- 1The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 2School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
- 3Department of Tumor High-intensity focused ultrasound (HIFU) Therapy, HwaMei Hospital, University of Chinese Academy of Sciences, Ningbo, China
Background: The effect of tobacco on breast cancer (BC) is controversial. The purpose of this study was to investigate the relationship between tobacco and BC.
Methods: A search was conducted in PubMed, EBSCO, Web of Science and Cochrane Library databases before February 2022. The adjusted odd ratio (OR) and corresponding 95% confidence interval (CI) were used to examine the relationship between active or passive smoking and BC risk.
Results: A total of 77 articles composed of 2,326,987 participants were included for this meta-analysis. Active (OR=1.15, 95% CI=1.11-1.20, p<0.001) and passive (OR=1.17, 95% CI=1.09-1.24, p<0.001) smoking increased the risk of BC in the female population, especially premenopausal BC (active smoking: OR=1.24, p<0.001; passive smoking: OR=1.29, p<0.001), but had no effect on postmenopausal BC (active smoking: OR=1.03, p=0.314; passive smoking: OR=1.13, p=0.218). Active smoking increased the risk of estrogen receptor-positive (ER+) BC risk (OR=1.13, p<0.001), but had no effect on estrogen receptor-negative (ER-) BC (OR=1.08, p=0.155). The risk of BC was positively associated with the duration and intensity of smoking, negatively associated with the duration of smoking cessation. Active smoking increased the risk of BC in the multiparous population (OR=1.13, p<0.001), but had no effect on the nulliparous population (OR=1.05, p=0.432), and smoking before the first birth (OR=1.22, 95% CI=1.17-1.27) had a greater impact on the risk of BC than smoking after the first birth (OR=1.08, 95% CI=1.04-1.12).
Conclusion: Smoking (active and passive) increased the risk of BC in women. The effect of smoking on BC was influenced by smoking-related factors (duration, intensity, years of quitting), population-related factors (fertility status), and BC subtypes.
Systematic Review Registration: identifier CRD42022322699.
Introduction
Breast cancer (BC) is the most common cancer in women worldwide (1). As a heterogeneous disease, its occurrence is influenced by both endogenous factors (such as heredity (2, 3), gene mutation (4, 5)) and exogenous factors (such as reproduction (6, 7), environment (8)). It is estimated that only 5-10% of BC cases are induced by genetic factors, while the remaining 90-95% are highly related to environmental factors or specific lifestyle (9, 10). Therefore, researchers are trying to provide better preventional strategies by adjusting exposure to BC protective or risky factors (1, 11). Evidence has shown that unhealthy lifestyle and some environmental factors are harmful to women (12–14), and eliminating these factors may help reduce the morbidity and mortality rate (15, 16).
The potential role of smoking in BC risk has been under intense discussion (17, 18). Although BC is not initially thought to be a tobacco-related cancer, over the past few decades, many chemicals contained in tobacco have been investigated to be a trigger of BC, such as 4-aminobiphenyl (19, 20) and benzopyrene (21, 22). In addition, evidence of the role of active smoking (23, 24) and secondhand smoke (25, 26) in the etiology of BC is accumulating, based on adequate animal trials (27, 28) and relevant epidemiological evidence (29). Recent trends have discovered smoking as one of the potential risk factors for BC (30).
Although many studies have shown that smoking may increase the risk of BC, a review of studies over the past 30 years has found that opinions among clinical researchers are still widely divided (17, 31). Firstly, some studies [e.g. Yingsong Lin et al. (32) and Chelsea Catsburg et al. (23)] failed to observe any association between smoking and BC incidence. Secondly, the results of subgroup analyses among different studies were high inconsistent (33, 34), or even reversed, such as subgroup analyses on menstrual status and BC subtypes. Third, published meta-analyses on the topic have also not reached consistent conclusions. Although most meta-analyses on active smoking suggest that smoking increases the incidence of BC, the conclusions of subgroup analyses are inconsistent (35, 36), and the meta-analyses on passive smoking are more inconsistent (37, 38). The last relevant meta-analysis was conducted and published in 2018. As of 2021, there are 153 million adult female smokers (including smoking, secondhand, and chewing) worldwide, accounting for 12% (39) of global smokers. Therefore, based on the inconsistency of previous studies, the large smoking population and the significant disease burden caused by tobacco (40), this study aimed to investigate the relationship between smoking and BC by conducting a systematic review and meta-analysis by searching for relevant observational studies. Therefore, it can provide a preventive reference for the female group and create greater value for the society.
Materials and methods
Search strategy
A comprehensive search of studies investigating the association between smoking and BC was carried out before February 2022 in electronic databases of PubMed, Web of science, EBSCO, and the Cochrane Library. The complete retrieval formula that was used to identify the related studies includes: (“breast cancer” OR “breast neoplasms” OR “BC”) AND (“smoking” OR “tobacco smoke pollution” OR “tobacco use” OR “tobacco products” OR “active smoking” OR “passive smoking” OR “secondhand smoking” OR “tobacco”). The reference lists of retrieved studies and conference records were also reviewed for potentially inclusive studies. When referring to duplicate literature, the original article was included if the study was published as an abstract or an original article. Also, if a study was continuously updated and reported, only the most recent or comprehensive articles were included. This meta-analysis was conducted according to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines (41). The population, intervention, comparison, outcome, and setting (PICOS) criteria were used to describe the research question. Participants in this study were people who had not previously been diagnosed with BC, the intervention was exposure to tobacco environments, including active and passive smoking, the comparison was a non-smoker, the outcome was the incidence of BC, and the setting was observational research. This meta-analysis’s prospero registration number was CRD42022322699.
Selection criteria
An eligible criterion was formulated. The specific criteria were as follows. Inclusion criteria: (1) all included studies are observational studies. (2) The main exposure of study was smoking including active and passive smoking, and the outcome was BC risk. (3) All studies included available data which reported the relationship between smoking and BC. Exclusion criteria: (1) the study was conducted on BC population and used mortality or recovery rate as the outcome. (2) The study was published in duplicate. (3) The study was not published in English.
Data collection and quality assessment
A jointly agreed data collection form was used to extract all data. Information was extracted as follows: the author’s name, year of publication, study type, age, exposure assessment, number of participants, number of BC cases, number of smokers, number of non-smokers, variables adjusted in the statistical analyses, and outcomes. To ensure the objectivity and accuracy of the data, two researchers independently extracted data from each study. Disagreements were resolved by consensus or consultation with a third researcher.
The quality of each included study was evaluated by the Newcastle-Ottawa Quality Assessment Scale (NOS) checklist, a tool used for quality assessment of non-randomized studies. NOS checklist is composed of eight items classified into three aspects, including selection, comparability, and outcome. The maximum scores of this checklist were nine, and scores between seven and nine were identified to be of higher study quality.
Objectives and endpoints
The primary objective was to explore the relationship between smoking and the incidence of BC. Secondary objectives were to explore the relationship between the incidence of BC and smoking subgroups (e.g. smoking pattern, smoking time, smoking frequency, smoking place, smoking cessation time, age of starting smoking), the relationship between smoking and BC in different populations (e.g. fertility status, menopausal status, race), and the association between smoking and different BC subtypes (e.g. estrogen receptor-positive (ER+) BC, estrogen receptor-negative (ER-) BC). The results after adjusting for relevant confounding factors were used consistently for the processing of relevant data from the included articles.
Statistical analysis
The Stata software version 12 (StataCorp, College Station, Texas, USA) was used to analyze the data. The confidence interval (CI) of odd ratio (OR) was set at 95% to examine the relationship between smoking and BC risk. Heterogeneity of included studies was tested by Q statistic and I2 statistic to quantitatively assess inconsistency. For statistical results, values of p<0.10 and I2>50% were considered to be representative of having statistically significant heterogeneity. Based on the heterogeneity of smoking intensity, smoking duration, race, BC subtype, etc. in different studies, in order to improve the reliability of the results, the random effects model was uniformly used in this study. When more than ten studies were included, sensitivity analysis and publication bias test were performed to evaluate the stability and reliability of their results. Publication bias was evaluated by the Begg’s test. Results with P-values less than 0.05 were considered to be statistically significant.
Results
Literature search
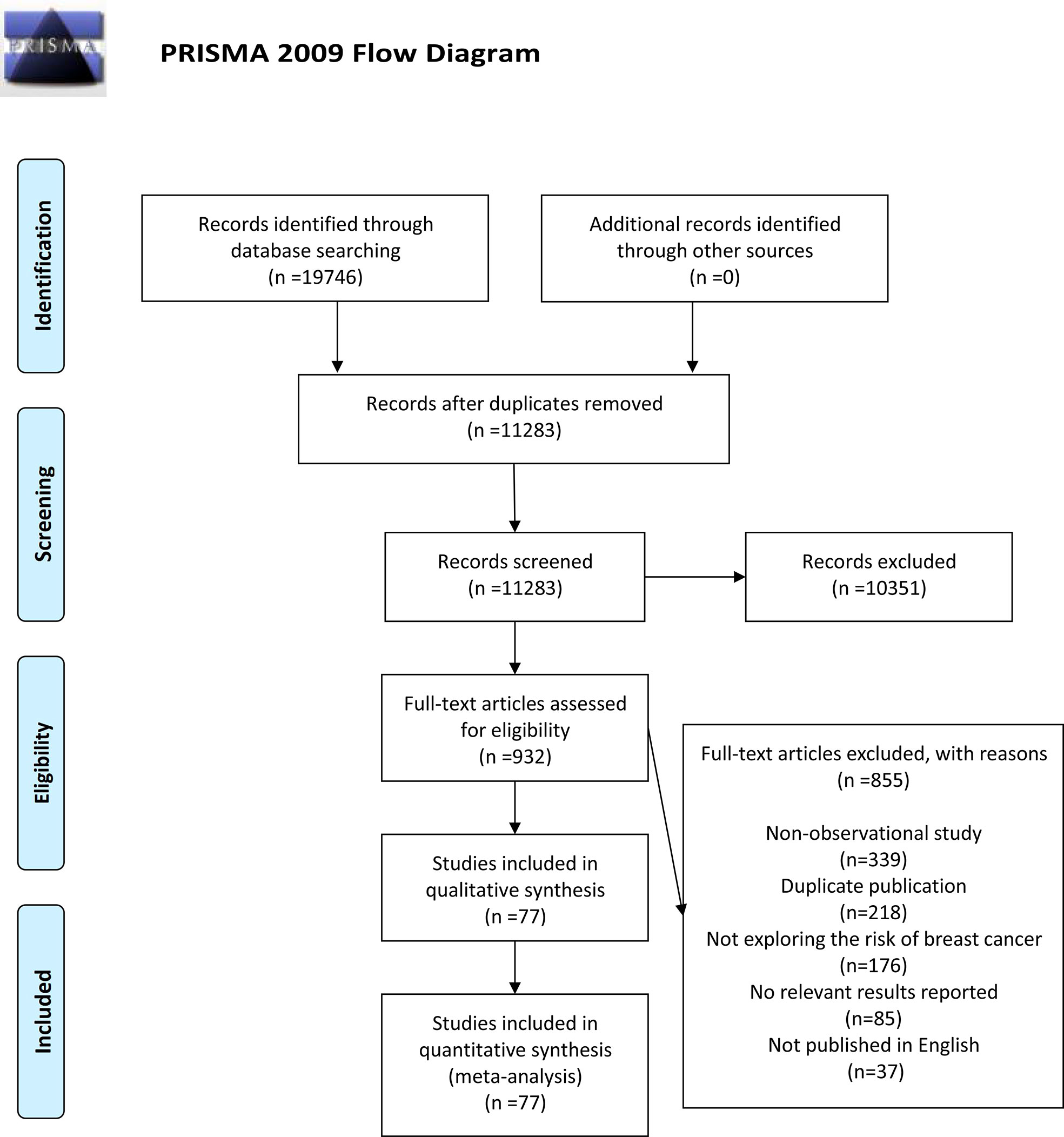
A total of 19,746 relevant articles were identified based on retrieval formula described in the methods section by initial search in PubMed, EBSCO, Web of Science, and Cochrane Library database. No additional records were identified through other sources. A total of 8,463 duplicate articles were deleted, and 11,283 articles were excluded due to the title or abstract. The remaining 932 articles were reviewed through full-text. Among them, 855 articles were eliminated because of being non-observational study (n=339), duplicate publication (n=218), not exploring the risk of BC (n=176), no relevant results reported (n=85), and not published in English (n=37). Eventually, 77 articles (13, 32–34, 42–115) composed of 2,326,987 participants were selected for this meta-analysis. The detailed search and study selection process was shown in Figure 1.
Characteristic of studies
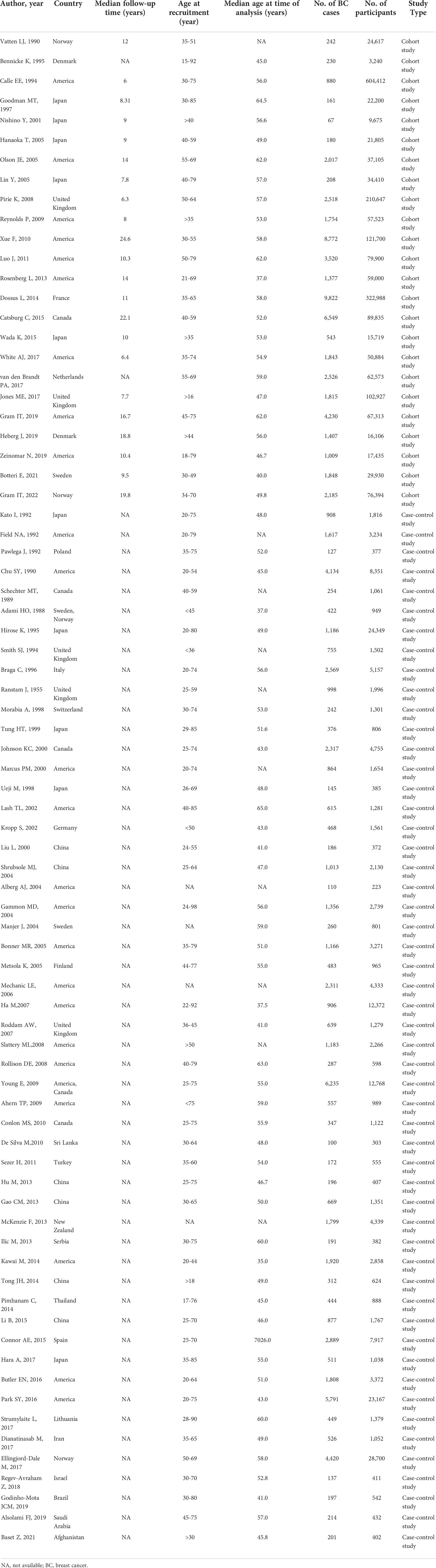
Of the 77 included studies, 24 were cohort studies (2,138,338 participants and 55,703 BC cases), 53 were case-control studies (188,649 controls and 58,859 BC cases). The participants in the two studies included men and women, and the rest were women. All studies were published between 1988 and 2022, with follow-up periods ranging from 6 to 24.6 years. Regarding age at recruitment, eight studies did not set the upper age limit, four studies did not set a lower age limit and four studies did not report the requirement for age. Among them, 30 studies were conducted in America, 24 were in Asia, 22 were in Europe, and 1 was in Oceania. Fifty-six studies investigated the association between active smoking and BC risk, 39 investigated the association between passive smoking and BC risk. The number of smokers (included active and passive smokers) was 1,326,603 in cohort studies and 108,175 in case-control studies. In order to collect data and evaluate relevant exposure factors, 59 studies chose questionnaire, 9 studies chose interview, and 9 studies chose questionnaire combined with interviews. In addition, the adjustment of potential confounding factors varied in different studies. Most of the adjustment parameters were age, body mass index (BMI), family history of BC, total energy intake, alcohol consumption, number of births, and physical activity. The characteristics of the included studies were shown in Table 1 and Supplementary Table 1.
Overall effect of active smoking
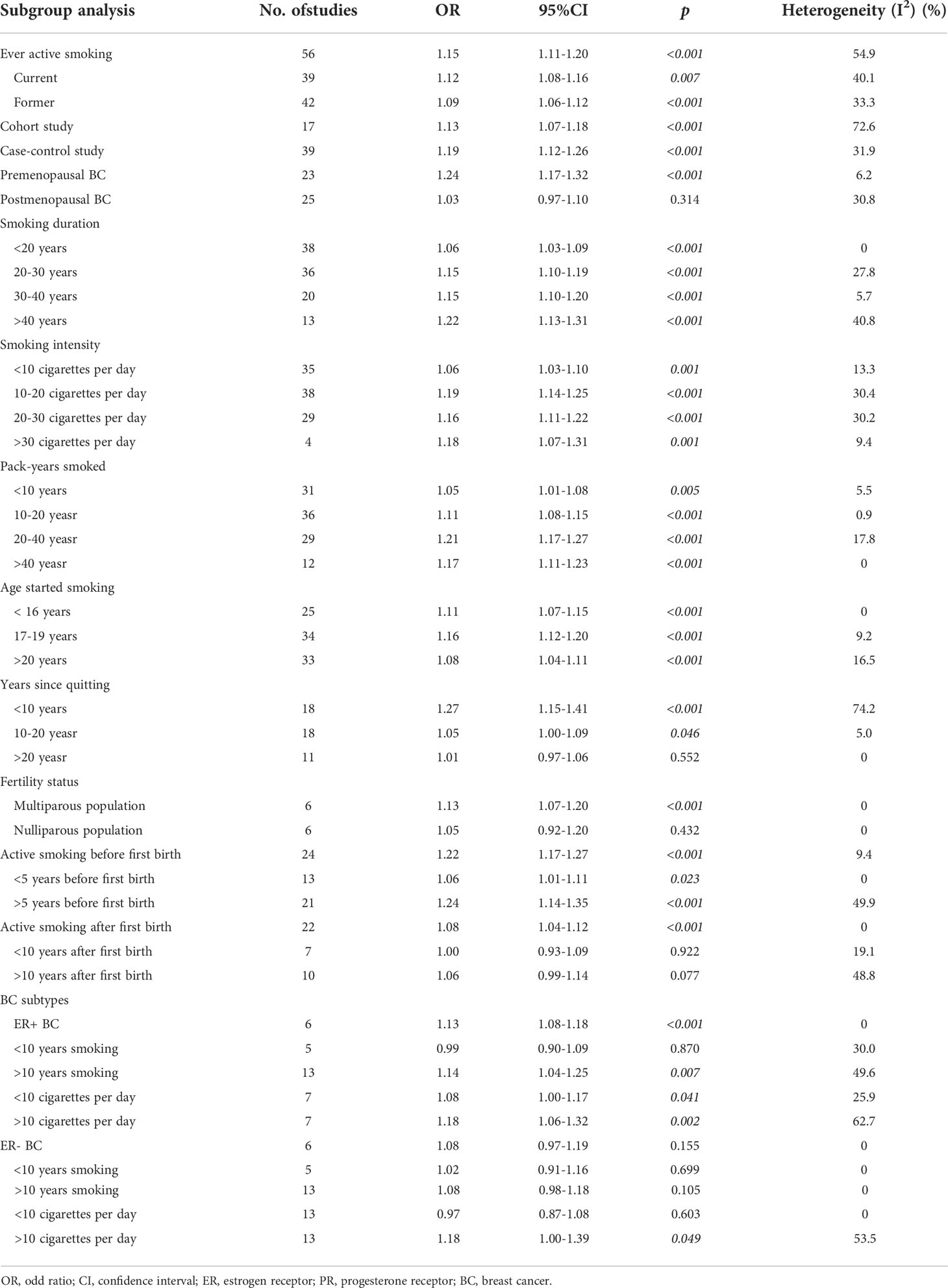
Fifty-six studies recorded data about active smoking in female population that was inducing BC. Studies had shown that women who actively smoked had a significantly higher incidence of BC than those who had never actively smoked (OR=1.15, 95% CI=1.11-1.20, p<0.001, I2 = 54.9%). Among them, current active smoking (OR=1.12, 95% CI=1.08-1.16, p=0.007, I2 = 40.1%) and former active smoking (OR=1.09, 95% CI=1.06-1.12, p<0.001, I2 = 33.3%) had a significantly increase on the incidence of BC, but current active smoking increased the incidence of BC more than former active smoking. In other words, active smoking is a risk factor for women, and the population who is still active smoking is under more risk than the population who quit smoking after active smoking. In addition, cohort studies (OR=1.13, p<0.001) and case-control studies (OR=1.19, p<0.001) had consistently concluded that active smoking increases the risk of BC in women. The detailed data was contained in Table 2.
Menopausal status
The correlation between smoking and BC is affected by menopausal status. Related data were available in 23 studies with premenopausal BC and 25 with postmenopausal BC. The analysis showed that active smoking increases the incidence of premenopausal BC (OR=1.24, 95% CI=1.17-1.32, p<0.001, I2 = 6.2%), but had no effect on postmenopausal BC (OR=1.03, 95% CI=0.97-1.10, p=0.314, I2 = 30.8%) with slight heterogeneity. The detailed data was contained in Table 2.
Smoking duration
Years were used to measure smoking duration in this study. The related data were divided into ‘<20 years group’, ‘20-30 years group’, ‘30-40 years group’, and ‘>40 years group’ according to the most studies. The results showed that women who smoked for less than 20 years (OR=1.06, p<0.001), 20-30 years (OR=1.15, p<0.001), 30-40 years (OR=1.15, p<0.001), and more than 40 years (OR=1.22, p<0.001) had a higher incidence of BC than those without smoking history. The incidence of BC was positively correlated with smoking duration. The detailed data was contained in Table 2.
Smoking intensity
Cigarettes per day were used to measure smoking intensity in this study. The data is grouped by 10 cigarettes per day, 20 cigarettes per day, and 30 cigarettes per day. Subgroup analysis showed smoking which less than 10 cigarettes per day (OR=1.06, p=0.001), between 10-20 cigarettes per day (OR=1.19, p<0.001), between 20-30 cigarettes per day (OR=1.16, p<0.001), and more than 30 cigarettes per day (OR=1.18, p=0.001) increased the incidence of BC with statistical significance. The incidence of BC increased with the increase of smoking intensity. The detailed data was contained in Table 2.
Pack-years smoked
Pack-years were used to simultaneously assess smoking duration and smoking intensity. Pack-years were defined as the product of the number of cigarettes smoked per day and the number of years of smoking. According to the grouping criteria of the included studies, this study divided the relevant data into ‘<10 pack-years group’, ‘10-20 pack-years group’, ‘20-40 pack-years group’, and ‘>40 pack-years group’. The analysis showed that women who smoke with less than 10 pack-years (OR=1.05, p=0.005), 10-20 pack-years (OR=1.11, p<0.001), 20-40 pack-years (OR=1.21, p<0.001), and >40 pack-years (OR=1.17, p<0.001) had a higher incidence of BC than those who had never smoked. The detailed data was contained in Table 2.
Age started smoking
In this study, smoking initiation age was divided into ‘<16 years group’, ‘17-19 years group’, and ‘>20 years group’. The results suggested that active smoking, regardless of the age at which smoking started is younger than 16 years old (OR=1.11, 95% CI=1.07-1.15), between 17-19 years old (OR=1.06, 95% CI=1.12-1.20), or older than 20 years old (OR=1.08, 95% CI=1.04-1.11), would significantly increase the incidence of BC in women with slight heterogeneity. The detailed data was contained in Table 2.
Years since quitting
Years of quitting smoking were used to measure the effect of smoking cessation in the participants. Data were grouped by 10- and 20-year cessation years. Subgroup analysis showed that previous smoking history remained a risk factor for BC among women who had quit smoking for less than 20 years. Among them, the harm of previous smoking history to women who quit smoking for less than 10 years (OR=1.27, 95% CI=1.15-41, p<0.001) is significantly greater than that to those who quit smoking for 10-20 years (OR=1.05, 95% CI=1.00-1.09, p=0.046). With increased time to quit smoking comes a reduction in the harm caused by previous smoking history. Previous smoking history was no longer an observable risk factor for BC in women who had quit smoking for more than 20 years (OR=1.01, 95% CI=0.97-1.06, p=0.552). The detailed data was contained in Table 2.
Fertility status
Six studies explored the association between active smoking and BC in different fertility statuses. The analysis showed that active smoking can increase the risk of BC in the multiparous population (OR=1.13, 95% CI=1.07-1.20, p<0.001), but had no effect on BC in the nulliparous population (OR=1.05, 95% CI=0.92-1.20, p=0.432) without heterogeneity. The detailed data was contained in Table 2.
Active smoking before/after the first birth
Regarding the relationship between active smoking and BC risk before/after the first birth, 24 studies contained data before the first birth and 22 studies contained data after the first birth. The results of the analysis showed that active smoking significantly increased the incidence of BC, regardless of whether the mother was smoking before the first birth (OR=1.22, 95% CI=1.17-1.27, p<0.001) or smoking after the first birth (OR=1.08, 95% CI=1.04-1.12, p<0.001), with slight heterogeneity. Furthermore, active smoking before the first birth had a greater impact on inducing BC than active smoking after the first birth. The detailed data was contained in Table 2.
Among those who actively smoked before the first birth, data were grouped by 5 years of smoking. Subgroup analysis showed that active smoking before the first birth increased the risk of BC whether the duration of smoking less than 5 years (OR=1.06, p=0.023) or more than 5 years (OR=1.24, p<0.001). There was a positive correlation between the smoking duration before the first birth and the risk of BC. Among those who have actively smoked after the first born, data were grouped by 10 years of smoking. Subgroup analysis showed that active smoking after the first birth had no effect on BC whether the duration of smoking less than 10 years (OR=1.00, p=0.922) or more than 10 years (OR=1.06, p=0.077). However, with the increase of smoking duration, active smoking had a tendency to harm the female population after the first birth by inducing BC. The detailed data was contained in Table 2.
BC subtypes
Six studies examined the association between active smoking and BC subtypes. The results showed that active smoking increased the incidence of ER+ BC (OR=1.13, 95% CI=1.08-1.18, p<0.001), but had no effect on ER- BC (OR=1.08, 95% CI=0.97-1.19, p=0.155), without heterogeneity. The detailed data was contained in Table 2.
BC subtypes and smoking duration
This study grouped data by 10-year active smoking aimed to investigate the correlation between different smoking duration and BC subtype. The analysis showed that active smoking for less than 10 years did not increase the incidence of BC, regardless of whether it was ER+ BC (OR=0.99, p=0.870) or ER- BC (OR=1.02, p=0.699). Active smoking for more than 10 years had no effect on ER- BC (OR=1.08, p=0.105), but could increase the incidence of ER+ BC (OR=1.14, p=0.007). The detailed data was contained in Table 2.
BC subtypes and smoking intensity
This study investigated the effect of smoking on BC subtypes at different smoking intensities by grouping data at 10 cigarettes per day boundaries. Subgroup analysis showed that smoking less than 10 cigarettes per day (OR=1.08, p=0.041) and more than 10 cigarettes per day (OR=1.18, p=0.002) could increase the risk of ER+ BC, and the risk was positively related to smoking intensity. For ER- BC, smoking less than 10 cigarettes per day had not been discovered as being effective (OR=0.97, p=0.603), However, smoking more than 10 cigarettes per day could increase the risk of suffering from ER- BC (OR=1.18, p=0.049). The results suggested that the occurrence of ER+ BC was more likely to be affected by active smoking than ER- BC. The detailed data was contained in Table 2.
Overall effect of passive smoking
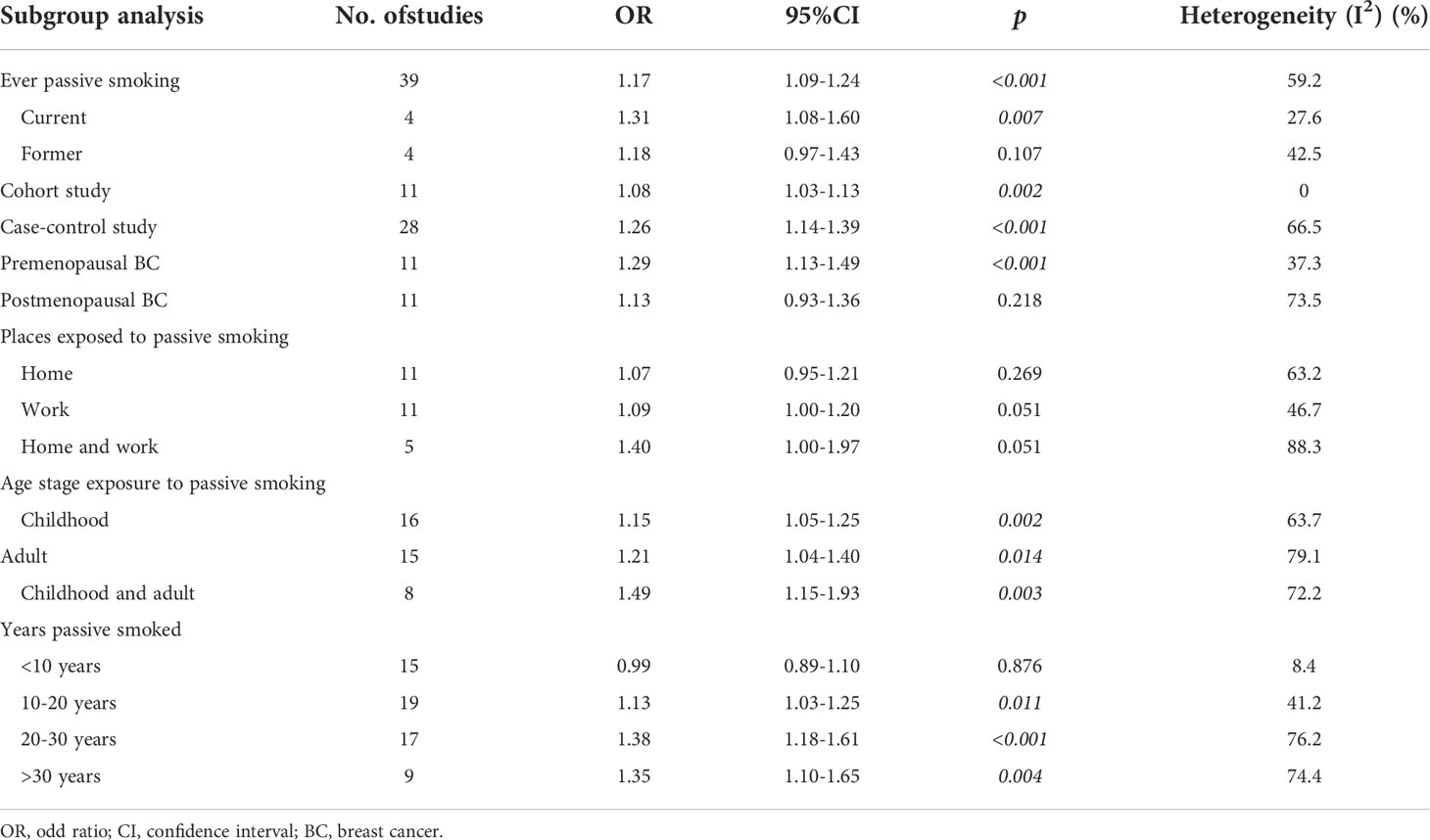
Thirty-nine studies documented BC risk data from passive smoking in women. The analysis showed that the risk of BC was significantly higher among women who passively smoked than those without passive smoking episode (OR=1.17, 95% CI=1.09-1.24, p<0.001, I2 = 59.2%). Among them, current passive smoking had a significant effect on BC (OR=1.31, 95% CI=1.08-1.60, p=0.007, I2 = 27.6%), but such history had no effect on BC (OR=1.18, 95% CI=0.97-1.43, p=0.107, I2 = 42.5%). This suggests that passive smoking, especially current passive smoking would increase the risk of BC. Furthermore, cohort studies (OR=1.08, 95% CI=1.03-1.13) and case-control studies (OR=1.15, 95% CI=1.14-1.39) had consistently concluded that passive smoking increases the risk of BC in women. The detailed data was shown in Table 3.
Menopausal status
Eleven studies included data on the relationship between passive smoking and BC in different menopausal states. The analysis showed that passive smoking increased the risk of premenopausal BC (OR=1.29, 95% CI=1.13-1.49, p<0.001, I2 = 37.3%), but had no effect on the incidence of postmenopausal BC (OR=1.13, 95% CI=0.93-1.36, p=0.218, I2 = 73.5%). The detailed data was contained in Table 3.
Places exposed to passive smoking
Regarding the relationship of passive smoking and BC in different exposure places, 11 studies had data on home exposure, 11 studies had data on work exposure, and 5 studies had data on both home and work exposure. Subgroup analysis showed no relationship between passive smoking and BC incidence in different passive smoking exposure settings. However, passive smoking exposure at work (OR=1.09, p=0.051) and exposure at both home and work (OR=1.40, p=0.051) had a trend of harm to female population. The detailed data was contained in Table 3.
Age stage exposure to passive smoking
In terms of the association between passive smoking and BC at different exposure ages, 16 studies had data on exposure in childhood, 15 studies had data on exposure in adult, and 8 studies had data on exposure in children and adult. Subgroup analyses showed that passive smoking increased BC risk regardless of exposure to childhood (OR=1.15, p=0.002), adult (OR=1.21, p=0.014), or both childhood and adult (OR=1.49, p=0.003). Among them, the increased risk of BC in those with simultaneous exposure in childhood and adult was significantly greater than that in those only with a single age group. The detailed data was contained in Table 3.
Years passive smoked
Years were used to measure the duration of passive smoking exposure in this study. The relevant data were divided into ‘less than 10 years group’, ‘10-20 years group’, ‘20-30 years group’, and ‘more than 30 years group’, in the way most studies were segmented. This study showed that passive smoking which duration was less than 10 years in female population had no effect on BC (OR=0.99, p=0.876), while passive smoking exposure for 10-20 years (OR=1.13, p=0.011), 20-30 years (OR=1.38, p<0.001) and more than 30 years (OR=1.35, p=0.004) had a significant impact on the incidence of BC, compared to women who had never smoked. In all, increased incidence was positively correlated with longer duration of passive smoking exposure. The detailed data was contained in Table 3.
Study quality
The NOS checklist was adopted to objectively evaluate the quality of included observational studies in this meta-study. 95.83% of the cohort studies were of high quality (NOS score >7), while 94.33% case-control studies were of high quality (NOS score >7). The quality ratings of cohort and case-control studies were listed in Supplementary Tables 2 and 3.
Publication bias and sensitivity analysis
Publication bias was evaluated by the Begg’s test. The results of Begg’s test indicated the absence of publication bias among included articles (p>0.05). Sensitivity analysis was used to assess whether the individual studies affected the overall results or not. The results indicated that the analysis was relatively stable.
Discussion
Through data analysis, this study found that smoking (active and passive) increases the risk of BC in women, with cohort and case-control studies showing consistent conclusions. Subgroup analysis of smoking-related factors showed that the effect of smoking on BC was positively correlated with smoking intensity and smoking duration. Among active smokers, current active smoking is more harmful to women than previous active smoking. With the increase of smoking cessation time, the harm of previous smoking history to the female population decreased. No differences were observed in the effect of smoking on BC at different starting ages. Among passive smokers, current passive smoking increases the incidence of BC, but past passive smoking does not. No differences in the effects of smoking on BC were observed between different passive smoking exposure sites and exposure age groups.
Subgroup analyses of population-related factors showed that smoking significantly increased the risk of BC in the multiparous population, but not in the nulliparous population. Smoking before the first birth has a greater effect on BC risk than smoking after the first birth. The risk of BC increases in women of different reproductive statuses with increasing duration of smoking.
Subgroup analysis of BC-related factors showed that smoking increases the risk of premenopausal BC, but has no effect on postmenopausal BC. At the same time, it can be clearly observed that smoking increases the risk of ER+ BC, and it is positively correlated with smoking duration and smoking intensity. For ER-BC, there was a trend of harm to women from smoking with increasing duration and intensity of smoking, but the difference did not reach statistical significance.
There is no consensus on the mechanism by which smoking increasing the risk of BC in women. The mainstream view is that smoking-specific DNA adducts (116, 117) (chemical carcinogens are activated by enzymes into electrophile and covalent combined with DNA, which are used to show DNA damage of specific carcinogens in human tissues (118)), mutations, and mal-regulated signaling pathways (119) represented by p53 [genes that inhibit cells from turning into cancer cells (75)] are the most important factors in BC (120). Animal and in vitro studies have shown that fat-soluble mutagenic compounds (121) in tobacco smoke, such as polycyclic hydrocarbons (122), aromatic amines (20) and N-nitrosamines (123), are major components of DNA adducts that can induce breast tumors (117) and have been detected in human milk (116). Compared with nonsmokers, detectable increases in cancer-causing DNA adducts were found in BC tissues and normal tissue adjacent to tumors in smokers (34, 124). In addition, studies have found that tobacco alters the incidence and spectrum of p53 mutations in breast cells, making smokers significantly more likely to carry p53 mutations (125). The potentially increased mutations affect related signaling pathways in smokers’ breast cells, hinder damage DNA repair and apoptosis, cause the body to be unable to respond to oncogenic signals, and ultimately induce tumors (126). The longer the exposure and the greater the intensity, the greater the effect (127). The starting point for these mechanisms is the compounds in tobacco smoke, which are present both in the smoke inhaled by smokers (mainly active smokers) and in the smoke exhaled by smokers and the end of lit cigarettes (mainly passive smokers) (128). This supports the conclusion in this study that both active smoking and passive smoking can induce BC in women, and confirms the biological plausibility of the positive correlation between BC risk and smoking intensity and duration. In addition, smoking status was correlated with the levels of carcinogenic DNA adducts in normal tissues adjacent to tumors, with a significant linear trend in the levels of carcinogenic DNA adducts in never-smokers, former smokers, and current smokers (19). When tobacco exposure was stopped, cancer cells became less active and the mutant gene was partially restored (34, 129). This supports our findings that the risk of current smoking is greater for women than previous smoking, and that the risk of BC from previous smoking decreases as the duration of cessation increases.
A relatively new view is that the harmful effects of smoking on BC depend on the antagonism of the estrogen-like and anti-estrogen-like effects of tobacco. According to previous studies, the health of the female breast is affected by the level and proportion of estrogen and progesterone (130, 131). Long-term exposure to estrogen or increased cell response to estrogen is an important risk factor for BC development (132, 133). On the one hand, carcinogenic metal-like metals in tobacco (106, 134), such as cadmium, chromium and arsenic, can induce estrogen receptor activation through hormone-binding domains and play estrogen-like roles in cell culture and animals (134). On the other hand, polycyclic aromatic hydrocarbons substances in tobacco play an anti-estrogen-like effect by competing with estrogen receptors or inducing hormone metabolism to reduce the level of active estrogen in the body (135, 136). At present, researchers tend to believe that the estrogen-like effect of tobacco and its carcinogenic effect are far superior to the breast protective effect brought by the anti-estrogen effect (114, 137). The anti-estrogen effect may cause breast cells to increase the number of estrogen receptors and enhance the sensitivity to estrogen, thus leading to the occurrence of hormone-sensitive tumors (138). There is accumulating evidence that ER+ and lobular BCs are more sensitive to ovarian hormones than are ER- and ductal cancers (139, 140). This may explain why smoking increases the incidence of ER+ BC, and the risk is positively correlated with the duration and intensity of smoking, but had no effect on ER- BC. In addition, premenopausal women have active gonadal function and secrete more estrogen (12, 129), which further aggravates the imbalance between estrogen and anti-estrogen effect on the basis of estrogen-like effect caused by tobacco, thus more likely to lead to the higher occurrence of BC (141). This supports the conclusion in this study that smoking increases the risk in premenopausal BC development, but not in postmenopausal BC development.
Based on the above two theories, tobacco exposure during the critical period is also considered to be an important factor affecting the occurrence of BC (100, 142). Animal models show that breast tissue is highly differentiated from puberty to the first full-term pregnancy, during which time the rapidly dividing cells are susceptible to malignant transformation due to carcinogens (143, 144). This period is therefore considered to be the period when tobacco smoke causes the greatest carcinogenic damage to breast tissue (145). During or after pregnancy, the second stage of BC carcinogenic damage is considered to be due to the onset of lactation, when breast cells are again active proliferation and vulnerable to tobacco smoke (146, 147). This may explain why smoking before the first birth had a greater impact on BC risk than smoking after the first birth. Unfortunately, no significant difference was observed in the subgroup analysis of the effect of smoking initiation on BC at different age in this study. In addition, increased exposure to estrogen (148), progesterone (149), and insulin-like growth factor (increased by growth hormone) (150) during pregnancy has been associated with promoting BC cell proliferation, which can trigger and/or promote tumors during continued tobacco exposure, known as “pregnancy-associated BC” (151–153). Epidemiological studies have found a higher incidence of BC in all multiparous women with, compared to all multiparous women regardless of their age (154–157). The higher incidence rate of BC in the multiparous population and the impact of tobacco exposure on estrogen levels in pregnant people may explain why smoking significantly increases the risk of BC in the multiparous population, but had no impact in the nulliparous population.
According to the above mechanisms and the characteristics of different included studies, we believe that the reasons for the differences between different studies may be as follows: First, each study has different assessment methods for exposure factors. Questionnaires and interviews both produce recall bias. The rigor of questionnaire design and the professionalism of interviewers will affect the validity of data collection, which makes researchers inevitably biased when exploring the relationship between smoking and BC; second, The duration of follow-up in the included studies varied considerably. The occurrence of BC often takes years to decades, and there is no exact number of years, but a longer follow-up period can often find more cases of BC, which can provide more abundant research data, conversely, a shorter follow-up period Time, not only limited the researchers’ discovery of the association between smoking and BC, but also prevented subgroup analyses; third, different studies defined smoking differently. According to World Health Organization (WHO) regulations, people who smoke continuously or cumulatively for 6 months or more are smokers in some studies, some studies extend the duration to 1 year, and some studies define smokers as long as they smoke. Different criteria make the baseline status of the control population different, and although the concentration of carcinogens in tobacco is not high, it may still have an impact on the final results with long-term follow-up. Therefore, we believe that the results of the study can be improved by shortening the time between two follow-up visits, increasing the number of follow-up visits, and updating them in a timely manner. In addition, large-scale cohort studies are still a feasible way to verify the conclusions of this study and narrow the differences between different studies.
Reviewing the same type of studies, A-sol Kim et al.’s study (158) reached a similar conclusion to the present study that passive smoking increases the risk of BC in women (OR=1.23, 95%CI=1.10-1.38). However, they did not perform subgroup analysis on population and smoking factors, thus could not provide reference to the female population from multiple aspects. Moreover, they only included those who had never smoked, did not consider those who had previously smoked and had successfully gone through smoking cessation. These may have led to their findings being overestimated and lacking reliability. The study by Lisa A DeRoo et al (159) did not find any association between smoking and BC. This may be due to the limited number of studies they included, or it may be that the low concentration of carcinogens in tobacco with a long latency to harm the breast make the relationship between smoking and BC not easily observed.
While this meta-analysis yielded comprehensive and objective conclusions, there were still some potential limitations to consider. Firstly, the design, study population, sample size, risk assessment, and adjustment for related confounding factors varied among the included studies, which may bias the results and reduce the confidence of the conclusions. Therefore, this study used a random-effects model to evaluate the effect of smoking on BC. Secondly, most studies used questionnaires to assess smoking exposure, and a few used the form of interviews or a combination of interviews and questionnaires, therefore inevitably led to evaluation bias or recall bias during the evaluation, especially the case-control studies nested in the cohort, which may bias the findings. Therefore, this study selected relevant data adjusted for the largest number of potential confounders for statistical analysis to improve the accuracy of the conclusions. Thirdly, some trials did not report more adequate subgroup data, such as BC type subgroup data, fertility status subgroup data, etc., which made it very difficult to conduct some subgroup analyses in this study.
Apart from its limitations, this meta-analysis had its own strengths. Firstly, this study included a large number of observational studies including more than 2.3 million participants in Asia, Europe, America, and Oceania. The larger observational population increases the reliability and authenticity of the conclusions of this study. Additionally, this study grouped the extracted data (by smoking related factors, population related factors, BC-related factors) and performed subgroup analysis to comprehensively explore the possibility of the effect of different kinds of smoking on different populations, different BC types from different aspects. Overall, this meta-analysis led to some meaningful conclusions that may provide a new reference for BC prevention in the female population.
Conclusion
This meta-analysis found that smoking (active and passive smoking) increases the risk of BC in the female population, especially premenopausal BC and ER+ BC, but had no effect on postmenopausal BC and ER- BC. The risk of BC was positively associated with the longer duration and stronger intensity of smoking, negatively associated with the duration of smoking cessation. Smoking increases BC risk in the multiparous population, but had no effect in the nulliparous population, where smoking before the first birth had a larger effect on BC risk than smoking after the first birth.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors helped to perform the research. YH and XL writing manuscript; YH and YS performing procedures and data analysis; JH and CY contribution to writing the manuscript; NH contribution to drafting conception and design. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.961970/full#supplementary-material
Abbreviations
BC, breast cancer; MOOSE, the meta-analysis of observational studies in epidemiology; PICOS, the population, intervention, comparison, outcome and setting criteria; NOS, the newcastle-ottawa quality assessment scale checklist; ER+, estrogen receptor-positive; ER-, estrogen receptor-negative; CI, confidence interval; OR, odd ratio; BMI, body mass index; WHO, world health organization.
References
1. Nagini S. Breast cancer: Current molecular therapeutic targets and new players. Anticancer Agents Med Chem (2017) 17(2):152–63. doi: 10.2174/1871520616666160502122724
2. Brignoni L, Cappetta M, Colistro V, Sans M, Artagaveytia N, Bonilla C, et al. Genomic diversity in sporadic breast cancer in a Latin American population. Genes (Basel) (2020) 11(11):1272. doi: 10.3390/genes11111272
3. Lee A, Moon BI, Kim TH. BRCA1/BRCA2 pathogenic variant breast cancer: Treatment and prevention strategies. Ann Lab Med (2020) 40(2):114–21. doi: 10.3343/alm.2020.40.2.114
4. Ambrosone CB, Hong CC, Goodwin PJ. Host factors and risk of breast cancer recurrence: Genetic, epigenetic and biologic factors and breast cancer outcomes. Adv Exp Med Biol (2015) 862:143–53. doi: 10.1007/978-3-319-16366-6_10
5. Kwong A, Shin VY, Ho JC, Kang E, Nakamura S, Teo SH, et al. Comprehensive spectrum of BRCA1 and BRCA2 deleterious mutations in breast cancer in Asian countries. J Med Genet (2016) 53(1):15–23. doi: 10.1136/jmedgenet-2015-103132
6. Rieder V, Salama M, Glockner L, Muhr D, Berger A, Tea MK, et al. Effect of lifestyle and reproductive factors on the onset of breast cancer in female BRCA 1 and 2 mutation carriers. Mol Genet Genomic Med (2016) 4(2):172–7. doi: 10.1002/mgg3.191
7. Zbuk K, Anand SS. Declining incidence of breast cancer after decreased use of hormone-replacement therapy: Magnitude and time lags in different countries. J Epidemiol Community Health (2012) 66(1):1–7. doi: 10.1136/jech.2008.083774
8. Park SY, Kolonel LN, Lim U, White KK, Henderson BE, Wilkens LR. Alcohol consumption and breast cancer risk among women from five ethnic groups with light to moderate intakes: The multiethnic cohort study. Int J Cancer (2014) 134(6):1504–10. doi: 10.1002/ijc.28476
9. Castello A, Martin M, Ruiz A, Casas AM, Baena-Canada JM, Lope V, et al. Lower breast cancer risk among women following the world cancer research fund and American institute for cancer research lifestyle recommendations: EpiGEICAM case-control study. PloS One (2015) 10(5):e0126096. doi: 10.1371/journal.pone.0126096
10. Ferrini K, Ghelfi F, Mannucci R, Titta L. Lifestyle, nutrition and breast cancer: facts and presumptions for consideration. Ecancermedicalscience (2015) 9:557. doi: 10.3332/ecancer.2015.557
11. Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, et al. Risk factors and preventions of breast cancer. Int J Biol Sci (2017) 13(11):1387–97. doi: 10.7150/ijbs.21635
12. Cordina-Duverger E, Koudou Y, Truong T, Arveux P, Kerbrat P, Menegaux F, et al. Night work and breast cancer risk defined by human epidermal growth factor receptor-2 (HER2) and hormone receptor status: A population-based case-control study in France. Chronobiol Int (2016) 33(6):783–7. doi: 10.3109/07420528.2016.1167709
13. Godinho-Mota JCM, Goncalves LV, Mota JF, Soares LR, Schincaglia RM, Martins KA, et al. Sedentary behavior and alcohol consumption increase breast cancer risk regardless of menopausal status: A case-control study. Nutrients (2019) 11(8):1871. doi: 10.3390/nu11081871
14. Lee J, Lee J, Lee DW, Kim HR, Kang MY. Sedentary work and breast cancer risk: A systematic review and meta-analysis. J Occup Health (2021) 63(1):e12239. doi: 10.1002/1348-9585.12239
15. Chlebowski RT, Aragaki AK, Anderson GL, Thomson CA, Manson JE, Simon MS, et al. Low-fat dietary pattern and breast cancer mortality in the women’s health initiative randomized controlled trial. J Clin Oncol (2017) 35(25):2919–26. doi: 10.1200/JCO.2016.72.0326
16. Goncalves RM, Delgobo M, Agnes JP, Neves das RN, Falchetti M, Casagrande T, et al. COX-2 promotes mammary adipose tissue inflammation, local estrogen biosynthesis, and carcinogenesis in high-sugar/fat diet treated mice. Cancer Lett (2021) 502:44–57. doi: 10.1016/j.canlet.2021.01.003
17. Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, Salmon AG, et al. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian expert panel on tobacco smoke and breast cancer risk (2009). Tob Control (2011) 20(1):e2. doi: 10.1136/tc.2010.035931
18. Reynolds P. Smoking and breast cancer. J Mammary Gland Biol Neoplasia (2013) 18(1):15–23. doi: 10.1007/s10911-012-9269-x
19. Faraglia B, Chen SY, Gammon MD, Zhang Y, Teitelbaum SL, Neugut AI, et al. Evaluation of 4-aminobiphenyl-DNA adducts in human breast cancer: The influence of tobacco smoke. Carcinogenesis (2003) 24(4):719–25. doi: 10.1093/carcin/bgg013
20. Sheikh IA, Beg MA, Yasir M. Molecular interactions of carcinogenic aromatic amines, 4-aminobiphenyl and 4,4’-diaminobiphenyl, with lactoperoxidase - insight to breast cancer. Anticancer Res (2017) 37(11):6245–9. doi: 10.21873/anticanres.12075
21. Amadou A, Praud D, Coudon T, Deygas F, Grassot L, Faure E, et al. Risk of breast cancer associated with long-term exposure to benzo[a]pyrene (BaP) air pollution: Evidence from the French E3N cohort study. Environ Int (2021) 149:106399. doi: 10.1016/j.envint.2021.106399
22. Sadikovic B, Rodenhiser DI. Benzopyrene exposure disrupts DNA methylation and growth dynamics in breast cancer cells. Toxicol Appl Pharmacol (2006) 216(3):458–68. doi: 10.1016/j.taap.2006.06.012
23. Catsburg C, Kirsh VA, Soskolne CL, Kreiger N, Rohan TE. Active cigarette smoking and the risk of breast cancer: A cohort study. Cancer Epidemiol (2014) 38(4):376–81. doi: 10.1016/j.canep.2014.05.007
24. Ghasemian A, Rezaei N, Saeedi Moghaddam S, Mansouri A, Parsaeian M, Delavari A, et al. Tobacco smoking status and the contribution to burden of diseases in Iran, 1990-2010: Findings from the global burden of disease study 2010. Arch Iran Med (2015) 18(8):493–501. doi: 10.015188/AIM.006
25. Carreras G, Lachi A, Boffi R, Clancy L, Gallus S, Fernandez E, et al. Burden of disease from breast cancer attributable to smoking and second-hand smoke exposure in Europe. Int J Cancer (2020) 147(9):2387–93. doi: 10.1002/ijc.33021
26. Malik A, Jeyaraj PA, Shankar A, Rath GK, Mukhopadhyay S, Kamal VK. Passive smoking and breast cancer - a suspicious link. Asian Pac J Cancer Prev (2015) 16(14):5715–9. doi: 10.7314/APJCP.2015.16.14.5715
27. Thomas RD, Vigerstad TJ. Use of laboratory animal models in investigating emphysema and cigarette smoking in humans. Regul Toxicol Pharmacol (1989) 10(3):264–71. doi: 10.1016/0273-2300(89)90053-6
28. Wehner AP, Dagle GE, Milliman EM, Phelps DW, Carr DB, Decker JR, et al. Inhalation bioassay of cigarette smoke in rats. Toxicol Appl Pharmacol (1981) 61(1):1–17. doi: 10.1016/0041-008X(81)90002-8
29. Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ Mol Mutagen (2002) 39(2-3):119–26. doi: 10.1002/em.10071
30. Izano M, Satariano WA, Hiatt RA, Braithwaite D. Smoking and mortality after breast cancer diagnosis: The health and functioning in women study. Cancer Med (2015) 4(2):315–24. doi: 10.1002/cam4.359
31. Castillo-Sanchez R, Villegas-Comonfort S, Galindo-Hernandez O, Gomez R, Salazar EP. Benzo-[a]-pyrene induces FAK activation and cell migration in MDA-MB-231 breast cancer cells. Cell Biol Toxicol (2013) 29(4):303–19. doi: 10.1007/s10565-013-9254-1
32. Zeinomar N, Knight JA, Genkinger JM, Phillips KA, Daly MB, Milne RL, et al. Alcohol consumption, cigarette smoking, and familial breast cancer risk: findings from the prospective family study cohort (ProF-SC). Breast Cancer Res (2019) 21(1):128. doi: 10.1186/s13058-019-1213-1
33. Hanaoka T, Yamamoto S, Sobue T, Sasaki S, Tsugane S, C Japan Public Health Center-Based Prospective Study on, et al. Active and passive smoking and breast cancer risk in middle-aged Japanese women. Int J Cancer (2005) 114(2):317–22. doi: 10.1002/ijc.20709
34. Luo J, Margolis KL, Wactawski-Wende J, Horn K, Messina C, Stefanick ML, et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: A prospective cohort study. BMJ (2011) 342:d1016. doi: 10.1136/bmj.d1016
35. Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J Natl Cancer Inst (2013) 105(8):515–25. doi: 10.1093/jnci/djt023
36. Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res Treat (2015) 154(2):213–24. doi: 10.1007/s10549-015-3628-4
37. Chen Z, Shao J, Gao X, Li X. Effect of passive smoking on female breast cancer in China: A meta-analysis. Asia Pac J Public Health (2015) 27(2):NP58–64. doi: 10.1177/1010539513481493
38. Yang Y, Zhang F, Skrip L, Wang Y, Liu S. Lack of an association between passive smoking and incidence of female breast cancer in non-smokers: Evidence from 10 prospective cohort studies. PloS One (2013) 8(10):e77029. doi: 10.1371/journal.pone.0077029
39. Burki TK. WHO releases latest report on the global tobacco epidemic. Lancet Oncol (2021) 22(9):1217. doi: 10.1016/S1470-2045(21)00464-2
40. Zhang K, Tartarone A, Perez-Rios M, Novello S, Mariniello A, Roviello G, et al. Smoking burden, MPOWER, future tobacco control and real-world challenges in China: Reflections on the WHO report on the global tobacco epidemic 2021. Transl Lung Cancer Res (2022) 11(1):117–21. doi: 10.21037/tlcr-22-27
41. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
42. Adami HO, Lund E, Bergstrom R, Meirik O. Cigarette smoking, alcohol consumption and risk of breast cancer in young women. Br J Cancer (1988) 58(6):832–7. doi: 10.1038/bjc.1988.320
43. Ahern TP, Lash TL, Egan KM, Baron JA. Lifetime tobacco smoke exposure and breast cancer incidence. Cancer Causes Control (2009) 20(10):1837–44. doi: 10.1007/s10552-009-9376-1
44. Alberg AJ, Daudt A, Huang HY, Hoffman SC, Comstock GW, Helzlsouer KJ, et al. N-acetyltransferase 2 (NAT2) genotypes, cigarette smoking, and the risk of breast cancer. Cancer Detect Prev (2004) 28(3):187–93. doi: 10.1016/j.cdp.2004.04.001
45. Alsolami FJ, Azzeh FS, Ghafouri KJ, Ghaith MM, Almaimani RA, Almasmoum HA, et al. Determinants of breast cancer in Saudi women from makkah region: A case-control study (breast cancer risk factors among Saudi women). BMC Public Health (2019) 19(1):1554. doi: 10.1186/s12889-019-7942-3
46. Baset Z, Abdul-Ghafar J, Parpio YN, Haidary AM. Risk factors of breast cancer among patients in a tertiary care hospitals in Afghanistan: A case control study. BMC Cancer (2021) 21(1):71. doi: 10.1186/s12885-021-07798-5
47. Bennicke K, Conrad C, Sabroe S, Sorensen HT. Cigarette smoking and breast cancer. BMJ (1995) 310(6992):1431–3. doi: 10.1136/bmj.310.6992.1431
48. Bonner MR, Nie J, Han D, Vena JE, Rogerson P, Muti P, et al. Secondhand smoke exposure in early life and the risk of breast cancer among never smokers (United states). Cancer Causes Control (2005) 16(6):683–9. doi: 10.1007/s10552-005-1906-x
49. Botteri E, Berstad P, Sandin S, Weiderpass E. Lifestyle changes and risk of cancer: experience from the Swedish women’s lifestyle and health cohort study. Acta Oncol (2021) 60(7):827–34. doi: 10.1080/0284186X.2021.1919756
50. Braga C, Negri E, La Vecchia C, Filiberti R, Franceschi S. Cigarette smoking and the risk of breast cancer. Eur J Cancer Prev (1996) 5(3):159–64. doi: 10.1097/00008469-199606000-00003
51. Butler EN, Tse CK, Bell ME, Conway K, Olshan AF, Troester MA. Active smoking and risk of luminal and basal-like breast cancer subtypes in the Carolina breast cancer study. Cancer Causes Control (2016) 27(6):775–86. doi: 10.1007/s10552-016-0754-1
52. Calle EE, Miracle-McMahill HL, Thun MJ, Heath CW. Cigarette smoking and risk of fatal breast cancer. Am J Epidemiol (1994) 139(10):1001–7. doi: 10.1093/oxfordjournals.aje.a116939
53. Catsburg C, Miller AB, Rohan TE. Active cigarette smoking and risk of breast cancer. Int J Cancer (2015) 136(9):2204–9. doi: 10.1002/ijc.29266
54. Chu SY, Stroup NE, Wingo PA, Lee NC, Peterson HB, Gwinn ML. Cigarette smoking and the risk of breast cancer. Am J Epidemiol (1990) 131(2):244–53. doi: 10.1093/oxfordjournals.aje.a115494
55. Conlon MS, Johnson KC, Bewick MA, Lafrenie RM, Donner A. Smoking (active and passive), n-acetyltransferase 2, and risk of breast cancer. Cancer Epidemiol (2010) 34(2):142–9. doi: 10.1016/j.canep.2010.02.001
56. Connor AE, Baumgartner KB, Baumgartner RN, Pinkston CM, Boone SD, John EM, et al. Cigarette smoking and breast cancer risk in Hispanic and non-Hispanic white women: The breast cancer health disparities study. J Womens Health (Larchmt) (2016) 25(3):299–310. doi: 10.1089/jwh.2015.5502
57. De Silva M, Senarath U, Gunatilake M, Lokuhetty D. Prolonged breastfeeding reduces risk of breast cancer in Sri Lankan women: A case-control study. Cancer Epidemiol (2010) 34(3):267–73. doi: 10.1016/j.canep.2010.02.012
58. Dianatinasab M, Fararouei M, Mohammadianpanah M, Zare-Bandamiri M, Rezaianzadeh A. Hair coloring, stress, and smoking increase the risk of breast cancer: A case-control study. Clin Breast Cancer (2017) 17(8):650–9. doi: 10.1016/j.clbc.2017.04.012
59. Dossus L, Boutron-Ruault MC, Kaaks R, Gram IT, Vilier A, Fervers B, et al. Active and passive cigarette smoking and breast cancer risk: Results from the EPIC cohort. Int J Cancer (2014) 134(8):1871–88. doi: 10.1002/ijc.28508
60. Ellingjord-Dale M, Vos L, Hjerkind KV, Hjartaker A, Russnes HG, Tretli S, et al. Alcohol, physical activity, smoking, and breast cancer subtypes in a Large, nested case-control study from the Norwegian breast cancer screening program. Cancer Epidemiol Biomarkers Prev (2017) 26(12):1736–44. doi: 10.1158/1055-9965.EPI-17-0611
61. Field NA, Baptiste MS, Nasca PC, Metzger BB. Cigarette smoking and breast cancer. Int J Epidemiol (1992) 21(5):842–8. doi: 10.1093/ije/21.5.842
62. Gammon MD, Eng SM, Teitelbaum SL, Britton JA, Kabat GC, Hatch M, et al. Environmental tobacco smoke and breast cancer incidence. Environ Res (2004) 96(2):176–85. doi: 10.1016/j.envres.2003.08.009
63. Gao CM, Ding JH, Li SP, Liu YT, Qian Y, Chang J, et al. Active and passive smoking, and alcohol drinking and breast cancer risk in chinese women. Asian Pac J Cancer Prev (2013) 14(2):993–6. doi: 10.7314/apjcp.2013.14.2.993
64. Goodman MT, Cologne JB, Moriwaki H, Vaeth M, Mabuchi K. Risk factors for primary breast cancer in Japan: 8-year follow-up of atomic bomb survivors. Prev Med (1997) 26(1):144–53. doi: 10.1006/pmed.1996.9979
65. Gram IT, Park SY, Maskarinec G, Wilkens LR, Haiman CA, Marchand Le L. Smoking and breast cancer risk by race/ethnicity and oestrogen and progesterone receptor status: The multiethnic cohort (MEC) study. Int J Epidemiol (2019) 48(2):501–11. doi: 10.1093/ije/dyy290
66. Gram IT, Wiik AB, Lund E, Licaj I, Braaten T. Never-smokers and the fraction of breast cancer attributable to second-hand smoke from parents during childhood: The Norwegian women and cancer study 1991–2018. Int J Epidemiol (2021) 50(6):1927–35. doi: 10.1093/ije/dyab153
67. Ha M, Mabuchi K, Sigurdson AJ, Freedman DM, Linet MS, Doody MM, et al. Smoking cigarettes before first childbirth and risk of breast cancer. Am J Epidemiol (2007) 166(1):55–61. doi: 10.1093/aje/kwm045
69. Hara A, Taira N, Mizoo T, et al. N-acetyltransferase 2 polymorphism and breast cancer risk with smoking: A case control study in Japanese women. Breast Cancer (2017) 24(2):254–62. doi: 10.1007/s12282-016-0696-1
70. Heberg J, Simonsen MK, Danielsen AK, Klausen TW, Zoffmann V, Thomsen T. Joint tobacco smoking and alcohol intake exacerbates cancer risk in women- the Danish nurse cohort. Eur J Oncol Nurs (2019) 43:101675. doi: 10.1016/j.ejon.2019.101675
71. Hirose K, Tajima K, Hamajima N, Inoue M, Takezaki T, Kuroishi T, et al. A large-scale, hospital-based case-control study of risk factors of breast cancer according to menopausal status. Jpn J Cancer Res (1995) 86(2):146–54. doi: 10.1111/j.1349-7006.1995.tb03032.x
72. Hu M, Han D, Sun S, Yan Y, Zhang J, Zhou Y. Bleomycin-induced mutagen sensitivity, passive smoking, and risk of breast cancer in Chinese women: A case-control study. Cancer Causes Control (2013) 24(4):629–36. doi: 10.1007/s10552-012-0137-1
73. Ilic M, Vlajinac H, Marinkovic J. Cigarette smoking and breast cancer: A case-control study in Serbia. Asian Pac J Cancer Prev (2014) 14(11):6643–7. doi: 10.7314/apjcp.2013.14.11.6643
74. Johnson KC, Hu J, Mao Y, et al. Passive and active smoking and breast cancer risk in Canada, 1994-97. Cancer Causes Control (2000) 11(3):211–21. doi: 10.1023/A:1008906105790
75. Jones ME, Schoemaker MJ, Wright LB, Ashworth A, Swerdlow AJ. Smoking and risk of breast cancer in the generations study cohort. Breast Cancer Res (2017) 19(1):118. doi: 10.1186/s13058-017-0908-4
76. Kato I, Miura S, Kasumi F, Iwase T, Tashiro H, Fujita Y, et al. A case-control study of breast cancer among Japanese women: With special reference to family history and reproductive and dietary factors. Breast Cancer Res Treat (1992) 24(1):51–9. doi: 10.1007/BF01832358
77. Kawai M, Malone KE, Tang MT, Li CI. Active smoking and the risk of estrogen receptor-positive and triple-negative breast cancer among women ages 20 to 44 years. Cancer (2014) 120(7):1026–34. doi: 10.1002/cncr.28402
78. Kropp S, Chang-Claude J. Active and passive smoking and risk of breast cancer by age 50 years among German women. Am J Epidemiol (2002) 156(7):616–26. doi: 10.1093/aje/kwf093
79. Lash TL, Aschengrau A. A null association between active or passive cigarette smoking and breast cancer risk. Breast Cancer Res Treat (2002) 75(2):181–4. doi: 10.1023/A:1019625102365
80. Li B, Wang L, Lu MS, et al. Passive smoking and breast cancer risk among non-smoking women: A case-control study in China. PloS One (2015) 10(4):e0125894. doi: 10.1371/journal.pone.0125894
81. Lin Y, Kikuchi S, Tamakoshi K, Wakai K, Kondo T, Niwa Y, et al. Active smoking, passive smoking, and breast cancer risk: findings from the Japan collaborative cohort study for evaluation of cancer risk. J Epidemiol (2008) 18(2):77–83. doi: 10.2188/jea.18.77
82. Liu L, Wu K, Lin X, Yin W, Zheng X, Tang X, et al. Passive smoking and other factors at different periods of life and breast cancer risk in Chinese women who have never smoked - a case-control study in chongqing, people’s republic of China. Asian Pac J Cancer Prev (2000) 1(2):131–7.
83. Manjer J, Johansson R, Lenner P. Smoking is associated with postmenopausal breast cancer in women with high levels of estrogens. Int J Cancer (2004) 112(2):324–8. doi: 10.1002/ijc.20409
84. Marcus PM, Newman B, Millikan RC, et al. The associations of adolescent cigarette smoking, alcoholic beverage consumption, environmental tobacco smoke, and ionizing radiation with subsequent breast cancer risk (United states). Cancer Causes Control (2000) 11(3):271–8. doi: 10.1023/A:1008911902994
85. McKenzie F, Ellison-Loschmann L, Jeffreys M, Firestone R, Pearce N, Romieu I. Cigarette smoking and risk of breast cancer in a new Zealand multi-ethnic case-control study. PloS One (2013) 8(4):e63132. doi: 10.1371/journal.pone.0063132
86. Mechanic LE, Millikan RC, Player J, de Cotret AR, Winkel S, Worley K, et al. Polymorphisms in nucleotide excision repair genes, smoking and breast cancer in African americans and whites: A population-based case-control study. Carcinogenesis (2006) 27(7):1377–85. doi: 10.1093/carcin/bgi330
87. Metsola K, Kataja V, Sillanpaa P, Siivola P, Heikinheimo L, Eskelinen M, et al. XRCC1 and XPD genetic polymorphisms, smoking and breast cancer risk in a Finnish case-control study. Breast Cancer Res (2005) 7(6):R987–97. doi: 10.1186/bcr1333
88. Morabia A, Bernstein M, Ruiz J, Heritier S, Berger Diebold S, Borisch B. Relation of smoking to breast cancer by estrogen receptor status. Int J Cancer (1998) 75(3):339–42. doi: 10.1002/(SICI)1097-0215(19980130)75:3<339::AID-IJC2>3.0.CO;2-3
89. Nishino Y, Tsubono Y, Tsuji I, Komatsu S, Kanemura S, Nakatsuka H, et al. Passive smoking at home and cancer risk: A population-based prospective study in Japanese nonsmoking women. Cancer Causes Control (2001) 12(9):797–802. doi: 10.1023/A:1012273806199
90. Olson JE, Vachon CM, Vierkant RA, Sweeney C, Limburg PJ, Cerhan JR, et al. Prepregnancy exposure to cigarette smoking and subsequent risk of postmenopausal breast cancer. Mayo Clin Proc (2005) 80(11):1423–8. doi: 10.4065/80.11.1423
91. Park SY, Palmer JR, Rosenberg L, Haiman CA, Bandera EV, Bethea TN, et al. A case-control analysis of smoking and breast cancer in African American women: findings from the AMBER consortium. Carcinogenesis (2016) 37(6):607–15. doi: 10.1093/carcin/bgw040
92. Pawlega J. Breast cancer and smoking, vodka drinking and dietary habits. a case-control study. Acta Oncol (1992) 31(4):387–92. doi: 10.3109/02841869209088276
93. Pimhanam C, Sangrajrang S, Ekpanyaskul C. Tobacco smoke exposure and breast cancer risk in Thai urban females. Asian Pac J Cancer Prev (2014) 15(17):7407–11. doi: 10.7314/APJCP.2014.15.17.7407
94. Pirie K, Beral V, Peto R, et al. Passive smoking and breast cancer in never smokers: Prospective study and meta-analysis. Int J Epidemiol (2008) 37(5):1069–79. doi: 10.1093/ije/dyn110
95. Ranstam J, Olsson H. Alcohol, cigarette smoking, and the risk of breast cancer. Cancer Detect Prev (1995) 19(6):487–93.
96. Regev-Avraham Z, Baron-Epel O, Hammond SK, et al. Passive smoking, NAT2 polymorphism, and breast cancer risk in Israeli Arab women: A case-control study. Breast Cancer (2018) 25(2):176–84. doi: 10.1007/s12282-017-0809-5
97. Reynolds P, Goldberg D, Hurley S, Nelson DO, Largent J, Henderson KD, et al. Passive smoking and risk of breast cancer in the California teachers study. Cancer Epidemiol Biomarkers Prev (2009) 18(12):3389–98. doi: 10.1158/1055-9965.EPI-09-0936
98. Roddam AW, Pirie K, Pike MC, Chilvers C, Crossley B, Hermon C, et al. Active and passive smoking and the risk of breast cancer in women aged 36-45 years: A population based case-control study in the UK. Br J Cancer (2007) 97(3):434–9. doi: 10.1038/sj.bjc.6603859
99. Rollison DE, Brownson RC, Hathcock HL, Newschaffer CJ. Case-control study of tobacco smoke exposure and breast cancer risk in Delaware. BMC Cancer (2008) 8:157. doi: 10.1186/1471-2407-8-157
100. Rosenberg L, Boggs DA, Bethea TN, Wise LA, Adams-Campbell LL, Palmer JR. A prospective study of smoking and breast cancer risk among African-American women. Cancer Causes Control (2013) 24(12):2207–15. doi: 10.1007/s10552-013-0298-6
101. Schechter MT, Miller AB, Howe GR, Baines CJ, Craib KJ, Wall C. Cigarette smoking and breast cancer: case-control studies of prevalent and incident cancer in the Canadian national breast screening study. Am J Epidemiol (1989) 130(2):213–20. doi: 10.1093/oxfordjournals.aje.a115327
102. Sezer H, Yilmaz M, Gurler H, Koyuncu A. Breast cancer risk factors in Turkey: a hospital-based case-control study. Asian Pac J Cancer Prev (2011) 12(9):2317–22.
103. Shrubsole MJ, Gao YT, Dai Q, Shu XO, Ruan ZX, Jin F, et al. Passive smoking and breast cancer risk among non-smoking Chinese women. Int J Cancer (2004) 110(4):605–9. doi: 10.1002/ijc.20168
104. Slattery ML, Curtin K, Giuliano AR, Sweeney C, Baumgartner R, Edwards S, et al. Active and passive smoking, IL6, ESR1, and breast cancer risk. Breast Cancer Res Treat (2008) 109(1):101–11. doi: 10.1007/s10549-007-9629-1
105. Smith SJ, Deacon JM, Chilvers CE. Alcohol, smoking, passive smoking and caffeine in relation to breast cancer risk in young women. UK national case-control study group. Br J Cancer (1994) 70(1):112–9. doi: 10.1038/bjc.1994.258
106. Strumylaite L, Kregzdyte R, Poskiene L, et al. Association between lifetime exposure to passive smoking and risk of breast cancer subtypes defined by hormone receptor status among non-smoking Caucasian women. PloS One (2017) 12(2):e0171198. doi: 10.1371/journal.pone.0171198
107. Tong JH, Li Z, Shi J, Li HM, Wang Y, Fu LY, et al. Passive smoking exposure from partners as a risk factor for ER+/PR+ double positive breast cancer in never-smoking Chinese urban women: A hospital-based matched case control study. PloS One (2014) 9(5):e97498. doi: 10.1371/journal.pone.0097498
108. Tung HT, Tsukuma H, Tanaka H, Kinoshita N, Koyama Y, Ajiki W, et al. Risk factors for breast cancer in Japan, with special attention to anthropometric measurements and reproductive history. Jpn J Clin Oncol (1999) 29(3):137–46. doi: 10.1093/jjco/29.3.137
109. Ueji M, Ueno E, Hyiaman DO, Saito T, Takahashi H, Kano K. Risk factors for breast cancer among Japanese women: A case-control study in ibaraki, Japan. Breast Cancer (1998) 5(4):351–8. doi: 10.1007/BF02967431
110. van den Brandt PA. A possible dual effect of cigarette smoking on the risk of postmenopausal breast cancer. Eur J Epidemiol (2017) 32(8):683–90. doi: 10.1007/s10654-017-0282-7
111. Vatten LJ, Kvinnsland S. Cigarette smoking and risk of breast cancer: A prospective study of 24,329 Norwegian women. Eur J Cancer (1990) 26(7):830–3. doi: 10.1016/0277-5379(90)90164-O
112. Wada K, Kawachi T, Hori A, et al. Husband’s smoking status and breast cancer risk in Japan: From the takayama study. Cancer Sci (2015) 106(4):455–60. doi: 10.1111/cas.12619
113. White AJ, D’Aloisio AA, Nichols HB, DeRoo LA, Sandler DP. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control (2017) 28(7):667–75. doi: 10.1007/s10552-017-0903-1
114. Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med (2011) 171(2):125–33. doi: 10.1001/archinternmed.2010.503
115. Young E, Leatherdale S, Sloan M, Kreiger N, Barisic A. Age of smoking initiation and risk of breast cancer in a sample of Ontario women. Tob Induc Dis (2009) 5(1):4. doi: 10.1186/1617-9625-5-4
116. Lissowska J, Brinton LA, Zatonski W, Blair A, Bardin-Mikolajczak A, Peplonska B, et al. Tobacco smoking, NAT2 acetylation genotype and breast cancer risk. Int J Cancer (2006) 119(8):1961–9. doi: 10.1002/ijc.22044
117. Terry PD, Rohan TE. Cigarette smoking and the risk of breast cancer in women: A review of the literature. Cancer Epidemiol Biomarkers Prev (2002) 11(10 Pt 1):953–71.
118. Hwa Yun B, Guo J, Bellamri M, et al. DNA Adducts: Formation, biological effects, and new biospecimens for mass spectrometric measurements in humans. Mass Spectrom Rev (2020) 39(1-2):55–82. doi: 10.1002/mas.21570
119. Ko KP, Kim SJ, Huzarski T, Gronwald J, Lubinski J, Lynch HT, et al. The association between smoking and cancer incidence in BRCA1 and BRCA2 mutation carriers. Int J Cancer (2018) 142(11):2263–72. doi: 10.1002/ijc.31257
120. Li D, Zhang W, Sahin AA, Hittelman WN. DNA Adducts in normal tissue adjacent to breast cancer: A review. Cancer Detect Prev (1999) 23(6):454–62. doi: 10.1046/j.1525-1500.1999.99059.x
121. Petrakis NL. Nipple aspirate fluid in epidemiologic studies of breast disease. Epidemiol Rev (1993) 15(1):188–95. doi: 10.1093/oxfordjournals.epirev.a036104
122. Pedersen JE, Strandberg-Larsen K, Andersson M, et al. Breast cancer among Danish women occupationally exposed to diesel exhaust and polycyclic aromatic hydrocarbons, 1964-2016. Scand J Work Environ Health (2021) 47(2):154–62. doi: 10.5271/sjweh.3923
123. Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of n-nitroso compounds and excess gastric acidity. J Natl Cancer Inst (2003) 95(13):948–60. doi: 10.1093/jnci/95.13.948
124. Perera FP, Estabrook A, Hewer A, et al. Carcinogen-DNA adducts in human breast tissue. Cancer Epidemiol Biomarkers Prev (1995) 4(3):233–8.
125. Conway K, Edmiston SN, Cui L, Drouin SS, Pang J, He M, et al. Prevalence and spectrum of p53 mutations associated with smoking in breast cancer. Cancer Res (2002) 62(7):1987–95.
126. Pilley S, Rodriguez TA, Vousden KH. Mutant p53 in cell-cell interactions. Genes Dev (2021) 35(7-8):433–48. doi: 10.1101/gad.347542.120
127. Firozi PF, Bondy ML, Sahin AA, et al. Aromatic DNA adducts and polymorphisms of CYP1A1, NAT2, and GSTM1 in breast cancer. Carcinogenesis (2002) 23(2):301–6. doi: 10.1093/carcin/23.2.301
128. Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. A tribute to Ernst l. wynder. Chem Res Toxicol (2001) 14(7):767–90. doi: 10.1021/tx000260u
129. Daly AA, Rolph R, Cutress RI, et al. A review of modifiable risk factors in young women for the prevention of breast cancer. Breast Cancer (Dove Med Press) (2021) 13:241–57. doi: 10.2147/BCTT.S268401
130. Mascarenhas M, Kamath MS, Chandy A, Kunjummen AT. Progesterone/Estradiol ratio as a predictor in the ART cycles with premature progesterone elevation on the day of hCG trigger. J Reprod Infertil (2015) 16(3):155–61.
131. Shalom-Paz E, Aslih N, Samara N, Michaeli M, Ellenbogen A. Late follicular progesterone to estradiol ratio is not influenced by protocols or gonadotropins used. Reprod Biol Endocrinol (2015) 13:119. doi: 10.1186/s12958-015-0116-y
132. Anisimov VN, Alimova IN, Baturin DA, Popovich IG, Zabezhinski MA, Manton KG, et al. The effect of melatonin treatment regimen on mammary adenocarcinoma development in HER-2/neu transgenic mice. Int J Cancer (2003) 103(3):300–5. doi: 10.1002/ijc.10827
133. Girgert R, Bartsch C, Hill SM, Kreienberg R, Hanf V. Tracking the elusive antiestrogenic effect of melatonin: A new methodological approach. Neuro Endocrinol Lett (2003) 24(6):440–4.
134. Martin MB, Reiter R, Johnson M, Shah MS, Iann MC, Singh B, et al. Effects of tobacco smoke condensate on estrogen receptor-alpha gene expression and activity. Endocrinology (2007) 148(10):4676–86. doi: 10.1210/en.2007-0208
136. Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol (1990) 162(2):502–14. doi: 10.1016/0002-9378(90)90420-C
137. Gram IT, Braaten T, Terry PD, et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev (2005) 14(1):61–6. doi: 10.1158/1055-9965.61.14.1
138. Chen C, Wang X, Wang L, Yang F, Tang G, Xing H, et al. Effect of environmental tobacco smoke on levels of urinary hormone markers. Environ Health Perspect (2005) 113(4):412–7. doi: 10.1289/ehp.7436
139. Beral V, Reeves G, Bull D, Green J, C Million Women Study. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst (2011) 103(4):296–305. doi: 10.1093/jnci/djq527
140. Reeves GK, Beral V, Green J, Gathani T, Bull D, C Million Women Study. Hormonal therapy for menopause and breast-cancer risk by histological type: A cohort study and meta-analysis. Lancet Oncol (2006) 7(11):910–8. doi: 10.1016/S1470-2045(06)70911-1
141. Pesch B, Harth V, Rabstein S, Baisch C, Schiffermann M, Pallapies D, et al. Night work and breast cancer - results from the German GENICA study. Scand J Work Environ Health (2010) 36(2):134–41. doi: 10.5271/sjweh.2890
142. Humans, IWGotEoCRt. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum (2004) 83:1–1438. doi: 10.5271/sjweh.2890
143. Althuis MD, Fergenbaum JH, Garcia-Closas M, et al. Etiology of hormone receptor-defined breast cancer: A systematic review of the literature. Cancer Epidemiol Biomarkers Prev (2004) 13(10):1558–68. doi: 10.1158/1055-9965.1558.13.10
144. Habel LA, Stanford JL. Hormone receptors and breast cancer. Epidemiol Rev (1993) 15(1):209–19. doi: 10.1093/oxfordjournals.epirev.a036107
145. Morabia A. Smoking (active and passive) and breast cancer: Epidemiologic evidence up to June 2001. Environ Mol Mutagen (2002) 39(2-3):89–95. doi: 10.1002/em.10046
146. Palmer JR, Rosenberg L, Clarke EA, et al. Breast cancer and cigarette smoking: A hypothesis. Am J Epidemiol (1991) 134(1):1–13. doi: 10.1093/oxfordjournals.aje.a115984
147. Tredaniel J, Boffetta P, Little J, Saracci R, Hirsch A. Exposure to passive smoking during pregnancy and childhood, and cancer risk: The epidemiological evidence. Paediatr Perinat Epidemiol (1994) 8(3):233–55. doi: 10.1111/j.1365-3016.1994.tb00455.x
148. Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer (2006) 6(4):281–91. doi: 10.1038/nrc1839
149. Pike MC, Krailo MD, Henderson BE, et al. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature (1983) 303(5920):767–70. doi: 10.1038/303767a0
150. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA (2002) 288(3):321–33. doi: 10.1001/jama.288.3.321
151. Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: When they collide. J Mammary Gland Biol Neoplasia (2009) 14(2):87–98. doi: 10.1007/s10911-009-9119-7
152. Schedin P, O’Brien J, Rudolph M, et al. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia (2007) 12(1):71–82. doi: 10.1007/s10911-007-9039-3
153. Tiede B, Kang Y. From milk to malignancy: The role of mammary stem cells in development, pregnancy and breast cancer. Cell Res (2011) 21(2):245–57. doi: 10.1038/cr.2011.11
154. Albrektsen G, Heuch I, Hansen S, et al. Breast cancer risk by age at birth, time since birth and time intervals between births: Exploring interaction effects. Br J Cancer (2005) 92(1):167–75. doi: 10.1038/sj.bjc.6602302
155. Chie WC, Hsieh C, Newcomb PA, Longnecker MP, Mittendorf R, Greenberg ER, et al. Age at any full-term pregnancy and breast cancer risk. Am J Epidemiol (2000) 151(7):715–22. doi: 10.1093/oxfordjournals.aje.a010266
156. Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med (1994) 331(1):5–9. doi: 10.1056/NEJM199407073310102
157. Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: Postpartum period with the highest risk (Sweden). Cancer Causes Control (2002) 13(4):299–305. doi: 10.1023/A:1015287208222
158. Kim AS, Ko HJ, Kwon JH, Lee JM. Exposure to secondhand smoke and risk of cancer in never smokers: A meta-analysis of epidemiologic studies. Int J Environ Res Public Health (2018) 15(9):1981. doi: 10.3390/ijerph15091981
Keywords: breast cancer, active smoking, passive smoking, incidence, meta-analysis selection criteria
Citation: He Y, Si Y, Li X, Hong J, Yu C and He N (2022) The relationship between tobacco and breast cancer incidence: A systematic review and meta-analysis of observational studies. Front. Oncol. 12:961970. doi: 10.3389/fonc.2022.961970
Received: 05 June 2022; Accepted: 24 August 2022;
Published: 15 September 2022.
Edited by:
Claudia Mello-Thoms, The University of Iowa, United StatesReviewed by:
Ernest Ekpo, The University of Sydney, AustraliaSadaf Alipour, Tehran University of Medical Sciences, Iran
Copyright © 2022 He, Si, Li, Hong, Yu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning He, YmNzbW9raW5nQDE2My5jb20=
 Yujing He1
Yujing He1