- 1Shengli Clinical Medical College of Fujian Medical University, Fuzhou, China
- 2Department of Surgical Oncology, Fujian Provincial Hospital, Fuzhou, China
- 3Department of VIP Clinic, Fujian Provincial Hospital, Fuzhou, China
Background: Laparoscopic total gastrectomy (LTG) with Roux-en-Y (RY) is often accompanied by a series of complications. Uncut RY (URY) can effectively reduce Roux stasis syndrome (RSS) in laparoscopic distal gastrectomy. To determine whether totally LTG (TLTG) with URY for gastric cancer (GC) can replace RY in short-term and long-term prognosis.
Methods: This comparative retrospective study selected GC patients from 2016 to 2022. The patients were divided into URY group and RY group. Cox multivariate proportional hazard regression analysis was used to explore the independent prognostic factors. Propensity score matching (PSM) was used to reduce bias.
Results: A total of 100 GC patients met the inclusion criteria. Compared to RY group, URY group showed significant advantages in operation time and length of hospital stay. In addition, URY group can significantly reduce short-term and long-term complications, especially RSS. The 1-, 3- and 5-year progression free survival (PFS) of URY group and RY group were 90.4% vs. 67.8% (P=0.005), 76.6% vs. 52.6% (P=0.009) and 76.6% vs. 32.8% (P<0.001), respectively. After PSM, the advantage of URY in PFS was verified again, while there was no significant difference in overall survival (OS) between the two groups. Cox multivariate analysis suggested that lower RSS was associated with better PFS.
Conclusions: TLTG with URY for GC helps control disease progression, speed up recovery and reduce short and long-term complications, especially RSS.
1 Introduction
According to the latest cancer statistics, gastric cancer (GC) is one of the most common cancers and the third leading cause of cancer-related deaths worldwide (1, 2). Radical surgery is the only possible cure for GC (3).
Total gastrectomy is the preferred surgical procedure for tumors located in the middle or proximal part of the stomach (4). With the popularization and application of laparoscopic technique in GC, assisted laparoscopic total gastrectomy (LTG) has gradually transformed into totally LTG (TLTG) (5, 6). The difficulty of this transformation lies in the reconstruction of the digestive tract under totally laparoscopy (7).
Roux-en-Y (RY) esophagojejunostomy (EJS) is still the most commonly used reconstruction method in total gastrectomy (8, 9). However, RY is often accompanied by a series of complications (10, 11). In addition to the improvement of survival, the quality of life (QoL) has also attracted more attention. In order to reduce the complications after total gastrectomy and improve the QoL of patients, researchers have been trying new anastomosis methods in recent decades. The uncut RY (URY) has been widely used in the reconstruction of digestive tract after distal gastrectomy (12). URY can effectively reduce Roux stasis syndrome (RSS) in laparoscopic distal gastrectomy (LDG) (13). Due to different reconstruction locations, whether TLTG with URY can reduce complications remain controversial. In addition, the existence of recanalization of jejunal input loops in TLTG is still a research hotspot (14, 15).
At present, there are few comparative studies on URY and RY under TLTG. Whether long-term complications after gastrointestinal reconstruction affect prognosis is a current research hotspot. Therefore, we retrospectively analyzed the data of GC patients to compare the short-term and long-term prognosis of TLTG with URY and RY for GC.
2 Materials and methods
2.1 Study population and grouping
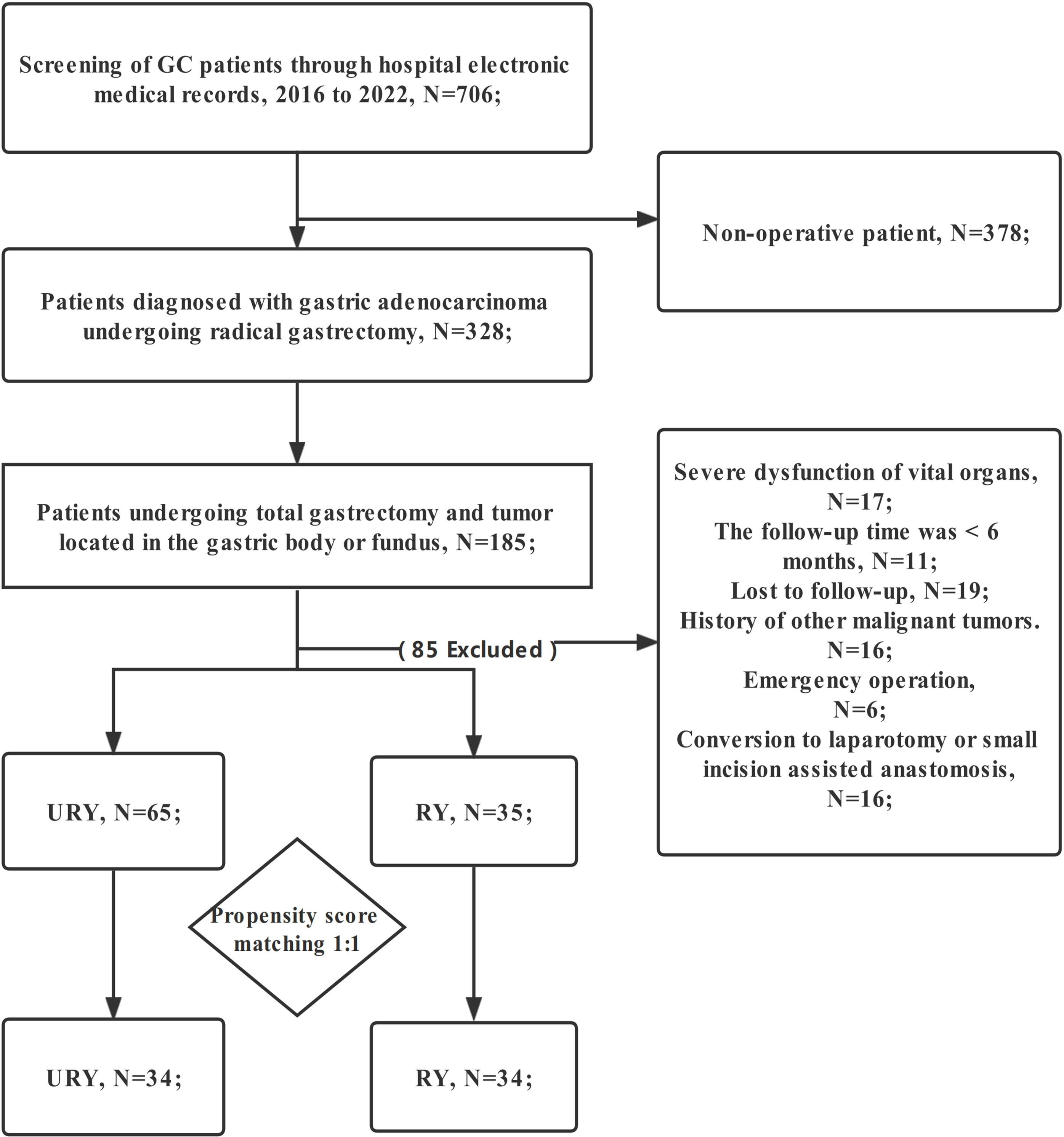
The clinical data of all GC patients who underwent TLTG + EJS in Fujian Provincial Hospital from January 2016 to January 2022 were retrospectively analyzed. Eligible patients were automatically divided into two groups for comparison: RY group and URY group. The choice of anastomosis was made randomly by the chief physician during the operation. The patient did not participate in the decision-making of anastomosis mode. Patients were screened strictly according to the following criteria: (1) Gastric adenocarcinoma was confirmed by postoperative pathological examination; (2) TNM stage I-III; (3) The patient informed consent and accepted TLTG+EJS; (4) The patient has complete clinical data (including endoscopy, CT, etc.); (5) Tumor located in the gastric body or fundus. Exclusion criteria: (1) Conversion to laparotomy or small incision assisted anastomosis; (2) History of other malignant tumors; (3) Emergency operation; (4) Preoperative chemotherapy; (5) Follow-up time less than 6 months; (6) Serious organ dysfunction; (7) Lost to follow-up (Figure 1). Written informed consent was obtained from all GC patients. This study was approved by the Medical Ethics Committee of Fujian Provincial Hospital.
2.2 Surgical procedure
All patients were given general anesthesia by endotracheal intubation. The patient was positioned with the head elevated and the feet low. Both groups were performed by the same surgical team. The chief physician had more than 500 laparoscopic radical gastrectomy experience. The “five-hole method” was used to establish the endoscopic hole. Abdominal exploration showed no obvious metastasis All patients underwent standard total gastrectomy and lymph node dissection (16). The digestive tract was reconstructed as follows:
2.2.1 Uncut-Roux-en-Y anastomosis
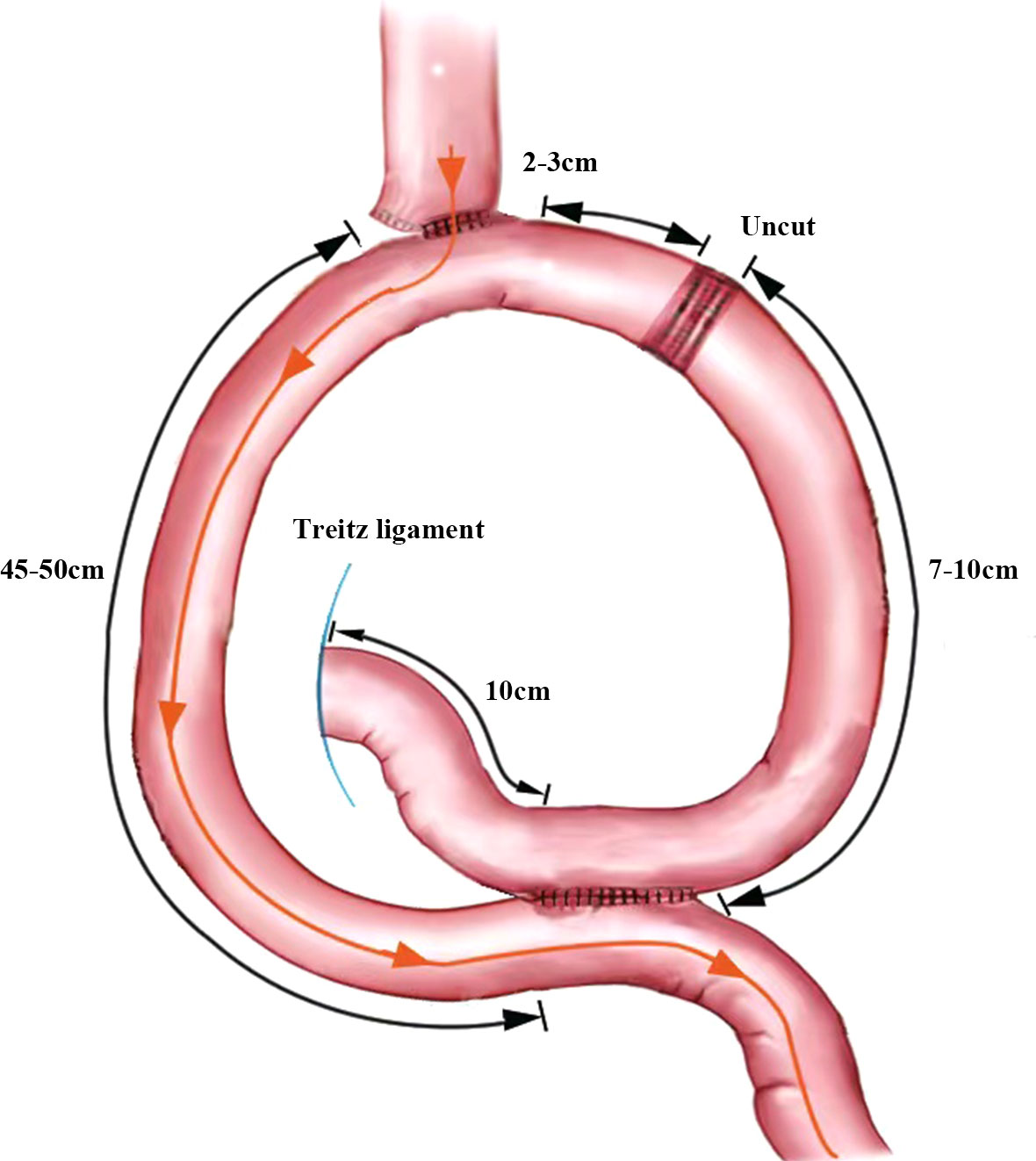
The jejunum, approximately 25 cm from the Treitz ligament, was raised to the lower esophagus, where a small incision was made on its lateral side. The two arms of the linear cutting closure are inserted through the esophageal, jejunal incision, respectively. And then the anastomosis is completed. The common opening of the esophagojejunal was finally closed with closure. A small incision was made into the distal jejunum approximately 45 cm from the esophagojejunostomy and the proximal jejunum approximately 10 cm from the Treitz ligament, respectively. A jejunal Braun anastomosis was then performed. The input loop jejunum 2-3 cm away from the esophagojejunostomy was closed with an uncut linear cutting closure (ATS45NK, Johnson & Johnson, USA). The specimen is placed into the specimen bag after completion of the URY anastomosis (Figure 2).
2.2.2 Roux-en-Y anastomosis
The jejunum, approximately 15-20 cm from the Treitz ligament, was cut with a linear cutting closure. The distal jejunum was raised to the esophageal. After stapler insertion in the esophagus and distal jejunum, an esophagojejunostomy was performed anterior to the colon. A small incision was made in the distal jejunum 45 cm away from the esophagojejunostomy. The proximal jejunum was anastomosed to the incision. Several stitches were added around each anastomosis. The specimen was placed into the specimen bag after completion of the RY anastomosis.
2.3 Definitions
The primary endpoint of this study was progression free survival (PFS). Secondary endpoints were RSS and other long-term complications related to EJS. PFS was defined as the time from TLTG to confirmed death or recurrence. Overall survival (OS) was defined as the time from TLTG to the last follow-up or death. Operation time: from skin incision to abdominal closure. Intraoperative anastomosis time: from the beginning of EJS to the end of all anastomosis. Postoperative complications were divided into short-term and long-term complications. Short-term complications were graded using the Clavien-Dindo classification (17). Definition of RSS was followed as: 1. Roux-en-Y anastomosis or Uncut-Roux-en-Y anastomosis was performed to reconstruct the digestive tract; 2. Intestinal obstruction such as nausea, vomiting, and abdominal distension still occurred 3 months after surgery; 3. Imaging or gastroscopy showed food retention in the loop of Roux. And the reasons for mechanical intestinal obstruction, anastomotic stenosis, ulcers, and tumor recurrence were excluded (13, 18–20). Our postoperative feeding protocol is as follows: Patients are allowed to drink water on the first day after gastrectomy. About 1 to 3 days after removal of the gastric tube, patients are allowed to eat liquid diet, such as rice soup. The specific time of liquid and semi-liquid diet was evaluated according to the recovery of intestinal function such as postoperative ventilation. About 4 to 7 days after removal of the gastric tube, patients are allowed to add a semi-liquid diet, such as porridge.
2.4 Data collection and follow-up
Baseline characteristics, perioperative information and complications information were obtained from the hospital electronic medical record systems. Follow-up until October 1, 2022, to obtain the survival status and long-term complications of GC patients. The follow-up protocol was in strict accordance with the guidelines and consensus, and clinical symptoms were recorded at each review. Each GC patient returned to the hospital every three months after TLTG to confirm disease progression by endoscopy, upper gastrointestinal contrast or CT. The follow-up interval was set to 6 months if the GC patient was progression-free in the initial 2 years. The frequency of follow-up was adjusted to once a year if the progression-free status was maintained over 5 years.
2.5 Statistical analysis
For categorical data, patient baseline characteristics are expressed as proportions. For continuous data, mean ± standard deviation is used. The Continuity correction or Pearson’s χ2 is used to compare the baseline characteristics or categorical data of the two groups. Differences in continuous variables between groups are assessed using the student’s t-test. OS and PFS of the two groups were estimated using the Kaplan–Meier method and compared using log-rank test. The Cox proportional hazard multiple regression model was established. Univariate analysis was conducted firstly, and related factors (P<0.1) were included in multivariate analysis. In the multivariate analysis, factors with P<0.05 were considered as independent predictors of OS and PFS.
To simulate randomization and further address confounding factors between the two groups, a propensity score matching (PSM) analysis was used in this study. The factors with statistical difference (T stage, N stage and TNM stage) in the two groups of baselines were put into the matching variables. Then, the two groups were formed using a one-to-one nearest neighbor caliper with a width of 0.05. All statistical analyses were performed using SPSS statistical software (version 25, SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Clinicopathological characteristics
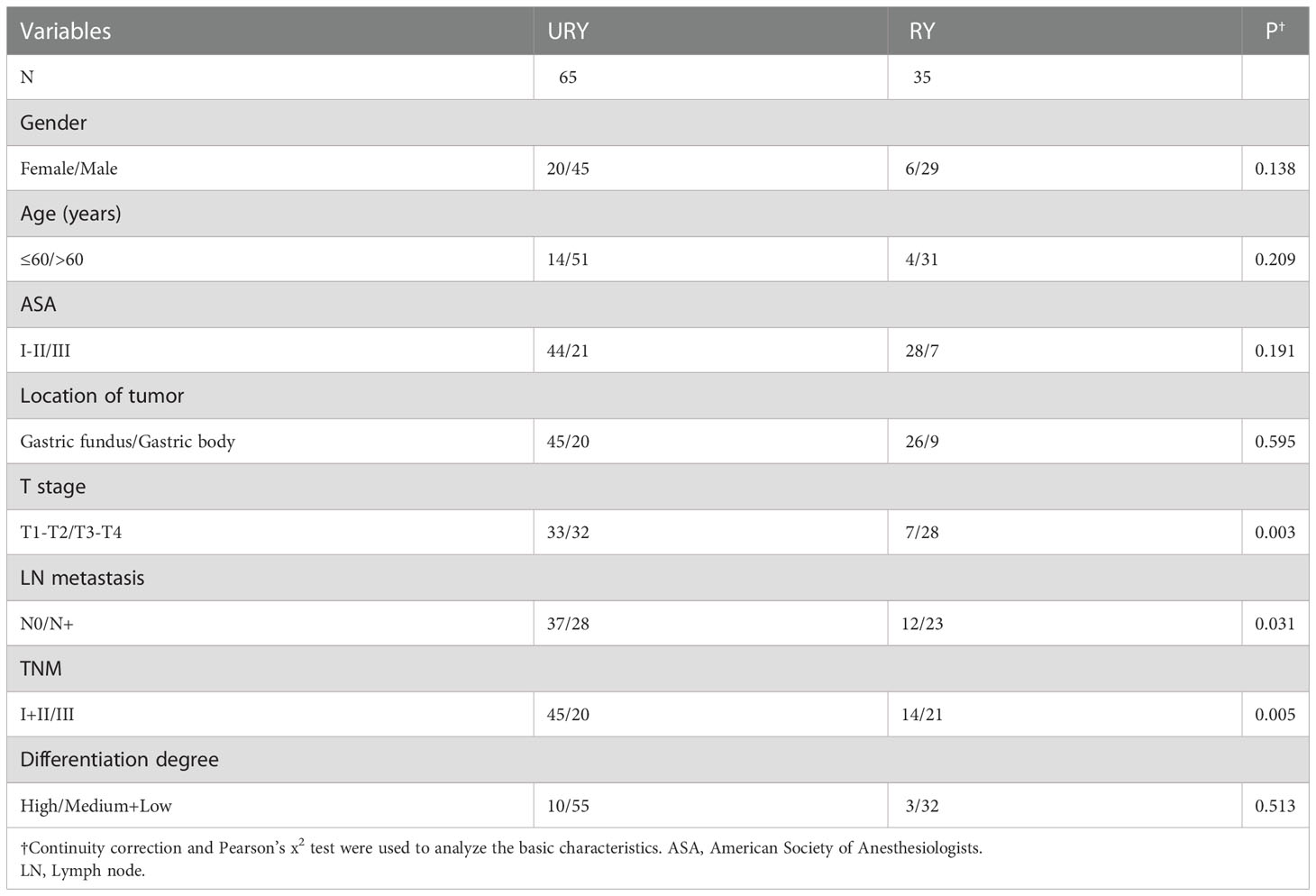
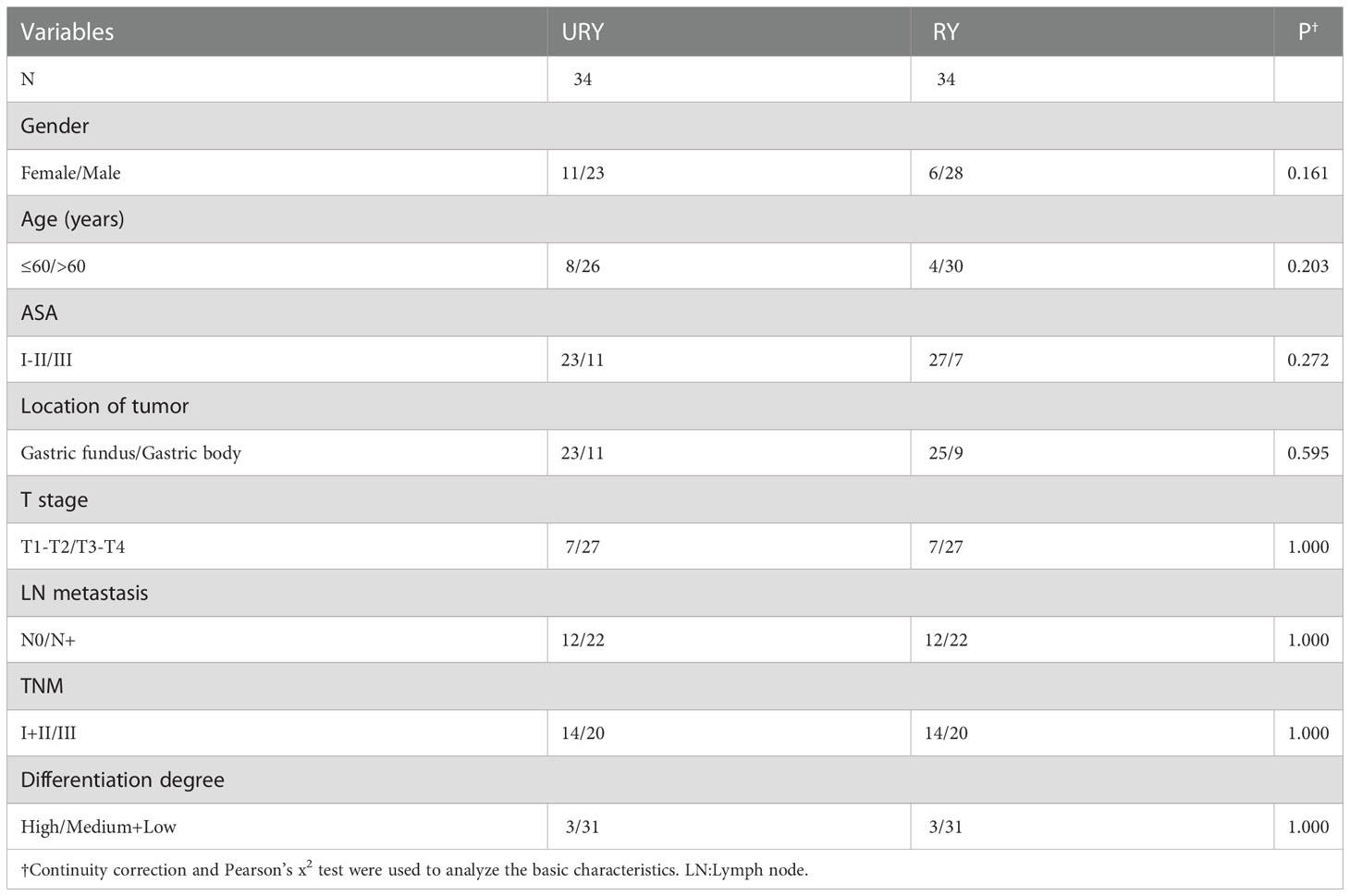
From 2016 to 2022, a total of 100 GC patients met the inclusion criteria. There were 65 patients in URY group and 35 patients in RY group. The clinical characteristics of the two groups are shown in Table 1. The median age of patients in URY group and RY group was 66.0 and 70.0 years, respectively. The ratio of female to male in the entire cohort was 26: 74. There were significant differences in T stage, N stage and TNM stage between the two groups. All patients had no preoperative radiotherapy or chemotherapy. Most patients are ASA I-II.
3.2 Surgical information and postoperative complications
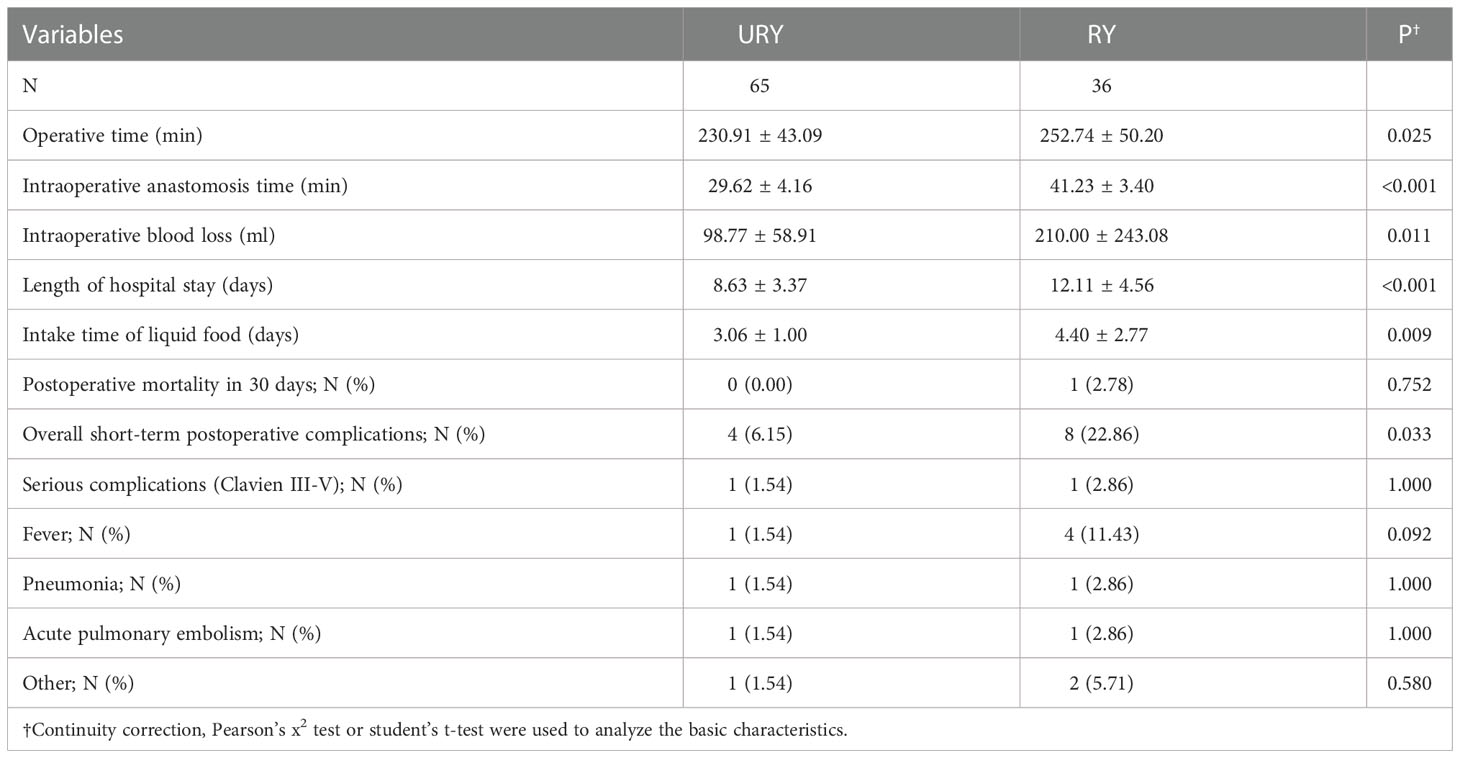
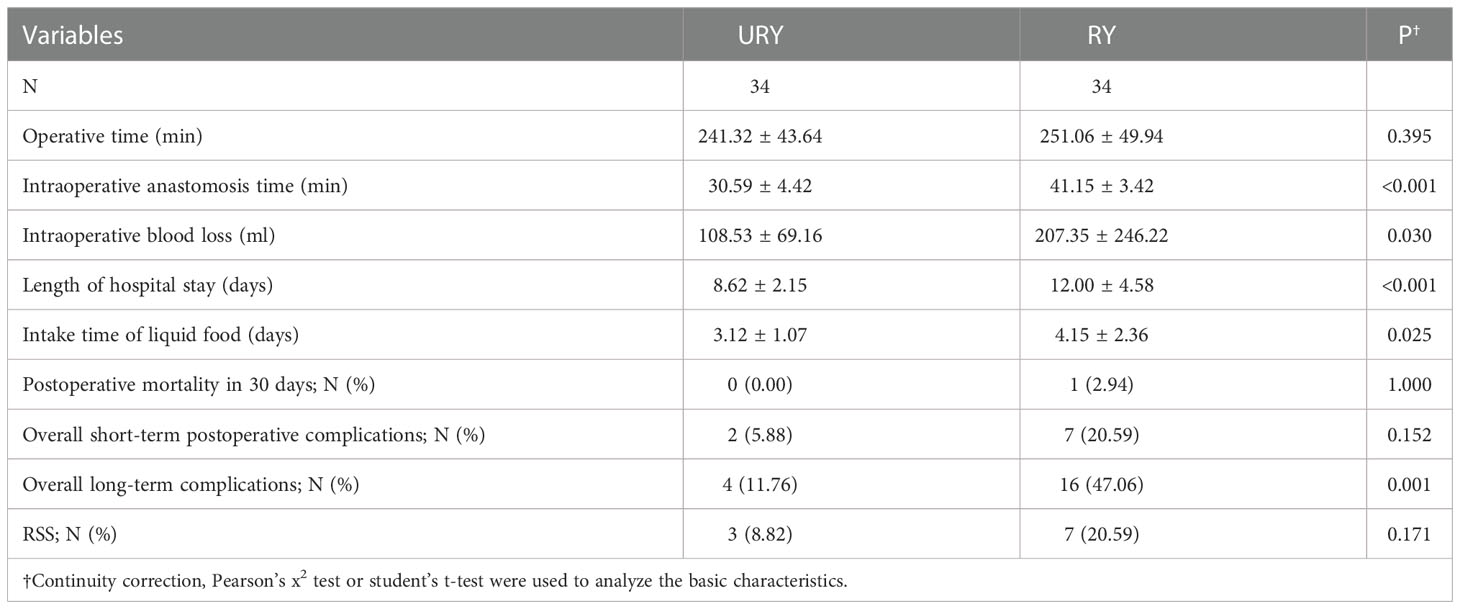
Both groups completed standard total gastrectomy and lymph node dissection. The perioperative conditions of TLTG for GC is shown in Table 2. All patients were not converted to laparotomy. The total operation time, anastomosis time, length of hospital stays and liquid food intake time in RY group were significantly longer than URY group (P=0.025, P<0.001, P<0.001 and P=0.009). The intraoperative blood loss in the RY group was also significantly more than URY group (mean 210.00 ml vs. 98.77 ml; P=0.011). One patient in the RY group died of complications within 30 days after surgery, whereas none in the URY group. The short-term postoperative complications in the RY group were significantly higher than URY group (22.86% vs. 6.15%, P=0.033).
Long-term postoperative complications are shown in Table 3. Compared to the overall short-term complications, the difference in long-term complications between the two groups was more obvious. The overall long-term complications in RY group were significantly higher than URY group (45.71% vs. 10.77%, P<0.001). Among them, RY group was significantly higher than URY group in RSS and Reflux esophagitis (P=0.036 and P=0.037). During the follow-up, no recanalization of the input loop was found in the URY group.
3.3 Survival analysis
Both groups completed R0 resection. All enrolled GC patients were reviewed strictly according to the follow-up protocol, with a median follow-up time of 27.5 months. This study uses PSM to solve the offset of the two groups of baselines. After PSM, we obtained a one-to-one matched cohort of URY and RY groups (34 GC patients per group) (Table 4). In the matching cohort, no significant differences in confounding factors were detected between the two groups. In addition, after PSM, compared to the RY group, the advantages of the URY group in intraoperative anastomosis time, intraoperative blood loss, length of hospital stay, short-term and long-term postoperative complications were verified again (Table 5).
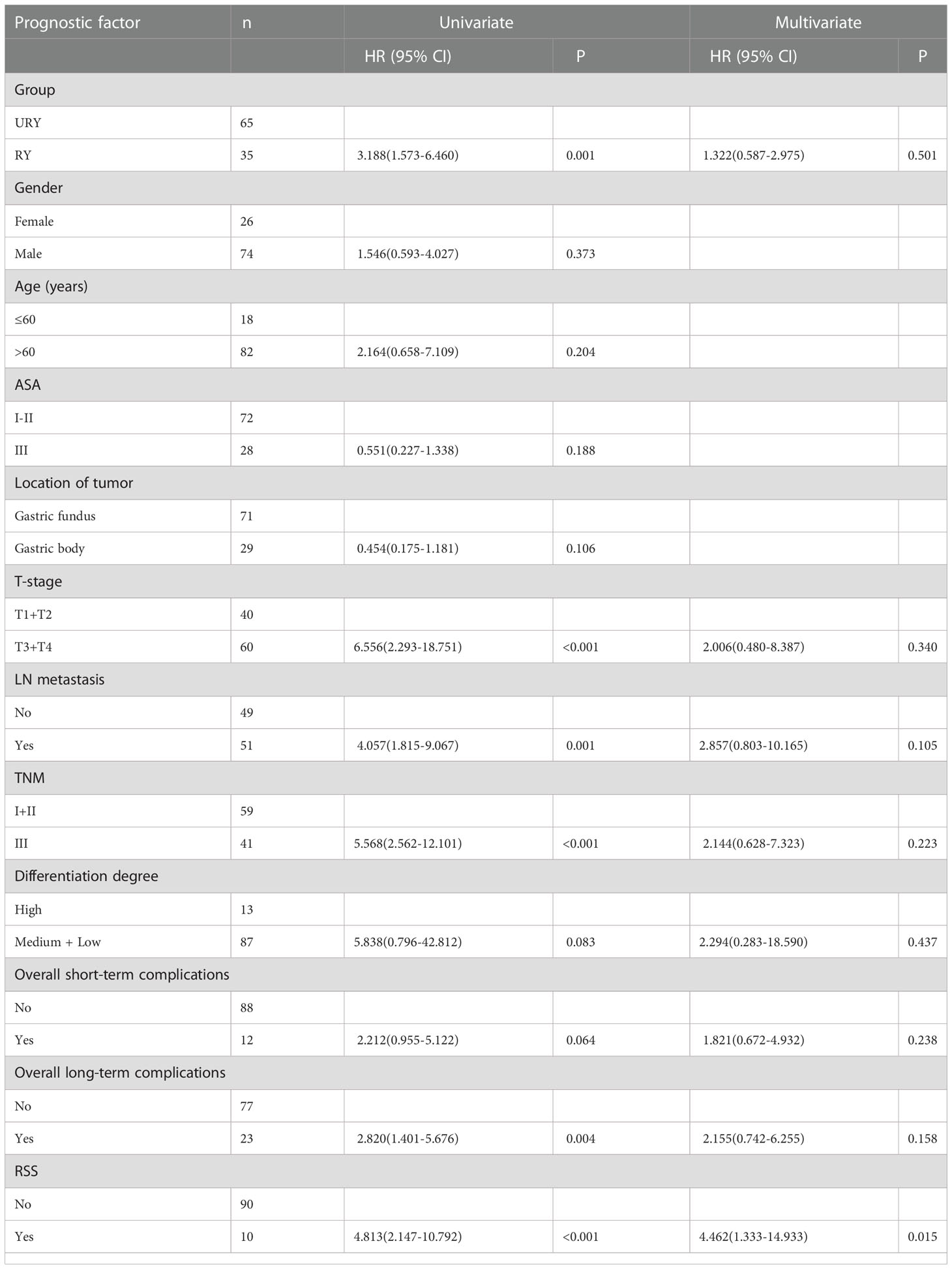
Compared to the RY group, PFS of GC patients in URY group was significantly improved before and after PSM (log-rank; P=0.001, and P=0.044) (Figures 3A, 3B). The 1-, 3-, 5-year PFS of URY group and RY group were 90.4% vs. 67.8% (P=0.005), 76.6% vs. 52.6% (P=0.009), 76.6% vs. 32.8% (P<0.001), respectively. After PSM, the 1-, 3-, 5-year PFS were 88.1% vs. 76.5% (P=0.072), 64.7% vs. 54.1% (P=0.310), 64.7% vs. 33.8% (P=0.049), respectively. Cox multivariate analysis showed that poor PFS was independently associated with the presence of RSS [hazard ratio (HR), 4.462; 95% confidence interval (CI): 1.333-14.933; P=0.015) (Table 6). PFS was not related to the type of anastomosis (P=0.501).
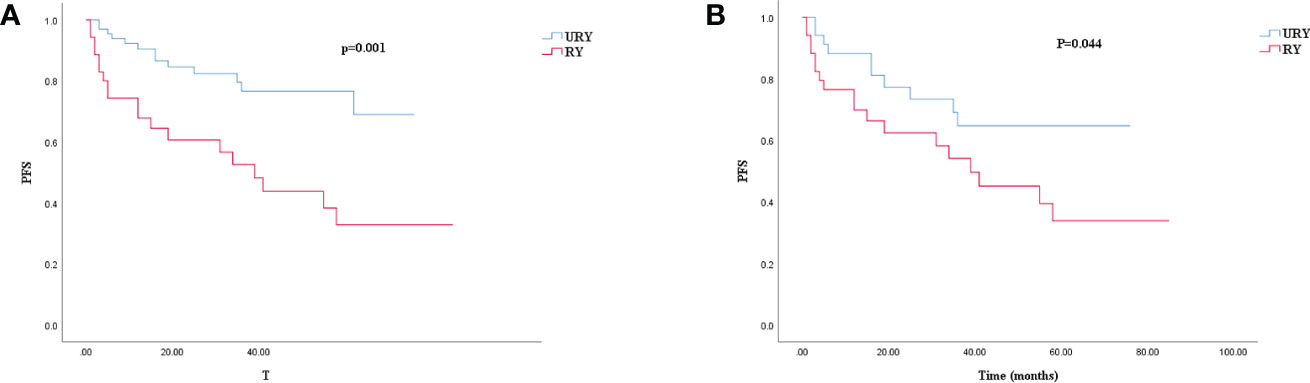
Figure 3. Kaplan–Meier survival curve for PFS of URY group and RY group. (A) was unmatched analyses and (B) was propensity-score-matched analyses.
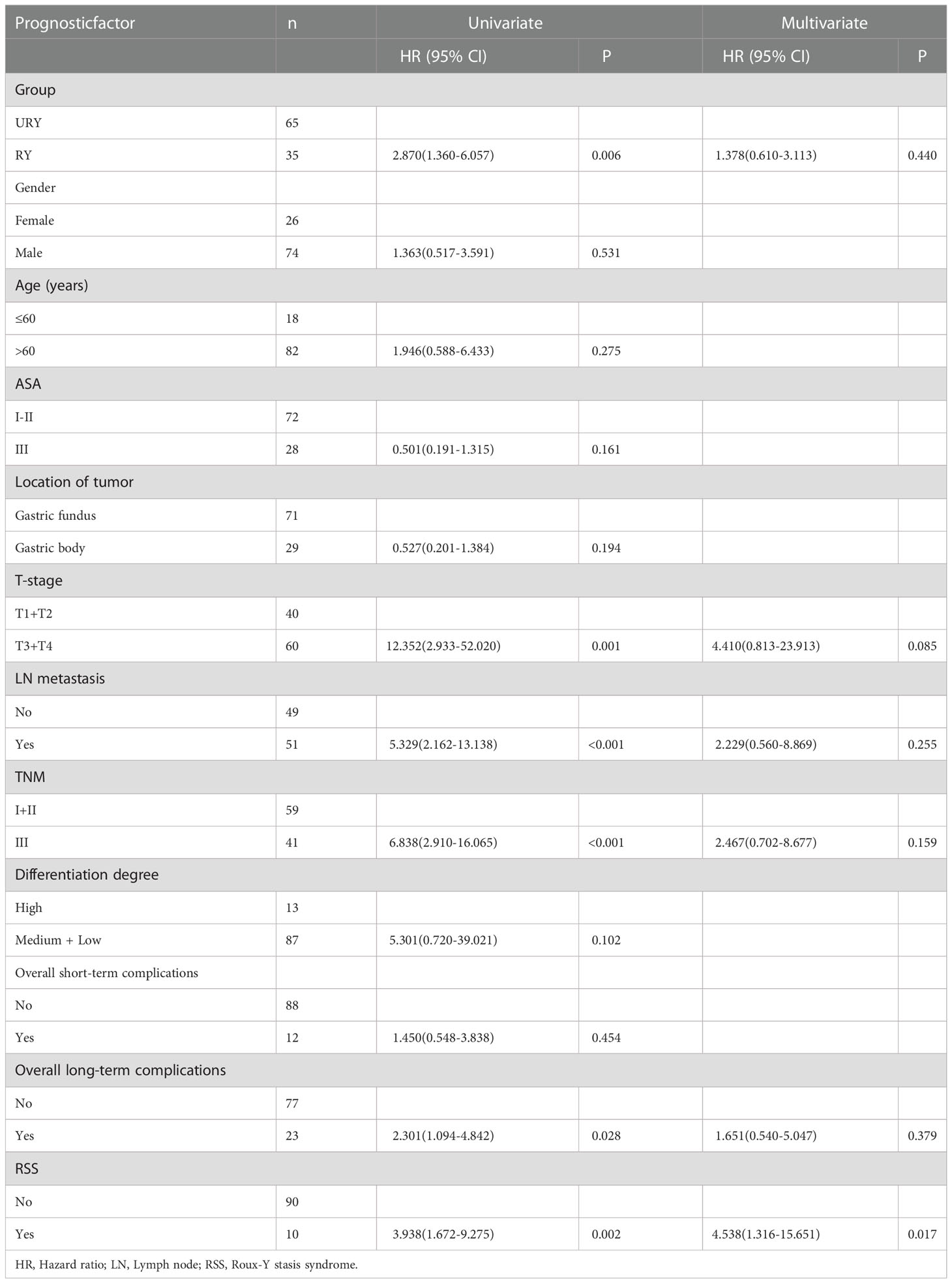
Compared to the RY group, the OS of GC patients in URY group was significantly improved before PSM (log-rank; P=0.004) (Figure 4A). However, there was no difference in OS between the two groups after PSM (log-rank; P=0.149) (Figure 4B). The 1-, 3-, 5-year OS of URY group and RY group were 95.3% vs. 73.7% (P=0.006), 80.6% vs. 58.2% (P=0.014), 74.4% vs. 49.2% (P=0.009), respectively. After PSM, the 1-, 3-, and 5-year OS were 90.8% vs. 76.0% (P=0.100), 71.8% vs. 59.9% (P=0.287), 62.5% vs. 50.7% (P=0.310), respectively. Cox multivariate analysis showed that poor OS was independently associated with the presence of RSS [HR, 5.538; 95% CI: 1.316-15.651; P=0.017) (Table 7). There was no correlation between OS and the type of anastomosis (P=0.440).
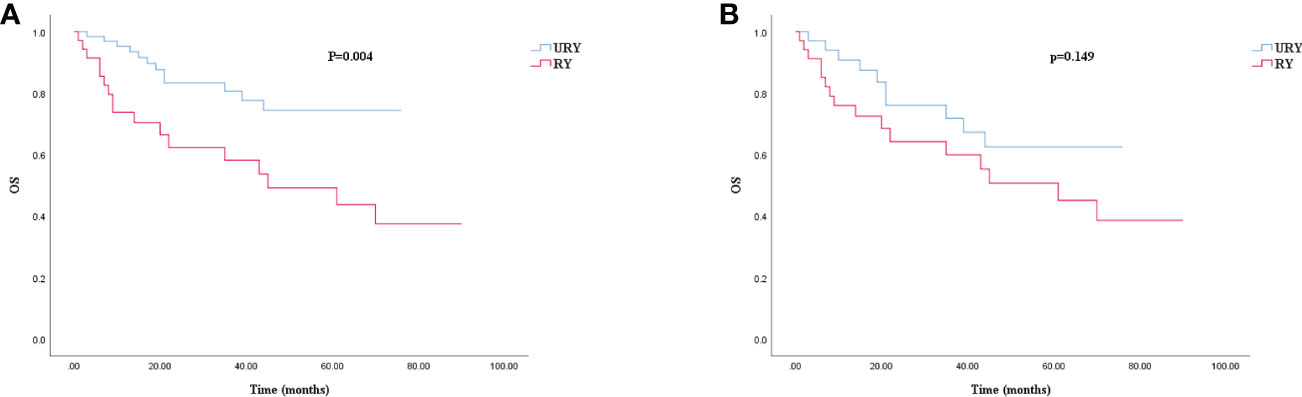
Figure 4. Kaplan–Meier survival curve for OS of URY group and RY group. (A) was unmatched analyses and (B) was propensity-score-matched analyses.
The PFS of the two groups was different before and after PSM, subgroup analysis was used to further study the effect of anastomosis on PFS under different factors (Figure 5). The results showed that PFS of URY group was better than RY group in most subgroups.
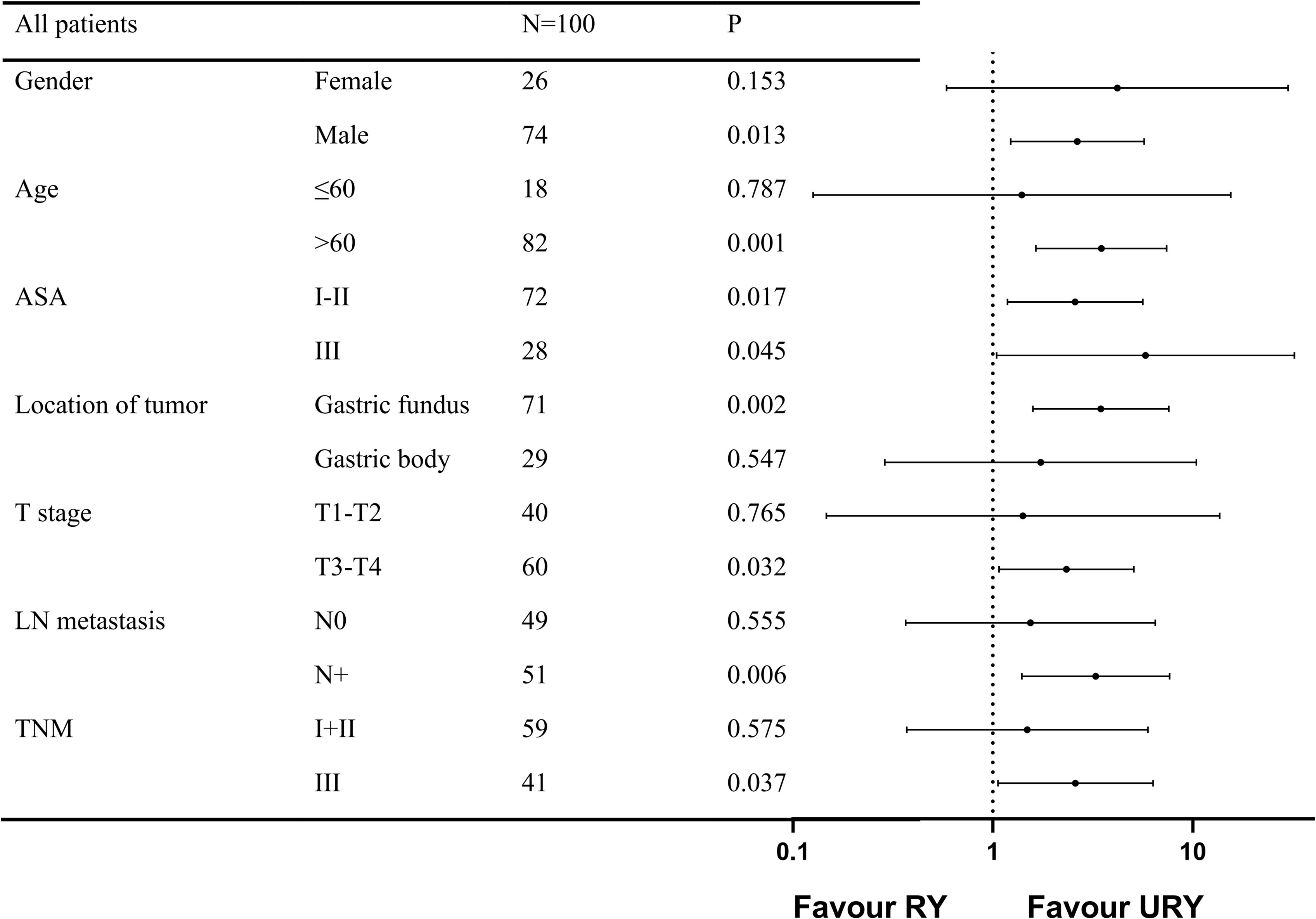
Figure 5. Forest plot evaluating the impact of TLTG with RY vs URY on PFS. TLTG: totally laparoscopic total gastrectomy; LN:Lymph node; RY: Roux-en-Y; URY: uncut RY.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The medical ethics committee of the Fujian Provincial Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All author contributed to the article and approved the submitted version. YZ-C, TZ, YF-C, YY-Z: material preparation, search and data collection. ST, SL-L, XJ-L and YH-Z: figure preparation. YZ-C, WJ-C, and YL-M: write original draft. ST-L, CS-Y and WH-L: supervision and conceptualization. WH-L: modify the draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network JNCCN (2022) 20(2):167–92. doi: 10.6004/jnccn.2022.0008
2. Siegel RL, Miller KD, Fuchs HE, and Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Zurleni T, Gjoni E, Altomare M, and Rausei S. Conversion surgery for gastric cancer patients: A review. World J gastrointestinal Oncol (2018) 10(11):398–409. doi: 10.4251/wjgo.v10.i11.398
4. Barbarisi A, Parisi V, Parmeggiani U, Cremona F, and Delrio P. Impact of surgical treatment on quality of life of patients with gastrointestinal tumors. Ann Oncol Off J Eur Soc Med Oncol (2001) 12 Suppl 3:27–30. doi: 10.1093/annonc/12.suppl_3.s27
5. Guo Z, Deng C, Zhang Z, Liu Y, Qi H, and Li X. Safety and effectiveness of overlap esophagojejunostomy in totally laparoscopic total gastrectomy for gastric cancer: A systematic review and meta-analysis. Int J Surg (London England) (2022) 102:106684. doi: 10.1016/j.ijsu.2022.106684
6. Muneoka Y, Ohashi M, Kurihara N, Fujisaki J, Makuuchi R, Ida S, et al. Short- and long-term oncological outcomes of totally laparoscopic gastrectomy versus laparoscopy-assisted gastrectomy for clinical stage I gastric cancer. Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2021) 24(5):1140–9. doi: 10.1007/s10120-021-01181-w
7. Antonakis PT, Ashrafian H, and Isla AM. Laparoscopic gastric surgery for cancer: Where do we stand? World J Gastroenterol (2014) 20(39):14280–91. doi: 10.3748/wjg.v20.i39.14280
8. Kitagami H, Morimoto M, Nakamura K, Watanabe T, Kurashima Y, Nonoyama K, et al. Technique of roux-En-Y reconstruction using overlap method after laparoscopic total gastrectomy for gastric cancer: 100 consecutively successful cases. Surg endoscopy (2016) 30(9):4086–91. doi: 10.1007/s00464-015-4724-6
9. Shen J, Ma X, Yang J, and Zhang JP. Digestive tract reconstruction options after laparoscopic gastrectomy for gastric cancer. World J gastrointestinal Oncol (2020) 12(1):21–36. doi: 10.4251/wjgo.v12.i1.21
10. Wei M, Wang N, Yin Z, Wu T, Zhou S, Dang L, et al. Short-term and quality of life outcomes of patients using linear or circular stapling in esophagojejunostomy after laparoscopic total gastrectomy. J gastrointestinal Surg Off J Soc Surg Alimentary Tract (2021) 25(7):1667–76. doi: 10.1007/s11605-020-04806-0
11. Lu J, Wu Z, Liu G, Wang B, and Shi L. The clinical effectiveness of establishing a proximal jejunum pouch after laparoscopic total gastrectomy: A propensity score-based analysis. Asian J Surg (2022) 45(1):425–30. doi: 10.1016/j.asjsur.2021.07.002
12. Wang J, Wang Q, Dong J, Yang K, Ji S, Fan Y, et al. Total laparoscopic uncut roux-En-Y for radical distal gastrectomy: An interim analysis of a randomized, controlled, clinical trial. Ann Surg Oncol (2021) 28(1):90–6. doi: 10.1245/s10434-020-08710-4
13. Park YS, Shin DJ, Son SY, Kim KH, Park DJ, Ahn SH, et al. Roux stasis syndrome and gastric food stasis after laparoscopic distal gastrectomy with uncut roux-En-Y reconstruction in gastric cancer patients: A propensity score matching analysis. World J Surg (2018) 42(12):4022–32. doi: 10.1007/s00268-018-4715-6
14. Wu F, Ni Z, Diao H, Huang C, Wang S, Ge B, et al. Recanalization in uncut roux-En-Y reconstruction: An animal experiment and a clinical study. Front Surg (2021) 8:644864. doi: 10.3389/fsurg.2021.644864
15. Morton JM, Lucktong TA, Trasti S, and Farrell TM. Bovine pericardium buttress limits recanalization of the uncut roux-En-Y in a porcine model. J gastrointestinal Surg Off J Soc Surg Alimentary Tract (2004) 8(1):127–31. doi: 10.1016/j.gassur.2003.09.024
16. Japanese Gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2021) 24(1):1–21. doi: 10.1007/s10120-020-01042-y
17. Dindo D, Demartines N, and Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
18. Sun C, Wang Y, Yao HS, and Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: Systematic review and meta-analysis. Int J Surg (London England) (2015) 13:102–10. doi: 10.1016/j.ijsu.2014.11.044
19. Yan Y, Wang D, Liu Y, Lu L, Wang X, Zhao Z, et al. Optimal reconstruction after laparoscopic distal gastrectomy: A single-center retrospective study. Cancer control J Moffitt Cancer Center (2022) 29:10732748221087059. doi: 10.1177/10732748221087059
20. Yang L, Xu H, Zhang DC, Li FY, Wang WZ, Li Z, et al. Uncut roux-En-Y reconstruction in a laparoscopic distal gastrectomy: A single-center study of 228 consecutive cases and short-term outcomes. Surg Innovation (2019) 26(6):698–704. doi: 10.1177/1553350619860964
21. Xing J, Wang Y, Shan F, Li S, Jia Y, Ying X, et al. Comparison of totally laparoscopic and laparoscopic assisted gastrectomy after neoadjuvant chemotherapy in locally advanced gastric cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol (2021) 47(8):2023–30. doi: 10.1016/j.ejso.2021.02.002
22. Zhong X, Wei M, Ouyang J, Cao W, Cheng Z, Huang Y, et al. Efficacy and safety of totally laparoscopic gastrectomy compared with laparoscopic-assisted gastrectomy in gastric cancer: A propensity score-weighting analysis. Front Surg (2022) 9:868877. doi: 10.3389/fsurg.2022.868877
23. Sun MM, Fan YY, and Dang SC. Comparison between uncut roux-En-Y and roux-En-Y reconstruction after distal gastrectomy for gastric cancer: A meta-analysis. World J Gastroenterol (2018) 24(24):2628–39. doi: 10.3748/wjg.v24.i24.2628
24. Li Y, Wang Q, Yang KL, Wang J, Jiang KW, and Ye YJ. Uncut roux-En-Y might reduce the rate of reflux gastritis after radical distal gastrectomy: An evidence mapping from a systematic review. Int J Surg (London England) (2022) 97:106184. doi: 10.1016/j.ijsu.2021.106184
25. Park JY and Kim YJ. Uncut roux-En-Y reconstruction after laparoscopic distal gastrectomy can be a favorable method in terms of gastritis, bile reflux, and gastric residue. J gastric Cancer (2014) 14(4):229–37. doi: 10.5230/jgc.2014.14.4.229
26. Yu C, Yang T, Yan Q, Li D, Wang Y, Yang X, et al. Application of laparoscopic gastric jejunum uncut roux-En-Y anastomosis. Gastroenterol Res Pract (2022) 2022:9496271. doi: 10.1155/2022/9496271
27. Coburn N, Cosby R, Klein L, Knight G, Malthaner R, Mamazza J, et al. Staging and surgical approaches in gastric cancer: A systematic review. Cancer Treat Rev (2018) 63:104–15. doi: 10.1016/j.ctrv.2017.12.006
28. Agnes A, Lirosi MC, Panunzi S, Santocchi P, Persiani R, and D'Ugo D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol (2018) 44(4):404–19. doi: 10.1016/j.ejso.2018.01.006
29. Nakanishi K, Kanda M, and Kodera Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J Gastroenterol (2019) 25(22):2743–51. doi: 10.3748/wjg.v25.i22.2743
30. Ma Z, Deng G, Meng Z, and Wu H. Hospitalization expenditures and out-of-Pocket expenses in patients with stroke in northeast China, 2015-2017: A pooled cross-sectional study. Front Pharmacol (2020) 11:596183. doi: 10.3389/fphar.2020.596183
31. Froelich MF, Schnitzer ML, Rathmann N, Tollens F, Unterrainer M, Rennebaum S, et al. Cost-effectiveness analysis of local ablation and surgery for liver metastases of oligometastatic colorectal cancer. Cancers (2021) 13(7):1507. doi: 10.3390/cancers13071507
32. Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, and Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg (2005) 242(3):326–41; discussion 41-3. doi: 10.1097/01.sla.0000179621.33268.83
33. Seese L, Sultan I, Gleason TG, Navid F, Wang Y, Thoma F, et al. The impact of major postoperative complications on long-term survival after cardiac surgery. Ann Thorac Surg (2020) 110(1):128–35. doi: 10.1016/j.athoracsur.2019.09.100
34. Fransen LFC, Berkelmans GHK, Asti E, van Berge Henegouwen MI, Berlth F, Bonavina L, et al. The effect of postoperative complications after minimally invasive esophagectomy on long-term survival: An international multicenter cohort study. Ann Surg (2021) 274(6):e1129–e37. doi: 10.1097/sla.0000000000003772
35. Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, et al. Changes of quality of life in gastric cancer patients after curative resection: A longitudinal cohort study in Korea. Ann Surg (2012) 256(6):1008–13. doi: 10.1097/SLA.0b013e31827661c9
36. Hangtian C, Huabing H, Tianhang L, Xiaoyi Y, and Guoen F. Isoperistaltic versus antiperistaltic uncut roux-En-Y anastomosis after distal gastrectomy for gastric cancer: A propensity score matched analysis. BMC Surg (2020) 20(1):274. doi: 10.1186/s12893-020-00936-z
37. Wang X, Li Y, and Yang W. Rapid rehabilitation program can promote the recovery of gastrointestinal function, speed up the postoperative rehabilitation process, and reduce the incidence of complications in patients undergoing radical gastrectomy. J Oncol (2022) 2022:1386382. doi: 10.1155/2022/1386382
38. Zhang Y, Chen H, Yu W, Jiang H, and Zhan C. The effects of uncut roux-En-Y anastomosis on laparoscopic radical gastrectomy patients' postoperative complications and quality of life. Am J Trans Res (2021) 13(8):9530–7.
39. Hersberger L, Bargetzi L, Bargetzi A, Tribolet P, Fehr R, Baechli V, et al. Nutritional risk screening (Nrs 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: Secondary analysis of a prospective randomised trial. Clin Nutr (Edinburgh Scotland) (2020) 39(9):2720–9. doi: 10.1016/j.clnu.2019.11.041
40. van Zanten ARH, De Waele E, and Wischmeyer PE. Nutrition therapy and critical illness: Practical guidance for the icu, post-icu, and long-term convalescence phases. Crit Care (London England) (2019) 23(1):368. doi: 10.1186/s13054-019-2657-5
41. Vancamelbeke M and Vermeire S. The intestinal barrier: A fundamental role in health and disease. Expert Rev Gastroenterol Hepatol (2017) 11(9):821–34. doi: 10.1080/17474124.2017.1343143
42. Farré R, Fiorani M, Abdu Rahiman S, and Matteoli G. Intestinal permeability, inflammation and the role of nutrients. Nutrients (2020) 12(4):1185. doi: 10.3390/nu12041185
Keywords: gastric cancer, total gastrectomy, laparoscope, uncut Roux-en-Y, digestive tract reconstruction
Citation: Chen Y, Zheng T, Chen Y, Zheng Y, Tan S, Liu S, Zhou Y, Lin X, Chen W, Mi Y, Lin S, Yang C and Li W (2022) Totally laparoscopic total gastrectomy with Uncut Roux-en-Y for gastric cancer may improve prognosis: A propensity score matching comparative study. Front. Oncol. 12:1086966. doi: 10.3389/fonc.2022.1086966
Received: 01 November 2022; Accepted: 07 December 2022;
Published: 22 December 2022.
Edited by:
Andras Papp, University of Pecs, HungaryReviewed by:
Karl-Hermann Fuchs, Julius Maximilian University of Würzburg, GermanyIstván Besznyák, Uzsoki Street Hospital, Hungary
Copyright © 2022 Chen, Zheng, Chen, Zheng, Tan, Liu, Zhou, Lin, Chen, Mi, Lin, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihua Li, bGl3aEBmam11LmVkdS5jbg==
†These authors share first authorship
 Yizhen Chen
Yizhen Chen Tao Zheng1,2†
Tao Zheng1,2† Yifan Chen
Yifan Chen Yuhang Zhou
Yuhang Zhou Weijie Chen
Weijie Chen Weihua Li
Weihua Li