- 1Department of Medical Oncology, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
- 2Breast Surgery Unit, Pierantoni-Morgagni Hospital Forlì and Santa Maria delle Croci Hospital Ravenna, Forli, Italy
- 3Radiotherapy Unit, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
Approximately 6% of metastatic breast cancers arise de novo. While systemic therapy (ST) remains the treatment backbone as for patients with metachronous metastases, locoregional treatment (LRT) of the primary tumor remains a controversial method. The removal of the primary has an established role for palliative purposes, but it is unclear if it could also determine a survival benefit. Retrospective evidence and pre-clinical studies seem to support the removal of the primary as an effective approach to improve survival. On the other hand, most randomized evidence suggests avoiding LRT. Both retrospective and prospective studies suffer several limitations, ranging from selection bias and outdated ST to a small sample of patients. In this review we discuss available data and try to identify subgroups of patients which could benefit the most from LRT of the primary, to facilitate clinical practice decisions, and to hypothesize future studies design on this topic.
Introduction
Approximately 6% of metastatic breast cancers (BC) arise de novo (1). In these patients, systemic therapy (ST), based on hormone receptor (HR) and HER2 expression, is the pillar of treatment as for patients with metachronous metastases. However, the presence of the primary tumor raises questions among clinicians about the potential benefit deriving from a local approach. Palliative removal of the primary is an established procedure as it can relieve BC patients from pain, skin ulceration, bleeding, and infections.
Surgery can also be useful to remove an ST-resistant primary tumor in presence of responsive metastatic disease.
On the other hand, it is unclear if surgery of the primary, with eventual lymph node dissection and consolidative radiotherapy, translates into a survival benefit that could justify such an invasive approach.
Pre-clinical data suggest that locoregional therapy (LRT) could be beneficial by several mechanisms. First of all, tumor burden reduction may increase CD4 and CD8 cells, improving immunologic response to cancer (2, 3). It can also minimize the dissemination of metastatic BC stem cells from the primary tumor which may act as a source of seeding (4, 5). Furthermore, some data suggest that mesenchymal stem cells released from the bone marrow may populate primary tumor more efficiently compared to metastatic sites, enhancing the metastatic potential of primary tumor cells (6).
These biological assumptions were also supported by retrospective studies that showed an association between primary tumor resection and improved survival in patients with synchronous metastases (7–10).
However, in addition to the intrinsic limitation of retrospective evidence, it is important to note that the timing of surgery is rarely specified. Patients which underwent LRT of the primary and are defined metastatic afterward because of post-operative systemic staging could have a better prognosis compared to patients who were diagnosed as metastatic before surgery. The potential influence of this stage migration bias is also outpointed by a retrospective study by Bafford et al. which highlighted a survival benefit only in those patients who underwent surgery of the primary before a diagnosis of metastatic disease (11). Consequently, randomized studies were designed to verify this hypothesis (Table 1).
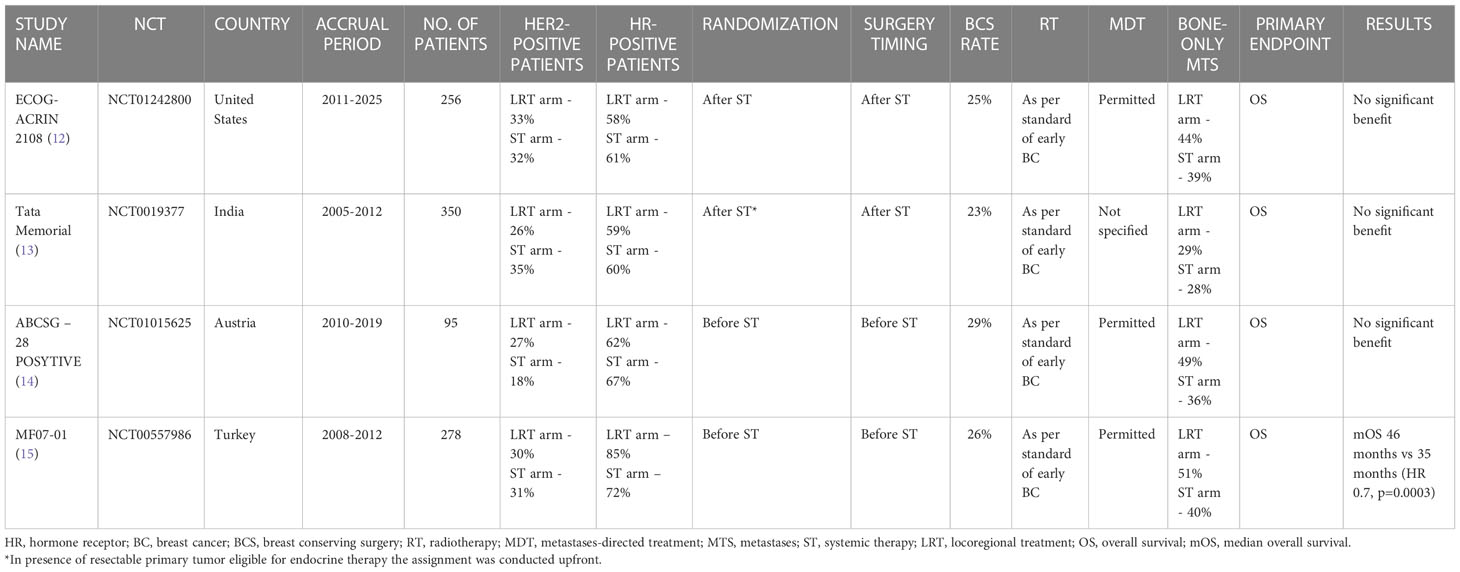
Table 1 Randomized controlled trials investigating the role of locoregional treatment of the primary tumor in de novo stage IV breast cancer patients.
Evidence from randomized trials
The most recent published study which investigated the impact of primary surgery on survival is the ECOG-ACRIN 2108. A total of 256 patients with metastatic BC who did not progress during 4-8 months of ST were assigned (from February 2011 to July 2015) to LRT of the primary plus ST or ST-only continuation. Overall Survival (OS) was chosen as the primary endpoint. The statistical analysis showed no difference in 3-year OS (68.4% vs 67.9%) (HR, 1.11; 90% CI, 0.82–1.52; p=0.57). No progression-free survival (PFS) difference was observed either; only locoregional progression was reduced in the LRT group (3-year rate: 16.3% v 39.8%; P < 0.001).
Subset analysis based on HR and HER2 status did not show any subgroup which benefited from the locoregional approach (16).
An open-label randomized controlled trial with a similar study design, conducted in Mumbai, compared LRT of the primary plus ST vs ST alone in a population of stage IV BC patients with de novo disease.
Patients with unresectable primary underwent chemotherapy before randomization while, in presence of a resectable primary tumor eligible for endocrine therapy, the assignment was conducted upfront. A total of 350 patients were randomized. The primary endpoint was OS as for the previous study. Even in this case, no statistically different median overall survival (mOS) was reported between the two groups: 19.2 months for the LRT group vs 20.5 months in the ST alone group (HR 1.04, 95% CI 0·81-1·34; p=0·79) (13).
However, it is worth noting that the reported mOS values were considerably lower in comparison to the previous trial, in which the mOS was about 55% in both groups (16). This discrepancy can be justified by the lack of tailored therapy in this Indian trial, such as HER2-directed therapy for HER-2 positive patients and endocrine therapy for HR-positive subtypes.
The ABCSG-28 POSYTIVE trial is another phase 3 randomized study with negative results but a different design. The random assignment of de novo stage IV BC patients was performed before ST and patients assigned to the intervention arm underwent upfront surgery followed by ST. Only 95 patients were included between 2011 and 2015. The mOS (primary endpoint) in the surgery plus ST arm was consistently lower compared to the ST-only arm (34.6 months vs 54.8 months, HR=0.0691, p=0.267). Whilst cT3 and cN2 tumors were more represented in the surgery arm (22.2% vs 6.7% and 15.6% vs 4.4% respectively), the two groups were balanced in relation to the ST schedule.
Even if the results of this trial seem unequivocal, it must be addressed that this study was stopped early due to poor recruitment, with consequently very low statistical power, and the control arm (ST alone) performed better than expected (54.8 months vs 24 months) (14).
The Turkish Federation’s MF07-01 trial is the unique randomized study that showed a survival benefit in favor of LRT.
Similarly to the ABCSG-28 POSYTIVE study, patients were randomized to upfront surgery followed by ST or ST alone. The statistical analysis demonstrated a benefit in OS at the median 40-months follow-up which was confirmed at 10-year follow-up: mOS for the LRT arm and ST-only arm was respectively 46 months and 35 months (HR 0.7, p=0.0003). However, the two groups were unbalanced for the BC subtype, as HR-positive disease was more represented in the LRT (86% vs 73%), and the control arm included more triple negative BC (18% vs 7%) (15, 17).
These data seem to rule out a potential role for LRT of the primary in de novo stage IV BC patients given that the majority of randomized studies did not show a survival benefit. However, these trials are not free from inherent limits, are heterogeneous and, last but not least, there are subgroups of patients which deserve in-depth analysis.
Oligometastatic vs polymetastatic disease
The oligometastatic disease is defined by the presence of no more than five metastatic lesions, assessed with high-resolution imaging and safely treatable with metastases-directed therapy (18).
The hypothesis that metastases-directed treatment (MDT) in oligometastatic disease could be beneficial is supported by retrospective and prospective data which showed long-term survival (19). The available randomized data rely only on two studies with conflicting results (20, 21). Waiting for data from numerous ongoing randomized trials, the current practice is to discuss oligometastatic BC patients in a multidisciplinary setting.
The chance to achieve long-term survival in oligometastatic BC patients legitimates an aggressive approach aimed at eradicating the detectable disease, making this subgroup of patients a suitable candidate for the surgery of the primary in case of de novo presentation.
Unfortunately, literature data regarding the survival impact of surgical resection of the primary in oligometastatic BC patients is lacking. This is probably due to the main use of LRT of the primary in clinical practice which is palliation.
In the ECOG-ACRIN 2108 trial, no survival difference was reported for oligometastatic patients (HR, 1.18; 95% CI, 0.38 to 3.67) which represented 16.3% of the study population (16).
A Similar result was shown in the Indian randomized trial in which 25% of patients had less than four metastases and were balanced between the intervention and control arm (13).
In the MF07-01 trial, the only randomized trial showing a survival benefit deriving from LRT, there was no clear distinction between oligometastatic and polymetastatic disease but no survival benefit for patients with solitary lung/liver metastases was reported for those treated with LRT, probably due to the poor representation of this subgroup (15).
However, when assessing the impact of local treatment of the primary in oligometastatic BC we cannot ignore if the limited metastases were treated with MDT. The aforementioned randomized trials generally did not specify this information, but it can be noted that MDT was generally permitted in accordance with clinical practice. In the Turkish trial it is only mentioned that irradiation rates and surgical interventions to metastatic sites were similar among the two arms (17).
On the other hand, even if the majority of randomized trials investigating MDT impact in oligometastatic BC does not include patients with uncontrolled primary (22–24), there are some exceptions (25, 26) in which it does not constitute an exclusion criterion if accessible to curative-intent treatment.
If the population of oligometastatic BC patients with synchronous metastases will be properly represented in these trials, we may have some insight into the potential survival benefit deriving from the combination of LRT of the primary and MDT with eradication intent.
Bone-only disease
Metastatic BC patients with bone-only disease have an excellent prognosis compared to those with visceral involvement, showing an mOS that can exceed 5 years after the detection of the metastases (27, 28), thus prompting clinicians to consider the possibility of primary tumor surgery during the therapeutic process.
The BOMET MF 14-01 is a prospective multicenter registry study that evaluated the role of LRT of the primary tumor in addition to ST in de novo stage IV BC patients with bone-only metastases. This study included 505 patients and highlighted a prolonged survival in the median 3-year follow-up in favor of LRT of the primary (HR 0.40, p<0.0001). At 34-months median follow-up, 85 (35.4%) patients in the ST-only group and 28 (10.5%) in the LRT group died (29).
The potential survival benefit of LRT is also suggested by retrospective evidence (30–32).
In a large cohort retrospective study including 3956 BC patients with bone metastases, surgery of the primary tumor in addition to ST significantly improved OS with a median survival of 50 months versus 31 months in ST-only patients (p<0.001) (33).
Regarding randomized trials, in the Turkish study, 51% and 40% of patients presented bone-only metastases in the LRT group and ST group respectively. Notably, 23% and 15% of patients had solitary bone metastasis in the LRT and ST groups respectively. At unplanned subgroup analysis patients with solitary bone, metastasis showed a lower risk of death if treated with LRT in addition to ST (15).
Conversely, in the ECOG-ACRIN 2108 trial, which did not demonstrate any benefit of LRT in addition to ST, patients with bone-only disease (37.7%) were less represented (12).
Even if available data are not enough to conclude that LRT of the primary tumor is beneficial among patients with bone-only disease, we can affirm that this population deserves more focus.
The STEREO-OS trial, which is aimed to demonstrate that Stereotactic Body Radiotherapy of the metastases can improve survival in patients with 1 to 3 bone metastases, will also include patients with a primary tumor accessible to curative-intent treatment and might provide some information in this regard.
What locoregional treatment modality should we prefer?
As previously mentioned, the rationale behind LRT of the primary tumor includes the reduction of tumor burden and the removal of cancer stem cells which may propagate the disease (7).
This implies that a complete removal of locoregional disease could be of utmost importance to achieve the best survival benefit, justifying surgery with clear margins and excision of involved axillary nodes.
In a retrospective study conducted by Rapiti et al. showing a survival benefit in metastatic BC patients treated with surgery of the primary, women with positive surgical margins exhibited the same survival as non-surgery ones (32).
The presence of free margins was generally associated with better survival in retrospective studies, while no clear difference was found between mastectomy and breast-conserving surgery (34–36).
Similarly, BC patients with synchronous metastases seem to benefit from axillary dissection in presence of nodal involvement even though evidence on this topic is lacking (32, 34).
As for surgery with clean margins and axillary dissection for patients with nodal metastases, local radiotherapy represents an important method in the pursuit of complete removal of locoregional disease in stage IV BC patients, considering its role in local relapse prevention and mortality reduction in early BC setting (37).
Some retrospective evidence pointed out that the omission of radiotherapy was associated with worse survival (36).
Looking at randomized studies, in the negative study published in 2022 by Khan et al., LRT consisted of surgery and radiotherapy as per standard of early-stage BC. Radiotherapy use followed NCCN guidelines and axillary dissection was reserved for patients with involved lymph nodes.
Among 107 patients which underwent surgery, 75 (70.1%) received mastectomy and 32 (29.9%) breast-conserving surgery. Radiotherapy has been employed in 44 patients (58.7%) after mastectomy and in 27 patients (84.4%) after breast-conserving surgery.
Notably, of 125 patients randomly assigned to the LRT arm, 18 (14.4%) did not receive it for various reasons, ranging from physician advice to progressive disease.
Furthermore, of 131 patients assigned to the ST-only arm, 22 (16.8%) received surgery, which was permitted for palliation, with postoperative radiotherapy in 10 cases (12). This displacement raises concerns about the negative result of the trial. LRT for primary tumor consisted of mastectomy or breast-conserving surgery with eventual postoperative radiotherapy also in the other three randomized trials.
In the Turkish trial 102 patients (74%) underwent a mastectomy, 36 (26%) breast-conserving surgery and the majority of patients received axillary dissection (92.8%) (15).
Given that also the timing of LRT could influence the outcomes, it is worth noting that in the ECOG-ACRIN 2108 trial surgery was carried out after a period of ST, while in the Turkish and ABCSG-28 POSYTIVE trials it was performed upfront.
Thus, considering the OS benefit reported in the Turkish trial (17), it might be thought that upfront surgery could provide some advantage over delayed one. In addition, this hypothesis is in accordance with previously reported biological assumptions. An upfront LRT could be convenient as it can stop the dissemination of metastatic BC stem cells from the primary earlier in the disease course (4, 5).
Surgery was performed upfront also in the ABCSG-28 POSYTIVE trial and no survival benefit for LRT was reported. However, it must be considered that this study was underpowered as it was stopped early due to poor recruitment (14).
Biological subtypes
It is well known that, between BC subtypes, HR-positive tumors feature the best prognosis (38). As HR-positive disease tends to progress with more indolence, it is not uncommon to consider primary surgery in de novo stage IV patients in clinical practice.
In confirmation of this trend, HR-positive de novo stage IV BC patients demonstrated to benefit the most from LRT in retrospective studies (11, 39–41).
Some retrospective evidence seems also to support the use of LRT in HER2-positive subtype (7, 40, 42, 43).
In the randomized trial by Soran et al., 86% of patients were HR-positive, 30% HER2-positive, and 7% triple negative in the LRT arm, while in the ST-only arm, 73% were HR-positive, 28% HER-2 positive, and 18% triple negative.
The imbalance in biological subtypes distribution, with aggressive ones being more represented in the ST-only arm, questions the positive result of this trial.
However, in accordance with retrospective evidence, an unplanned subgroup analysis showed a benefit in OS for HR-positive patients (15).
The exploratory post hoc subgroup analyses of the ECOG-ACRIN 2108 trial, which was well balanced for disease subtype distribution, reported similar results across all the subgroups except for disease subtype: LRT was clearly unfavorable for triple negative patients (HR 3.33) (12).
Based on this data, HR-positive BC seems to be the best candidate for LRT in presence of synchronous metastases, while for triple-negative tumors primary surgery could be even detrimental. Any opinion on HER2-positive patients must be weighed with caution as HER2-directed therapy was not used with the same frequency in these studies.
In addition, we should consider that the usual classification of BC subtypes is being revolutionized due to the introduction of HER2-low subtype, which is forcing us to reconsider the treatment approach in every setting of BC (44).
Modern therapy implications
ST for metastatic BC patients has dramatically evolved over the last twenty years for every disease subtype.
The recent introduction of cyclin-dependent kinase 4/6 inhibitors for metastatic HR-positive BC treatment has carried to PFS and OS improvement, further ameliorating the prognosis of this indolent subgroup (45).
Even if HER2 expression results in a more aggressive disease with a poor prognosis, the use of HER2-targeted therapy led to outstanding survival benefit in these patients. In particular, the combination of trastuzumab, pertuzumab, and docetaxel increased the number of HER2-positive long survivors with an 8-year survival rate of 37% for patients treated with dual HER-2 blockade therapy (46).
The breakthrough of antibody-drug conjugates is the best example of modern ST progress. Trastuzumab deruxtecan is changing the treatment paradigm of both HER2-positive (47, 48) and HER2-low (48) disease and sacituzumab govitecan are improving triple negative and HR-positive BC survival (49).
However, most retrospective and prospective studies investigating the role of LRT of the primary tumor in stage IV BC included patients treated with outdated ST.
The example of the open-label randomized trial conducted at Tata Memorial Hospital in India is explicative. In this study, anti-HER2 therapy was omitted in approximately 92% of HER2-positive patients (13).
On one hand, LRT of the primary tumor and modern ST seem the perfect partners for an aggressive approach aimed at eradicating the disease and reaching long-term survival. On the other hand, the development of highly effective systemic drugs may mitigate the benefits of primary tumor surgery, thus making it useless for survival benefit improvement. It is also possible that both hypotheses are true, but for different groups of patients.
The association of LRT and ST could also have a synergistic effect. Immune checkpoint inhibitors, which boost the immune response against cancer cells by targeting either programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1), are establishing themselves in triple-negative disease, becoming the first line of therapy in association with chemotherapy in case PD-L1 positive disease (50, 51).
Pre-clinical data suggest that tumor promotes metastasis by systemic inflammation and cytotoxic CD8+ T cell effector function suppression (52). At the same time surgery of the primary tumor led to the rebound of antibody and cell-mediated response, restoring immunocompetence and increasing CD4 and CD8 cells in mice with metastatic BC (2, 3). Consequently, the combination of immune checkpoint inhibitors and LRT of the primary tumor could determine a strong immune response with enhanced tumor response.
Final considerations
Retrospective studies seem consistent in supporting LRT for de novo stage IV BC patients (Table 2). However, retrospective data suffer from several limitations. The selection bias is one of the most relevant as patients who were candidates for LRT was younger, had better access to care, and a lower burden of disease (53). In addition, it is also plausible that these patients underwent more aggressive ST, thus unbalancing survival outcomes (54).
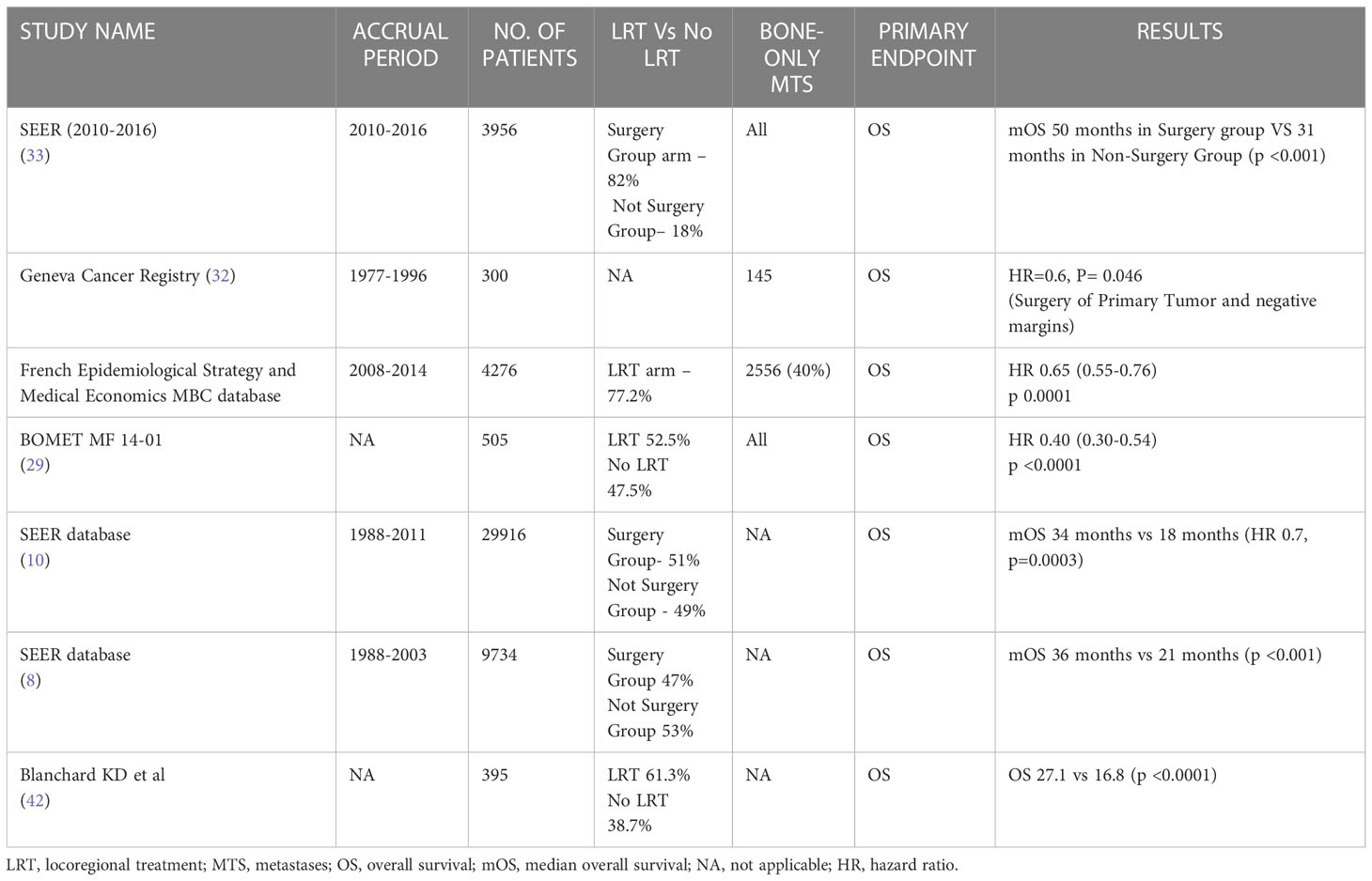
Table 2 Main Retrospective studies and outcome in locoregional treatment of the primary tumor in the novo stage IV breast cancer patients.
Even though some preclinical data provide a rationale for LRT of the primary there are also concerns about the possibility that surgery may lead to cancer cells shedding into the circulation (55), a hypothesis that seems consistent with the increased incidence of distant metastases in patients which underwent LRT, highlighted in the randomized trial by Badwe et al. (13).
Randomized trials did not support LRT of the primary altogether, as confirmed by a metanalysis by Reinhorm et al. (56), but, as previously discussed, they suffer major limitations as well, ranging from outdated ST to a small sample of patients.
We must take these results with caution, and we must not label LRT of the primary as a pointless technique, also considering that advances in ST and radiotherapy/surgery methodic require continuous testing of the possible benefit deriving from LRT in stage IV BC.
We should identify the best candidate for LRT and design randomized trials accordingly. Based on the retrospective evidence and the randomized Turkish trial, oligometastatic patients, with bone-only disease and HR-positive disease could be the best candidates for studies investigating the role of LRT in stage IV BC. Regarding oligometastatic patients, the combination of LRT of the primary and metastases-directed therapy, aimed at complete eradication of detectable disease, should be investigated. This aggressive approach in combination with highly effective modern ST could provide long-term survival and, in some cases, even the cure for metastatic BC patients (Figure 1).
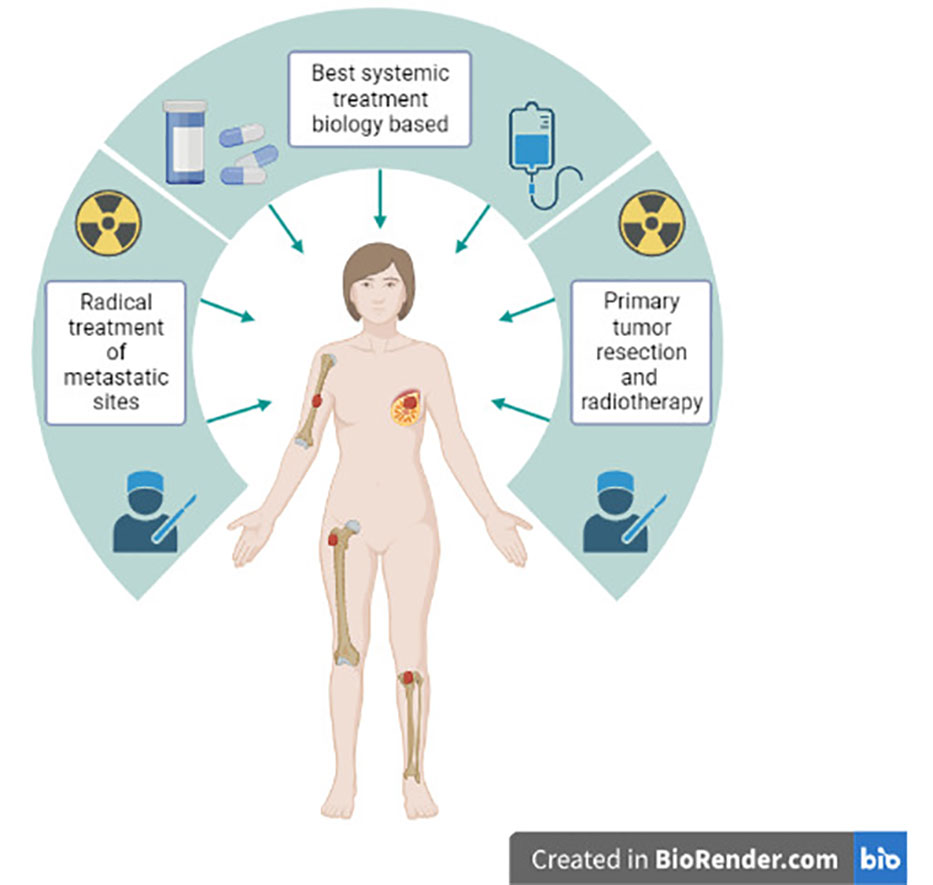
Figure 1 Aggressive approach combining modern systemic therapy, locoregional treatment of the primary tumor and metastases-directed therapy in stage IV de novo oligometastatic breast cancer patients.
The best timing for LRT of the primary remains an issue. Upfront surgery might be the correct approach according to the potential role of metastatic BC stem cells dissemination from the primary and the significant OS benefit observed in the randomized trial by Soran et al., in which metastatic BC patients underwent upfront surgery (4, 5, 15).
On the other hand, upfront surgery could represent an overtreatment for those patients destined to progress early in the disease course.
In this context, biomarkers, such as circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA), could help us characterize the metastatic disease.
In a retrospective analysis including 2436 patients with stage IV BC, a CTCs threshold of 5 cells per 7.5 ml was able to differentiate aggressive from indolent metastatic disease (57). ctDNA percentages were correlated with prognosis as well, with high levels being associated with shorter OS (58, 59).
Metastatic BC patients with high CTCs or ctDNA levels could be at higher risk of fast disease progression and, consequently, the rationale behind LRT of the primary tumor in those patients might be invalidated. Thus, the implementation of these biomarkers for patients’ stratification in future studies is suitable.
Results of two randomized trials investigating the role of LRT in de novo stage IV BC are awaited (60, 61). In addition, a single-arm trial investigating the role of palbociclib and LRT combination in HR-positive/HER2-negative metastatic BC is still recruiting (62).
Conclusions
The purpose of our review is to underline the limitations and strengths of LRT of the primary tumor, to design future randomized trials, more precisely and accurately. The design of new randomized clinical trials should include modern ST, a properly selected population, and new biomarkers are strongly encouraged.
Meanwhile, in the absence of robust evidence, LRT of the primary tumor should be discussed in a multidisciplinary context for every patient with de novo stage IV BC
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
UD received honoraria for advisory boards or speaker fees for Pfizer, BMS, MSD, PharmaMar, Astellas, Bayer, Ipsen, Roche, Novartis, Clovis, GSK, AstraZeneca, Institutional research grants from AstraZeneca, Sanofi and Roche. MP has received advisory board fees from Novartis.
All other authors confirm that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist (2005) 10(S3):20–9. doi: 10.1634/theoncologist.10-90003-20
2. Rashid OM, Nagahashi M, Ramachandran S, Graham L, Yamada A, Spiegel S, et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surg (United States) (2013) 153(6):771–8. doi: 10.1016/j.surg.2013.02.002
3. Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res (2004) 64(6):2205–11. doi: 10.1158/0008-5472.CAN-03-2646
4. Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci USA (2010) 107(42):18115–20. doi: 10.1073/pnas.1006732107
5. Norton L, Massagué J. Is cancer a disease of self-seeding? Nat Med (2006) 12(8):875–8. doi: 10.1038/nm0806-875
6. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature (2007) 449(7162):557–63:7162. doi: 10.1038/nature06188
7. Gera R, Chehade HELH, Wazir U, Tayeh S, Kasem A, Mokbel K. Locoregional therapy of the primary tumour in de novo stage IV breast cancer in 216 066 patients: A meta-analysis. Sci Rep (2020) 10(1):1–11. doi: 10.1038/s41598-020-59908-1
8. Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988-2003 SEER data. Ann Surg Oncol (2007) 14(8):2187–94. doi: 10.1245/s10434-007-9438-0
9. Lang JE, Tereffe W, Mitchell MP, Rao R, Feng L, Meric-Bernstam F, et al. Primary tumor extirpation in breast cancer patients who present with stage IV disease is associated with improved survival. Ann Surg Oncol (2013) 20(6):1893. doi: 10.1245/s10434-012-2844-y
10. Vohra NA, Brinkley J, Kachare S, Muzaffar M. Primary tumor resection in metastatic breast cancer: A propensity-matched analysis, 1988-2011 SEER data base. Breast J (2018) 24(4):549–54. doi: 10.1111/tbj.13005
11. Bafford AC, Burstein HJ, Barkley CR, Smith BL, Lipsitz S, Iglehart JD, et al. Breast surgery in stage IV breast cancer: impact of staging and patient selection on overall survival. Breast Cancer Res Treat (2008) 115(1):7–12. doi: 10.1007/s10549-008-0101-7
12. Khan SA, Zhao F, Solin LJ, Goldstein LJ, Cella D, Basik M, et al. A randomized phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: A trial of the ECOG-ACRIN research group (E2108). J Clin Oncol (2020) 38(18_suppl). doi: 10.1200/JCO.2020.38.18_suppl.LBA2
13. Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: An open-label randomised controlled trial. Lancet Oncol (2015) 16(13):1380–8. doi: 10.1016/S1470-2045(15)00135-7
14. Fitzal F, Bjelic-Radisic V, Knauer M, Steger G, Hubalek M, Balic M, et al. Impact of breast surgery in primary metastasized breast cancer: Outcomes of the prospective randomized phase III ABCSG-28 POSYTIVE trial. Ann Surg (2019) 269(6):1163–9. doi: 10.1097/SLA.0000000000002771
15. Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Primary surgery with systemic therapy in patients with de Novo stage IV breast cancer: 10-year follow-up; protocol MF07-01 randomized clinical trial. J Am Coll Surg (2021) 233(6):742–751.e5. doi: 10.1016/j.jamcollsurg.2021.08.686
16. Khan SA, Zhao F, Goldstein LJ, Cella D, Basik M, Golshan M, et al. Early local therapy for the primary site in De novo stage IV breast cancer: Results of a randomized clinical trial (EA2108). J Clin Oncol (2022) 40(9):978–87. doi: 10.1200/JCO.21.02006
17. Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: Protocol MF07-01. Ann Surg Oncol (2018) 25(11):3141–9. doi: 10.1245/s10434-018-6494-6
18. Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol (2020) 148:157–66. doi: 10.1016/j.radonc.2020.04.003
19. Makhlin I, Fox K. Oligometastatic breast cancer: Is this a curable entity? a contemporary review of the literature. vol. 22. Curr Oncol Rep (2020) 22(2):15. doi: 10.1007/s11912-020-0867-2
20. Palma DA, Olson R, Harrow S, Gaede S, v. LA, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol (2020) 38(25):2830–8. doi: 10.1200/JCO.20.00818
21. Chmura SJ, Winter KA, Al-Hallaq HA, Borges VF, Jaskowiak NT, Matuszak M, et al. NRG-BR002: A phase IIR/III trial of standard of care therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical ablation for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol (2019) 37(15_suppl):TPS1117–TPS1117. doi: 10.1200/JCO20193715_supplTPS1117
22. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic (1-3 metastases).
25. Thureau S, Marchesi V, Vieillard MH, Perrier L, Lisbona A, Leheurteur M, et al. Efficacy of extracranial stereotactic body radiation therapy (SBRT) added to standard treatment in patients with solid tumors (breast, prostate and non-small cell lung cancer) with up to 3 bone-only metastases: study protocol for a randomised phase III trial (STEREO-OS). BMC Cancer (2021) 21(1):117. doi: 10.1186/s12885-021-07828-2
26. Krug D, Vonthein R, Illen A, Olbrich D, Barkhausen J, Richter J, et al. Metastases-directed radiotherapy in addition to standard systemic therapy in patients with oligometastatic breast cancer: Study protocol for a randomized controlled multi-national and multi-center clinical trial (OLIGOMA). Clin Transl Radiat Oncol (2021) 28:90–6. doi: 10.1016/j.ctro.2021.03.012
27. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer (1987) 55(1):61–6. doi: 10.1038/bjc.1987.13
28. Zengel B, Kilic M, Tasli F, Simsek C, Karatas M, Ozdemir O, et al. Breast cancer patients with isolated bone metastases and oligometastatic bone disease show different survival outcomes. Sci Rep (2021) 11(1):20175. doi: 10.1038/s41598-021-99726-7
29. Soran A, Dogan L, Isik A, Ozbas S, Trabulus DC, Demirci U, et al. The effect of primary surgery in patients with De novo stage IV breast cancer with bone metastasis only (Protocol BOMET MF 14-01): A multi-center, prospective registry study. Ann Surg Oncol (2021) 28(9):5048–57. doi: 10.1245/s10434-021-09621-8
30. Pons-Tostivint E, Kirova Y, Lusque A, Campone M, Geffrelot J, Mazouni C, et al. Survival impact of locoregional treatment of the primary tumor in De novo metastatic breast cancers in a Large multicentric cohort study: A propensity score-matched analysis. Ann Surg Oncol (2018) 26(2):356–65:2. doi: 10.1245/s10434-018-6831-9
31. Wang K, Shi Y, Li ZY, Xiao YL, Li J, Zhang X, et al. Metastatic pattern discriminates survival benefit of primary surgery for de novo stage IV breast cancer: A real-world observational study. Eur J Surg Oncol (2019) 45(8):1364–72. doi: 10.1016/j.ejso.2019.02.013
32. Rapiti E, Verkooijen HM, Vlastos G, Fioretta G, Neyroud-Caspar I, Sappino AP, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol (2006) 24(18):2743–9. doi: 10.1200/JCO.2005.04.2226
33. Huang Z, Zhou X, Tong Y, Zhu L, Zhao R, Huang X. Surgery for primary tumor benefits survival for breast cancer patients with bone metastases: a large cohort retrospective study. BMC Cancer (2021) 21(1):222. doi: 10.1186/s12885-021-07964-9
34. Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery (2002) 132(4):620–7. doi: 10.1067/msy.2002.127544
35. Kommalapati A, Tella SH, Goyal G, Ganti AK, Krishnamurthy J, Tandra PK. A prognostic scoring model for survival after locoregional therapy in de novo stage IV breast cancer. Breast Cancer Res Treat (2018) 170(3):677–85. doi: 10.1007/s10549-018-4802-2
36. Arciero C, Liu Y, Gillespie T, Subhedar P. Surgery and survival in patients with stage IV breast cancer. Breast J (2019) 25(4):644–53. doi: 10.1111/tbj.13296
37. Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet (2011) 378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2
38. McAndrew NP, Finn RS. Clinical review on the management of hormone receptor–positive metastatic breast cancer. JCO Oncol Pract (2022) 18(5):319–27. doi: 10.1200/OP.21.00384
39. Tan Y, Li X, Chen H, Hu Y, Jiang M, Fu J, et al. Hormone receptor status may impact the survival benefit of surgery in stage iv breast cancer: a population-based study. Oncotarget (2016) 7(43):70991–1000. doi: 10.18632/oncotarget.11235
40. Neuman HB, Morrogh M, Gonen M, van Zee KJ, Morrow M, King TA. Stage IV breast cancer in the era of targeted therapy: does surgery of the primary tumor matter? Cancer (2010) 116(5):1226–33. doi: 10.1002/cncr.24873
41. Thomas A, Khan SA, Chrischilles EA, Schroeder MC. Initial surgery and survival in stage IV breast cancer in the united states, 1988–2011. JAMA Surg (2016) 151(5):424. doi: 10.1001/jamasurg.2015.4539
42. Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg (2008) 247(5):732–8. doi: 10.1097/SLA.0b013e3181656d32
43. Chen PY, Cheng SHC, Hung CF, Yu BL, Chen CM. Locoregional therapy in luminal-like and HER2-enriched patients with de novo stage IV breast cancer. Springerplus (2013) 2(1):589. doi: 10.1186/2193-1801-2-589
44. Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers (Basel) (2021) 13(5):1–18. doi: 10.3390/cancers13051015
45. Piezzo M, Chiodini P, Riemma M, Cocco S, Caputo R, Cianniello D, et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: A systematic review and meta-analysis. Int J Mol Sci (2020) 21(17):1–17. doi: 10.3390/ijms21176400
46. Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol (2020) 21(4):519–30. doi: 10.1016/S1470-2045(19)30863-0
47. Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med (2022) 386(12):1143–54. doi: 10.1056/NEJMoa2115022
48. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. New Engl J Med (2022) 387(1):9–20. doi: 10.1056/NEJMoa2203690
49. Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. New Engl J Med (2021) 384(16):1529–41. doi: 10.1056/NEJMoa2028485
50. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet (2020) 396(10265):1817–28. doi: 10.1016/S0140-6736(20)32531-9
51. Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2020) 21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8
52. Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: friend or foe? J Immunother Cancer (2017) 5(1):79. doi: 10.1186/s40425-017-0283-9
53. Cady B, Nathan NR, Michaelson JS, Golshan M, Smith BL. Matched pair analyses of stage IV breast cancer with or without resection of primary breast site. Ann Surg Oncol (2008) 15(12):3384–95:12. doi: 10.1245/s10434-008-0085-x
54. Khan SA. Primary tumor resection in stage IV breast cancer: consistent benefit, or consistent bias? Ann Surg Oncol (2007) 14(12):3285–7. doi: 10.1245/s10434-007-9547-9
55. Tohme S, Simmons RL, Tsung A. Surgery for cancer: A trigger for metastases. Cancer Res (2017) 77(7):1548–52. doi: 10.1158/0008-5472.CAN-16-1536
56. Reinhorn D, Mutai R, Yerushalmi R, Moore A, Amir E, Goldvaser H. Locoregional therapy in de novo metastatic breast cancer: Systemic review and meta-analysis. Breast (2021) 58:173–81. doi: 10.1016/j.breast.2021.05.003
57. Cristofanilli M, Pierga JY, Reuben J, Rademaker A, Davis AA, Peeters DJ, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit Rev Oncol / Hematol (2019) 134:39–45. doi: 10.1016/j.critrevonc.2018.12.004
58. Sant M, Bernat-Peguera A, Felip E, Margelí M. Role of ctDNA in breast cancer. Cancers (Basel) (2022) 14(2):310. doi: 10.3390/cancers14020310
59. Fiste O, Liontos M, Koutsoukos K, Terpos E, Dimopoulos MA, Zagouri F. Circulating tumor DNA-based predictive biomarkers in breast cancer clinical trials: a narrative review. Ann Transl Med (2020) 23):1603–3. doi: 10.21037/atm-20-1175
60. Compare the efficacy of surgery combined with systemic therapy and pure systemic therapy in patients with stage IV breast cancer.
61. Shien T, Mizutani T, Tanaka K, Kinoshita T, Hara F, Fujisawa T, et al. A randomized controlled trial comparing primary tumor resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (JCOG1017 PRIM-BC). J Clin Oncol (2017) 35(15_suppl):TPS588–8. doi: 10.1200/JCO20173515_supplTPS588
Keywords: breast cancer, stage IV, primary tumor, locoregional treatment, surgery, radiotherapy
Citation: Merloni F, Palleschi M, Gianni C, Casadei C, Curcio A, Romeo A, Rocchi M, Cima S, Sirico M, Sarti S, Cecconetto L, Mariotti M, Di Menna G and De Giorgi U (2023) Locoregional treatment of de novo stage IV breast cancer in the era of modern oncology. Front. Oncol. 13:1083297. doi: 10.3389/fonc.2023.1083297
Received: 28 October 2022; Accepted: 09 January 2023;
Published: 30 January 2023.
Edited by:
Benedetta Pellegrino, University of Parma, ItalyReviewed by:
René Aloisio Da Costa Vieira, Barretos Cancer Hospital, BrazilYi Ren, Duke University, United States
Veronica Jones, City of Hope National Medical Center, United States
Copyright © 2023 Merloni, Palleschi, Gianni, Casadei, Curcio, Romeo, Rocchi, Cima, Sirico, Sarti, Cecconetto, Mariotti, Di Menna and De Giorgi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filippo Merloni, ZmlsaXBwby5tZXJsb25pQGlyc3QuZW1yLml0
 Filippo Merloni
Filippo Merloni Michela Palleschi
Michela Palleschi Caterina Gianni
Caterina Gianni Chiara Casadei
Chiara Casadei Annalisa Curcio2
Annalisa Curcio2 Antonino Romeo
Antonino Romeo Marita Mariotti
Marita Mariotti Ugo De Giorgi
Ugo De Giorgi