- 1Department of Abdominal Cancer, Cancer Center, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Biotherapy, Cancer Center, West China Hospital of Sichuan University, Chengdu, China
Intra-abdominal desmoplastic small round cell tumor (IDSRCT) is a rare and highly malignant soft tissue neoplasm, which is characterized by rapid progression and poor prognosis. The mechanism underlying the development of this neoplasm remains elusive, but all cases are characterized by the chromosomal translocation t (11;22) (p13; q12), which results in a formation of EWSR1-WT1 gene fusion. The diagnosis of IDSRCT is often made with core-needle tissue biopsy specimens or laparoscopy or laparotomy. Immunohistochemical analyses have shown the co-expression of epithelial, neuronal, myogenic, and mesenchymal differentiation markers. FISH or reverse transcription polymerase chain reaction detecting EWS-WT1 fusion can be performed to assist in molecular confirmation. There is no standard of care for patients with IDSRCT currently, and majority of newly diagnosed patients received the aggressive therapy, which includes >90% resection of surgical debulking, high-dose alkylator-based chemotherapy, and radiotherapy. More recently, targeted therapy has been increasingly administered to recurrent IDSRCT patients and has been associated with improved survival in clinical conditions. Immunotherapy as a possible therapeutic strategy is being explored in patients with IDSRCT. In this review, we summarize currently available knowledge regarding the epidemiology, potential mechanisms, clinical manifestations, diagnosis, treatment, and prognosis of IDSRCT to assist oncologists in comprehensively recognizing and accurately treating this malignancy.
1. Introduction
Desmoplastic small round cell tumor (DSRCT), according to the International Classification of Disease for Oncology (2020), is categorized as a malignant tumor of uncertain differentiation. DSRCT typically occurs in the abdominal cavity, which is known as intra-abdominal desmoplastic small round cell tumor (IDSRCT) (1). Other primary sites have been reported (Figure 1). It is a rare and aggressive malignant soft tissue sarcoma that predominantly occurs in young male adults (20). IDSRCTs are associated with chromosomal translocation t (11;22) (p13; q12), which results in a formation of EWSR1-WT1 gene fusion (21, 22). IDSRCT mainly originates in the abdominopelvic cavity, involving the mesentery and retroperitoneum (23, 24). There is a lack of international consensus regarding the treatment of IDSRCT, and therapeutic regimens were derived from Ewing sarcoma’s (ES) treatment because of the involvement of the EWS gene and activation of similar oncogenic pathways in both ES and IDSRCT. The prognosis of IDSRCT is poor, with a median 5-year survival rate of 15%–25% (25). Because of the rarity of this malignancy, very few studies have investigated it, and most of these studies are case reports. This review summarizes the current knowledge on the epidemiology, potential mechanisms, clinical manifestations, diagnosis, treatment, and prognosis of IDSRCT, highlighting the modalities of treatment.
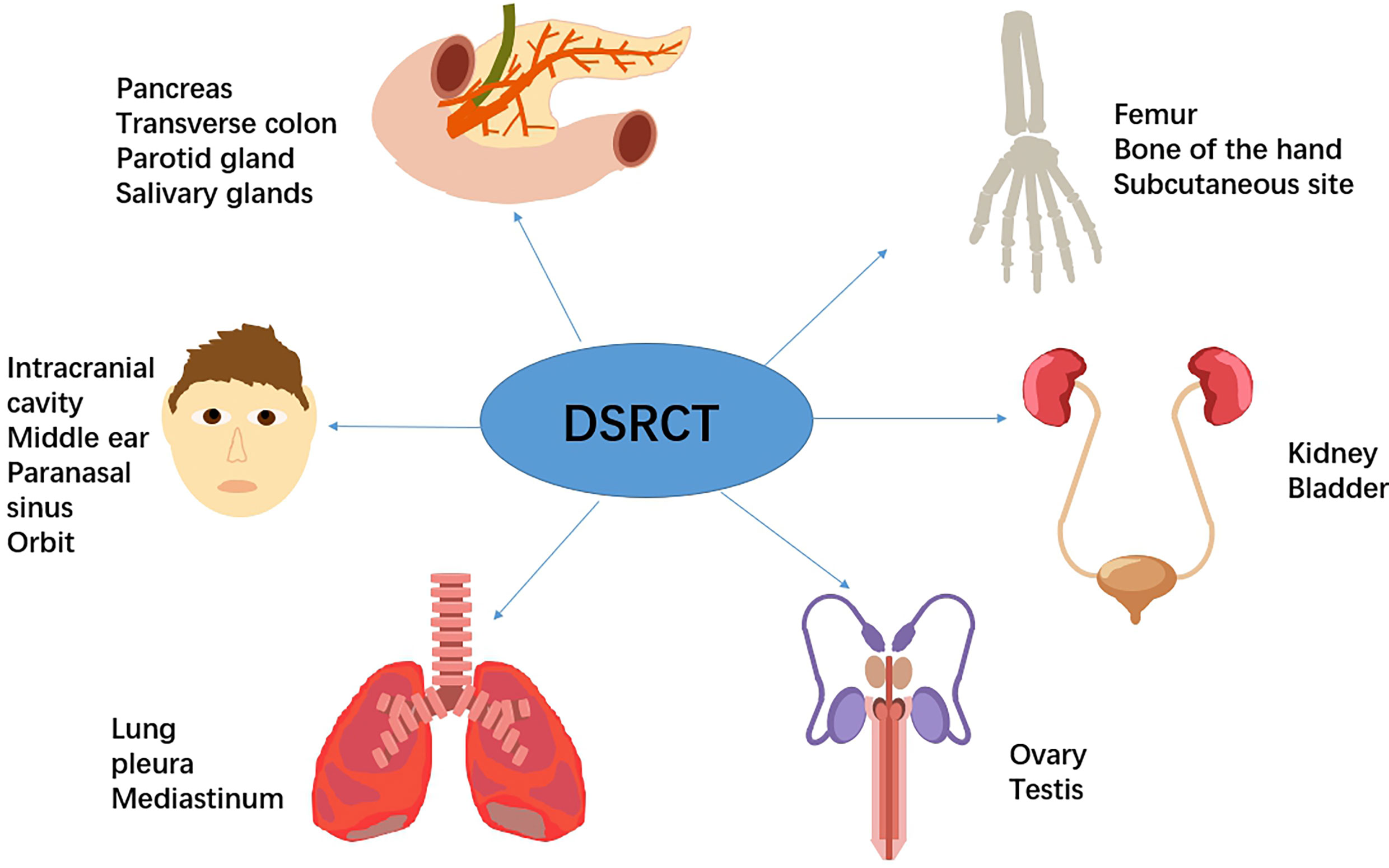
Figure 1 Previous case reports reported primary sites of DSRCT, including kidney (2), bladder (3), pancreas (4), transverse colon (5), testes (6), ovary (7), mediastinum (8), pleura (9), lung (10), parotid gland (11), salivary glands (12), middle ear (13), sinonasal (14), orbital (15), intracranial cavity (16), subcutaneous site (17), femur (18), and bone of the hand (19).
2. Methods
This review summarizes the available literature on IDSRCT. We used the terms “abdominal,” “desmoplastic small round cell tumor,” and “intra-abdominal desmoplastic small round cell tumor” in our literature search. Previous reviews, articles, clinical trials, and case reports were included, and our search was not limited by language. Studies published from 1989 to 2021 were analyzed in the current review.
3. Epidemiology
IDSRCT is a rare and aggressive soft-tissue sarcoma. Its morbidity ranges between 0.2 and 0.74 cases per million people per year (26–28). IDSRCT mainly affects adolescents or young adults (median age at diagnosis: 27.0 years; range: 16–45 years), with a marked predominance in males (4:1 male-to-female ratio) (29, 30). No specific risk factors associated with the occurrence or progression of IDSRCT have been reported.
4. Mechanism
The pathogenesis of IDSRCT remains elusive. However, it is uniquely characterized by chromosomal translocation t (11;22) (p13; q12), which results in the fusion of the EWS and WT1 genes. The wild-type WT1 gene encodes a zinc finger-containing protein, which acts as a repressor of transcription. With the fusion of the EWS and WT1 genes, the normal function of the zinc finger region of the WT1 gene is lost, leading to the transcriptional activation of at least 35 downstream target genes (31, 32). These target genes encode growth factors and growth factor receptors, such as platelet-derived growth factor (PDGF)-α, Type-1 insulin-like growth factor receptor (IGF-1-R), and epidermal growth factor receptor (EGFR) (33), which are related to tissue differentiation and the proliferation, adhesion, and metastasis of the tumor cells (34).
Recent genomics analysis of mutational profiles indicated that epithelial–mesenchymal transition (EMT), immune response, and the DNA damage response (DDR) are associated with gene deregulation in DSRCT (35). Whole-exome sequencing of six consecutive pre-treated DSRCT samples identified 137 unique somatic mutations: 133 mutated genes were case-specific, and only 2 genes were overlapping among two cases but in different locations, which reveals the heterogeneity of the DSRCT genome. They also discovered that 27% of the 135 mutated genes are associated with DDR and EMT/mesenchymal–epithelial reverse transition (MErT), which could result in tumor extreme heterogeneity followed by genomic instability, and consequently produce drug resistance (35). MErT/EMT plays a crucial role in the metastasis and associated invasiveness of the sarcoma (36). Jiang et al. reported two novel somatic mutations, one associated with c-Met tyrosine kinase and the other related to PIK3CA gene, in 10 advanced stage DSRCT patients (37). The latter mutation was involved in the activation of the PI3K/AKT/mTOR pathway, facilitating the growth and proliferation of tumor cells (38).
5. Clinical Manifestations
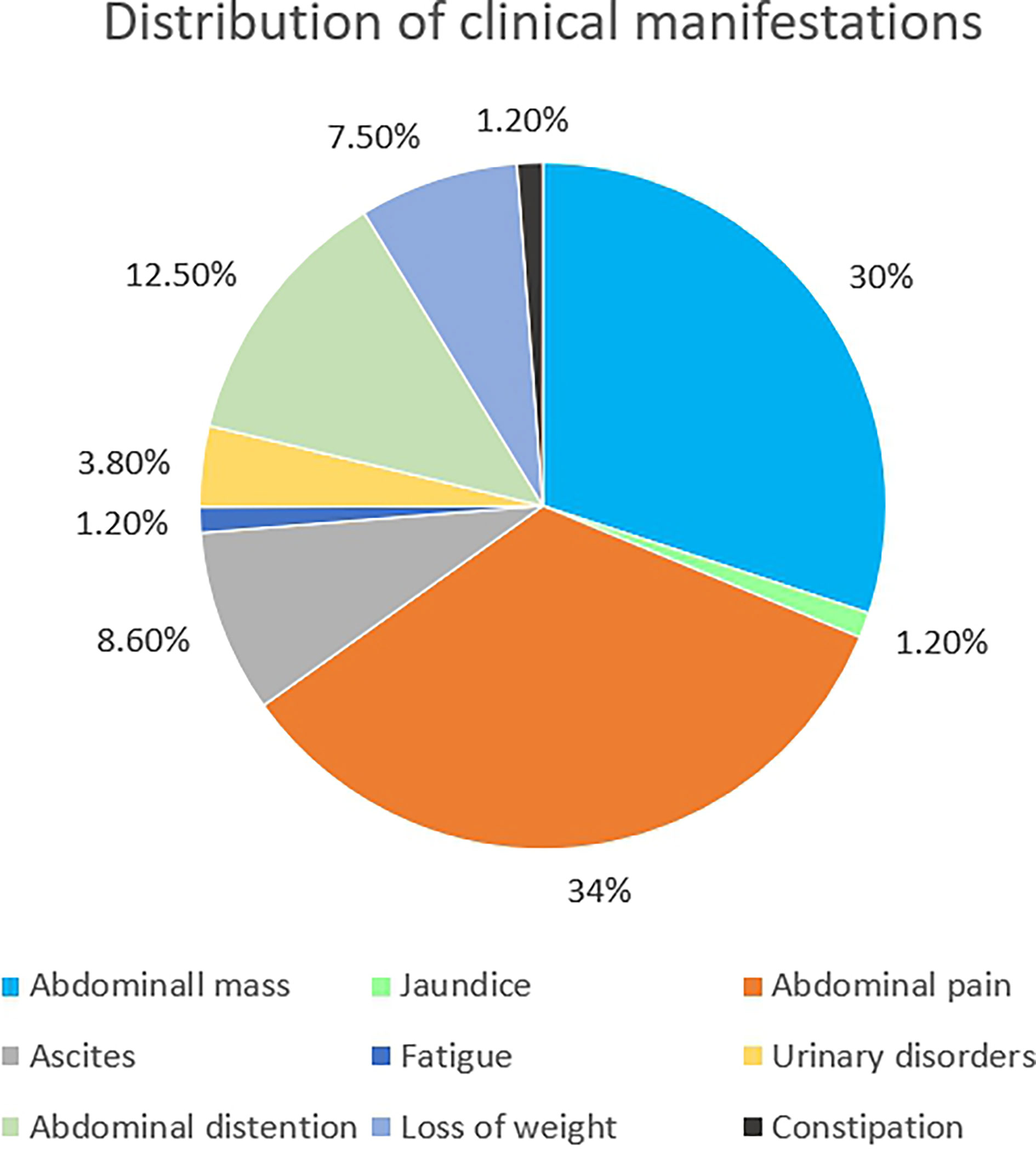
IDSRCT is commonly diagnosed at an advanced stage. Enlarged lymph nodes were seen in 50% of the patients, and distant metastasis was reported in 25% of the cases at the time of diagnosis (39). Widespread tumor nodules were also observed throughout the peritoneum, especially in the mesentery or omentum (40) (Table 1). The most common extraperitoneal metastasis is liver, followed by lymph node, bones, and lung (27, 28). The manifestations of IDSRCT are nonspecific and are related to the size, location, and speed of disease progression (40, 70, 71). It usually presents as a palpable abdominal mass with pain and other diverse symptoms, including distention, ascites, loss of weight, jaundice, fatigue, and constipation (Figure 2). Huge tumor masses can also cause compression symptoms, like intestinal obstruction and ureteral obstruction (72).
6. Diagnosis
The diagnosis of IDSRCT is often made with core-needle tissue biopsy specimens or laparoscopy or laparotomy (73). IDSRCT is thought to originate from progenitor cells with polyphenotypic potential (38). Macroscopically, IDSRCT is visible as a pale and firm mass in the peritoneal cavity with or without hemorrhage, necrosis, and cystic degeneration (20). Microscopically, histological examination shows uniform small round tumor cells grouped as clumps and nests with unclear cell borders, hyperchromatic nuclei, and inconspicuous nucleoli surrounded by hypocellular desmoplastic stroma. Glandular or rosette patterns or nuclear molding can be discovered. In addition, mitosis and apoptosis are common (71, 74, 75). However, a recent case report described a 12-year-old boy of IDSRCT characterized by spindle cells with scant cytoplasm and with no desmoplastic stromal reaction, which reveals the heterogeneity of morphologic features in IDSRCT (72). Immunohistochemical analyses of IDSRCT have shown the co-expression of epithelial (cytokeratin and epithelial membrane antigen), neuronal (NSE and CD56), myogenic (desmin), and mesenchymal (vimentin) differentiation markers and WT1 (C-terminus antibody) (Table 2). Notably, >90% of patients with DSRCT express desmin and EMA (80). Most IDSRCT patients typically do not express CD 99, HMB-45, S-100, and myogenin (75, 81)
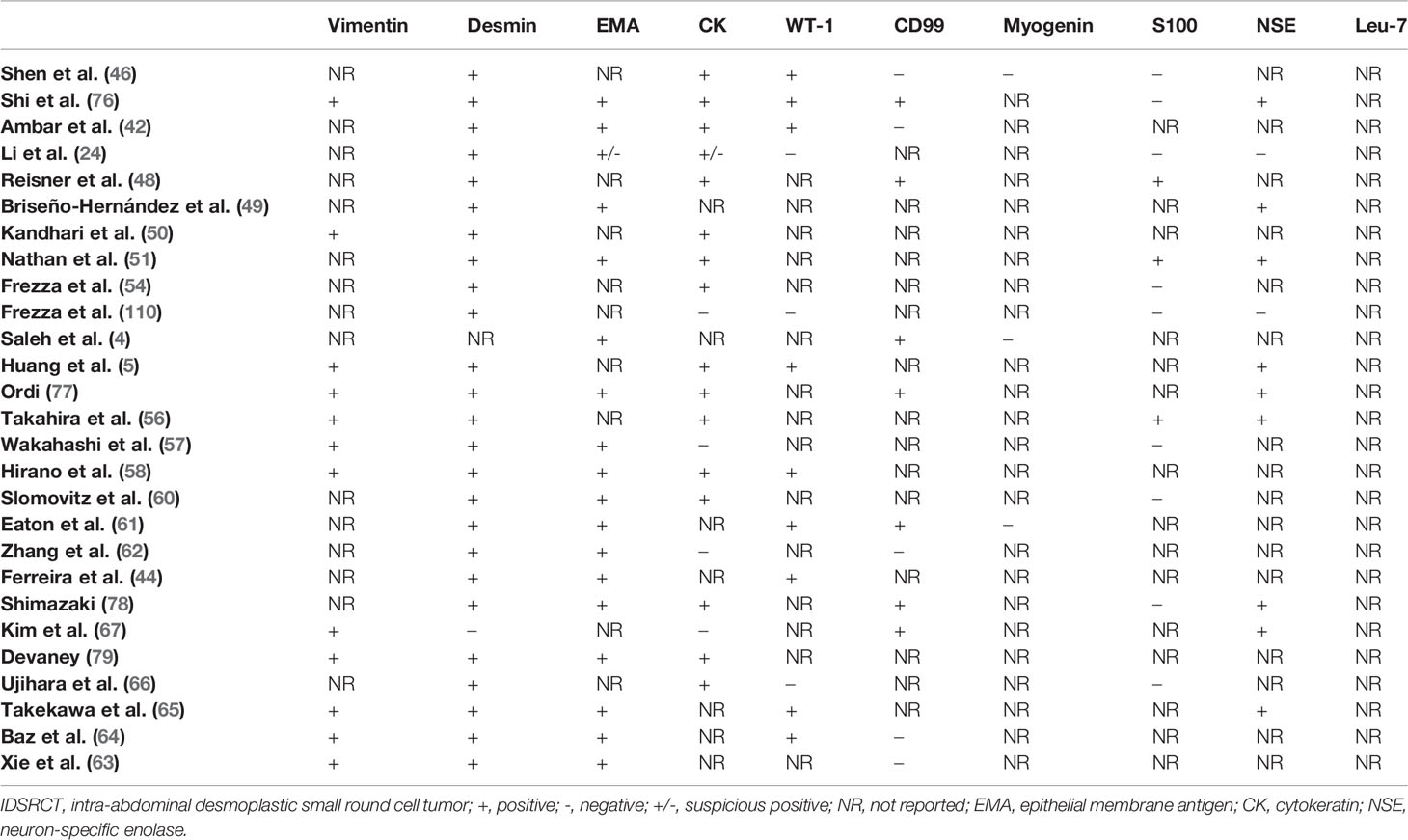
Table 2 The features of immunohistochemical staining of IDSRCT reviewed from published case reports and case series.
As for molecular characteristics of DSRCT, previous literature described that almost all DSRCTs exhibit nuclear positivity for the DNA binding domain (C-terminal portion) of WT1, which can distinguish DSRCT from Wilms tumor and Ewing sarcoma (80). The specific chromosomal translocation t(11;22) (p13; q12) of DSRCT produces a chimeric EWSR1-WT1 fusion gene that encodes an aberrant transcription regulatory factor consisting of the C-terminal portion of WT1 and the trans-activation domain (N-terminal portion) of EWS (72). The EWSR1-WT1 fusion gene generally consists of exon 7 of the EWSR1 gene on chromosome 22 and exons 8–10 of the WT1 gene on chromosome 11 (82). However, several studies reported various alternative breakpoints for the t (11;22) (p13; q12) translocation (72, 83–85). Those fusion gene variants generally contain additional exons from EWS with conservation of the WT1 complement (EWS-WT1 8/8, 9/8, 10/8, and 9/7). Due to the heterogeneity of immunohistochemical findings, molecular methods are essential to verify diagnosis of IDSRCT (86). Molecular analysis by fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction to detect EWSR1 rearrangement and the EWSR1-WT1 fusion gene, respectively, has also been used to confirm IDSRCT diagnosis along with clinical findings (86). The molecular methods have higher sensitivity than that of immunohistochemical tools (87).
No specific tumor markers have been identified for the diagnosis of IDSRCT, except elevated serum CA125 and NSE levels in some patients (3, 57, 71). For imaging information, ultrasound usually shows lobulated peritoneal masses with variable echogenicity, and dystrophic intratumoral calcification is discovered in 20% of cases (75). Computed tomography (CT) is considered as a primary auxiliary method for the assessment of response and follow-up of IDSRCT, which typically show multifocal masses originating from the retroperitoneum or abdominopelvic cavity with poorly defined boundaries and unevenly enhanced signals. Cystic changes in large masses with heterogeneous enhancement can be found after contrast. Some cases had evidence of punctate calcification in primary mass (34, 88, 89). Twenty percent of IDRCT patients show ascites, and lymph node involvement can be seen in 50% of cases (75). Magnetic resonance imaging (MRI) can detect potential lesions that are not observed by CT alone. Due to the presence of necrosis, hemorrhage, and fibrous stroma in IDSRCT, it often shows heterogeneous high-intensity signals on T2-weighted images and hypointense or isointense signals on T1-weighted images in MRI with heterogeneous enhancement after gadolinium (74, 75). Although CT and MRI can help to identify primary sites of IDSRCT, they have limited power to show the metabolic activity of tumors, which promotes the application of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) and CT (FDG PET/CT) in IDSRCT (90). A retrospective study indicated that FDG-PET may earlier predict histologic response to chemotherapy than macroscopic size change detected by CT. Although most patients obtain rapid symptom relief when treated with chemotherapy, the change of tumor size is generally minimal because of the presence of abundant stromal component in IDSRCT, while the great decrease of metabolic activity can be detected by FDG-PET earlier (91). Furthermore, the specificity of FDG PET/CT is as high as 97.4% in DSRCT lesions, especially for involved lymph node and bone metastatic lesions, allowing early discovery of recurrent IDRCT and change of treatment strategy (90, 92, 93). Thus, FDG PET-CT should be considered as the preferred imaging method for monitoring patients with IDSRCT (90).
7. Staging
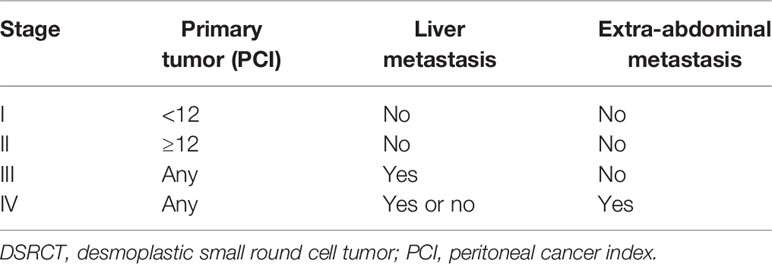
There is no definite staging system for IDSRCT. Green et al. (94) proposed a staging system based on primary tumor burden, liver metastasis, and extra-abdominal metastasis. Primary tumor burden was measured using the peritoneal cancer index (PCI) score, calculated by measuring the tumor diameter in 13 abdominopelvic regions (95). In this staging system, a lower PCI score (<12) without liver or extra-abdominal metastasis was defined as Stage I; a higher PCI score (≥12) without liver or extra-abdominal metastasis was defined as Stage II; liver metastasis without extra-abdominal metastasis was defined as Stage III; and extra-abdominal metastasis regardless of PCI score was defined as Stage IV (Table 3). However, this staging system requires further validation before being applied to all DSRCT types.
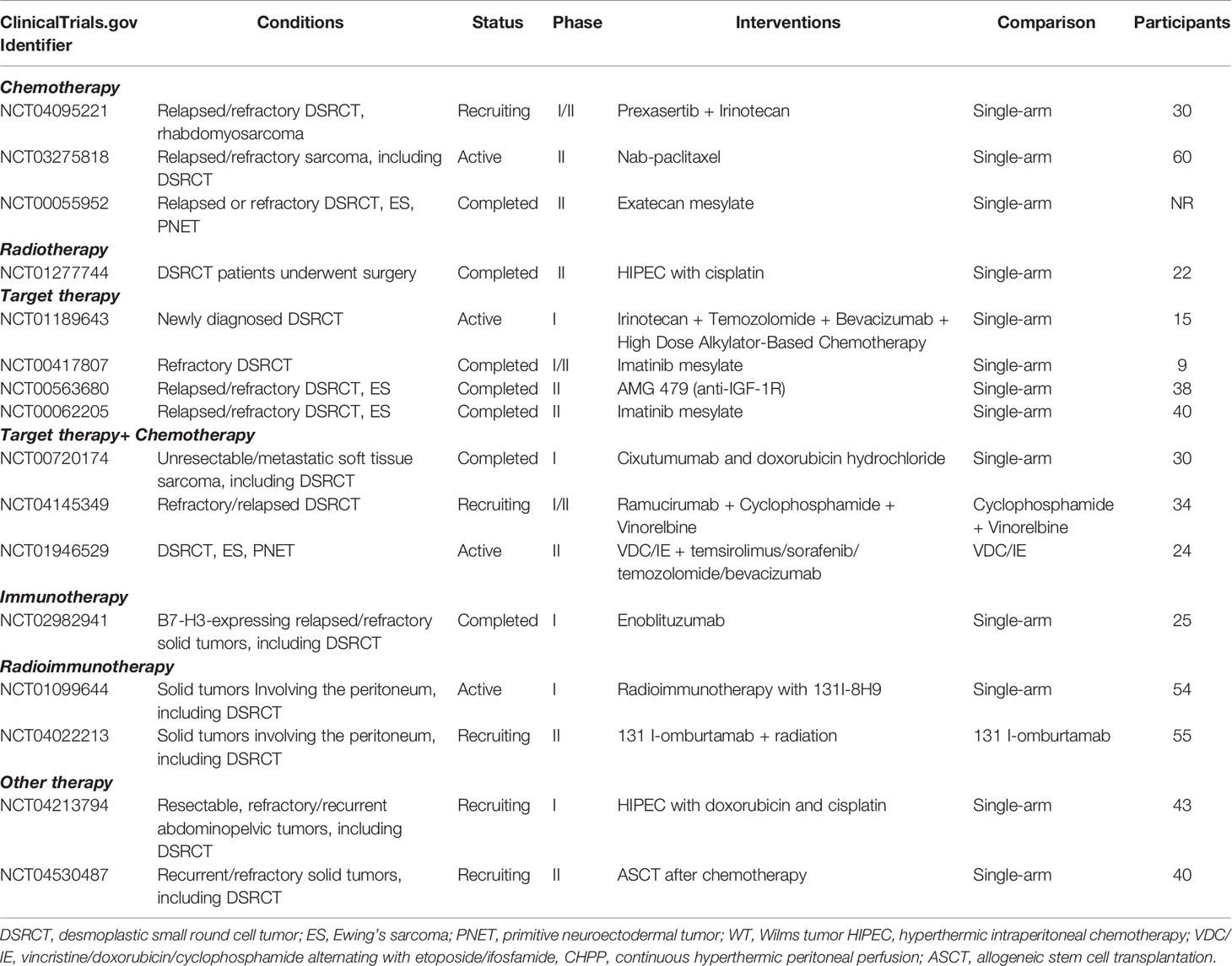
8. Treatments
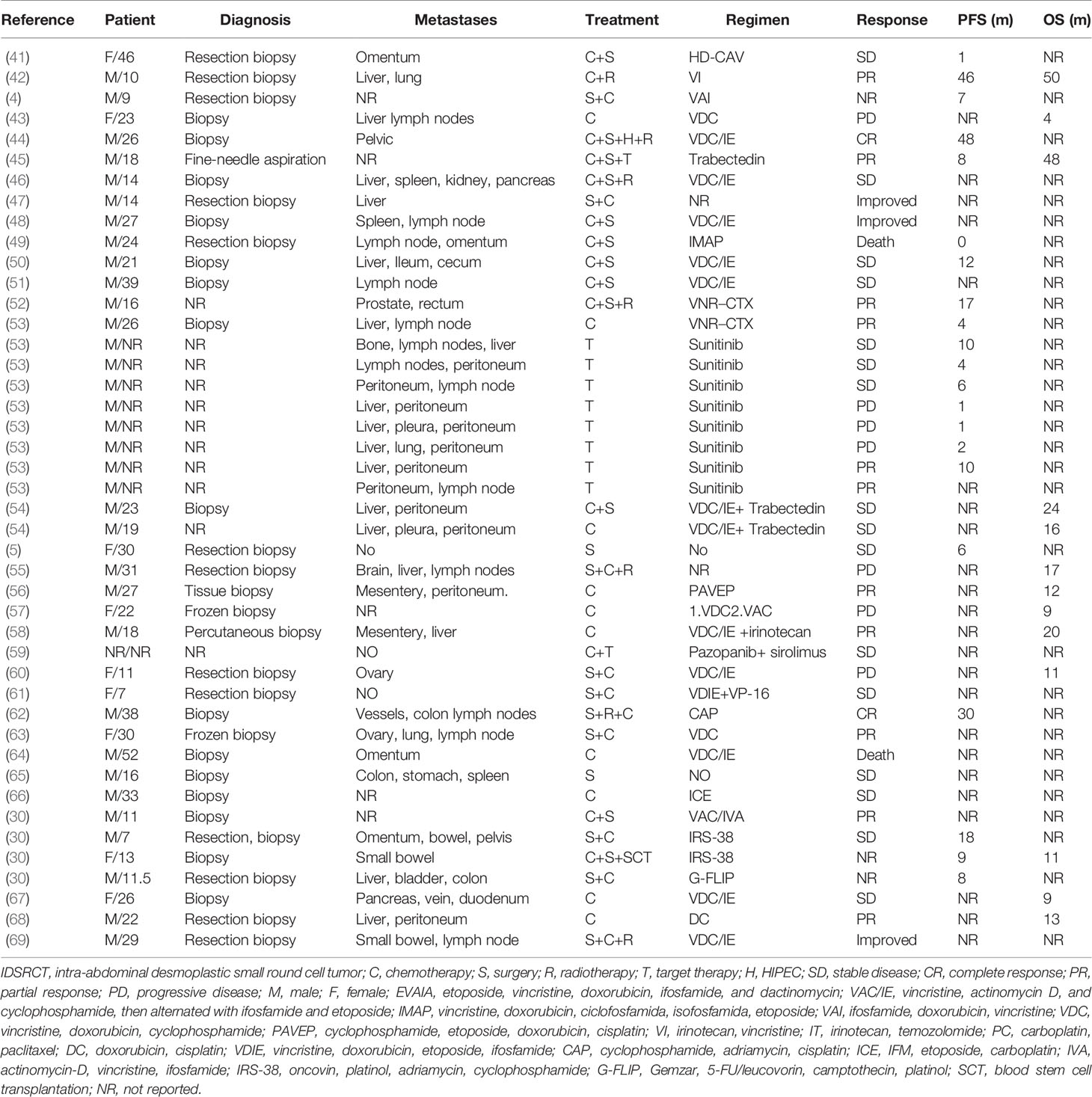
The therapeutic regimen for IDSRCT is mainly derived from ES treatment because of the similarity in the oncogenic pathways involved and EWS gene fusion in both malignancies (31). Although multimodal treatments have been proposed for IDSRCT patients, the prognosis remains poor (96). With the widespread utilization of targeted therapy, the prognosis of IDSRCT patients has markedly improved. Published case reports and case series of treatments administered to IDSRCT patients are summarized in Table 1.
8.1. Surgery
Radical surgical excision without residual disease is usually impossible because of the presence of multiple serosal tumor nodules and obscure boundary of IDSRCT (97). Thus, cytoreductive surgery (CRS) is regarded as the fundamental therapy for IDSRCT patients, which is defined as the resection of ≥90% of the tumor burden, preserving the non-invaded peritoneum macroscopically (70, 98). The lesions are generally confined to the serosal or superficial muscle layers, although there is dissemination throughout the peritoneal cavity, allowing the treatment of local tangential resection (99). The surgical resection extension is usually extensive, including the resection of primary disease with acceptable margins, peritonectomy, lymphadenectomy, and the resection of involved adjacent tissue (89). To preserve bowel length, wedge excision can be performed to remove masses that invade deeply into the bowel (99). According to the recent studies, patients treated with complete CRS have significantly improved survival compared with patients receiving insufficient or no surgery with macroscopic residual tumor (the 3-year survival rates 49.6% vs. 31.1% vs. 13.7%, p = 0.009) (70, 100). Complete resection of metastases combined with cytoreduction is essential in patients with extra-abdominal disease, while locoregional surgery alone typically cannot be considered if patients have extra-abdominal lesion. Moreover, it is not a first option for IDSRCT patients with extensive subdiaphragmatic lesion or unresectable periportal disease or widely infiltration or metastases of liver to received CRS (99). Based on the Sugarbaker completeness of cytoreduction (CR) score, the completeness of CRS was classified under four categories: CR 0, no macroscopically residual disease; CR 1, residual nodules smaller than 2.5 mm; CR2, residual nodule size ranging from 2.5 mm to 2.5 cm; and CR 3, residual nodule size larger than 2.5 cm (101). To achieve complete CRS, multivisceral resection is often needed, followed by various complications. Fifty-six percent of patients after surgery have mild complications, including urinary retention/urinary tract infection, wound infection, and ileus, while pelvic abscess, AKI, respiratory failure, and anastomotic dehiscence were reported in the residual 44% of patients who received surgery (102). Therefore, extensive surgical resection-associated complications should be considered before the administration of surgery.
8.2. Chemotherapy
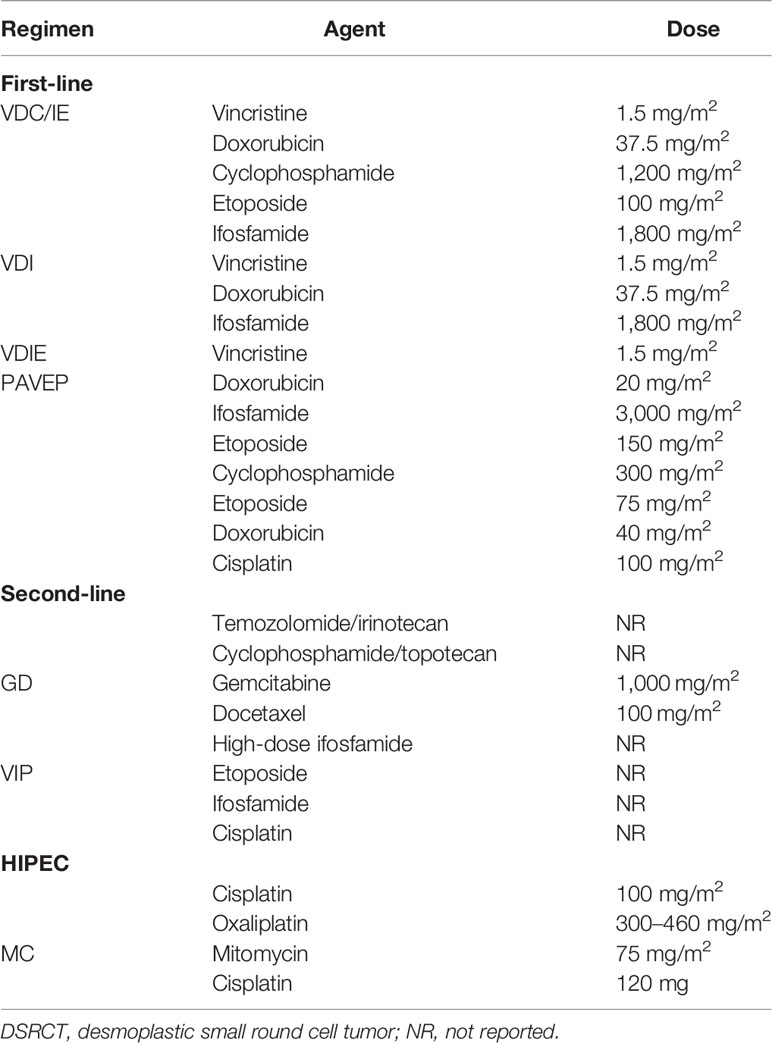
IDSRCT is sensitive to chemotherapy; therefore, neoadjuvant chemotherapy is usually recommended for patients with advanced-stage and unresectable IDSRCT (27). Several studies have suggested that IDSRCT patients who have evident efficacy to neoadjuvant chemotherapy have an improved overall survival (OS) than those who are resistant to neoadjuvant therapy (27, 103). However, IDSRCT treated with neoadjuvant chemotherapy may not have a significant reduction of size because of its large stromal component, which should not delay or prevent an attempt of administration of CRS (91). The chemotherapy regimen of IDSRCT mainly follows the same schedule as that used in ES (25). The P6 chemotherapy regimen, comprising vincristine/doxorubicin/cyclophosphamide alternating with etoposide/ifosfamide (VDC/IE), is the most common neoadjuvant chemotherapy regimen for IDSRCT patients (43, 69). Vincristine, doxorubicin, and ifosfamide are a reasonable alternative regimen for older people who may not tolerate the intense regimen (73). For P6 chemotherapy-resistant patients, temozolomide/irinotecan, cyclophosphamide/topotecan, gemcitabine/docetaxel, and high-dose ifosfamide can be considered as second- or third-line chemotherapy regimens (Table 4) (75, 80, 104). Some IDSRCT patients receive adjuvant chemotherapy (high-dose 5-FU, temozolomide/irinotecan-based therapy, and high-dose ifosfamide) (104–106) in combination with radiotherapy after CRS to increase the effectiveness of the surgery (105, 106). Palliative chemotherapy is administered to patients with metastatic tumors at the time of diagnosis (27). Several regimens, such as irinotecan in combination with vincristine (42), vinorelbine plus low-dose cyclophosphamide (52), and trabectedin (45, 54), have been reported to show clinical benefits for refractory IDSRCT patients. Specifically, trabectedin has been shown to interact with the minor groove of DNA, affecting several transcription factors, DNA repair molecules, and DNA-binding proteins; perturbing the cell cycle; and subsequently causing the death of cancer cells (107). Trabectedin has been deemed safe and effective in pre-treated IDSRCT patients who were refractory to conventional chemotherapy and resection (45, 54).
Other special chemotherapy forms, such as hyperthermic intraperitoneal chemotherapy (HIPEC), are also considered in patients with extensive metastasis over the abdominal cavity (70). However, the role of HIPEC remains controversial. A previous retrospective study has reported a significant improvement in the 3-year OS of patients treated with surgery and HIPEC (94), and other studies have shown that CRS followed by HIPEC may improve the disease control rate (DCR) in patients with peritoneal surface metastasis (106). Conversely, some studies have suggested that HIPEC is not associated with a better outcome (27, 70). Few patients who received CRS and HIPEC have been reported to need long-term parenteral nutrition or surgical intervention due to severe side effects, such as hemorrhagic cystitis, adhesive bowel obstruction, and sclerosing peritonitis (108). Therefore, assessing the actual effectiveness of HIPEC requires further studies.
8.3. Radiotherapy
Because of the multicentric growth tendency in the abdominopelvic cavity of IDRCT, whole-abdominopelvic radiotherapy (WAP RT) is a more effective treatment than locoregional radiotherapy (93). WAP RT is occasionally used as a consolidative therapy after CRS to remove microscopic or minimal residual disease (<2 cm) (88, 99). Treatment is performed with megavoltage photon beams by AP/PA fields into the entire peritoneal cavity. The median dose for patients without residual disease is 30 Gy in 1.5–1.55 Gy fractions, while for patients with residual lesions, the dose can be increased to 45–50 Gy (70, 99). IDSRCT patients treated with WAP RT combined with CRS and chemotherapy experienced prolonged survival (104). As for complications, gastrointestinal and hematological toxicities were the most common reported acute complications, while small bowel obstruction was the most common late toxicity in IDSRCT patients who underwent WAP RT (109). Acute complications can be treated with supportive care or symptomatic treatment, while surgical intervention is required in up to 10% of small bowel obstruction (106). Patients subjected to intensity-modulated radiation therapy (IMRT) reported a lower incidence of hematological toxicities compared to those who underwent WAP-RT (109). IMRT was found to selectively decrease the irradiated doses of adjacent normal organs (e.g., liver, kidneys, and pelvis), allowing even dose distribution to the peritoneal surfaces (106). Furthermore, there is no significant difference in OS between patients treated with IMRT and WART (109). In addition to being used as an adjunctive treatment, radiation can also be administered as a palliative treatment for recurrent IDSRCT (104).
8.4. Targeted Therapy
The formation of EWSR1-WT1 fusion gene can activate downstream signaling pathways, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and insulin growth factor (IGF)-1. Those pathways may be potential targets for treatment of IDSRCT (38). Until now, targeted therapy has shown its clinical benefit in IDSRCT patients who had tumor relapse or progression despite first-line or second-line treatment (38). Based on previous studies, tyrosine kinase inhibitors (TKI), including pazopanib (110), sunitinib (53), sorafenib (23), anlotinib (104), apatinib (76), imatinib (111), anti-VEGFR monoclonal antibody (e.g., bevacizumab) (68), IGF-1-R inhibitors (112, 113), mTOR inhibitors (114), and PARP inhibitors (100) were used for the therapies and were found to be effective in select IDSRCT cases. Targeted therapy-treated patients who were reviewed from case reports/series and our hospital are listed in Table 5. All ongoing clinical trials are summarized in Table 6.
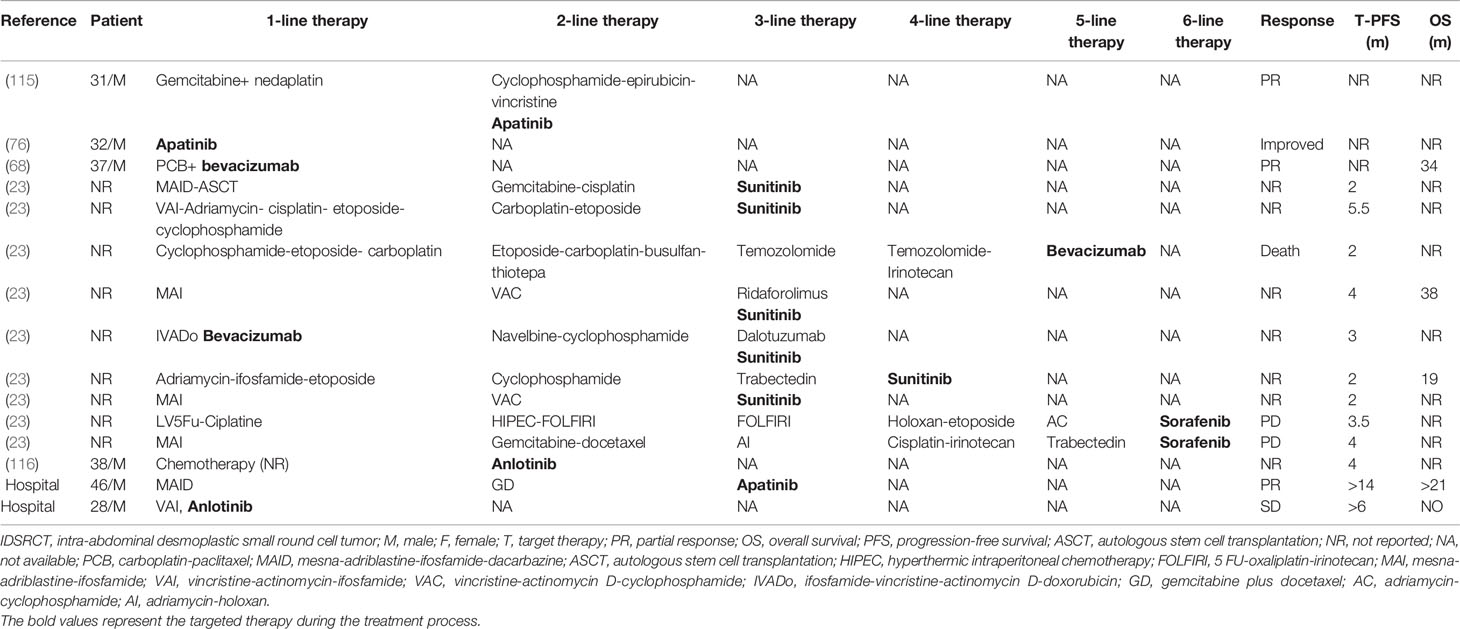
Table 5 Retrospectivec cases from published reports and our hospital of IDSRCT patients treated with target therapy.
8.4.1. Pazopanib
Pazopanib, a TKI, targets the stem cell factor receptor c-Kit, PDGFR-α and -β, and VEGFR-1, 2, and 3 to prohibit angiogenesis and tumor proliferation (117). Pazopanib has been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of patients with renal cell carcinoma (RCC) and sarcoma (118). IDSRCT overexpresses VEGF-A, VEGFR-2, and PDGF, which gives rationality to the administration of pazopanib in pretreated IDSRCT patients (119, 120). Pazopanib has been demonstrated to achieve a variable DCR of 61% and 78% for patients who received prior chemotherapy and those who did not, respectively. Median progression-free survival (PFS) of 9.2 months and OS of 15.4 months have also been observed with pazopanib treatment. The common adverse reactions following this treatment were neutropenia, hypertension, fatigue, and diarrhea (110, 121).
8.4.2. Sunitinib
Sunitinib has a mechanism of action like that of pazopanib, and it targets PDGFR, VEGFR, c-KIT, RET, colony-stimulating factor 1, and Flt3 (122). The FDA has approved sunitinib for the management of imatinib-refractory gastrointestinal stromal tumors (GISTs), RCC, and pancreatic neuroendocrine tumor (123). This agent has also shown clinical effectiveness in pretreated IDSRCT patients. In a case series comprising eight patients, six pretreated IDSRCT patients achieved an improved clinical outcome (partial remission [PR] and stable disease [SD]) after sunitinib treatment, and no severe adverse response was reported (53).
8.4.3. Sorafenib
Sorafenib, a multitargeted TKI, targets c-KIT, PDGFR-β, VEGFRs 2–3, and Flt-3. Sorafenib has been approved for treating hepatocellular carcinoma, RCC, and thyroid cancer (124). In a retrospective study, two progressive IDSRCT patients received sorafenib and progressed at 4 months. Of the two patients, one developed skin toxicity, while the other had to terminate the treatment due to severe abdominal pain (grade = 3) (23). The true efficacy of sorafenib treatment for DSRCT patients requires large sample data to further validate the results.
8.4.4. Apatinib
Apatinib, a novel multitargeted TKI, inhibits the expression of VEGFR-2, RET, c-Kit, and c-Src tyrosine kinases, selectively targeting VEGFR-2 (125). Apatinib is used for treating several tumor types, including gastric neoplasm (126), non-small cell lung cancer (NSCLC) (127), and colorectal cancer (CRC) (128). A case report showed that the tumor-related symptoms of IDSRCT patients were reduced quickly after the initiation of apatinib treatment (76). In another case report, one patient with IDSRCT received systemic chemotherapy (cyclophosphamide, epirubicin, and vincristine) plus apatinib and achieved PR after two cycles of treatment (115). Apatinib may be an alternative strategy for patients with IDSRCT. In a previous study, we presented the case of a 46-year-old man with metastatic IDSRCT admitted to our hospital. Although he had failed the first-line (MAID: mesna, doxorubicin, ifosfamide, and dacarbazine) and second-line chemotherapies (GD: gemcitabine plus docetaxel), he showed positive response to apatinib and quickly reached PR and achieved more than 14 months of PFS. This patient needed to reduce the dose of apatinib because of grade 3 hypertension and grade 2 digestive tract reaction. These adverse reactions resolved rapidly after dose reduction and usage of hypotensors.
8.4.5. Anlotinib
Anlotinib mainly targets VEGFRs 1–3, PDGFR-α and -β, fibroblast growth factor receptors (FGFRs) 1–4, EGFR, c-Kit, Met, and stem cell factor receptors (104, 116). Anlotinib plays a crucial role in the treatment of NSCLC and thyroid cancer (129). A case report showed that one IDSRCT patient who progressed after resection and chemotherapy (ifosfamide and liposomal doxorubicin) received anlotinib as the second-line therapy, which reduced invasive lymph node size with a PFS of 4 months. Only fatigue (grade = 1) and high triglyceride levels (grade = 1) were observed as side effects (104). In a previous study, we presented the case of a 28-year-old man admitted to our hospital with multiple metastatic and unresectable IDSRCT lesions. He received anlotinib plus chemotherapy (VAI) as the first-line treatment and achieved an SD of over 6 months.
8.4.6. Imatinib
Imatinib mesylate has been recommended as a treatment for GISTs and chronic myeloid leukemia (130). Notably, this agent also targets other tyrosine kinase receptors, such as PDGF-R and c-KIT (111). PDGF-A and PDGFR-β are related to tumor cell growth and proliferation (38) and are overexpressed in DSRCT (119). However, the efficacy of imatinib for IDSRCT has been found to be limited. Eight patients who were refractory to conventional treatment were administered imatinib, and only one patient achieved SD, while seven patients progressed rapidly 3 months after the initiation of imatinib. The toxicities were tolerated, and no severe adverse events were observed (111). Similarly, a phase II study suggested that imatinib was not associated with clinical benefits in pediatric patients with refractory DSRCT (131).
8.4.7. Monoclonal Antibody Targeting VEGF
Bevacizumab is a monoclonal antibody that targets VEGF-A. It has been recommended for the treatment of metastatic CRC, advanced NSCLC, advanced cervical cancer, and metastatic RCC (132). A previous study has found that VEGFR-2 and VEGFA are overexpressed in DSRCT cell lines and xenograft models (31). One IDSRCT patient who underwent prior partial resection received bevacizumab and achieved an OS of 34 months (68). Notably, bevacizumab in combination with WART can be used to eradicate residual disease after surgery because it increases the sensitivity of the tumor nodules to radiotherapy (106). An ongoing clinical trial (NCT01189643) is exploring the combination of irinotecan, temozolomide, and bevacizumab in combination with standard P6 regimen for treatment of newly diagnosed patients with DSRCT. Similarly, ramicirumab, an anti-VEGF-2 monoclonal antibody, in combination with chemotherapy, is being assessed in a phase II clinical trial on DSRCT (Table 4; NCT04145349).
8.4.8. IGF1R Inhibitor
IGF1R inhibitors can inhibit the proliferation of tumor cells by blocking the binding between IGF1R and IGF-1 and 2 (133). In addition, these inhibitors exert anti-angiogenic effects by targeting VEGF (134). Common IGF1R inhibitors include ganitumab and cixutumumab. IGF1R inhibitors are used for treating ES (135). In a Phase II study, 25% of clinical benefit (DCR ≥ 6 months) was achieved in DSRCT patients treated with ganitumab. This study suggested that ganitumab could prolong the survival of DSRCT patients with a median PFS of 19.0 months. Notably, five patients had to undergo dose reductions due to thrombocytopenia (grade = 3) caused by ganitumab. Other grade 3 toxicities, including hyperglycemia, leukopenia, neutropenia, and grade 4 thrombocytopenia, were also reported (112). In another study, SD was observed in two-thirds of pretreated patients who received cixutumumab in combination with temsirolimus (an mTOR inhibitor) (113).
8.4.9. mTOR Inhibitor
The PI3K/Akt/mTOR pathway is constitutively activated in DSRCT progression, mainly through mTORC-2 expression (136), and it is related to cancer cell growth and survival in sarcoma (137). One IDSRCT patient, who resisted chemotherapy and anti-androgen therapy, maintained an SD of 10 months when treated with temsirolimus (114). Furthermore, mTOR inhibitor plus pazopanib led to an SD of 11 months in one IDSRCT patient who progressed after treatment with pazopanib alone (59). Nevertheless, mTOR inhibitor plus IGF1R inhibitor showed limited efficacy in DSRCT treatment (138).
8.4.10. PARP Inhibitors
The DDR network may be a potential target for DSRCT due to the recent discovery that 27% of mutated genes of DSRCT are related to DDR (35). Poly (ADP-ribose) polymerase 1 (PARP1) is a key enzyme for base excision repair of single-strand DNA breaks, participating the recruitment of DNA repair proteins (139). PARP inhibitor has been reported to be effective in sarcoma with deficiency in DDR (38). Anke E. M. van Erp and coworkers discovered that PARP1 expression was observed in 100% of clinically derived DSRCT tumor tissues (100). They also found that DSRCT cells have a high sensitivity profile to the PARP inhibitor olaparib, and the combination of olaparib with temozolomide (an alkylating agent) in JN-DSRCT-1 cells results in synergistic effects, inducing cell cycle arrest followed by cell apoptosis, consequently leading to tumor reduction in vitro and in vivo.
IDSRCT patients who progress or relapse after first- and second-line treatment can first receive anti-VEGF monoclonal antibodies, like bevacizumab or TKI, that act on multiple pathways simultaneously (e.g., c-Kit, PDGFR-α and -β, and VEGFR-1, 2, and 3, Flt3, RET), including pazopanib, sunitinib, anlotinib, and apatinib. Among them, imatinib and sorafenib caused no benefit, which should not be considered in IDSRCT patients. Subsequent lines of management include IGF1R inhibitor or mTOR inhibitor. Notably, the effect of PARP inhibitor needs to be further verified in clinical studies. The existing preclinical and clinical data suggest some effect of targeted therapy in IDSRCT patients. However, the observed role predominantly is tumor stabilization rather than disease regression. Those potential targets are worthy of further exploration (23, 53, 68, 76, 100, 110–114).
8.5. Immunotherapy
Although immunotherapy has shown an unprecedented response in multiple tumor types, there is no clinical evidence of its efficacy in IDSRCT. Some potential targets for immunotherapy have been detected in IDSRCT. CD276, now called B7H3, and GD2 are expressed in 96% and 70% of DSRCT patients, respectively (140). B7H3 can lead to tumor cell immune evasion by inhibiting the effect of T cells, allowing tumor growth and metastasis (33). A phase I study indicated that intraperitoneal radioimmunotherapy with 131I-omburtamab (an anti-B7H3 monoclonal antibody) is well tolerated in patients with DSRCT (141). In addition, anti-B7H3 CAR-T cell immunotherapy has been evaluated in a phase I study for recurrent/refractory solid tumors, including DSRCT (Table 4; NCT04483778; NCT02982941). A clinical trial is studying the efficacy of intraperitoneal radioimmunotherapy with 131I-8H9 for patients with DSRCT (NCT01099644). The function of GD2 in IDSRCT has not been adequately identified. However, some studies have indicated that GD2 might increase the adhesion between extracellular matrix proteins and tumor cells and contribute to the metastasis of the neoplasm (33). Notably, GD3, an upstream molecule in the biosynthesis of GD2, has also been found in 70.0% of DSRCT patients. We speculate that GD3 may be a potential target of immunotherapy in IDSRCT patients (142). Immune checkpoint inhibitors targeting PD-1 and CTLA-4 have been demonstrated to prolong survival in several tumors, including NSCLC, RCC, and melanoma (143), but its clinical effect in IDSRCT remains unknown. Pembrolizumab, an anti-PD-1 agent, is now being tested in patients with rare sarcoma (including 6 patients with DSRCT) in a phase 2 clinical trial (NCT03012620).
8.6. Androgen Blockade Agent
Bulbul et al. reported higher expression of androgen receptor in DSRCT patients than in those with ES (144). Hence, androgen blockade agents were tested in IDSRCT patients, but its efficacy remains controversial. In a case report analyzing six patients treated with combined androgen blockade, 50% of the patients showed a positive response to the therapy (132), while in one patient, the tumor progressed after 2 months of treatment (114).
8.7. Autologous Stem Cell Transplant
ASCT has been performed in IDSRCT patients (145). In a case report, a complete response (CR) was seen in IDSRCT patients after resection, chemotherapy (VAC+VAP), and ASCT (146). Another study also found that ASCT could significantly improve OS in DSRCT patients who were in remission (147). Moreover, a retrospective study reported improved disease-free survival and OS in ASCT-treated DSRCT patients with residual tumors (148). Owing to the small sample size of the abovementioned studies, the true effectiveness of ASCT needs further validation in the future.
9. Palliative Care and Complications Management
Advanced IDSRCT patients with extensive systemic metastases who cannot tolerate surgery or systemic chemotherapy may consider targeted therapy with mild adverse reactions, including TKI inhibitors, mTOR inhibitors, or bevacizumab (53, 59, 68, 121). Nutritional care is essential for patients with advanced tumors. Pharmacological agents and pharmaconutrients have limited effects in patients with advanced cancer. If possible, patients with advanced tumor should engage in regular physical activity and adopt a prudent diet (149). For patients with tumor mass-associated compression symptoms, like intestinal obstruction and ureteral obstruction, palliative surgery can be considered, but the benefits and risks of palliative surgery must be balanced with special consideration in patients with advanced IDSRCT.
10. Prognosis and Follow-Up
The prognosis of IDSRCT is poor. Patients with IDSRCT had a dismal survival with 3- and 5-year survival rates from 44% to 15%–25% and a median OS of 1725 months (70, 75). Patients with hepatic/portal metastasis (150), resistance to neoadjuvant chemotherapy (27), and CD99 staining positive expression (100) have worse prognosis. Furthermore, local solitary lesions, no metastases, complete CRS, and adjuvant chemotherapy were independent risk factors for OS of IDSRCT (151). Some studies argued that some factors, such as sex, age, postoperative complications, lymph node metastases, the presence of extra-abdominal lesions at initial presentation, and tumor size (1, 29, 102), have no significant effect on the survival of IDSRCT. Despite the implementation of multimodal therapy, most IDSRCT patients experience quick relapses with a median PFS of 10–14 months (27, 75). Therefore, close follow-up is necessary after completion of treatment. If possible, PET-CT scan every 3 to 6 months is required, which can detect the change of metabolic activity prior to macroscopic neoplasm growth (90).
11. Future Perspectives
CRS combined with VDC/IE chemotherapy regimen and WAP-RT are regarded as the standard treatments for IDSRCT. Other therapies, such as molecularly targeted treatments, ASCT, and androgen blockade agents, can be considered when tumor progresses or relapses after standard therapy. Despite multimodal treatments, IDSRCT still has a dismal clinical outcome. The targeted agents for optimal effectiveness for IDSRCT remain unclear. Further research should be directed at exploring the potential effect of various targeted therapies in these patients with IDSRCT. Several potential targets like the DDR or MErT/EMT are waiting further exploration. Furthermore, immunotherapy as a possible therapeutic choice has limited extent effect in preclinical studies, which should be further verified in clinical conditions in the future.
12. Conclusion
IDSRCT refers to a rare and aggressive soft tissue malignancy that predominantly occurs in a young male population. It is often diagnosed with extensive peritoneal metastasis and has a dismal prognosis. Immunohistochemical analyses of IDSRCT are characterized by co-expression of epithelial, neuronal, and mesenchymal differentiation markers. FISH or reverse transcription polymerase chain reaction detecting EWS-WT1 fusion can be performed to assist in molecular confirmation. Multimodal treatments, including surgery, chemotherapy, and radiotherapy, in patients with IDSRCT have achieved improved outcome. Currently, targeted therapy and immunotherapy have expanded the treatment options for IDSRCT patients and are associated with improved survival rates in preclinical or clinical studies. Hepatic/portal metastasis and effect of neoadjuvant chemotherapy, number of lesions, and surgery types are associated with prognosis of IDSRCT. Due to the rarity and poor prognosis of this neoplasm, further studies are required to propose effective regimens for IDSRCT patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
GW reviewed the literature, and wrote the manuscript. XS wrote and revised the manuscript. YZ rechecked the manuscript. XL and XC assisted in drawing. MQ designed and revised the manuscript.
Funding
This work was supported by the research and development important project of the Science and Technology Bureau in Sichuan (2018SZ0188).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Honoré C, Atallah V, Mir O, Orbach D, Ferron G, LePéchoux C, et al. Abdominal Desmoplastic Small Round Cell Tumor Without Extraperitoneal Metastases: Is There a Benefit for HIPEC After Macroscopically Complete Cytoreductive Surgery? PloS One (2017) 12(2):e0171639. doi: 10.1371/journal.pone.0171639
2. Ertoy Baydar D, Armutlu A, Aydin O, Dagdemir A, Yakupoglu YK. Desmoplastic Small Round Cell Tumor of the Kidney: A Case Report. Diagn Pathol (2020) 15(1):95. doi: 10.1186/s13000-020-01015-w
3. Pickhardt PJ, Fisher AJ, Balfe DM, Dehner LP, Huettner PC. Desmoplastic Small Round Cell Tumor of the Abdomen: Radiologic-Histopathologic Correlation. Radiology (1999) 210(3):633–8. doi: 10.1148/radiology.210.3.r99mr42633
4. Saleh D, Al-Maghrabi S, Al-Maghrabi H, Al-Maghrabi J. Desmoplastic Small Round Cell Tumor of Pancreatic Origin in a Young Child: A Case Report and Review of Literature. Am J Case Rep (2020) 21:e922762. doi: 10.12659/AJCR.922762
5. Huang J, Sha L, Zhang H, Tang X, Zhang X. Desmoplastic Small Round Cell Tumor in Transverse Colon: Report of a Rare Case. Int Surg (2015) 100(5):809–13. doi: 10.9738/INTSURG-D-14-00134.1
6. Zhang GM, Zhu Y, Gan HL, Ye DW. Testicular Desmoplastic Small Round Cell Tumor: A Case Report and Review of Literature. World J Surg Oncol (2014) 12:227. doi: 10.1186/1477-7819-12-227
7. Vujić G, Mikuš M, Matak L, Bonevski A, Babić I, Planinić P, et al. Desmoplastic Small Round Cell Tumor of the Ovary: A Case Report With a New Modality of Treatment and Review of the Literature. Rev Bras Ginecol Obstet (2020) 42(5):297–302. doi: 10.1055/s-0040-1710350
8. Jin D, Chen M, Wang B, Gou Y. Mediastinal Desmoplastic Small Round Cell Tumor. Med (Baltimore) (2020) 99(44):e22921. doi: 10.1097/MD.0000000000022921
9. Ouarssani A, Atoini F, Ait Lhou F, Rguibi Idrissi M. Desmoplastic Small Round Cell Tumor of the Pleura. Thorac Cancer (2011) 2(3):117–9. doi: 10.1111/j.1759-7714.2011.00046.x
10. Syed S, Haque AK, Hawkins HK, Sorensen PH, Cowan DF. Desmoplastic Small Round Cell Tumor of the Lung. Arch Pathol Lab Med (2002) 126(10):1226–8. doi: 10.5858/2002-126-1226-DSRCTO
11. Cai Z, Zhang L, Karni RJ, Saluja K, Liu J, Zhu H. Desmoplastic Small Round Cell Tumor of Parotid Gland: A Rare Entity With Diagnostic Challenge. Int J Surg Pathol (2020) 28(7):782–6. doi: 10.1177/1066896920913109
12. Pang B, Leong CC, Salto-Tellez M, Petersson F. Desmoplastic Small Round Cell Tumor of Major Salivary Glands: Report of 1 Case and a Review of the Literature. Appl Immunohistochem Mol Morphol (2011) 19(1):70–5. doi: 10.1097/PAI.0b013e3181eec73c
13. Xu J, Yao M, Yang X, Liu T, Wang S, Ma D, et al. Desmoplastic Small Round Cell Tumor of the Middle Ear: A Case Report. Med (Baltimore) (2018) 97(17):e0494. doi: 10.1097/MD.0000000000010494
14. Tao Y, Shi L, Ge L, Yuan T, Shi L. Sinonasal Desmoplastic Small Round Cell Tumor: A Case Report and Review of the Literature. BMC Cancer (2019) 19(1):868. doi: 10.1186/s12885-019-6076-4
15. He XR, Liu Z, Wei J, Li WJ, Liu T. Primary Desmoplastic Small Round Cell Tumor in the Left Orbit: A Case Report and Literature Review. Int Ophthalmol (2019) 39(2):471–5. doi: 10.1007/s10792-018-0829-y
16. Thondam SK, du Plessis D, Cuthbertson DJ, Das KS, Javadpour M, MacFarlane IA, et al. Intracranial Desmoplastic Small Round Cell Tumor Presenting as a Suprasellar Mass. J Neurosurg (2015) 122(4):773–7. doi: 10.3171/2014.10.JNS132490
17. Asadbeigi SN, Zhang L, Linos K. Subcutaneous Desmoplastic Small Round-Cell Tumor: An Unusual Primary Location Expanding the Differential of Superficial Round-Cell Tumors. J Cutan Pathol (2020) 47(8):768–75. doi: 10.1111/cup.13703
18. Yoshida A, Edgar MA, Garcia J, Meyers PA, Morris CD, Panicek DM. Primary Desmoplastic Small Round Cell Tumor of the Femur. Skeletal Radiol (2008) 37(9):857–62. doi: 10.1007/s00256-008-0501-0
19. Adsay V, Cheng J, Athanasian E, Gerald W, Rosai J. Primary Desmoplastic Small Cell Tumor of Soft Tissues and Bone of the Hand. Am J Surg Pathol (1999) 23(11):1408–13. doi: 10.1097/00000478-199911000-00012
20. Thway K, Noujaim J, Zaidi S, Miah AB, Benson C, Messiou C, et al. Desmoplastic Small Round Cell Tumor: Pathology, Genetics, and Potential Therapeutic Strategies. Int J Surg Pathol (2016) 24(8):672–84. doi: 10.1177/1066896916668637
21. Gerald WL, Haber DA. The EWS-WT1 Gene Fusion in Desmoplastic Small Round Cell Tumor. Semin Cancer Biol (2005) 15(3):197–205. doi: 10.1016/j.semcancer.2005.01.005
22. Gedminas JM, Chasse MH, McBrairty M, Beddows I, Kitchen-Goosen SM, Grohar PJ. Desmoplastic Small Round Cell Tumor is Dependent on the EWS-WT1 Transcription Factor. Oncogenesis (2020) 9(4):41. doi: 10.1038/s41389-020-0224-1
23. Bétrian S, Bergeron C, Blay JY, Bompas E, Cassier PA, Chevallier L, et al. Antiangiogenic Effects in Patients With Progressive Desmoplastic Small Round Cell Tumor: Data From the French National Registry Dedicated to the Use of Off-Labeled Targeted Therapy in Sarcoma (OUTC’s). Clin Sarcoma Res (2017) 7:10. doi: 10.1186/s13569-017-0076-4
24. Li G, Wang HT, Gao Y, Cui XJ, Zhang GZ. Primary Abdominopelvic Desmoplastic Small Round Cell Tumor: CT and Correlated Clinicopathologic Features. Eur Rev Med Pharmacol Sci (2014) 18(18):2670–7.
25. Lal DR, Su WT, Wolden SL, Loh KC, Modak S, La Quaglia MP. Results of Multimodal Treatment for Desmoplastic Small Round Cell Tumors. J Pediatr Surg (2005) 40(1):251–5. doi: 10.1016/j.jpedsurg.2004.09.046
26. Lettieri CK, Garcia-Filion P, Hingorani P. Incidence and Outcomes of Desmoplastic Small Round Cell Tumor: Results From the Surveillance, Epidemiology, and End Results Database. J Cancer Epidemiol (2014) 2014:680126. doi: 10.1155/2014/680126
27. Subbiah V, Lamhamedi-Cherradi SE, Cuglievan B, Menegaz BA, Camacho P, Huh W, et al. Multimodality Treatment of Desmoplastic Small Round Cell Tumor: Chemotherapy and Complete Cytoreductive Surgery Improve Patient Survival. Clin Cancer Res (2018) 24(19):4865–73. doi: 10.1158/1078-0432.CCR-18-0202
28. Honoré C, Delhorme JB, Nassif E, Faron M, Ferron G, Bompas E, et al. Can We Cure Patients With Abdominal Desmoplastic Small Round Cell Tumor? Results of a Retrospective Multicentric Study on 100 Patients. Surg Oncol (2019) 29:107–12. doi: 10.1016/j.suronc.2019.04.002
29. Wong HH, Hatcher HM, Benson C, Al-Muderis O, Horan G, Fisher C, et al. Desmoplastic Small Round Cell Tumour: Characteristics and Prognostic Factors of 41 Patients and Review of the Literature. Clin Sarcoma Res (2013) 3(1):14. doi: 10.1186/2045-3329-3-14
30. Livaditi E, Mavridis G, Soutis M, Papandreou E, Moschovi M, Papadakis V, et al. Diffuse Intraabdominal Desmoplastic Small Round Cell Tumor: A Ten-Year Experience. Eur J Pediatr Surg (2006) 16(6):423–7. doi: 10.1055/s-2006-924736
31. Bulbul A, Fahy BN, Xiu J, Rashad S, Mustafa A, Husain H, et al. Desmoplastic Small Round Blue Cell Tumor: A Review of Treatment and Potential Therapeutic Genomic Alterations. Sarcoma (2017) 2017:1278268. doi: 10.1155/2017/1278268
32. Lee SB, Kolquist KA, Nichols K, Englert C, Maheswaran S, Ladanyi M, et al. The EWS-WT1 Translocation Product Induces PDGFA in Desmoplastic Small Round-Cell Tumour. Nat Genet (1997) 17(3):309–13. doi: 10.1038/ng1197-309
33. Loktev A, Shipley JM. Desmoplastic Small Round Cell Tumor (DSRCT): Emerging Therapeutic Targets and Future Directions for Potential Therapies. Expert Opin Ther Targets (2020) 24(4):281–5. doi: 10.1080/14728222.2020.1738392
34. Ito E, Honma R, Imai J, Azuma S, Kanno T, Mori S, et al. A Tetraspanin-Family Protein, T-Cell Acute Lymphoblastic Leukemia-Associated Antigen 1, is Induced by the Ewing’s Sarcoma-Wilms’ Tumor 1 Fusion Protein of Desmoplastic Small Round-Cell Tumor. Am J Pathol (2003) 163(6):2165–72. doi: 10.1016/S0002-9440(10)63573-0
35. Devecchi A, De Cecco L, Dugo M, Penso D, Dagrada G, Brich S, et al. The Genomics of Desmoplastic Small Round Cell Tumor Reveals the Deregulation of Genes Related to DNA Damage Response, Epithelial-Mesenchymal Transition, and Immune Response. Cancer Commun (Lond) (2018) 38(1):70. doi: 10.1186/s40880-018-0339-3
36. Yang J, Du X, Wang G, Sun Y, Chen K, Zhu X, et al. Mesenchymal to Epithelial Transition in Sarcomas. Eur J Cancer (2014) 50(3):593–601. doi: 10.1016/j.ejca.2013.11.006
37. Jiang Y, Subbiah V, Janku F, Ludwig JA, Naing A, Benjamin RS, et al. Novel Secondary Somatic Mutations in Ewing’s Sarcoma and Desmoplastic Small Round Cell Tumors. PloS One (2014) 9(8):e93676. doi: 10.1371/journal.pone.0093676
38. Bexelius TS, Wasti A, Chisholm JC. Mini-Review on Targeted Treatment of Desmoplastic Small Round Cell Tumor. Front Oncol (2020) 10:518. doi: 10.3389/fonc.2020.00518
39. Thomas R, Rajeswaran G, Thway K, Benson C, Shahabuddin K, Moskovic E. Desmoplastic Small Round Cell Tumour: The Radiological, Pathological and Clinical Features. Insights Imaging (2013) 4(1):111–8. doi: 10.1007/s13244-012-0212-x
40. Morani AC, Bathala TK, Surabhi VR, Yedururi S, Jensen CT, Huh WW, et al. Desmoplastic Small Round Cell Tumor: Imaging Pattern of Disease at Presentation. AJR Am J Roentgenol (2019) 212(3):W45–w54. doi: 10.2214/AJR.18.20179
41. Nacef K, Chaouch MA, Bouriga R, Khalifa MB, Chaouch A, Ghannouchi M, et al. A Case Report of Abdominal Desmoplastic Small Round Cell Tumor in a Young Tunisian Woman. J Gastrointest Cancer (2019) 50(3):568–71. doi: 10.1007/s12029-017-0048-1
42. Ambar NBD, de Seixas Alves MT, Lederman HM, Abib S, Duarte AAB, Caran EM. Irinotecan and Vincristine for the Treatment of Refractory Desmoplastic Small Round Cell Tumor in a Developing Country: A Case Report. J Med Case Rep (2019) 13(1):77. doi: 10.1186/s13256-019-1985-z
43. Altal OF, Aleshawi AJ, Tashtush NA, Alhowary A. A 23-Year-Old Joradanian Woman With a Desmoplastic Small Round Cell Tumor Involving the Ovary. Am J Case Rep (2019) 20:1675–8. doi: 10.12659/AJCR.919488
44. Ferreira EN, Barros BD, de Souza JE, Almeida RV, Torrezan GT, Garcia S, et al. A Genomic Case Study of Desmoplastic Small Round Cell Tumor: Comprehensive Analysis Reveals Insights Into Potential Therapeutic Targets and Development of a Monitoring Tool for a Rare and Aggressive Disease. Hum Genomics (2016) 10(1):36. doi: 10.1186/s40246-016-0092-0
45. López-González A, Cantos B, Tejerina E, Provencio M. Activity of Trabectidin in Desmoplastic Small Round Cell Tumor. Med Oncol (2011) 28:S644–6. doi: 10.1007/s12032-010-9687-9
46. Shen CJ, Loeb DM, Terezakis SA. Desmoplastic Small Round Cell Tumor: Postoperative Retroperitoneal Mass. Radiol Case Rep (2016) 11(3):248–50. doi: 10.1016/j.radcr.2016.05.007
47. Li X, Yu J, Fang S, Xing X, Zhao J. Desmoplastic Small Round Cell Tumor: A Case Report and Review of the Literature. World J Surg Oncol (2014) 12:9. doi: 10.1186/1477-7819-12-9
48. Reisner D, Brahee D, Patel S, Hartman M. A Case of Desmoplastic Small Round Cell Tumor. J Radiol Case Rep (2015) 9(8):1–7. doi: 10.3941/jrcr.v9i8.2526
49. Briseño-Hernández AA, Quezada-López DR, Corona-Cobián LE, Castañeda-Chávez A, Duarte-Ojeda AT, Macías-Amezcua MD. [Intra-Abdominal Desmoplastic Small Round Cell Tumour]. Cir Cir (2015) 83(3):243–8. doi: 10.1016/j.circir.2015.05.009
50. Kandhari C, Muddebihal U, Udupa V, Chandra S. Desmoplastic Small Round Cell Tumor (DSRCT): An Unusual Intra-Abdominal Tumor. Indian J Surg (2015) 77(1):67–9. doi: 10.1007/s12262-013-0990-5
51. Nathan JD, Gingalewski C, Salem RR. Intra-Abdominal Desmoplastic Small Round Cell Tumor. Yale J Biol Med (2001) 74(1):13–20.
52. Ferrari A, Grosso F, Stacchiotti S, Meazza C, Zaffignani E, Marchianò A, et al. Response to Vinorelbine and Low-Dose Cyclophosphamide Chemotherapy in Two Patients With Desmoplastic Small Round Cell Tumor. Pediatr Blood Cancer (2007) 49(6):864–6. doi: 10.1002/pbc.20682
53. Italiano A, Kind M, Cioffi A, Maki RG, Bui B. Clinical Activity of Sunitinib in Patients With Advanced Desmoplastic Round Cell Tumor: A Case Series. Target Oncol (2013) 8(3):211–3. doi: 10.1007/s11523-012-0251-8
54. Frezza AM, Whelan JS, Dileo P. Trabectedin for Desmoplastic Small Round Cell Tumours: A Possible Treatment Option? Clin Sarcoma Res (2014) 4:3. doi: 10.1186/2045-3329-4-3
55. Azami A, Takano Y, Honda M, Todate Y, Tada T, Waragai M, et al. [A Case of an Abdominal Desmoplastic Small Round Cell Tumor With Metastasis in the Medulla Oblongata]. Gan To Kagaku Ryoho (2016) 43(11):1409–12.
56. Takahira K, Ohi S, Fujii N, Matsuura Y, Sano M, Hanai H, et al. Intra-Abdominal Desmoplastic Small Round Cell Tumor (IDSRT). J Gastroenterol (2000) 35(9):712–6. doi: 10.1007/s005350070052
57. Wakahashi S, Sudo T, Ichida K, Sugita S, Hasegawa T, Nagao S, et al. Diagnosis of Desmoplastic Small-Round-Cell Tumor by Cytogenetic Analysis: A Case Report. Clin Case Rep (2016) 4(5):520–3. doi: 10.1002/ccr3.558
58. Hirano G, Irie M, Nakashima Y, Shakado S, Sohda T, Tanaka T, et al. Desmoplastic Small Round Cell Tumors in a Young Man. Intern Med (2013) 52(17):1909–14. doi: 10.2169/internalmedicine.52.0431
59. Katz D, Azraq Y, Eleyan F, Gill S, Peretz T, Merimsky O. Pazolimus: Pazopanib Plus Sirolimus Following Progression on Pazopanib, a Retrospective Case Series Analysis. BMC Cancer (2016) 16:616. doi: 10.1186/s12885-016-2618-1
60. Slomovitz BM, Girotra M, Aledo A, Saqi A, Soslow RA, Spigland NA, et al. Desmoplastic Small Round Cell Tumor With Primary Ovarian Involvement: Case Report and Review. Gynecol Oncol (2000) 79(1):124–8. doi: 10.1006/gyno.2000.5829
61. Eaton SH, Cendron MA. Primary Desmoplastic Small Round Cell Tumor of the Kidney in a 7-Year-Old Girl. J Pediatr Urol (2006) 2(1):52–4. doi: 10.1016/j.jpurol.2005.05.008
62. Zhang S, Zhang Y, Yu YH, Li J. Complete Response of Giant Desmoplastic Small Round Cell Tumor Treated With Chemoradiotherapy: A Case Report. Oncol Lett (2016) 11(2):1069–72. doi: 10.3892/ol.2015.4024
63. Xie YP, Shen YM. Ovarian Involvement of a Desmoplastic Small Round Cell Tumor of Unknown Primary Origin With Lymph Node and Lung Metastases: A Case Report. Oncol Lett (2016) 11(2):1125–9. doi: 10.3892/ol.2015.4012
64. Baz W, El-Soueidi R, Nakhl F, Aoun N, Chin N, Dhar M. Desmoplastic Small Round-Cell Tumor: An Adult With Previous Exposure to Agent Orange. Jpn J Clin Oncol (2010) 40(6):593–5. doi: 10.1093/jjco/hyq022
65. Takekawa Y, Ugajin W, Koide H, Nishio S, Yamamoto T, Sawada T. Pathologic, Cytologic and Immunohistochemical Findings of an Intra-Abdominal Desmoplastic Small Round Cell Tumor in a 15-Year-Old Male. Pathol Int (2000) 50(5):417–20. doi: 10.1046/j.1440-1827.2000.01051.x
66. Ujihara M, Ando T, Watanabe O, Asada T, Yamashita K, Ichihara T, et al. Treatment of Intraabdominal Desmoplastic Small Round Cell Tumor With Ifosfamide-Based Chemotherapy. Clin J Gastroenterol (2010) 3(1):25–9. doi: 10.1007/s12328-009-0131-7
67. Kim JW, Park JH, Cho HJ, Kwon JH, Koh Y, Kim SJ, et al. A Case of Desmoplastic Small Round Cell Tumor Diagnosed in a Young Female Patient. Cancer Res Treat (2009) 41(4):233–6. doi: 10.4143/crt.2009.41.4.233
68. de Araújo RA, Araújo BJ. Desmoplastic Small Round Cell Tumor: Report of 2 Cases Treated With Chemotherapy Alone or in Combination With Bevacizumab. Case Rep Oncol (2014) 7(1):102–8. doi: 10.1159/000359997
69. Koniari K, Mahera H, Nikolaou M, Chatzis O, Glezakou O, Magiasis V, et al. Intraabdominal Desmoplastic Small Round Cell Tumor: Report of a Case and Literature Review. Int J Surg Case Rep (2011) 2(8):293–6. doi: 10.1016/j.ijscr.2011.08.013
70. Honoré C, Amroun K, Vilcot L, Mir O, Domont J, Terrier P, et al. Abdominal Desmoplastic Small Round Cell Tumor: Multimodal Treatment Combining Chemotherapy, Surgery, and Radiotherapy is the Best Option. Ann Surg Oncol (2015) 22(4):1073–9. doi: 10.1245/s10434-014-4123-6
71. Tang Y, Song H, Bao Y, Zhi Y. Multimodal Treatment of Abdominal and Pelvic Desmoplastic Small Round Cell Tumor With Relative Good Prognosis. Int J Surg (2015) 16(Pt A):49–54. doi: 10.1016/j.ijsu.2015.02.015
72. Magro G, Broggi G, Zin A, Di Benedetto V, Meli M, Di Cataldo A, et al. Desmoplastic Small Round Cell Tumor With “Pure” Spindle Cell Morphology and Novel EWS-WT1 Fusion Transcript: Expanding the Morphological and Molecular Spectrum of This Rare Entity. Diagnostics (Basel) (2021) 11(3):545. doi: 10.3390/diagnostics11030545
73. Chicago Consensus Working Group.The Chicago Consensus on Peritoneal Surface Malignancies: Management of Desmoplastic Small Round Cell Tumor, Breast, and Gastrointestinal Stromal Tumors. Cancer (2020) 126(11):2566–70. doi: 10.1002/cncr.32856
74. Kis B, O’Regan KN, Agoston A, Javery O, Jagannathan J, Ramaiya NH. Imaging of Desmoplastic Small Round Cell Tumour in Adults. Br J Radiol (2012) 85(1010):187–92. doi: 10.1259/bjr/57186741
75. Martínez-Trufero J, Cruz Jurado J, Hernández-León CN, Correa R, Asencio JM, Bernabeu D, et al. Uncommon and Peculiar Soft Tissue Sarcomas: Multidisciplinary Review and Practical Recommendations. Spanish Group for Sarcoma Research (GEIS -GROUP). Part II. Cancer Treat Rev (2021) 99:102260. doi: 10.1016/j.ctrv.2021.102260
76. Shi C, Feng Y, Zhang LC, Ding DY, Yan MY, Pan L. Effective Treatment of Apatinib in Desmoplastic Small Round Cell Tumor: A Case Report and Literature Review. BMC Cancer (2018) 18(1):338. doi: 10.1186/s12885-018-4135-x
77. Ordi J, de Alava E, Torné A, Mellado B, Pardo-Mindan J, et al. Intraabdominal desmoplastic small round cell tumor with EWS/ERG fusion transcript. Am J Surg Pathol (1998) 22(8):026–32.
78. Shimazaki J, Motohashi G, Nishida K, Tabuchi T, Ubukuta H. Removal of an intra-abdominal desmoplastic small round cell tumor by repetitive debulking surgery: A case report and literature review. Oncol Lett (2014) 7(5):1464–8.
79. Devaney K. Intra-abdominal desmoplastic small round cell tumor of the peritoneum in a young man. Ultrastruct Pathol (1994) 18(3):389–98.
80. Hendricks A, Boerner K, Germer CT, Wiegering A. Desmoplastic Small Round Cell Tumors: A Review With Focus on Clinical Management and Therapeutic Options. Cancer Treat Rev (2021) 93:102140. doi: 10.1016/j.ctrv.2020.102140
81. Ordóñez NG. Desmoplastic Small Round Cell Tumor: II: An Ultrastructural and Immunohistochemical Study With Emphasis on New Immunohistochemical Markers. Am J Surg Pathol (1998) 22(11):1314–27. doi: 10.1097/00000478-199811000-00002
82. Gerald WL, Rosai J, Ladanyi M. Characterization of the Genomic Breakpoint and Chimeric Transcripts in the EWS-WT1 Gene Fusion of Desmoplastic Small Round Cell Tumor. Proc Natl Acad Sci U.S.A. (1995) 92(4):1028–32. doi: 10.1073/pnas.92.4.1028
83. Werner H, Idelman G, Rubinstein M, Pattee P, Nagalla SR, Roberts CT Jr. A Novel EWS-WT1 Gene Fusion Product in Desmoplastic Small Round Cell Tumor is a Potent Transactivator of the Insulin-Like Growth Factor-I Receptor (IGF-IR) Gene. Cancer Lett (2007) 247(1):84–90. doi: 10.1016/j.canlet.2006.03.027
84. Nakanishi Y, Oinuma T, Sano M, Fuchinoue F, Komatsu K, Seki T, et al. Coexpression of an Unusual Form of the EWS-WT1 Fusion Transcript and Interleukin 2/15 Receptor betamRNA in a Desmoplastic Small Round Cell Tumour. J Clin Pathol (2006) 59(10):1108–10. doi: 10.1136/jcp.2005.026245
85. Murphy AJ, Bishop K, Pereira C, Chilton-MacNeill S, Ho M, Zielenska M, et al. A New Molecular Variant of Desmoplastic Small Round Cell Tumor: Significance of WT1 Immunostaining in This Entity. Hum Pathol (2008) 39(12):1763–70. doi: 10.1016/j.humpath.2008.04.019
86. Mohamed M, Gonzalez D, Fritchie KJ, Swansbury J, Wren D, Benson C, et al. Desmoplastic Small Round Cell Tumor: Evaluation of Reverse Transcription-Polymerase Chain Reaction and Fluorescence in Situ Hybridization as Ancillary Molecular Diagnostic Techniques. Virchows Arch (2017) 471(5):631–40. doi: 10.1007/s00428-017-2207-y
87. Lae ME, Roche PC, Jin L, Lloyd RV, Nascimento AG. Desmoplastic Small Round Cell Tumor: A Clinicopathologic, Immunohistochemical, and Molecular Study of 32 Tumors. Am J Surg Pathol (2002) 26(7):823–35. doi: 10.1097/00000478-200207000-00001
88. Angarita FA, Hassan S, Cannell AJ, Dickson BC, Gladdy RA, Swallow CJ, et al. Clinical Features and Outcomes of 20 Patients With Abdominopelvic Desmoplastic Small Round Cell Tumor. Eur J Surg Oncol (2017) 43(2):423–31. doi: 10.1016/j.ejso.2016.08.017
89. Wang LL, Ji ZH, Gao Y, Chang H, Sun PP, Li Y. Clinicopathological Features of Desmoplastic Small Round Cell Tumors: Clinical Series and Literature Review. World J Surg Oncol (2021) 19(1):193. doi: 10.1186/s12957-021-02310-6
90. Ostermeier A, McCarville MB, Navid F, Snyder SE, Shulkin BL. FDG PET/CT Imaging of Desmoplastic Small Round Cell Tumor: Findings at Staging, During Treatment and at Follow-Up. Pediatr Radiol (2015) 45(9):1308–15. doi: 10.1007/s00247-015-3315-y
91. Magnan H, Abramson SJ, Price AP, Grewal RK, Merchant MS, LaQuaglia MP, et al. Positron Emission Tomography for Response Assessment in Desmoplastic Small Round Cell Tumor. J Pediatr Hematol Oncol (2013) 35(5):e190–3. doi: 10.1097/MPH.0b013e3182707d4c
92. Zhang WD, Li CX, Liu QY, Hu YY, Cao Y, Huang JH. CT, MRI, and FDG-PET/CT Imaging Findings of Abdominopelvic Desmoplastic Small Round Cell Tumors: Correlation With Histopathologic Findings. Eur J Radiol (2011) 80(2):269–73. doi: 10.1016/j.ejrad.2010.06.046
93. Shen XZ, Zhao JG, Wu JJ, Liu F. Clinical and Computed Tomography Features of Adult Abdominopelvic Desmoplastic Small Round Cell Tumor. World J Gastroenterol (2014) 20(17):5157–64. doi: 10.3748/wjg.v20.i17.5157
94. Hayes-Jordan A, Green H, Fitzgerald N, Xiao L, Anderson P. Novel Treatment for Desmoplastic Small Round Cell Tumor: Hyperthermic Intraperitoneal Perfusion. J Pediatr Surg (2010) 45(5):1000–6. doi: 10.1016/j.jpedsurg.2010.02.034
95. Harmon RL, Sugarbaker PH. Prognostic Indicators in Peritoneal Carcinomatosis From Gastrointestinal Cancer. Int Semin Surg Oncol (2005) 2(1):3. doi: 10.1186/1477-7800-2-3
96. Stuart-Buttle CE, Smart CJ, Pritchard S, Martin D, Welch IM. Desmoplastic Small Round Cell Tumour: A Review of Literature and Treatment Options. Surg Oncol (2008) 17(2):107–12. doi: 10.1016/j.suronc.2007.11.005
97. Cho KJ, Ro JY, Choi J, Choi SH, Nam SY, Kim SY. Mesenchymal Neoplasms of the Major Salivary Glands: Clinicopathological Features of 18 Cases. Eur Arch Otorhinolaryngol (2008) 265:S47–56. doi: 10.1007/s00405-007-0488-5
98. Dufresne A, Cassier P, Couraud L, Marec-Bérard P, Meeus P, Alberti L, et al. Desmoplastic Small Round Cell Tumor: Current Management and Recent Findings. Sarcoma (2012) 2012:714986. doi: 10.1155/2012/714986
99. Quaglia MP, Brennan MF. The Clinical Approach to Desmoplastic Small Round Cell Tumor. Surg Oncol (2000) 9(2):77–81. doi: 10.1016/S0960-7404(00)00024-4
100. Xiang T, Zhang SY, Wang SS, Fei RS, Li H. A Nationwide Analysis of Desmoplastic Small Round Cell Tumor. Med (Baltimore) (2020) 99(30):e21337. doi: 10.1097/MD.0000000000021337
101. Jacquet P, Sugarbaker PH. Clinical Research Methodologies in Diagnosis and Staging of Patients With Peritoneal Carcinomatosis. Cancer Treat Res (1996) 82:359–74. doi: 10.1007/978-1-4613-1247-5_23
102. Stiles ZE, Murphy AJ, Anghelescu DL, Brown CL, Davidoff AM, Dickson PV, et al. Desmoplastic Small Round Cell Tumor: Long-Term Complications After Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol (2020) 27(1):171–8. doi: 10.1245/s10434-019-07339-2
103. Schwarz RE, Gerald WL, Kushner BH, Coit DG, Brennan MF, La Quaglia MP. Desmoplastic Small Round Cell Tumors: Prognostic Indicators and Results of Surgical Management. Ann Surg Oncol (1998) 5(5):416–22. doi: 10.1007/BF02303860
104. Chen HM, Feng G. Use of Anlotinib in Intra-Abdominal Desmoplastic Small Round Cell Tumors: A Case Report and Literature Review. Onco Targets Ther (2019) 12:57–61. doi: 10.2147/OTT.S190333
105. Chang CC, Hsu JT, Tseng JH, Hwang TL, Chen HM, Jan YY. Combined Resection and Multi-Agent Adjuvant Chemotherapy for Desmoplastic Small Round Cell Tumor Arising in the Abdominal Cavity: Report of a Case. World J Gastroenterol (2006) 12(5):800–3. doi: 10.3748/wjg.v12.i5.800
106. Pinnix CC, Fontanilla HP, Hayes-Jordan A, Subbiah V, Bilton SD, Chang EL, et al. Whole Abdominopelvic Intensity-Modulated Radiation Therapy for Desmoplastic Small Round Cell Tumor After Surgery. Int J Radiat Oncol Biol Phys (2012) 83(1):317–26. doi: 10.1016/j.ijrobp.2011.06.1985
107. Herrero AB, Martín-Castellanos C, Marco E, Gago F, Moreno S. Cross-Talk Between Nucleotide Excision and Homologous Recombination DNA Repair Pathways in the Mechanism of Action of Antitumor Trabectedin. Cancer Res (2006) 66(16):8155–62. doi: 10.1158/0008-5472.CAN-06-0179
108. Deneve JL. ASO Author Reflections: Late-Term Toxicity After Cytoreductive Surgery/Hyperthermic Intraperitoneal Chemotherapy for Desmoplastic Small Round Cell Tumor Underscores the Need for Novel Drug Development and Clinical Trial Design. Ann Surg Oncol (2019) 26(Suppl 3):692–3. doi: 10.1245/s10434-019-07712-1
109. Desai NB, Stein NF, LaQuaglia MP, Alektiar KM, Kushner BH, Modak S, et al. Reduced Toxicity With Intensity Modulated Radiation Therapy (IMRT) for Desmoplastic Small Round Cell Tumor (DSRCT): An Update on the Whole Abdominopelvic Radiation Therapy (WAP-RT) Experience. Int J Radiat Oncol Biol Phys (2013) 85(1):e67–72. doi: 10.1016/j.ijrobp.2012.09.005
110. Frezza AM, Benson C, Judson IR, Litiere S, Marreaud S, Sleijfer S, et al. Pazopanib in Advanced Desmoplastic Small Round Cell Tumours: A Multi-Institutional Experience. Clin Sarcoma Res (2014) 4:7. doi: 10.1186/2045-3329-4-7
111. De Sanctis R, Bertuzzi A, Bisogno G, Carli M, Ferrari A, Comandone A, et al. Imatinib Mesylate in Desmoplastic Small Round Cell Tumors. Future Oncol (2017) 13(14):1233–7. doi: 10.2217/fon-2016-0305
112. Tap WD, Demetri G, Barnette P, Desai J, Kavan P, Tozer R, et al. Phase II Study of Ganitumab, a Fully Human Anti-Type-1 Insulin-Like Growth Factor Receptor Antibody, in Patients With Metastatic Ewing Family Tumors or Desmoplastic Small Round Cell Tumors. J Clin Oncol (2012) 30(15):1849–56. doi: 10.1200/JCO.2011.37.2359
113. Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. Insulin Growth Factor-Receptor (IGF-1R) Antibody Cixutumumab Combined With the mTOR Inhibitor Temsirolimus in Patients With Refractory Ewing’s Sarcoma Family Tumors. Clin Cancer Res (2012) 18(9):2625–31. doi: 10.1158/1078-0432.CCR-12-0061
114. Thijs AM, van der Graaf WT, van Herpen CM. Temsirolimus for Metastatic Desmoplastic Small Round Cell Tumor. Pediatr Blood Cancer (2010) 55(7):1431–2. doi: 10.1002/pbc.22755
115. Tian Y, Cheng X, Li Y. Chemotherapy Combined With Apatinib for the Treatment of Desmoplastic Small Round Cell Tumors: A Case Report. J Cancer Res Ther (2020) 16(5):1177–81. doi: 10.4103/jcrt.JCRT_589_20
116. Syed YY. Anlotinib: First Global Approval. Drugs (2018) 78(10):1057–62. doi: 10.1007/s40265-018-0939-x
117. van Geel RM, Beijnen JH, Schellens JH. Concise Drug Review: Pazopanib and Axitinib. Oncologist (2012) 17(8):1081–9. doi: 10.1634/theoncologist.2012-0055
118. Bukowski RM, Yasothan U, Kirkpatrick P. Pazopanib. Nat Rev Drug Discovery (2010) 9(1):17–8. doi: 10.1038/nrd3073
119. Zhang PJ, Goldblum JR, Pawel BR, Pasha TL, Fisher C, Barr FG. PDGF-A, PDGF-Rbeta, TGFbeta3 and Bone Morphogenic Protein-4 in Desmoplastic Small Round Cell Tumors With EWS-WT1 Gene Fusion Product and Their Role in Stromal Desmoplasia: An Immunohistochemical Study. Mod Pathol (2005) 18(3):382–7. doi: 10.1038/modpathol.3800264
120. Magnan HD, Chou T, LaQuaglia MP, Gerald W, Ladanyi M, Merchant MS. Elevated Expression of VEGFR-2 and VEGFA in Desmoplastic Small Round Cell Tumor (DSRCT) and Activity of Bevacizumab and Irinotecan in a Xenograft Model of DSRCT. (2009) 27: (15_suppl):10016. doi: 10.1200/jco.2009.27.15_suppl.10016
121. Menegaz BA, Cuglievan B, Benson J, Camacho P, Lamhamedi-Cherradi SE, Leung CH, et al. Clinical Activity of Pazopanib in Patients With Advanced Desmoplastic Small Round Cell Tumor. Oncologist (2018) 23(3):360–6. doi: 10.1634/theoncologist.2017-0408
122. Ferrari SM, Centanni M, Virili C, Miccoli M, Ferrari P, Ruffilli I, et al. Sunitinib in the Treatment of Thyroid Cancer. Curr Med Chem (2019) 26(6):963–72. doi: 10.2174/0929867324666171006165942
123. Bisht S, Feldmann G, Brossart P. Pharmacokinetics and Pharmacodynamics of Sunitinib for the Treatment of Advanced Pancreatic Neuroendocrine Tumors. Expert Opin Drug Metab Toxicol (2013) 9(6):777–88. doi: 10.1517/17425255.2013.791281
124. Hahn O, Stadler W. Sorafenib. Curr Opin Oncol (2006) 18(6):615–21. doi: 10.1097/01.cco.0000245316.82391.52
125. Scott AJ, Messersmith WA, Jimeno A. Apatinib: A Promising Oral Antiangiogenic Agent in the Treatment of Multiple Solid Tumors. Drugs Today (Barc) (2015) 51(4):223–9. doi: 10.1358/dot.2015.51.4.2320599
126. Scott LJ. Apatinib: A Review in Advanced Gastric Cancer and Other Advanced Cancers. Drugs (2018) 78(7):747–58. doi: 10.1007/s40265-018-0903-9
127. Xue JM, Astère M, Zhong MX, Lin H, Shen J, Zhu YX. Efficacy and Safety of Apatinib Treatment for Gastric Cancer, Hepatocellular Carcinoma and non-Small Cell Lung Cancer: A Meta-Analysis. Onco Targets Ther (2018) 11:6119–28. doi: 10.2147/OTT.S172717
128. Yu T, Wu C, Zhu C, He Y, Yang D, Cheng Y, et al. Oral Administration of Liposome-Apatinib and Locally Delivery of Docetaxel/MPEG-PCL by Fibrin Glue Synergistically Improve Therapeutic Effect in Colorectal Cancer. J BioMed Nanotechnol (2018) 14(12):2077–91. doi: 10.1166/jbn.2018.2651
129. Ruan X, Shi X, Dong Q, Yu Y, Hou X, Song X, et al. Antitumor Effects of Anlotinib in Thyroid Cancer. Endocr Relat Cancer (2019) 26(1):153–64. doi: 10.1530/ERC-17-0558
130. Waller CF. Imatinib Mesylate. Recent Results Cancer Res (2018) 212:1–27. doi: 10.1007/978-3-319-91439-8_1
131. Bond M, Bernstein ML, Pappo A, Schultz KR, Krailo M, Blaney SM, et al. A Phase II Study of Imatinib Mesylate in Children With Refractory or Relapsed Solid Tumors: A Children’s Oncology Group Study. Pediatr Blood Cancer (2008) 50(2):254–8. doi: 10.1002/pbc.21132
132. Li M, Kroetz DL. Bevacizumab-Induced Hypertension: Clinical Presentation and Molecular Understanding. Pharmacol Ther (2018) 182:152–60. doi: 10.1016/j.pharmthera.2017.08.012
133. Beltran PJ, Mitchell P, Chung YA, Cajulis E, Lu J, Belmontes B, et al. AMG 479, a Fully Human Anti-Insulin-Like Growth Factor Receptor Type I Monoclonal Antibody, Inhibits the Growth and Survival of Pancreatic Carcinoma Cells. Mol Cancer Ther (2009) 8(5):1095–105. doi: 10.1158/1535-7163.MCT-08-1171
134. Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The Insulin-Like Growth Factor-1 Receptor-Targeting Antibody, CP-751,871, Suppresses Tumor-Derived VEGF and Synergizes With Rapamycin in Models of Childhood Sarcoma. Cancer Res (2009) 69(19):7662–71. doi: 10.1158/0008-5472.CAN-09-1693
135. Guenther LM, Dharia NV, Ross L, Conway A, Robichaud AL, Catlett JL 2nd, et al. A Combination CDK4/6 and IGF1R Inhibitor Strategy for Ewing Sarcoma. Clin Cancer Res (2019) 25(4):1343–57. doi: 10.1158/1078-0432.CCR-18-0372
136. Subbiah V, Brown RE, Jiang Y, Buryanek J, Hayes-Jordan A, Kurzrock R, et al. Morphoproteomic Profiling of the Mammalian Target of Rapamycin (mTOR) Signaling Pathway in Desmoplastic Small Round Cell Tumor (EWS/WT1), Ewing’s Sarcoma (EWS/FLI1) and Wilms’ Tumor(WT1). PloS One (2013) 8(7):e68985. doi: 10.1371/journal.pone.0068985
137. Wan X, Helman LJ. The Biology Behind mTOR Inhibition in Sarcoma. Oncologist (2007) 12(8):1007–18. doi: 10.1634/theoncologist.12-8-1007
138. Wagner LM, Fouladi M, Ahmed A, Krailo MD, Weigel B, DuBois SG, et al. Phase II Study of Cixutumumab in Combination With Temsirolimus in Pediatric Patients and Young Adults With Recurrent or Refractory Sarcoma: A Report From the Children’s Oncology Group. Pediatr Blood Cancer (2015) 62(3):440–4. doi: 10.1002/pbc.25334
139. van Erp AEM, van Houdt L, Hillebrandt-Roeffen MHS, van Bree N, Flucke UE, Mentzel T, et al. Olaparib and Temozolomide in Desmoplastic Small Round Cell Tumors: A Promising Combination In Vitro and In Vivo. J Cancer Res Clin Oncol (2020) 146(7):1659–70. doi: 10.1007/s00432-020-03211-z
140. Modak S, Gerald W, Cheung NK. Disialoganglioside GD2 and a Novel Tumor Antigen: Potential Targets for Immunotherapy of Desmoplastic Small Round Cell Tumor. Med Pediatr Oncol (2002) 39(6):547–51. doi: 10.1002/mpo.10151
141. Modak S, Zanzonico P, Grkovski M, Slotkin EK, Carrasquillo JA, Lyashchenko SK, et al. B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study. J Clin Oncol (2020) 38(36):Jco2001974. doi: 10.1200/JCO.20.01974
142. Dobrenkov K, Ostrovnaya I, Gu J, Cheung IY, Cheung NK. Oncotargets GD2 and GD3 are Highly Expressed in Sarcomas of Children, Adolescents, and Young Adults. Pediatr Blood Cancer (2016) 63(10):1780–5. doi: 10.1002/pbc.26097
143. Johnson DB, Sullivan RJ, Menzies AM. Immune Checkpoint Inhibitors in Challenging Populations. Cancer (2017) 123(11):1904–11. doi: 10.1002/cncr.30642
144. Bulbul A, Shen JP, Xiu J, Tamayo P, Husain H. Genomic and Proteomic Alterations in Desmoplastic Small Round Blue-Cell Tumors. JCO Precis Oncol (2018) 2:PO.17.00170. doi: 10.1200/PO.17.00170
145. Fine RL, Shah SS, Moulton TA, Yu IR, Fogelman DR, Richardson M, et al. Androgen and C-Kit Receptors in Desmoplastic Small Round Cell Tumors Resistant to Chemotherapy: Novel Targets for Therapy. Cancer Chemother Pharmacol (2007) 59(4):429–37. doi: 10.1007/s00280-006-0280-z
146. Mazuryk M, Paterson AH, Temple W, Arthur K, Crabtree T, Stewart DA. Benefit of Aggressive Multimodality Therapy With Autologous Stem Cell Support for Intra-Abdominal Desmoplastic Small Round Cell Tumor. Bone Marrow Transplant (1998) 21(9):961–3. doi: 10.1038/sj.bmt.1701220
147. Bailey K, Roth M, Weiser D, Gill J. High-Dose Chemotherapy With Stem Cell Rescue in Desmoplastic Small Round Cell Tumor: A Single-Institution Experience and Review of the Literature. Sarcoma (2018) 2018:1948093. doi: 10.1155/2018/1948093
148. Cook RJ, Wang Z, Arora M, Lazarus HM, Kasow KA, Champagne MA, et al. Clinical Outcomes of Patients With Desmoplastic Small Round Cell Tumor of the Peritoneum Undergoing Autologous HCT: A CIBMTR Retrospective Analysis. Bone Marrow Transplant (2012) 47(11):1455–8. doi: 10.1038/bmt.2012.57
149. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin Nutr (2017) 36(1):11–48. doi: 10.1016/j.clnu.2016.07.015
150. Hayes-Jordan AA, Coakley BA, Green HL, Xiao L, Fournier KF, Herzog CE, et al. Desmoplastic Small Round Cell Tumor Treated With Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Results of a Phase 2 Trial. Ann Surg Oncol (2018) 25(4):872–7. doi: 10.1245/s10434-018-6333-9
Keywords: intra-abdominal desmoplastic small round cell tumor, EWS-WT1 gene, treatment, targeted therapy, immunotherapy
Citation: Wei G, Shu X, Zhou Y, Liu X, Chen X and Qiu M (2021) Intra-Abdominal Desmoplastic Small Round Cell Tumor: Current Treatment Options and Perspectives. Front. Oncol. 11:705760. doi: 10.3389/fonc.2021.705760
Received: 11 June 2021; Accepted: 25 August 2021;
Published: 15 September 2021.
Edited by:
Isacco Ferrarini, Brown University, United StatesReviewed by:
Giuseppe Broggi, University of Catania, ItalyBiswa Biswal, KPJ Ipoh Specialist Hospital, Malaysia
Copyright © 2021 Wei, Shu, Zhou, Liu, Chen and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Qiu, cWl1bWVuZ0B3Y2hzY3UuY24=
†These authors have contributed equally to this work
 Guixia Wei
Guixia Wei Xinyao Shu1†
Xinyao Shu1† Meng Qiu
Meng Qiu