- 1Seagen, Inc., Bothell, WA, United States
- 2Curta, Inc., Seattle, WA, United States
- 3Helen Diller Family Cancer Center, University of California San Francisco, San Francisco, CA, United States
Background: Urothelial carcinoma (UC) is a common malignancy with significant associated mortality. Recent clinical trials suggest an emerging role for HER2-targeted therapy. Testing for HER2 expression in UC is not part of current routine clinical practice. In consequence, the prevalence of HER2 expression in UC is not well defined.
Methods: A systematic literature review (SLR) was conducted to characterize HER2 expression in both locally advanced unresectable or metastatic (LA/mUC) and earlier stage UC, classified as HER2+, HER2-low, HER2-. HER2+ was defined as an immunohistochemistry (IHC) score of 3+ or IHC 2+ and ISH/FISH+. HER2-low was defined as an IHC score of 2+ and ISH/FISH- or IHC 1+. HER2- was defined as an IHC score of 0. Weighted averages were calculated to generate an estimate of the population prevalence.
Results: A total of 88 studies were identified, with 45, 30, and 13 studies investigating LA/mUC, earlier stage UC, and mixed stage/unspecified, respectively. The most common assays used were Dako HercepTest and Ventana Pathway anti-HER2/neu (4B5) for IHC to assess HER2 protein expression; Abbott PathVysion HER-2 DNA Probe Kit, FoundationOne CDx, and Guardant360 CDx for assessing HER2 gene amplification. The most frequently cited scoring guidelines were ASCO/CAP guidelines for breast cancer and gastric cancer, though most studies defined their own criteria for HER2 expression. Using the pre-specified definition, HER2+ prevalence ranged from 6.7% to 37.5% with a weighted average of 13.0% in LA/mUC. Only 1 study presented data that could be classified as HER2+ based on pre-specified criteria in earlier stage UC patients, and this study represented a likely outlier, at 76.0%.
Conclusion: The results from this SLR help to shed light on HER2 expression in UC, a potentially clinically relevant biomarker-driven subpopulation for emerging HER2-directed regimens. Results of this SLR illuminate the variability in how HER2+ status expression levels are being assessed and how HER2+ is defined. Consensus on standardized HER2 testing and scoring criteria is paramount to better understand the clinical relevance in patients with UC.
Introduction
Bladder cancer is a significant cause of mortality and morbidity globally, with 573,278 new cases and 212,536 deaths expected in 2020 (1). Histologically, approximately 90% of bladder tumors present as urothelial carcinoma (UC), and more than 90% of UC is located in the bladder (2). While the overall 5-year survival rate for all patients with UC is relatively high, at 68.6% in Europe and 77.1% in the US, the prognosis is worse for patients who have muscle-invasive disease at diagnosis (5-year survival rate of 37.5% in the US) or have distant metastases (5-year survival rate of 6.4% in the US) (3, 4).
Standard of care (SOC) for muscle-invasive UC is neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy with pelvic lymph node dissection (4, 5). An estimated 25% of patients with muscle-invasive disease develop metastatic disease (6). For patients with locally advanced unresectable or metastatic UC (LA/mUC), SOC is systemic therapy with a platinum-based regimen for eligible patients, followed by switch-maintenance avelumab immunotherapy for patients who experience clinical benefit (7–9). Despite high initial response rates, most LA/mUC patients who start a platinum-based regimen will eventually experience disease progression and will relapse.
In recent years, several checkpoint inhibitors have gained US Food and Drug Administration (FDA) approval and are recommended for the treatment of LA/mUC patients in the second-line setting following platinum-containing chemotherapy, in the first-line setting for cisplatin-ineligible patients who have high programmed death-ligand 1 (PD-L1) expression or are ineligible for any platinum-based therapy, and as first-line maintenance therapy (7, 10–13). However, only a minority of patients with LA/mUC who receive immunotherapy will derive a benefit. Objective response rates (ORR) for patients who receive anti-programmed death receptor-1 (PD-1)/L1 therapy remain low in both the first-line setting (23% to 29%), and in the second-line setting and beyond (15% to 20%) (14–16). More recently, enfortumab vedotin-ejfv, an antibody-drug conjugate (ADC), received FDA approval for the treatment of LA/mUC patients who have previously received a PD-1 or PD-L1 inhibitor and platinum-containing chemotherapy or are ineligible for cisplatin-containing chemotherapy and have previously received one or more prior lines of therapy (17). Other recent advances in this disease space include the accelerated approval of sacituzumab govitecan-hziy for patients progressing on platinum-based chemotherapy and an immune checkpoint inhibitor and approval of erdafitinib in patients whose tumors harbor FGFR3 alterations following progression on platinum-containing therapy (18, 19).
The low response rates with current treatment options in LA/mUC indicate that there remains a large unmet need for effective treatment options. Among potential therapeutic targets is the human epidermal growth factor receptor 2 (HER2) gene (also referred to as ERBB2), which plays a role in regulating cell growth, differentiation, and survival. While HER2 is known to be overexpressed in various tumors, there are currently conflicting data on whether HER2 overexpression is an oncogenic driver and whether it is a prognostic marker in UC, as it has been demonstrated in breast and gastric cancer (20–26). Targeting HER2 has led to substantial survival gains in HER2+ breast and gastric cancer. In metastatic breast cancer, the treatment landscape drastically changed with the introduction of HER2-targeted therapies, which have been successful in extending overall survival in patients with HER2-expressing tumors (27–32). Similarly, in gastric cancer, the ToGA trial critically demonstrated the efficacy of HER2 agents for patients with HER2+ advanced gastric and gastroesophageal junction cancer, and HER2-targeted agents are now considered standard of care for these patients (32). As such, HER2 testing has become a clinical routine and guidelines for HER2 testing have been established for breast and gastric cancer.
While the therapeutic value of HER2-targeted therapies has been demonstrated in breast and gastric cancer, conventional anti-HER2 therapies such as trastuzumab and tyrosine kinase inhibitors (apatinib, neratinib, and lapatinib) have failed to improve outcomes in UC (33). Multiple novel treatments have been or are currently being developed to target patients with HER2+ UC, such as ADCs (33, 34). For example, disitamab vedotin (DV), also known as RC48-ADC, a HER2-targeted ADC conjugated to the microtubule disrupting agent monomethyl auristatin E (MMAE) via a protease cleavable linker, recently received an FDA breakthrough designation for previously-treated, platinum-eligible patients with HER2 expressing advanced UC, and has demonstrated encouraging results (35, 36).
Testing for HER2 expression in UC is not part of current routine practice. Estimates of the proportion of UC patients who are HER2+—and subsequently may benefit from these targeted therapies—are uncertain. Available literature on this topic cites wide ranges for the proportion of patients with UC whose tumors express HER2 (37). Furthermore, there are no standardized UC criteria to assess HER2 status, and guidelines for breast cancer, gastric cancer, or other criteria are often relied on (38, 39). As such, a comprehensive review of existing literature is needed to determine the prevalence of HER2 expression in patients with UC to better understand the potential role that emerging HER2 directed regimens may play in this patient population. This SLR was conducted to determine (a) the prevalence of HER2 expression in UC, and specifically in both earlier stage UC and LA/mUC, classified as HER2+, HER2-low, or HER2-; (b) how HER2 expression and amplification were assessed; and (c) the concordance between results from different tests.
Materials and methods
SLR methods
The SLR was conducted in accordance with Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (40). Searches were focused on studies published from January 2000 to October 2021 in MEDLINE and Embase databases. Abstracts presented at the following conferences held between 2019 and 2021 were also searched: American Society of Clinical Oncology (ASCO), American Society of Clinical Oncology Genitourinary Cancers (ASCO-GU), European Society for Medical Oncology (ESMO), Society of Urologic Oncology (SUO), American Urological Association (AUA), and European Association of Urology (EAU).
Studies which reported HER2 protein expression or gene amplification data for UC tumor samples were included. Reviews, case reports, case series, letters, or editorials were excluded, as were non-English language studies. Clinical trials of HER2+ patients were included if HER2 expression was tested as part of enrollment criteria, and the proportion was reported. Studies which investigated cultured tumor cell lines or non-human samples were excluded.
Studies were screened for inclusion and exclusion criteria by two independent reviewers. Disagreements between the two reviewers were resolved through discussion, with a third reviewer making the final decision, if needed. Data were extracted by one reviewer and validated by an independent reviewer.
Definitions
HER2+ was defined as an immunohistochemistry (IHC) score of 3+ or IHC 2+ and in situ hybridization ISH+/fluorescence in situ hybridization FISH+. For studies to contribute data for HER2+, both IHC and ISH/FISH had to be conducted. In some studies, HER2+ was either defined differently (e.g., HER2+ as IHC 3+ or 2+ without confirmation by FISH) or left undefined. These studies were included in the category HER2+ (all studies). HER2-low was defined as an IHC of 2+ and ISH/FISH- or IHC 1+. HER2- was defined as an IHC score of 0.
Statistical analysis
Weighted averages were calculated for the proportion of patients who were HER2+, HER2-low, and HER2- to generate an estimate of the proportion of patients in each HER2 expression category. Weighted average calculations were considered the point estimate.
Results
Search results
Among 661 database references and 83 congress abstracts screened, 91 publications (representing 88 unique studies) were retained after full-text review (Figure 1). Of the 88 unique studies that reported data for HER2 status, 45 studies investigated LA/mUC patients, 30 studies investigated earlier stage UC (stage I-IIIA) patients, and 13 studies investigated a mixed earlier stage UC and LA/mUC patient population (n=7) or did not specify (n=6).
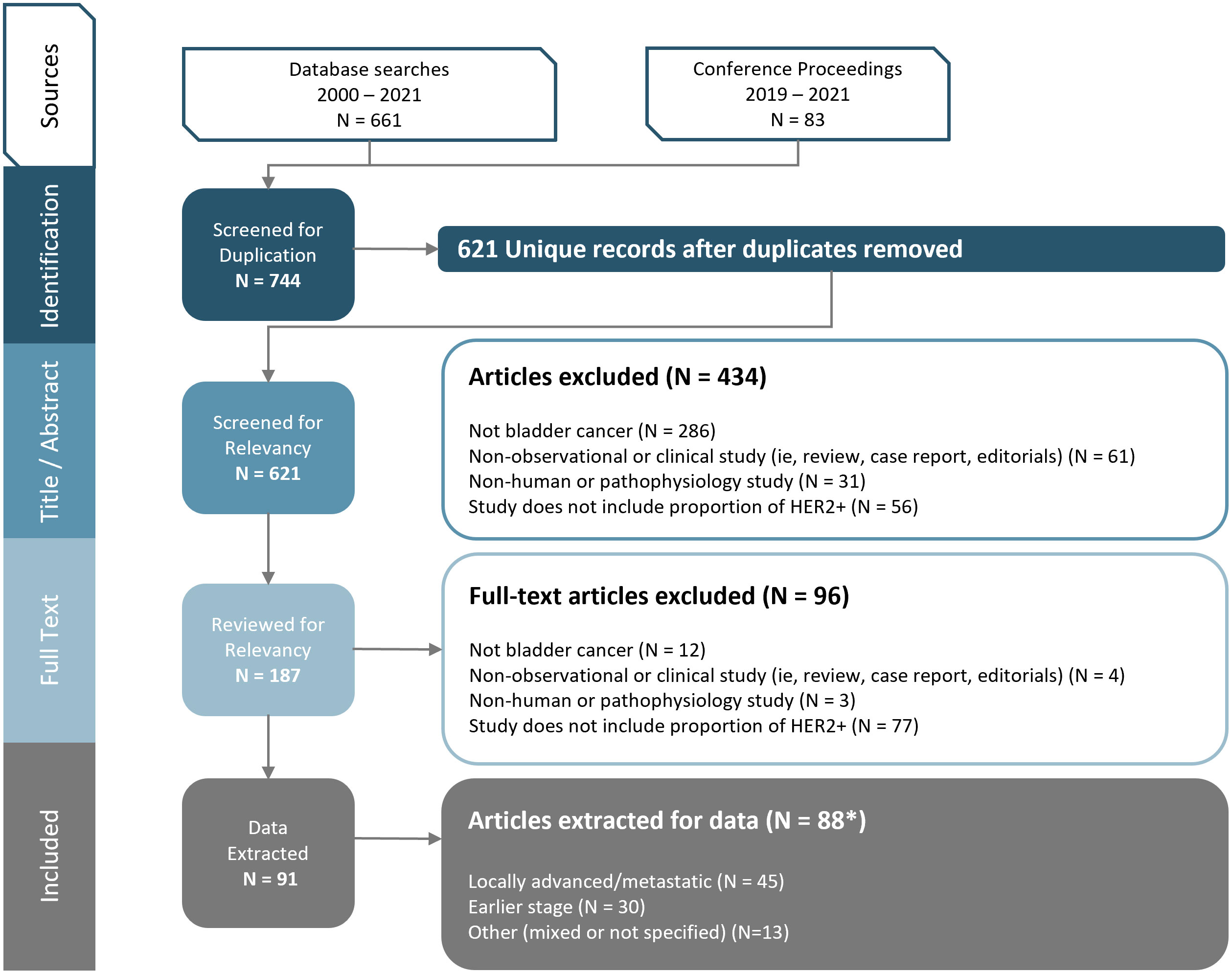
Figure 1 PRISMA Diagram. Figure footnote: *3 studies and 2 abstracts presented duplicate data, so data was extracted only once for each respective study.
The majority of publications that met inclusion criteria were observational studies (75 of 88), with the remaining 13 comprised of clinical trials or pooled analyses of clinical trial enrollment screening.
Assessment of HER2 expression and amplification
A total of 65 studies specified the type of test, assay, or antibody used. The most common IHC tests used were Dako HercepTest (IHC; n=18) and Ventana Pathway anti-Her2/neu (4B5) (IHC; n=6), and gene amplification tests were Abbott PathVysion HER-2 DNA Probe Kit (ISH; n=12), FoundationOne CDx (NGS; n=4), Guardant360 CDx (NGS; n=3), and Illumina NextSeq/MiSeq/HiSeq (NGS; n=3). A total of 41 studies used at least one assay other than the ones listed above, and 23 studies did not specify what test or assay was used. Assay counts were not mutually exclusive as some studies used multiple assays.
Various criteria were used to define HER2 expression, and most studies defined their own criteria (n=46) or did not mention criteria in the study methods (n=28). The most frequently cited scoring guidelines were ASCO/CAP guidelines for breast cancer (2007, 2013, and/or 2018) (n=13) and gastric cancer (n=5). Two studies cited ASCO/CAP guidelines but did not specify which guideline year was used. Only 1 study explicitly defined ERBB2 amplification (≥3.5 copies) (41). Study counts were not mutually exclusive as some studies used more than 1 criterion or guideline.
HER2 expression
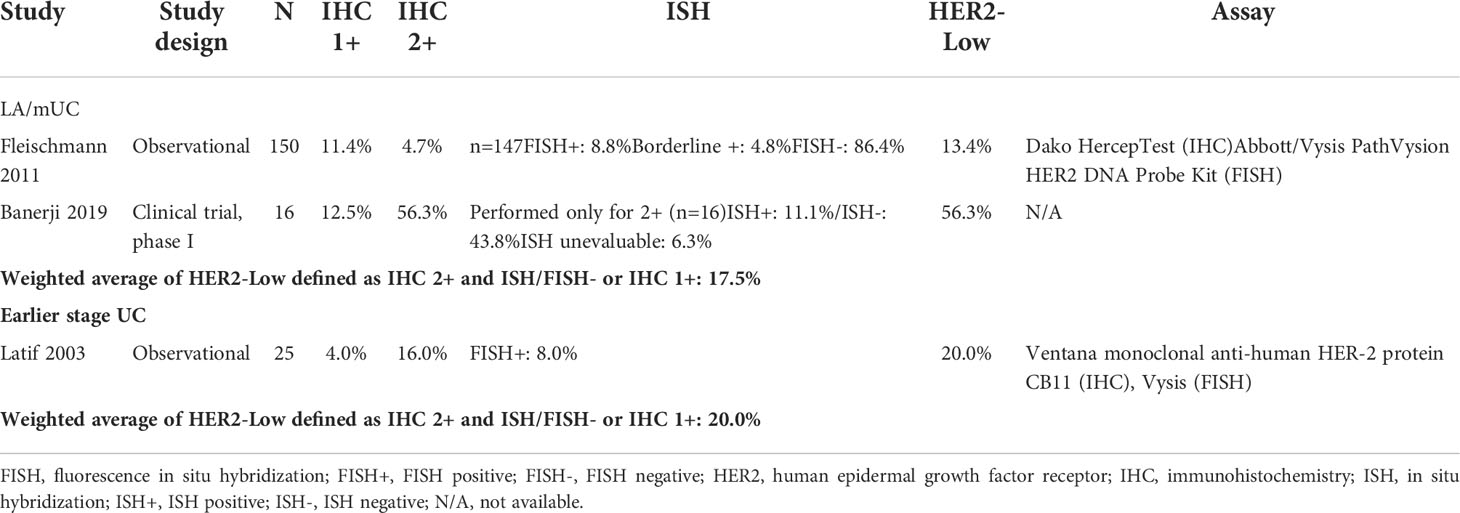
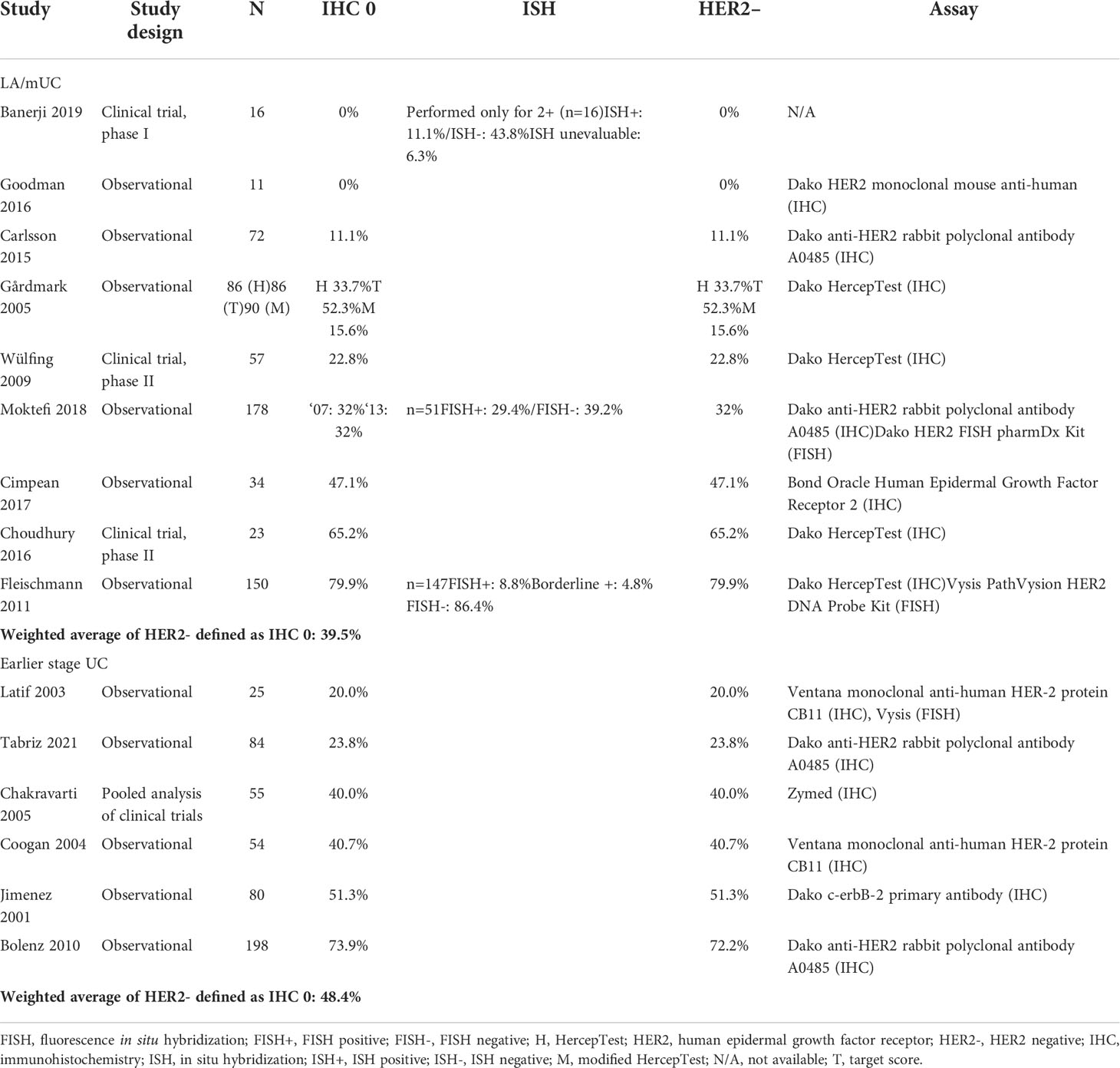
LA/mUC. Of the total 28 LA/mUC studies that conducted IHC, 11 presented data that could be categorized into HER2+, HER2-low, and HER2- using pre-defined criteria (22, 36, 41–49). HER2+ ranged from 6.7% to 37.5% with a weighted average of 13.0% (4 studies, n=862) (Table 1) (22, 36, 42, 43). HER2-low ranged from 13.4% to 56.3% with a weighted average of 17.5% (2 studies, n=166) (Table 2) (22, 43). HER2- ranged from 0% to 79.9% with a weighted average of 39.5% (9 studies, n=803) (Table 3) (22, 41, 43–49).
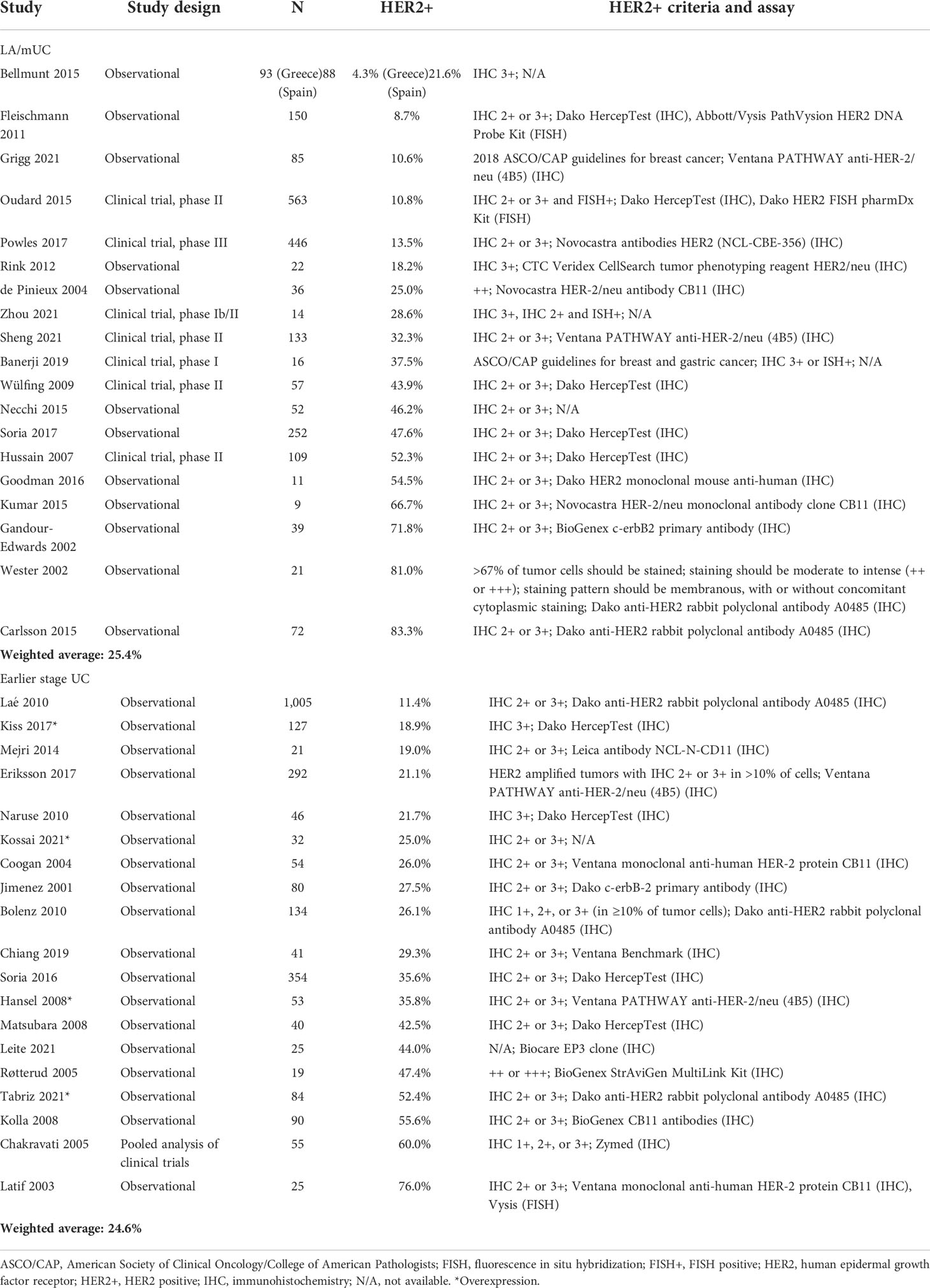
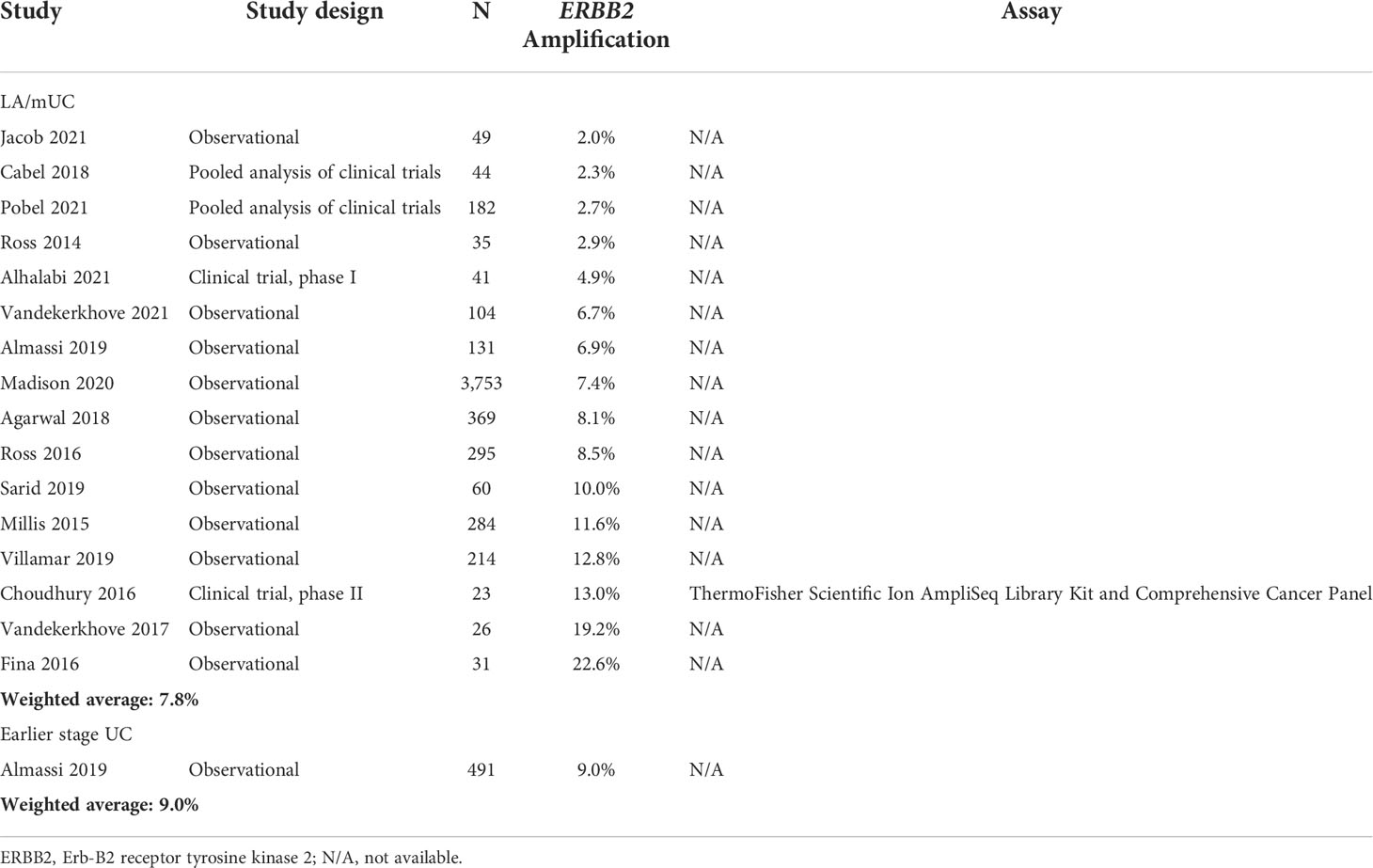
Across all studies (35 studies) reporting HER2+ in LA/mUC patients with or without pre-determined criteria, HER2+ ranged from 4.3% to 83.3% with a weighted average of 25.4% (19 studies, n=2,268) (Table 4) (21, 22, 26, 36, 42, 45, 47, 50–58). ERBB2 amplification ranged from 2.0% to 22.6% with a weighted average of 7.8% (16 studies, n=5,641) (Table 5) (41, 59–73).
Earlier stage UC. Of the 25 earlier stage UC studies that conducted IHC, 6 presented data that could be categorized into HER2+, HER2-low, and HER2- using pre-defined criteria (24, 74–78). Only 1 study (n=25) presented data that could be classified as HER2+ (60.0%) and HER2-low (20.0%) in earlier stage UC patients (Tables 1, 2) (74). HER2- ranged from 20.0% to 73.9% with a weighted average of 48.4% (6 studies, n=432) (Table 3) (24, 74–78).
Across all studies reporting HER2+ (17 earlier stage UC and 2 mixed early stage and LA/mUC which reported data for the earlier stage subgroup), HER2+ in earlier stage UC ranged from 11.4% to 76.0% with a weighted average of 24.6% (19 studies, n=2,577) (Table 1) (24, 37, 74–90). Only 1 study reported ERBB2 amplification in earlier stage UC at 9.0% (n=491) (Table 4) (91).
Concordance between HER2 overexpression and amplification
No studies identified in this SLR reported concordance for HER2 overexpression (IHC 3+) and gene amplification by NGS or gene amplification by ISH/FISH and NGS. Three studies outside of this SLR reported the level of concordance between HER2 overexpression and gene amplification, ranging from 71% to 91% (49, 70, 92). In patients with LA/mUC, 1 study reported tumor samples assessed with both IHC and ISH showed that 91% of tumors with IHC 3+ had HER2 gene amplification by ISH (70). In studies of UC overall, concordance between HER2 IHC 3+ and gene amplification by FISH and HER2 IHC 3+ and gene amplification by brightfield double in situ hybridization (BDISH), were 73.3% and 71%, respectively (49, 92).
Discussion
There is a growing body of literature that suggests the importance of the HER2 gene as a potentially clinically relevant biomarker in UC (33, 93, 94). To the best of our knowledge, this is the first study to systematically characterize the prevalence of HER2 expression in UC, as classified as HER2+, HER2-low, or HER2-. For patients with LA/mUC, we found published literature suggesting HER2+ to be present in 13-25% of patients and HER2-low status in up to 20%. This SLR only identified 1 earlier stage UC study that had available data to categorize into the pre-defined categories, reporting HER2+ prevalence of 60%, which is within the range that has been previously cited in literature (27.8%-85.2%) (93). However, reported ranges for HER2+ (for all studies regardless of criteria) were extremely variable for both LA/mUC (4%-83%) and earlier stage UC patients (11%-76%). As such, based on the findings of this SLR, HER2 expression did not seem to differ significantly between early stage and more advanced disease. It is notable however, that a recent, single-institution NGS study seemed to suggest that HER2 amplification occurred more frequently in patients with muscle invasive and metastatic BC than in patients with non-muscle invasive BC (95).
Wide ranges for HER2+ (all studies) can be attributed to the lack of optimized staining process and standardized criteria, specific for UC. The estimate for HER2+ using pre-defined criteria was lower than the estimate when all studies with HER2+ data, regardless of criteria, were included. Many studies used less rigorous criteria, such as IHC 2+ not confirmed by ISH/FISH, when defining HER2+. Had these studies tested for and confirmed patients by ISH/FISH, some likely would fall into the HER2-low classification, rather than HER2+. Overall, this highlights the importance of generating more rigorous data for HER2+ status in patients with urothelial cancer and its clinical relevance, using more optimized and standardized assays and scoring criteria.
Differences in scoring over time also likely contributed to the variability observed, as the same patients can be scored differently based on the guideline used. In one study, nearly half of patients were reclassified from IHC score 1+ to 2+ based on the shift in definitions from the 2007 to 2013 ASCO/CAP guidelines. The substantial shift in IHC scores from 1+ to 2+ directly affected the number of cases that were eligible for FISH testing, as 58 more patients—among a total of 98 patients—were FISH tested (48). Consequently, the proportion of patients who were HER2+ (IHC 3+ or IHC 2+/FISH+) increased from 15.3% using the 2007 guidelines to 28.6% using the 2013 guidelines.
There is a great need for optimization and standardization in testing methodology as well as algorithms for test result interpretation in UC. Published studies in breast and gastric cancer demonstrate the significance in validating and developing standardized testing in order to accurately select patients who may benefit from HER2-targeted therapies (96–98). While characteristic expression and HER2 scoring systems have been established in breast and gastric cancer, they have not in UC (99). It has been suggested that the pattern of HER2 staining on tumor cells in UC does not exactly mirror either breast or gastric staining patterns, but rather is a mix of the two—circular and patchy (100). Moreover, when assessing UC samples, FISH testing is rarely performed in routine clinical practice, unlike that for breast and gastric tumors. Additionally, it is important that studies assess factors contributing to variability in HER2 testing in order to reduce variation and improve validity (96). One study identified various factors, such as tumor location and Lauren classification, that affect HER2 testing results in gastric cancer; however, similar data in UC is currently unavailable (96). Overall, more comprehensive guidelines for HER2 testing in UC should be developed.
Very few studies identified in this SLR provided information on concordance between test results. In general, there is a dearth in published literature on concordance between detection methods in UC, with some studies focusing on reporting concordance between primary tumors and metastases (37, 79, 101). This SLR yielded 3 studies that reported concordance rates between differing techniques of assessing HER2 status, ranging from 71% to 91% (49, 70, 92). Overall, published literature seems to suggest that IHC 3+ and ISH/FISH produce similar results in UC. Future studies should aim to investigate concordance for HER2 overexpression and gene amplification by NGS, as adoption of NGS in clinical practice has increased in recent years.
A caveat of this SLR is the limited availability of IHC data. Though the presented definitions of HER2+, HER2-low, and HER2- are consistent with other tumor types, they require both IHC and ISH/FISH testing, which often limits the number of studies that could contribute data. ISH data were also often not presented, limiting the number of studies which contributed data to the estimates of HER2+ and HER2-low. These findings highlight the inconsistency in testing methods and assays currently used for HER2 testing in UC.
Differences in patient population, including staging and treatment history, as well as the timeframe and location of the included studies, may have also contributed to the wide ranges observed. For instance, location of primary tumor may affect HER2 overexpression. Patients with upper tract UC are more likely to have higher expression of HER2, with some studies reporting HER2 expression as an independent prognostic factor for patients with upper tract UC (102, 103). Heterogeneity of molecular subtypes may play a role as well. Luminal subtypes of bladder cancer are also characterized by overexpression of HER2 and are overrepresented in upper tract UC, which may help to explain higher expression rates in upper tract UC (104, 105). Additionally, geographic location and race/ethnicity of patients can affect HER2 status. One study found large differences in HER2 overexpression and/or amplification between Spanish and Greek patients and demonstrated that HER2 status varies between populations, suggesting etiologic heterogeneity (21). Lastly, an important difference to note is that some patients are more likely to get HER2 testing and more extensive path review of their tumors due to factors such as where patients receive care (e.g., academic institutions or community-based), geographic region, and insurance status.
Weighted averages were calculated in order to generate a summary estimate but do not account for heterogeneity across studies or statistical uncertainty in the pooled estimates.
Lastly, different studies used different tissue to assess HER2 status for LA/mUC, and some studies may have used primary tissue of the metastatic tissue to assess HER2 status even when results were reported as LA/mUC.
Conclusion
This review of published literature of HER2 expression in patients with LA/mUC revealed a wide reported range of prevalence, 6.7% to 37.5% for HER2+ and 13.4% to 56.3% for HER2-low. This variation may be attributed to heterogenous study populations, tissue samples collected, and testing methodologies. Results of this SLR help shed light on HER2 expression in UC, a potentially clinically relevant biomarker-driven population for emerging HER2-directed regimens, suggesting that up to half of all patients with UC may have some level of HER2 expression that could potentially be targeted. As these novel HER2-directed agents spark new interest in further understanding the role of HER2 expression, gene amplification, and mutations in UC, it is important to utilize well-characterized, optimized, and standardized assays as well as scoring, interpretation criteria, and data analysis for IHC, ISH/FISH, or NGS assays.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
ES made key contributions to the study design, helped draft the manuscript, and revised the submitted article for important intellectual content. AK made key contributions to the study design, performed analysis, assisted with interpretation of results, and helped draft the manuscript. LB made key contributions to the study design, assisted with interpretation of results, and helped draft the manuscript. VK helped draft the manuscript and revised the submitted article for important intellectual content. All authors read and approved the final manuscript. They are accountable for all aspects of the work and in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was sponsored by Seagen Inc., Bothell, WA.
Conflict of interest
AK and LB are employees of Curta, Inc., which received funding from Seagen Inc. in connection with this research. ES is an employee of Seagen Inc. and hold stock/stock options in Seagen Inc. VK has served in a consulting or advisory role for AstraZeneca, Clovis, Janssen, Pfizer, EMD Serono, Seagen, Astellas, Dendreon, Guidepoint, GLG and ExpertConnect; has received research funding for the institution from Endocyte, Nektar, Clovis, Janssen and Taiho.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GLOBOCAN. Cancer today (2021). Available at: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (Accessed September 24, 2021).
2. Hansel DE, Amin MB, Comperat E, Cote RJ, Knüchel R, Montironi R, et al. A contemporary update on pathology standards for bladder cancer: transurethral resection and radical cystectomy specimens. Eur Urol (2013) 63(2):321–32. doi: 10.1016/j.eururo.2012.10.008
3. Marcos-Gragera R, Mallone S, Kiemeney LA, Vilardell L, Malats N, Allory Y, et al. Urinary tract cancer survival in Europe 1999-2007: Results of the population-based study EUROCARE-5. Eur J Cancer (2015) 51(15):2217–30. doi: 10.1016/j.ejca.2015.07.028
4. National Cancer Institute. SEER cancer stat facts: Bladder cancer (2021). Available at: https://seer.cancer.gov/statfacts/html/urinb.html (Accessed September 24, 2021).
5. Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: A systematic review and two-step meta-analysis. Oncologist (2016) 21(6):708–15. doi: 10.1634/theoncologist.2015-0440
6. Bellmunt J, Petrylak DP. New therapeutic challenges in advanced bladder cancer. Semin Oncol (2012) 39(5):598–607. doi: 10.1053/j.seminoncol.2012.08.007
7. National Comprehensive Cancer Network. Bladder cancer (Version 4.2021) (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/bladder_blocks.pdf (Accessed September 27, 2021).
8. Bukhari N, Al-Shamsi HO, Azam F. Update on the treatment of metastatic urothelial carcinoma. ScientificWorldJournal (2018), 5682078. doi: 10.1155/2018/5682078
9. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med (2020) 383(13):1218–30. doi: 10.1056/NEJMoa2002788
10. Hepp Z, Shah SN, Smoyer K, Vadagam P. Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis. J Manage Care Spec Pharm (2021) 27(2):240–55. doi: 10.18553/jmcp.2020.20285
11. Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. NCCN guidelines insights: Bladder cancer, version 5.2018. J Natl Compr Canc Netw (2018) 16(9):1041–53. doi: 10.6004/jnccn.2018.0072
12. European Society for Medical Oncology. eUpdate: bladder cancer treatment recommendations (2020) (Accessed November 25, 2020).
13. Martini A, Raggi D, Fallara G, Nocera L, Schultz JG, Belladelli F, et al. Immunotherapy versus chemotherapy as first-line treatment for advanced urothelial cancer: A systematic review and meta-analysis. Cancer Treat Rev (2022) 104:102360. doi: 10.1016/j.ctrv.2022.102360
14. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet (2016) 387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4
15. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
16. Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet (2018) 391(10122):748–57. doi: 10.1016/S0140-6736(17)33297-X
17. PADCEV. (enfortumab vedotin-ejfv) [prescribing information]. Northbrook, Illinois: Seagen, Inc. Bothell, WA and Astellas Pharma US, Inc. (2022).
18. TRODELVY. (sacituzumab govitecan-hziy) [prescribing information]. Morris Plains, NM: Immunomedics, Inc. (2022).
20. Krüger S, Weitsch G, Büttner H, Matthiensen A, Böhmer T, Marquardt T, et al. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic implications. Int J Cancer (2002) 102(5):514–8. doi: 10.1002/ijc.10731
21. Bellmunt J, Werner L, Bamias A, Fay AP, Park RS, Riester M, et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med (2015) 4(6):844–52. doi: 10.1002/cam4.432
22. Fleischmann A, Rotzer D, Seiler R, Studer UE, Thalmann GN. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol (2011) 60(2):350–7. doi: 10.1016/j.eururo.2011.05.035
23. Grivas PD, Day M, Hussain M. Urothelial carcinomas: a focus on human epidermal receptors signaling. Am J Transl Res (2011) 3(4):362–73.
24. Coogan CL, Estrada CR, Kapur S, Bloom KJ. HER-2/neu protein overexpression and gene amplification in human transitional cell carcinoma of the bladder. Urology (2004) 63(4):786–90. doi: 10.1016/j.urology.2003.10.040
25. Latif Z, Watters AD, Dunn I, Grigor K, Underwood MA, Bartlett JM. HER2/neu gene amplification and protein overexpression in G3 pT2 transitional cell carcinoma of the bladder: a role for anti-HER2 therapy? Eur J Cancer (Oxford Engl 1990) (2004) 40(1):56–63. doi: 10.1016/j.ejca.2003.08.027
26. Wester K, Sjöström A, de la Torre M, Carlsson J, Malmström PU. HER-2–a possible target for therapy of metastatic urinary bladder carcinoma. Acta Oncol (2002) 41(3):282–8. doi: 10.1080/02841860260088836
27. Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer (2020) 126(19):4278–88. doi: 10.1002/cncr.33102
28. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med (2015) 372(8):724–34. doi: 10.1056/NEJMoa1413513
29. Krop IE, Kim SB, González-Martín A, LoRusso PM, Ferrero JM, Smitt M, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol (2014) 15(7):689–99. doi: 10.1016/S1470-2045(14)70178-0
30. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med (2012) 367(19):1783–91. doi: 10.1056/NEJMoa1209124
31. Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2-positive advanced breast cancer: Final results from MARIANNE. Cancer (2019) 125(22):3974–84. doi: 10.1002/cncr.32392
32. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (2010) 376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X
33. Patelli G, Zeppellini A, Spina F, Righetti E, Stabile S, Amatu A, et al. The evolving panorama of HER2-targeted treatments in metastatic urothelial cancer: A systematic review and future perspectives. Cancer Treat Rev (2022) 104:102351. doi: 10.1016/j.ctrv.2022.102351
34. Lambert JM, Morris CQ. Antibody-drug conjugates (ADCs) for personalized treatment of solid tumors: A review. Adv Ther (2017) 34(5):1015–35. doi: 10.1007/s12325-017-0519-6
35. Abel M, Burkenroad A, Sun A, Lu E, Stefanoudakis D, Drakaki A. The evolving landscape of antibody-drug conjugates for urothelial carcinoma. Clin Genitourin Cancer (2021) 19(3):183–93. doi: 10.1016/j.clgc.2020.11.006
36. Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo H, et al. Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin Cancer Res (2021) 27(1):43–51. doi: 10.1158/1078-0432.CCR-20-2488
37. Hansel DE, Swain E, Dreicer R, Tubbs RR. HER2 overexpression and amplification in urothelial carcinoma of the bladder is associated with MYC coamplification in a subset of cases. Am J Clin Pathol (2008) 130(2):274–81. doi: 10.1309/41VLTFX3YPP1HF6F
38. Wolff AC, Hammond M, Allison KH, Harvey BE, Mangu PB, Bartlett J, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical Oncology/College of American pathologists clinical practice guideline focused update. J Clin Oncol (2018) 36(20):2105–22. doi: 10.1200/JCO.2018.77.8738
39. Bartley AN, Washington MK, Ventura CB, Ismaila N, Colasacco C, Benson AB, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: Guideline from the college of American pathologists, American society for clinical pathology, and American society of clinical oncology. Arch Pathol Lab Med (2016) 140(12):1345–63. doi: 10.5858/arpa.2016-0331-CP
40. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
41. Choudhury NJ, Campanile A, Antic T, Yap KL, Fitzpatrick CA, Wade JL, et al. Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J Clin Oncol (2016) 34(18):2165–71. doi: 10.1200/JCO.2015.66.3047
42. Oudard S, Culine S, Vano Y, Goldwasser F, Théodore C, Nguyen T, et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer (2015) 51(1):45–54. doi: 10.1016/j.ejca.2014.10.009
43. Banerji U, van Herpen C, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol (2019) 20(8):1124–35. doi: 10.1016/S1470-2045(19)30328-6
44. Goodman AL, Osunkoya AO. Human epidermal growth factor receptor 2 expression in micropapillary urothelial carcinoma of the bladder: an analysis of 27 cases. Hum Pathol (2016) 57:160–4. doi: 10.1016/j.humpath.2016.07.014
45. Carlsson J, Wester K, de la Torre M, Malmström PU, Gårdmark T. EGFR-expression in primary urinary bladder cancer and corresponding metastases and the relation to HER2-expression. On possibility to target these receptors radionuclides Radiol Oncol (2015) 49(1):50–8. doi: 10.2478/raon-2014-0015
46. Gårdmark T, Wester K, de la Torre M, Carlsson J, Malmström PU. Analysis of HER2 expression in primary urinary bladder carcinoma and corresponding metastases. BJU Int (2005) 95(7):982–6. doi: 10.1111/j.1464-410X.2005.05452.x
47. Wülfing C, Machiels JP, Richel DJ, Grimm MO, Treiber U, De Groot MR, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer (2009) 115(13):2881–90. doi: 10.1002/cncr.24337
48. Moktefi A, Pouessel D, Liu J, Sirab N, Maille P, Soyeux P, et al. Reappraisal of HER2 status in the spectrum of advanced urothelial carcinoma: a need of guidelines for treatment eligibility. Mod Pathol (2018) 31(8):1270–81. doi: 10.1038/s41379-018-0023-9
49. Cimpean AM, Tarlui V, Cumpănaş AA, Bolintineanu S, Cumpănaş A, Raica M. Critical overview of HER2 assessement in bladder cancer: What is missing for a better therapeutic approach? Anticancer Res (2017) 37(9):4935–42. doi: 10.21873/anticanres.11903
50. Grigg CM, Livasy C, He J, Hartman A, Clark PE, Zhu J, et al. Human epidermal growth factor receptor 2 overexpression is frequently discordant between primary and metastatic urothelial carcinoma and is associated with intratumoral human epidermal growth factor receptor 2 heterogeneity. Hum Pathol (2021) 107:96–103. doi: 10.1016/j.humpath.2020.10.006
51. Rink M, Chun FK, Dahlem R, Soave A, Minner S, Hansen J, et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol (2012) 61(4):810–7. doi: 10.1016/j.eururo.2012.01.017
52. de Pinieux G, Colin D, Vincent-Salomon A, Couturier J, Amsellem-Ouazana D, Beuzeboc P, et al. Confrontation of immunohistochemistry and fluorescent in situ hybridization for the assessment of HER-2/ neu (c-erbb-2) status in urothelial carcinoma. Virchows Arch (2004) 444(5):415–9. doi: 10.1007/s00428-004-0986-4
53. Zhou L, Xu H, Yan X, Chi Z, Cui C, Si L, et al. RC48-ADC combined with toripalimab, an anti-PD-1 monoclonal antibody (Ab), in patients with locally advanced or metastatic urothelial carcinoma (UC): Preliminary results of a phase Ib/II study.2021 ASCO annual meeting; 2021 jun 4-8; Chicago, IL, USA. J Clin Oncol (2021) 30:4534. doi: 10.1200/JCO.2021.39.15_suppl.4534
54. Necchi A, Giannatempo P, Paolini B, Lo Vullo S, Marongiu M, Farè E, et al. Immunohistochemistry to enhance prognostic allocation and guide decision-making of patients with advanced urothelial cancer receiving first-line chemotherapy. Clin Genitourin Cancer (2015) 13(2):171–7.e1. doi: 10.1016/j.clgc.2014.08.002
55. Soria F, Moschini M, Haitel A, Wirth GJ, Karam JA, Wood CG, et al. HER2 overexpression is associated with worse outcomes in patients with upper tract urothelial carcinoma (UTUC). World J Urol (2017) 35(2):251–9. doi: 10.1007/s00345-016-1871-x
56. Hussain MH, MacVicar GR, Petrylak DP, Dunn RL, Vaishampayan U, Lara P. N. C.OMMAJ.R.X.X.X, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II national cancer institute trial. J Clin Oncol (2007) 25(16):2218–24. doi: 10.1200/JCO.2006.08.0994
57. Kumar S, Prajapati O, Vaiphei K, Parmar KM, Sriharsha AS, Singh SK. Human epidermal growth factor receptor 2/neu overexpression in urothelial carcinoma of the bladder and its prognostic significance: Is it worth hype? South Asian J Cancer (2015) 4(3):115–7. doi: 10.4103/2278-330X.173164
58. Gandour-Edwards R, Lara P. N. C.OMMAJ.R.X.X.X, Folkins AK, LaSalle JM, Beckett L, Li Y, et al. Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer (2002) 95(5):1009–15. doi: 10.1002/cncr.10808
59. Jacob J, Necchi A, Grivas P, Hughes M, Sanford T, Mollapour M, et al. Comprehensive genomic profiling of histologic subtypes of urethral carcinomas. Urol Oncol (2021) 39(10):731.e1–731.e15. doi: 10.1016/j.urolonc.2020.12.021
60. Cabel L, Fuerea A, Lacroix L, Baldini C, Martin P, Hollebecque A, et al. Efficacy of histology-agnostic and molecularly-driven HER2 inhibitors for refractory cancers. Oncotarget (2018) 9(11):9741–50. doi: 10.18632/oncotarget.24188
61. Pobel CJ, Teyssonneau D, Procureur A, Verlingue L, Facchinetti F, Roubaud G, et al. Outcomes according to genomic characteristics of patients with metastatic urothelial carcinoma in phase I/II trials. ESMO congress. Virtual Ann Oncol (2021) 2021:S718. doi: 10.1016/j.annonc.2021.08.107
62. Ross JS, Wang K, Al-Rohil RN, Nazeer T, Sheehan CE, Otto GA, et al. Advanced urothelial carcinoma: Next-generation sequencing reveals diverse genomic alterations and targets of therapy. Mod Pathol (2014) 27(2):271–80. doi: 10.1038/modpathol.2013.135
63. Alhalabi O, Hahn AW, Msaouel P, Andreev-Drakhlin AY, Meric-Bernstam F, Naing A, et al. Molecular profiling of metastatic bladder cancer early-phase clinical trial participants predicts patient outcomes. Mol Cancer Res (2021) 19(3):395–402. doi: 10.1158/1541-7786.MCR-20-0751
64. Vandekerkhove G, Lavoie JM, Annala M, Murtha AJ, Sundahl N, Walz S, et al. Plasma ctDNA is a tumor tissue surrogate and enables clinical-genomic stratification of metastatic bladder cancer. Nat Commun (2021) 12(1):184. doi: 10.1038/s41467-020-20493-6
65. Almassi N, Pietzak EJ, Funt SA, Schultz N, Dalbagni G, Bochner BH, et al. Characterization of actionable genomic alterations to guide targeted therapy for metastatic urothelial carcinoma. 2019 ASCO genitourinary cancers symposium; 2019 Feb 14-19; San Francisco, CA, USA. J Clin Oncol (2019) 37:407. doi: 10.1200/JCO.2019.37.7_suppl.407
66. Madison RW, Gupta SV, Elamin YY, Lin DI, Pal SK, Necchi A, et al. Urothelial cancer harbours EGFR and HER2 amplifications and exon 20 insertions. BJU Int (2020) 125(5):739–46. doi: 10.1111/bju.15006
67. Agarwal N, Pal SK, Hahn AW, Nussenzveig RH, Pond GR, Gupta SV, et al. Characterization of metastatic urothelial carcinoma via comprehensive genomic profiling of circulating tumor DNA. Cancer (2018) 124(10):2115–24. doi: 10.1002/cncr.31314
68. Ross JS, Wang K, Khaira D, Ali SM, Fisher HA, Mian B, et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer (2016) 122(5):702–11. doi: 10.1002/cncr.29826
69. Sarid DL, Berger R, Levertovsky M, Gadot M, Maurice-Dror C, Peer A, et al. Genomic analysis of urothelial cancer and associations with treatment choice and outcome. In: Ann. oncol, vol. 2019. Barcelona, Spain: ESMO Congress (2019). p. V377.
70. Millis SZ, Bryant D, Basu G, Bender R, Vranic S, Gatalica Z, et al. Molecular profiling of infiltrating urothelial carcinoma of bladder and nonbladder origin. Clin Genitourin Cancer (2015) 13(1):e37–49. doi: 10.1016/j.clgc.2014.07.010
71. Villamar DM, Price KS, Nagy R, Tagawa ST, Molina AM, Nanus DM, et al. Serial ctDNA tracking reveals clonal evolution dynamics in advanced urothelial carcinoma (UC). 2019 ASCO genitourinary cancers symposium; 2019 Feb 14-19; San Francisco, CA, USA. J Clin Oncol (2019) 37:401. doi: 10.1200/JCO.2019.37.7_suppl.401
72. Vandekerkhove G, Todenhöfer T, Annala M, Struss WJ, Wong A, Beja K, et al. Circulating tumor DNA reveals clinically actionable somatic genome of metastatic bladder cancer. Clin Cancer Res (2017) 23(21):6487–97. doi: 10.1158/1078-0432.CCR-17-1140
73. Fina E, Necchi A, Giannatempo P, Colecchia M, Raggi D, Daidone MG, et al. Clinical significance of early changes in circulating tumor cells from patients receiving first-line cisplatin-based chemotherapy for metastatic urothelial carcinoma. Bladder Cancer (2016) 2(4):395–403. doi: 10.3233/BLC-160069
74. Latif Z, Watters AD, Dunn I, Grigor KM, Underwood MA, Bartlett JM. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer (2003) 89(7):1305–9. doi: 10.1038/sj.bjc.6601245
75. Moradi Tabriz H, Nazar E, Ahmadi SA, Azimi E, Majidi F. Survivin and Her2 expressions in different grades of urothelial neoplasms of urinary bladder. Iran J Pathol (2021) 16(2):154–61. doi: 10.30699/IJP.2020.130859.2447
76. Chakravarti A, Winter K, Wu CL, Kaufman D, Hammond E, Parliament M, et al. Expression of the epidermal growth factor receptor and her-2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle-invading bladder cancers treated by concurrent radiation and cisplatin-based chemotherapy: a report from the radiation therapy oncology group. Int J Radiat Oncol Biol Phys (2005) 62(2):309–17. doi: 10.1016/j.ijrobp.2004.09.047
77. Jimenez RE, Hussain M, Bianco F. J. C.OMMAJ.R.X.X.X, Vaishampayan U, Tabazcka P, Sakr WA, et al. Her-2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res (2001) 7(8):2440–7.
78. Bolenz C, Shariat SF, Karakiewicz PI, Ashfaq R, Ho R, Sagalowsky AI, et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int (2010) 106(8):1216–22. doi: 10.1111/j.1464-410X.2009.09190.x
79. Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol (2010) 21(4):815–9. doi: 10.1093/annonc/mdp488
80. Kiss B, Wyatt AW, Douglas J, Skuginna V, Mo F, Anderson S, et al. Her2 alterations in muscle-invasive bladder cancer: Patient selection beyond protein expression for targeted therapy. Sci Rep (2017) 7:42713. doi: 10.1038/srep42713
81. Mejri N, Sellami R, Lamia C, Raoudha D, Hmida NB, Sriha B, et al. Status of Her2 over expression in muscle invasive urothelial bladder carcinoma: Report of 21 cases. Urol Ann (2014) 6(1):63–7. doi: 10.4103/0974-7796.127033
82. Eriksson P, Sjödahl G, Chebil G, Liedberg F, Höglund M. HER2 and EGFR amplification and expression in urothelial carcinoma occurs in distinct biological and molecular contexts. Oncotarget (2017) 8(30):48905–14. doi: 10.18632/oncotarget.16554
83. Naruse K, Yamada Y, Nakamura K, Aoki S, Taki T, Zennami K, et al. Potential of molecular targeted therapy of HER-2 and cox-2 for invasive transitional cell carcinoma of the urinary bladder. Oncol Rep (2010) 23(6):1577–83. doi: 10.3892/or_00000798
84. Kossai M, Radulescu C, Adam J, Dziegielewski A, Signolle N, Sibony M, et al. Plasmacytoid urothelial carcinoma (UC) are luminal tumors with similar immune microenvironment as compared to conventional UC2021 ASCO genitourinary cancers symposium; 2021 Feb 11-13; San Francisco, CA, USA J Clin Oncol (2021) 477. doi: 10.1200/JCO.2021.39.6_suppl.477
85. Chiang Y, Wang CC, Tsai YC, Huang CY, Pu YS, Lin CC, et al. Nuclear factor-κB overexpression is correlated with poor outcomes after multimodality bladder-preserving therapy in patients with muscle-invasive bladder cancer. J Clin Med (2019) 8(11):1954. doi: 10.3390/jcm8111954
86. Soria F, Moschini M, Haitel A, Wirth GJ, Gust KM, Briganti A, et al. The effect of HER2 status on oncological outcomes of patients with invasive bladder cancer. Urol Oncol (2016) 34(12):533.e1–533.e10. doi: 10.1016/j.urolonc.2016.07.006
87. Matsubara H, Yamada Y, Naruse K, Nakamura K, Aoki S, Taki T, et al. Potential for HER-2/neu molecular targeted therapy for invasive bladder carcinoma: comparative study of immunohistochemistry and fluorescentin situ hybridization. Oncol Rep (2008) 19(1):57–63.
88. Leite K, Borges LL, Filho LR, Chade D, Coelho RF, Cordeiro M, et al. Histological variants of urothelial carcinoma predict no response to neoadjuvant chemotherapy. Clin Genitourin Cancer (2022) 20(1):e1–6. doi: 10.1016/j.clgc.2021.07.011
89. Røtterud R, Nesland JM, Berner A, Fosså SD. Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int (2005) 95(9):1344–50. doi: 10.1111/j.1464-410X.2005.05497.x
90. Kolla SB, Seth A, Singh MK, Gupta NP, Hemal AK, Dogra PN, et al. Prognostic significance of Her2/neu overexpression in patients with muscle invasive urinary bladder cancer treated with radical cystectomy. Int Urol Nephrol (2008) 40(2):321–7. doi: 10.1007/s11255-007-9283-x
91. Almassi N, Pietzak E, Schultz N, Walasek A, Al-Ahmadie H, Teo MY, et al. Characterizing the landscape of actionable genomic alterations to identify opportunities for targeted therapy trials in patients with localized bladder cancer. American urological association’s 2019 annual meeting; 2019 may 3-6; Chicago, IL, USA. J Urol (2019) 201:e838. doi: 10.1097/01.JU.0000556759.93252.81
92. Nedjadi T, Al-Maghrabi J, Assidi M, Dallol A, Al-Kattabi H, Chaudhary A, et al. Prognostic value of HER2 status in bladder transitional cell carcinoma revealed by both IHC and BDISH techniques. BMC Cancer (2016) 16:653. doi: 10.1186/s12885-016-2703-5
93. Zhao J, Xu W, Zhang Z, Song R, Zeng S, Sun Y, et al. Prognostic role of HER2 expression in bladder cancer: a systematic review and meta-analysis. Int Urol Nephrol (2015) 47(1):87–94. doi: 10.1007/s11255-014-0866-z
94. Gan K, Gao Y, Liu K, Xu B, Qin W. The clinical significance and prognostic value of HER2 expression in bladder cancer: A meta-analysis and a bioinformatic analysis. Front Oncol (2021) 11:653491. doi: 10.3389/fonc.2021.653491
95. Lattanzi M, Niederhausern A, Zheng J, Bahadur N, Nichols C, Barton L, et al. Incidence and clinical outcomes of HER2-altered bladder cancer (BC) patients (pts). 2022 ASCO genitourinary cancers symposium; 2022 Feb 17-19; San Francisco, CA, USA. J Clin Oncol (2022) 40:556. doi: 10.1200/JCO.2022.40.6_suppl.556
96. Baretton G, Kreipe HH, Schirmacher P, Gaiser T, Hofheinz R, Berghäuser KH, et al. HER2 testing in gastric cancer diagnosis: insights on variables influencing HER2-positivity from a large, multicenter, observational study in Germany. Virchows Arch (2019) 474(5):551–60. doi: 10.1007/s00428-019-02541-9
97. Rüschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch (2010) 457(3):299–307. doi: 10.1007/s00428-010-0952-2
98. Dowsett M, Hanna WM, Kockx M, Penault-Llorca F, Rüschoff J, Gutjahr T, et al. Standardization of HER2 testing: results of an international proficiency-testing ring study. Mod Pathol (2007) 20(5):584–91. doi: 10.1038/modpathol.3800774
99. Lei H, Ling Y, Yuan P, Guo J, Sheng X, Zhou AP, et al. Assessment of a HER-2 scoring system and its correlation of HER2-targeting antibody-drug conjugate therapy in urothelial carcinoma 2022 ASCO annual meeting; 2022 jun 3-7; Chicago, IL, USA. J Clin Oncol (2022) 2022:4572. doi: 10.1200/JCO.2022.40.16_suppl.4572
100. Galsky M. HER-2 is new again in bladder cancer. 2022 ASCO annual meeting; 2022 jun 3-7; Chicago, IL, USA. J Clin Oncol (2022) 2022:4572.
101. Chen PC, Yu HJ, Chang YH, Pan CC. Her2 amplification distinguishes a subset of non-muscle-invasive bladder cancers with a high risk of progression. J Clin Pathol (2013) 66(2):113–9. doi: 10.1136/jclinpath-2012-200944
102. Li S, Wu X, Yan X, Zhou L, Xu H, Li J, et al. Prognostic value of HER2 expression levels for upper tract urothelial carcinoma2022 ASCO genitourinary cancers symposium; 2022 Feb 17-19; San Francisco, CA, USA. J clin Oncol (2022) 2022:557. doi: 10.1200/JCO.2022.40.6_suppl.557
103. Tsai YS, Tzai TS, Chow NH, Wu CL. Frequency and clinicopathologic correlates of ErbB1, ErbB2, and ErbB3 immunoreactivity in urothelial tumors of upper urinary tract. Urology (2005) 66(6):1197–202. doi: 10.1016/j.urology.2005.06.117
104. Dadhania V, Zhang M, Zhang L, Bondaruk J, Majewski T, Siefker-Radtke A, et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine (2016) 12:105–17. doi: 10.1016/j.ebiom.2016.08.036
Keywords: urothelial carcinoma, HER2, ErbB2, targeted therapy, systematic literature search
Citation: Scherrer E, Kang A, Bloudek LM and Koshkin VS (2022) HER2 expression in urothelial carcinoma, a systematic literature review. Front. Oncol. 12:1011885. doi: 10.3389/fonc.2022.1011885
Received: 04 August 2022; Accepted: 30 September 2022;
Published: 21 October 2022.
Edited by:
Sheldon L. Holder, Brown University, United StatesReviewed by:
Cynthia Cohen Shulman, School of Medicine, Emory University, United StatesLu Si, Department of Renal Cancer Melanoma Internal Medicine, Peking University, China
Copyright © 2022 Scherrer, Kang, Bloudek and Koshkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vadim S. Koshkin, VmFkaW0uS29zaGtpbkB1Y3NmLmVkdQ==
 Emilie Scherrer1
Emilie Scherrer1 Ashley Kang
Ashley Kang Vadim S. Koshkin
Vadim S. Koshkin