- 1Division of Medical Oncology, Department of Internal Medicine, The Ohio State University, Columbus, OH, United States
- 2Department of Medicine, University of Rochester Medical Center, Rochester, NY, United States
- 3Department of Surgery, University of Rochester Cancer Center National Cancer Institute (NCI) Community Oncology Research Program (NCORP) Research Base, Rochester, NY, United States
- 4Department of Radiation Oncology, School of Nursing, University of Rochester, Rochester, NY, United States
- 5Department of Radiation Oncology, City of Hope Comprehensive Cancer Center, Duarte, CA, United States
- 6West Cancer Center & Research Institute, Memphis, TN, United States
- 7Divisions of Hematology/Oncology and Geriatrics, Department of Medicine, University of California, San Francisco, San Francisco, CA, United States
- 8Division of Medical Oncology and Respiratory Medicine, Department of Internal Medicine, Shimane University Faculty of Medicine, Izumo, Japan
Introduction: More older adults die from lung cancer worldwide than breast, prostate, and colorectal cancers combined. Current lung cancer treatments may prolong life, but can also cause considerable treatment-related toxicity.
Objective: This study is a secondary analysis of a cluster-randomized clinical trial which evaluated whether providing a geriatric assessment (GA) summary and GA-guided management recommendations can improve grade 3-5 toxicity among older adults with advanced lung cancer.
Methods: We analyzed participants aged ≥70 years(y) with stage III & IV (advanced) lung cancer and ≥1 GA domain impairment starting a new cancer treatment with high-risk of toxicity within the National Cancer Institute’s Community Oncology Research Program. Community practices were randomized to the intervention arm (oncologists received GA summary & recommendations) versus usual care (UC: no summary or recommendations given). The primary outcome was grade 3-5 toxicity through 3 months post-treatment initiation. Secondary outcomes included 6-month (mo) and 1-year overall survival (OS), treatment modifications, and unplanned hospitalizations. Outcomes were analyzed using generalized linear mixed and Cox proportional hazards models with practice site as a random effect. Trial Registration: NCT02054741.
Results & Conclusion: Among 180 participants with advanced lung cancer, the mean age was 76.3y (SD 5.1), 39.4% were female and 82.2% had stage IV disease. The proportion of patients who experienced grade 3-5 toxicity was significantly lower in the intervention arm vs UC (53.1% vs 71.6%, P=0.01). More participants in the intervention arm received lower intensity treatment at cycle 1 (56.3% vs 35.3%; P<0.01). Even with a cycle 1 dose reduction, OS at 6mo and 1 year was not significantly different (adjusted hazard ratio [HR] intervention vs. UC: 6mo HR=0.90, 95% CI: 0.52-1.57, P=0.72; 1 year HR=0.89, 95% CI: 0.58-1.36, P=0.57). Frequent toxicity checks, providing education and counseling materials, and initiating direct communication with the patient’s primary care physician were among the most common GA-guided management recommendations. Providing a GA summary and management recommendations can significantly improve tolerability of cancer treatment among older adults with advanced lung cancer.
Introduction
Over 75% of all new non-small cell lung cancer (NSCLC) diagnoses are among adults ≥65 years of age (1). As lung cancer is generally a disease of older adults, cancer and aging research is significant because the population of older adults is large and growing. By 2030, nearly two-thirds of all cancer diagnoses will be among older adults (2, 3). More older adults die from lung cancer worldwide than any other cancer type (1). In the United States, among adults ≥65 years, 289 men and women per 100,000 will develop lung cancer (4). However, clinical trials include almost exclusively younger adults, thereby limiting external generalizability of clinical results to older adults, particularly in regard to toxicity and response to novel cancer drugs (5, 6). With the rapid approval of novel cancer drugs, the lack of evidence among older adults in pivotal trials continues to grow (7). A lack of clinical trial evidence perpetuates uncertainty for clinicians, patients, and families regarding important clinical outcomes such as treatment-related toxicity among older adults receiving lung cancer treatment within the community oncology setting. In addition, clinical trials include the healthiest older adults with little to no information on older adults with complex geriatric conditions.
Prior research has demonstrated that older adults with cancer have a high prevalence of characteristics that are associated with a greater risk of chemotherapy toxicity (8, 9). A geriatric assessment (GA) can identify areas of vulnerability (e.g., functional impairment, cognitive impairment, polypharmacy) and thus direct GA-guided management for older adults receiving cancer treatment (10–13). The GA has great potential to identify areas of vulnerability and develop recommendations that could help improve outcomes (e.g., treatment toxicity) among older adults with cancer (14–16). However, this type of evaluation is not routinely incorporated into the oncology clinical evaluation. A critical knowledge gap exists in respect to whether provision of GA information along with GA-guided management recommendations to the oncology treatment team would improve outcomes among older adults with advanced lung cancer receiving cancer treatment with a high risk of toxicity.
Balancing the benefits and risks of chemotherapy in the older adult patient population with advanced cancer is challenging because of the dearth of evidence-based data to guide these decisions (17, 18). Furthermore, older patients who are treated with chemotherapy are at high risk for adverse outcomes, including chemotherapy toxicity and functional and physical consequences (19–21). In addition, older adults are more susceptible to toxicity from combination chemotherapy plus newer immunotherapy or targeted kinase inhibitors (22–25). In a randomized controlled trial by Corre et al, GA-guided lung cancer treatment strategies have been shown to lower symptomatic toxicities and improve other clinical outcomes among older adults receiving chemotherapy for advanced lung cancer (26). There was no difference seen in overall survival between the GA-directed arm versus usual care; yet, 23% of the patients treated in the GA-directed arm did not receive chemotherapy. A more recent large cluster-randomized controlled trial (GAP-70+) demonstrated that GA-guided management recommendations could decrease the proportion of older adults who experienced a serious grade 3-5 toxicity from a new cancer treatment regimen for advanced cancer (>80% had stage IV disease) (27). A lower proportion of patients in the intervention arm experienced grade 3-5 toxicity (177/349; 50.7%) than in usual care (263/369; 71.3%); relative risk (RR) was 0.74 (95% CI: 0.64-0.86; p<0.001) (27). GA-guided recommendations can focus on managing symptomatic toxicities from cancer treatment among patients with functional impairments or can be interventions that are known to improve outcomes of older adults with geriatric syndromes (e.g., physical therapy and fall prevention education in patients who are falling or who are at risk for falling).
The primary goal of this secondary GAP-70+ analysis was to evaluate whether providing a GA summary and GA-guided management recommendations could decrease grade 3-5 toxicity specifically among older adults with advanced lung cancer.
Methods
Study Design
This is a secondary data analysis of the participants with lung cancer from the cluster-randomized clinical trial entitled “Geriatric Assessment for Patients 70 years and older (GAP-70+; NCT02054741).” Community oncology practices within the National Cancer Institute Community Oncology Research Program (NCORP) were randomized to the intervention arm (oncologists received GA summary & management recommendations) or usual care (UC: no summary or recommendations given; notifications were provided to oncologists for patients who screened positive for depression and severe cognitive impairment). NCORP practices were recruited through the University of Rochester National Cancer Institute (NCI) Research Base network (UR NCORP). NCORP is a national network of community cancer clinical trial practice sites in the United States (https://ncorp.cancer.gov/about/). Practice clusters were comprised of NCORP-affiliated community oncology practices. Participating practice clusters represent a large geographic area across the United States of which 33/40 practices enrolled patients with lung cancer. The UR Research Base coordinated study activities, but the UR did not enroll participants. The UR (Rochester, NY, USA) and all participating practice clusters obtained approval from their institutional review boards. All patients completed informed consent.
Participants
Participants were recruited from July 2014-March 2019. Participants aged ≥70 years(y) with advance solid tumors or lymphoma and ≥1 GA domain impairment (other than polypharmacy) starting a new cancer treatment regimen with a high risk of toxicity within 4 weeks of enrollment were included. Participants were required to be able to understand English and provide written informed consent independently or with a healthcare proxy. For inclusion in this secondary analysis, participants with advanced (non-surgical stage III/IV) lung cancer, either NSCLC or extensive stage small cell lung cancer (ES-SCLC), were selected. Treatment regimens had to include at least one chemotherapy agent or have a >50% prevalence of grade 3-5 toxicity as determined by the primary oncologist with review and approval by a clinical team blinded to study arm at the Research Base (27, 28). The treating oncologists selected the specific treatment regimen, dosing, and schedules.
Procedures
Community oncology practice clusters were randomized to the GA intervention versus UC arm, stratified by large or small based on prior accrual records. Participants in both arms completed a GA and were asked about proposed treatment plan before starting a new treatment regimen. Participants in the intervention arm were additionally given recommendations before starting a new treatment regimen. Oncologists in the intervention arm were provided with a tailored GA summary and GA-guided management recommendations before any cancer treatment was initiated. The GA evaluated 8 domains: comorbidity, cognition, physical performance, functional status, nutritional status, social support, polypharmacy, and psychological health. The recommendations provided based on GA domain impairment can be found in detail in the supplemental documents of Mohile et al. (27) Oncologists in the UC arm received notification for depression or severe cognitive impairment on screening tests, but no management recommendations were provided. There was no patient or provider blinding as this study evaluated a model of care rather than a particular treatment agent; however, all research investigators were blinded to the site assignment when the treatment and toxicity data were reviewed centrally.
Outcomes
The primary outcome was grade 3-5 toxicity within 3 months of starting a new treatment regimen. Secondary outcomes included unplanned hospitalizations, subsequent dose reduction, dose delay, treatment discontinuation, overall survival (OS) at 6-month (mo) and 1-year in addition to cycle 1 treatment intensity (standard vs reduced). Practice staff prospectively captured toxicities over 3 months using NCI’s Common Terminology Criteria for Adverse Events (V4.0). Blinded oncology clinicians reviewed medical records to verify all treatment and toxicity data. At UR NCORP, two blinded clinicians reviewed each enrolled patient’s medical record and treatment regimen and used guidelines and clinical trials to determine standard dosing and length for treatment regimens. We evaluated the proportion of patients who received a reduced intensity regimen (e.g., lower dose or omission of an agent compared to standard) at cycle one. Standard treatment was evaluated according to National Comprehensive Cancer Network guidelines (29) of published phase II/III clinical trials. The blinded clinicians also reviewed medical records to evaluate unplanned hospitalizations (an overnight hospital stay for any reason that was not scheduled), dose reductions, dose delays, and treatment discontinuation. These were assessed by comparing what the patient received compared to what was planned by the oncologist at the start of treatment. Outcomes captured those changes related to clinical reasons (e.g., toxicity, patient preference) but not logistical reasons (e.g., holiday).
Statistical Analysis
Descriptive statistics were performed to summarize demographics, GA measures, baseline clinical characteristics, and outcome measures. Bivariate analyses using chi-square tests for categorical variables and t tests for continuous variables were done to compare differences between study arms. A Generalized Linear Mixed Model (GLMM) was applied to analyze the primary outcome of grade 3-5 toxicity within 3 months with practice site as a random effect and study arm as a fixed effect. Proportions of patients who experienced grade 3-5 toxicity in the intervention vs UC arm were calculated by odds ratio adjusted for practice site. Kaplan-Meier method was used to estimate 6-month and 1-year OS and the effect of the intervention on OS was assessed by Cox Shared Frailty Model with practice sites as random effects. Similar to the primary outcome, GLMMs were applied to evaluate secondary outcomes (hospitalization, subsequent dose reduction, dose delay, treatment discontinuation, and reduced treatment intensity at cycle 1). Two-sided p values of <0.05 were considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
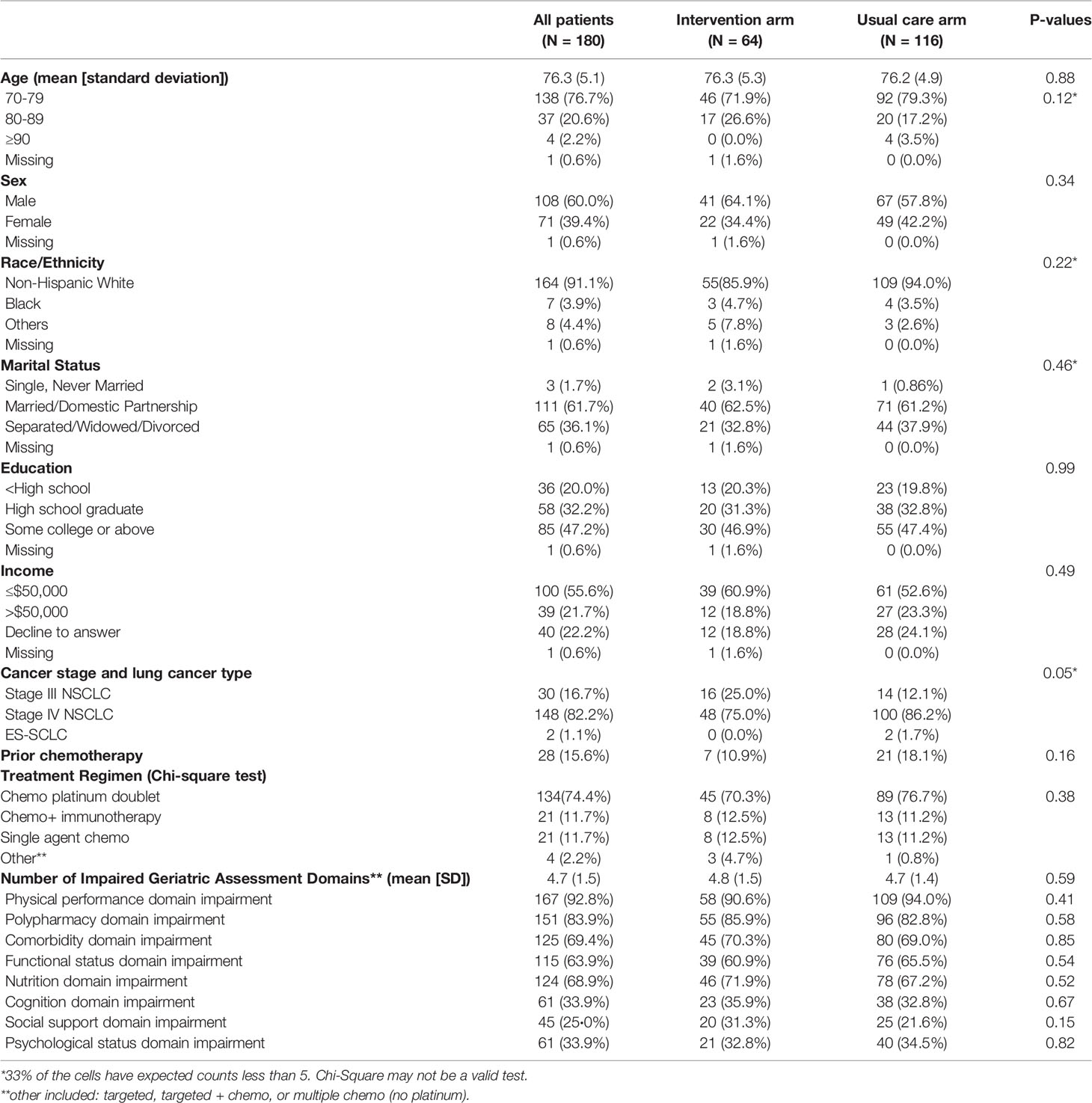
Among 180 participants with advanced lung cancer (NSCLC + ES-SCLC), the mean age was 76.3y (range 70-91, SD 5.1), 39.4% were female and 82.2% had stage IV disease. Patients in both arms (64 participants in the intervention and 116 participants in the UC arm) had similar baseline characteristics including age, sex, race/ethnicity, marital status, education, and income (Table 1). The majority of participants received platinum doublet chemotherapy (>70%). The GA domain impairments had similar distributions across arms. The mean number of geriatric impairments was 4.7 (SD: 1.5) and did not differ between study arms. The physical performance domain impairment was the most prevalent GA impaired domain (>90% in both arms). This was followed by polypharmacy, comorbidity, functional status, and nutritional domain impairment (Table 1).
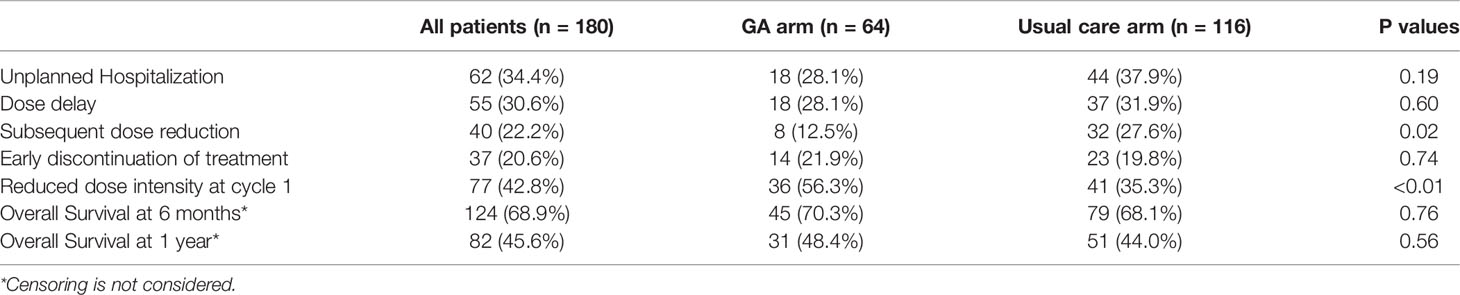
Sixty-five percent of all participants experienced a grade 3-5 toxicity (Figure 1). The proportion of patients who experienced grade 3-5 toxicity was lower in the intervention vs. UC arm (53.1% vs 71.6%, P=0.01). After accounting for practice sites as a random effect, the odds of any grade 3-5 toxicity were lower in the intervention vs. UC arm (Adjusted odds ratio=0.45 95% CI: 0.24-0.86, P=0.01, Figure 2). More participants in the intervention group received lower intensity treatment at cycle 1 (56.3% vs 35.3%; P<0.01). Unplanned hospitalizations, dose delay, and early discontinuation were similar across groups. Subsequent dose reduction post-C1 was significantly higher in the UC arm (P=0.02, Table 2).
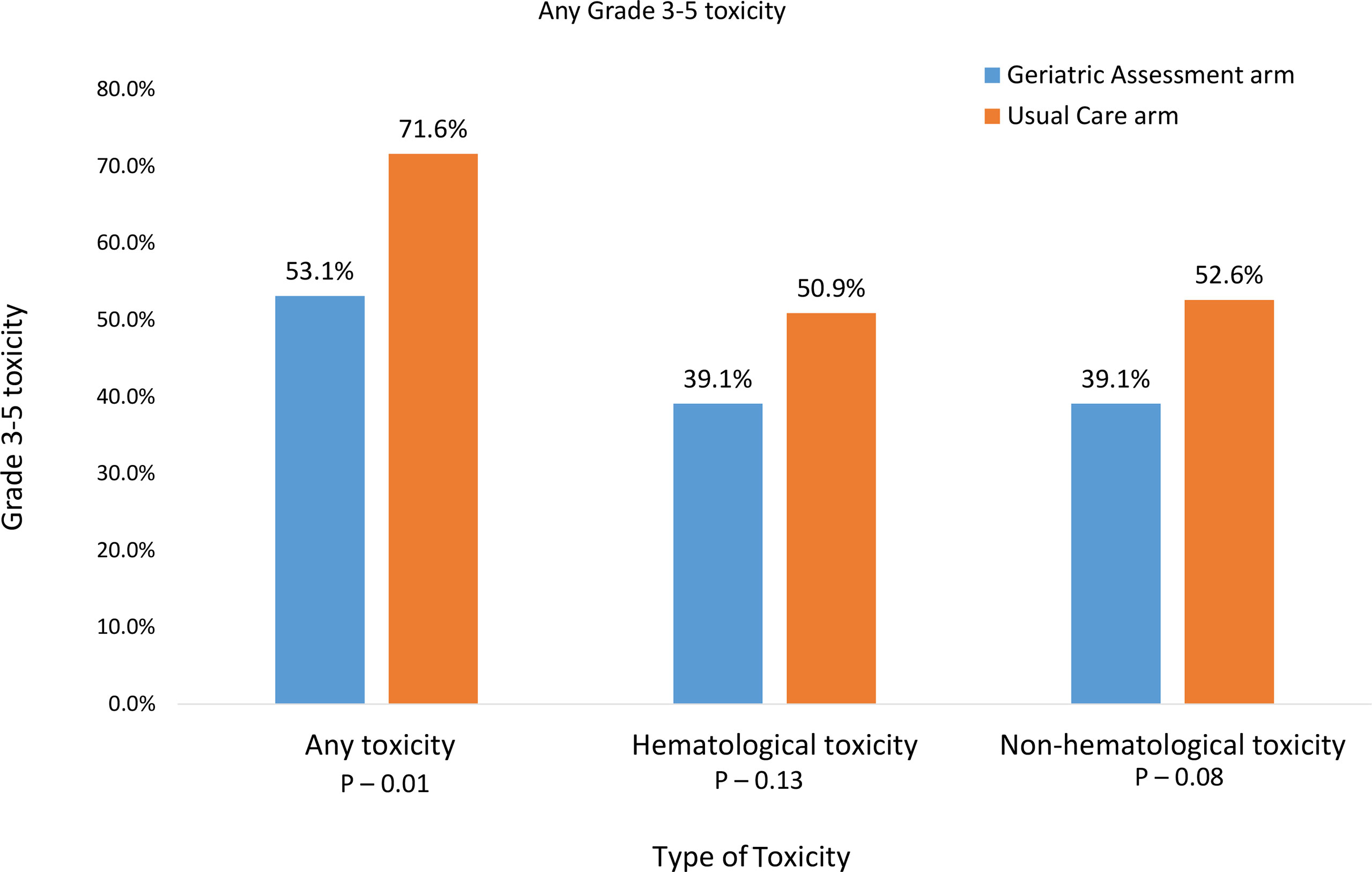
Figure 1 Prevalence of grade 3-5 toxicities over 3 months after the start of new treatment for advanced stage III/IV lung cancer.
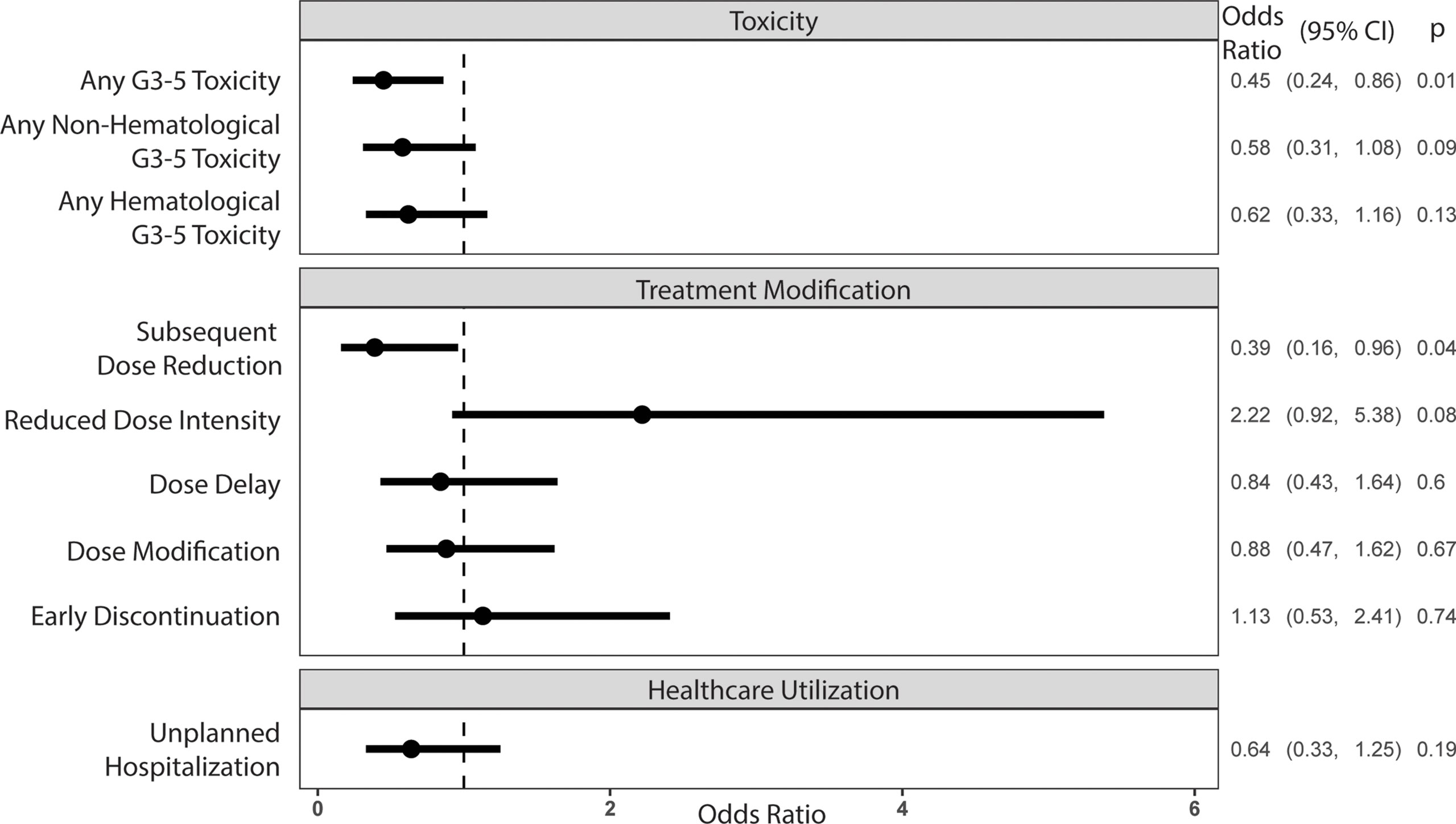
Figure 2 Odds ratios of outcome variables associated with the intervention arm, controlling for the site cluster (random effect)*. *All outcomes except reduced dose intensity at cycle 1 were assessed at 3 months of treatment.
The OS at 6mo and 1 year was not significantly different between arms (Figures 3A, B: adjusted hazard ratio [HR] interventions vs. UC: 6mo HR=0.90, 95% CI: 0.52-1.57, P=0.72; 1 year HR=0.89, 95% CI: 0.58-1.36, P=0.57). Frequent toxicity checks, providing education and counseling materials, and initiating direct communication with the patient’s primary care physician were among the most common GA-guided interventions recommended and acknowledged by the treating oncologist (Table 3).
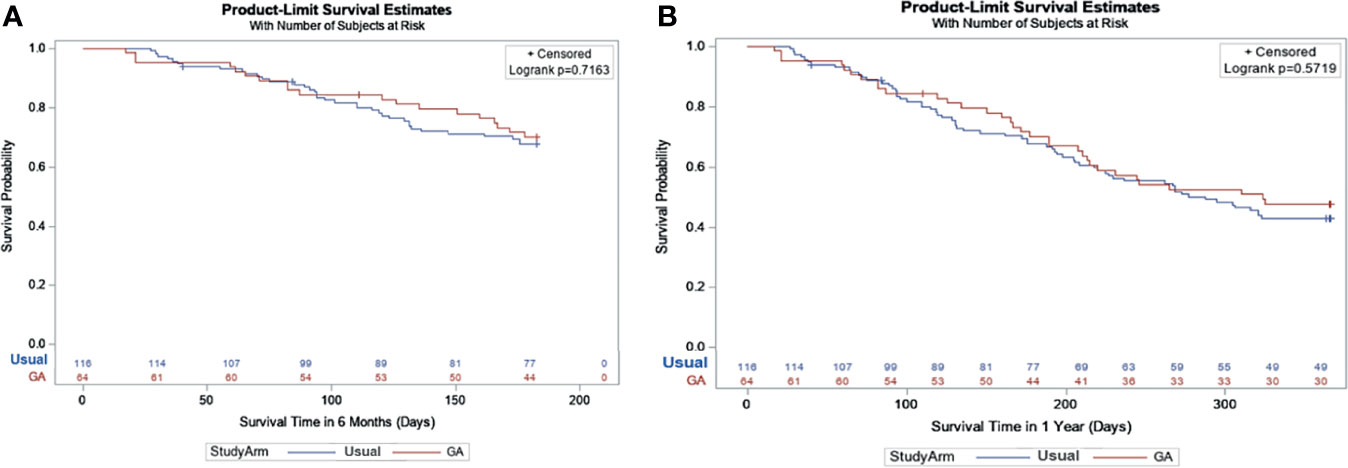
Figure 3 (A) Survival at 6 months based on Kaplan-Meier Estimates and Cox Model*. *Geriatric Assessment Intervention: 70.1% vs. Usual Care: 67.7%; Adjusted Hazard Ratio: 0.90 95% CI: (0.52-1.57), P = 0.72. (B) Survival at 1 year based on Kaplan-Meier Estimates and Cox Model*. *Geriatric Assessment Intervention: 47.8% vs. Usual Care: 43.1%; Adjusted Hazard Ratio: 0.89 95% CI: (0.58-1.36), P = 0.57.
Discussion
Providing GA information and recommendations can improve tolerability of cancer treatment among older adults with advanced lung cancer. Despite a significant difference in C1 dose reduction between arms (56.3% in the intervention arm versus 35.3% in the UC arm), there was no significant difference in 6-month or 1-year OS. However, there was a significantly decreased risk of grade 3-5 toxicity for the intervention arm. The majority of participants received a platinum-based chemotherapy regimen which is explained by the standard-of-care treatment at the time this study was conducted. The current standard-of-care is a platinum doublet with immunotherapy for most patients depending on PD-L1 status. Yet, our findings are still relevant to current treatment recommendations. With the addition of immunotherapy now to standard platinum doublets, the risk of toxicities is potentially even higher (30). Unfortunately, the proportion of older adults comprise only 41-55% of all patients with NSCLC included in the phase III clinical trials that led to the drug approvals (30), which are the healthiest of older adults. The incidence of high-grade toxicities among older adults with GA domain impairment receiving chemotherapy + immunotherapy is currently unknown.
This study confirms the utility of a GA among older adults with advanced lung cancer. The decrease in toxicity is similar to lung cancer outcome data presented by Corre et al. in the ESOGIA-GFPC-GECP 08-02 Study (26). Yet, a distinct difference is that GAP70+ is one of the first studies in the United States to provide geriatric domain-focused recommendations while letting the oncology team decide the final cancer treatment regimen. This is very distinct from the ESOGIA study that used the GA to dictate the lung cancer treatment regimen. The former approach is likely a much more palatable design for oncology clinicians in the United States, where personal and professional autonomy is culturally prioritized over algorithmic pathway approaches. This approach is also consistent with a current emphasis on shared patient-provider decision-making.
The majority of the GA was completed from patient-reported information. This may cause a barrier to implementation if the resources are not available either in-person or electronically to capture the patient-reported information. There are alternative GA tools (31, 32) such as the G8, the CARG, and CRASH tools that are shorter than the GA performed in this study; yet, many are not validated with the use of newer cancer therapeutics and do not include recommendations to the oncology team.
For advanced NSCLC in the United States, single agent immunotherapy (IO) is now a Food & Drug Administration-approved treatment option. Fewer patients who received single agent IO experienced grade 3-5 adverse events at 5 years of follow-up compared to those who received chemotherapy alone for PD-L1 positive (≥50%) disease (33). However, the trial comparing chemotherapy + IO versus IO alone (INSIGNA NCT NCT03793179) is ongoing. The PACIFIC study (34) also demonstrated an improvement in overall survival with the addition of durvalumab after concurrent chemoradiation. Unfortunately, over half of all older adults with advanced lung cancer are excluded from clinical trials (35). Future directions will hopefully explore GA-guided recommendations in a prospective clinical trial design among older adults with GA impairment receiving chemotherapy + IO for stage IV and chemoradiation therapy for stage III NSCLC. Whether to use concurrent versus sequential chemoradiation is controversial, but may have equivalent outcomes for older adults (36).
The majority of patients experienced impairment in physical performance and issues with polypharmacy. This is similar to the findings of Gomes et al. in a study of 70 older adults receiving IO for advanced NSCLC or malignant melanoma (37). Similarly, a study of over 200 older adults with lung cancer receiving treatment and GA demonstrated that handgrip strength was the most commonly impaired domain in octogenarians (38). Targeted interventions to improve both polypharmacy and physician impairment among other GA domain impairments are possible and should be incorporated into future research.
Limitations
A very small number of older adults with ES-SCLC were included, which is not representative of the percentage of patients with SCLC in the United States. The majority received a platinum-based chemotherapy regimen, which was standard-of-care treatment at the time this study was conducted. This high number of platinum doublet may be higher than that of other countries and may not be necessarily generalizable to other countries or geographic regions. The standard of care treatment has also changed since the study period, and now includes a combination of chemotherapy + IO; in addition, older adults may have received single agent IO, which would not have met the high-toxicity regimen inclusion criteria. There was a higher number of stage IIIB patients in the intervention than the usual care arm, which could affect the secondary survival endpoints. Future studies would need to use survival as the primary endpoint and stage as a stratification factor for randomization. This study required a full GA assessment, which is often not possible in routine clinical cancer care. Due to the nature of this secondary data analysis and small sample size of the subgroup of patients with lung cancer, the analysis focusing on hematologic and non-hematologic toxicities separately and the secondary endpoints analyses may be lacking sufficient statistical power. Future prospective properly powered study may be needed to confirm these promising results. These limitations may reduce the overall generalizability of the study results.
Conclusion
The use of a GA assessment and recommendations can result in upfront treatment dose reduction and a decrease in high-grade toxicity among older adults with advanced lung cancer without compromising survival outcomes. This is one of the first subset analyses in the United States to demonstrate the importance of GA recommendations in geriatric oncology treatment among older adults with advanced lung cancer.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The University of Rochester (Rochester, NY, USA) and all participating practice clusters obtained approval from their institutional review boards. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SM and CP: conceptualization. SM, EC, MF, and MM: data collection. MM, EC, RH, and DS: data analysis. CP and PV: drafting original version. CP and SM: supervision. All authors read and approved the final version of this manuscript.
Funding
This work was supported by the National Cancer Institute: R01CA177592, U01CA233167, UG1CA189961, The Ohio State University Comprehensive Cancer Center, and The National Institute of Aging (CP, 1K76AB074923-01, MW, K76AG064431, SM, K24AG056589, R33AG059206, DS, 1K01AG070310-01A1). Research reported in this publication was supported by The Ohio State University Comprehensive Cancer Center and the National Institutes of Health under grant number P30 CA016058.
Conflict of Interest
MW receives royalties from UpToDate and immediate family member is an employee of Genentech with stock ownership. Dr. Flannery reports grants from NIH NCI RO1 CA 177592, grants from NIH NCI UO1 CA 233167, grants from NIH NCI UG1 CA189961, during the conduct of the study. YT received grant and personal fees from Daiichi Sankyo Co., Ltd and AstraZeneca K.K. and personal fees from Chugai Pharmaceuticals Inc. outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to acknowledge the contributions of Kamila Jaroniec, M.F.A., Technical Editor, Div. of Medical Oncology, Dept. of Internal Medicine, The Ohio State University Wexner Medical Center, in the revision of this manuscript. We thank the patients for their participation.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. J Clin Oncol (2009) 27:2758–65. doi: 10.1200/JCO.2008.20.8983
3. Hurria A, Naylor M, Cohen H. Improving the Quality of Cancer Care in an Aging Population: Recommendations From an Iom Report. JAMA (2013) 310:1795–6. doi: 10.1001/jama.2013.280416
4. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2017. Bethesda, MD: National Cancer Institute (2021).
5. Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of Patients 65 Years of Age or Older in Cancer-Treatment Trials. N Engl J Med (1999) 341:2061–7. doi: 10.1056/NEJM199912303412706
6. Murthy VH, Krumholz HM, Gross CP. Participation in Cancer Clinical Trials: Race-, Sex-, and Age-Based Disparities. JAMA (2004) 291:2720–6. doi: 10.1001/jama.291.22.2720
7. Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, et al. Older Adult Participation in Cancer Clinical Trials: A Systematic Review of Barriers and Interventions. CA: A Cancer J Clin (2021) 71:78–92. doi: 10.3322/caac.21638
8. Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, et al. Association of a Cancer Diagnosis With Vulnerability and Frailty in Older Medicare Beneficiaries. J Natl Cancer Inst (2009) 101:1206–15. doi: 10.1093/jnci/djp239
9. Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, et al. Association of Cancer With Geriatric Syndromes in Older Medicare Beneficiaries. J Clin Oncol (2011) 29:1458–64. doi: 10.1200/JCO.2010.31.6695
10. Kenis C, Bron D, Libert Y, Decoster L, Van Puyvelde K, Scalliet P, et al. Relevance of a Systematic Geriatric Screening and Assessment in Older Patients With Cancer: Results of a Prospective Multicentric Study. Ann Oncol (2013) 24:1306–12. doi: 10.1093/annonc/mds619
11. Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, et al. Comprehensive Geriatric Assessment Adds Information to Eastern Cooperative Oncology Group Performance Status in Elderly Cancer Patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol (2002) 20:494–502. doi: 10.1200/JCO.2002.20.2.494
12. Kenis C, Decoster L, Van Puyvelde K, De Grève J, Conings G, Milisen K, et al. Performance of Two Geriatric Screening Tools in Older Patients With Cancer. J Clin Oncol (2014) 32:19–26. doi: 10.1200/JCO.2013.51.1345
13. Cuccia F, Mortellaro G, Mazzola R, Donofrio A, Valenti V, Tripoli A, et al. Prognostic Value of Two Geriatric Screening Tools in a Cohort of Older Patients With Early Stage Non-Small Cell Lung Cancer Treated With Hypofractionated Stereotactic Radiotherapy. J Geriatr Oncol (2020) 11:475–81. doi: 10.1016/j.jgo.2019.05.002
14. Rodin MB, Mohile SG. A Practical Approach to Geriatric Assessment in Oncology. J Clin Oncol (2007) 25:1936–44. doi: 10.1200/JCO.2006.10.2954
15. Hurria A, Cirrincione CT, Muss HB, Kornblith AB, Barry W, Artz AS, et al. Implementing a Geriatric Assessment in Cooperative Group Clinical Cancer Trials: CALGB 360401. J Clin Oncol (2011) 29:1290–6. doi: 10.1200/JCO.2010.30.6985
16. Pal SK, Katheria V, Hurria A. Evaluating the Older Patient With Cancer: Understanding Frailty and the Geriatric Assessment. CA Cancer J Clin (2010) 60:120–32. doi: 10.3322/caac.20059
17. Dale W, Mohile SG, Eldadah BA, Trimble EL, Schilsky RL, Cohen HJ, et al. Biological, Clinical, and Psychosocial Correlates at the Interface of Cancer and Aging Research. J Natl Cancer Inst (2012) 104:581–9. doi: 10.1093/jnci/djs145
18. Hurria A, Mohile SG, Dale W. Research Priorities in Geriatric Oncology: Addressing the Needs of an Aging Population. J Natl Compr Canc Netw (2012) 10:286–8. doi: 10.6004/jnccn.2012.0025
19. Hoppe S, Rainfray M, Fonck M, Hoppenreys L, Blanc JF, Ceccaldi J, et al. Functional Decline in Older Patients With Cancer Receiving First-Line Chemotherapy. J Clin Oncol (2013) 31:3877–82. doi: 10.1200/JCO.2012.47.7430
20. Kenis C, Decoster L, Bastin J, Bode H, Van Puyvelde K, De Grève J, et al. Functional Decline in Older Patients With Cancer Receiving Chemotherapy: A Multicenter Prospective Study. J Geriatr Oncol (2017) 8:196–205. doi: 10.1016/j.jgo.2017.02.010
21. Presley CJ, Arrato NA, Janse S, Shields PG, Carbone DP, Wong ML, et al. Functional Disability Among Older Versus Younger Adults With Advanced Non–Small-Cell Lung Cancer. JCO Oncol Practice:OP (2021) 17(6):e848–58. doi: 10.1200/OP.20.01004
22. Kyriakou F KP, Papamichael D. Targeted Agents: Review of Toxicity in the Elderly Metastatic Colorectal Cancer Patients. Targeted Oncool (2011) 6:245–51. doi: 10.1007/s11523-011-0198-1
23. Meoni G CFL, Lucherini E, Di Costanzo F. Medical Treatment of Advanced non-Small Cell Lung Cancer in Elderly Patients: A Review of the Role of Chemotherapy and Targeted Agents. J Geriatric Oncol (2013) 4:282–90. doi: 10.1016/j.jgo.2013.04.005
24. Zustovich F, Novara G. Advanced Kidney Cancer: Treating the Elderly. Expert Rev Anticancer Ther (2013) 13:1389–98. doi: 10.1586/14737140.2013.846095
25. Grothey A VCE, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet (2013) 381:303–12. doi: 10.1016/S0140-6736(12)61900-X
26. Corre R, Greillier L, Caër HL, Audigier-Valette C, Baize N, Bérard H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non–Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08-02 Study. J Clin Oncol (2016) 34:1476–83. doi: 10.1200/JCO.2015.63.5839
27. Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A, et al. Evaluation of Geriatric Assessment and Management on the Toxic Effects of Cancer Treatment (GAP70+): A Cluster-Randomised Study. Lancet (2021) 398(10314):1894–904. doi: 10.1016/S0140-6736(21)01789-X
28. Mohamed MR, Kyi K, Mohile SG, Xu H, Culakova E, Loh KP, et al. Prevalence of and Factors Associated With Treatment Modification at First Cycle in Older Adults With Advanced Cancer Receiving Palliative Treatment. J Geriatr Oncol (2021) 12(8):1208–13. doi: 10.1016/j.jgo.2021.06.007
29. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw (2021) 19:254–66. doi: 10.6004/jnccn.2021.0013
30. Presley CJ, Gomes F, Burd CE, Kanesvaran R, Wong ML. Immunotherapy in Older Adults With Cancer. J Clin Oncol (2021) 39:2115–27. doi: 10.1200/JCO.21.00138
31. Almodovar T, Teixeira E, Barroso A, Yin T, Liang Y. Elderly Patients With Advanced NSCLC: The Value of Geriatric Evaluation and the Feasibility of CGA Alternatives in Predicting Chemotherapy Toxicity. Pulmonology (2019) 25:40–50. doi: 10.1016/j.pulmoe.2018.07.004
32. Gridelli C, Balducci L, Ciardiello F, Di Maio M, Felip E, Langer C, et al. Treatment of Elderly Patients With Non-Small-Cell Lung Cancer: Results of an International Expert Panel Meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer (2015) 16:325–33. doi: 10.1016/j.cllc.2015.02.006
33. Brahmer JR, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. LBA51 KEYNOTE-024 5-Year OS Update: First-Line (1L) Pembrolizumab (Pembro) vs Platinum-Based Chemotherapy (Chemo) in Patients (Pts) With Metastatic NSCLC and PD-L1 Tumour Proportion Score (TPS) ≥50%. Ann Oncol (2020) 31:S1181–2. doi: 10.1016/j.annonc.2020.08.2284
34. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N Engl J Med (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
35. Tang M, Pearson SA, Schaffer AL, Lewis CR, John T, Simes RJ, et al. Are Clinical Trial Eligibility Criteria Representative of Older Patients With Lung Cancer? A Population-Based Data Linkage Study. J Geriatr Oncol (2021) 12:930–6. doi: 10.1016/j.jgo.2021.02.003
36. Maggiore RJ, Zahrieh D, McMurray RP, Feliciano JL, Samson P, Mohindra P, et al. Toxicity and Survival Outcomes in Older Adults Receiving Concurrent or Sequential Chemoradiation for Stage III Non-Small Cell Lung Cancer in Alliance Trials (Alliance A151812). J Geriatric Oncol (2020) 12(4):563–71. doi: 10.1016/j.jgo.2020.09.005
37. Gomes F, Lorigan P, Woolley S, Foden P, Burns K, Yorke J, et al. A Prospective Cohort Study on the Safety of Checkpoint Inhibitors in Older Cancer Patients – the ELDERS Study. ESMO Open (2021) 6(1):100042. doi: 10.1016/j.esmoop.2020.100042
Keywords: treatment toxicities, geriatric assessment, lung cancer, older adult, clinical trial
Citation: Presley CJ, Mohamed MR, Culakova E, Flannery M, Vibhakar PH, Hoyd R, Amini A, VanderWalde N, Wong ML, Tsubata Y, Spakowicz DJ and Mohile SG (2022) A Geriatric Assessment Intervention to Reduce Treatment Toxicity Among Older Adults With Advanced Lung Cancer: A Subgroup Analysis From a Cluster Randomized Controlled Trial. Front. Oncol. 12:835582. doi: 10.3389/fonc.2022.835582
Received: 14 December 2021; Accepted: 04 March 2022;
Published: 31 March 2022.
Edited by:
Jessica Desiree Menis, Integrated University Hospital Verona, ItalyReviewed by:
Francesco Cuccia, Sacro Cuore Don Calabria Hospital (IRCCS), ItalyAndrea Camerini, Azienda Usl Toscana Nord Ovest, Italy
Copyright © 2022 Presley, Mohamed, Culakova, Flannery, Vibhakar, Hoyd, Amini, VanderWalde, Wong, Tsubata, Spakowicz and Mohile. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolyn J. Presley, Y2Fyb2x5bi5wcmVzbGV5QG9zdW1jLmVkdQ==
 Carolyn J. Presley
Carolyn J. Presley Mostafa R. Mohamed
Mostafa R. Mohamed Eva Culakova3
Eva Culakova3 Arya Amini
Arya Amini Daniel J. Spakowicz
Daniel J. Spakowicz