- 1Research Center for Evidence-Based Medicine, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
- 2Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
- 3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
- 4Laboratory of Experimental Pharmacology, IRCCS Istituto Tumori Giovanni Paolo II, Bari, Italy
- 5Medical Oncology Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Tumori Giovanni Paolo II, Bari, Italy
- 6Department of Biomedical Sciences and Human Oncology, Aldo Moro University of Bari, Bari, Italy
- 7Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
As a unique population of tumor bulk, cancer stem cells have been implicated in tumor relapse and chemoresistance in triple-negative breast cancer (TNBC). Therefore, understanding the phenotype of cancer stem cells can pave the way for introducing novel molecular targeted therapies for treating TNBC patients. Preclinical studies have identified CD44+CD24-/low as a cancer stem cell phenotype; however, clinical studies have reported seemingly controversial results regarding the prognostic values of CD44 and CD44+CD24-/low phenotype in TNBC patients. To critically review the clinicopathological significance and prognostic values of CD44 and CD44+CD24-/low phenotype in TNBC patients, the Scopus, Embase, PubMed, and Web of Science databases were systematically searched to obtain the relevant records published before 20 October 2020. Based on nine included studies, CD44 and CD44+CD24-/low phenotype are associated with inferior prognosis in TNBC patients. Moreover, these cancer stem cell markers have been associated with advanced tumor stage, tumor size, higher tumor grade, tumor metastasis, and lymphatic involvement in TNBC patients. Our evidence has also indicated that, unlike the treatment-naïve TNBC patients, the tumoral cells of chemoradiotherapy-treated TNBC patients can upregulate the CD44+CD24-/low phenotype and establish an inverse association with androgen receptor (AR), leading to the inferior prognosis of affected patients. In summary, CD44 and CD44+CD24-/low phenotype can be utilized to determine TNBC patients’ prognosis in the pathology department as a routine practice, and targeting these phenotypes can substantially improve the prognosis of TNBC patients.
Introduction
Breast cancer is one of the frequently diagnosed cancers among females (1). TNBC, as one of the troublesome breast cancer subtypes, is characterized by the lack of expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) (2). TNBC can be further grouped into six subtypes, i.e., basal-like 1, basal like2, immunomodulatory, mesenchymal, mesenchymal stem-like, and luminal androgen receptor subtypes (3). Despite recent advances in treating breast cancer, the current therapeutic approaches have not resulted in desirable outcomes for TNBC patients. Therefore, there is a need to develop new approaches to treat TNBC patients (3).
Although cancer stem cells comprise a small tumor cell population, their self-renewal feature can facilitate rising progressive neoplasms. This unique tumor cell population is one of the culprits of developing chemoresistance and tumor relapse (4). Indeed, cancer stem cells share many features with normal stem cells; for instance, they can be divided asymmetrically and recapitulate tumor cells (5). Furthermore, cancer stem cells can stimulate the epithelial-to-mesenchymal transition (EMT) process to facilitate tumor metastasis (6).
Preclinical studies have indicated that CD44, as a transmembrane glycoprotein, is overexpressed in cancer stem cells and has been implicated in tumor development and migration (7, 8). The interaction between CD44 and hyaluronan can stimulate the epidermal growth factor receptor (EGFR)-related pathways and facilitate chemoresistance, tumor growth, and metastasis in various cancers (9). Indeed, CD44 has been implicated in the activation of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and the phosphatidylinositol-3-kinase and protein kinase B (PI3K/Akt) signaling pathways in tumoral cells (10, 11). The activation of the rat sarcoma (Ras)- rapidly accelerated fibrosarcoma (Raf)-extracellular signal-regulated kinase kinase (MEK)-ERK pathway has been associated with upregulated tumoral programmed death-ligand 1 (PD-L1) expression, which ultimately establishes an auto-inductive loop with PD-L1 (12–14). Therefore, CD44 can facilitate the immune evasion of tumoral cells via facilitating the activation of the Ras-Raf-MEK-ERK pathway. Indeed, recent findings have indicated that CD44 can promote the expression of tumoral PD-L1 in TNBC cells (15). Nam et al. have indicated that CD44 can promote the activation of the tyrosine-protein kinase Src (c-Src)/Akt signaling pathway, leading to the activation of c-Jun and transcription of c-Src. Therefore, CD44-mediated c-Src/Akt/c-Jun/c-Src signaling pathway can lead to the establishment of an auto-inductive, resulting in tumorigenesis and migration in breast cancer cells (16). Furthermore, the interaction of CD44 with its ligand, hyaluronic acid, has upregulated expression of multidrug resistance 1 (MDR-1) in Nanog/signal transducer and activator of transcription (STAT)-3-mediated fashion (17). The upregulation of STAT-3 has also been associated with increased expression of matrix metalloproteinase-2 (MMP)-2 and invasion in tumoral cells (18). Besides, CD44 can provide an activation site for Ezrin-Radixin-Moesin, leading to cytoskeletal modifications and migration (19). Therefore, preclinical studies have indicated CD44 has been implicated in tumorigenesis, chemoresistance, immune evasion, and migration in cancers.
In 2003, Al-Hajj et al. indicated that the CD44+/CD24-/Lin- phenotype can be linked to cancer stem cell features in breast cancer (20). In line with this, Taniuchi et al. have indicated that CD24 can inhibit the migration and metastasis of pancreatic cancer cells (21). Moreover, it has been reported that CD24 is less expressed in differentiated cells compared to progenitor cells (22). Pallegar et al. have shown that the activation of Raf can substantially downregulate the gene and protein expression of CD24 (23). Moreover, the activation of Ras has been associated with the generation of CD44+/CD24- cells from the CD44-/CD24+ cells in breast cancer (24). Consistent with these, recent data have shown that inhibiting ERK, which belongs to the Ras-Raf-MEK-ERK pathway, can substantially decrease the population of cells with CD44+/CD24- in TNBC (10). Thus, preclinical studies indicate that the CD44+/CD24- phenotype can be associated with tumor development and migration in breast cancer cells. However, the published clinical studies have not reached a consensus regarding the prognostic value of these phenotypes in TNBC patients (25–29).
Therefore, there is a need to clarify the prognostic role and clinical significance of these phenotypes in TNBC patients. This systematic review aimed to discuss the prognostic role and clinicopathological relevance of CD44 and CD44+CD24-/low phenotype in TNBC patients. Furthermore, this study intended to briefly review novel approaches to target CD44 to ameliorate the prognosis of TNBC patients.
Methods
This study was conducted under the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statements (30).
The Strategy of the Systematic Search
The Scopus, PubMed, Web of Science, and Embase databases were systematically searched to obtain the relevant studies published before 20 October 2020. For this purpose, the abovementioned databases were systematically searched with the following keywords: (“CD44” OR”CD 44” OR “HCAM” OR “homing cell adhesion molecule” OR “Pgp-1” OR “phagocytic glycoprotein-1” OR “phagocytic glycoprotein 1” OR “phagocytic glycoprotein1” OR “Hermes antigen” OR “lymphocyte homing receptor” OR “ECM-III” OR “HUTCH-1” OR “H-CAM” OR “Ly-24” OR “Cluster of Differentiation 44” OR “Cluster of Differentiation44”) and (“TNBC” OR “triple-negative” OR “triple negative” OR “triple-negative breast cancer” OR “triple negative breast cancer” OR “ER-negative PR-negative HER2-negative breast neoplasms” OR “ER negative PR negative HER2 negative breast neoplasms” OR “triple-negative breast cancers” OR “triple-negative breast neoplasm” OR “triple negative breast neoplasm” OR “triple-negative breast neoplasms” OR “ER-negative PR-negative HER2-negative breast cancer” OR “ER negative PR negative HER2 negative breast cancer” OR “triple negative breast cancer”).
Study Selection and Data Extraction
After the systematic search, the obtained studies were reviewed in two phases. In phase I, two authors (N.H and Z.A) independently screened records according to their titles and abstracts. In phase II, the same authors independently reviewed the full text of the remaining papers, along with their supplementary data. Any disagreements were resolved via consulting with B.B and consensus.
Data Extraction
The following data were extracted from the included studies: (1) the first author, (2) publication year, (3) the country, (4) the sample size, (5) the previous treatment of affected patients, (6) the prognostic values of CD44-CD44+/CD44-/low phenotype, e.g., progression-free survival (PFS), overall survival (OS), disease-free survival (DFS), breast cancer-specific survival (if reported), (7) the association between CD44-CD44/CD44 phenotypes with the clinicopathological features, and (8) the association between CD44-CD44/CD44 phenotypes with the EMT/metastasis factors.
Eligibility Criteria
Papers with the following eligibility criteria were included in our study: (1) human-based studies, (2) investigations with the objective of assessing the CD44-CD44+/CD44-/low phenotype in TNBC patients, (3) studies, which investigated the protein expression of CD44 and CD44+/CD44-/low phenotype TNBC patients, (4) studies, which demonstrated the prognostic value of CD44-CD44+/CD44-/low phenotype or the association between the clinicopathological characteristics with CD44-CD44+/CD44-/low phenotype in patients with TNBC, and (5) studies, which were published in English. Based on the following criteria, records were excluded from this study: (1) studies that failed to meet the aforementioned inclusion criteria, (2) duplicated studies, (3) review papers, (4) studies, which did not evaluate the protein expression of CD44-CD44+/CD44-/low phenotype, rather the gene expression, (5) conference abstracts, (6) cellular studies, and (7) animal studies.
Risk of Bias in Included Studies
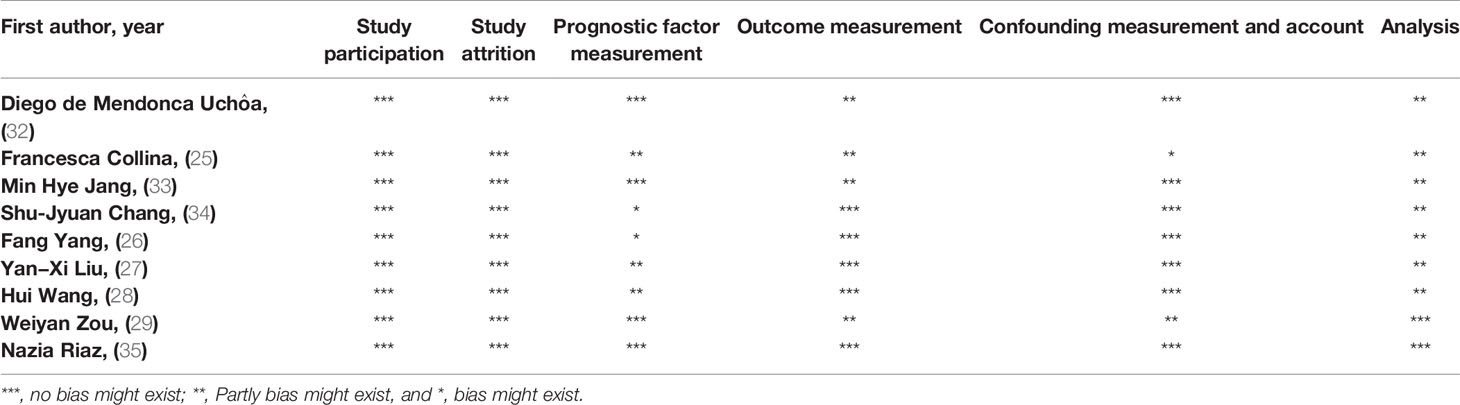
The methodologies of included investigations were assessed using Hayden et al. guidelines for assessing the quality of our included studies (31). Any disagreements were resolved via consulting with B.B. The evaluation is demonstrated in Table 1.
Results
Selected Studies
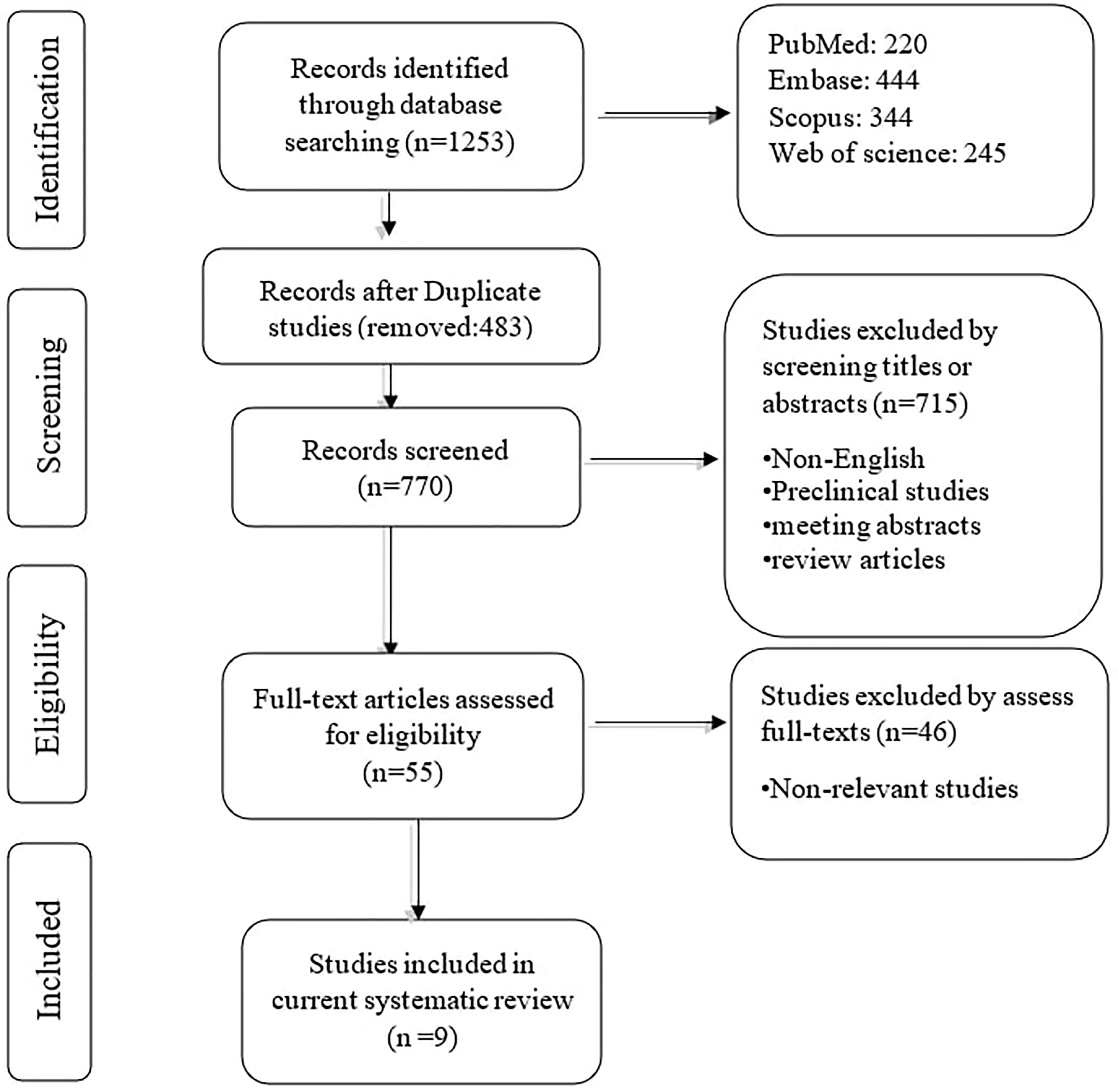
The systematic search retrieved 1253 records: PubMed (220), Embase (444), Scopus (344), and Web of Science (245). After removing duplication records, 770 records remained. In phase I, 715 studies were removed based on reviewing the title/abstract of the remaining records. In phase II, two authors reviewed the full text of 55 remaining studies, along with their supplementary data. Based on the second phase of reviewing, nine papers were included in the qualitative synthesis. The flowchart of literature identification, inclusion, and exclusion is demonstrated in Figure 1.
The Characteristic of Included Studies
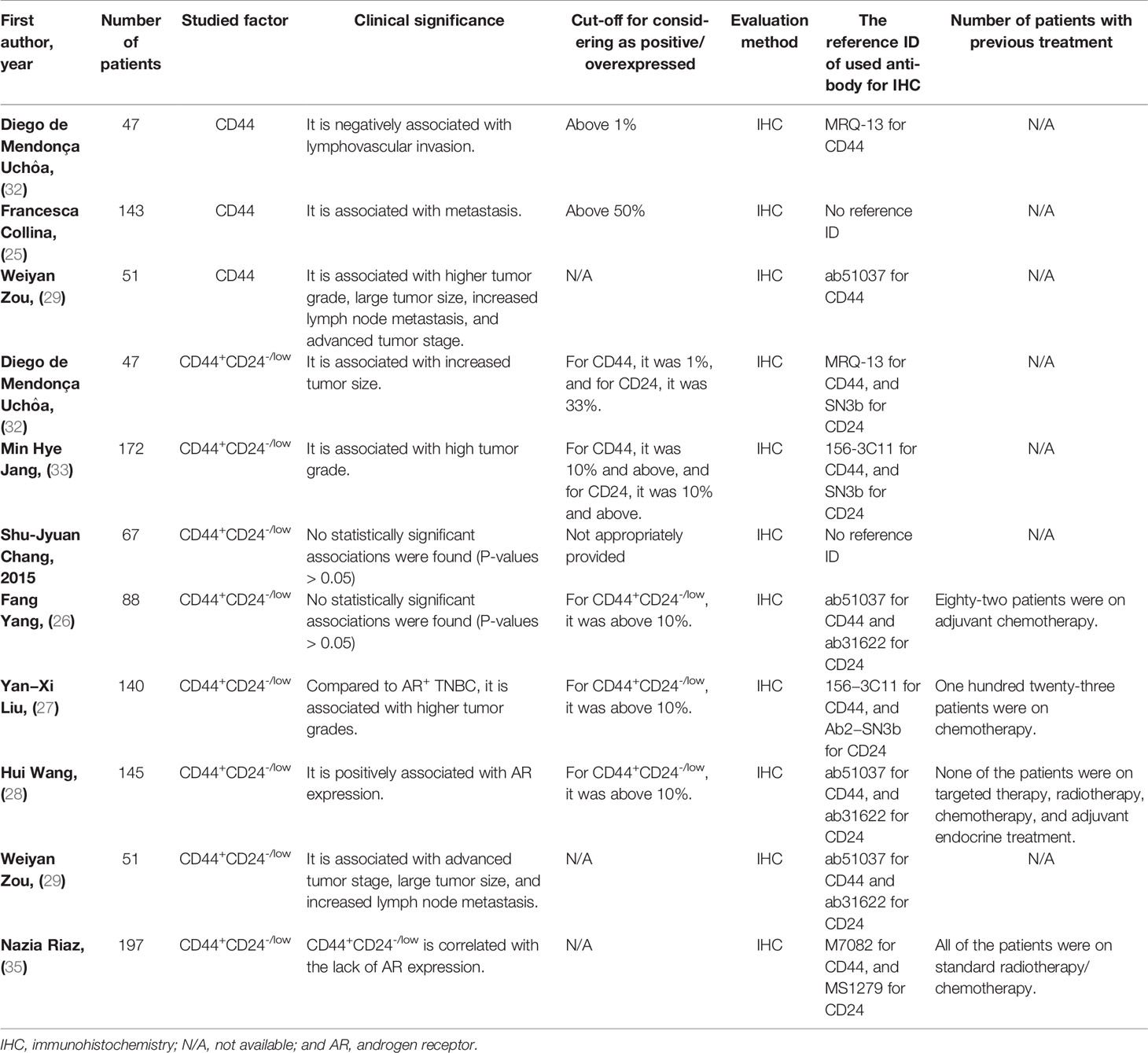
The nine clinical studies were published in English between 2014 and 2020. All investigations utilized immunohistochemistry (IHC) as the staining method. Regarding the clinico- pathological significance of CD44 in TNBC patients, CD44 has been associated with lymphovascular invasion, metastasis, higher tumor grade, lymph node metastasis, and advanced tumor stage in patients with TNBC (Table 2). Regarding the clinicopathological significance of CD44+CD24-/low phenotype in TNBC patients, CD44+CD24-/low phenotype has been associated with tumor grade, tumor stage, tumor size, histology classification, lymph node metastasis, and AR expression; however, this phenotype has been inversely associated with AR expression in TNBC patients treated with chemotherapy/radiotherapy (Table 2 for a better elucidation, refer to the discussion).
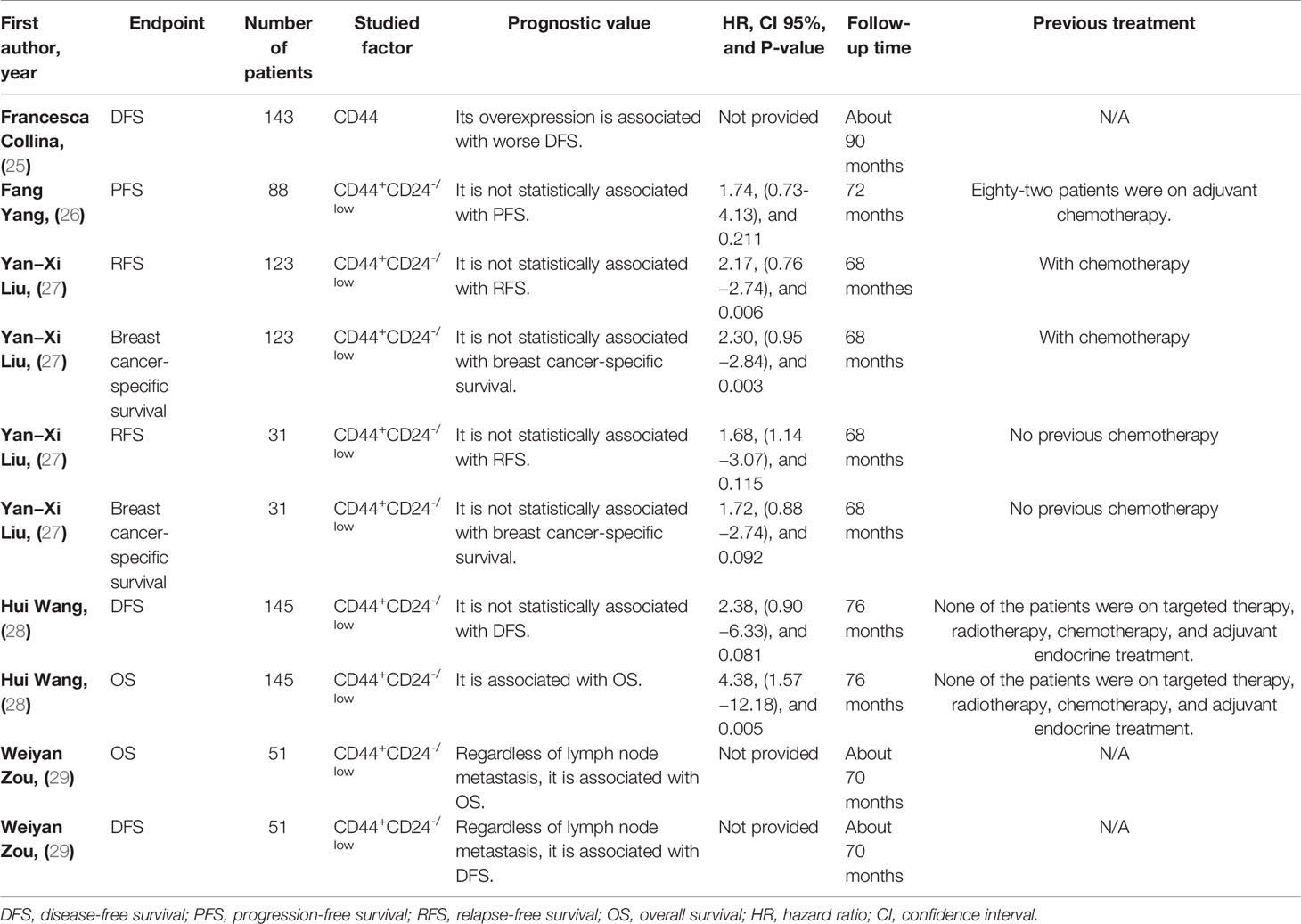
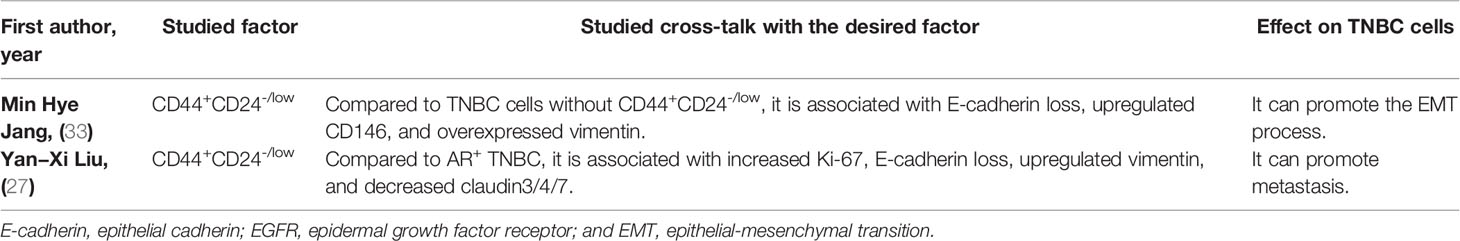
Regarding the prognostic value of CD44 in TNBC patients, CD44 has been associated with inferior DFS in affected patients (Table 3). Regarding the prognostic value of the CD44+CD24-/low phenotype in TNBC patients, this phenotype has been associated with inferior OS and DFS in affected patients (Table 3 for a better elucidation, refer to the discussion). Regarding the cross-talk between CD44+CD24-/low phenotype with TNBC development, this phenotype has been associated with epithelial cadherin (E-cadherin) loss, overexpressed CD146, upregulated vimentin, increased tumor necrosis, elevated Ki-67 level, higher EGFR expression, and downregulated claudin3/4/7 (Table 4).
The Risk of Bias in Included Studies
Based on the six items of Hayden et al. guidelines, the quality of the included studies was evaluated (Table 1). The study participation and attrition items were scored well according to the guideline. The main risk areas were prognostic factor measurement and analysis.
Discussion
The following sections are aimed to critically review the results of the including studies about the prognostic value of CD44 and CD44+CD24-/low phenotype, their association with the clinicopathological features of TNBC patients, and their associations with the EMT process, metastasis, chemoresistance, and tumor microenvironment of TNBC cells according to the preclinical studies to present a better picture of CD44 and CD44+CD24-/low phenotype in TNBC cells. Finally, we briefly review the current-evaluated preclinical approaches in targeting CD44 to inhibit TNBC development.
CD44
Collina et al. have reported that TNBC patients with upregulated expression of cytoplasmic CD44 might demonstrate worse PFS compared to the TNBC patients with low CD44 expression (25). The expression of CD44 has been substantially associated with higher tumor grade, lymph node metastasis, and advanced tumor stage in TNBC patients (29). Consistent with this, CD44 has been implicated in promoting lymphovascular invasion in TNBC patients (32). In line with this, there has been a remarkable association between CD44 expression and tumor metastasis in TNBC patients (25). Therefore, CD44 can be associated with advanced tumor stage, higher tumor grade, tumor metastasis, and lymphatic involvement in TNBC patients. Besides, CD44 overexpression might indicate an inferior prognosis in TNBC patients.
It has been reported that most TNBC cell lines are CD44-positive, making this factor a promising target for treating TNBC (36). A better understanding of its underlying cross-talk in chemoresistance, immunosuppression, and tumor migration is critical for treating TNBC patients. In TNBC cells, CD44 has been implicated in the upregulation of tumoral PD-L1 (15). Moreover, PD-L1 is required for the expression of CD44 in TNBC. Indeed, Lotfinejad et al. have indicated that PD-L1 silencing remarkably downregulates the expression of CD44 in TNBC cells (37). Zhang et al. have also reported a positive correlation between tumoral PD-L1 and CD44 in lung adenocarcinoma (38). It is well-established that PD-L1 can impede the development of anti-tumoral immune responses and result in tumor development (39). A recent meta-analysis has indicated a strong association between tumor-infiltrating lymphocytes and tumoral PD-L1 in TNBC patients (40).
In breast cancer patients, Zheng et al. have reported a strong positive association between CD44 and EGFR (41). Compared to AR+ TNBC cells, CD44+CD24-/low TNBC cells can upregulate EGFR expression (27). With the upregulation of EGFR in some TNBC cells, targeting EGFR via cetuximab administration has been a promising strategy for treating TNBC patients. Wenyan et al. have shown that delivering CD44-siRNA into EGFR+ TNBC cells can enhance the sensitivity of EGFR+ TNBC cells to cetuximab (42). EGFR and mucin 1 (MUC1), which are present in 90% of TNBC cells, can establish multiple immunosuppressive positive loops, resulting in the recruitment of myeloid-derived suppressor cells (MDSCs), leading to an immunosuppressive tumor microenvironment (43). Of interest, MUC-1 can also upregulate PD-L1 and promote tumor growth (44). Thus, in this intertwined network, CD44 is a critical factor for inducing immunosuppressive tumor microenvironment and tumor growth.
Regarding drug resistance, CD44-siRNA transfection can decrease clonogenicity and downregulate the expression of VEGF, MMP-9, and CXCR4 in MDA-MB-468 cells. Furthermore, the combination therapy of CD44-siRNA and doxorubicin has substantially decreased the half-maximal inhibitory concentration (IC50) of doxorubicin (45). Cheng et al. have shown that the doxorubicin-resistant MDA−MB−468 cells can considerably express CD44, and inhibiting STAT-3 can decrease the CD44+ cell population and enhance the chemosensitivity of MDA−MB−468 cells via the STAT-3/Oct-4/c-Myc pathway (46). There is growing evidence about the adverse effect of CD44 on the chemosensitivity of tumoral cells. In MCF-7/Adr cells, the interaction of CD44 with hyaluronan can activate the downstream signaling pathway of Erb-B2 receptor tyrosine kinase 2 (ErbB2), the PI3K pathway, which leads to the upregulation of MDR-1. Of interest, the stimulation of the PI3K signaling pathway results in hyaluronan production, leading to the establishment of an auto-inductive chemoresistant loop in breast cancer cells (47). Bourguignon et al. have shown that the interaction of hyaluronan with CD44 can stimulate the Nanog, leading to the upregulation of MDR-1 in STAT-3 dependent fashion. Moreover, hyaluronan interaction with CD44 has been implicated in efflux chemotherapeutic agents by facilitating the interaction of ankyrin with MDR-1 in tumoral cells (17). CD44 has also been implicated in promoting Nanog, metastasis, and tumorgenicity in head and neck squamous cell carcinoma (48). Moreover, it has been reported that the CD44 activation can upregulate Nanog and subsequently repress apoptosis in tumor cells (49). Collectively, CD44 might promote immunosuppressive tumor microenvironment, tumor growth, tumor migration, and chemoresistance in TNBC cells.
The CD44+CD24-/Low Phenotype in TNBC Patients: Untangling the Controversial Results
The CD44+CD24-/Low Phenotype and Its Prognostic Value in TNBC Patients
Zou et al. have reported that TNBC patients with the phenotype of CD44+CD24-/low have remarkably worse DFS and OS compared to the TNBC patients without the CD44+CD24-/low phenotype (29). Besides, TNBC patients with CD44+CD24-/low phenotype have experienced worse OS compared to CD44−/CD24− patients (HR = 4.38, CI 95%: 1.57−12.18, P-value = 0.005). However, compared to CD44−/CD24− TNBC patients, there has been no statistically significant association between DFS and CD44+CD24-/low phenotype (P-value = 0.081) (28). Compared to luminal A breast cancer patients, treatment-naïve CD44+CD24-/low TNBC patients have not have statistically significant worse relapse-free survival (RFS) and breast cancer-specific survival (both P-values > 0.05) (27). Although CD44+CD24-/low TNBC patients have not had statistically poor PFS in comparison to the CD133+ and/or aldehyde dehydrogenase 1 family member A1+ (ALDH1A1+) ones, the CD44+CD24-/low and/or ADLH1A1+ TNBC ones have had worse PFS in comparison with their counter partner TNBC patients (HR = 2.81, CI 95%: 1.26-6.24, P-value = 0.011) (26).
These seemingly conflicting results might be stemmed from the different references and relatively small sample sizes in these studies. In comparison with the CD44−/CD24− TNBC patients, there have been no statistically significant results for determining DFS of CD44+CD24-/low TNBC patients (P-value > 0.05) (28). Liu et al. have conducted the comparison between the luminal A patients with the CD44+CD24-/low TNBC patients, which have not led to statistically significant results regarding the RFS and breast cancer-specific survival (both P-values > 0.05) (27). In comparison with CD133+ and/or ALDH1A1+ TNBC patients, there have been no statistically significant results for determining the PFS of CD44+CD24-/low TNBC patients (P-value > 0.05) (26). Indeed, the comparison between the TNBC patients expressing CD44+CD24-/low phenotype with the TNBC patients not expressing CD44+CD24-/low can determine the prognostic value of CD44+CD24-/low phenotype in TNBC patients. Given this, regardless of lymph node metastasis, the CD44+CD24-/low phenotype can worsen DFS and OS of TNBC patients compared to TNBC patients without the CD44+CD24-/low phenotype (29). In line with this, breast cancer patients with high level of CD44+CD24-/low have demonstrated worse DFS and OS compared to breast cancer patients with low level of CD44+CD24-/low (HR = 1.890, CI 95%:1.217-3.464, P-value = 0.015, and HR = 1.92, CI 95%: 1.248-3.586, P-value = 0.017, respectively) (50). Thus, the CD44+CD24-/low phenotype can be associated with inferior survival in TNBC patients.
The CD44+CD24-/Low Phenotype and Its Association With Clinicopathological Features of TNBC Patients
The CD44+CD24-/low phenotype expression has been frequent in basal-like neoplasms than in non-basal-like neoplasms (33). Consistent with this, Riaz et al. have shed light on a correlation between CD44+CD24-/low phenotype and basal-like TNBC in chemotherapy and radiotherapy-experienced basal-like TNBC patients (35). Among the CD44/CD24 phenotypes, CD44+CD24-/low has been associated with more aggressive TNBC regarding the tumor size, TMN stage, and lymph node metastasis (29). Consistent with this, the CD44-/CD24+ phenotype has associated with less lymphovascular invasion in TNBC patients (32). Besides, the CD44+CD24-/low phenotype has been more frequent in high-grade TNBC cells (33). With the sample size of 67 TNBC patients, Chang et al. have failed to establish any statistically significant associations between CD44+CD24-/low phenotype with TNM stage, tumor grade, lymph node metastasis among the CD44/CD24 phenotypes (all P-values > 0.05) (34). These conflicting results might be due to the relatively small sample size of Chang’s study. Therefore, CD44+CD24-/low phenotype can be associated with tumor size, TMN stage, lymph node metastasis, and tumor grade in TNBC patients.
The CD44+CD24-/Low Phenotype in Treatment-Naïve and Treated Patients and Its Cross-Talk With Chemoresistance and Metastasis
Among the different CD44/CD24 phenotypes, CD44+CD24-/low cells have expressed a substantial AR in TNBC patients without previous chemotherapy and radiotherapy (28). However, in treated TNBC patients with standard chemotherapy and radiotherapy, the CD44+CD24-/low phenotype is inversely correlated with AR expression (35). Indeed, AR expression has been associated with improved OS and breast cancer-specific survival in treated TNBC patients (35). Consistent with this, the CD44+CD24-/low TNBC cells have exhibited a more aggressive histological pattern, high Ki67 score, increased vimentin, and upregulated EGFR, decreased E-cadherin, and downregulated claudin-3/4/7 compared to AR+ TNBC cells (27). Given this, the CD44+CD24-/low phenotype might decrease the AR expression and develop chemoresistance following chemo-and radiotherapy in TNBC patients (see below). Consistent with our observed results, Lehmann et al. have indicated that mesenchymal and mesenchymal stem-like subtypes, which are substantially enriched for the Wnt/β-catenin signaling pathway, predominantly stimulate the EMT and express CD44+CD24- phenotype. Mesenchymal and mesenchymal stem-like subtypes have been associated with inferior 5-year distant metastasis-free survival. Besides, the mesenchymal subtype has been associated with the inferior RFS of affected patients, and this subtype overexpresses proliferation-related genes. However, TNBC patients with luminal androgen receptor subtypes have shown improved RFS compared to patients with other subtypes (3).
Jang et al. have reported remarkable associations between CD44+CD24-/low phenotype with E-cadherin loss, CD146, and vimentin expression in TNBC cells (33). Recently, Vikram et al. have indicated that the CD44+CD24-/low phenotype can lead to the overexpression of the EMT/metastatic markers, e.g., Nanog and sex-determining region Y-related HMG box 2 (SOX2), in MDA-MB-231 cells. Indeed, the CD44+CD24-/low phenotype has been positively associated with tumor growth and migration in TNBC cells (51). Following doxorubicin treatment, doxorubicin-resistant MDA-MB-231 cells have substantially upregulated the CD44+CD24-/low phenotype compared to wild-type cells (52). Besides the TNBC cells, growing evidence indicates that the CD44+CD24-/low phenotype can promote EMT and chemoresistance in other cancers. In oral squamous cell carcinoma, the CD44+CD24-/low phenotype has promoted colony formation, tumor migration, and the expression of drug transporters, which can facilitate the EMT process and chemoresistance (53).
Lessons From the Past and the Road Ahead
Targeted therapy has become an ever-increasingly appealing approach for treating cancer patients. Based on our discussion, TNBC cells, in response to current chemotherapy, can lead to chemoresistance and tumor relapse, which the cancer stem cells have been implicated in promoting that. Therefore, it is pressingly needed to eradicate the cancer stem cells from tumor bulk. The following discussion intends to present novel paradigms for targeting CD44, as an essential cancer stem cell factor, in TNBC.
The miR-based therapy and small interfering RNA (siRNA)-based therapy can post-transcriptionally alter the expression of CD44. Preclinical studies have supported their efficacy in eradicating tumor cells. Vahidian et al. have demonstrated that the doxorubicin combination with CD44-siRNA can substantially decrease tumor growth, metastasis and increase apoptosis in MDA-MB-468 cells. Besides, CD44-siRNA has considerably decreased the IC50 of doxorubicin in MDA-MB-468 cells (45). In line with this, Van Phuc et al. have shown that the CD44+CD24- tumoral cells are resistant to doxorubicin, and targeting CD44 can substantially increase the sensitivity of breast cancer cells to doxorubicin (54). Eameema et al. have developed a drug delivery vehicle, which binds to CD44 via its anti-CD44 human antibody and delivers paclitaxel and salinomycin. They have demonstrated that this nanoparticle-based vehicle can specifically target CD44+ MDA-MB-231 cells and effectively eradicate the tumoral cells (55). Fu et al. have shown that the delivery of CD44-siRNA can substantially enhance the cetuximab sensitivity of TNBC cells, and the combined delivery of CD44-siRNA with cetuximab treatment can remarkably decrease tumor volume in mice bearing TNBCs (42). Targeting CD44 in TNBC cells has also been associated with increased survival of mice-bearing tumors, decreased tumor burden, and suppressed bone metastasis in aminal models (56). Consistent with these, the combined downregulation of CD44 with doxorubicin administration has considerably decreased tumor volume compared to animal models treated with doxorubicin (57). A liposomal-based vehicle, which delivers miR-34a to breast cancer cells, can downregulate ZEB1, Bmi1, and CD44 expression and eradicated breast cancer cells (58). Ahir et al. have designed a mesoporous silica nanoparticle vehicle, covered hyaluronic acid, to deliver miR-34a and antisense-miR-10b into TNBC cells. Their in vitro and in vivo results have shown promising outcomes regarding inhibition of tumor growth and metastasis (59). Al-Othman et al. have demonstrated that the transfection of miR-328-3p, which has been upregulated following the treatment of TNBC with 5α-dihydrotestosterone, can reduce CD44 expression and tumor migration in TNBC. Based on their study, 5α-dihydrotestosterone can downregulate CD44 expression via binding the AR/5α-dihydrotestosterone to CD44 promoter or upregulating the expression of miR-328-3p, which can inhibit post-transcriptionally decrease the expression of CD44 (60).
Moreover, the recent advances in immunotherapy have provided ample opportunities to ameliorate the prognosis of TNBC patients. Immunotherapeutic approaches are focused on stimulating anti-tumoral immune responses to reject tumoral cells. The PD-L1/programmed cell death protein 1 (PD-1) axis is a well-known inhibitory immune checkpoint axis that can substantially attenuate anti-tumoral immune responses (39, 61). This axis can be established between tumoral cells and effector immune cells and shield the tumoral cells from anti-tumoral immune responses (40). Recently, Lotfinejad et al. have shown that inhibiting tumoral PD-L1 can substantially decrease CD44 expression in TNBC cells (37). Besides, inhibiting CD44 has been associated with decreased expression of PD-L1 in TNBC cells (15). Consistent with these, it has been shown that selective inhibition and activation of the Wnt signaling pathway, which is enriched for cancer stem cell markers, can remarkably downregulate and upregulate PD-L1 expression in TNBC cells (62, 63). Thus, this positive association between CD44 and PD-L1 might provide the rationale for investigating the effect of monoclonal PD-L1/PD-1 antibodies administration on the CD44 expression and stemness of TNBC cells in affected patients.
The current systematic review has several strengths. First, given the controversial results of clinical studies accumulated between 2014 to 2020 regarding the prognostic values of the CD44+/CD24- phenotype in TNBC patients, our study has clarified its prognostic value in TNBC patients. Second, besides its prognostic value, we have clarified its clinicopathological significance in TNBC patients, which enables clinicians to determine the course of TNBC in affected patients. However, our systematic review has some limitations, as well. First, we only included the clinical studies that were published in English. Second, the population of our included studies was geographically and, presumably, ethnically diverse, which can lead to increase heterogeneity among the included studies. Third, the currently available evidence has used IHC staining for detecting protein expression; in light of the recent advances in mass-cytometry technologies, there might be a need to investigate the impact of CD44 and CD44+CD24- at the single-cell levels.
Conclusion
Since cancer stem cells are one of the daunting challenges of treating TNBC patients, identifying and categorizing them can provide valuable insights for targeted therapies. The current systematic review has demonstrated that CD44 and CD44+CD24-/low phenotype are associated with inferior prognosis in TNBC patients, and they are correlated with advanced tumor stage, tumor size, higher tumor grade, tumor metastasis, and lymphatic involvement in TNBC patients. These cancer stem cell factors can lead to chemoresistance, EMT activation, induction of immunosuppressive tumor microenvironment, and tumor growth in TNBC cells. The combined downregulation of CD44 and the administration of chemotherapeutic agents, e.g., doxorubicin, has shown promising results in preclinical studies. Besides, the combination of CD44-siRNA and specific tumor-suppressive miRs has been associated with enhanced chemosensitivity of TNBC cells to chemotherapeutic agents and decreased tumor growth both in vivo and in vitro studies. Therefore, siRNA/miR-based gene therapy and their combination with chemotherapeutic agents can provide ample opportunities to improve the prognosis of TNBC patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
MA and NeH contributed to the study selection. MA developed the systematic search, interpreted the results, and wrote the majority of the manuscript. AD and NiH have contributed to the assessment of included studies. NKA, ZA, PL, and OB have extracted the data from the included studies. NS and BB have supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the Research Center for Evidence-Based Medicine, Tabriz University of Medical Sciences, Tabriz, Iran (number: 67306).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This systematic review was approved by the Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. We appreciate the professional researchers of the Research Center for Evidence-Based Medicine, Tabriz University of Medical Sciences, Tabriz, Iran, for their support and guidance.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Hudis CA, Gianni L. Triple-Negative Breast Cancer: An Unmet Medical Need. Oncologist (2011) 16. doi: 10.1634/theoncologist.2011-S1-01
3. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J Clin Invest (2011) 121(7):2750–67. doi: 10.1172/JCI45014
4. Liu S, Wicha MS. Targeting Breast Cancer Stem Cells. J Clin Oncol (2010) 28(25):4006. doi: 10.1200/JCO.2009.27.5388
5. Baumann M, Krause M, Hill R. Exploring the Role of Cancer Stem Cells in Radioresistance. Nat Rev Cancer (2008) 8(7):545–54. doi: 10.1038/nrc2419
6. Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The Epithelial-Mesenchymal Transition Generates Cells With Properties of Stem Cells. Cell (2008) 133(4):704–15. doi: 10.1016/j.cell.2008.03.027
7. Xu H, Niu M, Yuan X, Wu K, Liu A. CD44 as a Tumor Biomarker and Therapeutic Target. Exp Hematol Oncol (2020) 9(1):1–14. doi: 10.1186/s40164-020-00192-0
8. Yaghobi Z, Movassaghpour A, Talebi M, Shadbad MA, Hajiasgharzadeh K, Pourvahdani S, et al. The Role of CD44 in Cancer Chemoresistance: A Concise Review. Eur J Pharmacol (2021) 174147. doi: 10.1016/j.ejphar.2021.174147
9. Yin J, Zhang H, Wu X, Zhang Y, Li J, Shen J, et al. CD44 Inhibition Attenuates EGFR Signaling and Enhances Cisplatin Sensitivity in Human EGFR Wild−Type non−Small−Cell Lung Cancer Cells. Int J Mol Med (2020) 45(6):1783–92. doi: 10.3892/ijmm.2020.4562
10. Wise R, Zolkiewska A. Metalloprotease-Dependent Activation of EGFR Modulates CD44+/CD24– Populations in Triple Negative Breast Cancer Cells Through the MEK/ERK Pathway. Breast Cancer Res Treat (2017) 166(2):421–33. doi: 10.1007/s10549-017-4440-0
11. Herishanu Y, Gibellini F, Njuguna N, Hazan-Halevy I, Keyvanfar K, Lee E, et al. CD44 Signaling via PI3K/AKT and MAPK/ERK Pathways Protects CLL Cells From Spontaneous and Drug Induced Apoptosis Through MCL-1. Leukemia Lymph (2011) 52(9):1758. doi: 10.3109/10428194.2011.569962
12. Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, et al. RAS/MAPK Activation is Associated With Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin Cancer Res (2016) 22(6):1499–509. doi: 10.1158/1078-0432.CCR-15-1125
13. Liu S, Chen S, Yuan W, Wang H, Chen K, Li D, et al. PD-1/PD-L1 Interaction Up-Regulates MDR1/P-Gp Expression in Breast Cancer Cells via PI3K/AKT and MAPK/ERK Pathways. Oncotarget (2017) 8(59):99901. doi: 10.18632/oncotarget.21914
14. Shadbad MA, Hajiasgharzadeh K, Derakhshani A, Silvestris N, Baghbanzadeh A, Racanelli V, et al. From Melanoma Development to RNA-Modified Dendritic Cell Vaccines: Highlighting the Lessons From the Past. Front Immunol (2021) 12:331. doi: 10.3389/fimmu.2021.623639
15. Kong T, Ahn R, Yang K, Zhu X, Fu Z, Morin G, et al. CD44 Promotes PD-L1 Expression and its Tumor-Intrinsic Function in Breast and Lung Cancers. Cancer Res (2020) 80(3):444–57. doi: 10.1158/0008-5472.CAN-19-1108
16. Nam K, Oh S, Lee K-m, Yoo S-a, Shin I. CD44 Regulates Cell Proliferation, Migration, and Invasion via Modulation of C-Src Transcription in Human Breast Cancer Cells. Cell Signalling (2015) 27(9):1882–94. doi: 10.1016/j.cellsig.2015.05.002
17. Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 Interaction Activates Stem Cell Marker Nanog, Stat-3-Mediated MDR1 Gene Expression, and Ankyrin-Regulated Multidrug Efflux in Breast and Ovarian Tumor Cells. J Biol Chem (2008) 283(25):17635–51. doi: 10.1074/jbc.M800109200
18. Xie T-x, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 Activation Regulates the Expression of Matrix Metalloproteinase-2 and Tumor Invasion and Metastasis. Oncogene (2004) 23(20):3550–60. doi: 10.1038/sj.onc.1207383
19. Chen C, Zhao S, Karnad A, Freeman JW. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J Hematol Oncol (2018) 11(1):1–23. doi: 10.1186/s13045-018-0605-5
20. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective Identification of Tumorigenic Breast Cancer Cells. Proc Natl Acad Sci (2003) 100(7):3983–8. doi: 10.1073/pnas.0530291100
21. Taniuchi K, Nishimori I, Hollingsworth MA. Intracellular CD24 Inhibits Cell Invasion by Posttranscriptional Regulation of BART Through Interaction With G3BP. Cancer Res (2011) 71(3):895–905. doi: 10.1158/0008-5472.CAN-10-2743
22. Jaggupilli A, Elkord E. Significance of CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity. Clin Dev Immunol (2012) 2012. doi: 10.1155/2012/708036
23. Pallegar NK, Ayre DC, Christian SL. Repression of CD24 Surface Protein Expression by Oncogenic Ras is Relieved by Inhibition of Raf But Not MEK or PI3K. Front Cell Dev Biol (2015) 3:47. doi: 10.3389/fcell.2015.00047
24. Morel A-P, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of Breast Cancer Stem Cells Through Epithelial-Mesenchymal Transition. PloS One (2008) 3(8):e2888. doi: 10.1371/journal.pone.0002888
25. Collina F, Di Bonito M, Li Bergolis V, De Laurentiis M, Vitagliano C, Cerrone M, et al. Prognostic Value of Cancer Stem Cells Markers in Triple-Negative Breast Cancer. BioMed Res Int (2015) 2015. doi: 10.1155/2015/158682
26. Yang F, Cao L, Sun Z, Jin J, Fang H, Zhang W, et al. Evaluation of Breast Cancer Stem Cells and Intratumor Stemness Heterogeneity in Triple-Negative Breast Cancer as Prognostic Factors. Int J Biol Sci (2016) 12(12):1568. doi: 10.7150/ijbs.16874
27. Liu YX, Wang KR, Xing H, Zhai XJ, Wang LP, Wang W. Attempt Towards a Novel Classification of Triple-Negative Breast Cancer Using Immunohistochemical Markers. Oncol Lett (2016) 12(2):1240–56. doi: 10.3892/ol.2016.4778
28. Wang H, Wang L, Song Y, Wang S, Huang X, Xuan Q, et al. CD44+/CD24−phenotype Predicts a Poor Prognosis in Triple−Negative Breast Cancer. Oncol Lett (2017) 14(5):5890–8. doi: 10.3892/ol.2017.6959
29. Zou W, Yang Y, Zheng R, Wang Z, Zeng H, Chen Z, et al. Association of CD44 and CD24 Phenotype With Lymph Node Metastasis and Survival in Triple-Negative Breast Cancer. Int J Clin Exp Pathol (2020) 13(5):1008.
30. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
31. Hayden JA, Côté P, Bombardier C. Evaluation of the Quality of Prognosis Studies in Systematic Reviews. Ann Internal Med (2006) 144(6):427–37. doi: 10.7326/0003-4819-144-6-200603210-00010
32. de Mendonça Uchôa D, Graudenz MS, Callegari-Jacques SM, Hartmann CR, Ferreira BP, Fitarelli-Kiehl M, et al. Expression of Cancer Stem Cell Markers in Basal and Penta-Negative Breast Carcinomas–a Study of a Series of Triple-Negative Tumors. Pathology-Res Pract (2014) 210(7):432–9. doi: 10.1016/j.prp.2014.03.005
33. Jang MH, Kim HJ, Kim EJ, Chung YR, Park SY. Expression of Epithelial-Mesenchymal Transition–Related Markers in Triple-Negative Breast Cancer: ZEB1 as a Potential Biomarker for Poor Clinical Outcome. Hum Pathol (2015) 46(9):1267–74. doi: 10.1016/j.humpath.2015.05.010
34. Chang S-J, Ou-Yang F, Tu H-P, Lin C-H, Huang S-H, Kostoro J, et al. Decreased Expression of Autophagy Protein LC3 and Stemness (CD44+/CD24–/low) Indicate Poor Prognosis in Triple-Negative Breast Cancer. Hum Pathol (2016) 48:48–55. doi: 10.1016/j.humpath.2015.09.034
35. Riaz N, Idress R, Habib S, Lalani E-N. Lack of Androgen Receptor Expression Selects for Basal-Like Phenotype and Is a Predictor of Poor Clinical Outcome in Non-Metastatic Triple Negative Breast Cancer. Front Oncol (2020) 10:1083. doi: 10.3389/fonc.2020.01083
36. Wang Z, Sau S, Alsaab HO, Iyer AK. CD44 Directed Nanomicellar Payload Delivery Platform for Selective Anticancer Effect and Tumor Specific Imaging of Triple Negative Breast Cancer. Nanomed: Nanotechnol Biol Med (2018) 14(4):1441–54. doi: 10.1016/j.nano.2018.04.004
37. Lotfinejad P, Kazemi T, Safaei S, Amini M, Baghbani E, Shotorbani SS, et al. PD-L1 Silencing Inhibits Triple-Negative Breast Cancer Development and Upregulates T-Cell-Induced Pro-Inflammatory Cytokines. Biomed Pharmacother (2021) 138:111436. doi: 10.1016/j.biopha.2021.111436
38. Zhang C, Wang H, Wang X, Zhao C, Wang H. CD44, a Marker of Cancer Stem Cells, is Positively Correlated With PD-L1 Expression and Immune Cells Infiltration in Lung Adenocarcinoma. Cancer Cell Int (2020) 20(1):1–8. doi: 10.1186/s12935-020-01671-4
39. Hosseinkhani N, Derakhshani A, Kooshkaki O, Abdoli Shadbad M, Hajiasgharzadeh K, Baghbanzadeh A, et al. Immune Checkpoints and CAR-T Cells: The Pioneers in Future Cancer Therapies? Int J Mol Sci (2020) 21(21):8305. doi: 10.3390/ijms21218305
40. Lotfinejad P, Asghari Jafarabadi M, Abdoli Shadbad M, Kazemi T, Pashazadeh F, Sandoghchian Shotorbani S, et al. Prognostic Role and Clinical Significance of Tumor-Infiltrating Lymphocyte (TIL) and Programmed Death Ligand 1 (PD-L1) Expression in Triple-Negative Breast Cancer (TNBC): A Systematic Review and Meta-Analysis Study. Diagnostics (2020) 10(9):704. doi: 10.3390/diagnostics10090704
41. Zheng Z, Shao N, Weng H, Li W, Zhang J, Zhang L, et al. Correlation Between Epidermal Growth Factor Receptor and Tumor Stem Cell Markers CD44/CD24 and Their Relationship With Prognosis in Breast Invasive Ductal Carcinoma. Med Oncol (2015) 32(1):275. doi: 10.1007/s12032-014-0275-2
42. Fu W, Sun H, Zhao Y, Chen M, Yang L, Yang X, et al. Targeted Delivery of CD44s-siRNA by ScFv Overcomes De Novo Resistance to Cetuximab in Triple Negative Breast Cancer. Mol Immunol (2018) 99:124–33. doi: 10.1016/j.molimm.2018.05.010
43. Shadbad MA, Hajiasgharzadeh K, Baradaran B. Cross-Talk Between Myeloid-Derived Suppressor Cells and Mucin1 in Breast Cancer Vaccination: On the Verge of a Breakthrough. Life Sci (2020) 118128. doi: 10.1016/j.lfs.2020.118128
44. Maeda T, Hiraki M, Jin C, Rajabi H, Tagde A, Alam M, et al. MUC1-C Induces PD-L1 and Immune Evasion in Triple-Negative Breast Cancer. Cancer Res (2018) 78(1):205–15. doi: 10.1158/0008-5472.CAN-17-1636
45. Vahidian F, Safarzadeh E, Mohammadi A, Najjary S, Mansoori B, Majidi J, et al. siRNA-Mediated Silencing of CD44 Delivered by Jet Pei Enhanced Doxorubicin Chemo Sensitivity and Altered miRNA Expression in Human Breast Cancer Cell Line (MDA-Mb468). Mol Biol Rep (2020) 47(12):9541–51. doi: 10.1007/s11033-020-05952-z
46. Cheng C-C, Shi L-H, Wang X-J, Wang S-X, Wan X-Q, Liu S-R, et al. Stat3/Oct-4/C-Myc Signal Circuit for Regulating Stemness-Mediated Doxorubicin Resistance of Triple-Negative Breast Cancer Cells and Inhibitory Effects of WP1066. Int J Oncol (2018) 53(1):339–48. doi: 10.3892/ijo.2018.4399
47. Misra S, Ghatak S, Toole BP. Regulation of MDR1 Expression and Drug Resistance by a Positive Feedback Loop Involving Hyaluronan, Phosphoinositide 3-Kinase, and Erbb2. J Biol Chem (2005) 280(21):20310–5. doi: 10.1074/jbc.M500737200
48. Huang C, Yoon C, Zhou X-H, Zhou Y-C, Zhou W-W, Liu H, et al. ERK1/2-Nanog Signaling Pathway Enhances CD44 (+) Cancer Stem-Like Cell Phenotypes and Epithelial-to-Mesenchymal Transition in Head and Neck Squamous Cell Carcinomas. Cell Death Dis (2020) 11(4):1–14. doi: 10.1038/s41419-020-2448-6
49. Najafzadeh B, Asadzadeh Z, Motafakker Azad R, Mokhtarzadeh A, Baghbanzadeh A, Alemohammad H, et al. The Oncogenic Potential of NANOG: An Important Cancer Induction Mediator. J Cell Physiol (2020). doi: 10.1002/jcp.30063
50. Chen Y, Song J, Jiang Y, Yu C, Ma Z. Predictive Value of CD44 and CD24 for Prognosis and Chemotherapy Response in Invasive Breast Ductal Carcinoma. Int J Clin Exp Pathol (2015) 8(9):11287.
51. Vikram R, Chou WC, Hung S-C, Shen C-Y. Tumorigenic and Metastatic Role of CD44–/low/CD24–/low Cells in Luminal Breast Cancer. Cancers (2020) 12(5):1239. doi: 10.3390/cancers12051239
52. Alkaraki A, Alshaer W, Wehaibi S, Gharaibeh L, Abuarqoub D, Alqudah DA, et al. Enhancing Chemosensitivity of Wild-Type and Drug-Resistant MDA-MB-231 Triple-Negative Breast Cancer Cell Line to Doxorubicin by Silencing of STAT 3, Notch-1, and β-Catenin Genes. Breast Cancer (2020) 1-10. doi: 10.1007/s12282-020-01098-9
53. Ghuwalewala S, Ghatak D, Das P, Dey S, Sarkar S, Alam N, et al. CD44highCD24low Molecular Signature Determines the Cancer Stem Cell and EMT Phenotype in Oral Squamous Cell Carcinoma. Stem Cell Res (2016) 16(2):405–17. doi: 10.1016/j.scr.2016.02.028
54. Van Phuc P, Nhan PLC, Nhung TH, Tam NT, Hoang NM, Tue VG, et al. Downregulation of CD44 Reduces Doxorubicin Resistance of CD44+ CD24– Breast Cancer Cells. OncoTargets Ther (2011) 4:71. doi: 10.2147/OTT.S21431
55. Muntimadugu E, Kumar R, Saladi S, Rafeeqi TA, Khan W. CD44 Targeted Chemotherapy for Co-Eradication of Breast Cancer Stem Cells and Cancer Cells Using Polymeric Nanoparticles of Salinomycin and Paclitaxel. Colloids Surfaces B: Biointerfaces (2016) 143:532–46. doi: 10.1016/j.colsurfb.2016.03.075
56. McFarlane S, Coulter JA, Tibbits P, O’Grady A, McFarlane C, Montgomery N, et al. CD44 Increases the Efficiency of Distant Metastasis of Breast Cancer. Oncotarget (2015) 6(13):11465. doi: 10.18632/oncotarget.3410
57. Van Pham P, Vu NB, Duong TT, Nguyen TT, Truong NH, Phan NLC, et al. Suppression of Human Breast Tumors in NOD/SCID Mice by CD44 shRNA Gene Therapy Combined With Doxorubicin Treatment. OncoTargets Ther (2012) 5:77. doi: 10.2147/OTT.S30609
58. Xie X, Li L, Wei W, Kong Y, Wu M, Yang L, et al. Abstract P5-10-10: The miR-34a is Down-Regulated in Breast Cancer and Breast Stem Cells and a Potential to Eradicating Breast Cancer via a Systemic Delivery of a VISA–miR-34a Nanoparticle System. AACR (2012). doi: 10.1158/0008-5472.SABCS12-P5-10-10
59. Ahir M, Upadhyay P, Ghosh A, Sarker S, Bhattacharya S, Gupta P, et al. Delivery of Dual miRNA Through CD44-Targeted Mesoporous Silica Nanoparticle for Enhanced and Effective Triple-Negative Breast Cancer Therapy. Biomater Sci (2020) 8. doi: 10.1039/D0BM00015A
60. Al-Othman N, Hammad H, Ahram M. Dihydrotestosterone Regulates Expression of CD44 via miR-328-3p in Triple-Negative Breast Cancer Cells. Gene (2018) 675:128–35. doi: 10.1016/j.gene.2018.06.094
61. Derakhshani A, Rostami Z, Safarpour H, Shadbad MA, Nourbakhsh NS, Argentiero A, et al. From Oncogenic Signaling Pathways to Single-Cell Sequencing of Immune Cells: Changing the Landscape of Cancer Immunotherapy. Molecules (2021) 26(8):2278. doi: 10.3390/molecules26082278
62. Castagnoli L, Cancila V, Cordoba-Romero SL, Faraci S, Talarico G, Belmonte B, et al. WNT Signaling Modulates PD-L1 Expression in the Stem Cell Compartment of Triple-Negative Breast Cancer. Oncogene (2019) 38(21):4047–60. doi: 10.1038/s41388-019-0700-2
Keywords: triple-negative breast cancer, cancer stem cell, cancer therapeutic resistance, CD44, CD44/CD24, prognosis
Citation: Abdoli Shadbad M, Hosseinkhani N, Asadzadeh Z, Derakhshani A, Karim Ahangar N, Hemmat N, Lotfinejad P, Brunetti O, Silvestris N and Baradaran B (2021) A Systematic Review to Clarify the Prognostic Values of CD44 and CD44+CD24- Phenotype in Triple-Negative Breast Cancer Patients: Lessons Learned and The Road Ahead. Front. Oncol. 11:689839. doi: 10.3389/fonc.2021.689839
Received: 01 April 2021; Accepted: 21 July 2021;
Published: 09 August 2021.
Edited by:
Guohong Hu, Shanghai Institute of Nutrition and Health (CAS), ChinaReviewed by:
Bruna Cerbelli, Sapienza University of Rome, ItalySofia Gameiro, National Cancer Institute (NCI), United States
Mariana Segovia, National Autonomous University of Mexico, Mexico
Copyright © 2021 Abdoli Shadbad, Hosseinkhani, Asadzadeh, Derakhshani, Karim Ahangar, Hemmat, Lotfinejad, Brunetti, Silvestris and Baradaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Behzad Baradaran, YmFyYWRhcmFuYkB0YnptZWQuYWMuaXI=; Nicola Silvestris, bi5zaWx2ZXN0cmlzQG9uY29sb2dpY28uYmFyaS5pdA==
†These authors have contributed equally to this work
‡These authors share last authorship
 Mahdi Abdoli Shadbad
Mahdi Abdoli Shadbad Negar Hosseinkhani
Negar Hosseinkhani Zahra Asadzadeh
Zahra Asadzadeh Afshin Derakhshani
Afshin Derakhshani Noora Karim Ahangar
Noora Karim Ahangar Nima Hemmat
Nima Hemmat Parisa Lotfinejad
Parisa Lotfinejad Oronzo Brunetti
Oronzo Brunetti Nicola Silvestris
Nicola Silvestris Behzad Baradaran
Behzad Baradaran