- Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, National Clinical Research Center for Obstetric and Gynecologic Diseases, Beijing, China
Low-grade endometrial stromal sarcoma (LG-ESS) is a rare and indolent malignancy. Hormone therapy has been reported as an adjuvant treatment for LG-ESS, although its effectiveness is controversial. Here we aimed to investigate the effects of postoperative hormone therapy on recurrence in patients with uterine LG-ESS. Between January 2010 and December 2019, a total of 152 patients (23 with and 129 without fertility-sparing) with a diagnosis of primary uterine LG-ESS confirmed by pathologists were enrolled in this study. In the cohort without fertility-sparing, 22 (17.7%) patients had recurrence, and the median disease-free survival (DFS) was 47 (2-130) months; only one of these patients died of LG-ESS. No significant difference was found in recurrence between the groups with and without hormone therapy (p=0.802). However, subgroup analysis showed that hormone therapy decreased the recurrence rate in stage II-IV (p=0.001, HR 0.144, 95% CI: 0.038-0.548), but not in stage I disease (p=0.256). High-dose progestins notably reduced recurrence (p=0.012, HR 0.154, 95% CI: 0.036-0.660), whereas non-progestin therapy marginally influenced recurrence (p=0.054) compared with no hormone therapy in stage II-IV disease. Moreover, hormone therapy within 12 months was effective in reducing recurrence (p=0.038, HR 0.241, 95% CI: 0.063-0.922). Ovarian preservation (p=0.004, HR 6.250, 95% CI: 1.786-21.874) and negative expression of ER/PR (p=0.000, HR 23.249, 95% CI: 4.912-110.026) were high-risk factors for recurrence in patients without fertility-sparing. In the fertility-sparing cohort, 15 (65.2%) patients experienced recurrence, and the median DFS was 24 (3-107) months. Six patients successfully delivered healthy fetuses, and five received hormone therapy. Twelve patients finally accepted hysterectomy after repeated recurrence, and only two of them had given birth before surgery. Patients who received hormone therapy showed longer DFS, although this difference was not statistically significant (p=0.466). In conclusion, postoperative hormone therapy reduces recurrence in patients with stage II–IV uterine LG-ESS without fertility-sparing, and high-dose treatment with progestins within 12 months is recommended. Bilateral oophorectomy can also reduce the risk of recurrence. Patients with fertility-sparing have a high risk of recurrence and poor pregnancy outcomes, and hormone therapy may be a reasonable choice in postoperative management.
Introduction
Endometrial stromal sarcoma (ESS) is a rare malignancy, accounting for approximately 20% of uterine sarcomas (1, 2). There are four categories of ESS: endometrial stromal nodule, low-grade endometrial stromal sarcoma (LG-ESS), high-grade endometrial stromal sarcoma (HG-ESS), and undifferentiated uterine sarcoma (UUS) (3). Among these, LG-ESS is the most common uterine stromal sarcoma (4). LG-ESS is an indolent disease, with a protracted interval to recurrence (2, 5). Hysterectomy is recommended in patients with LG-ESS, and ovarian preservation could be considered in premenopausal patients (2, 6). Due to the slow-growing nature LG-ESS, in most cases of early-stage disease (2, 7), fertility-sparing surgery is performed in patients with a desire to have children. The use of adjuvant hormone therapy, including high-dose progestins, aromatase inhibitors, and gonadotropin-releasing hormone agonists (GnRH-a), has been reported for the treatment because LG-ESS is considered as a hormone-dependent tumor (8–10). These different drugs work by different mechanisms. Progestins have an antioestrogenic effect and suppress stromal endometrial proliferation by binding to progesterone receptor (11). Besides, progestins also involve in cell cycle regulation by cyclin-dependent kinase (12). Aromatase inhibitors reduce estrogen levels by blocking aromatase activity in peripheral adipose and tumor tissue (13).GnRH-a suppress ovarian estrogen production by inhibiting the pituitary ovarian axis, leading to a “postmenopausal” status; moreover, GnRH-a may have an additive action by blocking the intra-tumoral GnRH receptor (11, 13). However, whether hormone therapy can reduce the recurrence of LG-ESS remains controversial (14, 15). Therefore, in this study, we aimed to investigate the impact of postoperative hormone therapy on recurrence in patients with uterine LG-ESS. In addition, patients with and without fertility-sparing were analyzed separately, to better understand the effects of hormone therapy.
Materials and Methods
Patients
This was a single-center retrospective cohort study. A total of 155 patients with primary uterine LG-ESS confirmed by pathologists at our hospital were enrolled, between January 2010 and December 2019. Among these patients, three with high-grade ESS elements in recurrent pathology were excluded; thus, a total of 152 patients were analyzed. The study was approved by the Institutional Review Board of Peking Union Medical College Hospital (No. S-K2016), and the requirement for informed consent was waived.
Patients were divided into two cohorts depending on whether they preserved fertility. In the cohort without fertility-sparing, all patients underwent hysterectomy with or without bilateral oophorectomy. In the fertility-sparing cohort, only resection of lesions was performed. The stage of LG-ESS was determined according to the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system. Postoperative hormone therapy included high-dose progestins [megestrol acetate (MA) 160-320 mg/day or medroxyprogesterone acetate (MPA) 250-500 mg/day)], letrozole (2.5 mg/day), and GnRH-a (3.75 mg/28 days). A levonorgestrel-releasing intrauterine device (LNG-IUD) was used in some fertility-sparing patients. Moreover, some patients received radiotherapy or chemotherapy after surgery.
Follow-Up and Measure Outcomes
After surgery, the patients were regularly followed up at our hospital, or other local hospitals. Follow-up methods included pelvic examination, blood tests, abdominopelvic ultrasonography, annual chest X-ray, and annual CT of the chest, abdomen, and pelvic cavity. Excluding those lost to follow-up, all patients were followed up until May 2021 by telephone or outpatient visits. The primary outcome was disease-free survival (DFS), defined as the time from surgery to recurrence or the last follow-up visit, whichever occurred first. Only one patient died of LG-ESS; therefore, we did not calculate overall survival. In addition, we also explored high-risk factors for recurrence in the cohort without fertility-sparing.
Statistical Analysis
All statistical analyses were performed using SPSS version 23.0. Survival analysis was performed using the Kaplan-Meier method and the log-rank test. Univariate and multivariate Cox regression analyses were used to analyze prognostic factors and estimate the hazard ratio (HR) with a 95% confidence interval (95% CI). GraphPad Prism version 9.3 was used to draw survival curves. Statistical significance was set at P < 0.05.
Results
Outcomes in the Cohort Without Fertility-Sparing
A total of 129 patients were enrolled in this cohort, and their epidemiological characteristics, treatment, and follow-up results are shown in Table 1. The median patient age was 43 (20–67) years, and the median body mass index (BMI) was 23.0 (17.2-37.3) kg/m2. The vast majority (93%) of patients were at premenopausal stage. Bilateral oophorectomy was performed in 80.6% (n = 104), whereas at least one ovary was preserved in 19.4% (n = 25) of patients. The tumor diameter was >5 cm in 60.5% of patients, and lymphovascular space involvement (LVSI) was positive in 34.9% of patients. Immunohistochemical staining for estrogen receptor/progesterone receptor (ER/PR) was positive in 76.7% (n=99), negative in 3.1% (n=4), and unknown in 20.2% (n=26) of patients. Only 18.6% of patients experienced elevated levels of serum CA125. According to the FIGO staging system, 69.8% (n=90) of patients had stage I and 30.2% (n=39) had stage II–IV disease. Post-surgery, 32 patients received radiotherapy whereas seven patients received chemotherapy. There were 75 patients that received postoperative hormone therapy. Among those, 53 received high-dose progestins, 13 received letrozole, and nine received GnRH-a or a combination of two drugs. In addition, 41.1% (n=53) of patients did not receive any hormone therapy. In terms of duration of hormone therapy, 44.0% (n=33) of patients were within 6 months, 26.7% (n=20) were between 6 and 12 months, and 28.0% (n=21) were over 12 months.
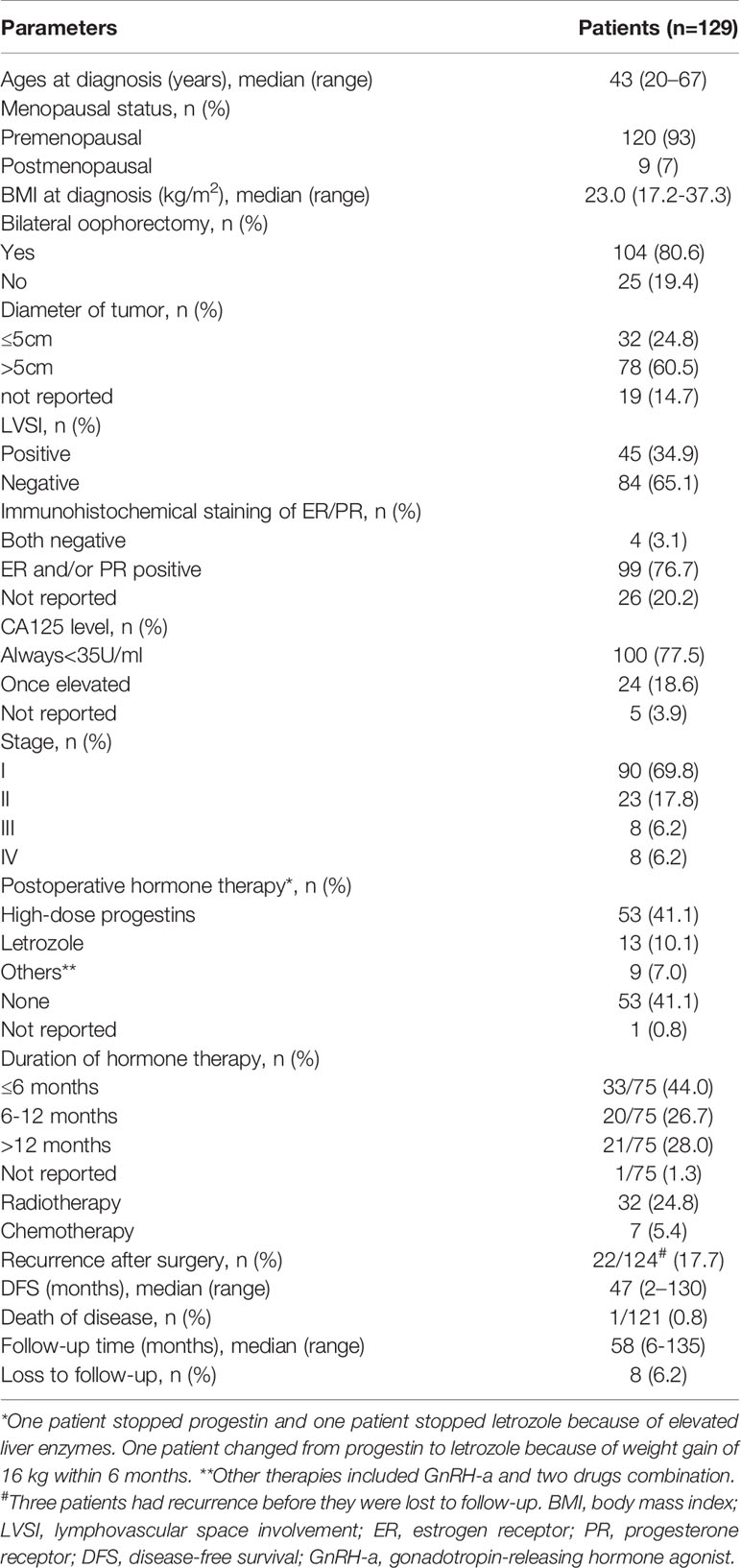
Table 1 Epidemiological characteristics, treatment, and follow-up of the cohort without fertility-sparing.
The median follow-up time was 58 (6–135) months, and eight (6.2%) patients were lost to follow-up. A total of 22 (17.7%) patients had disease recurrence, and the median DFS was 47 (2–130) months; only one patient died as a result of LG-ESS recurrence. There was no statistically significant difference in recurrence between the groups with and without hormone therapy (p=0.802) (Figure 1A). Multivariate Cox regression analysis showed that ovarian preservation (p=0.004) and negative expression of ER/PR (p=0.000) were high-risk factors for recurrence, and HR was 6.250 (95% CI: 1.786-21.874) and 23.249 (95% CI: 4.912-110.026), respectively (Table 2). Subgroup analysis revealed that hormone therapy reduced the recurrence rate only in patients with stage II–IV disease (p=0.001, HR 0.144, 95% CI: 0.038-0.548) (Supplementary Figure 1A and Table 3); no significant difference was found in patients with stage I disease (p=0.256) (Supplementary Figure 1B). Hormone therapy appeared to prolong DFS in patients with ovarian preservation; however, this effect was not significant (p=0.331) (Supplementary Figure 1C). In patients with stage II–IV disease, high-dose progestins could notably reduce recurrence compared with no hormone therapy (p=0.012, HR 0.154, 95% CI: 0.036-0.660), whereas the non-progestin group was marginally better than the group without hormone therapy (p=0.054) (Table 3). Moreover, hormone therapy within 12 months was a protective factor against recurrence (p=0.038, HR 0.241, 95% CI: 0.063-0.922).
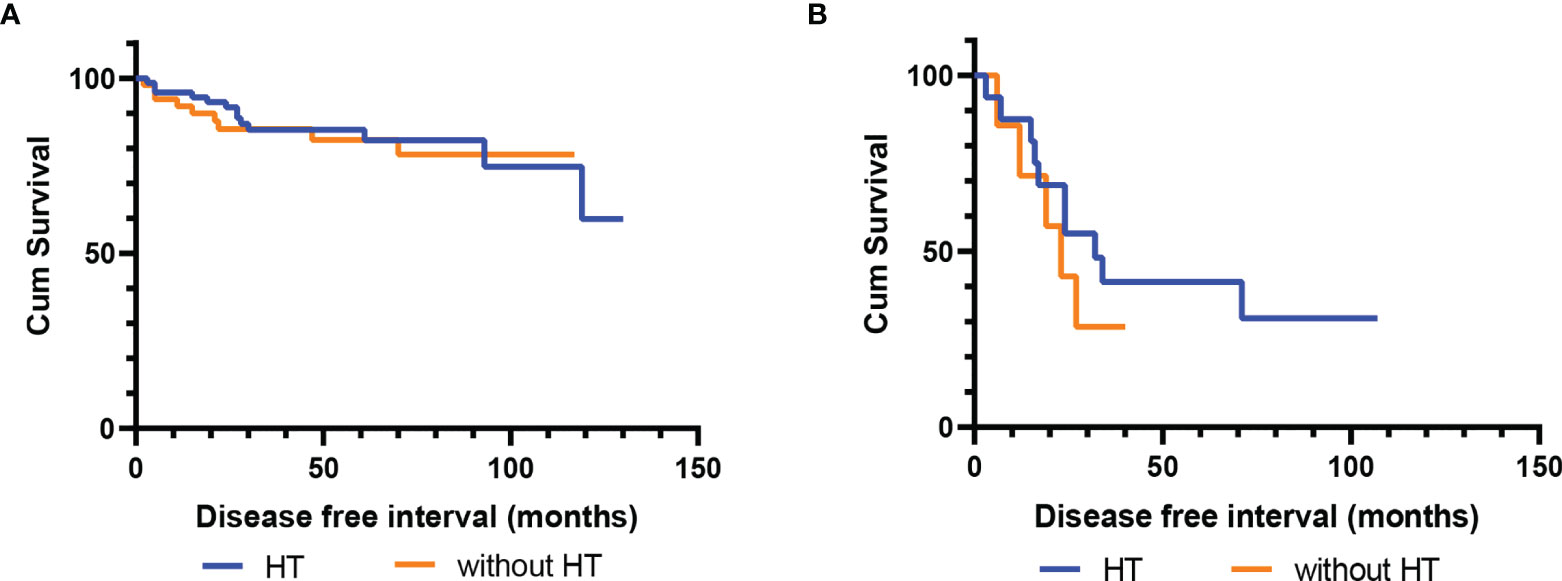
Figure 1 Disease-free survival (DFS) of patients with and without postoperative hormone therapy. (A) The cohort without fertility-sparing (p=0.802); (B) The fertility-sparing cohort (p=0.466).
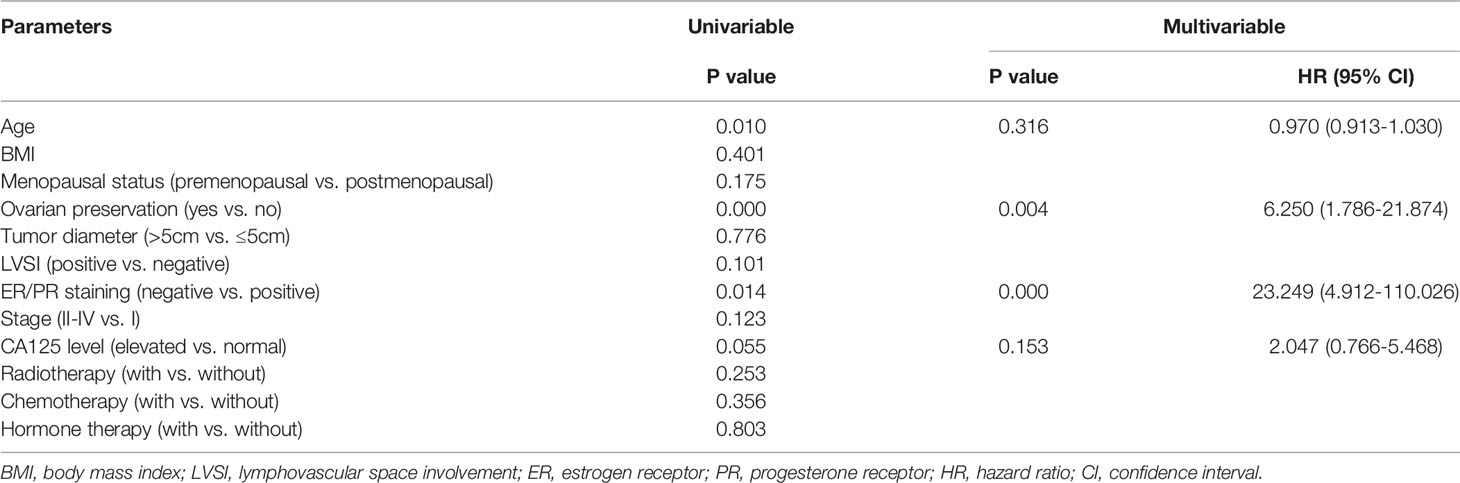
Table 2 Univariate and multivariate Cox regression of clinicopathological features for recurrence in the cohort without fertility-sparing.
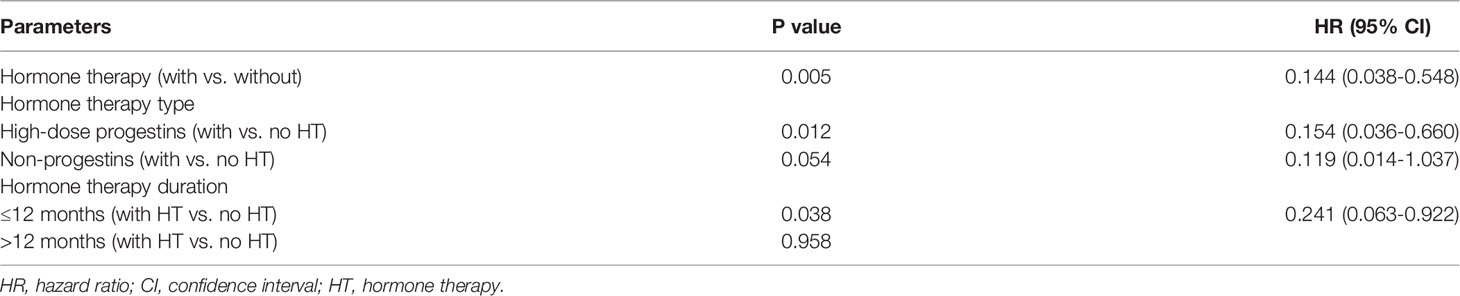
Table 3 Univariate Cox regression of hormone therapy parameters in patients with stage II-IV disease.
Outcomes in the Fertility-Sparing Cohort
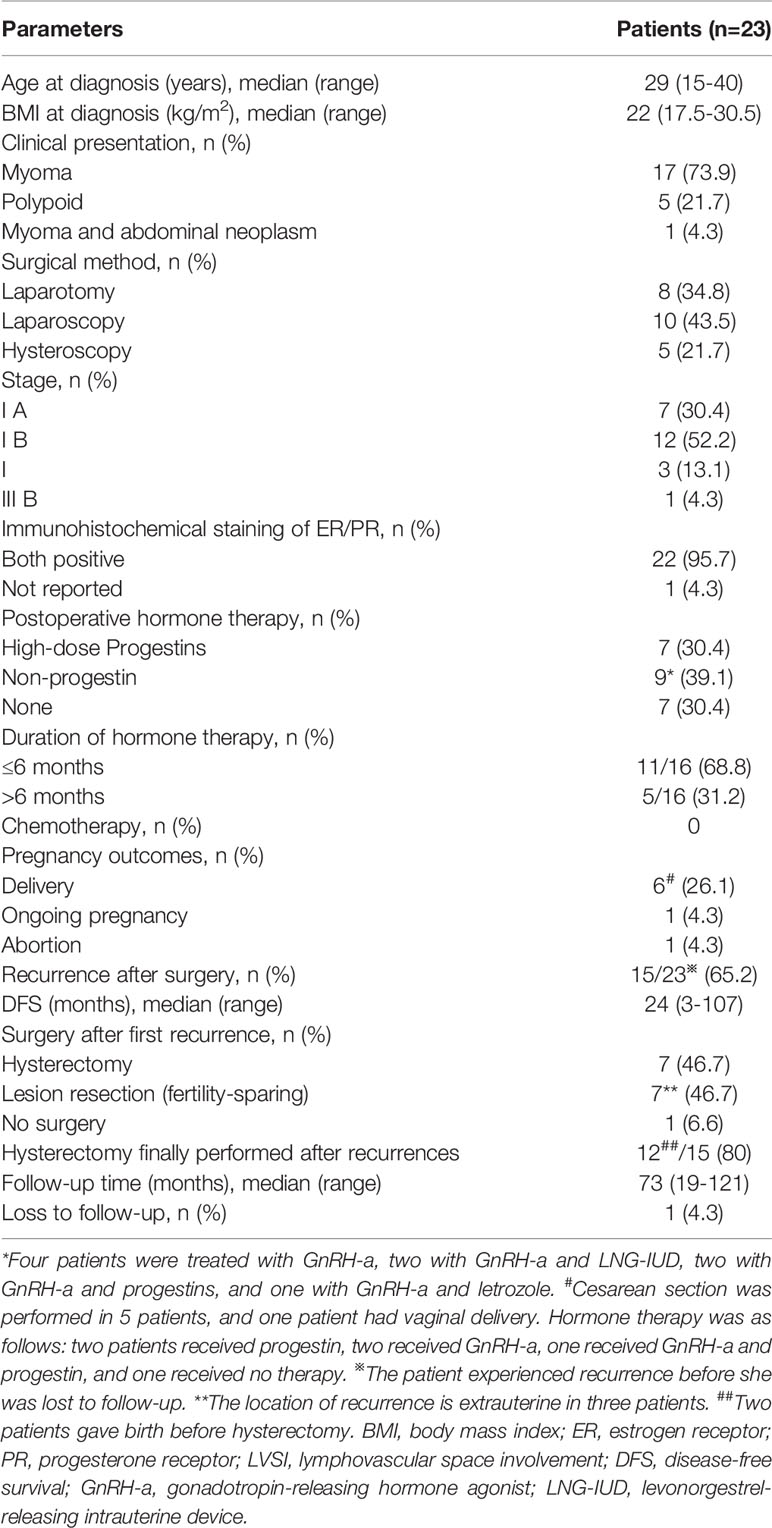
Table 4 summarizes the epidemiological characteristics, treatment, and follow-up results of the 23 patients in the fertility-sparing cohort. The median age and BMI were 29 (15–40) years and 22 (17.5-30.5) kg/m2, respectively. The most common clinical presentation was myoma (73.9%), followed by polypoid (21.7%); one patient had abdominal neoplasm. Resection of lesions by laparotomy was performed in 34.8%, by laparoscopy in 43.5%, and by hysteroscopy in 21.7% of patients. Almost all patients (95.7%) showed ER/PR expression. Twenty-two patients had stage I disease (7 with stage I A, and 12 with stage I B) and one patient had stage III B disease. In total, 16 patients (69.5%) received postoperative hormone therapy, including seven with only high-dose progestins and nine with non-progestin or combination therapy (all including GnRH-a). In terms of treatment duration, 11 patients (68.8%) received hormone therapy within 6 months, and five patients (31.2%) received over 6 months of therapy. None of these patients received chemotherapy after surgery.
Twenty-two patients finished follow-up, with only one patient being lost; however, this patient had disease recurrence before being lost to follow-up. A total of 15 patients (65.2%) experienced recurrence of LG-ESS, and the median DFS was 24 (3–107) months; no patient died of LG-ESS. Among these patients, seven (46.7%) continued to preserve fertility after the first recurrence. Finally, 12 (80%) patients gave up preserving fertility and underwent hysterectomy after repeated recurrence; only two of these patients had given birth before surgery. Six patients had successfully delivered a healthy fetus (five with cesarean section and one with vaginal delivery), one patient had ongoing pregnancy, and one patient had an abortion. Among patients with successful delivery, only one did not receive hormone therapy; the remaining patients received high-dose progestins (n=2), GnRH-a (n=2), or progestins with GnRH-a (n=1). The median follow-up duration was 73 (19–121) months. Patients who received postoperative hormone therapy had a longer DFS, although this observation was not statistically significant (p=0.466) (Figure 1B).
Discussion
LG-ESS is a rare gynecological malignancy that is generally diagnosed after surgery; thus, almost all studies are retrospective. To the best of our knowledge, this is the largest retrospective cohort study on the effect of hormone therapy on uterine LG-ESS recurrence. In particular, we analyzed patients with and without fertility-sparing.
Cohort Without Fertility-Sparing
According to our results, the recurrence rate was 17.7% and the median DFS was 47 months in the cohort without fertility-sparing. Only one patient suffered rapid recurrence leading to death. Approximately 70% of patients had stage I disease. This confirmed that LG-ESS is a slow-progressing malignancy with late recurrence, and reasonably good prognosis (2, 15, 16).
More than half of the patients received postoperative hormone therapy, with the most common drug used being high-dose progestins (MA or MPA). Our study revealed that hormone therapy had no impact on recurrence in the entire cohort and in the stage I subgroup. However, in patients with stage II–IV disease, hormone therapy could significantly reduce recurrence. This inconsistency in outcomes between the whole and partial cohorts may be due to the low proportion of patients with stage II–IV disease. Malouf et al. found that adjuvant treatment including hormone therapy was associated with the absence of recurrence in patients with stages I-II ESS (17). A cohort study that enrolled 37 patients with LG-ESS reported that hormone therapy was associated with a lower recurrence rate, even in patients with stage I disease (14). Zhang et al. showed that hormone therapy was a protective factor in patients with LG-ESS, accompanied by improved progression-free survival (PFS) (18). In addition, we confirmed a high proportion (76.7%) of ER/PR expression, which is consistent with previous studies (19, 20). This may reflect the potential validity of hormone therapy in patients with LG-ESS (21). However, discrepancies regarding the role of hormone therapy do exist. A multicenter study reported that PFS was comparable between LG-ESS patients with and without adjuvant hormone therapy (22). Moreover, Zhou et al. reported that hormone therapy had no significant impact on DFS in patients with LG-ESS (16). A meta-analysis including 10 retrospective LG-ESS studies indicated that patients with hormone therapy showed a significantly lower risk of recurrence; nevertheless, hormone therapy had little benefit in reducing the recurrence risk in stage III–IV patients (23).
In patients with stage II–IV disease, high-dose progestins significantly decreased the risk of recurrence, whereas non-progestin therapy (letrozole or GnRH-a) had a marginal effect on reducing recurrence. This suggests that high-dose progestin should be the hormone therapy of preference in this context, but non-progestin hormonal treatments may also be effective when there are contraindications or intolerable side effects to progestin. Moreover, hormone therapy for less than a year in duration was sufficiently effective. Mizuno et al. supported MPA as a therapeutic option for residual or recurrent LG-ESS, even as first-line therapy (24). Reich et al. found a high percentage of aromatase expression positivity in patients with LG-ESS, implying that aromatase inhibitors (e.g., letrozole) may play a role in the treatment of LG-ESS (25). Another study comparing aromatase inhibitors with progestins for LG-ESS treatment indicated that aromatase inhibitors were superior to progestins because of longer recurrence-free survival and fewer side effects in patients with stage II–IV disease; this, however, is not consistent with our observations (5).
Our study revealed that ovarian preservation is a high-risk factor for recurrence in patients with LG-ESS. LG-ESS is considered a hormone-dependent tumor that commonly occurs in premenopausal patients, which account for over 90% of the patients reported in our study (16, 26). The ovary is the main organ producing estrogen and progesterone hormones in these patients; therefore, ovarian preservation carries a potential risk of recurrence. In addition, hormone therapy cannot completely inhibit ovarian endocrine function based on our analysis. A meta-analysis of 17 studies with 786 patients reported that the ovarian preservation group had a significantly higher recurrence rate than the bilateral salpingo-oophorectomy group (27). However, the benefits of bilateral oophorectomy are controversial. Karataşlı et al. and Li et al. both reported that ovarian preservation did not affect the recurrence of stage I LG-ESS (4, 15). Negative ER/PR expression was another risk factor for recurrence in our study. Conversely, Cade et al. found that ER/PR positivity was related to survival benefit but did not significantly affect recurrence-free survival in ESS (28). Zhou et al. also stated that the ER/PR status did not influence DFS in patients with LG-ESS (16). We noted that ER/PR staining was unknown in 20.2% of patients, because it was not reported in our hospital in earlier years. However, we still reported this part to make results more completely. We did not observe any other high-risk factors for recurrence, including FIGO stage, radiotherapy, chemotherapy, LVSI, and menopausal status, which is in line with some previous studies (14, 16). Further research is needed to explore the role of age and serum CA125 levels in disease recurrence.
Fertility-Sparing Cohort
In the fertility-sparing cohort, the recurrence rate was 65.2% and the median DFS was 24 months. The prognosis was evidently worse than that in the cohort without fertility-sparing, even though almost all patients had stage I disease and expressed ER/PR. Most patients underwent lesion resection by laparoscopy or laparotomy, possibly because myoma was the most common clinical presentation.
The proportion of patients receiving postoperative hormone therapy was 69.5% in our study, whereas it was as high as 83.3%-100% in other studies (29–31). However, our cohort included a larger number of patients compared to these studies. Except for high-dose progestins, GnRH-a was a relatively common choice of hormone therapy in the fertility-sparing cohort; this was quite different from the cohort without fertility-sparing. The most common duration of hormone therapy was 6 months or less, which was shorter than that described in other studies (30–32). Hormone therapy seemed to prolong DFS in these patients, although this effect was not significant. Encouragingly, six patients successfully delivered healthy fetuses after fertility-sparing management. Among these patients, five received hormone therapy, including high-dose progestins and/or GnRH-a. In the few reports so far, patients with successful delivery used only progestins for hormone therapy (29–32). Thus, our findings suggest that GnRH-a is also a good option for fertility-sparing hormone therapy. However, we observed that nearly half of the patients chose hysterectomy after the first recurrence, and this proportion eventually reached 80% after repeated recurrence. Only two of those patients had given birth before hysterectomy. This reflects that the pregnancy outcomes of fertility-sparing patients are not optimistic; therefore, patients should be fully informed of the risks and make careful choices.
Our study has several limitations that need to be mentioned. First, it was a retrospective study conducted in a single-center. However, despite this, our study is still convincing because of the relatively large number of enrolled patients, relatively long follow-up times, and low rate of loss to follow-up. Second, the different types, doses, and durations of hormone therapy, may have interfered with the overall impact of hormone therapy on recurrence.
In conclusion, LG-ESS is an indolent malignancy with a generally good survival outcome. In patients without fertility-sparing, postoperative hormone therapy reduced recurrence in stage II–IV but not in stage I disease. Moreover, no more than one year of high-dose progestins is recommended in patients with stage II–IV disease. Ovarian preservation and negative ER/PR expression are high-risk factors for recurrence in patients without fertility-sparing. Patients with fertility-sparing have a high risk of recurrence and poor pregnancy outcomes; therefore, they should be fully informed of these risks. For patients that have intense fertility desire and choose fertility-sparing surgery, postoperative hormone therapy (high-dose progestins and/or GnRH-a) may be a reasonable choice because it showed the tendency to prolong DFS.
Data Availability Statement
The data underlying this article will be shared by the corresponding author, upon reasonable request. Requests to access these datasets should be directed to PP, cGVuZ3BlbmdAcHVtY2guY24=.
Ethics Statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of Peking Union Medical College Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
PP contributed to conception and design of the study. XH organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all enrolled patients for receiving our follow-up visits and sharing their information.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.922757/full#supplementary-material
References
1. Tropé CG, Abeler VM, Kristensen GB. Diagnosis and Treatment of Sarcoma of the Uterus. A Review. Acta Oncol (2012) 51(6):694–705. doi: 10.3109/0284186x.2012.689111
2. Amant F, Floquet A, Friedlander M, Kristensen G, Mahner S, Nam EJ, et al. Gynecologic Cancer Intergroup (Gcig) Consensus Review for Endometrial Stromal Sarcoma. Int J Gynecol Cancer (2014) 24(9 Suppl 3):S67–72. doi: 10.1097/igc.0000000000000205
3. Conklin CM, Longacre TA. Endometrial Stromal Tumors: The New Who Classification. Adv Anat Pathol (2014) 21(6):383–93. doi: 10.1097/pap.0000000000000046
4. Li AJ, Giuntoli RL 2nd, Drake R, Byun SY, Rojas F, Barbuto D, et al. Ovarian Preservation in Stage I Low-Grade Endometrial Stromal Sarcomas. Obstet Gynecol (2005) 106(6):1304–8. doi: 10.1097/01.AOG.0000185511.91694.1e
5. Deshmukh U, Black J, Perez-Irizarry J, Passarelli R, Levy K, Rostkowski A, et al. Adjuvant Hormonal Therapy for Low-Grade Endometrial Stromal Sarcoma. Reprod Sci (2019) 26(5):600–8. doi: 10.1177/1933719118778801
6. Nasioudis D, Mastroyannis SA, Latif NA, Ko EM, Haggerty AF, Kim SH, et al. Effect of Bilateral Salpingo-Oophorectomy on the Overall Survival of Premenopausal Patients With Stage I Low-Grade Endometrial Stromal Sarcoma; a National Cancer Database Analysis. Gynecol Oncol (2020) 157(3):634–8. doi: 10.1016/j.ygyno.2020.04.001
7. Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary Uterine Endometrial Stromal Neoplasms. A Clinicopathologic Study of 117 Cases. Am J Surg Pathol (1990) 14(5):415–38. doi: 10.1097/00000478-199005000-00002
8. Lim MC, Lee S, Seo SS. Megestrol Acetate Therapy for Advanced Low-Grade Endometrial Stromal Sarcoma. Onkologie (2010) 33(5):260–2. doi: 10.1159/000305661
9. Altal OF, Al Sharie AH, Halalsheh OM, Tashtush N, Shaban S, Alfaqih M, et al. Complete Remission of Advanced Low-Grade Endometrial Stromal Sarcoma After Aromatase Inhibitor Therapy: A Case Report. J Med Case Rep (2021) 15(1):262. doi: 10.1186/s13256-021-02838-x
10. Burke C, Hickey K. Treatment of Endometrial Stromal Sarcoma With a Gonadotropin-Releasing Hormone Analogue. Obstet Gynecol (2004) 104(5 Pt 2):1182–4. doi: 10.1097/01.AOG.0000133533.05148.aa
11. Maccaroni E, Lunerti V, Agostinelli V, Giampieri R, Zepponi L, Pagliacci A, et al. New Insights Into Hormonal Therapies in Uterine Sarcomas. Cancers (Basel) (2022) 14(4):921. doi: 10.3390/cancers14040921
12. Banno K, Kisu I, Yanokura M, Tsuji K, Masuda K, Ueki A, et al. Progestin Therapy for Endometrial Cancer: The Potential of Fourth-Generation Progestin (Review). Int J Oncol (2012) 40(6):1755–62. doi: 10.3892/ijo.2012.1384
13. Thanopoulou E, Judson I. Hormonal Therapy in Gynecological Sarcomas. Expert Rev Anticancer Ther (2012) 12(7):885–94. doi: 10.1586/era.12.74
14. Comert GK, Turkmen O, Kar I, Yucel O, Kilic C, Boran N, et al. Hormone Therapy Following Surgery in Low-Grade Endometrial Stromal Sarcoma: Is It Related to a Decrease in Recurrence Rate? J Chin Med Assoc (2019) 82(5):385–9. doi: 10.1097/JCMA.0000000000000039
15. Karataşlı V, Çakır İ, Can B, Erkılınç S, Karadeniz T, Kuru O, et al. Does Ovarian Preservation Have an Effect on Recurrence of Early Stage Low-Grade Endometrial Stromal Sarcoma? J Obstet Gynaecol (2021) 41(5):797–802. doi: 10.1080/01443615.2020.1803238
16. Zhou J, Zheng H, Wu SG, He ZY, Li FY, Su GQ, et al. Influence of Different Treatment Modalities on Survival of Patients With Low-Grade Endometrial Stromal Sarcoma: A Retrospective Cohort Study. Int J Surg (2015) 23(Pt A):147–51. doi: 10.1016/j.ijsu.2015.09.072
17. Malouf GG, Duclos J, Rey A, Duvillard P, Lazar V, Haie-Meder C, et al. Impact of Adjuvant Treatment Modalities on the Management of Patients With Stages I-II Endometrial Stromal Sarcoma. Ann Oncol (2010) 21(10):2102–6. doi: 10.1093/annonc/mdq064
18. Zhang Y, Li Y, Huang H, Yang J, Wu M, Jin Y, et al. Low-Grade Endometrial Stromal Sarcoma and Uterine Adenosarcoma: A Comparison of Clinical Manifestations and Outcomes. J Cancer (2019) 10(15):3352–60. doi: 10.7150/jca.30691
19. Reich O, Regauer S, Urdl W, Lahousen M, Winter R. Expression of Oestrogen and Progesterone Receptors in Low-Grade Endometrial Stromal Sarcomas. Br J Cancer (2000) 82(5):1030–4. doi: 10.1054/bjoc.1999.1038
20. Cui R, Yuan F, Wang Y, Li X, Zhang Z, Bai H. Clinicopathological Characteristics and Treatment Strategies for Patients With Low-Grade Endometrial Stromal Sarcoma. Med (Baltimore) (2017) 96(15):e6584. doi: 10.1097/md.0000000000006584
21. Katz L, Merino MJ, Sakamoto H, Schwartz PE. Endometrial Stromal Sarcoma: A Clinicopathologic Study of 11 Cases With Determination of Estrogen and Progestin Receptor Levels in Three Tumors. Gynecol Oncol (1987) 26(1):87–97. doi: 10.1016/0090-8258(87)90074-6
22. Stewart LE, Beck TL, Giannakopoulos NV, Rendi MH, Isacson C, Goff BA. Impact of Oophorectomy and Hormone Suppression in Low Grade Endometrial Stromal Sarcoma: A Multicenter Review. Gynecol Oncol (2018) 149(2):297–300. doi: 10.1016/j.ygyno.2018.03.008
23. Cui R, Cao G, Bai H, Zhang Z. The Clinical Benefits of Hormonal Treatment for Lg-Ess: A Meta-Analysis. Arch Gynecol Obstet (2019) 300(5):1167–75. doi: 10.1007/s00404-019-05308-4
24. Mizuno M, Yatabe Y, Nawa A, Nakanishi T. Long-Term Medroxyprogesterone Acetate Therapy for Low-Grade Endometrial Stromal Sarcoma. Int J Clin Oncol (2012) 17(4):348–54. doi: 10.1007/s10147-011-0299-y
25. Reich O, Regauer S. Aromatase Expression in Low-Grade Endometrial Stromal Sarcomas: An Immunohistochemical Study. Mod Pathol (2004) 17(1):104–8. doi: 10.1038/sj.modpathol.3800031
26. Cheng X, Yang G, Schmeler KM, Coleman RL, Tu X, Liu J, et al. Recurrence Patterns and Prognosis of Endometrial Stromal Sarcoma and the Potential of Tyrosine Kinase-Inhibiting Therapy. Gynecol Oncol (2011) 121(2):323–7. doi: 10.1016/j.ygyno.2010.12.360
27. Nasioudis D, Ko EM, Kolovos G, Vagios S, Kalliouris D, Giuntoli RL. Ovarian Preservation for Low-Grade Endometrial Stromal Sarcoma: A Systematic Review of the Literature and Meta-Analysis. Int J Gynecol Cancer (2019) 29(1):126–32. doi: 10.1136/ijgc-2018-000063
28. Cade TJ, Quinn MA, Rome RM, Polyakov A. Prognostic Significance of Steroid Receptor Positivity and Adjuvant Progestogen Use in Endometrial Stromal Sarcoma. Aust N Z J Obstet Gynaecol (2014) 54(5):453–6. doi: 10.1111/ajo.12245
29. Xie W, Cao D, Yang J, Jiang X, Shen K, Pan L, et al. Fertility-Sparing Surgery for Patients With Low-Grade Endometrial Stromal Sarcoma. Oncotarget (2017) 8(6):10602–8. doi: 10.18632/oncotarget.12491
30. Laurelli G, Falcone F, Scaffa C, Messalli EM, Del Giudice M, Losito S, et al. Fertility-Sparing Management of Low-Grade Endometrial Stromal Sarcoma: Analysis of an Institutional Series and Review of the Literature. Eur J Obstet Gynecol Reprod Biol (2015) 195:61–6. doi: 10.1016/j.ejogrb.2015.09.041
31. Zheng Y, Yin Q, Yang X, Dong R. Fertility-Sparing Management of Low-Grade Endometrial Stromal Sarcoma: Analysis of an Institutional Series, a Population-Based Analysis and Review of the Literature. Ann Transl Med (2020) 8(21):1358. doi: 10.21037/atm-20-2180
Keywords: low-grade endometrial stromal sarcoma, hormone therapy, Recurrence, Disease free survival, Fertility-sparing
Citation: Huang X and Peng P (2022) Hormone Therapy Reduces Recurrence in Stage II-IV Uterine Low-Grade Endometrial Stromal Sarcomas: A Retrospective Cohort Study. Front. Oncol. 12:922757. doi: 10.3389/fonc.2022.922757
Received: 18 April 2022; Accepted: 30 May 2022;
Published: 28 June 2022.
Edited by:
Annamaria Ferrero, Mauriziano Hospital, ItalyReviewed by:
Lorenzo Ceppi, Niguarda Ca’ Granda Hospital, ItalyElena Maccaroni, Ospedali Riuniti Umberto I, Italy
Copyright © 2022 Huang and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Peng, cGVuZ3BlbmdAcHVtY2guY24=
 Xiaodi Huang
Xiaodi Huang Peng Peng
Peng Peng