- 1Department of Breast and Thyroid Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Breast and Thyroid Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Introduction: Surgical management of lateral lymph nodes in papillary thyroid carcinoma, especially at level II, remains controversial. This study aimed to investigate the risk factors for level II lymph node metastasis in patients with papillary thyroid carcinoma and establish a prediction model to estimate the metastatic risk.
Materials and methods: A total of 768 patients with papillary thyroid carcinoma underwent thyroidectomy and central plus lateral lymph node dissection, including levels VI, II, III, and IV, at the First Affiliated Hospital of Chongqing Medical University from January 2016 to December 2018. Data on the clinicopathological characteristics were collected and analyzed. Univariate and multivariate analyses were performed to identify risk factors for level II lymph node metastasis. Subsequently, a predictive model was established based on the results of the multivariate analyses.
Results: The level II lymph node metastatic rate was 34.11% with the following features: largest tumor diameter >20 mm (Odds ratio=1.629, P=0.026), located in the upper pole (Odds ratio=4.970, P<0.001), clinical lymph node-positive (clinical central lymph node-positive: Odds ratio=1.797; clinical lateral lymph node-positive: Odds ratio=1.805, P=0.008), vascular invasion (Odds ratio=6.759, P=0.012), and rate of central lymph node metastasis (Odds ratio=2.498, P<0.001). Level III lymph node metastasis (Odds ratio=2.749, P<0.001) and level IV lymph node metastasis (Odds ratio=1.732, P=0.007) were independent of level II lymph node metastasis predictors. The prediction model’s areas under the receiver operating characteristic curve were 0.815 and 0.804, based on bootstrapping validation. Level II lymph node metastasis was associated with the tumor-free survival rate of patients with papillary thyroid carcinoma (P<0.001).
Conclusions: Largest tumor diameter >20 mm, located in the upper pole, clinical lymph node-positive, vascular invasion, rate of central lymph node metastasis, and levels III and IV lymph node metastases were independent level II lymph node metastasis predictors. We developed a prediction model for level II lymph node metastasis. Overall, level II lymph node metastasis dissection should be individualized according to clinicopathological data both preoperatively and intraoperatively.
Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy worldwide and its incidence has increased rapidly in recent decades (1–3). Most PTCs are mild and indolent with an excellent prognosis. However, lymph node metastasis is common in the early stages and is associated with an increased risk of recurrence and reoperation rate (4, 5). The regional nodal basins of the central and lateral neck are the first site of lymph node metastasis in PTC. Furthermore, the most common metastatic area is the central compartment (level VI), sequentially followed by levels III, IV, II, and V (6, 7).
The surgical management of lateral lymph nodes (LLNs) in PTC remains controversial. The American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) guidelines disagree on prophylactic lateral lymph node dissection (pLLND) (8, 9). The British Thyroid Association (BTA) guidelines suggest performing pLLND at the discretion of the patient when the central lymph node (CLN) is involved (10). Conversely, Chinese experts recommend prophylactic central lymph node dissection (pCLND) and elective LLND according to the number and proportion of central lymph node metastasis (CLNM) along with the location, size, and pathological type of PTC. However, the level II lymph node (LN-level II) metastatic rate can reach 45% (11–13), and occult metastases may also occur at level II (14, 15). Moreover, lymph node metastasis increases the risk of recurrence and reoperation rate, and most recurrences occur when LN level II is initially positive for metastatic disease (16). Albeit, blind dissection of LN-level II may increase the operative time and complications. Thus, it is necessary to carefully and accurately identify and manage patients with LN-level II positivity. This study investigated the risk factors for LN-level II metastasis in patients with PTC and aimed to establish a predictive model to estimate the risk of metastasis.
Materials and methods
Patient collection
The local institutional ethics committee approved this retrospective study verbally and informed consent was waived because there was no patient interest or privacy breach in the form of disclosure of personal information. A total of 2863 patients underwent surgery for thyroid diseases at the Department of Breast and Thyroid Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China, between January 2016 and December 2018. The inclusion criteria were as follows: proven PTC postoperatively and modified radical neck dissection with CLND and LLND, including levels II, III, and IV. Patients with any of the following conditions were excluded: reoperation, history of surgery for other malignant tumors, distant metastasis, adolescence, and incomplete clinical information. Ultimately, 768 eligible patients were included in this study.
Demographic and clinicopathological data
Data on the following clinicopathological variables were collected: sex, age, size, location, ultrasound-reported LN status (US-reported LN status), multifocality, laterality, capsule invasion, trachea/muscle/vascular/nerve invasion, Hashimoto thyroiditis (HT), and lymph node metastasis. The largest lesion diameter and location were recorded as the size and location. The presence of capsule invasion and trachea/muscle/vascular/nerve invasion was recorded in preoperative ultrasonography reports or intraoperative findings.
Recurrence was defined as the local or regional disease requiring treatment 6 months after the initial standard operation and diagnosed using ultrasound-guided fine-needle aspiration cytology or reoperation. The follow-ups were in an outpatient clinic. The patients were followed up using the electronic medical record system, telephone, or text messages. All of the 768 patients were effectively followed up.
Statistical analysis
The independent-sample t-test or Mann–Whitney U-test was used to compare the continuous variables. Categorical variables were compared using the Chi-square test or Fisher’s exact test. Statistical significance was set at P<0.05. Variables that showed a significant association in the univariate analysis (P<0.05) were included in the multivariable logistic regression model. Based on this model, a nomogram was developed to predict the probability of LN-level II metastasis, and the predictive ability of the nomogram was measured using the receiver operating characteristic (ROC) curve, concordance index (C-index), and calibration plot. Decision curve analysis (DCA) was performed to evaluate the clinical usefulness of the nomogram in calculating the net benefits at different threshold probabilities. The Kaplan–Meier method was used to estimate disease-free survival (DFS) rates. All statistical analyses were performed using SPSS (version 25.0; SPSS Inc., Chicago, IL, United States) and graphs were generated using R software version 4.2.0.
Results
Patient characteristics
A total of 768 patients were included in the analysis: 243 males and 525 females; mean age, 42.0 ± 12.7 years (range, 18–85); mean tumor size, 16.3 ± 10.0 mm (range, 3–60); mean rate of CLNM, 0.33 ± 0.28; mean rate of level III lymph node (LN-level III) metastasis, 0.16 ± 0.24; mean rate of level IV lymph node (LN-level IV) metastasis, 0.10 ± 0.19. Patient characteristics are summarized in Table 1. Overall, the LN-level II metastasis rate was 34.11%. In the univariate analyses, we found significant differences in size, location, US-reported LN status, trachea/vascular/nerve invasion, laterality, CLNM, LN-level III metastasis, and LN-level IV metastasis between the LN-level II positive and negative groups. No significant differences were observed in sex (P=0.404), age (P=0.532), capsule invasion (P=0.168), multifocality (P=0.057), muscle invasion (P=0.116), or HT (P=0.301).
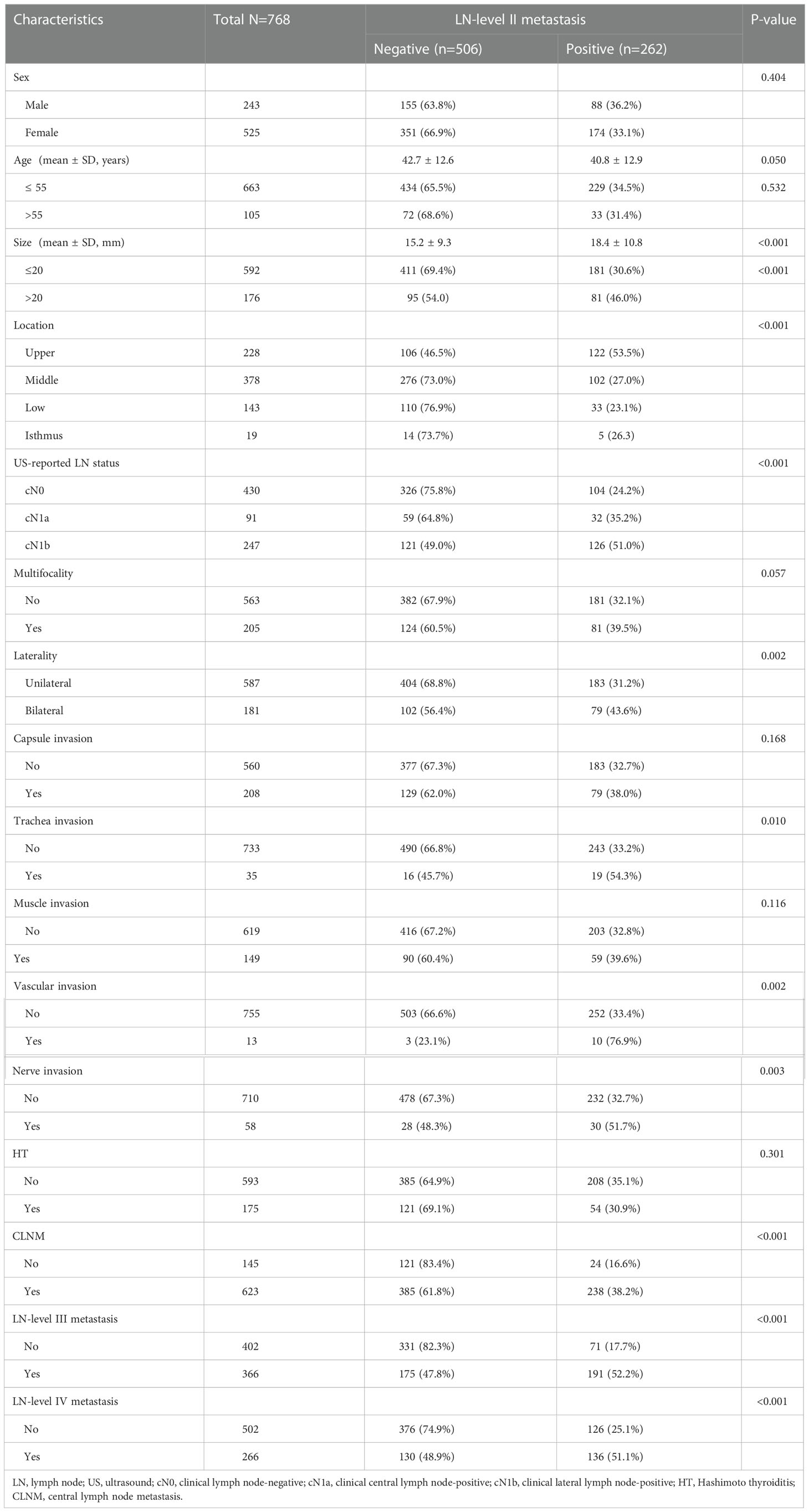
Table 1 Comparisons of clinicopathological factors between the negative and positive of LN-level II.
The ROC curve was used to analyze the diagnostic efficacy of the CLNM rate, LN level III metastasis, and LN level IV metastasis; the cut-off values are listed in Table 2.
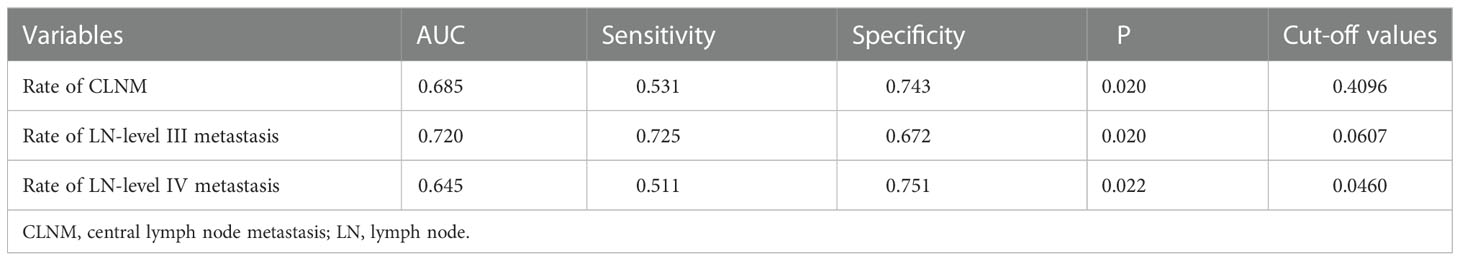
Table 2 The cut-off values of the Rate of different levels of LNM to predict LN-level II metastasis.
In the multivariate analyses, size (P=0.026), location (P<0.001), US-reported LN status (P=0.008), vascular invasion (P=0.012), rate of CLNM (P<0.001), LN-level III metastasis (P<0.001), and LN level IV metastasis (P=0.007) were independent predictors of LN-level II metastasis (Table 3).
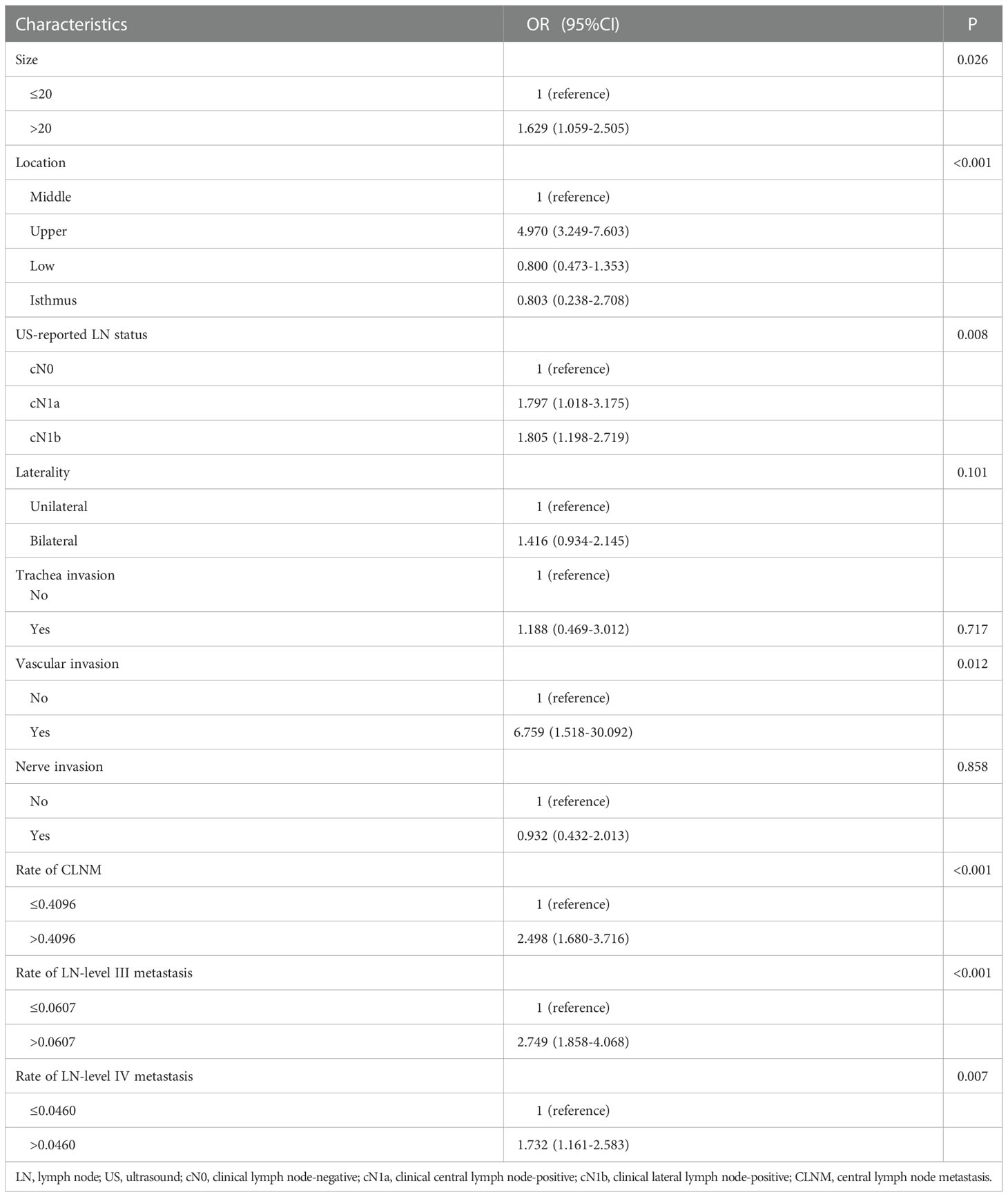
Table 3 Multivariate logistic regression analysis of factors between the negative and positive of LN-level II.
Prediction models
A predictive model for LN-level II metastasis was developed based on the multivariate logistic regression analysis. A nomogram incorporating the above seven predictors is shown in Figure 1. The C-index for the nomogram was 0.815 (95% CI, 0.784–0.846) and 0.804 by bootstrapping validation, which demonstrated good discriminative ability. The calibration plot revealed excellent agreement between the predicted and actual observations (Figure 2). These results showed that the nomogram had good efficacy in predicting the probability of LN-level II metastasis. The DCA results suggested that when the threshold probability was approximately 10–90%, compared to a “treat all” or “treat none” strategy, this nomogram yielded a greater net benefit, which indicated that it had good clinical value (Figure 3).
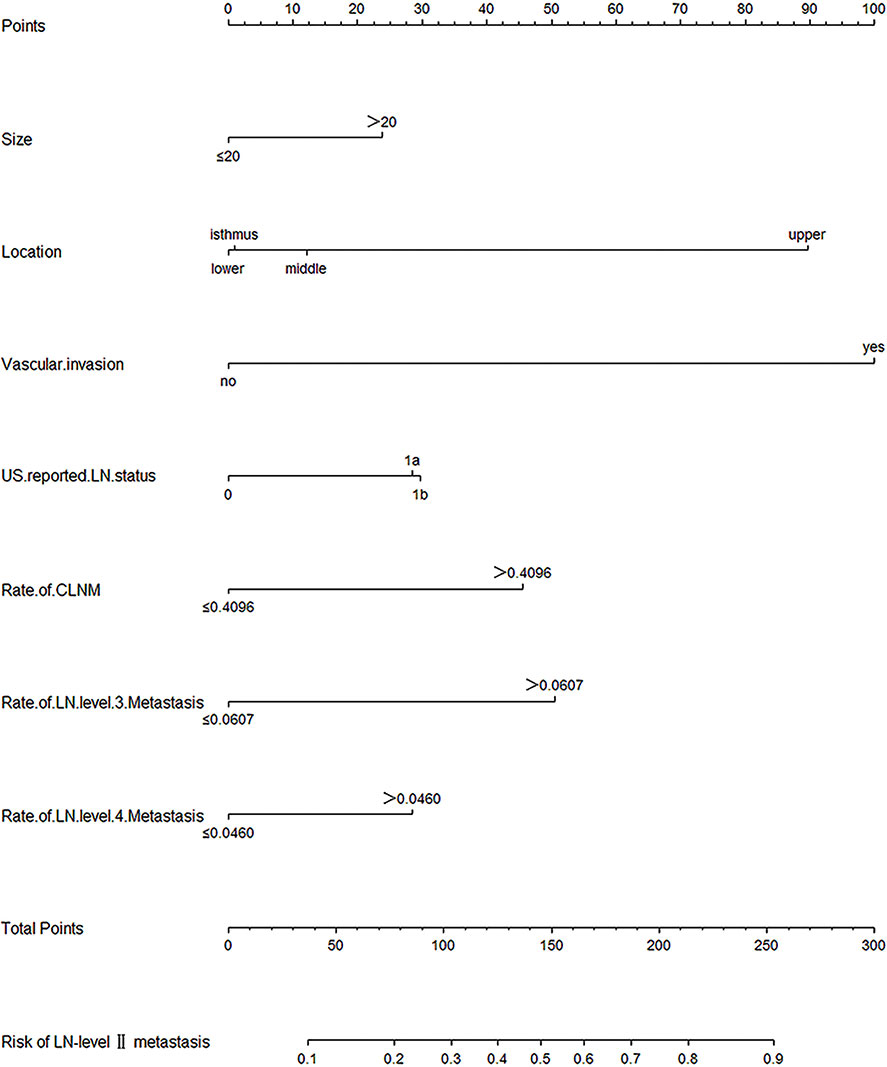
Figure 1 Nomogram for prediction of LN-level II metastasis. A line is drawn straight up to the point axis that corresponds with each patient variable to obtain the points. The sum of these points is located on the total score points axis. A line is drawn downwards to the risk axis to determine the possibility of LN-level II metastasis.
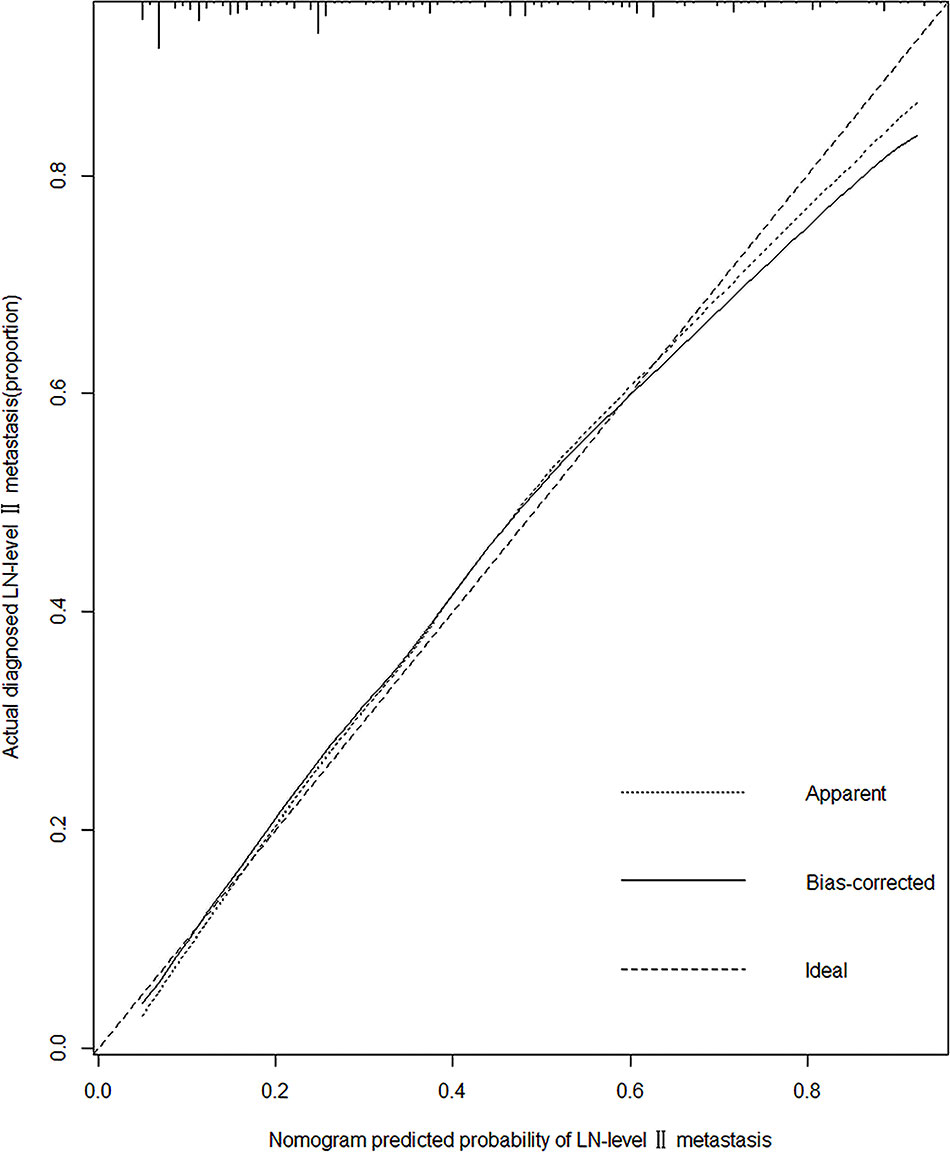
Figure 2 Calibration plot of the model. The calibration curve depicts the calibration of the model in terms of the agreement between the predicted risks of LN-level II metastasis and observed outcomes of LN-level II metastasis. The x-axis represents the predicted LN-level II metastasis risk. The y-axis represents the actual LN-level II metastasis rate. The diagonal dotted black line represents an ideal calibration model in which the predicted probabilities are identical to the actual outcomes. The dotted line represents the predictive performance of the nomogram; the closer the fit of the dotted line to the ideal line, the better the prediction. The black line represents the bias-corrected.
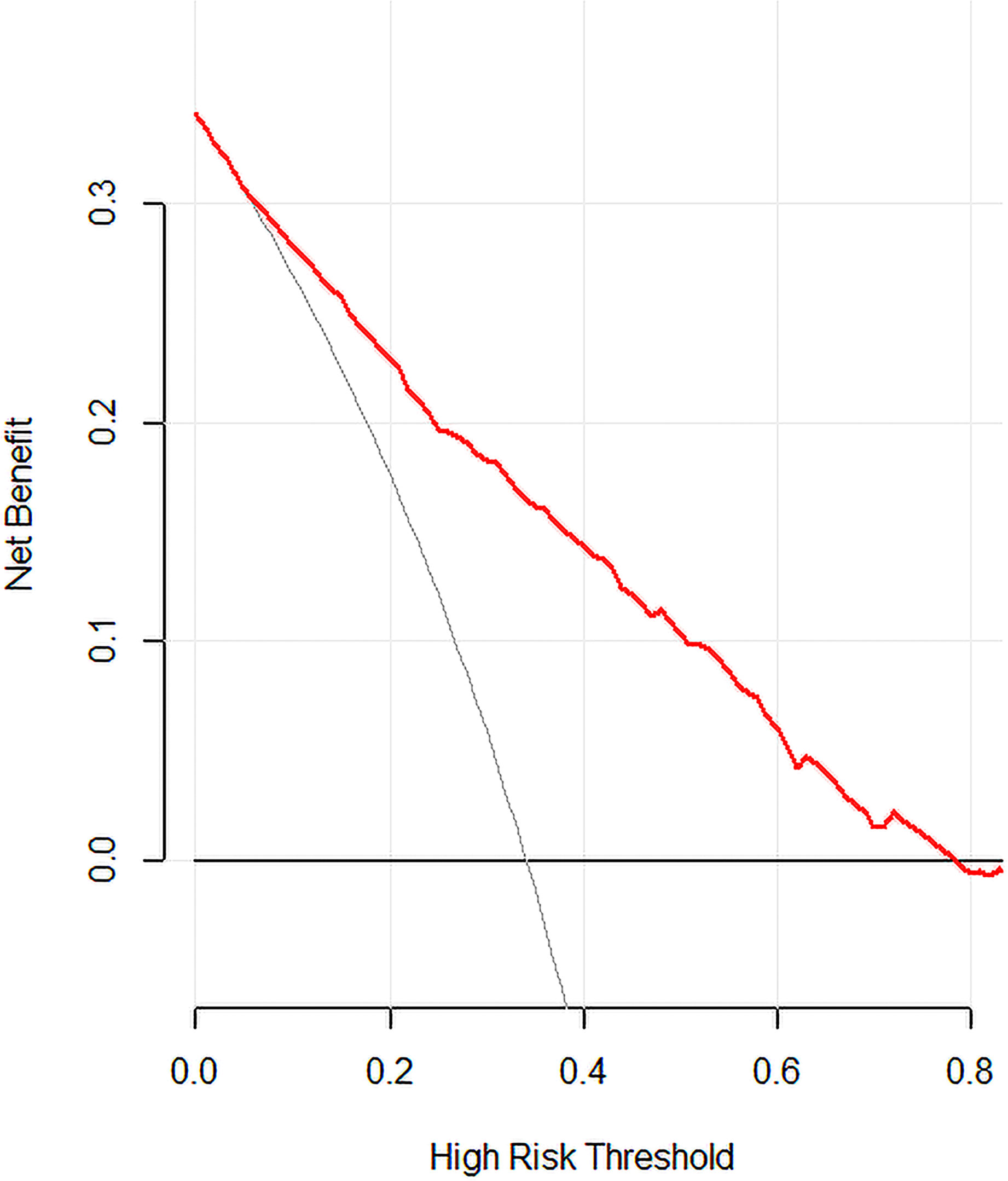
Figure 3 Decision curve analysis for the model. The y-axis measures the net benefit. The x-axis represents the threshold probability. The red solid line represents the nomogram. The gray line represents the hypothesis that all patients had LN-level II metastasis. The black solid line represents the hypothesis that none of the patients had LN-level II metastasis. The decision curve indicates that when the threshold probability is >10.0%, use of this predictive model would accrue greater benefit than that accruing from a treat-all or treat-none strategy.
Prognostic analysis
We investigated whether LN-level II metastasis affected recurrence. Patients were followed-up after the initial surgery until December 2021. The mean follow-up time was 51.5 ± 12.0 months (7–71 months). Among the 768 patients, 24 experienced recurrences, only five of whom were in the LN-level II negative group. Tumor-free survival of the two groups was compared and the prognosis in patients with LN-level II metastasis was worse than that of LN-level II negative (P<0.001) (Figure 4).
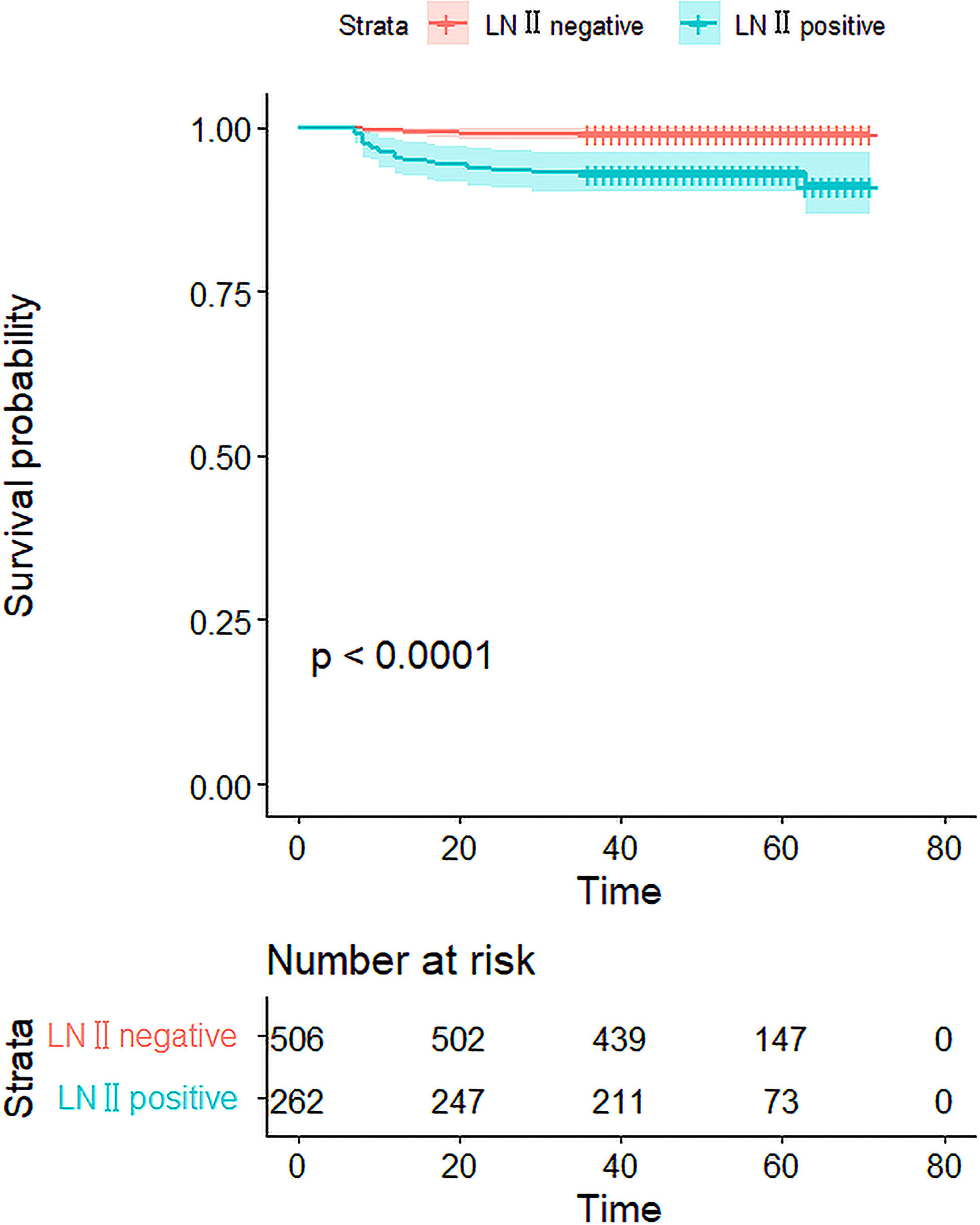
Figure 4 The Kaplan–Meier curves for the 768 patients. The curves show that the DFS rate was 96.9% in 768 patients, and there was a significant difference in the DFS rate between the LN-level negative and positive cohorts(p<0.001).
Discussion
The present study indicated that a tumor diameter of >20 mm, tumor located in the upper pole, clinical lymph node-positivity (cN1), vascular invasion, the rate of CLNM, and LN-levels III and IV metastasis, were all positively associated with the risk of LN-level II metastasis for PTC. However, sex, age, capsule invasion, multifocality, muscle/trachea/nerve invasion, laterality, and HT were not.
Several studies have reported the probability risk factors of LN-level II metastasis for PTC, and most have reported the factors of lateral lymph node metastasis. The present study has several advantages over previous studies. First, the predictors for LN-level II metastasis in recent years were evaluated in a large cohort of patients with PTC (overall 768). Second, we calculated the relationship between the LNM rate in each compartment and LN-level II metastasis, which is more objective than the number of LNM, considering the total number of dissected LNs and the number of metastatic LNs. Third, based on the seven risk factors available in preoperative clinicopathological features and intraoperative frozen analysis, we built a predictive model and nomogram, which is a reliable tool with high accuracy for predicting LN-level II metastasis for clinicians.
Many studies have shown that a maximum tumor diameter >2 cm is associated with lateral lymph node metastasis (LLNM) in PTC and size reflects solid tumor staging (17, 18). In this study, in cases of microcarcinoma, LN-level II metastasis was detected in 25.6%. Moreover, the risk of LN-level II metastasis increased when the diameter was >2 cm (odds ratio [OR] =1.629).
Previous studies have shown that PTC located in the upper pole is more prone to lateral lymph nodes and skip metastases (19, 20). Lee reported that all cases of skip metastases occurred in patients with “upper” thyroid tumors (21). In our study, 52 out of 675 patients had skip metastasis (52/675, 7.70%) and nine cases occurred only in level II. Among them, eight were located on the upper pole with one on the middle pole. The nomogram shows that the risk of LN-level II metastasis significantly increased when the tumor was located in the upper pole. According to Qubain et al. (22), different thyroid gland locations have different lymphatic drainage pathways. Lymphatic drainage occurs in the upper direction when the tumors are located in the upper third. Furthermore, the lymphatic drainage of the lower third or isthmus is primarily collected by the lymphatic vessels accompanying the inferior thyroid artery and flows into the lateral lymph nodes through the CLN. Lymphatic drainage is inferiorly and to the nearby lymph nodes when tumors are located in the middle third.
US is the most routinely recommended primary preoperative evaluation for lymph node metastases in patients with PTC (8, 9). Metastatic lymph nodes tend to be large, round, cystic, hypoechoic, calcified, and hypervascularized with a loss of hilar architecture (23, 24). US has higher sensitivities and specificities in the lateral neck than in the central neck, but the false-positive rates of US should not be ignored (25, 26). As reported by Patron et al., occult metastasis may occur at LN-level II (14). In our study, 26.10% (136/521) of patients with clinical lymph node-negative (cN0) and positive in the central compartment (cN1a) had LN-level II metastasis on postoperative pathological examinations. This was primarily because, once CLNM presence was confirmed on intraoperative frozen section biopsy, the extent of LND was further expanded. Notably, accuracy is limited by the experience of clinicians, the function of instruments, and the heterogeneity of patients. Therefore, we believe it is inappropriate to decide whether to dissect LN-level II only based on the US-reported LN status preoperatively.
The thyroid gland is supplied by a rich vascular network. Some studies have revealed that there appears to be a trend toward more lymph nodes and distant metastasis and poor prognosis when the vascular invasion is present (27–29). Similarly, vascular invasion is an independent risk factor for LLNM (30, 31). Our study demonstrated a significantly increased risk of LN level II metastasis when the vascular invasion was present (OR=6.759), as shown in the nomogram, with a high predictive value, and dissection of LN level II was necessary.
Most studies suggest that CLNM is associated with LLNM, whereas LLNM is associated with LN-level II metastasis (32–34). Thus, we calculated the correlation between the rate in each compartment and LN-level II metastasis. Koo et al. studied 52 patients with cN1b but no suspicions of metastasis before the operation and found that occult LN-level II metastasis was associated with positive LN-levels III and IV simultaneously (32). In contrast, their results showed that if preoperative US did not indicate LN-level III metastasis, LN-level II was considered negative. In our study, LN-level II was positive even though LN-level III was negative. The possible reasons for this anomaly are as follows. First, we included patients with cN1b that did not partition the lateral compartment; therefore, there were patients with preoperative LN-level II positivity. Second, the sample size was large. Nevertheless, it is undeniable that LN levels III and IV metastases are significantly correlated with LN-level II positivity.
This study demonstrated that the prognosis in patients with LN-level II metastasis was worse than in those who were LN-level II negative. Several studies have shown that lymph node metastasis is associated with an increased risk of recurrence and reoperation rate (5, 35). Recurrence may not necessarily affect mortality but may bring great psychological pressure to the patient. In addition, the risk of reoperation has increased. Therefore, it is necessary to identify the risk factors for LN-level II metastasis and formulate an appropriate surgical approach during the first operation.
We established a predictive model for LN-level II metastasis, based on the above risk factors and their effects on prognosis, with a C-index of 0.815 and 0.804 by bootstrapping validation; further demonstrating a high discriminative ability to guide clinicians. The limitation of the model is the lack of external center validation to evaluate the prediction model’s accuracy.
We have gone through extensive dissection and rough zoning for precise assessment and selective cleaning. In this process, we continue to summarize and strive for more precise dissection to minimize recurrence and complications.
This study had several limitations. Several clinicopathological features, such as the histological subtype and BRAF mutation, were not identified. This was a single-center retrospective study with a lack of prospective validation. Furthermore, we are currently examining more detailed pathological reports and follow-up data.
Our study found that the largest tumor diameter >20 mm, located in the upper pole, with cN1 and vascular invasion. The rates of CLNM, LN-levels III, and IV metastases were independent of LN-level II metastatic predictors. Subsequently, we developed a prediction model to better guide lymph node dissection in patients with PTC. LN-level II dissection should be individualized according to preoperative and intraoperative clinicopathological data.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors made substantial contributions to the conception or design of the work. Material preparation, data collection, and analysis were performed by CH, YZ. The first draft of the manuscript was written by CH, XS and DH revised it critically for important intellectual content. All author contributed to the article and approved the submitted version.
Acknowledgments
The authors appreciate the support of the staff that was involved in the preparation of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang JY, Yu FF, Shang YN, Ping ZG, Liu L. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine (2020) 68(1):163–73. doi: 10.1007/s12020-020-02207-6
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the united states, 1974–2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
4. Mazzaferri EL, Massoll N. Management of papillary and follicular (differentiated) thyroid cancer: New paradigms using recombinant human thyrotropin. Endocr Relat Cancer (2002) 9:227–47. doi: 10.1677/erc.0.0090227
5. Yuksel UM, Turanli S, Acar Y, Berberoglu U. The prognostic factors for clinical N1b patients in thyroid papillary carcinoma. J Cancer Res Ther (2019) 15(3):681–5. doi: 10.4103/jcrt.JCRT_1011_16
6. King JM, Corbitt C, Miller FR. Management of lateral cervical metastases in papillary thyroid cancer: patterns of lymph node distribution. Ear Nose Throat J (2011) 90:386–9. doi: 10.1177/014556131109000814
7. Sivanandan R, Soo KC. Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg (2001) 88(9):1241–4. doi: 10.1046/j.0007-1323.2001.01843.x
8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
9. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN guidelines insights: Thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw (2018) 16(12):1429–40. doi: 10.6004/jnccn.2018.0089
10. Perros P, Boelaert K, Colley S, Evans C, Williams GR. British Thyroid association guidelines for the management of thyroid cancer. Clin Endocrinol (2014) 81:1–122. doi: 10.1111/cen.12515
11. Lee BJ, Wang SG, Lee JC, Son SM, Kim IJ, Kim YK. Level IIb lymph node metastasis in neck dissection for papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg (2007) 133(10):1028–30. doi: 10.1001/archotol.133.10.1028
12. Cho GS, Han MW, Sang YK, Nam SY, Choi SH. Predictive factors of level II lymph node metastasis in N1b papillary thyroid carcinoma patients. Korean J Otorhinolaryngology-Head Neck Surg (2009) 52(11):899–904. doi: 10.3342/kjorl-hns.2009.52.11.899
13. Keum HS, Ji YB, Kim JM, Choi WH, Ahn YH, Tae K. Optimal surgical extent of lateral and central neck dissection for papillary carcinoma located in one lobe with clinical lateral lymph node metastasis. World J Surg Oncol (2012) 10(1):221–6. doi: 10.1186/1477-7819-10-221
14. Patron V, Bedfert C, Le CG, Aubry K, Jegoux F. Pattern of lateral neck metastases in N0 papillary thyroid carcinoma. BMC Cancer (2011) 11:8. doi: 10.1186/1471-2407-11-8
15. Kang BC, Roh JL, Lee JH, Cho KJ. Candidates for limited lateral neck dissection among patients with metastatic papillary thyroid carcinoma. World J Surg (2014) 38(4):863–71. doi: 10.1007/s00268-013-2361-6
16. Caron NR, Tan YY, Ogilvie JB, Triponez F, Reiff ES, Kebebew E, et al. Selective modified radical neck dissection for papillary thyroid cancer-is level I, II and V dissection always necessary? World J Surg (2006) 30(5):833–40. doi: 10.1007/s00268-005-0358-5
17. Song M, Huang Z, Wang S, Huang J, Wu Z. Predictive factors of lateral lymph node metastasis in conventional papillary thyroid carcinoma. Gland Surg (2020) 9(4):1000–7. doi: 10.21037/gs-20-482
18. Patron V, Hitier M, Bedfert C, Metreau A, Dugue A, Jegoux F. Predictive factors for lateral occult lymph node metastasis in papillary thyroid carcinoma. Eur Arch Otorhinolaryngol (2013) 270(7):2095–100. doi: 10.1007/s00405-012-2305-z
19. Dou Y, Hu D, Chen Y, Xiong W, Xiao Q, Su X. PTC located in the upper pole is more prone to lateral lymph node metastasis and skip metastasis. World J Surg Oncol (2020) 18(1):188. doi: 10.1186/s12957-020-01965-x
20. Back K, Kim JS, Kim JH, Choe JH. Superior located papillary thyroid microcarcinoma is a risk factor for lateral lymph node metastasis. Ann Surg Oncol (2019) 26(12):3992–4001. doi: 10.1245/s10434-019-07587-2
21. Lee YS, Shin SC, Lim YS, Lee JC, Wang SG, Son SM, et al. Tumor location-dependent skip lateral cervical lymph node metastasis in papillary thyroid cancer. Head Neck (2014) 36(6):887–991. doi: 10.1002/hed.23391
22. Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery (2002) 131(3):249–56. doi: 10.1067/msy.2002.120657
23. Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab (2007) 92(9):3590–4. doi: 10.1210/jc.2007-0444
24. Rosário PWS, de Faria S, Bicalho L, Alves MFG, Borges MAR, Purisch S, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med (2005) 24(10):1385–9. doi: 10.7863/jum.2005.24.10.1385
25. Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol (2013) 39(2):191–6. doi: 10.1016/j.ejso.2012.07.119
26. Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: Comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid (2008) 18(4):411–8. doi: 10.1089/thy.2007.0269
27. Gardner RE, Tuttle RM, Burman KD, Haddady S, Truman C, Sparling YH, et al. Prognostic importance of vascular invasion in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg (2000) 126:309–12. doi: 10.1001/archotol.126.3.309
28. Falvo L, Catania A, D’Andrea V, Marzullo A, Giustiniani MC, de Antoni E. Prognostic importance of histologic vascular invasion in papillary thyroid carcinoma. Ann Surg (2005) 241(4):640–6. doi: 10.1097/01.sla.0000157317.60536.08
29. Xu MS, Li J, Wiseman SM. Major vessel invasion by thyroid cancer: a comprehensive review. Expert Rev Anticancer Ther (2019) 19(2):191–203. doi: 10.1080/14737140.2019.1559059
30. Fraser S, Zaidi N, Norlen O, Glover A, Kruijff S, Sywak M, et al. Incidence and risk factors for occult level 3 lymph node metastases in papillary thyroid cancer. Ann Surg Oncol (2016) 23(11):3587–92. doi: 10.1245/s10434-016-5254-8
31. So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
32. Koo BS, Seo ST, Lee GH, Kim JM, Choi EC, Lim YC, et al. Prophylactic lymphadenectomy of neck level II in clinically node-positive papillary thyroid carcinoma. Ann Surg Oncol (2010) 17:1637–41. doi: 10.1245/s10434-010-0958-7
33. Xiao GZ, Gao L. Central lymph node metastasis: is it a reliable indicator of lateral node involvement in papillary thyroid carcinoma? World J Surg (2010) 34(2):237–41. doi: 10.1007/s00268-009-0347-1
34. Lan X, Sun W, Zhang H, Dong W, Wang Z, Zhang T. A meta-analysis of central lymph node metastasis for predicting lateral involvement in papillary thyroid carcinoma. Otolaryngol Head Neck Surg (2015) 153:731–8. doi: 10.1177/0194599815601412
Keywords: papillary thyroid carcinoma, level II, lymph node metastasis, risk factors, prediction model
Citation: Huang C, Hu D, Zhuang Y and Su X (2022) Risk factors and prediction model of level II lymph node metastasis in papillary thyroid carcinoma. Front. Oncol. 12:984038. doi: 10.3389/fonc.2022.984038
Received: 01 July 2022; Accepted: 07 December 2022;
Published: 20 December 2022.
Edited by:
Ting Zhang, The First Affiliated Hospital of China Medical University, ChinaReviewed by:
Ruifeng Liu, Capital Medical University, ChinaHao Zhang, China Medical University, China
Copyright © 2022 Huang, Hu, Zhuang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinliang Su, c3V4aW5saWFuZ0AyMWNuLmNvbQ==
 Chun Huang
Chun Huang Daixing Hu
Daixing Hu Yuchen Zhuang1
Yuchen Zhuang1 Xinliang Su
Xinliang Su