- 1Pediatric Critical Care Medicine, University of Texas at MD Anderson Cancer Center, Houston, TX, United States
- 2Pediatric Stem Cell Transplantation and Cellular Therapy and CARTOX Program, University of Texas at MD Anderson Cancer Center, Houston, TX, United States
Hematopoietic Cell Transplantation (HCT) is a potentially curative therapy for children and adolescent/young adults (AYA) with high-risk malignancies as well as some non-malignant genetic diseases. However, HCT may be associated with endotheliopathies and/or organ dysfunction that may progress to pediatric multi-organ dysfunction syndrome (pMODS) and require critical care intervention. Discipline specific scoring systems may be used to characterize individual organ dysfunction, but the extent to which they are used to prospectively monitor HCT patients with mild dysfunction is unknown. Further, separate scoring systems may be used to define risk of mortality and inform prognostication among those who require critical care support. Our understanding of the epidemiology, risk factors, morbidity, mortality, required monitoring, optimal prevention strategies and appropriate management of children undergoing HCT who develop organ dysfunction, endotheliopathies and/or progress to pMODS is poor. Discipline-specific registries and clinical studies have described improving outcomes for children undergoing HCT, including those who require critical care support; however, longitudinal studies/prospective registries that capture common data elements among HCT patients with and without organ dysfunction, endotheliopathies and pMODS are needed to facilitate inter-disciplinary collaboration and optimally characterize the risk profiles, define screening and prophylaxis regimens and mitigate toxicity.
Introduction
Advancements in transplantation and supportive care have led to marked improvement in outcomes of hematopoietic cell transplant (HCT) patients who require pediatric intensive care unit (PICU) support (1). Endothelial cell activation post-HCT may trigger complications such as capillary leak syndrome, engraftment syndrome, transplant-associated microangiopathy (TMA), diffuse alveolar hemorrhage (DAH), idiopathic pneumonia syndrome (IPS) and sinusoidal obstructive syndrome (SOS) (2). Clinical manifestations of these disorders may overlap with each other as well as with other post-HCT complications such as graft-versus-host-disease (GVHD) (2). Thus, the individual contribution of these disorders to organ dysfunction and subsequent failure have been poorly defined. While some studies have characterized the clinical progression of HCT patients with pediatric multi-organ dysfunction syndrome (pMODS) once in the PICU, there is a paucity of information regarding predisposition, incidence, spectrum of severity and overall and functional outcomes of HCT patients with organ dysfunction who do not require critical care support (3–9).
Improved characterization of post-HCT complications that do not escalate to require PICU admission may facilitate more precise risk stratification. Use of well-defined screening and diagnostic criteria may allow for a clear-cut comparison among HCT patients who do and do not require PICU admission. Further, application of discipline specific organ screening criteria may improve inter-disciplinary collaboration to follow the progression of asymptomatic and/or severe organ dysfunction over time. Accurate recognition of predisposing factors and prompt diagnosis of endotheliopathies and organ dysfunction may mitigate the progression to pMODS and need for critical care support and/or improve survival following PICU admission (3–9).
Here, we review existing pediatric screening and diagnostic algorithms for organ toxicity and provide a framework for prospective comprehensive screening of pediatric-AYA patients undergoing HCT, which incorporates the use of internationally accepted discipline specific standard definitions.
Overview of HCT and Associated Toxicities
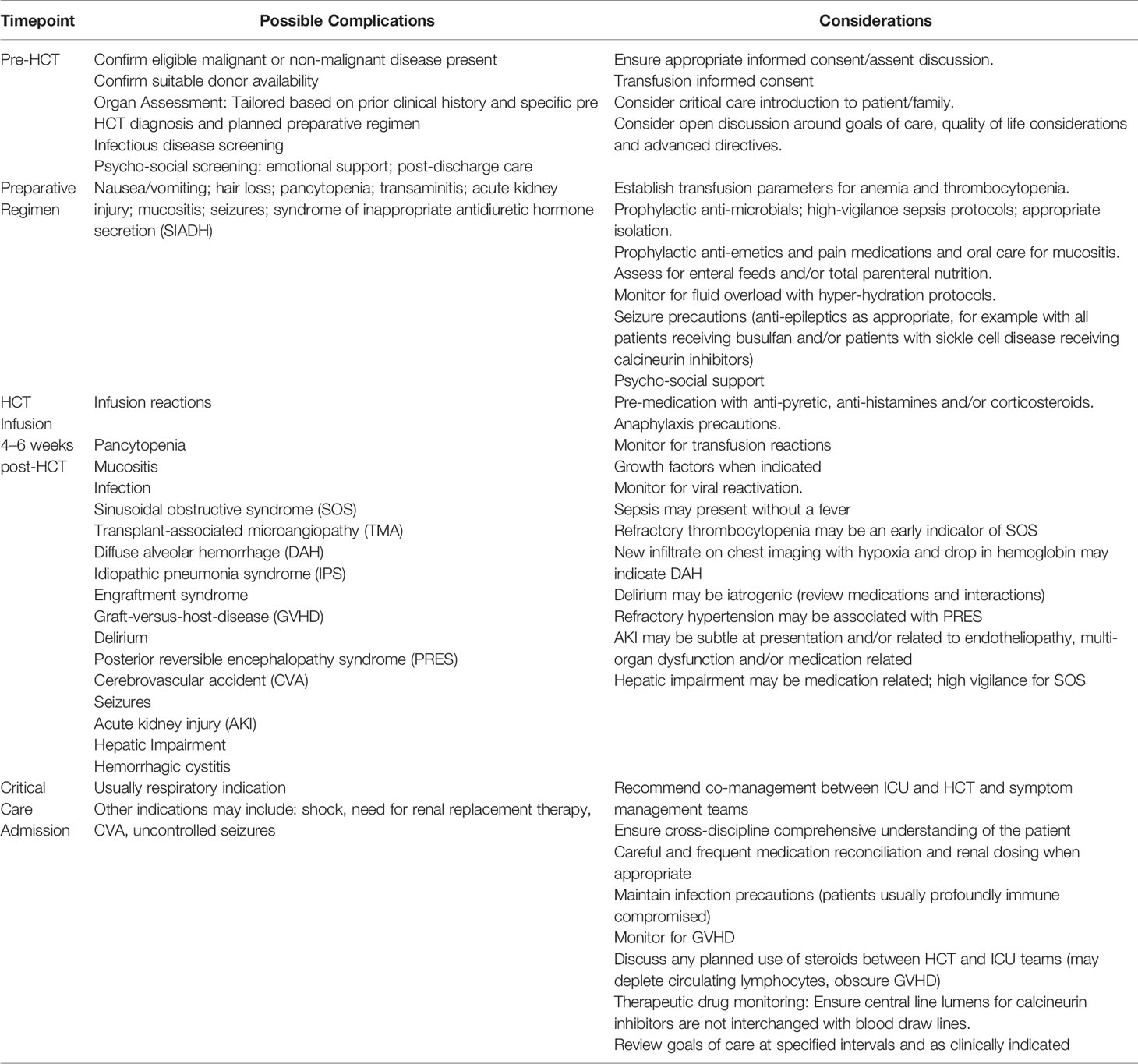
HCT is a potentially curative treatment for children with malignant and non-malignant diseases (10).Patients receive a preparative regimen (chemo and/or radiotherapy) that may vary in intensity based on disease indications and/or host factors that is followed by infusion of either autologous (patient's own) or allogeneic (donor's) stem cells. Administration of the preparative regimen prior to HCT, may result in tissue damage, associated cytokine release and activation of endothelial cells. Translocation of endotoxins across damaged mucosa, infections, white blood cell recovery and engraftment (in particular, allogeneic donor cell engraftment) all contribute to a pro-inflammatory cytokine milieu which may exacerbate endothelial dysfunction (11). Infection is a significant cause of HCT morbidity and mortality, especially in the early neutropenic phase. Graft-versus-host-disease (GVHD) is a complication of allogeneic HCT, where a donor's allo-reactive T-lymphocytes view the recipient's healthy cells as foreign and damage them. Poorly controlled infections, GVHD and post-HCT endotheliopathies have the potential to evolve into multi-organ dysfunction syndrome (MODS). MODS is defined as the presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without intervention (12). It is characterized by the concurrent dysfunction of two or more organs or systems including respiratory, cardiovascular, hematological, neurological, gastrointestinal, hepatic and renal (9, 13–15). An overview of HCT and possible toxicities and important considerations are presented in Table 1.
Post-HCT Endotheliopathies
Post-HCT endotheliopathies include capillary leak syndrome (CLS), engraftment syndrome (ES), transplant-associated microangiopathy (TMA), diffuse alveolar hemorrhage (DAH), idiopathic pneumonia syndrome (IPS) and sinusoidal obstructive syndrome (SOS). 2 CLS is characterized by the loss of intravascular fluids into interstitial spaces and is dominated by sudden weight gain, generalized edema unresponsive to diuresis and hypotension, which may eventually lead to cardiovascular shock with respiratory and pre‐renal insufficiency (16). ES is characterized by fever, skin rash, pulmonary edema, weight gain, liver and renal dysfunction in addition to encephalopathy and it occurs at the time of neutrophil recovery after HCT (17, 18). TMA may be diagnosed using published diagnostic criteria that includes (1) lactate dehydrogenase (LDH) elevated above the upper limit of normal for age (2) de novo thrombocytopenia with a platelet count <50 × 109/L or a ≥50% decrease in the platelet count (3) de novo anemia with a hemoglobin below the lower limit of normal or anemia requiring transfusion support (4) microangiopathic changes defined as the presence of schistocytes in the peripheral blood or histologic evidence of microangiopathy on a tissue specimen and (5) absence of a coagulopathy and a negative Coombs test. Laboratory criteria must occur concurrently, and criteria 1 to 4 should be documented on at least two consecutive tests to be classified as positive. ADAMTS13 activity should be measured to exclude a diagnosis of thrombotic thrombocytopenic purpura (19, 20). SOS, formerly referred to as hepatic veno-occlussive disease (VOD), may be diagnosed by modified EBMT Pediatric Criteria which require two or more of the following: unexplained consumptive and transfusion-refractory thrombocytopenia; otherwise unexplained weight gain on three consecutive days despite the use of diuretics, or a weight gain of more than 5% above baseline value within 72 h; bilirubin increasing from baseline on three consecutive days, or bilirubin 2 mg/dl or more within 72; hepatomegaly (best if supported by imaging) above baseline value; and ascites (best if supported by imaging) above baseline (21). DAH is a devastating non-infectious complication following HCT defined as a syndrome of hypoxia, dyspnea, infiltrates on chest radiograph, and progressively bloodier bronchoalveolar lavage or the presence of hemosiderin-laden macrophages on microscopy (22, 23). IPS is defined as the presence of multi-lobar infiltrates by chest radiograph or computed tomography scan, need for supplemental oxygenation with declining pulse oximetry and no identifiable pulmonary infection (24).
Organ Dysfunction Syndromes Post-HCT
Respiratory
Pulmonary complications post-HCT are common, with respiratory failure the leading cause of PICU admission among this population, and a significant source of non-relapse mortality. Respiratory complications post-HCT can be categorized into infectious and non-infectious, with imperfect diagnostic strategies that too often rely upon the presence of a constellation of overlapping clinical symptoms and diagnoses of exclusion. The Pediatric Acute Lung Injury Consensus Conference (PALICC) established criteria for diagnosis and severity grading of pediatric acute respiratory distress syndrome (PARDS) in children (25). Among children post-HCT who develop respiratory illness, there is a very high risk of developing severe PARDS irrespective of the underlying cause of pulmonary dysfunction, with a mortality rate of 40% to 60%. Emerging data suggest that longer duration of respiratory distress, increased use of non-invasive ventilation, and/or supplemental use of oxygen prior to intubation are associated with higher mortality for these children (4, 26).
Respiratory insufficiency in children post-HCT may result from a variety of causes such as post-HCT endotheliopathies, acute fluid overload and/or infection. The true incidence of respiratory insufficiency in pediatric HCT patients encompassing those who do and do not progress to failure is unknown. Emergency response systems such as the pediatric early warning system (PEWS) monitor indicators, such as heart rate, respiratory rate, systolic blood pressure, capillary refill time, work of breathing, oxygen therapy, and transcutaneous oxygen saturation (27). Among pediatric oncology and HCT patients, critical deterioration (defined as unplanned PICU transfer requiring life-sustaining interventions within 12 h) is preceded by a long duration of abnormal vital signs, making it potentially preventable through prompt recognition (28). Understanding the relative contributions of post-HCT complications to development of respiratory failure may further improve risk mitigation strategies.
Cardiovascular
Arrhythmias and cardiac dysfunction are not insignificant post-HCT complications in children. Known risk factors include exposure to anthracycline-based chemotherapeutic regimens and/or prior thoracic or total body irradiation. Prospective data that assesses the impact of degree of acute fluid overload and/or blood pressure variations, incidence and significance of arrhythmias, pericardial effusions as well as the role of potential biomarkers such as brain natriuretic peptide (BNP) and galectin-3, with regard to post-HCT outcomes may inform future monitoring and management strategies (29, 30). The New York Heart Association (NYHA) Heart Failure classification is not applicable to most of the pediatric population. The Ross Heart Failure classification, developed to assess severity in infants and subsequently modified to apply to all pediatric ages, provides a numeric score comparable with the NYHA classification for adults (31).While the general principles of heart failure management may be similar to adults, there is a compelling need for larger and higher-quality studies regarding cardiac failure in children undergoing HCT to provide a more robust evidence base (32).
Renal
Known risk factors for acute kidney injury (AKI) in the pediatric HCT population include allogeneic HCT, sinusoidal obstructive syndrome (SOS), use of nephrotoxic medications thrombotic microangiopathy (TMA), prior history of AKI, decreased baseline glomerular filtration rate (GFR), total body irradiation, and myeloablative chemotherapy conditioning regimens (33–42). DiCarlo and Alexander described the evolution of organ dysfunction in pediatric HCT as a result of a cytokine driven process, which may first manifest as fluid accumulation (38).Acute fluid overload above specific thresholds may be associated with high mortality rates in pediatric HCT patients who require PICU admission and detection of AKI often occurs well after the window for potentially successful mitigation strategies have passed (43, 44). The true incidence and precise impact of acute fluid overload among pediatric HCT patients who do not require PICU admission remains poorly characterized and clinical and physiological studies to date, demonstrate that the ideal fluid strategy in AKI has not been developed. Serum creatinine is insufficiently sensitive to detect “renal angina” and has been especially problematic for clinical research in pediatric AKI. The Kidney Disease Improving Global Outcomes (KDIGO) definition and staging of AKI was recently adopted into the severity staging for pediatric SOS and may be used to guide clinical care based on consensus of pediatric nephrology experts (21, 45). The renal angina index (RAI), determined by a composite of vasopressor use, invasive mechanical ventilation, percent fluid overload, and estimated creatinine clearance, may improve prediction of subsequent severe AKI (KDIGO Stage 2 to 3) in critically ill children when compared with an increase in serum creatinine alone (46, 47). These classification systems require prospective validation among pediatric HCT patients which may inform optimal strategies for AKI mitigation, fluid management (restrictive versus permissive), diuresis, thresholds for initiation of renal replacement therapy and placement of fluid drains.
Hepatic
Pediatric patients undergoing HCT are at risk for liver dysfunction from exacerbation of pre-existing co-morbidities such as prior infection, iron overload, SOS and/or hepatotoxic drugs, preparative regimens as well as allo-reactivity. No reliable tools exist to predict hepatic injury and overall survival or death in patients with pediatric acute liver failure. Existing liver failure scoring systems include the Child-Pugh score, Model for End-Stage Liver Disease and Pediatric End-Stage Liver Disease score, and the Liver Injury Unit score (48–51). These scoring systems incorporate ascites, encephalopathy, bilirubin kinetics, and coagulopathy and together resemble elements included in the current severity grading for sinusoidal obstructive syndrome in pediatric patients now routinely used among pediatric HCT patients (21). Vigilance for SOS in pediatric HCT patients based on current diagnostic criteria may facilitate early recognition of hepatic injury and inform future prophylaxis and treatment strategies. Sheer wave elastography ultrasound studies may represent a promising strategy to augment emerging vigilance protocols (52). Indeed, prospective data regarding the true incidence and spectrum of hepatic injury in pediatric HCT, including progression to pMODS are needed.
Neurologic
Neurological complications post-HCT may results from infection, metabolic derangements (due to medication or organ dysfunction), anatomical or metabolic abnormalities associated with the underlying diagnosis, or cerebrovascular events and are a significant cause of HCT-related mortality (53). Presenting symptoms may include delirium, seizure, encephalopathy, altered mental status, headache, or focal neurological signs. While little is known regarding true predisposition, a history of a neurological event prior to HCT has been associated with an increased risk of neurological complications post-HCT. Posterior reversible encephalopathy syndrome (PRES) may be one of the most common neurological complications in HCT patients that prompts transfer to the PICU. PRES may present with headache, acute mental status changes, visual changes including cortical blindness, and seizures, generally in association with an acute rise in blood pressure. Signal abnormalities on FLAIR imaging in the posterior regions of the brain, reflective of vasogenic edema may be present on magnetic resonance imaging (MRI). Cerebral vascular dysregulation, in response to elevated blood pressure or to endothelial activation, may cause vasogenic edema, commonly in the parieto-occipital regions, but may be found elsewhere. PRES has most commonly been associated with the administration of calcineurin inhibitors, sirolimus and dexamethasone. Usually reversible, PRES may be associated with severe morbidity and mortality if unrecognized. CNS infections in HCT patients are relatively rare but associated with potentially severe sequelae (54–56). For example, progressive multifocal leukoencephalopathy (PML) is a demyelinating disease due to JC virus infection that is found late (months to years) post-HCT. Optimal screening algorithms for neurologic toxicity post-HCT may reduce progression to severe complications. Screening and other mitigation strategies for endotheliopathies, GVHD and infection may result in prompt recognition and appropriate management. Early clinical signs and symptoms of neurological complications may be subtle. The Cornell Assessment of Pediatric Delirium (CAPD) provides a validated screening tool for delirium, which may be an early symptom associated with neurological complications (57, 58).The CAPD scoring system is a familiar tool used on pediatric HCT units since it has been incorporated into diagnostic and severity grading criteria for immune-effector cell associated neurotoxicity (ICANS) as well as SOS severity grading (21, 59).
MODS
As delineated above, pediatric patients undergoing HCT are at risk for organ dysfunction that may progress to pMODS. Over three decades, several scoring methods have emerged to define pediatric MODS, including the Pediatric Logistic Organ Dysfunction (PELOD) Score, Pediatric Multiple Organ Dysfunction Score (pMODS), and the pediatric Sequential Organ Failure Assessment (pSOFA) score (60–62). Also during this time, several studies reported pediatric MODS incidence rates ranging between 6% and 57% in the general PICU population, across all diagnoses (63–69). As mentioned previously, HCT is a well-described risk factor for pediatric MODS (5, 7, 62, 70).Additionally some studies suggest that the pediatric HCT subpopulation may display more severe and/or unique manifestations of organ dysfunction syndromes, including acute respiratory failure, sepsis and delirium, compared to the general pediatric population (71–73).
Discussion
Currently, no ideal scoring system exists to appropriately define organ dysfunction and risk of severity in pediatric HCT patients. Many of the existing clinical algorithms identify patients at risk for rapid clinical deterioration once admitted to the PICU but do not pre-emptively identify patients with mild abnormalities at risk for pMODS; none are specific to HCT patients, where consideration of the cumulative effect of mild dysfunction in multiple organs may be important. A pre-transplant HCT Comorbidity Index score >3 as commonly used in adult patients, has been associated with inferior survival of patients undergoing allogeneic HCT for some non-malignant diseases (74). However, 69% of pediatric patients in these cohorts may have a score of 0 prior to transplantation and the index does not provide dynamic and prospective considerations following HCT (74).
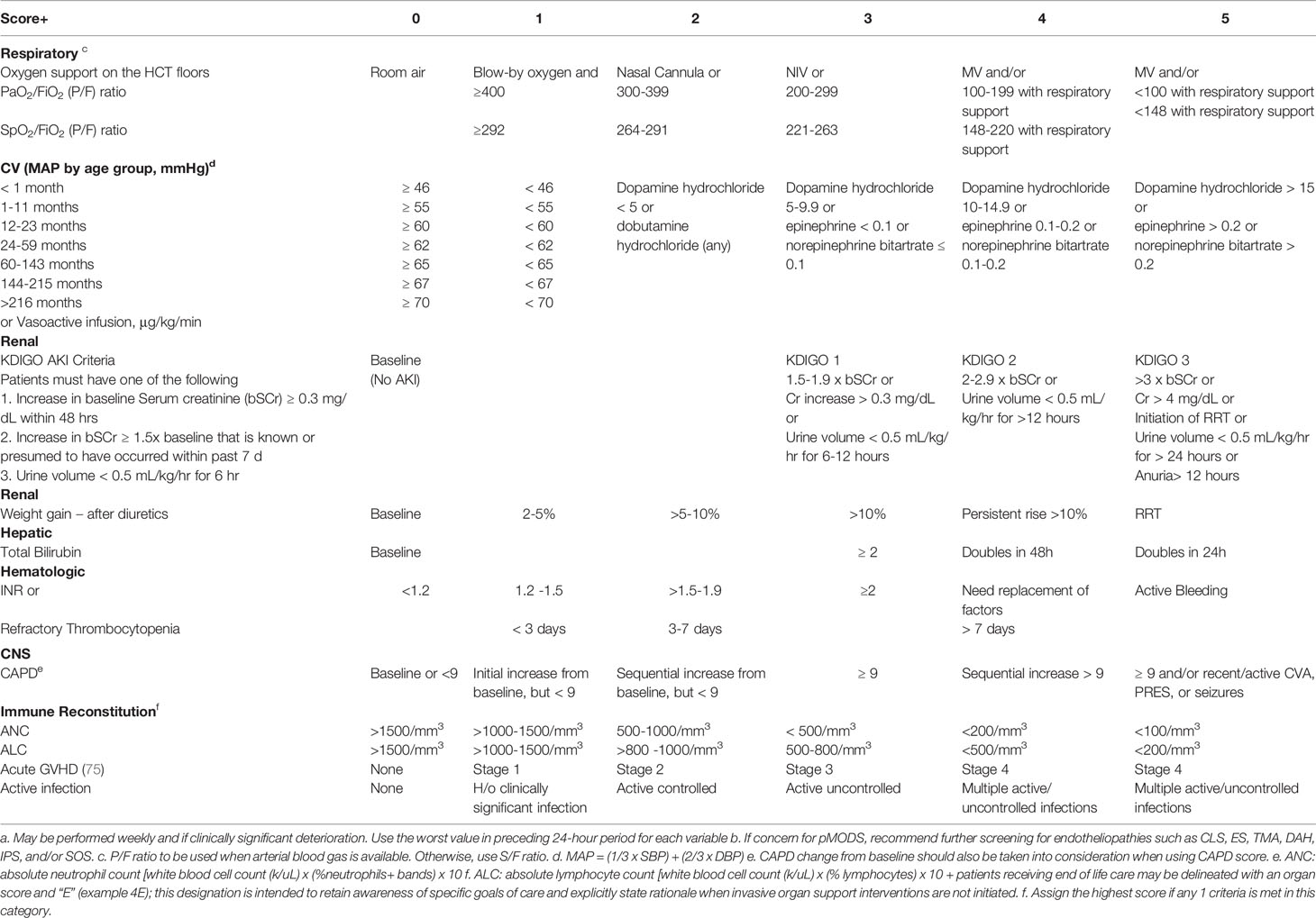
Binary descriptors of normal function versus dysfunction do not reflect cumulative decrements in organ function which may indicate progressive increase in mortality. Herein, as shown in Table 2, we provide a framework for prospective toxicity scoring tool that highlights progressive organ and immune dysfunction that can be used throughout an HCT admission (including PICU). Use of cross-discipline scoring systems that have been adapted to the expected ranges for pediatric HCT patients, is meant to facilitate a broader understanding of the patient’s clinical status among inter-disciplinary clinical specialties. Incorporation of discipline specific elements such as KDIGO and CAPD with standard HCT variables may foster a broader inter-disciplinary approach to the pediatric HCT patient. We have also included notation to reflect patients who have organ dysfunction and are receiving end-of-life-care. Goals of care may change during a patient’s clinical course and it is important that respective disciplines are aware. In the future, prospective validation of such a tool may help delineate cumulative toxicity scores and/or thresholds that warrant specific interventions. We hope that this tool stimulates inter-disciplinary conversation and prompts an effort for validation of this or a similar scoring system. The scores for individual observation points are meant to highlight severity of that system only (which is not always appreciated outside of specific disciplines). For example, KDIGO monitoring may not be routinely performed on HCT units and its significance may not be promptly appreciated by an HCT clinical team. Similarly, the impact of absolute lymphocyte count or higher grade graft-versus-host-disease may not be promptly recognized by a critical care team. The impact of cumulative toxicity scores among multiple observation points will require further investigation. In the interim, we hope that tools such as this, will promote a more comprehensive approach to the HCT patient.
The use of clinical scoring systems that encompass discipline specific tools may facilitate cross-talk, promote inter-disciplinary research and advance our understanding of the complex interactions involved in pMODS. Given the rarity of childhood diseases and enrollment on clinical studies as compared to adults, it is essential that collaborations aimed at collecting broad clinical data to compliment biospecimen collection are developed in pediatric HCT (76). The Center for International Blood and Marrow Transplant Research (CIBMTR) and Virtual Pediatric Systems capture discipline specific data in disparate registries. Attempts to merge these registries to advance our understanding of pediatric HCT patients who require PICU care have been an important step forward to improve our working knowledge of the factors influencing the progression of critical illness in pediatric allogeneic HCT patients (77). However, even these efforts are limited by the limitations of current data fields which may not capture detailed organ specific information, in particular among Pediatric HCT patients who do not require PICU admission or who do become critically ill but proceed to palliative care in lieu of escalation of care.
Attention to changes in clinical variables that inform organ assessments among pediatric HCT and PICU patients such as used in SOS severity and pSOFA scoring may normalize the use of common discipline specific variables among inter-disciplinary teams (21, 64). In a validation study by Matics and Sanchez-Pinto, the pSOFA score was adapted to pediatrics by modifying age-dependent cardiovascular and renal variables of the original SOFA score using validated cutoffs from the PELOD-2 scoring system, and also by expanding the respiratory subscore to include the SpO2/FiO2 (S/F) ratio as an alternative surrogate for PaO2/FiO2 (P/F) ratio to assess lung injury (64). The derivation of S/F ratio to impute for P/F ratio in the respiratory subscore has been validated in other studies (65). Pediatric SOS severity scores includes common CTCAE organ toxicity assessments with adjustments for pediatric ages when appropriate (21). This includes use of discipline specific variables such as CAPD and KDIGO organ assessments. Given the overlap between pSOFA and SOS scoring, we depict in Table 2, our proposed screening for all pediatric HCT patients at baseline, serially (perhaps weekly during inpatient transplant hospitalization) and during sentinel events (including development of endotheliopathy, PICU admission, transition to end-of-life care). Capture of raw data from such screening into a multi-center prospective registry as planned by PALISI-Network centers should serve as an invaluable resource for inter-disciplinary collaboration.
Advancements in cellular therapy and regenerative medicine may allow for therapeutic interventions that may mitigate organ toxicity. For example, mesenchymal stem cells (MSC) may be used to control heart failure in patients with anthracycline induced cardiomyopathy (78).Umbilical cord blood and MSC infusions are currently under investigation in the treatment of some pediatric brain injuries, including autism (79). Rapid advancement in potential therapeutics must be matched by innovative approaches to optimize available clinical data in small pediatric cohorts. Innovative approaches that integrate a “big data” inter-disciplinary platform for pediatric HCT patients are desperately needed. Herein, we propose a framework to screen for organ toxicity and express support for a broad pediatric HCT registry that is linked to a bio-specimen repository.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
AA—First authorship, initial draft and revisions, and primary literature review. KM—Last authorship, revisions and new content, and secondary literature review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Duncan CN, Lehmann LE, Cheifetz IM, Greathouse K, Haight AE, Hall MW, et al. Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med (2013) 14(3):261–7. doi: 10.1097/PCC.0b013e3182720601
2. Pagliuca S, Michonneau D, Sicre de Fontbrune F, Sutra Del Galy A, Xhaard A, Robin M, et al. Allogeneic reactivity-mediated endothelial cell complications after HSCT: a plea for consensual definitions. Blood Adv (2019) 3(15):2424–35. doi: 10.1182/bloodadvances.2019000143
3. Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest (1996) 109(4):1033–7. doi: 10.1378/chest.109.4.1033
4. Rowan CM, McArthur J, Hsing DD, Gertz SJ, Smith LS, Loomis A, et al. Acute Respiratory Failure in Pediatric Hematopoietic Cell Transplantation: A Multicenter Study. Crit Care Med (2018) 46(10):e967–e74. doi: 10.1097/ccm.0000000000003277
5. Afessa B, Tefferi A, Hoagland HC, Letendre L, Peters SG. Outcome of recipients of bone marrow transplants who require intensive-care unit support. Mayo Clin Proc (1992) 67(2):117–22. doi: 10.1016/s0025-6196(12)61310-x
6. Pillon M, Amigoni A, Contin A, Cattelan M, Carraro E, Campagnano E, et al. Risk Factors and Outcomes Related to Pediatric Intensive Care Unit Admission after Hematopoietic Stem Cell Transplantation: A Single-Center Experience. Biol Blood Marrow Transplant (2017) 23(8):1335–41. doi: 10.1016/j.bbmt.2017.04.016
7. Azoulay E, Thiéry G, Chevret S, Moreau D, Darmon M, Bergeron A, et al. The prognosis of acute respiratory failure in critically ill cancer patients. Med (Baltimore) (2004) 83(6):360–70. doi: 10.1097/01.md.0000145370.63676.fb
8. Azoulay E, Mokart D, Lambert J, Lemiale V, Rabbat A, Kouatchet A, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med (2010) 182(8):1038–46. doi: 10.1164/rccm.201001-0018OC
9. Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med (2007) 35(3):808–14. doi: 10.1097/01.Ccm.0000256846.27192.7a
10. D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant (2020) 26(8):e177–e82. doi: 10.1016/j.bbmt.2020.04.013
11. Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant (2011) 46(12):1495–502. doi: 10.1038/bmt.2011.65
12. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med (1992) 20(6):864–74. doi: 10.1097/00003246-199206000-00025
13. Gebara BM. Values for systolic blood pressure. Pediatr Crit Care Med (2005) 6(4):500. doi: 10.1097/01.pcc.0000164344.07588.83
14. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med (2005) 6(1):2–8. doi: 10.1097/01.Pcc.0000149131.72248.E6
15. Villeneuve A, Joyal JS, Proulx F, Ducruet T, Poitras N, Lacroix J. Multiple organ dysfunction syndrome in critically ill children: clinical value of two lists of diagnostic criteria. Ann Intensive Care (2016) 6(1):40. doi: 10.1186/s13613-016-0144-6
16. Lucchini G, Willasch AM, Daniel J, Soerensen J, Jarisch A, Bakhtiar S, et al. Epidemiology, risk factors, and prognosis of capillary leak syndrome in pediatric recipients of stem cell transplants: a retrospective single-center cohort study. Pediatr Transplant (2016) 20(8):1132–6. doi: 10.1111/petr.12831
17. Spitzer TR. Engraftment syndrome: double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant (2015) 50(4):469–75. doi: 10.1038/bmt.2014.296
18. Mutahar E, Al-Anazi KA. Engraftment Syndrome: An Updated Review. J Stem Cell Biol Transplant (2017) 01(03):e1–e5. doi: 10.21767/2575-7725.100016
19. Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation (2010) 90(8):918–26. doi: 10.1097/TP.0b013e3181f24e8d
20. Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood (2014) 124(4):645–53. doi: 10.1182/blood-2014-03-564997
21. Mahadeo KM, Bajwa R, Abdel-Azim H, Lehmann LE, Duncan C, Zantek N, et al. Diagnosis, grading, and treatment recommendations for children, adolescents, and young adults with sinusoidal obstructive syndrome: an international expert position statement. Lancet Haematol (2020) 7(1):e61–e72. doi: 10.1016/s2352-3026(19)30201-7
22. Heggen J, West C, Olson E, Olson T, Teague G, Fortenberry J, et al. Diffuse alveolar hemorrhage in pediatric hematopoietic cell transplant patients. Pediatrics (2002) 109(5):965–71. doi: 10.1542/peds.109.5.965
23. Fan K, McArthur J, Morrison RR, Ghafoor S. Diffuse Alveolar Hemorrhage After Pediatric Hematopoietic Stem Cell Transplantation. Front Oncol (2020) 10:1757(1757):e1–e15 doi: 10.3389/fonc.2020.01757
24. Keates-Baleeiro J, Moore P, Koyama T, Manes B, Calder C, Frangoul H. Incidence and outcome of idiopathic pneumonia syndrome in pediatric stem cell transplant recipients. Bone Marrow Transplant (2006) 38(4):285–9. doi: 10.1038/sj.bmt.1705436
25. Jouvet P, Thomas NJ, Wilson DF, Erickson S, Khemani R, Zimmerman J, et al. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med (2015) 16(5):428–39. doi: 10.1097/pcc.0000000000000350
26. Rowan CM, Gertz SJ, McArthur J, Fitzgerald JC, Nitu ME, Loomis A, et al. Invasive Mechanical Ventilation and Mortality in Pediatric Hematopoietic Stem Cell Transplantation: A Multicenter Study. Pediatr Crit Care Med (2016) 17(4):294–302. doi: 10.1097/pcc.0000000000000673
27. Gawronski O, Ciofi Degli Atti ML, Di Ciommo V, Cecchetti C, Bertaina A, Tiozzo E, et al. Accuracy of Bedside Paediatric Early Warning System (BedsidePEWS) in a Pediatric Stem Cell Transplant Unit. J Pediatr Oncol Nurs (2016) 33(4):249–56. doi: 10.1177/1043454215600154
28. Agulnik A, Gossett J, Carrillo AK, Kang G, Morrison RR. Abnormal Vital Signs Predict Critical Deterioration in Hospitalized Pediatric Hematology-Oncology and Post-hematopoietic Cell Transplant Patients. Front Oncol (2020) 10:(354):e1–e9. doi: 10.3389/fonc.2020.00354
29. Christenson RH, Duh SH, Wu AH, Smith A, Abel G, deFilippi CR, et al. Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem (2010) 43(7-8):683–90. doi: 10.1016/j.clinbiochem.2010.02.001
30. Mavinkurve-Groothuis AM, Kapusta L, Nir A, Groot-Loonen J. The role of biomarkers in the early detection of anthracycline-induced cardiotoxicity in children: a review of the literature. Pediatr Hematol Oncol (2008) 25(7):655–64. doi: 10.1080/08880010802244001
31. Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol (1992) 13(2):72–5. doi: 10.1007/bf00798207
32. Jayaprasad N. Heart Failure in Children. Heart Views (2016) 17(3):92–9. doi: 10.4103/1995-705x.192556
33. Kist-van Holthe JE, Goedvolk CA, Brand R, van Weel MH, Bredius RG, van Oostayen JA, et al. Prospective study of renal insufficiency after bone marrow transplantation. Pediatr Nephrol (2002) 17(12):1032–7. doi: 10.1007/s00467-002-0989-9
34. Hazar V, Gungor O, Guven AG, Aydin F, Akbas H, Gungor F, et al. Renal function after hematopoietic stem cell transplantation in children. Pediatr Blood Cancer (2009) 53(2):197–202. doi: 10.1002/pbc.22030
35. Ileri T, Ertem M, Ozcakar ZB, Ince EU, Biyikli Z, Uysal Z, et al. Prospective evaluation of acute and chronic renal function in children following matched related donor hematopoietic stem cell transplantation. Pediatr Transplant (2010) 14(1):138–44. doi: 10.1111/j.1399-3046.2009.01182.x
36. Benoit SW, Dixon BP, Goldstein SL, Bennett MR, Lane A, Lounder DT, et al. A novel strategy for identifying early acute kidney injury in pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant (2019) 54(9):1453–61. doi: 10.1038/s41409-018-0428-6
37. Koh KN, Sunkara A, Kang G, Sooter A, Mulrooney DA, Triplett B, et al. Acute Kidney Injury in Pediatric Patients Receiving Allogeneic Hematopoietic Cell Transplantation: Incidence, Risk Factors, and Outcomes. Biol Blood Marrow Transplant (2018) 24(4):758–64. doi: 10.1016/j.bbmt.2017.11.021
38. DiCarlo J, Alexander SR. Acute kidney injury in pediatric stem cell transplant recipients. Semin Nephrol (2008) 28(5):481–7. doi: 10.1016/j.semnephrol.2008.05.008
39. Raina R, Herrera N, Krishnappa V, Sethi SK, Deep A, Kao WM, et al. Hematopoietic stem cell transplantation and acute kidney injury in children: A comprehensive review. Pediatr Transplant (2017) 21(4):e12935. doi: 10.1111/petr.12935
40. Didsbury MS, Mackie FE, Kennedy SE. A systematic review of acute kidney injury in pediatric allogeneic hematopoietic stem cell recipients. Pediatr Transplant (2015) 19(5):460–70. doi: 10.1111/petr.12483
41. Hingorani S. Renal Complications of Hematopoietic-Cell Transplantation. N Engl J Med (2016) 374(23):2256–67. doi: 10.1056/NEJMra1404711
42. Sawinski D. The kidney effects of hematopoietic stem cell transplantation. Adv Chronic Kidney Dis (2014) 21(1):96–105. doi: 10.1053/j.ackd.2013.08.007
43. Goldstein SL. Fluid management in acute kidney injury. J Intensive Care Med (2014) 29(4):183–9. doi: 10.1177/0885066612465816
44. Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol (2004) 19(1):91–5. doi: 10.1007/s00467-003-1313-z
45. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. Kidney Int Suppl (2012) 2:1. doi: 10.1038/kisup.2012.1
46. Goldstein SL, Chawla LS. Renal angina. Clin J Am Soc Nephrol (2010) 5(5):943–9. doi: 10.2215/CJN.07201009
47. Chawla LS, Goldstein SL, Kellum JA, Ronco C. Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care (2015) 19(1):93. doi: 10.1186/s13054-015-0779-y
48. Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol (2001) 96(7):1968–76. doi: 10.1111/j.1572-0241.2001.03964.x
49. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology (2001) 33(2):464–70. doi: 10.1053/jhep.2001.22172
50. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg (1973) 60(8):646–9. doi: 10.1002/bjs.1800600817
51. Liu E, MacKenzie T, Dobyns EL, Parikh CR, Karrer FM, Narkewicz MR, et al. Characterization of acute liver failure and development of a continuous risk of death staging system in children. J Hepatol (2006) 44(1):134–41. doi: 10.1016/j.jhep.2005.06.021
52. Chan SS, Robinson AL, Staggs VS, Goyal RK, Goette MJ, Dodd NA, et al. Using Ultrasound Elastography to Predict Which Pediatric HSCT Patients Will Develop Severe Sinusoidal Obstruction Syndrome. Transplant Cell Ther Meet (2019). 25(3):S43–44. doi: 10.1016/j.bbmt.2018.12.119
53. Dulamea AO, Lupescu IG. Neurological complications of hematopoietic cell transplantation in children and adults. Neural Regener Res (2018) 13(6):945–54. doi: 10.4103/1673-5374.233431
54. Morris EB, Laningham FH, Sandlund JT, Khan RB. Posterior reversible encephalopathy syndrome in children with cancer. Pediatr Blood Cancer (2007) 48(2):152–9. doi: 10.1002/pbc.20703
55. Khan RB, Sadighi ZS, Zabrowski J, Gajjar A, Jeha S. Imaging Patterns and Outcome of Posterior Reversible Encephalopathy Syndrome During Childhood Cancer Treatment. Pediatr Blood Cancer (2016) 63(3):523–6. doi: 10.1002/pbc.25790
56. Przybylyski A, Esper P. Early Recognition and Management of Posterior Reversible Encephalopathy Syndrome: A Newly Recognized Complication in Patients Receiving Tyrosine Kinase Inhibitors. Clin J Oncol Nurs (2016) 20(3):305–8. doi: 10.1188/16.CJON.305-308
57. Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med (2014) 42(3):656–63. doi: 10.1097/CCM.0b013e3182a66b76
58. Traube C, Ariagno S, Thau F, Rosenberg L, Mauer EA, Gerber LM, et al. Delirium in Hospitalized Children with Cancer: Incidence and Associated Risk Factors. J Pediatr (2017) 191:212–7. doi: 10.1016/j.jpeds.2017.08.038
59. Mahadeo KM, Khazal SJ, Abdel-Azim H, Fitzgerald JC, Taraseviciute A, Bollard CM, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol (2019) 16(1):45–63. doi: 10.1038/s41571-018-0075-2
60. Watson RS, Crow SS, Hartman ME, Lacroix J, Odetola FO. Epidemiology and Outcomes of Pediatric Multiple Organ Dysfunction Syndrome. Pediatr Crit Care Med (2017) 18(3_suppl Suppl 1):S4–S16. doi: 10.1097/PCC.0000000000001047
61. Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet (2003) 362(9379):192–7. doi: 10.1016/s0140-6736(03)13908-6
62. Graciano AL, Balko JA, Rahn DS, Ahmad N, Giroir BP. The Pediatric Multiple Organ Dysfunction Score (P-MODS): development and validation of an objective scale to measure the severity of multiple organ dysfunction in critically ill children. Crit Care Med (2005) 33(7):1484–91. doi: 10.1097/01.ccm.0000170943.23633.47
63. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
64. Matics TJ, Sanchez-Pinto LN. Adaptation and Validation of a Pediatric Sequential Organ Failure Assessment Score and Evaluation of the Sepsis-3 Definitions in Critically Ill Children. JAMA Pediatr (2017) 171(10):e172352. doi: 10.1001/jamapediatrics.2017.2352
65. Pandharipande PP, Shintani AK, Hagerman HE, St Jacques PJ, Rice TW, Sanders NW, et al. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med (2009) 37(4):1317–21. doi: 10.1097/CCM.0b013e31819cefa9
66. Peres Bota D, Melot C, Lopes Ferreira F, Nguyen Ba V, Vincent JL. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med (2002) 28(11):1619–24. doi: 10.1007/s00134-002-1491-3
67. Gogia P, Koreti S, Patel GS. SOFA (Sequential Organ Failure Assessment) and PELOD (Pediatric Logistic Organ Dysfunction). Sch J App Med Sci (2015) 3(4A):1645–8. doi: 10.36347/sjams
68. Probst L, Schalk E, Liebregts T, Zeremski V, Tzalavras A, von Bergwelt-Baildon M, et al. Prognostic accuracy of SOFA, qSOFA and SIRS criteria in hematological cancer patients: a retrospective multicenter study. J Intensive Care (2019) 7:41. doi: 10.1186/s40560-019-0396-y
69. Wilkinson JD, Pollack MM, Glass NL, Kanter RK, Katz RW, Steinhart CM. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr (1987) 111(3):324–8. doi: 10.1016/s0022-3476(87)80448-1
70. Zinter MS, Holubkov R, Steurer MA, Dvorak CC, Duncan CN, Sapru A, et al. Pediatric Hematopoietic Cell Transplant Patients Who Survive Critical Illness Frequently Have Significant but Recoverable Decline in Functional Status. Biol Blood Marrow Transplant (2018) 24(2):330–6. doi: 10.1016/j.bbmt.2017.10.036
71. van Dorst M, van Gestel JPJ, van Grotel M, Versluijs B, van den Heuvel-Eibrink MM, Nijman J, et al. PICU Admission Rates in Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients Receiving High-flow Nasal Cannula Oxygen Therapy on the General Ward. J Pediatr Hematol Oncol (2020) 42(1):e1–6. doi: 10.1097/mph.0000000000001649
72. Hensley MK, Donnelly JP, Carlton EF, Prescott HC. Epidemiology and Outcomes of Cancer-Related Versus Non-Cancer-Related Sepsis Hospitalizations. Crit Care Med (2019) 47(10):1310–6. doi: 10.1097/CCM.0000000000003896
73. Winsnes K, Sochacki P, Eriksson C, Shereck E, Recht M, Johnson K, et al. Delirium in the pediatric hematology, oncology, and bone marrow transplant population. Pediatr Blood Cancer (2019) 66(6):e27640. doi: 10.1002/pbc.27640
74. Thakar MS, Broglie L, Logan B. The Hematopoietic Cell Transplant Comorbidity Index predicts survival after allogeneic transplant for nonmalignant diseases. Blood (2019) 133(7):754–62. doi: 10.1182/blood-2018-09-876284
75. Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol (1997) 97(4):855–64. doi: 10.1046/j.1365-2141.1997.1112925.x
76. Shores DR, Everett AD. Children as Biomarker Orphans: Progress in the Field of Pediatric Biomarkers. J Pediatr (2018) 193:14–20.e31. doi: 10.1016/j.jpeds.2017.08.077
77. Zinter MS, Logan BR, Fretham C, Sapru A, Abraham A, Aljurf MD, et al. Comprehensive Prognostication in Critically Ill Pediatric Hematopoietic Cell Transplant Patients: Results from Merging the Center for International Blood and Marrow Transplant Research (CIBMTR) and Virtual Pediatric Systems (VPS) Registries. Biol Blood Marrow Transplant (2020) 26(2):333–42. doi: 10.1016/j.bbmt.2019.09.027
78. Olson AL. Donor Bone Marrow Derived Mesenchymal Stem Cells in Controlling Heart Failure in Patients With Cardiomyopathy Caused by Anthracyclines. MD Anderson Cancer Center. (2020) NCT02962661.
79. Dawson G, Sun JM, Baker J, Carpenter K, Compton S, Deaver M, et al. A Phase II Randomized Clinical Trial of the Safety and Efficacy of Intravenous Umbilical Cord Blood Infusion for Treatment of Children with Autism Spectrum Disorder. J Pediatr (2020) 222:164–73.e5. doi: 10.1016/j.jpeds.2020.03.011
Keywords: pediatric stem cell transplantation, pediatric critical care, multiple organ dysfunction, pediatric critical care illness severity scores, pediatric multi-organ dysfunction syndrome
Citation: Ahmad AH and Mahadeo KM (2021) Perspective: A Framework to Screen Pediatric and Adolescent Hematopoietic Cellular Therapy Patients for Organ Dysfunction: Time for a Multi-Disciplinary and Longitudinal Approach. Front. Oncol. 11:622630. doi: 10.3389/fonc.2021.622630
Received: 28 October 2020; Accepted: 05 January 2021;
Published: 24 February 2021.
Edited by:
Brandon Triplett, St. Jude Children's Research Hospital, United StatesReviewed by:
Katia Perruccio, University of Perugia, ItalyNirali Shah, National Institutes of Health (NIH), United States
Copyright © 2021 Ahmad and Mahadeo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kris M. Mahadeo, a21tYWhhZGVvQG1kYW5kZXJzb24ub3Jn
 Ali H. Ahmad
Ali H. Ahmad Kris M. Mahadeo
Kris M. Mahadeo