- 1Department of Oncology, Henan Provincial People's Hospital, Zhengzhou University People's Hospital, Zhengzhou, China
- 2Department of Radiation Oncology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Introduction: It has been believed that breast-conserving therapy (lumpectomy plus adjuvant radiation, Lum + RT) and mastectomy without radiation (Mast + NoRT) have equivalent survival outcomes. However, there is a need to re-evaluate the role of lumpectomy plus adjuvant radiation due to changed breast cancer management over time. This study aimed to conduct a population-based study that compare long-term oncologic survival outcomes after Lum + RT vs Mast + NoRT.
Methods: The Surveillance, Epidemiology and End Results database was used to identify female breast cancer patients with a primary localized breast cancer diagnosis from 1988 to 2018. The standardized incidence/mortality ratio (SIR/SMR) for breast cancer recurrence (BCR) and breast cancer-specific death (BSD) was estimated by the SEER*Stat program. Cumulative incidences of BCR and BSD were assessed using Gray’s method. We evaluated the effects of Lum + RT vs. Mast + NoRT on breast cancer recurrence-free survival (BRFS) and breast cancer-specific survival (BCSS). Fine-Gray competing risk model analyses, propensity score-adjusted Kaplan-Meier analyses and Cox proportional hazards model analyses were applied.
Results: A total of 205,788 women were included in the study. Patients who underwent Lum + RT had higher SIR of BCR (4.14 [95% confidence interval, CI: 3.94-4.34] vs. 1.11 [95% CI: 1.07-1.14]) and lower SMR (9.89 [95% CI: 9.71-10.08] vs. 17.07 [95% CI: 16.82-17.33]) than patients who underwent Mast + NoRT. Lum + RT was associated with higher competing risk of BCR (adjusted hazard ratio [HR]: 1.996, 95% CI: 1.925-2.069, p < 0.001) and lower competing risk of BSD when compared to Mast + RT (adjusted HR: 0.584, 95% CI: 0.572-0.597, p < 0.001). Multivariate Cox regression analysis revealed similar results (adjusted HR after PSW for BRFS: 1.792, 95% CI 1.716-1.871, p < 0.001; adjusted HR after PSW for BCSS: 0.706, 95% CI 0.688-0.725, p < 0.001). These findings persisted in the sensitivity and subgroup analyses.
Discussion: The present study further confirmed superior long-term survival with lumpectomy plus adjuvant radiation over mastectomy independent of patient characteristics including age, race, time period, historic subtype, tumor size, historic grade and stage, indicating that this benefit may result from the treatment itself.
Introduction
Breast-conserving therapy (lumpectomy plus adjuvant radiotherapy) has become the primary treatment option for patients with early-stage breast cancer since a National Institutes of Health (NIH) Consensus Statement released in 1991 recommended that breast conserving therapy (BCT) offered equivalent survival compared with mastectomy (1). The use of BCT has increased rapidly during the last 2–3 decades as it provides superior cosmetic and quality-of-life outcomes (2–4).
However, mastectomy has recently re-emerged to play an essential role in breast cancer treatment for limited radiotherapy resources, patient preference, improvements in breast reconstruction techniques, and increased use of BRCA testing (5–9). In addition, radiotherapy after breast-conserving surgery has raised concerns about an increased risk of locoregional recurrence (10–14).
Some clinical trials and observational studies have compared oncological outcomes of mastectomy versus BCT (13, 15–25). and have not yielded conclusive results. Several reports also pointed out the need to re-evaluate the role of BCT because of recent advances in treating breast cancer with radiotherapy (26–28). Moreover, most of these studies used the Kaplan-Meier (KM) survival method, which is generally meant to describe time to a single type of event and is often unsuitable when comparing cause-specific outcomes in patients with multiple potential outcomes (29–31). The competing risk methodology may be more suitable to adjust for the influence of competing events because estimating the incidence of events of interest in the competing risk model is conditional on the composite event rate of all events of interest and those competing events (32–34). A recent study applied this method to data reported by the Early Breast Cancer Trialists Collaborative Group (EBCTCG), which confirmed that KM-based methods led to biased risk estimates in early breast cancer studies, especially for uncommon outcomes such as local recurrence (35).
In the present study, we adopted the competing risk method in population-based data from Surveillance, Epidemiology, and End Results (SEER). We aimed to compare the long-term oncological outcomes including breast cancer recurrence-free survival (BRFS) and breast cancer-specific survival (BCSS) between lumpectomy plus adjuvant radiation (Lum + RT) and mastectomy without radiation (Mast + NoRT). Furthermore, adjusted KM estimates and Cox proportional hazards models using a propensity score weighting (PSW) method were applied to balance the observed covariates between patients who underwent Lum + RT and those who underwent Mast + NoRT.
Materials and methods
Data source and study population
Data was obtained from the SEER database, the largest publicly available cancer dataset, covering approximately 47.9 percent of the U.S. population. The exact dataset we used for this study was SEER Program Research Data (1988–2018), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, based on the November 2021 submission (36).
We used SEER* Stat 8.4.0 software to retrieve demographic and clinical information of female patients with breast cancers diagnosed between 1988 and 2018 from the SEER database. Patients diagnosed before 1988 were not included in this study because data regarding lymph node involvement were not uniformly recorded in the SEER database before this point. The following inclusion criteria were applied (1): ICD WHO site recode of ‘breast’ (2); age 18 years or greater at the time of diagnosis (3); primary site of breast cancer (4); localized and regional SEER historic stage. We excluded patients of unknown race or stage, or diagnosed by death certificates or autopsy.
Treatment modalities for breast cancer
Patients treated with lumpectomy and postoperative radiation were included in the Lum + RT group. Lumpectomy was identified by site-specific surgery codes 10–28 or surgery of primary site codes 20-24. The Mast + NoRT group was composed of patients who underwent mastectomy alone. Mastectomy was identified by site-specific surgery codes 30-90 or surgery of primary site codes 30-90. We excluded patients who received other types of treatment interventions.
Study variables and outcomes
The following demographic and clinical variables were extracted from the SEER database: age at diagnosis, race, year of diagnosis, historic subtype, tumor size, historic grade, chemotherapy, ER/PR status, HER2 status. Tumor staging was derived according to the American Joint Committee on Cancer (AJCC) 7th edition staging systems using data on tumor size, lymph node involvement, and distant metastasis (37). Laterality was categorized into three groups according to the lateralities of the primary breast cancer and the recurrence breast cancer (ipsilateral, contralateral, and bilateral/unspecified).
The primary endpoints of our study were BRFS and BCSS. Follow-up began 12 months after breast cancer surgery, ensuring patients who had undergone adjuvant radiotherapy or chemotherapy. BRFS was defined as the period from the date of diagnosis to the date of any breast cancer recurrence, and BCSS was measured from the date of diagnosis to the date of death from breast cancer. Patients with second primary cancers or who died due to causes other than breast cancer or with an unknown cause of death were excluded from the analysis.
Statistical analysis
Patients’ demographic and tumor characteristics were compared between the treatment groups. Comparisons of categorical variables were performed using the Chi-square test, and continuous variables were compared using Student’s t test. The standardized incidence ratio (SIR) of recurrence and breast cancer-specific standardized mortality ratio (SMR) among breast cancer survivors was estimated by the SEER*Stat MP-SIR session. The SIR/SMR was calculated by dividing the observed incidence of recurrence or observed cancer-specific mortality by the expected incidence of recurrence or expected cancer-specific mortality [observed/expected (O/E) ratio] in the U.S. general population. Stratified SIR/SMRs were calculated based on attained age, calendar year, and latency (interval time from primary breast cancer diagnosis to recurrence or cancer-specific death). The cumulative incidence function (CIF) was adopted to estimate the probability of breast cancer recurrence (BCR) and breast cancer-specific death (BSD) by the Gray’s subdistribution hazard method (38). Univariable and multivariable competing risk regression models by Fine and Gray were used to assess the risk of BRFS and BCSS in patients who received different treatment modalities (39). A new PSW approach based on overlap weight (OW) was applied to balance observed covariates between the treatment groups. The popular inverse probability weighting method is limited by biased estimates induced by extreme weights when the propensity score distributions between the treatment groups lack overlap (40, 41). The OW method overcomes essential limitations of traditional weighting approaches by emphasizing the target population with the most overlap (42, 43). Survival function estimations for BCR and BSD were adopted using KM estimates and the log-rank test. Univariate and multivariate Cox proportional hazards models were performed to analyze the association between patient treatments with BRFS and BCSS before and after PSW. Recurrence laterality was not included as only patients with BCR had this information, nor were ER/PR and HER2 status included because these data were not routinely reported to SEER registries prior to 1990 or 2010. Sensitivity analyses were conducted in patients with different ER/PR statuses. Stratified analyses were also carried out to examine the impact of covariates. Hazard ratios (HRs) and 95% CIs adjusted by the PSW method were estimated separately for these subgroups. All p values were two-sided and p < 0.05 was considered statistically significant. Data analysis was performed using R software (version 4.1.2).
Results
Patient characteristics
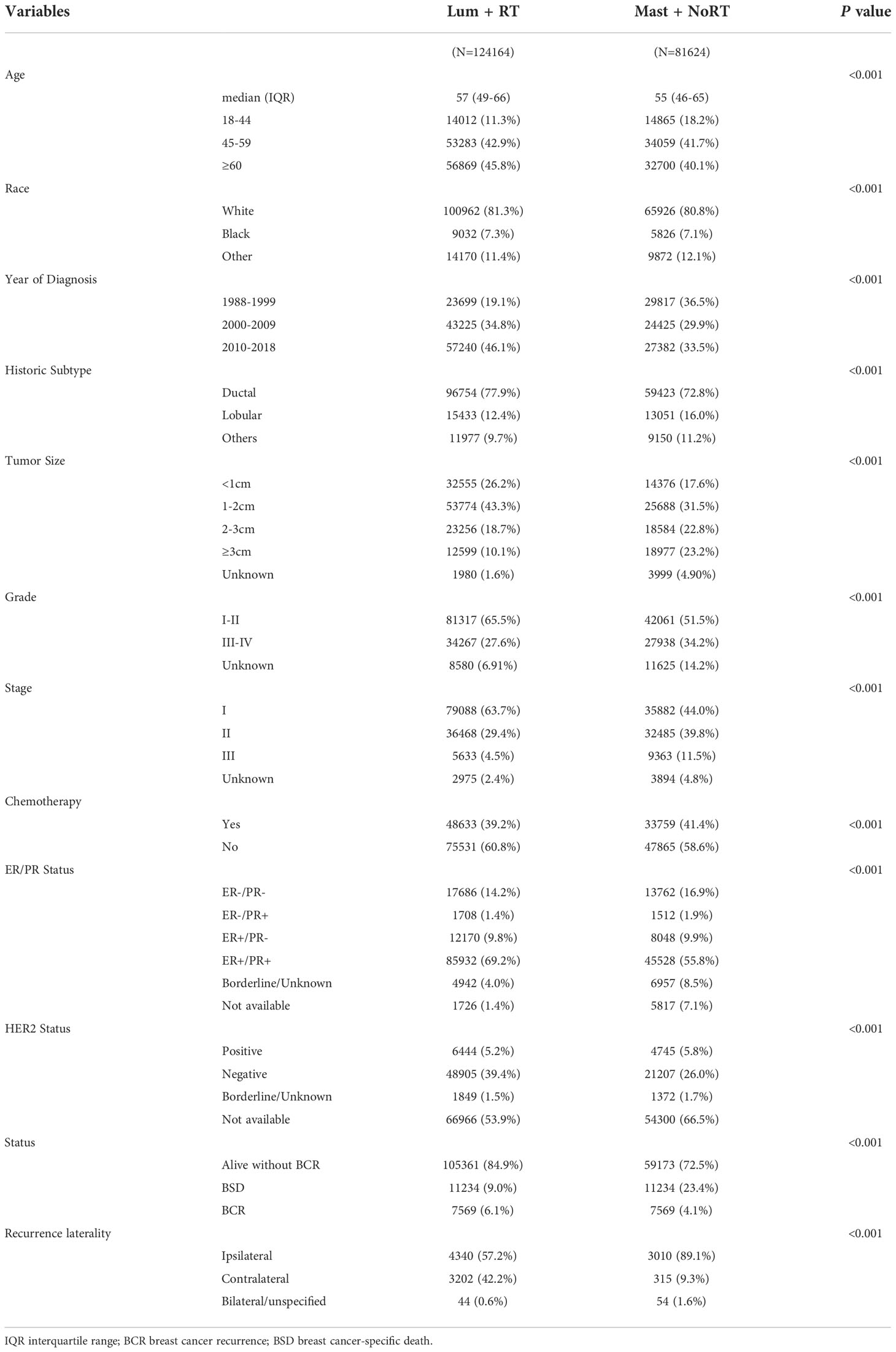
Within the SEER database, we identified 532,616 female patients with breast cancer diagnosed between 1988 and 2019. Among these patients, 235,101 were excluded. Consequently, a total of 205,788 patients were included in the final cohort. Detailed patient selection flowchart is given in Figure S1. The final study population consisted of 124,164 patients treated with Lum + RT and 81,624 patients treated with Mast + NoRT. The median age at diagnosis for all patients was 56 (48–66) years. The median follow-up duration was 114 months in total, and 112 and 116 months for the Lum + RT group and the Mast + NoRT group, respectively. Between 1988 and 1995, mastectomy without radiation was more common in female breast cancer patients. The use of lumpectomy plus adjuvant radiation became more frequent and had increased over the last two decades (1996 to 2018) (Figure S2A).
Table 1 shows significant differences in the clinical characteristics of patients between the treatment groups. There were 10,913 (5.3%) breast cancer recurrences and 30,341 (14.7%) breast cancer-specific deaths in total. The overall median BRFS time was 115 months and the overall median BCSS time was 68 months. The median BRFS and BCSS time were longer for patients treated with Lum + RT than those treated with Mast + NoRT (118 vs. 108 months and 77 vs. 64 months, respectively) (Figure S2B).
Standardized incidence ratio of BCR and Standardized mortality ratio of BSD
Table 2 presents the SIRs of BCR and the SMRs of BSD. The overall SIR of BCR for patients who received Lum + RT was 4.14 (95% CI: 3.94-4.34), and the overall SIR of BCR for patients who received Mast + NoRT was much lower (SIR, 1.11; 95% CI: 1.07-1.14). The same trends were observed in the SIRs stratified by attained age, calendar year, and latency. On the contrary, the SMRs of BCD for patients in the Lum + RT group were significantly lower than that for patients in the Mast + NoRT group (overall SMR: 9.89 [95% CI: 9.71-10.08] vs. 17.07 [95% CI: 16.82-17.33]), similar results were seen in subgroup analyses (Table 2).
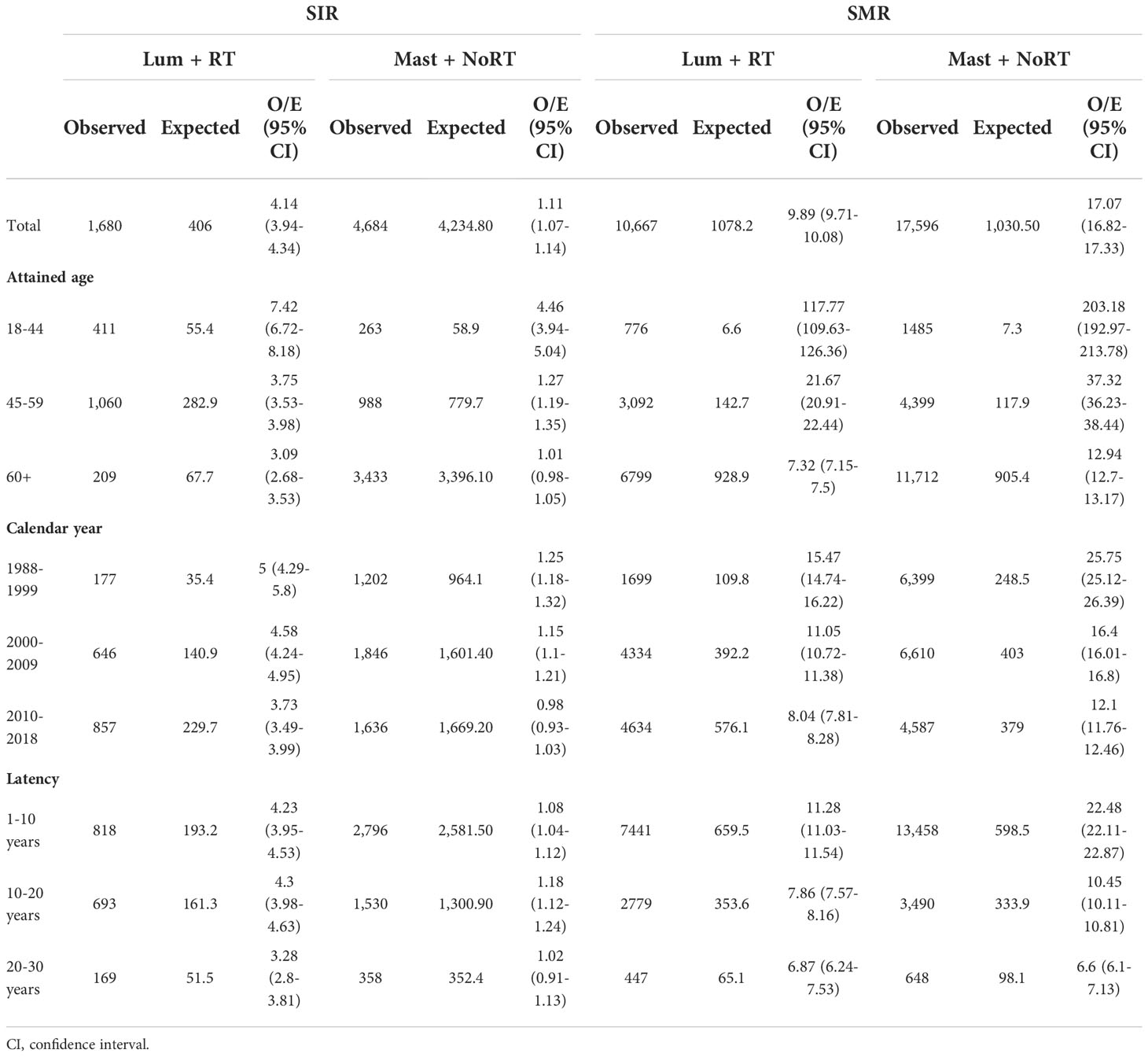
Table 2 Standardized incidence ratios (SIRs) for breast cancer recurrence and standardized mortality ratios (SMRs) for breast cancer-specific death by treatment.
Cumulative incidence of BCR and BSD
The 30-year CI of BCR was 18.44% in patients who received Lum + RT, while the incidence of BCR in patients who received Mast + NoRT was significantly lower (8.25%; p < 0.001) (Figure 1). On the contrary, the 30-year CI of BSD was significantly higher in the Mast + NoRT group as compared with the Lum + RT group (21.28% vs. 36.72%; p < 0.001). CIF curves were plotted across patient subgroups to evaluate the impact of patients’ characteristics (Figure S3). Similar patterns of association between treatment modalities and incidences of BCR and BSD were observed (all p < 0.001).
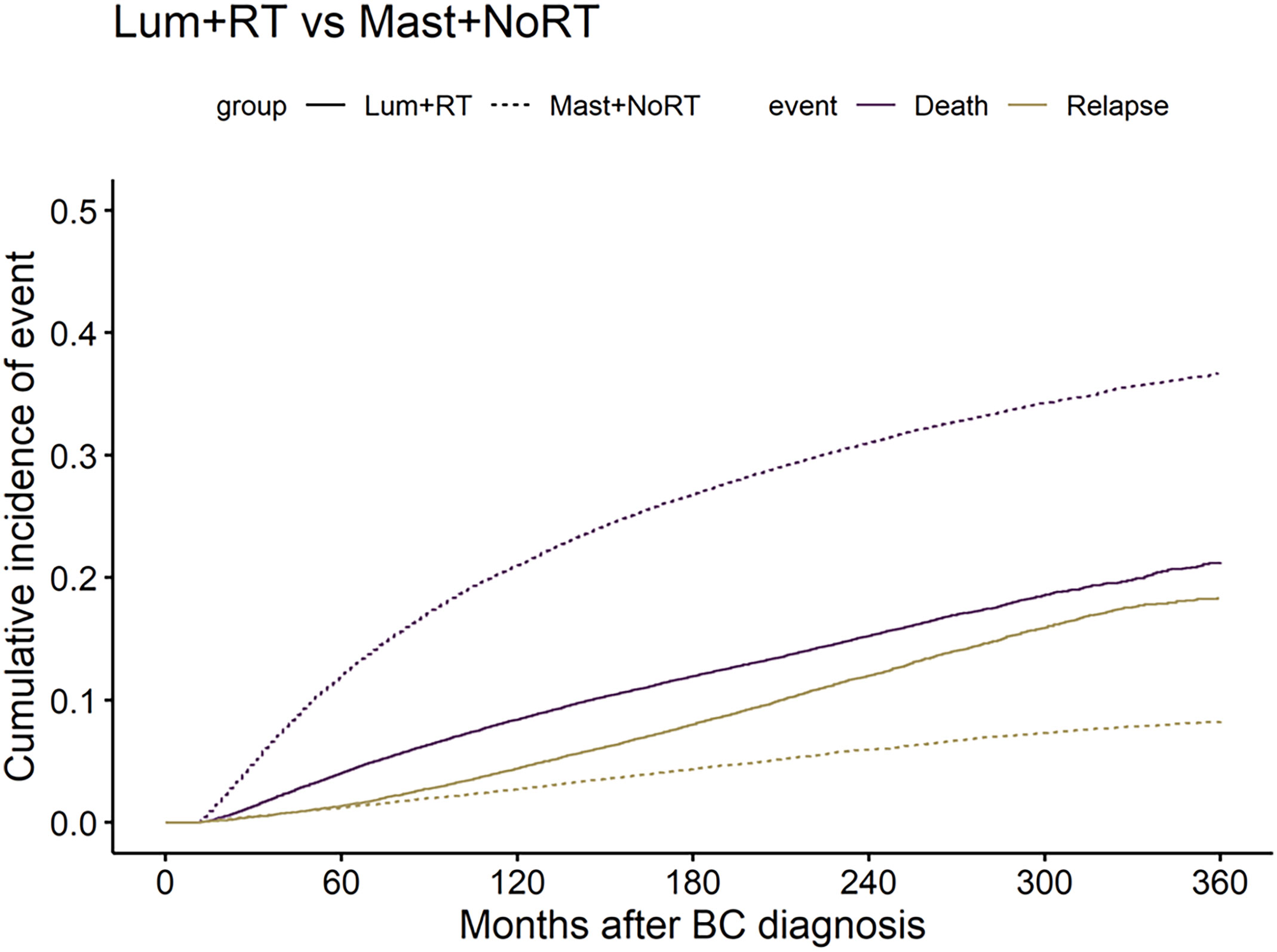
Figure 1 Comparisons of cumulative incidence of breast cancer recurrence (BCR) and breast cancer-specific death (BSD) between patients who received lumpectomy with adjuvant radiation (Lum + RT) and patients received mastectomy without radiation (Mast + NoRT). The solid lines represent Lum + RT, the dotted lines represent Mast + NoRT; The yellow lines represent BCR, the purple lines represent BSD. BC, breast cancer.
Competing risk for BCR and BSD
In univariate competing risk analysis, the incidence of recurrence was significantly greater in patients who received Lum + RT (HR: 1.900, 95% CI: 1.830–1.960, p < 0.001). After adjustment for covariates, the association between Lum + RT and higher incidence of BCR persisted (adjusted HR: 1.996, 95% CI: 1.925-2.069, p < 0.001) (Table 3). For competing risk analyses of BSD, Lum + RT was significantly associated with lower incidence of BSD before (HR: 0.408, 95% CI: 0.400-0.416, p < 0.001) and after (HR: 0.584, 95% CI: 0.572-0.597, p < 0.001) adjustment for covariates (Table 3).
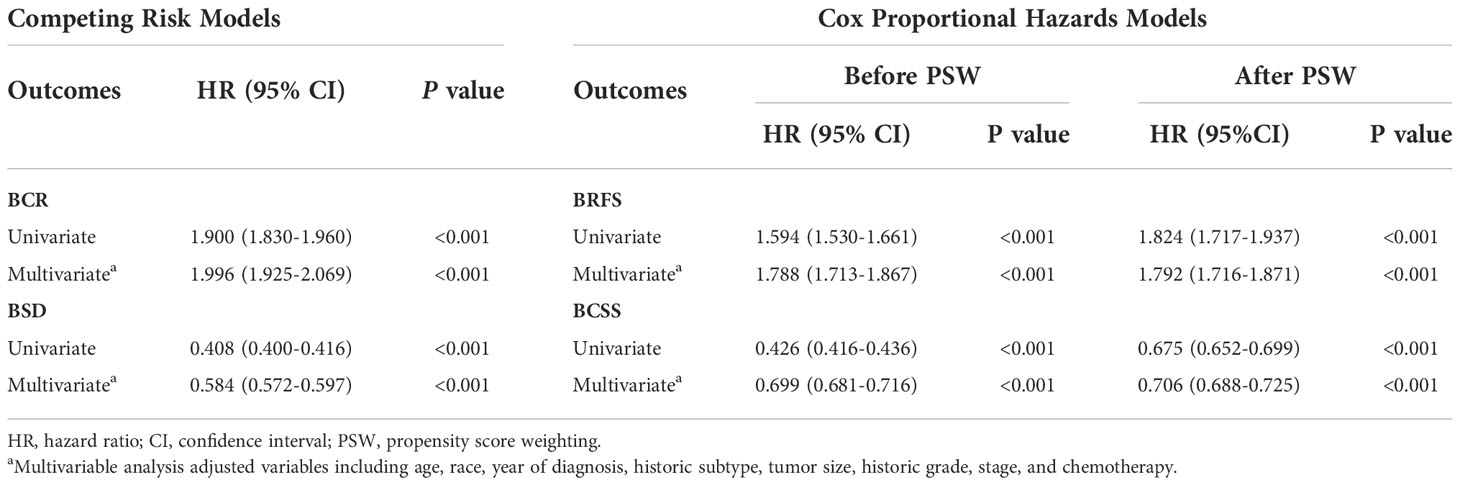
Table 3 Risk of breast cancer recurrence (BCR) and breast cancer-specific death (BSD) and risk of breast cancer recurrence free survival (BRFS) and breast cancer-specific survival without recurrence (BCSS).
Survival analysis
PSW-adjusted standardized differences were all less than 0.1 (Figure S4), indicating a good balance of covariates between the Lum + RT group and the Mast + NoRT group. The 30-year survival probability for BRFS estimated by the Kaplan-Meier method was 82.8%. The 30-year survival probability for BCSS was 69.1%. Lum + RT was associated with a significantly worse BRFS (log-rank p < 0.001 before and after PSW; Figures 2A, B), and a significant better BCSS when compared with Mast + NoRT (log-rank p <0.001 before and after PSW; Figures 2C, D).
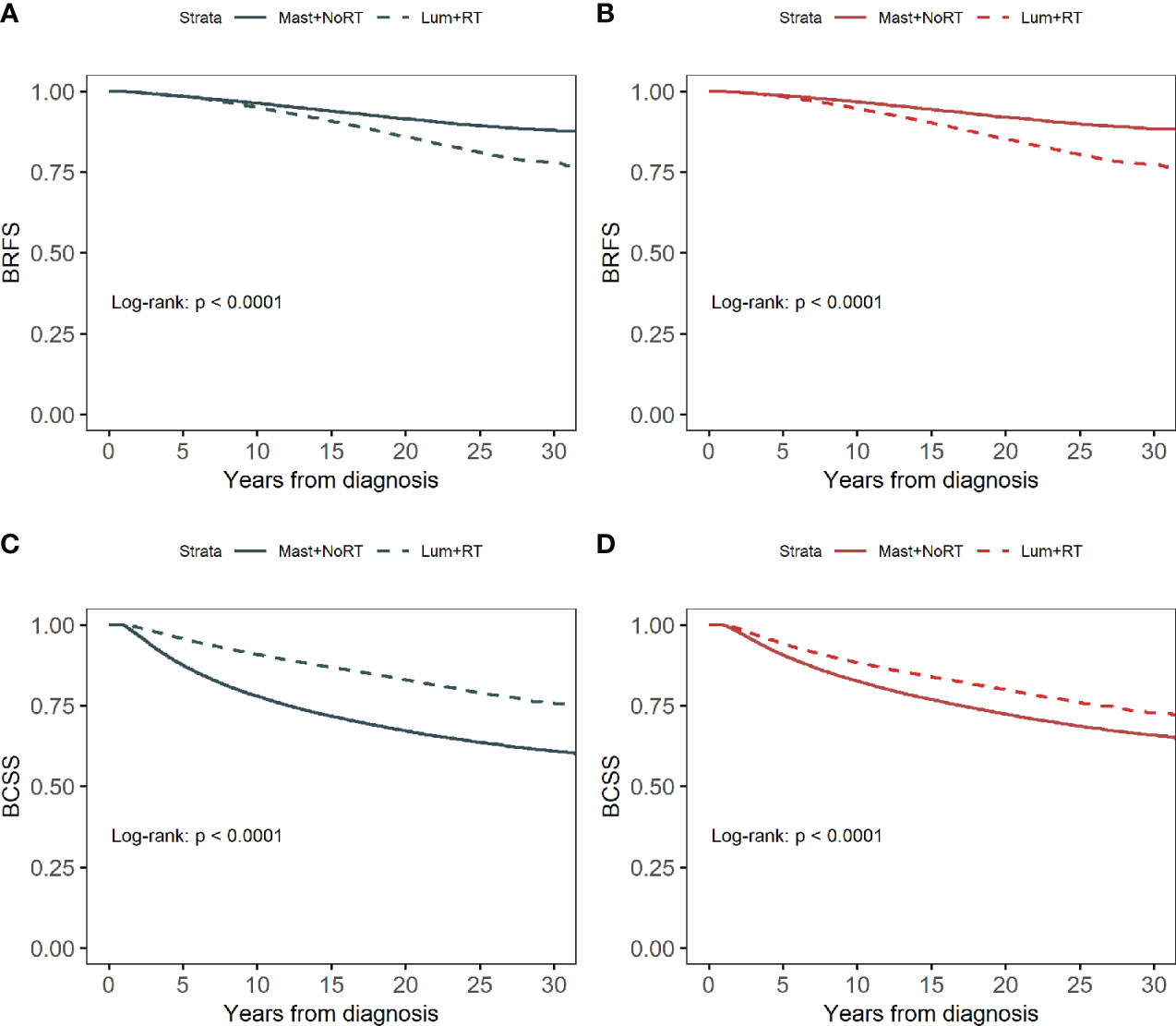
Figure 2 Kaplan–Meier curves for breast cancer recurrence-free survival (BRFS) by treatment before (A) and after (B) propensity score weighting. Kaplan–Meier curves for breast cancer-specific survival (BCSS) by treatment before (C) and after (D) propensity score weighting.
Table 3 shows the unadjusted and adjusted HRs and CIs for BRFS and BCSS estimated from Cox regression models. Patients in the Lum + RT group had significantly worse BRFS (adjusted HRs before/after PSW: 1.788 [95% CI 1.713-1.867] and 1.792 [95% CI 1.716-1.871], respectively). In contrast, Lum + RT was associated with better BCSS when compared with Mast + NoRT (adjusted HRs before/after PSW: 0.699 [95% CI 0.681-0.716] and 0.706 [95% CI 0.688-0.725], respectively). Detailed HRs and 95% CIs for Cox model covariates have been provided in Table S1 and Table S2.
Sensitivity analyses and stratified analyses
Competing risk regression analyses and Cox regression analyses in patients with different ER/PR statuses yielded similar results. Patients in the Lum + RT group had worse BRFS and better BCSS independent of ER/PR statuses (Table S3). Stratified analyses with PSW adjustment were carried out to assess whether treatment differences in survival outcomes depend on certain characteristics of patients. The weighted HRs were all < 1, showing an benefit for the Mast + NoRT treatment in these patient groups (Figure S5A). On the contrary, the weighted HRs for BCSS were all > 1, indicating a worse breast cancer-specific survival for patients who received Mast + NoRT (Figure S5B).
Discussion
The use of lumpectomy plus adjuvant radiation had increased since the 1990s in our data, consistent with previous reports (2–4). We also noticed different trends by age. Lum + RT initially raised in the 2000s and then declined in the 2010s for women aged 18 to 44 years. A reverse trend was observed for mastectomy. For women aged over 60 years, mastectomy had been falling and Lum + RT had been increasing since the 1990s (data not shown). These trends were similar to a recent report (4).
In the early 1980s, large randomized controlled trials demonstrated that BCT provided long-term survival rates equivalent to that obtained after mastectomy for patients with early-stage invasive breast cancer (18–20). However, in agreement with previous studies (21–25, 44–51), our results observed long-term survival benefits in patients receiving Lum + RT compared to those receiving mastectomy. A previous study, which used SEER registry data on 83,776 women with breast cancer diagnosed between 1988 and 1997, found the best survival rates with combined lumpectomy and radiation (44). Our findings confirmed the superiority of lumpectomy plus adjuvant radiation to mastectomy alone using the updated SEER data, indicating that the survival benefit associated with Lum + RT had not changed over time. This finding was consistent across multiple analyses and all the predefined risk factors in our data. A registry-based study also demonstrated that women treated with primary mastectomy had a hazard ratio of 1.64 (95% CI 1.43-1.88) for breast cancer death compared with women treated with primary Lum + RT after adjusting for the year of diagnosis, age at diagnosis, stage, histology, and grade (47). Some had claimed that higher death rates in women treated with mastectomy was due to unfavorable prognostic patient characteristics such as preexisting comorbidities or older age (52). But this conflicted with the fact that elderly patients received more BCT treatment than mastectomy in recent years, which was also seen in our data. Also, a study conducted in Swedish national data from 48,986 breast cancer patients demonstrated that Lum+RT yielded better survival than mastectomy irrespective of RT despite adjustment for covariates including comorbidity burden (50). Lum + RT was associated with better overall survival and BCSS even in patients with triple-negative breast cancer tumors, which were generally more aggressive and associated with a worse prognosis (51).
Our results showed that patients receiving lumpectomy plus adjuvant radiation had a higher risk of locoregional recurrences than those receiving mastectomy alone. However, previous studies yielded inconsistent findings (13–19, 53, 54). Early trials suggested no significant difference in locoregional recurrence rates with BCT compared with mastectomy (15–17). Some studies found lower recurrence rates in patients who received BCT (18, 53, 54), yet others demonstrated similar findings to ours (13, 14, 19). One possible hypothesis for explaining higher recurrence rates with BCT is multifocality and multicentricity in young breast cancer patients, who constitute most of the patients treated with BCT (19). Nevertheless, based on previous researches, local recurrence in BCT did not seem to lead to worse survival (55, 56). Recurrence in the BCT was characterized by longer disease-free interval and related to a better prognosis (56). Our results also confirmed late recurrence in the Lum + RT group (Figure S2B). Thus, we believed that higher recurrence rates would not offset the survival advantage of BCT.
We acknowledge several limitations in the present study. The SEER registry dose not collect clinical data such as coexisting comorbidities, which may have influenced the treatment choice. Furthermore, the excess burden of comorbidities is related to shortened life expectancy. Nevertheless, this factor alone could not account for worse survival for patients who underwent mastectomy after adjusting for age and tumor characteristics. In addition, the current study lacks information on the details of treatments such as radiation dose, chemotherapy regimens, endocrine therapy and the specifics of radiotherapy, including dose, fields and type of radiation. Another concern has been the migration between SEER geographic area, which may lead to a loss of follow-up and an underestimation of the incidence of locoregional recurrences of breast cancer.
Our study has several strengths. To our knowledge, the present study is the first to examine oncologic outcomes between BCT and mastectomy in women with early-stage breast cancer from the population-based SEER registry by modern competing risk techniques. Competing risks arise when individuals are exposed to many causes of failure, and the occurrence of one failure hinders the occurrence of other failure events. Traditional survival analysis techniques such as the Kaplan-Meier curve and the Cox proportional hazard model treat failures from competing risks as censored, which may lead to overestimating the probability of outcomes of interest and biased results (30, 31, 57). The competing risk analyses and Cox proportional hazard model analyses yielded similar results in the current study, indicating a consistent independent association between BCT and better cancer-specific survival outcome. Moreover, we estimated long-term treatment-associated outcomes using data over the past 30 years. Risk estimation from short-term follow-up might be biased when a time-dependent risk factor is present. In addition, we applied the PSW method to adjust confounding factors between different treatment groups. The OW approach allows one to analyze an observational study to mimic a randomized experiment by modeling the assignment process of participants (58, 59).
Conclusions
The analysis of the SEER registry data over the past 30 years (1988–2018) indicated that lumpectomy plus adjuvant radiation was associated with superior long-term breast cancer-specific survival and a higher risk of breast cancer recurrence when compared to mastectomy alone, independent of age, race, time period, historic subtype, tumor size, historic grade and stage. Because the survival advantage of Lum + RT cannot be explained by heterogeneity in patient characteristics, it may result from the treatment itself.
Data availability statement
The datasets generated and/or analyzed during the present study are available in the SEER repository (https://seer.cancer.gov/).
Ethics statement
The studies involving human participants were reviewed and approved by SEER. The ethics committee waived the requirement of written informed consent for participation. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SK: Study conceptualization, design and manuscript writing. WW: Data analysis, and manuscript editing. BL and XF: Data analysis, and manuscript editing. DY: Data analysis: JL: Study conceptualization, design, supervision, and manuscript writing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Miss Zheping Yuan for offering guidance on data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1032063/full#supplementary-material
Abbreviations
SEER, Surveillance, Epidemiology, and End Results; Lum + RT, Lumpectomy plus adjuvant radiation; Mast + NoRT, Mastectomy without radiation; SIR, Standardized incidence ratio; SMR, Standardized mortality ratio; BCR, Breast cancer recurrence; BSD, Breast cancer-specific death; BRFS, Breast cancer recurrence-free survival; BCSS, Breast cancer-specific survival; HR, Hazard ratio; CI, Confidence interval; PSW, Propensity score weighting; NIH, National Institutes of Health; BCT, Breast-conserving therapy; KM, Kaplan-Meier; EBCTCG, Early Breast Cancer Trialists Collaborative Group; DCCPS, National Cancer Institute’s Division of Cancer Control and Population Sciences; ICD, International Classification of Diseases; WHO, World Health Organization; ER, Estrogen receptor; PR, Progesterone receptor; HER2, Human epidermal growth factor receptor 2; AJCC, American Joint Committee on Cancer; O/E, Observed/expected ratio; CIF, Cumulative incidence function; OW, Overlap weight; CI, Cumulative incidence.
References
1. Consensus statement: treatment of early-stage breast cancer. national institutes of health consensus development panel. J Natl Cancer Inst Monogr (1992) 11):1–5.
2. Gilligan MA, Kneusel RT, Hoffmann RG, Greer AL, Nattinger AB. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care (2002) 40(3):181–9. doi: 10.1097/00005650-200203000-00002
3. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol (2009) 27(5):713–9. doi: 10.1200/JCO.2008.17.9234
4. Nelson JA, Rubenstein RN, Haglich K, Chu JJ, Yin S, Stern CS, et al. Analysis of a trend reversal in US lumpectomy rates from 2005 through 2017 using 3 nationwide data sets. JAMA Surg (2022) 157(8):702–11. doi: 10.1001/jamasurg.2022.2065
5. Balch CM, Jacobs LK. Mastectomies on the rise for breast cancer: “the tide is changing”. Ann Surg Oncol (2009) 16(10):2669–72. doi: 10.1245/s10434-009-0634-y
6. Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the united states? J Clin Oncol (2010) 28(21):3437–41. doi: 10.1200/JCO.2009.27.6774
7. Sisco M, Kyrillos AM, Lapin BR, Wang CE, Yao KA. Trends and variation in the use of nipple-sparing mastectomy for breast cancer in the united states. Breast Cancer Res Treat (2016) 160(1):111–20. doi: 10.1007/s10549-016-3975-9
8. Lim DW, Metcalfe KA, Narod SA. Bilateral mastectomy in women with unilateral breast cancer: A review. JAMA Surg (2021) 156(6):569–76. doi: 10.1001/jamasurg.2020.6664
9. Gomez SL, Lichtensztajn D, Kurian AW, Telli ML, Chang ET, Keegan TH, et al. Increasing mastectomy rates for early-stage breast cancer? population-based trends from California. J Clin Oncol (2010) 28(10):e155–7; author reply e8. doi: 10.1200/JCO.2009.26.1032
10. van der Sangen MJ, van de Wiel FM, Poortmans PM, Tjan-Heijnen VC, Nieuwenhuijzen GA, Roumen RM, et al. Are breast conservation and mastectomy equally effective in the treatment of young women with early breast cancer? long-term results of a population-based cohort of 1,451 patients aged </= 40 years. Breast Cancer Res Treat (2011) 127(1):207–15. doi: 10.1007/s10549-010-1110-x
11. Jones HA, Antonini N, Hart AA, Peterse JL, Horiot JC, Collin F, et al. Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol (2009) 27(30):4939–47. doi: 10.1200/JCO.2008.21.5764
12. Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol (2001) 19(6):1688–97. doi: 10.1200/JCO.2001.19.6.1688
13. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European organization for research and treatment of cancer 10801 trial. J Natl Cancer Inst (2000) 92(14):1143–50. doi: 10.1093/jnci/92.14.1143
14. Arriagada R, Le MG, Guinebretiere JM, Dunant A, Rochard F, Tursz T. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol (2003) 14(11):1617–22. doi: 10.1093/annonc/mdg452
15. van Dongen JA, Bartelink H, Fentiman IS, Lerut T, Mignolet F, Olthuis G, et al. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Inst Monogr (1992) 11):15–8.
16. Arriagada R, Le MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. institut gustave-roussy breast cancer group. J Clin Oncol (1996) 14(5):1558–64. doi: 10.1200/JCO.1996.14.5.1558
17. Jacobson JA, Danforth DN, Cowan KH, d’Angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med (1995) 332(14):907–11. doi: 10.1056/NEJM199504063321402
18. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med (2002) 347(16):1233–41. doi: 10.1056/NEJMoa022152
19. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med (2002) 347(16):1227–32. doi: 10.1056/NEJMoa020989
20. Litiere S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Van Limbergen E, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol (2012) 13(4):412–9. doi: 10.1016/S1470-2045(12)70042-6
21. Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer (2013) 119(7):1402–11. doi: 10.1002/cncr.27795
22. Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg (2014) 149(3):267–74. doi: 10.1001/jamasurg.2013.3049
23. Fisher S, Gao H, Yasui Y, Dabbs K, Winget M. Survival in stage I-III breast cancer patients by surgical treatment in a publicly funded health care system. Ann Oncol (2015) 26(6):1161–9. doi: 10.1093/annonc/mdv107
24. Onitilo AA, Engel JM, Stankowski RV, Doi SA. Survival comparisons for breast conserving surgery and mastectomy revisited: Community experience and the role of radiation therapy. Clin Med Res (2015) 13(2):65–73. doi: 10.3121/cmr.2014.1245
25. Hartmann-Johnsen OJ, Karesen R, Schlichting E, Nygard JF. Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: A registry-based follow-up study of Norwegian women primary operated between 1998 and 2008. Ann Surg Oncol (2015) 22(12):3836–45. doi: 10.1245/s10434-015-4441-3
26. Takano S, Omura M, Suzuki R, Tayama Y, Matsui K, Hashimoto H, et al. Intensity-modulated radiation therapy using TomoDirect for postoperative radiation of left-sided breast cancer including lymph node area: comparison with TomoHelical and three-dimensional conformal radiation therapy. J Radiat Res (2019) 60(5):694–704. doi: 10.1093/jrr/rrz052
27. Corradini S, Krug D, Meattini I, Matuschek C, Bolke E, Francolini G, et al. Preoperative radiotherapy: A paradigm shift in the treatment of breast cancer? a review of literature. Crit Rev Oncol Hematol (2019) 141:102–11. doi: 10.1016/j.critrevonc.2019.06.003
28. Haussmann J, Corradini S, Nestle-Kraemling C, Bolke E, Njanang FJD, Tamaskovics B, et al. Recent advances in radiotherapy of breast cancer. Radiat Oncol (2020) 15(1):71. doi: 10.1186/s13014-020-01501-x
29. Pepe MS, Mori M. Kaplan-Meier, Marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med (1993) 12(8):737–51. doi: 10.1002/sim.4780120803
30. Southern DA, Faris PD, Brant R, Galbraith PD, Norris CM, Knudtson ML, et al. Kaplan-Meier Methods yielded misleading results in competing risk scenarios. J Clin Epidemiol (2006) 59(10):1110–4. doi: 10.1016/j.jclinepi.2006.07.002
31. Lacny S, Wilson T, Clement F, Roberts DJ, Faris P, Ghali WA, et al. Kaplan-Meier Survival analysis overestimates cumulative incidence of health-related events in competing risk settings: a meta-analysis. J Clin Epidemiol (2018) 93:25–35. doi: 10.1016/j.jclinepi.2017.10.006
32. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med (2007) 26(11):2389–430. doi: 10.1002/sim.2712
33. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med (1999) 18(6):695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O
34. Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer (2004) 91(7):1229–35. doi: 10.1038/sj.bjc.6602102
35. Saleh RR, Nadler MB, Desnoyers A, Rodin DL, Abdel-Qadir H, Amir E. Influence of competing risks on estimates of recurrence risk and breast cancer-specific mortality in analyses of the early breast cancer trialists collaborative group. Sci Rep (2020) 10(1):4091. doi: 10.1038/s41598-020-61093-0
36. Surveillance E, and End Results (SEER) Program Research Data (1975-2019), National CancerInstitute, DCCPS. Surveillance research program, surveillance systems branch, released April 15, 2022, based on the November 2021 submission . Available at: http://wwwseercancergov (Accessed April, 2022).
37. Byrd DR, Carducci M, Compton C, Fritz A, Greene F. AJCC cancer staging manual. New York, NY: Springer (2010).
38. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat (1988) 16(3):1141–54. doi: 10.1214/aos/1176350951
39. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc (1999) 94(446):496–509. doi: 10.1080/01621459.1999.10474144
40. Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res (2017) 26(4):1654–70. doi: 10.1177/0962280215584401
41. Zhou Y, Matsouaka RA, Thomas L. Propensity score weighting under limited overlap and model misspecification. Stat Methods Med Res (2020) 29(12):3721–56. doi: 10.1177/0962280220940334
42. Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol (2019) 188(1):250–7. doi: 10.1093/aje/kwy201
43. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ (2019) 367:l5657. doi: 10.1136/bmj.l5657
44. Vinh-Hung V, Burzykowski T, Van de Steene J, Storme G, Soete G. Post-surgery radiation in early breast cancer: survival analysis of registry data. Radiother Oncol (2002) 64(3):281–90. doi: 10.1016/S0167-8140(02)00105-6
45. Hofvind S, Holen A, Aas T, Roman M, Sebuodegard S, Akslen LA. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol (2015) 41(10):1417–22. doi: 10.1016/j.ejso.2015.07.002
46. Chen K, Liu J, Zhu L, Su F, Song E, Jacobs LK. Comparative effectiveness study of breast-conserving surgery and mastectomy in the general population: A NCDB analysis. Oncotarget (2015) 6(37):40127–40. doi: 10.18632/oncotarget.5394
47. Chen QX, Wang XX, Lin PY, Zhang J, Li JJ, Song CG, et al. The different outcomes between breast-conserving surgery and mastectomy in triple-negative breast cancer: a population-based study from the SEER 18 database. Oncotarget (2017) 8(3):4773–80. doi: 10.18632/oncotarget.13976
48. van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol (2016) 17(8):1158–70. doi: 10.1016/S1470-2045(16)30067-5
49. De la Cruz Ku G, Karamchandani M, Chambergo-Michilot D, Narvaez-Rojas AR, Jonczyk M, Principe-Meneses FS, et al. Does breast-conserving surgery with radiotherapy have a better survival than mastectomy? a meta-analysis of more than 1,500,000 patients. Ann Surg Oncol (2022) 29(10):6163–88. doi: 10.1245/s10434-022-12133-8
50. de Boniface J, Szulkin R, Johansson ALV. Survival after breast conservation vs mastectomy adjusted for comorbidity and socioeconomic status: A Swedish national 6-year follow-up of 48986 women. JAMA Surg (2021) 156(7):628–37. doi: 10.1001/jamasurg.2021.1438
51. Saifi O, Chahrour MA, Li Z, Hoballah J, Panoff J, Vallow LA, et al. Is breast conservation superior to mastectomy in early stage triple negative breast cancer? Breast (2022) 62:144–51. doi: 10.1016/j.breast.2022.02.006
52. Minicozzi P, Van Eycken L, Molinie F, Innos K, Guevara M, Marcos-Gragera R, et al. Comorbidities, age and period of diagnosis influence treatment and outcomes in early breast cancer. Int J Cancer (2019) 144(9):2118–27. doi: 10.1002/ijc.31974
53. Abdulkarim BS, Cuartero J, Hanson J, Deschenes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol (2011) 29(21):2852–8. doi: 10.1200/JCO.2010.33.4714
54. Corradini S, Reitz D, Pazos M, Schonecker S, Braun M, Harbeck N, et al. Mastectomy or breast-conserving therapy for early breast cancer in real-life clinical practice: Outcome comparison of 7565 cases. Cancers (Basel) (2019) 11(2):160. doi: 10.3390/cancers11020160
55. van Tienhoven G, Voogd AC, Peterse JL, Nielsen M, Andersen KW, Mignolet F, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC breast cancer cooperative group and the Danish breast cancer cooperative group. Eur J Cancer (1999) 35(1):32–8. doi: 10.1016/S0959-8049(98)00301-3
56. Kurtz JM, Amalric R, Brandone H, Ayme Y, Spitalier JM. Local recurrence after breast-conserving surgery and radiotherapy. Helv Chir Acta (1989) 55(6):837–42.
57. Mell LK, Jeong JH. Pitfalls of using composite primary end points in the presence of competing risks. J Clin Oncol (2010) 28(28):4297–9. doi: 10.1200/JCO.2010.30.2802
58. Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc (2018) 113(521):390–400. doi: 10.1080/01621459.2016.1260466
Keywords: breast cancer, recurrence, cancer-specific survival, competing risk, propensity score weighting, SEER
Citation: Ke S, Wang W, Li B, Feng X, Yan D and Liu J (2023) Superior survival for breast-conserving therapy over mastectomy in patients with breast cancer: A population-based SEER database analysis across 30 years. Front. Oncol. 12:1032063. doi: 10.3389/fonc.2022.1032063
Received: 30 August 2022; Accepted: 29 November 2022;
Published: 04 January 2023.
Edited by:
Cynthia Aristei, University of Perugia, ItalyReviewed by:
Paramita Dasgupta, Cancer Council Queensland, AustraliaAngel Montero, HM Madrid Hospital, Spain
Copyright © 2023 Ke, Wang, Li, Feng, Yan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianbo Liu, ZHJsaXVqaWFuYm9AMTYzLmNvbQ==
 Shanbao Ke1
Shanbao Ke1 Jianbo Liu
Jianbo Liu