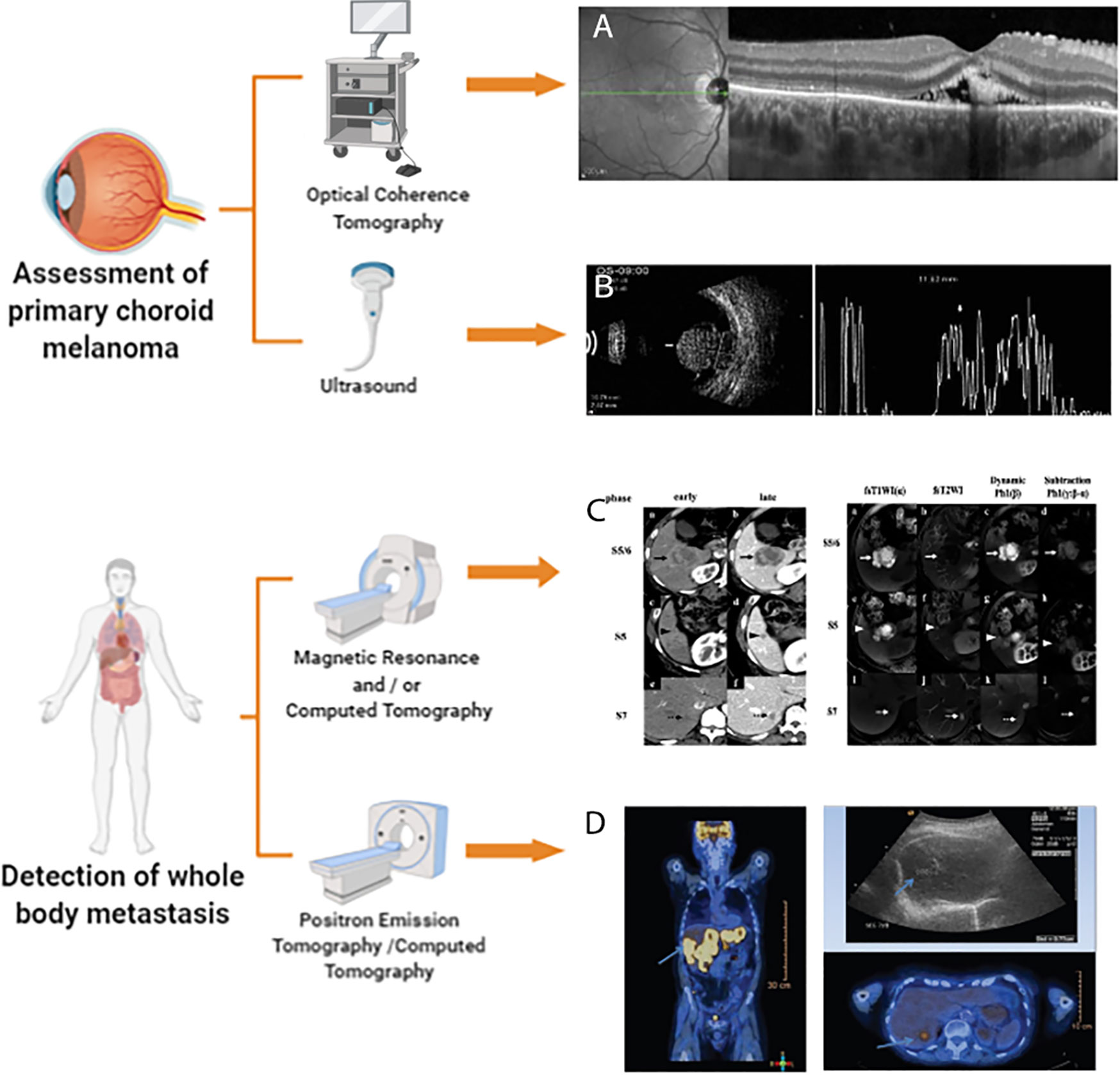
Figure 1 Multimodal and Molecular imaging techniques to assess choroidal melanoma. This illustration is created by Biorender.com (A) enhanced depth imaging (EDI)-OCT image is reprinted with permission from ref (42).Copyright © 2015 Ann Q. Tran et al. (B) Ultrasound image is reprinted with permission from ref (3).Copyright © 2012 Singh P and Singh A (C) magnetic resonance imaging (MRI) and CT image is reprinted with permission from ref (66).Copyright © 2020 The Author(s) (D) PET/CT image is reprinted with permission from ref (75).Copyright © 2018 Middle East African Journal of Ophthalmology.
Discussion
Choroidal melanomas are insidious tumors prone to extraocular extension and metastasis. Therefore, early diagnosis of choroidal melanomas and timely detection of metastases are particularly important. Early choroidal melanomas can easily be confused with choroidal nevi, so accurate identification of choroidal nevi is needed. Pathological examination is considered the gold standard for cancer diagnosis; thus far, invasive examination is not recommended for early choroidal melanomas. Therefore, non-invasive imaging methods are critical for the assessment of patients with suspected choroidal melanoma. Ultrasound is an important method for the diagnosis and monitoring of choroidal melanomas; in combination with other imaging techniques, ultrasound enables real-time observation of tumors and their posterior structures. Compared with ultrasound, OCT methods (especially EDI-OCT) provide more accurate and detailed observations of the morphology and blood supply of choroidal melanomas, offering valuable information for the diagnosis and monitoring of these tumors. MRI and CT are of limited value during diagnosis of the primary tumor, but are indispensable for tumor staging and metastasis detection. PET/CT is a powerful supplement to the above imaging methods for the staging of choroidal melanomas; it can identify metastases that not detected in a timely manner by conventional imaging methods.
Current technology allows the use of PET/CT to assess the risks of tumor recurrence and metastasis at the cytological level, prior to histological changes, by monitoring the metabolic behavior of melanoma cells. This information provides a reference for medical decisions and has the potential to greatly improve the survival and quality of life for affected patients. At the same time, there is an unavoidable challenge that the use of SPECT (such as 123IMP-SPECT) has the higher detection rate than 18FDG-PET/CT (83). Therefore, more effective radioactive tracer should be actively sought for PET/CT imaging of choroidal melanoma, so as to improve the sensitivity of PET/CT to the detection of micro-liver metastasis.
Author Contributions
The co-first authors of this article, XL and LW, were responsible for the conception and design of the manuscript, and conducted data collection and analysis, made valuable and constructive changes to the content of the manuscript, confirmed the final version of this article, and agreed responsible for all matters related to the publication of this manuscript. The corresponding author, XW, who is in charge of the publishing process and established the core idea of this manuscript. Two co-authors, LZ and FT, provided many valuable suggestions and sources and made some crucial amendments to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Natural Science Foundation of China (No.82070954), The Applied Basic Research Programs of Science and Technology Commission Foundation of Sichuan Province (No.19YYJC0790), and The Innovative Spark Grant of Sichuan University (No.2018SCUH0062).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol (2017) 101:38–44. doi: 10.1136/bjophthalmol-2016-309034
2. Chattopadhyay C, Kim DW, Gombos DS, Oba J, Qin Y, Williams MD, et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer (2016) 122:2299–312. doi: 10.1002/cncr.29727
3. Singh P, Singh A. Choroidal melanoma. Oman J Ophthalmol (2012) 5:3–9. doi: 10.4103/0974-620X.94718
4. Augsburger JJ, Brooks CC, Correa ZM. Isolated choroidal melanocytosis: clinical update on 37 cases. Graefes Arch Clin Exp Ophthalmol (2020) 258:2819–29. doi: 10.1007/s00417-020-04919-x
5. Brooks CC, Augsburger JJ, Correa ZM. Unilateral retinoblastoma with contralateral isolated choroidal Melanocytosis: case report of an unexpected presentation. BMC Ophthalmol (2018) 18:251. doi: 10.1186/s12886-018-0916-x
6. Messineo D, Barile D, Morrone S. Meta-analysis on the utility of radiotherapy for the treatment of Ocular Melanoma. Clin Ter (2020) 170:89–98. doi: 10.7417/CT.2020.2195
7. Thornton S, Coupland SE, Heimann H, Hussain R, Groenewald C, Kacperek A, et al. Effects of plaque brachytherapy and proton beam radiotherapy on prognostic testing: a comparison of uveal melanoma genotyped by microsatellite analysis. Br J Ophthalmol (2020) 104:1462–6. doi: 10.1136/bjophthalmol-2019-315363
8. Afshar AR, Damato BE, Stewart JM, Zablotska LB, Roy R, Olshen AB, et al. Next-Generation Sequencing of Uveal Melanoma for Detection of Genetic Alterations Predicting Metastasis. Transl Vis Sci Technol (2019) 8:18. doi: 10.1167/tvst.8.2.18
9. Damato B, Eleuteri A, Taktak AFG, Coupland SE. Estimating prognosis for survival after treatment of choroidal melanoma. Prog Retin Eye Res (2011) 30:285–95. doi: 10.1016/j.preteyeres.2011.05.003
10. Bagger M, Andersen MT, Andersen KK, Heegaard S, Andersen MK, Kiilgaard JF. The Prognostic Effect of American Joint Committee on Cancer Staging and Genetic Status in Patients With Choroidal and Ciliary Body Melanoma. Invest Ophthalmol Vis Sci (2015) 56:438–44. doi: 10.1167/iovs.14-15571
11. Dogrusöz M, Jager MJ. Genetic prognostication in uveal melanoma. Acta Ophthalmol (Copenh) (2018) 96:331–47. doi: 10.1111/aos.13580
12. Yavuzyigitoglu S, Koopmans AE, Verdijk RM, Vaarwater J, Eussen B, van Bodegom A, et al. Uveal Melanomas with SF3B1 Mutations. Ophthalmology (2016) 123:1118–28. doi: 10.1016/j.ophtha.2016.01.023
13. Field MG, Decatur CL, Kurtenbach S, Gezgin G, van der Velden PA, Jager MJ, et al. PRAME as an Independent Biomarker for Metastasis in Uveal Melanoma. Clin Cancer Res (2016) 22:1234–42. doi: 10.1158/1078-0432.CCR-15-2071
14. Walter SD, Chao DL, Feuer W, Schiffman J, Char DH, Harbour JW. Prognostic Implications of Tumor Diameter in Association With Gene Expression Profile for Uveal Melanoma. JAMA Ophthalmol (2016) 134:734–40. doi: 10.1001/jamaophthalmol.2016.0913
15. Binkley E, Triozzi PL, Rybicki L, Achberger S, Aldrich W, Singh A. A prospective trial of adjuvant therapy for high-risk uveal melanoma: assessing 5-year survival outcomes. Br J Ophthalmol (2020) 104:524–8. doi: 10.1136/bjophthalmol-2019-314461
16. Cunha Rola A, Taktak A, Eleuteri A, Kalirai H, Heimann H, Hussain R, et al. Multicenter External Validation of the Liverpool Uveal Melanoma Prognosticator Online: An OOG Collaborative Study. Cancers (2020) 12: 477. doi: 10.3390/cancers12020477
17. Damato B, Eleuteri A, Hussain R, Kalirai H, Thornton S, Taktak A, et al. Parsimonious Models for Predicting Mortality from Choroidal Melanoma. Invest Opthalmol Vis Sci (2020) 61:35. doi: 10.1167/iovs.61.4.35
18. Eleuteri A, Taktak AFG, Coupland SE, Heimann H, Kalirai H, Damato B. Prognostication of metastatic death in uveal melanoma patients: A Markov multi-state model. Comput Biol Med (2018) 102:151–6. doi: 10.1016/j.compbiomed.2018.09.024
19. Yan B, Fu T, Liu Y, Wei W, Dai H, Fang W, et al. Tc-99m-3PRGD2 single-photon emission computed tomography/computed tomography for the diagnosis of choroidal melanoma A preliminary STROBE-compliant observational study. Med (Baltimore) (2018) 97:e12441. doi: 10.1097/MD.0000000000012441
20. Damato B. Ocular treatment of choroidal melanoma in relation to the prevention of metastatic death - A personal view. Prog Retin Eye Res (2018) 66:187–99. doi: 10.1016/j.preteyeres.2018.03.004
21. Ndulue J K, Mashayekhi AL, Shields C. Ciliary Body Seeding after Pars Plana Transvitreal Fine-Needle Aspiration Biopsy of Choroidal Melanoma. J Ophthalmic Vis Res (2020) 15:252–5. doi: 10.18502/jovr.v15i2.6744
22. Singh AD, Kalyani P, Topham A. Estimating the Risk of Malignant Transformation of a Choroidal Nevus. Ophthalmology (2005) 112:1784–9. doi: 10.1016/j.ophtha.2005.06.011
23. Lüke J, Grisanti S, Tura A. Aktuelle Diagnostik bei choroidalen Nävi. Klin Monatsblätter Für Augenheilkd (2018) 235:730–9. doi: 10.1055/s-0043-102592
24. Chien JL, Sioufi K, Surakiatchanukul T, Shields JA, Shields CL. Choroidal nevus: a review of prevalence, features, genetics, risks, and outcomes. Curr Opin Ophthalmol (2017) 28:228–37. doi: 10.1097/ICU.0000000000000361
25. Shields CL, Dalvin LA, Yu MD, Ancona-Lezama D, Di Nicola M, Williams BK, et al. CHOROIDAL NEVUS TRANSFORMATION INTO MELANOMA PER MILLIMETER INCREMENT IN THICKNESS USING MULTIMODAL IMAGING IN 2355 CASES: The 2019 Wendell L. Hughes Lecture. Retina (2019) 39:1852–60. doi: 10.1097/IAE.0000000000002508
26. Shields CL, Kaliki S, Rojanaporn D, Ferenczy SR, Shields JA. Enhanced Depth Imaging Optical Coherence Tomography of Small Choroidal Melanoma: Comparison With Choroidal Nevus. Arch Ophthalmol (2012) 130:850. doi: 10.1001/archophthalmol.2012.1135
27. Dalvin LA, Shields CL, Ancona-Lezama DA, Yu MD, Di Nicola M, Williams BK Jr., et al. Combination of multimodal imaging features predictive of choroidal nevus transformation into melanoma. Br J Ophthalmol (2019) 103:1441–7. doi: 10.1136/bjophthalmol-2018-312967
28. Shields CL, Lim L-AS, Dalvin LA, Shields JA. Small choroidal melanoma: detection with multimodal imaging and management with plaque radiotherapy or AU-011 nanoparticle therapy. Curr Opin Ophthalmol (2019) 30:206–14. doi: 10.1097/ICU.0000000000000560
29. Shields CL, Dalvin LA, Ancona-Lezama D, Yu MD, Di Nicola M, Williams BK, et al. CHOROIDAL NEVUS IMAGING FEATURES IN 3,806 CASES AND RISK FACTORS FOR TRANSFORMATION INTO MELANOMA IN 2,355 CASES: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina (2019) 39:1840–51. doi: 10.1097/IAE.0000000000002440
30. Pellegrini M, Corvi F, Say EAT, Shields CL, Staurenghi G. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY FEATURES OF CHOROIDAL NEOVASCULARIZATION ASSOCIATED WITH CHOROIDAL NEVUS. Retina (2018) 38:1338–46. doi: 10.1097/IAE.0000000000001730
31. Francis JH, Pang CE, Abramson DH, Milman T, Folberg R, Mrejen S, et al. Swept-Source Optical Coherence Tomography Features of Choroidal Nevi. Am J Ophthalmol (2015) 159:169–76.e1. doi: 10.1016/j.ajo.2014.10.011
32. Shah SU, Kaliki S, Shields CL, Ferenczy SR, Harmon SA, Shields JA. Enhanced Depth Imaging Optical Coherence Tomography of Choroidal Nevus in 104 Cases. Ophthalmology (2012) 119:1066–72. doi: 10.1016/j.ophtha.2011.11.001
33. Ghassemi F, Mirshahi R, Fadakar K, Sabour S. Optical coherence tomography angiography in choroidal melanoma and nevus. Clin Ophthalmol (2018) 12:207–14. doi: 10.2147/OPTH.S148897
34. Valverde-Megías A, Say EAT, Ferenczy SR, Shields CL. DIFFERENTIAL MACULAR FEATURES ON OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN EYES WITH CHOROIDAL NEVUS AND MELANOMA. Retina (2017) 37:731–40. doi: 10.1097/IAE.0000000000001233
35. Kivelä T, Jager MJ, Desjardins L, Kivelä T, Damato B. Diagnosis of Uveal Melanoma. In: Developments in Ophthalmology. Basel: KARGER. Available at: https://www.karger.com/Article/FullText/330613 (Accessed September 12, 2020).
36. Girbardt C, Rehak M, Wiedemann P. Diagnostisches Vorgehen bei Verdacht auf Aderhautmelanom. Klin Monatsblätter Für Augenheilkd (2018) 235:1393–7. doi: 10.1055/s-0042-123827
37. Walter U, Niendorf T, Graessl A, Rieger J, Krüger P-C, Langner S, et al. Ultrahigh field magnetic resonance and colour Doppler real-time fusion imaging of the orbit – a hybrid tool for assessment of choroidal melanoma. Eur Radiol (2014) 24:1112–7. doi: 10.1007/s00330-014-3101-5
38. Jacobsen BH, Ricks C, Harrie RP. Ocular ultrasound versus MRI in the detection of extrascleral extension in a patient with choroidal melanoma. BMC Ophthalmol (2018) 18:320. doi: 10.1186/s12886-018-0990-0
39. Thakkar H, Tyagi M, Bharwada R, Billore P, Mithal K. Role of echography in diagnostic dilemma in choroidal masses. Indian J Ophthalmol (2014) 62:167. doi: 10.4103/0301-4738.128626
40. Lemaître S, Zmuda M, Jacomet PV, Lévy-Gabriel C, Dendale R, Berges O, et al. Small Choroidal Melanoma Revealed by a Large Extrascleral Extension. Ocul Oncol Pathol (2017) 3:240–6. doi: 10.1159/000455870
41. Krema H, Habal S, Gonzalez JE, Pavlin CJ. ROLE OF OPTICAL COHERENCE TOMOGRAPHY IN VERIFYING THE SPECIFICITY OF ULTRASONOGRAPHY IN DETECTING SUBTLE SUBRETINAL FLUID ASSOCIATED WITH SMALL CHOROIDAL MELANOCYTIC TUMORS. Retina (2014) 34:360–5. doi: 10.1097/IAE.0b013e3182993dd9
42. Tran AQ, Eadie JA, Altaweel MM. Shaggy photoreceptors with subfoveal fluid associated with a distant choroidal melanoma. Case Rep Ophthalmol Med (2015) 2015:187542. doi: 10.1155/2015/187542
43. Vishnevskia-Dai V, Zur D, Yaacobi S, Moroz I, Newman H, Neudorfer M. Optical Coherence Tomography: An Adjunctive Tool for Differentiating between Choroidal Melanoma and Metastasis. J Ophthalmol (2016) 2016:1–7. doi: 10.1155/2016/9803547
44. Ung C, Laíns I, Silverman RF, Woods R, Lane AM, Papakostas TD, et al. Evaluation of choroidal lesions with swept-source optical coherence tomography. Br J Ophthalmol (2019) 103:88–93. doi: 10.1136/bjophthalmol-2017-311586
45. Sayanagi K, Pelayes DE, Kaiser PK, Singh AD. 3D Spectral Domain Optical Coherence Tomography Findings in Choroidal Tumors. Eur J Ophthalmol (2011) 21:271–5. doi: 10.5301/EJO.2010.5848
46. Samuelsson D, Sznage M, Engelsberg K, Wittström E. Clinical, optical coherence tomography, and fundus autofluorescence findings in patients with intraocular tumors. Clin Ophthalmol (2016) 10:1953–64. doi: 10.2147/OPTH.S109222
47. Myakoshina EB, Saakyan SV. Optical coherence tomography in diagnostics of small choroidal melanoma. Vestn Oftalmol (2020) 136:56. doi: 10.17116/oftalma202013601156
48. Pellegrini M, Staurenghi G. Swept-source Optical Coherence Tomography Angiography Imaging in a Case of Uveal Melanoma. Ophthalmology (2017) 124:729. doi: 10.1016/j.ophtha.2016.11.017
49. Cennamo G, Romano MR, Breve MA, Velotti N, Reibaldi M, de Crecchio G, et al. Evaluation of choroidal tumors with optical coherence tomography: enhanced depth imaging and OCT-angiography features. Eye (2017) 31:906–15. doi: 10.1038/eye.2017.14
50. Lee CS, Cho A, Lee KS, Lee SC. Association of high metabolic activity measured by positron emission tomography imaging with poor prognosis of choroidal melanoma. Br J Ophthalmol (2011) 95:1588–91. doi: 10.1136/bjo.2010.198085
51. Soret M, Bacharach SL, Buvat I. Partial-Volume Effect in PET Tumor Imaging. J Nucl Med (2007) 48:932–45. doi: 10.2967/jnumed.106.035774
52. Keramida G, Peters AM. Fasting hepatic glucose uptake is higher in men than women. Physiol Rep (2017) 5:e13174. doi: 10.14814/phy2.13174
53. Calcagni ML, Mattoli MV, Blasi MA, Petrone G, Sammarco MG, Indovina L, et al. A prospective analysis of 18F-FDG PET/CT in patients with uveal melanoma: comparison between metabolic rate of glucose (MRglu) and standardized uptake value (SUV) and correlations with histopathological features. Eur J Nucl Med Mol Imaging (2013) 40:1682–91. doi: 10.1007/s00259-013-2488-6
54. Matsuo T, Ogino Y, Ichimura K, Tanaka T, Kaji M. Clinicopathological correlation for the role of fluorodeoxyglucose positron emission tomography computed tomography in detection of choroidal malignant melanoma. Int J Clin Oncol (2014) 19:230–9. doi: 10.1007/s10147-013-0538-5
55. Lee JH, Lee SC, Cho A, Keum KC, Suh Y-G, Lee CS. Association Between Choroidal Thickness and Metabolic Activity on Positron Emission Tomography in Eyes With Choroidal Melanoma. Am J Ophthalmol (2015) 160:1111–5.e2. doi: 10.1016/j.ajo.2015.08.031
56. Papastefanou VP, Islam S, Szyszko T, Grantham M, Sagoo MS, Cohen VML. Metabolic activity of primary uveal melanoma on PET/CT scan and its relationship with monosomy 3 and other prognostic factors. Br J Ophthalmol (2014) 98:1659–65. doi: 10.1136/bjophthalmol-2014-305304
57. Romero-Aroca P, Montero-Jaime M, Intriago B, Riu F, Peña-Gonzalez KB, Almena-Garcia M. 18 FDG-PET/CT Assessing the Absence of Cell Viability and Excluding Metastatic Disease in a Case of Necrotic Choroidal Melanoma. Eur J Ophthalmol (2012) 22:288–92. doi: 10.5301/ejo.5000016
58. Sabate M, García JR, Valls E, Moragas M, Soler M, Riera E, et al. Utilidad de la PET-TC con 18F-FDG en un caso de recidiva del melanoma de coroides. Rev Esp Med Nucl E Imagen Mol (2012) 31:167–8. doi: 10.1016/j.remn.2011.10.009
59. Balasubramanya R, Selvarajan SK, Cox M, Joshi G, Deshmukh S, Mitchell DG, et al. Imaging of ocular melanoma metastasis. Br J Radiol (2016) 89:20160092. doi: 10.1259/bjr.20160092
60. Piperno-Neumann S, Servois V, Mariani P, Plancher C, Lévy-Gabriel C, Lumbroso-Le Rouic L, et al. Prospective study of surveillance testing for metastasis in 100 high-risk uveal melanoma patients. J Fr Ophtalmol (2015) 38:526–34. doi: 10.1016/j.jfo.2015.04.005
61. Francis JH, Catalanotti F, Landa J, Barker CA, Shoushtari AN, Abramson DH. Hepatic abnormalities identified by staging MRI and accuracy of MRI of patients with uveal melanoma. Br J Ophthalmol (2019) 103:1266–71. doi: 10.1136/bjophthalmol-2018-312612
62. Lorenzo D, Piulats JM, Ochoa M, Arias L, Gutiérrez C, Català J, et al. Clinical predictors of survival in metastatic uveal melanoma. Jpn J Ophthalmol (2019) 63:197–209. doi: 10.1007/s10384-019-00656-9
63. Patel M. Characterization of Computed Tomography Scan Abnormalities in Patients With Biopsy-Proven Hepatic Metastases From Uveal Melanoma. Arch Ophthalmol (2011) 129:1576. doi: 10.1001/archophthalmol.2011.263
64. Khoja L, Atenafu EG, Suciu S, Leyvraz S, Sato T, Marshall E, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol (2019) 30:1370–80. doi: 10.1093/annonc/mdz176
65. Mariani P, Dureau S, Savignoni A, Rouic LL-L, Levy-Gabriel C, Piperno-Neumann S, et al. Development of a Prognostic Nomogram for Liver Metastasis of Uveal Melanoma Patients Selected by Liver MRI. Cancers (2019) 11:863. doi: 10.3390/cancers11060863
66. Choudhary MM, Gupta A, Bena J, Emch T, Singh AD. Hepatic Ultrasonography for Surveillance in Patients With Uveal Melanoma. JAMA Ophthalmol (2016) 134:174. doi: 10.1001/jamaophthalmol.2015.4810
67. Muraki R, Morita Y, Ida S, Kitajima R, Furuhashi S, Kiuchi R, et al. Multimodal therapy with surgery and adjuvant nivolumab for late-onset multiple liver metastases of choroidal malignant melanoma: a case report. Surg Case Rep (2020) 6:187. doi: 10.1186/s40792-020-00948-0
68. Bellerive C, Ouellet E, Kamaya A, Singh AD. Liver Imaging Techniques: Recognition of Uveal Melanoma Metastases. Ocul Oncol Pathol (2018) 4:254–60. doi: 10.1159/000485424
69. Marshall E, Romaniuk C, Ghaneh P, Wong H, McKay M, Chopra M, et al. MRI in the detection of hepatic metastases from high-risk uveal melanoma: a prospective study in 188 patients. Br J Ophthalmol (2013) 97:159–63. doi: 10.1136/bjophthalmol-2012-302323
70. Altenbernd J, Wetter A, Forsting M, Umutlu L. Dual-energy CT of liver metastases in patients with uveal melanoma. Eur J Radiol Open (2016) 3:254–8. doi: 10.1016/j.ejro.2016.10.003
71. Davanzo JM, Binkley EM, Bena JF, Singh AD. Risk-stratified systemic surveillance in uveal melanoma. Br J Ophthalmol (2019) 103:1868–71. doi: 10.1136/bjophthalmol-2018-313569
72. Rantala ES, Peltola E, Helminen H, Hernberg M, Kivelä TT. Hepatic Ultrasonography Compared With Computed Tomography and Magnetic Resonance Imaging at Diagnosis of Metastatic Uveal Melanoma. Am J Ophthalmol (2020) 216:156–64. doi: 10.1016/j.ajo.2020.03.049
73. Rodríguez-Marco NA, Caicedo-Zamudio C, Solanas-Álava S, Gil-Arnaiz I, Córdoba-Iturriagagoitia A, Andonegui-Navarro J. PET/TC de cuerpo completo para la detección de metástasis de melanoma coroideo. Sist Sanit Navar (2014) 37:293–8. doi: 10.4321/S1137-66272014000200013
74. Finger PT. Whole body PET/CT for initial staging of choroidal melanoma. Br J Ophthalmol (2005) 89:1270–4. doi: 10.1136/bjo.2005.069823
75. Donaldson MJ, Pulido JS, Mullan BP, Inwards DJ, Cantrill H, Johnson MR, et al. Combined positron emission tomography/computed tomography for evaluation of presumed choroidal metastases. Clin Experiment Ophthalmol (2006) 34:846–51. doi: 10.1111/j.1442-9071.2006.01364.x
76. Orcurto V, Denys A, Voelter V, Schalenbourg A, Schnyder P, Zografos L, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography and magnetic resonance imaging in patients with liver metastases from uveal melanoma: results from a pilot study. Melanoma Res (2012) 22:63–9. doi: 10.1097/CMR.0b013e32834d3dcb
77. Cohen VL, Pavlidou E, Costa J, Arora A, Szyszko T, Sagoo M, et al. Staging uveal melanoma with whole-body positron-emission tomography/computed tomography and abdominal ultrasound: Low incidence of metastatic disease, high incidence of second primary cancers. Middle East Afr J Ophthalmol (2018) 25:91. doi: 10.4103/meajo.MEAJO_96_18
78. Freton A, Chin KJ, Raut R, Tena LB, Kivelä T, Finger PT. Initial PET/CT Staging for Choroidal Melanoma: AJCC Correlation and Second Nonocular Primaries in 333 Patients. Eur J Ophthalmol (2012) 22:236–43. doi: 10.5301/ejo.5000049
79. Servois V, Mariani P, Malhaire C, Petras S, Piperno-Neumann S, Plancher C, et al. Preoperative staging of liver metastases from uveal melanoma by magnetic resonance imaging (MRI) and fluorodeoxyglucose-positron emission tomography (FDG-PET). Eur J Surg Oncol EJSO (2010) 36:189–94. doi: 10.1016/j.ejso.2009.08.010
80. Murphy G, Hussey D, Metser U. Non-cutaneous melanoma: is there a role for 18 F-FDG PET-CT? Br J Radiol (2014) 87:20140324. doi: 10.1259/bjr.20140324
81. Breazzano MP, Daniels AB. Initial staging imaging for uveal melanoma: what’s necessary and what’s extraneous? Invest Ophthalmol Vis Sci (2017) 58:4412.
82. Klingenstein A, Haug AR, Nentwich MM, Tiling R, Schaller UC. Whole-body F-18-fluoro-2-deoxyglucose positron emission tomography/computed tomography imaging in the follow-up of metastatic uveal melanoma. Melanoma Res (2010) 20:511–6. doi: 10.1097/CMR.0b013e3283403d6c
Keywords: choroidal melanoma, multimodal imaging, diagnosis, staging, positron-emission tomography/computed tomography scan
Citation: Li X, Wang L, Zhang L, Tang F and Wei X (2021) Application of Multimodal and Molecular Imaging Techniques in the Detection of Choroidal Melanomas. Front. Oncol. 10:617868. doi: 10.3389/fonc.2020.617868
Received: 15 October 2020; Accepted: 16 December 2020;
Published: 01 February 2021.
Edited by:
Changqiang Wu, North Sichuan Medical College, ChinaReviewed by:
Peng Lv, Lanzhou University Second Hospital, ChinaRong Li, The First Affiliated Hospital of Xi’an Medical University, China
Copyright © 2021 Li, Wang, Zhang, Tang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wei, d2VpeGluXzE5ODJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xuying Li
Xuying Li Lixiang Wang1†
Lixiang Wang1† Li Zhang
Li Zhang Fei Tang
Fei Tang Xin Wei
Xin Wei