- 1Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China
- 2Department of Radiation Oncology, the First Affiliated Hospital of Xiamen University, Xiamen, China
Background: This study aimed to construct a nomogram for Breast sarcoma (BS) to predict the prognosis of patients with BS accurately and provide a theoretical basis for individualized treatment.
Methods: Patients selected from the Surveillance, Epidemiology and End Results (SEER) database from 2000 to 2018 were assigned to a training group (TG, n = 696) and an internal validation group (IVG, n = 299) at a 7:3 ratio. Cox regression analysis was performed on the TG, and statistically significant factors were used to establish a nomogram to predict 3-, 5-, and 10-year overall survival (OS). The nomogram’s predictive power was validated using data from patients who attended our institution as the external validation group (EVG, n =79).
Results: Cox regression analysis identified five factors, which were used to construct the nomogram. Good prediction accuracy was demonstrated using calibration curves. The concordance (C) indices for TG = 0.804 (95% confidence interval (CI) 0.777–0.831) and IVG = 0.761 (0.716–0.806) were higher than those based on 8th American Joint Committee on Cancer (AJCC8) stage: TG = 0.695 (0.660–0.730), IVG = 0.637 (0.584–0.690). The EVG also had a high C-index: 0.844 (0.768–0.920). Decision curve analysis showed that nomogram has larger net benefits than the AJCC8. The Kaplan–Meier curves of the nomogram-based risk groups showed significant differences (p < 0.001).
Conclusions: The nomogram could accurately predict 3-, 5-, and 10-year OS and provided nomogram-based risk stratification, which could help physicians to personalize treatment plans for patients with BS.
Introduction
Breast sarcoma (BS) is a rare malignancy derived from mesenchymal cells, which can be divided into primary and secondary BS. Compared with the more common breast carcinoma, the natural history, treatment, and prognosis of BS are unique (1). According to clinical statistics, primary BS represents < 1% of all breast tumors and < 5% of all sarcomas (2). Research on BS is limited because of its rarity, and comprises mostly small retrospective studies and case studies; moreover, treatment strategies for BS are mostly based on the extrapolated research data from soft tissue sarcomas of the extremities (1). Currently, BS staging principally relies on the 8th American Joint Committee on Cancer (AJCC8) for soft tissue sarcomas. The staging system is based on tumor size (T), lymph node involvement (N), metastasis (M), and histological grade (3). However, studies have shown that age (4), histological type (1), and treatment all have different effects on the prognosis of BS.
A nomogram is a tool that predicts a certain clinical outcome or the probability of a certain event based on the values of multiple clinical indicators or biological parameters. It predicts the probability of a specific outcome for patients at any given time by incorporating a variety of disease indicators. Nomograms have the advantages of convenience, simplicity, accuracy, reliability, and practicality. They have been used widely to predict prognosis in various tumors, e.g., oropharyngeal carcinoma (5), breast cancer (6), and cervical cancer (7). However, to the best of our knowledge, a nomogram to predict the survival of patients with BS has not been reported.
Therefore, this study aimed to develop a nomogram that could predict the overall survival (OS) of patients with BS using data from the Surveillance, Epidemiology, and End Results database (SEER). Further SEER data was used for internal validation and data from patients attending the Cancer Center of Sun Yat-sen University (SYSUCC) were used for external verification. Ultimately, we aimed to provide a nomogram that could assist clinicians to accurately predict patient prognosis and that formed a theoretical foundation to formulated individualized clinical treatment strategies.
Materials and methods
Patient selection and study variables
Data for the analysis were obtained from the SEER database from 2000 to 2018 and from SYSUCC patients’ data from 2000 to 2015.
The inclusion criteria for patients from the SEER database were: (1) IDC-O-3, malignant = 8800/3-9581/3; (2) site record = breast; (3) first malignant primary indicator = yes. The exclusion criteria comprised: (1) Diagnosed at autopsy or from a death certificate; (2) Follow-up time < 1 month or the patient died within one month; (3) Lack of necessary information including ethnicity, tumor size, lymph node status, and surgery type. (4) Tumors with sizes < 0.5 cm and > 40 cm were also excluded (because of the low incidence of tumors in this size range) (3). Finally, the study included 995 patients.
Variables analyzed included ethnicity, sex, age at diagnosis, marital status, histological subtype, grade, laterality, tumor size, lymph node status, metastasis, axillary lymph node surgery (biopsy or dissection), surgery, radiotherapy, chemotherapy, and follow-up time. To adjust the nonlinear relationship between age and death risks, the best cutoff value of age was determined using the restricted cubic spline (RCS) with five knots. The best cutoff of tumor size was determined using X-tile (Yale School of medicine, New Haven, CT, USA). According to the above information, N stage and AJCC stage were determined according to AJCC8 staging standards. The endpoint of the study was overall survival (OS).
A total of 95 patients with BS attended SYSUCC from 2000 to 2015, and after following the same inclusion/exclusion criteria used for the SEER data, 79 of these patients were assigned to the external validation group (EVG).
Nomogram development and risk stratification
The 995 patients from SEER were assigned randomly to the training group (TG, n = 696) and the internal validation group (IVG, n = 299) according to a ratio of 7:3. Univariate and multivariate Cox regression analyses were carried out on the TG data to identify statistically significant factors (P < 0.05). Next, backward stepwise regression was performed to calculate the Akaike information criterion (AIC): The lower the AIC value, the better the model (8). Finally, based on the selected variables, a nomogram to predict the OS of patients at 3, 5, and 10 years was constructed, and the IVG and EVG data were used to test the reliability and generalizability of the developed model. Based on the nomogram-derived scores of different variables, we calculated the total score for each patient. This allowed the patients to be assigned to three risk groups (low, middle, and high). X-tile was used to determine the optimal cut-off value of risk stratification, and for the three risk groups, Kaplan–Meier survival curves in the TG, IVG and EVG were drawn, respectively, and survival analysis was performed using the log-rank test.
The concordance index (C-index), receiver operating characteristic curve (ROC) analysis, and the area under the curve (AUC) of the nomogram and of the AJCC8 stage at 3, 5, and 10 years were calculated to assess the discriminative power of the nomogram. The correlation between the actual and predicted results was compared using calibration curves. After determining that the nomogram was accurate, the clinical utility of the nomogram was compared with that of AJCC8 using decision curve analysis (DCA).
All statistical analyses were performed using the R software v4.1.1. (https://www.R-project.org). P < 0.05 was considered to be a statistically significant difference.
Results
Patients characteristics
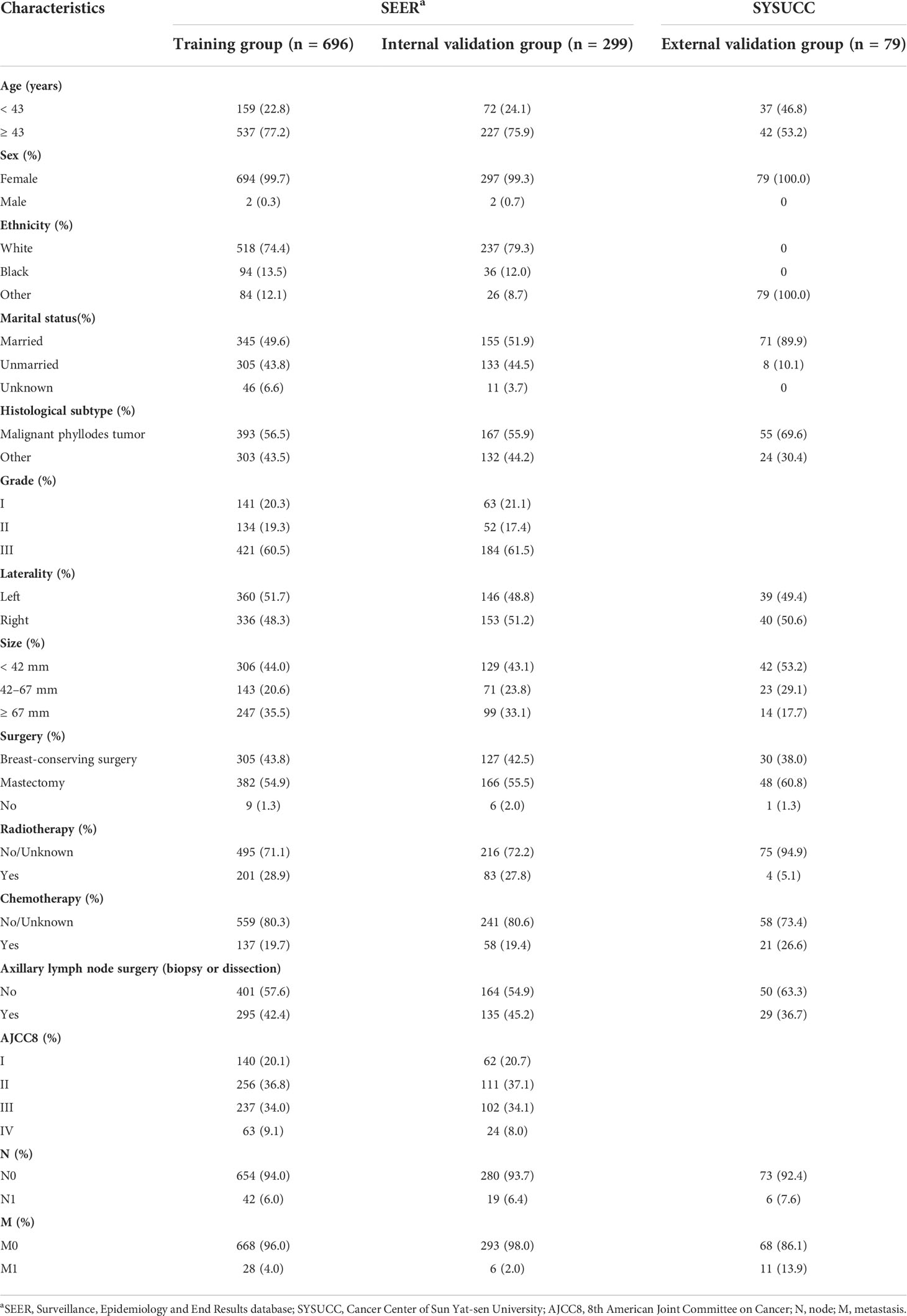
In the SEER database, after following strict inclusion and exclusion criteria, a total of 995 patients were included in the study and were randomized to the TG (n = 696) and IVG (n = 299). Cases from SYSUCC were classified as the EVG (n = 79). The median age at diagnosis of patients from the SEER database was 54 years old, (interquartile range (IQR), 43.5–64), the median tumor size was 4.6 cm (IQR 2.95–8), and the follow-up time was 81 months (range: 31–129 months). The median age at diagnosis, tumor size, and follow-up time of patients from the EVG were 43 years (37–53), 4 cm (3–7.5), and 84 months (34–128), respectively. The detailed demographic and disease characteristics of the patients are presented in Table 1.
Nomogram variable screening
Univariate and multivariate Cox proportional hazards regression analysis identified five independent prognostic factors for OS in the TG, comprising age at diagnosis, histological subtype, tumor size, surgery, and M stage. As calculated using stepwise regression, the five variables produced the smallest AIC value; therefore, there was no need to eliminate the variables. Full details are provided in Table 2.
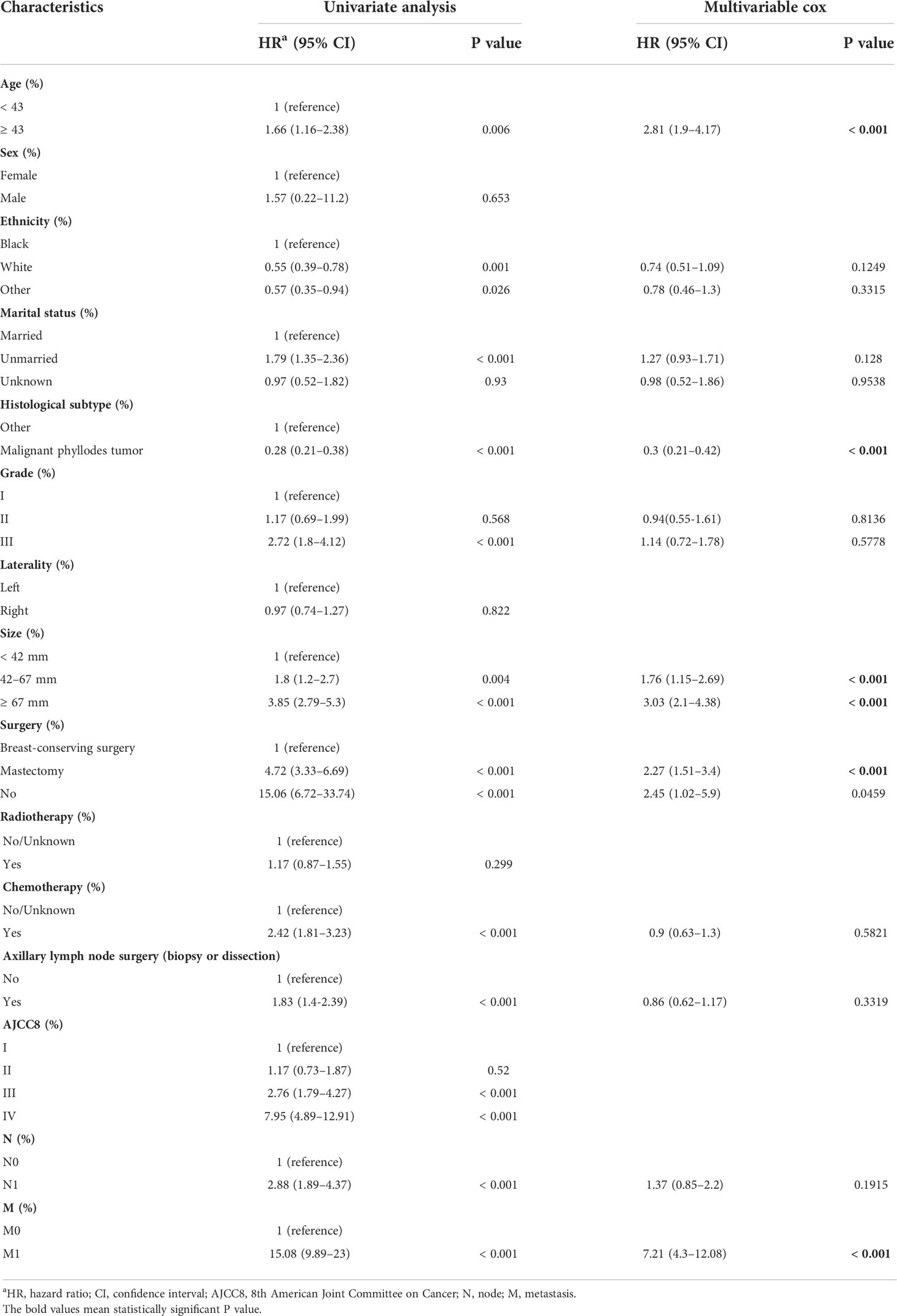
Table 2 Analysis of overall survival using univariate and multivariate Cox analysis in the training set.
Nomogram construction and validation
Based on the screening results above, we established a nomogram to predict 3-, 5-, and 10-year OS (Figure 1). Each predictor was assigned a score, age < 43 years = 0 points; age ≥ 43 years = 50; malignant phyllodes tumor = 0; other histological subtype = 61; tumor size < 4.2 cm = 0; 4.2 ≤ tumor size < 6.7 cm = 29; tumor size ≥ 6.7 cm = 59; breast-conserving surgery = 0; mastectomy = 40; no surgery = 50; M0 stage = 0; M1 stage = 100. The scores for each variable were added to obtain the 3-, 5-, and 10-year OS probability of a patient.
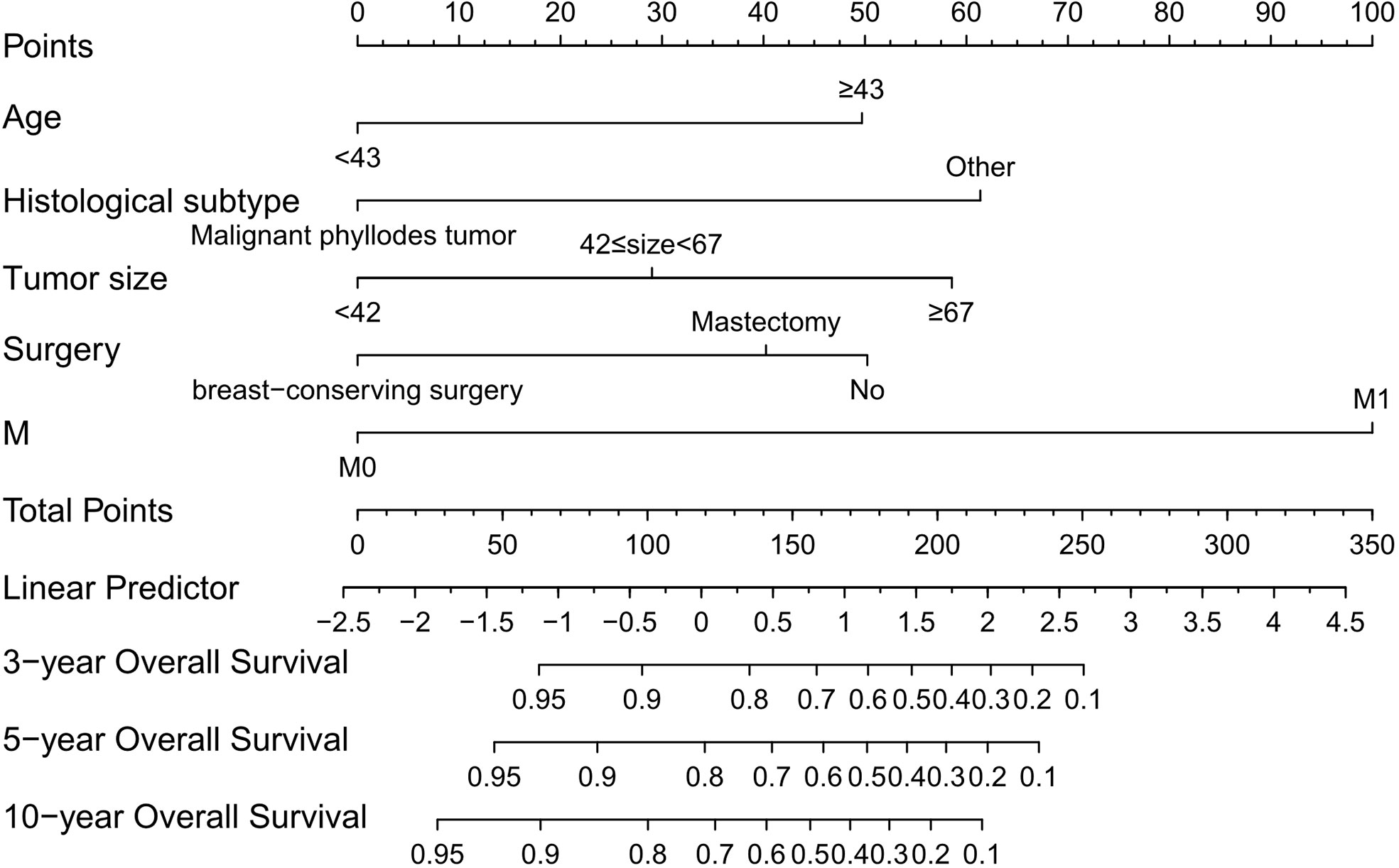
Figure 1 Nomogram that can predict the 3-, 5-, and 10-year overall survival of patients with primary breast sarcoma. M, metastasis.
The actual and predicted results showed good agreement based on calibration plots of the nomogram for 3-, 5-, and 10-year OS in the TG, IVG, and EVG (Figure 2).
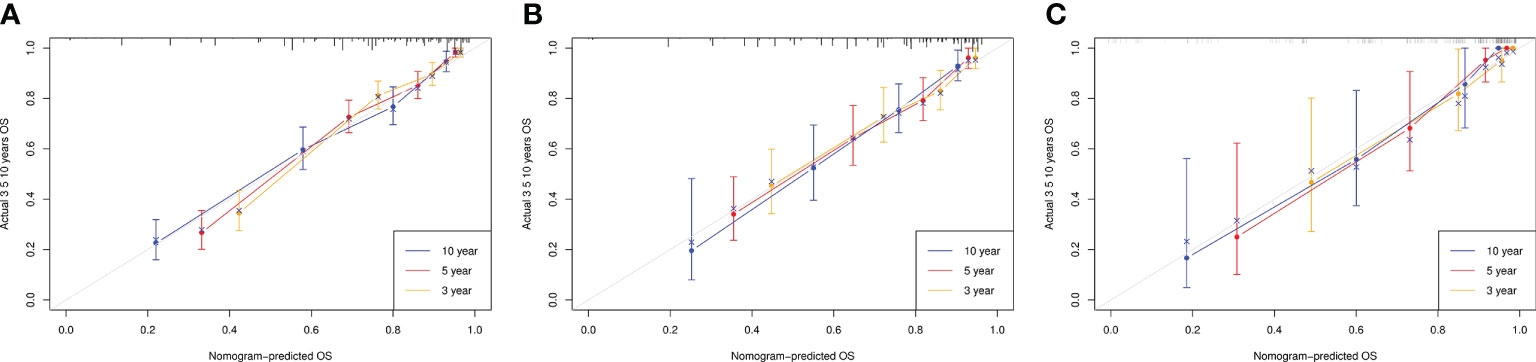
Figure 2 Calibration curves for overall survival (OS) at 3, 5, and 10 years in the training, internal-validation, and external-validation groups (A–C).
The C-indices to predict OS based on the nomogram were: TG = 0.804 (95% confidence interval (CI) 0.777–0.831) and IVG = 0.761 (95% CI 0.716–0.806), which were higher than those of the AJCC8 model: TG = 0.695 (95% CI 0.660–0.730), IVG = 0.637 (95% CI 0.5840.690), indicating the nomogram’s better discriminative ability. The results also showed an ideal predictive value in the EVG, with the C-index = 0.844 (95% CI 0.768–0.920). In addition, compared with the traditional AJCC8 staging model, the nomogram showed a better discrimination ability at different time points: the AUCs at 3, 5, and 10 years for the nomogram were 0.853, 0.855, and 0.827 in the TG; 0.776, 0.801, and 0.877 in the IVG. For the AJCC8 stage, the AUCs at 3, 5, and 10 years were 0.746, 0.723, and 0.675 in the TG; and 0.672, 0.655, and 0.673 in the IVG, respectively (Figure 3). These results suggested that the nomogram has a better power to predict OS than the AJCC8 stage. In addition, the AUCs at 3, 5, and 10 years for the nomogram to predict OS in the EVG were 0.863, 0.900, and 0.860, respectively (Figure 4), indicating that the nomogram model also has extremely high accuracy in practice (when AUC = [0.85, 0.95], the predicting effects are ideal).
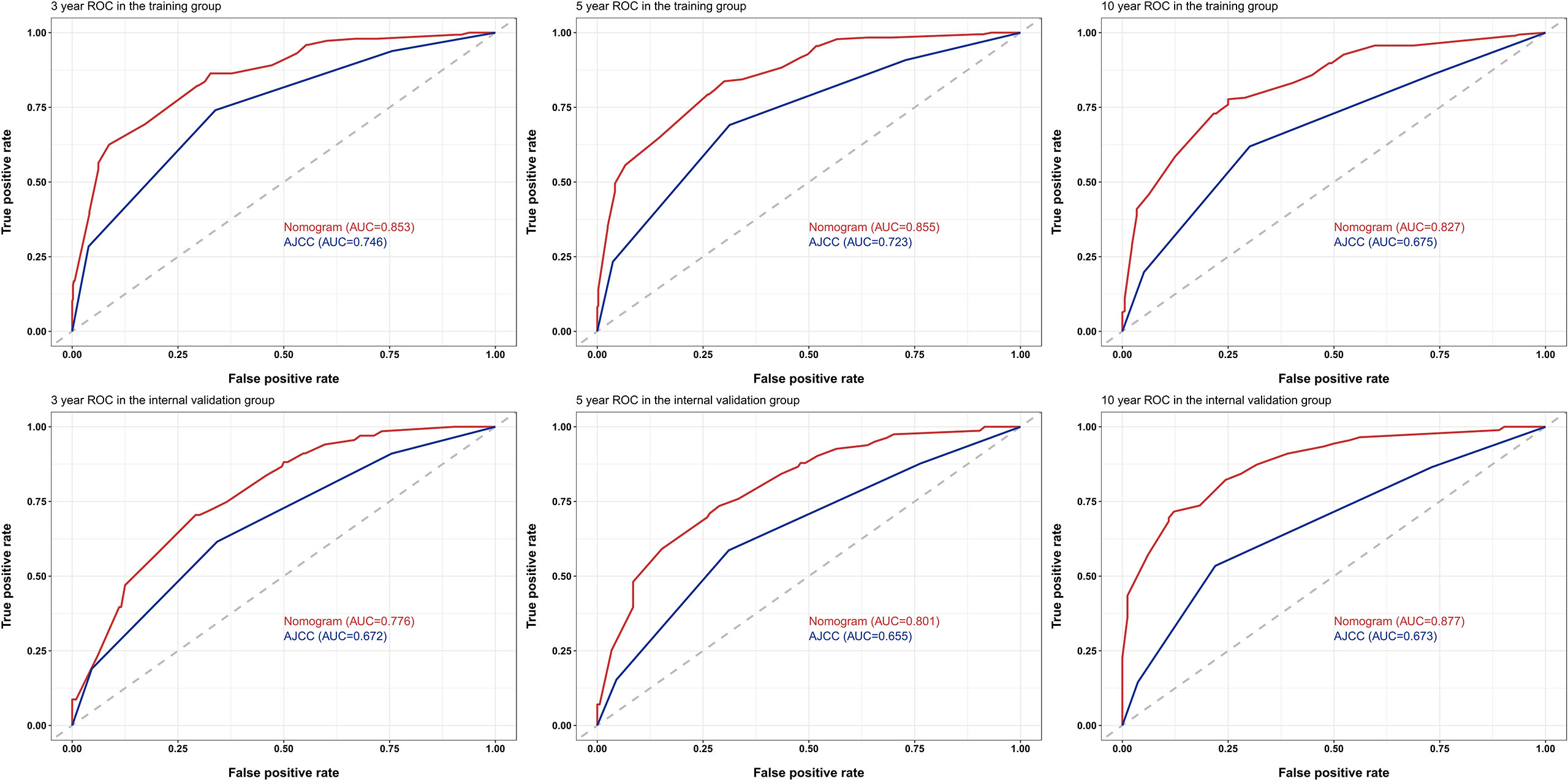
Figure 3 Receiver operating characteristic (ROC) curves for survival at 3, 5, and 10 years using the nomogram compared with AJCC8 data in the training and internal-validation groups. AUC, area under the curve; AJCC, American Joint Committee on Cancer.
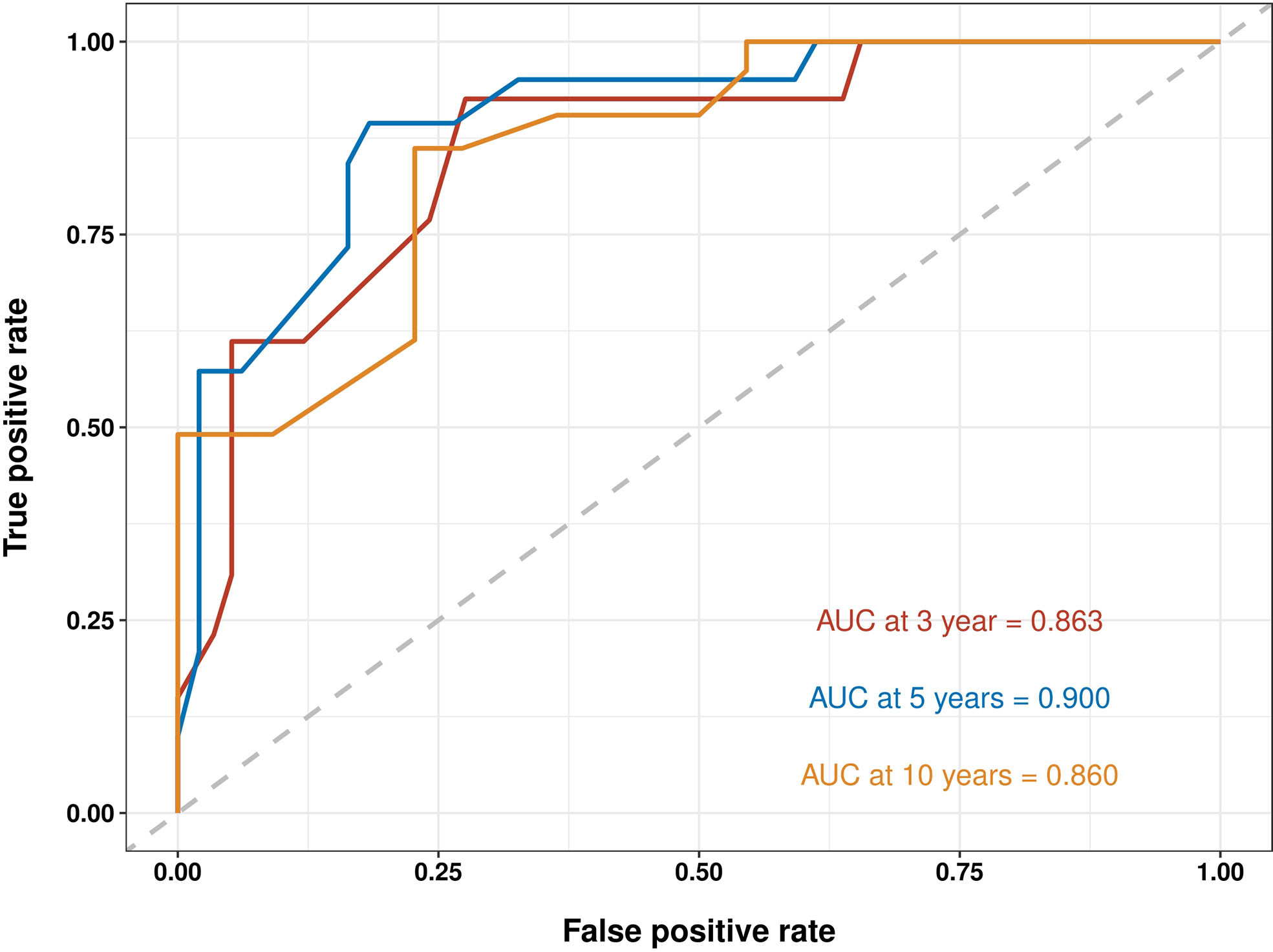
Figure 4 Receiver operating characteristic (ROC) curves for survival at 3, 5, and 10 years using nomogram in the external-validation group. AUC, area under the curve.
The benefits and clinical utility and benefits of the nomogram and AJCC8 were compared using DCA, and the areas under the DCA of the nomogram at 3, 5, and 10 years were larger than those of the AJCC8 staging, indicating that the nomogram can obtain a higher net benefit than the AJCC8 model (Figure 5).
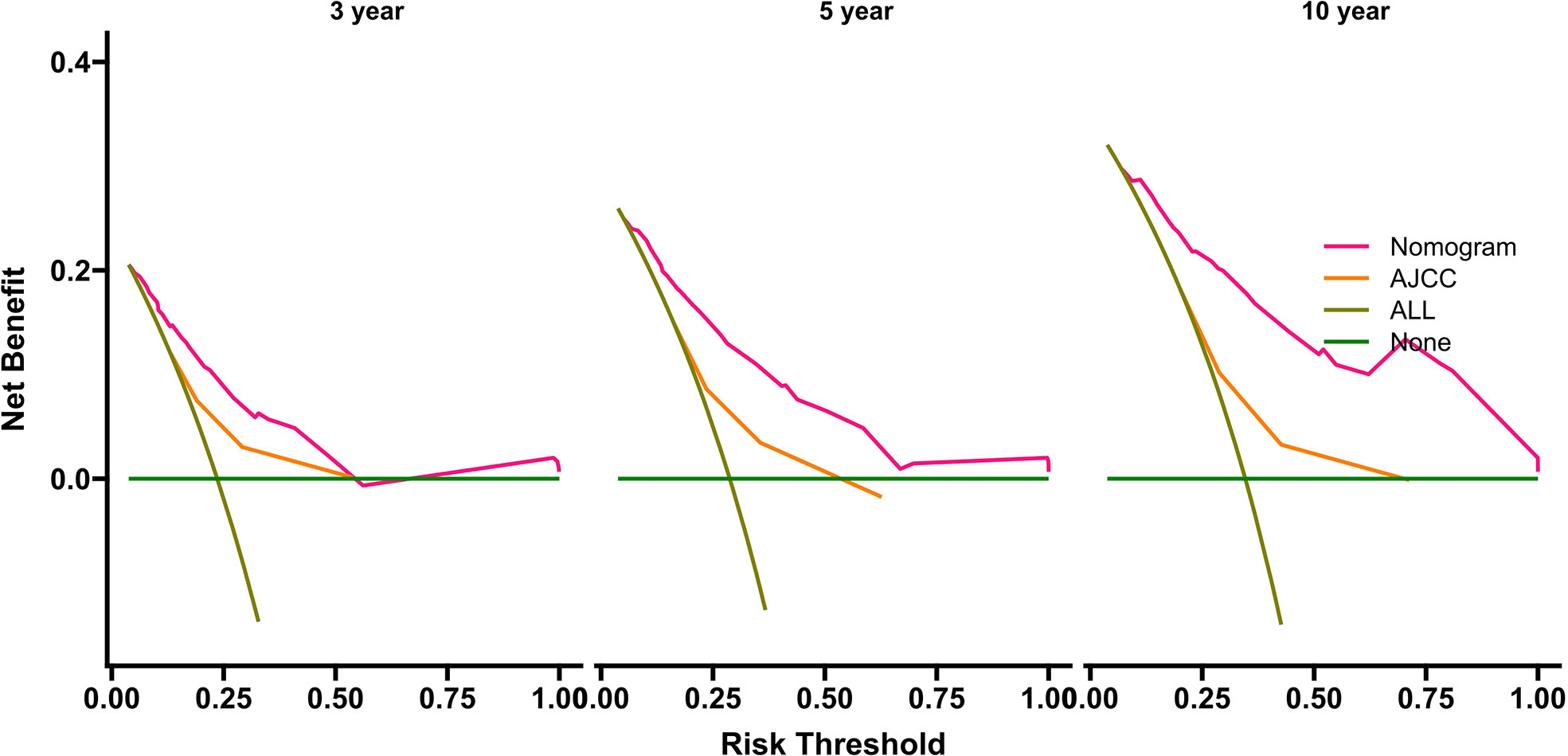
Figure 5 Decision curve analyses for survival at 3, 5, and 10 years using the nomogram compared with AJCC data in the internal-validation group. AJCC, American Joint Committee on Cancer.
Nomogram-based risk stratification
The total score calculated by the nomogram was used for risk stratification: the best cut-off value was obtained by calculating the total score of all patients using the X-tile software. A patient’s probability of OS could be divided into three nomogram-based risk groups: 0 ≤ low risk < 130; 130 ≤ medium risk < 180; and high-risk ≥ 280. Kaplan–Meier survival curves for the three nomogram-based risk groups were then drawn. The new risk model displayed significant stratification power (p<0.001). To further validate the risk stratification ability of the nomogram, Kaplan–Meier survival curves for risk stratification in the IVG and EVG were drawn, and good results were obtained (p > 0.001) (see Figure 6 and Table 3 for details.)
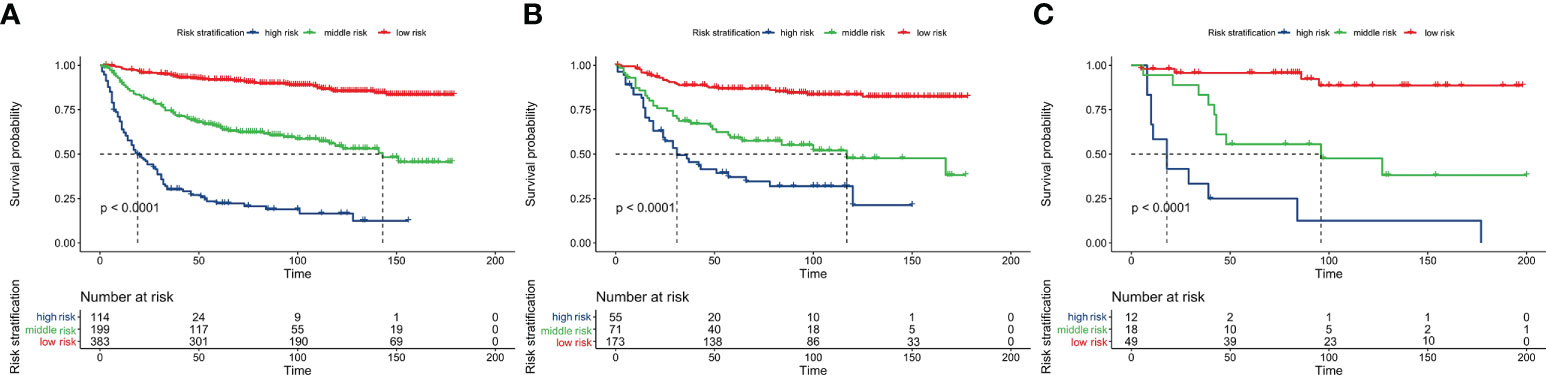
Figure 6 Overall survival analysis using Kaplan-Meier curves from the nomogram-based risk stratification in the training, internal-validation, and external-validation groups (A–C).
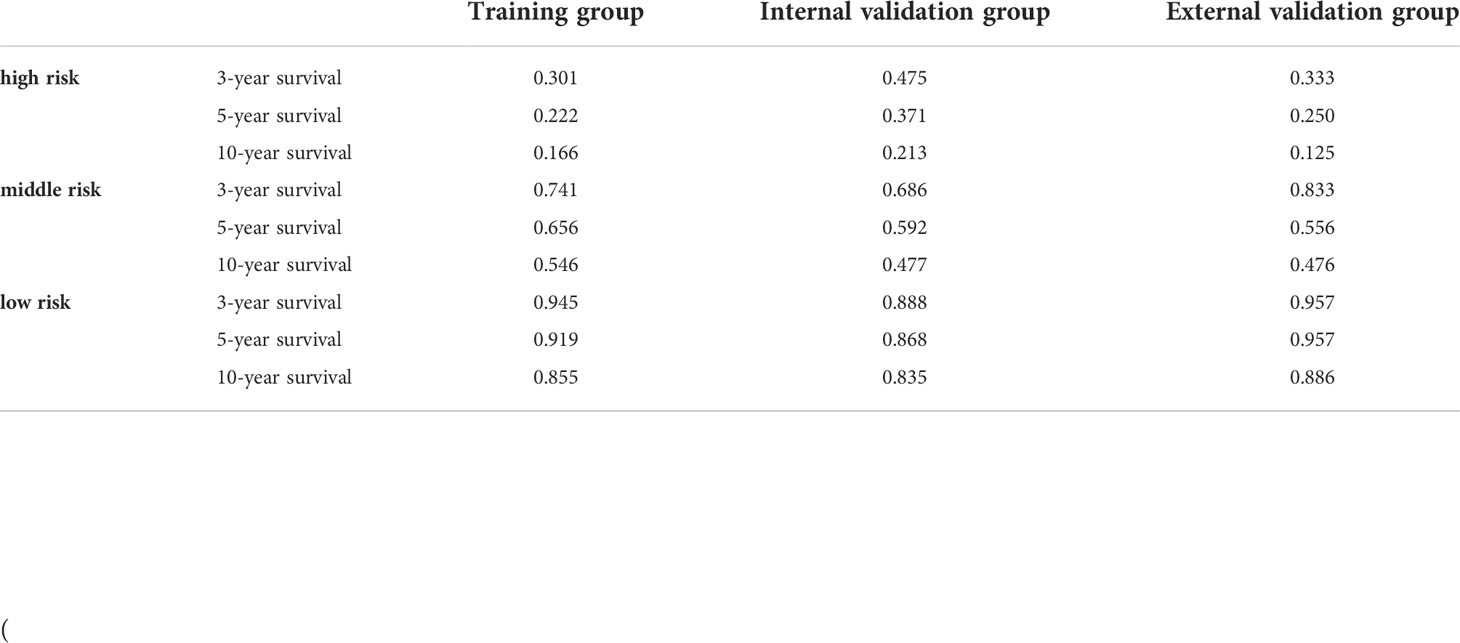
Table 3 3-, 5-, and 10-year overall survival in high-, intermediate-, and low-risk groups for each cohort.
Discussion
Primary breast sarcoma is rare and has diverse histological differentiation; therefore, it is difficult to evaluate its prognosis. Currently, the principles of its diagnosis, treatment, and prognosis evaluation of malignancy mainly refer to soft tissue sarcoma occurring elsewhere in the body. Therefore, a reliable method to predict the prognosis of patients with primary BS and treatment guidance is urgently required. Currently, the most widely used tool to assess prognosis is the AJCC8 staging system; however, it lacks information on important predictors such as demographics and treatment. Nomograms are currently considered to be more scientific tools to predict prognosis; therefore, we constructed a nomogram to solve this clinical problem. To the best of our knowledge, this is the first study to construct a nomogram to predict the OS of patients with primary BS based on the big data SEER cohort, including validation of the accuracy of the model using retrospective data from an external single-center institution.
By analyzing variables such as demographics, clinicopathology, and treatment regimens, five variables were screened as predictors of OS, and the C-index, AUC, and DCA values of the nomogram were higher than those of the AJCC8 system, suggesting its superior discrimination ability and clinical significance. When using the nomogram to calculate the OS of EVG, it also showed a high C-index and AUC, demonstrating that the nomogram has high accuracy and practicability in predicting prognosis, and can help clinicians to personalize the evaluation of patient prognosis. Interestingly, over time, the AUC value and net benefit in the DCA of the nomogram increased compared with those of the AJCC8 system, suggesting the nomogram might useful to predict long-term survival. In addition, this study also calculated the total score of the five variables using the OS nomogram, used X-tile to calculate the best cut-off value, and then divided the patients’ probability of OS into three risk groups: high, medium, and low. Since high-risk patients may be at increased risk of tumor-related mortality, intense systemic therapy might be the preferred strategy for high-risk patients. This would aid clinicians to provide patients with personalized diagnosis and treatment methods and follow-up measures, which could avoid under- or over-treatment for patients with BS.
As with breast carcinoma of epithelial origin, the vast majority of patients with BS are female (9); however, patients with BS are generally younger (median 54 years old in SEER) and have larger tumors (median 4.6 cm in SEER) (10–12), and neither marital status nor ethnicity were independent prognostic factors (9, 13). Our study showed that age, tumor size, and metastasis are risk factors for OS in patients with BS, which is similar to other studies of sarcoma (12, 14–17). In addition, in this study, histological grade, although statistically significant in the univariate Cox analysis, was excluded in the multivariate Cox regression analysis (p ≥ 0.05), which was different from other studies (2, 12, 18, 19). Similarly, most previous studies reported that malignant phyllodes tumor (MPT) accounted for the most cases in BS, and in this study, MPT comprised 55.6% of cases in the TG and 69.6% in the EVG; angiosarcoma was the highest histological subtype after MPT (9% in the SEER database) (1, 20).
Although nearly half of the patients underwent axillary lymph node biopsy or dissection (43.2% in the SEER database and 36.7% in the EVG), our study showed that lymph node involvement was not an independent prognostic factor for OS, possibly because BS mainly metastasizes through blood, but rarely through lymph nodes. While most of the lymphadenopathy in clinical practice is reactive, lymph node dissection does not bring survival benefits to patients with BS (1, 11, 12, 21). Interestingly, although mastectomy is widely regarded as the gold standard for BS treatment (11), our study showed that patients receiving mastectomy did not have a better prognosis than those receiving conservative surgery, which was consistent with recent reports (1, 10, 14, 22). Therefore, more prospective studies are needed to explore whether mastectomy can provide additional benefits for patients with BS Studies suggest that adequate resection is an important factor for the long-term survival of patients, rather than the surgical method (1, 2, 23–26). In addition, like most studies (1, 27, 28), this study suggested that neither radiotherapy nor chemotherapy, or other adjuvant treatments, could significantly improve the OS of the patients; however, a study by Johnstone et al. showed that postoperative radiotherapy can improve the tumor specific survival of patients (20). Therefore, adjuvant radiotherapy is recommended for patients with BS with a large tumor volume or a particularly aggressive subtype (19, 29). Given the divergence in adjuvant therapy for BS, more prospective studies are needed to explore whether adjuvant therapy can improve patient outcomes.
The following limitations of the study need to be pointed out. First, although we randomly divided the SEER data into the TG and IVG and used an external data cohort as the EVG for validation, larger multicenter data are needed to confirm the results of the study. Second, some the SEER database lacked potentially important parameters and specific information associated with prognosis, including chemotherapy, radiotherapy, vascular invasion, and details of the surgical margin status. These important variables need to be considered in future studies. Furthermore, although research has demonstrated that histological grade is not an independent risk factor for the construction of nomograms, this information is lacking in the EVG patient data. In addition, the study was retrospective in nature and was thus inevitably biased.
Conclusion
In this study, we constructed and validated a nomogram to predict OS in patients with BS, the reliability and clinical applicability of which were higher than those of the currently used AJCC8 staging system. We also developed a universal risk stratification model for clinical use, which provides clinicians with an accurate prognostic evaluation tool and will help to formulate the best individualized treatment strategy for patients with BS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the hospital ethics committee at Sun Yat-sen University Cancer Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YC and PZ designed the study. YH and JS drafted the manuscript. RT, FC, and LZ analyzed the data. S-GW and ZH were responsible for the critical revision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81872459), the Natural Science Foundation of Guangdong Province (No. 2018A030313666) and the Guangdong Medical Science and Technology Research Fund A2020516.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Confavreux C, Lurkin A, Mitton N, Blondet R, Saba C, Ranchère D, et al. Sarcomas and malignant phyllodes tumours of the breast – a retrospective study. Eur J Cancer (2006) 42:2715–21. doi: 10.1016/j.ejca.2006.05.040
2. Lim SZ, Ong KW, Tan BKT, Selvarajan S, Tan PH. Sarcoma of the breast: an update on a rare entity. J Clin Pathol (2016) 69:373–81. doi: 10.1136/jclinpath-2015-203545
3. Cates JMM. The AJCC 8th edition staging system for soft tissue sarcoma of the extremities or trunk: A cohort study of the SEER database. J Natl Compr Cancer Netw (2018) 16:144–52. doi: 10.6004/jnccn.2017.7042
4. Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun (2020) 40:301–12. doi: 10.1002/cac2.12067
5. Fakhry C, Zhang Q, Nguyen-Tân PF, Rosenthal DI, Weber RS, Lambert L, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. JCO (2017) 35:4057–65. doi: 10.1200/JCO.2016.72.0748
6. Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: Results from combined analysis of national surgical adjuvant breast and bowel project b-18 and b-27. JCO (2012) 30:3960–6. doi: 10.1200/JCO.2011.40.8369
7. Qiu J-J, Sun S-G, Tang X-Y, Lin Y-Y, Hua K-Q. Extracellular vesicular Wnt7b mediates HPV E6-induced cervical cancer angiogenesis by activating the β-catenin signaling pathway. J Exp Clin Cancer Res (2020) 39:260. doi: 10.1186/s13046-020-01745-1
8. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: More than meets the eye. Lancet Oncol (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
9. Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJL, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet (2011) 378:1461–84. doi: 10.1016/S0140-6736(11)61351-2
10. Fields RC, Aft RL, Gillanders WE, Eberlein TJ, Margenthaler JA. Treatment and outcomes of patients with primary breast sarcoma. Am J Surg (2008) 196:559–61. doi: 10.1016/j.amjsurg.2008.06.010
11. Al-Benna S, Poggemann K, Steinau H-U, Steinstraesser L. Diagnosis and management of primary breast sarcoma. Breast Cancer Res Treat (2010) 122:619–26. doi: 10.1007/s10549-010-0915-y
12. Bousquet G, Confavreux C, Magné N, de Lara CT, Poortmans P, Senkus E, et al. Outcome and prognostic factors in breast sarcoma: A multicenter study from the rare cancer network. Radiother Oncol (2007) 85:355–61. doi: 10.1016/j.radonc.2007.10.015
13. DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state: Breast cancer statistics, 2017. CA: A Cancer J Clin (2017) 67:439–48. doi: 10.3322/caac.21412
14. Yin M, Mackley HB, Drabick JJ, Harvey HA. Primary female breast sarcoma: clinicopathological features, treatment and prognosis. Sci Rep (2016) 6:31497. doi: 10.1038/srep31497
15. Xiao Y, Jiang Y, Xiong Y, Ruan S, Huang T. Pediatric malignant phyllodes tumors of the breast: Characteristics and outcomes based on the surveillance epidemiology and end results database. J Surg Res (2020) 249:205–15. doi: 10.1016/j.jss.2019.12.031
16. Li Y, Song Y, Lang R, Shi L, Gao S, Liu H, et al. Retrospective study of malignant phyllodes tumors of the breast: Younger age, prior fibroadenoma surgery, malignant heterologous elements and surgical margins may predict recurrence. Breast (2021) 57:62–70. doi: 10.1016/j.breast.2021.03.001
17. Pastorelli F, Di Silvestre M, Plasmati R, Michelucci R, Greggi T, Morigi A, et al. The prevention of neural complications in the surgical treatment of scoliosis: The role of the neurophysiological intraoperative monitoring. Eur Spine J (2011) 20 Suppl 1:S105–114. doi: 10.1007/s00586-011-1756-z
18. Voutsadakis IA, Zaman K, Leyvraz S. Breast sarcomas: Current and future perspectives. Breast (2011) 20:199–204. doi: 10.1016/j.breast.2011.02.016
19. Zelek L, Llombart-Cussac A, Terrier P, Pivot X, Guinebretiere JM, Le Pechoux C, et al. Prognostic factors in primary breast sarcomas: A series of patients with long-term follow-up. J Clin Oncol (2003) 21:2583–8. doi: 10.1200/JCO.2003.06.080
20. Li GZ, Raut CP, Hunt KK, Feng M, Chugh R. Breast sarcomas, phyllodes tumors, and desmoid tumors: Epidemiology, diagnosis, staging, and histology-specific management considerations. Am Soc Clin Oncol Educ Book (2021), 41:390–404. doi: 10.1200/EDBK_321341
21. Adem C, Reynolds C, Ingle JN, Nascimento AG. Primary breast sarcoma: clinicopathologic series from the Mayo clinic and review of the literature. Br J Cancer (2004) 91:237–41. doi: 10.1038/sj.bjc.6601920
22. Pasta V, Monti M, Cialini M, Vergine M, Urciuoli P, Iacovelli A, et al. Primitive sarcoma of the breast: New insight on the proper surgical management. J Exp Clin Cancer Res (2015) 34:72. doi: 10.1186/s13046-015-0190-1
23. Trovik CS, Bauer HCF, AlvegaÊ TA, Anderson H, Blomqvist C, Berlin O, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian sarcoma group register. Eur J Cancer (2000) 36(6):710–6. doi: 10.1016/s0959-8049(99)00287-7
24. Duncan MA, Lautner MA. Sarcomas of the breast. Surg Clinics North America (2018) 98:869–76. doi: 10.1016/j.suc.2018.03.013
25. Zhou Z-R, Wang C-C, Sun X-J, Yang Z-Z, Chen X-X, Shao Z-M, et al. Prognostic factors in breast phyllodes tumors: A nomogram based on a retrospective cohort study of 404 patients. Cancer Med (2018) 7:1030–42. doi: 10.1002/cam4.1327
26. Byerly S, Chopra S, Nassif NA, Chen P, Sener SF, Eisenberg BL, et al. The role of margins in extremity soft tissue sarcoma: Margins in extremity soft tissue sarcoma. J Surg Oncol (2016) 113:333–8. doi: 10.1002/jso.24112
27. Zhao W, Tian Q, Zhao A, Wang B, Yang J, Wang L, et al. The role of adjuvant radiotherapy in patients with malignant phyllodes tumor of the breast: a propensity-score matching analysis. Breast Cancer (2021) 28:110–8. doi: 10.1007/s12282-020-01135-7
28. Blanchard DK, Reynolds CA, Grant CS, Donohue JH. Primary nonphylloides breast sarcomas. Am J Surg (2003) 186:359–61. doi: 10.1016/S0002-9610(03)00269-1
Keywords: breast neoplasms, nomogram, sarcoma, prognosis, survival rate
Citation: Cheng Y, Zhang P, Huang Y, Tang R, Zhang L, Sun J, Chi F, Wu S-G and He Z (2022) Construction and validation of a nomogram to predict overall survival in patients with breast sarcoma. Front. Oncol. 12:899018. doi: 10.3389/fonc.2022.899018
Received: 07 April 2022; Accepted: 22 September 2022;
Published: 06 October 2022.
Edited by:
Xiaosong Chen, Shanghai Jiao Tong University, ChinaReviewed by:
Lu Cao, Shanghai Jiao Tong University, ChinaShiva Basnet, Southern University of Science and Technology, China
Copyright © 2022 Cheng, Zhang, Huang, Tang, Zhang, Sun, Chi, Wu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenyu He, aGV6aHlAc3lzdWNjLm9yZy5jbg==; San-Gang Wu, d3VzZ0B4bXUuZWR1LmNu
†These authors have contributed equally to this work
 Yixin Cheng
Yixin Cheng Pengkun Zhang1†
Pengkun Zhang1† Lei Zhang
Lei Zhang Jiayuan Sun
Jiayuan Sun Zhenyu He
Zhenyu He