- 1Department of General Practice, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 3Department of Pathology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Multiple neuroendocrine tumors (M-NETs) are rare in the rectum and there is no consensus on their characteristics and treatments. Here, we report 15 cases of rectal M-NETs and review the previous literature. We discuss the clinical characteristics, endoscopic features and pathological features of rectal M-NETs, aiming to analyze the treatments and follow-up strategies in combination with these characteristics. We retrospectively reviewed and analyzed the data of 15 patients with rectal M-NETs who were diagnosed and treated at Beijing Friendship Hospital, Capital Medical University. Their clinical data, endoscopic findings, pathological features and treatments were analyzed. Follow-up evaluations and literature review were performed. In all, 14 male (93.3%) and 1 female (6.7%) were recruited. The average age at diagnosis was 55.7 years. The clinical manifestations include asymptomatic in 9 patients (60.0%), defecation habits changes in 2 patients (13.3%), anal distension in 2 patients (13.3%), and abdominal distension in 2 patient (13.3%). The largest tumor diameter ≤10mm was found in 13 patients (86.7%) and >10mm in 2 patients (13.3%). All of the lesions originated from the mucous or submucosa layer. WHO grades were all NET G1. The number of tumors diagnosed by pathology in 13 patients was consistent with that observed by endoscopy, while more lesions were observed by pathology than endoscopy in two patients. Lymph node metastasis occurred in 1 patient (6.7%), and vascular or lymphatic invasion occurred in 9 patients (60.0%). Among the 13 patients with the largest tumor diameter being ≤10mm, lymphovascular invasion occurred in 8 patients (61.5%). And among the 2 patients with the largest tumor diameter of >10mm, lymphovascular invasion occurred in 1 patient (50.0%). 14 patients underwent endoscopic resection and 1 underwent surgical excision. Postoperative follow-up was achieved in 13 patients and no recurrence or metastasis was found. The true number of rectal M-NETs may be more than seen under endoscopy. Rectal M-NETs is associated with a high risk of metastasis; therefore, treatment and surveillance strategies should be more radical than single lesion.
Introduction
The incidence of neuroendocrine neoplasm (NEN) is increasing in the world. Gastrointestinal and pancreatic neuroendocrine neoplasm (GEP-NEN) is the most common. Rectal NENs (r-NENs) represent 12%–27% of all gastrointestinal NENs and 1%–2% of all rectal tumors are neuroendocrine (1). According to the degree of differentiation, NEN can be divided into well differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs) (2). Most of rectal NETs are single; multiple neuroendocrine tumors (M-NETs) are rare. It is reported that the incidence of rectal M-NETs is between 2% and 4.5% (3).
Rectal NETs are usually accidentally found in screening endoscopies and only a few patients have symptoms. Common symptoms include changes in defecation habits, hematochezia and abdominal pain, but these symptoms may have nothing to do with tumors (4). There are no specific physical exams and lab results for M-NETs. The choice of treatment for rectal NETs depends on the size of the tumors, the depth of invasion and the presence of distant metastasis (3). However, due to the rarity of rectal M-NETs, most of the literature related to M-NETs in the past appeared in the form of case reports. There is no consensus on their characteristics and treatments. Therefore, it is necessary to accumulate similar cases. In this study, we will report 15 cases of rectal M-NETs in our hospital and review the previous literature, aiming to discuss treatments and follow-up strategies in combination with clinical, endoscopic and pathological features.
Materials and methods
This study was approved by the ethics committees of Beijing Friendship Hospital, Capital Medical University. We retrospectively reviewed and analyzed the data of patients with rectal M-NETs found under endoscopy and confirmed by pathology and treated in Beijing Friendship Hospital, Capital Medical University between November 2012 and May 2022. A total of 15 patients were included in this study. We collected clinical data of the cases, including gender, age at diagnosis, clinical symptoms, tumor marker test results, endoscopic features, pathological and immunohistochemistry results and treatments. The number, location, size and invasion depth of the lesions were evaluated by professional endoscopists under electronic colonoscopy and endoscopic ultrasonography. Biopsy or surgical specimens were evaluated by pathologists and immunohistochemical staining and special staining were performed to confirm the diagnosis and evaluate lymphovascular invasion and lymph node metastasis.
Patients were advised to follow up in the outpatient department after operation and the follow-up period depends on the characteristics of the lesions. Recurrence and metastasis were monitored by abdominal and pelvic enhanced CT or electronic colonoscopy. The follow-up results were based on the latest outpatient medical record or electronic examination report, and the patients without postoperative reexamination records were followed up by telephone to find out the specific situation. The follow-up time was from surgical treatment to the latest outpatient visit.
We reviewed the literature related to rectal M-NETs in PubMed up to May 2022. The search command used in the search was (neuroendocrine tumors) and (multiple) and (rectal or rectum). Cases with complete clinical data and pathologically confirmed as M-NETs were included. When there was no full text of the article, or the clinical data of the cases reported in the article was incomplete or the patient were not pathologically diagnosed as M-NETs, it was excluded.
Results
Patient demographics and clinical characteristics
Detailed demographics and clinical characteristics are summarized in Table 1. Of the fifteen cases, 93.3% (14/15) of the patients were male. The age at diagnosis ranged from 44 to 80 years, with an average age of 55.7 years. Among them, 9 cases (60.0%) were asymptomatic and lesions were accidentally found during routine physical examination, 2 cases (13.3%) (case 3 and 15) showed changes in defecation habits, 2 cases (13.3%) (case 2 and 10) showed a feeling of anal distension, and the other 2 cases (13.3%) (case 11 and case14) showed abdominal distension. None of the 15 patients had a family history of neuroendocrine tumors or digestive tract tumors. One case (case12) had undergone partial hepatectomy and orthotopic liver transplantation for hepatocellular carcinoma.
Laboratory and imaging features
Serum tumor markers were examined in all 15 patients, including 11cases in the normal range, 1 case of elevated CA125 (case5), 1 case of elevated CA199 (case10), 1 case of elevated AFP (case12), and 1 case of elevated CA724 (case13). All patients underwent abdominal-pelvic enhanced CT or abdominal MRI, where no pancreatic or upper gastrointestinal lesions were found, and no lymph node or distant metastasis were found.
Endoscopic findings
Among the 15 patients, only case15 had lesions in both the rectum and sigmoid colon, and the remaining 14 patients had lesions confined to the rectum. The location of the lesions ranged from 2 to 12 cm from the anal verge, with a median distance of 8 cm. The number of lesions under endoscopy ranged from 2 to 8, with a median number of 3. The size of lesions ranged from 2 to 14mm. The largest tumor diameter ≤10mm was found in 13 cases (86.7%), except that the maximum diameter of case 2 was 14mm and case 14 was 12mm. Most lesions showed as a yellow or white bulge under endoscopy, and the surface mucosa was smooth (Figure 1). Uniquely in case 15, one adenocarcinoma lesion and 5 small smooth polyps with yellow and smooth surface mucosa were scattered in the rectum and sigmoid colon. Endoscopic ultrasonography was performed in case1, 2, 4, 6, 7, 9, 10, 12, 13 and 14, and the results showed that all of the lesions originated from the mucous layer or submucosa, and the internal echo was homogeneous, showing low or moderately low echo, and the boundary was clear (Figure 2).
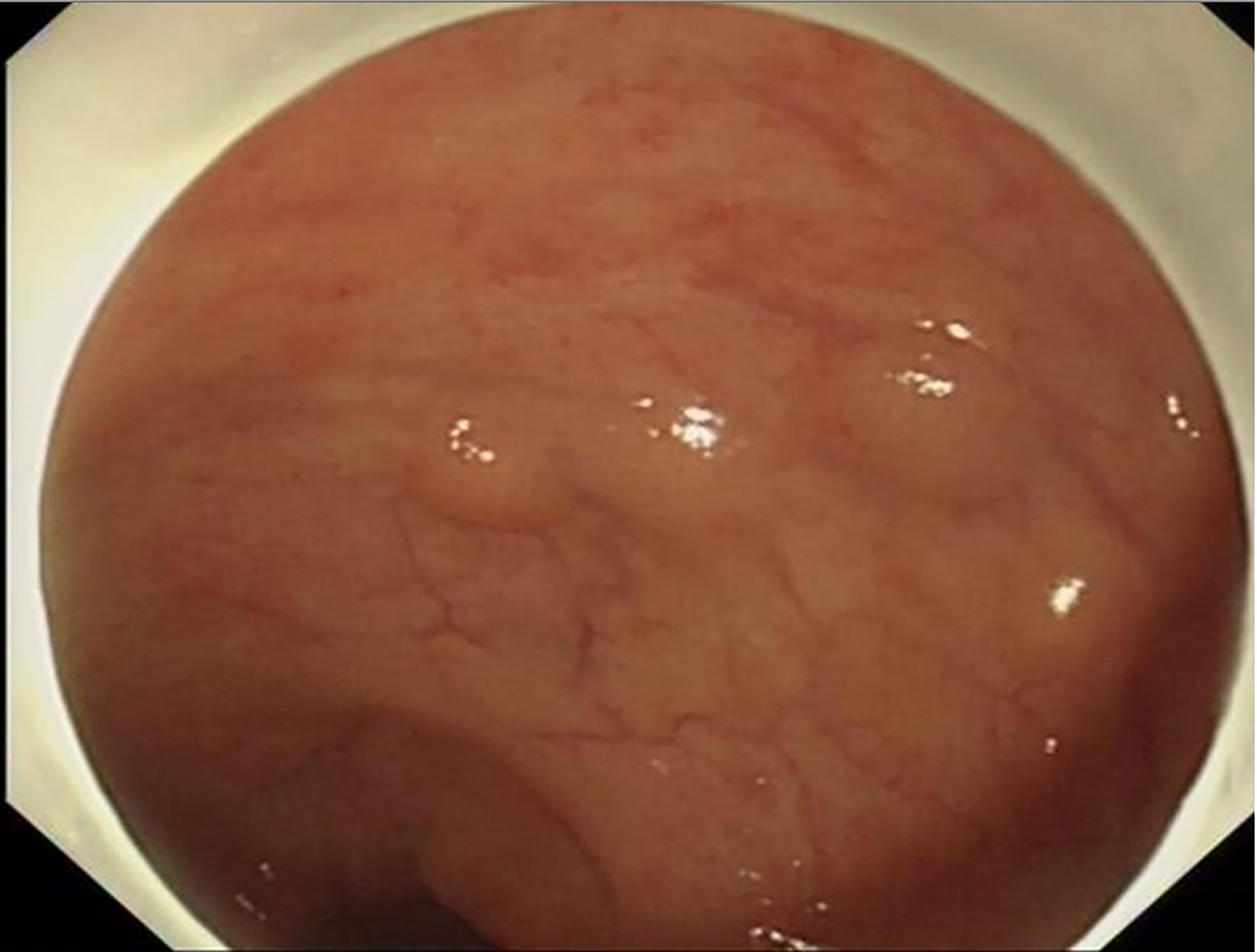
Figure 1 Most NET lesions showed as yellow or white bulge under endoscopy, and the surface mucosa was smooth.
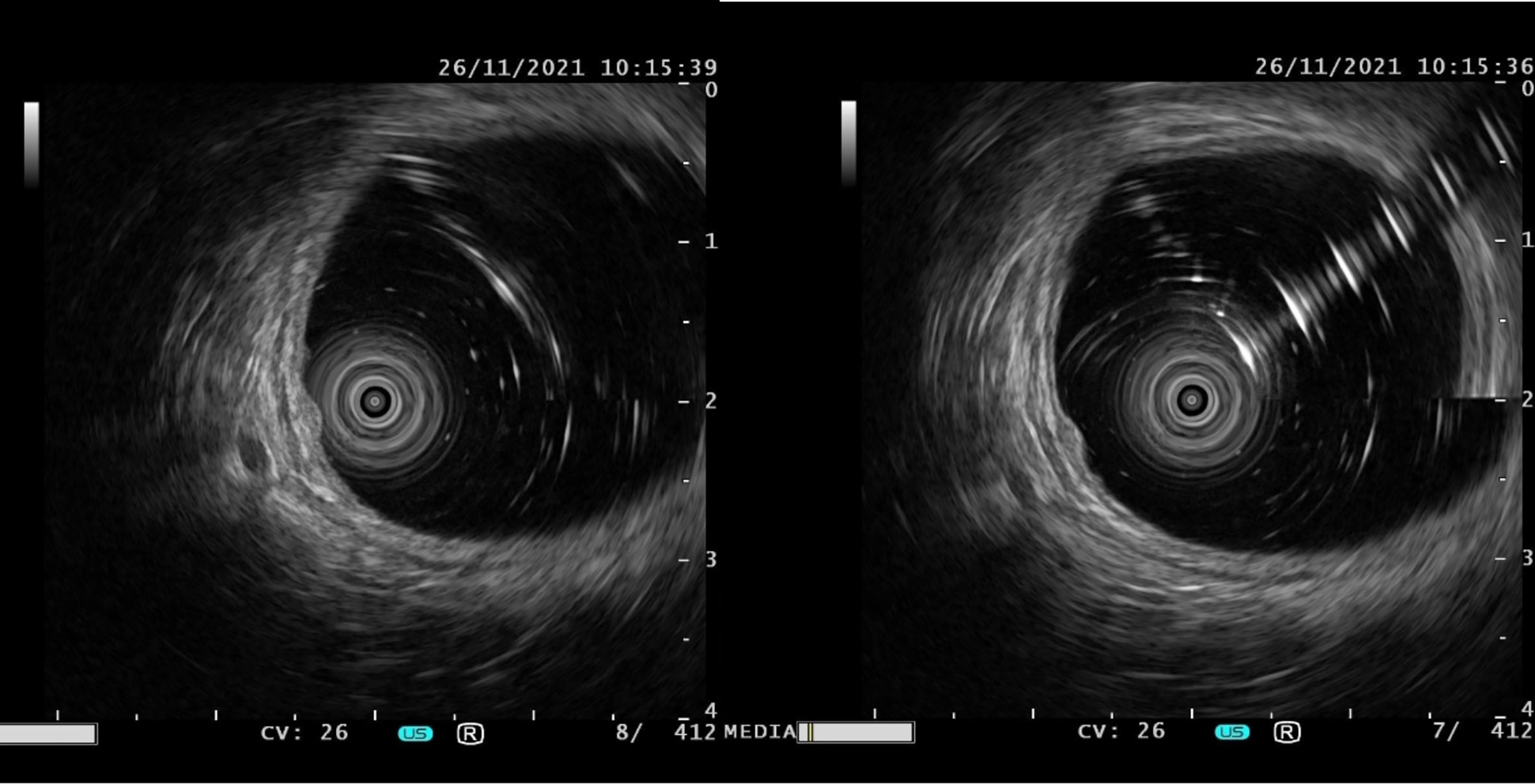
Figure 2 The typical manifestations of lesions under endoscopic ultrasonography were originated from the mucous layer or submucosa, and the internal echo was homogeneous, showing low or moderately low echo, and the boundary was clear.
Pathological, immunohistochemical and special staining findings
The NETs in all patients were limited to the mucosa and submucosa, and the postoperative pathological WHO grades were all NET G1. Uniquely, the adenocarcinoma lesion of case 15 was consisted of moderately differentiated adenocarcinoma and NET, with a clear boundary between the two components. The number of tumors found by pathology was not completely consistent with that seen by endoscopy. Specifically, case 11 showed 7 bulges under endoscopy, and an additional small lesion was observed by pathology. There were 6 lesions scattered in the rectum of case 15, and more lesions were found in the postoperative pathological examination. Only case 15 showed lymph node metastasis and neuroendocrine tumors (G1) were found in 11 of 31 periintestinal lymph nodes. The rate of lymph node metastasis was 6.7% (1/15). Among the 15 patients, lymphovascular invasion occurred in 9 patients (60.0%), including vascular invasion in 7 patients (46.7%) and lymphatic invasion in 7 patients (46.7%). Among the 13 patients with the largest tumor diameter ≤10mm, lymphovascular invasion occurred in 8 patients (61.5%). And among the 2 patients with the largest tumor diameter >10mm, lymphovascular invasion occurred in 1 patient (50.0%).
Treatment and follow-up
Eleven patients were treated by endoscopic submucosal dissection (ESD), one patient was treated by endoscopic mucosal resection (EMR) (case 8), one patient was treated by endoscopic submucosal excavation (ESE) (case 12), one patient was treated by EMR and ESD (case 14), and one patient was treated by total mesorectal excision (TME) and transanal endoscopic microsurgery (TEM) (case 15).
Two patients lost follow-up (case 8 and 12). Postoperative follow-up was achieved in the rest thirteen patients. Seven patients were re-examined by abdominal-pelvic enhanced CT or electronic colonoscopy regularly, and no recurrence or metastasis was found. Five patients (case 1, 9, 10, 11 and 14) did not undergo any re-examination after treatment, some because the re-examination time was not reached, and some were unable to see a doctor because of the epidemic of novel coronavirus. One patient (case 13) underwent partial enterectomy in other hospital five months after our treatment and the re-examination time has not come until the deadline of this article.
Discussion
With the popularity of colonoscopy screening, the detection rate of early rectal NET has increased significantly in recent years. 80-90% of rectal NET are diagnosed incidentally by colonoscopy (5). Rectal NET usually occurs singly, while multiple tumors are rare. Although progress has been made in the study of rectal NEN, many aspects of the disease are still unclear, in part due to its rarity. To study the clinical characteristics of rectal M-NETs, we reviewed the previous case reports and found nineteen articles reporting a total of 27 cases with relatively complete clinical data. The data of the patients in the literature are shown in Table 2. The number of M-NETs in these cases ranged from 2 to 69, except that some cases only describe “numerous”. Most patients were treated with local resection. Among them, 2 cases complicated with UC, 6 cases complicated with other gastrointestinal malignancies.
General and clinical characteristics
Only a small number of patients have carcinoid syndrome, and often are related to peptides and hormones secreted from the primary site. Most M-NETs patients have no typical symptoms, it is difficult to predict accurate prevalence (8). Only one case (18) was clearly reported with carcinoid syndrome. Rectal NET can occur at any age, but it is more common in middle-aged and elderly patients. The average age at diagnosis was 58.2 years old, which is about 10 years younger than other types of tumors (22). In previous case reports of M-NETs, the age of onset ranged from 32 to 70 years, and the average age was 55.0 years, which was almost consistent with the 15 patients we reported (55.7 years), and was close to the median age of onset of NET. The male-to-female ratio of rectal NET is about 1.06, and the African American to white ratio is about 0.34 (22). But these ratios in M-NETs have not been reported by large sample experiment. The male-to-female ratio in the previous reports we reviewed was 22:5, and the ratio of our study was 14:1, which is quite different from the male-to-female ratio of single NET. Perhaps we can boldly speculate that men are more likely to develop rectal M-NETs. A case-control study had reported that family history of cancer was an important risk factor for NETs, and that genetic factors may contribute the most to their occurrence (23). However, only one article (14) reported M-NETs in monozygotic twins, and none of our patients had a family history of cancer. This may be attributed to the small sample size of our study.
Tumor markers
Rectal M-NETs have not been reported to be associated with specific tumor markers. A recent study collected six serum tumor markers, including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9), cancer antigen 72-4 (CA72-4), cytokeratin 19 fragment 21-1 (Cyfra21-1) and neuron-specific enolase (NSE), and compared the distribution of all these serum tumor markers in the study participants. Among the six serum tumor markers, only NSE was significantly associated with the histologic grades in GEP-NETs (24). Only one case (5) reported the increase of CEA. Including our 15 patients, there was no increase in NSE, which may be related to the small sample size. The relationship between tumor markers and M-NETs remains to be supported by more data.
Endoscopic characteristics
Rectal M-NETs have unique appearance under endoscopy, but there is also the possibility of misdiagnosis and missed diagnosis. Typically, NETs appear as smooth, round submucosal nodules with yellow or white mucosa under endoscope. A few present as sessile polyps (25). Rectal NETs are often located in the mid-rectum (4 to 8 cm from the anorectal junction) (26), which is consistent with our data of M-NETs. Therefore, if a lesion is observed at this location, the endoscopist needs to be alert to whether the lesion is a neuroendocrine tumor and to carefully observe whether there are more lesions. About 80% of NETs are 10 mm or less in size and are contained within the submucosa at the time of diagnosis (27). Multiple biopsies had been reported to be the initial diagnosis of M-NETs. Additionally, rectal M-NETs may have more lesions that cannot be recognized by endoscope. In 12 previously reported cases (Table 2), the number of lesions under endoscopy was less than the actual number; this is largely because lesions were in the initial or intermediate stages of carcinoid tumor development (defined as micronests) with small size and almost normal mucosa and difficult to find under endoscopy (7). The compression of gas or water injection during colonoscopy procedure may also be one of the reasons for the differences. In our report, case 11 and case 15 were also observed more lesions by pathology. When rectal NET is found by accident, the possibility of multiple lesions should be considered, and other sites should be carefully examined.
Risk factors of lymph node metastasis
Initial diagnosis and assessment of risk factors of metastasis might be important consideration in rectal NETs, as early detection and prompt prediction of metastasis can help patients prolong their survival time. Risk factors associated with lymph node metastasis of rectal NETs mentioned in previous studies mainly include number of lesions, size of the tumor (larger than 10 mm), invasion depth, and the lymphovascular invasion. In 1987, Kanter (21) first proposed that the number of rectal NETs is related to lymphatic metastasis. Patients with multiple tumors are at a high risk for lymph node metastasis regardless of tumor size. Compared with isolated tumors less than 1 cm in size, multiple rectal carcinoid tumors measuring less than 1 cm have a higher incidence of lymph node metastasis (10% to 22.7%) (28). These conclusions were later confirmed in a single-center retrospective study (29). Kasuga (30) et al. found that lymph node metastasis occurred in 11.7% of cases (limited to the mucosa or submucosa) and 87.5% of cases (into or through the muscularis propria). The likelihood of lymph node metastasis increases with tumor size. In tumors smaller than 10mm, lymph node metastasis is a rare result, with an incidence of 1% ~ 10% (31–33). The rectal NEN with a maximum diameter of 1-2 cm has a lymph node metastasis rate of about 30% at the time of diagnosis, and about 40% in those ≥20 mm (27, 34). In the latest multicenter retrospective study, diameter > 11.5 mm and vascular infiltration were independently correlated with nodal involvement (35). It is reported that 22.7% of patients with multiple rectal NETs whose diameter is less than 10mm have lymphatic metastasis (36). Even in tumors 5 to 9 mm in size, 8.4% of metastases were reported (31). As a result, even if the rectal NETs are small, CT, MRI, positron emission tomography, and endoscopic ultrasonography may be necessary to evaluate lymph nodes and distant metastases. In a multivariate analysis of patients with small rectal NETs (≤10 mm), only venous invasion was independently associated with metastasis (30).
In our reported patients and previously reported cases, no distant metastases occurred. Lymph node metastasis occurred in 6 patients in the past reports (Table 2) and 1 patient in our study. The tumor sizes of these 7 cases were all ≤10mm and were limited to the submucosa. Among the 7 patients, only 2 cases had lymphovascular invasion, although the incidence of lymphovascular invasion was high in patients with rectal M-NETs. And the number of lesions ranged from 9 to 69. This result supports the conclusion that the number of rectal NET is related to Lymph node metastasis, regardless of the size of the lesions.
Treatment strategies
Since M-NETs is associated with a high risk of lymph node metastasis, monitoring and treatment strategies should be different from those of single rectal NETs (3). For single rectal NET, small lesions (≤10 mm) without muscularis propria invasion and no metastases found on imaging, complete resection with negative margins under endoscopy is the first choice for treatment (37). However, treatment for multiple rectal neuroendocrine tumors measuring ≤10 mm is not clear. Multiple rectal neuroendocrine tumors even if they are ≤10 mm in size without muscularis propria invasion may have malignant potential and can be effectively treated via radical resection (13). For rectal NETs larger than 20 mm, surgical resection is recommended due to the high metastatic risk and involvement of muscularis propria (34). The metastasis risk of rectal NETs of 10-19mm is reported to be 10-15%, and the treatment is controversial (26). The tumor should be fully evaluated by endoscopy and imaging examination, and then appropriate treatment can be discussed. Patients with lymphatic or venous invasion are at high risk of lymph node metastasis, so surgery resection with lymph node dissection should be performed as a radical therapy no matter the size and number of tumors (29).
There are various surgical methods, including EMR, ESD, TEM, anterior resection (AR), abdominoperineal resection (APR) and so on. EMR and ESD are considered to be good options for small NETs. Most NETs occur deep in the epithelial glands and form nodular lesions in the submucosa after penetrating the muscularis mucosa. Therefore, conventional endoscopic polypectomy (EMR and ESD) may be incomplete (16). In contrast, TEM enables wider resection and ensures better tumor outcomes for lesions with malignant potential (16). Among the 6 previously reported patients with lymph node metastasis (Table 2), only one patient (7) was treated with EMR, and the rest were treated with surgical radical resection. All patients with lesions >10mm, with lymphatic or venous invasion underwent surgical radical resection. In our study, 1 patient underwent radical enterectomy because of adenocarcinoma, 1 patient underwent enterectomy because of lymphovascular invasion and 13 patients underwent endoscopic resection. Among the 13 patients underwent endoscopic resection, lymphatic or venous invasion occurred in 7 patients, and endoscopic resection may be not enough for them. Fortunately, none of them relapsed until the last re-examination (some patients did not arrive for the re-examination time). As the follow-up time increases, we may prove that radical treatment may not be necessary.
Prognosis and surveillance
In order to reduce the psychological burden of patients and improve the overall quality of life, surveillance protocol is necessary. The diameter of the primary neoplasm, the number of positive nodes (≥ 5 as a cut-off) and the depth of invasion with the invasion of the muscolaris propria are considered to be the main independent predictors of metastatic spread and dismal prognosis (1). Patients with small (≤1 cm) and confined to the submucosa rectal NET have a 5-year survival of 98–100% and post-treatment monitoring is not recommended currently. While those with regional and distant metastases have a survival of 54–74% and 15–37% respectively. If a tumor has distant metastasis or regional lymph node metastasis, invades peritoneum or other organs, invades muscularis propria or size more than 2 cm should have 6-monthly surveillance for 5-10 years with CT of the chest, abdomen and pelvis. For rectal NETs 1–2cm in size, colonoscopy, EUS and MRI at 12 months is necessary (27). There is no statistical report on the survival rate and no surveillance protocol of M-NETs. However, it should be noted that in the case of M-NETs, relatively short-term endoscopy may be needed to avoid missing other residual NET lesions, and for highly invasive tumors, long-term rather than frequent monitoring is emphasized. In the recorded reports, the follow-up period ranged from 0 to 240 months, with a median follow-up time of 12 months. And all patients survived except 1 patient who died of a ruptured dissecting aneurysm. In our patients, 7 patients underwent regular postoperative re-examination. Our median follow-up time was 13.5 months, and no recurrence was found in all patients. 4 of the 9 patients with lymph node metastasis or lymphovascular invasion were followed up regularly after surgery, with an average follow-up time of 13.5 months. No metastasis or recurrence occurred. Rectal NET is a tumor with relatively slow growth. One of the disadvantages of our study is that the follow-up time is short and the reference value for evaluating patient survival rate is limited.
Multiple rectal neuroendocrine tumors co-occur with other disease
Rectal M-NETs can co-occur with other malignant tumors. Multiple primary malignant tumors (MPMN) refers to two or more kinds of malignant tumors in an individual, and there is no relationship between these tumors (38). In recent years, the number of reported cases has been increasing. However, there are relatively rare reports of rectal carcinoid and other malignant tumors. In fact, it is reported that up to 55% of patients with gastrointestinal NETs suffer from second primary malignant tumor (39). 6 patients in the articles we reviewed and our case 11 and 15 suffered from MPMN. Treatment is primarily focused on the tumor type that is more histologically aggressive or has predominant behavioural features.
Inflammatory bowel disease (IBD) may promote the development of NETs. Patients with IBD have increased risk of colorectal adenocarcinoma (40). In contrast, colorectal NETs are rarely reported in the context of IBD. Previous reports have questioned whether IBD and NET are related because the incidence of NETs in IBD patients is considered to be similar to that in non-IBD patients, and tumors mainly occur in parts of the intestine not affected by IBD (41). However, recent literature suggests that NETs may be more common in IBD patients than previously thought, and they may be responsive phenomena in IBD to various factors (42). It remains unclear how and to what extent inflammation promotes the development of NETs. In the literature we reviewed, there were two cases complicated with UC. But none of the 15 patients in our study had UC.
Conclusions
In summary, the true number of rectal M-NETs may be more than seen under endoscopy, mainly because some lesions were in the initial stage of carcinoid tumor development, small and similar to normal mucosa, so it’s difficult to find under endoscopy. Since M-NETs is associated with a high risk of lymph node metastasis, treatment and surveillance strategies should be more radical.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committees of Beijing Friendship Hospital, Capital Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SP, YZ, YWu and PL were responsible for the conception and design of the study. SP made data collection, data analysis and interpretation, and drafted the manuscript. YZ participated in the idea, protocol design, made English grammar corrections and critically revised important intellectual content. YWu and PL critically revised the important intellectual content and finally approved the version to be released. KZ reevaluated the pathological results. YZ, HZ, YWa, JW and CL were the endoscopists that performed the surgery and did data collection, data analysis, and interpretation. All authors agree to be accountable for the content of the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Digestive Medical Coordinated Development Center of Beijing Hospitals Authority No. XXT08. The funder had no role in study design, data collection, analysis and interpretation, decision to submit the article for publication, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gallo C, Rossi RE, Cavalcoli F, Barbaro F, Boškoski I, Invernizzi P, et al. Rectal neuroendocrine tumors: Current advances in management, treatment, and surveillance. World J Gastroenterol (2022) 28(11):1123–38. doi: 10.3748/wjg.v28.i11.1123
2. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol (2017) 29:11–6. doi: 10.1016/j.anndiagpath.2017.04.005
3. Park SS, Han N, Lee J, Chang HJ, Oh JH, Sohn DK. Multiple small, rectal neuroendocrine tumors with numerous micronests. J Dig Dis (2018) 19(9):572–5. doi: 10.1111/1751-2980.12645
4. Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol (2008) 9(1):61–72. doi: 10.1016/S1470-2045(07)70410-2
5. Park CS, Lee SH, Kim SB, Kim KO, Jang BI. Multiple rectal neuroendocrine tumors: Report of five cases. Korean J Gastroenterol (2014) 64(2):103–9. doi: 10.4166/kjg.2014.64.2.103
6. Manhal K, Christophe R, Radu B, Daniel L, Jeremy S, Alex K. Case report of multiple rectal neuroendocrine tumors in a context of ulcerative colitis. Int J Surg Case Rep (2022) 91:106760. doi: 10.1016/j.ijscr.2022.106760
7. Sasou S, Suto T, Satoh T, Tamura G, Kudara N. Multiple carcinoid tumors of the rectum: Report of two cases suggesting the origin of carcinoid tumors. Pathol Int (2012) 62(10):699–703. doi: 10.1111/j.1440-1827.2012.02852.x
8. Xie R, Fu KI, Chen SM, Tuo BG, Wu HC. Neurofibromatosis type 1-associated multiple rectal neuroendocrine tumors: A case report and review of the literature. World J Gastroenterol (2018) 24(33):3806–12. doi: 10.3748/wjg.v24.i33.3806
9. Ghassemi KA, Ou H, Roth BE. Multiple rectal carcinoids in a patient with neurofibromatosis. Gastrointest Endosc (2010) 71(1):216–8. doi: 10.1016/j.gie.2009.06.026
10. Saxe DH. Multiple carcinoid of the rectum; case report. Am J Surg (1953) 86(5):553–5. doi: 10.1016/0002-9610(53)90355-9
11. Maruyama M, Fukayama M, Koike MA. Case of multiple carcinoid tumors of the rectum with extraglandular endocrine cell proliferation. Cancer (1988) 61(1):131–6. doi: 10.1002/1097-0142(19880101)61:1<131::aid-cncr2820610123>3.0.co;2-g
12. Rigdon RH, Fletcher DE. Multiple argentaffin tumors (Carcinoids) of the rectum. Am J Surg (1946) 71:822–2. doi: 10.1016/0002-9610(46)90231-0
13. Maeda K, Koide Y, Katsuno H, Matsuoka H. Malignant potential of multiple rectal carcinoid tumors measuring ≤=10 mm. Asian J Surg (2020) 43(10):1033–4. doi: 10.1016/j.asjsur.2020.06.008
14. Doi M, Ikawa O, Taniguchi H, Kawamura T, Katsura K. Multiple rectal carcinoid tumors in monozygotic twins. Clin J Gastroenterol (2016) 9(4):215–21. doi: 10.1007/s12328-016-0662-7
15. Haraguchi M, Kinoshita H, Koori M, Tsuneoka N, Kosaka T, Ito Y, et al. Multiple rectal carcinoids with diffuse ganglioneuromatosis. World J Surg Oncol (2007) 5:19. doi: 10.1186/1477-7819-5-19
16. Zhou JL, Lin GL, Zhao DC, Zhong GX, Qiu HZ. Resection of multiple rectal carcinoids with transanal endoscopic microsurgery: Case report. World J Gastroenterol (2015) 21(7):2220–4. doi: 10.3748/wjg.v21.i7.2220
17. Colbert PM. Primary carcinoids of the ileum and rectum. a simultaneous occurrence. JAMA (1976) 236(19):2201–3. doi: 10.1001/jama.1976.03270200039028
18. McNeely B, Owen DA, Pezim M. Multiple microcarcinoids arising in chronic ulcerative colitis. Am J Clin Pathol (1992) 98(1):112–6. doi: 10.1093/ajcp/98.1.112
19. Zhu Y, Wang M. [Double primary carcinoma of rectum: A case report]. Zhonghua Bing Li Xue Za Zhi (2006) 35(7):431. doi: 10.3760/j.issn:0529-5807.2006.07.016
20. Okamoto Y, Fujii M, Tateiwa S, Sakai T, Ochi F, Sugano M, et al. Treatment of multiple rectal carcinoids by endoscopic mucosal resection using a device for esophageal variceal ligation. Endoscopy (2004) 36(5):469–70. doi: 10.1055/s-2004-814386
21. Kanter M, Lechago J. Multiple malignant rectal carcinoid tumors with immunocytochemical demonstration of multiple hormonal substances. Cancer (1987) 60(8):1782–6. doi: 10.1002/1097-0142(19871015)60:8<1782::aid-cncr2820600819>3.0.co;2-t
22. Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer (1997) 79(4):813–29. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2
23. Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer (2008) 123(4):867–73. doi: 10.1002/ijc.23529
24. Li Y, Wu ZQ, Xu Q, Goyal H, Xu HG. Development and validation of novel nomograms using serum tumor markers for the prediction of preoperative histologic grades in gastroenteropancreatic neuroendocrine tumors. Front Oncol (2021) 11:681149. doi: 10.3389/fonc.2021.681149
25. Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology (2005) 128(6):1717–51. doi: 10.1053/j.gastro.2005.03.038
26. Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, Maione R, et al. Diagnosis and management of rectal neuroendocrine tumors (Nets). Diagn (Basel) (2021) 11(5):771–83. doi: 10.3390/diagnostics11050771
27. Basuroy R, Haji A, Ramage JK, Quaglia A, Srirajaskanthan R. Review article: The investigation and management of rectal neuroendocrine tumours. Aliment Pharmacol Ther (2016) 44(4):332–45. doi: 10.1111/apt.13697
28. Sumida T, Nagata S, Nagata S, Ougoshi H, Ishida Y, Kuwahara T, Tatsugami M, et al. Multiple rectal carcinoid tumors treated by endoscopic mucosal resection-report of a case. Gastroenterol Endosc (2005) 47(7):1419–24. doi: 10.11280/gee1973b.47.1419
29. Nishikawa Y, Chino A, Ide D, Saito S, Igarashi M, Takamatsu M, et al. Clinicopathological characteristics and frequency of multiple rectal neuroendocrine tumors: A single-center retrospective study. Int J Colorectal Dis (2019) 34(11):1887–94. doi: 10.1007/s00384-019-03405-z
30. Kasuga A, Chino A, Uragami N, Kishihara T, Igarashi M, Fujita R, et al. Treatment strategy for rectal carcinoids: A clinicopathological analysis of 229 cases at a single cancer institution. J Gastroenterol Hepatol (2012) 27(12):1801–7. doi: 10.1111/j.1440-1746.2012.07218.x
31. Takatsu Y, Fukunaga Y, Nagasaki T, Akiyoshi T, Konishi T, Fujimoto Y, et al. Short- and long-term outcomes of laparoscopic total mesenteric excision for neuroendocrine tumors of the rectum. Dis Colon Rectum (2017) 60(3):284–9. doi: 10.1097/DCR.0000000000000745
32. Al Natour RH, Saund MS, Sanchez VM, Whang EE, Sharma AM, Huang Q, et al. Tumor size and depth predict rate of lymph node metastasis in colon carcinoids and can be used to select patients for endoscopic resection. J Gastrointest Surg (2012) 16(3):595–602. doi: 10.1007/s11605-011-1786-1
33. Shin S, Maeng YI, Jung S, Small YCSA. Low-grade rectal neuroendocrine tumor with lateral pelvic lymph node metastasis: A case report. Ann Coloproctol (2022). doi: 10.3393/ac.2021.00899.0128
34. Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, et al. Enets consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology (2016) 103(2):139–43. doi: 10.1159/000443166
35. Ricci AD, Pusceddu S, Panzuto F, Gelsomino F, Massironi S, De Angelis CG, et al. Assessment of the risk of nodal involvement in rectal neuroendocrine neoplasms: The novara score, a multicentre retrospective study. J Clin Med (2022) 11(3):713–22. doi: 10.3390/jcm11030713
36. Tanaka H. Multiple rectal carcinoid tumors with 31 lesions, report of a case. STOMACH AND INTESTINE (2003) 38(8):1193–9.
37. Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, et al. Neuroendocrine and adrenal tumors, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(7):839–68. doi: 10.6004/jnccn.2021.0032
38. Copur MS, Manapuram S. Multiple primary tumors over a lifetime. Oncol (Williston Park) (2019) 33(7):629384.
39. Habal N, Sims C, Bilchik AJ. Gastrointestinal carcinoid tumors and second primary malignancies. J Surg Oncol (2000) 75(4):310–6. doi: 10.1002/1096-9098(200012)75:4<306::aid-jso14>3.0.co;2-3
40. Cohen-Mekelburg S, Schneider Y, Gold S, Scherl E, Steinlauf A. Advances in the diagnosis and management of colonic dysplasia in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) (2017) 13(6):357–62.
41. Greenstein AJ, Balasubramanian S, Harpaz N, Rizwan M, Sachar DB. Carcinoid tumor and inflammatory bowel disease: A study of eleven cases and review of the literature. Am J Gastroenterol (1997) 92(4):682–5.
Keywords: rectum, multiple, neuroendocrine tumors, metastasis, diagnosis, treatment, prognosis
Citation: Pang S, Zong Y, Zhang K, Zhao H, Wang Y, Wang J, Liu C, Wu Y and Li P (2022) Multiple rectal neuroendocrine tumors: An analysis of 15 cases and literature review. Front. Oncol. 12:996306. doi: 10.3389/fonc.2022.996306
Received: 17 July 2022; Accepted: 24 August 2022;
Published: 14 September 2022.
Edited by:
Jacques Waisberg, Faculdade de Medicina do ABC, BrazilReviewed by:
Weiguang Qiao, Southern Medical University, ChinaSara Massironi, San Gerardo Hospital, Italy
Copyright © 2022 Pang, Zong, Zhang, Zhao, Wang, Wang, Liu, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongdong Wu, d3V5b25nZG9uZzIwMThAc2luYS5jb20=; Peng Li, bGlwZW5nQGNjbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Shu Pang
Shu Pang Ye Zong2†
Ye Zong2† Yongjun Wang
Yongjun Wang Junxiong Wang
Junxiong Wang Peng Li
Peng Li