- 1Post Graduation Program A.C. Camargo Cancer Center, São Paulo, Brazil
- 2Nucleus of Epidemiology and Statistics in Cancer, A. C. Camargo Cancer Center, São Paulo, Brazil
Objective: To analyze factors affecting 1-year overall survival and burden of gastric adenocarcinoma in a single-institution cohort.
Methods: A prospective cohort study of gastric adenocarcinoma patients from a cancer center in São Paulo, Brazil, was conducted between February 2016 and July 2019. Overall survival was analyzed at 12 months post-diagnosis using the Kaplan–Meier method. A log-rank test was applied to compare curves. Sociodemographic and clinicopathological features were assessed to detect prognostic factors using univariate and multivariable Cox regression analyses to calculate hazard ratio (HR) and its confidence intervals (CIs). Disability-adjusted life years (DALY) constituted the sum of years of life lost (YLL) plus years lived with disability (YLD). YLL represented the sum of years lost before the age of 76.6 years. YLD was calculated as the number of cases multiplied by the duration and burden of the disease. YLL per death was calculated as the mean YLL for each individual.
Results: Overall survival at 1-year follow-up was 80.8%. The multivariable model adjusted for age and sex identified cerebrovascular disease (HR 8.5, 95% CI 3.3–21.8), stage III/IV (HR 5.7, 95% CI 2.3–13.7), diabetes (HR 3.2, 95% CI 1.5–6.6), and<9 years of education (HR 2.9, 95% CI 1.5–5.8) as prognostic factors. Out of the 214 treated cases, there was 700.72 DALY during the first year, of which 90.55% corresponded to YLL and 9.45% to YLD. The average YLL per death was 15.48 and was higher among women (19.24 YLL per death).
Conclusion: At a single cancer center, 1-year overall survival probability was approximately 80% in patients with gastric adenocarcinoma. Patients with a higher risk of death had cerebrovascular disease, advanced clinical staging, diabetes, and/or lower educational level. Approximately 700 years of DALY was documented, with women having the highest YLL per death. Because this study was conducted at a single cancer center, the results might not be representative of a general population. To the best of our knowledge, this study was the first to assess gastric adenocarcinoma DALY, YLL, and YLL per death in the first year of follow-up in a hospital cohort in Brazil.
Introduction
In 2020, there were approximately 1.1 million new cases of gastric cancer and 769,000 related deaths globally (1). In Brazil, gastric cancer is the fourth and sixth most common type in men (13,360 new cases) and women (7,870 new cases), respectively. In 2019, gastric cancer was responsible for 7.9% of cancer deaths in men and 5% in women (2). Most cases of gastric cancer are adenocarcinomas (3, 4); the main risk factors of gastric adenocarcinoma are age >60 years (3, 5), male gender (5–7), low socioeconomic status, occupational exposure (high temperatures and dust, asbestos, X and gamma radiation, inhalation of hexavalent chromium, and inorganic lead compounds) (8), smoking, alcohol consumption, obesity, gastroesophageal reflux disease, Helicobacter pylori infection, high consumption of salt and nitrites, and low consumption of fresh fruit and vegetables (3, 4, 9). Prognostic factors include the presence of comorbidities (10, 11), smoking, alcohol consumption (12), clinical stage (13–15), and clinical and molecular characteristics of the tumors (15–17).
Gastric cancer survival remains low in most countries, with 5-year survival (2010–2014) rates of 20%–40% (18) and 1-year survival rates of 59.7% in the United States (19) and 57% in Denmark (20). Survival probability is even lower when based on the clinical stage. In the United States, for cases diagnosed at clinical stage I, overall survival is 80.6% in the first year, 64.9% at 3 years, and 56.7% at 5 years (15). For clinical stage IV cases, overall survival was 28.3% in the first year, 7.8% after 3 years, and 5.0% after 5 years (15). A single-institution experience in Brazil has overall survival, ranging from 87.3% to 76.5% in the first year of follow-up for early tumors (stages I and II) and falling to 27.2% in metastatic cases (stage IV) (21).
Gastric cancer has a major social and economic impact. In 2017, it accounted for 19.1 million disability-adjusted life years (DALY), of which 98% was due to years of life lost (YLL) and 2% was due to years lived with disability (YLD). This disease represents the third leading cause of DALY for cancer in men (5). In Brazil, the Global Burden of Disease Study estimated that gastric cancer was responsible for 261.54 DALY per 100,000 population, compared with 323.29 DALY per 100,000 in Colombia, 472.18 DALY per 100,000 in Chile, 107.75 DALY per 100,000 in South Africa, 118.23 DALY per 100,000 in the United States, and 165.60 DALY per 100,000 in Denmark (22).
In general, survival studies focus on elucidating the severity of disease and prognostic factors, whereas pooled analyses of DALY, YLL, YLD, and YLL per death provide information on the burden of disease. Analyzing these indicators together in a hospital cohort provides key insights into the burden and severity of gastric adenocarcinoma in populations with access to healthcare. This is important, as few studies have investigated this issue in Brazil. The main purpose of this study was to estimate overall survival from gastric adenocarcinoma at 1-year follow-up, along with prognostic factors. Within this framework, DALY, YLL, and YLL per death were evaluated in patients with gastric adenocarcinoma.
Materials and methods
Data source
This is a prospective cohort of cases in São Paulo (Brazil), which forms part of a larger multicenter case–control study. This study included 214 patients recruited from A. C. Camargo Cancer Center in São Paulo, Brazil, between February 2016 and July 2019. São Paulo is the largest city in Brazil and is one of the most populous areas in Latin America, with 12,396,372 inhabitants, and it had a human development index of 0.805 in 2010 (23). The study data were drawn from the module “Epidemiology of Gastric Adenocarcinomas in Brazil”, which is part of the project “Epidemiology and Genomics of Gastric Adenocarcinomas in Brazil”. A. C. Camargo Cancer Center was founded 69 years ago and is a referral hospital for cancer treatment in Brazil and South America, serving patients from both public and private or health insurance. The cancer center runs a post-doctoral training program and oncologic training in surgery, radiotherapy, chemotherapy, and clinical trials. The center also conducts translational research to support cancer patients (24).
Patients
Cases were interviewed by a trained nurse or nutritionist. All patients completed an epidemiological questionnaire to collect sociodemographic, lifestyle, and clinical information. Study data were stored on the REDCap platform hosted by the A. C. Camargo Cancer Center (25, 26). The inclusion criteria were cases with gastric adenocarcinoma (ICD O3 C16, M-8140/3) (27) confirmed by histology without previous cancer diagnoses, except for non-melanoma skin cancer and patients aged 18–75 years. The exclusion criterion was patients with physical or psychological limitations in answering the questionnaire.
Vital status was recorded through passive and active follow-up. Passive follow-up involved using the most recent information available from medical records (day, month, and year). Active follow-up was obtained from the Regional Electoral Court from the death certification registry held by the state and federal governments for all patients. Death was coded as death from cancer, death by other causes, or lost to follow-up when the sources searched yielded no information. There were no losses in the first year of follow-up in this study.
Variables
The assessed variables were gender (female and male), ethnicity (white and non-white, with the latter being composed of black, brown, and yellow ethnicity), education (grouped into<9 years corresponding to illiterate and elementary and >10 years—high school and university), access to the public health service (Brazilian national health service—SUS) or private or health insurance, tumor location (cardia or non-cardia), and clinical stage (categorized into three groups: I/II, III, and IV). These three stage groupings were used to analyze the YLL due to death and were grouped into I/II and III/IV in the survival analysis.
The parameters assessed in the survival analysis were age (<60 and ≥60 years), marital status (married or unmarried, with the latter including single, widowed, and divorced), tobacco consumption (smokers/ex-smokers and non-smokers), alcohol consumption (no or yes), body mass index (BMI) (classified as underweight [<18.5 kg/m2], normal weight [18.5–24.99 kg/m2], overweight [25–29.99 kg/m2], and obese [>30 kg/m2]) (28), Helicobacter pylori infection (negative or positive), Lauren’s histological type (intestinal, diffuse, mixed, or not otherwise specified (NOS) adenocarcinoma) (29), histological grade (well differentiated, moderately differentiated, poorly differentiated, undifferentiated, or information not available), human epidermal growth factor receptor 2 (HER2) (negative for results 0 and 1+, doubtful for 2+, and positive for 3+) (30), treatment (yes or no), and presence of comorbidities before cancer diagnosis (using the Charlson Comorbidity Index [CCI]). This index was grouped into three categories based on scores (0, 1 or 2, and ≥3), where the criteria “any tumor” (2 points) and “metastatic solid tumor” (6 points) were not included in the sum (31).
Statistical analysis
Descriptive data analysis was performed using absolute and relative frequencies. For group comparisons, Pearson’s chi-square test or Fisher’s exact test was used, as appropriate.
Overall survival was defined as the interval in months between the date of diagnosis and the date of final information or death from any cause. The Kaplan–Meier product-limit estimator and log-rank test were applied to evaluate curves. Cox semiparametric proportional hazard model was used to assess the prognostic potential of each variable and to calculate hazard ratio (HR) and its 95% confidence interval (95% CI). The proportional hazards assumption was based on Schoenfeld residuals. Variables with p< 0.20 were selected for multivariable analysis, in which those with p ≤ 0.05 and those that contributed to the goodness of fit of the model were retained. For epidemiological criteria, age and sex were retained to adjust the model.
Disability-adjusted life years (DALY) is a summary measure that combines YLL and YLD (32, 33). YLL is the sum of years of life lost for each individual. It is obtained by subtracting the age at death from life expectancy and was used for 2019 in the state of São Paulo (76.6 years) (34) with the following formula (1) (33, 35):
The calculation of YLD was performed by multiplying the number of cases (n) by the mean duration of the disease (t) and the weight factor that reflects the severity of the disease (pd), with the following formula (2) (32, 33, 35):
Disease duration for individuals who survived the first year of follow-up corresponded to 1 year (study period), and the burden of the disease was 0.288 for cases of localized gastric adenocarcinoma at diagnosis and 0.451 for metastatic cases (32, 33).
Years of life lost per individual (YLL per death) was calculated by dividing YLL by the total number of deaths for each variable. Survival analyses, as well as graphs and images, were implemented in RStudio version 1.3.959. DALY, YLL, and YLD calculations were performed using the statistical program SPSS version 22.0. Excel was used to construct graphs.
Results
Of the initial 223 patients, nine were excluded. Of the nine excluded cases, one had no confirmation of adenocarcinoma in the histological analyses, seven had esophageal adenocarcinoma, and one asked to withdraw from the study, giving a final total of 214 cases that met the study criteria and were included (Figure 1). Of these 214 cases (epidemiological characteristics are described in Table 1), 63.1% (135/214) were male, 72.0% (154/214) had <9 years of education, mean age was 57.5 years (standard deviation 11.1), 58.4% (127/214) had comorbidities, and 10.3% (22/214) had a CCI score ≥3. The most frequent conditions were peripheral vascular disease (33.6%; 72/214) and diabetes (14.5%; 31/214). H. pylori infection was positive in 19.2% (41/187) of cases, while 47.2% (101/214) had diffuse Lauren’s subtype tumor, 67.8% (145/214) had non-cardia tumors, 55.6% (119/214) had clinical stage III/IV, and 97.2% (208/214) were treated in the institution (Table 1).
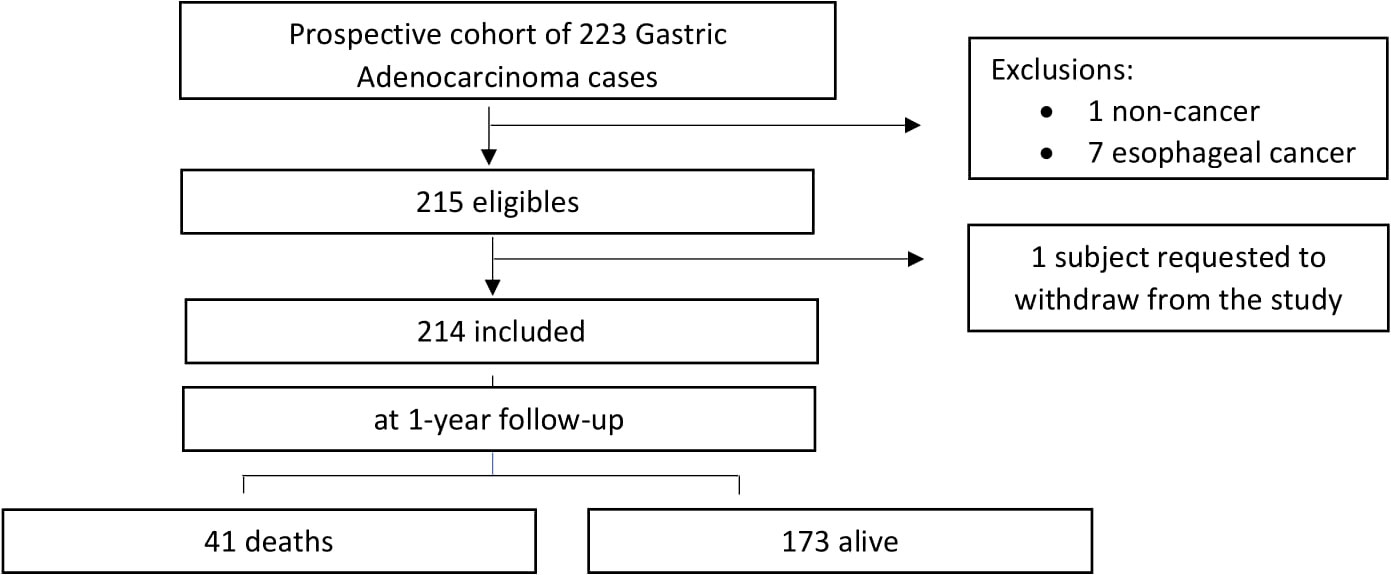
Figure 1 Summary of Gastric Adenocarcinoma cases status after one year follow-up and flowchart of exclusion criteria.
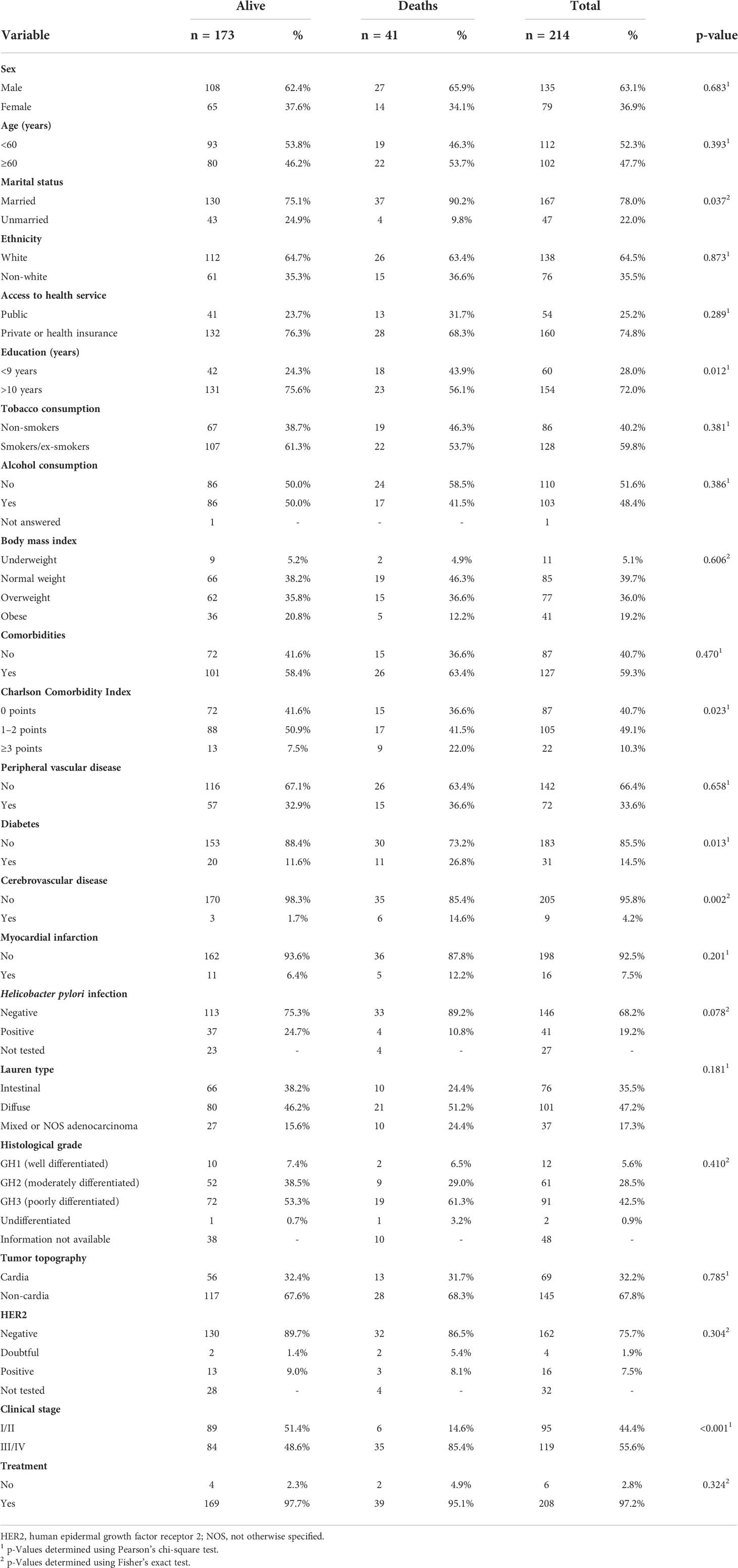
Table 1 Sociodemographic, lifestyle, and clinical characteristics of 214 patients with gastric adenocarcinoma according to vital status at 1-year follow-up.
At 1-year follow-up, 41 patients (19.2%) had died (Figure 1). Of these deaths, 65.9% (27/41) were male, 43.9% (18/41) had<9 years of education, and 46.3% (19/41) were <60 years. CCI score was ≥3 in 21.9% (9/41) of cases, and the most frequent comorbidities were peripheral vascular disease (36.6%; 15/41) and diabetes (26.8%; 11/41). H. pylori infection was positive in 10.8% (4/37) of cases, 65.9% (27/41) had non-cardia tumors, 51.2% (21/41) had the diffuse subtype, 86.5% (32/37) had negative HER2 receptor, 85.4% (35/41) were at clinical stage III/IV, and 95.1% (39/41) received treatment in the institution (Supplementary Table 1). Data comparing patients by vital status at 1-year follow-up revealed that deceased patients were more likely to be married (90.2% vs 75.1%, p = 0.037) and had lower education (<9 years; 43.9% vs 24.3%, p = 0.012), more comorbidities (CCI score ≥ 3; 22% vs 7.5%, p = 0.023), diabetes (26.8% vs 11.6%, p = 0.013), cerebrovascular disease (14.6% vs 1.7%, p = 0.002), and clinical stage III/IV (85.4% vs 48.6%, p< 0.001) (Table 1).
Overall survival was 80.8% (95% CI 75.7–86.3%). A significantly lower survival probability was observed in patients with <9 years of education (70.0%), CCI score ≥3 (59.1%), cerebrovascular disease (33.3%), diabetes (64.5%), and clinical stage III/IV (70.6%) (Figure 2; Table 2).
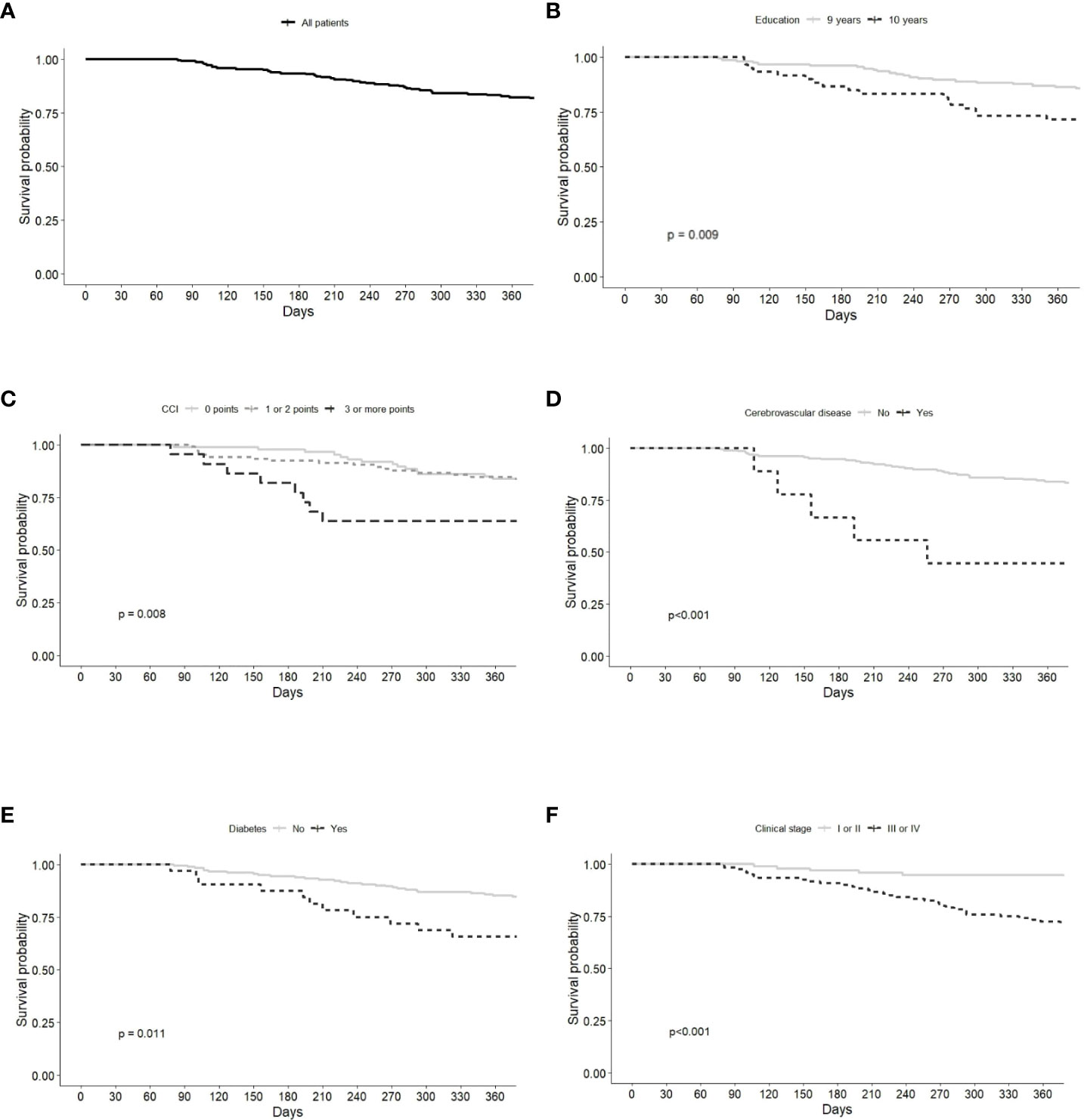
Figure 2 Kaplan-Meier survival curves of Gastric Adenocarcinoma patients grouped by (A) all patients, (B) education, (C) Charlson Comorbidity index (CCI), (D) cerebrovascular disease, (E) diabetes, (F) clinical stage.
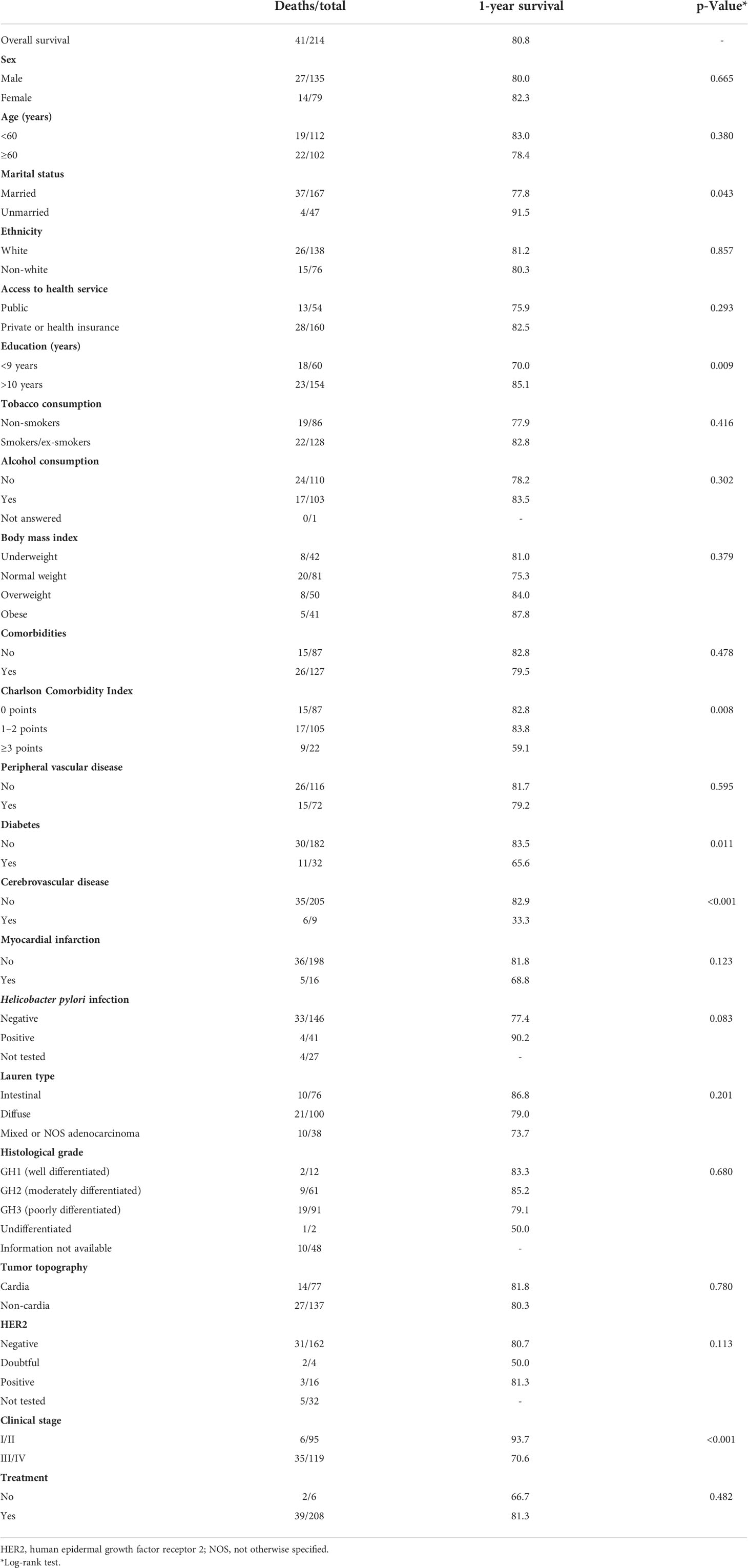
Table 2 One-year overall survival probability of 41 gastric adenocarcinoma patients based on Kaplan–Meier curves with log-rank test.
Cox univariate regression analyses showed that the risk of death clearly increased in groups with lower education, CCI score ≥3, cerebrovascular disease, diabetes, and clinical stage III/IV. After age and sex were adjusted in the multivariable model, prognostic factors were cerebrovascular disease (HR 8.54, 95% CI 3.35–21.8), stage III/IV (HR 5.67, 95% CI 2.34–13.72), diabetes (HR 3.18, 95% CI 1.53–6.6), and <9 years of education (HR 2.92, 95% CI 1.48–5.8) (Figure 3; Table 3).
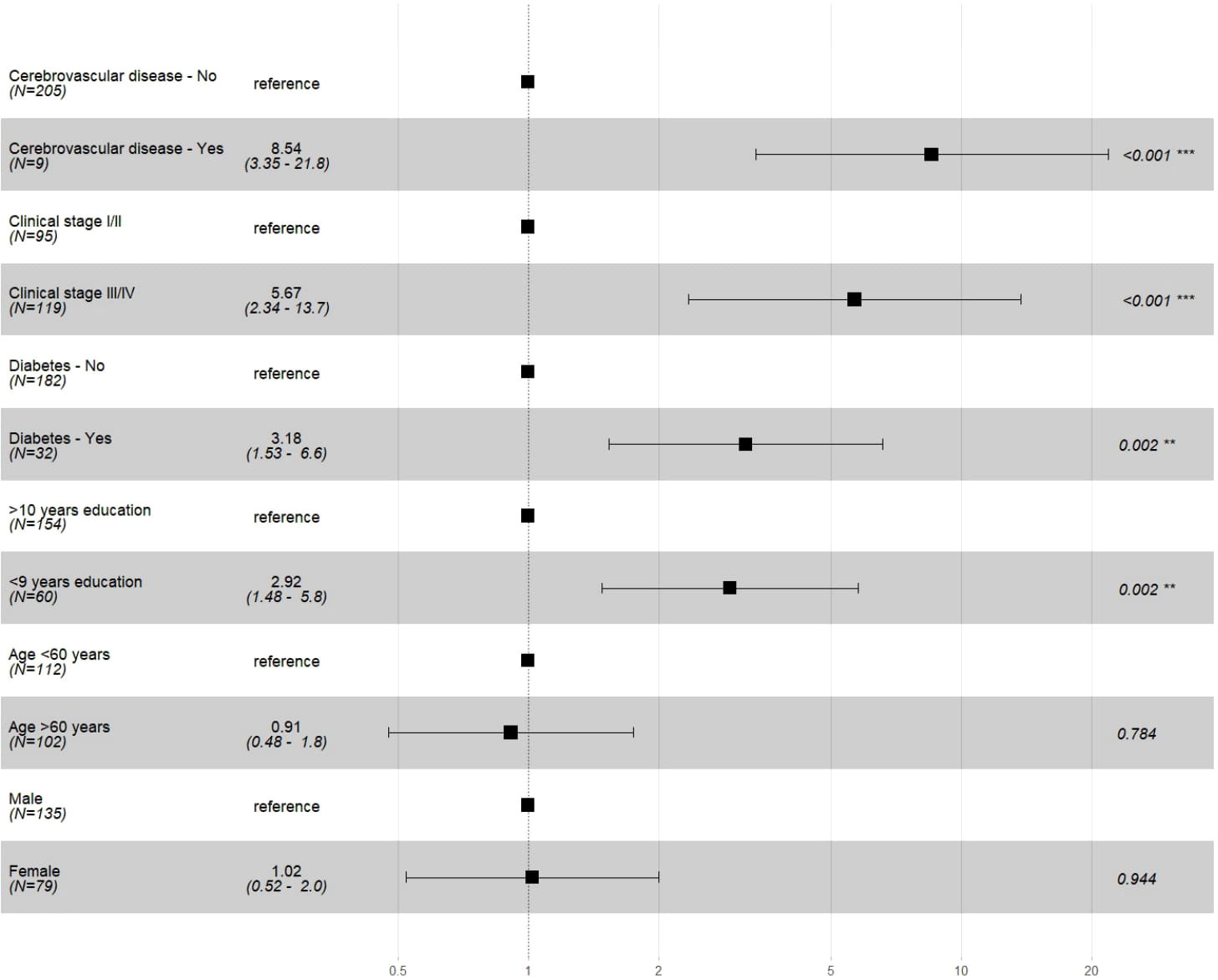
Figure 3 Multivariable Cox regression analysis of selected prognostic factors in Gastric Adenocarcinoma cases. * Schoenfeld risk proportionality test of the multiple model p=0.990; model adjusted for variables age and sex.
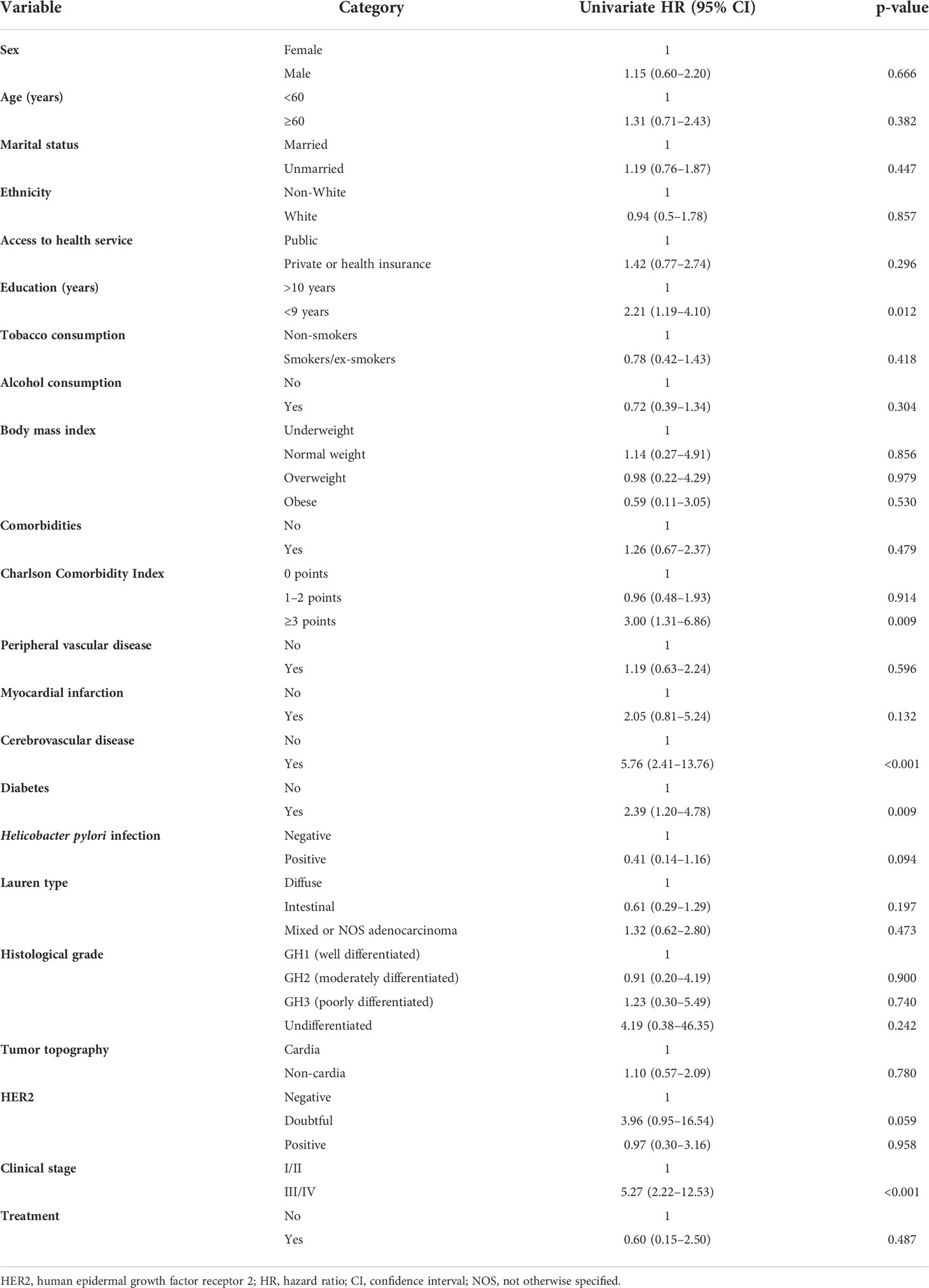
Table 3 Cox univariate regression analyses of 1-year overall survival prognostic factors of 41 patients with gastric adenocarcinoma.
Of the 214 participants in the study, at 1-year follow-up, there was 700.72 DALY, of which 634.51 (90.55%) corresponded to YLL and 66.21 (9.45%) to YLD. An average of 15.48 years was lost per death, and YLL per death was higher in individuals who were female (19.24 YLL per death), had >10 years of education (18.99 YLL per death), had clinical stage IV (17.44 YLL per death), and were non-white (16.68 YLL per death) (Figure 4).
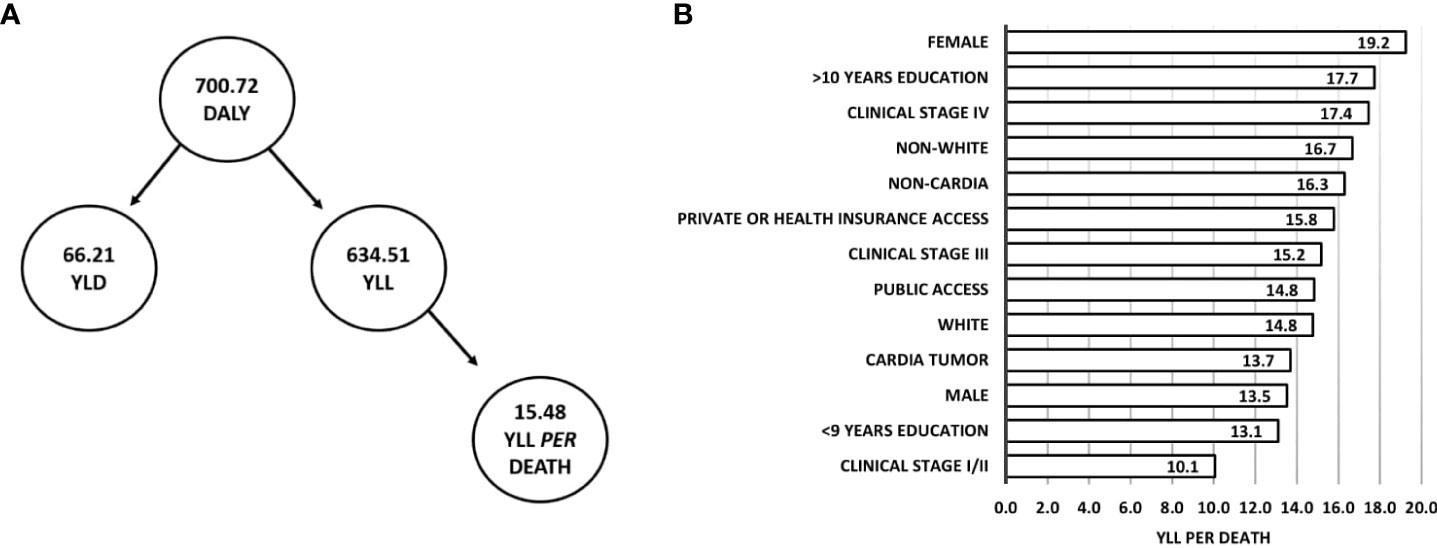
Figure 4 DALY (YLD plus YLL) and YLL per death at 1-year follow-up of Gastric Adenocarcinoma. (A) Flowchart of DALY composition and average YLL per death. (B) YLL per death according to clinical and sociodemographic characteristics.
Gender comparison showed that clinical stage III/IV was more frequent in women (92.9% vs 81.5%), whereas alcohol consumption (55.6% vs 14.3%) and tobacco consumption (70.4% vs 21.4%) were more frequent in men (Table 4).
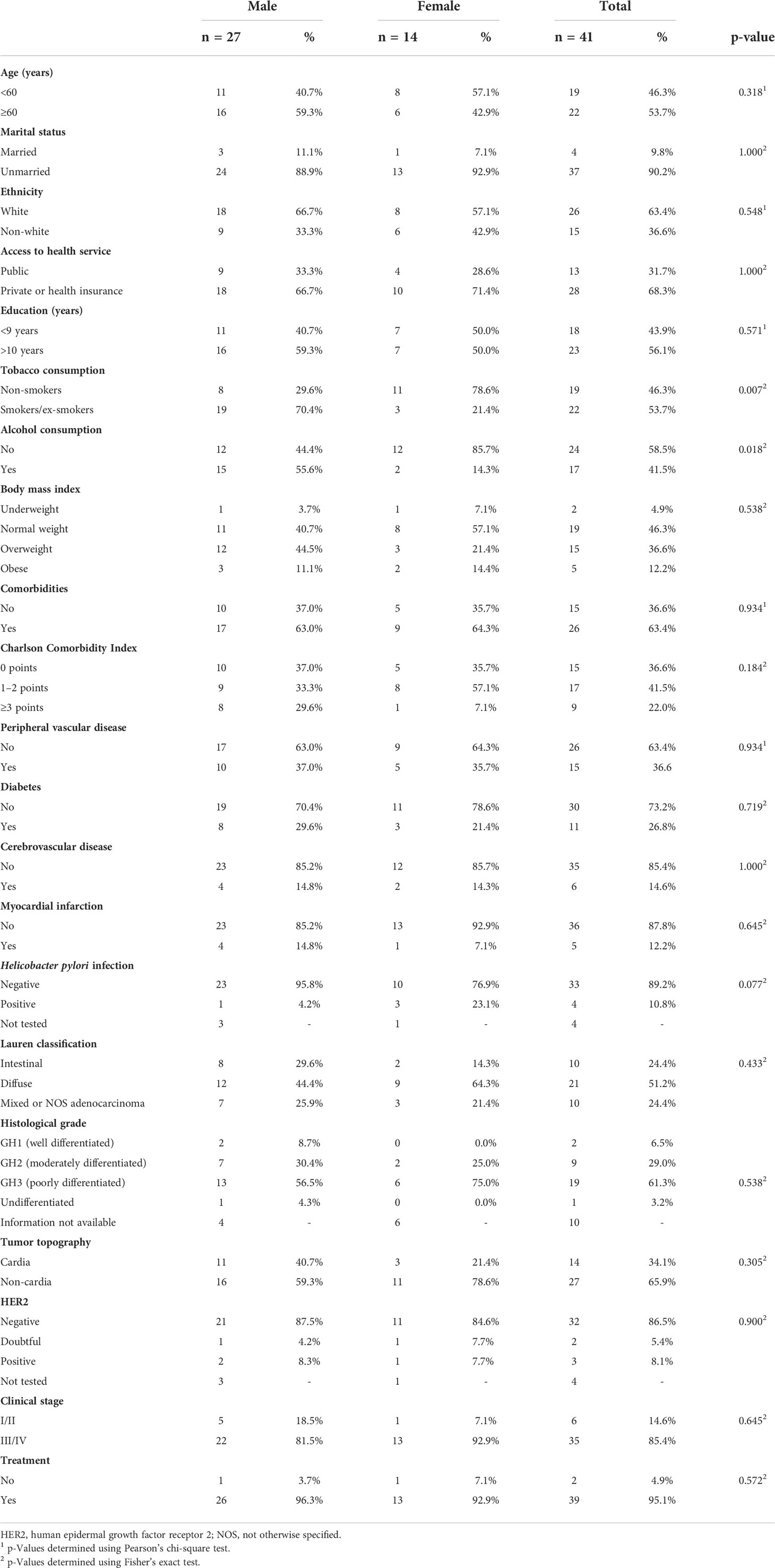
Table 4 Sociodemographic, lifestyle, and clinical characteristics of 41 patients with gastric adenocarcinoma who died in the first year of follow-up, by sex.
Discussion
This study reported the status of gastric adenocarcinoma patients at 1-year follow-up for overall survival probability and prognostic factors, DALY, YLL, YLD, and YLL per death. Overall survival was 80.8% (95% CI 75.7%–86.3%). Survival probability was lower in patients with <9 years of education, CCI score ≥3, cerebrovascular disease, diabetes, and clinical stage III/IV. In the first year post-diagnosis, there was 700.72 DALY, of which 90.55% was YLL, an average of 15.48 years was lost per death, and women had the highest YLL per death.
This study showed that 1-year overall survival was higher compared to that described by Poonyam et al. (2021) in Thailand (36) and that of Carneseca et al. (2013) in another study in Brazil (37). However, the overall survival was comparable to rates reported in Holland and Portugal (71% and 82%, respectively) (38, 39). The contrasting results of the present study could be explained by advances in cancer treatment (40) and the specific sociodemographic profile of the population investigated. In a representative sample of the Brazilian population, 56.2% self-declared as non-white, 51.1% had an educational level of elementary or lower (<9 years of education), and only one-third (28.5%) had private health insurance (23, 41). By contrast, in the present cohort, patients were predominantly of white ethnicity, had >10 years of education, and had access to the healthcare service by private or health insurance, differing from the national average.
Patients with lower educational levels tend to have lower survival rates (4) and, consequently, poorer prognosis (42). Education is also associated with socioeconomic level, eating habits, sanitary conditions (3), and access to diagnosis and treatment (43). Lower educational level was also associated with lower access to private or health insurance, lower survival rate, and poorer prognosis in the current study; socioeconomic factors have an impact on the prognosis of gastric adenocarcinoma.
The clinical stage is typically associated with a poorer prognosis (13–15, 44–46). For instance, in this study, stage III/IV gastric adenocarcinoma cases had an increased risk of death as compared to stage I/II patients, and lower overall survival. Similarly, a study performed in northern Brazil reported 86.4% overall survival in patients with early-stage cancer in the first year and 44.6% overall survival in advanced cases (47). Moreover, studies carried out in São Paulo by Ramos et al. (2018) and Costa et al. (2015) recorded 87.3% overall survival for stage I and 27.2% overall survival for stage IV (21) in the first year. Furthermore, after neoadjuvant therapy, 95.4% and 78.9% overall survival were recorded for stages I/II and III/IV (48), respectively. Comorbidities were also associated with an increased risk of death in patients with gastric cancer (10), and a CCI score ≥3 was associated with lower overall survival, supporting previous studies (11, 49). This result confirmed that comorbidities exist at gastric adenocarcinoma diagnosis and that the type and severity of the comorbidity are important for therapeutic planning. In our study, diabetes and cerebrovascular disease impacted prognosis. According to Shimada et al. (2020), patients who died by the first year of follow-up had a higher rate of cerebrovascular diseases before cancer diagnosis (49). This relationship was corroborated by the findings of the current study. Although cerebrovascular diseases are associated with a poorer prognosis in gastric adenocarcinoma cases, studies investigating the impact of cerebrovascular diseases are scarce. Previous studies on sarcoma, colorectal, and breast cancers found an association between comorbidities and therapeutic indication, which could affect survival (50–53). The role of diabetes in gastric cancer risk and prognosis is uncertain; however, some studies suggested that diabetes promotes cell growth, proliferation, and chemoresistance of gastric adenocarcinoma (54, 55). A large cohort study by Dabo et al. (2021) concluded that, while diabetes is associated with cardia gastric cancer, it is not related to gastric cancer overall (56). In the current study, diabetes before gastric adenocarcinoma was correlated with lower overall survival probability, higher risk of death, and, consequently, poorer prognosis, supporting existing epidemiological studies (57–60).
In 2016, approximately 17.9 million years of life was lost due to gastric cancer worldwide, of which 98% was due to YLL (5). This rate was slightly higher than that obtained in our study, in which approximately 90% of DALY corresponded to a total YLL of 634.51 years. These data might reflect differences in access to treatment at a referral cancer center and completeness of follow-up. Although men between the ages of 30 and 70 are more likely to develop gastric cancer (one in 32 men vs one in 81 women) (5), women in this cohort had higher YLL per death. Kim et al. (2016) found an association between gastric adenocarcinoma diagnosis in young women and poorer prognosis (59). In this cohort, female gender was not a prognostic factor; however, the exploratory analysis revealed that most women were diagnosed at stage III/IV and were younger than 60, explaining the higher YLL per death in women. Similar results were obtained in population-based studies conducted in the United States (11.6 YLL and 12.5 YLL per death for men and women, respectively) (61) and Japan (12.0 YLL and 13.5 YLL per death for men and women, respectively) (62). The hospital-based findings for the cohort of the current study reflected the population profile.
The findings of the current study were consistent with other population-based studies. However, the small sample size and the number of events (deaths) could have reduced the power and widened the confidence interval. Furthermore, the current study was conducted at a single center, limiting population-level inferences. Given the low number of events, if an interaction between any of the variables was present, it was a natural phenomenon whose likelihood is unaffected by our sample size.
At a single cancer center, 1-year overall survival probability was approximately 80% in patients with gastric adenocarcinoma. Patients with a higher risk of death had cerebrovascular disease, advanced clinical staging, diabetes, and/or lower educational level. Approximately 700 years of DALY was documented, with women having the highest YLL per death. Because this study was conducted at a single cancer center, the results might not be representative of the general population. To the best of our knowledge, this study was the first to assess gastric adenocarcinoma DALY, YLL, and YLL per death at the first year of follow-up in a hospital cohort in Brazil.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics and Research Committee of the AC Camargo Cancer Center under protocol n°2169/165. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors participated in this study. TT, GF, and MC, concepts and design. TT, GF, and MC, data acquisition. TT, GF, and MC, data analysis and interpretation. GF and MC, study supervision. TT, GF, and MC, writing, review, and revision of manuscripts. All authors contributed to the article and approved the submitted version.
Funding
The project “Epidemiology and Genomics of Gastric Adenocarcinomas in Brazil” was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP under grant 2014/26897-0. The present study received financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Acknowledgments
We thank the staff of the Group of Epidemiology and Statistics on Cancer, A. C. Camargo Cancer Center, São Paulo, Brazil, for supporting the study.
Conflict of interest
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.918833/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2020 : incidência de câncer no brasil / instituto nacional de câncer josé alencar gomes da Silva. Rio de Janeiro: INCA (2019). p. 120.
3. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev (2014) 23:700–13. doi: 10.1158/1055-9965.EPI-13-1057
4. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol (2019) 14:26–38. doi: 10.5114/pg.2018.80001
5. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016 a systematic analysis for the global burden of disease study global burden o. JAMA Oncol (2018) 4:1553–68. doi: 10.1001/jamaoncol.2018.2706
6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
7. Cavatorta O, Scida S, Miraglia C, Barchi A, Nouvenne A, Leandro G, et al. Epidemiology of gastric cancer and risk factors. Acta BioMed (2018) 89:82–7. doi: 10.23750/abm.v89i8-S.7966
8. International Agency for Research on Cancer. List of classifications by cancer sites with sufficient or limited evidence in humans , volumes 1 to 113 * cancer site carcinogenic agents with sufficient evidence in humans agents with limited evidence in humans lip , oral cavity , and pharynx list of cl (2020). Available at: https://monographs.iarc.fr/agents-classified-by-the-iarc/ (Accessed January 10, 2022).
9. World Cancer Research Fund/American Institute for Cancer Research. Diet , nutrition , physical activity and stomach cancer. UK:WCRF International, Upper Ground Floor, 140 Pentonville Road, London N1 9FW (2018). dietandcancerreport.org
10. Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of comorbidities on survival in gastric. Colorectal Lung Cancer Patients (2019) 29:110–5. doi: 10.2188/jea.JE20170241
11. Lin J-X, Huang Y-Q, Xie J-W, Wang J-B, Lu J, Chen Q-Y, et al. Age-adjusted charlson comorbidity index (ACCI) is a significant factor for predicting survival after radical gastrectomy in patients with gastric cancer. BMC Surg (2019) 19:53. doi: 10.1186/s12893-019-0513-9
12. Kim SA, Choi BY, Song KS, Park CH, Eun CS, Han DS, et al. Prediagnostic smoking and alcohol drinking and gastric cancer Survival : A Korean. Korean J Gastroenterol (2019) 73:141–51. doi: 10.4166/kjg.2019.73.3.141
13. Fang W, Huang K, Chen M, Liu C, Hung Y-P, Chao Y, et al. Comparative study of the 7th and 8th AJCC editions for gastric cancer patients after curative surgery. PloS One (2017) 12:e0187626. doi: 10.1371/journal.pone.0187626
14. Coimbra FJF, de Jesus VHF, Ribeiro HSC, Diniz AL, de Godoy AL, de Farias IC, et al. Impact of ypT, ypN, and adjuvant therapy on survival in gastric cancer patients treated with perioperative chemotherapy and radical surgery. Ann Surg Oncol (2019) 26:3618–26. doi: 10.1245/s10434-019-07454-0
15. Ajani JA, In H, Sano T, Gaspar LE, Erasmus JJ, Tang LH, et al. “Stomach.,”. In: AJCC cancer staging manual. Cham: Springer International Publishing (2017). p. 203–20. doi: 10.1007/978-3-319-40618-3_17
16. Aguiar Junior PN, Artigiani Neto R, Forones NM. HER2 expression as a prognostic factor in metastatic gastric cancer. Arq Gastroenterol (2016) 53:62–7. doi: 10.1590/S0004-28032016000200003
17. Kim H, Seo S, Kim K, Park YH, An M, Baik H, et al. Prognostic significance of human epidermal growth factor receptor-2 expression in patients with resectable gastric adenocarcinoma. World J Surg Oncol (2019) 17:1–9. doi: 10.1186/s12957-019-1652-2
18. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (London England) (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
19. SEER*Explorer: An interactive website for SEER cancer statistics, in: Surveill res program, natl cancer institute . Available at: https://seer.cancer.gov/explorer (Accessed August 16, 2021).
20. Danckert B, Ferlay J, Engholm G, Hansen H, Johannesen T, Khan S, et al. NORDCAN: Cancer incidence, mortality, prevalence and survival in the Nordic countries, version 9.0 (01.03.2021), in: Assoc nord cancer regist cancer regist Norway. Available at: https://nordcan.iarc.fr (Accessed August 16, 2021).
21. Ramos MFKP, Pereira MA, Yagi OK, Dias AR, Charruf AZ, de Oliveira RJ, et al. Surgical treatment of gastric cancer: a 10-year experience in a high-volume university hospital. Clinics (Sao Paulo) (2018) 73:e543s. doi: 10.6061/clinics/2018/e543s
22. Global Burden of Disease Collaborative Network. Global burden of disease study 2019 (GBD 2019) results. Seattle, United States (2020). Available at: http://ghdx.healthdata.org/gbd-results-tool.
23. IBGE. Características gerais dos domicílios e dos moradores 2019 - PNAD contínua. In: Pesqui nac por amostra domicílios contínua, vol. 8. (2020). Rio de Janeiro:IBGE, Coordenação de Trabalho e Rendimento
24. Available at: https://www.accamargo.org.br/sobre-o-cancer/noticias/accamargo-cancer-center-uma-historia-de-muitas-conquistas.
25. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J BioMed Inform (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
26. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J BioMed Inform (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
27. Organização Mundial da Saúde. CID-O – classificação internacional de doenças para oncologia. 3rd ed. São Paulo: Edusp – Editora da Universidade de São Paulo (2005). p. 248.
28. World Health Organization [WHO]. Obesity preventing and managing the global epidemic. Geneva:World Health Organization (1998). p. 275.
29. Lauren P. The two histological main types of gastric carcinoma diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand (1965) 64:31–49. doi: 10.1111/apm.1965.64.1.31
30. Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology (2008) 52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x
31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
32. Devleesschauwer B, Havelaar AH, Maertens De Noordhout C, Haagsma JA, Praet N, Dorny P, et al. Calculating disability-adjusted life years to quantify burden of disease. Int J Public Health (2014) 59:565–9. doi: 10.1007/s00038-014-0552-z
33. World Health Organization. WHO methods and data sources for global burden of disease estimates 2000-2019 (2020). Available at: http://www.who.int/gho/mortality_burden_disease/en/index.html.For.
34. IBGE. Tábua completa de mortalidade para o brasil: análises e tabelas (2020). Available at: https://agenciadenoticias.ibge.gov.br/media/com_mediaibge/arquivos/65c3023462edaabf0d7318c1a0f80ca4.pdf.
35. Gordis L. “The occurrence of disease: II. In: Mortality and other measures of disease impact.,” epidemiology. Philadelphia:Elsevier/Saunders (2014). p. 61–87.
36. Poonyam P, Aumpan N, Vilaichone R-K. Prognostic factors for survival in patients with gastric adenocarcinoma. Cancer Rep (Hoboken NJ) (2021) 4:e1305. doi: 10.1002/cnr2.1305
37. Carneseca EC, Mauad EC, de Araujo MAA, Dalbó RM, Longatto Filho A, Vazquez V de L. The hospital de câncer de barretos registry: an analysis of cancer survival at a single institution in Brazil over a 10-year period. BMC Res Notes (2013) 6:141. doi: 10.1186/1756-0500-6-141
38. Van Der Werf LR, Wijnhoven BPL, Fransen LFC, Van Sandick JW, Nieuwenhuijzen GAP, Busweiler LAD, et al. A national cohort study evaluating the association between short-term outcomes and long-term survival after esophageal and gastric cancer surgery. Ann Surg (2019) 270:868–76. doi: 10.1097/SLA.0000000000003520
39. Peyroteo M, Martins PC, Canotilho R, Correia AM, Baía C, Sousa A, et al. Impact of the 8th edition of the AJCC TNM classification on gastric cancer prognosis-study of a western cohort. Ecancermedicalscience (2020) 14:1124. doi: 10.3332/ecancer.2020.1124
40. Epistola RJ, Chao J. Systemic therapy for advanced gastroesophageal cancers: Progress and pitfalls. Transl Gastroenterol Hepatol (2020) 5:1–11. doi: 10.21037/tgh.2020.01.10
41. Ministério da Saúde. Pesquisa nacional de saúde: 2019: ciclos de vida. Brasil / IBGE: Rio de Janeiro (2013). p. 139. Available at: http://biblioteca.ibge.gov.br/visualizacao/livros/liv91110.pdf.
42. Fontana V, Decensi A, Orengo MA, Parodi S, Torrisi R, Puntoni R. Socioeconomic status and survival of gastric cancer patients. Eur J Cancer (1998) 34:537–42. doi: 10.1016/S0959-8049(97)10098-3
43. Kim NY, Oh JS, Choi Y, Shin J, Park EC. Relationship between socioeconomic status and accessibility for endoscopic resection among gastric cancer patients: using national health insurance cohort in Korea: poverty and endoscopic resection. Gastric Cancer (2017) 20:61–9. doi: 10.1007/s10120-016-0597-1
44. Zhao L-L, Huang H, Wang Y, Wang T-B, Zhou H, Ma F-H, et al. Lifestyle factors and long-term survival of gastric cancer patients: A large bidirectional cohort study from China. World J Gastroenterol (2020) 26:1613–27. doi: 10.3748/wjg.v26.i14.1613
45. Lu J, Chen Y, Liu Y, Ding J, Piao Z, Liu W. Clinical significance of prognostic score based on age , tumor size , and grade in gastric cancer after gastrectomy. Cancer Manag Res (2018) 10:4279–86. doi: 10.2147/CMAR.S171663
46. Ueno D, Matsumoto H, Kubota H, Higashida M, Akiyama T, Shiotani A. Prognostic factors for gastrectomy in elderly patients with gastric cancer. World J Surg Onc (2017) 15, 59. doi: 10.1186/s12957-017-1131-6.
47. Moreira-Nunes C de FA, Martins CN de SAT, Feio D, Lima IK, Lamarão LM, de Souza CRT, et al. PD-L1 expression associated with Epstein-Barr virus status and patients’ survival in a Large cohort of gastric cancer patients in northern Brazil. Cancers (Basel) (2021) 13:3107. doi: 10.3390/cancers13133107
48. da Costa WL, Coimbra FJF, Ribeiro HSC, Diniz AL, de Godoy AL, de Farias IC, et al. Total gastrectomy for gastric cancer: An analysis of postoperative and long-term outcomes through time: Results of 413 consecutive cases in a single cancer center. Ann Surg Oncol (2015) 22:750–7. doi: 10.1245/s10434-014-4212-6
49. Shimada T, Yamagata T, Kanno Y, Ohira T, Harada Y, Koike Y, et al. Predictive factors for short-term survival after non-curative endoscopic submucosal dissection for early gastric cancer. Digestion (2020) 0824:1–10. doi: 10.1159/000510165
50. Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA (2005) 294(6):716–24. doi: 10.1001/jama.294.6.716
51. Parise CA, Caggiano V. The influence of comorbidity on treatment and survival of triple-negative breast cancer. Breast J (2020) 26:1729–35. doi: 10.1111/tbj.13924
52. Loong HHF, Wong CKH, Wei Y, Kwan SSC, Zhang Y, Tse T, et al. Prevalence and prognostic impact of comorbidities and peripheral blood indices in sarcomas. ESMO Open (2020) 5:1–9. doi: 10.1136/esmoopen-2020-001035
53. Biscond M, Guimbaud R, Digue L, Cirilo-cassaigne I, Bousser V, Oum-Sack E, et al. How does comorbidity affect colon cancer patients’ care trajectory? results from the French EvaCCoR cohort study. Clin Res Hepatol Gastroenterol (2021) 45:101422. doi: 10.1016/j.clinre.2020.03.022
54. Zhou Y, Wang Y, Wang S, Shen L. Hyperglycemia promotes human gastric carcinoma progression via aquaporin 3. Dig Dis Sci (2015) 60:2338–45. doi: 10.1007/s10620-015-3625-9
55. Zhao W, Chen R, Zhao M, Li L, Fan L, Che X-M. High glucose promotes gastric cancer chemoresistance in vivo and in vitro. Mol Med Rep (2015) 12:843–50. doi: 10.3892/mmr.2015.3522
56. Dabo B, Pelucchi C, Rota M, Jain H, Bertuccio P, Bonzi R, et al. The association between diabetes and gastric cancer: Results from the Stomach Cancer Pooling Project Consortium. Eur J Cancer Prev (2022) 31(3)260–9. doi: 10.1097/cej.0000000000000703
57. Wei Z-W, Li J-L, Wu Y, Xia G-K, Schwarz RE, He Y-L, et al. Impact of pre-existing type-2 diabetes on patient outcomes after radical resection for gastric cancer: A retrospective cohort study. Dig Dis Sci (2014) 59:1017–24. doi: 10.1007/s10620-013-2965-6
58. Tsai M-S, Wang Y-C, Kao Y-H, Jeng L-B, Kao C-H. Preexisting diabetes and risks of morbidity and mortality after gastrectomy for gastric cancer: A nationwide database study. Med (Baltimore) (2015) 94:e1467. doi: 10.1097/MD.0000000000001467
59. Kim HW, Kim J-H, Lim BJ, Kim H, Kim H, Park JJ, et al. Sex disparity in gastric cancer: Female sex is a poor prognostic factor for advanced gastric cancer. Ann Surg Oncol (2016) 23:4344–51. doi: 10.1245/s10434-016-5448-0
60. Karlin NJ, Buras MR, Kosiorek HE, Verona PM, Cook CB. Glycemic control and survival of patients with coexisting diabetes mellitus and gastric or esophageal cancer. Futur Sci OA (2019) 5(6):FSO397. doi: 10.2144/fsoa-2019-0038
61. Song M, Hildesheim A, Shiels MS. Premature years of life lost due to cancer in the united states in 2017. Cancer Epidemiol Biomarkers Prev (2020) 29:2591–8. doi: 10.1158/1055-9965.EPI-20-0782
Keywords: gastric adenocarcinoma, survival, prognostic factor, years of life lived with disability, years of life lost
Citation: Tiengo T, Fernandes GA and Curado MP (2022) Gastric adenocarcinoma: 1-year overall survival, disability-adjusted life years, years of life lost, and prognostic factors—a single-institution experience. Front. Oncol. 12:918833. doi: 10.3389/fonc.2022.918833
Received: 12 April 2022; Accepted: 26 July 2022;
Published: 08 September 2022.
Edited by:
Clement Adebamowo, University of Maryland, United StatesReviewed by:
Leila Allahqoli, Iran University of Medical Sciences, IranAmir Tiyuri, Iran University of Medical Sciences, Iran
Copyright © 2022 Tiengo, Fernandes and Curado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Paula Curado, bXAuY3VyYWRvQGFjY2FtYXJnby5vcmcuYnI=; Tatiane Tiengo, dGF0aWVuZ29AZ21haWwuY29t
 Tatiane Tiengo
Tatiane Tiengo Gisele Aparecida Fernandes
Gisele Aparecida Fernandes Maria Paula Curado
Maria Paula Curado