- 1Centre de Recherche en Automatique Nancy France - UMR 7039 - BioSiS Department, Faculty of Medicine, Université de Lorraine, Vandoeuvre-lès-Nancy, France
- 2Neurology Departement, Neurooncology Unit, CHRU, Nancy, France
- 3Department of Neurosurgery, Gui de Chauliac Hospital, Montpellier University Medical Center, Montpellier, France
- 4Team “Plasticity of Central Nervous System, Stem Cells and Glial Tumors”, National Institute for Health and Medical Research (INSERM), U1191 Laboratory, Institute of Functional Genomics, University of Montpellier, Montpellier, France
Introduction
Neurooncology is a young specialty which initially dealt mostly with glioblastoma patients with a short overall survival (OS). Yet, recently the scope gradually expanded by taking care of lower-grade glioma (LGG) patients with a longer OS (1). Historically, these patients were managed with a “wait and see attitude” claiming “benignity” despite 6 to 7 years OS (2, 3). Therefore, quality of care was mainly based on physician’s subjectivity and not on the natural history, leading to beliefs that early surgery was not adapted due to “normal neurological examination”.
Twenty years later, it is now admitted that (i) beyond seizures, LGG patients suffer from cognitive and behavioral deficits at diagnosis even in incidental cases (4) (ii) this tumor will inescapably transform in higher grades, explaining the use of “lower-grade glioma” (mixing II/III) expression (5) (iii) early surgery is a main therapeutic factor (significant correlation between extent of resection and OS) (6, 7) (iv) early radiotherapy, at least given alone, is not associated with decreased mortality (6, 8). These changes resulted in a longer life expectancy now over 15 to 16 years (9–11).
Moreover, neurooncologists had to pay more attention to quality of life (QoL) for patients who must learn to live with a chronic neoplastic disease.
On the other hand, because LGG will systematically recur, further adapted treatments have to be administrated (12). However, heterogeneity of progression patterns (13) makes the prediction of timescales of proliferation, migration, and degeneration at the individual level impossible.
To provide more reliable prognostic factors, advances in molecular biology led to a new classification designed for more appropriate decisions (14). Surprisingly, although genetics was initially a tool to better dissociate types of LGG with distinct prognosis, molecular biology rapidly became the first parameter in guidelines (15). Although useful, by taking mostly account of genetics criteria and extrapolating a correlation to specific OS based upon statistical analysis, there is a risk to neglect tumor-host interactions, patient’s wishes, and long-term QoL.
Here, the main purpose is to redefine what “best quality of care” means by considering both tumor characteristics and patient’s personal criteria. The ultimate goal is to give the choice of therapeutic orientation at each step thanks to honest although complex and time-consuming information highlighting oncofunctional balance and various strategies individually adapted over time in parallel with changes in tumor behavior and patient’s expectations.
Toward Hegemony of Precision Medicine Based on Glioma Molecular Profile: The Risk to Impose a “Unique Solution”
Official guidelines, elaborated on EBM and mostly relying on randomized controlled trials (RCTs), were primarily designed to help physicians within a framework facilitating decision making and thus defining a “quality of care”.
Particularly, progress allowed a refinement of the WHO classification increasingly based on genetic profiling (14, 16). This praiseworthy initiative gradually drifts toward more drastic molecular recommendations. Such a so-called precision EBM (17), glioma, and not patient-centered, is questionable. First, the 2016 classification (14) was built on few parameters (e.g., 1p19q, IDH, and MGMT status) too simplistic to capture complex glioma behavior and host interactions. Because improved knowledge will still take a considerable time, it is difficult to understand how “quality of care” can be determined on preliminary criteria. For example, IDH wild-type glioma were considered as molecular glioblastoma (15), whereas by integrating markers, such as TERT or EGFR, distinct groups exhibiting different prognosis (18–20) are now identified. Thus, many patients dogmatically receive and continue to receive RT-CT, whereas it would be more adapted to follow some of them by integrating parameters, such as growth rate (21, 22), and wonder about the multimodal heterogeneity. Similarly, because response rate to CT is statistically higher in oligodendrogliomas, it was peremptorily postulated that upfront, CT was not indicated in astrocytomas by neglecting that stabilization or shrinkage was nonetheless possible (23), thus opening the door to surgery which can have a major impact on prognosis. Thereby, tumor genetics represent an important but not exclusive part of the story (24).
These examples illustrate the drift in the utilization of EBM originally defined as “the conscientious, explicit, and judicious use of the current best evidence in making decisions about the care of individual patients” (25). Yet, the power of population-based observational studies based on real-life data collected in clinical routine was progressively denied for the benefit of exclusive RCTs. Nevertheless, they suffer from serious limitations (26, 27), first the inclusion of selected patients not reflecting the daily practice [e.g., young age in Stupp et al. trial (28)], or the fact that factors like extent of resection are overlooked (29), whereas a meta-analysis confirmed a strong correlation to OS (7) even after adjustment for molecular markers (6). Currently, a statistical result identified by RCTs is erected as a rigid law to be applied to each patient, without considering the inter-subject multimodal variability (30). If RCTs are the most convincing and effective strategy for answering a simple therapeutic question with measured short-term effects, they remain unsuitable to the current neuro-oncological issues. Indeed, the challenge in this era is rather to know what kind of patients will respond effectively to a therapeutic strategy and not to determine the best treatment among highly selected patients. Even if statistical tools as interaction tests used in RCTs design could give results of subgroup analyses, they remain insufficient because of a lack of statistical power and never allow conclusion. In fact, when the clinical questions and situations are not compatible with the use of RCTs, the importance of observational studies should be reconsidered. If they are conducted with a methodological rigor (long follow-up, sufficient size, few missing data) and analyzed with statistical tools limiting biases, they could provide reliable evidence and enable a better understanding of the long-term evolution. Besides, a Cochrane review (31) highlighted that the results of observational studies and RCTs are most often in agreement.
Third, EBM was not designed to validate a multistep strategy over years. Indeed, time-scales are different between the long life expectancy of patients and many RCTs with only a short follow-up which optionally use surrogates (such as progression-free survival [PFS] moreover often not accurately assessed) to demonstrate within the time allowed a significant difference regarding investigated parameters. This “reality of the moment” does not reflect long-term OS and QoL, e.g., early RT may have an impact on PFS but not on OS (8) while generating delayed and sometimes major cognitive deterioration (32, 33) not observed with too short a follow-up. It was the case in the RCT trial by Buckner et al. (29) within which (i) contrast enhancement was noted for approximately 50% of patients which is quite atypical for LGG (ii) surgical status is mainly represented by biopsies or partial surgeries in opposition to specialized teams practices mainly carrying out subtotal or total resections (iii) IDH status is only accessible in less than half of the cases and 1p19q in a quarter of them (iv) and cognitive analysis was only based on the MMSE (designed for dementia patients) with a longitudinal partial completion (Table 1 for a critical review of RCTs).
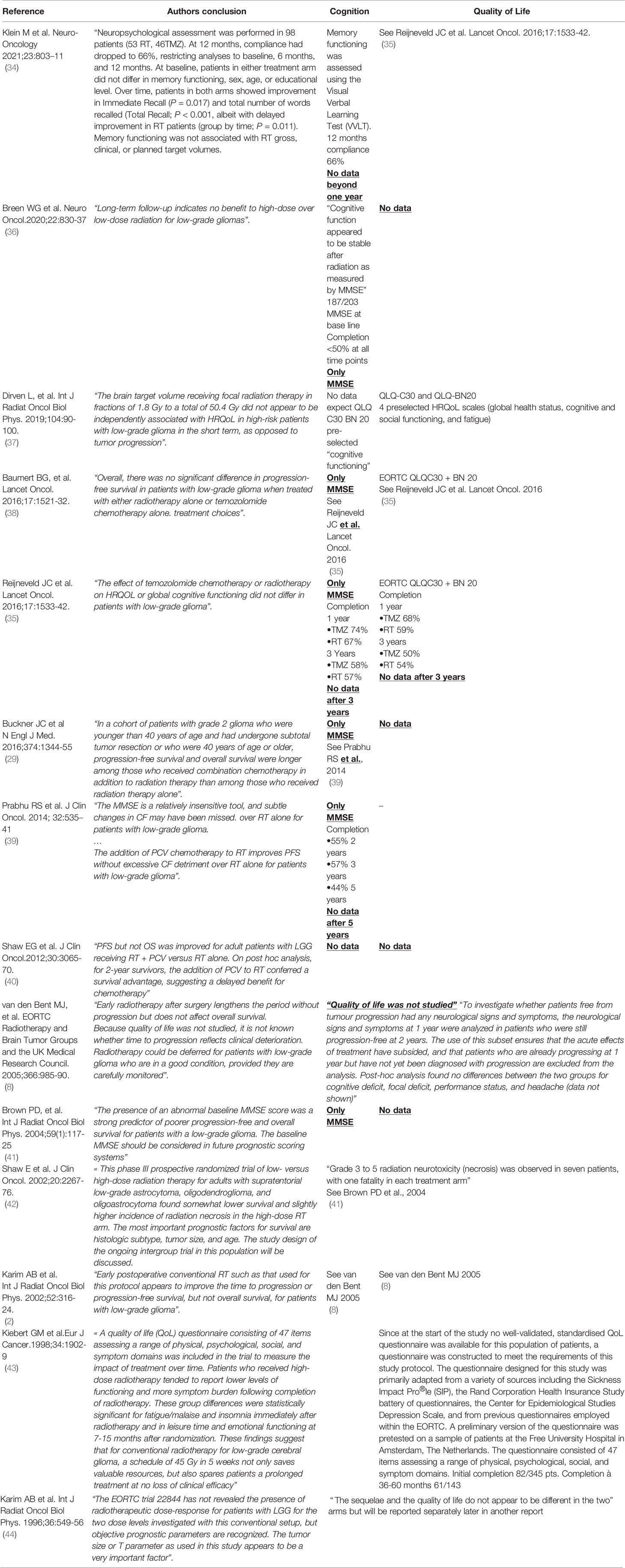
Table 1 Main RCTs in medical neurooncology for LGGs: critical review of cognition and quality of life data.
Fourthly, whereas the quality of care relying on RCT depends on a reductionist panel of criteria, the selection of parameters “officially recognized” as decreed under the guise of EBM is questionable. For example, velocity expansion diameter is not incorporated in trials while it is an independent prognostic marker not correlated to molecular profile (45) and more reliable than 2007 WHO classification to predict OS (46, 47). Moreover, a main weakness of the 2016 WHO classification is arbitrarily to not consider intra-tumoral heterogeneity (19, 48, 49). Indeed, although areas of malignant transformation are frequently identified in the middle of LGG, especially after extensive surgery, they are not recognized as “foci of grade III/IV” within a grade II glioma but condition the final grading for the entire tumor. This oversimplification leads to a monolithic strategy, namely to administrate RT-CT, while efficient alternative exists, particularly to delay adjuvant treatments following maximal resection with a 95% survival rate at 5 years (50).
To sum up, due to a new orientation of EBM different from the Sackett et al. seminal concept (25) this “precision-medicine” risks to indirectly impose a “unique solution” based upon few molecular markers unable to reflect the complex glioma-host interactions. This simplistic inflexible attitude does not really represent the “informed consent” of the patient.
The Alterative Way of Multimodal and Adaptive Individual Decision Making Aiming To Anticipate the Story Years in Advance
Because LGG patients live one to two decades, neurooncologists should learn to anticipate functional considerations. Indeed, a major lack of “precision-medicine” in gliomas is to prioritize analysis of PFS and OS as first endpoints at the expense of QoL. However, if a patient is doing well, this means that he/she is still alive, while the reverse is not true. Therefore, QoL should be more systematically considered as the main endpoint since LGG patients should have an active life (30). Yet, physicians are usually content with a basic neurological examination optionally with a simplistic neuropsychological assessment (e.g. MMSE) and a performance scale score (15). Nonetheless, to enjoy an optimal lifestyle (social investment, sexuality, childbirth, work) preservation of higher-order cognitive, emotional, and behavioral functions is mandatory (12). Neurosurgeons developed intraoperative awake mapping and monitoring of conation, cognition, and personality, resulting in a connectome-based resection according to a real-time investigation of neural networks and taking account of neuroplasticity (51–53). This led to a decrease of morbidity with stabilization or even improvement of postoperative neuropsychological scores (4) and over 97% of return to employment (54). By contrast, these types of high-level parameters have never been reported in CT/RT randomized study.
Beyond the lack of cognitive or QoL parameters framing each treatment in RCTs for LGG, and criticisms concerning tools (MMSE or QoL questionnaires tailored for malignant rapidly evolving tumors), these criteria are nonetheless essential to elaborate new guidelines paving the way for “quality of care.” Neuro-oncologists should ask the patient to define his/her own expectations and adjust the management accordingly (12, 33), e.g., awake surgery with identification of eloquent networks à la carte (55). Indeed, the patient must understand during the first meeting that therapeutic reserve is not inexhaustible. Typically, early RT may improve glioma control for years but entire re-irradiation is not possible at progression. This issue should be clearly explained to anticipate next stages. Moreover, because RT may induce delayed cognitive deteriorations, the onco-functional balance must be extensively discussed by tailoring a real patient-centered attitude (12, 56). The ultimate aim should be to use the good treatment(s) at the optimal moment(s) according not only to the tumor genetics but also other prognostic parameters and patient’s expectations over time. Remarkably, recent series showed that applying this concept led to OS over 16 to 17 years while preserving the QoL for over one decade (10, 11).
Conclusions
Beyond the fundamental opposition between precision medicine relying on molecular EBM and individualized multistep therapeutic approach adapted over years, “best quality of care” starts by giving the choice to the patient and family and by honestly detailing both philosophies. This approach of complexity is time-consuming and poorly suited to productionist practices of our care systems. It is, nevertheless, possible, independent of the socio-cultural level of each patient, and it represents the condition of a true interactive patient-centered medicine, far from a “unique solution” dogma.
The other risk of a single thought is to disempower the physicians who will not continue to actively discuss the best therapeutic option tailored to each patient but only passively apply a “standardized protocol”. This could lead to an impoverishment of knowledge, failing to see the full picture if all alternatives are not critically considered anymore. The ultimate danger would be to end up with strategies exclusively dictated by processing of large databanks with pre-defined reductive parameters or to use artificial intelligence methods disconnected from clinical practice and real life: this may turn doctors into uncritical executing agents.
Therefore, official recommendations should only be a guide, and tumor boards should provide consultative proposals but not become too oppressive (particularly for medico-legal issues); otherwise, a rigid EBM might kill innovation, which is still essential because glioma patients cannot yet be cured.
In summary, although efforts have been made to excavate different molecular subtypes from the formerly not well-defined mix of gliomas LGG (57, 58), more refined instruments measuring QoL are still lacking. Overcoming the problem of an overbalance of molecular marker can only be counteracted by triggering high-quality multicentric studies focusing on imaging and QoL issues.
Author Contributions
LT and HD contributed to conception and design of the study. HD wrote the first draft of the manuscript. LT and TO wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Karim AB, Afra D, Cornu P, Bleehan N, Schraub S, De Witte O, et al. Randomized Trial on the Efficacy of Radiotherapy for Cerebral Low-Grade Glioma in the Adult: European Organization for Research and Treatment of Cancer Study 22845 With the Medical Research Council Study BRO4: An Interim Analysis. Int J Radiat Oncol Biol Phys (2002) 52:316–24. doi: 10.1016/S0360-3016(01)02692-X
3. Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, et al. European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group. Prognostic Factors for Survival in Adult Patients With Cerebral Low-Grade Glioma. J Clin Oncol (2002) 20:2076–84. doi: 10.1200/JCO.2002.08.121
4. Ng S, Herbet G, Lemaitre AL, Cochereau J, Moritz-Gasser S, Duffau H. Neuropsychological Assessments Before and After Awake Surgery for Incidental Low-Grade Gliomas. J Neurosurg (2020), 1–10. doi: 10.3171/2020.7.JNS201507 Online ahead of print.
5. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med (2015) 372:2481–98. doi: 10.1056/NEJMoa1402121
6. Jakola AS, Skjulsvik AJ, Myrmel KS, Sjåvik K, Unsgård G, Torp SH, et al. Surgical Resection Versus Watchful Waiting in Low-Grade Gliomas. Ann Oncol (2017) 28:1942–8. doi: 10.1093/annonc/mdx230
7. Brown TJ, Bota DA, van Den Bent MJ, Brown PD, Maher E, Aregawi D, et al. Management of Low-Grade Glioma: A Systematic Review and Meta-Analysis. Neurooncol Pract (2019) 6:249–58. doi: 10.1093/nop/npy034
8. van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, et al. Long-Term Efficacy of Early Versus Delayed Radiotherapy for Low-Grade Astrocytoma and Oligodendroglioma in Adults: The EORTC 22845 Randomised Trial. Lancet (2005) 366:985–90. doi: 10.1016/S0140-6736(05)67070-5
9. Capelle L, Fontaine D, Mandonnet E, Taillandier L, Golmard JL, Bauchet L, et al. Spontaneous and Therapeutic Prognostic Factors in Adult Hemispheric World Health Organization Grade II Gliomas: A Series of 1097 Cases. J Neurosurg (2013) 118:1157–68. doi: 10.3171/2013.1.JNS121
10. Obara T, Blonski M, Brzenczek C, Mézières S, Gaudeau Y, Pouget C, et al. Adult Diffuse Low-Grade Gliomas: 35-Year Experience Nancy France Neurooncology Unit. Front Oncol (2020) 10:574679. doi: 10.3389/fonc.2020.574679
11. Hamdan N, Duffau H. Extending the Multistage Surgical Strategy for Recurrent Initially Low-Grade Gliomas: Functional and Oncological Outcomes in 31 Consecutive Patients Who Underwent a Third Resection Under Awake Mapping. J Neurosurg.
12. Duffau H, Taillandier L. New Concepts in the Management of Diffuse Low-Grade Glioma: Proposal of a Multistage and Individualized Therapeutic Approach. Neuro Oncol (2015) 17:332–42. doi: 10.1093/neuonc/nou153
13. Ferracci FX, Michaud K, Duffau H. The Landscape of Postsurgical Recurrence Patterns in Diffuse Low-Grade Gliomas. Crit Rev Oncol Hematol (2019) 138:148–55. doi: 10.1016/j.critrevonc.2019.04.009
14. Louis DN, Wiestler OD, Cavenee WK. Who Classification of Tumours of the Central Nervous System. 4th edition. Lyon: International Agency for Research on Cancer (IARC (2016).
15. Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, et al. European Association for Neuro-Oncology (EANO) Guideline on the Diagnosis and Treatment of Adult Astrocytic and Oligodendroglial Gliomas. Lancet Oncol (2017) 18:e315–29. doi: 10.1016/S1470-2045(17)30194-8
16. Louis DN, Wesseling P, Aldape K, Brat DJ, Capper D, Cree IA, et al. cIMPACT-NOW Update 6: New Entity and Diagnostic Principle Recommendations of the cIMPACT-Utrecht Meeting on Future CNS Tumor Classification and Grading. Brain Pathol (2020) 30:844–56. doi: 10.1111/bpa.12832
17. Levine VA. Personalized Medicine in Neuro-Oncology. CNS Oncol (2016) 5:55–8. doi: 10.2217/cns-2016-0006
18. Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA. et al: Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell (2016) 164:550–63. doi: 10.1016/j.cell.2015.12.028
19. Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y. et al: Mutational Landscape and Clonal Architecture in Grade II and III Gliomas. Nat Genet (2015) 47:458–68. doi: 10.1038/ng.3273
20. Zhang ZY, Chan AK, Ding XJ, Qin ZY, Hong CS, Chen LC, et al. TERT Promoter Mutations Contribute to IDH Mutations in Predicting Differential Responses to Adjuvant Therapies in WHO Grade II and III Diffuse Gliomas. Oncotarget (2015) 6:24871–83. doi: 10.18632/oncotarget.4549
21. Poulen G, Gozé C, Rigau V, Duffau H. Huge Heterogeneity in Survival in a Subset of Adult Patients With Resected, Wild-Type Isocitrate Dehydrogenase Status, WHO Grade II Astrocytomas. J Neurosurg (2019) 130:1289–98. doi: 10.3171/2017.10.JNS171825
22. Di Carlo DT, Duffau H, Cagnazzo F, Benedetto N, Morganti R, Perrini P. IDH Wild-Type WHO Grade II Diffuse Low-Grade Gliomas. A Heterogeneous Family With Different Outcomes. Systematic Review and Meta-Analysis. Neurosurg Rev (2020) 43:383–95. doi: 10.1007/s10143-018-0996-3
23. Blonski M, Taillandier L, Herbet G, Maldonado IL, Beauchesne P, Fabbro M, et al. Combination of Neoadjuvant Chemotherapy Followed by Surgical Resection as a New Strategy for WHO Grade II Gliomas: A Study of Cognitive Status and Quality of Life. J Neurooncol (2012) 106:353–66. doi: 10.1007/s11060-011-0670-x
24. The European Low-Grade Glioma Network. Evidence-Based Medicine in Glioma: Molecular Biology is Only Part of the Story. Lancet Oncol (2017) 18:e429. doi: 10.1016/S1470-2045(17)30510-7
25. Sackett D, Rosenberg W, Gray M, Haynes RB, Richardson WS. Evidence Based Medicine: What it is and What it Isn’t. BMJ (1996) 312:71–2. doi: 10.1136/bmj.312.7023.71
26. Bothwell LE, Greene JA, Podolsky SH, Jones DS. Assessing the Gold Standard-Lesson From the History of Rcts. N Eng J Med (2016) 374:2175–81. doi: 10.1056/NEJMms1604593
27. Mielke D, Rohde V. Randomized Controlled Trials-a Critical Re-Appraisal. Neurosurg Rev (2020). doi: 10.1007/s10143-020-01401-4
28. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med (2005) 352:987–96. doi: 10.1056/NEJMoa043330
29. Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Bargeret GR. Al. Radiation Plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med (2016) 374:1344–55. doi: 10.1056/NEJMoa1500925
30. Duffau H. Paradoxes of Evidence-Based Medicine in Lower-Grade Glioma: To Treat the Tumor or the Patient? Neurology (2018) 91:657–62. doi: 10.1212/WNL.0000000000006288
31. Anglemyer A, Horvath HT, Bero L. Healthcare Outcomes Assessed With Observational Study Designs Compared With Those Assessed in Randomized Trials. Cochrane Database Syst Rev (2014) 4):MR000034. doi: 10.1002/14651858.MR000034.pub2
32. Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJB, Aaronson NK, et al. Cognitive and Radiological Effects of Radiotherapy in Patients With Low-Grade Glioma: Long-Term Follow-Up. Lancet Neurol (2009) 8:810–8. doi: 10.1016/S1474-4422(09)70204-2
33. Duffau H. Why Brain Radiation Therapy Should Take Account of the Individual Structural and Functional Connectivity: Toward an Irradiation “À La Carte”. Crit Rev Oncol Hematol (2020) 154:103073. doi: 10.1016/j.critrevonc.2020.103073
34. Klein M, Drijver AJ, van den Bent MJ, Bromberg JC, Hoang-Xuan K, Taphoorn MJB, et al. Memory in Low-Grade Glioma Patients Treated With Radiotherapy or Temozolomide: A Correlative Analysis of EORTC Study 22033-26033. Neuro Oncol (2021) 23:803–11. doi: 10.1093/neuonc/noaa252
35. Reijneveld JC, Taphoorn MJB, Coens C, Bromberg JEC, Mason WP, Hoang-Xuan K, et al. Health-Related Quality of Life in Patients With High-Risk Low-Grade Glioma (EORTC 22033-26033): A Randomised, Open-Label, Phase 3 Intergroup Study. Lancet Oncol (2016) 17:1533–42. doi: 10.1016/S1470-2045(16)30305-9
36. Breen WG, Anderson SK, Carrero XW, Brown PD, Ballman KV, O’Neill BP, et al. Final Report From Intergroup Ncctg 86-72-51 (Alliance): A Phase III Randomized Clinical Trial of High-Dose Versus Low-Dose Radiation for Adult Low-Grade Glioma. Neuro Oncol (2020) 22:830–7. doi: 10.1093/neuonc/noaa021
37. Dirven L, Reijneveld JC, Taphoorn MJB, Coens C, El-Badawy SA, Tzuk-Shina T, et al. Impact of Radiation Target Volume on Health-Related Quality of Life in Patients With Low-Grade Glioma in the 2-Year Period Post Treatment: A Secondary Analysis of the EORTC 22033-26033. Int J Radiat Oncol Biol Phys (2019) 104:90–100. doi: 10.1016/j.ijrobp.2019.01.003
38. Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, et al. Temozolomide Chemotherapy Versus Radiotherapy in High-Risk Low-Grade Glioma (EORTC 22033-26033): A Randomised, Open-Label, Phase 3 Intergroup Study. Lancet Oncol (2016) 17:1521–32. doi: 10.1016/S1470-2045(16)30313-8
39. Prabhu RS, Won M, Shaw EG, Hu C, Brachman DG, Buckner JC, et al. Effect of the Addition of Chemotherapy to Radiotherapy on Cognitive Function in Patients With Low-Grade Glioma: Secondary Analysis of RTOG 98-02. J Clin Oncol (2014) 32:535–41. doi: 10.1200/JCO.2013.53.1830
40. Shaw EG, Wang M, Coons SW, Brachman DG, Buckner JC, Stelzer KJ, et al. Randomized Trial of Radiation Therapy Plus Procarbazine, Lomustine, and Vincristine Chemotherapy for Supratentorial Adult Low-Grade Glioma: Initial Results of RTOG 9802. J Clin Oncol (2012) 30:3065–70. doi: 10.1200/JCO.2011.35.8598
41. Brown PD, Buckner JC, O’Fallon JR, Iturria NL, O’Neill BP, Brown CA, et al. Importance of Baseline Mini-Mental State Examination as a Prognostic Factor for Patients With Low-Grade Glioma. Int J Radiat Oncol Biol Phys (2004) 59:117–25. doi: 10.1016/j.ijrobp.2003.10.040
42. Shaw E, Arusell R, Scheithauer B, O’Fallon J, O’Neill B, Dinapoli R, et al. Prospective Randomized Trial Of Low- Versus High-Dose Radiation Therapy In Adults With Supratentorial Low-Grade Glioma: Initial Report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group Study. J Clin Oncol (2002) 20:2267–76. doi: 10.1200/JCO.2002.09.126
43. Kiebert GM, Curran D, Aaronson NK, Bolla M, Menten J, Rutten EH, et al. Quality of Life After Radiation Therapy of Cerebral Low-Grade Gliomas of The Adult: Results of a Randomised Phase III Trial on Dose Response (EORTC Trial 22844). EORTC Radiotherapy Co-operative Group. Eur J Cancer (1998) 34:1902–9. doi: 10.1016/S0959-8049(98)00268-8
44. Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, et al. A Randomized Trial on Dose-Response in Radiation Therapy Of Low-Grade Cerebral Glioma: European Organization for Research and Treatment of Cancer (Eortc) Study 22844. Int J Radiat Oncol Biol Phys (1996) 36:549–56. doi: 10.1016/S0360-3016(96)00352-5
45. Gozé C, Blonski M, Le Maistre G, Bauchet L, Dezamis E, Page P, et al. Imaging Growth and Isocitrate Dehydrogenase 1 Mutation are Independent Predictors for Diffuse Low-Grade Gliomas. Neuro Oncol (2014) 16:1100–9. doi: 10.1093/neuonc/nou085
46. Pallud J, Mandonnet E, Duffau H, Kujas M, Guillevin R, Galanaud D, et al. Prognostic Value of Initial Magnetic Resonance Imaging Growth Rates for World Health Organization Grade II Gliomas. Ann Neurol (2006) 60:380–3. doi: 10.1002/ana.20946
47. Pallud J, Blonski M, Mandonnet E, Audureau E, Fontaine D, Sanai N, et al. Velocity of Tumor Spontaneous Expansion Predicts Long-Term Outcomes for Diffuse Low-Grade Gliomas. Neuro-Oncol (2013) 15:595–606. doi: 10.1093/neuonc/nos331
48. Pedeutour-Braccini Z, Burel-Vandenbos F, Gozé C, Roger C, Bazin A, Costes-Martineau V, et al. Microfoci of Malignant Progression in Diffuse Low-Grade Gliomas: Towards the Creation of an Intermediate Grade in Glioma Classification? Virchows Arch (2015) 466:433–44. doi: 10.1007/s00428-014-1712-5
49. Augustus M, Pineau D, Aimond F, Azar S, Lecca D, Scamps F, et al. Identification of CRYAB+ Kcnn3+ SOX9+ Astrocyte-Like and EGFR+ Pdgfra+ OLIG1+ Oligodendrocyte-Like Tumoral Cells in Diffuse Idh1-Mutant Gliomas and Implication of NOTCH1 Signalling in Their Genesis. Cancers (Basel) (2021) 13:2107. doi: 10.3390/cancers13092107
50. Darlix A, Rigau V, Fraisse J, Gozé C, Fabbro M, Duffau H. Postoperative Follow-Up for Selected Diffuse Low-Grade Gliomas With WHO Grade III/IV Foci. Neurology (2020) 94:e830–41. doi: 10.1212/WNL.0000000000008877
51. Duffau H. Stimulation Mapping of White Matter Tracts to Study Brain Functional Connectivity. Nat Rev Neurol (2015) 11:255–65. doi: 10.1038/nrneurol.2015.51
52. Herbet G, Duffau H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol Rev (2020) 100:1181–228. doi: 10.1152/physrev.00033.2019
53. Herbet G, Maheu M, Costi E, Lafargue G, Duffau H. Mapping Neuroplastic Potential in Brain-Damaged Patients. Brain (2016) 139:829–44. doi: 10.1093/brain/awv394
54. Ng S, Herbet G, Moritz-Gasser S, Duffau H. Return to Work Following Surgery for Incidental Diffuse Low-Grade Glioma: A Prospective Series With 74 Patients. Neurosurgery (2020) 87:720–9. doi: 10.1093/neuros/nyz513
55. Duffau H. New Philosophy, Clinical Pearls, and Methods for Intraoperative Cognition Mapping and Monitoring “À La Carte” in Brain Tumor Patients. Neurosurgery (2021) 88:919–30. doi: 10.1093/neuros/nyaa363
56. Mandonnet E, Duffau H. An Attempt to Conceptualize the Individual Onco-Functional Balance: Why a Standardized Treatment is an Illusion for Diffuse Low-Grade Glioma Patients. Crit Rev Oncol Hematol (2018) 122:83–91. doi: 10.1016/j.critrevonc.2017.12.008
57. Fujimoto K, Arita H, Satomi K, Yamasaki K, Matsushita Y, Nakamura T, et al. TERT Promoter Mutation Status is Necessary and Sufficient to Diagnose IDH-wildtype Diffuse Astrocytic Glioma With Molecular Features of Glioblastoma. Acta Neuropathol (2021) 142(2):323–38. doi: 10.1007/s00401-021-02337-9
Keywords: glioma, quality of life, evidence-based medicine, precision medicine, awake surgery, chemotherapy, radiation therapy
Citation: Taillandier L, Obara T and Duffau H (2021) What Does Quality of Care Mean in Lower-Grade Glioma Patients: A Precision Molecular-Based Management of the Tumor or an Individualized Medicine Centered on Patient’s Choices? Front. Oncol. 11:719014. doi: 10.3389/fonc.2021.719014
Received: 01 June 2021; Accepted: 02 July 2021;
Published: 20 July 2021.
Edited by:
Marie-Therese Forster, University Hospital Frankfurt, GermanyReviewed by:
Joerg Tonn, LMU Munich University Hospital, GermanyCopyright © 2021 Taillandier, Obara and Duffau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hugues Duffau, aC1kdWZmYXVAY2h1LW1vbnRwZWxsaWVyLmZy
 Luc Taillandier
Luc Taillandier Tiphaine Obara
Tiphaine Obara Hugues Duffau
Hugues Duffau