- 1Faculty of Medicine and Health Sciences, UCSI University, Kuala Lumpur, Malaysia
- 2Centre of Cancer and Stem Cells Research, International Medical University, Kuala Lumpur, Malaysia
- 3Institute for Research, Development and Innovation, International Medical University, Kuala Lumpur, Malaysia
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy that raises public health concerns in endemic countries. Despite breakthroughs in therapeutic strategies, late diagnosis and drug resistance often lead to unsatisfactory clinical outcomes in NPC patients. The tumor microenvironment (TME) is a complex niche consisting of tumor-associated cells, such as fibroblasts, endothelial cells, leukocytes, that influences tumor initiation, progression, invasion, and metastasis. Cells in the TME communicate through various mechanisms, of note, exosomes, ligand-receptor interactions, cytokines and chemokines are active players in the construction of TME, characterized by an abundance of immune infiltrates with suppressed immune activities. The NPC microenvironment serves as a target-rich niche for the discovery of potential promising predictive or diagnostic biomarkers and the development of therapeutic strategies. Thus, huge efforts have been made to exploit the role of the NPC microenvironment. The whole picture of the NPC microenvironment remains to be portrayed to understand the mechanisms underlying tumor biology and implement research into clinical practice. The current review discusses the recent insights into the role of TME in the development and progression of NPC which results in different clinical outcomes of patients. Clinical interventions with the use of TME components as potential biomarkers or therapeutic targets, their challenges, and future perspectives will be introduced. This review anticipates to provide insights to the researchers for future preclinical, translational and clinical research on the NPC microenvironment.
Introduction
Nasopharyngeal carcinoma (NPC) is a squamous cell neoplasm that originated from the nasopharyngeal mucosal lining, commonly found at the fossa of Rosenmüller (1, 2). In 2020, the International Agency for Research on Cancer (IARC) reported 133,354 incidences of NPC globally, which accounted for 0.7% of all cancers diagnosed (Figure 1A) (4). Intriguingly, NPC demonstrated a remarkable geographical distribution, where over 77% of NPC incidences were found in Eastern and South-Eastern Asia (Figure 1B). In South-Eastern Asia, NPC ranked the 10th most common cancer among the entire population (Figure 1C) (3). Brunei had the highest age-standardized incidence rate of NPC (13.35 per 100,000 in males; 6.44 per 100,000 in females), followed by Maldives (10.67 per 100,000 in males; 3.3 per 100,000 in females), Indonesia (10.71 per 100,000 in males; 3.03 per 100,000 in females), Malaysia (9.53 per 100,000 in males; 3.05 per 100,000 in females) and Vietnam (8.12 per 100,000 in males; 2.79 per 100,000 in females) (5, 6). China had an age-standardized rate of 3.0 per 100,000, whereby the incidences are more prevalent among the Cantonese-speaking population which resided in southern China, including Guangzhou province (13.9 per 100,000 in males; 5.2 per 100,000 in females) and Hong Kong (12.8 per 100,000 in males; 4.0 per 100,000 in females).
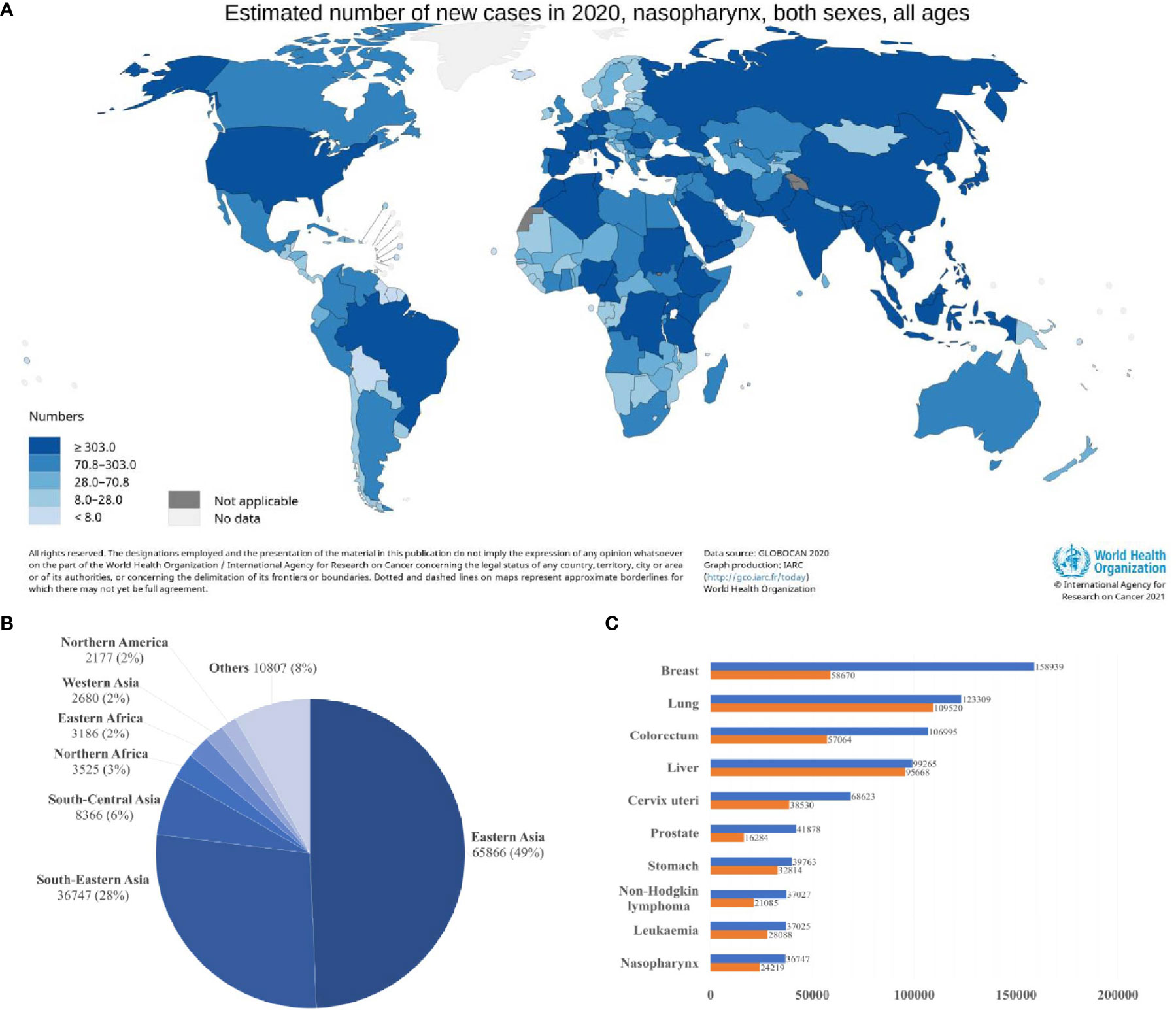
Figure 1 Global distribution of nasopharyngeal carcinoma (A) Estimated age-standardized incidence rate (ASR; world) (B) Estimated number of new cases in different world areas. (C) Estimated number of incident cases and deaths in South-Eastern Asia. Data source: GLOBOCAN 2020 (3).
The incidence of NPC is higher in males (96,371) than in females (36,983), with a ratio of about 2 to 3:1 (4). This phenomenon can be explained by the inheritance of genetic susceptibility associated with X chromosomes, leading to male predominance in the incidence of NPC (7). Sex hormones, such as estrogen, may play a protective role against NPC in females (8). Viral infection with Epstein-Barr virus (EBV) is the most common aetiologic factor in NPC, 100% of non-keratinizing NPCs are detected with EBV infection. Several environmental factors, including dietary consumption of preserved food, and social practice such as tobacco smoking and alcohol consumption, are associated with increased risk of NPC (1, 2, 9). Taken together, EBV infection, genetic and environmental factors contribute to the pathogenesis of NPC.
The application of intensity-modulated radiotherapy (IMRT) alone or with chemotherapy for treatment of NPC significantly improves the clinical outcome of NPC patients. NPC patients diagnosed with early-stage (stage I to II) have a 5-year overall survival (OS) as high as 94% (10–12). Tragically, despite the advances in medical treatment, over 60% of newly diagnosed patients have poor clinical outcomes as they are often diagnosed at late-stage, due to non-specific clinical presentation and lack of biomarkers for early detection (12). Early manifestations of NPC, such as headache, cervical mass, nasal obstruction, and epistaxis, are non-specific, leading to a misdiagnosis rate of approximately 43%, which ultimately results in delay in treatment and poor prognosis (10). When patients are diagnosed with late-stage (stage III-IV) NPC, the 5-year survival rate declines drastically, which is lower than 80% (10). The mortality risk of patients who were diagnosed with stage IV NPC is 3.41-fold higher in comparison with those diagnosed at early-stage (13). Other challenges include locoregional or distant recurrence, which affects 20 to 30% of patients, and therapeutic resistance (1, 12). Thus, it is imperative for the development of high specificity, high sensitivity, and non-invasive laboratory tests for the diagnosis and prognosis of NPC and the development of new therapeutic strategies to reduce morbidity and mortality of the disease.
The tumor microenvironment (TME) is a specialized niche made up of resident malignant and infiltrated cells, metabolites, and extracellular matrix, characterized by its heterogeneity and complexity (Figure 2). Stephen Paget’s “seed and soil” theory suggested the preference of tumor cells (the “seed”) to grow in a favourable microenvironment (the “soil”) (14). The Tissue Organization Field Theory supported the above hypothesis by suggesting that communication between the microenvironment and cell through biophysical and biochemical cues drives phenotypic transformation of cells, which eventually leads to cancer (15). The heterogenous TME further contributes to the progression of cancer by facilitating the acquisition of a series of hallmark capabilities, including [1] sustained proliferative signaling; [2] evasion of growth suppressors; [3] apoptotic resistance; [4] immortal replication; [5] angiogenesis; [6] invasion and metastasis; [7] reprogramming energy metabolism and [8] evasion from immune surveillance, as enumerated by Hanahan and Weinberg (16). These theories open a door towards a new era of medical research targeting the TME. Since the last few decades, several efforts have been made to explore the NPC microenvironment by immunohistological analysis of NPC biopsies. With the advancement of technologies, researchers can delineate the complex TME and cellular crosstalk at single-cell resolution, thereby facilitating the development of diagnostic, prognostic, and therapeutic tools against NPC (17–21).
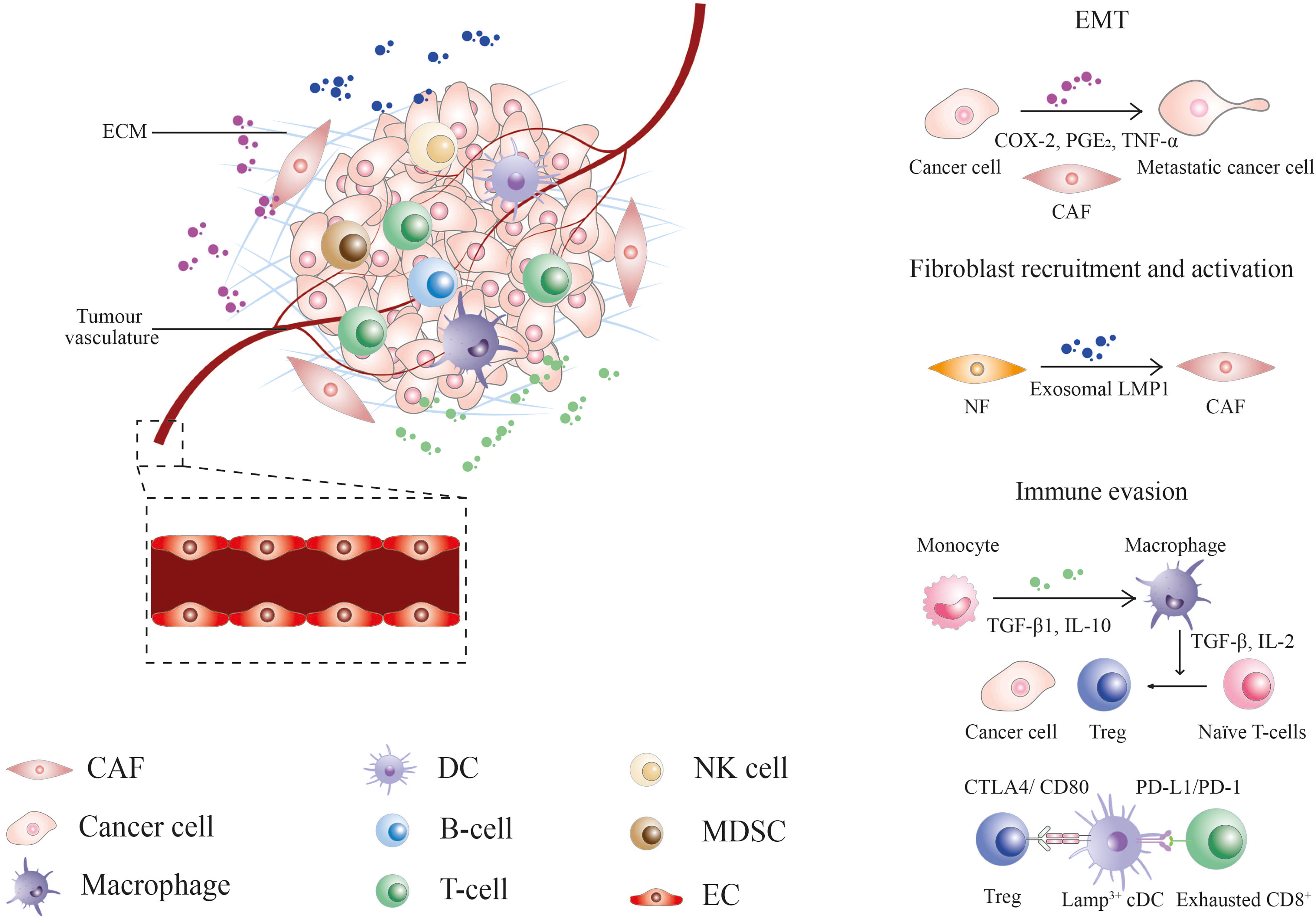
Figure 2 The nasopharyngeal carcinoma tumor microenvironment. CAF, cancer-associated fibroblast; COX-2, cyclooxygenase-2; CTLA4, cytotoxic T-lymphocyte associated protein 4; DC, dendritic cell; EC, endothelial cell; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; Exo-LMP, exosome-packaged latent membrane protein; IL, interleukin; MDSCs, myeloid-derived suppressor cells; NF, normal fibroblast; NK, natural killer; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PGE2, prostaglandin E2; TGF, tumor growth factor; TNF, tumor necrosis factor; Treg, regulatory T-cell.
As a requisite in tumor initiation, progression, and metastasis, the TME components and their interplay with cancer cells offer a variety of promising targets for the development of anti-cancer therapy against NPC. This review introduces the cellular and acellular players, their roles in the NPC microenvironment, current and potential biomarkers, and therapeutic strategies targeting TME.
Components in Tumor Microenvironment
The NPC microenvironment is complex and highly heterogeneous, its components can be classified into cellular and acellular components. Cellular components include [1] tumor endothelial cells (TECs), which forms blood and lymphatic vascular networks for transportation of nutrients and oxygen, [2] cancer-associated fibroblasts (CAFs), which support tumor growth, survival, invasion, and migration, and [3] immune cells, which are involved in immune reactions in the TME (22–25). On the other hand, acellular components include the extracellular matrix (ECM), which facilitates tumor development, progression, and metastasis (26, 27).
Tumor-Infiltrating Immune Cells
The NPC microenvironment is characterized by the intense filtration of tumor-infiltrating immune cells (TIICs), which constitute 40 – 50% of the tumor mass in NPC, with EBV-negative CD3+ T-lymphocytes as the commonest infiltrate (28–30). Despite its abundance, lack of effective immune response, and the presence of immunosuppressive infiltrates such as regulatory T-cells (Tregs), M2 macrophages, and myeloid-derived suppressor cells (MDSCs) leads to immunotolerance, promoting tumor progression. TIICs can be further classified based on lymphoid and myeloid lineage. Tumor-infiltrating lymphocytes (TILs), including T-cells and B-cells, are abundant in the NPC microenvironment as a result of an immune response against EBV. On the other hand, tumor-infiltrating myeloid cells (TIMs) consist of various cell types, including mature mast cells, monocytes and macrophages, dendritic cells (DCs), and pathologically activated immature MDSCs (17, 31, 32).
Tumor-Infiltrating Lymphocytes
TILs, including T-cells and B-cells, are most predominant in the NPC microenvironment. Tumor-associated T-cells consisted of several subpopulations, namely naïve T-cells, cytotoxic T-cells (CTLs), exhausted T-cells, and Tregs (17–21). CD3+ T-lymphocytes, which are comprised of CD4+ T-helper cells and CD8+ T-suppressor cells, is the predominant lymphocytes infiltrating the NPC microenvironment. The majority of these lymphocytes express the activation marker OKT10, however, restriction on human leukocyte antigen (HLA) and T-cell receptor gene expression might contribute to tumor evasion from immune surveillance (29, 30, 33, 34). Consistent with these findings, transcriptomic studies reported that CD4+ and CD8+ T-cells clusters in NPC are highly activated and exhausted, as they co-express exhaustion markers (LAG3, TIGIT, PDCD1, HAVCR2, CTLA4, TOX) and effector molecules (GZMB, GZMK, INFG, NKG7, GNLY, and IL2) (17–21). Exhausted T-cell clusters display intermediate-to-high cytotoxic activity, implying a dynamic transitional process from activated T-cells to exhausted T-cells (17). Both intrinsic and extrinsic mechanisms are involved in the exhaustion or dysfunction of T-cells. The intrinsic mechanisms include [1] expression of inhibitory receptors, including programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte activation gene (LAG-3), B and T lymphocyte attenuator (BTLA) and T-cell immunoglobulin and ITIM domain (TIGIT), [2] decreased cytokine signaling pathways which suppresses the production of interleukin (IL)-2, tumor necrosis factor (TNF)-α, interferon (IFN)-γ and granzyme B (GzmB) in a hierarchical manner, and [3] transcription factors such as the nuclear factor of activated T-cells 1 (NFATC1) (20, 35). Extrinsic mechanisms, on the other hand, include [1] inhibitory effects exerted by regulatory cells including Treg cells, DCs, tumor-associated macrophages (TAMs), MDSCs, [2] immunosuppressive cytokines, such as transforming growth factor (TGF)-β and IL-10, and [3] interaction with major histocompatibility complex (MHC) class II-expressing NPC cells (18, 35).
Tregs are immunosuppressive cells expressing CD4+CD25highFoxp3+ markers (18, 36). Tregs mediate tumor escape from immune surveillance by [1] involvement of immune checkpoint molecules, such as PD-1 and CTLA-4, [2] secretion of cytokines, for instance, IL-10 and TGF-β, which elicits inhibitory effects on immunoreactive cell proliferation, [3] induction of T-cells apoptosis through secretion of cytotoxic perforin and GzmB or ligand-receptor interaction through Fas/FasL, [4] cell contact-dependent suppression of naïve T-cells proliferation, [5] metabolic modulation through enhancing immunosuppressive metabolites, such as indoleamine 2,3-dioxygenase (IDO), in the TME (37–39). Studies revealed enhanced expression level of LGALS1 in NPC-derived Tregs, which was reported to mediate immunosuppression by upregulation of cell-surface programmed death-ligand 1 (PD-L1) and galectin-9 (Gal-9) in head and neck cancer (18, 40). Physical or pharmacologic depletion of Tregs targeting drugs removes their suppression effect and restores EBV-specific CD8+ T-cells (41).
NK cells are the first line of defense against viral infections and neoplasms. Studies reported diminished expression of inhibitory surface receptors of NK cells, including NK group 2, member D (NKG2D), NKp30, and NKp46 as a mechanism for immune evasion. Expression of metastasis-associated in colon cancer-1 (MACC1) gene on NPC cells and enhanced level of soluble MHC class I chain-related molecular A (MICA) is correlated with the downregulation of NKG2D surface expression on NK cells, contributing to tumor progression (42–44). Moreover, the expression of inhibitory receptors such as IL-18-induced PD-1 also mitigates the anti-tumor effect of NK cells (45). Paradoxically, in a recent transcriptomic study of NPC-infiltrating cells, Gong et al. reported expression of cytotoxic genes and chemokine encoding genes in NK cells of the NPC microenvironment, indicating their role as a positive regulator of the immune response against the malignancy (17).
B-cells represent the second largest and most diverse cells in the NPC microenvironment. In particular, EBV positivity is correlated with an increase in B-cell abundance and diversity. The various phenotypes of tumor-infiltrating B-cells include memory B-cells, germinal center B-cells, plasmablast-like cells and plasma cells. B-cells are recruited into tertiary lymphoid structures by tumor-derived PD-1+ exhausted CD4+ T- cells through the CXCL13/CXCR5 axis (20, 46, 47). CD19+ B-cells positively correlated with better prognosis in EBV-positive NPC patients (48). Consistent with this finding, higher expression of B-cell-associated gene signatures such as CD79A, MS4A1, IGHD and FCRL4 favored the survival in NPC patients, suggesting potential anti-NPC immunity by NPC-infiltrating B-cells (17). In contrast, IL-10+ B-cells are immunosuppressive B-cells induced by NPC-derived miRNA-21 (49).
Tumor-Infiltrating Myeloid Cells
MDSCs, macrophages and DCs are the three major myeloid-lineage subtypes in the NPC microenvironment. Under the influence of tumors, alteration of myelopoiesis and impairment of progenitor cells differentiation leads to accumulation of MDSCs. Metabolic alteration by EBV-encoded latent membrane protein (LMP)1 enhances the secretion of IL-1β, IL-16 and GM-CSF through NLRP3 inflammasome, cyclooxygenase-2 (COX-2) and P-p65, which subsequently activates signal transducer and activator of transcription (STAT)-3 signaling pathway to induce MDSCs accumulation (50, 51). Many signature genes of MDSCs are associated with exhaustion and cell cycle arrest of immune cells, implying their role in the induction of immunosuppression through inhibition of T-cell proliferation, promotion of T-cell anergy, and induction of T-cell apoptosis (17, 51). Other than that, MDSCs directly promote NPC cell migration, invasion, and metastasis via contact-dependent induction of epithelial-mesenchymal transition (EMT) in NPC cells in vitro through upregulation of COX-2 expression and activation of β-catenin/TCF4 pathway. Clinically, HLA-DR-CD33+ MDSCs and COX-2 predict poor disease-free survival (DFS) in NPC patients (32).
Monocytes and macrophages are the most predominant cell types in TIMs, which account for around 50% of TIMs in NPC (52). Macrophages may replace T-lymphocytes in anti-tumor immune response and preventing lymphatic spread (53). Macrophages demonstrate a high degree of plasticity when exposed to signals from the TME (52). They can be classically polarized into inflammatory M1 macrophages or activated into immunosuppressive M2 macrophages (54). NPC cells induce polarization of CD163+ M2 macrophages via TGF-β1 and IL-10, which subsequently recruits Foxp3+ Tregs through induction of conversion from naïve T-cells, via TGF-β and IL-2, and chemotaxis, leading to immune escape. Tregs, in return, secrete TGF-β1 and IL-10 to promote M2 macrophage differentiation, forming a positive feedback loop that favors immune escape in NPC (55). Single-cell transcriptomic analysis via RNA sequencing reported co-expression of M1 and M2 gene signatures in NPC-derived TAMs, suggesting an M1-M2 coupled activation pattern in TMEs which gives rise to a unique phenotype that exhibits both pro-inflammatory and pro-tumoral functions (18). The potential anti-tumor capacity of NPC-derived macrophage is exhibited by its high expression of CXC chemokine ligands CXCL9 and CXCL10 which recruit CXCR3+ NK cells and CD8+ T-cells into the TME (20). In contrast, pro-tumorigenic TAMs display expression of angiogenic signature SPP1, which is typically associated with poor prognosis (31). Furthermore, exosomal miR-18a derived from M2 macrophages promote NPC progression, invasion, and tumor growth in in vitro and in vivo animal models via TGF-β signaling pathway by repression of transforming growth factor-beta III receptor (TGFBR3) (56).
DCs initiate antigen-specific immune responses by recognition and presentation of antigen to T-cells. Early studies reported the presence of T-zone histiocytes such as DCs in about half of NPC tissues, their densities significantly correlates with favorable prognosis in NPC patients, implying their role in anti-tumor immunity (57, 58). Tumor-infiltrating DCs can be immunogenic or regulatory, depending on the environmental signals. Different subtypes of DCs are present in the TME, for instance, classical dendritic cells (cDCs) and plasmacytoid dendritic cells (pDCs). The proportion of pDCs is high in NPC compared to other malignancies. pDCs are associated with favorable prognostic value, which implies their potential roles in the induction of anti-tumor immune response. Consistent with this finding, GZMB, a gene that encodes pro-apoptotic enzyme GzmB, is highly expressed in pDCs (20). On the other hand, cDCs can be further divided into three distinct subsets, including classical CLEC9A+ cDC1s and CDC1C+ cDC2s and a mature phenotype LAMP3+ cDCs. LAMP3+ cDCs can be derived from both cDC1s and cDC2s, resulting in LAMP3+ cDCs with different transcriptomic properties and might exhibit diverse functions. LAMP3+ cDCs represent a group of regulatory DCs demonstrating a high level of differentiation and apoptosis but low antigen presentation, with elevated expression of immune-suppressive related genes, such as CD274 (PD-L1), PDCD1LG2 (PD-L2), IDO1, and CD200. These cells potentially exert regulatory activities via recruitment of Tregs, secretion of immunosuppressive molecules, and ligand-receptor inhibition of T-cell activities (21, 31).
Intercellular interactions among LAMP3+ DCs, Treg cells, exhausted CD8+ T-cells, and malignant cells nurture an immunosuppressive NPC microenvironment. LAMP3+ DCs potentially recruit peripheral Tregs through C-C motif chemokine ligand (CCL)/C-C motif chemokine receptor (CCR) interactions such as the CCL17/CCR4 and CCL22/CCR4 signalling pathways. Furthermore, LAMP3+ DCs elicit suppressive activities on CD8+ T-cells via CD200/CD200R signalling and PD-L1/PD-1, leading to the exhaustion of CD8+ T-cells. On the other hand, Treg cells expressing CTLA-4 interacts with CD80/CD86 on LAMP3+ DCs, whereby the interaction might restrain the antigen presentation of DCs and promote their secretion of IDO1 to induce the proliferation of Treg cells (21).
The proportion of mast cells is relatively higher in NPC when compared to other malignancies. NPC-derived mast cells have a high anti-tumor TNF to angiogenic VEGFA ratio, implying high anti-tumor capacity, whereas mast cells in other malignancies are generally pro-tumorigenic. Hence, mast cells are correlated with a survival advantage in NPC. The anti-tumor phenotype of mast cells is possibly driven by the presence of IL1B+ macrophages via IL1B/ADRB2 ligand-receptor interaction (31, 59).
Cancer-Associated Fibroblasts
CAFs, also termed tumor-associated fibroblasts, are found abundantly in the cancer stroma. CAFs have elongated spindle morphology, displayed mesenchymal biomarkers, such as alpha-smooth muscle actin (α-SMA), platelet-derived growth factor receptors (PDGFRs), vimentin and fibroblast activation protein (FAP), and lack of genetic mutations (60, 61). The CAFs predecessors are recruited from diverse origins, which include resident tissue fibroblasts, peri-tumoral adipocytes, endothelial cells through endothelial-mesenchymal transition (EndMT), epithelial cells through EMT, bone-marrow-derived mesenchymal stem cells and haematopoietic stem cells (25, 60, 62). In NPC, extracellular vesicle-packaged EBV-encoded LMP1 can promote the activation of normal fibroblasts into CAFs via the nuclear factor-kappa B (NF-κB) p65 pathway (63). α-SMA expression in CAFs predicted an adverse prognosis in patients with NPC. A high density of CAFs is correlated with shorter OS and lower 5-year survival rates, suggesting CAF density as an independent prognostic factor for NPC patients, suggesting their role in tumorigenesis (64). Consistent with this finding, several studies reported that CAFs promote neoangiogenesis, metastasis, and therapeutic resistance in NPC (65–67).
CAFs stimulate neoangiogenesis in NPC. Genes correlated with endothelial cells abundance were highly expressed by fibroblasts, implying their potential roles in endothelial cell recruitment and angiogenesis (18). The immunohistochemical method revealed high expression of α-SMA fibroblasts in NPC stroma, together with increased intensities of the chemokine stroma-derived factor-1 (SDF-1, also known as CXCL12), a mediator of the recruitment of endothelial progenitor cells, and its receptor CXCR4 in NPC cells. Vascular endothelial growth factor (VEGF) was detected in both cancer and stromal cells, indicating secretion of a significant amount of VEGF in these cells. Prominin 1 (PROM 1, or CD133) and VEGF receptor (VEGFR)-2 double-positive endothelial progenitor cells or CD34 positive cells were observed in the stroma, suggesting tumor-associated neoangiogenesis. Statistical analyses revealed a positive correlation between α-SMA and endothelial antigens CD34, suggesting that CAFs and NPC tumor cells may enhance neoangiogenesis in a VEGF- and SDF-1-dependent manner by the recruitment endothelial progenitor cells from the bone marrow into the tumor stroma (65).
CAFs support tumor metastasis in NPC. Increased density of α-SMA-expressing CAFs at metastatic sites of NPC compared with primary sites, along with upregulation of COX-2 or prostaglandin-endoperoxide synthase (PTGS2) in CAFs, indicated the involvement of fibroblasts and COX-2 in NPC metastasis. High expression of COX-2 catalysed CAF-secreted prostaglandin E2 (PGE2), which induces EMT, thereby promoting NPC cell migration and invasiveness in vitro. Furthermore, COX-2 in host fibroblasts promotes lung metastasis and correlated with the expression of TNF-α expression in mouse models, suggesting that high expression of COX-2 in fibroblasts promotes NPC metastasis through the COX-2-PGE2-TNF-α axis. Consistent with this finding, the expression of COX-2 in CAF was positively correlated with N stage, relapse, and poor survival in patients with NPC (66).
CAFs promote therapeutic resistance and immune evasion. CAFs induced the formation of radioresistance and promoted NPC cell survival following irradiation via the IL-8/NF-κB pathway to reduce irradiation-induced DNA damage. Moreover, CAFs express immunosuppressive factor IDO1, which encodes the enzyme IDO. IDO catalyses the production of L-kynurenine (Kyn), which subsequently promote the generation or differentiation of tolerogenic immune cells by interacting with aryl hydrocarbon receptors on immune cells. High expression of IDO is inversely correlated with the density of CD3+ T-cells and predicted poor survival outcomes in NPC patients. Hence, CAFs may promote immune suppression in NPC by the expression of IDO (18, 68).
CAFs exhibit a supportive role in tumor progression through the remodeling of the ECM (25). NPC-derived fibroblasts express genes that encode ECM components, including COL1A1, COL1A2, LUM, FN1. This suggests the complexity of ECM in the NPC microenvironment and the possible interaction with tumors and stromal cells via integrin signaling, indicating integrin receptors on tumor and immune cells as potential therapeutic targets for disruption of ECM-dependent tumor progression and suppressive immunomodulation (17). Increased production and crosslinking of collagen, such as COL1A1, increase the stiffness of ECM, leading to the promotion of tumor progression through increased integrin signaling (25, 27). On the other hand, fibronectin 1 (FN1) is shown to increase migration and invasion of NPC cells by upregulation of matrix metalloproteinase 9 (MMP9) and MMP2, which are ECM-digesting enzymes mainly produced by CAFs. FN1 also suppresses NPC cell apoptosis via the NF-κB pathway by upregulation of the expression of BCL2 and P65 (25, 69). CAFs mediated directional migration of cancer cells by assembling a fibronectin-rich ECM with anisotropic fiber orientation through increased non-muscle myosin II- and PDGFRα-mediated contractility and traction forces (62, 70).
Angiogenesis, Tumor Endothelial Cells and Hypoxia
Angiogenesis, the formation of blood vessels from existing vasculature in response to a hypoxic condition, is one of the hallmarks of cancer that contributes to tumor growth, development and metastases (71). The vascular niche is associated with stem cell-like NPC at the invasive front (72). The tumor vasculature is lined internally by TECs and surrounded externally by perivascular cells. TECs are characterized by their genetic instability, which contributes to the development of distinct phenotypes that promote therapeutic resistance, tumor progression and metastasis. TECs also interact with tumor cells by secretion of angiocrine factors, contributing to tumor migration and metastasis (73, 74). Drug-resistant human microvasculature endothelial cells (HMECs) are reported to promote progression, EMT and chemoresistance in NPC through secretion of exosomes (75). In NPC, TECs demonstrated enhanced capacity to recruit CTLs and Tregs via CXCL9–CXCR3 and CXCL10–CXCR3 while simultaneously inhibiting CTLs through PDL2–PD1 interaction, suggesting their role in the mediation of an immunosuppressive niche in the NPC microenvironment (18).
Hypoxia was detected in over 80% of NPC tumors as a result of the high requirement of oxygen due to increased proliferation and metabolism. It was reported to play an important role in NPC by regulating its apoptotic activity, metastasis, angiogenesis, metabolic adaptation, and therapeutic resistance, thereby representing a potential target for the treatment of NPC. Hypoxic stress prevents cell survival by inducing the upregulation of anti-apoptotic proteins such as growth arrest and DNA-damage-inducible beta (Gadd45β) and immediate early response 3 (IER3) (76). Hypoxia promotes tumor cell migration by EMT stimulation and ECM remodeling through Notch signaling (27). Lysyl oxidase (LOX), an extracellular matrix-remodeling enzyme, was significantly upregulated in hypoxic conditions and predicted poor prognosis in patients with NPC (76, 77). In addition, hypoxia induces the upregulation of VEGF, which is responsible for neoangiogenesis. Furthermore, hypoxia mediates metabolic adaptation and acidosis in NPC (76). A metabolic shift in NPC infiltrating lymphocytes was reported to induce the exhausted phenotype of T-cells through overexpression of miR-24. miR-24 overexpression suppresses the expression of MYC and FG11 in TILs and disrupts MFN1-mediated mitochondrial fusion, thereby inducing T-cell exhaustion (78).
Various molecular signals and tumor-derived factors are involved in angiogenesis. VEGF is a pro-angiogenic factor that activates and stimulates proliferation and migration in VEGFR expressing endothelial cells. VEGF also alters vascular permeability by loosening the junctions between endothelial cells, favouring tumor intravasation (79). Besides hypoxia, increased VEGF production can be induced by EBV-encoded LMP1 through COX-2 expression in NPC cells (80). High expression of tissue VEGF is associated with reduced OS and DFS in NPC patients (81).
Intercellular Communications in Nasopharyngeal Carcinoma Microenvironment
Intercellular communication between malignant and stromal cells in the NPC microenvironment is established via paracrine mechanisms involving cytokines and chemokines, extracellular vesicles including exosomes and ligand-receptor interactions. The NPC microenvironment is primarily comprised of heavy immune infiltrates, with CD3+ T-lymphocytes as the most abundant lymphocyte infiltrates in NPC biopsies (37, 82). EBV-encoded proteins, such as LMP1, initiate immune cell recruitment by the regulation of multiple signalling pathways associated with cytokine and chemokine secretion from tumor and immune cells. Immunosuppression, mediated by cytokines and regulatory cells, facilitates malignant cells to escape from anti-tumor immune response and promotes tumor growth and progression. Tumor-derived exosomes, carrying viral proteins and immunosuppressive substances, and ligand-receptor interactions, such as PD-1/PD-L1 signalling pathway, also contribute to the construction of an immunosuppressive TME (28, 83).
Chemokines and Cytokines
EBV-infected nasopharyngeal epithelial cells construct the NPC microenvironment, which is characterized by heavy infiltration of immune cells with suppressed immune activities, by secretion of EBV-encoded products that activate several inflammatory-associated signalling pathways. A variety of cytokines, such as IL-6, IL-8, interferon-inducible protein 10 (IP-10), TNF-α, VEGF, and macrophage inflammatory protein (MIP)-3α are elevated in NPC patients (84). LMP1 recruit immune cells into the tumor site by upregulating several cytokines through NF-κB and STAT3 signaling pathways (82, 85). These include regulatory cells with immunosuppressive influence in the TME. NPC cells induce polarization of macrophage into immunosuppressive M2-like phenotype through secretion of interferon-stimulated gene 15 (ISG15) (18). EBV nuclear antigen 1 (EBNA1) induce the production of CCL20, or MIP-3α to promote chemotaxis of Tregs to the tumor site (86, 87). In addition, EBV-encoded small RNAs (EBERs) activates the toll-like receptor 3 (TLR3) pathway to produce inflammatory cytokines such as CXCL8 which recruit and activate TAMs. The inflammatory responses in NPC cells are amplified by a positive feedback loop consisting of EBER, LMP1 and NF-κB (88). Metabolic reprogramming by LMP1 leads to alteration in Nod-like receptor family protein 3 (NLRP3) inflammasome, COX-2 and P-p65 signaling pathways, which results in the release of cytokines, including IL-1β, IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF), contributing to the expansion of MDSCs (50). Stromal cells, including TECs, can attract CTLs and Tregs via chemokine-receptor interactions such as CXCL9/CXCR3 and CXCL10/CXCR3, however, cytotoxic activities of CTLs are inhibited by PD-L2/PD1 interaction (18).
Chemokines and cytokines facilitate tumor elusion from immune surveillance. EBER, LMP1 and EBV lytic transactivator, Zta, are positively correlated with the production of IL-10. BCRF1, or viral IL-10, share immunosuppressive properties with human IL-10. IL-10 induces immunosuppressive activities by inhibition of antigen-specific T-cell proliferation, induction of T-cell apoptosis and inhibition of IFN-γ secretion from NK cells. LMP1 upregulates IL-18 and IP-10 (CXCL10), which take part in the recruitment and immunosuppression of CXCR3+ NK cells and T-cells (45, 82, 85, 89). IL-6 and TNF-α mediate the expression of IDO, which demonstrates an immunosuppressive role by suppression of T-cell proliferation and impairment of CD8+ T-cells cytotoxic activities. Pre-treatment serum levels of IL-6 and TNF-α are negatively correlated with the 2-year survival rate in NPC patients (90, 91). Increased levels of IL-18 in NPC induces NK cells exhaustion via upregulation of expression of PD-1 on NK cells (45).
Furthermore, chemokines induce metastasis and promote tumor progression. IL-8 induce EMT by activating AKT signalling, enhancing migration and invasion of NPC cells. Enhanced level of IL-8 predicts adverse OS, DFS, and DMFS of NPC patients (92).
Exosomes
Exosomes are small (30-100 nm) extracellular vesicles that originated from the plasma membrane. They are often involved in the intercellular interactions in the TME, influencing various cellular processes, including cell growth, angiogenesis, EMT, metastasis, immune tolerance, and therapeutic resistance (93). Exosomes may play a significant role in the formation of a premetastatic milieu by acting as a vehicle for tumor-derived factors that modulate pre-metastatic sites (15). Exosome contents include transcription factors, enzymes, ECM proteins, lipids, and nucleic acids, such as DNA, mRNA, and non-coding RNA. Based on their parental cells, exosomes in NPC can be classified into nasopharyngeal carcinoma-derived exosomes (NPC-Exo) and EBV-related exosomes (94–96).
NPC-Derived Exosomes
NPC-derived exosomes (NPC-Exos) [1] mediate the tumor immune microenvironment, [2] enhance angiogenesis, [3] induce EMT and [4] promotes resistance towards chemoradiotherapy as summarized in Tables 1 and 2. NPC-Exo facilitates tumor progression by promoting tumor evasion from immune surveillance. NPC-Exo carries clusters of microRNAs (miRNAs) associated with the downregulation of the MARK1 signaling pathway, including miR-24-3p, miR-891a, miR-106a-5p, miR-20a-5p, and miR-1908, leading to altered T-cell proliferation and differentiation. Hypoxia-induced exosomal miR-24-3p modulates the phosphorylation of ERK and STAT protein by repression of FG11 expression, leading to impeded T-cell proliferation, induction of Foxp3+ Tregs differentiation and inhibition of Th1 and Th17 differentiation. Low levels of exosomal serum miR-24-3p and high FGF11 expression predicts favorable DFS in NPC patients and may serve as potential prognostic biomarkers in NPC. Furthermore, NPC-Exo enhances the levels of pro-inflammatory cytokines IL-1β, IL-6 and IL-10 and reduce the levels of IFN-γ, IL-2 and IL-17 in CD4+ and CD8+ T-cells (113, 114, 125). Exosome-packaged CCL20 expands the Tregs population by promoting Tregs recruitment and conversion of conventional T-cells into inhibitory Tregs in the TME. CCL20 also enhances the suppressive effect of Tregs by inducing the overexpression of TNFRSF4, SELL, ICAM1, TBX21, CCR6, TNF, GZMB, TGFB1, IL10, IL2 and IL15, which are associated with Treg phenotype, properties, and recruitment capacity (107, 113). Moreover, exosomes also transport Gal-9, which is associated with myeloid lineage-mediated immunosuppression through regulation of the expression of pro-inflammatory cytokines for the expansion of MDSCs by attenuating STING signaling (100).
Exosomes mediate angiogenesis by the transportation of pro-angiogenic and angiogenic-suppressive proteins and miRNAs, which mediate multiple angiogenesis-associated pathways (99). miR-17-5p and miR-23a are angiogenesis promoters, targeting BAMBI and testis-specific gene antigen (TSGA10) respectively (108, 109). In contrast, miR-9 inhibits angiogenesis by downregulating MDK and regulating PDK/Akt pathway.
Exosomes are also involved in the promotion of tumor invasion and metastasis. For instance, miR-301a-3p, which targets B-cell translocation gene 1 (BTG1) mRNA, promotes proliferation, migration, invasion and EMT of NPC (115). Under hypoxic condition, exosomes containing MMP13promotes metastasis by inducing EMT of malignant cells or mediating the TME by interacting with stromal fibroblasts and endothelial cells, hence promoting tumor invasion (105, 106).
Exosomes contributed to the development of resistance towards chemotherapy and radiotherapy. Taxol-resistant NPC cells can transfer dead-box helicase 53 (DDX53) into normal NPC cells to promote resistance to Taxol through upregulation of multidrug resistance 1 (MDR1) (126). circMYC is a circulating exosomal circular RNA (circRNA) associated with cell survival from radiotherapy. circMYC could sponge tumor suppressing miR-20b-5p and let-7e-5p, hence influencing their downstream targets, argonaute RISC component 1 (AGO1) and cryptochrome circadian regulator 2 (CRY2), affecting tumor progression (103).
EBV-Related Exosomes
EBV-infected NPC cells promote tumor growth by transferring viral oncoprotein such as LMP1, signal transduction molecules, and virus-encoded miRNA through exosomes (127). It is reported that recipient cells internalized exosomes derived from EBV-infected cells via caveola-dependent endocytosis (128).
LMP1 is an EBV-encoded gene product that is detected in almost all primary NPC specimens. A small amount of LMP1 facilitates tumor progression, whereas high LMP1 expression induced growth inhibition and cell apoptosis. LMP1 exerts its oncogenic activity by activating several signaling cascades including NF-κB, PI3K-AKT, ERK-MAPK, JNK, JAK-STAT and p38/MAPK signaling pathways. Activation of NF-κB and STAT3 pathway results in increase secretion of immunomodulatory molecules, for instance, IL-18 and IP-10, which recruit and suppress CXCR3+ T-cells and macrophages. LMP1 and IFN-γ upregulate immune checkpoint PD-1/PD-L1 synergistically, leading to suppression of anti-tumor activities. Furthermore, LMP1 induce the Warburg effect in NPC through upregulation of hypoxia-inducible factor (HIF)-1α and hexokinase 2, leading to enhance malignant transformation, tumor progression and resistance to radiotherapy. In addition, LMP1 facilitates metastasis by inducing EMT, through upregulation of Twist, Snail and SatB1 pathways, and remodeling of ECM, by induction of MMP9. LMP1 also promote angiogenesis, cell growth and cell survival through induction of pro-angiogenic factors, growth factors and anti-apoptotic proteins (85, 129). LMP-1 activated NF-κB induces cellular proliferation, EMT and metastasis by inhibition of tumor suppressing miR-203 (130).
EBNA1 promotes immunosuppression by converting naïve T-cells into Treg cells and promotes chemotactic migration of Treg cells by upregulating TGF-β1. Upregulation of the TGF-β1-SMAD3-PI3K-AKT-c-JUN axis induces the production of CXCL12 which recruit Treg cells by binding to CXCR4. On the other hand, TGF-β1 downregulates miR-200a, a negative regulator of CXCL12 by enhancing the SMAD3/c-Jun complex. CXCL12 also exerts an inhibitory effect on miR-200a via the c-Jun-miR-200a-CXCL12-c-JUN feedback loop (36). Enhanced CCL20 production in EBNA1-expressed tumor cells increased Tregs migration. Polarized-M2 macrophages by EBNA1 expression cells converted naïve T-cells into Tregs (86).
EBV non-coding RNAs (ncRNAs) include EBERs and miRNAs. EBERs mediate inflammatory response through interferon regulatory factor 3 (IRF3) and NF-κB signaling pathways by targeting RIG-I, leading to tumor progression. EBERs trigger MCP-1 and M-CSF which recruit macrophages into the TME and promote their differentiation into pro-tumorigenic TAMs (131, 132). EBER-1 expression was upregulated in NPC tissues, a high level of EBER-1 correlated with better prognosis in NPC patients (133). The EBV miRNA precursors are clustered in two main regions, which are Bam HI fragment H rightward open reading frame 1 (BHRF1) and Bam HI-A region rightward transcript (BART). EBV-miRNAs assist NPC progression by promotion of immune evasion, cell proliferation, inhibition of apoptosis and promotion of invasion and metastasis (134, 135).
Targeting Tumor Microenvironment in Nasopharyngeal Carcinoma: Current and Future Perspectives
Immunotherapy: Boosting T-Cell Immunity
NPC is a promising candidate for immunotherapy owing to its immunosuppressive property and expression of immunogenic EBV antigen. Several approaches which include immune checkpoint inhibitors and cellular-based immunotherapy have been employed to reinvigorate the exhausted immune cells in the NPC microenvironment (83) (Table 2). PD-1/PD-L1 axis has been exploited as a potential therapeutic target for reversing T-cells exhaustion and restoration of their anti-tumor function (136, 137). PD-1 is an immune checkpoint encoded by the PDCD1 gene and expressed on the T-cell surface, whereas PD-L1 is expressed on tumor cells and immune cells. Studies reported expression of PD-L1 in approximately 70 - 90% of NPC tissues and its prognostic significance. The difference in interpretational methods leads to inconsistency of results, suggesting the need for further large-scale study (138–141).
PD-1/PD-L1 blockade therapies with anti-PD-1 antibodies, such as nivolumab, pembrolizumab, cemiplimab and anti-PD-L1 antibodies, including atezolizumab, avelumab and duravulumab have been implicated in immunotherapy against various neoplasms (136, 137). Anti-PD-1 and anti-PD-L1 therapies are generally safer than chemotherapy, with a lower incidence of treatment-related adverse events (142, 143). Single-agent studies involving PD-L1 antibodies showed lower overall response rate (ORR), OS and progression-free survival (PFS) when compared with studies with PD-1 antibodies, likely due to alternative binding of PD-1 to PD-L2 following blockade of PD-L1. Combination therapy of chemoradiation and PD-1/PD-L1 promote a synergistic anti-tumor immunity by enhancing host recognition, elimination of tumor cells and preventing T-cell apoptosis. For instance, a phase I trial investigating anti-PD1 antibody camrelizumab achieved an ORR of 34%, whereas a combination trial of camrelizumab with gemcitabine and cisplatin achieved an ORR of 91%. In spite of that, combination therapy exhibited a higher incidence of treatment-related adverse events when compared with anti-PD-1 monotherapy, suggesting synergistic toxicity (142, 143). There is also limited anti-tumor response rate towards PD-1/PD-L1 blockade therapy. Resistance to PD-1/PD-L1 therapy involves multiple mechanisms, including low tumor immunogenicity, T-cell dysfunction by other immune checkpoint receptors and modulation by noncoding RNAs.
Strategies to overcome the challenge include the incorporation of PD-L1/PD-1 inhibitors with other anti-tumor therapy. Ongoing clinical trials propose the combination of PD-1/PD-L1 blockade with other immune checkpoint inhibitors such as anti-CTLA-4 or anti-angiogenic agents such as anti-VEGFR inhibitors as potential therapeutic strategies for NPC (144, 145). Besides that, the identification of predictive biomarkers of PD-1 inhibitors will allow the selection of appropriate patients for PD-1/PD-L1 targeted treatment (136). Other immune regulatory checkpoints, for instance, the Gal-9/TIM-3 (HAVCR2) interaction represents a promising alternative as they are prominently expressed in recurrent NPC (20, 146).
Two approaches of cellular-based immunotherapy include adoptive immunotherapy, which is the infusion of ex vivo generated activated effector cells, such as EBV-specific CTLs, chimeric antigen receptor (CAR) engineered T-cells and cytokine-induced killer cells (CIKs) (147–150). An increase in the frequency of MDSCs, and immunosuppressive cytokines, such as CCL2 and CXCL10, underlie the development of resistance towards adoptive immunotherapy. Chemotherapeutic agents, such as gemcitabine and carboplatin may alleviate the resistance by limiting the expansion of MDSCs, secretion of pro-inflammatory cytokines and depletion of immune checkpoint molecules (151). A phase II study combining chemotherapy with adoptive immunotherapy using engineered EBV-specific CTLs as first-line treatment for metastatic or recurrent NPC patients yield a satisfactory result, with a 2-year OS of 62.9% (118). On the other hand, active immunotherapy or tumor vaccines aim to enhance recognition by the immune system by the delivery of tumor-specific antigens through antigen-presenting cells or viral vectors. These include LMP2 expressing DCs and recombinant modified vaccinia Ankara vaccine (MVA-EL), which has been proved to be safe and well-tolerated (152, 153). Besides targeting EBV-encoded oncoproteins, a peptide vaccine targeting non-EBV associated tumor specific antigen, four-jointed box 1 (FJX1), was also designed in a preclinical setting (154).
Further research should consider [1] large-scale studies for the evaluation of clinical efficacy and the optimization of dosage for the above therapy; [2] combination of therapies to augment the clinical responses, for example, combining immune checkpoint blockade with adoptive T-cell therapy (155–157); [3] development of personalized immunotherapy targeting neoantigens (158); and [4] localization of NPC-specific CTLs at the malignant site, for example, via chemokine-receptor interactions (159). Other alternatives, for example, CAR-NK, an emerging cellular immunotherapy strategy for cancer, should be evaluated in NPC, given its promising clinical efficacy in other settings (160).
Tumor Infiltrating Immune Cells as Prognostic Markers
Immune score, established based upon the density of lymphocyte populations, is predictive of disease progression and distant metastases. High immune scores predict better OS, DFS and distant-metastasis free survival (DMFS) of NPC patients (37). Among TILs, CD3+, CD4+, and CD8+ T-cells as well as CD56+ NK cells are good prognostic factors for NPC patients (161–163). Despite its immunosuppressive role, high Foxp3+ T-cells to CD8+ T-cells ratio was associated with favorable PFS in early-stage NPC patients (164). Similarly, Ooft et al. reported Foxp3 as an independent predictor for better OS in NPC patients (165). On the other hand, CD68+ TAM density predicted favorable DFS, whereas stromal CD163+ M2-like TAMs correlated with poor OS and PFS in NPC patients (20, 166, 167).
Nonetheless, there are discrepancies among studies investigating the prognostic significance for different TIICs, due to different methodologies used. Thus, further studies should consider the development of a standardized method for the evaluation of TIICs as an indicator or predictor for the progression and therapeutic response of NPC patients.
Anti-angiogenic Therapy
Angiogenesis is important to meet the huge demand for oxygen and nutrients by tumor cells, thus, anti-angiogenic therapy using angiogenic inhibitors is a promising anti-cancer therapeutic strategy. Conventional chemotherapeutic agents, for instance, epirubicin, elicits inhibitory activity on angiogenesis by inducing non-specific vascular toxicity (168). On the other hand, hypericin-mediated photodynamic therapy exerts its antitumor activity by targeting the tumor vasculature (169). Several small-molecule inhibitors of VEGFR such as apatinib are employed in angiogenesis-targeting therapy for NPC patients (170, 171). The inhibition of angiogenesis could enhance the effectiveness of standard chemoradiotherapy against NPC. Combination of VEGF inhibitor bevacizumab with chemotherapy promoted microvasculature maturation, enhanced immune infiltration and exhibited promising tumor response (172). Combination therapy of apatinib with cisplatin showed a synergistic effect in inhibition of tumor growth, repression of VEGFR-2 expression and reduction in microvascular density in preclinical models (173). On the other hand, combining apatinib and radiation enhances the anti-angiogenic effect and increases hypoxia in tumor tissues, leading to an anti-tumor effect (174). Endostatin, an endogenous angiogenesis inhibitor is another anti-tumor agent tested in clinical trials. Endostar, a recombinant endostatin, sensitized NPC towards radiation therapy by induction of endothelial cell and tumor cell apoptosis and repression of radiation-induced pro-angiogenic factors (175). Phase II multicentre randomized controlled combining Endostar therapy with standard chemoradiation reported an improved remission rate of cervical lymph node metastasis, with a slight improvement in objective response rate (121). To sum, anti-angiogenic agents targeting VEGF and VEGFR can enhance the effectiveness of chemoradiotherapy through inhibition of blood vessels formation, normalization of blood vessels, which restore vascular function, improve tumor perfusion, and drug delivery, and activation of immune response (176).
Currently, anti-angiogenic therapy faces the challenge of the development of resistance, which leads to limited survival benefits. The resistance is likely due to hypoxic events as a consequence of vascular depletion, resulting in the promotion of cancer invasion and metastasis, activation of alternative pro-angiogenic signalling pathways, VEGF-independent vascular mimicry, and increased recruitment of pro-angiogenic cells (176, 177). Studies unveiled upregulation of several proangiogenic factors, including VEGF, TNF-α, IFN-α, and basic fibroblast growth factor (bFGF), following hypericin-mediated photodynamic therapy. This indicates the need of combination therapies such as photodynamic therapies with angiogenesis inhibitors to enhance the efficacy of treatment (169, 178). Other challenges include toxicity effects, which include haemorrhage, hypertension, and thrombosis (79). Several strategies direct targeting the tumor vessels are introduced to increase the effectiveness of the therapy and minimise the side effects. Nanoparticles might serve as vectors for the delivery of anti-angiogenic drugs to the target site (79). Given the high effectivity for DNA enzyme (DNAzyme) to cleave targeted sequence with high specificity, DNAzyme also serves as a potential therapeutic agent for anti-angiogenic therapy. DNAzyme targeting VEGFR-1 mRNA significantly downregulated VEGFR-1, inhibited angiogenesis and altered the vascular permeability. No toxicity effect was observed in vivo, indicating anti-VEGFR-1 DNAzyme as potential drug candidates for further clinical evaluation (179).
Targeting Hypoxic Condition in Nasopharyngeal Carcinoma
The increased oxygen demand by the highly proliferative and metabolically active tumor cells with inadequate oxygen supply from the impaired vasculature leads to hypoxia, a condition that mediates tumor progression, metastasis, and therapeutic resistance. This tumor-specific event has been studied extensively for the development of anti-tumor therapy. Several strategies targeting hypoxia are employed, which include a cytotoxic approach with hypoxic selectivity. In the hypoxic TME, hypoxia-activated prodrugs such as tirapazamine are preferentially activated into cytotoxic drugs and eliminate hypoxic tumor cells with high selectivity. The second approach direct inhibits hypoxia-inducible proteins. HIF-1α siRNA and PX-478 directly target HIF-1α and elicit anti-tumor activity. Hypoxia-modifying therapy improves the hypoxic condition in tumors by increasing the blood oxygen level through blood transfusion, carbogen, and nicotinamide treatment (76).
The combination of hypoxia-targeting therapy and radiotherapy exhibited a synergistic effect. Topotecan is reported to enhance the radiosensitivity of tumors by inhibiting topoisomerase I and subsequently downregulating the expression of HIF-1α target genes (180, 181). Nanotechnology is also employed to enhance the therapeutic efficacy of hypoxia-targeting therapy. In a preclinical study, nanoparticle delivering siRNA targeting HIF-1α significantly suppressed tumor growth in in vivo animal models of NPC (182). In recent studies, hypoxia-responsive nanoparticles have been designed for the selective release of drugs in the hypoxic tumor environment, thereby prolonging the bioavailability and improving the effectiveness of the drug (183). These nanomedicines are still in the early phase, their mechanism, delivery efficacy and safety in human bodies remain to be studied.
Targeting Chemokines and Cytokines
Chemokines and cytokines are potential targets for the development of diagnostic biomarkers or therapeutic strategies. CCL5, also known as RANTES (regulated upon activation, normal T-cell expressed and presumably secreted), is a pro-angiogenic chemokine that can be detected in NPC patients’ plasma with 90.07% sensitivity and 56.34% specificity. Increased screening efficacy was observed when combining CCL5 with EBV viral capsid antigen (VCA-IgA) or EBV DNA assay. Inhibition of the CCL5 receptor, CCR5 with its antagonist maraviroc could suppress CCL5-associated migration of NPC cells (184, 185). Leukaemia inhibitory factor (LIF), a member of the IL-6 type cytokine family, is remarkably increased in the TME and NPC patients’ serum. Secreted by tumor cells and inflammatory infiltrates, LIF mediates the mTOR pathway in NPC, which leads to tumor growth and enhanced radioresistance. Elevated serum LIF in NPC patients is predictive of local recurrence and radiosensitivity. The blockade of the LIF signalling pathway through a LIF antagonist, soluble LIF receptor, or mTOR inhibitor, rapamycin, could sensitize NPC towards irradiation (186, 187).
The development of anti-tumor therapeutics targeting chemokines or their receptors need to consider [1] the optimal dosage of drugs with concern on their safety and efficacy; [2] evaluation of synergism effect when combined with conventional therapy and immunotherapy; [3] development of drugs with high affinity and high specificity; and [4] identification of tumor-specific targets, with minimal effects on non-tumor-associated cells.
Clinical Applications of Exosomes
Tumor-derived exosomes are involved in a wide range of biological and pathological processes as well as intercellular crosstalk and thus are promising targets for the development of biomarkers and anti-cancer therapies. Exosomes are highly stable and can be detected in patients’ body fluids, such as urine, saliva, cerebrospinal fluid, and serum, and hence can be used in liquid biopsies as non-invasive biomarkers (96). Sera exosome concentrations have clinical significance and prognostic value in NPC patients, whereby a positive correlation between tumor lymph node metastasis and shorter disease-free survival was observed in NPC patients.
Nasopharyngeal brush or swab is a less invasive alternative for the collection of tumor tissue samples when compared to the conventional biopsy method. Elevation of EBV miRNAs, including mir-BART1-5p, mir-BART5, mir-BART6-5p, mir-BART17-5p, were reported in nasopharyngeal brush samples from NPC patients. miR-BART1-5p shows potential as a diagnostic indicator of NPC, with 93.5% sensitivity and 100% specificity, its expression level positively correlated with tumor progression (188).
In addition, miR-BART7 and miR-BART13 are significantly elevated in plasma specimens from NPC patients, the combination of both markers offers a 90% prediction of NPC. Furthermore, the level of miR-BART7 is associated with metastatic progression. Diminished levels of miR-BART7 and miR-BART13 were reported after radiotherapy, suggesting their potential as biomarkers for the monitoring of therapeutic efficacy (189). Post-treatment detection of circulating miR-BART17-5p is a potential biomarker of a poor prognosis (190). Plasma EBV-miR-BART7-3p showed 96.1% sensitivity and 96.7% specificity, whereas miR-BART13-3p has a sensitivity of 97.9% and specificity of 96.7% for the detection of NPC. miR-BART7-3p is a potential prognostic biomarker, whereby prominent levels of pre-treatment miR-BART7-3p in plasma indicated a higher risk of distant metastasis whereas post-treatment miR-BART7-3p is associated with short DMFS and OS (191, 192). Significant upregulation of miR-1301-3p was detected in the exosomes from early-stage NPC patients’ plasma, indicating their potential application as diagnostic biomarkers in early-stage NPC (193).
Upregulation of circulating exosomal circRNA circMYC is correlated with increase cell proliferation and resistance to radiotherapy in NPC patients. Circulating circMYC is a potential biomarker for the differentiation of radiosensitive and radioresistant patients with 90.24% sensitivity and 94.51% specificity (103). The detection of exosomal cyclophilin A (CYPA) combined with EBV VCA-IgA could increase the accuracy of diagnosis, indicating the utility of exosomal CYPA as a potential non-invasive biomarker for the diagnosis of NPC (194).
Besides serving as biomarkers, exosomes are potential vehicles for the delivery of cancer drugs to the target molecules. iRGD-tagged exosomes targeting αvβ3 integrin-positive NPC cells containing antagomir-BART10-5p and antagomir-18a showed remarkable anti-angiogenic efficacy in in vitro and in vivo NPC models (110). Exosomes are also employed as vehicles for tumor antigen in cancer vaccines, however, to our knowledge, exosome vaccines have not been tested specifically in NPC yet (195).
While exploiting exosomes as therapeutic and diagnostic tools, there are some challenges that need to be addressed. These include [1] time and cost consumption; [2] unsatisfactory yield, purity, and quality, which could affect [3] the sensitivity and specificity of assays, [4] absence of endogenous control for miRNA quantification, and [5] limited sample size in current studies, which affect the reliability of these studies (196, 197). On the other hand, several problems need to be considered when using exosomes as therapeutic vehicles or targets: [1] enhancing the specificity of therapy to prevent undesirable adverse events; [2] prolonging the half-life of exosomes after administration into the body; [3] effective and safe therapeutic dose of exosomes; [4] effective drug loading into the exosomes; [5] cost and time effectiveness in the production of bioengineered exosomes (93, 196).
Overall, exosomes play a pivotal role in the interplay communication between EBV-infected NPC cells and stromal cells, which contribute to the construction of the TME that favours tumor progression, development, invasion, and metastasis. Further investigations should consider [1] understanding the mechanisms underlying cellular crosstalk via exosomes, including the biogenesis and internalization of exosomes; [2] development of standard methods for scalable isolation and purification of exosomes; [3] large-scale clinical studies for the evaluation of exosome as reliable cancer biomarkers.
Cancer-Associated Fibroblasts
The vast influence of CAFs on tumor development and progression made them promising therapeutic targets. Several strategies for CAF-directed anti-cancer therapies have been tested in preclinical settings, but only a few studies are focusing on CAFs in NPC (198). Disulfiram/copper exhibited anti-tumor activity by targeting both cancer cells and CAFs. It could induce apoptosis and inactivate CAFs by inhibiting the expression of α-SMA (199). Treatment with CAF inhibitor, for instance, Tranilast, inhibits CAFs from activating the NF-κB pathway, thus sensitizing NPC cells to irradiation (67). The targeting of CAFs is challenging as they demonstrate complexity in intercellular signaling, phenotype and source and lack of specific surface marker (198). Thus, further understanding of CAFs, with regards to their different phenotype and respective protumorigenic or tumor suppressive properties in TME is required for CAF-targeting therapies.
NF-κB Signaling Pathway
The NF-κB signaling pathway is constitutively activated in 90% of EBV-associated NPC by EBV oncoproteins and genetic mutations. This pathway plays a pivotal role in the intercellular communication and regulation of immune cells in the TME, which renders it a promising target for anti-cancer therapy against NPC (200–202). Strategies targeting the NF-κB signaling pathway include anti-inflammatory compounds such as aspirin, inhibition of IκB kinase (IKK) and proteasome inhibition (130, 203, 204). Acting as an NF-κB inhibitor, aspirin reverses LMP1-induced EMT by suppressing NF-κB-exosomal secretion of LMP1 and promoting miR-203 expression (130). Inhibition of lung metastasis by aspirin is also observed in mouse models. PS1145, an inhibitor of IKK, could abrogate the NF-κB signaling pathway and subsequently inhibit the production of pro-inflammatory cytokine and cell proliferation. PS1145 significantly inhibits the tumor growth of NPC in in vitro cell lines and in vivo xenograft models (203). Bortezomib, a proteasome inhibitor, targeting STAT1 and NF-κB, could relieve the immune tolerance in NPC (204). Vitexin, a natural flavonoid glycoside targeting NF-κB, displayed promising anti-tumor activity against NPC in preclinical studies (205). Restoration of Ras-like estrogen-regulated growth inhibitor (RERG), an NF-κB inhibitor, by 5-Aza-2’-deoxycytidine and trichostatin A attenuated ERK/NF-κB signaling pathway, resulting in the inhibition of tumor growth and angiogenesis in vivo. Therefore, RERG might be employed as a target molecule in cancer therapy (206).
Whilst translating these bench findings into bedside application, several issues need to be addressed. As the NF-κB signaling pathway regulates multiple physiological processes, the development of therapeutic tools should consider developing drug delivery strategies with high specificity to prevent undesirable adverse events. It is reported that the NF-κB complex p50/p50/Bcl3 is prevalent in NPC but seldom found in a normal cell. Thus, Bcl3 inhibitors may represent promising therapeutic agents against NPC (202, 207). Other than that, the route of administration and dosage of NF-κB inhibitors should consider their bioavailability and safety.
Concluding Thoughts
The advances in multi-omics technology allow researchers to unravel the complex intercellular communication in the NPC microenvironment, which contributes to the growth, development, progression, and metastasis of this malignancy. Nevertheless, there is a lack of thorough studies on the players in the NPC microenvironment, particularly, B-cells, NK cells, cancer stem cells and the ECM. Of note, spindle-shaped NPC cells predominantly located at the invasive margin of the tumor site display stem cell-like properties and are significantly associated with EMT. Further studies targeting these neoplastic spindle cells might shed light on the understanding of the mechanism underlying tumor cell dissemination, and thus facilitating future development of predictive biomarker and preventive medicine for NPC metastasis (72, 208, 209). Other than that, it is suggested that future studies look into the spatial heterogeneity of the NPC microenvironment to gain further insights into tumor heterogeneity and discover new opportunities for the development of theragnostic tools (210).
Several anti-cancer drugs targeting TME have been tested in clinical trials, however, several pre-clinical and clinical barriers remain to be overcome. Preclinically, models for cancer drugs are inadequate to visualize the complexity of TME. Cell and tissue-engineered models with 3-dimensional co-culture systems could be utilized to recapitulate the cellular organization, growth kinetics, cellular heterogeneity, intra- and intercellular interactions in vitro to improve translation and reduce animal testing (211). Clinically, given the inter-patient heterogeneity in genetic and epigenetic factors, responses towards drugs are highly variable among individuals. Thus, an optimal combination of therapeutic strategies should be designed based on the integration of individual information on TME landscape, genomic and molecular profile to ensure precision, safety, and effectiveness of cancer therapies, which ensure better health outcomes of NPC patients. Moreover, to reduce the adverse events of therapeutic and enhance therapeutic efficacy, strategies to improve specificity in targeting, delivery, and release of drugs against TME should be considered. These include the engineering of nanoparticles or exosomes with specific ligands as vehicles for the delivery of drugs into the target cells. Several delivery systems which could enhance the effectiveness of delivery have been developed, which include arginine-modified hydroxyapatite nanoparticles and fusion-based NPC-specific lipid nanoparticles (212, 213).
On the other hand, there is a need for the discovery of tumor-specific molecules as targets for the specific delivery of therapeutic agents or disease diagnosis via liquid biopsy. The biomarker should have high specificity, sensitivity, accuracy, precision, and reliability, inexpensive and timely. While diagnostic biomarkers allow the early detection of NPC, prognostic biomarkers will predict the disease progression of patients and facilitate the development of prophylactic drugs. Advancement in molecular technologies, together with enormous databases integrating molecular and clinical data sets, will accelerate the research findings and their translation into clinical use.
In conclusion, the NPC microenvironment consists of cellular and acellular players which serve as targets for the development of therapeutic, diagnostic, and prognostic tools. Despite that, most of them are only tested in preclinical or early phases of clinical studies. Hence, large-scale clinical studies should be considered to evaluate the reliability of TME components as diagnostic tools, and guidelines or standards should be developed to ensure safe clinical use of therapeutic tools.
Author Contributions
ZS, PS, and S-CC were involved in the conceptualization and design of the content. ZS wrote and revised the manuscript. PS, C-OL, and S-CC reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grant from the Ministry of Higher Education (MOHE) through Fundamental Research Grant Scheme (FGRS/1/2020/SKK0/UCSI/02/2) and UCSI University Research Excellence and Innovation (REIG-FMS-2020/009).
Conflict of Interest
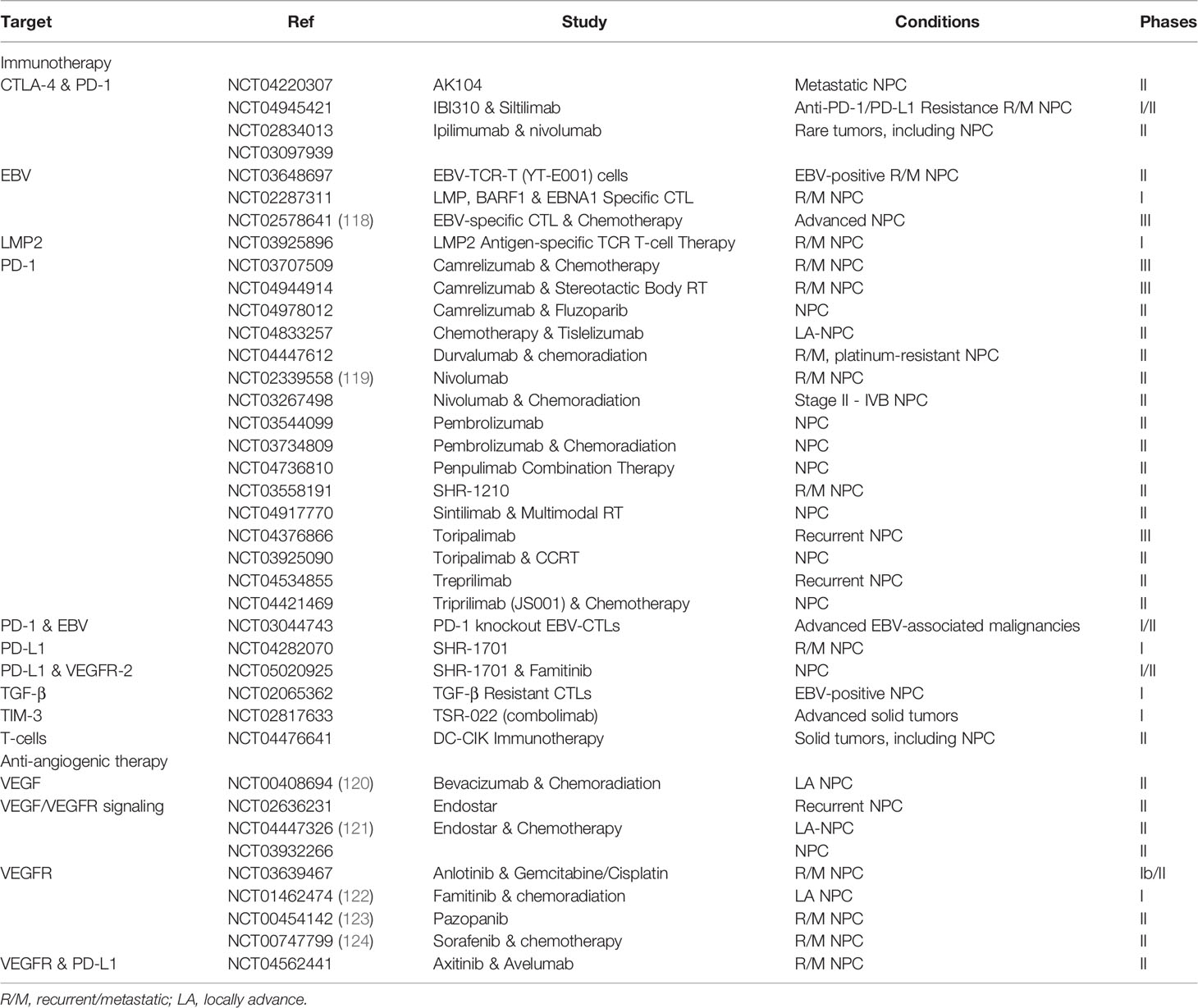
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal Carcinoma. Lancet (2019) 394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0
2. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal Carcinoma. Lancet (2016) 387(10022):1012–24. doi: 10.1016/S0140-6736(15)00055-0
3. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today (2020). Available at: https://gco.iarc.fr/today/home.
4. Sung H, Ferlay J, Soerjomataram I, Siegel RL, Bray F, Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21660
5. Bray F, Colombet M, Mery L, Pineros M, Znaor A, Zanetti R, et al. Cancer Incidence in Five Continents Volume Xi. Lyon: World Health Organization (2021).
6. Chang ET, Ye W, Zeng Y-X, Adami H-O. The Evolving Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol Biomark Prev (2021) 30(6):1035–47. doi: 10.1158/1055-9965.EPI-20-1702
7. Zuo X-Y, Feng Q-S, Sun J, Wei P-P, Chin Y-M, Guo Y-M, et al. X-Chromosome Association Study Reveals Genetic Susceptibility Loci of Nasopharyngeal Carcinoma. Biol Sex Differ (2019) 10(1):13–24. doi: 10.1186/s13293-019-0227-9
8. Xie SH, Yu ITS, Tse LA, Mang OWK, Yue L. Sex Difference in the Incidence of Nasopharyngeal Carcinoma in Hong Kong 1983-2008: Suggestion of a Potential Protective Role of Oestrogen. Eur J Cancer (2013) 49(1):150–5. doi: 10.1016/j.ejca.2012.07.004
9. Linton RE, Daker M, Khoo AS-B, Chung YCD, Viljoen M, Neilsen PM. Nasopharyngeal Carcinoma Among the Bidayuh of Sarawak, Malaysia: History and Risk Factors (Review). Oncol Lett (2021) 22(1):514–36. doi: 10.3892/ol.2021.12775
10. Wu Z-X, Xiang L, Rong J-F, He H-L, Li D. Nasopharyngeal Carcinoma With Headaches as the Main Symptom: A Potential Diagnostic Pitfall. J Cancer Res Ther (2016) 12(1):209–14. doi: 10.4103/0973-1482.157334
11. Jen C-W, Tsai Y-C, Wu J-S, Chen P-L, Yen J-H, Chuang W-K, et al. Prognostic Classification for Patients With Nasopharyngeal Carcinoma Based on American Joint Committee on Cancer Staging System T and N Categories. Ther Radiol Oncol (2020) 4(0):2–13. doi: 10.21037/tro.2020.02.01
12. Pok MH, Lung HL, Wu M, Tsang CM, Wong K-L, Mak NK, et al. Targeting Epstein-Barr Virus in Nasopharyngeal Carcinoma. Front Oncol (2020) 10:600. doi: 10.3389/fonc.2020.00600
13. Siti-Azrin AH, Norsa’adah B, Naing NN. Prognostic Factors of Nasopharyngeal Carcinoma Patients in a Tertiary Referral Hospital: A Retrospective Cohort Study. BMC Res Notes (2017) 10(1):705–12. doi: 10.1186/s13104-017-2990-1
14. Langley RR, Fidler IJ. The Seed and Soil Hypothesis Revisited-The Role of Tumor-Stroma Interactions in Metastasis to Different Organs. Int J Cancer (2011) 128(11):2527–35. doi: 10.1002/ijc.26031
15. Smythies J. Intercellular Signaling in Cancer-The SMT and TOFT Hypotheses, Exosomes, Telocytes and Metastases: Is the Messenger in the Message? J Cancer (2015) 6(7):604–9. doi: 10.7150/jca.12372
16. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
17. Gong L, Lai-Wan Kwong D, Dai W, Wu P, Li S, Yan Q, et al. Comprehensive Single-Cell Sequencing Reveals the Stromal Dynamics and Tumor-Specific Characteristics in the Microenvironment of Nasopharyngeal Carcinoma. Nat Commun (2021) 12(1):1540–58. doi: 10.1038/s41467-021-21795-z
18. Jin S, Li R, Chen MY, Yu C, Tang LQ, Liu YM, et al. Single-Cell Transcriptomic Analysis Defines the Interplay Between Tumor Cells, Viral Infection, and the Microenvironment in Nasopharyngeal Carcinoma. Cell Res (2020) 30(11):950–65. doi: 10.1038/s41422-020-00402-8
19. Zhao J, Guo C, Xiong F, Yu J, Ge J, Wang H, et al. Single Cell RNA-Seq Reveals the Landscape of Tumor and Infiltrating Immune Cells in Nasopharyngeal Carcinoma. Cancer Lett (2020) 477:131–43. doi: 10.1016/j.canlet.2020.02.010
20. Chen Y-P, Yin J-H, Li W-F, Li H-J, Chen D-P, Zhang C-J, et al. Single-Cell Transcriptomics Reveals Regulators Underlying Immune Cell Diversity and Immune Subtypes Associated With Prognosis in Nasopharyngeal Carcinoma. Cell Res (2020) 30(11):1024–42. doi: 10.1038/s41422-020-0374-x
21. Liu Y, He S, Wang X-L, Peng W, Chen Q-Y, Chi D-M, et al. Tumour Heterogeneity and Intercellular Networks of Nasopharyngeal Carcinoma at Single Cell Resolution. Nat Commun (2021) 12(1):741–59. doi: 10.1038/s41467-021-21043-4
22. Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of Tumor Microenvironment in Tumorigenesis. J Cancer (2017) 8(5):761–73. doi: 10.7150/jca.17648
24. Tao L, Huang G, Song H, Chen Y, Chen L. Cancer Associated Fibroblasts: An Essential Role in the Tumor Microenvironment (Review). Oncol Lett (2017) 14(3):2611–20. doi: 10.3892/ol.2017.6497
25. Liu T, Zhou L, Li D, Andl T, Zhang Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front Cell Dev Biol (2019) 7:60–74. doi: 10.3389/fcell.2019.00060
26. Eble JA, Niland S. The Extracellular Matrix in Tumor Progression and Metastasis. Clin Exp Metastasis (2019) 36(3):171–98. doi: 10.1007/s10585-019-09966-1
27. Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the Physical Microenvironment on Tumor Progression and Metastasis. Curr Opin Biotechnol (2016) 40:41–8. doi: 10.1016/j.copbio.2016.02.007
28. Gourzones C, Barjon C, Busson P. Host-Tumor Interactions in Nasopharyngeal Carcinomas. Semin Cancer Biol (2012) 22(2):127–36. doi: 10.1016/j.semcancer.2012.01.002
29. Lai M-M, Cheng PNM, Tsao SY, Lai KN. Immunohistological Characteristics of the Infiltrating Lymphoid Cells and Expression of HLA Class I and II Antigens in Nasopharyngeal Carcinoma. Virchows Arch A Pathol Anat (1990) 417(4):347–52. doi: 10.1007/BF01605787
30. Chong PY, Chew CT, Chan KC, Ow CK. Tumour Infiltrating Lymphocytes in Nasopharyngeal Carcinoma. Ann Acad Med Singapore (1988) 17(2):238–42.
31. Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A Pan-Cancer Single-Cell Transcriptional Atlas of Tumor Infiltrating Myeloid Cells. Cell (2021) 184(3):792–809.e23. doi: 10.1016/j.cell.2021.01.010
32. Li Z-L, Ye S-B, Ouyang L-Y, Zhang H, Chen Y-S, He J, et al. COX-2 Promotes Metastasis in Nasopharyngeal Carcinoma by Mediating Interactions Between Cancer Cells and Myeloid-Derived Suppressor Cells. Oncoimmunology (2015) 4(11):e1044712. doi: 10.1080/2162402X.2015.1044712
33. Chen Y, Chew CT, Chan SH. T-Cell Receptor Gene Expression in Tumour-Infiltrating Lymphocytes and Peripheral Blood Lymphocytes of Patients With Nasopharyngeal Carcinoma. Br J Cancer (1995) 72(1):117–22. doi: 10.1038/bjc.1995.286
34. Herait P, Ganem G, Lipinski M, Carlu C, Micheau C, Schwaab G, et al. Lymphocyte Subsets in Tumour of Patients With Undifferentiated Nasopharyngeal Carcinoma: Presence of Lymphocytes With the Phenotype of Activated T Cells. Br J Cancer (1987) 55(2):135–9. doi: 10.1038/bjc.1987.28
35. Jiang Y, Li Y, Zhu B. T-Cell Exhaustion in the Tumor Microenvironment. Cell Death Dis (2015) 6(6):e1792. doi: 10.1038/cddis.2015.162
36. Huo S, Luo Y, Deng R, Liu X, Wang J, Wang L, et al. EBV-EBNA1 Constructs an Immunosuppressive Microenvironment for Nasopharyngeal Carcinoma by Promoting the Chemoattraction of Treg Cells. J Immunother Cancer (2020) 8(2):e001588. doi: 10.1136/jitc-2020-001588
37. Yang L, Liu G, Li Y, Pan Y. The Emergence of Tumor-Infiltrating Lymphocytes in Nasopharyngeal Carcinoma: Predictive Value and Immunotherapy Implications. Genes Dis (2021). doi: 10.1016/j.gendis.2021.07.002
38. Ohue Y, Nishikawa H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells be a New Therapeutic Target? Cancer Sci (2019) 110(7):2080–9. doi: 10.1111/cas.14069
39. Li J, Huang Z-F, Xiong G, Mo H-Y, Mai H-Q, Chen Q-Y, et al. Distribution, Characterization, and Induction of CD8 + Regulatory T Cells and IL-17-Producing CD8 + T Cells in Nasopharyngeal Carcinoma. J Transl Med (2011) 9:189. doi: 10.1186/1479-5876-9-189
40. Nambiar DK, Aguilera T, Cao H, Kwok S, Kong C, Bloomstein J, et al. Galectin-1-Driven T Cell Exclusion in the Tumor Endothelium Promotes Immunotherapy Resistance. J Clin Invest (2019) 129(12):5553–67. doi: 10.1172/JCI129025
41. Fogg M, Murphy JR, Lorch J, Posner M, Wang F. Therapeutic Targeting of Regulatory T Cells Enhances Tumor-Specific CD8+ T Cell Responses in Epstein–Barr Virus Associated Nasopharyngeal Carcinoma. Virology (2013) 441(2):107–13. doi: 10.1016/j.virol.2013.03.016
42. Zhao C, Liu Y, Liang Z, Feng H, Xu S, Xu E. MACC1 Facilitates the Escape of Nasopharyngeal Carcinoma Cells From Killing by Natural Killer Cells MACC1 Facilitates the Escape of Nasopharyngeal Carcinoma Cells From Killing by Natural Killer Cells. Biotechnol Biotechnol Equip (2019) 33(1):579–88. doi: 10.1080/13102818.2019.1596041
43. Xu Y, Zhou R, Huang C, Zhang M, Li J, Zong J, et al. Analysis of the Expression of Surface Receptors on NK Cells and NKG2D on Immunocytes in Peripheral Blood of Patients With Nasopharyngeal Carcinoma. Asian Pac J Cancer Prev (2018) 19(3):661–5. doi: 10.22034/APJCP.2018.19.3.661
44. Arij BC, Nesrine O, Hayet D, Fayza A, Hajer A, Tesnim M, et al. Soluble MICA and Anti-MICA Antibodies as Biomarkers of Nasopharyngeal Carcinoma Disease. Immunol Invest (2019) 49(5):498–509. doi: 10.1080/08820139.2019.1690506
45. Liou AK-F, Soon G, Tan L, Peng Y, Meng Cher B, Cher Goh B, et al. Elevated IL18 Levels in Nasopharyngeal Carcinoma Induced PD-1 Expression on NK Cells in TILS Leading to Poor Prognosis. Oral Oncol (2020) 104:104616. doi: 10.1016/j.oraloncology.2020.104616
46. Li J-P, Wu C-Y, Chen M-Y, Liu S-X, Yan S-M, Kang Y-F, et al. PD-1 + CXCR5 – CD4 + Th-CXCL13 Cell Subset Drives B Cells Into Tertiary Lymphoid Structures of Nasopharyngeal Carcinoma. J Immunother Cancer (2021) 9(7):2101. doi: 10.1136/jitc-2020-002101
47. Selitsky SR, Marron D, Mose LE, Parker JS, Dittmer DP. Epstein-Barr Virus-Positive Cancers Show Altered B-Cell Clonality. mSystems (2018) 3(5):e00081–18. doi: 10.1128/mSystems.00081-18
48. Xu T, Huang Z, Su B, Wang S, Wang D, Wang C, et al. Prognostic Significance of Circulating CD19+ B Lymphocytes in EBV-Associated Nasopharyngeal Carcinoma. Med Oncol (2014) 31(10):198. doi: 10.1007/s12032-014-0198-y
49. Miao B-P, Zhang R-S, Li M, Fu Y-T, Zhao M, Liu Z-G, et al. Nasopharyngeal Cancer-Derived microRNA-21 Promotes Immune Suppressive B Cells. Cell Mol Immunol (2015) 12(6):750–6. doi: 10.1038/cmi.2014.129
50. Cai T-T, Ye S-B, Liu Y-N, He J, Chen Q-Y, Mai H-Q, et al. LMP1-Mediated Glycolysis Induces Myeloid-Derived Suppressor Cell Expansion in Nasopharyngeal Carcinoma. PloS Pathog (2017) 13(7):e1006503. doi: 10.1371/journal.ppat.1006503
51. Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression Mediated by Myeloid-Derived Suppressor Cells (MDSCs) During Tumour Progression. Br J Cancer (2019) 120(1):16–25. doi: 10.1038/s41416-018-0333-1
52. Kim J, Bae JS. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm (2016) 2016:6058147. doi: 10.1155/2016/6058147
53. Chen HC, Kawamura A, Murata M, Hamajima K, Osono M. A Histopathological Study of Lymphoid Tissue Reaction to Metastatic Nasopharyngeal Carcinoma in Nude Mice. IARC Sci Publ (1978) 20:65–84.
54. Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res (2019) 79(18):4557–67. doi: 10.1158/0008-5472.CAN-18-3962
55. Wang J, Huang H, Lu J, Bi P, Wang F, Liu X, et al. Tumor Cells Induced-M2 Macrophage Favors Accumulation of Treg in Nasopharyngeal Carcinoma. Int J Clin Exp Pathol (2017) 10(8):8389–401.
56. Peng Y, Li X, Liu H, Deng X, She C, Liu C, et al. microRNA-18a From M2 Macrophages Inhibits TGFBR3 to Promote Nasopharyngeal Carcinoma Progression and Tumor Growth via TGF-β Signaling Pathway. Nanoscale Res Lett (2020) 15(1):196. doi: 10.1186/s11671-020-03416-8
57. Gallo O, Bianchi S, Giannini A, Gallina E, Libonati GA, Gini-Storchi O. Correlations Between Histopathological and Biological Findings in Nasopharyngeal Carcinoma and Its Prognostic Significance. Laryngoscope (1991) 101(5):487–93. doi: 10.1288/00005537-199105000-00008
58. Nomori H, Watanabe S, Nakajima T, Shimosato Y, Kameya T. Histiocytes in Nasopharyngeal Carcinoma in Relation to Prognosis. Cancer (1986) 57(1):100–5. doi: 10.1002/1097-0142(19860101)57:1<100::AID-CNCR2820570121>3.0.CO;2-X
59. Lu J, Chen XM, Huang HR, Zhao FP, Wang F, Liu X, et al. Detailed Analysis of Inflammatory Cell Infiltration and the Prognostic Impact on Nasopharyngeal Carcinoma. Head Neck (2018) 40(6):1245–53. doi: 10.1002/hed.25104
60. Ping Q, Yan R, Cheng X, Wang W, Zhong Y, Hou Z, et al. Cancer-Associated Fibroblasts: Overview, Progress, Challenges, and Directions. Cancer Gene Ther (2021) 28(9):984–99. doi: 10.1038/s41417-021-00318-4
61. Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In Search of Definitions: Cancer-Associated Fibroblasts and Their Markers. Int J Cancer (2019) 146(4):895–905. doi: 10.1002/ijc.32193
62. Liao Z, Tan ZW, Zhu P, Tan NS. Cancer-Associated Fibroblasts in Tumor Microenvironment – Accomplices in Tumor Malignancy. Cell Immunol (2019) 343:103729. doi: 10.1016/j.cellimm.2017.12.003
63. Wu X, Zhou Z, Xu S, Liao C, Chen X, Li B, et al. Extracellular Vesicle Packaged LMP1-Activated Fibroblasts Promote Tumor Progression via Autophagy and Stroma-Tumor Metabolism Coupling. Cancer Lett (2020) 478:93–106. doi: 10.1016/j.canlet.2020.03.004
64. Chen J, Yang P, Xiao Y, Zhang Y, Liu J, Xie D, et al. Overexpression of α-Sma-Positive Fibroblasts (CAFs) in Nasopharyngeal Carcinoma Predicts Poor Prognosis. J Cancer (2017) 8(18):3897–902. doi: 10.7150/jca.20324
65. Wang S, Ma N, Kawanishi S, Hiraku Y, Oikawa S, Xie Y, et al. Relationships of Alpha-SMA-Positive Fibroblasts and SDF-1-Positive Tumor Cells With Neoangiogenesis in Nasopharyngeal Carcinoma. BioMed Res Int (2014) 2014:507353. doi: 10.1155/2014/507353
66. Zhu Y, Shi C, Zeng L, Liu G, Jiang W, Zhang X, et al. High COX-2 Expression in Cancer-Associated Fibiroblasts Contributes to Poor Survival and Promotes Migration and Invasiveness in Nasopharyngeal Carcinoma. Mol Med (2019) 59(3):265–80. doi: 10.1002/mc.23150
67. Huang W, Zhang L, Yang M, Wu X, Wang X, Huang W, et al. Cancer-Associated Fibroblasts Promote the Survival of Irradiated Nasopharyngeal Carcinoma Cells via the NF-κb Pathway. J Exp Clin Cancer Res (2021) 40(1):87–101. doi: 10.1186/s13046-021-01878-x
68. Ben-Haj-Ayed A, Moussa A, Ghedira R, Gabbouj S, Miled S, Bouzid N, et al. Prognostic Value of Indoleamine 2,3-Dioxygenase Activity and Expression in Nasopharyngeal Carcinoma. Immunol Lett (2016) 169:23–32. doi: 10.1016/j.imlet.2015.11.012
69. Wang J, Deng L, Huang J, Cai R, Zhu X, Liu F, et al. High Expression of FIbronectin 1 Suppresses Apoptosis Through the NF-κb Pathway and Is Associated With Migration in Nasopharyngeal Carcinoma. Am J Transl Res (2017) 9(10):4502–11.
70. Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, et al. Cancer-Associated Fibroblasts Promote Directional Cancer Cell Migration by Aligning Fibronectin. J Cell Biol (2017) 216(11):3799–817. doi: 10.1083/jcb.201704053
71. Wakisaka N, Wen Q-H, Yoshizaki T, Nishimura T, Furukawa M, Kawahara E, et al. Association of Vascular Endothelial Growth Factor Expression With Angiogenesis and Lymph Node Metastasis in Nasopharyngeal Carcinoma From the Department of Otolaryngology. Laryngoscope (1999) 109(5):810–4. doi: 10.1097/00005537-199905000-00024
72. Luo W, Li S, Peng B, Ye Y, Deng X. Embryonic Stem Cells Markers SOX2, OCT4 and Nanog Expression and Their Correlations With Epithelial-Mesenchymal Transition in Nasopharyngeal Carcinoma. PloS One (2013) 8(2):56324. doi: 10.1371/journal.pone.0056324
73. Maishi N, Hida K. Tumor Endothelial Cells Accelerate Tumor Metastasis. Cancer Sci (2017) 108(10):1921–6. doi: 10.1111/cas.13336
74. Hida K, Maishi N, Annan DA, Hida Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int J Mol Sci (2018) 19(5):1272–84. doi: 10.3390/ijms19051272
75. Huang L, Hu C, Chao H, Zhang Y, Li Y, Hou J, et al. Drug-Resistant Endothelial Cells Facilitate Progression, EMT and Chemoresistance in Nasopharyngeal Carcinoma via Exosomes. Cell Signal (2019) 63:109385. doi: 10.1016/j.cellsig.2019.109385
76. Hong B, Lui VWY, Hashiguchi M, Hui EP, Chan AT-C. Targeting Tumor Hypoxia in Nasopharyngeal Carcinoma. Head Neck (2011) 35(1):133–45. doi: 10.1002/hed.21877
77. Hua YJ, Wang HY, Tang LQ, Chen QY, Shao JY, Mai HQ. LOX Expression in Primary Nasopharyngeal Carcinoma: Correlation With Prognostic Parameters and Outcome. Oncotarget (2016) 7(7):8200–7. doi: 10.18632/oncotarget.6996
78. Liu YN, Yang JF, Huang DJ, Ni HH, Zhang CX, Zhang L, et al. Hypoxia Induces Mitochondrial Defect That Promotes T Cell Exhaustion in Tumor Microenvironment Through MYC-Regulated Pathways. Front Immunol (2020) 11:1906. doi: 10.3389/fimmu.2020.01906
79. Mukherjee A, Madamsetty VS, Paul MK, Mukherjee S. Recent Advancements of Nanomedicine Towards Antiangiogenic Therapy in Cancer. Int J Mol Sci (2019) 21(2):455–82. doi: 10.3390/ijms21020455
80. Murono S, Inoue H, Tanabe T, Joab I, Yoshizaki T, Furukawa M, et al. Induction of Cyclooxygenase-2 by Epstein-Barr Virus Latent Membrane Protein 1 is Involved in Vascular Endothelial Growth Factor Production in Nasopharyngeal Carcinoma Cells. Proc Natl Acad Sci USA (2001) 98(12):6905–10. doi: 10.1073/pnas.121016998
81. Liu X, Wang F, Peng L, Wang Y. A Meta-Analysis of Vascular Endothelial Growth Factor for Nasopharyngeal Cancer Prognosis. Front Oncol (2018) 8:486. doi: 10.3389/fonc.2018.00486
82. Chor S, Huang M, Tsao SW, Tsang CM. Interplay of Viral Infection, Host Cell Factors and Tumor Microenvironment in the Pathogenesis of Nasopharyngeal Carcinoma. Cancers (Basel) (2018) 10(4):106. doi: 10.3390/cancers10040106
83. Renaud S, Lefebvre A, Mordon S, Moralès O, Delhem N. Novel Therapies Boosting T Cell Immunity in Epstein Barr Virus-Associated Nasopharyngeal Carcinoma. Int J Mol Sci (2020) 21(12):4292. doi: 10.3390/ijms21124292
84. Chang KP, Chang YT, Wu CC, Liu YL, Chen MC, Tsang NM, et al. Multiplexed Immunobead-Based Profiling of Cytokine Markers for Detection of Nasopharyngeal Carcinoma and Prognosis of Patient Survival. Head Neck (2011) 33(6):886–97. doi: 10.1002/hed.21557
85. Kwok-Fung Lo A, Dawson CW, Lok Lung H, Wong K-L, Young LS. The Role of EBV-Encoded LMP1 in the NPC Tumor Microenvironment: From Function to Therapy. Front Oncol (2021) 11:640207. doi: 10.3389/fonc.2021.640207
86. Wang J, Luo Y, Bi P, Lu J, Wang F, Liu X, et al. Mechanisms of Epstein-Barr Virus Nuclear Antigen 1 Favor Tregs Accumulation in Nasopharyngeal Carcinoma. Cancer Med (2020) 9(15):5598–608. doi: 10.1002/cam4.3213
87. Tang M, Ou N, Li C, Lu A, Li J, Ma L, et al. Expression and Prognostic Significance of Macrophage Inflammatory Protein-3 Alpha and Cystatin A in Nasopharyngeal Carcinoma. BioMed Res Int (2015) 2015:617143. doi: 10.1155/2015/617143
88. Li Z, Duan Y, Cheng S, Chen Y, Hu Y, Zhang L, et al. EBV-Encoded RNA via TLR3 Induces Inflammation in Nasopharyngeal Carcinoma. Oncotarget (2015) 6(27):24291–303. doi: 10.18632/oncotarget.4552
89. Lee C-H, Yeh T-H, Lai H-C, Wu S-Y, Su I-J, Takada K, et al. Epstein-Barr Virus Zta-Induced Immunomodulators From Nasopharyngeal Carcinoma Cells Upregulate Interleukin-10 Production From Monocytes. J Virol (2011) 85(14):7333–42. doi: 10.1128/JVI.00182-11
90. Adel FA-K, Omminea AA, Mamdouh ZA, Manal MH, Mohamed FS, Dalia M, et al. Pre-Treatment Serum Inflammatory Cytokines as Survival Predictors of Patients With Nasopharyngeal Carcinoma Receiving Chemoradiotherapy. Mol Clin Oncol (2016) 5(6):811–6. doi: 10.3892/mco.2016.1041
91. Liu W, Lin Y, Xiao H, Xing S, Chen H, Chi P, et al. Epstein-Barr Virus Infection Induces Indoleamine 2,3-Dioxygenase Expression in Human Monocyte-Derived Macrophages Through P38/Mitogen-Activated Protein Kinase and NF-κb Pathways: Impairment in T Cell Functions. J Virol (2014) 88(12):6660–71. doi: 10.1128/JVI.03678-13
92. Li X-J, Peng L-X, Shao J-Y, Lu W-H, Zhang J-X, Chen S, et al. As an Independent Unfavorable Prognostic Factor, IL-8 Promotes Metastasis of Nasopharyngeal Carcinoma Through Induction of Epithelial-Mesenchymal Transition and Activation of AKT Signaling. Carcinogenesis (2012) 33(7):1302–9. doi: 10.1093/carcin/bgs181
93. Luo H, Yi B. The Role of Exosomes in the Pathogenesis of Nasopharyngeal Carcinoma and the Involved Clinical Application. Int J Biol Sci (2021) 17(9):2147–56. doi: 10.7150/ijbs.59688
94. Mashouri L, Yousefi H, Reza Aref A, mohammad Ahadi A, Molaei F, Alahari SK. Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol Cancer (2019) 18(1):75–89. doi: 10.1186/s12943-019-0991-5
95. Li I, Nabet BY. Exosomes in the Tumor Microenvironment as Mediators of Cancer Therapy Resistance. Mol Cancer (2019) 18(1):32–42. doi: 10.1186/s12943-019-0975-5
96. Zhou Y, Xia L, Lin J, Wang H, Oyang L, Tan S, et al. Exosomes in Nasopharyngeal Carcinoma. J Cancer (2018) 9(5):767–77. doi: 10.7150/jca.22505
97. You B, Cao X, Shao X, Ni H, Shi S, Shan Y, et al. Clinical and Biological Significance of HAX-1 Overexpression in Nasopharyngeal Carcinoma. Oncotarget (2016) 7(11):12505–24. doi: 10.18632/oncotarget.7274
98. Zhang K, Liu D, Zhao J, Shi S, He X, Da P, et al. Nuclear Exosome HMGB3 Secreted by Nasopharyngeal Carcinoma Cells Promotes Tumour Metastasis by Inducing Angiogenesis. Cell Death Dis (2021) 12(6):554. doi: 10.1038/s41419-021-03845-y
99. Chan Y-K, Zhang H, Liu P, Tsao S-W, Li Lung M, Mak N-K, et al. Proteomic Analysis of Exosomes From Nasopharyngeal Carcinoma Cell Identifies Intercellular Transfer of Angiogenic Proteins. UICC Int J Cancer IJC (2015) 137(8):1830–41. doi: 10.1002/ijc.29562
100. Zhang C, Huang D, Baloche V, Zhang L, Xu J, Li B, et al. Galectin-9 Promotes a Suppressive Microenvironment in Human Cancer by Enhancing STING Degradation. Oncogenesis (2020) 9(7):65. doi: 10.1038/s41389-020-00248-0
101. Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal Hif1α Supports Invasive Potential of Nasopharyngeal Carcinoma-Associated LMP1-Positive Exosomes. Oncogene (2014) 33(37):4613–22. doi: 10.1038/onc.2014.66
102. Zhou SK, Gao F, Zhong ZS, Yao H. Long Non-Coding RNA Colon Cancer Associated Transcript-2 From Nasopharyngeal Carcinoma-Derived Exosomes Promotes Angiogenesis. Chin J Otorhinolaryngol Head Neck Surg (2020) 55(10):944–51. doi: 10.3760/cma.j.cn115330-20200423-00322
103. Luo Y, Ma J, Liu F, Guo J, Gui R. Diagnostic Value of Exosomal circMYC in Radioresistant Nasopharyngeal Carcinoma. Head Neck (2020) 42(12):3702–11. doi: 10.1002/hed.26441
104. Gu M, Li L, Zhang Z, Chen J, Zhang W, Zhang J, et al. PFKFB3 Promotes Proliferation, Migration and Angiogenesis in Nasopharyngeal Carcinoma. J Cancer (2017) 8(18):3887–96. doi: 10.7150/jca.19112
105. You Y, Shan Y, Chen J, Yue H, You B, Shi S, et al. Matrix Metalloproteinase 13-Containing Exosomes Promote Nasopharyngeal Carcinoma Metastasis. Cancer Sci (2015) 106(12):1669–77. doi: 10.1111/cas.12818
106. Shan Y, You B, Shi S, Shi W, Zhang Z, Zhang Q, et al. Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes From Nasopharyngeal Carcinoma Enhances Metastases. Cell Death Dis (2018) 9(3):382. doi: 10.1038/s41419-018-0425-0
107. Mrizak D, Martin N, Barjon C, Jimenez-Pailhes A-S, Mustapha R, Niki T, et al. Effect of Nasopharyngeal Carcinoma-Derived Exosomes on Human Regulatory T Cells. J Natl Cancer Inst (2015) 107(1):363. doi: 10.1093/jnci/dju363
108. Duan B, Shi S, Yue H, You B, Shan Y, Zhu Z, et al. Exosomal miR-17-5p Promotes Angiogenesis in Nasopharyngeal Carcinoma via Targeting BAMBI. J Cancer (2019) 10(26):6681–92. doi: 10.7150/jca.30757
109. Bao L, You B, Shi S, Shan Y, Zhang Q, Yue H, et al. Metastasis-Associated miR-23a From Nasopharyngeal Carcinoma-Derived Exosomes Mediates Angiogenesis by Repressing a Novel Target Gene TSGA10. Oncogene (2018) 37(21):2873–89. doi: 10.1038/s41388-018-0183-6
110. Wang J, Jiang Q, Faleti OD, Tsang CM, Zhao M, Wu G, et al. Exosomal Delivery of AntagomiRs Targeting Viral and Cellular MicroRNAs Synergistically Inhibits Cancer Angiogenesis. Mol Ther - Nucleic Acids (2020) 22:153–65. doi: 10.1016/j.omtn.2020.08.017
111. Tian X, Liu Y, Wang Z, Wu S. miR-144 Delivered by Nasopharyngeal Carcinoma-Derived EVs Stimulates Angiogenesis Through the FBXW7/HIF-1α/VEGF-A Axis. Mol Ther - Nucleic Acids (2021) 24:1000–11. doi: 10.1016/j.omtn.2021.03.016
112. Lu J, Liu QH, Wang F, Tan JJ, Deng YQ, Peng XH, et al. Exosomal miR-9 Inhibits Angiogenesis by Targeting MDK and Regulating PDK/AKT Pathway in Nasopharyngeal Carcinoma. J Exp Clin Cancer Res (2018) 37(1):147. doi: 10.1186/s13046-018-0814-3
113. Ye S-B, Li Z-L, Luo D-H, Huang B-J, Chen Y-S, Zhang X-S, et al. Tumor-Derived Exosomes Promote Tumor Progression and T-Cell Dysfunction Through the Regulation of Enriched Exosomal microRNAs in Human Nasopharyngeal Carcinoma. Oncotarget (2014) 5:5439–52. doi: 10.18632/oncotarget.2118
114. Ye S-B, Zhang H, Cai T-T, Liu Y-N, Ni J-J, He J, et al. Exosomal miR-24-3p Impedes T-Cell Function by Targeting FGF11 and Serves as a Potential Prognostic Biomarker for Nasopharyngeal Carcinoma. J Pathol (2016) 240(3):329–40. doi: 10.1002/path.4781
115. Cheng Q, Li Q, Xu L, Jiang H. Exosomal microRNA-301a-3p Promotes the Proliferation and Invasion of Nasopharyngeal Carcinoma Cells by Targeting BTG1 mRNA. Mol Med Rep (2021) 23(5):328. doi: 10.3892/mmr.2021.11967
116. Wan F-Z, Chen K-H, Sun Y-C, Chen X-C, Liang R-B, Chen L, et al. Exosomes Overexpressing miR-34c Inhibit Malignant Behavior and Reverse the Radioresistance of Nasopharyngeal Carcinoma. J Transl Med (2020) 18(1):12. doi: 10.1186/s12967-019-02203-z
117. Yin H, Qiu X, Shan Y, You B, Xie L, Zhang P, et al. HIF-1α Downregulation of miR-433-3p in Adipocyte-Derived Exosomes Contributes to NPC Progression via Targeting SCD1. Cancer Sci (2021) 2021(112):1457–70. doi: 10.1111/cas.14829
118. Chia WK, Teo M, Wang WW, Lee B, Ang SF, Tai WM, et al. Adoptive T-Cell Transfer and Chemotherapy in the First-Line Treatment of Metastatic and/or Locally Recurrent Nasopharyngeal Carcinoma. Mol Ther (2014) 22(1):132–9. doi: 10.1038/mt.2013.242
119. Ma BBY, Lim W-T, Goh B-C, Hui EP, Lo K-W, Pettinger A, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol (2018) 36(14):1412–8. doi: 10.1200/JCO.2017.77.0388
120. Lee NY, Zhang E, Pfister DG, Kim J, Garden AS, Mechalakos J, et al. Phase II Study of the Addition of Bevacizumab to Standard Chemoradiation for Loco-Regionally Advanced Nasopharyngeal Carcinoma: Radiation Therapy Oncology Group (RTOG) Trial 0615. Lancet Oncol (2012) 13(2):172. doi: 10.1016/S1470-2045(11)70303-5
121. Li Y, Tian Y, JIn F, Wu W, Long J, Ouyang J, et al. A Phase II Multicenter Randomized Controlled Trial to Compare Standard Chemoradiation With or Without Recombinant Human Endostatin Injection (Endostar) Therapy for the Treatment of Locally Advanced Nasopharyngeal Carcinoma: Long-Term Outcomes Update. Curr Probl Cancer (2020) 44(1):100492. doi: 10.1016/j.currproblcancer.2019.06.007
122. Chen Q, Tang L, Liu N, Han F, Guo L, Guo S, et al. Famitinib in Combination With Concurrent Chemoradiotherapy in Patients With Locoregionally Advanced Nasopharyngeal Carcinoma: A Phase 1, Open-Label, Dose-Escalation Study. Cancer Commun (2018) 38(1):66. doi: 10.1186/s40880-018-0330-z
123. Lim W-T, Ng Q-S, Ivy P, Leong S-S, Singh O, Chowbay B, et al. A Phase II Study of Pazopanib in Asian Patients With Recurrent/Metastatic Nasopharyngeal Carcinoma. Clin Cancer Res (2011) 17(16):5481–9. doi: 10.1158/1078-0432.CCR-10-3409
124. Xue C, Huang Y, Huang PY, Yu QT, Pan JJ, Liu LZ, et al. Phase II Study of Sorafenib in Combination With Cisplatin and 5-Fluorouracil to Treat Recurrent or Metastatic Nasopharyngeal Carcinoma. Ann Oncol (2013) 24(4):1055–61. doi: 10.1093/annonc/mds581
125. Wang X, Xiang Z, Tsao GSW, Tu W. Exosomes Derived From Nasopharyngeal Carcinoma Cells Induce IL-6 Production From Macrophages to Promote Tumorigenesis. Cell Mol Immunol (2021) 18(2):501–3. doi: 10.1038/s41423-020-0420-0
126. Yuan F, Zhou Z-F. Exosomes Derived From Taxol-Resistant Nasopharyngeal Carcinoma (NPC) Cells Transferred DDX53 to NPC Cells and Promoted Cancer Resistance to Taxol. Eur Rev Med Pharmacol Sci (2021) 25(1):127–38. doi: 10.26355/eurrev_202101_24375
127. Meckes DG, Shair KHY, Marquitz AR, Kung C-P, Edwards RH, Raab-Traub N. Human Tumor Virus Utilizes Exosomes for Intercellular Communication. Proc Natl Acad Sci USA (2010) 107(47):20370–5. doi: 10.1073/pnas.1014194107
128. Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes Derived From Epstein-Barr Virus-Infected Cells Are Internalized via Caveola-Dependent Endocytosis and Promote Phenotypic Modulation in Target Cells. J Virol (2013) 87(18):19334–47. doi: 10.1128/JVI.01310-13
129. Yoshizaki T, Kondo S, Endo K, Nakanishi Y, Hatano M, Ueno T, et al. Modulation of the Tumor Microenvironment by Epstein-Barr Virus Latent Membrane Protein 1 in Nasopharyngeal Carcinoma. Cancer Sci (2017) 109(2):272–8. doi: 10.1111/cas.13473
130. Zuo L, Xie Y, Tang J, Xin S, Liu L, Zhang S, et al. Targeting Exosomal EBV-LMP1 Transfer and miR-203 Expression via the NF-κb Pathway: The Therapeutic Role of Aspirin in NPC. Mol Ther - Nucleic Acids (2019) :17:175–84. doi: 10.1016/j.omtn.2019.05.023
131. Duan Y, Li Z, Cheng S, Chen Y, Zhang L, He J, et al. Nasopharyngeal Carcinoma Progression is Mediated by EBER-Triggered Inflammation via the RIG-I Pathway. Cancer Lett (2015) 361(1):67–74. doi: 10.1016/j.canlet.2015.02.037
132. Takada K. Role of EBER and BARF1 in Nasopharyngeal Carcinoma (NPC) Tumorigenesis. Semin Cancer Biol (2012) 22(2):162–5. doi: 10.1016/j.semcancer.2011.12.007
133. Zeng Z, Fan S, Zhang X, Li S, Zhou M, Xiong W, et al. Epstein-Barr Virus-Encoded Small RNA 1 (EBER-1) Could Predict Good Prognosis in Nasopharyngeal Carcinoma. Clin Transl Oncol (2016) 18(2):206–11. doi: 10.1007/s12094-015-1354-3
134. Fan C, Tang Y, Wang J, Guo C, Wang Y, Xiang B, et al. The Emerging Role of Epstein-Barr Virus Encoded microRNAs in Nasopharyngeal Carcinoma. J Cancer (2018) 9(16):2852–64. doi: 10.7150/jca.25460
135. Michiel Pegtel D, Cosmopoulos K, Thorley-Lawson DA, Van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, et al. Functional Delivery of Viral miRNAs via Exosomes. Proc Natl Acad Sci USA (2010) 107(14):6328–33. doi: 10.1073/pnas.0914843107
136. Hamanishi J, Masaki M, Noriomi M, Abiko K, Baba T, Konishi I. PD-1/PD-L1 Blockade in Cancer Treatment: Perspectives and Issues. Int J Clin Oncol (2016) 21(3):462–73. doi: 10.1007/s10147-016-0959-z
137. Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in Cancer Immunotherapy: Clinical Implications and Future Considerations. Hum Vaccin Immunother (2019) 15(5):1111–22. doi: 10.1080/21645515.2019.1571892
138. Hsu C, Lee S-H, Ejadi S, Even C, Cohen RB, Le TC, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1–Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol (2017) 35(36):4050–6. doi: 10.1200/JCO.2017.73.3675
139. Li Y, Ding J, Liao L, Zhang Z, Liao S, Wu Y, et al. Expression of Programmed Death Ligand-1 Predicts Poor Outcome in Nasopharyngeal Carcinoma. Mol Clin Oncol (2017) 7(3):378–82. doi: 10.3892/mco.2017.1318/abstract
140. Huang Z-L, Liu S, Wang G-N, Zheng S-H, Ding S-R, Tao Y-L, et al. The Prognostic Significance of PD-L1 and PD-1 Expression in Patients With Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis. Cancer Cell Int (2019) 19:141. doi: 10.1186/s12935-019-0863-5
141. Zhu Q, Cai MY, Chen CL, Hu H, Lin HX, Li M, et al. Tumor Cells PD-L1 Expression as a Favorable Prognosis Factor in Nasopharyngeal Carcinoma Patients With Pre-Existing Intratumor-Infiltrating Lymphocytes. Oncoimmunology (2017) 6(5):e1312240. doi: 10.1080/2162402X.2017.1312240
142. Lv J-W, Li J-Y, Luo L-N, Wang Z-X, Chen Y-P. Comparative Safety and Efficacy of Anti-PD-1 Monotherapy, Chemotherapy Alone, and Their Combination Therapy in Advanced Nasopharyngeal Carcinoma: Findings From Recent Advances in Landmark Trials. J Immunother Cancer (2019) 7(1):159. doi: 10.1186/s40425-019-0636-7
143. Dong H, De L, Cruz-Merino L, Inman BA, Du J, Huang Y-F, et al. Comparative Safety of PD-1/PD-L1 Inhibitors for Cancer Patients: Systematic Review and Network Meta-Analysis. Front Oncol (2019) 9:972. doi: 10.3389/fonc.2019.00972
144. Masterson L, Howard J, Gonzalez-Cruz J, Jackson C, Barnett C, Overton L, et al. Immune Checkpoint Inhibitors in Advanced Nasopharyngeal Carcinoma: Beyond an Era of Chemoradiation? Int J Cancer (2020) 146(8):2305–14. doi: 10.1002/ijc.32869
145. Johnson D, Ma BBY. Targeting the PD-1/PD-L1 Interaction in Nasopharyngeal Carcinoma. Oral Oncol (2021) 113:105127. doi: 10.1016/j.oraloncology.2020.105127
146. Chen T-C, Chen C-H, Wang C-P, Lin P-H, Yang T-L, Lou P-J, et al. The Immunologic Advantage of Recurrent Nasopharyngeal Carcinoma From the Viewpoint of Galectin-9/Tim-3-Related Changes in the Tumour Microenvironment. Sci Rep (2017) 7(1):10349. doi: 10.1038/s41598-017-10386-y
147. Huang J, Fogg M, Wirth LJ, Daley H, Ritz J, Posner MR, et al. Epstein-Barr Virus-Specific Adoptive Immunotherapy for Recurrent, Metastatic Nasopharyngeal Carcinoma. Cancer (2017) 123(14):2642–50. doi: 10.1002/cncr.30541
148. Tang X, Zhou Y, Li W, Tang Q, Chen R, Zhu J, et al. T Cells Expressing a LMP1-Specific Chimeric Antigen Receptor Mediate Antitumor Effects Against LMP1-Positive Nasopharyngeal Carcinoma Cells In Vitro and In Vivo. J BioMed Res (2014) 28(6):468–75. doi: 10.7555/JBR.28.20140066
149. Zhu Q, Zhao G, Li Y, Talakatta G, Mai H, Le Q, et al. Advances in Pathogenesis and Precision Medicine for Nasopharyngeal Carcinoma. MedComm (2021) 2(2):175–206. doi: 10.1002/mco2.32
150. Li Y, Pan K, Liu L, Li Y, Gu M, Zhang H, et al. Sequential Cytokine-Induced Killer Cell Immunotherapy Enhances the Efficacy of the Gemcitabine Plus Cisplatin Chemotherapy Regimen for Metastatic Nasopharyngeal Carcinoma. PloS One (2015) 10(6):e0130620. doi: 10.1371/journal.pone.0130620
151. Hopkins R, Xiang W, Marlier D, Au VB, Ching Q, Wu LX, et al. Monocytic Myeloid-Derived Suppressor Cells Underpin Resistance to Adoptive T Cell Therapy in Nasopharyngeal Carcinoma. Mol Ther (2021) 29(2):734–43. doi: 10.1016/j.ymthe.2020.09.040
152. Taylor GS, Jia H, Harrington K, Lee LW, Turner J, Ladell K, et al. A Recombinant Modified Vaccinia Ankara Vaccine Encoding Epstein-Barr Virus (EBV) Target Antigens: A Phase I Trial in UK Patients With EBV-Positive Cancer. Clin Cancer Res (2014) 20(19):5009–22. doi: 10.1158/1078-0432.CCR-14-1122-T
153. Zeng Y, Si YF, Lan GP, Wang Z, Zhou L, Tang MZ, et al. LMP2-DC Vaccine Elicits Specific EBV-LMP2 Response to Effectively Improve Immunotherapy in Patients With Nasopharyngeal Cancer. BioMed Environ Sci (2020) 33(11):849–56. doi: 10.3967/bes2020.115
154. Jiun Chai S, Yeow Yap Y, Ching Foo Y, Fah Yap L, Ponniah S, Hwang Teo S, et al. Identification of Four-Jointed Box 1 (FJX1)-Specific Peptides for Immunotherapy of Nasopharyngeal Carcinoma. PloS One (2015) 10(11):e0130464. doi: 10.1371/journal.pone.0130464
155. Smith C, McGrath M, Neller MA, Matthews KK, Crooks P, Le Texier L, et al. Complete Response to PD-1 Blockade Following EBV-Specific T-Cell Therapy in Metastatic Nasopharyngeal Carcinoma. Precis Oncol (2021) 5(1):24. doi: 10.1038/s41698-021-00162-7
156. Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual Blockade of PD-1 and CTLA-4 Combined With Tumor Vaccine Effectively Restores T-Cell Rejection Function in Tumors. Cancer Res (2013) 73(12):3591–603. doi: 10.1158/0008-5472.CAN-12-4100
157. Foy SP, Sennino B, dela Cruz T, Cote JJ, Gordon EJ, Kemp F, et al. Poxvirus-Based Active Immunotherapy With PD-1 and LAG-3 Dual Immune Checkpoint Inhibition Overcomes Compensatory Immune Regulation, Yielding Complete Tumor Regression in Mice. PloS One (2016) 11(2):150084. doi: 10.1371/journal.pone.0150084
158. Lin M, Zhang X-L, You R, Yang Q, Zou X, Yu K, et al. Neoantigen Landscape in Metastatic Nasopharyngeal Carcinoma. Theranostics (2021) 11(13):6427–44. doi: 10.7150/thno.53229
159. Parsonage G, MacHado LR, Hui JWY, McLarnon A, Schmaler T, Balasothy M, et al. CXCR6 and CCR5 Localize T Lymphocyte Subsets in Nasopharyngeal Carcinoma. Am J Pathol (2012) 180(3):1215–22. doi: 10.1016/j.ajpath.2011.11.032
160. Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine (2020) 59:102975. doi: 10.1016/j.ebiom.2020.102975
161. Liu W, Chen G, Zhang C, Liao X, Xie J, Liang T, et al. Prognostic Significance of Tumor-Infiltrating Lymphocytes and Macrophages in Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis. Eur Arch Oto-Rhino-Laryngol (2021) 279(1):25–35. doi: 10.1007/s00405-021-06879-2
162. Al-Rajhi N, Soudy H, Ahmed SA, Elhassan T, Mohammed SF, Khoja HA, et al. Cd3+T-Lymphocyte Infiltration is an Independent Prognostic Factor for Advanced Nasopharyngeal Carcinoma. BMC Cancer (2020) 20):240–53. doi: 10.1186/s12885-020-06757-w
163. Ooft ML, Van Ipenburg JA, Braunius WW, Zuur CI, Koljenovic S, Koljenovic K, et al. Prognostic Role of Tumor Infiltrating Lymphocytes in EBV Positive and EBV Negative Nasopharyngeal Carcinoma. Oral Oncol (2017) 71:16–25. doi: 10.1016/j.oraloncology.2017.05.015
164. Zhang Y-L, Li J, Mo H-Y, Zheng L-M, Qian C-N, Zeng Y-X. Different Subsets of Tumor Infiltrating Lymphocytes Correlate With NPC Progression in Different Ways. Mol Cancer (2010) 9:4. doi: 10.1186/1476-4598-9-4
165. Ooft ML, van Ipenburg JA, Sanders ME, Kranendonk M, Hofland I, de Bree R, et al. Prognostic Role of Tumour-Associated Macrophages and Regulatory T Cells in EBV-Positive and EBV-Negative Nasopharyngeal Carcinoma. J Clin Pathol (2018) 71(3):267–74. doi: 10.1136/jclinpath-2017-204664
166. Chen Y-L. Prognostic Significance of Tumor-Associated Macrophages in Patients With Nasopharyngeal Carcinoma A Meta-Analysis. Med (2020) 99(39):e21999. doi: 10.1097/MD.0000000000021999
167. Huang H, Liu X, Zhao F, Lu J, Zhang B, Peng X, et al. M2-Polarized Tumour-Associated Macrophages in Stroma Correlate With Poor Prognosis and Epstein–Barr Viral Infection in Nasopharyngeal Carcinoma. Acta Otolaryngol (2017) 137(8):888–94. doi: 10.1080/00016489.2017.1296585
168. Zhang X-S, Zhu X-F, Gao J-S, Ye Y-L, Feng Q-S, Liu Z-C, et al. Variable Sensitivity of Endothelial Cells to Epirubicin in Xenografts of Human Nasopharyngeal Carcinoma CNE-2 Cells. Cancer Biol Ther (2002) 1(3):263–5. doi: 10.4161/cbt.78
169. Bhuvaneswari R, Gan YYY, Yee KKL, Soo KC, Olivo M. Effect of Hypericin-Mediated Photodynamic Therapy on the Expression of Vascular Endothelial Growth Factor in Human Nasopharyngeal Carcinoma. Int J Mol Med (2007) 20(4):421–8. doi: 10.3892/ijmm.20.4.421/abstract
170. Li L, Kong F, Zhang L, Li X, Fu X, Wang X, et al. Apatinib, a Novel VEGFR-2 Tyrosine Kinase Inhibitor, for Relapsed and Refractory Nasopharyngeal Carcinoma: Data From an Open-Label, Single-Arm, Exploratory Study. Invest New Drugs (2020) 38(6):1847–53. doi: 10.1007/s10637-020-00925-2
171. Ruan X, Liang J-H, Pan Y, Cai R, Zhang RJ, He Z, et al. Apatinib for the Treatment of Metastatic or Locoregionally Recurrent Nasopharyngeal Carcinoma After Failure of Chemotherapy: A Multicenter, Single-Arm, Prospective Phase 2 Study. Cancer (2021) 127(17):3163–71. doi: 10.1002/cncr.33626
172. Chong WQ, CMi L, AK S, CS T, Chan GHJ, Huang Y, et al. Integration of Antiangiogenic Therapy With Cisplatin and Gemcitabine Chemotherapy in Patients With Nasopharyngeal Carcinoma. Clin Cancer Res (2020) 26(20):5320–8. doi: 10.1158/1078-0432.CCR-20-1727
173. Peng Q-X, Han Y-W, Zhang Y-L, Hu J, Fan J, Fu S-Z, et al. Apatinib Inhibits VEGFR-2 and Angiogenesis in an In Vivo Murine Model of Nasopharyngeal Carcinoma. Oncotarget (2017) 8(32):52813–22. doi: 10.18632/oncotarget.17264
174. Liu S, Wu F, Zhang Y, Qin R, Zhu N, Li Y, et al. Apatinib Combined With Radiotherapy Enhances Antitumor Effects in an In Vivo Nasopharyngeal Carcinoma Model. Cancer Control (2020) 27(1):1073274820922553. doi: 10.1177/1073274820922553
175. Wen Q-L, Meng M-B, Yang B, Tu L-L, Jia L, Zhou L, et al. Endostar, a Recombined Humanized Endostatin, Enhances the Radioresponse for Human Nasopharyngeal Carcinoma and Human Lung Adenocarcinoma Xenografts in Mice. Cancer Sci (2009) 100(8):1510–9. doi: 10.1111/j.1349-7006.2009.01193.x
176. Lugano R, Mohanraj R, Dimberg A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell Mol Life Sci (2020) 77(9):1745–70. doi: 10.1007/s00018-019-03351-7
177. Xiang T, Lin Y-X, Ma W, Zhang H-J, Chen K-M, He G-P, et al. Vasculogenic Mimicry Formation in EBV-Associated Epithelial Malignancies. Nat Commun (2018) 9(1):5009. doi: 10.1038/s41467-018-07308-5
178. Bhuvaneswari R, Gan YY, Lucky SS, Chin WW, Ali SM, Soo KC, et al. Molecular Profiling of Angiogenesis in Hypericin Mediated Photodynamic Therapy (2008). Available at: http://www.molecular-cancer.com/content/7/1/56.
179. Shen L, Zhou Q, Wang Y, Liao W, Chen Y, Xu Z, et al. Antiangiogenic and Antitumoral Effects Mediated by a Vascular Endothelial Growth Factor Receptor 1 (VEGFR-1)- Targeted DNAzyme. Mol Med (2013) 19(1):377–86. doi: 10.2119/molmed.2013.00090
180. Guérin E, Raffelsberger W, Pencreach E, Maier A, Neuville A, Schneider A, et al. In Vivo Topoisomerase I Inhibition Attenuates the Expression of Hypoxia-Inducible Factor 1α Target Genes and Decreases Tumor Angiogenesis. Mol Med (2012) 181. 2011 Oct 1918(1):83–94. doi: 10.2119/molmed.2011.00120
181. Zhang Y, Chen X, Ren P, Su Z, Cao H, Zhou J, et al. Synergistic Effect of Combination Topotecan and Chronomodulated Radiation Therapy on Xenografted Human Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys (2013) 87(2):356–62. doi: 10.1016/j.ijrobp.2013.05.047
182. Chen Y, Xu G, Zheng Y, Yan M, Li Z, Zhou Y, et al. Nanoformulation of D-α-Tocopheryl Polyethylene Glycol 1000 Succinate-B-Poly(ϵ-Caprolactone-Ran- Glycolide) Diblock Copolymer for sirNa Targeting hIF-1α for Nasopharyngeal Carcinoma Therapy. Int J Nanomed (2015) 10:1375–86. doi: 10.2147/IJN.S76092
183. Li Y, Ding J, Xu X, Shi R, Saw PE, Wang J, et al. Dual Hypoxia-Targeting RNAi Nanomedicine for Precision Cancer Therapy. Nano Lett (2020) 20(7):4857–63. doi: 10.1021/acs.nanolett.0c00757
184. Ma W, Feng L, Zhang S, Zhang H, Zhang X, Qi X, et al. Induction of Chemokine (C-C Motif) Ligand 5 by Epstein-Barr Virus Infection Enhances Tumor Angiogenesis in Nasopharyngeal Carcinoma. Cancer Sci (2018) 109(5):1710–22. doi: 10.1111/cas.13584
185. Lin SJ, Chang KP, Hsu CW, Chi LM, Chien KY, Liang Y, et al. Low-Molecular-Mass Secretome Profiling Identifies C-C Motif Chemokine 5 as a Potential Plasma Biomarker and Therapeutic Target for Nasopharyngeal Carcinoma. J Proteom (2013) 94:186–201. doi: 10.1016/j.jprot.2013.09.013
186. Liu S-C, Chang Y-S. Role of Leukemia Inhibitory Factor in Nasopharyngeal Carcinogenesis. Mol Cell Oncol (2014) 1(1):e29900. doi: 10.4161/mco.29900
187. Liu S-C, Tsang N-M, Chiang W-C, Chang K-P, Hsueh C, Liang Y, et al. Leukemia Inhibitory Factor Promotes Nasopharyngeal Carcinoma Progression and Radioresistance. J Clin Invest (2013) 123(12):5269–83. doi: 10.1172/JCI63428
188. Zheng X-H, Lu L-X, Cui C, Chen M-Y, Li X-Z, Jia W-H. Epstein-Barr Virus Mir-Bart1-5p Detection via Nasopharyngeal Brush Sampling is Effective for Diagnosing Nasopharyngeal Carcinoma. Oncotarget (2015) 7(4):4972–80. doi: 10.18632/oncotarget.6649
189. Zhang G, Zong J, Lin S, Verhoeven RJA, Tong S, Chen Y, et al. Circulating Epstein-Barr Virus microRNAs miR-BART7 and miR-BART13 as Biomarkers for Nasopharyngeal Carcinoma Diagnosis and Treatment. Authors Int J Cancer (2015) 136(5):E301–12. doi: 10.1002/ijc.29206
190. Hirai N, Wakisaka N, Kondo S, Aga M, Moriyama-Kita M, Ueno T, et al. Potential Interest in Circulating miR-BART17-5p As a Post-Treatment Biomarker for Prediction of Recurrence in Epstein-Barr Virus-Related Nasopharyngeal Carcinoma. PloS One (2016) 11(9):e0163609. doi: 10.1371/journal.pone.0163609
191. Lu T, Guo Q, Lin K, Chen H, Chen Y, Xu Y, et al. Circulating Epstein-Barr Virus microRNAs BART7-3p and BART13-3p as Novel Biomarkers in Nasopharyngeal Carcinoma. Cancer Sci (2020) 111(5):1711–23. doi: 10.1111/cas.14381
192. Siak PY, Khoo AS-B, Leong CO, Hoh B-P, Cheah S-C. Current Status and Future Perspectives About Molecular Biomarkers of Nasopharyngeal Carcinoma. Cancers (Basel) (2021) 13(14):3490. doi: 10.3390/cancers13143490
193. Zheng W, Ye W, Wu Z, Huang X, Xu Y, Chen Q, et al. Identification of Potential Plasma Biomarkers in Early-Stage Nasopharyngeal Carcinoma-Derived Exosomes Based on RNA Sequencing. Cancer Cell Int (2021) 21(1):185. doi: 10.1186/s12935-021-01881-4
194. Liu L, Zuo L, Yang J, Xin S, Zhang J, Zhou J, et al. Exosomal Cyclophilin A as a Novel Noninvasive Biomarker for Epstein-Barr Virus Associated Nasopharyngeal Carcinoma. Cancer Med (2019) 8(6):3142–51. doi: 10.1002/cam4.2185
195. Xu Z, Zeng S, Gong Z, Yan Y. Exosome-Based Immunotherapy: A Promising Approach for Cancer Treatment. Mol Cancer (2020) 19:160. doi: 10.1186/s12943-020-01278-3
196. Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, et al. Comparative Analysis of Exosome Isolation Methods Using Culture Supernatant for Optimum Yield, Purity and Downstream Applications. Sci Rep (2019) 9(1):5335. doi: 10.1038/s41598-019-41800-2
197. Ludwig N, Whiteside TL, Reichert TE. Challenges in Exosome Isolation and Analysis in Health and Disease. Int J Mol Sci (2019) 20(19):4684. doi: 10.3390/ijms20194684
198. Chen X, Song E. Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat Rev Drug Discov (2019) 18(2):99–115. doi: 10.1038/s41573-018-0004-1
199. Li Y, Chen F, Chen J, Chan S, He Y, Liu W, et al. Disulfiram/Copper Induces Antitumor Activity Against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts Through ROS/MAPK and Ferroptosis Pathways. Cancers (Basel) (2020) 12(1):138. doi: 10.3390/cancers12010138
200. Bruce JP, To K-F Y, Lui VW, Y Chung GT, Chan Y-Y, Man Tsang C, et al. Whole-Genome Profiling of Nasopharyngeal Carcinoma Reveals Viral-Host Co-Operation in Inflammatory NF-κb Activation and Immune Escape. Nat Commun (2021) 12(1):4193. doi: 10.1038/s41467-021-24348-6
201. Li YY, Chung GTY, Lui VWY, To K-F, Ma BBY, Woo JKS, et al. Exome and Genome Sequencing of Nasopharynx Cancer Identifies NF-kB Pathway Activating Mutations. Nat Commun (2017) 8:14121. doi: 10.1038/ncomms14121
202. Chung GTY, Lou WPK, Chow C, To KF, Choy KW, Leung AWC, et al. Constitutive Activation of Distinct NF-κb Signals in EBV-Associated Nasopharyngeal Carcinoma. J Pathol (2013) 231(3):311–22. doi: 10.1002/path.4239
203. Lung HL, Kan R, Yin Chau W, Man Y, Mak K, Fong CH, et al. The Anti-Tumor Function of the iKK Inhibitor PS1145 and High Levels of P65 and KLF4 are Associated With the Drug Resistance in Nasopharyngeal Carcinoma Cells. Sci Rep (2019) 9(1):12064. doi: 10.1038/s41598-019-48590-7
204. Jiang G-M, Wang H-S, Du J, Ma W-F, Wang H, Qiu Y, et al. Bortezomib Relieves Immune Tolerance in Nasopharyngeal Carcinoma via STAT1 Suppression and Indoleamine 2,3-Dioxygenase Downregulation. Cancer Immunol Res (2017) 5(1):42–51. doi: 10.1158/2326-6066.CIR-16-0102
205. Wang W, Cheng H, Gu X, Yin X. The Natural Flavonoid Glycoside Vitexin Displays Preclinical Antitumor Activity by Suppressing NF-κb Signaling in Nasopharyngeal Carcinoma. Onco Targets Ther (2019) 12:4461–8. doi: 10.2147/OTT.S210077
206. Zhao W, Ma N, Wang S, Mo Y, Zhang Z, Huang G, et al. RERG Suppresses Cell Proliferation, Migration and Angiogenesis Through ERK/NF-κb Signaling Pathway in Nasopharyngeal Carcinoma. J Exp Clin Cancer Res (2017) 36(1):88. doi: 10.1186/s13046-017-0554-9
207. Soukupová J, Bordoni C, Turnham DJ, Yang WW, Seaton G, Gruca A, et al. The Discovery of a Novel Antimetastatic Bcl3 Inhibitor. Mol Cancer Ther (2021) 20(5):775–86. doi: 10.1158/1535-7163.MCT-20-0283
208. Luo WR, Chen XY, Li SY, Wu AB, Yao KT. Neoplastic Spindle Cells in Nasopharyngeal Carcinoma Show Features of Epithelial-Mesenchymal Transition. Histopathology (2012) 61(1):113–22. doi: 10.1111/j.1365-2559.2012.04205.x
209. Luo W, Yao K. Molecular Characterization and Clinical Implications of Spindle Cells in Nasopharyngeal Carcinoma: A Novel Molecule-Morphology Model of Tumor Progression Proposed. PloS One (2013) 8(12):e83135. doi: 10.1371/journal.pone.0083135
210. Yuan Y. Spatial Heterogeneity in the Tumor Microenvironment. Cold Spring Harb Perspect Med (2016) 6(8):a026583. doi: 10.1101/cshperspect.a026583
211. Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting Tumor Microenvironment for Cancer Therapy. Int J Mol Sci (2019) 20(4):840–71. doi: 10.3390/ijms20040840
212. Luo H, Lu L, Yang F, Wang L, Yang X, Luo Q, et al. Nasopharyngeal Cancer-Specific Therapy Based on Fusion Peptide- Functionalized Lipid Nanoparticles. ACS Nano (2014) 8(5):4334–47. doi: 10.1021/nn405989n
Keywords: nasopharyngeal carcinoma, tumor microenvironment, Epstein-Barr virus, immunotherapy, exosomes
Citation: Su ZY, Siak PY, Leong C-O and Cheah S-C (2022) Nasopharyngeal Carcinoma and Its Microenvironment: Past, Current, and Future Perspectives. Front. Oncol. 12:840467. doi: 10.3389/fonc.2022.840467
Received: 21 December 2021; Accepted: 11 February 2022;
Published: 02 March 2022.
Edited by:
Pranav Gupta, Albert Einstein College of Medicine, United StatesReviewed by:
Olivier Morales, CNRS, FranceWeiren Luo, The Second Affiliated hospital of Southern University of Science and Technology, China
Copyright © 2022 Su, Siak, Leong and Cheah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiau-Chuen Cheah, Y2hlYWhzY0B1Y3NpdW5pdmVyc2l0eS5lZHUubXk=
 Zhi Yi Su
Zhi Yi Su Pui Yan Siak
Pui Yan Siak Chee-Onn Leong
Chee-Onn Leong Shiau-Chuen Cheah
Shiau-Chuen Cheah