- 1Department of Gastroenterology, The Suqian Clinical College of Xuzhou Medical University, Suqian, Jiangsu, China
- 2Cancer Institute, The Affiliated People’s Hospital, Jiangsu University, Zhenjiang, Jiangsu, China
- 3Department of General Surgery, The Suqian Clinical College of Xuzhou Medical University, Suqian, Jiangsu, China
Background: Frailty as a common geriatric syndrome can affect the clinical outcomes in patients with gastric cancer. However, the impact of frailty on survival and readmission patients with gastric cancer has not been well-characterised.
Objectives: To investigate the impact of frailty on survival and readmission in patients with gastric cancer undergoing gastrectomy by conducting a meta-analysis.
Methods: Eligible studies were identified by searching the PubMed, Web of Science, Cochrane Library, and Embase databases until 2 September 2022. Observational studies that evaluated the value of frailty in predicting adverse outcomes in gastric cancer patients undergoing gastrectomy were included. The outcomes of interest were overall survival, disease-specific survival (death from gastric cancer), and readmission. Adjusted hazard ratios (HR) with 95% confidence intervals (CI) were pooled to calculate the association of frailty with adverse outcomes.
Results: Eight studies reported on nine articles with 2,792 patients with gastric cancer were included. A fixed-effect meta-analysis indicated that frailty was associated with a reduced in-hospital overall survival (HR 2.08; 95% CI 1.46–2.95), long-term overall survival (HR 1.84; 95% CI 1.37–2.47), and disease-specific survival (HR 1.94; 95% CI 1.34–2.83). In addition, frailty was associated with increased risk of readmission within 1 year (HR 3.63; 95% CI 1.87–7.06).
Conclusions: Frailty was associated with a reduced overall survival and disease-specific survival and an increased risk of readmission in patients with gastric cancer undergoing gastrectomy. Frail status may play an important role in the risk stratification of gastric cancer after gastrectomy.
Introduction
Gastric cancer is the fifth most common malignancy that is responsible for more than 1 million new cases annually worldwide (1). Gastrectomy is the main curative treatment for gastric cancer (2). Despite the advancement in surgical treatment, the mortality rate from gastric cancer remains substantially high (3). Gastric cancer frequently occurs in elderly people. The incidence rates for gastric cancer increase with advanced age (4).Therefore, comprehensive geriatric assessment may help in improving the risk classification of gastric cancer.
Frailty is a geriatric syndrome characterised by a decline in physiological reserve and functioning across multiple-organ systems (5, 6). This geriatric syndrome increases with aging (7). Frailty is a promising predictor for adverse health outcomes in older patients with cancer (8, 9). Frailty is associated with increased risk of postoperative morbidity and mortality among malignant or benign diseases in the stomach among patients who underwent gastrectomy (10). An early systematic review only described the association between frailty and adverse outcomes in patients with gastric cancer undergoing gastrectomy (11). Subsequently, several new articles (12–17) that investigated the predictive utility of frailty in patients with gastric cancer have pursued a meta-analysis that applies accumulating clinical evidence. Nevertheless, the value of the frailty in predicting overall survival remains conflicting in patients with gastric cancer (17, 18).
To address these knowledge gaps, we performed this more focused meta-analysis to examine the impact of frailty on survival and readmission in patients with gastric cancer undergoing gastrectomy.
Materials and methods
Search strategy
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (19). Two independent authors comprehensively searched the studies indexed in PubMed, Web of Science, Cochrane Library, and Embase databases until 2 September 2022. The following combined keywords were used for literature search: “frailty” OR “frail” AND “gastric cancer” OR “stomach cancer” AND “gastrectomy” OR “gastric surgery” (Supplemental Text S1). A manual search of reference lists of included studies and pertinent reviews were performed to identify any additional studies.
Study selection
Studies that met the following criteria were included: 1) population: patients with gastric cancer undergoing gastrectomy; 2) predictor: frailty assessed using a valid tool before gastric surgery; 3) comparison: individuals with frailty versus those without; 4) outcome measures: overall survival, disease-specific survival (defined by death from the gastric cancer), or readmission; 5) study design: retrospective or prospective cohort studies published in peer-reviewed journals; and 6) reported the risk estimate associated frailty in multivariate regression analyses. For multiple articles from the same population, only articles with the most comprehensive data were included. The exclusion criteria were as follows: 1) not restricted in patients with gastric cancer; 2) outcome measures were not of interest; and 3) conference abstract, letter, or unpublished studies.
Data extraction and quality assessment
Two authors independently extracted the last name of the first author, year of publication, country, study design, sample size, age of patients, gender distribution, surgical type, assessment of frail tool, outcome measures, follow-up duration, fully adjusted risk summary associated with frailty, and variable adjustment from eligible studies. The Newcastle–Ottawa Scale (NOS) for cohort studies was used to assess the risk of bias of the included studies (20). A total score of seven points or over indicates a low risk of bias (high-quality). Any disagreements were settled through a discussion between two authors until consensus was reached. When information was not reported in the original article, we contacted the corresponding author by e-mail for the missing data.
Data analysis
Stata software 12.0 (Stata, College Station, TX, USA) was used for all meta-analyses.
If the relative risk of an outcome is reported as odds ratio (OR), we calculated the hazard ratio (HR) from the OR by using the following formula: HR = OR/[(1-Prevalence non-exposed group) + (Prevalence non-exposed group× OR)]. The impact of frailty on adverse outcomes was expressed by pooling the fully adjusted HR with 95% confidence intervals (CI) from individual studies. Between-study heterogeneity was investigated using the I2 statistics and Cochrane Q test. A fixed-effect model was selected in the absence of significant heterogeneity (p-value >0.10 of the Cochrane Q test or I2 statistic value <50%). Sensitivity analysis was performed by sequentially removing an individual study for each turn. Begg’s test (21) and Egger’s test (22) were scheduled to investigate publication bias when the outcomes were reported in more than 10 studies. The overall certainty of evidence was summarised using the GRADE framework.
Results
Search results and study characteristics
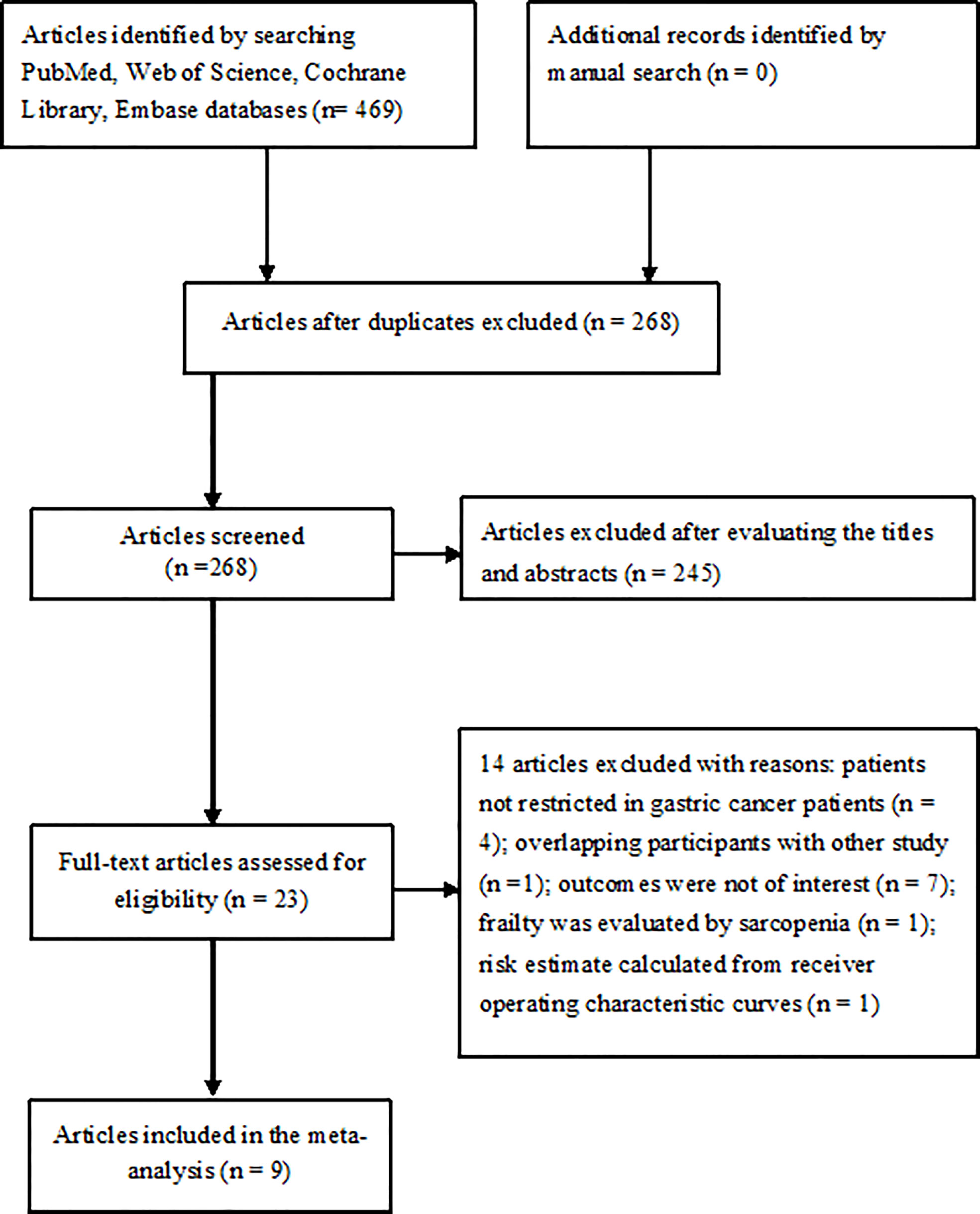
The literature search yielded 469 potentially relevant articles, of which 201 articles were directly excluded as duplicate. After evaluating the titles and abstracts, 23 articles were retrieved for full-text evaluation. Two articles (12, 23) were obtained from the same population and reported the different clinical outcomes. Finally, eight studies reported on nine articles (12, 13, 15–18, 23–25) were included in the meta-analysis (Figure 1).
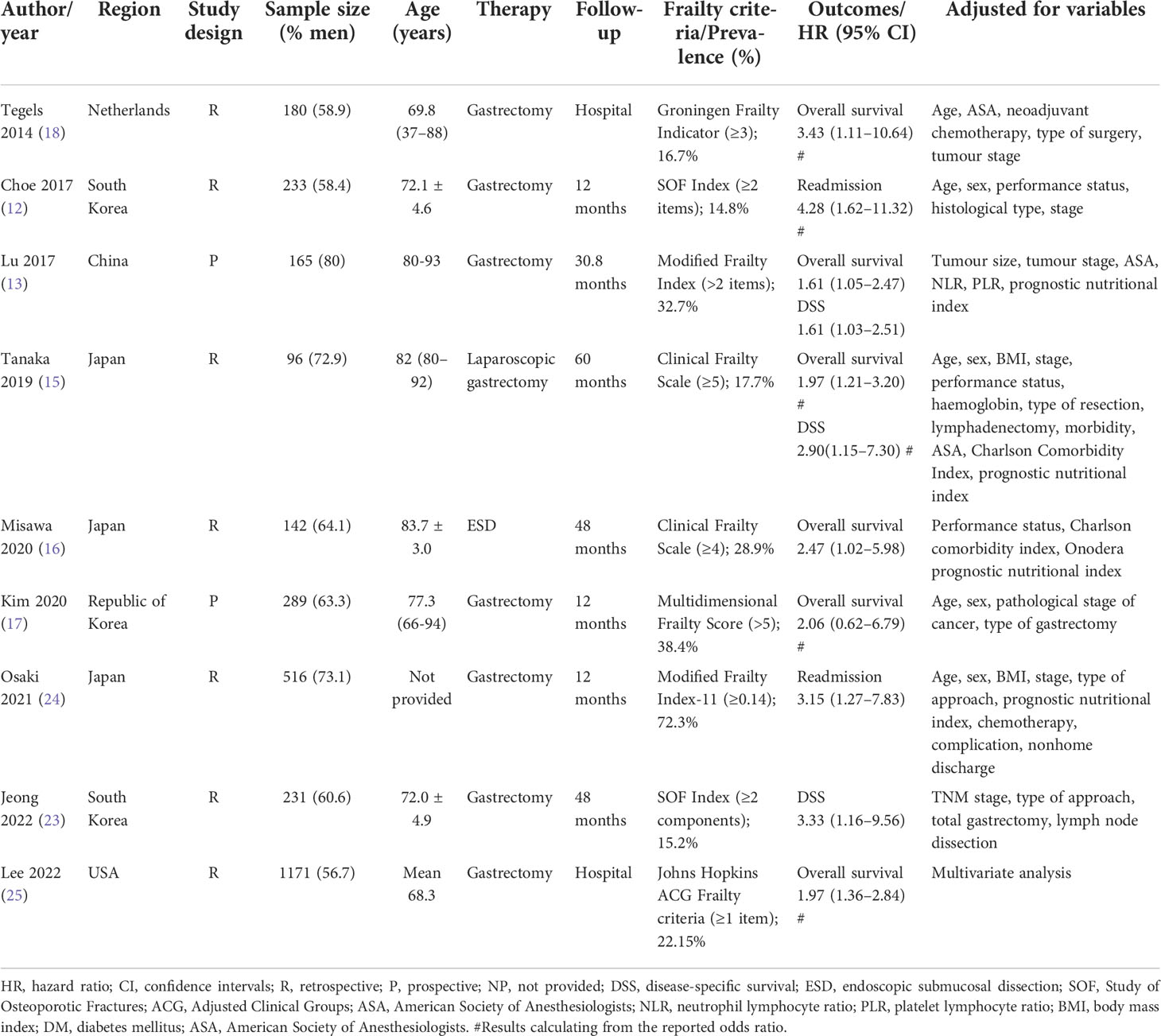
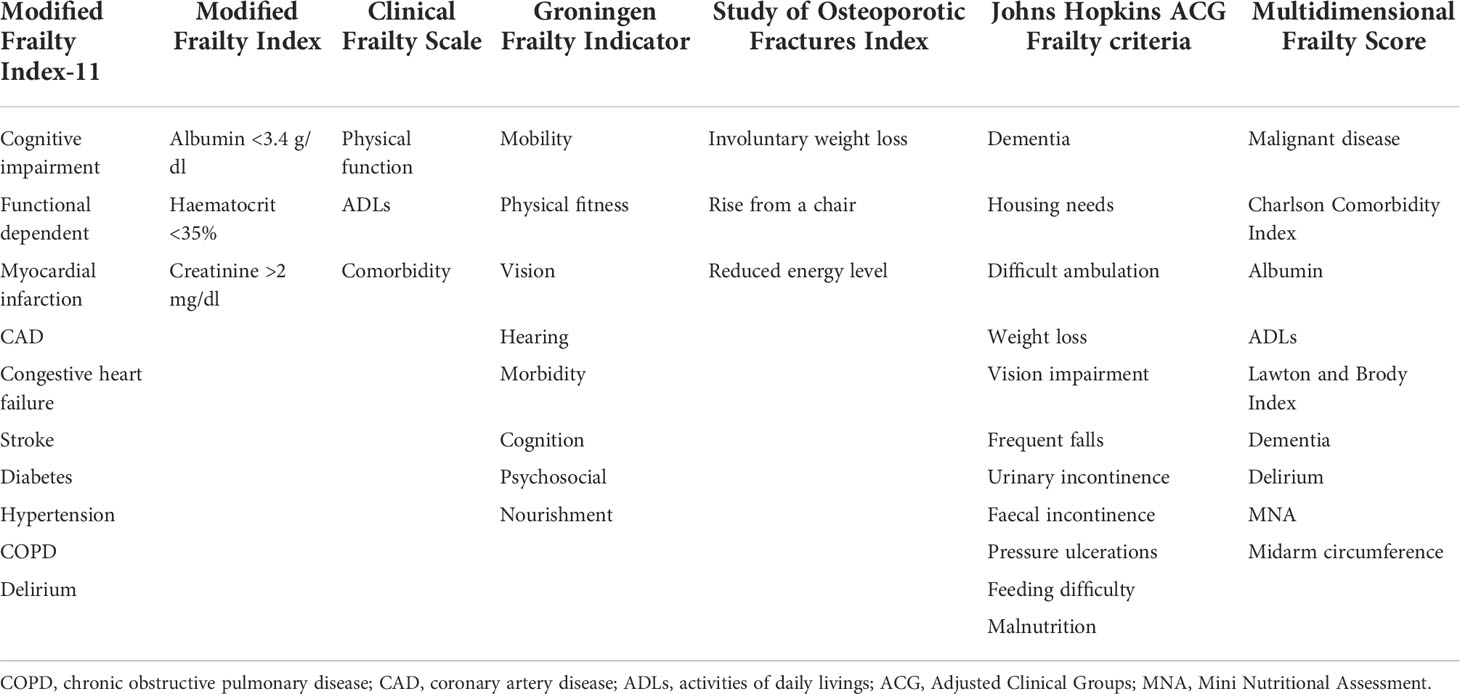
The major characteristic of the included studies is summarised in Table 1. These studies were published from 2014 to 2022 and conducted in South Korea (12, 23), Republic of Korea (17), Japan (15, 16), The Netherlands (18), USA (25), and China (13). Two articles (13, 17) adopted a prospective design, while the others adopted a retrospective design. The sample sizes of individual studies ranged from 96 to 1,173, with a total of 2,792 patients with gastric cancer. The Clinical Frailty Scale (15, 16), Modified Frailty Index (13, 24), Groningen Frailty Indicator (18), Study of Osteoporotic Fractures Index (12, 23), Johns Hopkins Adjusted Clinical Groups Frailty criteria (25), and Multidimensional Frailty Score (17) tools were used to assess frailty. The detailed components used to assess frailty are summarised in Table 2. According to the frailty tool used, the prevalence of frailty varied from 14.8% to 72.3%. The duration of follow-up was up to 60 months. Based on the NOS criteria, all the included articles were grouped to have high-quality (Supplemental Table S1).
Overall survival
Six studies (13, 15–18, 25) reported the impact of frailty on overall survival. As shown in Figure 2, no heterogeneity was observed across studies (I2 = 0%, p = 0.847). A fixed-effect model meta-analysis showed that frailty was associated with a reduced overall survival (HR 1.94; 95% CI 1.55–2.42). Subgroup analysis on the duration of follow-up showed that frailty was associated with a reduced in-hospital overall survival (HR 2.08; 95% CI 1.46–2.95; Figure 2A) and long-term overall survival (HR 1.84; 95% CI 1.37–2.47; Figure 2B). After removing the most influential study (Lu 2017), the pooled HR of long-term overall survival was 1.84 (95% CI 1.37–2.47). The results of the leave-out one study sensitivity analysis showed that the pooled HR of long-term overall survival varied from 1.77 to 2.07 (all p-value <0.05). Begg’s test (n = 0.452) and Egger’s test (n = 0.142) suggested no evidence of publication bias.
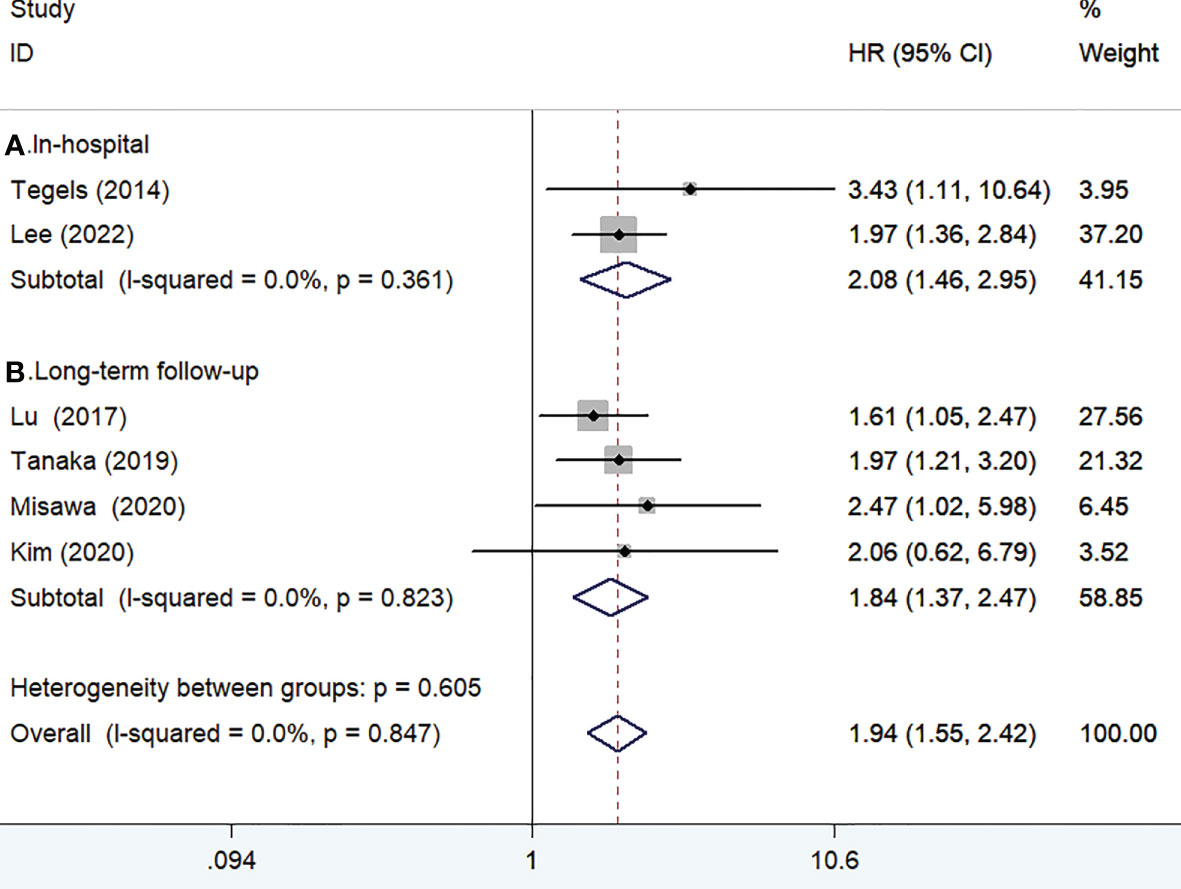
Figure 2 Forest plots showing the pooled HR with 95% CI of in-hospital (A) and long-term overall survival for the frail patients.
Disease-specific survival
Three studies (13, 15, 23) reported the impact of frailty on disease-specific survival. As shown in Figure 3, no significant heterogeneity was observed between studies (I2 = 17.0%, p = 0.300). A fixed-effect model meta-analysis showed that frailty was associated with a reduced disease-specific survival (HR 1.94; 95% CI 1.34–2.83). After removing the most influential study (Lu 2017), the pooled HR was 3.08 (95% CI 1.54–6.17) for disease-specific survival. The results of the leave-out one study sensitivity analysis suggested that the pooled HR of disease-specific survival ranged from 1.80 to 3.08 (all p-values <0.05).Two studies (12, 24) reported the impact of frailty on readmission within 1 year. As shown in Figure 4, no heterogeneity (I2 = 0%, p = 0.652) was observed between studies. A fixed-effect model meta-analysis showed that frailty was associated with an increased risk of readmission (HR 3.63; 95% CI 1.87–7.06).
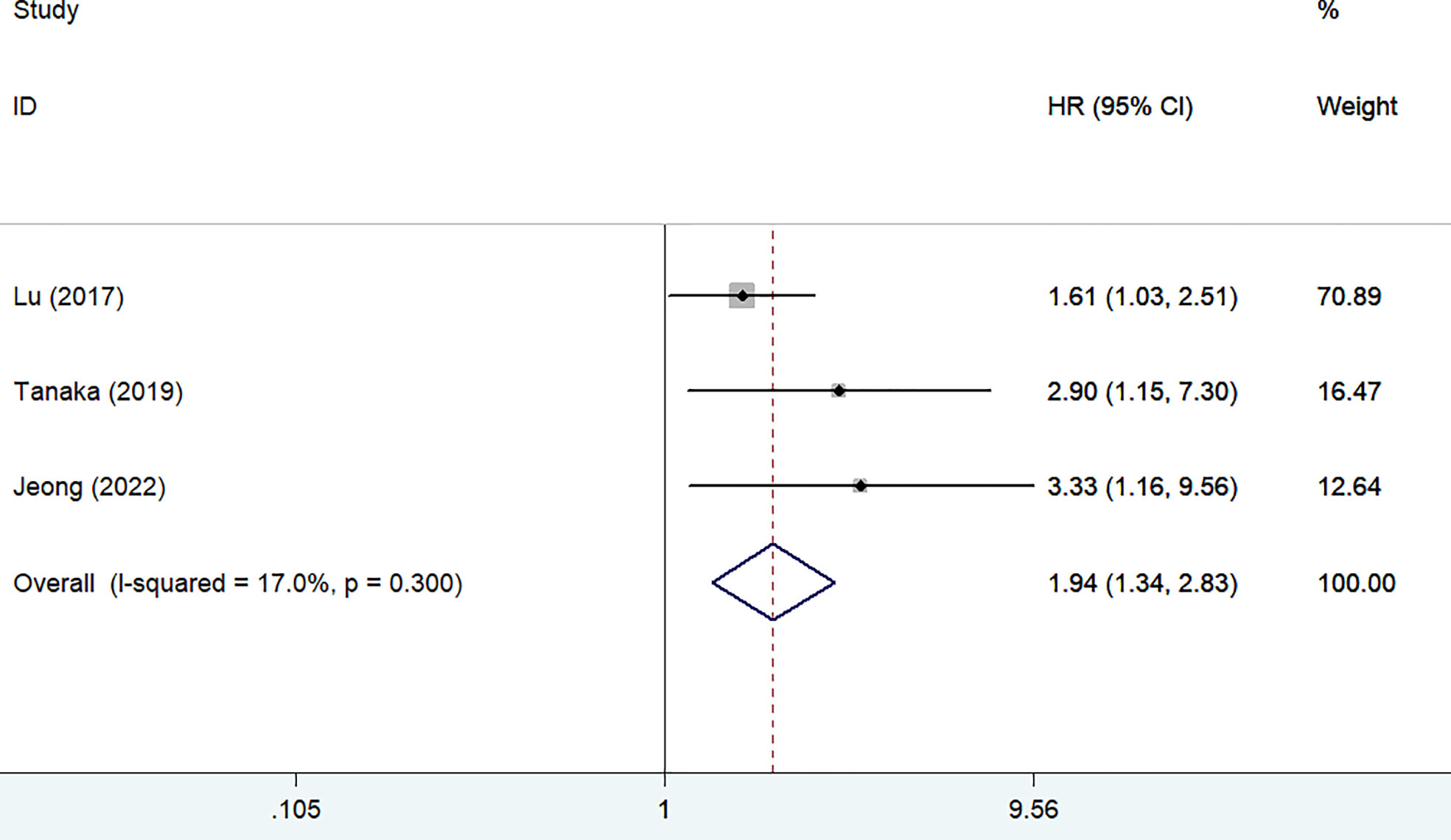
Figure 3 Forest plots showing the pooled HR with 95% CI of disease-specific survival for the frail patients.
GRADE certainty of evidence
The overall certainty of evidence for overall survival was high, and that for disease-specific survival/readmission was low (Supplemental Table S2). The downgrading evidence quality for disease-specific survival/readmission can be attributed to imprecision (small number of participants) and unclear risk of publication bias.
Discussion
The current meta-analysis focused on the impact of preoperative frailty on adverse clinical outcomes in patients with gastric cancer undergoing gastrectomy. The meta-analysis results confirmed that frailty was associated with a reduced overall survival and disease-specific survival among these patients. Patients with gastric cancer undergoing gastrectomy having frailty conferred a 2.08-fold and 84% poor in-hospital and long-term follow-up overall survival. Frailty was also associated with 94% poor disease-specific survival. In addition, patients with gastric cancer having frailty had a 3.63-fold higher risk of readmission within 1 year follow-up. Therefore, frailty may provide important prognostic information in patients with gastric cancer.
Apart from the abovementioned outcomes, frailty, as defined by the Modified Frailty Index, was an independent predictor of non-home discharge (26) and postoperative pulmonary infection (27) in elderly patients with gastric cancer who underwent gastrectomy. In patients with gastric cancer above the age of 80 years, frailty independently predicted the recurrence-free survival (13). Frailty was associated with a higher risk of postoperative complications in patients with gastric cancer after surgery (14, 18). Moreover, frailty, as defined by the Johns Hopkins Adjusted Clinical Groups criteria, conferred a 40% higher risk of length of the hospital stay in patients undergoing gastrectomy for gastric cancer (25). These findings further supported frailty as an important prognostic indicator in patients with gastric cancer undergoing gastrectomy.
Seven frailty tools were used in the current meta-analysis. However, the frailty tools with the superior predictive value cannot be compared because of the small number of studies included. Future prospective studies are required to determine which frailty tool can accurately predict adverse outcomes in patients with gastric cancer. No frailty tool has been well accepted for use among patients with gastric cancer. Nutritional status is an important aspect in patients with gastric cancer because their intake is usually restricted by mechanical obstruction. Malnutrition estimated by a low prognostic nutritional index (28) or higher controlling nutritional status score (29) is associated with a poor prognosis following gastrectomy for gastric cancer. Therefore, a combination of self-reported items and nutritional status should be considered to estimate the frailty in a clinical setting for patients with gastric cancer. A valid frailty tool needs to be developed from a cancer-specific geriatric assessment in older adults with gastric cancer.
Depending on the tool of frailty, the prevalence of frailty varied from 14.8% to 72.3% among patients with gastric cancer before gastrectomy. Our meta-analysis highlights the incorporation of frailty assessment before surgery can improve risk stratification in patients with gastric cancer. A thorough assessment of frail status can help oncologists to identify patients with gastric cancer at risk for mortality, postoperative complications, and other adverse outcomes. Frailty may also affect the clinical decision-making and individualised treatment strategies. Early intervention of frailty may help to improve survival and reduce admission in patients with gastric cancer.
The management of patients with gastric cancer faces many challenges during the COVID-19 pandemic. The COVID-19 outbreak has affected the diagnosis and treatment of many patients with gastric cancer (30, 31). Delayed diagnosis and extended waiting times for surgery could advance the cancer stage and deteriorate the survival outcome (32). Therefore, strategies should be developed for gastric cancer patients with frailty during the COVID-19 pandemic.
The strengths of our meta-analysis included the inclusion of high-quality studies and low heterogeneity between these studies. However, it has several limitations. First, majority of the included studies were of retrospective designs, which carried an inherent selection bias. Second, various methods of frailty assessment across studies remarkably limit the results. An inadequate definition of frailty may have affected the prognostic utility of frailty. Third, subgroup analysis was not performed according to the gender, type of surgery (open procedures or laparoscopy), or gastrectomy (partial or total) because of insufficient such data. Future studies should further investigate whether the prognostic value of frailty is affected by these factors. Only two studies were included in the analysis of readmission. Therefore, this outcome should be interpreted with caution because meta-analysis that involves two studies may result in biased information. Fourth, the result of the publication bias for overall survival may be potentially unreliable because the number of studies was less than the recommended arbitrary minimum number of 10 (33). Moreover, a publication test for disease-specific survival and readmission outcomes was not carried out because of the small number of studies included. Fifth, surgical procedure (17), pre-morbid functional status or cancer stage (34), and number and site of lymph node invasions (35) are associated with the prognosis of patients with gastric cancer. Lack of adjustment in these factors could have an effect on the prognostic utility of frailty. Finally, our meta-analysis could not determine the frailty tool with the best predictive value in patients with gastric cancer undergoing gastrectomy. Future well-designed studies are required to directly compare the predictive utility of different frailty tools in this population.
Conclusions
Frailty was associated with a reduced overall survival and disease-specific survival and increased risk of readmission in patients with gastric cancer undergoing gastrectomy. Frail status may play an important role in risk stratification of gastric cancer after gastrectomy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YF and DG contributed to study design and guaranteed the integrity of study. XW, YS, and PW searched the literature, extracted the data, assessed the study quality, and conducted the statistical analysis. GL drafted the manuscript. XW revised/edited the manuscript. All the authors have read and approved the final version of manuscript.
Funding
This work is supported by the Suqian Science and Technology Support Project Fund (K202014), the Zhenjiang Key Research and Development Fund (SH2021038), and the Jiangsu 333 Talent Fund (WSW205, WSW236).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materials
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.972287/full#supplementary-material.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Kawaguchi Y, Akaike H, Shoda K, Furuya S, Hosomura N, Amemiya H, et al. Is surgery the best treatment for elderly gastric cancer patients? World J Gastrointest Surg (2021) 13(11):1351–60. doi: 10.4240/wjgs.v13.i11.1351
3. Wong MCS, Huang J, Chan PSF, Choi P, Lao XQ, Chan SM, et al. Global incidence and mortality of gastric cancer, 1980-2018. JAMA Netw Open (2021) 4(7):e2118457. doi: 10.1001/jamanetworkopen.2021.18457
4. Thrift AP, Nguyen TH. Gastric cancer epidemiology. Gastrointest Endosc Clin N Am (2021) 31(3):425–39. doi: 10.1016/j.giec.2021.03.001
5. Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm–issues and controversies. journals gerontology Ser A Biol Sci Med Sci (2007) 62(7):731–7. doi: 10.1093/gerona/62.7.731
6. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (2013) 381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9
7. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet (2019) 394(10206):1365–75. doi: 10.1016/S0140-6736(19)31786-6
8. Huisingh-Scheetz M, Walston J. How should older adults with cancer be evaluated for frailty? J Geriatr Oncol (2017) 8(1):8–15. doi: 10.1016/j.jgo.2016.06.003
9. Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol (2015) 26(6):1091–101. doi: 10.1093/annonc/mdu540
10. Zorbas KA, Velanovich V, Esnaola NF, Karachristos A. Modified frailty index predicts complications and death after non-bariatric gastrectomies. Transl Gastroenterol Hepatol (2021) 6:10. doi: 10.21037/tgh.2020.01.07
11. Shen Y, Hao Q, Zhou J, Dong B. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: a systematic review and meta-analysis. BMC Geriatr (2017) 17(1):188. doi: 10.1186/s12877-017-0569-2
12. Choe YR, Joh JY, Kim YP. Association between frailty and readmission within one year after gastrectomy in older patients with gastric cancer. J Geriatr Oncol (2017) 8(3):185–9. doi: 10.1016/j.jgo.2017.02.002
13. Lu J, Cao LL, Zheng CH, Li P, Xie JW, Wang JB, et al. The preoperative frailty versus inflammation-based prognostic score: Which is better as an objective predictor for gastric cancer patients 80 years and older? Ann Surg Oncol (2017) 24(3):754–62. doi: 10.1245/s10434-016-5656-7
14. Lu J, Zheng HL, Li P, Xie JW, Wang JB, Lin JX, et al. High preoperative modified frailty index has a negative impact on short- and long-term outcomes of octogenarians with gastric cancer after laparoscopic gastrectomy. Surg Endosc (2018) 32(5):2193–200. doi: 10.1007/s00464-018-6085-4
15. Tanaka T, Suda K, Inaba K, Umeki Y, Gotoh A, Ishida Y, et al. Impact of frailty on postoperative outcomes for laparoscopic gastrectomy in patients older than 80 years. Ann Surg Oncol (2019) 26(12):4016–26. doi: 10.1245/s10434-019-07640-0
16. Misawa N, Higurashi T, Tachikawa J, Tanabe H, Yoshihara T, Ashikari K, et al. Clinical impact of evaluation of frailty in endoscopic submucosal dissection for early gastric cancer in elderly patients. Geriatr Gerontol Int (2020) 20(5):461–6. doi: 10.1111/ggi.13905
17. Kim G, Min SH, Won Y, Lee K, Youn SI, Tan BC, et al. Frailty in elderly gastric cancer patients undergoing gastrectomy. Dig Surg (2020) 8:1–7. doi: 10.1159/000511895
18. Tegels JJ, de Maat MF, Hulsewe KW, Hoofwijk AG, Stoot JH. Value of geriatric frailty and nutritional status assessment in predicting postoperative mortality in gastric cancer surgery. J Gastrointest Surg (2014) 18(3):439–45. doi: 10.1007/s11605-013-2443-7
19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol (2009) 62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006S0895-4356(09)00180-2
20. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed September 2, 2022).
21. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101.
22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
23. Jeong JR, Choi JW, Ryu SY, Choe YR. Relationship between frailty and mortality after gastrectomy in older patients with gastric cancer. J Geriatr Oncol (2022) 13(1):67–73. doi: 10.1016/j.jgo.2021.06.010
24. Osaki T, Saito H, Miyauchi W, Shishido Y, Miyatani K, Matsunaga T, et al. The type of gastrectomy and modified frailty index as useful predictive indicators for 1-year readmission due to nutritional difficulty in patients who undergo gastrectomy for gastric cancer. BMC Surg (2021) 21(1):445. doi: 10.1186/s12893-021-01450-6
25. Lee DU, Kwon J, Han J, Fan GH, Hastie DJ, Lee KJ, et al. The clinical impact of frailty on the postoperative outcomes of patients undergoing gastrectomy for gastric cancer: a propensity-score matched database study. Gastric Cancer (2022) 25(2):450-458. doi: 10.1007/s10120-021-01265-7
26. Osaki T, Saito H, Shimizu S, Murakami Y, Miyatani K, Matsunaga T, et al. Modified frailty index is useful in predicting non-home discharge in elderly patients with gastric cancer who undergo gastrectomy. World J Surg (2020) 44(11):3837–44. doi: 10.1007/s00268-020-05691-z
27. Meng Y, Zhao P, Yong R. Modified frailty index independently predicts postoperative pulmonary infection in elderly patients undergoing radical gastrectomy for gastric cancer. Cancer Manag Res (2021) 13:9117–26. doi: 10.2147/CMAR.S336023
28. Li J, Xu R, Hu DM, Zhang Y, Gong TP, Wu XL. Prognostic nutritional index predicts outcomes of patients after gastrectomy for cancer: A systematic review and meta-analysis of nonrandomized studies. Nutr Cancer (2019) 71(4):557–68. doi: 10.1080/01635581.2019.1577986
29. Takagi K, Domagala P, Polak WG, Buettner S, Wijnhoven BPL, Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing gastrectomy for gastric cancer: a systematic review and meta-analysis. BMC Surg (2019) 19(1):129. doi: 10.1186/s12893-019-0593-6
30. Park H, Seo SH, Park JH, Yoo SH, Keam B, Shin A. The impact of COVID-19 on the screening of colorectal, gastric, breast and cervical cancer in Korea. Epidemiol Health (2022) 44:e2022053. doi: 10.4178/epih.e2022053
31. Hesary FB, Salehiniya H. The impact of the COVID-19 epidemic on diagnosis, treatment, concerns, problems, and mental health in patients with gastric cancer. J Gastrointest Cancer (2022) 53(3):797–804. doi: 10.1007/s12029-021-00692-0
32. Ma J, Zhu C, Li W, Qiu Z, Yang J, Ge L, et al. The effect of delayed oncology surgery on survival outcomes for patients with gastric cancer during the COVID-19 pandemic: Evidence-based strategies. Front Oncol (2022) 12:780949. doi: 10.3389/fonc.2022.780949
33. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj (2006) 333(7568):597–600. doi: 10.1136/bmj.333.7568.597
34. Giannotti C, Sambuceti S, Signori A, Ballestrero A, Murialdo R, Romairone E, et al. Frailty assessment in elective gastrointestinal oncogeriatric surgery: Predictors of one-year mortality and functional status. J Geriatr Oncol (2019) 10(5):716–23. doi: 10.1016/j.jgo.2019.04.017
Keywords: frailty, gastric cancer, gastrectomy, overall survival, disease-specific survival, meta-analysis
Citation: Wang X, Sun Y, Wang P, Jie Y, Liu G, Gong D and Fan Y (2022) Impact of frailty on survival and readmission in patients with gastric cancer undergoing gastrectomy: A meta-analysis. Front. Oncol. 12:972287. doi: 10.3389/fonc.2022.972287
Received: 18 June 2022; Accepted: 26 September 2022;
Published: 31 October 2022.
Edited by:
Jinqiu Jacky Yuan, Seventh Affiliated Hospital, Sun Yat-sen University, ChinaReviewed by:
Gagan Matta, Gurukul Kangri Vishwavidyalaya, IndiaYanfei Li, Lanzhou University, China
Xie Peng, Seventh Affiliated Hospital, Sun Yat-sen University, China
Vinodhkumar Obli Rajendran, Indian Veterinary Research Institute (IVRI), India
Marco Materazzo, Policlinico Tor Vergata, Italy
Copyright © 2022 Wang, Sun, Wang, Jie, Liu, Gong and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Fan, anN6amZhbnl1QDE2My5jb20=; Dandan Gong, Z29uZ2RhbmRhbnpoakAxMjYuY29t
†These authors have contributed equally to this work
 Xiaoyan Wang
Xiaoyan Wang Yimeng Sun
Yimeng Sun Pei Wang2†
Pei Wang2† Dandan Gong
Dandan Gong Yu Fan
Yu Fan