- 1Department of Internal Medicine, Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang, South Korea
- 2Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, South Korea
- 3Department of Gastroenterology and Hepatology, Soonchunhyang University Bucheon Hospital, Bucheon, South Korea
- 4Department of Gastroenterology and Hepatology, Soonchunhyang University Seoul Hospital, Seoul, South Korea
- 5Department of Internal Medicine, Healthcare Research Institute, Gangnam Healthcare Center, Seoul National University Hospital, Seoul, South Korea
- 6Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 7Department of Biostatistics, College of Medicine, The Soongsil University, Seoul, South Korea
- 8Supportive Care Center/Department of Family Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 9Department of Clinical Research Design and Evaluation, Samsung Advanced Institute for Health Science and Technology (SAIHST), Sungkyunkwan University, Seoul, South Korea
- 10Department of Digital Health, Samsung Advanced Institute for Health Science and Technology (SAIHST), Sungkyunkwan University, Seoul, South Korea
The positive association between metabolic syndrome (MetS) and hepatocellular carcinoma (HCC) has been suggested. However, no studies have yet looked at how the risk of developing HCC varies with changes in MetS status. Therefore, we aimed to investigate the association between changes in MetS and subsequent HCC development. Data were obtained from the Korean National Health Insurance Service. In this study, 5,975,308 individuals who participated in health screenings both in 2009–2010 and 2011–2012 were included. Individuals with preexisting viral hepatitis, liver cirrhosis, or cancer diagnoses were excluded. Subjects were divided into four groups according to change in MetS status during the 2-year interval screening (from 2009 to 2011): sustained non-MetS, transition to MetS, transition to non-MetS, and sustained MetS. Cox regression analysis was used to examine the hazard ratios of HCC. The subjects were followed through December 31, 2018. During a median of 7.3 years of follow-up, 25,880 incident HCCs were identified. Compared to the sustained non-MetS group, age, sex, smoking, alcohol, regular exercise, and body mass index-adjusted hazard ratios (95% confidence interval) for HCC development were 1.01 (0.97–1.05) for the transition to MetS group, 1.05 (1.003–1.09) for the transition to non-MetS group, and 1.07 (1.03–1.10) for the sustained MetS group. Stratified analyses according to age, sex, smoking, alcohol intake, exercise, diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease showed similar results. A significantly increased HCC risk was observed in the sustained MetS and transition to non-MetS groups. The baseline status of MetS was associated with the risk of HCC development. Strategies to improve MetS, especially targeting insulin resistance, might prevent HCC development.
Introduction
Worldwide, hepatocellular carcinoma (HCC) is the third most frequent malignancy (1), and the incidence of HCC has increased dramatically in the last two decades in many of the developed countries of the world (2). The etiologies of HCC include hepatitis B virus or hepatitis C virus infection and alcohol. However, 5%−30% of the HCC cases do not have an identifiable risk factor, which is called “cryptogenic HCC” (3). A trend toward the higher proportion of nonalcoholic fatty liver disease (NAFLD) patients with cryptogenic HCC has been reported (4). This suggests that increased proportion of risk factors associated with NAFLD may have contributed to the development of cryptogenic HCC (5).
The metabolic syndrome (MetS) is a cluster of metabolically related risk factors for cardiovascular disease (CVD) (6). NAFLD is also known as a feature of MetS (7). Recent experimental translational studies are available as an evidence that supports that the components of MetS including central obesity, dyslipidemia, and insulin resistance might be the important factors for HCC (8–10).
Recently, experts have suggested that “metabolic (dysfunction)-associated fatty liver disease (MAFLD)” might be a more appropriate term to describe fatty liver diseases associated with metabolic dysfunction (11). This novel term emphasizes the role of metabolic dysfunction on clinical outcome of patients with fatty liver disease, which may identify subjects at a higher risk of hepatic outcomes (12). Some epidemiologic studies have reported the positive association between MetS and HCC (13–16). However, the temporal and probably causative relation between MetS and HCC remains open to discussion.
We hypothesized that the temporal changes in MetS status might be the significant factor for HCC. Thus, this nationwide population-based study investigated whether the temporal changes in MetS status or the status of MetS components impact on the incidence of HCC.
Materials and Methods
Data Source
The Korean government has a single mandatory health insurance system that covers nearly 97% of South Koreans. The remaining 3% are covered by the Medical Aid program. The Korean National Health Insurance Services (NHIS) manages all administrative processes and reimburses medical providers and pharmacies based on their claims data. Korean NHIS is a mandatory social insurance that covers virtually all Koreans except for Medicaid beneficiaries in the lowest income bracket (approximately 3% of the population).
It conducts biennial health examinations for all Korean employees of any age and those aged 40 or older. This prevention program aims to detect and treat CVD-related health conditions including hypertension, diabetes, and dyslipidemia early to reduce the burden of CVD and offers subsequent educational counseling or treatment referral for participants with identified health problems. The examinations consist of anthropometric measurements, laboratory tests (lipid profiles, blood glucose, etc.), and questionnaires on lifestyle behaviors (smoking, alcohol consumption, and physical activity). Therefore, the Korean NHIS database includes health information from all Korean people (~50 million) based on eligibility (age, sex, place of residence, income level, etc.), medical utilization (diagnosis code, diagnostic and therapeutic procedures, prescription, medical expenses), and results from health examinations. This database has been widely used for various epidemiologic studies (17, 18).
Ethics Statement
This study was approved by the institutional review board of Seoul National University Hospital (IRB No. E-1912-024-1085). Anonymized and de-identified information was used for analyses; therefore, informed consent was not required. The database is open to all researchers whose study protocols are approved by the official review committee. All the methods were performed in accordance with relevant guidelines and regulations.
Study Population
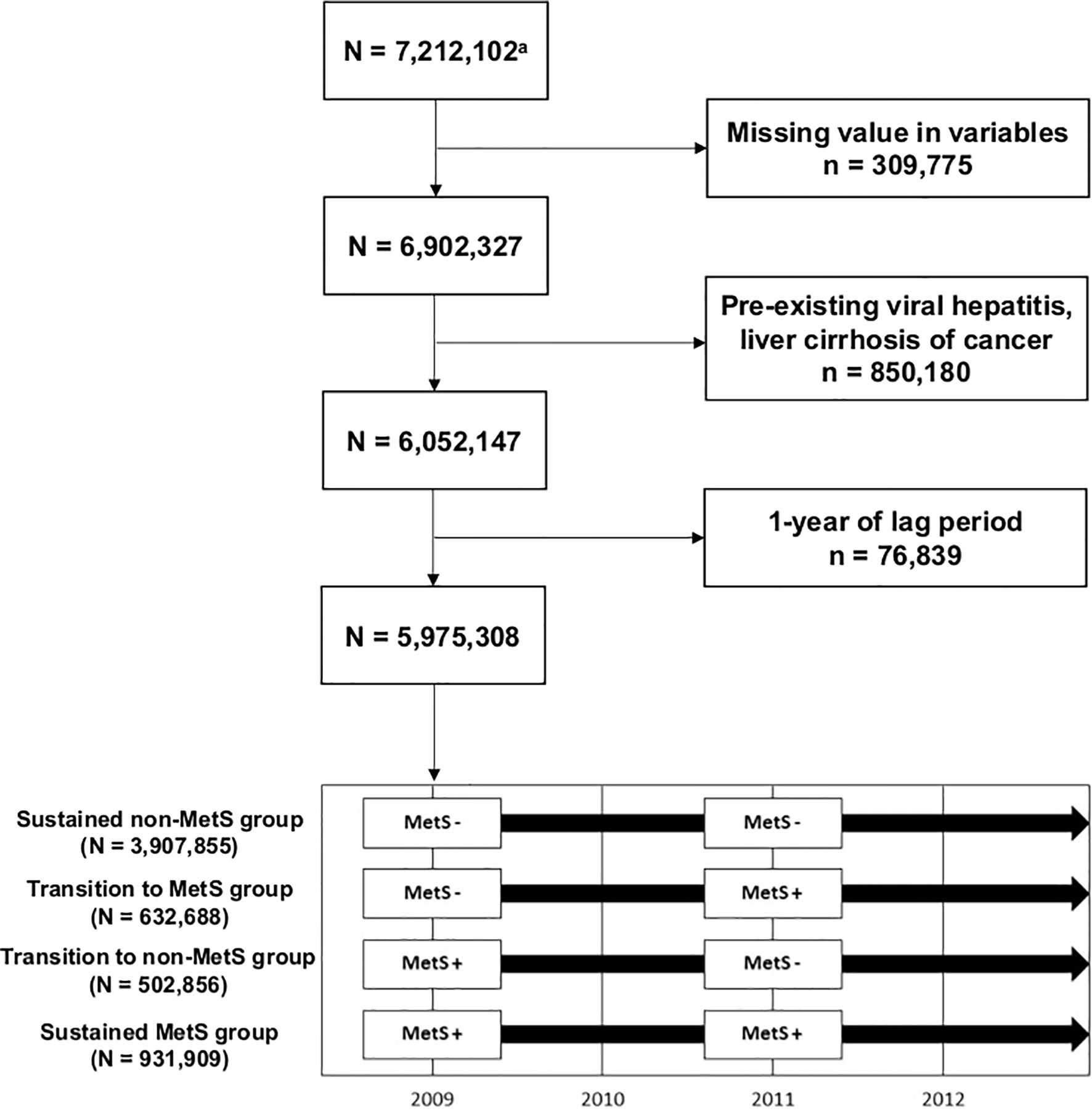
Among 7,212,102 subjects (age ≥20 years) who participated in health screenings both in 2009–2010 and 2011–2012, we excluded individuals with missing data (n = 309,775) and those with preexisting viral hepatitis [defined by International Classification of Disease version 10 (ICD-10) B15–B19], liver cirrhosis (LC, defined by ICD-10 K703, K746), or cancer diagnoses (n = 850,180). As subjects who developed HCC immediately after health examination may have an unclear temporal relationship with the MetS status identified at the health examination, we gave a 1-year lag time and further excluded 12,567 subjects diagnosed with HCC within a year after their second health examination. Therefore, 5,975,308 subjects were included in the final study population (Figure 1). The subjects were followed through December 31, 2018.
Exposure Variable: Metabolic Syndrome Status
The definition of MetS followed the 2009 agreement of the International Diabetes Federation and American Heart Association/National Heart, Lung, and Blood Institute (6). By definition, the presence of three or more out of five risk factors constituted a MetS diagnosis: triglyceride (TG) ≥150 mg/dl or patient was taking lipid-lowering medication; high-density lipoprotein (HDL) <40 mg/dl in men and <50 mg/dl in women or use of lipid-lowering medication; systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg or use of antihypertensive medication; fasting glucose ≥100 mg/dl or use of hypoglycemic agents; and abdominal obesity. Abdominal obesity was defined as waist circumference ≥90 cm for men and ≥85 cm for women according to the definition from the Korean Society for the Study of Obesity. (19) We compared 2009–2010 and 2011–2012 National Health Screening Program (NHSP) results. Using MetS change during biennial screening, we divided participants into four groups: sustained non-MetS, transition to MetS, transition to non-MetS, and sustained MetS.
Outcome Variable: Hepatocellular Carcinoma
HCCs were identified using the following diagnoses from the ICD-10: Hepatocellular Carcinoma [C22.0]. In addition to ICD-10 codes, we confirmed cases of HCC through the registration program for critically ill or incurable diseases. Since 2005, the Korean government has provided co-payment reduction for registered cancer patients, and only patients whose cancer diagnoses were confirmed by physicians (after thorough evaluation) could be registered in this program.
Covariates
Household income was classified into quartiles according to health insurance premium. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. Waist circumference was measured at the midpoint between the lower margin of the ribs at the mid-axillary plane and the top of the iliac crest.
Health behavior, including smoking, alcohol consumption, and physical activity, was evaluated by self-reporting questionnaires. Smoking history was classified as never, former, and current smoker. Alcohol consumption was divided into three levels: none, mild-to-moderate (<30 g of alcohol/day), and heavy (≥30 g/day). Regular physical activity was defined as moderate physical activity for more than 30 min daily and more than 5 days per week over the past week. Income status was divided into quartiles based on the amount of health insurance premiums paid (Korean premiums are determined by income level), where those who received medical aid (the poorest 3%) were merged with the lowest income quartile.
Hypertension was defined as any of the following: systolic blood pressure ≥140 mmHg; diastolic blood pressure ≥90 mmHg; or treatment with an antihypertensive medication that was linked to the hypertension ICD-10 codes (I10–I13 and I15) and resulted in at least one claim in a year. Diabetes mellitus (DM) was defined as a blood glucose level ≥126 mg/dl or history of a hypoglycemic medication prescription that was linked to a diabetes ICD-10 code (E11–E14) and resulted in at least one claim in a year. Dyslipidemia was defined as total cholesterol ≥240 mg/dl or history of a lipid-lowering medication that was associated with an ICD-10 code (E78). Each medication code is described in Supplementary Table S1. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate <60 ml/min per 1.73 m² of body surface area.
Statistical Analysis
The comparison of baseline characteristics according to the change in MetS was conducted using independent t-tests for continuous variables and the chi-square test for categorical variables. The incidence rates of HCC were assessed as the incident cases divided by 1,000 person-years. Cox proportional hazards regression was performed to estimate the risk of HCC for the four MetS change groups (sustained non-MetS, transition to MetS, transition to non-MetS, and sustained MetS). Multivariable analyses were adjusted for age, sex, smoking history, alcohol consumption, physical activity, and BMI. Stratified analyses were performed according to age (<65 vs. ≥65 years old), sex (men vs. women), smoking, alcohol (average <30 g/day vs. heavy drinking ≥30 g/day), regular exercise (≥30 min of moderate physical activity ≥five times per week or ≥20 min of strenuous physical activity ≥three times per week). The statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). In this study, p-values <0.05 were considered statistically significant.
Results
Characteristics of Study Subjects
A total of 5,975,308 subjects were enrolled in the study. Among the total subject group, 3,907,855 (65.4%) remained normal during the first and the second NHSP (sustained non-MetS group). Newly developed MetS was seen in 632,688 (10.6%) in the second screening (transition to MetS group), and 502,856 (8.4%) had MetS at the first screening that normalized at the second screening (transition to non-MetS group). Sustained MetS was noted for 931,909 (15.6%) during the two screenings (sustained MetS group).
All characteristics at 2011–2012 national health examinations were significantly different among the four groups (p < 0.001) (Table 1). Heavy drinking was higher in the transition to MetS group (9.3%), transition to non-MetS group (8.6%), and sustained MetS group (8.6%) than that in the sustained non-MetS group (6.6%). The regular exercise rate was higher in the transition to non-MetS group (22.3%) than in other groups. DM (32.0%), hypertension (66.0%), dyslipidemia (29.8%), and CKD (10.3%) were higher in the sustained MetS group than in other groups. The baseline characteristics of the study population at the first national health examinations are described in Supplementary Table S2.
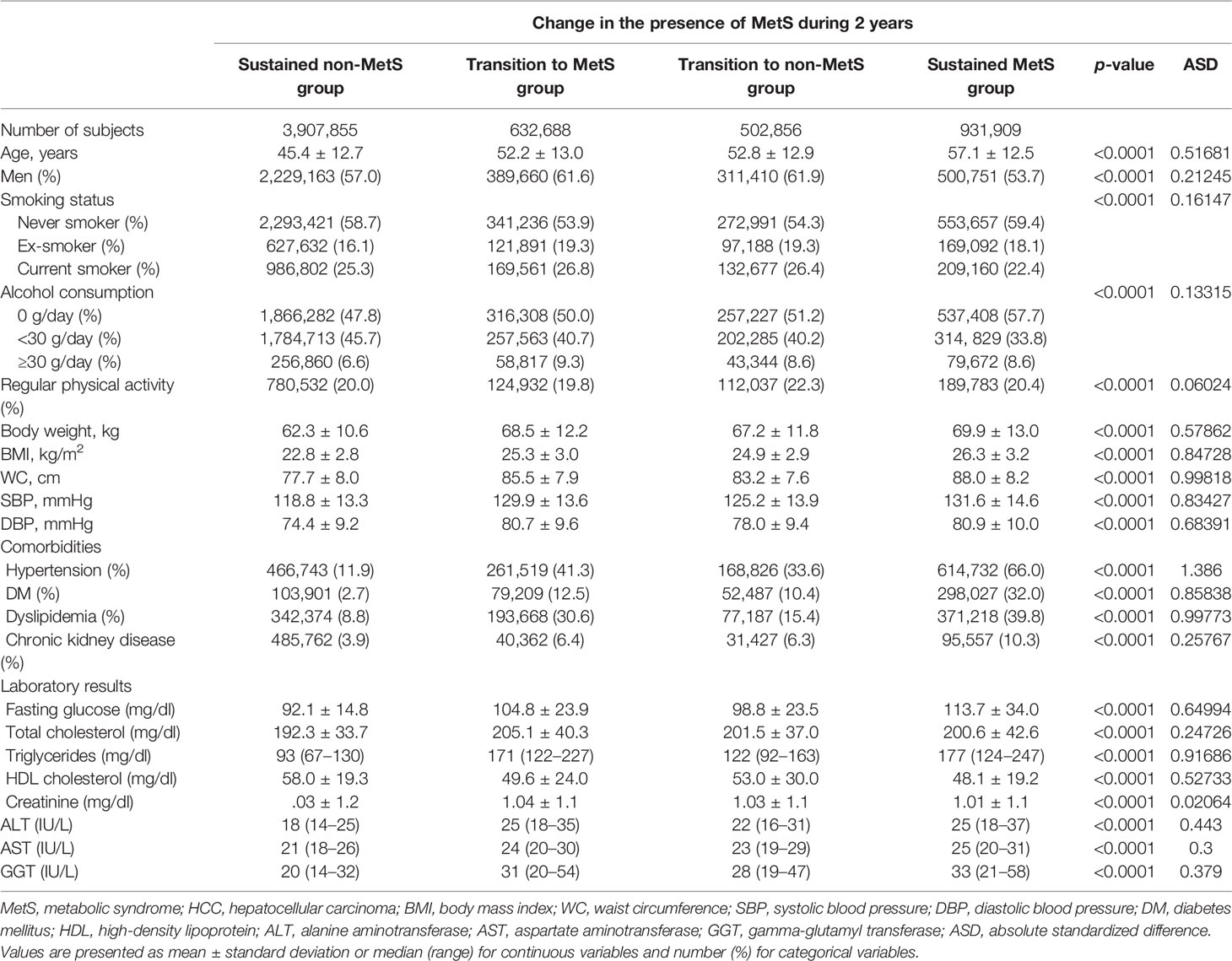
Table 1 Baseline characteristics of the study population (at the time of second national health examinations).
Incidence of Hepatocellular Carcinoma According to Baseline Metabolic Syndrome and Components
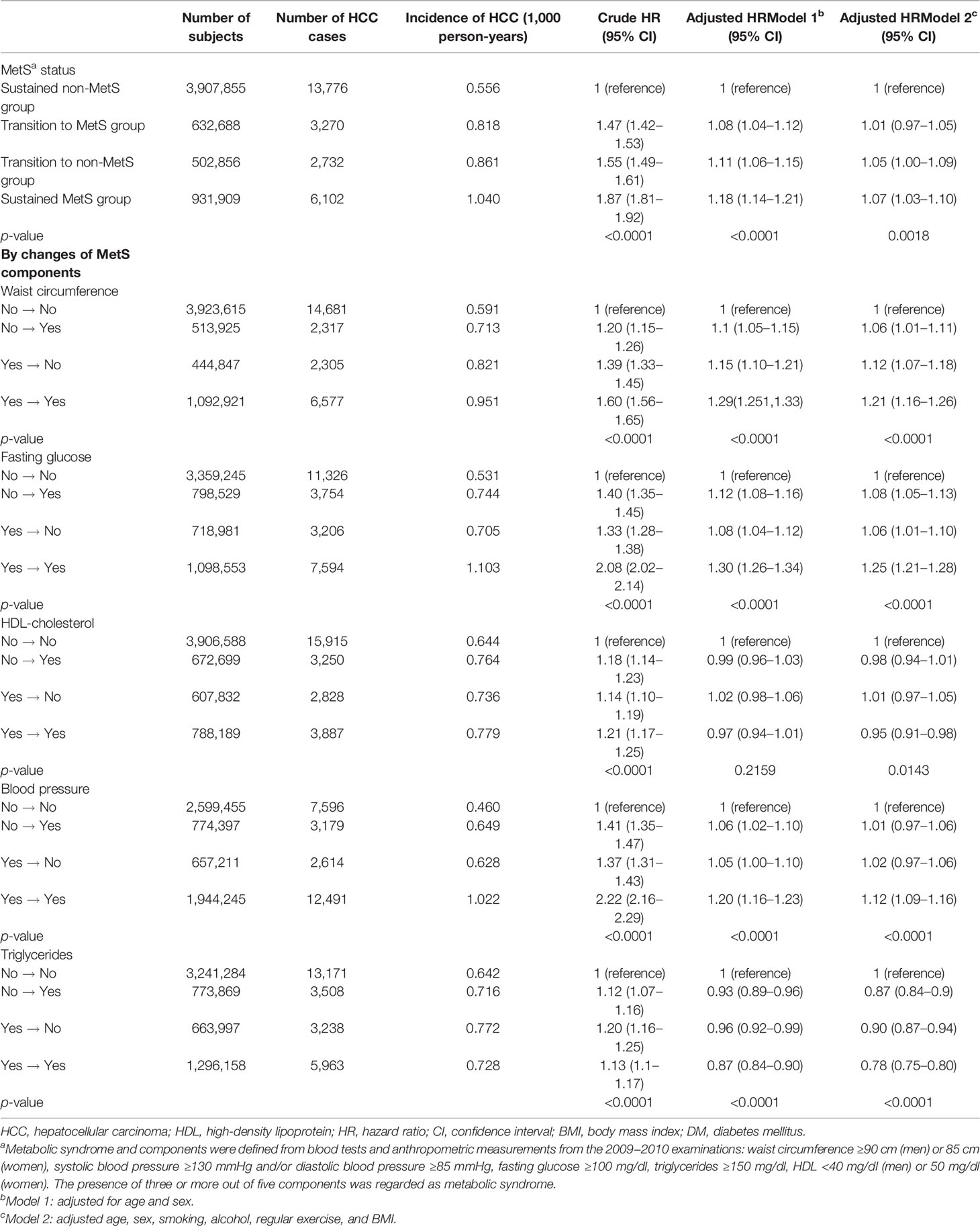
Median follow-up duration of the study population was 7.3 years. The HCC incidence rate for the non-MetS group was 0.59 cases per 1,000 person-years and 0.95 cases in the MetS group (Table 2). HCC development risk was higher in the MetS group [adjusted hazard ratio (aHR), 1.03; 95% confidence interval (CI), 1.004–1.06]. Without adjustment, all MetS components also showed higher risk of HCC development. After adjustment for age, sex, smoking, alcohol, regular exercise, and BMI, waist circumference (aHR 1.12, 95% CI 1.08–1.15), fasting glucose (aHR 1.17, 95% CI 1.14–1.20), and the presence of hypertension (aHR 1.09, 95% CI 1.06–1.12) were the significant factors for developing HCC. However, low HDL (aHR 0.96, 95% CI 0.96–0.99) and high TG (aHR 0.83, 95% CI 0.81–0.85) were inversely associated with HCC risk.
Incidence of Hepatocellular Carcinoma According to Change in Metabolic Syndrome and Components
Compared to the sustained non-MetS group, other groups showed a higher risk of HCC development. The risk for HCC did not significantly increase for transition to MetS group (aHR 1.01; 95% CI, 0.97–1.05) compared with sustained non-MetS group. However, the risk for HCC was significantly increased among both transition to non-MetS (aHR 1.05; 95% CI, 1.003–1.09) and sustained MetS group (aHR 1.07; 95% CI, 1.03–1.10).
By individual components, those who developed high waist circumference (aHR 1.06; 95% CI, 1.01–1.11) and fasting glucose (aHR 1.08; 95% CI, 1.05–1.13) showed slightly increased risk for HCC compared to sustained non-MetS group. Those who showed sustained high waist circumference (aHR 1.21, 95% CI 1.16–1.26), fasting glucose (aHR 1.25, 95% CI 1.21–1.28), and blood pressure (aHR 1.12, 95% CI 1.09–1.16) were associated with higher HCC risk compared to sustained non-MetS group. In comparison, those who showed normalization of waist circumference (aHR 1.12, 95% CI 1.07–1.18 vs. aHR 1.21, 95% CI 1.16–1.26), blood pressure (aHR 1.02, 95% CI 0.97–1.06 vs. aHR 1.12, 95% CI 1.09–1.16), and fasting glucose (aHR 1.06, 95% CI 1.01–1.10 vs. aHR 1.25, 95% CI 1.21–1.28) showed an elevated risk for HCC compared to sustained non-MetS group, but the risks were lower than those in the sustained high group. For HDL cholesterol, sustained low HDL cholesterol group showed a slightly lower risk than that of sustained non-MetS group (aHR 0.95, 95% CI 0.91–0.98). For TG, all of transition to normal (aHR 0.87, 95% CI 0.84–0.90), transition to high TG (aHR 0.90, 95% CI 0.87–0.94), and sustained high TG group (aHR 0.78, 95% CI 0.75–0.80) showed a lower risk of HCC than the sustained normal TG group (Table 3). When we changed the reference group as sustained MetS group, the sustained non-MetS group (aHR 0.94, 95% CI 0.91–0.97) showed a lower risk of HCC incidence compared with that of the sustained MetS group (Supplementary Table S3).
Stratified Analyses
We performed stratified analyses according to age (<65 vs. ≥65 years), sex (men vs. women), smoking, alcohol (<30 vs. ≥30 g/day), and regular exercise (Figure 2). Stratified analyses also showed generally similar associations between the change in MetS and with the risk of HCC development (Supplementary Table S4). However, the association between sustained MetS and HCC risk was larger in older person (aHR 1.14, 95% CI 1.08–1.21 vs. aHR 1.06, 95% CI 1.01–1.11 in younger person), men (aHR 1.15, 95% CI 1.10–1.2 vs. aHR 1.00, 95% CI 0.94–1.06 in women), and heavy drinker (aHR 1.26, 95% CI 1.14–1.40 vs. aHR 1.05, 95% CI 1.01–1.09 in non- or moderate drinker).
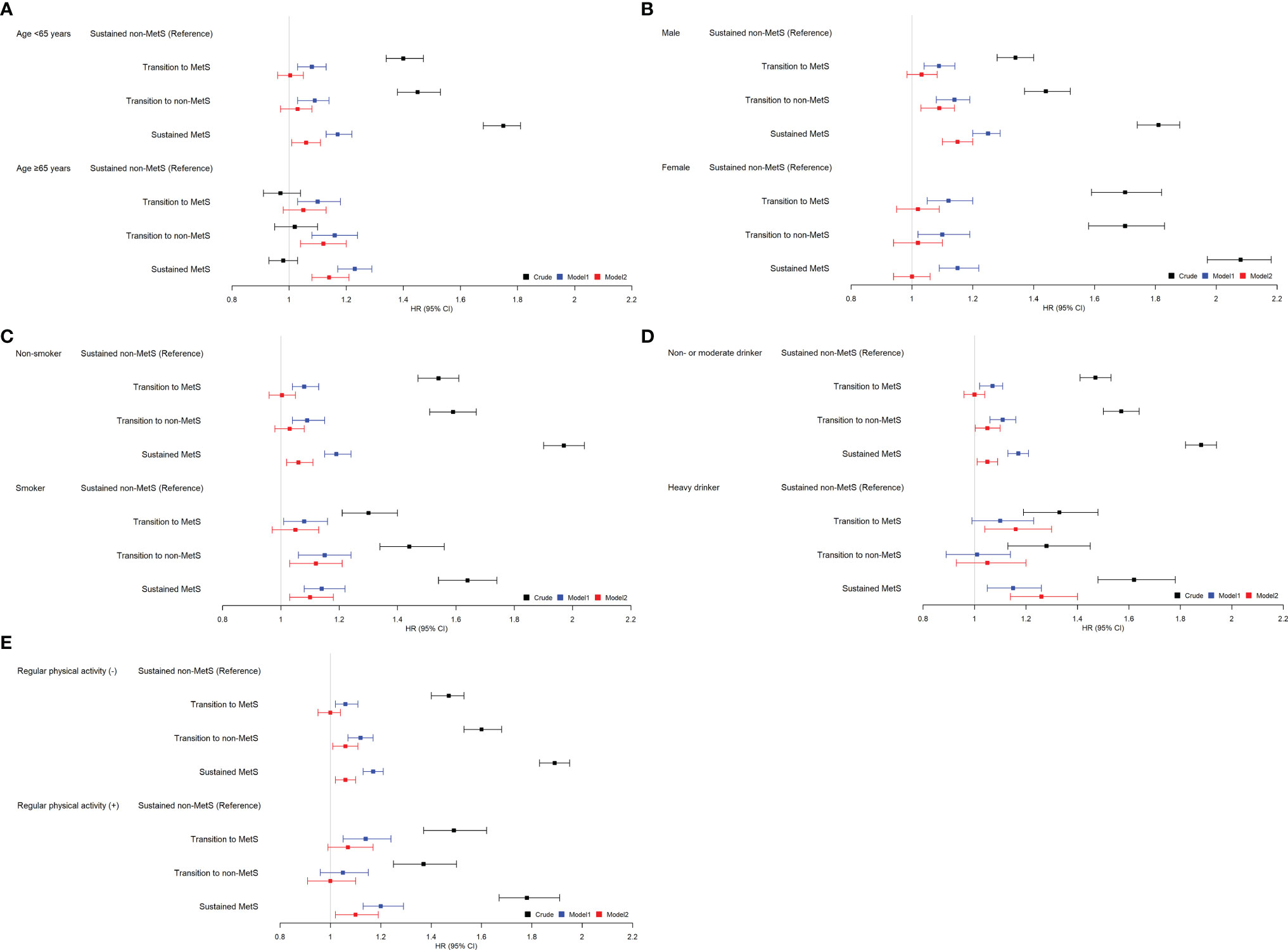
Figure 2 Relationship between the change in metabolic syndrome (MetS) status and hepatocellular carcinoma (HCC) incidence stratified by patient characteristics. (A) Age, (B) sex, (C) smoking, (D) alcohol, (E) regular physical activity. *Adjusted for age, sex, smoking, alcohol, regular exercise, and body mass index (BMI).
We also additionally performed stratified analyses according to age subgroups (<45, 45–55, >55 years). Interestingly, among subjects with <45 and 45–55 years old, there is no significant difference in HRs for the incidence of HCC among the four MetS change groups (Supplementary Table S5). Only among subjects >55 years old, the sustained MetS group showed a higher risk for HCC incidence compared to that of the sustained non-MetS group (aHR 1.18, 95% CI 1.1–1.26).
Discussion
To the best of our knowledge, this is the first population-based study that examined the HCC risks according to MetS state changes. The uniqueness of our study is that we measured MetS status twice with 2-year interval, thus enabling estimating the HCC risk by the changes of metabolic status. We found that MetS was significantly associated with the risk of HCC, and the risk was greater when the MetS was sustained. Among the components of MetS, central obesity, impaired fasting glucose, and high blood pressure were associated with an increased risk of HCC. However, the risk of HCC development was lower in people who returned to non-MetS status than that of those who had sustained MetS status.
Our study is consistent with previous studies that show a positive association between MetS and the HCC risk. A previous prospective European cohort study reported that MetS increases the risk of HCC with adjusted relative risk of 1.35 (95% CI 1.12−1.61) (15). A population-based case-control study in the United States also showed that MetS increases the risk of HCC, with odds ratio of 2.13 (95% CI 1.96−2.31) (14). Those studies reported a slightly higher risk of HCC than that in our study probably due to including patients with underlying chronic liver disease (e.g., chronic viral hepatitis, alcohol-related liver disease). In addition, we showed that sustained MetS was associated with 7% higher HCC risk, further suggesting a causal association between MetS and HCC. The baseline status of MetS increased HCC risk by 3%, which is lower than that of sustained MetS group. The status of MetS can change dynamically, (20) so one time measurement would not be optimal. A previous cohort study using data from the Korean National Insurance Service from 2002 failed to observe a significantly increased risk of liver cancer among individuals with a high-risk metabolic profile (aHR 0.93; 95% CI, 0.75–1.16 for men, aHR 1.18; 95% CI, 0.78–1.77 for women) (21). Interestingly, our study including individuals who participated in health screenings both in 2009–2010 and 2011–2012 is the first to show an increased HCC risk among individuals with sustained MetS, which might reflect the temporal changes of HCC epidemiology in Korea.
This study’s uniqueness is that we investigated the impact of MetS temporal changes on HCC development. Our data show that if subjects have had MetS even once, the risk of HCC increased. Actually, the mean age of non-viral causes of HCC is higher than that of viral causes of HCC, (22) which is one of the pieces of evidence that a long exposure period of MetS influences the incidence of HCC (23). However, when we look at the risk by changes of individual component, transition to central obesity (aHR 1.06), impaired fasting glucose (aHR 1.08) showed significant elevation of HCC risk. We expect that the risk of HCC in this transition to MetS group might increase significantly in the future long-term follow-up study, as other previous studies have reported (24, 25). The results from our study also support that the at-risk population for HCC may be far larger than estimated and highlight the need defining appropriate strategies for HCC risk stratification among those with MetS and early detection in individuals without LC.
The incidence of MetS and HCC continues to increase worldwide (25). Previous studies have suggested that there is a link between the increase in HCC and MetS (26, 27). MetS might promote HCC development in multiple ways. Insulin resistance leads to an increase in insulin-like growth factor (IGF)-1, the most powerful activator of cellular proliferation including cancer cells (28). The aberrant activation of IGF signaling pathways is an important mechanism in the development of HCC. IGF-1 and IGF-2 interact with specific receptors. IGF-2 may also act in early hepatocarcinogenesis (29). Another key mechanism involves peroxisome proliferator-activated receptor (PPAR)-γ that is most highly expressed in white or brown adipose tissue and fatty liver. The inhibitory action of PPAR-γ on hepatocarcinogenesis via the upregulation of plasminogen activator factor 1 was reported (30). MetS also increases several adipokines, hormone levels, and the signaling pathways that create the ideal tumor microenvironment for developing steatosis and hepatic inflammation (31). Adipokines, such as leptin, may mediate HCC development through their effects on angiogenesis (32). Furthermore, MetS may lead to a state of chronic inflammation. Excess consumption of fatty acids and glucose can lead to the increased expression of several signaling molecules with known importance in carcinogenesis, invasion, and metastasis, including nuclear factor-κB, epidermal growth factor, and fibroblast growth factor (33).
Our data show that the risk of HCC development in this transition to non-MetS group was lower in the sustained MetS group, suggesting that there might be a preventive effect for HCC development by improving MetS status. The difference in relative risk between transition to non-MetS group and sustained MetS group was even more prominent when compared by individual components, e.g., waist circumference (aHR 1.12 vs. 1.21), fasting glucose (1.06 vs. 1.25), and blood pressure (1.02 vs. 1.12). This suggests the need to reverse MetS to prevent the risk of HCC. Efforts should start focusing at controlling MetS components such as DM, hypertension, dyslipidemia, and obesity (34). Weight control losing >5%–7% (35) is the key factor in managing MetS with a combination of reduced total caloric intake more than 500 kcal per day and increased physical activity (36). Pharmacological hypoglycemic treatments including PPAR-γ agonists (e.g., pioglitazone) (37), metformin (38), or glucagon-like peptide-1 agonists [e.g., liraglutide (39) or semaglutide (40)] have shown histological improvement in nonalcoholic steatohepatitis (NASH) patients. Also, well-established pharmacological treatments for hypertension and dyslipidemia, such as statins, have shown a positive preventive effect on the progression of cirrhosis and HCC development. A multidimensional approach for managing MetS might reduce HCC risk.
Interestingly, high TG levels were inversely associated with HCC risk in this study, which is consistent with our previous report (41). Dysregulation of cholesterol metabolism itself might act as a part of hepatocarcinogenesis. Cholesterol is involved in numerous biochemical pathways that are potentially relevant in HCC development, including several cytokine and signaling pathways. Interleukin-6, tumor necrosis factor-α, and interleukin-1 inhibit TG synthesis. These proinflammatory cytokines could act as carcinogens or cofactors for hypocholesterolemia and hepatocarcinogenesis.
Subjects with hypertension showed increased risk of HCC development. Essential hypertension is associated with MetS, which is characterized by insulin resistance and is strongly correlated with the development of NAFLD (42). Essential hypertension has been associated with an increased HCC mortality but the mechanisms of this positive relationship are not well known. The effects of high blood pressure might influence the progression of hepatic fibrosis. In animal models, hypertension was a potential risk factor for liver injury through glucose intolerance and decreased anti-inflammatory mechanism (43). Recently, Feng et al. reported that renin-angiotensin inhibitor was associated with longer time-to-recurrence and overall survival of HCC patients with essential hypertension after hepatectomy (44). Arterial hypertension might play a fundamental role in liver injury and progression of hepatic fibrosis leading to increased risk of HCC development.
Several limitations of this study must be acknowledged. Importantly, in this study, there is the chance that cirrhosis is not identified by Korean NHIS. Different definitions of cirrhosis might carry essentially misclassification bias. In real-life clinical practice, physicians use different methods to diagnose cirrhosis, which may affect coding the diagnosis in the medical record system. Liver biopsy is not a routine clinical practice, and liver stiffness measurement is not always feasible. It is possible that some of those who had unrecognized cirrhosis due to radiological ambiguity of the diagnosis of cirrhosis were included in this analysis. Second, the time period analyzed during our study is relatively short. Regarding that patients with NASH and fibrosis can progress to cirrhosis from 0% at 5 years to 12% over 8 years, (45) it is difficult to determine how the duration of risk factor exposure influences the development of HCC. However, it is reported that a third of NASH-related HCC develops in a non-cirrhotic liver, (46) which might lead to the positive findings of this study. Third, as with all epidemiological studies, our study cannot establish a causal relationship. Fourth, subjects with MetS (especially sustained MetS group) were significantly older than others. However, age-matching analysis was hard to apply in our 4-group analyses. Instead, we performed stratified analysis according to age group to minimize the confounding effect of age. Only among subjects >55 years old, sustained MetS group showed a higher risk for HCC incidence compared to that of the sustained non-MetS group. This might be due to the difference in exposure time to MetS. The effect of MetS status on hepatocarcinogenesis might be more prominent in older subjects (>55 years) who have been exposed to MetS for a relatively longer period of time than younger subjects (<55 years). Regarding that NAFLD-related HCC tends to occur in older individuals than viral hepatitis-related HCC (47), the result of this study is consistent with those of previous reports (48, 49).
In conclusion, this study demonstrated that MetS was significantly associated with the risk of HCC, and the risk was greater when MetS was sustained. Among the components of MetS, central obesity, impaired fasting glucose, and high blood pressure were associated with increased risk of HCC, suggesting the role of insulin resistance in the development of HCC. However, the risk of HCC development in the transition to non-MetS group was lower than that of the sustained MetS group, suggesting that there might be a preventive effect for HCC development by improving MetS status. Strategies to improve MetS, especially targeting insulin resistance, might help to prevent HCC development.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board of Seoul National University Hospital (IRB No. E-1912-024-1085). The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
SJY and DWS have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. YuC, EJC, JJY, YoC, GEC, and IYC collected data. YuC, EJC, SHP, KH, SJY, and DWS analyzed and interpreted data. YuC, SJY, and DWS wrote the article. All authors contributed to the interpretation and discussion of the results and read and approved the final article.
Funding
This work was supported by grants from the Seoul National University Hospital Research Fund (06-2020-4150), Liver Research Foundation of Korea as part of the Bio Future Strategies Research Project, and the National Research Foundation of Korea grant funded by the Korea government (2021R1A2C4001401).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was performed using a database from the National Health Insurance System (NHIS).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.863352/full#supplementary-material
References
1. Llovet JM, Burroughs A, Bruix J. Hepatocellular Carcinoma. Lancet (2003) 362:1907–17. doi: 10.1016/S0140-6736(03)14964-1
2. Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular Carcinoma: Epidemiology, Risk Factors and Pathogenesis. World J Gastroenterol (2008) 14:4300–8. doi: 10.3748/wjg.14.4300
3. El-Serag HB, Rudolph KL. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology (2007) 132:2557–76. doi: 10.1053/j.gastro.2007.04.061
4. Oh KC, Park SH, Park JC, Jin DK, Park CS, Kim KO, et al. [Is the Prevalence of Cryptogenic Hepatocellular Carcinoma Increasing in Korea?]. Korean J Gastroenterol (2005) 45:45–51.
5. Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD May Be a Common Underlying Liver Disease in Patients With Hepatocellular Carcinoma in the United States. Hepatology (2002) 36:1349–54. doi: 10.1002/hep.1840360609
6. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
7. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic Fatty Liver Disease: A Feature of the Metabolic Syndrome. Diabetes (2001) 50:1844–50. doi: 10.2337/diabetes.50.8.1844
8. Ioannou GN, Splan MF, Weiss NS, Mcdonald GB, Beretta L, Lee SP. Incidence and Predictors of Hepatocellular Carcinoma in Patients With Cirrhosis. Clin Gastroenterol Hepatol (2007) 5:938–45 945 e931–934. doi: 10.1016/j.cgh.2007.02.039
9. Zhou JR, Blackburn GL, Walker WA. Symposium Introduction: Metabolic Syndrome and the Onset of Cancer. Am J Clin Nutr (2007) 86:s817–819. doi: 10.1093/ajcn/86.3.817S
10. Okuno T, Kakehashi A, Ishii N, Fujioka M, Gi M, Wanibuchi H. mTOR Activation in Liver Tumors Is Associated With Metabolic Syndrome and Non-Alcoholic Steatohepatitis in Both Mouse Models and Humans. Cancers (Basel) (2018) 10:465. doi: 10.3390/cancers10120465
11. Kang SH, Cho Y, Jeong SW, Kim SU, Lee JW, Korean NSG. From Nonalcoholic Fatty Liver Disease to Metabolic-Associated Fatty Liver Disease: Big Wave or Ripple? Clin Mol Hepatol (2021) 27:257–69. doi: 10.3350/cmh.2021.0067
12. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J Hepatol (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
13. Russo A, Autelitano M, Bisanti L. Metabolic Syndrome and Cancer Risk. Eur J Cancer (2008) 44:293–7. doi: 10.1016/j.ejca.2007.11.005
14. Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, Mcglynn KA. Metabolic Syndrome Increases the Risk of Primary Liver Cancer in the United States: A Study in the SEER-Medicare Database. Hepatology (2011) 54:463–71. doi: 10.1002/hep.24397
15. Borena W, Strohmaier S, Lukanova A, Bjorge T, Lindkvist B, Hallmans G, et al. Metabolic Risk Factors and Primary Liver Cancer in a Prospective Study of 578,700 Adults. Int J Cancer (2012) 131:193–200. doi: 10.1002/ijc.26338
16. Kasmari AJ, Welch A, Liu G, Leslie D, Mcgarrity T, Riley T. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased With Diabetes and Metabolic Syndrome. Am J Med (2017) 130:746 e741–746 e747. doi: 10.1016/j.amjmed.2016.12.029
17. Jeong SM, Jang W, Shin DW. Association of Statin Use With Parkinson's Disease: Dose-Response Relationship. Mov Disord (2019) 34:1014–21. doi: 10.1002/mds.27681
18. Soh H, Im JP, Han K, Park S, Hong SW, Moon JM, et al. Crohn's Disease and Ulcerative Colitis Are Associated With Different Lipid Profile Disorders: A Nationwide Population-Based Study. Aliment Pharmacol Ther (2020) 51:446–56. doi: 10.1111/apt.15562
19. Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, et al. 2014 Clinical Practice Guidelines for Overweight and Obesity in Korea. Endocrinol Metab (Seoul) (2014) 29:405–9. doi: 10.3803/EnM.2014.29.4.405
20. Park S, Han K, Kim DK. Altered Risk for Cardiovascular Events With Changes in the Metabolic Syndrome Status. Ann Intern Med (2020) 172:707–8. doi: 10.7326/L20-0076
21. Ko S, Yoon SJ, Kim D, Kim AR, Kim EJ, Seo HY. Metabolic Risk Profile and Cancer in Korean Men and Women. J Prev Med Public Health (2016) 49:143–52. doi: 10.3961/jpmph.16.021
22. Tokushige K, Hashimoto E, Horie Y, Taniai M, Higuchi S. Hepatocellular Carcinoma Based on Cryptogenic Liver Disease: The Most Common Non-Viral Hepatocellular Carcinoma in Patients Aged Over 80 Years. Hepatol Res (2015) 45:441–7. doi: 10.1111/hepr.12372
23. Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O, et al. Temporal Trends, Clinical Patterns and Outcomes of NAFLD-Related HCC in Patients Undergoing Liver Resection Over a 20-Year Period. Aliment Pharmacol Ther (2017) 46:856–63. doi: 10.1111/apt.14261
24. Bugianesi E. Review Article: Steatosis, the Metabolic Syndrome and Cancer. Aliment Pharmacol Ther (2005) 22 Suppl 2:40–3. doi: 10.1111/j.1365-2036.2005.02594.x
25. Siegel AB, Zhu AX. Metabolic Syndrome and Hepatocellular Carcinoma: Two Growing Epidemics With a Potential Link. Cancer (2009) 115:5651–61. doi: 10.1002/cncr.24687
26. Baffy G, Brunt EM, Caldwell SH. Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: An Emerging Menace. J Hepatol (2012) 56:1384–91. doi: 10.1016/j.jhep.2011.10.027
27. Simon TG, King LY, Chong DQ, Nguyen LH, Ma Y, Vopham T, et al. Diabetes, Metabolic Comorbidities, and Risk of Hepatocellular Carcinoma: Results From Two Prospective Cohort Studies. Hepatology (2018) 67:1797–806. doi: 10.1002/hep.29660
28. Ampuero J, Romero-Gomez M. Prevention of Hepatocellular Carcinoma by Correction of Metabolic Abnormalities: Role of Statins and Metformin. World J Hepatol (2015) 7:1105–11. doi: 10.4254/wjh.v7.i8.1105
29. Scalera A, Tarantino G. Could Metabolic Syndrome Lead to Hepatocarcinoma via Non-Alcoholic Fatty Liver Disease? World J Gastroenterol (2014) 20:9217–28.
30. Pang X, Wei Y, Zhang Y, Zhang M, Lu Y, Shen P. Peroxisome Proliferator-Activated Receptor-Gamma Activation Inhibits Hepatocellular Carcinoma Cell Invasion by Upregulating Plasminogen Activator Inhibitor-1. Cancer Sci (2013) 104:672–80. doi: 10.1111/cas.12143
31. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic Fatty Liver Disease and Hepatocellular Carcinoma: A Weighty Connection. Hepatology (2010) 51:1820–32. doi: 10.1002/hep.23594
32. Siegel AB, Goyal A, Salomao M, Wang S, Lee V, Hsu C, et al. Serum Adiponectin Is Associated With Worsened Overall Survival in a Prospective Cohort of Hepatocellular Carcinoma Patients. Oncology (2015) 88:57–68. doi: 10.1159/000367971
33. Balkwill F, Mantovani A. Inflammation and Cancer: Back to Virchow? Lancet (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
34. Nishida N. Metabolic Disease as a Risk of Hepatocellular Carcinoma. Clin Mol Hepatol (2021) 27:87–90. doi: 10.3350/cmh.2020.0302
35. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology (2015) 149:367–378 e365; quiz e314-365. doi: 10.1053/j.gastro.2015.04.005
36. Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, et al. KASL Clinical Practice Guidelines: Management of Nonalcoholic Fatty Liver Disease. Clin Mol Hepatol (2021) 27:363–401. doi: 10.3350/cmh.2021.0178
37. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A Placebo-Controlled Trial of Pioglitazone in Subjects With Nonalcoholic Steatohepatitis. N Engl J Med (2006) 355:2297–307. doi: 10.1056/NEJMoa060326
38. Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A Randomized Controlled Trial of Metformin Versus Vitamin E or Prescriptive Diet in Nonalcoholic Fatty Liver Disease. Am J Gastroenterol (2005) 100:1082–90. doi: 10.1111/j.1572-0241.2005.41583.x
39. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide Safety and Efficacy in Patients With Non-Alcoholic Steatohepatitis (LEAN): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Study. Lancet (2016) 387:679–90. doi: 10.1016/S0140-6736(15)00803-X
40. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med (2021) 384:1113–24. doi: 10.1056/NEJMoa2028395
41. Cho Y, Cho EJ, Yoo JJ, Chang Y, Chung GE, Jeong SM, et al. Association Between Lipid Profiles and the Incidence of Hepatocellular Carcinoma: A Nationwide Population-Based Study. Cancers (Basel) (2021) 13:1599. doi: 10.3390/cancers13071599
42. Brookes MJ, Cooper BT. Hypertension and Fatty Liver: Guilty by Association? J Hum Hypertens (2007) 21:264–70. doi: 10.1038/sj.jhh.1002148
43. Arima S, Uto H, Ibusuki R, Kumamoto R, Tanoue S, Mawatari S, et al. Hypertension Exacerbates Liver Injury and Hepatic Fibrosis Induced by a Choline-Deficient L-Amino Acid-Defined Diet in Rats. Int J Mol Med (2014) 33:68–76. doi: 10.3892/ijmm.2013.1544
44. Feng LH, Sun HC, Zhu XD, Zhang SZ, Li KS, Li XL, et al. Renin-Angiotensin Inhibitors Were Associated With Improving Outcomes of Hepatocellular Carcinoma With Primary Hypertension After Hepatectomy. Ann Transl Med (2019) 7:739. doi: 10.21037/atm.2019.11.131
45. Day CP. Natural History of NAFLD: Remarkably Benign in the Absence of Cirrhosis. Gastroenterology (2005) 129:375–8. doi: 10.1053/j.gastro.2005.05.041
46. Fan JG, Kim SU, Wong VW. New Trends on Obesity and NAFLD in Asia. J Hepatol (2017) 67:862–73. doi: 10.1016/j.jhep.2017.06.003
47. Huang DQ, El-Serag HB, Loomba R. Global Epidemiology of NAFLD-Related HCC: Trends, Predictions, Risk Factors and Prevention. Nat Rev Gastroenterol Hepatol (2021) 18:223–38. doi: 10.1038/s41575-020-00381-6
48. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of Nonalcoholic Fatty Liver Disease (NAFLD) With Hepatocellular Carcinoma (HCC) in the United States From 2004 to 2009. Hepatology (2015) 62:1723–30. doi: 10.1002/hep.28123
Keywords: hepatocellular carcinoma, epidemiology, nonalcoholic fatty liver disease, obesity, metabolic syndrome
Citation: Cho Y, Cho EJ, Yoo J-J, Chang Y, Chung GE, Choi IY, Park S-H, Han K, Kim YJ, Yoon J-H, Shin DW and Yu SJ (2022) The Importance of Metabolic Syndrome Status for the Risk of Non-Viral Hepatocellular Carcinoma: A Nationwide Population-Based Study. Front. Oncol. 12:863352. doi: 10.3389/fonc.2022.863352
Received: 27 January 2022; Accepted: 23 March 2022;
Published: 04 May 2022.
Edited by:
Takahiro Kodama, Osaka University, JapanReviewed by:
Hyo Geun Choi, Hallym University, South KoreaHosui Atsushi, Osaka Rosai Hospital, Japan
Yuji Eso, Kyoto University, Japan
Copyright © 2022 Cho, Cho, Yoo, Chang, Chung, Choi, Park, Han, Kim, Yoon, Shin and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su Jong Yu, c3Vqb25neXVAZ21haWwuY29t; Dong Wook Shin, ZHdzaGluLm1kQGdtYWlsLmNvbQ==
 Yuri Cho
Yuri Cho Eun Ju Cho
Eun Ju Cho Jeong-Ju Yoo
Jeong-Ju Yoo Young Chang4
Young Chang4 Goh Eun Chung
Goh Eun Chung Dong Wook Shin
Dong Wook Shin Su Jong Yu
Su Jong Yu