- 1Department of General Surgery, the Third People’s Hospital of Dalian, Dalian Medical University, Dalian, China
- 2Department of Central Laboratory, the Third People’s Hospital of Dalian, Dalian Medical University, Dalian, China
- 3Department of Critical Care Medicine, The Second Affiliated Hospital of Dalian Medical University, Dalian, China
- 4Department of Endocrinology, The Second Affiliated Hospital of Dalian Medical University, Dalian, China
- 5Department of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, China
Hepatocellular carcinoma (HCC) is the most common primary liver tumor. It is ranked the sixth most common neoplasm and the third most common cause of cancer mortality. At present, the most common treatment for HCC is surgery, but the 5-year recurrence rates are still high. Patients with early stage HCC with few nodules can be treated with resection or radiofrequency ablation (RFA); while for multinodular HCC, transarterial chemoembolization (TACE) has been the first-line treatment. In recent years, based on medical engineering cooperation, nanotechnology has been increasingly applied to the treatment of cancer. Photodynamic therapy and photothermal therapy are effective for cancer. This paper summarizes the latest progress of photodynamic therapy and photothermal therapy for HCC, with the aim of providing new ideas for the treatment of HCC.
Introduction
Cancer is the second most common cause of death among all diseases (1). Hepatocellular carcinoma (HCC) is a common digestive system tumor and the sixth most common type of cancer worldwide (2). Treatment includes radical surgery (3), molecular targeted therapies (4) and neoadjuvant therapy (5). Although progress has been made in the treatment of HCC, the prognosis of HCC patients is still poor and the 5-year survival rate is only about 18% (6). Therefore, new treatment methods are urgently needed to change this situation.
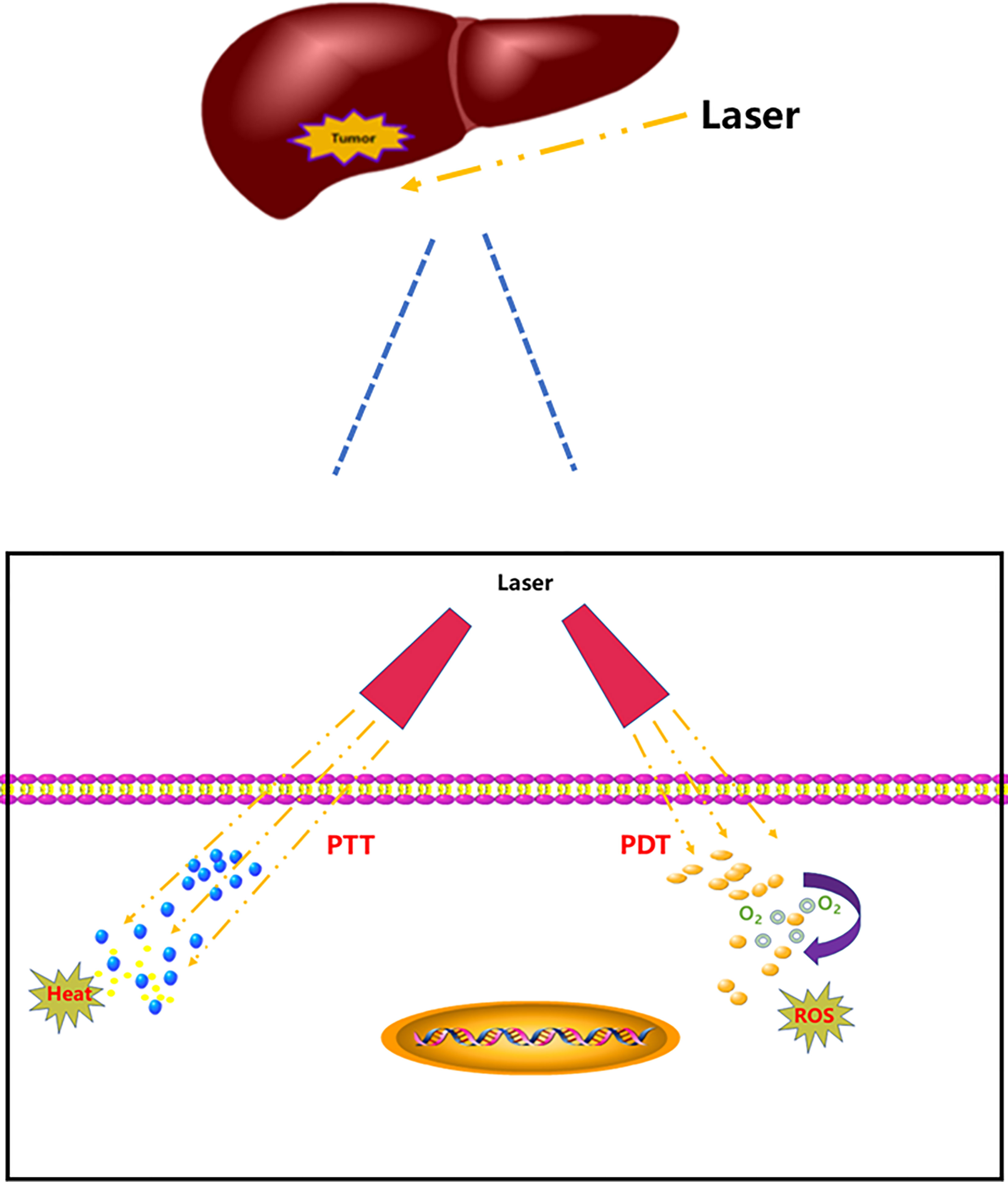
The toxicity and adverse effects of antitumor drugs have led researchers to seek new tumor treatment strategies (7) and photothermal therapy (PTT) and photodynamic therapy (PDT) have gradually emerged because of their specific spatial selectivity and lower invasiveness and initial resistance (8–10). PTT is a tumor treatment strategy that utilizes photothermal agents to induce thermal energy by laser. Absorbed light energy can be transformed into heat energy to achieve thermal ablation of tumor cells; therefore, tumors can be killed in the high temperature environment (11–13). PDT takes advantage of the active metabolism of tumor tissue; whereby non-toxic photosensitizers accumulate in tumor tissue after injection. When the tumor tissue is irradiated with harmless visible light, the activated photosensitizer transfers its energy to surrounding intracellular oxygen that forms reactive oxygen species (ROS), which specifically destroy the tumor cells and neovascularization (14–17) (Figure 1).
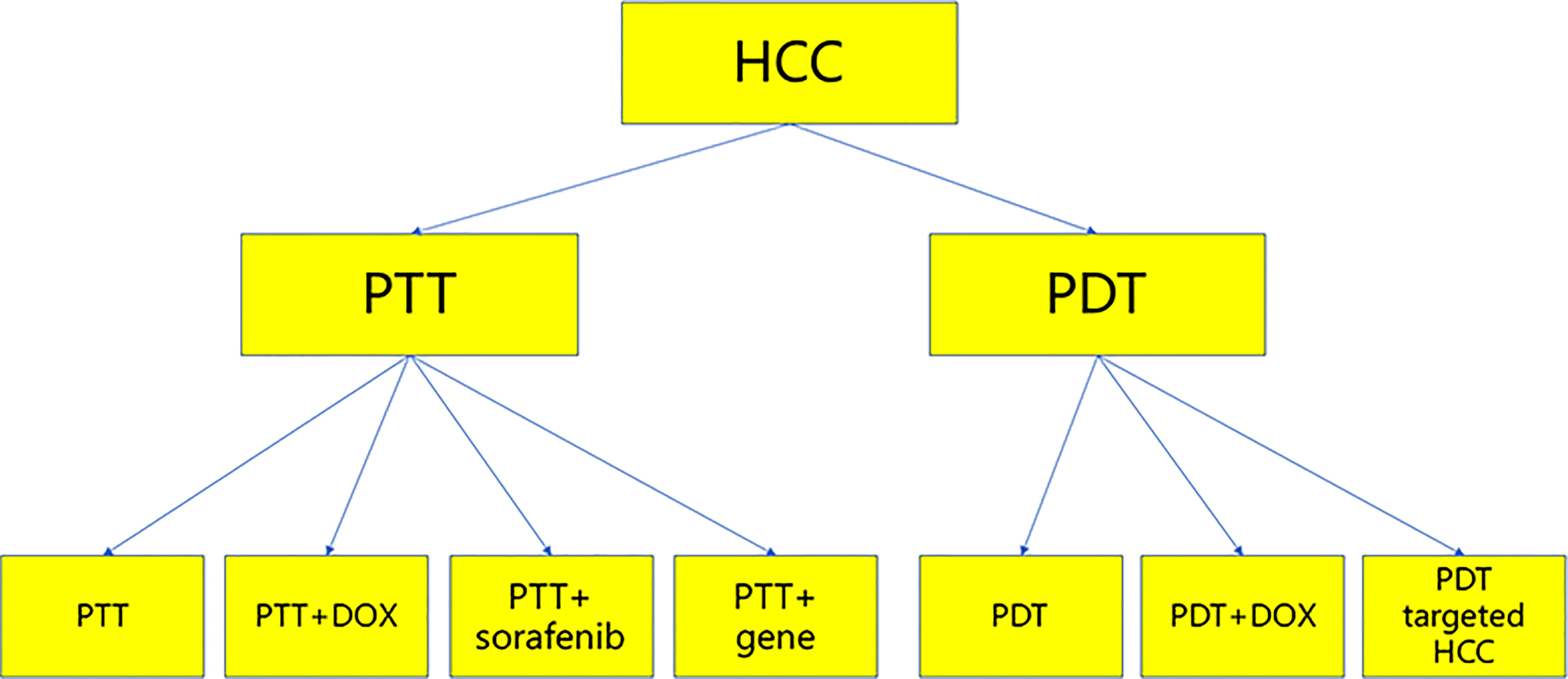
PTT and PDT have played a significant role in the treatment of tumors, and they have been used to treat HCC. This paper reviews recent studies on the treatment of HCC by PDT and PTT, with the aim of exploring new ideas and strategies for the treatment of HCC (Figure 2).
PTT
PTT for HCC
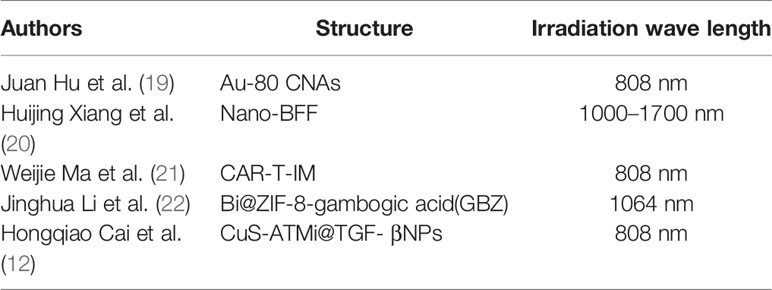
The application of light to heat energy conversion in tumor diagnosis and treatment has attracted the extensive attention of researchers (18) (Table 1). Metal nanoparticles play an important role in the diagnosis and treatment of tumors (23). Strong near-infrared light absorption is the basis of metal nanoparticles in PTT. Compared with traditional treatment, metal nanoparticles have the characteristics of high selectivity and efficiency and they are minimally invasive (24). Gold nanomaterials are the most widely used (25) because they have tunable surface plasma resonance properties and strong photothermal conversion efficiency (26). Juan Hu et al. synthesized cubic gold nanoparticles with different sizes, which could be excited by near-infrared light at 808 nm wavelength, showed strong near-infrared light absorption, optical stability, photothermal effect and high biocompatibility and were effective for treating liver cancer cells and animal models (19).
The absorption wavelength of near-infrared light in zone I is 650–1000 nm, which has poor tissue penetration. The tissue penetration of near-infrared light in zone II is good, with a wavelength of 1000–1700 nm, but it is rarely used at present (27). Huijing Xiang et al. polymerized and self-assembled boron difluoride formazanate dye to turn it into a two zone near-infrared dye. In vivo and in vitro studies confirmed that the nanoparticles had deep tissue penetration of light in zone II and inhibited HCC (20).
Chimeric antigen receptor (CAR) T-cell therapy is an important emerging treatment for tumors. T cells of tumor patients are modified in vitro to carry tumor specific antigens, and then injected into patients to attack tumor cells (28). CAR-T-cell therapy has shown clinical efficacy and safety for hematological malignancies and solid tumors (29). CAR-T cells can specifically recognize tumor-associated antigens and eliminate tumor cells through the single chain variable region. This region is derived from the heavy and light chains of polyclonal antibodies and can be expressed on the cell membrane of CAR-T cells (30). Weijie Ma et al. synthesized mesoporous silicon with 2-[2-(2-chloro-3-[(1,3-dihydro-3,3-dimethyl-2h-1-propyl-indole-2-subunits) ethylidene]-1-cyclohexene-1-base) vinyl]-3,3-dimethyl-1-propyl indole Weng iodide.
After T cells were transfected with heparin sulfate proteoglycan-3 (GPC3)–CAR lentivirus, the cell membrane of T cells was separated to form the CAR-T capsule (CAR-Tc). Finally, the CAR-Tc and IM were assembled to form the CAR-Tc–IM, which showed a good photothermal effect on liver cancer cells and it killed HCC cells (21).
Traditional photothermal agents (PTAs) perform hyperthermia ablation via activation of near-infrared I region, but the penetration depth is not high. At the same time, the heat resistance caused by heat shock protein also restricts the therapeutic effect of PTT on tumors (31, 32). At present, the cost of PTAs used is often expensive. Bismuth is a classical semi-metallic element and a hot spot of scientific research (33, 34) because it is cheap and non-toxic (35). Most ZIF-8 nanodrug carriers are used for intravenous drug delivery, and are considered to be promising drug release and controlled release platforms (36). Jinghua Li et al. combined Bi and ZIF-8 through a one-step reduction method (Bi@ZIF-8), added gambogic acid to Bi@ZIF-8 to form Bi@ZIF-8–gambogic acid (GBZ), while gambogic acid could be an inhibitor of Hsp90. In addition to good biocompatibility, GBZ is important because the temperature of PTT is low, the damage to surrounding normal tissues is small and it has a good killing effect on HCC cells (22). Hongqiao Cai et al. noted the adverse effects of heat damage to normal tissues near tumors (11). They synthesized hollow structure CuS nanoparticles with ataxia telangiectasia mutated (ATM) inhibitor loaded with surface modified TGF-β antibody (CuS-ATMi@TGF- βNPs). The nanoparticles not only achieved low-temperature PTT, but also caused less damage to normal tissue, and had sufficient targeting and biocompatibility (12).
PTT Plus Doxorubicin Treatment for HCC
PTT and chemotherapy often play a synergistic role. However, low targeting and poor drug delivery capacity are still the common shortcomings of photosensitizers and chemotherapeutic drugs. Therefore, it is of importance to design an effective nanodrug delivery platform to transport and control chemotherapeutic drugs and the accurate targeting of photothermal preparations (37, 38). Due to the depth of penetration, PTT often cannot eradicate tumors; therefore, PTT is often combined with chemotherapeutic drugs to achieve synergistic therapeutic effects and fewer adverse effects (39, 40). Doxorubicin is a classical chemotherapeutic drug with anthracycline structure. It has been used in the treatment of a variety of tumors, but there are many adverse effects, which affect its widespread application (41, 42).
The targeted and controlled release of drugs in the tumor area is the main difficulty in the treatment of HCC. In order to overcome this problem, Long Wu et al. designed a platelet cell membrane encapsulated polypyrole and doxorubicin nanoparticles (PLT PPy–DOX). These nanoparticles have photothermal activity because of PPy and chemotherapeutic activity because of doxorubicin. This platelet-simulated drug delivery system shows a good therapeutic effect on orthotopic HCC (43).
Polyethylene glycol (PEG), doxorubicin, mesoporous silica nanoparticles (MSNs) and CuS can be synthesized into nanoparticles (PEG–DOX–MSN@CuS nanoparticles), which have photothermal and chemotherapeutic effects on HCC. Specifically, CuS is irradiated by near-infrared, PTT can destroy MSNs, and then doxorubicin is released to kill HCC cells (44).
To focus on the anti-HCC effect and avoid adverse effects, Huili Li et al. synthesized PEG–hyaluronic acid (HA) 4–gold nanocages (AuNCs)–Dox (PEG–HA4–AuNCs–Dox) nanoparticles. PEG–HA4–AuNCs–Dox play the role of photosensitizers; doxorubicin is a classic chemotherapeutic drug; HA controls drug release into the tumor microenvironment; and PEG acts as a surfactant and increases the circulation time of nanoparticles (45).
Indocyanine green (ICG) has been approved by the US FDA for medical diagnosis and treatment (46). IR-820 is a cheaper analog of ICG (47). IR-820 and doxorubicin are hydrophilic molecules. For the treatment of liver cancer, their disadvantages are less circulating time in the body and rapid internal disappearance (48). Yue Jiang et al. has solved the above problems. Lactosylated IR-820 is assembled with doxorubicin to form LA-IR-820/DOX nanoparticles. Lactose IR-820 has the characteristics of liver cancer targeting and photosensitizer (49), and doxorubicin can lead to immune cell death and consolidate the effect of PTT (50).
Multidrug resistance (MDR) occurs in the treatment of various tumors and is a major challenge in tumor treatment (51). P-glycoprotein (P-gp) is overexpressed in many MDR cell lines, resulting in an increase of MDR (52).
Weiping Wang et al. found that anti-mir-21 can effectively inhibit P-gp and upregulate expression of PTEN to enhance sensitivity to chemotherapeutic drugs. Therefore, a novel nanoparticle system was synthesized, HA/anti-miR-21/PPAuNCs (HA-conjugated, anti-miR-21-loaded, PEI-modified PEGylated AuNCs). In addition to enhancing the sensitivity of HepG2/ADR cell line to chemotherapy, AuNCs can also play the role of PTT by mild near-infrared irradiation (53).
The 5-year recurrence rate of patients with liver cancer is 70–80%, which urgently needs to be resolved. Theoretically, the treatment of recurrent liver cancer is repeat hepatectomy or liver transplantation. The results of repeat hepatectomy, transarterial chemoembolization and radiofrequency ablation are poor (54). The combination of PTT and chemotherapy has an obvious synergistic antitumor effect (55). In order to treat recurrent liver cancer by PTT and chemotherapy, a homotypic tumor cell membrane drug delivery platform thermosensitive liposome–HCC cell membrane (HepM–TSL) was synthesized. This platform consists of thermosensitive liposome vesicles and HCC cell membranes, and ICG and doxorubicin are encapsulated by the above platform (ICG–DOX–HepM–TSL). ICG–DOX–HepM–TSL can avoid the immune system and directly target recurrent HCC. Excitation at 808 nm can lead to the decomposition of TSL, and the photothermal and chemotherapeutic effects of ICG and doxorubicin can be realized. At the same time, this platform also has good therapeutic effects and few adverse effects (56).
Tumor thermal ablation has become an effective method for local treatment of HCC, but it is not recommended for HCC with local recurrence > 3 cm (57). MoS2 has become an ideal PTT reagent because of its excellent surface plasmon resonance characteristics, photothermal conversion efficiency and biocompatibility (58). 300 nm diameter hollow MoS2 nanoparticles were established, and then doxorubicin was embedded (DOX@MoS2). The antitumor effect of the nanoparticles was confirmed by in vitro and in vivo experiments (59).
PTT Plus Sorafenib Treatment for HCC
Sorafenib, a type of multikinase inhibitor, is the first-line drug treatment for advanced HCC approved by the United States FDA (60). However, sorafenib’s disadvantages include poor drug targeting and poor water solubility of oral sorafenib (61). With the emergence of nanotechnology, sorafenib has become more effective for treatment of liver cancer.
Tianjun Zhou et al. designed nanoparticles of SP94–PB–SF–Cy5.5, which included sorafenib (SF), Prussian blue porous metal organic frame (PB), HCC-specific targeting peptide SP94, and near-infrared dye cyanine 5.5 (Cy5.5) (62). PB is an FDA-approved drug for thallium poisoning (63). It can be designed as a metal organic framework to carry drugs and combine with fluorescent dyes (64). Sp94 is an HCC-specific targeting polypeptide that can achieve specific binding between nanoparticles and HCC cells. Cy5.5 is a near-infrared dye that can be excited by 808 nm visible light (65). SP94–PB–SF–Cy5.5 achieved no recurrence of HCC in a HepG2 cell line nude mouse liver cancer model (62).
A macrophage–cancer cell membrane hybrid has been constructed. The membrane packages hollow CuS nanoparticles that contain sorafenib; and the membrane is surface modified with anti-VEGFR antibodies (CuS-SF@CMV NPs). The anti-VEGFR antibody can kill tumor cells by inhibiting angiogenesis via PI3K/AKT pathways. The nanoparticles avoid the immune system through immune escape, accurately locate HCC cells through liver cancer targeting, and kill tumor cells through PTT and kinase inhibition (66, 67).
PTT Plus Gene Therapy for HCC
MSNs are widely used because of their high specific surface area, controllable shape and easy surface functionalization (68, 69). Silica nanoparticles have a sharp surface, which has strong plasmid DNA binding ability and transfection performance (70). Mesoporous silica nanoparticles (MSNs) and Au NR core can be synthesized into Au@MSNs, and addition of PEG forms Au@MSN–PGEA. Au@MSN–PGEA, SF, and P53 gene can be synthesized into Au@MSN–PGEA@SF@P53 nanoparticles. besides PTT and targeted therapy, Au@MSN–PGEA@SF@P53 nanoparticles also increase the role of gene therapy for HCC (11).
PDT
PDT for HCC
PDT has been widely used for cancer. During PDT, reactive oxygen species (ROS) are generated, such as singlet oxygen, that can damage cancer cells (71). The principle of PDT is that a photosensitizer is excited by a specific excitation wavelength of light, converts energy into oxygen molecules in cell to form ROS, and ROS act on tumor cells (72), which can directly induce cell death, disturb tumor vasculature and activate the innate immune system (73).
As a second-generation photosensitizer, Radachlorin has a strong absorption band at 662 nm and has excellent physical and chemical properties, such as weak dark toxicity and rapid in vivo metabolic rate (74). Hamidreza Mirzaei et al. found that Radachlorin can induce HepG2 cell apoptosis through PDT, but it has no obvious harmful effect on HFLF-PI4 cells (75).
Metal phthalocyanines are photosensitizers that have been used in the treatment of tumors. Jingwei Shao et al. synthesized photocyanine and a series of analogs: tetra-triethyleneoxysulfonyl zinc phthalocyanines (ZnPcs). When photocyanine is activated by 670 nm excitation, it promotes apoptosis and necrosis of HepG2 cells by producing ROS, activating caspase-3 and stagnating cells in G2/M phase (76). ZnPc is also used in PDT of HCC cells. It can inhibit mitogen-activated protein kinase and extracellular signal-regulated kinase pathways through PDT, and upregulate Bax and downregulate Bcl-2 to destroy cancer cells (77). Gold nanoparticles combined with photosensitizer can be used for PDT of liver cancer cells. Pu-18-N–butylimide–N-methyl-D-glucamine (NMGA) is a new photosensitizer that combines with gold nanoparticles to form Pu-18-N–butylimide–NMGA–GNP. It can significantly reduce transplanted liver cancer under excitation light of 640–710 nm (78). Lactosomes are core-shell nanoparticles including amphiphilic polymeric micelles. ICG lactosomes were injected into male BALB/c nude mice through the caudal vein for 48 h. After xenograft tumors were stimulated by near infrared laser (AVL-15), a large number of apoptotic tumor cells could be observed (79).
Tumor tissue is different from non-tumor tissue in many biological and chemical aspects, and the tumor microenvironment is more likely to be acidic (pH 6.5–6.8) (80); therefore, an acidic environment is often used for activation of pH-responsive photosensitizer (81). However, the acidic activation pH of most pH-responsive photosensitizers is < 6 (82), which means that not all photosensitizers are pH responsive. Some photosensitizers can obtain pH-responsive function through being modified by pH-responsive groups, such as phthalocyanine dimer modified by an acid-sensitive unit (83), polysaccharide/Ce6 conjugate modified by pH-induced functional group (84) and cyclometalated iridium (III) complexes modified by benzimidazole (85). The activation efficiency of the above photosensitizers is not high, which limits their application (86). Layered double hydroxides (LDHs) have attracted much attention because of their ability to carry drugs or genes, as well as acid sensitivity and anion exchange properties (87, 88). ZnPcS8 has high photosensitivity efficiency, but it has the shortcomings of aggregation and rapid metabolism in the body. In order to overcome these shortcomings, Xingshu Li et al. synthesized LDH–ZnPcS8. The pH response of LDH–ZnPcS8 is reflected in high quenching effects at pH 7.4 and high reactivating effects at pH 6.5. There were strong PDT effects on HepG2 cells with LDH–ZnPcS8 at pH 6.0/6.5 compared with at pH 7.4 (86).
Metal-organic frameworks (MOFs) have been used for PDT research on tumor cells. Due to the low-oxygen environment in tumor cells, MOFs are not efficient at converting oxygen molecules in tumors into singlet oxygen. Platinum nanozymes can be decorated to MOFs to form high catalase-like activity that could produce a more efficient PDT effect (89).
PDT Plus Doxorubicin Treatment for HCC
Doxorubicin is a classic chemotherapeutic drug that has been used in the treatment of many types of tumors, but its adverse effects are serious and affect its application (90). Sulfonated aluminum phthalocyanine (AlPcS) has the following characteristics: good water solubility, strong absorption band in the red light region, and high singlet oxygen output rate (91). However, the sulfonated group in AlPcS reduces the affinity of AlPcS for the cell membrane (92). AlPcS–DOX conjugates can increase the uptake of AlPcS by HCC cells (hepatology cell line 7701), doxorubicin can act on the DNA of HCC cells, and AlPcS-mediated PDT targets lysosomes to kill HCC cells (93).
PDT Targeted HCC
Mitoxantrone, a type II topoisomerase inhibitor, is an antitumor drug (94). At the same time, it is also an efficient photosensitizer with two major absorption peaks at 610 and 660 nm (95). Epithelial cell adhesion molecule (EpCAM) is considered to be an important marker of cancer stem cells (96), and is associated with poor outcomes of HCC (97). Yong Han et al. grafted mitoxantrone with anti-EpCAM antibody to synthesize anti-EpCAM nano-micelles, which can recognize the EpCAM of HCC cells and have targeting properties, and then mitoxantrone exhibits excellent chemotherapeutic and PDT effects (95).
Folate receptor (FR) expression is lower in normal cells but higher in tumor cells. Folic acid (FA) can bind to its specific receptor (98). Porphyrin MOFs consist of porphyrin and metal ions, and have excellent biocompatibility and good dispersibility, as well as being effective for PDT (99). Gd-MOFs are synthesized in combination with FA. These nanoparticles can be recognized by fluorescence and magnetic resonance imaging, and can specifically target FR-positive cancer cells. Once inside the cell, the effect of PDT is highlighted (100).
Integrin αvβ3 is an angiogenesis driver in malignant tumors, and plays an important role in HCC (101). A hydrophilic and targeted peptide (cRGD) can be recognized by integrin αvβ3 via receptor-mediated endocytosis (102). Fluorogens with aggregation-induced emission (AIE) have been used in biotechnology. Fluorogen derivatives with AIE (TPETS nanodots) can be used to treat cancer cells and ROS are generated by visible light irradiation (103). Yang Gao et al. modified cRGD on TPETS nanodots, which had the ability to target cRGD to be recognized by integrin αvβ3, but also has a PDT effect on HCC cells (104–106).
Conclusion and Future Prospects
HCC is a malignant tumor with poor prognosis and high mortality, and is difficult to detect in the early stage, which seriously endangers human health. Research efforts have focused on finding an effective treatment. Over the years, surgical treatment and chemotherapy, as well as the current emerging targeted therapies and immunotherapy, have been shown to have therapeutic effects on HCC. In recent years, the combination of medical and engineering methods as a treatment strategy for liver cancer began to achieve results. However, most of the current studies are based on basic research, and there are still few clinical PTT- or PDT-based HCC studies. Maybe there are good strategies combining immunotherapy/targeted therapy with PTT/PDT; at the same time, accelerating the transformation of basic research into clinical research and the promotion of clinical research into clinical application are effective approaches for the treatment of HCC, With the development of science and technology and the deepening of research, effective treatment of liver cancer will improve.
Author Contributions
ZF, CZ, SW, and YZ contributed to conception and design of the article. ZF organized the database. ZF wrote the first draft of the manuscript. CZ and SW wrote sections of the manuscript. YZ revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study received financial support from the National Natural Science Foundation of China (NO. 81701965, 81872255); Natural Science Foundation of Liaoning Province (NO. 20180550116, 2019-MS-069); Doctoral Research Initiation Fund of Liaoning Province (2020-BS-187).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Zhou C, Zhang X, Peng Y, Zhou K, Hu J, Yu L, et al. Surgical Resection Plus Radiofrequency Ablation Versus Radical Surgery for Hepatocellular Carcinoma: A Propensity Score Matching Analysis. J Cancer (2019) 10(17):3933–40. doi: 10.7150/jca.29501
4. Wang H, Hou W, Perera A, Bettler C, Beach JR, Ding X, et al. Targeting Epha2 Suppresses Hepatocellular Carcinoma Initiation and Progression by Dual Inhibition of JAK1/STAT3 and AKT Signaling. Cell Rep (2021) 34(8):108765. doi: 10.1016/j.celrep.2021.108765
5. Pinato DJ, Cortellini A, Sukumaran A, Cole T, Pai M, Habib N, et al. PRIME-HCC: Phase Ib Study of Neoadjuvant Ipilimumab and Nivolumab Prior to Liver Resection for Hepatocellular Carcinoma. BMC Cancer (2021) 21(1):301. doi: 10.1186/s12885-021-08033-x
6. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA: Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
7. Lv R, Yang P, He F, Gai S, Li C, Dai Y, et al. A Yolk-Like Multifunctional Platform for Multimodal Imaging and Synergistic Therapy Triggered by a Single Near-Infrared Light. ACS Nano (2015) 9(2):1630–47. doi: 10.1021/nn5063613
8. Turan IS, Yildiz D, Turksoy A, Gunaydin G, Akkaya EU. A Bifunctional Photosensitizer for Enhanced Fractional Photodynamic Therapy: Singlet Oxygen Generation in the Presence and Absence of Light. Angew Chem (2016) 55(8):2875–8. doi: 10.1002/anie.201511345
9. Zhuang X, Ma X, Xue X, Jiang Q, Song L, Dai L, et al. A Photosensitizer-Loaded DNA Origami Nanosystem for Photodynamic Therapy. ACS Nano (2016) 10(3):3486–95. doi: 10.1021/acsnano.5b07671
10. Feng Z, Guo J, Liu X, Song H, Zhang C, Huang P, et al. Cascade of Reactive Oxygen Species Generation by Polyprodrug for Combinational Photodynamic Therapy. Biomaterials (2020) 255:120210. doi: 10.1016/j.biomaterials.2020.120210
11. Chen X, Zhang Q, Li J, Yang M, Zhao N, Xu FJ. Rattle-Structured Rough Nanocapsules With in-Situ-Formed Gold Nanorod Cores for Complementary Gene/Chemo/Photothermal Therapy. ACS Nano (2018) 12(6):5646–56. doi: 10.1021/acsnano.8b01440
12. Cai H, Dai X, Guo X, Zhang L, Cao K, Yan F, et al. Ataxia Telangiectasia Mutated Inhibitor-Loaded Copper Sulfide Nanoparticles for Low-Temperature Photothermal Therapy of Hepatocellular Carcinoma. Acta Biomater (2021) 127:276–86. doi: 10.1016/j.actbio.2021.03.051
13. Zhao L, Zhang X, Wang X, Guan X, Zhang W, Ma J. Recent Advances in Selective Photothermal Therapy of Tumor. J Nanobiotechnol (2021) 19(1):335. doi: 10.1186/s12951-021-01080-3
14. Cheng L, Kamkaew A, Sun H, Jiang D, Valdovinos HF, Gong H, et al. Dual-Modality Positron Emission Tomography/Optical Image-Guided Photodynamic Cancer Therapy With Chlorin E6-Containing Nanomicelles. ACS Nano (2016) 10(8):7721–30. doi: 10.1021/acsnano.6b03074
15. Abdel Fadeel D, Al-Toukhy GM, Elsharif AM, Al-Jameel SS, Mohamed HH, Youssef TE. Improved Photodynamic Efficacy of Thiophenyl Sulfonated Zinc Phthalocyanine Loaded in Lipid Nano-Carriers for Hepatocellular Carcinoma Cancer Cells. Photodiagnosis Photodyn Ther (2018) 23:25–31. doi: 10.1016/j.pdpdt.2018.06.003
16. Guo R, Yang G, Feng Z, Zhu Y, Yang P, Song H, et al. Glutathione-Induced Amino-Activatable Micellar Photosensitization Platform for Synergistic Redox Modulation and Photodynamic Therapy. Biomater Sci (2018) 6(5):1238–49. doi: 10.1039/c8bm00094h
17. Kumar A, Morales O, Mordon S, Delhem N, Boleslawski E. Could Photodynamic Therapy be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers (2021) 13(20):5176. doi: 10.3390/cancers13205176
18. Jung HS, Verwilst P, Sharma A, Shin J, Sessler JL, Kim JS. Organic Molecule-Based Photothermal Agents: An Expanding Photothermal Therapy Universe. Chem Soc Rev (2018) 47(7):2280–97. doi: 10.1039/c7cs00522a
19. Hu J, Zhu X, Li H, Zhao Z, Chi X, Huang G, et al. Theranostic Au Cubic Nano-Aggregates as Potential Photoacoustic Contrast and Photothermal Therapeutic Agents. Theranostics (2014) 4(5):534–45. doi: 10.7150/thno.8188
20. Xiang H, Zhao L, Yu L, Chen H, Wei C, Chen Y, et al. Self-Assembled Organic Nanomedicine Enables Ultrastable Photo-to-Heat Converting Theranostics in the Second Near-Infrared Biowindow. Nat Commun (2021) 12(1):218. doi: 10.1038/s41467-020-20566-6
21. Ma W, Zhu D, Li J, Chen X, Xie W, Jiang X, et al. Coating Biomimetic Nanoparticles With Chimeric Antigen Receptor T Cell-Membrane Provides High Specificity for Hepatocellular Carcinoma Photothermal Therapy Treatment. Theranostics (2020) 10(3):1281–95. doi: 10.7150/thno.40291
22. Li J, Zhu D, Ma W, Yang Y, Wang G, Wu X, et al. Rapid Synthesis of a Bi@ZIF-8 Composite Nanomaterial as a Near-Infrared-II (NIR-II) Photothermal Agent for the Low-Temperature Photothermal Therapy of Hepatocellular Carcinoma. Nanoscale (2020) 12(32):17064–73. doi: 10.1039/d0nr03907a
23. Wang S, Huang P, Nie L, Xing R, Liu D, Wang Z, et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv Mater (2013) 25(22):3055–61. doi: 10.1002/adma.201204623
24. Zha Z, Yue X, Ren Q, Dai Z. Uniform Polypyrrole Nanoparticles With High Photothermal Conversion Efficiency for Photothermal Ablation of Cancer Cells. Adv Mater (2013) 25(5):777–82. doi: 10.1002/adma.201202211
25. Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The Golden Age: Gold Nanoparticles for Biomedicine. Chem Soc Rev (2012) 41(7):2740–79. doi: 10.1039/c1cs15237h
26. Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, et al. Gold Nanocages: From Synthesis to Theranostic Applications. Accounts Chem Res (2011) 44(10):914–24. doi: 10.1021/ar200061q
27. Zhu S, Tian R, Antaris AL, Chen X, Dai H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv Mater (2019) 31(24):e1900321. doi: 10.1002/adma.201900321
28. Fournier C, Martin F, Zitvogel L, Kroemer G, Galluzzi L, Apetoh L. Trial Watch: Adoptively Transferred Cells for Anticancer Immunotherapy. Oncoimmunology (2017) 6(11):e1363139. doi: 10.1080/2162402X.2017.1363139
29. Siddiqui RS, Sardar M. A Systematic Review of the Role of Chimeric Antigen Receptor T (CAR-T) Cell Therapy in the Treatment of Solid Tumors. Cureus (2021) 13(4):e14494. doi: 10.7759/cureus.14494
30. Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. New Engl J Med (2013) 368(16):1509–18. doi: 10.1056/NEJMoa1215134
31. Mimun LC, Ajithkumar G, Pokhrel M, Yust BG, Elliott ZG, Pedraza F, et al. Bimodal Imaging Using Neodymium Doped Gadolinium Fluoride Nanocrystals With Near-Infrared to Near-Infrared Downconversion Luminescence and Magnetic Resonance Properties. J Mater Chem B (2013) 1(41):5702–10. doi: 10.1039/C3TB20905A
32. Zhang G, Gou H, Liu Y, Xi K, Jiang D, Jia X. Ph-Responsive PEG-Chitosan/Iron Oxide Hybrid Nanoassemblies for Low-Power Assisted PDT/PTT Combination Therapy. Nanomedicine (2020) 15(11):1097–112. doi: 10.2217/nnm-2020-0022
33. Song L, Dong X, Zhu S, Zhang C, Yin W, Zhang X, et al. Bi2 S3 -Tween 20 Nanodots Loading PI3K Inhibitor, LY294002, for Mild Photothermal Therapy of Lovo Cells In Vitro and In Vivo. Adv Healthcare Mater (2018) 7(22):e1800830. doi: 10.1002/adhm.201800830
34. Wang S, Wang H, Song C, Li Z, Wang Z, Xu H, et al. Synthesis of Bi2WO6-X Nanodots With Oxygen Vacancies as an All-in-One Nanoagent for Simultaneous CT/IR Imaging and Photothermal/Photodynamic Therapy of Tumors. Nanoscale (2019) 11(32):15326–38. doi: 10.1039/c9nr05236d
35. Liu J, Wang P, Zhang X, Wang L, Wang D, Gu Z, et al. Rapid Degradation and High Renal Clearance of Cu3BiS3 Nanodots for Efficient Cancer Diagnosis and Photothermal Therapy In Vivo. ACS Nano (2016) 10(4):4587–98. doi: 10.1021/acsnano.6b00745
36. Kota D, Kang L, Rickel A, Liu J, Smith S, Hong Z, et al. Low Doses of Zeolitic Imidazolate Framework-8 Nanoparticles Alter the Actin Organization and Contractility of Vascular Smooth Muscle Cells. J Hazard Mater (2021) 414:125514. doi: 10.1016/j.jhazmat.2021.125514
37. Kong Y, Li X, Liu X, Pang J, Mu X, Liu W. Galactosylated Chitosan Modified Magnetic Mesoporous Silica Nanoparticles Loaded With Nedaplatin for the Targeted Chemo-Photothermal Synergistic Therapy of Cancer. J Nanosci Nanotechnol (2021) 21(9):4553–64. doi: 10.1166/jnn.2021.19142
38. Zhang G, Wang W, Zou H, Wei Z, Song C, Ran J, et al. Novel Semiconducting Nano-Agents Incorporating Tirapazamine for Imaging Guided Synergistic Cancer Hypoxia Activated Photo-Chemotherapy. J Mater Chem B (2021) 9(26):5318–28. doi: 10.1039/d1tb00659b
39. Li X, Liu L, Li S, Wan Y, Chen JX, Tian S, et al. Biodegradable Pi-Conjugated Oligomer Nanoparticles With High Photothermal Conversion Efficiency for Cancer Theranostics. ACS Nano (2019) 13(11):12901–11. doi: 10.1021/acsnano.9b05383
40. Dibaba ST, Caputo R, Xi W, Zhang JZ, Wei R, Zhang Q, et al. NIR Light-Degradable Antimony Nanoparticle-Based Drug-Delivery Nanosystem for Synergistic Chemo-Photothermal Therapy In Vitro. ACS Appl Mater Interfaces (2019) 11(51):48290–9. doi: 10.1021/acsami.9b20249
41. Karabekmez ME, Taymaz-Nikerel H, Eraslan S, Kirdar B. Time-Dependent Re-Organization of Biological Processes by the Analysis of the Dynamic Transcriptional Response of Yeast Cells to Doxorubicin. Mol Omics (2021) 17(4):572–82. doi: 10.1039/d1mo00046b
42. Xu L, Wang C, Zou Z, Wu Z. Ozone Attenuated H9c2 Cell Injury Induced by Doxorubicin. J Cardiovasc Pharmacol (2021) 78(1):e86–93. doi: 10.1097/FJC.0000000000001043
43. Wu L, Xie W, Zan HM, Liu Z, Wang G, Wang Y, et al. Platelet Membrane-Coated Nanoparticles for Targeted Drug Delivery and Local Chemo-Photothermal Therapy of Orthotopic Hepatocellular Carcinoma. J Mater Chem B (2020) 8(21):4648–59. doi: 10.1039/d0tb00735h
44. Wu L, Wu M, Zeng Y, Zhang D, Zheng A, Liu X, et al. Multifunctional PEG Modified DOX Loaded Mesoporous Silica Nanoparticle@Cus Nanohybrids as Photo-Thermal Agent and Thermal-Triggered Drug Release Vehicle for Hepatocellular Carcinoma Treatment. Nanotechnology (2015) 26(2):25102. doi: 10.1088/0957-4484/26/2/025102
45. Li H, Li H, Yu W, Huang S, Liu Y, Zhang N, et al. Pegylated Hyaluronidase/NIR Induced Drug Controlled Release System for Synergetic Chemo-Photothermal Therapy of Hepatocellular Carcinoma. Eur J Pharm Sci: Off J Eur Fed Pharm Sci (2019) 133:127–36. doi: 10.1016/j.ejps.2019.02.022
46. Fan Z, Zong J, Lau WY, Zhang Y. Indocyanine Green and Its Nanosynthetic Particles for the Diagnosis and Treatment of Hepatocellular Carcinoma. Am J Trans Res (2020) 12(6):2344–52.
47. Della Pelle G, Delgado Lopez A, Salord Fiol M, Kostevsek N. Cyanine Dyes for Photo-Thermal Therapy: A Comparison of Synthetic Liposomes and Natural Erythrocyte-Based Carriers. Int J Mol Sci (2021) 22(13):6914. doi: 10.3390/ijms22136914
48. Xia B, Zhang Q, Shi J, Li J, Chen Z, Wang B. Co-Loading of Photothermal Agents and Anticancer Drugs Into Porous Silicon Nanoparticles With Enhanced Chemo-Photothermal Therapeutic Efficacy to Kill Multidrug-Resistant Cancer Cells. Colloids Surf B Biointerfaces (2018) 164:291–8. doi: 10.1016/j.colsurfb.2018.01.059
49. Jiang Y, Huang C, Luan Y. Lactosylated IR820/DOX Co-Assembled Nanodrug for Synergetic Antitumour Therapy. Int J Nanomed (2020) 15:4431–40. doi: 10.2147/IJN.S247617
50. Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat Commun (2019) 10(1):3349. doi: 10.1038/s41467-019-11269-8
51. Ling S, Li J, Shan Q, Dai H, Lu D, Wen X, et al. USP22 Mediates the Multidrug Resistance of Hepatocellular Carcinoma via the SIRT1/AKT/MRP1 Signaling Pathway. Mol Oncol (2017) 11(6):682–95. doi: 10.1002/1878-0261.12067
52. Kim J, Kim J, Jeong C, Kim WJ. Synergistic Nanomedicine by Combined Gene and Photothermal Therapy. Adv Drug Deliv Rev (2016) 98:99–112. doi: 10.1016/j.addr.2015.12.018
53. Wang W, Huang S, Yuan J, Xu X, Li H, Lv Z, et al. Reverse Multidrug Resistance in Human Hepg2/ADR by Anti-Mir-21 Combined With Hyperthermia Mediated by Functionalized Gold Nanocages. Mol Pharm (2018) 15(9):3767–76. doi: 10.1021/acs.molpharmaceut.8b00046
54. Ho CM, Lee PH, Shau WY, Ho MC, Wu YM, Hu RH. Survival in Patients With Recurrent Hepatocellular Carcinoma After Primary Hepatectomy: Comparative Effectiveness of Treatment Modalities. Surgery (2012) 151(5):700–9. doi: 10.1016/j.surg.2011.12.015
55. Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-Photothermal Therapy Combination Elicits Anti-Tumor Immunity Against Advanced Metastatic Cancer. Nat Commun (2018) 9(1):1074. doi: 10.1038/s41467-018-03473-9
56. Sun Y, Zhai W, Liu X, Song X, Gao X, Xu K, et al. Homotypic Cell Membrane-Cloaked Biomimetic Nanocarrier for the Accurate Photothermal-Chemotherapy Treatment of Recurrent Hepatocellular Carcinoma. J Nanobiotechnol (2020) 18(1):60. doi: 10.1186/s12951-020-00617-2
57. Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Aggressive Intrasegmental Recurrence of Hepatocellular Carcinoma After Radiofrequency Ablation: Risk Factors and Clinical Significance. Radiology (2015) 276(1):274–85. doi: 10.1148/radiol.15141215
58. Teng L, Yu C, Min G, Chao L, Qian C, Song G, et al. Ultra-Small Mos2 Nanodots With Rapid Body Clearance for Photothermal Cancer Therapy. 纳米研究(英文版) (2016) 9(010):3003–17. doi: 10.1007/s12274-016-1183-x
59. Li S, Tan L, Xu W, Liu C, Wu Q, Fu C, et al. Doxorubicin-Loaded Layered Mos(2) Hollow Spheres and Its Photothermo-Chemotherapy on Hepatocellular Carcinoma. J Biomed Nanotechnol (2017) 13(11):1557–64. doi: 10.1166/jbn.2017.2461
60. Guan DX, Shi J, Zhang Y, Zhao JS, Long LY, Chen TW, et al. Sorafenib Enriches Epithelial Cell Adhesion Molecule-Positive Tumor Initiating Cells and Exacerbates a Subtype of Hepatocellular Carcinoma Through TSC2-AKT Cascade. Hepatology (2015) 62(6):1791–803. doi: 10.1002/hep.28117
61. Liu C, Chen Z, Chen Y, Lu J, Li Y, Wang S, et al. Improving Oral Bioavailability of Sorafenib by Optimizing the “Spring” and “Parachute” Based on Molecular Interaction Mechanisms. Mol Pharm (2016) 13(2):599–608. doi: 10.1021/acs.molpharmaceut.5b00837
62. Zhou T, Liang X, Wang P, Hu Y, Qi Y, Jin Y, et al. A Hepatocellular Carcinoma Targeting Nanostrategy With Hypoxia-Ameliorating and Photothermal Abilities That, Combined With Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano (2020) 14(10):12679–96. doi: 10.1021/acsnano.0c01453
63. Sandal N, Mittal G, Bhatnagar A, Pathak DP, Singh AK. Preparation, Characterization, and In Vivo Pharmacoscintigraphy Evaluation of an Intestinal Release Delivery System of Prussian Blue for Decorporation of Cesium and Thallium. J Drug Deliv (2017) 2017:4875784. doi: 10.1155/2017/4875784
64. Qin Z, Li Y, Gu N. Progress in Applications of Prussian Blue Nanoparticles in Biomedicine. Adv Healthcare Mater (2018) 7(20):e1800347. doi: 10.1002/adhm.201800347
65. Song R, Ruan M, Dai J, Xue W. Biomimetic Magnetofluorescent Ferritin Nanoclusters for Magnetic Resonance and Fluorescence-Dual Modal Imaging and Targeted Tumor Therapy. J Mater Chem B (2021) 9(10):2494–504. doi: 10.1039/d0tb02175j
66. Kudo M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers (2018) 10(11):412. doi: 10.3390/cancers10110412
67. Ji B, Cai H, Yang Y, Peng F, Song M, Sun K, et al. Hybrid Membrane Camouflaged Copper Sulfide Nanoparticles for Photothermal-Chemotherapy of Hepatocellular Carcinoma. Acta Biomater (2020) 111:363–72. doi: 10.1016/j.actbio.2020.04.046
68. Jat SK, Bhattacharya J, Sharma MK. Nanomaterial Based Gene Delivery: A Promising Method for Plant Genome Engineering. J Mater Chem B (2020) 8(19):4165–75. doi: 10.1039/d0tb00217h
69. Candela-Noguera V, Vivo-Llorca G, Diaz de Grenu B, Alfonso M, Aznar E, Orzaez M, et al. Gene-Directed Enzyme Prodrug Therapy by Dendrimer-Like Mesoporous Silica Nanoparticles Against Tumor Cells. Nanomaterials (2021) 11(5):1298. doi: 10.3390/nano11051298
70. Song H, Yu M, Lu Y, Gu Z, Yang Y, Zhang M, et al. Plasmid Dna Delivery: Nanotopography Matters. J Am Chem Soc (2017) 139(50):18247–54. doi: 10.1021/jacs.7b08974
71. Yang X, Zhang W, Jiang W, Kumar A, Zhou S, Cao Z, et al. Nanoconjugates to Enhance PDT-Mediated Cancer Immunotherapy by Targeting the Indoleamine-2,3-Dioxygenase Pathway. J Nanobiotechnol (2021) 19(1):182. doi: 10.1186/s12951-021-00919-z
72. Robertson CA, Evans DH, Abrahamse H. Photodynamic Therapy (PDT): A Short Review on Cellular Mechanisms and Cancer Research Applications for PDT. J Photochem Photobiol B Biol (2009) 96(1):1–8. doi: 10.1016/j.jphotobiol.2009.04.001
73. Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes With Immune Checkpoint Blockade to Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J Am Chem Soc (2016) 138(51):16686–95. doi: 10.1021/jacs.6b09538
74. Vargas F, Díaz Y, Yartsev V, Marcano A, Lappa A. Photophysical Properties of Novel PDT Photosensitizer Radachlorin in Different Media. CIEN (2004) 12:70–7.
75. Mirzaei H, Djavid GE, Hadizadeh M, Jahanshiri-Moghadam M, Hajian P. The Efficacy of Radachlorin-Mediated Photodynamic Therapy in Human Hepatocellular Carcinoma Cells. J Photochem Photobiol B Biol (2015) 142:86–91. doi: 10.1016/j.jphotobiol.2014.11.007
76. Shao J, Xue J, Dai Y, Liu H, Chen N, Jia L, et al. Inhibition of Human Hepatocellular Carcinoma Hepg2 by Phthalocyanine Photosensitiser PHOTOCYANINE: ROS Production, Apoptosis, Cell Cycle Arrest. Eur J Cancer (2012) 48(13):2086–96. doi: 10.1016/j.ejca.2011.10.013
77. Ogbodu RO, Nitzsche B, Ma A, Atilla D, Gurek AG, Hopfner M. Photodynamic Therapy of Hepatocellular Carcinoma Using Tetra-Triethyleneoxysulfonyl Zinc Phthalocyanine as Photosensitizer. J Photochem Photobiol B Biol (2020) 208:111915. doi: 10.1016/j.jphotobiol.2020.111915
78. Kwon JG, Song IS, Kim MS, Lee BH, Kim JH, Yoon I, et al. Pu-18-N-Butylimide-NMGA-GNP Conjugate Is Effective Against Hepatocellular Carcinoma. Integr Med Res (2013) 2(3):106–11. doi: 10.1016/j.imr.2013.05.001
79. Tsuda T, Kaibori M, Hishikawa H, Nakatake R, Okumura T, Ozeki E, et al. Near-Infrared Fluorescence Imaging and Photodynamic Therapy With Indocyanine Green Lactosome has Antineoplastic Effects for Hepatocellular Carcinoma. PloS One (2017) 12(8):e0183527. doi: 10.1371/journal.pone.0183527
80. Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, et al. A Nanoparticle-Based Strategy for the Imaging of a Broad Range of Tumours by Nonlinear Amplification of Microenvironment Signals. Nat Mater (2014) 13(2):204–12. doi: 10.1038/nmat3819
81. Zhang B, Wan S, Peng X, Zhao M, Li S, Pu Y, et al. Human Serum Albumin-Based Doxorubicin Prodrug Nanoparticles With Tumor Ph-Responsive Aggregation-Enhanced Retention and Reduced Cardiotoxicity. J Mater Chem B (2020) 8(17):3939–48. doi: 10.1039/d0tb00327a
82. Meng LB, Zhang W, Li D, Li Y, Hu XY, Wang L, et al. Ph-Responsive Supramolecular Vesicles Assembled by Water-Soluble Pillar[5]Arene and a BODIPY Photosensitizer for Chemo-Photodynamic Dual Therapy. Chem Commun (2015) 51(76):14381–4. doi: 10.1039/c5cc05785j
83. Ke MR, Ng DK, Lo PC. A Ph-Responsive Fluorescent Probe and Photosensitiser Based on a Self-Quenched Phthalocyanine Dimer. Chem Commun (2012) 48(72):9065–7. doi: 10.1039/c2cc34327d
84. Park SY, Baik HJ, Oh YT, Oh KT, Youn YS, Lee ES. A Smart Polysaccharide/Drug Conjugate for Photodynamic Therapy. Angew Chem (2011) 50(7):1644–7. doi: 10.1002/anie.201006038
85. He L, Li Y, Tan CP, Ye RR, Chen MH, Cao JJ, et al. Cyclometalated Iridium(Iii) Complexes as Lysosome-Targeted Photodynamic Anticancer and Real-Time Tracking Agents. Chem Sci (2015) 6(10):5409–18. doi: 10.1039/c5sc01955a
86. Li X, Zheng BY, Ke MR, Zhang Y, Huang JD, Yoon J. A Tumor-Ph-Responsive Supramolecular Photosensitizer for Activatable Photodynamic Therapy With Minimal In Vivo Skin Phototoxicity. Theranostics (2017) 7(10):2746–56. doi: 10.7150/thno.18861
87. Park DH, Cho J, Kwon OJ, Yun CO, Choy JH. Biodegradable Inorganic Nanovector: Passive Versus Active Tumor Targeting in Sirna Transportation. Angew Chem (2016) 55(14):4582–6. doi: 10.1002/anie.201510844
88. Li XS, Ke MR, Huang W, Ye CH, Huang JD. A Ph-Responsive Layered Double Hydroxide (LDH)-Phthalocyanine Nanohybrid for Efficient Photodynamic Therapy. Chemistry (2015) 21(8):3310–7. doi: 10.1002/chem.201404514
89. Zhang Y, Wang F, Liu C, Wang Z, Kang L, Huang Y, et al. Nanozyme Decorated Metal-Organic Frameworks for Enhanced Photodynamic Therapy. ACS Nano (2018) 12(1):651–61. doi: 10.1021/acsnano.7b07746
90. Rahim MA, Madni A, Tahir N, Jan N, Shah H, Khan S, et al. Mild Hyperthermia Responsive Liposomes for Enhanced In Vitro and In Vivo Anticancer Efficacy of Doxorubicin Against Hepatocellular Carcinoma. Pharmaceutics (2021) 13(8):1310. doi: 10.3390/pharmaceutics13081310
91. Chou KL, Won N, Kwag J, Kim S, Chen JY. Femto-Second Laser Beam With a Low Power Density Achieved a Two-Photon Photodynamic Cancer Therapy With Quantum Dots. J Mater Chem B (2013) 1(36):4584–92. doi: 10.1039/c3tb20928h
92. Zhao JF, Chen JY, Mi L, Wang PN, Peng Q. Enhancement of Intracellular Delivery of Anti-Cancer Drugs by the Tat Peptide. Ultrastructural Pathol (2011) 35(3):119–23. doi: 10.3109/01913123.2011.557522
93. Qin YL, Huang X, Chen JY. Conjugation of Sulfonated Aluminum Phthalocyanine to Doxorubicin can Improve the Efficacy of Photodynamic Cancer Therapy. Anti-cancer Drugs (2012) 23(10):1047–53. doi: 10.1097/CAD.0b013e3283569759
94. Shukla S, Marks I, Church D, Chan SK, Pokorski JK, Steinmetz NF. Tobacco Mosaic Virus for the Targeted Delivery of Drugs to Cells Expressing Prostate-Specific Membrane Antigen. RSC Adv (2021) 11(33):20101–8. doi: 10.1039/d1ra03166j
95. Han Y, An Y, Jia G, Wang X, He C, Ding Y, et al. Theranostic Micelles Based on Upconversion Nanoparticles for Dual-Modality Imaging and Photodynamic Therapy in Hepatocellular Carcinoma. Nanoscale (2018) 10(14):6511–23. doi: 10.1039/C7NR09717D
96. Mani SK, Zhang H, Diab A, Pascuzzi PE, Lefrancois L, Fares N, et al. Epcam-Regulated Intramembrane Proteolysis Induces a Cancer Stem Cell-Like Gene Signature in Hepatitis B Virus-Infected Hepatocytes. J Hepatol (2016) 65(5):888–98. doi: 10.1016/j.jhep.2016.05.022
97. Park DJ, Sung PS, Kim JH, Lee GW, Jang JW, Jung ES, et al. Epcam-High Liver Cancer Stem Cells Resist Natural Killer Cell-Mediated Cytotoxicity by Upregulating CEACAM1. J Immunother Cancer (2020) 8(1):e000301. doi: 10.1136/jitc-2019-000301
98. Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA Methylation: A Review of Molecular Mechanisms and the Evidence for Folate’s Role. Adv Nutr (2012) 3(1):21–38. doi: 10.3945/an.111.000992
99. Zheng H, Zhang Y, Liu L, Wan W, Guo P, Nystrom AM, et al. One-Pot Synthesis of Metal-Organic Frameworks With Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J Am Chem Soc (2016) 138(3):962–8. doi: 10.1021/jacs.5b11720
100. Chen Y, Liu W, Shang Y, Cao P, Cui J, Li Z, et al. Folic Acid-Nanoscale Gadolinium-Porphyrin Metal-Organic Frameworks: Fluorescence and Magnetic Resonance Dual-Modality Imaging and Photodynamic Therapy in Hepatocellular Carcinoma. Int J Nanomed (2019) 14:57–74. doi: 10.2147/IJN.S177880
101. Wang R, Qi B, Dong YW, Cai QQ, Deng NH, Chen Q, et al. Sulfatide Interacts With and Activates Integrin Alphavbeta3 in Human Hepatocellular Carcinoma Cells. Oncotarget (2016) 7(24):36563–76. doi: 10.18632/oncotarget.9095
102. Li M, Zhang W, Wang B, Gao Y, Song Z, Zheng QC. Ligand-Based Targeted Therapy: A Novel Strategy for Hepatocellular Carcinoma. Int J Nanomed (2016) 11:5645–69. doi: 10.2147/IJN.S115727
103. Yuan Y, Xu S, Cheng X, Cai X, Liu B. Bioorthogonal Turn-on Probe Based on Aggregation-Induced Emission Characteristics for Cancer Cell Imaging and Ablation. Angew Chem (2016) 55(22):6457–61. doi: 10.1002/anie.201601744
104. Gao Y, Zheng QC, Xu S, Yuan Y, Cheng X, Jiang S, et al. Theranostic Nanodots With Aggregation-Induced Emission Characteristic for Targeted and Image-Guided Photodynamic Therapy of Hepatocellular Carcinoma. Theranostics (2019) 9(5):1264–79. doi: 10.7150/thno.29101
105. Cao C, Ge W, Yin J, Yang D, Wang W, Song X, et al. Mesoporous Silica Supported Silver-Bismuth Nanoparticles as Photothermal Agents for Skin Infection Synergistic Antibacterial Therapy. Small (2020) 16(24):e2000436. doi: 10.1002/smll.202000436
Keywords: photodynamic therapy, photothermal therapy, hepatocellular carcinoma, treatment, review
Citation: Fan Z, Zhuang C, Wang S and Zhang Y (2021) Photodynamic and Photothermal Therapy of Hepatocellular Carcinoma. Front. Oncol. 11:787780. doi: 10.3389/fonc.2021.787780
Received: 01 October 2021; Accepted: 22 November 2021;
Published: 07 December 2021.
Edited by:
Fan Feng, The 302nd Hospital of PLA, ChinaReviewed by:
Chuanshan Xu, Guangzhou Medical University, ChinaXiaochen Dong, Nanjing Tech University, China
Copyright © 2021 Fan, Zhuang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengjun Zhuang, cWlsZTE5ODNAaG90bWFpbC5jb20=; Shuang Wang, d2FuZ2RhbGlhbnNAaG90bWFpbC5jb20=; Yewei Zhang, emhhbmd5ZXdlaUBuam11LmVkdS5jbg==
 Zhe Fan
Zhe Fan Chengjun Zhuang
Chengjun Zhuang Shuang Wang
Shuang Wang Yewei Zhang
Yewei Zhang