- 1College of Pharmacy, Heilongjiang University of Chinese Medicine, Heilongjiang, Haerbin, China
- 2Chinese Medicine Research Institute, Heilongjiang University of Chinese Medicine, Heilongjiang, Haerbin, China
The existence of malignant tumors has been a threat to human life, health, and safety. Although the rapid development of radiotherapy, drug therapy, surgery, and local therapy has improved the quality of life of tumor patients, there are still some risks. Natural compounds are widely used in cancer because they are easy to obtain, have a good curative effects and have no obvious side effects, and play a vital role in the prevention and treatment of various cancers. Phenolic, flavonoids, terpenoids, alkaloids, and other natural components of traditional Chinese medicine have certain anti-tumor activities, which can promote apoptosis, anti-proliferation, anti-metastasis, inhibit angiogenesis, change the morphology of cancer cells and regulate immune function, etc., and have positive effects on breast cancer, liver cancer, lung cancer, gastric cancer, rectal cancer and so on. To better understand the effects of natural compounds on cancer, this paper screened out four important pathways closely related to cancer, including cell death and immunogenic cell death, immune cells in the tumor microenvironment, inflammation and related pathways and tumor metastasis, and systematically elaborated the effects of natural compounds on cancer.
1 Introduction
Cancer is a multifaceted disease that is influenced by environmental factors and genetic factors. According to the World Health Organization’s global Cancer report, breast cancer and lung cancer are serious threat to human life and health, and the cases of colorectal cancer, prostate cancer, gastric cancer, and liver cancer are also on the rise. By 2040, the cancer burden is projected to increase by 50%, with nearly 30 million new cancer cases (1). Although there are many ways to treat cancer, the results are not ideal. Some chemotherapy drugs, such as doxorubicin, cisplatin, doxorubicin and even radiotherapy, are commonly used in the treatment of most cancers, which will cause serious adverse reactions and produce a series of toxic side effects. The limitation of radiotherapy is that it will cause memory, learning, and reasoning dysfunction, and make brain function decline in late radiotherapy (2). In addition, chemotherapy-induced secondary tumors and normal tissue damage also pose clinical problems for patients with cancer survivors. In the course of chemotherapy, most chemotherapy drugs can cause bone marrow suppression, resulting in immunosuppression or decline (3). Some chemotherapy drugs can also cause liver damage toxicity, kidney toxicity, cardiotoxicity, and so on. For example, cisplatin can cause nausea and vomiting, acute kidney injury, neurotoxicity, and ototoxicity (4). Even if some cancer cells have very low activity, this means that chemotherapy has little effect and has no effect on overall survival, which may have a significant impact on prognosis.In recent years, natural compounds play a key role in the prevention and treatment of cancer, including phenols (curcumin, quercetin, resveratrol, capsaicin, etc.), flavonoids (quercetin, tanshensin IIa, icariin, etc.), terpenoids (andrographolide, artesunate, atractylodes, etc.), alkaloids (matrine, berberine, piperine, etc.) and other natural components, all of which can be used by anti-inflammatory, Promote cell apoptosis, avoid invasion and metastasis, achieve immune destruction and other markers of tumor occurrence, and can resist lung cancer, breast cancer and ovarian cancer (5). These classic tumor landmark events are manifested as a new environment created by cancer cells through the secretion of various cytokines, namely the tumor microenvironment (TME), in which some innate immune cells have different activation states and thus affect the progression of the tumor (6). Cancer stem cells (CSC) also exist in TME and regulate the self-renewal and drive of tumors (7). There is evidence that chronic inflammation driven by immune cells and related pathways enhances human susceptibility to cancer, and 25% of cancers are associated with inflammation (8). Tumor metastasis is the main cause of cancer death. In the process of tumor development, metastatic tumors spread from the primary site to other sites, aggravating the deterioration of the tumor. Recent studies have shown that RNA plays an important role in tumor metastasis and is involved in almost all human cancers (9). So far, there is no widely accepted optimal treatment for cancer, and it is necessary to find new therapeutic approaches. Therefore, this paper elucidates the therapeutic effects of natural compounds on the tumor from the perspectives of apoptosis and immunogenic cell death, immune cells in the tumor microenvironment, inflammation and related pathways, and tumor metastasis. As shown in Figure 1 and Tab. 1.
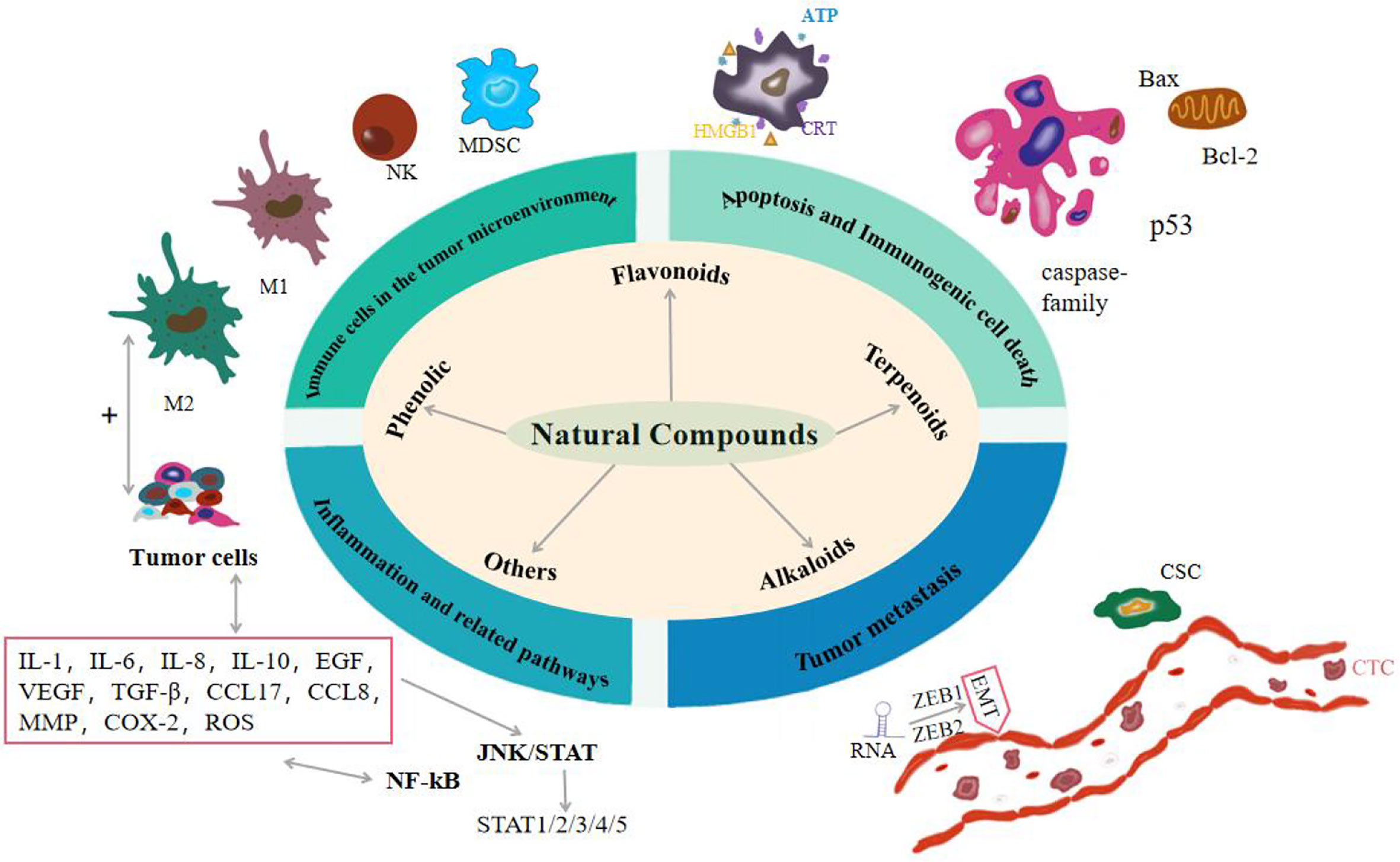
Figure 1 Effects of natural compounds on cancer from four aspects: apoptosis and immunogenic cell death, immune cells in the tumor microenvironment, inflammation and related pathways, and tumor metastasis.
Apoptosis and Immunogenic cell death
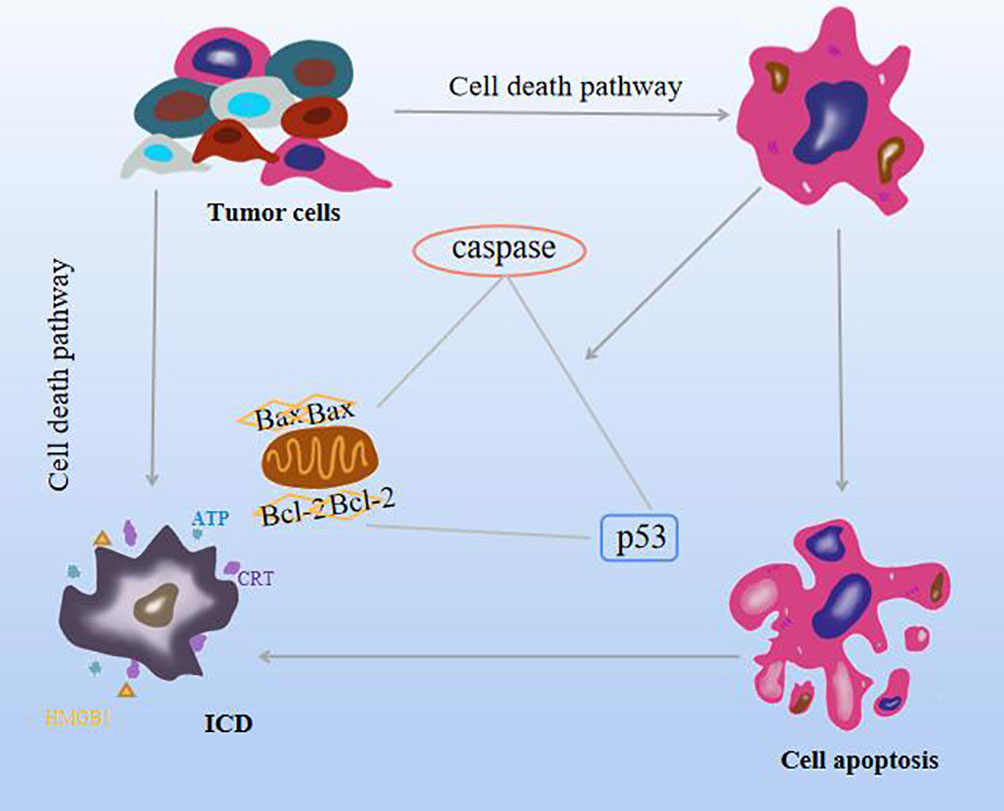
The cell death pathway includes apoptosis, autophagy, iron death, and necrotizing apoptosis. Apoptosis is an important mechanism of tumor inhibition in all stages of cancer progression. Different from cell necrosis, programmed death, which is the autonomous control of cells, is actively expressed for the body to better adapt to the environment. The generation of this mechanism is regarded as an effective therapeutic approach (5). The mechanism of apoptosis is triggered mainly by two pathways. Mitochondria is the first stronghold of apoptosis, and it is the transition of cell membrane permeability characterized by the breakdown of mitochondrial transmembrane potential. This process is caused by the family of pro-apoptotic proteins (Bax) and anti-apoptotic protein (Bcl-2) activity. The second pathway is initiated by members of the tumor death factor (TNF) receptor family, TNF, and Fas (10, 11). In addition, it is found that caspase family can directly induce apoptosis or change Bax, Bcl-2, and intracellular oxidation levels after activation of death signal receptors to promote apoptosis, and the tumor suppressor p53 is involved in the cell cycle. The ability of cell death to trigger an immune response is called immunogenic cell death (ICD), which is the transition from a non-immunogenic agent to an immunogenic agent and is the product of a balanced combination of certain factors and tumor-associated antigens (12). In the tumor microenvironment (TME), ICD can stimulate the antigenic immune response of dead cells, including cancer cells, and has the effect of killing cancer cells and fighting solid tumors while driving autoimmunity (13, 14), not only limited to inhibiting primary tumors but also playing a role in distant or metastatic tumors (15). ICD is induced by the stress effect of the lateral endoplasmic reticulum, with exposure to the release of numerous damage-associated molecular patterns (DAMPs), including calreticulin (CRT), secretion of adenosine triphosphate (ATP), type I interferon (IL-1) and high migration group box 1 (HMGB1), the effects of these processes on tumors have some prognostic value and a powerful adjuvant effect on dying cancer cells (16). In addition, iron death can release various DAMPs, stimulate antigen-antibody response to induce ICD, and enhance anti-tumor immunity (17). Necrotizing apoptosis, like ICD, release TNF-α, IL-6, IL-1β, and other cytokines to induce inflammation (18). As shown in Figure 2.
1.1. Natural compounds
1.1.1. Phenolic
Curcumin, a polyphenolic compound in the plant turmeric, has significant pharmacological activities with anticancer, antimalarial, antioxidant, antimutagenic, antiangiogenic, and anti-inflammatory properties. A newly developed curcumin-like compound (2E,6E)-2,6-bis(2,3-dimethoxy benzyl aniline)cyclohexanone (DMCH) in colon cancer HT29 and SW620 cell lines Analysis of the apoptotic panel of the group showed that Bax protein expression was up-regulated, and it was found that more apoptotic cells were observed in the treated SW620 cell line than in the HT29 cell line (19). Another study showed that total phenolics of grape leaves the increased expression of apoptosis-promoting gene Bax, reduced expression of anti-apoptotic gene BCL, and regulated expression of MCF-7 and human hepatoma cells in human breast cancer cells (20). Curcumin can synergize with metformin to increase apoptosis, cytotoxicity, and Bax gene expression levels, and this positive effect suggests that the combination may be a candidate drug for the treatment of prostate cancer (21). It has also been found that capsaicin can slow tumor growth and act as an ICD inducer in human bladder cancer by inducing the release of CRT and ATP (22).
1.1.2. Flavonoids
Quercetin in bupleurum chinensis, mulberry leaf, and Pseudoacacia chinensis can significantly reduce the expression of the Bax gene in MCD-7 human breast cancer cell lines and reduce the apoptosis index (23), and its combination with propiolactone has anti-tumor immunity, which induces cytotoxicity by inducing ICD. As well as regulating the immunosuppressive TME significantly inhibited the growth of rectal cancer tumors (24). Icariin, as the main component of Icarii, inhibits the proliferation of breast cancer cells and induces apoptosis in concentration and time-dependent, and upregulates the ratio of Bax/Bcl-2 and reactive oxygen species through mitochondrial pathways (25). Modern pharmacological studies have shown that hyperoside has strong analgesia, protection of heart, brain, and liver, anti-myocardial hypoxia injury, and protection of cerebral ischemia injury, etc, one of the flavonoid glycosides, increases the levels of Bcl-2 and cleaved caspase-3 (caspase-3) in breast cancer cell lines and subcutaneous allograft mouse models high, which reduces the level of Bax and induces apoptosis (26). In addition to lowering blood glucose, anti-mutation, and eliminating free radicals in the body, myricetin also has anticancer potential, which prevents cell cycle progression in A549 lung cancer cells, enhances p53 expression and ROS-dependent mitochondria-mediated death, showing certain cytotoxicity (27).
1.1.3. Terpenoids
Ginsenoside Rg3 is a highly active trace component of the Chinese herb ginseng, which plays an anti-tumor role in many cancer models, such as lung, liver, and breast cancer (28). Compared with monotherapy, administration of Rg3 via the hepatic artery combined with local transarterial embolization can more significantly inhibit liver tumor growth and induce caspase-dependent apoptosis (29). Betulinic acid is an extract of Betula bark, and it also exists in traditional Chinese medicine such as jujube kernel and prunella. It has anti-inflammatory and antiviral activities and is most famous for its anti-tumor activity. In ovarian cancer cell line A2780 treated with this treatment, the percentage of apoptotic cells and nuclear condensation was increased in a concentration-dependent manner, and the expressions of caspase 8, caspase 3, caspase 9, and Bax were increased, while the expression of Bcl-2 was decreased. It was demonstrated that betulinic acid could induce apoptosis of ovarian cancer cells through mitochondrial pathways and other independent pathways (30). Gemmanone, one of the natural compounds in the traditional Chinese medicine Radix Curcumae, inhibited the proliferation of gastric cancer cell line BGC823 by inducing cell cycle arrest in the G2/M phase and promoting apoptosis as well as mitochondria-mediated apoptosis (31). Paclitaxel induces CRT exposure, ATP secretion, and HMB1 release to induce ICD production in ovarian cancer (32). Atractylodes ketone derived from atractylodes atractylodes or atractylodes atractylodes, is widely used for liver protection, antibacterial, and antiviral, can reduce mitochondrial membrane potential, increase ROS level, inhibit the expression of Bcl-2, promote the expression of Bax and caspase-3 to induce hepatocellular carcinoma apoptosis, and inhibit EMT to inhibit the metastasis and invasion of hepatocellular carcinoma (33).
1.1.4. Alkaloids
Both oxymatrine and matrine are derived from the Chinese herb Sophora flavescens, which are bactericidal, anti-inflammatory, heat-clearing, and moistening. By detecting cell apoptosis, it was found that oxymatrine can enhance the expression of cleaved asparaginase 3 and Bax, and attenuate the expression of Bcl-2, which further leads to the increase of apoptosis, indicating that oxymatrine can increase the vulvar scale. Antitumor effect of vulva squamous cell carcinoma(VSCC)cells (34). Similarly, matrine not only reduces the expression level of Bcl-2 and increases the expression of caspase-8, but also inhibits the viability, and migration and induces apoptosis of ovarian cancer cells by up-regulating the p38MAPK and JNK pathways (35). Chelidonine of Chelidonium, a plant in the poppy family, is well known for its anti-tumor and analgesic effects, despite its toxicity, and is the most useful. It increased the expression of p53 and cleaved caspase 3 protein in BxPC-3 and MIA PaCa-2 pancreatic cancer cell lines and induced apoptosis of pancreatic cancer cells (36). In human lung cancer cell lines A549 and H1299, evodiamine has the effects of stomach-strengthening, analgesic, and anti-vomiting, and also has certain efficacy in the treatment of early senile dementia and stroke. It effectively increased mitochondrial membrane depolarization, increased Bax/Bcl-2 ratio, and promoted lung cancer cell apoptosis independently of the p53 pathway (37).
1.1.5. Others
Plumbagin has anti-diabetic properties and has a powerful anti-cancer effect on many types of cancer. Plumbagin and Dihydrotanshinone I are ICD inducers of liver cancer cells. Studies have shown that the growth of liver cancer tumors can be significantly inhibited after being sequestered with nanoparticles of polylactic acid-coglycolic acid (38). Alternol, a mutagenic strain of yew tree bark, induces ICD in prostate cancer and releases CRT, ATP, and a series of pro-inflammatory factors leading to delayed tumor growth and prolonged survival (39). Hinesol, a compound extracted from Atractylodes Rhizoma application in the study of non-small cell lung cancer, induces proliferation, inhibition, and apoptosis in non-small cell lung cancer cell lines by up-regulating Bax and inhibiting the expression of Bcl-2 (40). Atractylenolide III is a kind of anti-inflammatory and anti-tumor active substance, that induces apoptosis of human colon cancer HCT-116 cells by promoting apoptosis-related genes, regulating the Bax/Bcl-2 apoptosis signaling pathway, and the expression of caspase-3 and p53 (41).
2. Immune cells in the TME
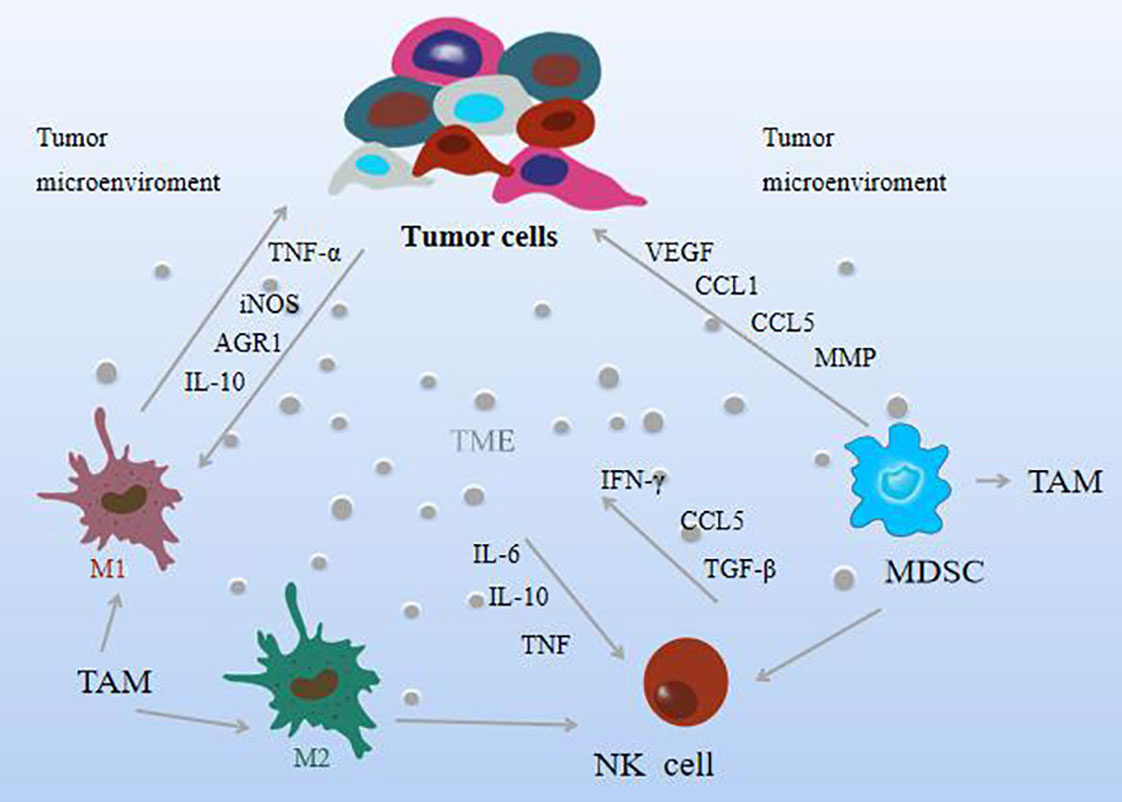
The TME refers to the complex environment in which tumor cells are located, which is composed of some immune cells and extracellular components. Immune cells serve as an important component of the tumor stroma, including macrophages, natural killer (NK)cells, and myeloid-derived suppressor cells, called innate immune cells, that mediate immune tolerance or elicit tumor-targeted immune responses. The environment in which these cells in the TME are located is defined as the tumor immune microenvironment (TIME) (42), and the activation status of immune cells in TIME may vary (43, 44). Interactions between tumor cells and immune cells lead to the formation of an environment that promotes tumor growth and metastasis and generally exerts tumorigenic effects by stimulating uncontrolled cell proliferation before carcinogenesis. It is widely recognized today that TME plays an essential role in tumorigenesis and malignant progression. Tumor-associated macrophages (TAM) are macrophages located in tumor microenvironments. Numerous studies have shown that macrophages are key mediators of tissue homeostasis, which can regulate the degree of tumor growth and enable tumor necrosis factor (TNF-α) level and high expression of nitric oxide synthase (iNOS), which can inhibit tumor growth; make the pro-inflammatory factors interleukin-10 (IL-10) and arginase-1 (AGR1) play a role in promoting role in tumor growth (44). TAM can be divided into two forms, M1 and M2, according to the polarization state, and in TAM, the M1 subtype has a tumor-suppressing effect, while the M2 subtype plays a tumor-promoting role (45). Moreover, specific anti-tumor immunity and inhibition of tumor metastasis are crucial for the process of M2-to-M1-type transformation (46). When the ratio of M1/M2 is low, angiogenesis is inhibited, immune function is enhanced, and tumor invasion and metastasis are also inhibited.
NK cells are the frontier cells for the development of tumor therapy. They have cytotoxic activity against a variety of tumor cells, can recognize and kill tumor cells, and are considered to be key effectors in cancer immune detection, transplant rejection, or early viral immunity. Cytokines such as IL-6, IL-10, and TGF-β produced by tumor cells in the TME hinder the activation of NK cells (47). As the main effector cells of cancer, NK cells are highly heterogeneous and can be processed in a different way to kill tumor cells. Its activation is driven by the balance between activating and inhibitory signals, and it can generate anti-tumor responses without sensitization. By interacting with tumor cells or extracellular matrix, it can achieve anti-tumor immunity and control tumor growth (48, 49). It produces a series of chemokines and cytokines that regulate immunity, including IFN-γ, TNF, IL-6, and CCL5, which have the function of regulating immune response and anti-tumor and are related to the improvement of the overall survival rate of patients (50). It has also been found that reduced mitochondrial mass or increased ROS production is an important limitation of NK cell function in the TME (51). And when immune cells remove immunogenic malignant cells, they shape tumors and select aggressive variants, preferentially selecting clones that produce mutations that make them resistant to immunity, a process known as “cancer immunity” “Edit” hinders NK cells from exerting tumor eradication effect (52). The activation of NK cells is not only inhibited by TAMs, but also by myeloid-derived suppressor cells (MDSCs), which are heterogeneous cells derived from the bone marrow with potent immunosuppressive activity that can migrate to peripheral lymphoid organs or tumors, promote the formation of TME. It is divided into two subtypes: monocyte MDSC (M-MDSC) and granulocyte polymorphonuclear MDSC (PMN-MDSC), both of which can act on CSC. When they enter the TME, most M-MDSCs differentiate into immunosuppressive TAMs, but this process is impaired by the mediation of inflammation, a typical manifestation of tumor progression (53, 54). M-MDSCs are recruited to tumors via a CCL1, CCL5-induced chemokine cascade that is disseminated by tumor cells, and CCL3 produced by TAMs is retained in the primary tumor (55, 56). In addition, MDSCs can promote vascular re-formation by producing VEGF, and promote tumor invasion and metastasis by producing matrix metalloproteinases (MMP), thereby mediating immune or non-immune mechanisms to promote tumor growth and development (57). Furthermore, it was found to have certain associations with macrophages and NK cells, mainly by isolating an amino acid, cysteine, which is essential for T cell activation, MDSC polarizes macrophages to a tumor-promoting phenotype, and also Transfers macrophages to an M2 phenotype with immunosuppressive features and low IL-12 production (58), while inhibiting NK-mediated tumor cell lysis (59–61), production of the immunosuppressive cytokines IL-10 and TGF -β affects NK cell function. Helper cells in the TME that contribute to tumor acquisition of a phenotype are thought to be immune to genetic instability and mutational reprogramming to enhance tumor-promoting activity. Instead, these cancer-associated fibroblasts, immune cells, endothelial cells, and pericytes of the tumor vasculature are hypothesized to undergo epigenetic reprogramming after being recruited by soluble and physical factors of the solid TME. It can be expected that the multi-omics analysis techniques currently applied to cancer cells will be increasingly applied to helper (stromal) cells in tumors to elucidate how normal cells are altered to become functionally supportive of tumor development and progression. As shown in Figure 3.
2.1. Natural compounds
2.1.1. Phenolic
Resveratrol, mainly derived from knotweed, has anti-aging, cardiovascular disease prevention, immune regulation, and anti-tumor effects, and exerts antitumor effects by regulating M2 macrophages and inhibiting vascular endothelial cell-induced migration and invasion (62). Dan Liu et al. observed that curcumin reduced MDSC in lung tumor tissue by inhibiting tumor growth in Lewis lung cancer genes, and promoted MDSC maturation and differentiation, down-regulating reactive oxygen species and IL-6 (63). The extract of Goji berries has certain immunomodulatory functions, among which phenolic substances have shown effective antioxidant, anti-diabetes, regulation of intestinal flora, and anti-cancer effects (64) improve the vitality and proliferation of NK cells, and have a certain ability to recognize and eliminate colon cancer cells (65). Green tea polyphenol EGCG has antibacterial, antiviral, antioxidant, and anticancer pharmacological activities, and can inhibit the accumulation of MDSC to inhibit the growth of breast cancer cells (66). Chlorogenic acid, an antibacterial and antiviral component in honeysuckle and Eucommia ulmoides, can reduce the growth of glioma by transforming macrophages from M2-type to M1-type markers (67).
2.1.2. Flavonoids
Baicalein induces the transformation of macrophages from M2 type to M1 type and increases the number of M1 phenotypes, and at the same time reduces the tendency of TGF-β to promote tumor expression, thereby inhibiting the activity of breast cancer cells (68). In addition, it can initiate cell cycle arrest and inhibit metastasis through cell apoptosis to play an anticancer role (69). Icariin and its derivatives reduced the proportion of MDSCs in tumor-bearing mice, and also reduced the formation of NO and ROS, delaying tumor development (70).
2.1.3. Terpenoids
More and more evidence shows that ginsenoside-Rh2 is an effective therapeutic drug for improving lung cancer. The mechanism is that ginsenoside-Rh2 can effectively regulate the differentiation of the M2 subtype of macrophages into the M1 subtype, and relieve the blood vessels in lung cancer cells. expression of factors associated with generation and invasion (71). Astragaloside has anti-inflammatory, immune regulation, antiviral and anti-tumor activities, and has a wide range of clinical applications. A study of astragaloside III and the photodynamic therapy (PDT) agent Chloro-e6 combined with immunotherapy in the treatment of colon cancer showed that the combination of the two effectively activated NK cells and inhibited tumor cell proliferation (72). Artemisinin has significant antitumor activity, and studies have shown that artemisinin can reduce the growth of breast cancer in antiretroviral therapy dependent on the inhibition of MDSC and prolong the survival period (73). Another study found that andrographolide sulfonate can inhibit 5-fluorouracil (5-FU)-induced MDSC, synergistically enhance the anti-tumor effect and improve tumor immunity, to solve the problem of 5-FU resistance in the treatment of colorectal cancer. Question (74).
2.1.4. Alkaloids
Ailanthus altissima has the function of clearing heat and drying dampness, retracting the astringent stop zone, and stopping bleeding. The alkaloid 9-hydroxyferimidone isolated from the stem bark of Ailanthus altissima has certain cytotoxicity and can inhibit M2 phenotype markers and inflammation such as MMP and VEGF in macrophages developed under ovarian cancer conditions factor (75). Chelandrubine effectively reduces the in vivo survival time of Dalton lymphoma-bearing mice and enhances the function of tumor-associated NK cells (76).
2.1.5. Others
Polysaccharide is a kind of natural polymer that is formed by aldose or ketolose through a glycosidic bond. Polysaccharides can regulate the immune system, inhibit tumors, delay aging, resist fatigue, reduce blood sugar, and so on.Through quantitative RT-PCR, cell migration assay, and immunofluorescence staining to study the biological activity of double-strand starch, it was found that endophytic exopolysaccharide of plant Codonopsis pilosula can activate macrophages to inhibit the proliferation and migration of cancer cells (77). Ganoderma lucidum polysaccharide is one of the active components of Ganoderma lucidum. Its inhibition of rat glioma tumor-bearing is by enhancing the activity of NK cells and T cells, and increasing the concentration of serum interleukin-2 (IL-2) and TNF-α. Produces antitumor and immunomodulatory effects (78). Salvia miltiorrhiza polysaccharide induces enhanced immunity in gastric cancer rats by enhancing the killing activity of NK cells, respectively promoting and inhibiting the production of anti-inflammatory cytokines IL-2, IL-4, and pro-inflammatory cytokines IL-6 and TNF-α (79). Astragalus polysaccharide can reduce the number of MDSCs and the expression of MDSC-related Arg-1 and TGF-β, thereby controlling tumor growth in melanoma-bearing mice (80).
3. Inflammation and related pathways
Inflammation, a critical node in cancer development, is marked by the activation of most of the core cellular and molecular capabilities required for tumorigenesis. During the early stages of inflammation, immune cells recognize pathogen-associated molecular patterns and pro-inflammatory cytokines, and chemokines are activated, enhancing the tumor immune response (81). Chronic inflammation and cancer restrict each other, and the ROS generated by inflammation lead to genetic instability, induces DNA damage, and promote the malignant transformation of cancer. Inflammation promotes the development of cancer. Cancer can easily cause inflammation. After chemotherapy or drug treatment, the immune system of the body is damaged, and various pathogens are easy to cause inflammation. This is mainly related to the release of inflammatory mediators, the persistent activation of inflammatory oncogenes in inflamed tissues, with the appearance of persistent aberrant cell replication and proliferation, angiogenesis, metastasis, and suppression of innate immune responses (82, 83). Inflammatory factors can come from different pathways, some from tumor cells and some from immune cell mediators. The former includes TNF-α, cytokines, chemokines, and growth factors, and the latter includes M2-type TAM, lymphocytes, dendritic cells, neutrophils, etc. It is activated in the environment to interfere with the development and metastasis of tumor cells (84, 85). Among them IL-1, IL-6, epidermal growth factor (EGF) help tumor cells survive, VEGF and IL-8 promote angiogenesis, M2 macrophages, and MDSCs antagonize inflammatory responses, IL-10, TGF-β, C-C motif chemokine ligand (CCL17) inhibits tumor immune function, and TGF-β and MMP promote tumor metastasis (86, 87). In addition, cyclooxygenase-2 (COX-2), ROS, thromboxane, and inflammasome are also involved in the progression of inflammation. Oncogenes continue to develop in this environment. Based on this, targeting inflammation is an important approachtor anti-tumor therapy. In addition, NF-kB and STAT3 are also common factors in tumor severity, and in the process of inflammation discovery, TNF-α and IL-1 were found to promote the pro-inflammatory phenotype of endothelial cells and fibroblasts by activating the NF-kB pathway (88, 89). NF-kB is involved in the immune inflammatory response, cell growth and apoptosis, and tumor development. The NF-kB signaling pathway is activated by some extracellular pro-inflammatory factors, such as TNF-α, IL-6, IL-2, IL-12, COX-2, and some chemokines. In turn, the expression of these signaling factors increases due to the activation of the NF-kB signaling pathway, and the cycle repeats, promoting the initiation of inflammatory responses (90). The NF-kB pathway has also been shown to be activated by a few members of the TNF receptor family. It has also been found that the innate immune response expressed by immune cells such as macrophage immune cells activates NF-kB, resulting in the production of pathogen-associated molecular patterns (PAMPs) and DAMPs that re-induce the expression of pro-inflammatory factors (91). Canonical NF-kB also regulates VEGF, and CCL8 promotes angiogenesis and promotes tumor invasion. In addition to this, NF-kB also promotes cancer progression by influencing MMP to control epithelial-mesenchymal metastasis (92). The Janus kinase (JAK)signal transducer and activator of transcription (STAT) is an important signaling pathway involved in hematopoiesis, cell proliferation, differentiation, angiogenesis, and apoptosis, as well as immune regulation and tumor development. JAK and STAT were thought to be associated with malignancies as early as the 1990s (93). JAK-mediated phosphorylation activates STAT expression. There are seven proteins in the STAT family, among which STAT1 and STAT2 play an important role in anti-tumor immune response, STAT3 and STAT5 are related to tumorigenesis, especially STAT3 is closely related to cancer cell survival, immunosuppression, and persistent inflammation (94). Regulates the expression of multiple genes in response to cellular stimuli, helping cancer cells to survive, proliferate, and progress, and, like NF-kB, has been identified as a key factor in the communication between inflammation and tumors (95). There is evidence that STAT4 knockout mice can both enable IL-2-induced interferon (IFN-γ) cell proliferation and increased NK cytotoxicity (96). STAT3 is a responsive factor activated by IL-6, which significantly increases the activity of NF-kB by enhancing its acetylation. Cytokines produced by immune T cells can activate STAT3 in tumor tissues and affect tumorigenesis (97). Although STAT showed a certain cancer-promoting effect, it also had a certain protective effect on tumors. The elimination of tumor immune response is closely related to interferon, during which immune response destroys malignant tumor cells, and INF is mostly mediated by STAT1 (98, 99). As shown in Figure 4.
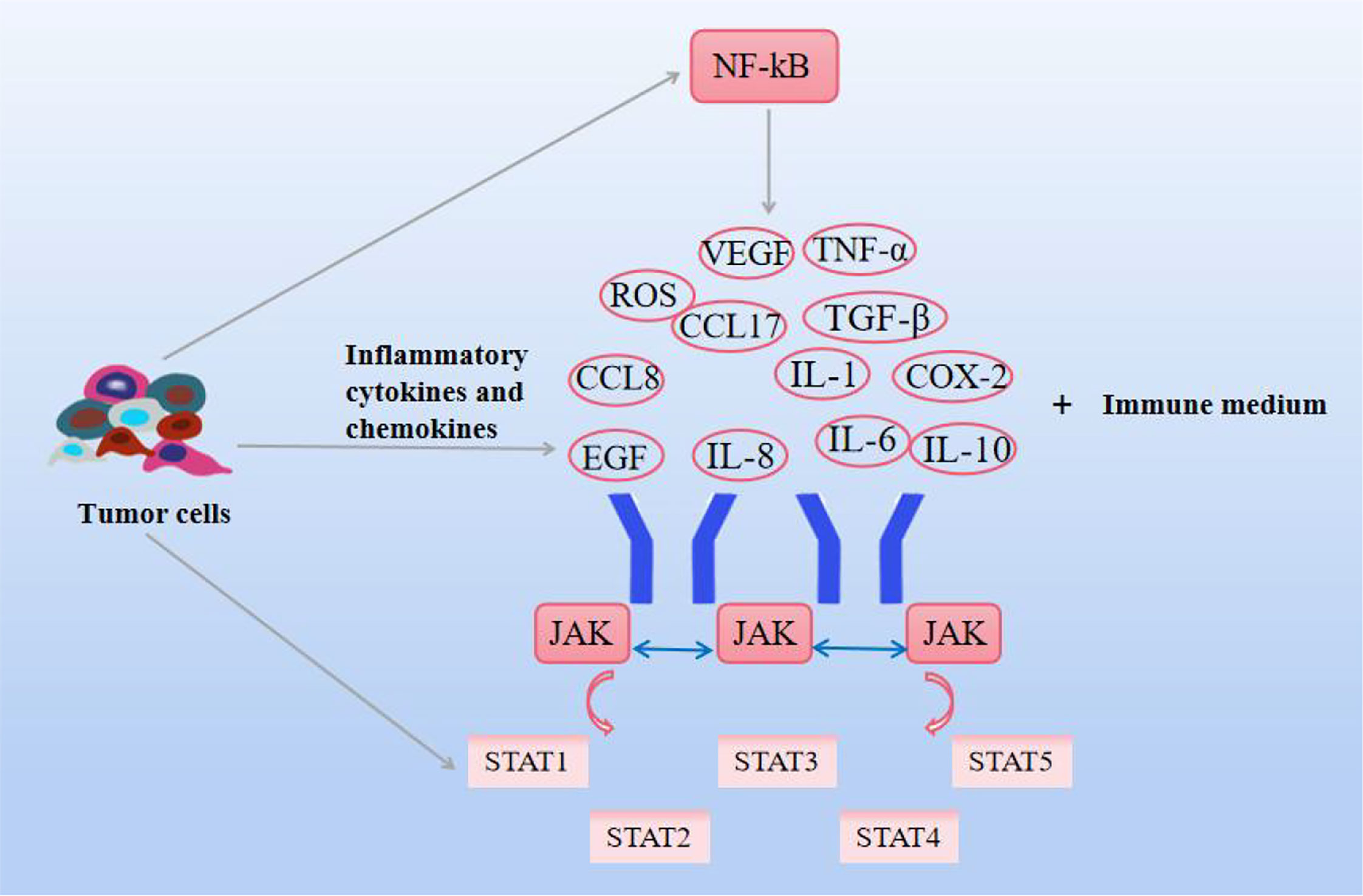
Figure 4 Effects of natural compounds on inflammation and related pathways. Inflammatory cytokines and chemokines themselves, as well as JAK-STAT and NF-KB signaling pathways, interact to promote cancer cell growth.
3.1. Natural compounds
3.1.1. Phenolic
Resveratrol showed a dose-dependent inhibition of TGF-β1-induced EMT-induced cell migration in the human MDA231 breast cancer cell line, decreased the expression levels of MMP-2 and MMP-9, and effectively inhibited MDA231 human breast cancer lung metastasis of cancer (100). Similarly, resveratrol inhibited STAT3 inactivation during M2 polarization of macrophages in mouse lung cancer xenografts (101), in addition, resveratrol effectively inhibited lung metastasis of breast cancer, which effectively Inactivation of STAT3 blocks the function of regulatory B cells and blocks the production of TGF-β (102). As an active compound in green tea, gallocatechin gallate (EGCG) has antioxidant and anti-inflammatory properties and inhibits the activity of STAT3, which can inhibit the growth and migration of pancreatic cancer cells by interfering with the STAT3 signaling pathway (95). Notably, curcumin can inhibit the proliferation and invasion of cervical cancer cells by impairing the NF-kB signaling pathway (103), and its derivatives inhibit STAT3 which in turn inhibits breast tumor cell growth, angiogenesis, and metastasis, and induces TAM by M2-type transformation to M1-type, to achieve anti-tumor effect (104). Capsaicin promotes the anti-proliferative ability of breast cancer tissue by inhibiting the NF-kB pathway mediated by the oncogene FBI-1 and induces tissue cell apoptosis at the same time (105).
3.1.2. Flavonoids
Quercetin has a significant antitumor effect, inhibiting the proliferation of colon cancer by regulating the formation of ROS in a dose-dependent manner and attenuating the infiltration and hyperproliferation of inflammatory cells induced by DMH (106). Scutellarin can down-regulate the Wnt/β-cyclone in the signaling pathway and decrease the expression of TNF-α and IL-6 in serum, improving colitis-related colorectal cancer (107). Tanshinone IIA, the active ingredient of Salvia miltiorrhiza, inhibits cell proliferation in human colon cancer cell lines by targeting TGF-β1, reducing the expression of VEGF, and inhibiting tumor growth and angiogenesis (108). The activity of chronic lymphocytic leukemia cells can be reversed by wogonin-mediated TNF-α via the transfer of T-cell leukemia cells and inhibits TNF-α-induced NF-kB pathway activity (109). Studies have shown that EGCG, the main component of catechin, inhibits programmed cell death ligand 1 (PD-L1) in lung cancer cells induced by interferon (INF-γ) and EGF, even JAK2/STAT1 expression (110).
3.1.3. Terpenoids
VEGF, as a vascular endothelial growth factor, can stimulate tumor angiogenesis, and andrographolide, As a kind of diterpenoid lactone, Andrographolide is good for the gallbladder, protects the liver, regulates the body’s immunity, and is used in meningitis, pneumonia, upper respiratory tract infection and has anti-tumor effect, inhibits angiogenesis through the VEGF pathway and inhibits the expression of COX-2 at the protein and mRNA levels. expression, showed a significant antitumor effect from the inflammatory pathway, thereby significantly inhibiting breast cancer proliferation (111). β-Elemene is an effective component extracted from the ambulatorium of the ginger plant. It can inhibit cell proliferation, induce cell apoptosis and play an anti-angiogenesis and metastasis role to achieve an inhibitory effect on lung cancer, brain cancer, breast cancer,and ovarian cancer (112). It was found that it has a certain effect on non-small cell lung cancer, mainly by activating the NF-kB/iNOS signaling pathway. And decreased the expression of EMT and CSC markers (113). Artesunate is suitable for the rescue of cerebral malaria and all kinds of severe and critical malaria, can promote nitrosodiethylamine-mediated up-regulation of IL-6, JAK-2, and STAT3 expression, and down-regulation of caspase-3 expression, inducing the production of anti-tumor effect of liver cancer cells (114). Atractylodesin inhibited STAT1/3 protein phosphorylation in a dose-dependent manner and moderately inhibited the expression of NF-kB protein in cholangiocarcinoma-related cell lines (115).
3.1.4. Alkaloids
Recent studies have shown that matrine has a protective effect on rectal cancer by reducing the expression levels of IL-6 and TNF-α (116). Berberine, also known as berberine, has certain antioxidant and anti-inflammatory effects. Studies have found that berberine can inhibit the migration of cells in breast cancer cell lines in scratch injury, and also inhibit the expression of TNF-α and IL-6. Increased expression interferes with breast cancer progression (117). Another study found that Crinamine, an alkaloid of Amaryllidaceae, achieved anti-angiogenesis by inhibiting the secretion of VEGF in cervical cancer cells and also inhibited the migration of cervical cancer cells by inhibiting EMT (118). Sanguinarine inhibits cell proliferation and induces apoptosis by down-regulating JAK/STAT signaling pathway in non-small cell lung cancer, silencing STAT3 expression in non-small cell lung cancer, and further increasing Bax/Bcl-2 to promote cysteine The activity of winter enzyme can inhibit tumor (119).
4. Tumor metastasis
Tumor metastasis is also the deadliest feature of cancer progression, with several molecular pathways coordinating biological cellular events in the metastatic cascade. The following events mainly occur during the metastatic process. Specifically, particular epithelial-derived tumor cells are required to be aggressive and migratory due to cell-to-cell junctions, extracellular matrix (ECM) contacts, and loss of normal epithelial polarity, promoting epithelialization. Derived tumor cells migrate and colonize distant organs and form metastases, a process known as epithelial-mesenchymal transition (EMT) (120). As a key step in the early stage of cancer metastasis, it is associated with invasion, recurrence, and cancer resistance to therapy. Plasma-epithelial transition (MET) is the reversal of EMT and is also evident in the process of tumor metastasis (121). Tumor cells also further invade the surrounding stroma or ECM, survive in the blood circulation, and hematogenous spread is considered to be the predominant form of metastasis to distant organs, infiltrating the blood and into the lymphatic circulatory system. Cancer cells in the blood circulation are called circulating tumor cells (CTCs) (122, 123). Although CTCs originate from tumor cells, they have EMT-transforming properties. In a prospective study of 39 patients with invasive breast cancer, most of the heterogeneous CTC phenotypes exhibited EMT plasticity (124). Finally, it aggregates into complexes with platelets, adheres distal to the primary tumor, extravasates through vascular endothelial cells and leaves the circulatory system, and colonizes and proliferates in new tissue sites to form secondary tumors. Evidence suggests that EMT is regulated by transcription factors microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), mainly performed by the SNAIL, TWIST, and ZEB families (120), which in turn interact with components in the TME, Affects the EMT process (125). In addition to this, miRNA can be involved in various stages of cell development, adhesion, differentiation, EMT, apoptosis, and metabolism, and its abnormal expression can disrupt many signaling pathways and trigger cancer cell metastasis, mainly by inhibiting tumor cell proliferation or reducing invasion, promote tumor cell apoptosis (126–128). H119 is a cancer marker and is associated with the formation of various cancers, and inhibiting the expression of H119 can inhibit cancer development (129, 130). Studies have shown that H19 is highly expressed in lung cancer tissues and cell lines, promotes lung cancer cell growth, migration, and invasion, and upregulates the expression of ZEB1 and ZEB2 to further promote EMT. Another study reported that the high expression of lncRNA HAGLR in NSCLC patients was positively correlated with the high detection rate of CTCs (131). RNA is involved in cancer metastasis and progression by affecting EMT, CTC, or as a predictive biomarker. Besides, it is found that in the early stage of metastasis, cancer cells and normal stem cells have similar gene expression patterns, and CSC is also the main factor leading to cancer cell metastasis. The metastasis of cancer cells starts from the most characteristic stem cells and is also transformed from EMT with non-stem cell characteristics to cells with stem cell characteristics (132). EMT induced by different factors also greatly increased the levels of stem cell-related genes, leading to cancer cell metastasis. Not only that, some studies reported that the transcription factors, Oct-4 and Nanog in CSC can positively regulate the EMT and metastasis process of breast cancer patients, Nanog induces squamous cell carcinoma metastasis through EMT-related promoters such as ZEB1 and ZEB2 (133, 134). Furthermore, there are views that bone marrow mesenchymal stem cells may become CSCs and trigger cancer metastasis due to their ability to promote survival, migration, and differentiation (135). Notably, inflammatory cytokines such as interferon, TNF, IL-6, and IL-17 can play a role in the induction of CSCs. Currently, a series of cell surface markers, including CD44, CD177, CD133, CD29, and EpCAM, are used to identify breast cancer, lung cancer, prostate cancer, etc. As shown in Figure 5.
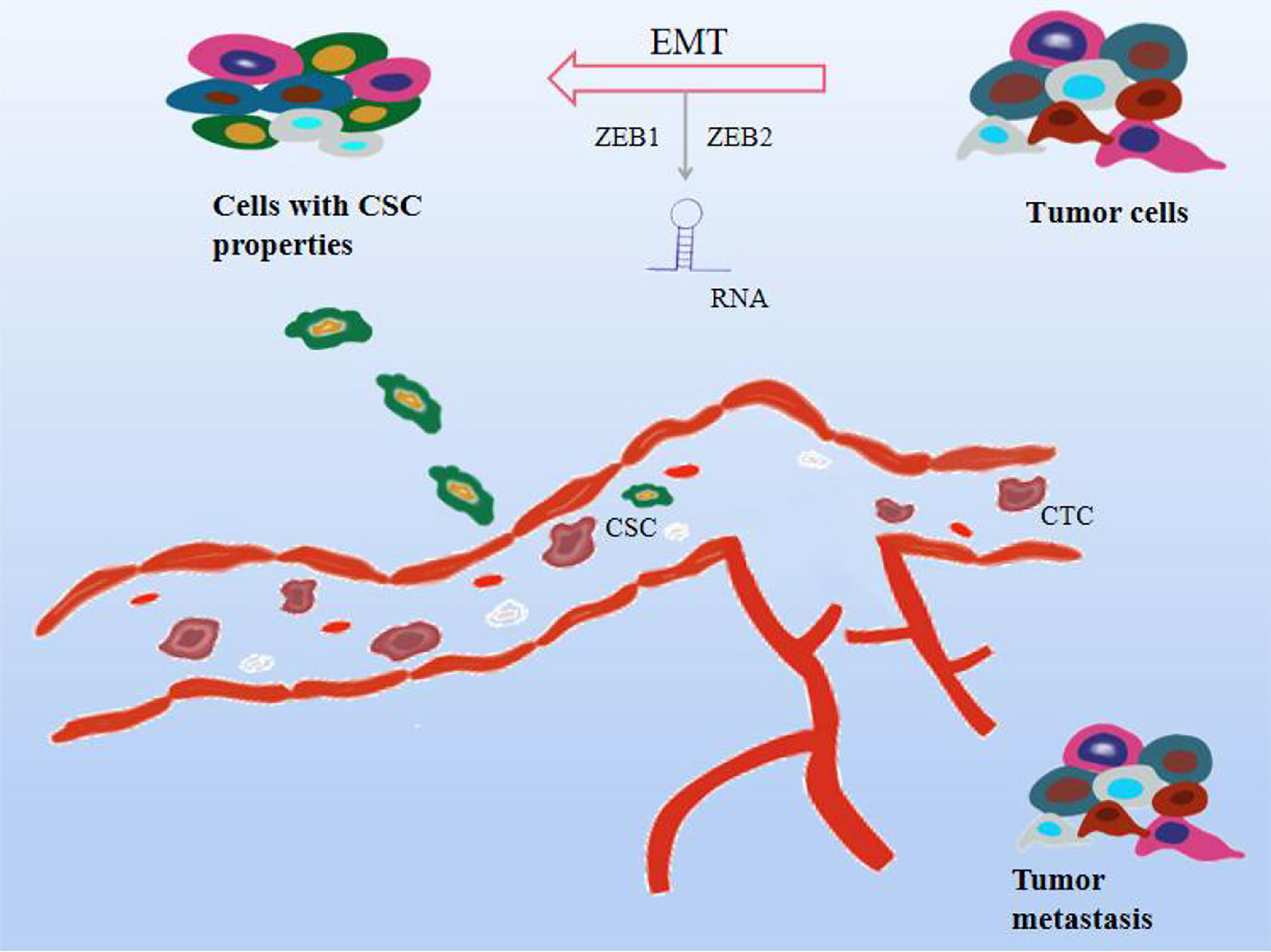
Figure 5 Effect mechanism of natural compounds on tumor metastasis. Cancer cells can proliferate indefinitely, lose their original cell function, and migrate easily. As the blood circulates, they cause cancer to metastasize. Natural compounds can inhibit tumor metastasis and exert anticancer effects through a variety of mechanisms, including 1. Epithelial-mesenchymal transition (EMT); 2. Circulating tumor cells (CTC); 3. Cancer stem cells (CSC) 4.RNA regulation.
4.1. Natural compounds
4.1.1. Phenolics
One of the potential markers of stage 1 non-small cell lung cancer is the lncRNA MALAT1, which can be significantly down-regulated after resveratrol treatment, suggesting that resveratrol may be an entry point for the treatment of non-small cell carcinoma (136). Pterostilbene and resveratrol can reverse the miRNA-mediated regulation of oncogenes in prostate cancer, resulting in the reduction of tumor genes in vivo (137). miR-141 is a specific tumor suppressor miRNA, ZEB2 is a target of miR-141 and is an important regulator in EMT and CSC, and honokiol, a natural product of magnolia plants, mainly used to eliminate chest and abdominal congestion, calm the central nervous system, anti-inflammatory and antibacterial, can be mediated by miR-141. The miR-141/ZEB2 axis produces anti-tumor effects and regulates EMT and CSC to significantly inhibit renal cell carcinoma (138). Gingerol can increase the number of apoptotic cells, resulting in a decrease in primary tumor volume and CTC number, and enhance activity against triple-negative breast cancer (139). Curcumin is mainly derived from the ginger plant, which has antioxidant, anti-inflammatory, and anti-angiogenic effects. The inhibitory effect on breast tumor cells is associated with anti-cancer stem cells and EMT processes (140). Another study reported that resveratrol effectively reduced the self-renewal of CSCs and inhibited the expression of CD133+347, suggesting that targeting CSCs could be helpful in the treatment of ovarian cancer (141). Polyphenols extracted from Artemisia annua can inhibit the phenotype of CSC, mediate the phosphorylation of MMP-9 and STAT3, and exhibit anticancer effects in human breast cancer cells (142).
4.1.2. Flavonoids
Luteolin, a natural herbal flavonoid, can reduce inflammation, anti-allergy, anti-tumor, and antiviral mainly used to reduce blood fat and cholesterol, significantly reversed EMT and inactivated the AKT/mTOR signaling pathway leading to metastasis or proliferation of androgen receptor-positive TNBC (143). Silibinin has the effect of soothing the liver and relieving depression, clearing heat and detoxifying, enhancing the gallbladder, dispelling dampness, and a variety of anti-tumor activities, can eliminate CSC and reduce EMT by reducing the expression of N-cadherin and reducing EMT-related markers to prevent rectal cancer (144), and can also affect tumors through the JAK2/STAT3 pathway the ability of the tissue to migrate and invade (145). Isoliquiritigenin has a good effect on anti-tumor and AIDS and inhibits angiogenic TGF-β and VEGF signaling through miR-194-5p and lncRNA NEAT1, providing a new therapeutic strategy for human glioma (146). The nucleoside erythroid-2-related factor 2 (Nrf2), a nuclear factor that maintains CSC survival and anti-stress, can be regulated by Brusatol from the mature seeds of brucei Chinensis and the extract of brucei japonica can inhibit the surface marker CD133 of CSC in hepatoma cells and EpCAM expression (147, 148). Quercetin has anticancer properties in vitro and in vivo. In pancreatic cancer cells, quercetin can inhibit the expression of pancreatic cancer stem cell surface markers CD24 and CD133, and also induce its differentiation through β-cyclonectin (149, 150). Isoliquiritigenin inhibits the invasion, metastasis, and growth of oral squamous cell carcinoma and reduces the expression of CSC markers such as ALDH1 and CD44 (151).
4.1.3. Terpenoids
Ursolic acid has sedative, anti-inflammatory, antibacterial, and other biological functions. In recent years, ursolic acid has been found to have anti-cancer, pro-differentiation, differentiation induction, anti-angiogenesis, and other effects, which is expected to become a low-toxicity and efficient anti-cancer drug. Not only inhibits the expression of Bcl-2, increases the expression of Bax, and induces caspase-dependent apoptosis, but also inhibits EMT and affects the growth of colon cancer RKO cells (152). It has the functions of dispelling wind and dehumidification, relieving pain through the meridian, promoting blood circulation and detoxifying, and treating rheumatic joint pain, limb numbness, headache, toothache, hernia, dysmenorrhea, and other diseases. 28-Hydroxy-3-oxoolean-12-en-29-oic acid, a triterpene acid from the extract of Rhododendron Chinensis, inhibits the migration of SGC-7901 and BGC-823 gastric cancer cells in a dose-dependent manner and invasion while reducing the expression levels of EMT and MMP in gastric cancer tumor cells (153). Tanshinone IIA has antibacterial, anti-inflammatory, activating blood stasis, promoting wound healing, and other effects, which can reduce the production of TNF-α by cells and reduce the activity of IL-6 while increasing the expression of miR-155 as a target for the prevention of colon cancer (154). Celastrol It has anti-oxidation, anti-rheumatoid, anti-Alzheimer’s, and anti-cancer properties, induces cell cycle arrest, up-regulates the expression of caspase-3, caspase-8, and Bax, down-regulates the expression of Bcl-2 to induce apoptosis, inhibits the expression of STAT3 and IL-6, and inhibits the properties of CSCs. Inhibits ovarian cancer tumor development (155). Esculentoside A induces mammary CSC apoptosis by blocking the expression of IL-6 and STAT3 pathway proteins and the up-regulation of caspase-3 and Bax/Bcl-2 ratio (156). Saikosaponin-d inhibits the growth of prostate cancer cells by reversing the expression and activity of EMT and MMP2/9 in a dose-dependent manner (157).
4.1.4. Alkaloids
miR-345-5p is considered to be an anticancer factor, and studies have found that matrine can increase the expression of miR-345-5p to resist the development-promoting effect of circ_0027345 on the development of liver cancer cells while inhibiting the migration and invasion of liver cancer cells (158). Sinomenine hydrochloride is naturally extracted from the rhizomes of caprophyaceae, and can inhibit breast cancer metastasis because it can inhibit EMT and CSC properties while inhibiting the activation of NF-kB and the expression of MMP, reversing endogenous and exogenous EMT, reversing the inflammatory microenvironment, and then inhibiting human Metastasis of glioblastoma cells (159, 160). In breast cancer, CTC spreads to the lungs through the circulatory system, causing complications of breast cancer. Palmatine can just interfere with lung metastasis of breast cancer and increase the tumor suppressor factor p53 (161). Piperine can reverse the biomarkers of EMT and inhibit the regulator of EMT, while the activation of STAT3 is down-regulated by piperine, which further inhibits the migration and invasion ability of rectal cancer (162). In addition, matrine-derived compounds can inhibit the development of hepatocellular carcinoma by reducing the expression of Bcl-2, inducing cell cycle arrest, reducing the number of EpCAM and CD133 cells, and inhibiting the expression of CSC markers (163). Berberine was found to downregulate CSC-like features, inhibit GLI1 signaling-induced EMT, and control ovarian cancer cell metastasis (164). The Sonic hedgehog signaling pathway is activated in pancreatic tumor CSCs, and sanguinarine has been shown to play a role in the secondary pathway, while sanguinarine upregulates E-cadherin and inhibits N-cadherin to inhibit the EMT process and prevent pancreatic cancer progression. Process (165).
5 Discussion
In a word, cancer formation requires the growth and development of early tumor cells through the micro-osmosis of neighboring organs and tissues to provide nutrients. When nutrients are insufficient for themselves, various angiogenesis factors and inhibitory factors interact to form blood vessels that provide nutrients for tumors. Some tumor cells secrete factors that can increase their movement first into the vasculature, then into the blood circulation, through the tube wall to escape the blood vessels into the surrounding tissues to form new metastatic cancer lesions. Therefore, in this paper, from promoting the death of tumor cells at the very beginning, to further requiring more nutrients and cytokines to trigger the interaction of immune cells in the tumor environment, inflammation, and some pathway changes, and finally inhibiting tumor metastasis to form metastasis, these four important pathways of tumor formation indicate that natural compounds play an active role in the fight against cancer. Phenols, flavonoids, terpenoids, and alkaloids have antioxidant, anti-inflammatory, and antiviral activities in addition to anti-tumor ability. Curcumin, capsaicin, quercetin, icariin, matrine, resveratrol, EGCG, berberine, and blood root line (SNG) have been cited many times in this paper. It can be seen that natural compounds have a wide range of anti-cancer pathways, and there may be a certain correlation between these pathways. In TME, immune cells MDSC can achieve tumor suppression by regulating TAM, and down-regulate STAT3, which is related to inflammation (166, 167). Inhibition of STAT3 phosphorylation was also found to induce programmed death of colon cancer cells and down-regulate the apoptotic protein Bcl-XL (168). CSC acts on TAM surface receptors by secreting chemokines and TGF-β, and activates STAT3 and NF-kB, leading to the immune escape of tumor cells. NK cells can induce inflammation, including the activation of DAMPs in one death, necrotizing apoptosis, and ICD, which appear to be critical to the immune response against tumors, However, most STAT-targeting drugs are still in the clinical stage, and magnolol can inhibit induced STAT3 activation while inhibiting Bcl-2 mRNA expression (169). In contrast, the presence of natural compounds makes up for the side effects caused by drugs such as cisplatin, doxorubicin, and fluorouracil, reducing the toxicity to some extent. For example, phenolic compounds are effective against adriamycin-induced cardiotoxicity in vitro and in vivo (170). Although this paper takes the important process of cancer as the entry point to explain the relationship between natural compounds and tumors, natural compounds have the advantage of lower toxicity side effects, but there are some unexplored other pathways, and there are some limitations. In future studies, it can be combined with classical anti-cancer prescriptions to interpret the anti-cancer effects of natural compounds from an overall perspective. At the same time, the separation technology of natural compounds is not complete, and the development of advanced technology can be effectively preserved to further improve the effectiveness of natural compounds. In addition, natural compounds have the advantage of synergies when combined with traditional anti-tumor therapies targeting tumors. The progress and development of science and technology will be more conducive to the discovery of natural compounds, bring hope to the research and development of anti-tumor drugs, and also provide a chance for the survival of cancer patients.
Author contributions
YN, HS and BZ prepared the original draft. YN conceptualized the study and framed the article. SL supervised the draft. YN, HS and BZ contribute equally to this work. All authors contributed to the article and approved the submitted version.
Funding
National Natural Science Foundation of China. Youth Science Foundation. NO.82204562. Hei longjiang University of Chinese Medicine PhD Innovation Fund (No. 2015bs05).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel R L, Laversanne M, Soerjomataram L, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Ongnok B, Chattipakorn N, Chattipakorn SC. Doxorubicin and cisplatin induced cognitive impairment: the possible mechanisms and interventions. Exp Neurol (2020) 324:113118. doi: 10.1016/j.expneurol.2019.113118
3. Speth PAJ, Van Hoesel Q, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet (1988) 15(1):15–31. doi: 10.2165/00003088-198815010-00002
4. Desilets A, Adam JP, Soulières D. Management of cisplatin-associated toxicities in bladder cancer patients. Curr Opin Support Palliat Care (2020) 14(3):286–92. doi: 10.1097/SPC.0000000000000505
5. Cao K, Tait SWG. Apoptosis and cancer: force awakens, phantom menace, or both? Int Rev Cell Mol Biol (2018) 337:135–52. doi: 10.1016/bs.ircmb.2017.12.003
6. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
7. CMurata M. Inflammation and cancer. Environ Health Prev Med (2018) 23(1):1–8. doi: 10.1186/s12199-018-0740-1
8. Li J, Meng H, Bai Y, Wang K. Regulation of lncRNA and its role in cancer metastasis. Oncol Res (2016) 23(5):205. doi: 10.3727/096504016X14549667334007
9. Deng LJ, Qi M, Li N, Lei YH, Zhang DM, Chen JX, et al. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J Leukocyte Biol (2020) 108(2):493–508. doi: 10.1002/JLB.3MR0320-444R
10. Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, et al. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med (1996) 184(3):1155–60. doi: 10.1084/jem.184.3.1155
11. Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, caenorhabditis elegans. Dev Biol (1977) 56(1):110–56. doi: 10.1016/0012-1606(77)90158-0
12. Catanzaro E, Feron O, Skirtach A, Krysko V. Immunogenic cell death and role of nanomaterials serving as therapeutic vaccine for personalized cancer immunotherapy. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.925290
13. Ribatti D. The concept of immune surveillance against tumors: The first theories. Oncotarget (2017) 8(4):7175. doi: 10.18632/oncotarget.12739
14. Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angewandte Chemie Int Edition (2019) 58(3):670–80. doi: 10.1002/anie.201804882
15. Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z, et al. Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med (2019) 23(8):4854–65. doi: 10.1111/jcmm.14356
16. Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis (2020) 11(11):1–13. doi: 10.1038/s41419-020-03221-2
17. Shi L, Liu Y, Li M, Luo Z. Emerging roles of ferroptosis in the tumor immune landscape: from danger signals to anti-tumor immunity. FEBS J (2022) 289(13):3655–65. doi: 10.1111/febs.16034
18. Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Different (2019) 26(1):99–114. doi: 10.1038/s41418-018-0212-6
19. Rahim NFC, Hussin Y, Aziz MNM, Mohamad NE, Yeap SK, Masarudin MJ, et al. Cytotoxicity and apoptosis effects of curcumin analogue (2E, 6E)-2, 6-bis (2, 3-dimethoxybenzylidine) cyclohexanone (DMCH) on human colon cancer cells HT29 and SW620 in vitro. Molecules (2021) 26(5):1261. doi: 10.3390/molecules26051261
20. Ferhi S, Santaniello S, Zerizer S, Cruciani S, Fadda A, Sanna D, et al. Total phenols from grape leaves counteract cell proliferation and modulate apoptosis-related gene expression in MCF-7 and HepG2 human cancer cell lines. Molecules (2019) 24(3):612. doi: 10.3390/molecules24030612
21. Eslami SS, Jafari D, Montazeri H, Sadeghizadeh M, Tarighi P. Combination of curcumin and metformin inhibits cell growth and induces apoptosis without affecting the cell cycle in LNCaP prostate cancer cell line. Nutr Cancer (2021) 73(6):1026–39. doi: 10.1080/01635581.2020.1783327
22. D’Eliseo D, Manzi L, Velotti F. Capsaicin as an inducer of damage-associated molecular patterns (DAMPs) of immunogenic cell death (ICD) in human bladder cancer cells. Cell Stress Chaperones (2013) 18(6):801–8. doi: 10.1007/s12192-013-0422-2
23. Khorsandi L, Orazizadeh M, Niazvand F, Abbaspour MR, Mansouri E, Khodadadi A, et al. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratislava Med J (2017) 118(2):123–8. doi: 10.4149/BLL_2017_025
24. Zhang J, Shen L, Li X, Song W, Liu Y, Huang L, et al. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano (2019) 13(11):12511–24. doi: 10.1021/acsnano.9b02875
25. Song L, Chen X, Mi L, Liu C, Zhu S, Yang T, et al. Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci (2020) 111(11):4242–56. doi: 10.1111/cas.14648
26. Qiu J, Zhang T, Zhu X, Yang C, Wang Y, Zhou N, et al. Hyperoside induces breast cancer cells apoptosis via ROS-mediated NF-κB signaling pathway. Int J Mol Sci (2019) 21(1):131. doi: 10.3390/ijms21010131
27. Rajendran P, Maheshwari U, Muthukrishnan A, et al. Myricetin: Versatile plant based flavonoid for cancer treatment by inducing cell cycle arrest and ROS–reliant mitochondria-facilitated apoptosis in A549 lung cancer cells and in silico prediction. Mol Cell Biochem (2021) 476(1):57–68. doi: 10.1007/s11010-020-03885-6
28. Joo EJ, Chun J, Ha YW, Ko HJ, Xu MY, Kim YS, et al. Novel roles of ginsenoside Rg3 in apoptosis through downregulation of epidermal growth factor receptor. Chemico-Biol Interact (2015) 233:25–34. doi: 10.1016/j.cbi.2015.03.016
29. Yu Y, Zhang C, Liu L, Li X. Hepatic arterial administration of ginsenoside Rg3 and transcatheter arterial embolization for the treatment of VX2 liver carcinomas. Exp Ther Med (2013) 5(3):761–6. doi: 10.3892/etm.2012.873
30. Lee D, Lee SR, Kang KS, Ko Y, Pang C, Yamabe N, et al. Betulinic acid suppresses ovarian cancer cell proliferation through induction of apoptosis. Biomolecules (2019) 9(7):257. doi: 10.3390/biom9070257
31. Wu L, Wang L, Tian X, Zhang J, Feng H. Germacrone exerts anti-cancer effects on gastric cancer through induction of cell cycle arrest and promotion of apoptosis. BMC complement Med therapies (2020) 20(1):1–9. doi: 10.1186/s12906-019-2810-3
32. Lau TS, Chan LKY, Man GCW, Wong CH, Lee JHS, Yim SF, et al. Paclitaxel induces immunogenic cell death in ovarian cancer via TLR4/IKK2/SNARE-dependent ExocytosisPaclitaxel induces ICD via TLR4 in cancer cells. Cancer Immunol Res (2020) 8(8):1099–111. doi: 10.1158/2326-6066.CIR-19-0616
33. Cheng Y, Chen T, Yang X, Xue J, Chen J. Atractylon induces apoptosis and suppresses metastasis in hepatic cancer cells and inhibits growth in vivo. Cancer Manage Res (2019) 11:5883–94. doi: 10.2147/CMAR.S194795
34. Wang Y, Yang S, Zhang S, Wu X. Oxymatrine inhibits proliferation and migration of vulvar squamous cell carcinoma cells via attenuation of the RAS/RAF/MEK/ERK pathway. Cancer Manage Res (2020) 12:2057. doi: 10.2147/CMAR.S245696
35. Liang X, Ju J. Matrine inhibits ovarian cancer cell viability and promotes apoptosis by regulating the ERK/JNK signaling pathway via p38MAPK. Oncol Rep (2021) 45(5):1–10. doi: 10.3892/or.2021.8033
36. Jang HJ, Yang JH, Hong E, Jo E, Lee S, Lee S, et al. Chelidonine induces apoptosis via GADD45a-p53 regulation in human pancreatic cancer cells. Integr Cancer Ther (2021) 20:15347354211006191. doi: 10.1177/15347354211006191
37. Mohan V, Agarwal R, Singh RP. A novel alkaloid, evodiamine causes nuclear localization of cytochrome-c and induces apoptosis independent of p53 in human lung cancer cells. Biochem Biophys Res Commun (2016) 477(4):1065–71. doi: 10.1016/j.bbrc.2016.07.037
38. Han S, Bi S, Guo T, et al. Nano co-delivery of plumbagin and dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J Controlled Release (2022) 348:250–63. doi: 10.1016/j.jconrel.2022.05.057
39. Li C, Zhang Y, Yan S, Zhang G, Wei W, Qi Z, et al. Alternol triggers immunogenic cell death via reactive oxygen species generation. Oncoimmunology (2021) 10(1):1952539. doi: 10.1080/2162402X.2021.1952539
40. Guo W, Liu S, Ju X, Du J, Xu B, Yuan H, et al. The antitumor effect of hinesol, extract from atractylodes lancea (Thunb.) DC. by proliferation, inhibition, and apoptosis induction via MEK/ERK and NF-κB pathway in non–small cell lung cancer cell lines A549 and NCI-H1299. J Cell Biochem (2019) 120(11):18600–7.
41. Zhang D, Li X, Song D, Chen S, Zhang Z, Cao S, et al. Atractylenolide III induces apoptosis by regulating the Bax/Bcl-2 signaling pathway in human colorectal cancer HCT-116 cells in vitro and in vivo. Anti-Canc Drugs (2022) 33(1):30–47. doi: 10.1097/CAD.0000000000001136
42. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol (2013) 14(10):1014–22. doi: 10.1038/ni.2703
43. Ginefra P, Lorusso G, Vannini N. Innate immune cells and their contribution to T-cell-based immunotherapy. Int J Mol Sci (2020) 21(12):4441. doi: 10.3390/ijms21124441
44. Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett (2017) 387:61–8. doi: 10.1016/j.canlet.2016.01.043
45. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol (2019) 19(6):369–82. doi: 10.1038/s41577-019-0127-6
46. Yuan R, Li S, Geng H, Wang X, Guan Q, Li X, et al. Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis. Int Immunopharmacol (2017) 49:30–7. doi: 10.1016/j.intimp.2017.05.014
47. Konjević GM, Vuletić AM, Martinović KMM, Larsen AK, Jurišić VB. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine (2019) 117:30–40. doi: 10.1016/j.cyto.2019.02.001
48. Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer (2020) 19(1):1–26. doi: 10.1186/s12943-020-01238-x
49. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol (2016) 17(9):1025–36. doi: 10.1038/ni.3518
50. Sungur CM, Murphy WJ. Positive and negative regulation by NK cells in cancer. Crit Rev Oncog (2014) 19(1-2):57–66. doi: 10.1615/CritRevOncog.2014010805
51. Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD, et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res (2019) 7(2):335–46. doi: 10.1158/2326-6066.CIR-18-0481
52. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (2011) 331(6024):1565–70. doi: 10.1126/science.1203486
53. Sambi M, Bagheri L, Szewczuk MR. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol (2019) 2019:4508794. doi: 10.1155/2019/4508794
54. Escors D. Tumour immunogenicity, antigen presentation, and immunological barriers in cancer immunotherapy. New J Sci (2014) 2014:734515. doi: 10.1155/2014/734515
55. Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med (2015) 212(7):1043–59. doi: 10.1084/jem.20141836
56. Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol (2013) 190(7):3783–97. doi: 10.4049/jimmunol.1201449
57. Yang L, Yu H, Dong S, Zhong Y, Hu S. Recognizing and managing on toxicities in cancer immunotherapy. Tumor Biol (2017) 39(3):1010428317694542. doi: 10.1177/1010428317694542
58. Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer (2019) 120(1):16–25. doi: 10.1038/s41416-018-0333-1
59. Umansky V, Blattner C, Gebhardt C, Utikal J. CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol Immunother (2017) 66(8):1015–23. doi: 10.1007/s00262-017-1988-9
60. Karakasheva TA, Waldron TJ, Eruslanov E, Kim SB, Lee JS, O'Brien S, et al. CD38-expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal CancerCD38 function in MDSCs. Cancer Res (2015) 75(19):4074–85. doi: 10.1158/0008-5472.CAN-14-3639
61. Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, et al. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+ Gr1+ cells. J Immunol (2011) 187(11):6120–9. doi: 10.4049/jimmunol.1101225
62. Kimura Y, Sumiyoshi M. Resveratrol prevents tumor growth and metastasis by inhibiting lymphangiogenesis and M2 macrophage activation and differentiation in tumor-associated macrophages. Nutr Cancer (2016) 68(4):667–78. doi: 10.1080/01635581.2016.1158295
63. Liu D, You M, Xu Y, Li F, Zhang D, Li X, et al. Inhibition of curcumin on myeloid-derived suppressor cells is requisite for controlling lung cancer. Int Immunopharmacol (2016) 39:265–72. doi: 10.1016/j.intimp.2016.07.035
64. Jiang Y, Fang Z, Leonard W, Zhang P. Phenolic compounds in lycium berry: Composition, health benefits and industrial applications. J Funct Foods (2021) 77:104340. doi: 10.1016/j.jff.2020.104340
65. Kwaśnik P, Lemieszek MK, Rzeski W. Impact of phytochemicals and plant extracts on viability and proliferation of NK cell line NK-92–a closer look at immunomodulatory properties of goji berries extract in human colon cancer cells. Ann Agric Environ Med (2021) 28(2):291. doi: 10.26444/aaem/133801
66. Xu P, Yan F, Zhao Y, Chen X, Sun S, Wang Y, et al. Green tea polyphenol EGCG attenuates MDSCs-mediated immunosuppression through canonical and non-canonical pathways in a 4T1 murine breast cancer model. Nutrients (2020) 12(4):1042. doi: 10.3390/nu12041042
67. Xue N, Zhou Q, Ji M, Jin J, Lai F, Chen J, et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci Rep (2017) 7(1):1–11. doi: 10.1038/srep39011
68. Zhao X, Qu J, Liu X, Wang J, Ma X, Zhao X, et al. Baicalein suppress EMT of breast cancer by mediating tumor-associated macrophages polarization. Am J Cancer Res (2018) 8(8):1528.
69. Wang L, Feng T, Su Z, Pi C, Wei Y, Zhao L, et al. Latest research progress on anticancer effect of baicalin and its aglycone baicalein. Arch Pharmacal Res (2022), 45(8):535–57. doi: 10.1007/s12272-022-01397-z
70. Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, et al. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol (2011) 11(7):890–8. doi: 10.1016/j.intimp.2011.01.007
71. Li H, Huang N, Zhu W, Wu J, Yang X, Teng W, et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer (2018) 18(1):1–12. doi: 10.1186/s12885-018-4299-4
72. Wu X, Yang H, Chen X, Gao J, Duan Y, Wei D, et al. Nano-herb medicine and PDT induced synergistic immunotherapy for colon cancer treatment. Biomaterials (2021) 269:120654. doi: 10.1016/j.biomaterials.2021.120654
73. Cao Y, Feng YH, Gao LW, Li XY, Jin QX, Wang YY, et al. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int Immunopharmacol (2019) 70:110–6. doi: 10.1016/j.intimp.2019.01.041
74. Xu L, Cai P, Li X, Wu X, Gao J, Liu W, et al. Inhibition of NLRP3 inflammasome activation in myeloid-derived suppressor cells by andrographolide sulfonate contributes to 5-FU sensitization in mice. Toxicol Appl Pharmacol (2021) 428:115672. doi: 10.1016/j.taap.2021.115672
75. Jeong M, Kim HM, Ahn JH, Lee KT, Jang DS, Choi JH, et al. 9-Hydroxycanthin-6-one isolated from stem bark of ailanthus altissima induces ovarian cancer cell apoptosis and inhibits the activation of tumor-associated macrophages. Chemico-biol Interact (2018) 280:99–108. doi: 10.1016/j.cbi.2017.12.011
76. Kumar S, Tomar MS, Acharya A. Chelerythrine delayed tumor growth and increased survival duration of dalton’s lymphoma bearing BALB/c h 2d mice by activation of NK cells in vivo. J Cancer Res Ther (2015) 11(4):904. doi: 10.4103/0973-1482.143342
77. Chen M, Li Y, Liu Z, Qu Y, Zhang H, Li D, et al. Exopolysaccharides from a codonopsis pilosula endophyte activate macrophages and inhibit cancer cell proliferation and migration. Thorac Cancer (2018) 9(5):630–9. doi: 10.1111/1759-7714.12630
78. Wang C, Shi S, Chen Q, Lin S, Wang R, Wang S, et al. Antitumor and immunomodulatory activities of ganoderma lucidum polysaccharides in glioma-bearing rats. Integr Cancer therapies (2018) 17(3):674–83. doi: 10.1177/1534735418762537
79. Wang N, Yang J, Lu J, Qiao Q, Wu T, Du X, et al. A polysaccharide from salvia miltiorrhiza bunge improves immune function in gastric cancer rats. Carbohydr Polymers (2014) 111:47–55. doi: 10.1016/j.carbpol.2014.04.061
80. Ding G, Gong Q, Ma J, Liu X, Wang Y, Cheng X, et al. Immunosuppressive activity is attenuated by astragalus polysaccharides through remodeling the gut microenvironment in melanoma mice. Cancer Sci (2021) 112(10):4050. doi: 10.1111/cas.15078
81. Almatroodi SA, Almatroudi A, Khan AA, Alhumaydhi FA, Alsahli MA, Rahmani AH, et al. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules (2020) 25(14):3146. doi: 10.3390/molecules25143146
82. Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell (2020) 183(3):771–85. doi: 10.1016/j.cell.2020.09.058
83. Stanislavovich Rogovskii V. The linkage between inflammation and immune tolerance: interfering with inflammation in cancer. Curr Cancer Drug Targets (2017) 17(4):325–32. doi: 10.2174/1568009617666170109110816
84. Nissinen L, Kähäri VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta (BBA)-General Subj (2014) 1840(8):2571–80. doi: 10.1016/j.bbagen.2014.03.007
85. Deng S, Hu B, Shen KP, Xu L. Inflammation, macrophage in cancer progression and chinese herbal treatment. J Basic Clin Pharm (2012) 3(2):269.
86. Colotta F, Allavena P, Sica A, Mantovani A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis (2009) 30(7):1073–81. doi: 10.1093/carcin/bgp127
87. Tu Y, Wu Z, Tan B, Yang A, Fang Z. Emodin: Its role in prostate cancer-associated inflammation. Oncol Rep (2019) 42(4):1259–71. doi: 10.3892/or.2019.7264
88. Steinke JW, Borish L. 3. cytokines and chemokines. J Allergy Clin Immunol (2006) 117(2):S441–5. doi: 10.1016/j.jaci.2005.07.001
89. Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol (2007) 7(10):803–15. doi: 10.1038/nri2171
90. Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell (2013) 4(3):176–85. doi: 10.1007/s13238-013-2084-3
91. Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol (2020) 20(2):95–112. doi: 10.1038/s41577-019-0215-7
92. Huber MA, Azoitei N, Baumann B, Grünert S, Sommer A, Pehamberger H, et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest (2004) 114(4):569–81. doi: 10.1172/JCI200421358
93. Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol (1998) 16:293. doi: 10.1146/annurev.immunol.16.1.293
94. Villarino AV, Gadina M, O’Shea JJ, Kanno Y. SnapShot: Jak-STAT signaling II. Cell (2020) 181(7):1696–6. doi: 10.1016/j.cell.2020.04.052
95. Wang Y, Ren X, Deng C, Yang L, Yan E, Guo T, et al. Mechanism of the inhibition of the STAT3 signaling pathway by EGCG. Oncol Rep (2013) 30(6):2691–6. doi: 10.3892/or.2013.2743
96. Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature (1996) 382(6587):174–7. doi: 10.1038/382174a0
97. Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, et al. IL-22+ CD4+ T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity (2014) 40(5):772–84. doi: 10.1016/j.immuni.2014.03.010
98. Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol (2006) 6(11):836–48. doi: 10.1038/nri1961
99. Chen E, Beer PA, Godfrey AL, Ortmann CA, Li J, Costa-Pereira AP, et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell (2010) 18(5):524–35. doi: 10.1016/j.ccr.2010.10.013
100. Sun Y, Zhou QM, Lu YY, Zhang H, Chen QL, Zhao M, et al. Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-β1-induced epithelial-mesenchymal transition. Molecules (2019) 24(6):1131. doi: 10.3390/molecules24061131
101. Sun L, Chen B, Jiang R, Li J, Wang B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell Immunol (2017) 311:86–93. doi: 10.1016/j.cellimm.2016.11.002
102. Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel M, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory b cells. J Immunol (2013) 191(8):4141–51. doi: 10.4049/jimmunol.1300606
103. Ghasemi F, Shafiee M, Banikazemi Z, Pourhanifeh MH, Khanbabaei H, Shamshirian A, et al. Curcumin inhibits NF-kB and wnt/β-catenin pathways in cervical cancer cells. Pathology-Res Pract (2019) 215(10):152556. doi: 10.1016/j.prp.2019.152556
104. Shiri S, Alizadeh AM, Baradaran B, Farhanghi B, Shanehbandi D, Khodayari S, et al. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pacific J Cancer Prev (2015) 16(9):3917–22. doi: 10.7314/APJCP.2015.16.9.3917
105. Chen M, Xiao C, Jiang W, Yang W, Qin Q, Tan Q, et al. Capsaicin inhibits proliferation and induces apoptosis in breast cancer by down-regulating FBI-1-mediated NF-κB pathway. Drug Design Dev Ther (2021) 15:125. doi: 10.2147/DDDT.S269901
106. Shree A, Islam J, Sultana S. Quercetin ameliorates reactive oxygen species generation, inflammation, mucus depletion, goblet disintegration, and tumor multiplicity in colon cancer: Probable role of adenomatous polyposis coli, β-catenin. Phytotherapy Res (2021) 35(4):2171–84. doi: 10.1002/ptr.6969
107. Zeng S, Chen L, Sun Q, Zhao H, Yang H, Ren S, et al. Scutellarin ameliorates colitis-associated colorectal cancer by suppressing wnt/β-catenin signaling cascade. Eur J Pharmacol (2021) 906:174253. doi: 10.1016/j.ejphar.2021.174253
108. Sui H, Zhao J, Zhou L, Wen H, Deng W, Li C, et al. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett (2017) 403:86–97. doi: 10.1016/j.canlet.2017.05.013
109. Dürr C, Hanna BS, Schulz A, Lucas F, Zucknick M, Benner A, et al. Tumor necrosis factor receptor signaling is a driver of chronic lymphocytic leukemia that can be therapeutically targeted by the flavonoid wogonin. haematologica (2018) 103(4):688. doi: 10.3324/haematol.2017.177808
110. Rawangkan A, Wongsirisin P, Namiki K, et al. Green tea catechin is an alternative immune checkpoint inhibitor that inhibits PD-L1 expression and lung tumor growth. Molecules (2018) 23(8):2071. doi: 10.3390/molecules23082071
111. Peng Y, Wang Y, Tang N, Sun D, Lan Y, Yu Z, et al. Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway. J Exp Clin Cancer Res (2018) 37(1):1–14. doi: 10.1186/s13046-018-0926-9
112. Zhai B, Zeng Y, Zeng Z, Zhang N, Li C, Zeng Y, et al. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. Int J nanomed (2018) 13:6279. doi: 10.2147/IJN.S174527
113. Zou K, Li Z, Zhang Y, Mu L, Chen M, Wang R, et al. β-elemene enhances radiosensitivity in non-small-cell lung cancer by inhibiting epithelial–mesenchymal transition and cancer stem cell traits via prx-1/NF-kB/iNOS signaling pathway. Aging (Albany NY) (2021) 13(2):2575. doi: 10.18632/aging.202291
114. Ilamathi M, Prabu PC, Ayyappa KA, Sivaramakrishnan V. Artesunate obliterates experimental hepatocellular carcinoma in rats through suppression of IL-6-JAK-STAT signalling. Biomed Pharmacother (2016) 82:72–9. doi: 10.1016/j.biopha.2016.04.061
115. Mathema VB, Chaijaroenkul W, Na-Bangchang K. Cytotoxic activity and molecular targets of atractylodin in cholangiocarcinoma cells. J Pharm Pharmacol (2019) 71(2):185–95. doi: 10.1111/jphp.13024
116. Fan H, Jiang C, Zhong B, Sheng J, Chen T, Chen Q, et al. Matrine ameliorates colorectal cancer in rats via inhibition of HMGB1 signaling and downregulation of IL-6, TNF-α, and HMGB1. J Immunol Res (2018) 2018:5408324. doi: 10.1155/2018/5408324
117. Zhao L, Zhang C. Berberine inhibits MDA-MB-231 cells by attenuating their inflammatory responses. BioMed Res Int (2020) 2020:3617514. doi: 10.1155/2020/3617514
118. Khumkhrong P, Piboonprai K, Chaichompoo W, Pimtong W, Khongkow M, Namdee K, et al. Crinamine induces apoptosis and inhibits proliferation, migration, and angiogenesis in cervical cancer SiHa cells. Biomolecules (2019) 9(9):494. doi: 10.3390/biom9090494
119. Prabhu KS, Bhat AA, Siveen KS, Kuttikrishnan S, Raza SS, Raheed T, et al. Sanguinarine mediated apoptosis in non-small cell lung cancer via generation of reactive oxygen species and suppression of JAK/STAT pathway. Biomed Pharmacother (2021) 144:112358. doi: 10.1016/j.biopha.2021.112358
120. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol (2018) 13(1):395–412. doi: 10.1146/annurev-pathol-020117-043854
121. Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol (2020) 30(10):764–76. doi: 10.1016/j.tcb.2020.07.003
122. Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B (2015) 5(5):402–18. doi: 10.1016/j.apsb.2015.07.005
123. Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol (2010) 22(5):697–706. doi: 10.1016/j.ceb.2010.08.015
124. Tashireva LA, Savelieva OE, Grigoryeva ES, Nikitin YV, Denisov EV, Vtorushin SV, et al. Heterogeneous manifestations of epithelial–mesenchymal plasticity of circulating tumor cells in breast cancer patients. Int J Mol Sci (2021) 22(5):2504. doi: 10.3390/ijms22052504
125. Cao M, Jiang Y, Tang Y, Liang XH. The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial-mesenchymal plasticity. Oncotarget (2017) 8(7):12472. doi: 10.18632/oncotarget.13957
126. Yan S, Wang H, Chen X, Liang C, Shang W, Wang L, et al. MiR-182-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-c. Cancer Lett (2020) 488:18–26. doi: 10.1016/j.canlet.2020.04.021
127. Zhang X, Zhang L, Chen M, Liu D. miR-324-5p inhibits gallbladder carcinoma cell metastatic behaviours by downregulation of transforming growth factor beta 2 expression. Artif Cells Nanomed Biotechnol (2020) 48(1):315–24. doi: 10.1080/21691401.2019.1703724
128. Xia H, Zhao Y. miR-155 is high-expressed in polycystic ovarian syndrome and promotes cell proliferation and migration through targeting PDCD4 in KGN cells. Artif cells nanomed Biotechnol (2020) 48(1):197–205. doi: 10.1016/j.biopha.2019.109774
129. Zhang L, Zhou Y, Huang T, et al. The interplay of LncRNA-H19 and its binding partners in physiological process and gastric carcinogenesis. Int J Mol Sci (2017) 18(2):450. doi: 10.3390/ijms18020450
130. Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: roles in tumorigenesis. Biomed Pharmacother (2020) 123:109774. doi: 10.1016/j.biopha.2019.109774
131. Zhuo W, Liu Y, Li S, Long noncoding RNA GMAN. Up-regulated in gastric cancer tissues, is associated with metastasis in patients and promotes translation of ephrin A1 by competitively binding GMAN-AS. Gastroenterology (2019) 156(3):676–691.e11. doi: 10.1053/j.gastro.2018.10.054
132. Espinoza I, Miele L. Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett (2013) 341(1):41–5. doi: 10.1016/j.canlet.2013.08.027
133. Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei X, et al. Oct-4 and nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget (2014) 5(21):10803. doi: 10.18632/oncotarget.2506
134. Palla AR, Piazzolla D, Alcazar N, Cañamero M, Graña O, Gómez-López G, et al. The pluripotency factor NANOG promotes the formation of squamous cell carcinomas. Sci Rep (2015) 5(1):1–13. doi: 10.1038/srep10205
135. Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, et al. Origins of the tumor microenvironment: Quantitative assessment of adipose-derived and bone marrow–derived stroma. PloS One (2012) 7(2):e30563. doi: 10.1371/journal.pone.0030563
136. Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated wnt/β-catenin signal pathway. PloS One (2013) 8(11):e78700. doi: 10.1371/journal.pone.0078700
137. Dhar S, Kumar A, Rimando AM, Zhang X, Levenson A. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget (2015) 6(29):27214. doi: 10.18632/oncotarget.4877
138. Li W, Wang Q, Su Q, Ma D, An C, Ma L, et al. Honokiol suppresses renal cancer cells’ metastasis via dual-blocking epithelial-mesenchymal transition and cancer stem cell properties through modulating miR-141/ZEB2 signaling. Mol Cells (2014) 37(5):383.
139. Baptista Moreno Martin AC, Tomasin R, Luna-Dulcey L, Graminha AE, Naves MA, Tele RHG, et al. -gingerol improves doxorubicin anticancer activity and decreases its side effects in triple negative breast cancer models. Cell Oncol (2020) 43(5):915–29. doi: 10.1007/s13402-020-00539-z
140. Hu C, Li M, Guo T, Wang S, Huang W, Yang K, et al. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine (2019) 58:152740. doi: 10.1016/j.phymed.2018.11.001
141. Seino M, Okada M, Shibuya K, Seino S, Suzuki S, Takeda S, et al. Differential contribution of ROS to resveratrol-induced cell death and loss of self-renewal capacity of ovarian cancer stem cells. Anticancer Res (2015) 35(1):85–96.
142. Ko YS, Jung EJ, Go S, Jeong BK, Kim GS, Jung GM, et al. Polyphenols extracted from artemisia annua l. exhibit anti-cancer effects on radio-resistant MDA-MB-231 human breast cancer cells by suppressing stem cell phenotype, β-catenin, and MMP-9. Molecules (2020) 25(8):1916.
143. Wu HT, Lin J, Liu YE, Chen HF, Hsu KW, Lin S H, et al. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine (2021) 81:153437. doi: 10.1016/j.phymed.2020.153437
144. Sameri S, Saidijam M, Bahreini F, Najafi R. Cancer chemopreventive activities of silibinin on colorectal cancer through regulation of e-cadherin/β-catenin pathway. Nutr Cancer (2021) 73(8):1389–99. doi: 10.1080/01635581.2020.1800764
145. Binienda A, Ziolkowska S, Pluciennik E. The anticancer properties of silibinin: its molecular mechanism and therapeutic effect in breast cancer. Anti-Canc Agents Med Chem (Formerly Curr Med Chemistry-Anti-Canc Agents) (2020) 20(15):1787–96. doi: 10.2174/1871520620666191220142741
146. Wang C, Chen Y, Wang Y, Liu X, Liu Y, Li Y, et al. Inhibition of COX-2, mPGES-1 and CYP4A by isoliquiritigenin blocks the angiogenic akt signaling in glioma through ceRNA effect of miR-194-5p and lncRNA NEAT1. J Exp Clin Cancer Res (2019) 38(1):1–14. doi: 10.1186/s13046-019-1361-2
147. Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci (2011) 108(4):1433–8. doi: 10.1073/pnas.1014275108
148. Chen JH, Kim SH, Fan PW, Fan PW, Liu CY, Hsieh CH, et al. The aqueous extract of Chinese medicinal herb brucea javanica suppresses the growth of human liver cancer and the derived stem-like cells by apoptosis. Drug Design Dev Ther (2016) 10:2003–13.
149. Cao C, Sun L, Mo W, Sun L, Luo J, Yang Z, et al. Quercetin mediates β-catenin in pancreatic cancer stem-like cells. Pancreas (2015) 44(8):1334–9. doi: 10.1097/MPA.0000000000000400
150. Hoca M, Becer E, Kabadayı H, Yücecan S, Vatansever HS. The effect of resveratrol and quercetin on epithelial-mesenchymal transition in pancreatic cancer stem cell. Nutr Cancer (2020) 72(7):1231–42. doi: 10.1080/01635581.2019.1670853
151. Hu FW, Yu CC, Hsieh PL, Liao YW, Lu MY, Chu PM, et al. Targeting oral cancer stemness and chemoresistance by isoliquiritigenin-mediated GRP78 regulation. Oncotarget (2017) 8(55):93912. doi: 10.18632/oncotarget.21338
152. Zheng JL, Wang SS, Shen KP, Chen L, Peng X, Chen J, et al. Ursolic acid induces apoptosis and anoikis in colorectal carcinoma RKO cells. BMC complement Med therapies (2021) 21(1):1–8. doi: 10.1186/s12906-021-03232-2
153. Chu Z, Wang H, Ni T, Tao L, Xiang L, Zhou Z, et al. 28-Hydroxy-3-oxoolean-12-en-29-oic acid, a triterpene acid from celastrus orbiculatus extract, inhibits the migration and invasion of human gastric cancer cells in vitro. Molecules (2019) 24(19):3513. doi: 10.3390/molecules24193513
154. Tu J, Xing Y, Guo Y, Tang F, Guo L, Xi Y, et al. TanshinoneIIA ameliorates inflammatory microenvironment of colon cancer cells via repression of microRNA-155. Int Immunopharmacol (2012) 14(4):353–61. doi: 10.1016/j.intimp.2012.08.015
155. Li X, Wang H, Ding J, Nie S, Wang L, Zhang L, et al. Celastrol strongly inhibits proliferation, migration and cancer stem cell properties through suppression of Pin1 in ovarian cancer cells. Eur J Pharmacol (2019) 842:146–56. doi: 10.1016/j.ejphar.2018.10.043
156. Liu C, Dong L, Sun Z, Wang L, Wang Q, Li H, et al. Esculentoside a suppresses breast cancer stem cell growth through stemness attenuation and apoptosis induction by blocking IL-6/STAT3 signaling pathway. Phytotherapy Res (2018) 32(11):2299–311. doi: 10.1002/ptr.6172
157. Zhong D, Zhang H, Jiang Y, Wu P, Qi H, Cai C, et al. Saikosaponin-d: a potential chemotherapeutics in castration resistant prostate cancer by suppressing cancer metastases and cancer stem cell phenotypes. Biochem Biophys Res Commun (2016) 474(4):722–9. doi: 10.1016/j.bbrc.2016.05.017
158. Lin S, Zhuang J, Zhu L, Jiang Z. Matrine inhibits cell growth, migration, invasion and promotes autophagy in hepatocellular carcinoma by regulation of circ_0027345/miR-345-5p/HOXD3 axis. Cancer Cell Int (2020) 20(1):1–12. doi: 10.1186/s12935-020-01293-w
159. Li X, Li P, Liu C, Ren Y, Tang X, Wang K, et al. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial-mesenchymal transition and cancer stemness. Oncotarget (2017) 8(8):13560. doi: 10.18632/oncotarget.14593
160. Jiang Y, Jiao Y, Liu Y, Zhang M, Wang Z, Li Y, et al. Sinomenine hydrochloride inhibits the metastasis of human glioblastoma cells by suppressing the expression of matrix metalloproteinase-2/-9 and reversing the endogenous and exogenous epithelial-mesenchymal transition. Int J Mol Sci (2018) 19(3):844. doi: 10.3390/ijms19030844
161. Danquah CA, Ativui S, Ossei PPS, Ofori M. Palmatine attenuates metastatic lung colonization of triple negative breast cancer cells. Front Pharmacol (2022) 1167. doi: 10.3389/fphar.2022.853230
162. Song L, Wang Y, Zhen Y, Li D, He X, Yang H, et al. Piperine inhibits colorectal cancer migration and invasion by regulating STAT3/Snail-mediated epithelial–mesenchymal transition. Biotechnol Lett (2020) 42(10):2049–58. doi: 10.1007/s10529-020-02923-z
163. Liu Y, Qi Y, Bai Z, Ni C, Ren Q, Xu W, et al. A novel matrine derivate inhibits differentiated human hepatoma cells and hepatic cancer stem-like cells by suppressing PI3K/AKT signaling pathways. Acta Pharmacol Sin (2017) 38(1):120–32. doi: 10.1038/aps.2016.104
164. Zhao Y, Yang X, Zhao J, Gao M, Zhang M, Shi M, et al. Berberine inhibits chemotherapy-exacerbated ovarian cancer stem cell-like characteristics and metastasis through GLI1. Eur J Pharmacol (2021) 895:173887. doi: 10.1016/j.ejphar.2021.173887
165. Ma Y, Yu W, Shrivastava A, Alemi F, Lankachandra K, Srivastava RK, et al. Sanguinarine inhibits pancreatic cancer stem cell characteristics by inducing oxidative stress and suppressing sonic hedgehog-Gli-Nanog pathway. Carcinogenesis (2017) 38(10):1047–56. doi: 10.1093/carcin/bgx070
166. Liu R, Peng J, Wang H, Li L, Wen X, Tan Y, et al. Oxysophocarpine retards the growth and metastasis of oral squamous cell carcinoma by targeting the Nrf2/HO-1 axis. Cell Physiol Biochem (2018) 49(5):1717–33. doi: 10.1159/000493615
167. Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, et al. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity (2016) 44(2):303–15. doi: 10.1016/j.immuni.2016.01.014
168. Maeda Y, Takahashi H, Nakai N, Yanagita T, Ando N, Okubo T, et al. Apigenin induces apoptosis by suppressing bcl-xl and mcl-1 simultaneously via signal transducer and activator of transcription 3 signaling in colon cancer. Int J Oncol (2018) 52(5):1661–73. doi: 10.3892/ijo.2018.4308
169. Yin Q, Wang L, Yu H, Chen D, Zhu W, Sun C, et al. Pharmacological effects of polyphenol phytochemicals on the JAK-STAT signaling pathway. frontiers in pharmacology. (2021) 12:-2350:. doi: 10.3389/fphar.2021.716672
Keywords: anticancer natural compounds, potential cancer target, cell death, immune cells, tumor metastasis
Citation: Nan Y, Su H, Zhou B and Liu S (2023) The function of natural compounds in important anticancer mechanisms. Front. Oncol. 12:1049888. doi: 10.3389/fonc.2022.1049888
Received: 21 September 2022; Accepted: 30 November 2022;
Published: 04 January 2023.
Edited by:
Leilei Fu, Southwest Jiaotong University, ChinaReviewed by:
Bo Han, Chengdu University of Traditional Chinese Medicine, ChinaJin Zhang, Shenzhen University, China
Copyright © 2023 Nan, Su, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shumin Liu, a2VqaS1saXVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yang Nan
Yang Nan Hongchan Su1†
Hongchan Su1† Shumin Liu
Shumin Liu