- 1Department of Pathology, Robert J. (R.J) Tomsich Pathology and Laboratory Medicine Institute, Cleveland Clinic, Cleveland, OH, United States
- 2Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 3Imaging Institute, Cardiovascular and Thoracic Radiology, Cleveland Clinic, Cleveland, OH, United States
- 4Department of Thoracic Surgery, Cleveland Clinic, Cleveland, OH, United States
Thymomas are derived from the epithelial component of the thymus and constitute the most common tumor of the anterior mediastinum. These neoplasms are considered malignant for their potential for invasion and metastases. Several histopathologic subclassification schemes have been proposed over the years, however, correlation of histotypes with prognosis remains controversial. In contrast, studies invariably have shown that staging and resection status correlate with oncologic behavior and disease outcomes. In this regard, several staging systems have been presented, though transcapsular invasion and degree of involvement of adjacent anatomic structures are common denominators of all schemes. Involvement of the great vessels and heart most commonly results from direct invasion, which may lead to unusual clinical presentations such as superior vena cava syndrome. Moreover, intravascular and intracardiac growth with or without direct mural invasion rarely occurs. We provide an overview of thymomas with intravascular and intracardiac involvement.
Introduction
Thymomas are malignant neoplasms derived from the epithelial component of the thymus. While they constitute the most common malignancy primary to the mediastinum, thymomas are rare, with a reported age standardized rate of 0.15 to 0.19/100,000 (1).
Given the complex histologic morphology and architecture of the normal thymus, thymomas are histologically characterized by their morphologic heterogeneity and have remained difficult to categorize by conventional histologic findings. Similarly, while treatment modalities, oncologic behavior, and disease outcomes are delineated by the clinical and pathologic staging, this parameter did not escape from the thymoma controversies in the literature.
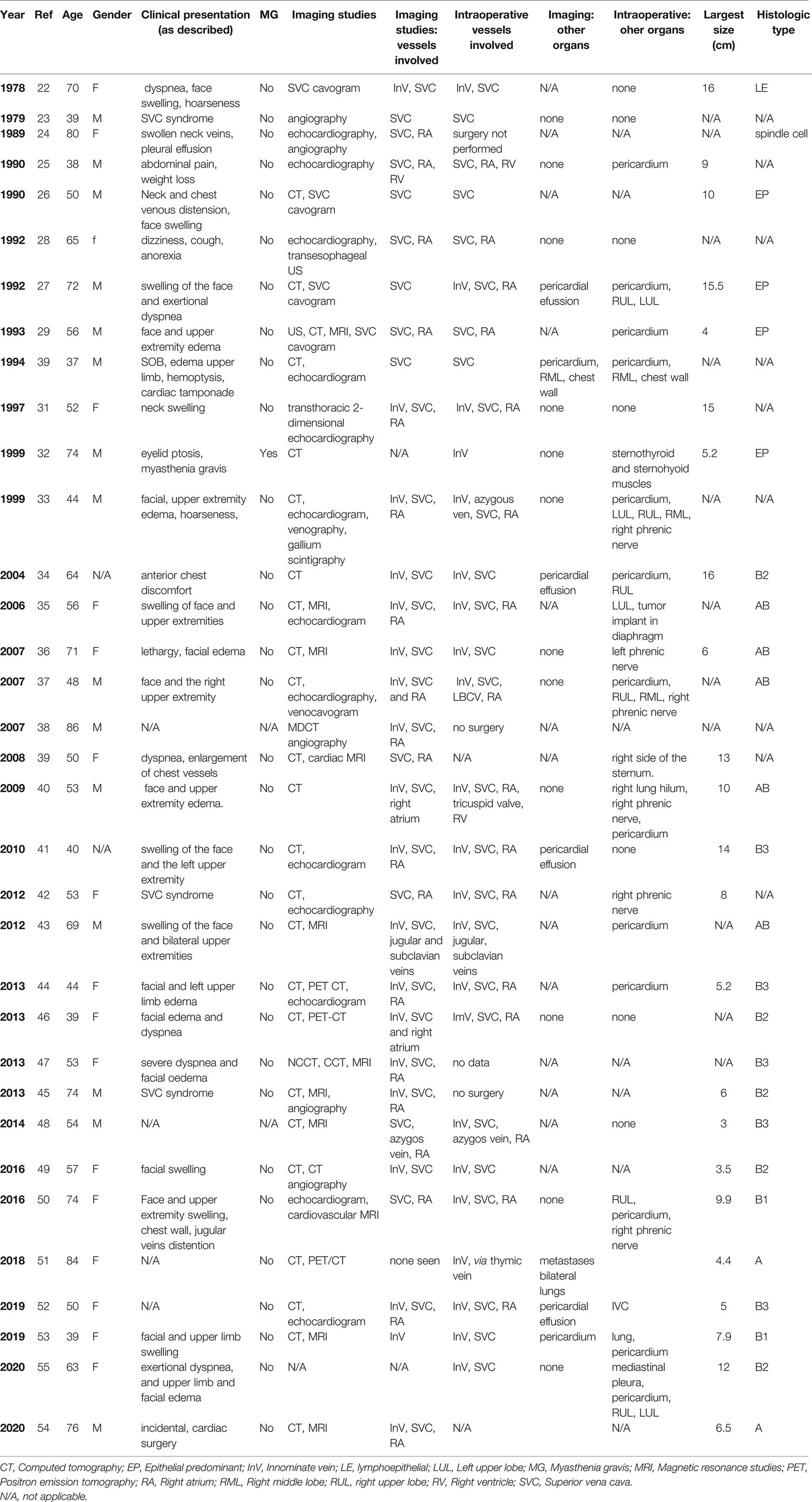
Notably, disparity and lack of granularity amongst the current staging systems creates some challenges in the studies of locally advanced thymomas with vascular or cardiac involvement. In the Masaoka-Koga staging system (2) these tumors would classify as stage III. However, there is no differentiation in stage based on organ involvement type thus innominate vein (InV) involvement (often resectable) and direct myocardial involvement (often unresectable) are staged similarly (Table 1). The latest version of the TNM staging system does provide greater clarity. Macroscopic invasion into neighboring organs has been further differentiated in the T classification (3). The T3 group includes invasion into adjacent structures that are typically considered resectable and include InV, superior vena cava (SVC), chest wall, phrenic nerve, and extracardiac pulmonary vessels. On the other hand, T4 includes invasion into structures that are classically not considered resectable and include aorta, arch vessels, main pulmonary artery, myocardium, trachea or esophagus (Table 2). However, with advances in cardiac and great vessel surgery, paradigms are evolving with respect to determination of resectability. For example, focal great vessel involvement is no longer necessarily considered a formidable challenge.
Invasion of the great vessels, particularly InV and SVC, is not uncommon in advanced thymomas, however, in most cases, stage III (Masaoka-Koga) or T3 (TNM system) result from invasion of extravascular structures, i.e. lung and/or pericardium (4–8). Furthermore, 2 patterns of vascular invasion may be found. Vessels may be involved by contiguous extension (Figure 1 Left), or, rarely, by downstream endoluminal growth into the large vessels and heart (Figure 1 Right).
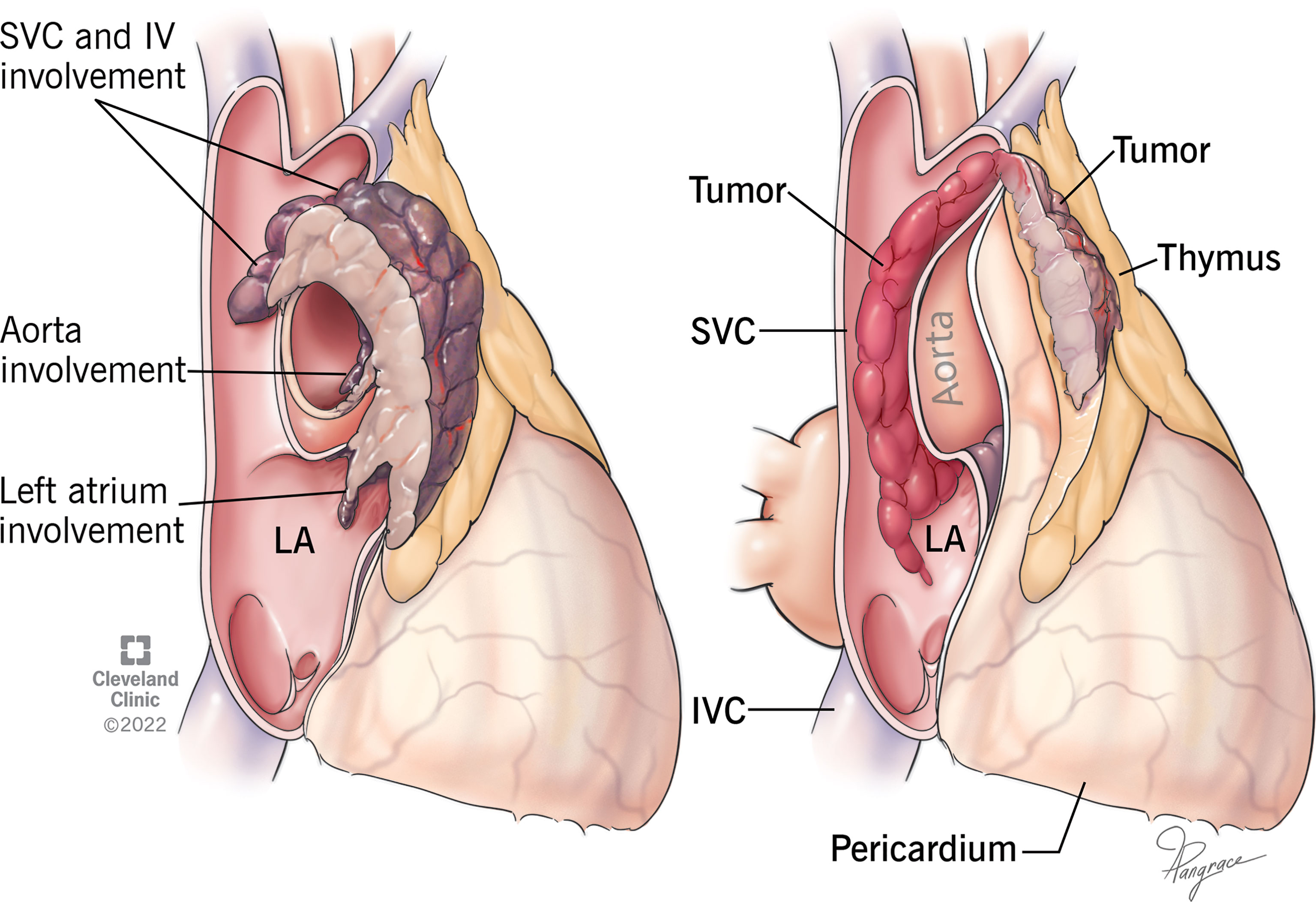
Figure 1 Drawing of 2 different types of vascular invasion. Left: Thymoma invades superior vena cava (SVC), innominate vein (IV), aorta, and left atrium (LA) by contiguous infiltration. Right: Thymoma shows a single point of wall invasion into the vessel with intravascular downstream extension into SVC and LA without contiguous involvement.
In this report, we summarize the cases in the English medical literature that provide detail of thymic tumors with intravascular/thrombotic growth protruding into the SVC, its tributaries, and heart, without evidence of contiguous extension through their walls. Our purpose is to highlight this unusual pattern of vascular invasion and generate the base for the identification and further evaluation of these cases.
Design
A search of thymomas with vascular involvement was performed in the PubMed database (National Medical Library) using the following search terms: “thymoma” and “vascular” and/or each of the terms “intravascular”, and/or “superior vena cava”, “innominate vein”, “brachiocephalic vein”, “atrium”, “cardiac”, “heart”, “intracardiac”, “thrombosis”.
All pertinent articles in the English language medical literature were reviewed. Only those studies with confirmed endovascular growth as the pattern of vascular involvement (Figure 1 Left) were included. Articles referring to cases with vascular involvement resulting from adjacent contiguous invasion, or without detailed data regarding the pattern of vascular invasion were excluded (4–7, 9–21).
The clinical, radiographic, and surgical data were gathered and tabulated for each publication.
Results
Thirty-five publications of patients with confirmed radiographic and/or surgical description of intravascular/intracardiac spread of thymoma without evidence of direct connection were found after thorough review of the numerous articles from the English medical literature. Each publication reported 1 patient with these characteristics (22–55).
The detailed results are shown in Table 3. In summary, there were a total of 34 patients (17 females and 15 males, gender not available in 2 cases) with an average age of 58 years old. Twenty-six cases (87%) presented with swelling of the veins of the face, neck, upper extremities, and/or chest wall, characteristic of SVC syndrome, 7 of them associated with dyspnea. SVC syndrome was not present in 5 (17%) patients, 1 was an incidental finding during cardiac surgery, and 4 presented with dizziness and cough, abdominal pain and distension, ptosis, pain and weight loss, ptosis, and chest discomfort respectively. The patient with ptosis was the only one with evidence of myasthenia gravis. Clinical presentation was not provided in 4 patients.
Patients received a wide range of diagnostic imaging studies, with all but 5 having either magnetic resonance imaging (MRI) and/or computed tomography (CT) imaging performed as part of the evaluation. All 5 patients who did not undergo CT or MRI were evaluated in 1997 or earlier and had echocardiography or catheter-based venography. By imaging studies, invasion of the SVC was seen in 28 (85%) patients, 21 of whom with involvement of the right atrium (RA) and 15 with involvement of SVC tributaries, mostly InV. Vascular invasion was not detected radiographically in 1 patient. Radiographic data was not available in 2 patients. Both intraoperative and imaging data were provided in 27 cases. Of those, concordant findings were present in 20 cases and in 7 cases, 1 or more involved vessels were encountered intraoperatively.
The average size of the thymomas (available in 23 cases) was 8.9 cm, ranging from 3 to 16 cm. The histologic type was reported in 27 cases: WHO types A in 3, AB in 5, B1 in 2, and B2 and B3 in 5. Two cases were reported as epithelial-predominant, ‘mixed” in 1, “type II” in 1, and lymphoepithelial in 1.
Discussion
Thymomas are the most common primary tumor occurring in the anterior mediastinum, with an annual incidence 0.15 to 0.19/100,000 (1). All age groups are affected, but most commonly they occur in middle-aged adults (40-50 years). Patients may present with symptoms due to mass effect, autoimmune or paraneoplastic syndromes or metastases, and in a subset of patients, thymomas are found incidentally during thoracic imaging. Autoimmune or paraneoplastic symptoms are most commonly, but are not limited to, neuromuscular disorders (myasthenia gravis), immunodeficiency disorders (hypogammaglobulinemia), or hematologic diseases (pure cell aplasia, hemolytic anemia).
Once a diagnosis is made, a multidisciplinary treatment approach with clinicians experienced with thymoma/thymic carcinoma is vital. Tumor type, stage, extent of invasiveness, potential phrenic nerve involvement, and the physiologic status of the patient are all essential considerations when determining the appropriate multi-modal treatment plan. In the circumstance of potential vascular or cardiac involvement, surgery may still play a role in the patient management depending on degree and location of involvement.
At the time of presentation, most tumors (65%) are Masaoka-Koga stage I or II, 25% are stage III, and around 10% are stage IV (8). Half of all presentations have invasion into surrounding structures but remain candidates for multimodal therapy including surgical resection. This emphasizes the importance of multidisciplinary management. Unfortunately, the Masaoka-Koga staging system makes no differentiation in stage based on organ involvement type thus innominate vein involvement (often resectable) and direct myocardial involvement (often unresectable) are staged similarly (Table 1). This is better reflected in the latest version of the TNM staging system, in which invasion of mediastinal organs is further divided into T3 (invasion or lung, InV, SVC, phrenic nerve, chest wall or extrapericardial pulmonary arteries) and T4 (invasion of aorta, arch vessels, intrapericardial pulmonary artery, myocardium, trachea and/or esophagus) (Table 2) (3).
We present a review of thymoma cases reported in the English medical literature that demonstrated vascular invasion through endovascular and intracardiac growth not associated with contiguous extension from the main mass (Figure 1). SVC syndrome was the most common presentation of these patients. In most cases, imaging studies were able to demonstrate endovascular growth with good correlation with the intraoperative findings. These cases are staged as Masaoka-Koga III or TNM system T3, however they have an unusual unifying component, the intravascular extension of tumor. It is hypothesized that thymomas enter the great veins through small vessels, such as the thymic veins, or focal transmural invasion, analogous to other angioinvasive malignancies such as renal cell carcinoma, leading to its growth along the venous stream down into the larger veins and atrium. Protrusion of the tumor into the thymic veins was noted intraoperatively in some of the cases in this review.
The WHO classification is currently the primary scheme used for the histologic typification of thymomas (56). Types A, AB, B1, B2, and B3 are divided based on cell morphology and progressive loss of the background population of immature thymic lymphocytes. While the prognosis of thymomas is mainly dependent on stage and resection status, Weiss et al. showed that recurrence rate was correlated with the WHO histotypes but not overall survival (57). However, the relevance of the pathologic classification as a prognostic indicator for recurrence and overall survival has been the subject of numerous studies, with discrepant results. Furthermore, several other histologic schemes have been proposed in the literature, and the controversy regarding which one is the most reproducible, applicable to, and reflective of clinical behavior is ongoing.
Histologic data was available in 25 cases and included 14 cases B2 and B3 (3 older cases classified as epithelial predominant were converted to B3 in this review). Surprisingly, types A, AB, and B1 grouped together were reported at frequencies comparable to types B2 and B3.
Imaging plays a central role in the diagnosis and staging of thymomas. Thymomas typically present in an anterior mediastinal location, arising from one side of the thymus with well-defined margins, smooth or lobulated contours, and locations varying from thoracic inlet to cardiophrenic angle (58). Use of intravenous contrast is indicated whenever feasible, as this allows for improved assessment of vascular involvement, as well as enhancement characterization of the mass. Vascular invasion is suggested by alteration of vessel lumen contour, encasement or obliteration of vessel, or soft tissue intravascular extension, which may also extend to pericardium or cardiac chambers (59). Use of ECG synchronized imaging, either by CT or MRI may allow for improved delineation of cardiac involvement.
Involvement of the mediastinal vessels, in particular InV and/or SVC, is not uncommon in locally advanced thymomas (about 15%), however, lung and pericardium are the most frequently involved organs in Masaoka-Koga stage III or TNM T3 tumors (4–8). The impact on tumor behavior of vascular involvement remains unclear, however, recurrence rates tend to be higher, and disease-free survival shorter, in cases with invasion of the great vessels compared to those without (4, 6, 7). More so, almost no studies evaluate the significance of differentiating specific vessel involvement. In this regard, in a study of clinicopathologic correlation of 250 thymoma cases by Moran et al. the InV was stratified separately from the other great vessels and heart as stage IIA and stage IIC respectively. This stratification did not result statistically significant, however, the number of cases in the study was low, with only 56 tumors stage IIA and 2 tumors stage IIC. Nonetheless, these groups were still included in the proposed staging system. The authors manifest that such stratification is important for the possibility of advances in surgical techniques, additional therapy, and future larger studies. (Table 4) (60). For instance, extended involvement of the surrounding anatomic structures (Masaoka stage III or TNM system T3) makes radical surgery unfeasible in up to 30% to 40% of invasive thymomas and thymic carcinomas grouped together (20). Involvement of the InV, however, can be addressed with simple vein resection with or without reconstruction, and involvement of the SVC can be addressed surgically in certain circumstances although cardiopulmonary bypass may be necessary. Thus, there is likely significantly variability in the determination of resectability based on surgeon and center level experience.
Notably, Moran et al. also recognized that vascular invasion in thymomas may follow 2 different patterns, either direct wrapping/extension into the vascular wall, or spread within the vessel itself, as the cases reviewed in this article. Whether staging should be different for these tumors is uncertain. The significance of specific vessel differentiation in the stratification of staging systems, and the pattern of vascular involvement would need to be elucidated.
Limitations
In this study, we present cases of thymoma with intravascular/thrombotic pattern of vascular invasion based on a review of the literature. We excluded the cases that presented vascular invasion by contiguity, as well as those we deemed confusing or ambiguous. However, we recognize that our study is based on a retrospective literature review, and as such, accuracy of the data might be difficult to assess in some cases, especially older reports, which constitutes the main limitation of our study. Nonetheless, we believe that our study could set the base for the identification of these cases and development of future studies to delineate their significance.
Conclusion
We provide a descriptive analysis of thymoma cases with vascular invasion resulting from downstream polypoid and/or thrombotic intravascular growth detected with imaging studies and/or intraoperatively. intravascular spread is rare among thymomas, regardless of histologic type or staging, and may create uncertainties regarding management. We acknowledge that precise assessment of the incidence of this phenomenon is challenging due to the ambiguity in defining the pattern of vascular invasion in most studies. Importantly, vital to the most appropriate intra-operative planning and perioperative support in managing patients with thymomas is an ongoing, multidisciplinary evaluation, appropriate physiologic assessment of the patient, and precise staging.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AA contributed to conception and design of the study, literature search using the MedHub database, extraction of data from the literature, and writing the first draft of the manuscript. JD, MB, and DR wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hsu C-H, Chan JK, Yin C-H, Lee C-C, Chern C-U, Liao C-I. Trends in the Incidence of Thymoma, Thymic Carcinoma, and Thymic Neuroendocrine Tumor in the United States. PloS One (2019) 14(12):e0227197. doi: 10.1371/journal.pone.0227197
2. Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T, et al. A Review of 79 Thymomas: Modification of Staging System and Reappraisal of Conventional Division Into Invasive and non-Invasive Thymoma. Pathol Int (1994) 44(5):359–67. doi: 10.1111/j.1440-1827.1994.tb02936.x
3. Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an Evidence-Based Stage Classification System for the Forthcoming (8th) Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2014) 9(9 Suppl 2):S65–72. doi: 10.1097/JTO.0000000000000290
4. Okumura M, Miyoshi S, Takeuchi Y, Yoon H-E, Minami M, Takeda S-I, et al. Results of Surgical Treatment of Thymomas With Special Reference to the Involved Organs. J Thorac Cardiovasc Surg (1999) 117(3):605–12. doi: 10.1016/S0022-5223(99)70343-0
5. Wright CD, Wain JC, Wong DR, Donahue DM, Gaissert HA, Grillo HC, et al. Predictors of Recurrence in Thymic Tumors: Importance of Invasion, World Health Organization Histology, and Size. J Thorac Cardiovasc Surg (2005) 130(5):1413–21. doi: 10.1016/j.jtcvs.2005.07.026
6. Utsumi T, Shiono H, Matsumura A, Maeda H, Ohta M, Tada H, et al. Stage III Thymoma: Relationship of Local Invasion to Recurrence. J Thorac Cardiovasc Surg (2008) 136(6):1481–5. doi: 10.1016/j.jtcvs.2008.05.012
7. Comacchio GM, Dell’Amore A, Marino MC, Russo MD, Schiavon M, Mammana M, et al. Vascular Involvement in Thymic Epithelial Tumors: Surgical and Oncological Outcomes. Cancers (2021) 13(13):3355. doi: 10.3390/cancers13133355
8. Detterbeck FC, Zeeshan A. Thymoma: Current Diagnosis and Treatment. Chin Med J (Engl) (2013) 126(11):2186–91. doi: 10.3760/cma.j.issn.0366-6999.20130177
9. Dartevelle P, Chapelier A, Navajas M, Levasseur P, Rojas A, Khalife J, et al. Replacement of the Superior Vena Cava With Polytetrafluoroethylene Grafts Combined With Resection of Mediastinal-Pulmonary Malignant Tumors. J Thorac Cardiovasc Surg (1987) 94(3):361–6. doi: 10.1016/S0022-5223(19)36248-8
10. Freedberg. The Evaluation of MRI in the Evaluation of Intracardiac Tumors Diagnosed by Ecocardiography. Circulation (1988) 77:96. doi: 10.1161/01.CIR.77.1.96
11. Dartevelle PG, Chapelier AR, Pastorino U, Corbi P, Lenot B, Cerrina J, et al. Long-Term Follow-Up After Prosthetic Replacement of the Superior Vena Cava Combined With Resection of Mediastinal-Pulmonary Malignant Tumors. J Thorac Cardiovasc Surg (1991) 102(2):259–65. doi: 10.1016/S0022-5223(19)36558-4
12. Shimizu N, Moriyama S, Aoe M. The Surgical Treatment of Invasive Thymoma. J Thorac Cardiovasc Surg Vol. 103. (1992). pp. 414–20. doi: 10.1016/S0022-5223(19)34979-7
13. Yagi K, Hirata T, Fukuse T, Yokomise H, Inui K, Ike O, et al. Surgical Treatment for Invasive Thymoma, Especially When the Superior Vena Cava is Invaded. Ann Thorac Surg (1996) 61(2):521–4. doi: 10.1016/0003-4975(95)00983-3
14. Bacha EA, Chapelier AR, Macchiarini P, Fadel E, Dartevelle PG. Surgery for Invasive Primary Mediastinal Tumors. Ann Thorac Surg (1998) 66(1):234–9. doi: 10.1016/S0003-4975(98)00350-6
15. Lanuti M, De Delva PE, Gaissert HA, Wright CD, Wain JC, Allan JS, et al. Review of Superior Vena Cava Resection in the Management of Benign Disease and Pulmonary or Mediastinal Malignancies. Ann Thorac Surg (2009) 88(2):392–7. doi: 10.1016/j.athoracsur.2009.04.068
16. Marulli G, Lucchi M, Margaritora S, Cardillo G, Mussi A, Cusumano G, et al. Surgical Treatment of Stage III Thymic Tumors: A Multi-Institutional Review From Four Italian Centers☆. Eur J Cardiothorac Surg (2011) 39(3):e1–7. doi: 10.1016/j.ejcts.2010.11.026
17. De Giacomo T, Patella M, Mazzesi G, Venuta F. Successful Resection of Thymoma Directly Invading the Right Atrium Under Cardiopulmonary Bypass. Eur J Cardiothorac Surg (2015) 48(2):332–3. doi: 10.1093/ejcts/ezu376
18. Dai W, Dong J, Zhang H, Yang X, Li Q. Superior Vena Cava Replacement Combined With Venovenous Shunt for Lung Cancer and Thymoma: A Case Series. J Thorac Dis (2018) 10(1):363–70. doi: 10.21037/jtd.2017.12.130
19. Maurizi G, Poggi C, D’Andrilli A, Vanni C, Ciccone AM, Ibrahim M, et al. Superior Vena Cava Replacement for Thymic Malignancies. Ann Thorac Surg (2019) 107(2):386–92. doi: 10.1016/j.athoracsur.2018.08.060
20. Bertolaccini L, Prisciandaro E, Galetta D, Casiraghi M, Guarize J, Petrella F, et al. Outcomes and Safety Analysis in Superior Vena Cava Resection for Extended Thymic Epithelial Tumors. Ann Thorac Surg (2021) 112(1):271–7. doi: 10.1016/j.athoracsur.2020.07.069
21. Okereke OU, Nzewi OC, Chikwendu VC, Odigwe E, Onuigbo WB. Radical Excision of Invasive Thymoma With Intracardiac Extension. J Cardiovasc Surg (Torino) (1994) 35(4):355–8.
22. Arai T, Inagaki K, Hata E, Hirata M, Onoue Y, Morimoto K. Reconstruction of the Superior Vena Cava in a Patient With a Thymoma. Chest (1978) 73(2):230–1. doi: 10.1378/chest.73.2.230
23. Tanabe T, Kubo Y, Hashimoto M, Sugie S. Patch Angioplasty of the Superior Vena Caval Obstruction (Case Reports With Long Follow-Up Results). J Cardiovasc Surg (Torino) (1979) 20(5):519–22.
24. Korobkin M, Gasano VA. Intracaval and Intracardiac Extension of Malignant Thymoma: CT Diagnosis. J Comput Assist Tomogr (1989) 13(2):348–50. doi: 10.1097/00004728-198903000-00035
25. Airan. Malignant Thymoma Presenting Intracardiac Tumor and SVC Obstruction. Ann Thorac Surg (1990) 50:989–91. doi: 10.1016/0003-4975(90)91143-Y
26. Chiou GTJ, Chen C-L, Wei J, Hwang W-S. Reconstruction of Superior Vena Cava in Invasive Thymoma. Chest (1990) 97(2):502–3. doi: 10.1378/chest.97.2.502
27. Yokoi K, Miyazawa N, Mori K, Saito Y, Tominaga K, Imura G, et al. Invasive Thymoma With Intracaval Growth Into the Right Atrium. Ann Thorac Surg (1992) 9(53):507–9.
28. Missault L, Duprez D, De Buyzere M, Cambier B, Adang L, Clement D. Right Atrial Invasive Thymoma With Protrusion Through the Tricuspid Valve. Eur Heart J (1992) 13(12):1726–7. doi: 10.1093/oxfordjournals.eurheartj.a060132
29. Futami S, Yamasaki T, Minami R, Matsuba K, Inoue K, Nishimoto S, et al. Intracaval and Intracardiac Extension of Malignant Thymoma. Intern Med (1993) 32(3):257–60. doi: 10.2169/internalmedicine.32.257
30. Dib H, Friedman B, Khouli H. Malignant Thymoma*A Complicated Triad of SVC Syndrome, Cardiac Tamponade, and DIC. Chest (1993) 105:941–2. doi: 10.1378/chest.105.3.941
31. Filippone G, Savona I, Tomasello V, Guzzetta P, Zarcone N, Agate V. Radical Excision of Invasive Thymoma With Intracaval and Intracardial Extension: A Successful Case Report. J Cardiovasc Surg (Torino) (1997) 38(5):547–9.
32. Nomori H, Horio H, Morinaga S, Suemasu K. Minimally Invasive Thymoma With Extensive Intravascular Growth. Jpn J Clin Oncol (1999) 29(12):630–2. doi: 10.1093/jjco/29.12.630
33. Minato N, Rikitake K, Ohnishi H, Takarabe K, Ishida H. Invasive Thymoma With Intracaval Growth Extending and Directly Invading the Right Atrium. J Cardiovasc Surg (Torino) (1999) 40(6):915–7.
34. Terada Y, Ono N, Noguchi T, Kamakari K, Kitano M. A Case of Thymoma Protruding Into the Superior Vena Cava Through the Thymic Vein. Ann Thorac Surg (2004) 77(3):1088–90. doi: 10.1016/S0003-4975(03)01172-X
35. Ichimura H, Usui S, Okazaki H, Konishi T, Osaka M, Jikuya T, et al. Excision After Chemoradiotherapy of Invasive Thymoma Extending Into the Right Atrium: Report of a Case. Surg Today (2006) 36(6):534–7. doi: 10.1007/s00595-006-3188-7
36. Konstantinov IE, Saxena P, Koniuszko M, Ghosh S, Low VHS, Khor TS, et al. Superior Vena Cava Obstruction by Tumour Thrombus in Invasive Thymoma: Diagnosis and Surgical Management. Heart Lung Circ (2007) 16(6):462–4. doi: 10.1016/j.hlc.2006.08.010
37. Shudo Y, Takahashi T, Ohta M, Ikeda N, Matsue H, Taniguchi K. Radical Operation for Invasive Thymoma With Intracaval, Intracardiac, and Lung Invasion. J Card Surg (2007) 22(4):330–2. doi: 10.1111/j.1540-8191.2007.00417.x
38. Alladin M, Bozlar U, Turba UC, Kron IL, Ahmed H, Hagspiel KD. Mediastinal Thymoma Invading the Superior Vena Cava and the Right Atrium. Acta Cardiol (2007) (5):517–8. doi: 10.2143/AC.62.5.2023417
39. Dursun M, Sarvar S, Cekrezi B, Kaba E, Bakir B, Toker A. Cardiac Metastasis From Invasive Thymoma Via the Superior Vena Cava: Cardiac MRI Findings. Cardiovasc Intervent Radiol (2008) 31(S2):209–12. doi: 10.1007/s00270-007-9146-y
40. Amirghofran H, Emaminia A, Rayatpisheh S, Malek-Hosseini S, Attaran Y, et al. Intracardiac Invasive Thymoma Presenting as Superior Vena Cava Syndrome. Ann Thorac Surg (2009) 87:1616–8. doi: 10.1016/j.athoracsur.2008.09.052
41. Li W, Chen X, Lv X, Guo X, Yang Y, Ni Y, et al. Resection of an Invasive Thymoma Extending Into the Superior Vena Cava and Right Atrium. J Card Surg (2010) 25(5):515–7. doi: 10.1111/j.1540-8191.2010.01036.x
42. Toker A, Tireli E, Tanju S, Kaya S. Transcaval Invasion of Right Atrium by Thymoma: Resection via Transient Cava-Pulmonary Shunt. Eur J Cardiothorac Surg (2012) 41(5):1175–7. doi: 10.1093/ejcts/ezr261
43. Tsutsui S, Ashizawa K, Tagawa T, Nagayasu T, Hayashi T, Uetani M. Invasive Thymoma With Venous Intraluminal Extension: CT and MRI Findings. Clin Imaging (2012) 36(6):854–7. doi: 10.1016/j.clinimag.2012.01.033
44. Rizzardi G, Arena V, Passera E, Bortolotti L. Radical Surgery in an Unusual Case of Thymoma With Intraluminal Growth Into the Superior Vena Cava and Right Atrium. Interact Cardiovasc Thorac Surg (2013) 17(6):1054–5. doi: 10.1093/icvts/ivt397
45. Kurata A, Saji H, Ikeda N, Kuroda M. Intracaval and Intracardiac Extension of Invasive Thymoma Complicated by Superior and Inferior Vena Cava Syndrome: Intracardiac Extension of Thymoma. Pathol Int (2013) 63(1):56–62. doi: 10.1111/pin.12023
46. Kim HJ, Cho SY, Cho WH, Lee DH, Lim DH, Seo PW, et al. An Unusual Case of Superior Vena Cava Syndrome Caused by the Intravascular Invasion of an Invasive Thymoma. Tuberc Respir Dis (2013) 75(5):210–3. doi: 10.4046/trd.2013.75.5.210
47. Gümüstas S, Akça A, Inan N, Akgül AG, Liman ST. Characterization of Malignant Thrombus in an Invasive Thymoma With Intravascular Growth. J Radiol Case Rep (2013) 7(2):17–23. doi: 10.3941/jrcr.v7i2.1115
48. Melloni G, Bandiera A, Castiglioni A, Zannini P. Thymoma With Intravascular Extension Into the Right Atrium. Eur J Cardiothorac Surg (2014) 45(4):e126–6. doi: 10.1093/ejcts/ezt618
49. Ohata K, Yoshimura T, Matsubara Y, Yasuhara Y, Terada Y. Invasive Thymoma Protruding Into the Superior Vena Cava Through the Thymic Vein. Ann Thorac Cardiovasc Surg (2016) 22(2):122–4. doi: 10.5761/atcs.cr.15-00149
50. Afzal A, Wong I, Korniyenko A, Ivanov A, Worku B, Gulkarov I. Superior Vena Cava Syndrome From an Invasive Thymoma With Transcaval Invasion to the Right Atrium. J Surg Case Rep (2016) 2016(4):rjw044. doi: 10.1093/jscr/rjw044
51. Kawakita N, Kondo K, Toba H, Yoneda A, Takizawa H, Tangoku A. A Case of Atypical Type A Thymoma With Vascular Invasion and Lung Metastasis. Gen Thorac Cardiovasc Surg (2018) 66(4):239–42. doi: 10.1007/s11748-017-0794-9
52. Yang T, Hui R, Wu Q, Tian J, Chen H. An Invasive Thymoma Extending Into the Superior Vena Cava and Right Atrium. Ann Transl Med (2019) 7(18):498. doi: 10.21037/atm.2019.08.59
53. Sena A, Ferreira R, Gonçalves J, Nobre Â. Surgical Treatment of an Advanced Stage Thymoma in a Good's Syndrome Patient - Case Report. Rev Port Cir Cardiotorac Vasc (2019) 26(4):269–71.
54. Singh N, Nand P, Alison P. Complex Redo Surgery to Treat a Large Thymoma Invading the Right Atrium. J Card Surg (2020) 35(6):1368–70. doi: 10.1111/jocs.14571
55. Shen W, Cao Y, Wang X, Zhang P, Zhou Q. Invasive Thymoma With Intravascular Growth Into the Great Veins and Right Atrium: A Case Report. Thorac Cancer (2020) 11(5):1326–9. doi: 10.1111/1759-7714.13242
56. WHO Classification of Tumours Editorial Board. Tumours of the Thymus, Thymoma. In: WHO Classification of Tumours Thoracic Tumours, 5th ed, vol. 5. Lyon, France: International Agency for Research on Cancer (2021). p. 319–49.
57. Weis C-A, Yao X, Deng Y, Detterbeck FC, Marino M, Nicholson AG, et al. The Impact of Thymoma Histotype on Prognosis in a Worldwide Database. J Thorac Oncol (2015) 10(2):367–72. doi: 10.1097/JTO.0000000000000393
58. Benveniste MFK, Rosado-de-Christenson ML, Sabloff BS, Moran CA, Swisher SG, Marom EM. Role of Imaging in the Diagnosis, Staging, and Treatment of Thymoma. RadioGraphics (2011) 31(7):1847–61. doi: 10.1148/rg.317115505
59. Rosado-de-Christenson ML, Strollo DC, Marom EM. Imaging of Thymic Epithelial Neoplasms. Hematol Oncol Clin North Am (2008) 22(3):409–31. doi: 10.1016/j.hoc.2008.03.011
Keywords: invasive thymoma, staging, intravascular growth, superior vena cava syndrome, intravascular growth pattern
Citation: Arrossi AV, Dermawan JK, Bolen M and Raymond D (2022) Thymomas With Intravascular and Intracardiac Growth. Front. Oncol. 12:881553. doi: 10.3389/fonc.2022.881553
Received: 22 February 2022; Accepted: 05 May 2022;
Published: 24 June 2022.
Edited by:
Cesar Moran, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Niccolò Daddi, University of Bologna, ItalyMichael Ried, University Hospital Regensburg, Germany
Copyright © 2022 Arrossi, Dermawan, Bolen and Raymond. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Valeria Arrossi, YXJyb3NzYUBjY2Yub3Jn
 Andrea Valeria Arrossi
Andrea Valeria Arrossi Josephine K. Dermawan
Josephine K. Dermawan Michael Bolen3
Michael Bolen3